User login
Predicting Platinum Efficacy
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
E-cigarette flavorings foster cardiovascular dysfunction
Flavored tobacco products are popular among current smokers, including youth, and the flavorings have been deemed ingestible, but their impact on heart health has not been studied, wrote Jennifer Fetterman, PhD, of Boston University, and her colleagues. The report was published in Arteriosclerosis, Thrombosis, and Vascular Biology.
The researchers studied nine types of flavorings used in alternative tobacco products to assess their impact on cardiovascular health.
The first part of the study comprised a population of nine nonsmokers, six nonmenthol cigarette smokers, and six menthol cigarette smokers without cardiovascular disease. The researchers isolated venous endothelial cells from each participant.
Overall, cells from both nonmenthol and menthol cigarette smokers had significantly lower nitric oxide production compared with nonsmokers (P = .003 and P = .012, respectively). In addition, the flavoring compounds menthol and eugenol impaired nitric oxide production in the cells of healthy individuals.
“Increased inflammation and a loss of nitric oxide are some of the first changes to occur leading up to cardiovascular disease and events like heart attacks and stroke, so they are considered early predictors of heart disease,” Dr. Fetterman said in a statement, adding that the “findings suggest that these flavoring additives may have serious health consequences.”
All nine flavorings induced cell death at the highest concentration tested, ranging from 10 to 100 mmol/L).
The study findings were limited by several factors, primarily a lack of data on how heating the flavorings in the in vitro part of the study might have affected toxicity in the body, the researchers noted.
“Future studies will focus on how the toxicity of the flavorings is altered with heating and characterization of the levels obtained in the circulation after use of an e-cigarette,” they said.
However, data support the need for regulation and limits on the level of flavorings used in e-cigarettes and other tobacco products, they emphasized.
“These findings suggest that flavoring compounds induce endothelial cell dysfunction in human cells similarly to the abnormal function in active cigarette smokers,” the researchers noted.
The study was funded by the National Heart, Lung, and Blood Institute; Food and Drug Administration Center for Tobacco Products; and the American Heart Association. The researchers had no financial conflicts to disclose.
SOURCE: Fetterman J et al. Arterioscler Thromb Vasc Biol. 2018. doi: 10.1161/ATVBAHA.118.311156.
Flavored tobacco products are popular among current smokers, including youth, and the flavorings have been deemed ingestible, but their impact on heart health has not been studied, wrote Jennifer Fetterman, PhD, of Boston University, and her colleagues. The report was published in Arteriosclerosis, Thrombosis, and Vascular Biology.
The researchers studied nine types of flavorings used in alternative tobacco products to assess their impact on cardiovascular health.
The first part of the study comprised a population of nine nonsmokers, six nonmenthol cigarette smokers, and six menthol cigarette smokers without cardiovascular disease. The researchers isolated venous endothelial cells from each participant.
Overall, cells from both nonmenthol and menthol cigarette smokers had significantly lower nitric oxide production compared with nonsmokers (P = .003 and P = .012, respectively). In addition, the flavoring compounds menthol and eugenol impaired nitric oxide production in the cells of healthy individuals.
“Increased inflammation and a loss of nitric oxide are some of the first changes to occur leading up to cardiovascular disease and events like heart attacks and stroke, so they are considered early predictors of heart disease,” Dr. Fetterman said in a statement, adding that the “findings suggest that these flavoring additives may have serious health consequences.”
All nine flavorings induced cell death at the highest concentration tested, ranging from 10 to 100 mmol/L).
The study findings were limited by several factors, primarily a lack of data on how heating the flavorings in the in vitro part of the study might have affected toxicity in the body, the researchers noted.
“Future studies will focus on how the toxicity of the flavorings is altered with heating and characterization of the levels obtained in the circulation after use of an e-cigarette,” they said.
However, data support the need for regulation and limits on the level of flavorings used in e-cigarettes and other tobacco products, they emphasized.
“These findings suggest that flavoring compounds induce endothelial cell dysfunction in human cells similarly to the abnormal function in active cigarette smokers,” the researchers noted.
The study was funded by the National Heart, Lung, and Blood Institute; Food and Drug Administration Center for Tobacco Products; and the American Heart Association. The researchers had no financial conflicts to disclose.
SOURCE: Fetterman J et al. Arterioscler Thromb Vasc Biol. 2018. doi: 10.1161/ATVBAHA.118.311156.
Flavored tobacco products are popular among current smokers, including youth, and the flavorings have been deemed ingestible, but their impact on heart health has not been studied, wrote Jennifer Fetterman, PhD, of Boston University, and her colleagues. The report was published in Arteriosclerosis, Thrombosis, and Vascular Biology.
The researchers studied nine types of flavorings used in alternative tobacco products to assess their impact on cardiovascular health.
The first part of the study comprised a population of nine nonsmokers, six nonmenthol cigarette smokers, and six menthol cigarette smokers without cardiovascular disease. The researchers isolated venous endothelial cells from each participant.
Overall, cells from both nonmenthol and menthol cigarette smokers had significantly lower nitric oxide production compared with nonsmokers (P = .003 and P = .012, respectively). In addition, the flavoring compounds menthol and eugenol impaired nitric oxide production in the cells of healthy individuals.
“Increased inflammation and a loss of nitric oxide are some of the first changes to occur leading up to cardiovascular disease and events like heart attacks and stroke, so they are considered early predictors of heart disease,” Dr. Fetterman said in a statement, adding that the “findings suggest that these flavoring additives may have serious health consequences.”
All nine flavorings induced cell death at the highest concentration tested, ranging from 10 to 100 mmol/L).
The study findings were limited by several factors, primarily a lack of data on how heating the flavorings in the in vitro part of the study might have affected toxicity in the body, the researchers noted.
“Future studies will focus on how the toxicity of the flavorings is altered with heating and characterization of the levels obtained in the circulation after use of an e-cigarette,” they said.
However, data support the need for regulation and limits on the level of flavorings used in e-cigarettes and other tobacco products, they emphasized.
“These findings suggest that flavoring compounds induce endothelial cell dysfunction in human cells similarly to the abnormal function in active cigarette smokers,” the researchers noted.
The study was funded by the National Heart, Lung, and Blood Institute; Food and Drug Administration Center for Tobacco Products; and the American Heart Association. The researchers had no financial conflicts to disclose.
SOURCE: Fetterman J et al. Arterioscler Thromb Vasc Biol. 2018. doi: 10.1161/ATVBAHA.118.311156.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Nitric oxide production was impaired in cells exposed to compounds used in alternative tobacco products.
Major finding: Nitric oxide products were significantly lower in nonmenthol and menthol cigarette smokers compared with nonsmokers (P = .003 and P = .012, respectively).
Study details: The data come from nine nonsmokers, six menthol cigarette smokers, and six nonmenthol cigarette smokers, plus in vitro cells.
Disclosures: The study was funded by the National Heart, Lung, and Blood Institute; Food and Drug Administration Center for Tobacco Products; and the American Heart Association. The researchers had no financial conflicts to disclose.
Source: Fetterman J et al. Arterioscler Thromb Vasc Biol. 2018. doi: 10.1161/ATVBAHA.118.311156.
Ivosidenib active in R/R IDH1-mutated AML patients
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
FDA grants pembrolizumab accelerated approval for PMBCL
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
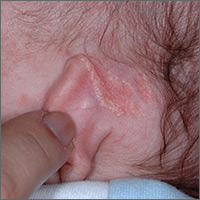
Growth behind infant’s ear
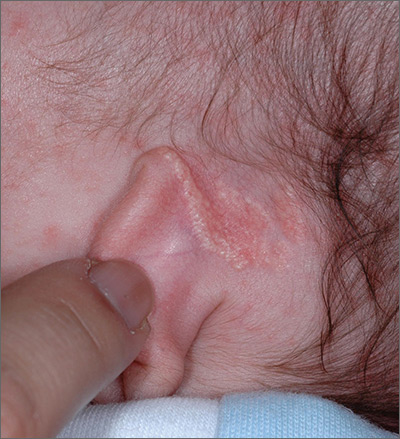
The FP recognized the growth as an early nevus sebaceous (NS).
NS may be present at birth or noted in early childhood and occurs in males and females equally. In early stages of development, it appears skin-colored and waxy. Because of the potential for malignant transformation, particularly following puberty, many authors have recommended early complete plastic surgical excision. However, in a retrospective analysis of 757 cases of NS from 1996 to 2002 in children <16 years, investigators found no malignancies and questioned the need for prophylactic surgical removal.
The FP emphasized that this condition was benign and would likely get darker and more raised over time. He said that he would keep an eye on it during future visits and that the boy could choose to have it removed when he became an adult. The FP explained that reasons for removal include cosmetic issues and the prevention of malignant changes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the growth as an early nevus sebaceous (NS).
NS may be present at birth or noted in early childhood and occurs in males and females equally. In early stages of development, it appears skin-colored and waxy. Because of the potential for malignant transformation, particularly following puberty, many authors have recommended early complete plastic surgical excision. However, in a retrospective analysis of 757 cases of NS from 1996 to 2002 in children <16 years, investigators found no malignancies and questioned the need for prophylactic surgical removal.
The FP emphasized that this condition was benign and would likely get darker and more raised over time. He said that he would keep an eye on it during future visits and that the boy could choose to have it removed when he became an adult. The FP explained that reasons for removal include cosmetic issues and the prevention of malignant changes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the growth as an early nevus sebaceous (NS).
NS may be present at birth or noted in early childhood and occurs in males and females equally. In early stages of development, it appears skin-colored and waxy. Because of the potential for malignant transformation, particularly following puberty, many authors have recommended early complete plastic surgical excision. However, in a retrospective analysis of 757 cases of NS from 1996 to 2002 in children <16 years, investigators found no malignancies and questioned the need for prophylactic surgical removal.
The FP emphasized that this condition was benign and would likely get darker and more raised over time. He said that he would keep an eye on it during future visits and that the boy could choose to have it removed when he became an adult. The FP explained that reasons for removal include cosmetic issues and the prevention of malignant changes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Mobile app engages individuals with opioid use disorders
SAN DIEGO – Individuals with opioid use disorder generally embraced the idea of using a novel mobile application to learn about recovery options and medication-assisted treatment, according to results from a pilot study.
“We found that, after participants engaged with our mobile app, their interest for recovery and attitudes about recovery improved,” lead study author Patricia Cavazos-Rehg, PhD, of the department of psychiatry at Washington University in St. Louis, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We were not expecting results to be so positive, and these findings are very promising because they suggest that individuals may not have interest in or be reluctant to engage in treatment. But we can engage them with a tool that will get them interested and move them toward treatment recovery.”
Dr. Cavazos-Rehg and her associates set out to examine the effectiveness of a mobile application called “uMAT-R,” which includes evidence-based health information about opioid use disorder recovery and medication-assisted treatment (MAT). The app is being develop in partnership with iTether, an Arizona-based mobile health software company. Material for the user is derived from the Substance Abuse and Mental Health Services Administration’s online handbook, “Decisions in Recovery: Treatment for Opioid Disorders,” and is divided into consumer-friendly content modules that provide information on MAT, including costs, latest research findings, and potential side effects.
For the study, researchers recruited 161 individuals 18 years of age and older from three opioid-focused groups on Reddit to pilot the use of uMAT-R. To be eligible, they had to be a U.S. resident, be fluent in English, have misused opioids in the past 30 days, and not currently be using medication-assisted treatment. Participants had access to the app for 1 month, and the researchers administered a baseline questionnaire that assessed demographics, opioid dependence, and history of treatment. They also administered pre- and postapplication engagement questionnaires that assessed attitudes toward MAT, treatment interest, and usefulness of the app.
Of the 161 invited participants, 44 downloaded the app, and 26 completed all content modules and all pre- and postapp assessments. The median age of the 26 participants was 28 years; 65% were male, and 88% were non-Hispanic white. Nearly all (96%) met criteria for opioid dependence, and 12% had received treatment for opioid misuse in the past 6 months. Dr. Cavazos-Rehg and her associates found that only 32% expressed interest in starting treatment for their opioid use disorder before using the app, compared with 48% after using the app, a difference that reached statistical significance (P = 0.046). Feedback from participants was mostly positive. For example, 92% agreed that the app includes useful tips on how to make life better; 88% would consult the app if they had to make a decision about their recovery; 88% believed the app has a positive outlook; 84% said the app helped them have a better understanding of options for recovery, and 84% said they learned something new from the app.
In addition, scores on the MAT attitudes scale rose from a mean of 3.3 to a mean of 3.5 (P = 0.044).
“,” Dr. Cavazos-Rehg said. “We want to develop a mobile app that will teach individuals who have opioid use disorder about their recovery options so that we can nudge them towards considering recovery, and our findings indicate some success with this goal.”
Next, she and her associates plan to pair use of the app with traditional in-person care among pregnant women with opioid use disorders. “Even if people are engaged in treatment, they still only see a therapist or a clinician once or twice a week,” she said. “We want to be able to use a mobile health treatment to support their needs while they are outside of treatment.”
The study was funded by a grant from the National Institutes of Health. Dr. Cavazos-Rehg reported having no financial disclosures.
SAN DIEGO – Individuals with opioid use disorder generally embraced the idea of using a novel mobile application to learn about recovery options and medication-assisted treatment, according to results from a pilot study.
“We found that, after participants engaged with our mobile app, their interest for recovery and attitudes about recovery improved,” lead study author Patricia Cavazos-Rehg, PhD, of the department of psychiatry at Washington University in St. Louis, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We were not expecting results to be so positive, and these findings are very promising because they suggest that individuals may not have interest in or be reluctant to engage in treatment. But we can engage them with a tool that will get them interested and move them toward treatment recovery.”
Dr. Cavazos-Rehg and her associates set out to examine the effectiveness of a mobile application called “uMAT-R,” which includes evidence-based health information about opioid use disorder recovery and medication-assisted treatment (MAT). The app is being develop in partnership with iTether, an Arizona-based mobile health software company. Material for the user is derived from the Substance Abuse and Mental Health Services Administration’s online handbook, “Decisions in Recovery: Treatment for Opioid Disorders,” and is divided into consumer-friendly content modules that provide information on MAT, including costs, latest research findings, and potential side effects.
For the study, researchers recruited 161 individuals 18 years of age and older from three opioid-focused groups on Reddit to pilot the use of uMAT-R. To be eligible, they had to be a U.S. resident, be fluent in English, have misused opioids in the past 30 days, and not currently be using medication-assisted treatment. Participants had access to the app for 1 month, and the researchers administered a baseline questionnaire that assessed demographics, opioid dependence, and history of treatment. They also administered pre- and postapplication engagement questionnaires that assessed attitudes toward MAT, treatment interest, and usefulness of the app.
Of the 161 invited participants, 44 downloaded the app, and 26 completed all content modules and all pre- and postapp assessments. The median age of the 26 participants was 28 years; 65% were male, and 88% were non-Hispanic white. Nearly all (96%) met criteria for opioid dependence, and 12% had received treatment for opioid misuse in the past 6 months. Dr. Cavazos-Rehg and her associates found that only 32% expressed interest in starting treatment for their opioid use disorder before using the app, compared with 48% after using the app, a difference that reached statistical significance (P = 0.046). Feedback from participants was mostly positive. For example, 92% agreed that the app includes useful tips on how to make life better; 88% would consult the app if they had to make a decision about their recovery; 88% believed the app has a positive outlook; 84% said the app helped them have a better understanding of options for recovery, and 84% said they learned something new from the app.
In addition, scores on the MAT attitudes scale rose from a mean of 3.3 to a mean of 3.5 (P = 0.044).
“,” Dr. Cavazos-Rehg said. “We want to develop a mobile app that will teach individuals who have opioid use disorder about their recovery options so that we can nudge them towards considering recovery, and our findings indicate some success with this goal.”
Next, she and her associates plan to pair use of the app with traditional in-person care among pregnant women with opioid use disorders. “Even if people are engaged in treatment, they still only see a therapist or a clinician once or twice a week,” she said. “We want to be able to use a mobile health treatment to support their needs while they are outside of treatment.”
The study was funded by a grant from the National Institutes of Health. Dr. Cavazos-Rehg reported having no financial disclosures.
SAN DIEGO – Individuals with opioid use disorder generally embraced the idea of using a novel mobile application to learn about recovery options and medication-assisted treatment, according to results from a pilot study.
“We found that, after participants engaged with our mobile app, their interest for recovery and attitudes about recovery improved,” lead study author Patricia Cavazos-Rehg, PhD, of the department of psychiatry at Washington University in St. Louis, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We were not expecting results to be so positive, and these findings are very promising because they suggest that individuals may not have interest in or be reluctant to engage in treatment. But we can engage them with a tool that will get them interested and move them toward treatment recovery.”
Dr. Cavazos-Rehg and her associates set out to examine the effectiveness of a mobile application called “uMAT-R,” which includes evidence-based health information about opioid use disorder recovery and medication-assisted treatment (MAT). The app is being develop in partnership with iTether, an Arizona-based mobile health software company. Material for the user is derived from the Substance Abuse and Mental Health Services Administration’s online handbook, “Decisions in Recovery: Treatment for Opioid Disorders,” and is divided into consumer-friendly content modules that provide information on MAT, including costs, latest research findings, and potential side effects.
For the study, researchers recruited 161 individuals 18 years of age and older from three opioid-focused groups on Reddit to pilot the use of uMAT-R. To be eligible, they had to be a U.S. resident, be fluent in English, have misused opioids in the past 30 days, and not currently be using medication-assisted treatment. Participants had access to the app for 1 month, and the researchers administered a baseline questionnaire that assessed demographics, opioid dependence, and history of treatment. They also administered pre- and postapplication engagement questionnaires that assessed attitudes toward MAT, treatment interest, and usefulness of the app.
Of the 161 invited participants, 44 downloaded the app, and 26 completed all content modules and all pre- and postapp assessments. The median age of the 26 participants was 28 years; 65% were male, and 88% were non-Hispanic white. Nearly all (96%) met criteria for opioid dependence, and 12% had received treatment for opioid misuse in the past 6 months. Dr. Cavazos-Rehg and her associates found that only 32% expressed interest in starting treatment for their opioid use disorder before using the app, compared with 48% after using the app, a difference that reached statistical significance (P = 0.046). Feedback from participants was mostly positive. For example, 92% agreed that the app includes useful tips on how to make life better; 88% would consult the app if they had to make a decision about their recovery; 88% believed the app has a positive outlook; 84% said the app helped them have a better understanding of options for recovery, and 84% said they learned something new from the app.
In addition, scores on the MAT attitudes scale rose from a mean of 3.3 to a mean of 3.5 (P = 0.044).
“,” Dr. Cavazos-Rehg said. “We want to develop a mobile app that will teach individuals who have opioid use disorder about their recovery options so that we can nudge them towards considering recovery, and our findings indicate some success with this goal.”
Next, she and her associates plan to pair use of the app with traditional in-person care among pregnant women with opioid use disorders. “Even if people are engaged in treatment, they still only see a therapist or a clinician once or twice a week,” she said. “We want to be able to use a mobile health treatment to support their needs while they are outside of treatment.”
The study was funded by a grant from the National Institutes of Health. Dr. Cavazos-Rehg reported having no financial disclosures.
REPORTING FROM CPDD 2018
Key clinical point: Social media can be leveraged to connect individuals with opioid use disorder to a mobile app that encourages recovery.
Major finding:. Only 32% expressed interest in starting treatment for their opioid use disorder before using the app, compared with 48% after using the app, a difference that reached statistical significance (P = 0.046).
Study details: A study of 26 opioid users who piloted the use of a mobile app.
Disclosures: The study was funded by a grant from the National Institutes of Health. Dr. Cavazos-Rehg reported having no financial disclosures.
Type 2 diabetes may promote Parkinson development
Type 2 diabetes mellitus may be associated with an increased risk of Parkinson’s disease, according to a large retrospective cohort study published online June 13 in Neurology.
Researchers accessed the linked English national Hospital Episode Statistics and mortality data from 1999-2011 to compare data from 2,017,115 individuals admitted to hospital who had a diagnostic code for type 2 diabetes with data from a reference cohort of 6,173,208 individuals admitted for a range of other minor procedures.
They found a significant 32% higher incidence of Parkinson’s disease among individuals with type 2 diabetes, compared with the reference cohort (95% confidence interval, 1.29-1.35; P less than .001).
The incidence was particularly high among younger individuals with type 2 diabetes; those aged 25-44 years at the time of admission had a 3.8-fold higher rate of Parkinson’s disease, compared with the reference group (P less than .001). Individuals aged 45-64 years with type 2 diabetes had 71% greater rate of Parkinson, those aged 65-74 years had a 40% higher incidence, and those aged 75 years or over had an 18% higher rate.
Individuals with complicated type 2 diabetes, defined as the presence of diabetic neuropathy, nephropathy, or retinopathy, had a 49% higher incidence of Parkinson disease than did the reference cohort.
Eduardo De Pablo-Fernandez, MD, from the University College London Institute of Neurology, and his coauthors suggested that the interaction between the two, apparently unconnected, diseases may be a function of both genetics and shared pathogenic pathways.
“The magnitude of risk in our study was greater in younger individuals, whereby genetic factors may relatively exert more of an effect, and more than 400 genes, previously identified through genome-wide association studies, have been closely linked to both conditions using integrative network analysis,” the authors wrote.
“However, the association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes.”
They proposed that disrupted brain insulin signaling could lead to neuroinflammation, mitochondrial dysfunction, and increased oxidative stress that could contribute to the development of Parkinson’s disease.
The findings were similar to those seen in a previous meta-analysis of five studies, although the authors commented that there was significant heterogeneity among the studies included in that analysis.
The authors of this study also noted that their study did not adjust for potential confounders such as smoking or antidiabetic medication use.
The study was supported by the National Institute for Health Research Biomedical Research Centre at the University of Oxford, England, and the NIHR Biomedical Research Centre at University College London. Two authors declared funding and payments from private industry outside the submitted work, but no other conflicts of interest were declared.
SOURCE: De Pablo Fernandez E et al. Neurology. 2018 June 13. doi: 10.1212/WNL.0000000000005771.
Type 2 diabetes mellitus may be associated with an increased risk of Parkinson’s disease, according to a large retrospective cohort study published online June 13 in Neurology.
Researchers accessed the linked English national Hospital Episode Statistics and mortality data from 1999-2011 to compare data from 2,017,115 individuals admitted to hospital who had a diagnostic code for type 2 diabetes with data from a reference cohort of 6,173,208 individuals admitted for a range of other minor procedures.
They found a significant 32% higher incidence of Parkinson’s disease among individuals with type 2 diabetes, compared with the reference cohort (95% confidence interval, 1.29-1.35; P less than .001).
The incidence was particularly high among younger individuals with type 2 diabetes; those aged 25-44 years at the time of admission had a 3.8-fold higher rate of Parkinson’s disease, compared with the reference group (P less than .001). Individuals aged 45-64 years with type 2 diabetes had 71% greater rate of Parkinson, those aged 65-74 years had a 40% higher incidence, and those aged 75 years or over had an 18% higher rate.
Individuals with complicated type 2 diabetes, defined as the presence of diabetic neuropathy, nephropathy, or retinopathy, had a 49% higher incidence of Parkinson disease than did the reference cohort.
Eduardo De Pablo-Fernandez, MD, from the University College London Institute of Neurology, and his coauthors suggested that the interaction between the two, apparently unconnected, diseases may be a function of both genetics and shared pathogenic pathways.
“The magnitude of risk in our study was greater in younger individuals, whereby genetic factors may relatively exert more of an effect, and more than 400 genes, previously identified through genome-wide association studies, have been closely linked to both conditions using integrative network analysis,” the authors wrote.
“However, the association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes.”
They proposed that disrupted brain insulin signaling could lead to neuroinflammation, mitochondrial dysfunction, and increased oxidative stress that could contribute to the development of Parkinson’s disease.
The findings were similar to those seen in a previous meta-analysis of five studies, although the authors commented that there was significant heterogeneity among the studies included in that analysis.
The authors of this study also noted that their study did not adjust for potential confounders such as smoking or antidiabetic medication use.
The study was supported by the National Institute for Health Research Biomedical Research Centre at the University of Oxford, England, and the NIHR Biomedical Research Centre at University College London. Two authors declared funding and payments from private industry outside the submitted work, but no other conflicts of interest were declared.
SOURCE: De Pablo Fernandez E et al. Neurology. 2018 June 13. doi: 10.1212/WNL.0000000000005771.
Type 2 diabetes mellitus may be associated with an increased risk of Parkinson’s disease, according to a large retrospective cohort study published online June 13 in Neurology.
Researchers accessed the linked English national Hospital Episode Statistics and mortality data from 1999-2011 to compare data from 2,017,115 individuals admitted to hospital who had a diagnostic code for type 2 diabetes with data from a reference cohort of 6,173,208 individuals admitted for a range of other minor procedures.
They found a significant 32% higher incidence of Parkinson’s disease among individuals with type 2 diabetes, compared with the reference cohort (95% confidence interval, 1.29-1.35; P less than .001).
The incidence was particularly high among younger individuals with type 2 diabetes; those aged 25-44 years at the time of admission had a 3.8-fold higher rate of Parkinson’s disease, compared with the reference group (P less than .001). Individuals aged 45-64 years with type 2 diabetes had 71% greater rate of Parkinson, those aged 65-74 years had a 40% higher incidence, and those aged 75 years or over had an 18% higher rate.
Individuals with complicated type 2 diabetes, defined as the presence of diabetic neuropathy, nephropathy, or retinopathy, had a 49% higher incidence of Parkinson disease than did the reference cohort.
Eduardo De Pablo-Fernandez, MD, from the University College London Institute of Neurology, and his coauthors suggested that the interaction between the two, apparently unconnected, diseases may be a function of both genetics and shared pathogenic pathways.
“The magnitude of risk in our study was greater in younger individuals, whereby genetic factors may relatively exert more of an effect, and more than 400 genes, previously identified through genome-wide association studies, have been closely linked to both conditions using integrative network analysis,” the authors wrote.
“However, the association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes.”
They proposed that disrupted brain insulin signaling could lead to neuroinflammation, mitochondrial dysfunction, and increased oxidative stress that could contribute to the development of Parkinson’s disease.
The findings were similar to those seen in a previous meta-analysis of five studies, although the authors commented that there was significant heterogeneity among the studies included in that analysis.
The authors of this study also noted that their study did not adjust for potential confounders such as smoking or antidiabetic medication use.
The study was supported by the National Institute for Health Research Biomedical Research Centre at the University of Oxford, England, and the NIHR Biomedical Research Centre at University College London. Two authors declared funding and payments from private industry outside the submitted work, but no other conflicts of interest were declared.
SOURCE: De Pablo Fernandez E et al. Neurology. 2018 June 13. doi: 10.1212/WNL.0000000000005771.
FROM NEUROLOGY
Key clinical point: Type 2 diabetes may be associated with an increased risk of Parkinson’s disease.
Major finding: The incidence of Parkinson’s disease is 32% higher in people with type 2 diabetes.
Study details: Retrospective cohort study including 2,017,115 individuals with type 2 diabetes and 6,173,208 controls.
Disclosures: The study was supported by the National Institute for Health Research Biomedical Research Centre at the University of Oxford, England, and the NIHR Biomedical Research Centre at University College London. Two authors declared funding and payments from private industry outside the submitted work, but no other conflicts of interest were declared.
Source: De Pablo-Fernandez E et al. Neurology. 2018 June 13. doi: 10.1212/WNL.0000000000005771.
Diagnosing pulmonary tuberculosis in the hospital
An uncommon but serious problem in certain populations
Case
A 40-year-old Indian immigrant presented to the emergency department with hemoptysis. He had had an intermittent productive cough for the past 4 weeks with increasing fatigue and lack of appetite. He also had intermittent fever with drenching night sweats. Chest radiograph and CT scan showed a left upper lobe cavitary lesion with infiltrate. He was admitted to the hospital with concern for pneumonia and to rule out possible active pulmonary tuberculosis.
Background
Active pulmonary tuberculosis (APTB) remains an important but often missed diagnosis in hospitalized patients in the Western world.1,2 Because of its relative rarity, the diagnosis of APTB often is delayed in the United States, which can lead hospitalized patients to nosocomial transmission, unnecessary exposures, patient harm,3 and potentially avoidable cost to the health care system.4 The diagnosis and management can be challenging involving isolation needs, sputum clearance, treatment strategy, and criteria for discharge to home.
Diagnosis
Any patient with risk factors presenting with signs and/or symptoms of APTB such as productive cough for more than 4 weeks, night sweats, weight loss, low-grade fevers, upper lobe cavitary lesions, or hemoptysis should be suspected. The diagnostic work-up for APTB should always begin with a thorough medical and social history. A chest radiograph or a CT scan should always be obtained. Risk factors for infection and for progression to active pulmonary TB are listed below.
Risk factors for TB infection:
- Close contacts of a person with APTB.
- Health care workers.
- Immigrants from high-burden countries.
- Homeless people.
- Individuals who have been incarcerated.
- International travelers.
- HIV patients.
- Intravenous drug users.
Risk factors for progression to APTB:
- HIV infection.
- Intravenous drug use.
- Silicosis.
- Younger than 5 years of age.
- Immunosuppressed.
All patients with suspected or confirmed APTB who cannot be safely discharged home (see discharge considerations below) should be kept in negative-pressure airborne isolation rooms. Isolation can be discontinued once APTB has been ruled out or the patient is determined to be noninfectious based on three consecutive negative sputum smears.
Although rapid and inexpensive, acid-fast bacilli (AFB) smear microscopy has poor sensitivity (45%-80%, with culture-confirmed APTB cases) and poor positive predictive value (50%-80%) for TB in settings in which nontuberculous mycobacteria are commonly isolated. This makes an AFB smear nondiagnostic in the early diagnosis of APTB. The burden of mycobacteria seen in the sputum smear correlates with infectivity.
To improve sensitivity of testing, it is strongly recommended that three AFB smears be completed in 8- to 24-hour intervals and positive smears be accompanied by nucleic acid amplification (NAA) testing.5 If APTB is suspected, but the patient is unable to expectorate, induced sputum samples should be obtained, and, if unable to induce sputum samples, flexible bronchoscopy sampling should be pursued especially for the high-risk populations described above.
The Centers for Disease Control and Prevention recommends that at least one sputum specimen be tested with NAA to expedite the time to diagnosis of APTB. A negative NAA does NOT rule out TB. The turnaround time for this test is about 24-48 hours. NAA has better positive predictive value (greater than 95%) with AFB smear-positive specimens in settings in which nontuberculous mycobacteria are common. The ability to confirm rapidly the presence of Mycobacterium tuberculosis is 50%-80% in AFB smear-negative, culture-positive specimens.6
In patients with clinical or radiologic suspicion of APTB who are unable to produce sputum or have negative sputum smear microscopy results, bronchoscopy is a safe and reliable method for the diagnosis of pulmonary tuberculosis. For the diagnosis of tuberculosis, bronchoalveolar lavage has a sensitivity and specificity of 60% and 100%, respectively. Adding transbronchial biopsy further increases the sensitivity to 84%, and post-bronchoscopy sputum smear microscopy increases the sensitivity to 94%.6
In 2005, the CDC released guidelines for using interferon-gamma release assays (IGRA) to test for M. tuberculosis infection.7 Both tuberculin skin testing (TST) and IGRAs assess lymphocytes’ response to M. tuberculosis. Although these tests can be supportive of a previous tuberculosis infection, they are not diagnostic tests for APTB. Neither an IGRA nor a TST can distinguish latent from active tuberculosis.
Sputum AFB culture remains the preferred method for laboratory confirmation of APTB. Once APTB is confirmed, it is essential for susceptibility testing and genotyping. However, in the absence of a positive culture, APTB can be diagnosed based on signs and symptoms alone in a high-risk patient.
Treatment
A multidisciplinary, patient-centered approach involving the patient, providers, and public health officials is required to accomplish the following treatment goals: eradicating Mycobacterium infection, eliminating the risk of transmission, avoiding the disease, and preventing drug resistance.8
Infectious disease consultation is mandatory in all HIV-positive and suspected or confirmed multidrug-resistant cases. Directly observed therapy is an essential component of APTB treatment to ensure compliance in many situations.
Admission and discharge
Admission to a hospital is not required unless a patient meets criteria for admission independent of APTB diagnosis, or proper risk stratification and assessment cannot be completed in a timely manner. A patient with suspected APTB should be placed in airborne isolation. All staff should wear N95 disposable masks or respirators while inside the patient’s room.9
Discharge considerations are listed below:
- Inform the department of health (DOH).
- Establish proper isolation precautions to minimize exposure.
- Ensure ability to stay at home until DOH and physician determines noninfectivity.
- Educate the patient about length of therapy, directly observed therapy, side effects and importance of compliance.
- Coordinate discharge with the DOH.
- Make sure proper follow-up is scheduled.
Back to the case
Our patient was placed on airborne respiratory isolation immediately upon admission and sputum was sent for AFB. Sputum smear was positive for AFB as well as a positive nucleic acid testing for Mycobacterium tuberculosis. HIV antibody testing was negative. Once the sputum AFB was determined to be positive, the department of health was informed. He was started on the intensive phase of therapy with pyrazinamide, rifampin, ethambutol, and isoniazid along with pyridoxine. He tolerated his medications well and had no immediate reactions. His family and close contacts were screened and advised to be tested.
The patient was discharged after proper follow-up with primary care doctor was scheduled. The department of health arranged for directly observed therapy. He received information about the importance of taking all of his medications and staying at home except for medical visits until the DOH had deemed him to be noninfectious.
Bottom line
APTB in the hospital is an uncommon but serious problem in certain populations. It requires a high index of suspicion and a multidisciplinary approach for effective treatment and prevention of transmission.
Dr. Mallampalli is an attending physician in hospital medicine at Geisinger in Danville, Pa., and clinical assistant professor at Temple University, Philadelphia. Dr. Velidi is an attending physician in hospital medicine at Geisinger. Dr. Courtney is associate director of the department of hospital medicine at Geisinger.
References
1. Miller AC et al. Missed opportunities to diagnose tuberculosis are common among hospitalized patients and patients seen in emergency departments. Open Forum Infectious Diseases. 2015. doi. org/10.1093/ofid/ofv171.
2. Greenaway C et al and the Canadian Collaborative Group in Nosocomial Transmission of Tuberculosis. Delay in diagnosis among hospitalized patients with active tuberculosis–predictors and outcomes. Am J Respir Crit Care Med. 2002 Apr;165:927-33.
3. Medrano BA et al. A missed tuberculosis diagnosis resulting in hospital transmission. Infect Control Hosp Epidemiol. 2014 May;35(5):534-7.
4. Kelly AM et al. Delayed tuberculosis diagnosis and costs of contact investigations for hospital exposure: New York City, 2010-2014. Am J Infect Control. 2017 May 1;45(5):483-6.
5. Lewinsohn DM et al. Official ATS/IDSA/CDC Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan;64:111-5.
6. Mazurek GH et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005 Dec 16;54(RR-15):49-55.
7. CDC. Trends in tuberculosis – United States, 2010. MMWR Morb Mortal Wkly Rep. 2011 Mar 25;60(11):333-7.
8. Jensen PA et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005 Dec 30;54(RR-17):1-141.
9. Siegel JD et al and the Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007 Dec;35(10 Suppl 2):S65-164.
An uncommon but serious problem in certain populations
An uncommon but serious problem in certain populations
Case
A 40-year-old Indian immigrant presented to the emergency department with hemoptysis. He had had an intermittent productive cough for the past 4 weeks with increasing fatigue and lack of appetite. He also had intermittent fever with drenching night sweats. Chest radiograph and CT scan showed a left upper lobe cavitary lesion with infiltrate. He was admitted to the hospital with concern for pneumonia and to rule out possible active pulmonary tuberculosis.
Background
Active pulmonary tuberculosis (APTB) remains an important but often missed diagnosis in hospitalized patients in the Western world.1,2 Because of its relative rarity, the diagnosis of APTB often is delayed in the United States, which can lead hospitalized patients to nosocomial transmission, unnecessary exposures, patient harm,3 and potentially avoidable cost to the health care system.4 The diagnosis and management can be challenging involving isolation needs, sputum clearance, treatment strategy, and criteria for discharge to home.
Diagnosis
Any patient with risk factors presenting with signs and/or symptoms of APTB such as productive cough for more than 4 weeks, night sweats, weight loss, low-grade fevers, upper lobe cavitary lesions, or hemoptysis should be suspected. The diagnostic work-up for APTB should always begin with a thorough medical and social history. A chest radiograph or a CT scan should always be obtained. Risk factors for infection and for progression to active pulmonary TB are listed below.
Risk factors for TB infection:
- Close contacts of a person with APTB.
- Health care workers.
- Immigrants from high-burden countries.
- Homeless people.
- Individuals who have been incarcerated.
- International travelers.
- HIV patients.
- Intravenous drug users.
Risk factors for progression to APTB:
- HIV infection.
- Intravenous drug use.
- Silicosis.
- Younger than 5 years of age.
- Immunosuppressed.
All patients with suspected or confirmed APTB who cannot be safely discharged home (see discharge considerations below) should be kept in negative-pressure airborne isolation rooms. Isolation can be discontinued once APTB has been ruled out or the patient is determined to be noninfectious based on three consecutive negative sputum smears.
Although rapid and inexpensive, acid-fast bacilli (AFB) smear microscopy has poor sensitivity (45%-80%, with culture-confirmed APTB cases) and poor positive predictive value (50%-80%) for TB in settings in which nontuberculous mycobacteria are commonly isolated. This makes an AFB smear nondiagnostic in the early diagnosis of APTB. The burden of mycobacteria seen in the sputum smear correlates with infectivity.
To improve sensitivity of testing, it is strongly recommended that three AFB smears be completed in 8- to 24-hour intervals and positive smears be accompanied by nucleic acid amplification (NAA) testing.5 If APTB is suspected, but the patient is unable to expectorate, induced sputum samples should be obtained, and, if unable to induce sputum samples, flexible bronchoscopy sampling should be pursued especially for the high-risk populations described above.
The Centers for Disease Control and Prevention recommends that at least one sputum specimen be tested with NAA to expedite the time to diagnosis of APTB. A negative NAA does NOT rule out TB. The turnaround time for this test is about 24-48 hours. NAA has better positive predictive value (greater than 95%) with AFB smear-positive specimens in settings in which nontuberculous mycobacteria are common. The ability to confirm rapidly the presence of Mycobacterium tuberculosis is 50%-80% in AFB smear-negative, culture-positive specimens.6
In patients with clinical or radiologic suspicion of APTB who are unable to produce sputum or have negative sputum smear microscopy results, bronchoscopy is a safe and reliable method for the diagnosis of pulmonary tuberculosis. For the diagnosis of tuberculosis, bronchoalveolar lavage has a sensitivity and specificity of 60% and 100%, respectively. Adding transbronchial biopsy further increases the sensitivity to 84%, and post-bronchoscopy sputum smear microscopy increases the sensitivity to 94%.6
In 2005, the CDC released guidelines for using interferon-gamma release assays (IGRA) to test for M. tuberculosis infection.7 Both tuberculin skin testing (TST) and IGRAs assess lymphocytes’ response to M. tuberculosis. Although these tests can be supportive of a previous tuberculosis infection, they are not diagnostic tests for APTB. Neither an IGRA nor a TST can distinguish latent from active tuberculosis.
Sputum AFB culture remains the preferred method for laboratory confirmation of APTB. Once APTB is confirmed, it is essential for susceptibility testing and genotyping. However, in the absence of a positive culture, APTB can be diagnosed based on signs and symptoms alone in a high-risk patient.
Treatment
A multidisciplinary, patient-centered approach involving the patient, providers, and public health officials is required to accomplish the following treatment goals: eradicating Mycobacterium infection, eliminating the risk of transmission, avoiding the disease, and preventing drug resistance.8
Infectious disease consultation is mandatory in all HIV-positive and suspected or confirmed multidrug-resistant cases. Directly observed therapy is an essential component of APTB treatment to ensure compliance in many situations.
Admission and discharge
Admission to a hospital is not required unless a patient meets criteria for admission independent of APTB diagnosis, or proper risk stratification and assessment cannot be completed in a timely manner. A patient with suspected APTB should be placed in airborne isolation. All staff should wear N95 disposable masks or respirators while inside the patient’s room.9
Discharge considerations are listed below:
- Inform the department of health (DOH).
- Establish proper isolation precautions to minimize exposure.
- Ensure ability to stay at home until DOH and physician determines noninfectivity.
- Educate the patient about length of therapy, directly observed therapy, side effects and importance of compliance.
- Coordinate discharge with the DOH.
- Make sure proper follow-up is scheduled.
Back to the case
Our patient was placed on airborne respiratory isolation immediately upon admission and sputum was sent for AFB. Sputum smear was positive for AFB as well as a positive nucleic acid testing for Mycobacterium tuberculosis. HIV antibody testing was negative. Once the sputum AFB was determined to be positive, the department of health was informed. He was started on the intensive phase of therapy with pyrazinamide, rifampin, ethambutol, and isoniazid along with pyridoxine. He tolerated his medications well and had no immediate reactions. His family and close contacts were screened and advised to be tested.
The patient was discharged after proper follow-up with primary care doctor was scheduled. The department of health arranged for directly observed therapy. He received information about the importance of taking all of his medications and staying at home except for medical visits until the DOH had deemed him to be noninfectious.
Bottom line
APTB in the hospital is an uncommon but serious problem in certain populations. It requires a high index of suspicion and a multidisciplinary approach for effective treatment and prevention of transmission.
Dr. Mallampalli is an attending physician in hospital medicine at Geisinger in Danville, Pa., and clinical assistant professor at Temple University, Philadelphia. Dr. Velidi is an attending physician in hospital medicine at Geisinger. Dr. Courtney is associate director of the department of hospital medicine at Geisinger.
References
1. Miller AC et al. Missed opportunities to diagnose tuberculosis are common among hospitalized patients and patients seen in emergency departments. Open Forum Infectious Diseases. 2015. doi. org/10.1093/ofid/ofv171.
2. Greenaway C et al and the Canadian Collaborative Group in Nosocomial Transmission of Tuberculosis. Delay in diagnosis among hospitalized patients with active tuberculosis–predictors and outcomes. Am J Respir Crit Care Med. 2002 Apr;165:927-33.
3. Medrano BA et al. A missed tuberculosis diagnosis resulting in hospital transmission. Infect Control Hosp Epidemiol. 2014 May;35(5):534-7.
4. Kelly AM et al. Delayed tuberculosis diagnosis and costs of contact investigations for hospital exposure: New York City, 2010-2014. Am J Infect Control. 2017 May 1;45(5):483-6.
5. Lewinsohn DM et al. Official ATS/IDSA/CDC Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan;64:111-5.
6. Mazurek GH et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005 Dec 16;54(RR-15):49-55.
7. CDC. Trends in tuberculosis – United States, 2010. MMWR Morb Mortal Wkly Rep. 2011 Mar 25;60(11):333-7.
8. Jensen PA et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005 Dec 30;54(RR-17):1-141.
9. Siegel JD et al and the Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007 Dec;35(10 Suppl 2):S65-164.
Case
A 40-year-old Indian immigrant presented to the emergency department with hemoptysis. He had had an intermittent productive cough for the past 4 weeks with increasing fatigue and lack of appetite. He also had intermittent fever with drenching night sweats. Chest radiograph and CT scan showed a left upper lobe cavitary lesion with infiltrate. He was admitted to the hospital with concern for pneumonia and to rule out possible active pulmonary tuberculosis.
Background
Active pulmonary tuberculosis (APTB) remains an important but often missed diagnosis in hospitalized patients in the Western world.1,2 Because of its relative rarity, the diagnosis of APTB often is delayed in the United States, which can lead hospitalized patients to nosocomial transmission, unnecessary exposures, patient harm,3 and potentially avoidable cost to the health care system.4 The diagnosis and management can be challenging involving isolation needs, sputum clearance, treatment strategy, and criteria for discharge to home.
Diagnosis
Any patient with risk factors presenting with signs and/or symptoms of APTB such as productive cough for more than 4 weeks, night sweats, weight loss, low-grade fevers, upper lobe cavitary lesions, or hemoptysis should be suspected. The diagnostic work-up for APTB should always begin with a thorough medical and social history. A chest radiograph or a CT scan should always be obtained. Risk factors for infection and for progression to active pulmonary TB are listed below.
Risk factors for TB infection:
- Close contacts of a person with APTB.
- Health care workers.
- Immigrants from high-burden countries.
- Homeless people.
- Individuals who have been incarcerated.
- International travelers.
- HIV patients.
- Intravenous drug users.
Risk factors for progression to APTB:
- HIV infection.
- Intravenous drug use.
- Silicosis.
- Younger than 5 years of age.
- Immunosuppressed.
All patients with suspected or confirmed APTB who cannot be safely discharged home (see discharge considerations below) should be kept in negative-pressure airborne isolation rooms. Isolation can be discontinued once APTB has been ruled out or the patient is determined to be noninfectious based on three consecutive negative sputum smears.
Although rapid and inexpensive, acid-fast bacilli (AFB) smear microscopy has poor sensitivity (45%-80%, with culture-confirmed APTB cases) and poor positive predictive value (50%-80%) for TB in settings in which nontuberculous mycobacteria are commonly isolated. This makes an AFB smear nondiagnostic in the early diagnosis of APTB. The burden of mycobacteria seen in the sputum smear correlates with infectivity.
To improve sensitivity of testing, it is strongly recommended that three AFB smears be completed in 8- to 24-hour intervals and positive smears be accompanied by nucleic acid amplification (NAA) testing.5 If APTB is suspected, but the patient is unable to expectorate, induced sputum samples should be obtained, and, if unable to induce sputum samples, flexible bronchoscopy sampling should be pursued especially for the high-risk populations described above.
The Centers for Disease Control and Prevention recommends that at least one sputum specimen be tested with NAA to expedite the time to diagnosis of APTB. A negative NAA does NOT rule out TB. The turnaround time for this test is about 24-48 hours. NAA has better positive predictive value (greater than 95%) with AFB smear-positive specimens in settings in which nontuberculous mycobacteria are common. The ability to confirm rapidly the presence of Mycobacterium tuberculosis is 50%-80% in AFB smear-negative, culture-positive specimens.6
In patients with clinical or radiologic suspicion of APTB who are unable to produce sputum or have negative sputum smear microscopy results, bronchoscopy is a safe and reliable method for the diagnosis of pulmonary tuberculosis. For the diagnosis of tuberculosis, bronchoalveolar lavage has a sensitivity and specificity of 60% and 100%, respectively. Adding transbronchial biopsy further increases the sensitivity to 84%, and post-bronchoscopy sputum smear microscopy increases the sensitivity to 94%.6
In 2005, the CDC released guidelines for using interferon-gamma release assays (IGRA) to test for M. tuberculosis infection.7 Both tuberculin skin testing (TST) and IGRAs assess lymphocytes’ response to M. tuberculosis. Although these tests can be supportive of a previous tuberculosis infection, they are not diagnostic tests for APTB. Neither an IGRA nor a TST can distinguish latent from active tuberculosis.
Sputum AFB culture remains the preferred method for laboratory confirmation of APTB. Once APTB is confirmed, it is essential for susceptibility testing and genotyping. However, in the absence of a positive culture, APTB can be diagnosed based on signs and symptoms alone in a high-risk patient.
Treatment
A multidisciplinary, patient-centered approach involving the patient, providers, and public health officials is required to accomplish the following treatment goals: eradicating Mycobacterium infection, eliminating the risk of transmission, avoiding the disease, and preventing drug resistance.8
Infectious disease consultation is mandatory in all HIV-positive and suspected or confirmed multidrug-resistant cases. Directly observed therapy is an essential component of APTB treatment to ensure compliance in many situations.
Admission and discharge
Admission to a hospital is not required unless a patient meets criteria for admission independent of APTB diagnosis, or proper risk stratification and assessment cannot be completed in a timely manner. A patient with suspected APTB should be placed in airborne isolation. All staff should wear N95 disposable masks or respirators while inside the patient’s room.9
Discharge considerations are listed below:
- Inform the department of health (DOH).
- Establish proper isolation precautions to minimize exposure.
- Ensure ability to stay at home until DOH and physician determines noninfectivity.
- Educate the patient about length of therapy, directly observed therapy, side effects and importance of compliance.
- Coordinate discharge with the DOH.
- Make sure proper follow-up is scheduled.
Back to the case
Our patient was placed on airborne respiratory isolation immediately upon admission and sputum was sent for AFB. Sputum smear was positive for AFB as well as a positive nucleic acid testing for Mycobacterium tuberculosis. HIV antibody testing was negative. Once the sputum AFB was determined to be positive, the department of health was informed. He was started on the intensive phase of therapy with pyrazinamide, rifampin, ethambutol, and isoniazid along with pyridoxine. He tolerated his medications well and had no immediate reactions. His family and close contacts were screened and advised to be tested.
The patient was discharged after proper follow-up with primary care doctor was scheduled. The department of health arranged for directly observed therapy. He received information about the importance of taking all of his medications and staying at home except for medical visits until the DOH had deemed him to be noninfectious.
Bottom line
APTB in the hospital is an uncommon but serious problem in certain populations. It requires a high index of suspicion and a multidisciplinary approach for effective treatment and prevention of transmission.
Dr. Mallampalli is an attending physician in hospital medicine at Geisinger in Danville, Pa., and clinical assistant professor at Temple University, Philadelphia. Dr. Velidi is an attending physician in hospital medicine at Geisinger. Dr. Courtney is associate director of the department of hospital medicine at Geisinger.
References
1. Miller AC et al. Missed opportunities to diagnose tuberculosis are common among hospitalized patients and patients seen in emergency departments. Open Forum Infectious Diseases. 2015. doi. org/10.1093/ofid/ofv171.
2. Greenaway C et al and the Canadian Collaborative Group in Nosocomial Transmission of Tuberculosis. Delay in diagnosis among hospitalized patients with active tuberculosis–predictors and outcomes. Am J Respir Crit Care Med. 2002 Apr;165:927-33.
3. Medrano BA et al. A missed tuberculosis diagnosis resulting in hospital transmission. Infect Control Hosp Epidemiol. 2014 May;35(5):534-7.
4. Kelly AM et al. Delayed tuberculosis diagnosis and costs of contact investigations for hospital exposure: New York City, 2010-2014. Am J Infect Control. 2017 May 1;45(5):483-6.
5. Lewinsohn DM et al. Official ATS/IDSA/CDC Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan;64:111-5.
6. Mazurek GH et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005 Dec 16;54(RR-15):49-55.
7. CDC. Trends in tuberculosis – United States, 2010. MMWR Morb Mortal Wkly Rep. 2011 Mar 25;60(11):333-7.
8. Jensen PA et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005 Dec 30;54(RR-17):1-141.
9. Siegel JD et al and the Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007 Dec;35(10 Suppl 2):S65-164.
CRP apheresis: A promising new STEMI therapy
PARIS – Removal of circulating C-reactive protein (CRP) via selective extracorporeal apheresis shows considerable promise as a safe and effective adjunctive therapy for ST-segment elevation MI in an interim analysis of an ongoing, multicenter, German, randomized, proof-of-concept trial, Christoph D. Garlichs, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
In the first 64 of 80 scheduled, randomized ST-segment elevation MI (STEMI) patients participating in the CRP Apheresis in the Acute Myocardial Infarction (CAMI 1) trial, CRP apheresis plus standard European Society of Cardiology guideline-recommended STEMI therapy, including complete coronary revascularization, resulted in a 47% reduction in infarct area by cardiac MRI performed 2-7 days post infarct, compared with standard therapy alone, which is the primary study endpoint, reported Dr. Garlichs, a cardiologist and head of the internal medicine clinic at Deaconess Hospital in Flensburg, Germany.
Cardiac MRI will be repeated in all 80 CAMI 1 participants 12 weeks post-MI, Dr. Garlichs added at a session on outside-the-box technologies in the cardiovascular pipeline.
The theory behind CRP apheresis in the treatment of MI is that high serum levels of CRP during acute cardiac events are a key player in the resultant myocardial damage because CRP activates the complement system, promoting destruction of stressed cardiac myocytes by macrophages. This is CRP as a mediator of myocardial tissue damage, not in the protein’s more familiar role as a systemic inflammation marker reflecting damage to coronary arteries, which is a whole different story.
“If you remove CRP from the system, complement will not be activated and myocardial cells regain viability,” Dr. Garlichs explained.
This has been established in animal studies, with elucidation of the specific mechanisms involved. The same process figures prominently in cerebral damage from stroke, he continued.
The device utilized for CRP apheresis in CAMI 1 is the PentraSorb CRP adsorber, produced by Henningsdorf, Germany–based Pentracor and already approved for marketing throughout Europe. The treatment regimen consists of two treatment sessions, each lasting roughly 4 hours. The first begins 24 hours after STEMI symptom onset, the second comes 24 hours later. If the serum CRP is above 30 mg/L a few hours after the second session, a third session is given 24 hours later.
In each session, 6,000 mL of plasma gets filtered via peripheral venous access. CRP levels were reduced by an average of 70%, and there have been no side effects or safety issues to date.
Discussant and entrepreneur Peter J. Fitzgerald, MD, PhD, said that if the magnitude of infarct size reduction, as measured by cardiac MRI, holds up in the final results, the device would be approvable for treatment of acute MI based upon Food and Drug Administration criteria.
Dr. Garlichs said that once CAMI 1 is finished, a similar randomized trial in stroke patients is planned.
“But you’ve got to get it approved,” said Dr. Fitzgerald, a cardiologist who has founded and/or been a principal in more than a dozen U.S. medical device start-up companies and serves as director of the Center for Cardiovascular Technology at Stanford (Calif.) University. “You can’t jump to the head until you get the heart approved.”
Towards that goal, he suggested that the investigators focus heavily on STEMI patients who have large anterior MIs, which he called “the tombstones.”
“I don’t care what the therapy is, it doesn’t work unless you look at anterior MIs,” said Dr. Fitzgerald.
As it turns out, Dr. Garlichs said, in fact most of the STEMI patients enrolled in CAMI 1 have had anterior MIs.
Asked if CRP apheresis might have a potential role in the treatment of RA and other autoimmune disorders marked by elevated CRP, Dr. Garlichs said indeed it does. But he noted that, in order to utilize the procedure on a regular long-term basis to treat a chronic disease such as RA as opposed to an acute cardiac or cerebrovascular event, a slew of new clinical trials will be required.
Dr. Garlichs serves as a consultant to Pentracor, which sponsored CAMI 1.
PARIS – Removal of circulating C-reactive protein (CRP) via selective extracorporeal apheresis shows considerable promise as a safe and effective adjunctive therapy for ST-segment elevation MI in an interim analysis of an ongoing, multicenter, German, randomized, proof-of-concept trial, Christoph D. Garlichs, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
In the first 64 of 80 scheduled, randomized ST-segment elevation MI (STEMI) patients participating in the CRP Apheresis in the Acute Myocardial Infarction (CAMI 1) trial, CRP apheresis plus standard European Society of Cardiology guideline-recommended STEMI therapy, including complete coronary revascularization, resulted in a 47% reduction in infarct area by cardiac MRI performed 2-7 days post infarct, compared with standard therapy alone, which is the primary study endpoint, reported Dr. Garlichs, a cardiologist and head of the internal medicine clinic at Deaconess Hospital in Flensburg, Germany.
Cardiac MRI will be repeated in all 80 CAMI 1 participants 12 weeks post-MI, Dr. Garlichs added at a session on outside-the-box technologies in the cardiovascular pipeline.
The theory behind CRP apheresis in the treatment of MI is that high serum levels of CRP during acute cardiac events are a key player in the resultant myocardial damage because CRP activates the complement system, promoting destruction of stressed cardiac myocytes by macrophages. This is CRP as a mediator of myocardial tissue damage, not in the protein’s more familiar role as a systemic inflammation marker reflecting damage to coronary arteries, which is a whole different story.
“If you remove CRP from the system, complement will not be activated and myocardial cells regain viability,” Dr. Garlichs explained.
This has been established in animal studies, with elucidation of the specific mechanisms involved. The same process figures prominently in cerebral damage from stroke, he continued.
The device utilized for CRP apheresis in CAMI 1 is the PentraSorb CRP adsorber, produced by Henningsdorf, Germany–based Pentracor and already approved for marketing throughout Europe. The treatment regimen consists of two treatment sessions, each lasting roughly 4 hours. The first begins 24 hours after STEMI symptom onset, the second comes 24 hours later. If the serum CRP is above 30 mg/L a few hours after the second session, a third session is given 24 hours later.
In each session, 6,000 mL of plasma gets filtered via peripheral venous access. CRP levels were reduced by an average of 70%, and there have been no side effects or safety issues to date.
Discussant and entrepreneur Peter J. Fitzgerald, MD, PhD, said that if the magnitude of infarct size reduction, as measured by cardiac MRI, holds up in the final results, the device would be approvable for treatment of acute MI based upon Food and Drug Administration criteria.
Dr. Garlichs said that once CAMI 1 is finished, a similar randomized trial in stroke patients is planned.
“But you’ve got to get it approved,” said Dr. Fitzgerald, a cardiologist who has founded and/or been a principal in more than a dozen U.S. medical device start-up companies and serves as director of the Center for Cardiovascular Technology at Stanford (Calif.) University. “You can’t jump to the head until you get the heart approved.”
Towards that goal, he suggested that the investigators focus heavily on STEMI patients who have large anterior MIs, which he called “the tombstones.”
“I don’t care what the therapy is, it doesn’t work unless you look at anterior MIs,” said Dr. Fitzgerald.
As it turns out, Dr. Garlichs said, in fact most of the STEMI patients enrolled in CAMI 1 have had anterior MIs.
Asked if CRP apheresis might have a potential role in the treatment of RA and other autoimmune disorders marked by elevated CRP, Dr. Garlichs said indeed it does. But he noted that, in order to utilize the procedure on a regular long-term basis to treat a chronic disease such as RA as opposed to an acute cardiac or cerebrovascular event, a slew of new clinical trials will be required.
Dr. Garlichs serves as a consultant to Pentracor, which sponsored CAMI 1.
PARIS – Removal of circulating C-reactive protein (CRP) via selective extracorporeal apheresis shows considerable promise as a safe and effective adjunctive therapy for ST-segment elevation MI in an interim analysis of an ongoing, multicenter, German, randomized, proof-of-concept trial, Christoph D. Garlichs, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
In the first 64 of 80 scheduled, randomized ST-segment elevation MI (STEMI) patients participating in the CRP Apheresis in the Acute Myocardial Infarction (CAMI 1) trial, CRP apheresis plus standard European Society of Cardiology guideline-recommended STEMI therapy, including complete coronary revascularization, resulted in a 47% reduction in infarct area by cardiac MRI performed 2-7 days post infarct, compared with standard therapy alone, which is the primary study endpoint, reported Dr. Garlichs, a cardiologist and head of the internal medicine clinic at Deaconess Hospital in Flensburg, Germany.
Cardiac MRI will be repeated in all 80 CAMI 1 participants 12 weeks post-MI, Dr. Garlichs added at a session on outside-the-box technologies in the cardiovascular pipeline.
The theory behind CRP apheresis in the treatment of MI is that high serum levels of CRP during acute cardiac events are a key player in the resultant myocardial damage because CRP activates the complement system, promoting destruction of stressed cardiac myocytes by macrophages. This is CRP as a mediator of myocardial tissue damage, not in the protein’s more familiar role as a systemic inflammation marker reflecting damage to coronary arteries, which is a whole different story.
“If you remove CRP from the system, complement will not be activated and myocardial cells regain viability,” Dr. Garlichs explained.
This has been established in animal studies, with elucidation of the specific mechanisms involved. The same process figures prominently in cerebral damage from stroke, he continued.
The device utilized for CRP apheresis in CAMI 1 is the PentraSorb CRP adsorber, produced by Henningsdorf, Germany–based Pentracor and already approved for marketing throughout Europe. The treatment regimen consists of two treatment sessions, each lasting roughly 4 hours. The first begins 24 hours after STEMI symptom onset, the second comes 24 hours later. If the serum CRP is above 30 mg/L a few hours after the second session, a third session is given 24 hours later.
In each session, 6,000 mL of plasma gets filtered via peripheral venous access. CRP levels were reduced by an average of 70%, and there have been no side effects or safety issues to date.
Discussant and entrepreneur Peter J. Fitzgerald, MD, PhD, said that if the magnitude of infarct size reduction, as measured by cardiac MRI, holds up in the final results, the device would be approvable for treatment of acute MI based upon Food and Drug Administration criteria.
Dr. Garlichs said that once CAMI 1 is finished, a similar randomized trial in stroke patients is planned.
“But you’ve got to get it approved,” said Dr. Fitzgerald, a cardiologist who has founded and/or been a principal in more than a dozen U.S. medical device start-up companies and serves as director of the Center for Cardiovascular Technology at Stanford (Calif.) University. “You can’t jump to the head until you get the heart approved.”
Towards that goal, he suggested that the investigators focus heavily on STEMI patients who have large anterior MIs, which he called “the tombstones.”
“I don’t care what the therapy is, it doesn’t work unless you look at anterior MIs,” said Dr. Fitzgerald.
As it turns out, Dr. Garlichs said, in fact most of the STEMI patients enrolled in CAMI 1 have had anterior MIs.
Asked if CRP apheresis might have a potential role in the treatment of RA and other autoimmune disorders marked by elevated CRP, Dr. Garlichs said indeed it does. But he noted that, in order to utilize the procedure on a regular long-term basis to treat a chronic disease such as RA as opposed to an acute cardiac or cerebrovascular event, a slew of new clinical trials will be required.
Dr. Garlichs serves as a consultant to Pentracor, which sponsored CAMI 1.
REPORTING FROM EUROPCR 2018
PREOPANC-1: Early findings suggest benefit with preop chemo in pancreatic cancer
CHICAGO – Preoperative chemotherapy improves outcomes in patients with resectable or borderline resectable pancreatic cancer, preliminary findings from the phase 3 PREOPANC-1 trial suggest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Overall survival in 127 patients randomized to immediate surgery followed by adjuvant chemotherapy was 13.7 months vs. 17.1 months in 119 patients randomized to receive preoperative chemoradiotherapy and postoperative adjuvant chemotherapy, Geertjan van Tienhoven, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The difference did not quite reach statistical significance, but final analysis requires an additional 26 events, Dr. van Tienhoven of Academic Medical Center, Amsterdam explained in a video interview at the meeting.
Other differences between the groups, which included disease-free survival, local control, and metastasis-free survival, did differ significantly in favor of preoperative chemotherapy, he said.
Of note, 72% and 62% of patients in the immediate surgery and preoperative chemoradiotherapy groups, respectively, underwent resection and a greater proportion of patients in the latter group achieved microscopically complete resection, he said (63% vs. 31%).
Should these results hold up in the final analysis, particularly if the difference in overall survival reaches statistical significance, “then this is a proof of principle and practice-changing trial,” Dr. van Tienhoven said.
Dr. van Tienhoven reported having no disclosures.
SOURCE: van Tienhoven et al. ASCO 2108, Abstract LBA4002.
CHICAGO – Preoperative chemotherapy improves outcomes in patients with resectable or borderline resectable pancreatic cancer, preliminary findings from the phase 3 PREOPANC-1 trial suggest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Overall survival in 127 patients randomized to immediate surgery followed by adjuvant chemotherapy was 13.7 months vs. 17.1 months in 119 patients randomized to receive preoperative chemoradiotherapy and postoperative adjuvant chemotherapy, Geertjan van Tienhoven, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The difference did not quite reach statistical significance, but final analysis requires an additional 26 events, Dr. van Tienhoven of Academic Medical Center, Amsterdam explained in a video interview at the meeting.
Other differences between the groups, which included disease-free survival, local control, and metastasis-free survival, did differ significantly in favor of preoperative chemotherapy, he said.
Of note, 72% and 62% of patients in the immediate surgery and preoperative chemoradiotherapy groups, respectively, underwent resection and a greater proportion of patients in the latter group achieved microscopically complete resection, he said (63% vs. 31%).
Should these results hold up in the final analysis, particularly if the difference in overall survival reaches statistical significance, “then this is a proof of principle and practice-changing trial,” Dr. van Tienhoven said.
Dr. van Tienhoven reported having no disclosures.
SOURCE: van Tienhoven et al. ASCO 2108, Abstract LBA4002.
CHICAGO – Preoperative chemotherapy improves outcomes in patients with resectable or borderline resectable pancreatic cancer, preliminary findings from the phase 3 PREOPANC-1 trial suggest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Overall survival in 127 patients randomized to immediate surgery followed by adjuvant chemotherapy was 13.7 months vs. 17.1 months in 119 patients randomized to receive preoperative chemoradiotherapy and postoperative adjuvant chemotherapy, Geertjan van Tienhoven, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The difference did not quite reach statistical significance, but final analysis requires an additional 26 events, Dr. van Tienhoven of Academic Medical Center, Amsterdam explained in a video interview at the meeting.
Other differences between the groups, which included disease-free survival, local control, and metastasis-free survival, did differ significantly in favor of preoperative chemotherapy, he said.
Of note, 72% and 62% of patients in the immediate surgery and preoperative chemoradiotherapy groups, respectively, underwent resection and a greater proportion of patients in the latter group achieved microscopically complete resection, he said (63% vs. 31%).
Should these results hold up in the final analysis, particularly if the difference in overall survival reaches statistical significance, “then this is a proof of principle and practice-changing trial,” Dr. van Tienhoven said.
Dr. van Tienhoven reported having no disclosures.
SOURCE: van Tienhoven et al. ASCO 2108, Abstract LBA4002.
REPORTING FROM ASCO 2018