User login
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.


ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).

ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.

- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.


ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).

ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.

Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.


ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).

ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.

- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
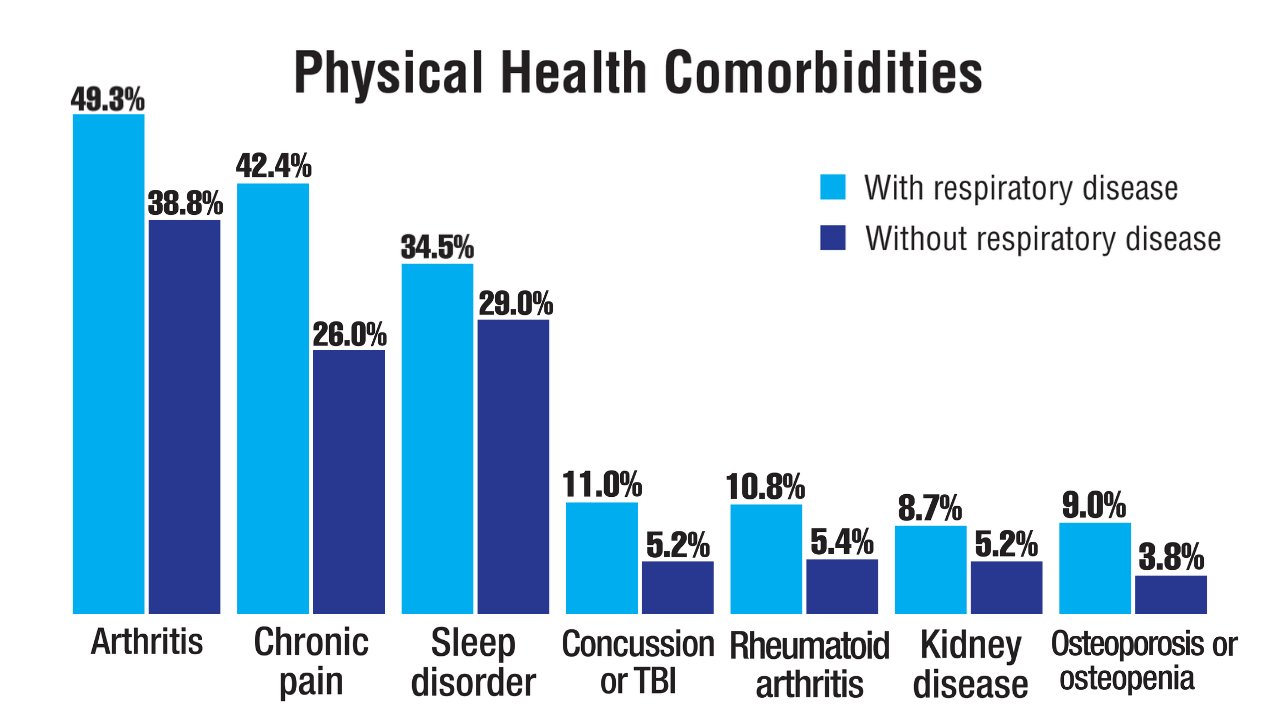
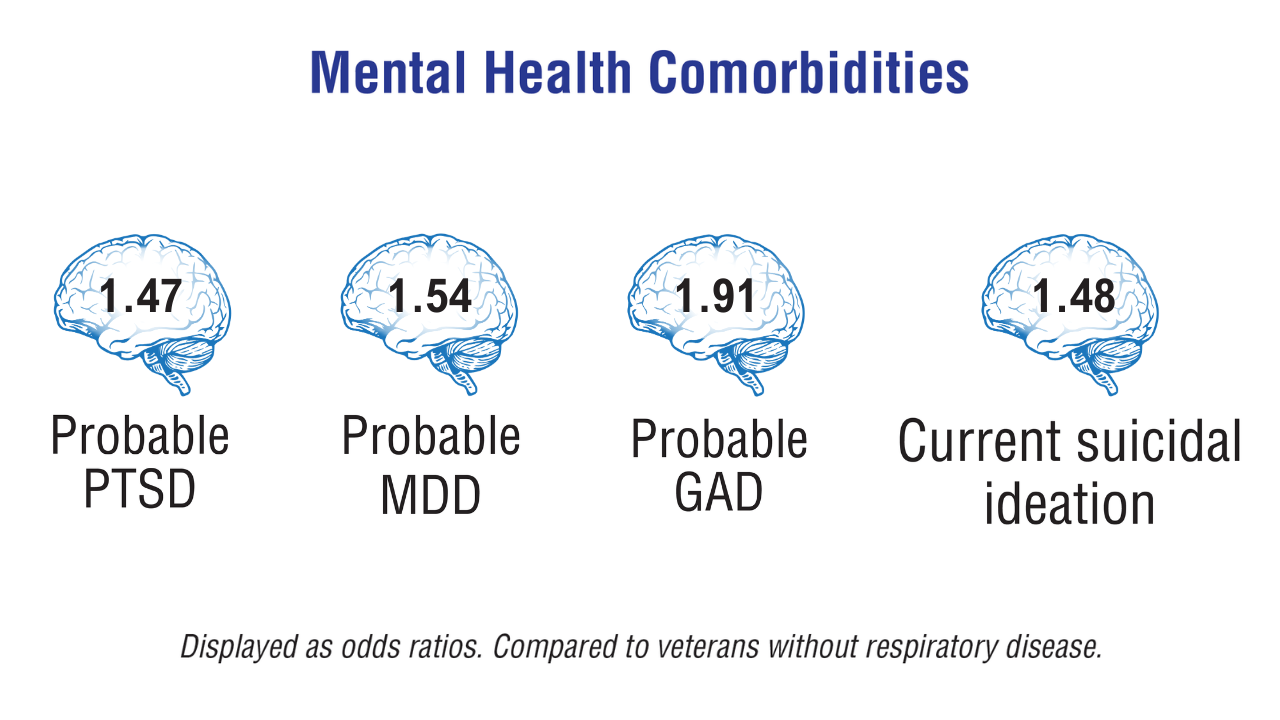
Military Deployment Raises Respiratory Disease Risk
Individuals who served in Iraq or Afghanistan had significantly higher rates of new-onset respiratory diseases after deployment compared to non-deployed control peers, based on data from more than 48,000 veterans. The findings were presented at the American College of Allergy, Asthma, and Immunology (ACAAI) 2025 Annual Meeting.
“Veterans deployed to Iraq and Afghanistan were often exposed to airborne hazards such as burn pits and dust storms,” said Patrick Gleeson, MD, an allergist at the University of Pennsylvania Perelman School of Medicine, Philadelphia, in a press release.
“We found that these exposures may have long-term health impacts, particularly for respiratory diseases that can affect quality of life for years after service,” said Gleeson, who presented the results at the meeting.
Gleeson and colleagues used data from the Veterans Affairs Corporate Data Warehouse and Observational Medical Outcomes Partnership to identify veterans with a single deployment as part of Operation Iraqi Freedom or Operation Enduring Freedom. Participants had at least one outpatient visit prior to deployment with no baseline history of asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis. The mean age of the participants at deployment was 26.7 years, 84% were male, 75% were White, and 11% were Hispanic or Latino. Each was matched with a similar non-deployed veteran control.
The primary outcome was outpatient diagnoses or problem list entries for asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis.
Compared to non-deployed peers, deployed veterans had a 55% increased risk of asthma, a 48% increased risk of nasal polyposis, a 41% increased risk of chronic rhinitis, and a 27% increased risk of chronic rhinosinusitis, based on Cox proportional hazards models (P < .0005 for all).
The findings were limited by the retrospective design. However, “Recognizing the link between deployment and respiratory disease can help guide medical support, policy, and preventive strategies for those affected,” Gleeson said in the press release.
The study received no outside funding. The researchers disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Individuals who served in Iraq or Afghanistan had significantly higher rates of new-onset respiratory diseases after deployment compared to non-deployed control peers, based on data from more than 48,000 veterans. The findings were presented at the American College of Allergy, Asthma, and Immunology (ACAAI) 2025 Annual Meeting.
“Veterans deployed to Iraq and Afghanistan were often exposed to airborne hazards such as burn pits and dust storms,” said Patrick Gleeson, MD, an allergist at the University of Pennsylvania Perelman School of Medicine, Philadelphia, in a press release.
“We found that these exposures may have long-term health impacts, particularly for respiratory diseases that can affect quality of life for years after service,” said Gleeson, who presented the results at the meeting.
Gleeson and colleagues used data from the Veterans Affairs Corporate Data Warehouse and Observational Medical Outcomes Partnership to identify veterans with a single deployment as part of Operation Iraqi Freedom or Operation Enduring Freedom. Participants had at least one outpatient visit prior to deployment with no baseline history of asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis. The mean age of the participants at deployment was 26.7 years, 84% were male, 75% were White, and 11% were Hispanic or Latino. Each was matched with a similar non-deployed veteran control.
The primary outcome was outpatient diagnoses or problem list entries for asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis.
Compared to non-deployed peers, deployed veterans had a 55% increased risk of asthma, a 48% increased risk of nasal polyposis, a 41% increased risk of chronic rhinitis, and a 27% increased risk of chronic rhinosinusitis, based on Cox proportional hazards models (P < .0005 for all).
The findings were limited by the retrospective design. However, “Recognizing the link between deployment and respiratory disease can help guide medical support, policy, and preventive strategies for those affected,” Gleeson said in the press release.
The study received no outside funding. The researchers disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Individuals who served in Iraq or Afghanistan had significantly higher rates of new-onset respiratory diseases after deployment compared to non-deployed control peers, based on data from more than 48,000 veterans. The findings were presented at the American College of Allergy, Asthma, and Immunology (ACAAI) 2025 Annual Meeting.
“Veterans deployed to Iraq and Afghanistan were often exposed to airborne hazards such as burn pits and dust storms,” said Patrick Gleeson, MD, an allergist at the University of Pennsylvania Perelman School of Medicine, Philadelphia, in a press release.
“We found that these exposures may have long-term health impacts, particularly for respiratory diseases that can affect quality of life for years after service,” said Gleeson, who presented the results at the meeting.
Gleeson and colleagues used data from the Veterans Affairs Corporate Data Warehouse and Observational Medical Outcomes Partnership to identify veterans with a single deployment as part of Operation Iraqi Freedom or Operation Enduring Freedom. Participants had at least one outpatient visit prior to deployment with no baseline history of asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis. The mean age of the participants at deployment was 26.7 years, 84% were male, 75% were White, and 11% were Hispanic or Latino. Each was matched with a similar non-deployed veteran control.
The primary outcome was outpatient diagnoses or problem list entries for asthma, chronic rhinitis, chronic rhinosinusitis, or nasal polyposis.
Compared to non-deployed peers, deployed veterans had a 55% increased risk of asthma, a 48% increased risk of nasal polyposis, a 41% increased risk of chronic rhinitis, and a 27% increased risk of chronic rhinosinusitis, based on Cox proportional hazards models (P < .0005 for all).
The findings were limited by the retrospective design. However, “Recognizing the link between deployment and respiratory disease can help guide medical support, policy, and preventive strategies for those affected,” Gleeson said in the press release.
The study received no outside funding. The researchers disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACAAI 2025
Metastatic Pulmonary LCNEC With Pancreatic Involvement in a Young Non-Smoker: An Unusual Presentation
Background
Pulmonary large-cell neuroendocrine carcinoma (LCNEC) is a rare, aggressive lung cancer subtype, comprising ~3% of lung malignancies. It commonly affects older, heavy smokers and presents at an advanced stage. Prognosis is poor, with a 5-year survival rate of 15–25% in metastatic disease.
Case Presentation
A 33-year-old previously healthy male presented with a month of abdominal and lower back pain, along with significant weight loss. Lab tests revealed elevated lipase (378), and he was initially treated for acute pancreatitis. Imaging revealed a 1.9 cm pancreatic head mass and three hypodense hepatic lesions. MRI confirmed these findings but remained inconclusive. An incidental 8 mm right lower lobe pulmonary nodule led to chest CT, identifying a dominant left lower lobe mass and mediastinal lymphadenopathy, raising suspicion for primary lung malignancy. The patient was discharged but returned three days later with worsening symptoms and a lipase of 754. Endoscopic biopsy of the pancreatic mass was deferred due to ongoing pancreatitis. A liver biopsy revealed neuroendocrine differentiation, positive for CK AE1/AE3, CK7, CK19, and synaptophysin. Molecular profiling showed PD-L1 (TPS 50%), low tumor mutational burden, microsatellite stability, and high loss of heterozygosity. Bronchoscopy revealed a left hilar mass, and lymph node biopsy confirmed LCNEC (CK7+, chromogranin+, TTF- 1+, synaptophysin+), establishing a diagnosis of stage IV pulmonary LCNEC with pancreatic and liver metastases. The patient began treatment with bevacizumab, paclitaxel, carboplatin, and atezolizumab, resulting in improvement in hilar, hepatic, and pancreatic lesions on further imagings. The patient was continued on chemoimmunotherapy.
Discussion
This case highlights an uncommon presentation of LCNEC in a young, non-smoking male, initially manifesting as pancreatitis due to pancreatic metastasis. The absence of pulmonary symptoms complicated the diagnosis. Histopathology and immunohistochemistry were essential. While no standardized treatment exists for LCNEC, platinum-based chemotherapy with immunotherapy remains the mainstay. PD-L1 expression may guide immunotherapy decisions.
Conclusions
Pulmonary LCNEC should be considered in metastatic neuroendocrine tumors, even in young, non-smoking patients without pulmonary symptoms. Early tissue diagnosis and molecular profiling are key to guiding management.
Background
Pulmonary large-cell neuroendocrine carcinoma (LCNEC) is a rare, aggressive lung cancer subtype, comprising ~3% of lung malignancies. It commonly affects older, heavy smokers and presents at an advanced stage. Prognosis is poor, with a 5-year survival rate of 15–25% in metastatic disease.
Case Presentation
A 33-year-old previously healthy male presented with a month of abdominal and lower back pain, along with significant weight loss. Lab tests revealed elevated lipase (378), and he was initially treated for acute pancreatitis. Imaging revealed a 1.9 cm pancreatic head mass and three hypodense hepatic lesions. MRI confirmed these findings but remained inconclusive. An incidental 8 mm right lower lobe pulmonary nodule led to chest CT, identifying a dominant left lower lobe mass and mediastinal lymphadenopathy, raising suspicion for primary lung malignancy. The patient was discharged but returned three days later with worsening symptoms and a lipase of 754. Endoscopic biopsy of the pancreatic mass was deferred due to ongoing pancreatitis. A liver biopsy revealed neuroendocrine differentiation, positive for CK AE1/AE3, CK7, CK19, and synaptophysin. Molecular profiling showed PD-L1 (TPS 50%), low tumor mutational burden, microsatellite stability, and high loss of heterozygosity. Bronchoscopy revealed a left hilar mass, and lymph node biopsy confirmed LCNEC (CK7+, chromogranin+, TTF- 1+, synaptophysin+), establishing a diagnosis of stage IV pulmonary LCNEC with pancreatic and liver metastases. The patient began treatment with bevacizumab, paclitaxel, carboplatin, and atezolizumab, resulting in improvement in hilar, hepatic, and pancreatic lesions on further imagings. The patient was continued on chemoimmunotherapy.
Discussion
This case highlights an uncommon presentation of LCNEC in a young, non-smoking male, initially manifesting as pancreatitis due to pancreatic metastasis. The absence of pulmonary symptoms complicated the diagnosis. Histopathology and immunohistochemistry were essential. While no standardized treatment exists for LCNEC, platinum-based chemotherapy with immunotherapy remains the mainstay. PD-L1 expression may guide immunotherapy decisions.
Conclusions
Pulmonary LCNEC should be considered in metastatic neuroendocrine tumors, even in young, non-smoking patients without pulmonary symptoms. Early tissue diagnosis and molecular profiling are key to guiding management.
Background
Pulmonary large-cell neuroendocrine carcinoma (LCNEC) is a rare, aggressive lung cancer subtype, comprising ~3% of lung malignancies. It commonly affects older, heavy smokers and presents at an advanced stage. Prognosis is poor, with a 5-year survival rate of 15–25% in metastatic disease.
Case Presentation
A 33-year-old previously healthy male presented with a month of abdominal and lower back pain, along with significant weight loss. Lab tests revealed elevated lipase (378), and he was initially treated for acute pancreatitis. Imaging revealed a 1.9 cm pancreatic head mass and three hypodense hepatic lesions. MRI confirmed these findings but remained inconclusive. An incidental 8 mm right lower lobe pulmonary nodule led to chest CT, identifying a dominant left lower lobe mass and mediastinal lymphadenopathy, raising suspicion for primary lung malignancy. The patient was discharged but returned three days later with worsening symptoms and a lipase of 754. Endoscopic biopsy of the pancreatic mass was deferred due to ongoing pancreatitis. A liver biopsy revealed neuroendocrine differentiation, positive for CK AE1/AE3, CK7, CK19, and synaptophysin. Molecular profiling showed PD-L1 (TPS 50%), low tumor mutational burden, microsatellite stability, and high loss of heterozygosity. Bronchoscopy revealed a left hilar mass, and lymph node biopsy confirmed LCNEC (CK7+, chromogranin+, TTF- 1+, synaptophysin+), establishing a diagnosis of stage IV pulmonary LCNEC with pancreatic and liver metastases. The patient began treatment with bevacizumab, paclitaxel, carboplatin, and atezolizumab, resulting in improvement in hilar, hepatic, and pancreatic lesions on further imagings. The patient was continued on chemoimmunotherapy.
Discussion
This case highlights an uncommon presentation of LCNEC in a young, non-smoking male, initially manifesting as pancreatitis due to pancreatic metastasis. The absence of pulmonary symptoms complicated the diagnosis. Histopathology and immunohistochemistry were essential. While no standardized treatment exists for LCNEC, platinum-based chemotherapy with immunotherapy remains the mainstay. PD-L1 expression may guide immunotherapy decisions.
Conclusions
Pulmonary LCNEC should be considered in metastatic neuroendocrine tumors, even in young, non-smoking patients without pulmonary symptoms. Early tissue diagnosis and molecular profiling are key to guiding management.
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
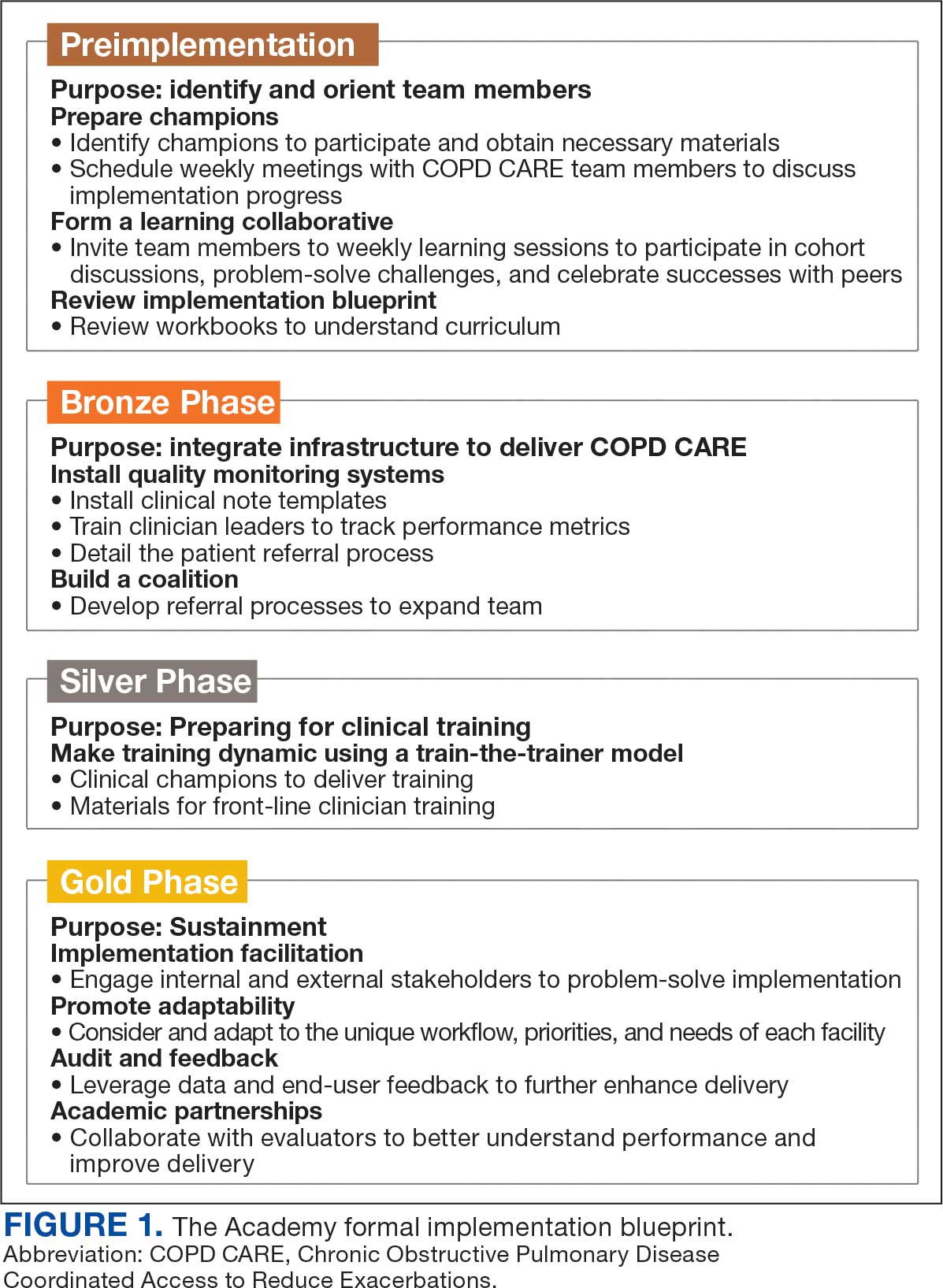
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.
Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
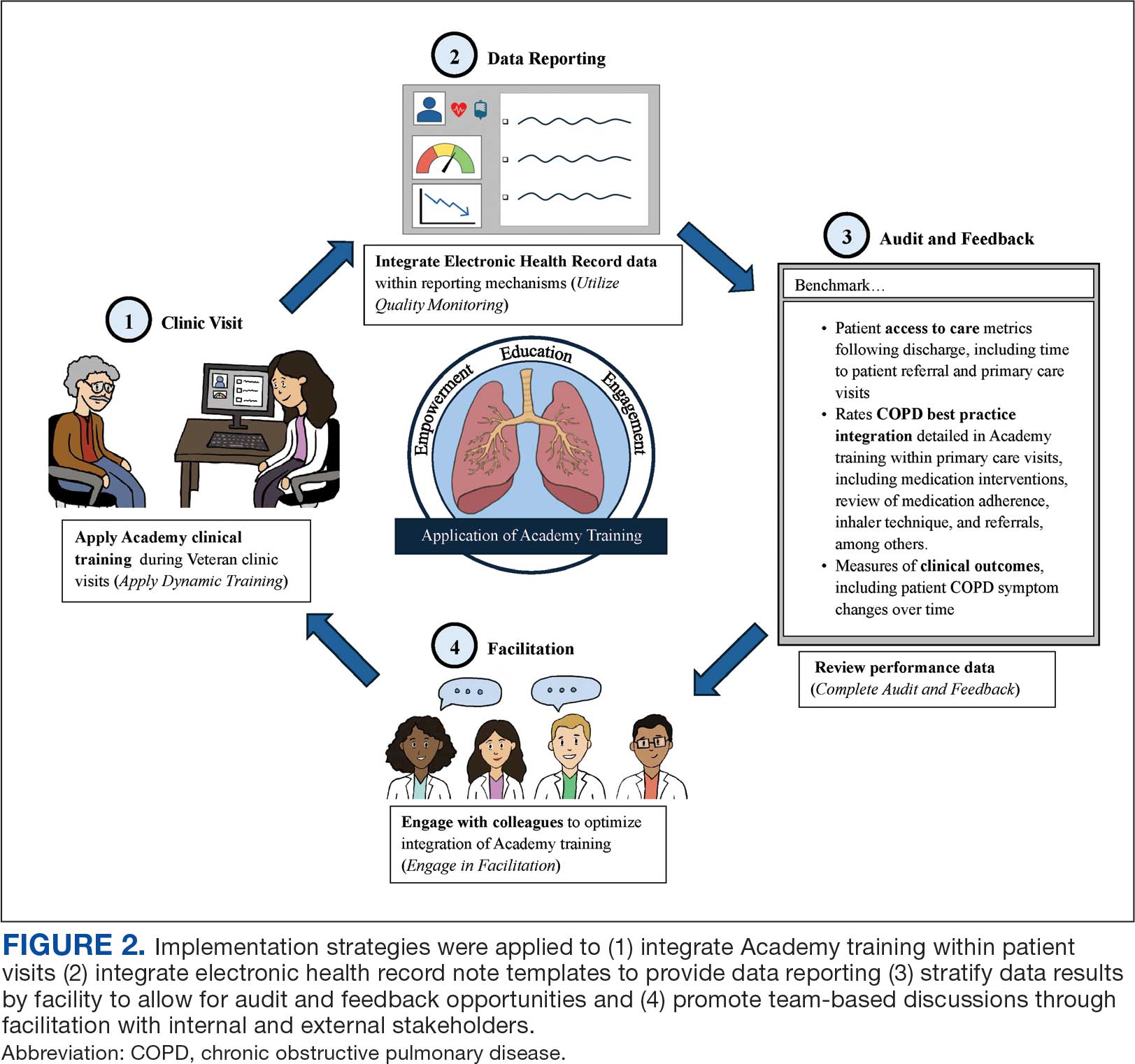
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.
While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Data Trends 2025: Pulmonology
Data Trends 2025: Pulmonology
Click to view more from Federal Health Care Data Trends 2025.
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
Data Trends 2025: Pulmonology
Data Trends 2025: Pulmonology
Older Veterans May Be at Risk for Cannabis Use Disorder
Older Veterans May Be at Risk for Cannabis Use Disorder
Research on cannabis use disorder (CUD) has mainly focused on individuals aged < 65 years, but a recently published study in JAMA Network Open found one-third of older veterans who had used cannabis in the previous 30 days screened positive for CUD.
The cross-sectional study of 4503 veterans aged 65 to 84 years from the US Department of Veterans Affairs (VA) Cannabis and Aging Cohort found 57% of participants reported lifetime cannabis use, with 29% citing medical reasons, usually for pain management. About 10% reported using cannabis in the previous 30 days, with 52% reporting use for ≥ 20 days in a month. The odds of CUD were higher among men, respondents aged < 76 years, individuals with anxiety, and individuals who reported any illicit drug use or frequent cannabis use.
In 2019, 9.8% of veterans reported using cannabis in the previous year. In 2019 to 2020, > 20% of veterans aged 18 to 44 years said they had used cannabis in the previous 6 months. According to VA Health Systems Research, about 1 in 11 veterans had used cannabis in the previous year. Compared to the general US population, recent cannabis use was similar or slightly lower among veterans. Among those with previous year use, however, the percentage of veterans using cannabis for medical purposes was more than double that of the general population.
Older veterans are particularly at risk for CUD. Cannabis use can increase the chance of neuropsychiatric disorders, respiratory symptoms, and cardiovascular outcomes—all leading causes of death in older adults. They also have an elevated risk of suicidal ideation and therefore may be particularly susceptible to adverse effects of cannabis, even if used for therapeutic purposes.
In addition to CUD, older veterans may be at risk for tetrahydrocannabinol (THC) intoxication if they are unable to tolerate cannabis potency or the latent THC components found in products marketed as only having cannabidiol. THC is the primary psychoactive compound found in the cannabis plant and interacts with brain cannabinoid receptors to affect mood, perception, and various bodily functions. Cannabis potency has increased from about 3% in the 1980s to about 15% in recent years; the average THC-to-CBD ratio has increased substantially over the past decade.
Unlike veterans aged 18 to 25 years, those aged ≥ 65 years are less likely to use recreational cannabis, are more likely to use medicinal cannabis recommended by a health care professional, and report use for pain management, insomnia, and mental health (including posttraumatic stress disorder [PTSD]). Some research indicates that rates of cannabis use and CUD are particularly elevated among veterans with PTSD and major depressive disorder who may use cannabis as a means of coping with negative affect and sleep disturbances. PTSD is recognized as a qualifying condition by states that have legalized medicinal cannabis.
Sleep disturbance, especially in conjunction with PTSD, is associated with CUD among veterans. According to the VA, research does not support cannabis as an effective PTSD treatment, a reason the 2023 VA/DoD Clinical Practice Guideline for PTSD does not recommend it for that use. In 2020, lifetime prevalence of CUD among veterans was 9.2%; the prevalence of past-6-month CUD diagnoses among veterans was 2.7%. Among veterans with PTSD, however, CUD rates were much higher (12.1%).
Current VA guidelines recommend that patients with CUD be offered referral to mental health services for evidence-based treatments, including motivational interviews, contingency management, and cognitive behavioral therapy. The JAMA Network Open study notes the importance of screening and informing older veterans about the risks of cannabis use: “Unidentified, patients cannot be offered existing evidence-based treatments. Despite increasing cannabis use among older adults, there is an inadequate evidence base on therapeutic benefits and potential harms from cannabis use among older people.”
Research on cannabis use disorder (CUD) has mainly focused on individuals aged < 65 years, but a recently published study in JAMA Network Open found one-third of older veterans who had used cannabis in the previous 30 days screened positive for CUD.
The cross-sectional study of 4503 veterans aged 65 to 84 years from the US Department of Veterans Affairs (VA) Cannabis and Aging Cohort found 57% of participants reported lifetime cannabis use, with 29% citing medical reasons, usually for pain management. About 10% reported using cannabis in the previous 30 days, with 52% reporting use for ≥ 20 days in a month. The odds of CUD were higher among men, respondents aged < 76 years, individuals with anxiety, and individuals who reported any illicit drug use or frequent cannabis use.
In 2019, 9.8% of veterans reported using cannabis in the previous year. In 2019 to 2020, > 20% of veterans aged 18 to 44 years said they had used cannabis in the previous 6 months. According to VA Health Systems Research, about 1 in 11 veterans had used cannabis in the previous year. Compared to the general US population, recent cannabis use was similar or slightly lower among veterans. Among those with previous year use, however, the percentage of veterans using cannabis for medical purposes was more than double that of the general population.
Older veterans are particularly at risk for CUD. Cannabis use can increase the chance of neuropsychiatric disorders, respiratory symptoms, and cardiovascular outcomes—all leading causes of death in older adults. They also have an elevated risk of suicidal ideation and therefore may be particularly susceptible to adverse effects of cannabis, even if used for therapeutic purposes.
In addition to CUD, older veterans may be at risk for tetrahydrocannabinol (THC) intoxication if they are unable to tolerate cannabis potency or the latent THC components found in products marketed as only having cannabidiol. THC is the primary psychoactive compound found in the cannabis plant and interacts with brain cannabinoid receptors to affect mood, perception, and various bodily functions. Cannabis potency has increased from about 3% in the 1980s to about 15% in recent years; the average THC-to-CBD ratio has increased substantially over the past decade.
Unlike veterans aged 18 to 25 years, those aged ≥ 65 years are less likely to use recreational cannabis, are more likely to use medicinal cannabis recommended by a health care professional, and report use for pain management, insomnia, and mental health (including posttraumatic stress disorder [PTSD]). Some research indicates that rates of cannabis use and CUD are particularly elevated among veterans with PTSD and major depressive disorder who may use cannabis as a means of coping with negative affect and sleep disturbances. PTSD is recognized as a qualifying condition by states that have legalized medicinal cannabis.
Sleep disturbance, especially in conjunction with PTSD, is associated with CUD among veterans. According to the VA, research does not support cannabis as an effective PTSD treatment, a reason the 2023 VA/DoD Clinical Practice Guideline for PTSD does not recommend it for that use. In 2020, lifetime prevalence of CUD among veterans was 9.2%; the prevalence of past-6-month CUD diagnoses among veterans was 2.7%. Among veterans with PTSD, however, CUD rates were much higher (12.1%).
Current VA guidelines recommend that patients with CUD be offered referral to mental health services for evidence-based treatments, including motivational interviews, contingency management, and cognitive behavioral therapy. The JAMA Network Open study notes the importance of screening and informing older veterans about the risks of cannabis use: “Unidentified, patients cannot be offered existing evidence-based treatments. Despite increasing cannabis use among older adults, there is an inadequate evidence base on therapeutic benefits and potential harms from cannabis use among older people.”
Research on cannabis use disorder (CUD) has mainly focused on individuals aged < 65 years, but a recently published study in JAMA Network Open found one-third of older veterans who had used cannabis in the previous 30 days screened positive for CUD.
The cross-sectional study of 4503 veterans aged 65 to 84 years from the US Department of Veterans Affairs (VA) Cannabis and Aging Cohort found 57% of participants reported lifetime cannabis use, with 29% citing medical reasons, usually for pain management. About 10% reported using cannabis in the previous 30 days, with 52% reporting use for ≥ 20 days in a month. The odds of CUD were higher among men, respondents aged < 76 years, individuals with anxiety, and individuals who reported any illicit drug use or frequent cannabis use.
In 2019, 9.8% of veterans reported using cannabis in the previous year. In 2019 to 2020, > 20% of veterans aged 18 to 44 years said they had used cannabis in the previous 6 months. According to VA Health Systems Research, about 1 in 11 veterans had used cannabis in the previous year. Compared to the general US population, recent cannabis use was similar or slightly lower among veterans. Among those with previous year use, however, the percentage of veterans using cannabis for medical purposes was more than double that of the general population.
Older veterans are particularly at risk for CUD. Cannabis use can increase the chance of neuropsychiatric disorders, respiratory symptoms, and cardiovascular outcomes—all leading causes of death in older adults. They also have an elevated risk of suicidal ideation and therefore may be particularly susceptible to adverse effects of cannabis, even if used for therapeutic purposes.
In addition to CUD, older veterans may be at risk for tetrahydrocannabinol (THC) intoxication if they are unable to tolerate cannabis potency or the latent THC components found in products marketed as only having cannabidiol. THC is the primary psychoactive compound found in the cannabis plant and interacts with brain cannabinoid receptors to affect mood, perception, and various bodily functions. Cannabis potency has increased from about 3% in the 1980s to about 15% in recent years; the average THC-to-CBD ratio has increased substantially over the past decade.
Unlike veterans aged 18 to 25 years, those aged ≥ 65 years are less likely to use recreational cannabis, are more likely to use medicinal cannabis recommended by a health care professional, and report use for pain management, insomnia, and mental health (including posttraumatic stress disorder [PTSD]). Some research indicates that rates of cannabis use and CUD are particularly elevated among veterans with PTSD and major depressive disorder who may use cannabis as a means of coping with negative affect and sleep disturbances. PTSD is recognized as a qualifying condition by states that have legalized medicinal cannabis.
Sleep disturbance, especially in conjunction with PTSD, is associated with CUD among veterans. According to the VA, research does not support cannabis as an effective PTSD treatment, a reason the 2023 VA/DoD Clinical Practice Guideline for PTSD does not recommend it for that use. In 2020, lifetime prevalence of CUD among veterans was 9.2%; the prevalence of past-6-month CUD diagnoses among veterans was 2.7%. Among veterans with PTSD, however, CUD rates were much higher (12.1%).
Current VA guidelines recommend that patients with CUD be offered referral to mental health services for evidence-based treatments, including motivational interviews, contingency management, and cognitive behavioral therapy. The JAMA Network Open study notes the importance of screening and informing older veterans about the risks of cannabis use: “Unidentified, patients cannot be offered existing evidence-based treatments. Despite increasing cannabis use among older adults, there is an inadequate evidence base on therapeutic benefits and potential harms from cannabis use among older people.”
Older Veterans May Be at Risk for Cannabis Use Disorder
Older Veterans May Be at Risk for Cannabis Use Disorder
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.
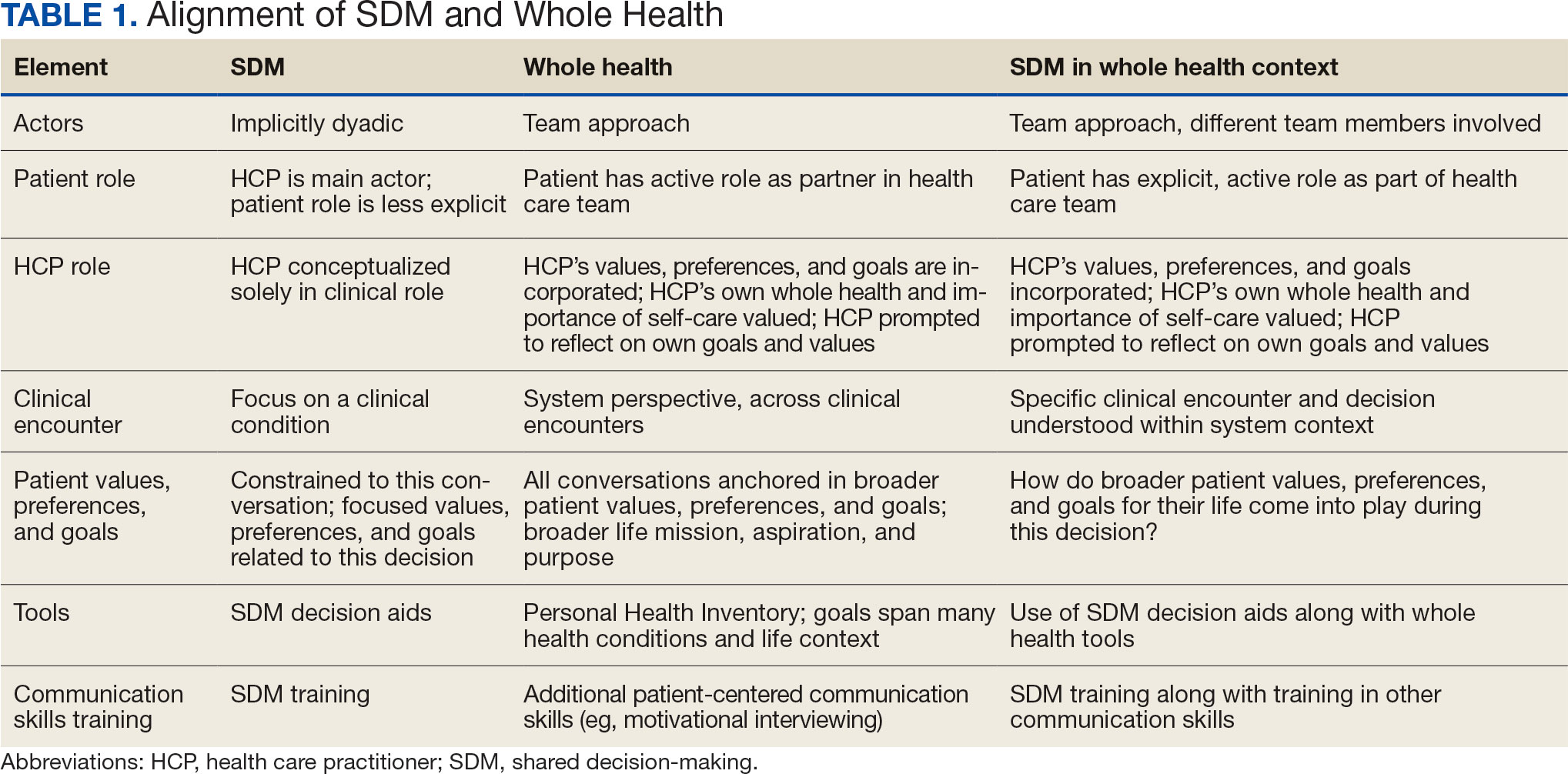

STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
Open Clinical Trials for Patients With Chronic Obstructive Pulmonary Disease
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
Patients With Asthma and COPD At Increased Cancer Risk From Microplastics
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.