User login
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
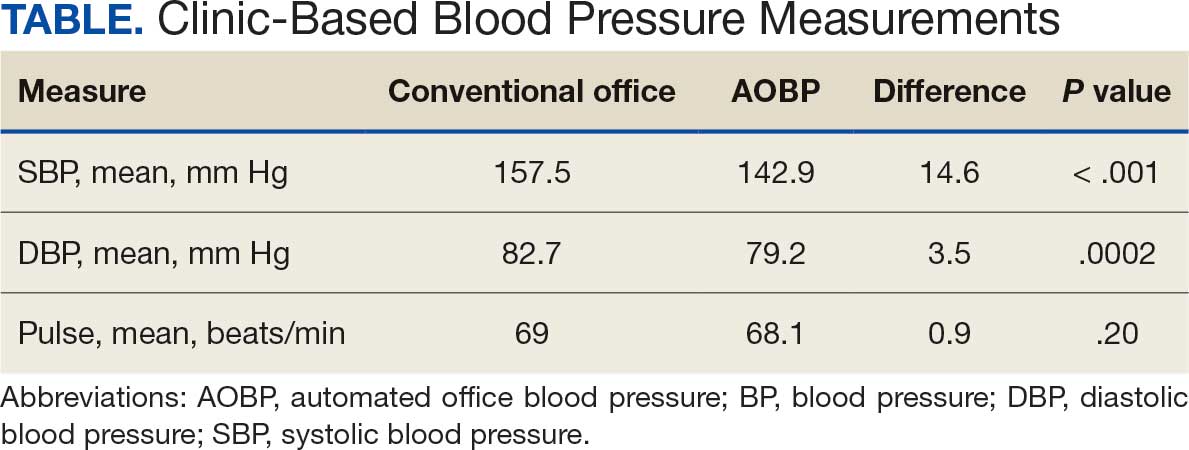
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).
A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).
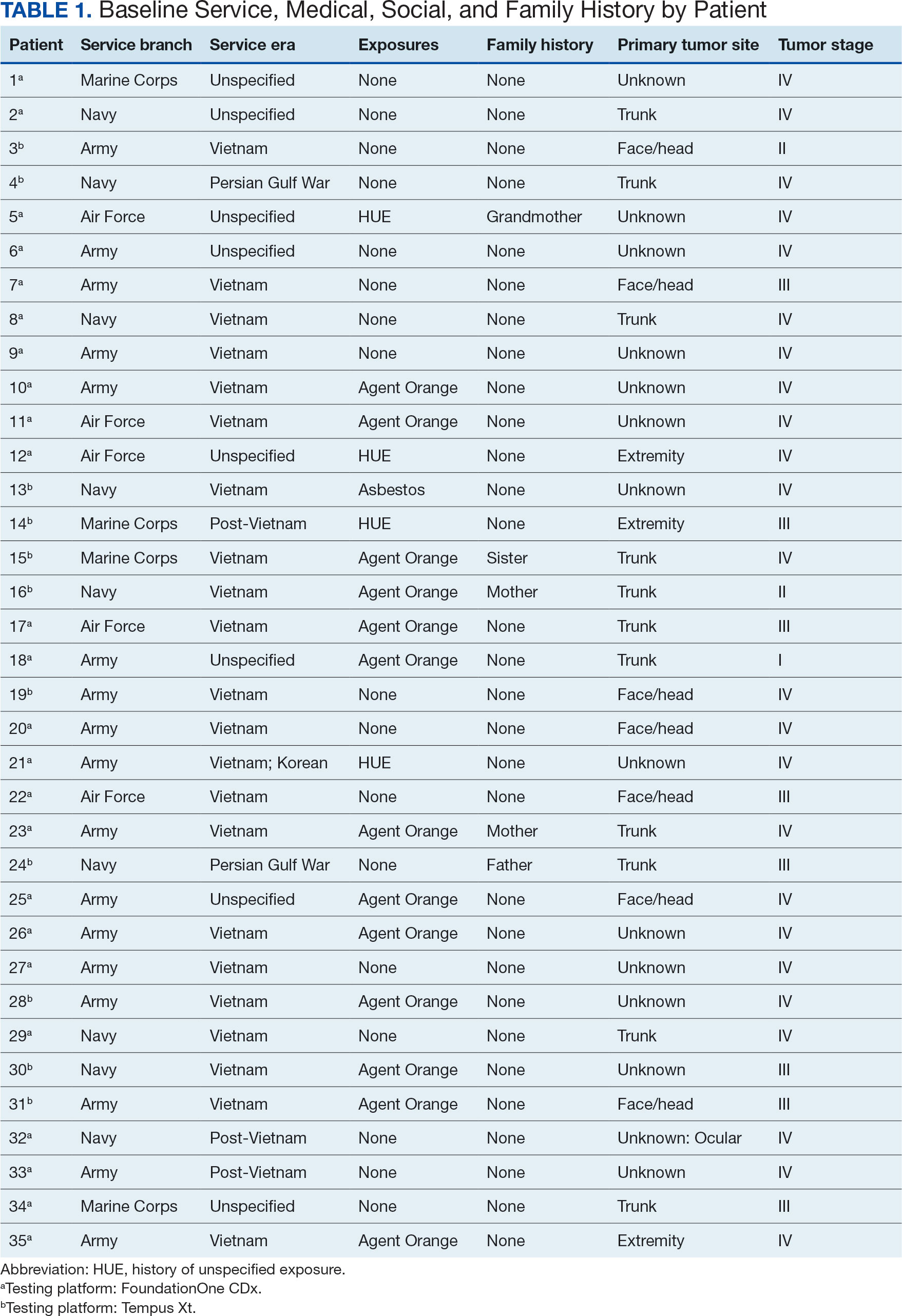
Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.
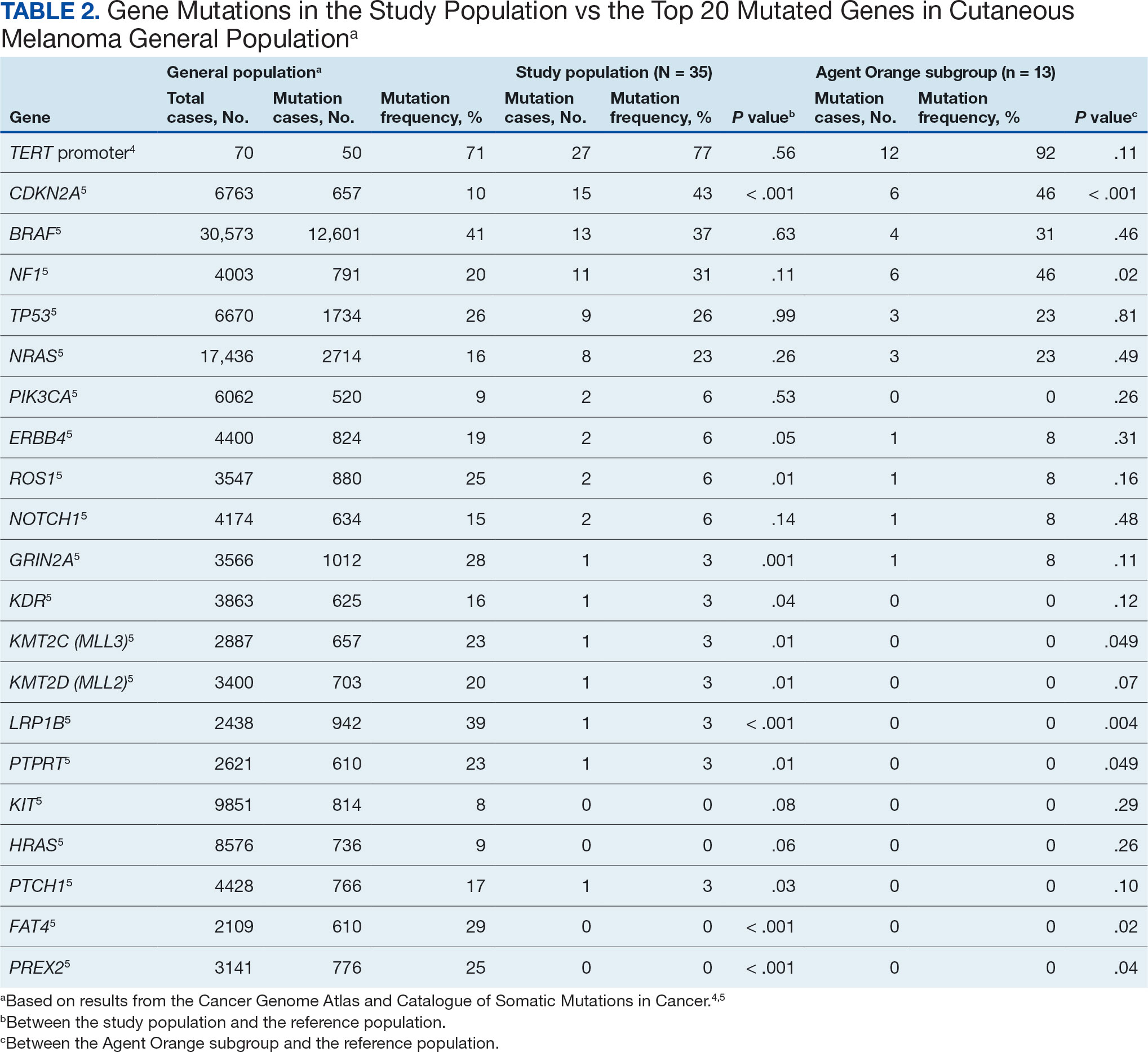
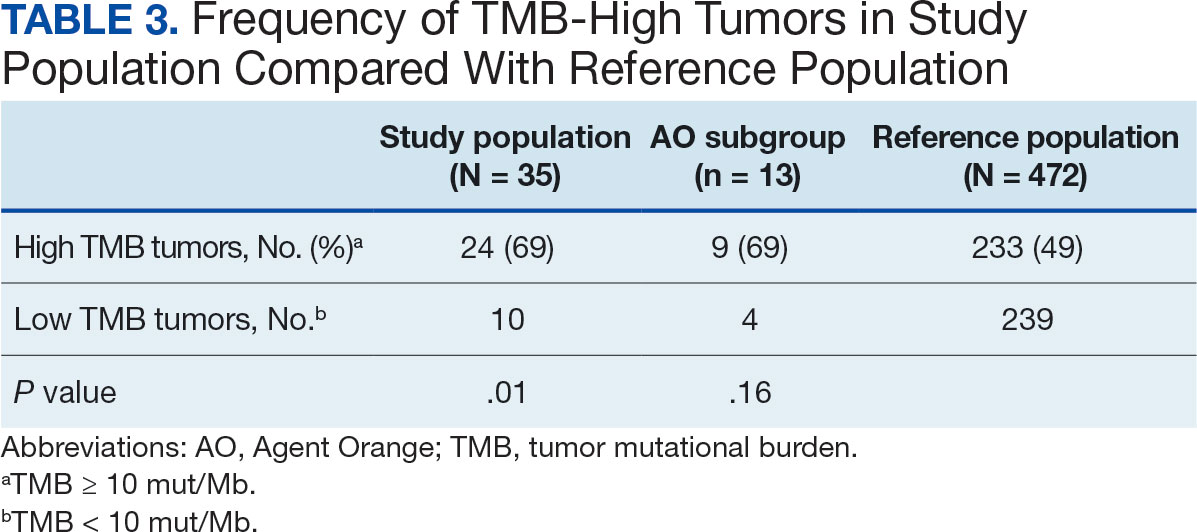
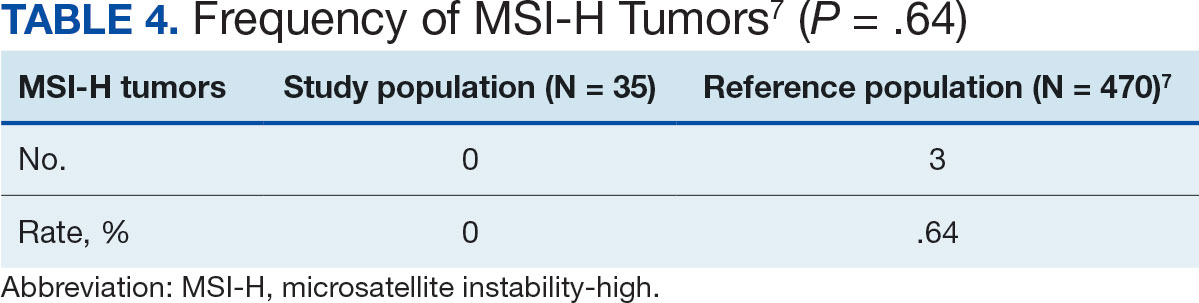
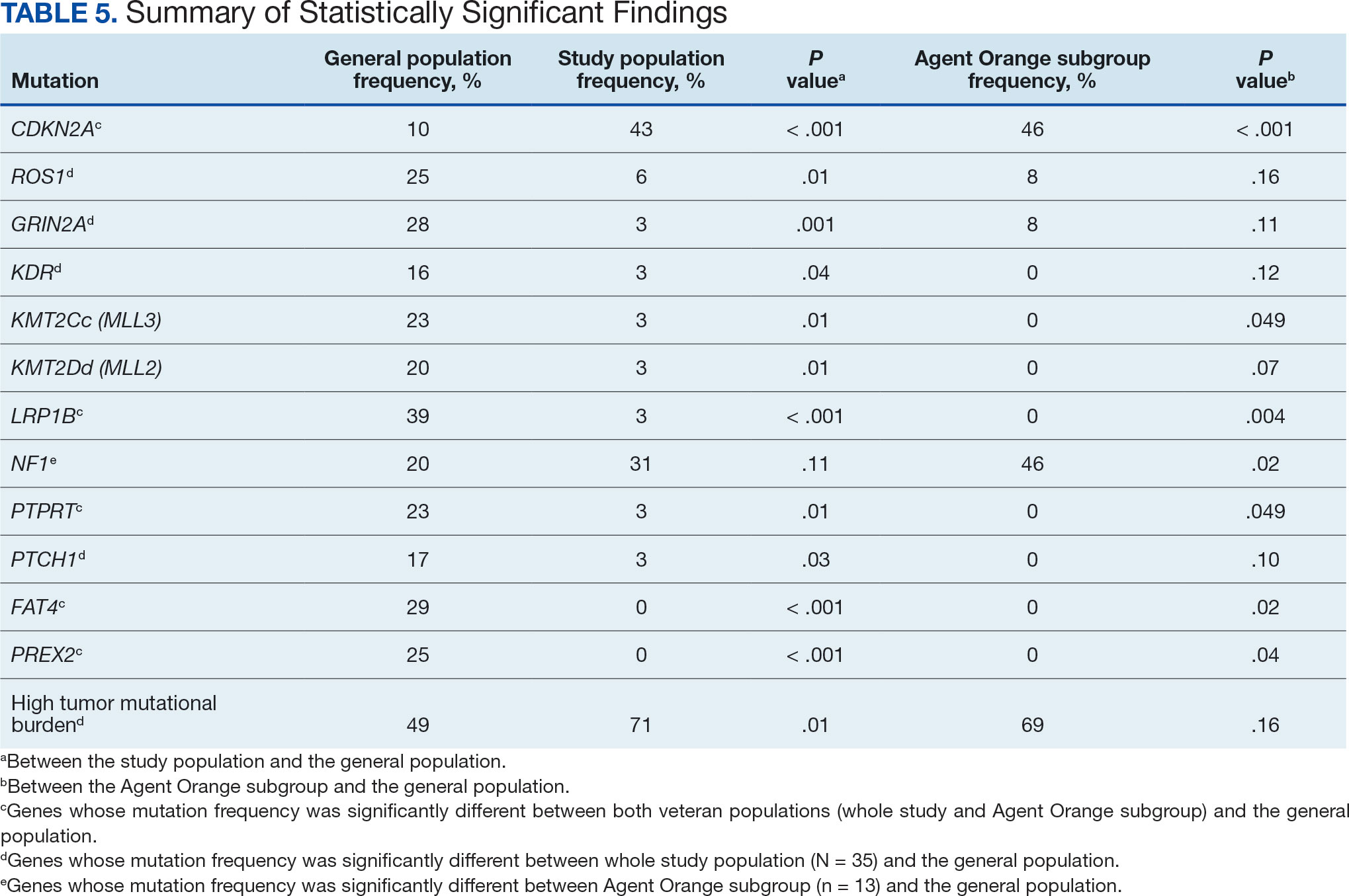
Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
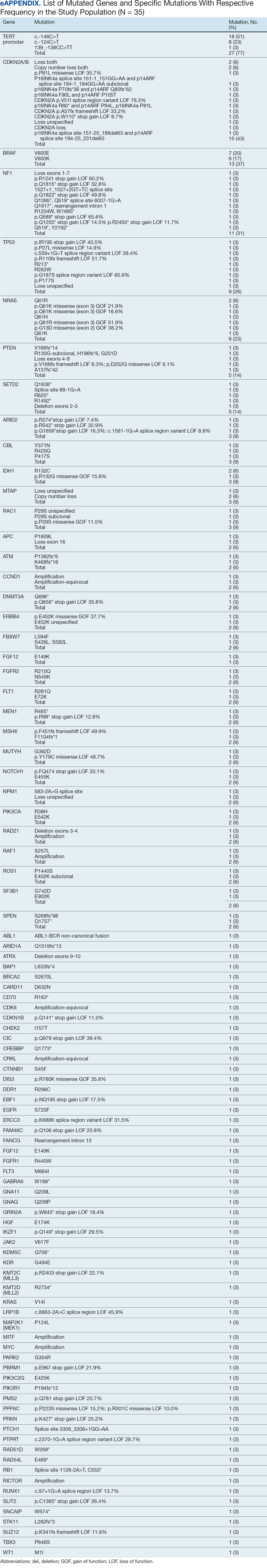
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).
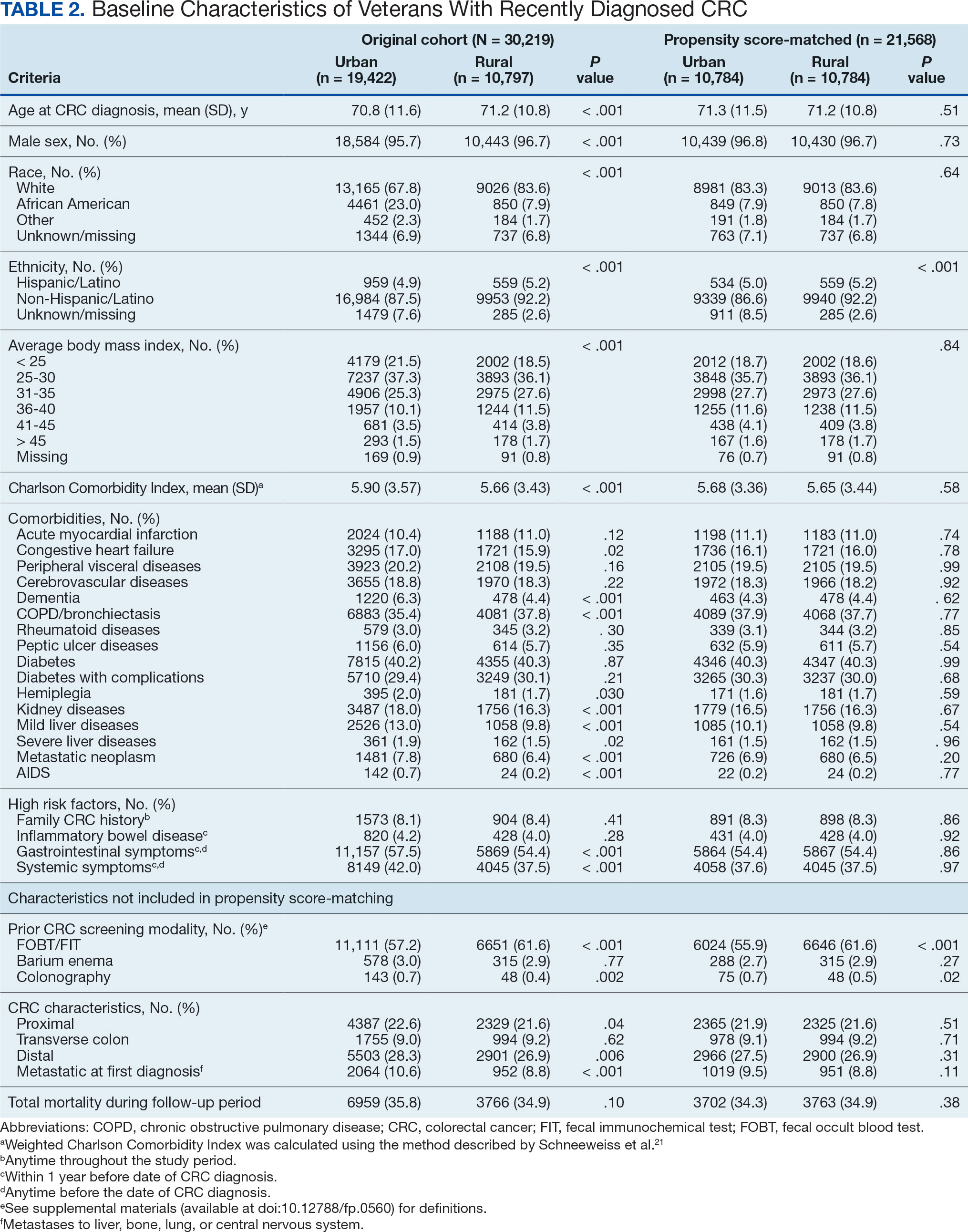
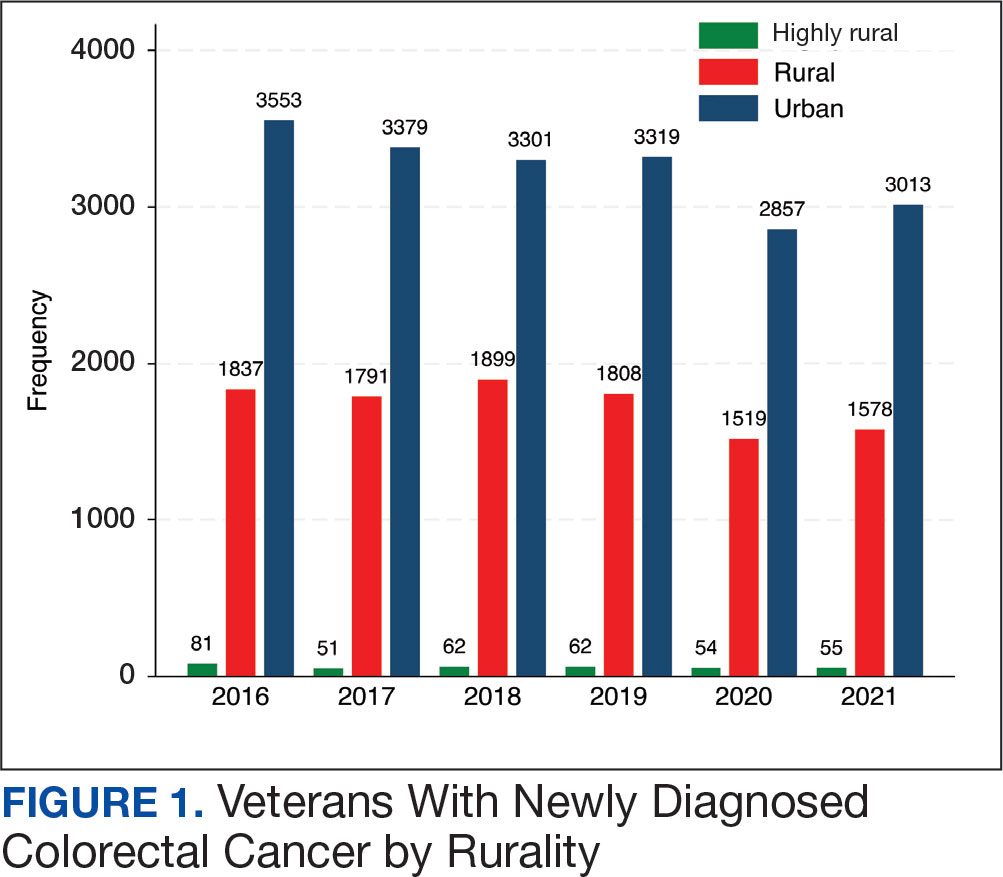
There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).
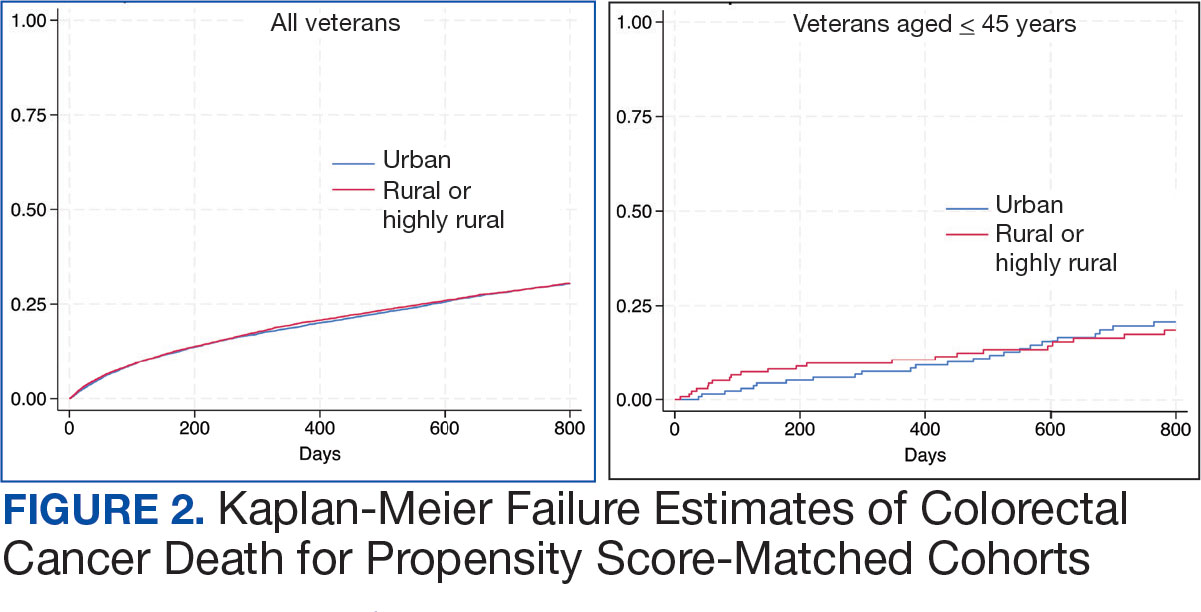
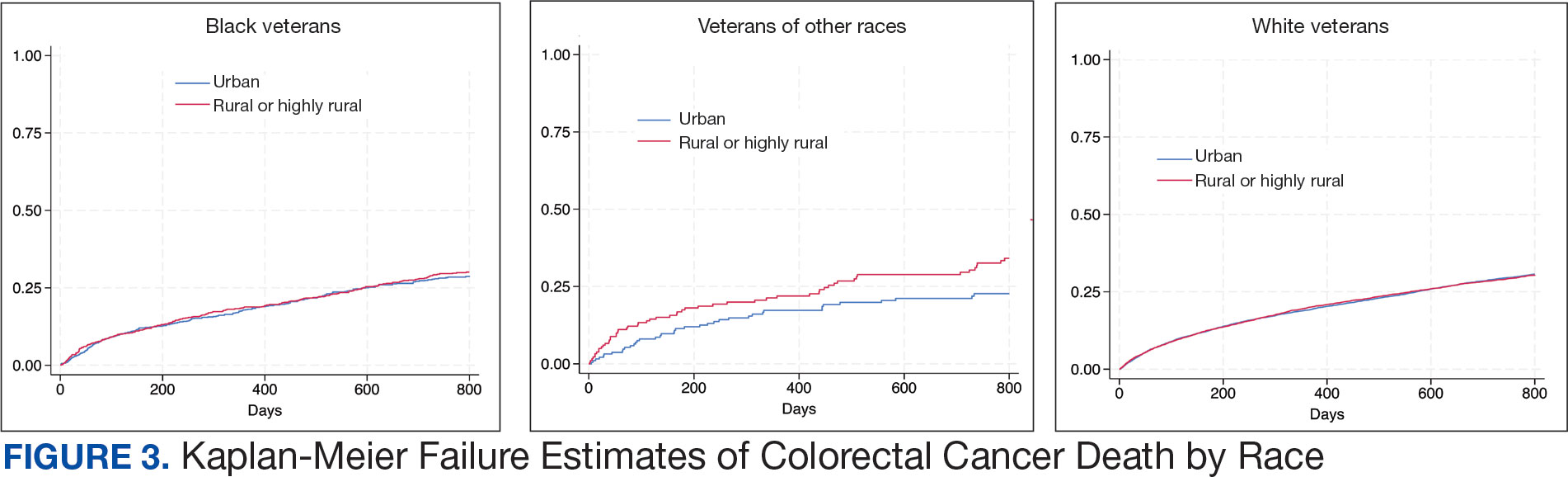
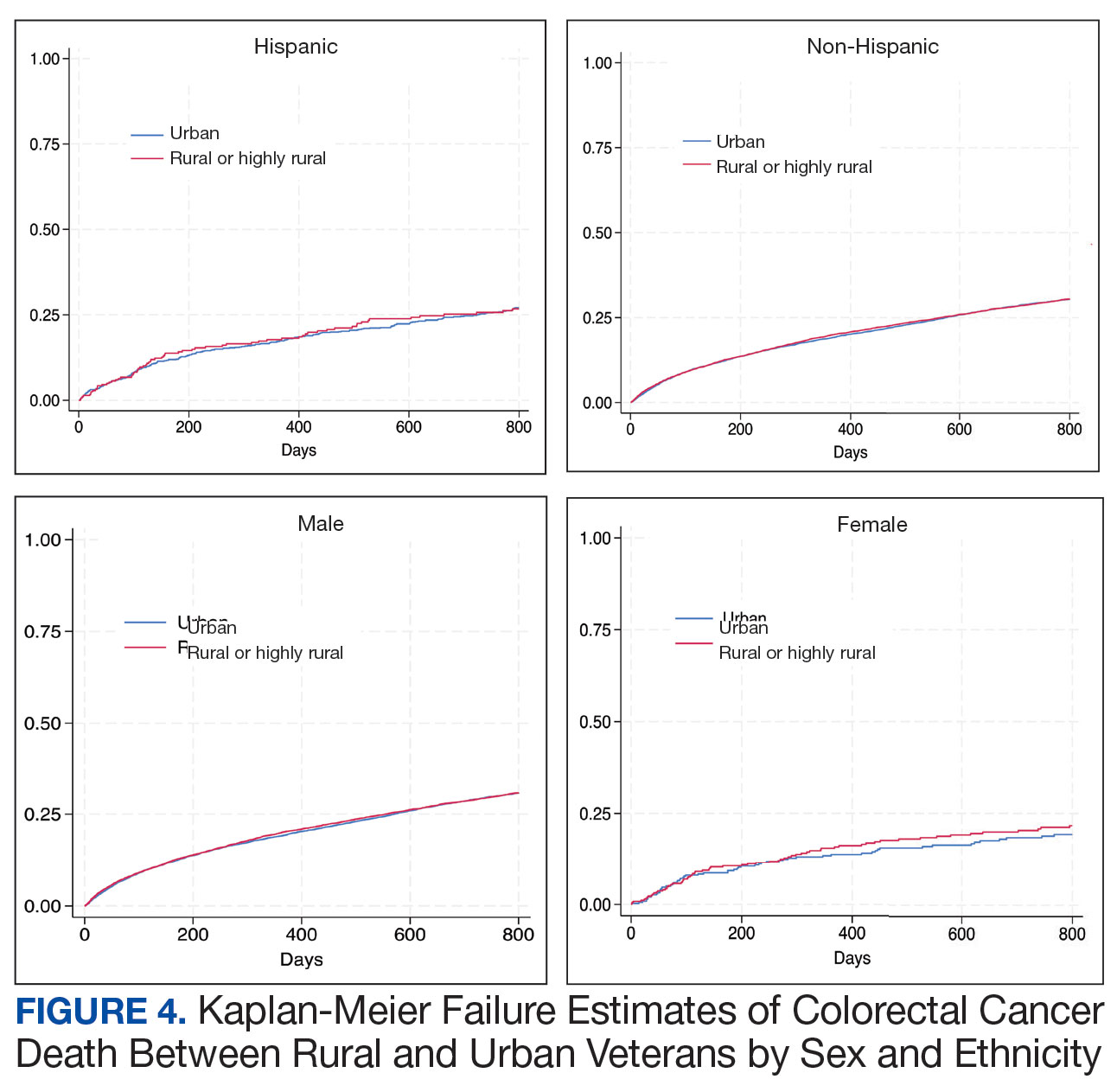
Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).
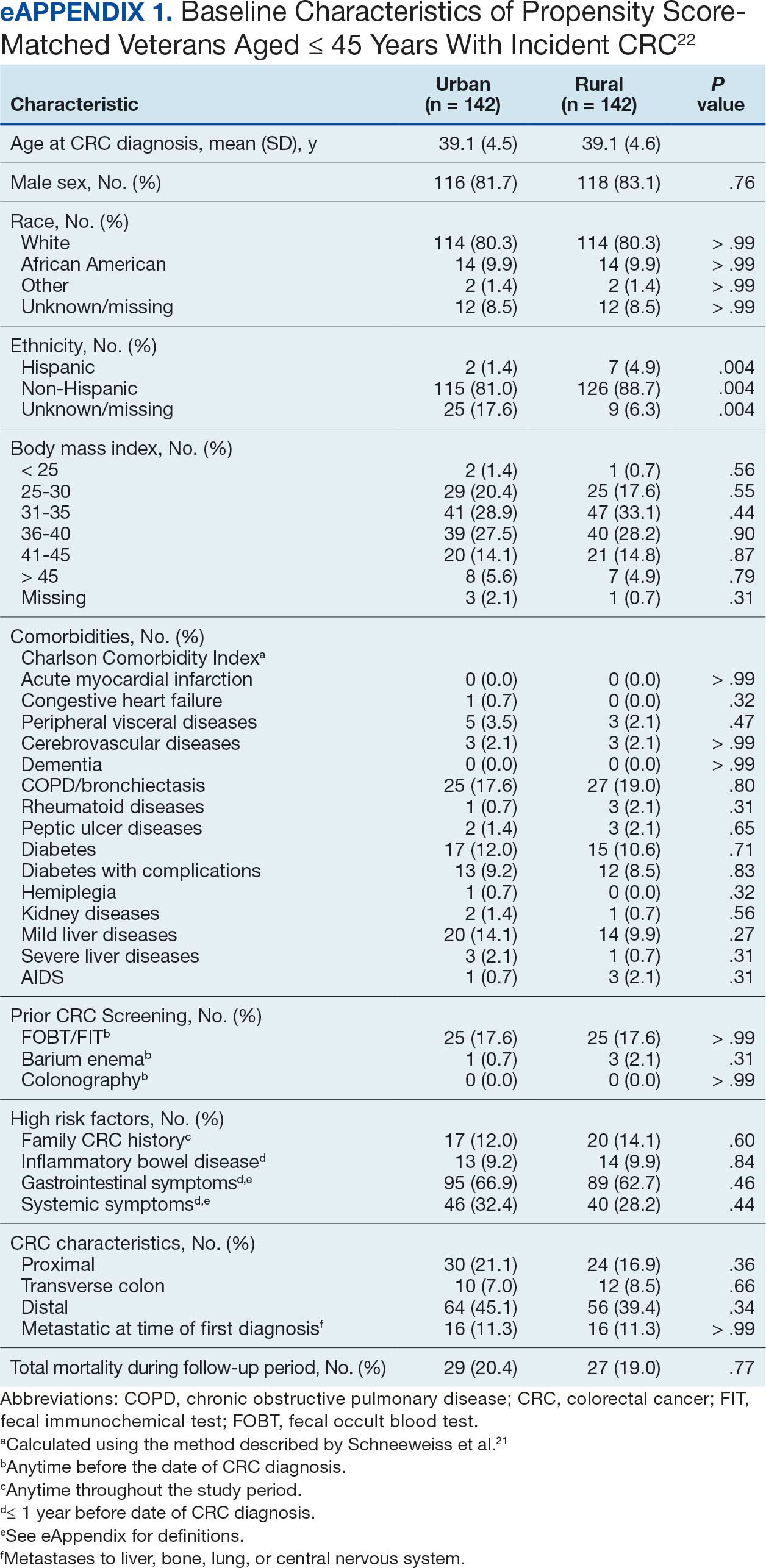
There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).
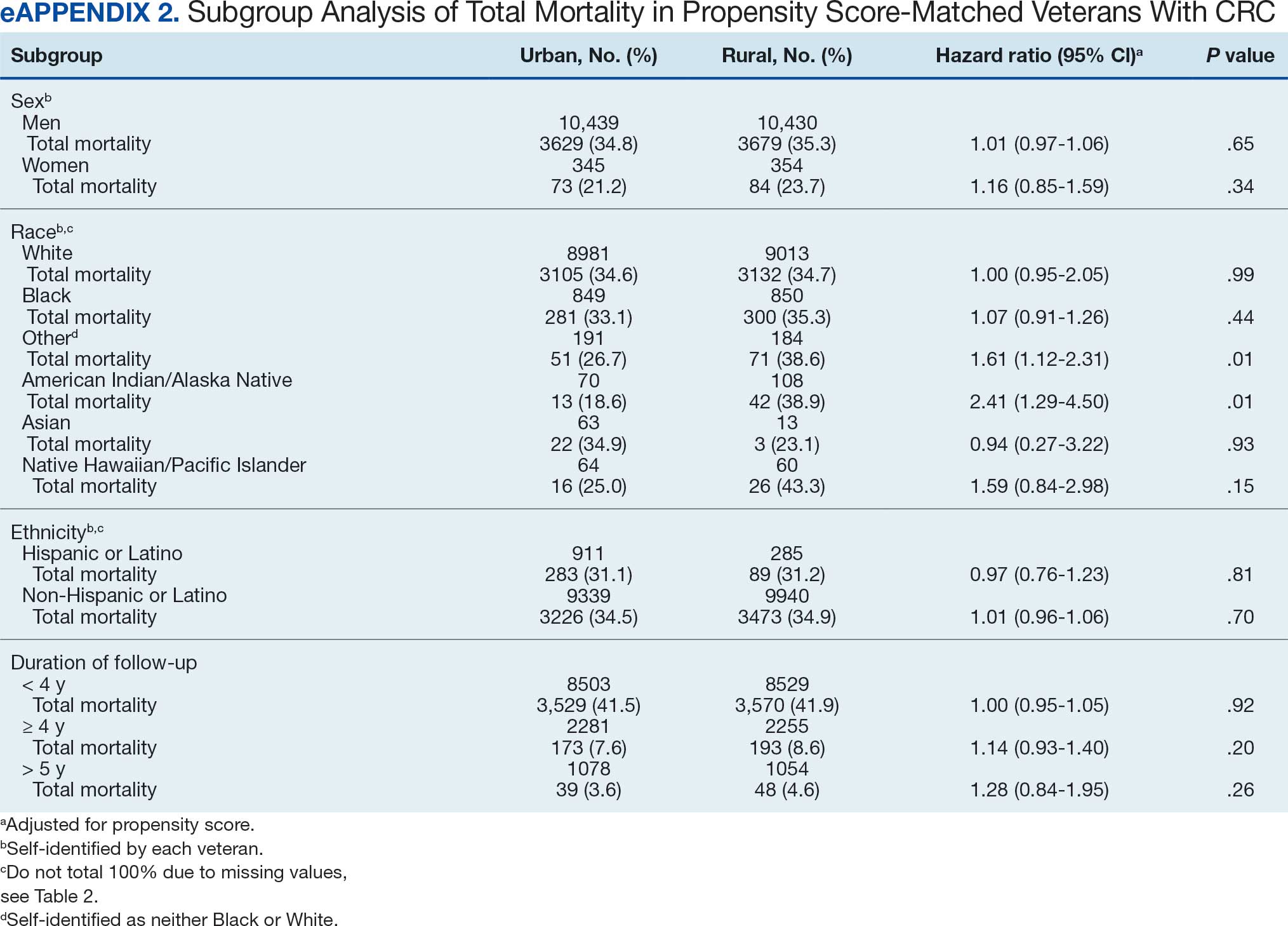
Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Continuous Glucose Monitoring vs Fingerstick Monitoring for Hemoglobin A1c Control in Veterans
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.
This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.
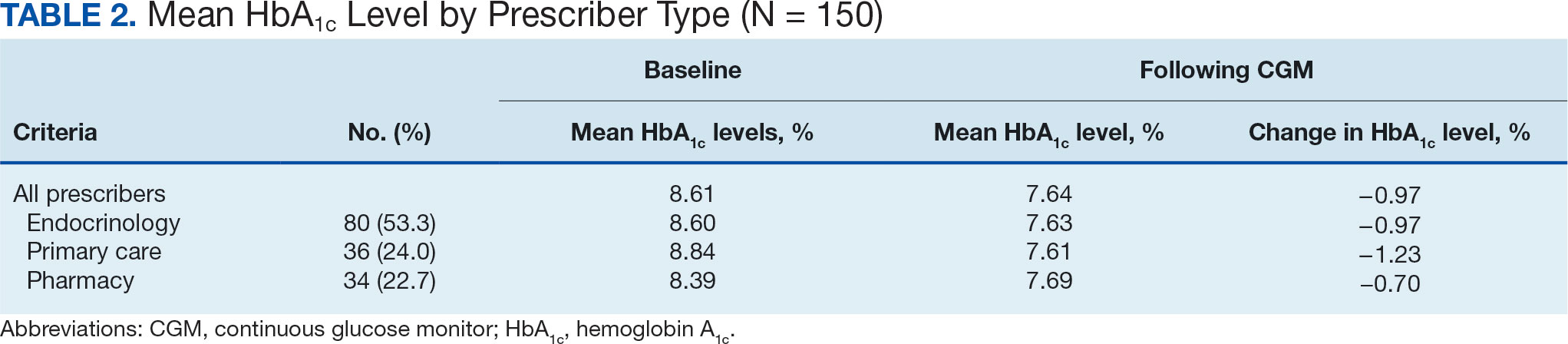
The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.
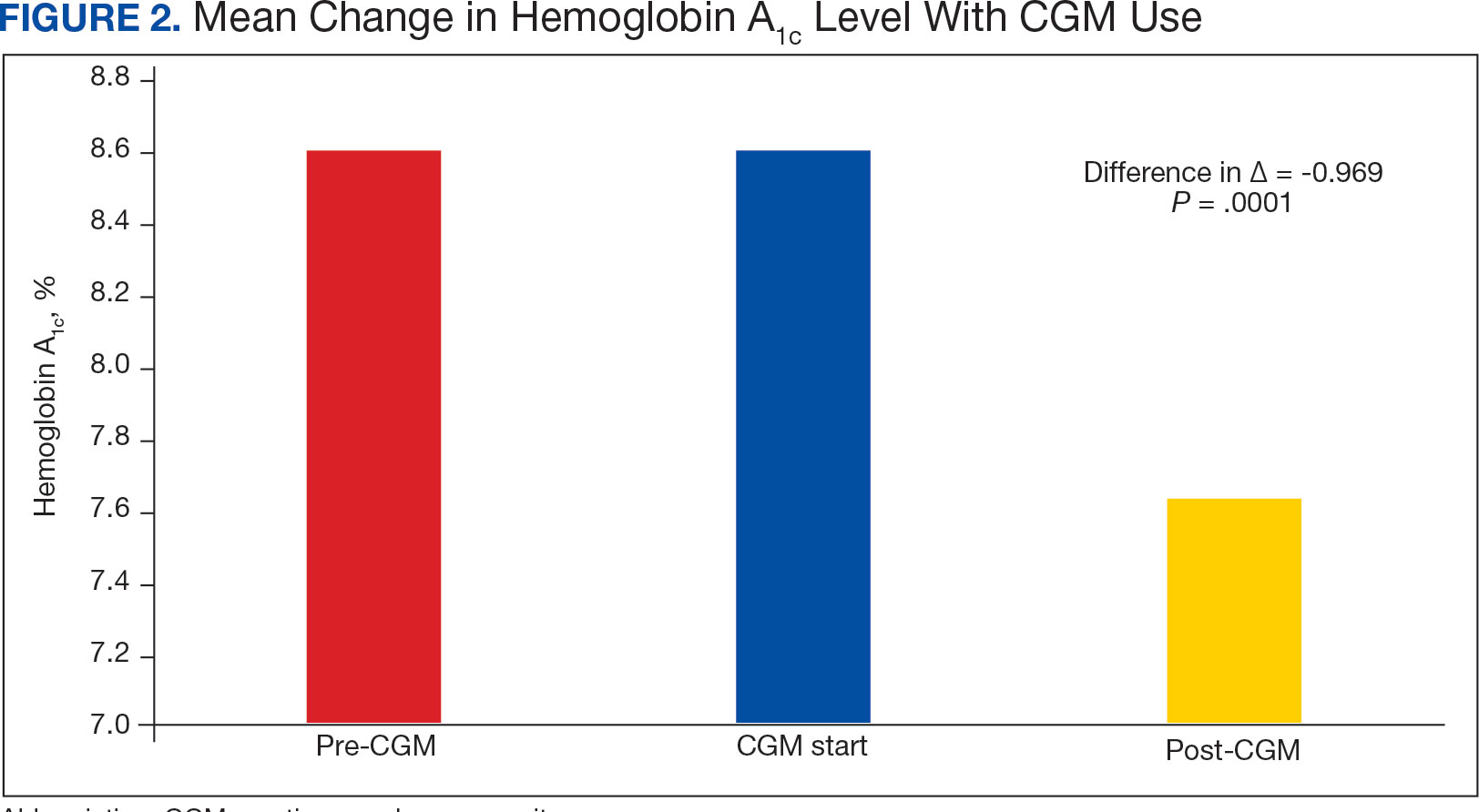
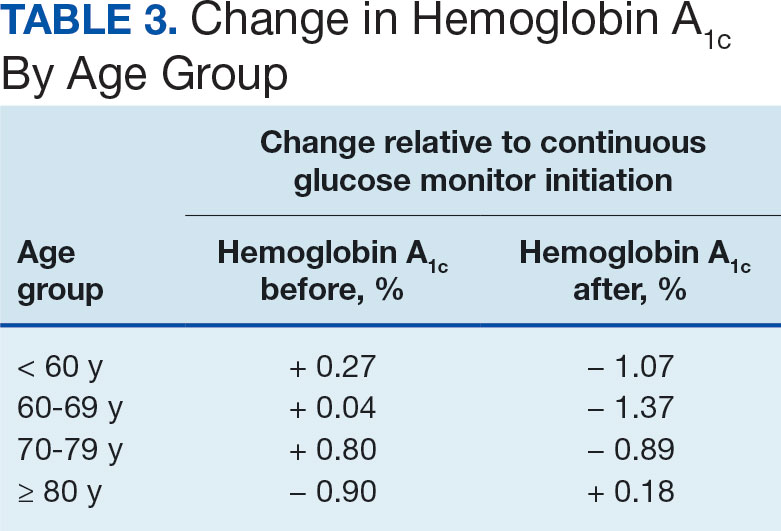
Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
VA Cancer Clinical Trials as a Strategy for Increasing Accrual of Racial and Ethnic Underrepresented Groups
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.

Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.
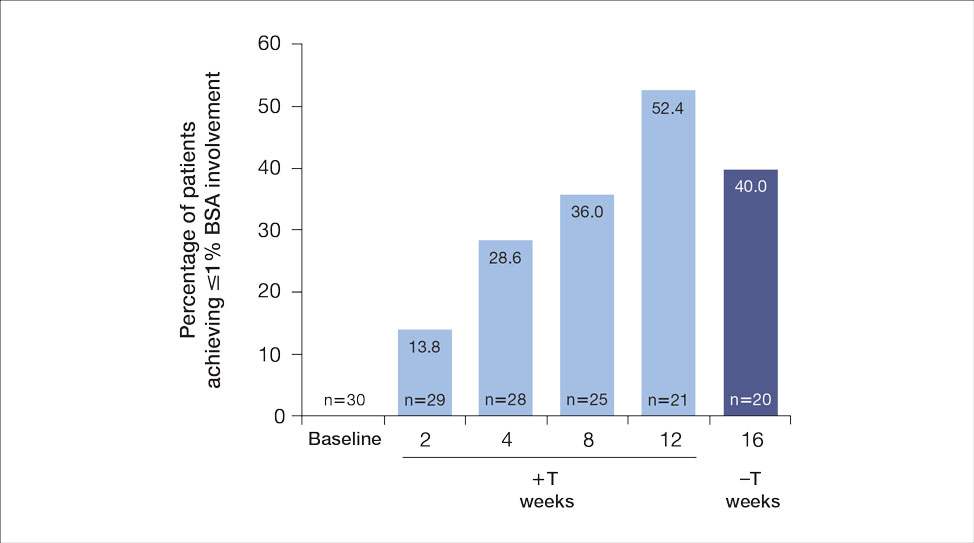
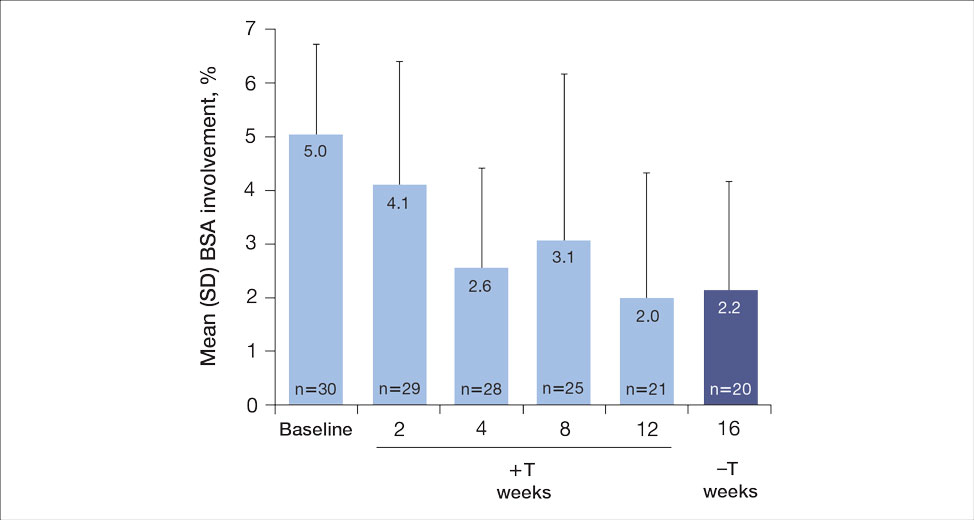
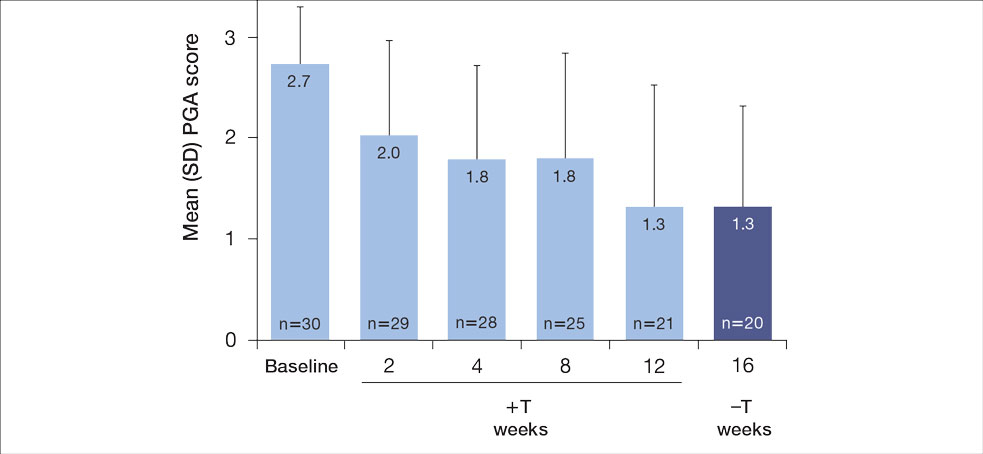
After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.
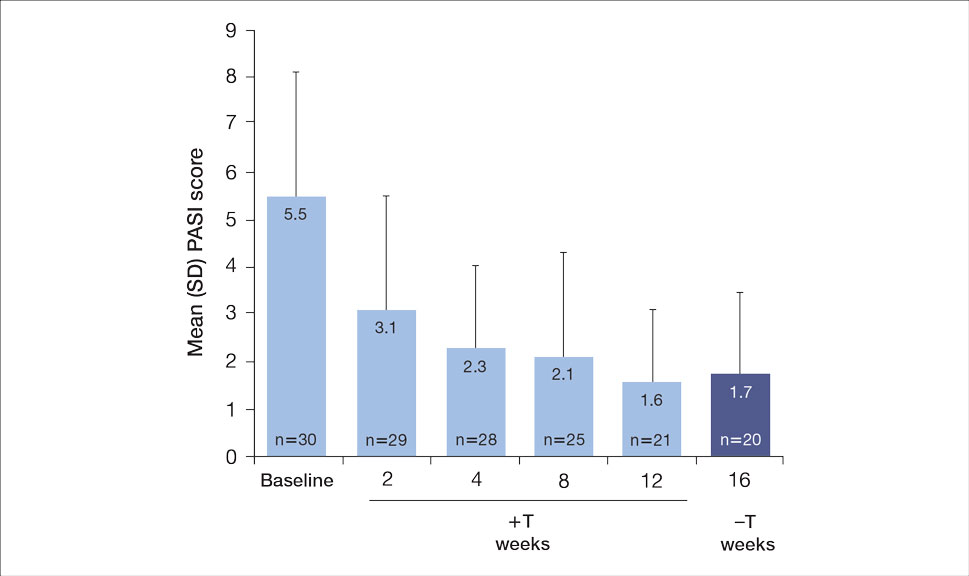
Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.
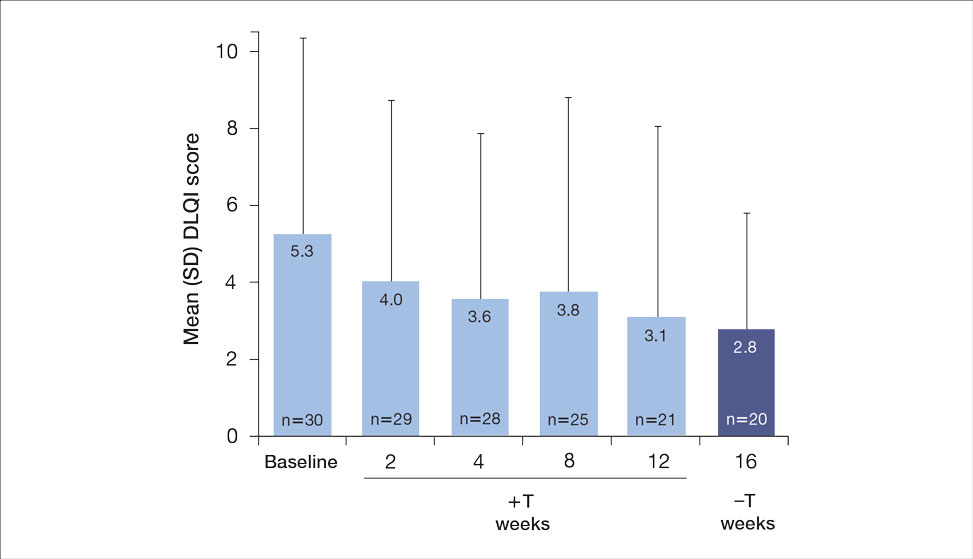

A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.
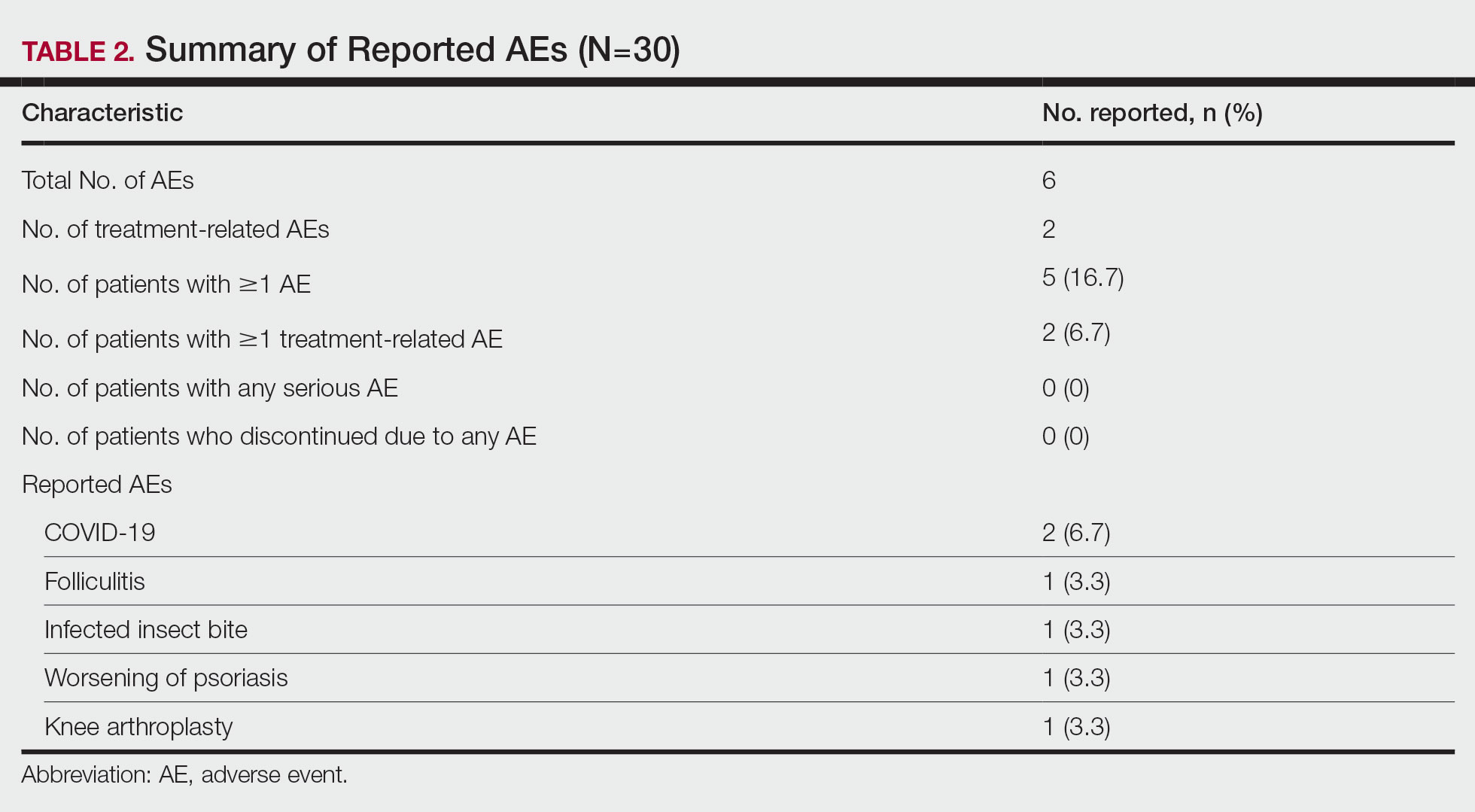
Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Practice Points
- Patients with moderate to severe psoriasis do not always reach treatment goals with biologic therapy alone.
- Adjunctive use of nonsteroidal tapinarof cream 1% may enhance the effects of ongoing biologic therapy in patients with moderate to severe plaque psoriasis, possibly avoiding the need to switch to another biologic.
- Patients with moderate to severe plaque psoriasis who are not adequately responding to biologics may benefit from adding tapinarof cream 1% to their current regimen.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).
The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).
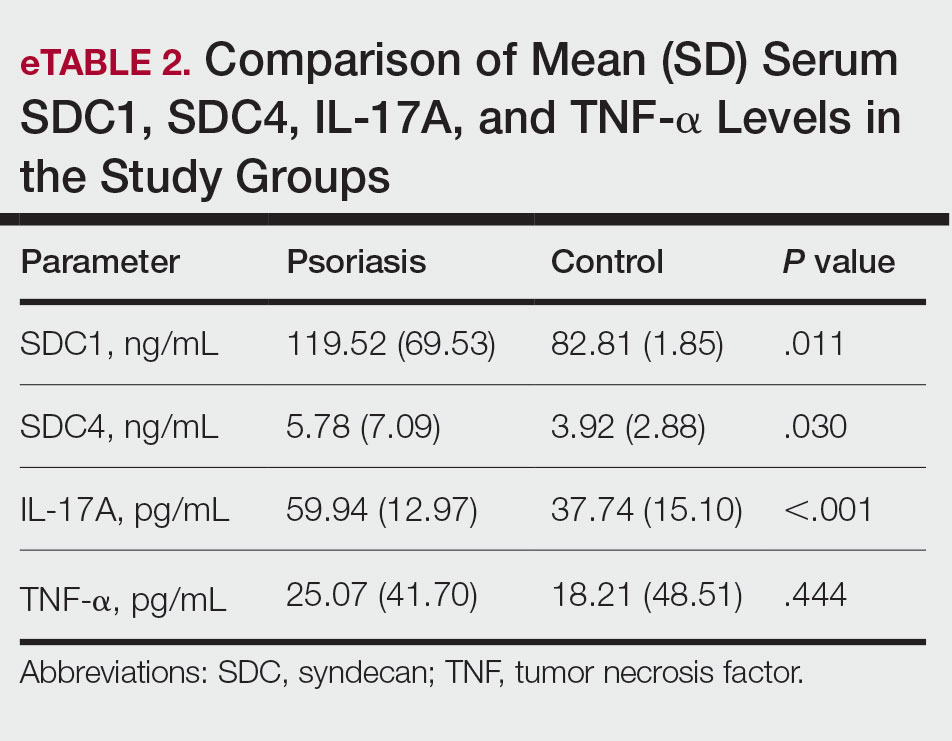
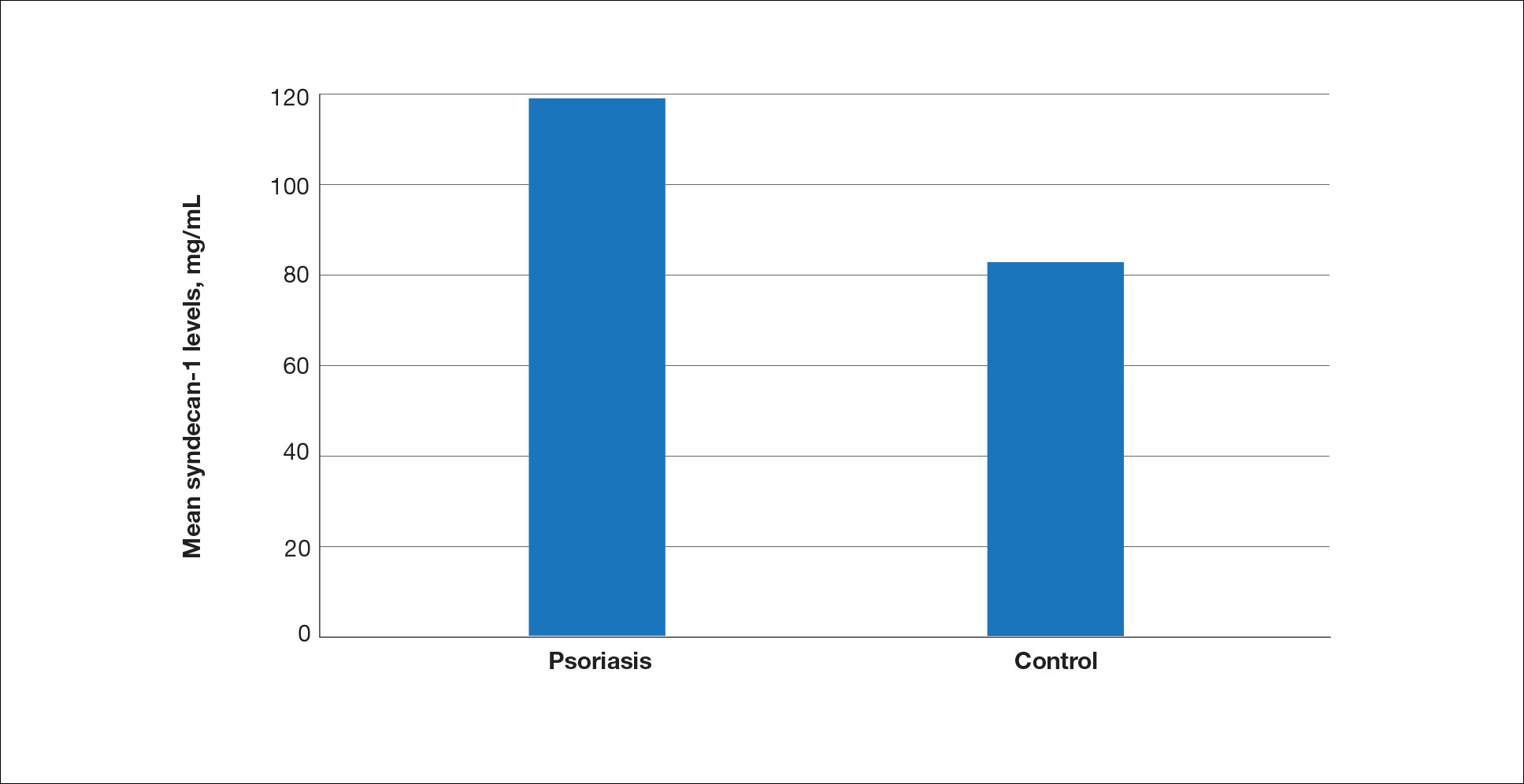
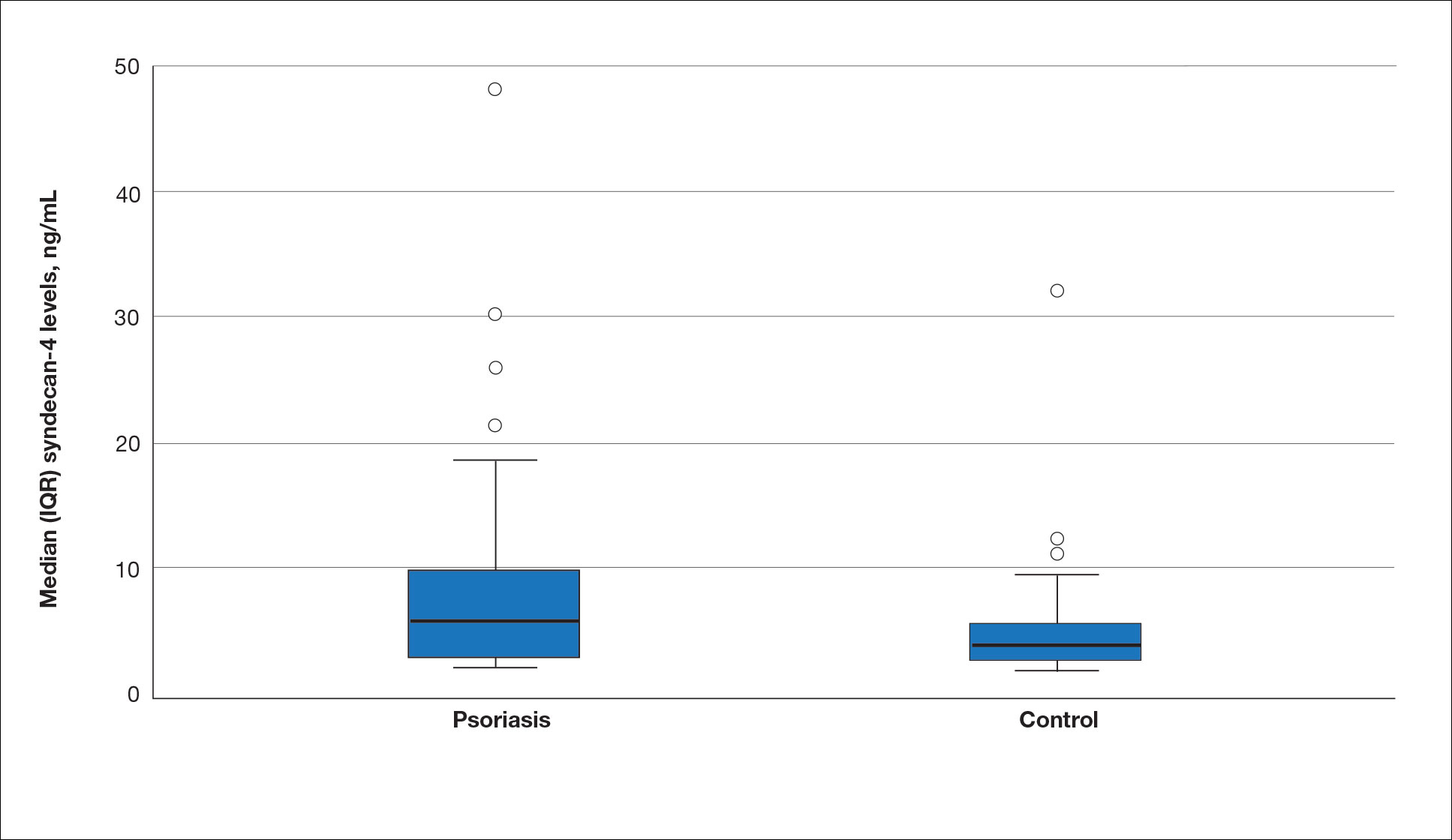
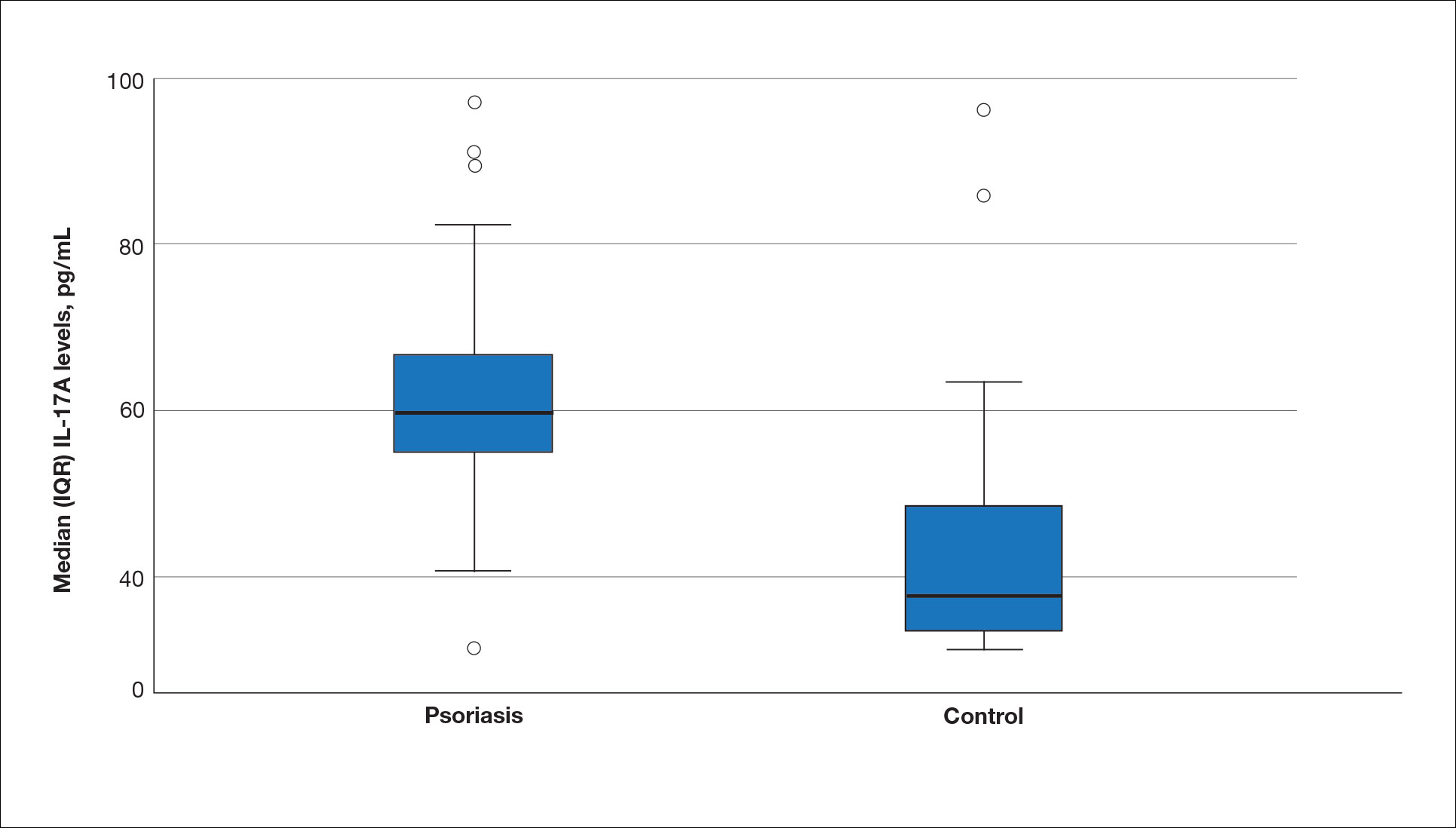
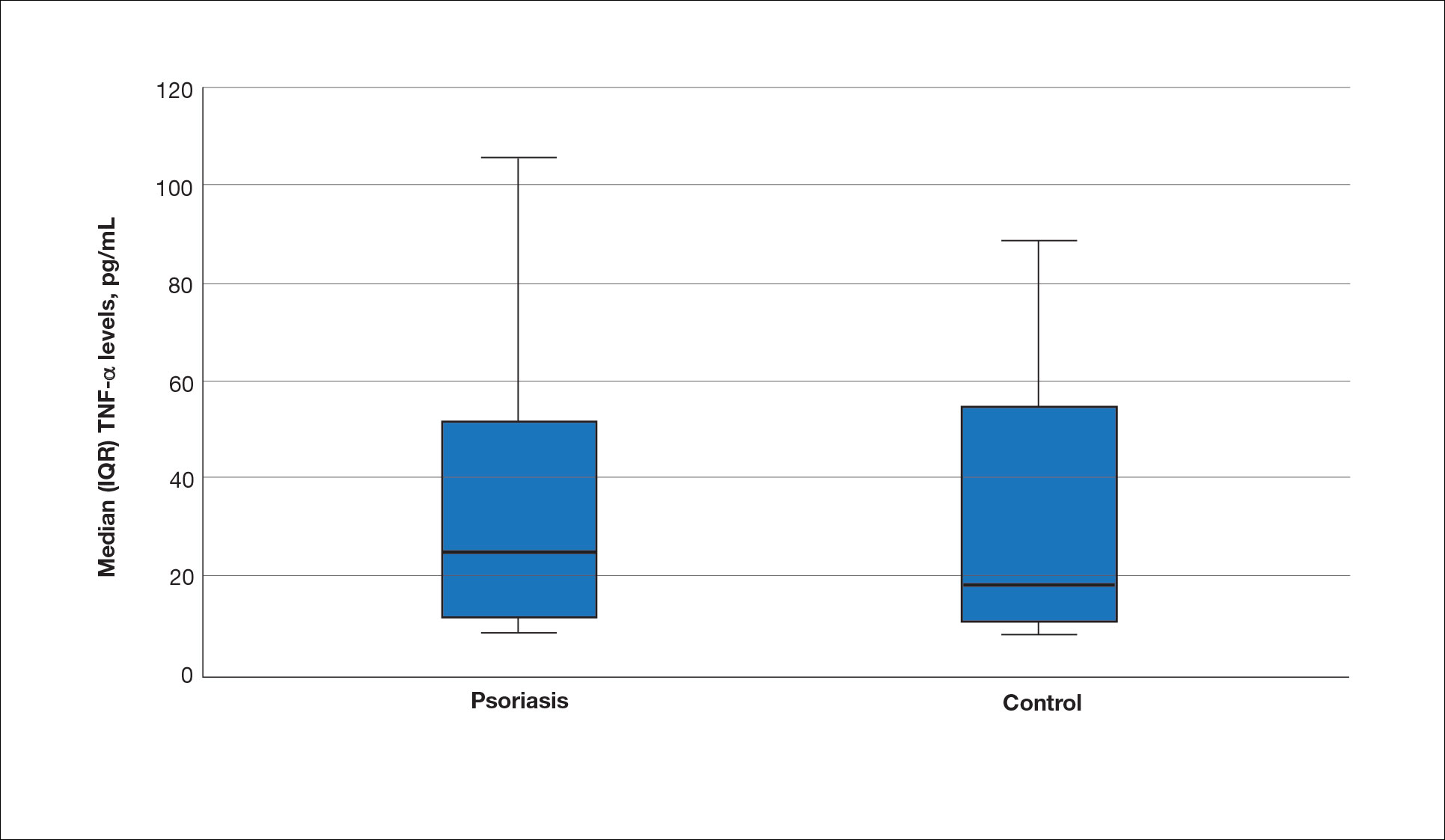
A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).
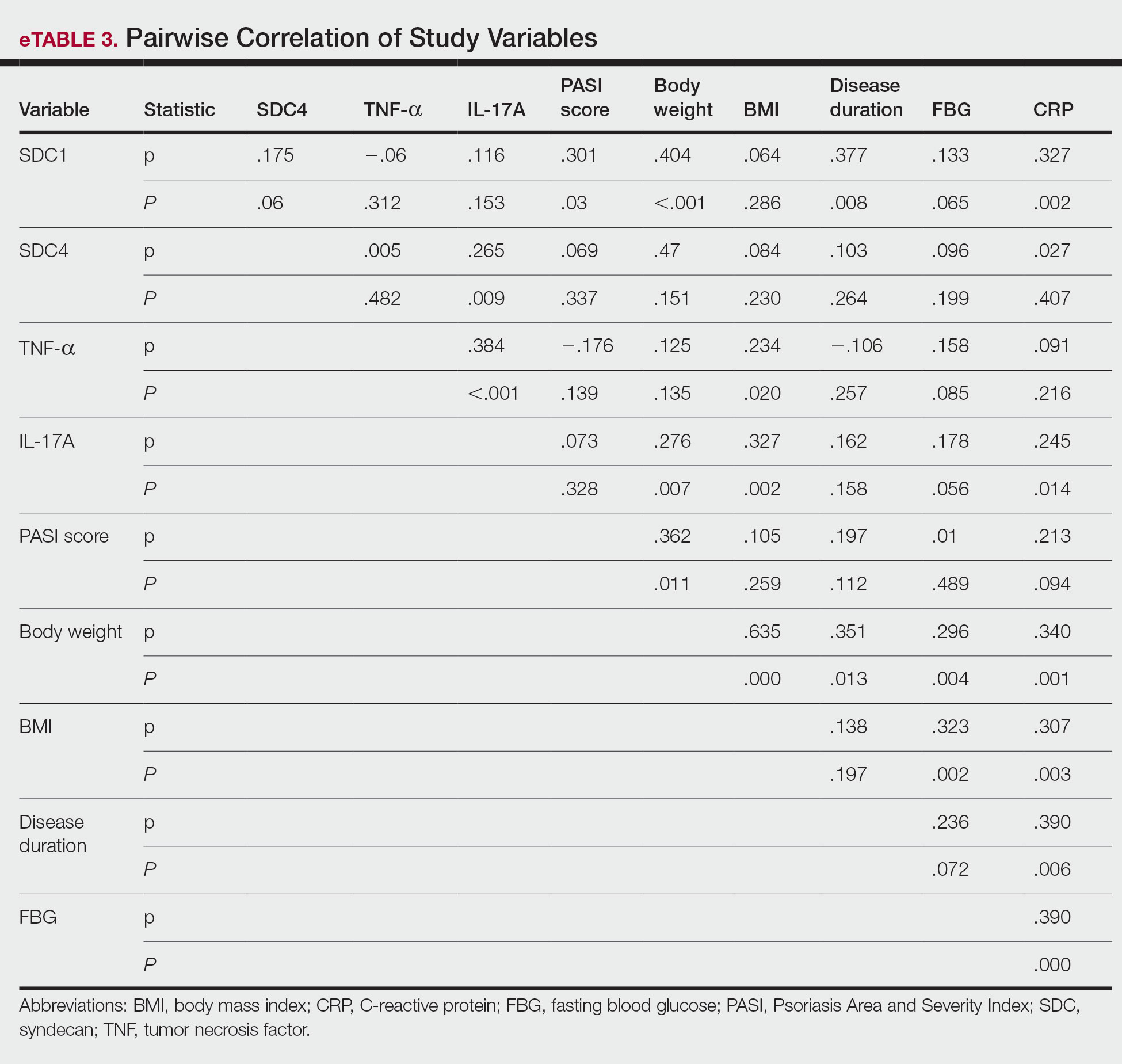
Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).
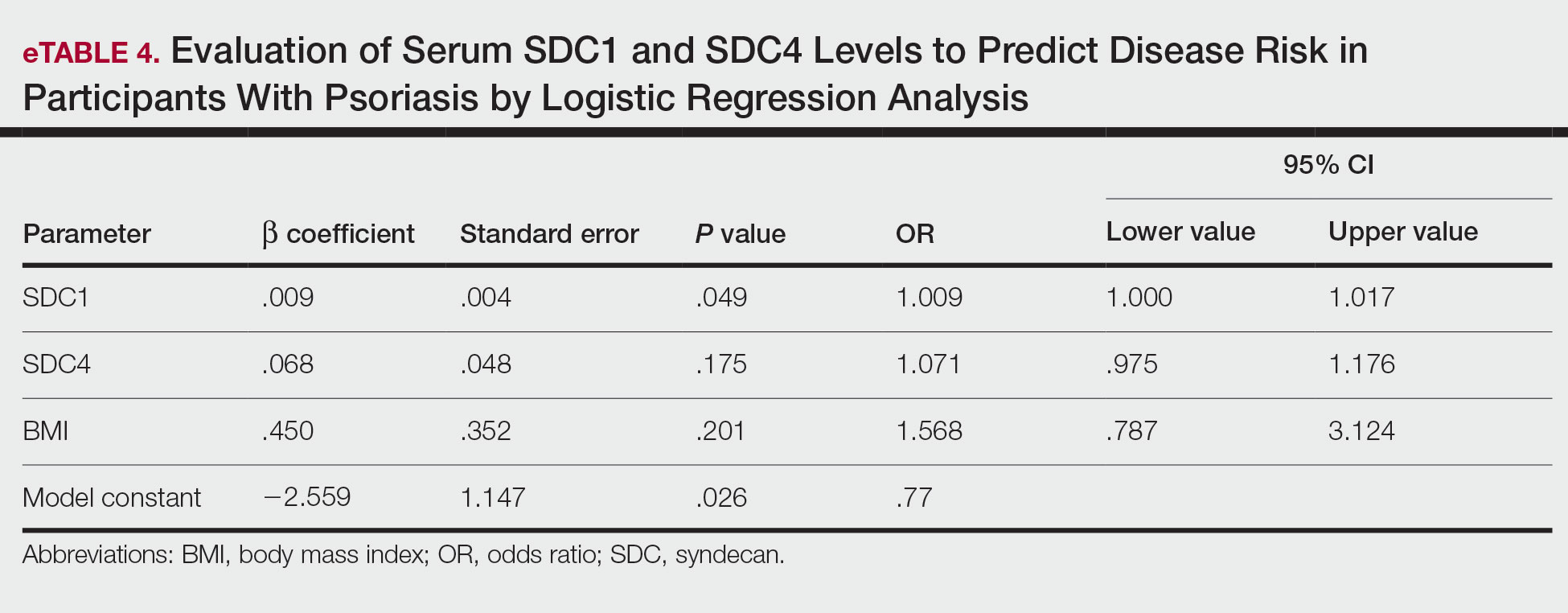
Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
PRACTICE POINTS
- Improved understanding of psoriasis pathogenesis has enabled the development of targeted treatments, although the mediators driving the disease have not yet been fully identified.
- Based on the findings of this study and existing literature, we suggest that syndecan-1 and syndecan-4 may play a role in the pathogenesis of psoriasis; however, further studies are needed to elucidate their precise mechanisms of action.
Primary Care Clinician and Patient Knowledge, Interest, and Use of Integrative Treatment Options for Chronic Low Back Pain Management
Primary Care Clinician and Patient Knowledge, Interest, and Use of Integrative Treatment Options for Chronic Low Back Pain Management
More than 50 million US adults report experiencing chronic pain, with nearly 7% experiencing high-impact chronic pain.1-3 Chronic pain negatively affects daily function, results in lost productivity, is a leading cause of disability, and is more prevalent among veterans compared with the general population.1,2,4-6 Estimates from 2021 suggest the prevalence of chronic pain among veterans exceeds 30%; > 11% experienced high-impact chronic pain.1
Primary care practitioners (PCPs) have a prominent role in chronic pain management. Pharmacologic options for treating pain, once a mainstay of therapy, present several challenges for patients and PCPs, including drug-drug interactions and adverse effects.7 The US opioid epidemic and shift to a biopsychosocial model of chronic pain care have increased emphasis on nonpharmacologic treatment options.8,9 These include integrative modalities, which incorporate conventional approaches with an array of complementary health approaches.10-12
Integrative therapy is a prominent feature in whole person care, which may be best exemplified by the US Department of Veterans Affairs (VA) Whole Health System of care.13-14 Whole health empowers an individual to take charge of their health and well-being so they can “live their life to the fullest.”14 As implemented in the Veterans Health Administration (VHA), whole health includes the use of evidence-based
METHODS
Using a cross-sectional survey design, PCPs and patients with chronic back pain affiliated with the VA Ann Arbor Healthcare System were invited to participate in separate but similar surveys to assess knowledge, interest, and use of nonpharmacologic integrative modalities for the treatment of chronic pain. In May, June, and July 2023, 78 PCPs received 3 email
Both survey instruments are available upon request, were developed by the study team, and included a mix of yes/no questions, “select all that apply” items, Likert scale response items, and open-ended questions. For one question about which modalities they would like available, the respondent was instructed to select up to 5 modalities. The instruments were extensively pretested by members of the study team, which included 2 PCPs and a nonveteran with chronic back pain.
The list of integrative modalities included in the survey was derived from the tier 1 and tier 2 complementary and integrative health modalities identified in a VHA Directive on complementary and integrative health.15,16 Tier 1 approaches are considered to have sufficient evidence and must be made available to veterans either within a VA medical facility or in the community. Tier 2 approaches are generally considered safe and may be made available but do not have sufficient evidence to mandate their provision. For participant ease, the integrative modalities were divided into 5 subgroups: manual therapies, energy/biofield therapies, mental health therapies, nutrition counseling, and movement therapies. The clinician survey assessed clinicians’ training and interest, clinical and personal use, and perceived barriers to providing integrative modalities for chronic pain. Professional and personal demographic data were also collected. Similarly, the patient survey assessed use of integrative therapies, perceptions of and interest in integrative modalities, and potential barriers to use. Demographic and health-related information was also collected.
Data analysis included descriptive statistics (eg, frequency counts, means, medians) and visual graphic displays. Separate analyses were conducted for clinicians and patients in addition to a comparative analysis of the use and potential interest in integrative modalities. Analysis were conducted using R software. This study was deemed nonresearch quality improvement by the VA Ann Arbor Healthcare System facility research oversight board and institutional review board approval was not solicited.
RESULTS
Twenty-eight clinicians completed the survey, yielding a participation rate of 36%. Participating clinicians had a median (IQR) age of 48 years (9.5), 15 self-identified as White (54%), 8 as Asian (29%), 15 as female (54%), 26 as non-Hispanic (93%), and 25 were medical doctors or doctors of osteopathy (89%). Nineteen (68%) worked at the main hospital outpatient clinic, and 9 practiced at community-based outpatient clinics (CBOCs). Thirteen respondents (46%) reported having no formal education or training in integrative approaches. Among those with prior training, 8 clinicians had nutrition counseling (29%) and 7 had psychologic therapy training (25%). Thirteen respondents (46%) also reported using integrative modalities for personal health needs: 8 used psychological therapies, 8 used movement therapies, 10 used integrative modalities for stress management or relaxation, and 8 used them for physical symptoms (Table 1).
Overall, 85 of 200 patients (43%) responded to the study survey. Two patients indicated they did not have chronic back pain and were excluded. Patients had a median (IQR) age of 66 (20) years, with 66 self-identifying as White (80%), 69 as male (83%), and 66 as non-Hispanic (80%). Forty-four patients (53%) received care at CBOCs. Forty-seven patients reported excellent, very good, or good overall health (57%), while 53 reported excellent, very good, or good mental health (64%). Fifty-nine patients reported back pain duration > 5 years (71%), and 67 (81%) indicated experiencing back pain flare-ups at least once per week over the previous 12 months. Sixty patients (72%) indicated they were somewhat or very interested in using integrative therapies as a back pain treatment; however, 40 patients (48%) indicated they had not received information about these therapies. Among those who indicated they had received information, the most frequently reported source was their PCP (41%). Most patients (72%) also reported feeling somewhat to very comfortable discussing integrative medicine therapies with their PCP.
Integrative Therapy Recommendations and Use
PCPs reported recommending multiple integrative modalities: 23 (82%) recommended cognitive-behavioral therapy, 22 (79%) recommended acupuncture, 21 (75%) recommended chiropractic, 19 (68%) recommended battlefield acupuncture, recommended massage 18 (64%), 17 (61%) recommended meditation or mindfulness, and 15 (54%) recommended movement therapies such as yoga or tai chi/qigong (Figure 1). The only therapies used by at least half of the patients were chiropractic used by 59 patients (71%) and acupuncture by 42 patients (51%). Thirty-eight patients (46%) reported massage use and 21 patients (25%) used cognitive-behavioral therapy (Table 2).
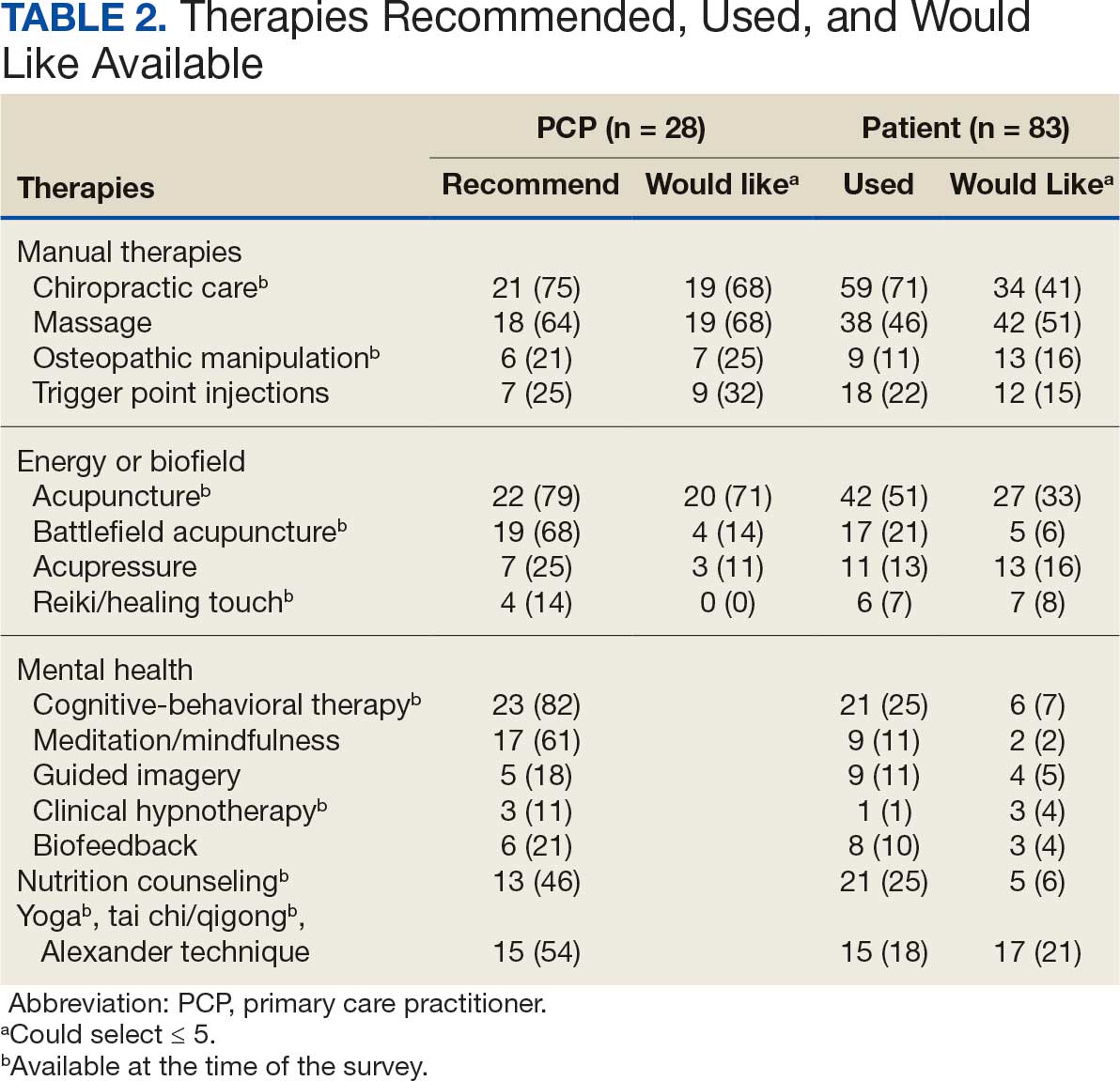
Integrative Therapies Desired
A majority of PCPs identified acupuncture (n = 20, 71%), chiropractic (n = 19, 68%), and massage (n = 19, 68%) as therapies they would most like to have available for patients with chronic pain (Figure 2). Similarly, patients identified massage (n = 42, 51%), chiropractic (n = 34, 41%), and acupuncture (n = 27, 33%) as most desired. Seventeen patients (21%) expressed interest in movement therapies.
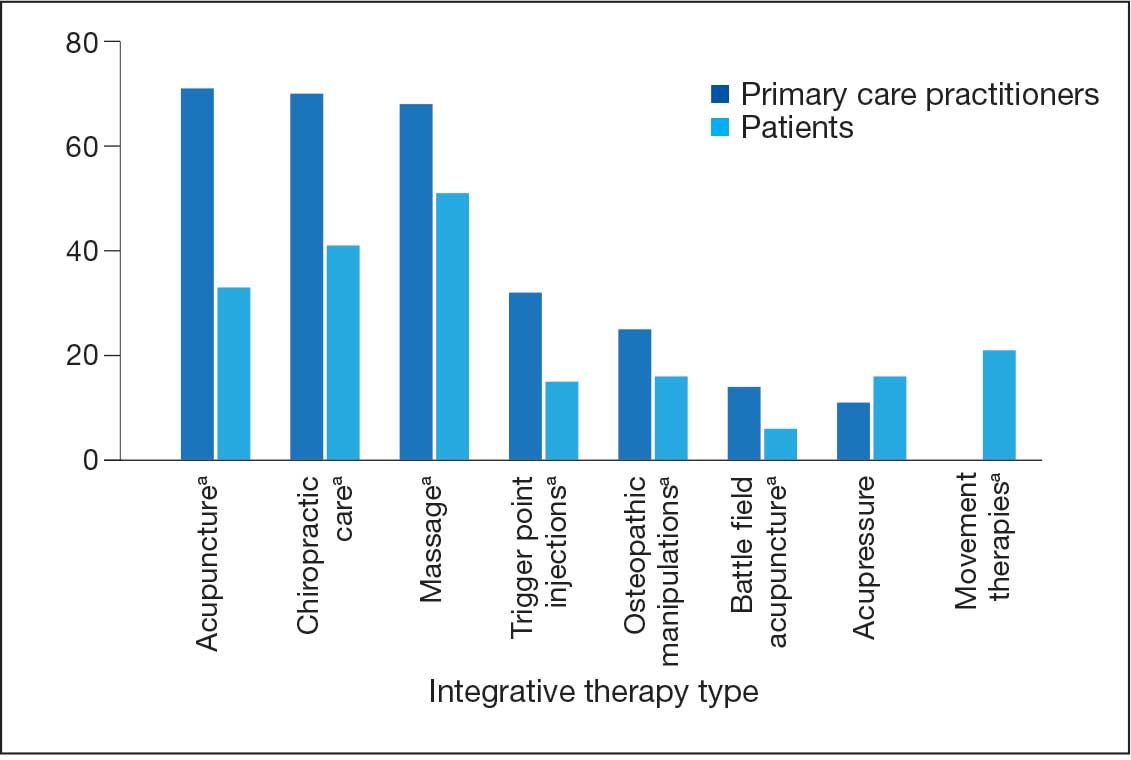
Barriers to Integrative Therapies Use
When asked about barriers to use, 26 PCPs (93%) identified access to services as a somewhat or extremely likely barrier, and 22 identified time constraints (79%) (Table 3). However, 17 PCPs (61%) noted lack of familiarity, and 18 (64%) noted a lack of scientific evidence as barriers to recommending integrative modalities. Among patients, 33 (40%) indicated not knowing what services were available at their facility as a barrier, 32 (39%) were not familiar with specific therapies, and 21 (25%) indicated a lack of clarity about the benefits of a specific therapy. Only 14 patients (17%) indicated that there were no obstacles to use.
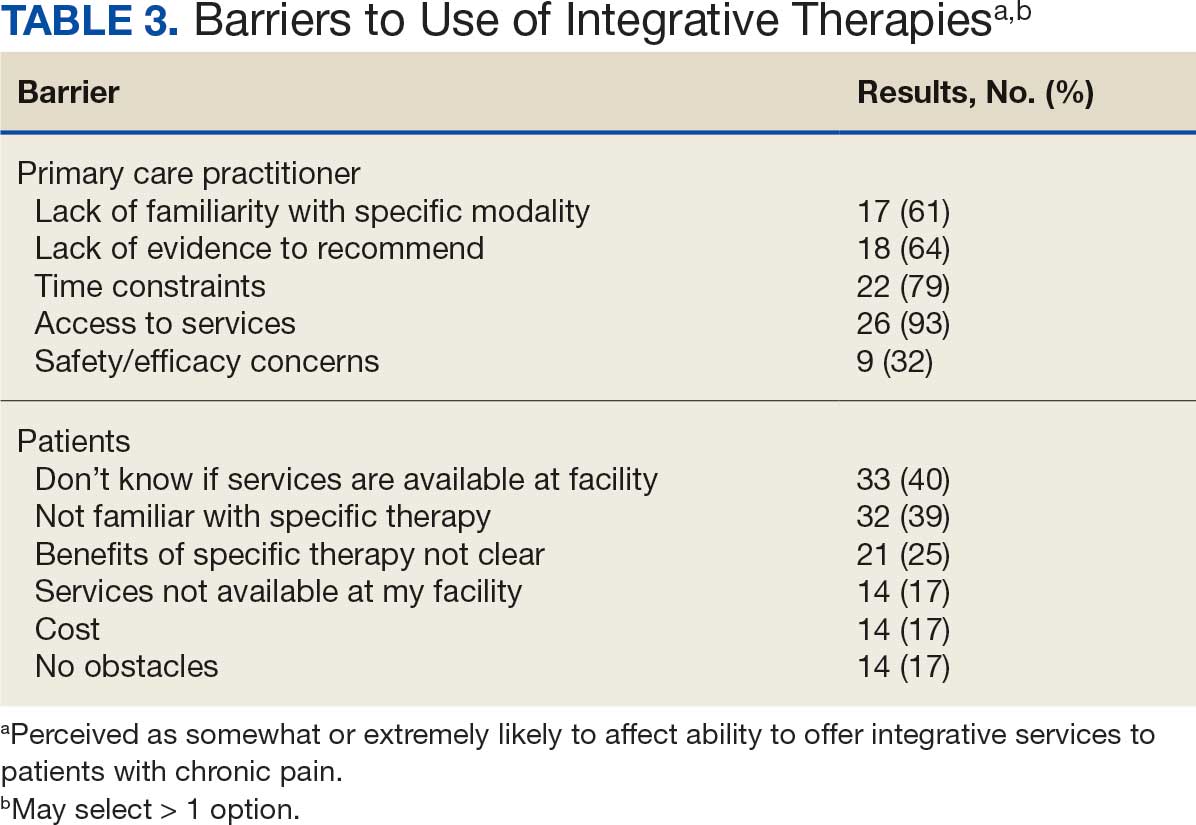
DISCUSSION
Use of integrative therapies, including complementary treatments, is an increasingly important part of chronic pain management. This survey study suggests VA PCPs are willing to recommend integrative therapies and patients with chronic back pain both desire and use several therapies. Moreover, both groups expressed interest in greater availability of similar therapies. The results also highlight key barriers, such as knowledge gaps, that should be addressed to increase the uptake of integrative modalities for managing chronic pain.
An increasing number of US adults are using complementary health approaches, an important component of integrative therapy.12 This trend includes an increase in use for pain management, from 42.3% in 2002 to 49.2% in 2022; chiropractic care, acupuncture, and massage were most frequently used.12 Similarly, chiropractic, acupuncture and massage were most often used by this sample of veterans with chronic back pain and were identified by the highest percentages of PCPs and patients as the therapies they would most like available.
There were areas where the opinions of patients and clinicians differed. As has been seen previously reported, clinicians largely recommended cognitive-behavioral therapy while patients showed less interest.17 Additionally, while patients expressed interest in the availability of movement therapies, such as yoga, PCPs expressed more interest in other strategies, such as trigger point injections. These differences may reflect true preference or a tendency for clinicians and patients to select therapies with which they are more familiar. Additional research is needed to better understand the acceptability and potential use of integrative health treatments across a broad array of therapeutic options.
Despite VHA policy requiring facilities to provide certain complementary and integrative health modalities, almost all PCPs identified access to services as a major obstacle.15 Based on evidence and a rigorous vetting process, services currently required on-site, via telehealth, or through community partners include acupuncture and battlefield acupuncture (battlefield auricular acupuncture), biofeedback, clinical hypnosis, guided imagery, medical massage therapy, medication, tai chi/qigong, and yoga. Optional approaches, which may be made available to veterans, include chiropractic and healing touch. Outside the VHA, some states have introduced or enacted legislation mandating insurance coverage of nonpharmacological pain treatments.18 However, these requirements and mandates do not help address challenges such as the availability of trained/qualified practitioners.19,20 Ensuring access to complementary and integrative health treatments requires a more concerted effort to ensure that supply meets demand. It is also important to acknowledge the budgetary and physical space constraints that further limit access to services. Although expansion and integration of integrative medicine services remain a priority within the VA Whole Health program, implementation is contingent on available financial and infrastructure resources.
Time was also identified by PCPs as a barrier to recommending integrative therapies to patients. Developing and implementing time-efficient communication strategies for patient education such as concise talking points and informational handouts could help address this barrier. Furthermore, leveraging existing programs and engaging the entire health care team in patient education and referral could help increase integrative and complementary therapy uptake and use.
Although access and time were identified as major barriers, these findings also suggest that PCP and patient knowledge are another target area for enhancing the use of complementary and integrative therapies. Like prior research, most clinicians identified a lack of familiarity with certain services and a lack of scientific evidence as extremely or somewhat likely to affect their ability to offer integrative services to patients with chronic pain.21 Likewise, about 40% of patients identified being unfamiliar with a specific therapy as one of the major obstacles to receiving integrative therapies, with a similar number identifying PCPs as a source of information. The lack of familiarity may be due in part to the evolving nomenclature, with terms such as alternative, complementary, and integrative used to describe approaches outside what is often considered conventional medicine.10 On the other hand, there has also been considerable expansion in the number of therapies within this domain, along with an expanding evidence base. This suggests a need for targeted educational strategies for clinicians and patients, which can be rapidly deployed and continuously adapted as new therapies and evidence emerge.
Limitations
There are some inherent limitations with a survey-based approach, including sampling, non-response, and social desirability biases. In addition, this study only included PCPs and patients affiliated with a single VA medical center. Steps to mitigate these limitations included maintaining survey anonymity and reporting information about respondent characteristics to enhance transparency about the representativeness of the study findings.
CONCLUSIONS
Expanding the use of nonpharmacological pain treatments, including integrative modalities, is essential for safe and effective chronic pain management and reducing opioid use. Our findings show that VA PCPs and patients with chronic back pain are interested in and have some experience with certain integrative therapies. However, even within the context of a health care system that supports the use of integrative therapies for chronic pain as part of whole person care, increasing uptake will require addressing access and time-related constraints as well as ongoing clinician and patient education.
- Rikard SM, Strahan AE, Schmit KM, et al. Chronic pain among adults — United States, 2018-2021. MMWR Morb Mortal Wkly Rep. 2023;72:379-385. doi:10.15585/mmwr.mm7215a1
- Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163:E328-E332. doi:10.1097/j.pain.0000000000002291
- Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020. JAMA Netw Open. 2023;6:e2313563. doi:10.1001/jamanetworkopen.2023.13563
- Ferrari AJ, Santomauro DF, Aali A, et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2133-2161. doi:10.1016/S0140-6736(24)00757-8 5.
- Qureshi AR, Patel M, Neumark S, et al. Prevalence of chronic non-cancer pain among military veterans: a systematic review and meta-analysis of observational studies. BMJ Mil Health. 2025;171:310-314. doi:10.1136/military-2023-002554
- Feldman DE, Nahin RL. Disability among persons with chronic severe back pain: results from a nationally representative population-based sample. J Pain. 2022;23:2144-2154. doi:10.1016/j.jpain.2022.07.016
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi:10.7326/M16-2367
- van Erp RMA, Huijnen IPJ, Jakobs MLG, Kleijnen J, Smeets RJEM. Effectiveness of primary care interventions using a biopsychosocial approach in chronic low back pain: a systematic review. Pain Practice. 2019;19:224-241. doi:10.1111/papr.12735
- Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505. doi:10.7326/M16-2459
- Complementary, alternative, or integrative health: what’s in a name? National Institutes of Health, National Center for Complementary and Integrative Health. Updated April 2021. Accessed December 15, 2025. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name.
- Taylor SL, Elwy AR. Complementary and alternative medicine for US veterans and active duty military personnel promising steps to improve their health. Med Care. 2014;52:S1-S4. doi:10.1097/MLR.0000000000000270.
- Nahin RL, Rhee A, Stussman B. Use of complementary health approaches overall and for pain management by US adults. JAMA. 2024;331:613-615. doi:10.1001/jama.2023.26775
- Gantt CJ, Donovan N, Khung M. Veterans Affairs’ Whole Health System of Care for transitioning service members and veterans. Mil Med. 2023;188:28-32. doi:10.1093/milmed/usad047
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the Whole Health System of Care. Health Serv Res. 2022;57:53-65. doi:10.1111/1475-6773.13938
- Department of Veterans Affairs VHA. VHA Policy Directive 1137: Provision of Complementary and Integrative Health. December 2022. Accessed December 15, 2025. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=10072
- Giannitrapani KF, Holliday JR, Miake-Lye IM, Hempel S, Taylor SL. Synthesizing the strength of the evidence of complementary and integrative health therapies for pain. Pain Med. 2019;20:1831-1840. doi:10.1093/pm/pnz068
- Belitskaya-Levy I, David Clark J, Shih MC, Bair MJ. Treatment preferences for chronic low back pain: views of veterans and their providers. J Pain Res. 2021;14:161-171. doi:10.2147/JPR.S290400
- Onstott TN, Hurst S, Kronick R, Tsou AC, Groessl E, McMenamin SB. Health insurance mandates for nonpharmacological pain treatments in 7 US states. JAMA Netw Open. 2024;7:E245737. doi:10.1001/jamanetworkopen.2024.5737
- Sullivan M, Leach M, Snow J, Moonaz S. The North American yoga therapy workforce survey. Complement Ther Med. 2017;31:39-48. doi:10.1016/j.ctim.2017.01.006
- Bolton R, Ritter G, Highland K, Larson MJ. The relationship between capacity and utilization of nonpharmacologic therapies in the US Military Health System. BMC Health Serv Res. 2022;22. doi:10.1186/s12913-022-07700-4
- Stussman BJ, Nahin RL, Barnes PM, Scott R, Feinberg T, Ward BW. Reasons office-based physicians in the United States recommend common complementary health approaches to patients: an exploratory study using a national survey. J Integr Complement Med. 2022;28:651-663. doi:10.1089/jicm.2022.0493
More than 50 million US adults report experiencing chronic pain, with nearly 7% experiencing high-impact chronic pain.1-3 Chronic pain negatively affects daily function, results in lost productivity, is a leading cause of disability, and is more prevalent among veterans compared with the general population.1,2,4-6 Estimates from 2021 suggest the prevalence of chronic pain among veterans exceeds 30%; > 11% experienced high-impact chronic pain.1
Primary care practitioners (PCPs) have a prominent role in chronic pain management. Pharmacologic options for treating pain, once a mainstay of therapy, present several challenges for patients and PCPs, including drug-drug interactions and adverse effects.7 The US opioid epidemic and shift to a biopsychosocial model of chronic pain care have increased emphasis on nonpharmacologic treatment options.8,9 These include integrative modalities, which incorporate conventional approaches with an array of complementary health approaches.10-12
Integrative therapy is a prominent feature in whole person care, which may be best exemplified by the US Department of Veterans Affairs (VA) Whole Health System of care.13-14 Whole health empowers an individual to take charge of their health and well-being so they can “live their life to the fullest.”14 As implemented in the Veterans Health Administration (VHA), whole health includes the use of evidence-based
METHODS
Using a cross-sectional survey design, PCPs and patients with chronic back pain affiliated with the VA Ann Arbor Healthcare System were invited to participate in separate but similar surveys to assess knowledge, interest, and use of nonpharmacologic integrative modalities for the treatment of chronic pain. In May, June, and July 2023, 78 PCPs received 3 email
Both survey instruments are available upon request, were developed by the study team, and included a mix of yes/no questions, “select all that apply” items, Likert scale response items, and open-ended questions. For one question about which modalities they would like available, the respondent was instructed to select up to 5 modalities. The instruments were extensively pretested by members of the study team, which included 2 PCPs and a nonveteran with chronic back pain.
The list of integrative modalities included in the survey was derived from the tier 1 and tier 2 complementary and integrative health modalities identified in a VHA Directive on complementary and integrative health.15,16 Tier 1 approaches are considered to have sufficient evidence and must be made available to veterans either within a VA medical facility or in the community. Tier 2 approaches are generally considered safe and may be made available but do not have sufficient evidence to mandate their provision. For participant ease, the integrative modalities were divided into 5 subgroups: manual therapies, energy/biofield therapies, mental health therapies, nutrition counseling, and movement therapies. The clinician survey assessed clinicians’ training and interest, clinical and personal use, and perceived barriers to providing integrative modalities for chronic pain. Professional and personal demographic data were also collected. Similarly, the patient survey assessed use of integrative therapies, perceptions of and interest in integrative modalities, and potential barriers to use. Demographic and health-related information was also collected.
Data analysis included descriptive statistics (eg, frequency counts, means, medians) and visual graphic displays. Separate analyses were conducted for clinicians and patients in addition to a comparative analysis of the use and potential interest in integrative modalities. Analysis were conducted using R software. This study was deemed nonresearch quality improvement by the VA Ann Arbor Healthcare System facility research oversight board and institutional review board approval was not solicited.
RESULTS
Twenty-eight clinicians completed the survey, yielding a participation rate of 36%. Participating clinicians had a median (IQR) age of 48 years (9.5), 15 self-identified as White (54%), 8 as Asian (29%), 15 as female (54%), 26 as non-Hispanic (93%), and 25 were medical doctors or doctors of osteopathy (89%). Nineteen (68%) worked at the main hospital outpatient clinic, and 9 practiced at community-based outpatient clinics (CBOCs). Thirteen respondents (46%) reported having no formal education or training in integrative approaches. Among those with prior training, 8 clinicians had nutrition counseling (29%) and 7 had psychologic therapy training (25%). Thirteen respondents (46%) also reported using integrative modalities for personal health needs: 8 used psychological therapies, 8 used movement therapies, 10 used integrative modalities for stress management or relaxation, and 8 used them for physical symptoms (Table 1).

Overall, 85 of 200 patients (43%) responded to the study survey. Two patients indicated they did not have chronic back pain and were excluded. Patients had a median (IQR) age of 66 (20) years, with 66 self-identifying as White (80%), 69 as male (83%), and 66 as non-Hispanic (80%). Forty-four patients (53%) received care at CBOCs. Forty-seven patients reported excellent, very good, or good overall health (57%), while 53 reported excellent, very good, or good mental health (64%). Fifty-nine patients reported back pain duration > 5 years (71%), and 67 (81%) indicated experiencing back pain flare-ups at least once per week over the previous 12 months. Sixty patients (72%) indicated they were somewhat or very interested in using integrative therapies as a back pain treatment; however, 40 patients (48%) indicated they had not received information about these therapies. Among those who indicated they had received information, the most frequently reported source was their PCP (41%). Most patients (72%) also reported feeling somewhat to very comfortable discussing integrative medicine therapies with their PCP.
Integrative Therapy Recommendations and Use
PCPs reported recommending multiple integrative modalities: 23 (82%) recommended cognitive-behavioral therapy, 22 (79%) recommended acupuncture, 21 (75%) recommended chiropractic, 19 (68%) recommended battlefield acupuncture, recommended massage 18 (64%), 17 (61%) recommended meditation or mindfulness, and 15 (54%) recommended movement therapies such as yoga or tai chi/qigong (Figure 1). The only therapies used by at least half of the patients were chiropractic used by 59 patients (71%) and acupuncture by 42 patients (51%). Thirty-eight patients (46%) reported massage use and 21 patients (25%) used cognitive-behavioral therapy (Table 2).


Integrative Therapies Desired
A majority of PCPs identified acupuncture (n = 20, 71%), chiropractic (n = 19, 68%), and massage (n = 19, 68%) as therapies they would most like to have available for patients with chronic pain (Figure 2). Similarly, patients identified massage (n = 42, 51%), chiropractic (n = 34, 41%), and acupuncture (n = 27, 33%) as most desired. Seventeen patients (21%) expressed interest in movement therapies.

Barriers to Integrative Therapies Use
When asked about barriers to use, 26 PCPs (93%) identified access to services as a somewhat or extremely likely barrier, and 22 identified time constraints (79%) (Table 3). However, 17 PCPs (61%) noted lack of familiarity, and 18 (64%) noted a lack of scientific evidence as barriers to recommending integrative modalities. Among patients, 33 (40%) indicated not knowing what services were available at their facility as a barrier, 32 (39%) were not familiar with specific therapies, and 21 (25%) indicated a lack of clarity about the benefits of a specific therapy. Only 14 patients (17%) indicated that there were no obstacles to use.

DISCUSSION
Use of integrative therapies, including complementary treatments, is an increasingly important part of chronic pain management. This survey study suggests VA PCPs are willing to recommend integrative therapies and patients with chronic back pain both desire and use several therapies. Moreover, both groups expressed interest in greater availability of similar therapies. The results also highlight key barriers, such as knowledge gaps, that should be addressed to increase the uptake of integrative modalities for managing chronic pain.
An increasing number of US adults are using complementary health approaches, an important component of integrative therapy.12 This trend includes an increase in use for pain management, from 42.3% in 2002 to 49.2% in 2022; chiropractic care, acupuncture, and massage were most frequently used.12 Similarly, chiropractic, acupuncture and massage were most often used by this sample of veterans with chronic back pain and were identified by the highest percentages of PCPs and patients as the therapies they would most like available.
There were areas where the opinions of patients and clinicians differed. As has been seen previously reported, clinicians largely recommended cognitive-behavioral therapy while patients showed less interest.17 Additionally, while patients expressed interest in the availability of movement therapies, such as yoga, PCPs expressed more interest in other strategies, such as trigger point injections. These differences may reflect true preference or a tendency for clinicians and patients to select therapies with which they are more familiar. Additional research is needed to better understand the acceptability and potential use of integrative health treatments across a broad array of therapeutic options.
Despite VHA policy requiring facilities to provide certain complementary and integrative health modalities, almost all PCPs identified access to services as a major obstacle.15 Based on evidence and a rigorous vetting process, services currently required on-site, via telehealth, or through community partners include acupuncture and battlefield acupuncture (battlefield auricular acupuncture), biofeedback, clinical hypnosis, guided imagery, medical massage therapy, medication, tai chi/qigong, and yoga. Optional approaches, which may be made available to veterans, include chiropractic and healing touch. Outside the VHA, some states have introduced or enacted legislation mandating insurance coverage of nonpharmacological pain treatments.18 However, these requirements and mandates do not help address challenges such as the availability of trained/qualified practitioners.19,20 Ensuring access to complementary and integrative health treatments requires a more concerted effort to ensure that supply meets demand. It is also important to acknowledge the budgetary and physical space constraints that further limit access to services. Although expansion and integration of integrative medicine services remain a priority within the VA Whole Health program, implementation is contingent on available financial and infrastructure resources.
Time was also identified by PCPs as a barrier to recommending integrative therapies to patients. Developing and implementing time-efficient communication strategies for patient education such as concise talking points and informational handouts could help address this barrier. Furthermore, leveraging existing programs and engaging the entire health care team in patient education and referral could help increase integrative and complementary therapy uptake and use.
Although access and time were identified as major barriers, these findings also suggest that PCP and patient knowledge are another target area for enhancing the use of complementary and integrative therapies. Like prior research, most clinicians identified a lack of familiarity with certain services and a lack of scientific evidence as extremely or somewhat likely to affect their ability to offer integrative services to patients with chronic pain.21 Likewise, about 40% of patients identified being unfamiliar with a specific therapy as one of the major obstacles to receiving integrative therapies, with a similar number identifying PCPs as a source of information. The lack of familiarity may be due in part to the evolving nomenclature, with terms such as alternative, complementary, and integrative used to describe approaches outside what is often considered conventional medicine.10 On the other hand, there has also been considerable expansion in the number of therapies within this domain, along with an expanding evidence base. This suggests a need for targeted educational strategies for clinicians and patients, which can be rapidly deployed and continuously adapted as new therapies and evidence emerge.
Limitations
There are some inherent limitations with a survey-based approach, including sampling, non-response, and social desirability biases. In addition, this study only included PCPs and patients affiliated with a single VA medical center. Steps to mitigate these limitations included maintaining survey anonymity and reporting information about respondent characteristics to enhance transparency about the representativeness of the study findings.
CONCLUSIONS
Expanding the use of nonpharmacological pain treatments, including integrative modalities, is essential for safe and effective chronic pain management and reducing opioid use. Our findings show that VA PCPs and patients with chronic back pain are interested in and have some experience with certain integrative therapies. However, even within the context of a health care system that supports the use of integrative therapies for chronic pain as part of whole person care, increasing uptake will require addressing access and time-related constraints as well as ongoing clinician and patient education.
More than 50 million US adults report experiencing chronic pain, with nearly 7% experiencing high-impact chronic pain.1-3 Chronic pain negatively affects daily function, results in lost productivity, is a leading cause of disability, and is more prevalent among veterans compared with the general population.1,2,4-6 Estimates from 2021 suggest the prevalence of chronic pain among veterans exceeds 30%; > 11% experienced high-impact chronic pain.1
Primary care practitioners (PCPs) have a prominent role in chronic pain management. Pharmacologic options for treating pain, once a mainstay of therapy, present several challenges for patients and PCPs, including drug-drug interactions and adverse effects.7 The US opioid epidemic and shift to a biopsychosocial model of chronic pain care have increased emphasis on nonpharmacologic treatment options.8,9 These include integrative modalities, which incorporate conventional approaches with an array of complementary health approaches.10-12
Integrative therapy is a prominent feature in whole person care, which may be best exemplified by the US Department of Veterans Affairs (VA) Whole Health System of care.13-14 Whole health empowers an individual to take charge of their health and well-being so they can “live their life to the fullest.”14 As implemented in the Veterans Health Administration (VHA), whole health includes the use of evidence-based
METHODS
Using a cross-sectional survey design, PCPs and patients with chronic back pain affiliated with the VA Ann Arbor Healthcare System were invited to participate in separate but similar surveys to assess knowledge, interest, and use of nonpharmacologic integrative modalities for the treatment of chronic pain. In May, June, and July 2023, 78 PCPs received 3 email
Both survey instruments are available upon request, were developed by the study team, and included a mix of yes/no questions, “select all that apply” items, Likert scale response items, and open-ended questions. For one question about which modalities they would like available, the respondent was instructed to select up to 5 modalities. The instruments were extensively pretested by members of the study team, which included 2 PCPs and a nonveteran with chronic back pain.
The list of integrative modalities included in the survey was derived from the tier 1 and tier 2 complementary and integrative health modalities identified in a VHA Directive on complementary and integrative health.15,16 Tier 1 approaches are considered to have sufficient evidence and must be made available to veterans either within a VA medical facility or in the community. Tier 2 approaches are generally considered safe and may be made available but do not have sufficient evidence to mandate their provision. For participant ease, the integrative modalities were divided into 5 subgroups: manual therapies, energy/biofield therapies, mental health therapies, nutrition counseling, and movement therapies. The clinician survey assessed clinicians’ training and interest, clinical and personal use, and perceived barriers to providing integrative modalities for chronic pain. Professional and personal demographic data were also collected. Similarly, the patient survey assessed use of integrative therapies, perceptions of and interest in integrative modalities, and potential barriers to use. Demographic and health-related information was also collected.
Data analysis included descriptive statistics (eg, frequency counts, means, medians) and visual graphic displays. Separate analyses were conducted for clinicians and patients in addition to a comparative analysis of the use and potential interest in integrative modalities. Analysis were conducted using R software. This study was deemed nonresearch quality improvement by the VA Ann Arbor Healthcare System facility research oversight board and institutional review board approval was not solicited.
RESULTS
Twenty-eight clinicians completed the survey, yielding a participation rate of 36%. Participating clinicians had a median (IQR) age of 48 years (9.5), 15 self-identified as White (54%), 8 as Asian (29%), 15 as female (54%), 26 as non-Hispanic (93%), and 25 were medical doctors or doctors of osteopathy (89%). Nineteen (68%) worked at the main hospital outpatient clinic, and 9 practiced at community-based outpatient clinics (CBOCs). Thirteen respondents (46%) reported having no formal education or training in integrative approaches. Among those with prior training, 8 clinicians had nutrition counseling (29%) and 7 had psychologic therapy training (25%). Thirteen respondents (46%) also reported using integrative modalities for personal health needs: 8 used psychological therapies, 8 used movement therapies, 10 used integrative modalities for stress management or relaxation, and 8 used them for physical symptoms (Table 1).

Overall, 85 of 200 patients (43%) responded to the study survey. Two patients indicated they did not have chronic back pain and were excluded. Patients had a median (IQR) age of 66 (20) years, with 66 self-identifying as White (80%), 69 as male (83%), and 66 as non-Hispanic (80%). Forty-four patients (53%) received care at CBOCs. Forty-seven patients reported excellent, very good, or good overall health (57%), while 53 reported excellent, very good, or good mental health (64%). Fifty-nine patients reported back pain duration > 5 years (71%), and 67 (81%) indicated experiencing back pain flare-ups at least once per week over the previous 12 months. Sixty patients (72%) indicated they were somewhat or very interested in using integrative therapies as a back pain treatment; however, 40 patients (48%) indicated they had not received information about these therapies. Among those who indicated they had received information, the most frequently reported source was their PCP (41%). Most patients (72%) also reported feeling somewhat to very comfortable discussing integrative medicine therapies with their PCP.
Integrative Therapy Recommendations and Use
PCPs reported recommending multiple integrative modalities: 23 (82%) recommended cognitive-behavioral therapy, 22 (79%) recommended acupuncture, 21 (75%) recommended chiropractic, 19 (68%) recommended battlefield acupuncture, recommended massage 18 (64%), 17 (61%) recommended meditation or mindfulness, and 15 (54%) recommended movement therapies such as yoga or tai chi/qigong (Figure 1). The only therapies used by at least half of the patients were chiropractic used by 59 patients (71%) and acupuncture by 42 patients (51%). Thirty-eight patients (46%) reported massage use and 21 patients (25%) used cognitive-behavioral therapy (Table 2).


Integrative Therapies Desired
A majority of PCPs identified acupuncture (n = 20, 71%), chiropractic (n = 19, 68%), and massage (n = 19, 68%) as therapies they would most like to have available for patients with chronic pain (Figure 2). Similarly, patients identified massage (n = 42, 51%), chiropractic (n = 34, 41%), and acupuncture (n = 27, 33%) as most desired. Seventeen patients (21%) expressed interest in movement therapies.

Barriers to Integrative Therapies Use
When asked about barriers to use, 26 PCPs (93%) identified access to services as a somewhat or extremely likely barrier, and 22 identified time constraints (79%) (Table 3). However, 17 PCPs (61%) noted lack of familiarity, and 18 (64%) noted a lack of scientific evidence as barriers to recommending integrative modalities. Among patients, 33 (40%) indicated not knowing what services were available at their facility as a barrier, 32 (39%) were not familiar with specific therapies, and 21 (25%) indicated a lack of clarity about the benefits of a specific therapy. Only 14 patients (17%) indicated that there were no obstacles to use.

DISCUSSION
Use of integrative therapies, including complementary treatments, is an increasingly important part of chronic pain management. This survey study suggests VA PCPs are willing to recommend integrative therapies and patients with chronic back pain both desire and use several therapies. Moreover, both groups expressed interest in greater availability of similar therapies. The results also highlight key barriers, such as knowledge gaps, that should be addressed to increase the uptake of integrative modalities for managing chronic pain.
An increasing number of US adults are using complementary health approaches, an important component of integrative therapy.12 This trend includes an increase in use for pain management, from 42.3% in 2002 to 49.2% in 2022; chiropractic care, acupuncture, and massage were most frequently used.12 Similarly, chiropractic, acupuncture and massage were most often used by this sample of veterans with chronic back pain and were identified by the highest percentages of PCPs and patients as the therapies they would most like available.
There were areas where the opinions of patients and clinicians differed. As has been seen previously reported, clinicians largely recommended cognitive-behavioral therapy while patients showed less interest.17 Additionally, while patients expressed interest in the availability of movement therapies, such as yoga, PCPs expressed more interest in other strategies, such as trigger point injections. These differences may reflect true preference or a tendency for clinicians and patients to select therapies with which they are more familiar. Additional research is needed to better understand the acceptability and potential use of integrative health treatments across a broad array of therapeutic options.
Despite VHA policy requiring facilities to provide certain complementary and integrative health modalities, almost all PCPs identified access to services as a major obstacle.15 Based on evidence and a rigorous vetting process, services currently required on-site, via telehealth, or through community partners include acupuncture and battlefield acupuncture (battlefield auricular acupuncture), biofeedback, clinical hypnosis, guided imagery, medical massage therapy, medication, tai chi/qigong, and yoga. Optional approaches, which may be made available to veterans, include chiropractic and healing touch. Outside the VHA, some states have introduced or enacted legislation mandating insurance coverage of nonpharmacological pain treatments.18 However, these requirements and mandates do not help address challenges such as the availability of trained/qualified practitioners.19,20 Ensuring access to complementary and integrative health treatments requires a more concerted effort to ensure that supply meets demand. It is also important to acknowledge the budgetary and physical space constraints that further limit access to services. Although expansion and integration of integrative medicine services remain a priority within the VA Whole Health program, implementation is contingent on available financial and infrastructure resources.
Time was also identified by PCPs as a barrier to recommending integrative therapies to patients. Developing and implementing time-efficient communication strategies for patient education such as concise talking points and informational handouts could help address this barrier. Furthermore, leveraging existing programs and engaging the entire health care team in patient education and referral could help increase integrative and complementary therapy uptake and use.
Although access and time were identified as major barriers, these findings also suggest that PCP and patient knowledge are another target area for enhancing the use of complementary and integrative therapies. Like prior research, most clinicians identified a lack of familiarity with certain services and a lack of scientific evidence as extremely or somewhat likely to affect their ability to offer integrative services to patients with chronic pain.21 Likewise, about 40% of patients identified being unfamiliar with a specific therapy as one of the major obstacles to receiving integrative therapies, with a similar number identifying PCPs as a source of information. The lack of familiarity may be due in part to the evolving nomenclature, with terms such as alternative, complementary, and integrative used to describe approaches outside what is often considered conventional medicine.10 On the other hand, there has also been considerable expansion in the number of therapies within this domain, along with an expanding evidence base. This suggests a need for targeted educational strategies for clinicians and patients, which can be rapidly deployed and continuously adapted as new therapies and evidence emerge.
Limitations
There are some inherent limitations with a survey-based approach, including sampling, non-response, and social desirability biases. In addition, this study only included PCPs and patients affiliated with a single VA medical center. Steps to mitigate these limitations included maintaining survey anonymity and reporting information about respondent characteristics to enhance transparency about the representativeness of the study findings.
CONCLUSIONS
Expanding the use of nonpharmacological pain treatments, including integrative modalities, is essential for safe and effective chronic pain management and reducing opioid use. Our findings show that VA PCPs and patients with chronic back pain are interested in and have some experience with certain integrative therapies. However, even within the context of a health care system that supports the use of integrative therapies for chronic pain as part of whole person care, increasing uptake will require addressing access and time-related constraints as well as ongoing clinician and patient education.
- Rikard SM, Strahan AE, Schmit KM, et al. Chronic pain among adults — United States, 2018-2021. MMWR Morb Mortal Wkly Rep. 2023;72:379-385. doi:10.15585/mmwr.mm7215a1
- Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163:E328-E332. doi:10.1097/j.pain.0000000000002291
- Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020. JAMA Netw Open. 2023;6:e2313563. doi:10.1001/jamanetworkopen.2023.13563
- Ferrari AJ, Santomauro DF, Aali A, et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2133-2161. doi:10.1016/S0140-6736(24)00757-8 5.
- Qureshi AR, Patel M, Neumark S, et al. Prevalence of chronic non-cancer pain among military veterans: a systematic review and meta-analysis of observational studies. BMJ Mil Health. 2025;171:310-314. doi:10.1136/military-2023-002554
- Feldman DE, Nahin RL. Disability among persons with chronic severe back pain: results from a nationally representative population-based sample. J Pain. 2022;23:2144-2154. doi:10.1016/j.jpain.2022.07.016
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi:10.7326/M16-2367
- van Erp RMA, Huijnen IPJ, Jakobs MLG, Kleijnen J, Smeets RJEM. Effectiveness of primary care interventions using a biopsychosocial approach in chronic low back pain: a systematic review. Pain Practice. 2019;19:224-241. doi:10.1111/papr.12735
- Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505. doi:10.7326/M16-2459
- Complementary, alternative, or integrative health: what’s in a name? National Institutes of Health, National Center for Complementary and Integrative Health. Updated April 2021. Accessed December 15, 2025. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name.
- Taylor SL, Elwy AR. Complementary and alternative medicine for US veterans and active duty military personnel promising steps to improve their health. Med Care. 2014;52:S1-S4. doi:10.1097/MLR.0000000000000270.
- Nahin RL, Rhee A, Stussman B. Use of complementary health approaches overall and for pain management by US adults. JAMA. 2024;331:613-615. doi:10.1001/jama.2023.26775
- Gantt CJ, Donovan N, Khung M. Veterans Affairs’ Whole Health System of Care for transitioning service members and veterans. Mil Med. 2023;188:28-32. doi:10.1093/milmed/usad047
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the Whole Health System of Care. Health Serv Res. 2022;57:53-65. doi:10.1111/1475-6773.13938
- Department of Veterans Affairs VHA. VHA Policy Directive 1137: Provision of Complementary and Integrative Health. December 2022. Accessed December 15, 2025. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=10072
- Giannitrapani KF, Holliday JR, Miake-Lye IM, Hempel S, Taylor SL. Synthesizing the strength of the evidence of complementary and integrative health therapies for pain. Pain Med. 2019;20:1831-1840. doi:10.1093/pm/pnz068
- Belitskaya-Levy I, David Clark J, Shih MC, Bair MJ. Treatment preferences for chronic low back pain: views of veterans and their providers. J Pain Res. 2021;14:161-171. doi:10.2147/JPR.S290400
- Onstott TN, Hurst S, Kronick R, Tsou AC, Groessl E, McMenamin SB. Health insurance mandates for nonpharmacological pain treatments in 7 US states. JAMA Netw Open. 2024;7:E245737. doi:10.1001/jamanetworkopen.2024.5737
- Sullivan M, Leach M, Snow J, Moonaz S. The North American yoga therapy workforce survey. Complement Ther Med. 2017;31:39-48. doi:10.1016/j.ctim.2017.01.006
- Bolton R, Ritter G, Highland K, Larson MJ. The relationship between capacity and utilization of nonpharmacologic therapies in the US Military Health System. BMC Health Serv Res. 2022;22. doi:10.1186/s12913-022-07700-4
- Stussman BJ, Nahin RL, Barnes PM, Scott R, Feinberg T, Ward BW. Reasons office-based physicians in the United States recommend common complementary health approaches to patients: an exploratory study using a national survey. J Integr Complement Med. 2022;28:651-663. doi:10.1089/jicm.2022.0493
- Rikard SM, Strahan AE, Schmit KM, et al. Chronic pain among adults — United States, 2018-2021. MMWR Morb Mortal Wkly Rep. 2023;72:379-385. doi:10.15585/mmwr.mm7215a1
- Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163:E328-E332. doi:10.1097/j.pain.0000000000002291
- Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020. JAMA Netw Open. 2023;6:e2313563. doi:10.1001/jamanetworkopen.2023.13563
- Ferrari AJ, Santomauro DF, Aali A, et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2133-2161. doi:10.1016/S0140-6736(24)00757-8 5.
- Qureshi AR, Patel M, Neumark S, et al. Prevalence of chronic non-cancer pain among military veterans: a systematic review and meta-analysis of observational studies. BMJ Mil Health. 2025;171:310-314. doi:10.1136/military-2023-002554
- Feldman DE, Nahin RL. Disability among persons with chronic severe back pain: results from a nationally representative population-based sample. J Pain. 2022;23:2144-2154. doi:10.1016/j.jpain.2022.07.016
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi:10.7326/M16-2367
- van Erp RMA, Huijnen IPJ, Jakobs MLG, Kleijnen J, Smeets RJEM. Effectiveness of primary care interventions using a biopsychosocial approach in chronic low back pain: a systematic review. Pain Practice. 2019;19:224-241. doi:10.1111/papr.12735
- Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505. doi:10.7326/M16-2459
- Complementary, alternative, or integrative health: what’s in a name? National Institutes of Health, National Center for Complementary and Integrative Health. Updated April 2021. Accessed December 15, 2025. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name.
- Taylor SL, Elwy AR. Complementary and alternative medicine for US veterans and active duty military personnel promising steps to improve their health. Med Care. 2014;52:S1-S4. doi:10.1097/MLR.0000000000000270.
- Nahin RL, Rhee A, Stussman B. Use of complementary health approaches overall and for pain management by US adults. JAMA. 2024;331:613-615. doi:10.1001/jama.2023.26775
- Gantt CJ, Donovan N, Khung M. Veterans Affairs’ Whole Health System of Care for transitioning service members and veterans. Mil Med. 2023;188:28-32. doi:10.1093/milmed/usad047
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the Whole Health System of Care. Health Serv Res. 2022;57:53-65. doi:10.1111/1475-6773.13938
- Department of Veterans Affairs VHA. VHA Policy Directive 1137: Provision of Complementary and Integrative Health. December 2022. Accessed December 15, 2025. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=10072
- Giannitrapani KF, Holliday JR, Miake-Lye IM, Hempel S, Taylor SL. Synthesizing the strength of the evidence of complementary and integrative health therapies for pain. Pain Med. 2019;20:1831-1840. doi:10.1093/pm/pnz068
- Belitskaya-Levy I, David Clark J, Shih MC, Bair MJ. Treatment preferences for chronic low back pain: views of veterans and their providers. J Pain Res. 2021;14:161-171. doi:10.2147/JPR.S290400
- Onstott TN, Hurst S, Kronick R, Tsou AC, Groessl E, McMenamin SB. Health insurance mandates for nonpharmacological pain treatments in 7 US states. JAMA Netw Open. 2024;7:E245737. doi:10.1001/jamanetworkopen.2024.5737
- Sullivan M, Leach M, Snow J, Moonaz S. The North American yoga therapy workforce survey. Complement Ther Med. 2017;31:39-48. doi:10.1016/j.ctim.2017.01.006
- Bolton R, Ritter G, Highland K, Larson MJ. The relationship between capacity and utilization of nonpharmacologic therapies in the US Military Health System. BMC Health Serv Res. 2022;22. doi:10.1186/s12913-022-07700-4
- Stussman BJ, Nahin RL, Barnes PM, Scott R, Feinberg T, Ward BW. Reasons office-based physicians in the United States recommend common complementary health approaches to patients: an exploratory study using a national survey. J Integr Complement Med. 2022;28:651-663. doi:10.1089/jicm.2022.0493
Primary Care Clinician and Patient Knowledge, Interest, and Use of Integrative Treatment Options for Chronic Low Back Pain Management
Primary Care Clinician and Patient Knowledge, Interest, and Use of Integrative Treatment Options for Chronic Low Back Pain Management
Development and Validation of an Administrative Algorithm to Identify Veterans With Epilepsy
Development and Validation of an Administrative Algorithm to Identify Veterans With Epilepsy
Epilepsy affects about 4.5 million people in the United States and 150,000 new individuals are diagnosed each year.1,2 In 2019, epilepsy-attributable health care spending for noninstitutionalized people was around $5.4 billion and total epilepsy-attributable and epilepsy or seizure health care-related costs totaled $54 billion.3
Accurate surveillance of epilepsy in large health care systems can potentially improve health care delivery and resource allocation. A 2012 Institute of Medicine (IOM) report identified 13 recommendations to guide public health action on epilepsy, including validation of standard definitions for case ascertainment, identification of epilepsy through screening programs or protocols, and expansion of surveillance to better understand disease burden.4
A systematic review of validation studies concluded that it is reasonable to use administrative data to identify people with epilepsy in epidemiologic research. Combining The International Classification of Diseases (ICD) codes for epilepsy (ICD-10, G40-41; ICD-9, 345) with antiseizure medications (ASMs) could provide high positive predictive values (PPVs) and combining symptoms codes for convulsions (ICD-10, R56; ICD-9, 780.3, 780.39) with ASMs could lead to high sensitivity.5 However, identifying individuals with epilepsy from administrative data in large managed health care organizations is challenging.6 The IOM report noted that large managed health care organizations presented varying incidence and prevalence estimates due to differing methodology, geographic area, demographics, and definitions of epilepsy.
The Veterans Health Administration (VHA) is the largest integrated US health care system, providing care to > 9.1 million veterans.7 To improve the health and well-being of veterans with epilepsy (VWEs), a network of sites was established in 2008 called the US Department of Veterans Affairs (VA) Epilepsy Centers of Excellence (ECoE). Subsequent to the creation of the ECoE, efforts were made to identify VWEs within VHA databases.8,9 Prior to fiscal year (FY) 2016, the ECoE adopted a modified version of a well-established epilepsy diagnostic algorithm developed by Holden et al for large managed care organizations.10 The original algorithm identified patients by cross-matching ASMs with ICD-9 codes for an index year. But it failed to capture a considerable number of stable patients with epilepsy in the VHA due to incomplete documentation, and had false positives due to inclusion of patients identified from diagnostic clinics. The modified algorithm the ECoE used prior to FY 2016 considered additional prior years and excluded encounters from diagnostic clinics. The result was an improvement in the sensitivity and specificity of the algorithm. Researchers evaluating 500 patients with epilepsy estimated that the modified algorithm had a PPV of 82.0% (95% CI, 78.6%-85.4%).11
After implementation of ICD-10 codes in the VHA in FY 2016, the task of reliably and efficiently identifying VWE led to a 3-tier algorithm. This article presents a validation of the different tiers of this algorithm after the implementation of ICD-10 diagnosis codes and summarizes the surveillance data collected over the years within the VHA showing the trends of epilepsy.
Methods
The VHA National Neurology office commissioned a Neurology Cube dashboard in FY 2021 in collaboration with VHA Support Service Center (VSSC) for reporting and surveillance of VWEs as a quality improvement initiative. The Neurology Cube uses a 3-tier system for identifying VWE in the VHA databases. VSSC programmers extract data from the VHA Corporate Data Warehouse (CDW) and utilize Microsoft SQL Server and Microsoft Power BI for Neurology Cube reports. The 3-tier system identifies VWE and divides them into distinct groups. The first tier identifies VWE with the highest degree of confidence; Tiers 2 and 3 represent identification with successively lesser degrees of confidence (Figure 1).
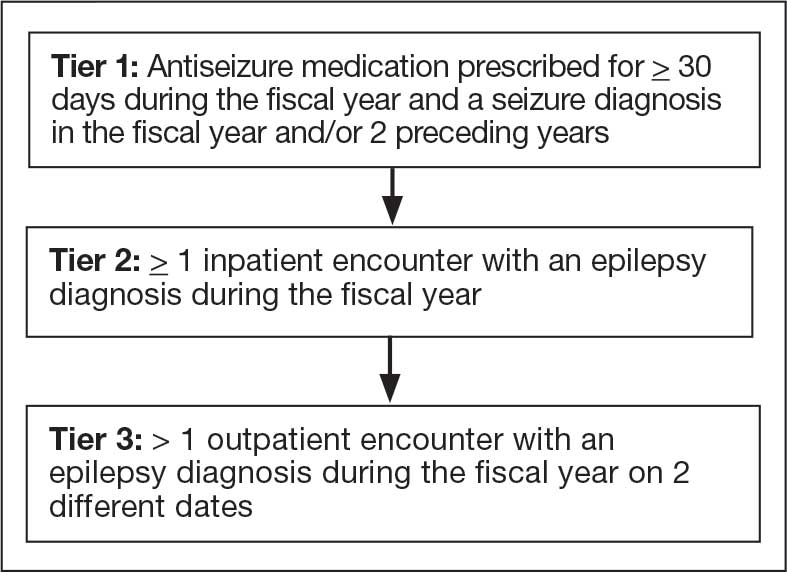
Tier 1
Definition. For a given index year and the preceding 2 years, any of following diagnosis codes on ≥ 1 clinical encounter are considered: 345.xx (epilepsy in ICD-9), 780.3x (other convulsions in ICD-9), G40.xxx (epilepsy in ICD-10), R40.4 (transient alteration of awareness), R56.1 (posttraumatic seizures), or R56.9 (unspecified convulsions). To reduce false positive rates, EEG clinic visits, which may include long-term monitoring, are excluded. Patients identified with ICD codes are then evaluated for an ASM prescription for ≥ 30 days during the index year. ASMs are listed in Appendix 1.
Validation. The development and validation of ICD-9 diagnosis codes crossmatched with an ASM prescription in the VHA has been published elsewhere.11 In FY 2017, after implementation of ICD-10 diagnostic codes, Tier 1 development and validation was performed in 2 phases. Even though Tier 1 study phases were conducted and completed during FY 2017, the patients for Tier 1 were identified from evaluation of FY 2016 data (October 1, 2015, to September 30, 2016). After the pilot analysis, the Tier 1 definition was implemented, and a chart review of 625 randomized patients was conducted at 5 sites for validation. Adequate preliminary data was not available to perform a sample size estimation for this study. Therefore, a practical target of 125 patients was set for Tier 1 from each site to obtain a final sample size of 625 patients. This second phase validated that the crossmatch of ICD-10 diagnosis codes with ASMs had a high PPV for identifying VWE.
Tiers 2 and 3
Definitions. For an index year, Tier 2 includes patients with ≥ 1 inpatient encounter documentation of either ICD-9 345.xx or ICD-10 G40.xxx, excluding EEG clinics. Tier 3 Includes patients who have had ≥ 2 outpatient encounters with diagnosis codes 345.xx or G40.xxx on 2 separate days, excluding EEG clinics. Tiers 2 and 3 do not require ASM prescriptions; this helps to identify VWEs who may be getting their medications outside of VHA or those who have received a new diagnosis.
Validations. Tiers 2 and 3 were included in the epilepsy identification algorithm in FY 2021 after validation was performed on a sample of 8 patients in each tier. Five patients were subsequently identified as having epilepsy in Tier 2 and 6 patients were identified in Tier 3. A more comprehensive validation of Tiers 2 and 3 was performed during FY 2022 that included patients at 5 sites seen during FY 2019 to FY 2022. Since yearly trends showed only about 8% of total patients were identified as having epilepsy through Tiers 2 and 3 we sought ≥ 20 patients per tier for the 5 sites for a total of 200 patients to ensure representation across the VHA. The final count was 126 patients for Tier 2 and 174 patients for Tier 3 (n = 300).
Gold Standard Criteria for Epilepsy Diagnosis
We used the International League Against Epilepsy (ILAE) definition of epilepsy for the validation of the 3 algorithm tiers. ILAE defines epilepsy as ≥ 2 unprovoked (or reflex) seizures occurring > 24 hours apart or 1 unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (≥ 60%) after 2 unprovoked seizures, occurring over the next 10 years.12
A standard protocol was provided to evaluators to identify patients using the VHA Computerized Patient Record System (Appendix 1). After review, evaluators categorized each patient in 1 of 4 ways: (1) Yes, definite: The patient’s health care practitioner (HCP) believes the patient has epilepsy and is treating with medication; (2) Yes, uncertain: The HCP has enough suspicion of epilepsy that a medication is prescribed, but uncertainty is expressed of the diagnosis; (3) No, definite: The HCP does not believe the patient has epilepsy and is therefore not treating with medication for seizure; (4) No, uncertain: The HCP is not treating with medication for epilepsy, because the diagnostic suspicion is not high enough, but there is suspicion for epilepsy.
As a quality improvement operational project, the Epilepsy National Program Office approved this validation project and determined that institutional review board approval was not required.
Statistical Analysis
Counts and percentages were computed for categories of epilepsy status. PPV of each tier was estimated with asymptotic 95% CIs.
Results
ICD-10 codes for 480 patients were evaluated in Tier 1 phase 1; 13.8% were documented with G40.xxx, 27.9% with R56.1, 34.4% with R56.9, and 24.0% with R40.4 (Appendix 2). In total, 68.1% fulfilled the criteria of epilepsy, 19.2% did not, and 12.7% were uncertain). From the validation of Tier 1 phase 2 (n = 625), the PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) was 85.1% (95% CI, 82.1%-87.8%) (Table).
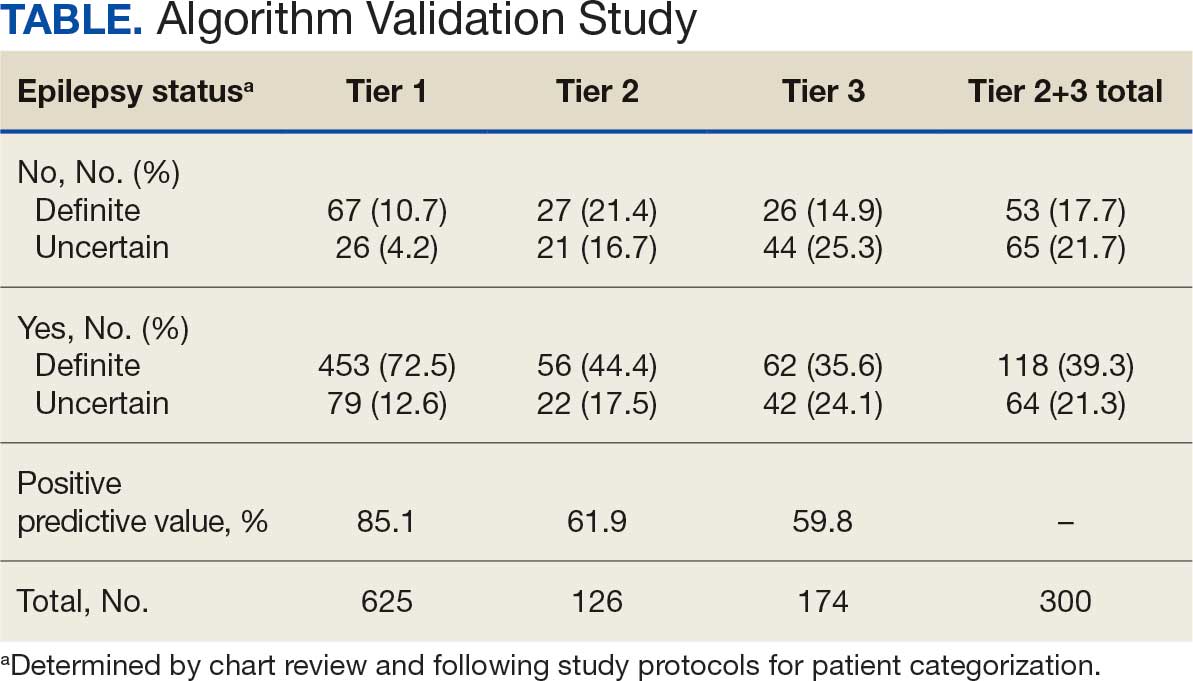
Of 300 patients evaluated, 126 (42.0%) were evaluated for Tier 2 with a PPV of 61.9% (95% CI, 53.4%-70.4%), and 174 (58.0%) patients were evaluated for Tier 3 with a PPV of 59.8% (95% CI, 52.5%-67.1%. The PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) were combined to calculate the PPV. Estimates of VHA VWE counts were computed for each tier from FY 2014 to FY 2023 using the VSSC Neurology Cube (Figure 2). For all years, > 92% patients were classified using the Tier 1 definition.
Discussion
The development and validation of the 3-tier diagnostic algorithm represents an important advancement in the surveillance and management of epilepsy among veterans within the VHA. The validation of this algorithm also demonstrates its practical utility in a large, integrated health care system.
Specific challenges were encountered when attempting to use pre-existing algorithms; these challenges included differences in the usage patterns of diagnostic codes and the patterns of ASM use within the VHA. These challenges prompted the need for a tailored approach, which led to the development of this algorithm. The inclusion of additional ICD-10 codes led to further revisions and subsequent validation. While many of the basic concepts of the algorithm, including ICD codes and ASMs, could work in other institutions, it would be wise for health care organizations to develop their own algorithms because of certain variables, including organizational size, patient demographics, common comorbidities, and the specific configurations of electronic health records and administrative data systems.
Studies have shown that ICD-10 codes for epilepsy (G40.* and/or R56.9) perform well in identifying epilepsy whether they are assigned by neurologists (sensitivity, 97.7%; specificity, 44.1%; PPV, 96.2%; negative predictive value, 57.7%), or in emergency department or hospital discharges (PPV, 75.5%).13,14 The pilot study of the algorithm’s Tier 1 development (phase 1) evaluated whether the selected ICD-10 diagnostic codes accurately included the VWE population within the VHA and revealed that while most codes (eg, epilepsy [G40.xxx]; posttraumatic seizures [R56.1]; and unspecified convulsions [R56.9]), had a low false positive rate (< 16%), the R40.4 code (transient alteration of awareness) had a higher false positivity of 42%. While this is not surprising given the broad spectrum of conditions that can manifest as transient alteration of awareness, it underscores the inherent challenges in diagnosing epilepsy using diagnosis codes.
In phase 2, the Tier 1 algorithm was validated as effective for identifying VWE in the VHA system, as its PPV was determined to be high (85%). In comparison, Tiers 2 and 3, whose criteria did not require data on VHA prescribed ASM use, had lower tiers of epilepsy predictability (PPV about 60% for both). This was thought to be acceptable because Tiers 2 and 3 represent a smaller population of the identified VWEs (about 8%). These VWEs may otherwise have been missed, partly because veterans are not required to get ASMs from the VHA.
Upon VHA implementation in FY 2021, this diagnostic algorithm exhibited significant clinical utility when integrated within the VSSC Neurology Cube. It facilitated an efficient approach to identifying VWEs using readily available databases. This led to better tracking of real-time epilepsy cases, which facilitated improving current resource allocation and targeted intervention strategies such as identification of drug-resistant epilepsy patients, optimizing strategies for telehealth and patient outreach for awareness of epilepsy care resources within VHA. Meanwhile, data acquired by the algorithm over the decade since its development (FY 2014 to FY 2023) contributed to more accurate epidemiologic information and identification of historic trends. Development of the algorithm represents one of the ways ECoEs have led to improved care for VWEs. ECoEs have been shown to improve health care for veterans in several metrics.15
A strength of this study is the rigorous multitiered validation process to confirm the diagnostic accuracy of ICD-10 codes against the gold standard ILAE definition of epilepsy to identify “definite” epilepsy cases within the VHA. The use of specific ICD codes further enhances the precision of epilepsy diagnoses. The inclusion of ASMs, which are sometimes prescribed for conditions other than epilepsy, could potentially inflate false positive rates.16
This study focused exclusively on the identification and validation of definite epilepsy cases within the VHA VSSC database, employing more stringent diagnostic criteria to ensure the highest level of certainty in ascertaining epilepsy. It is important to note there is a separate category of probable epilepsy, which involves a broader set of diagnostic criteria. While not covered in this study, probable epilepsy would be subject to future research and validation, which could provide insights into a wider spectrum of epilepsy diagnoses. Such future research could help refine the algorithm’s applicability and accuracy and potentially lead to more comprehensive surveillance and management strategies in clinical practice.
This study highlights the inherent challenges in leveraging administrative data for disease identification, particularly for conditions such as epilepsy, where diagnostic clarity can be complex. However, other conditions such as multiple sclerosis have noted similar success with the use of VHA administrative data for categorizing disease.17
Limitations
The algorithm discussed in this article is, in and of itself, generalizable. However, the validation process was unique to the VHA patient population, limiting the generalizability of the findings. Documentation practices and HCP attitudes within the VHA may differ from those in other health care settings. Identifying people with epilepsy can be challenging because of changing definitions of epilepsy over time. In addition to clinical evaluation, EEG and magnetic resonance imaging results, response to ASM treatment, and video-EEG monitoring of habitual events all can help establish the diagnosis. Therefore, studies may vary in how inclusive or exclusive the criteria are. ASMs such as gabapentin, pregabalin, carbamazepine, lamotrigine, topiramate, and valproate are used to treat other conditions, including headaches, generalized pain, and mood disorders. Consequently, including these ASMs in the Tier 1 definition may have increased the false positive rate. Additional research is needed to evaluate whether excluding these ASMs from the algorithm based on specific criteria (eg, dose of ASM used) can further refine the algorithm to identify patients with epilepsy.
Further refinement of this algorithm may also occur as technology changes. Future electronic health records may allow better tracking of different epilepsy factors, the integration of additional diagnostic criteria, and the use of natural language processing or other forms of artificial intelligence.
Conclusions
This study presents a significant step forward in epilepsy surveillance within the VHA. The algorithm offers a robust tool for identifying VWEs with good PPVs, facilitating better resource allocation and targeted care. Despite its limitations, this research lays a foundation for future advancements in the management and understanding of epilepsy within large health care systems. Since this VHA algorithm is based on ASMs and ICD diagnosis codes from patient records, other large managed health care systems also may be able to adapt this algorithm to their data specifications.

- Kobau R, Luncheon C, Greenlund K. Active epilepsy prevalence among U.S. adults is 1.1% and differs by educational level-National Health Interview Survey, United States, 2021. Epilepsy Behav. 2023;142:109180. doi:10.1016/j.yebeh.2023.109180
- GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78:165-176. doi:10.1001/jamaneurol.2020.4152
- Moura LMVR, Karakis I, Zack MM, et al. Drivers of US health care spending for persons with seizures and/or epilepsies, 2010-2018. Epilepsia. 2022;63:2144-2154. doi:10.1111/epi.17305
- Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. The National Academies Press; 2012. Accessed November 11, 2025. www.nap.edu/catalog/13379
- Mbizvo GK, Bennett KH, Schnier C, Simpson CR, Duncan SE, Chin RFM. The accuracy of using administrative healthcare data to identify epilepsy cases: A systematic review of validation studies. Epilepsia. 2020;61:1319-1335. doi:10.1111/epi.16547
- Montouris GD. How will primary care physicians, specialists, and managed care treat epilepsy in the new millennium? Neurology. 2000;55:S42-S44.
- US Department of Veterans Affairs. Veterans Health Administration: About VHA. Accessed November 11, 2025. https://www.va.gov/health/aboutvha.asp
- Veterans’ Mental Health and Other Care Improvements Act of 2008, S 2162, 110th Cong (2008). Accessed November 11, 2025. https://www.congress.gov/bill/110th-congress/senate-bill/2162
- Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of Veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
- Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8:1-14. doi:10.1089/dis.2005.8.1
- Rehman R, Everhart A, Frontera AT, et al. Implementation of an established algorithm and modifications for the identification of epilepsy patients in the Veterans Health Administration. Epilepsy Res. 2016;127:284-290. doi:10.1016/j.eplepsyres.2016.09.012
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi:10.1111/epi.12550
- Smith JR, Jones FJS, Fureman BE, et al. Accuracy of ICD-10-CM claims-based definitions for epilepsy and seizure type. Epilepsy Res. 2020;166:106414. doi:10.1016/j.eplepsyres.2020.106414
- Jetté N, Reid AY, Quan H, et al. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51:62-69. doi:10.1111/j.1528-1167.2009.02201.x
- Kelly P, Chinta R, Privitera G. Do centers of excellence reduce health care costs? Evidence from the US Veterans Health Administration Centers for Epilepsy. Glob Bus Organ Excell. 2015;34:18-29.
- Haneef Z, Rehman R, Husain AM. Association between standardized mortality ratio and utilization of care in US veterans with drug-resistant epilepsy compared with all US veterans and the US general population. JAMA Neurol. 2022;79:879-887. doi:10.1001/jamaneurol.2022.2290
- Culpepper WJ, Marrie RA, Langer-Gould A, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92:e1016-e1028 doi:10.1212/WNL.0000000000007043
Epilepsy affects about 4.5 million people in the United States and 150,000 new individuals are diagnosed each year.1,2 In 2019, epilepsy-attributable health care spending for noninstitutionalized people was around $5.4 billion and total epilepsy-attributable and epilepsy or seizure health care-related costs totaled $54 billion.3
Accurate surveillance of epilepsy in large health care systems can potentially improve health care delivery and resource allocation. A 2012 Institute of Medicine (IOM) report identified 13 recommendations to guide public health action on epilepsy, including validation of standard definitions for case ascertainment, identification of epilepsy through screening programs or protocols, and expansion of surveillance to better understand disease burden.4
A systematic review of validation studies concluded that it is reasonable to use administrative data to identify people with epilepsy in epidemiologic research. Combining The International Classification of Diseases (ICD) codes for epilepsy (ICD-10, G40-41; ICD-9, 345) with antiseizure medications (ASMs) could provide high positive predictive values (PPVs) and combining symptoms codes for convulsions (ICD-10, R56; ICD-9, 780.3, 780.39) with ASMs could lead to high sensitivity.5 However, identifying individuals with epilepsy from administrative data in large managed health care organizations is challenging.6 The IOM report noted that large managed health care organizations presented varying incidence and prevalence estimates due to differing methodology, geographic area, demographics, and definitions of epilepsy.
The Veterans Health Administration (VHA) is the largest integrated US health care system, providing care to > 9.1 million veterans.7 To improve the health and well-being of veterans with epilepsy (VWEs), a network of sites was established in 2008 called the US Department of Veterans Affairs (VA) Epilepsy Centers of Excellence (ECoE). Subsequent to the creation of the ECoE, efforts were made to identify VWEs within VHA databases.8,9 Prior to fiscal year (FY) 2016, the ECoE adopted a modified version of a well-established epilepsy diagnostic algorithm developed by Holden et al for large managed care organizations.10 The original algorithm identified patients by cross-matching ASMs with ICD-9 codes for an index year. But it failed to capture a considerable number of stable patients with epilepsy in the VHA due to incomplete documentation, and had false positives due to inclusion of patients identified from diagnostic clinics. The modified algorithm the ECoE used prior to FY 2016 considered additional prior years and excluded encounters from diagnostic clinics. The result was an improvement in the sensitivity and specificity of the algorithm. Researchers evaluating 500 patients with epilepsy estimated that the modified algorithm had a PPV of 82.0% (95% CI, 78.6%-85.4%).11
After implementation of ICD-10 codes in the VHA in FY 2016, the task of reliably and efficiently identifying VWE led to a 3-tier algorithm. This article presents a validation of the different tiers of this algorithm after the implementation of ICD-10 diagnosis codes and summarizes the surveillance data collected over the years within the VHA showing the trends of epilepsy.
Methods
The VHA National Neurology office commissioned a Neurology Cube dashboard in FY 2021 in collaboration with VHA Support Service Center (VSSC) for reporting and surveillance of VWEs as a quality improvement initiative. The Neurology Cube uses a 3-tier system for identifying VWE in the VHA databases. VSSC programmers extract data from the VHA Corporate Data Warehouse (CDW) and utilize Microsoft SQL Server and Microsoft Power BI for Neurology Cube reports. The 3-tier system identifies VWE and divides them into distinct groups. The first tier identifies VWE with the highest degree of confidence; Tiers 2 and 3 represent identification with successively lesser degrees of confidence (Figure 1).

Tier 1
Definition. For a given index year and the preceding 2 years, any of following diagnosis codes on ≥ 1 clinical encounter are considered: 345.xx (epilepsy in ICD-9), 780.3x (other convulsions in ICD-9), G40.xxx (epilepsy in ICD-10), R40.4 (transient alteration of awareness), R56.1 (posttraumatic seizures), or R56.9 (unspecified convulsions). To reduce false positive rates, EEG clinic visits, which may include long-term monitoring, are excluded. Patients identified with ICD codes are then evaluated for an ASM prescription for ≥ 30 days during the index year. ASMs are listed in Appendix 1.
Validation. The development and validation of ICD-9 diagnosis codes crossmatched with an ASM prescription in the VHA has been published elsewhere.11 In FY 2017, after implementation of ICD-10 diagnostic codes, Tier 1 development and validation was performed in 2 phases. Even though Tier 1 study phases were conducted and completed during FY 2017, the patients for Tier 1 were identified from evaluation of FY 2016 data (October 1, 2015, to September 30, 2016). After the pilot analysis, the Tier 1 definition was implemented, and a chart review of 625 randomized patients was conducted at 5 sites for validation. Adequate preliminary data was not available to perform a sample size estimation for this study. Therefore, a practical target of 125 patients was set for Tier 1 from each site to obtain a final sample size of 625 patients. This second phase validated that the crossmatch of ICD-10 diagnosis codes with ASMs had a high PPV for identifying VWE.
Tiers 2 and 3
Definitions. For an index year, Tier 2 includes patients with ≥ 1 inpatient encounter documentation of either ICD-9 345.xx or ICD-10 G40.xxx, excluding EEG clinics. Tier 3 Includes patients who have had ≥ 2 outpatient encounters with diagnosis codes 345.xx or G40.xxx on 2 separate days, excluding EEG clinics. Tiers 2 and 3 do not require ASM prescriptions; this helps to identify VWEs who may be getting their medications outside of VHA or those who have received a new diagnosis.
Validations. Tiers 2 and 3 were included in the epilepsy identification algorithm in FY 2021 after validation was performed on a sample of 8 patients in each tier. Five patients were subsequently identified as having epilepsy in Tier 2 and 6 patients were identified in Tier 3. A more comprehensive validation of Tiers 2 and 3 was performed during FY 2022 that included patients at 5 sites seen during FY 2019 to FY 2022. Since yearly trends showed only about 8% of total patients were identified as having epilepsy through Tiers 2 and 3 we sought ≥ 20 patients per tier for the 5 sites for a total of 200 patients to ensure representation across the VHA. The final count was 126 patients for Tier 2 and 174 patients for Tier 3 (n = 300).
Gold Standard Criteria for Epilepsy Diagnosis
We used the International League Against Epilepsy (ILAE) definition of epilepsy for the validation of the 3 algorithm tiers. ILAE defines epilepsy as ≥ 2 unprovoked (or reflex) seizures occurring > 24 hours apart or 1 unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (≥ 60%) after 2 unprovoked seizures, occurring over the next 10 years.12
A standard protocol was provided to evaluators to identify patients using the VHA Computerized Patient Record System (Appendix 1). After review, evaluators categorized each patient in 1 of 4 ways: (1) Yes, definite: The patient’s health care practitioner (HCP) believes the patient has epilepsy and is treating with medication; (2) Yes, uncertain: The HCP has enough suspicion of epilepsy that a medication is prescribed, but uncertainty is expressed of the diagnosis; (3) No, definite: The HCP does not believe the patient has epilepsy and is therefore not treating with medication for seizure; (4) No, uncertain: The HCP is not treating with medication for epilepsy, because the diagnostic suspicion is not high enough, but there is suspicion for epilepsy.
As a quality improvement operational project, the Epilepsy National Program Office approved this validation project and determined that institutional review board approval was not required.
Statistical Analysis
Counts and percentages were computed for categories of epilepsy status. PPV of each tier was estimated with asymptotic 95% CIs.
Results
ICD-10 codes for 480 patients were evaluated in Tier 1 phase 1; 13.8% were documented with G40.xxx, 27.9% with R56.1, 34.4% with R56.9, and 24.0% with R40.4 (Appendix 2). In total, 68.1% fulfilled the criteria of epilepsy, 19.2% did not, and 12.7% were uncertain). From the validation of Tier 1 phase 2 (n = 625), the PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) was 85.1% (95% CI, 82.1%-87.8%) (Table).

Of 300 patients evaluated, 126 (42.0%) were evaluated for Tier 2 with a PPV of 61.9% (95% CI, 53.4%-70.4%), and 174 (58.0%) patients were evaluated for Tier 3 with a PPV of 59.8% (95% CI, 52.5%-67.1%. The PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) were combined to calculate the PPV. Estimates of VHA VWE counts were computed for each tier from FY 2014 to FY 2023 using the VSSC Neurology Cube (Figure 2). For all years, > 92% patients were classified using the Tier 1 definition.

Discussion
The development and validation of the 3-tier diagnostic algorithm represents an important advancement in the surveillance and management of epilepsy among veterans within the VHA. The validation of this algorithm also demonstrates its practical utility in a large, integrated health care system.
Specific challenges were encountered when attempting to use pre-existing algorithms; these challenges included differences in the usage patterns of diagnostic codes and the patterns of ASM use within the VHA. These challenges prompted the need for a tailored approach, which led to the development of this algorithm. The inclusion of additional ICD-10 codes led to further revisions and subsequent validation. While many of the basic concepts of the algorithm, including ICD codes and ASMs, could work in other institutions, it would be wise for health care organizations to develop their own algorithms because of certain variables, including organizational size, patient demographics, common comorbidities, and the specific configurations of electronic health records and administrative data systems.
Studies have shown that ICD-10 codes for epilepsy (G40.* and/or R56.9) perform well in identifying epilepsy whether they are assigned by neurologists (sensitivity, 97.7%; specificity, 44.1%; PPV, 96.2%; negative predictive value, 57.7%), or in emergency department or hospital discharges (PPV, 75.5%).13,14 The pilot study of the algorithm’s Tier 1 development (phase 1) evaluated whether the selected ICD-10 diagnostic codes accurately included the VWE population within the VHA and revealed that while most codes (eg, epilepsy [G40.xxx]; posttraumatic seizures [R56.1]; and unspecified convulsions [R56.9]), had a low false positive rate (< 16%), the R40.4 code (transient alteration of awareness) had a higher false positivity of 42%. While this is not surprising given the broad spectrum of conditions that can manifest as transient alteration of awareness, it underscores the inherent challenges in diagnosing epilepsy using diagnosis codes.
In phase 2, the Tier 1 algorithm was validated as effective for identifying VWE in the VHA system, as its PPV was determined to be high (85%). In comparison, Tiers 2 and 3, whose criteria did not require data on VHA prescribed ASM use, had lower tiers of epilepsy predictability (PPV about 60% for both). This was thought to be acceptable because Tiers 2 and 3 represent a smaller population of the identified VWEs (about 8%). These VWEs may otherwise have been missed, partly because veterans are not required to get ASMs from the VHA.
Upon VHA implementation in FY 2021, this diagnostic algorithm exhibited significant clinical utility when integrated within the VSSC Neurology Cube. It facilitated an efficient approach to identifying VWEs using readily available databases. This led to better tracking of real-time epilepsy cases, which facilitated improving current resource allocation and targeted intervention strategies such as identification of drug-resistant epilepsy patients, optimizing strategies for telehealth and patient outreach for awareness of epilepsy care resources within VHA. Meanwhile, data acquired by the algorithm over the decade since its development (FY 2014 to FY 2023) contributed to more accurate epidemiologic information and identification of historic trends. Development of the algorithm represents one of the ways ECoEs have led to improved care for VWEs. ECoEs have been shown to improve health care for veterans in several metrics.15
A strength of this study is the rigorous multitiered validation process to confirm the diagnostic accuracy of ICD-10 codes against the gold standard ILAE definition of epilepsy to identify “definite” epilepsy cases within the VHA. The use of specific ICD codes further enhances the precision of epilepsy diagnoses. The inclusion of ASMs, which are sometimes prescribed for conditions other than epilepsy, could potentially inflate false positive rates.16
This study focused exclusively on the identification and validation of definite epilepsy cases within the VHA VSSC database, employing more stringent diagnostic criteria to ensure the highest level of certainty in ascertaining epilepsy. It is important to note there is a separate category of probable epilepsy, which involves a broader set of diagnostic criteria. While not covered in this study, probable epilepsy would be subject to future research and validation, which could provide insights into a wider spectrum of epilepsy diagnoses. Such future research could help refine the algorithm’s applicability and accuracy and potentially lead to more comprehensive surveillance and management strategies in clinical practice.
This study highlights the inherent challenges in leveraging administrative data for disease identification, particularly for conditions such as epilepsy, where diagnostic clarity can be complex. However, other conditions such as multiple sclerosis have noted similar success with the use of VHA administrative data for categorizing disease.17
Limitations
The algorithm discussed in this article is, in and of itself, generalizable. However, the validation process was unique to the VHA patient population, limiting the generalizability of the findings. Documentation practices and HCP attitudes within the VHA may differ from those in other health care settings. Identifying people with epilepsy can be challenging because of changing definitions of epilepsy over time. In addition to clinical evaluation, EEG and magnetic resonance imaging results, response to ASM treatment, and video-EEG monitoring of habitual events all can help establish the diagnosis. Therefore, studies may vary in how inclusive or exclusive the criteria are. ASMs such as gabapentin, pregabalin, carbamazepine, lamotrigine, topiramate, and valproate are used to treat other conditions, including headaches, generalized pain, and mood disorders. Consequently, including these ASMs in the Tier 1 definition may have increased the false positive rate. Additional research is needed to evaluate whether excluding these ASMs from the algorithm based on specific criteria (eg, dose of ASM used) can further refine the algorithm to identify patients with epilepsy.
Further refinement of this algorithm may also occur as technology changes. Future electronic health records may allow better tracking of different epilepsy factors, the integration of additional diagnostic criteria, and the use of natural language processing or other forms of artificial intelligence.
Conclusions
This study presents a significant step forward in epilepsy surveillance within the VHA. The algorithm offers a robust tool for identifying VWEs with good PPVs, facilitating better resource allocation and targeted care. Despite its limitations, this research lays a foundation for future advancements in the management and understanding of epilepsy within large health care systems. Since this VHA algorithm is based on ASMs and ICD diagnosis codes from patient records, other large managed health care systems also may be able to adapt this algorithm to their data specifications.


Epilepsy affects about 4.5 million people in the United States and 150,000 new individuals are diagnosed each year.1,2 In 2019, epilepsy-attributable health care spending for noninstitutionalized people was around $5.4 billion and total epilepsy-attributable and epilepsy or seizure health care-related costs totaled $54 billion.3
Accurate surveillance of epilepsy in large health care systems can potentially improve health care delivery and resource allocation. A 2012 Institute of Medicine (IOM) report identified 13 recommendations to guide public health action on epilepsy, including validation of standard definitions for case ascertainment, identification of epilepsy through screening programs or protocols, and expansion of surveillance to better understand disease burden.4
A systematic review of validation studies concluded that it is reasonable to use administrative data to identify people with epilepsy in epidemiologic research. Combining The International Classification of Diseases (ICD) codes for epilepsy (ICD-10, G40-41; ICD-9, 345) with antiseizure medications (ASMs) could provide high positive predictive values (PPVs) and combining symptoms codes for convulsions (ICD-10, R56; ICD-9, 780.3, 780.39) with ASMs could lead to high sensitivity.5 However, identifying individuals with epilepsy from administrative data in large managed health care organizations is challenging.6 The IOM report noted that large managed health care organizations presented varying incidence and prevalence estimates due to differing methodology, geographic area, demographics, and definitions of epilepsy.
The Veterans Health Administration (VHA) is the largest integrated US health care system, providing care to > 9.1 million veterans.7 To improve the health and well-being of veterans with epilepsy (VWEs), a network of sites was established in 2008 called the US Department of Veterans Affairs (VA) Epilepsy Centers of Excellence (ECoE). Subsequent to the creation of the ECoE, efforts were made to identify VWEs within VHA databases.8,9 Prior to fiscal year (FY) 2016, the ECoE adopted a modified version of a well-established epilepsy diagnostic algorithm developed by Holden et al for large managed care organizations.10 The original algorithm identified patients by cross-matching ASMs with ICD-9 codes for an index year. But it failed to capture a considerable number of stable patients with epilepsy in the VHA due to incomplete documentation, and had false positives due to inclusion of patients identified from diagnostic clinics. The modified algorithm the ECoE used prior to FY 2016 considered additional prior years and excluded encounters from diagnostic clinics. The result was an improvement in the sensitivity and specificity of the algorithm. Researchers evaluating 500 patients with epilepsy estimated that the modified algorithm had a PPV of 82.0% (95% CI, 78.6%-85.4%).11
After implementation of ICD-10 codes in the VHA in FY 2016, the task of reliably and efficiently identifying VWE led to a 3-tier algorithm. This article presents a validation of the different tiers of this algorithm after the implementation of ICD-10 diagnosis codes and summarizes the surveillance data collected over the years within the VHA showing the trends of epilepsy.
Methods
The VHA National Neurology office commissioned a Neurology Cube dashboard in FY 2021 in collaboration with VHA Support Service Center (VSSC) for reporting and surveillance of VWEs as a quality improvement initiative. The Neurology Cube uses a 3-tier system for identifying VWE in the VHA databases. VSSC programmers extract data from the VHA Corporate Data Warehouse (CDW) and utilize Microsoft SQL Server and Microsoft Power BI for Neurology Cube reports. The 3-tier system identifies VWE and divides them into distinct groups. The first tier identifies VWE with the highest degree of confidence; Tiers 2 and 3 represent identification with successively lesser degrees of confidence (Figure 1).

Tier 1
Definition. For a given index year and the preceding 2 years, any of following diagnosis codes on ≥ 1 clinical encounter are considered: 345.xx (epilepsy in ICD-9), 780.3x (other convulsions in ICD-9), G40.xxx (epilepsy in ICD-10), R40.4 (transient alteration of awareness), R56.1 (posttraumatic seizures), or R56.9 (unspecified convulsions). To reduce false positive rates, EEG clinic visits, which may include long-term monitoring, are excluded. Patients identified with ICD codes are then evaluated for an ASM prescription for ≥ 30 days during the index year. ASMs are listed in Appendix 1.
Validation. The development and validation of ICD-9 diagnosis codes crossmatched with an ASM prescription in the VHA has been published elsewhere.11 In FY 2017, after implementation of ICD-10 diagnostic codes, Tier 1 development and validation was performed in 2 phases. Even though Tier 1 study phases were conducted and completed during FY 2017, the patients for Tier 1 were identified from evaluation of FY 2016 data (October 1, 2015, to September 30, 2016). After the pilot analysis, the Tier 1 definition was implemented, and a chart review of 625 randomized patients was conducted at 5 sites for validation. Adequate preliminary data was not available to perform a sample size estimation for this study. Therefore, a practical target of 125 patients was set for Tier 1 from each site to obtain a final sample size of 625 patients. This second phase validated that the crossmatch of ICD-10 diagnosis codes with ASMs had a high PPV for identifying VWE.
Tiers 2 and 3
Definitions. For an index year, Tier 2 includes patients with ≥ 1 inpatient encounter documentation of either ICD-9 345.xx or ICD-10 G40.xxx, excluding EEG clinics. Tier 3 Includes patients who have had ≥ 2 outpatient encounters with diagnosis codes 345.xx or G40.xxx on 2 separate days, excluding EEG clinics. Tiers 2 and 3 do not require ASM prescriptions; this helps to identify VWEs who may be getting their medications outside of VHA or those who have received a new diagnosis.
Validations. Tiers 2 and 3 were included in the epilepsy identification algorithm in FY 2021 after validation was performed on a sample of 8 patients in each tier. Five patients were subsequently identified as having epilepsy in Tier 2 and 6 patients were identified in Tier 3. A more comprehensive validation of Tiers 2 and 3 was performed during FY 2022 that included patients at 5 sites seen during FY 2019 to FY 2022. Since yearly trends showed only about 8% of total patients were identified as having epilepsy through Tiers 2 and 3 we sought ≥ 20 patients per tier for the 5 sites for a total of 200 patients to ensure representation across the VHA. The final count was 126 patients for Tier 2 and 174 patients for Tier 3 (n = 300).
Gold Standard Criteria for Epilepsy Diagnosis
We used the International League Against Epilepsy (ILAE) definition of epilepsy for the validation of the 3 algorithm tiers. ILAE defines epilepsy as ≥ 2 unprovoked (or reflex) seizures occurring > 24 hours apart or 1 unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (≥ 60%) after 2 unprovoked seizures, occurring over the next 10 years.12
A standard protocol was provided to evaluators to identify patients using the VHA Computerized Patient Record System (Appendix 1). After review, evaluators categorized each patient in 1 of 4 ways: (1) Yes, definite: The patient’s health care practitioner (HCP) believes the patient has epilepsy and is treating with medication; (2) Yes, uncertain: The HCP has enough suspicion of epilepsy that a medication is prescribed, but uncertainty is expressed of the diagnosis; (3) No, definite: The HCP does not believe the patient has epilepsy and is therefore not treating with medication for seizure; (4) No, uncertain: The HCP is not treating with medication for epilepsy, because the diagnostic suspicion is not high enough, but there is suspicion for epilepsy.
As a quality improvement operational project, the Epilepsy National Program Office approved this validation project and determined that institutional review board approval was not required.
Statistical Analysis
Counts and percentages were computed for categories of epilepsy status. PPV of each tier was estimated with asymptotic 95% CIs.
Results
ICD-10 codes for 480 patients were evaluated in Tier 1 phase 1; 13.8% were documented with G40.xxx, 27.9% with R56.1, 34.4% with R56.9, and 24.0% with R40.4 (Appendix 2). In total, 68.1% fulfilled the criteria of epilepsy, 19.2% did not, and 12.7% were uncertain). From the validation of Tier 1 phase 2 (n = 625), the PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) was 85.1% (95% CI, 82.1%-87.8%) (Table).

Of 300 patients evaluated, 126 (42.0%) were evaluated for Tier 2 with a PPV of 61.9% (95% CI, 53.4%-70.4%), and 174 (58.0%) patients were evaluated for Tier 3 with a PPV of 59.8% (95% CI, 52.5%-67.1%. The PPV of the algorithm for patients presumed to have epilepsy (definite and uncertain) were combined to calculate the PPV. Estimates of VHA VWE counts were computed for each tier from FY 2014 to FY 2023 using the VSSC Neurology Cube (Figure 2). For all years, > 92% patients were classified using the Tier 1 definition.

Discussion
The development and validation of the 3-tier diagnostic algorithm represents an important advancement in the surveillance and management of epilepsy among veterans within the VHA. The validation of this algorithm also demonstrates its practical utility in a large, integrated health care system.
Specific challenges were encountered when attempting to use pre-existing algorithms; these challenges included differences in the usage patterns of diagnostic codes and the patterns of ASM use within the VHA. These challenges prompted the need for a tailored approach, which led to the development of this algorithm. The inclusion of additional ICD-10 codes led to further revisions and subsequent validation. While many of the basic concepts of the algorithm, including ICD codes and ASMs, could work in other institutions, it would be wise for health care organizations to develop their own algorithms because of certain variables, including organizational size, patient demographics, common comorbidities, and the specific configurations of electronic health records and administrative data systems.
Studies have shown that ICD-10 codes for epilepsy (G40.* and/or R56.9) perform well in identifying epilepsy whether they are assigned by neurologists (sensitivity, 97.7%; specificity, 44.1%; PPV, 96.2%; negative predictive value, 57.7%), or in emergency department or hospital discharges (PPV, 75.5%).13,14 The pilot study of the algorithm’s Tier 1 development (phase 1) evaluated whether the selected ICD-10 diagnostic codes accurately included the VWE population within the VHA and revealed that while most codes (eg, epilepsy [G40.xxx]; posttraumatic seizures [R56.1]; and unspecified convulsions [R56.9]), had a low false positive rate (< 16%), the R40.4 code (transient alteration of awareness) had a higher false positivity of 42%. While this is not surprising given the broad spectrum of conditions that can manifest as transient alteration of awareness, it underscores the inherent challenges in diagnosing epilepsy using diagnosis codes.
In phase 2, the Tier 1 algorithm was validated as effective for identifying VWE in the VHA system, as its PPV was determined to be high (85%). In comparison, Tiers 2 and 3, whose criteria did not require data on VHA prescribed ASM use, had lower tiers of epilepsy predictability (PPV about 60% for both). This was thought to be acceptable because Tiers 2 and 3 represent a smaller population of the identified VWEs (about 8%). These VWEs may otherwise have been missed, partly because veterans are not required to get ASMs from the VHA.
Upon VHA implementation in FY 2021, this diagnostic algorithm exhibited significant clinical utility when integrated within the VSSC Neurology Cube. It facilitated an efficient approach to identifying VWEs using readily available databases. This led to better tracking of real-time epilepsy cases, which facilitated improving current resource allocation and targeted intervention strategies such as identification of drug-resistant epilepsy patients, optimizing strategies for telehealth and patient outreach for awareness of epilepsy care resources within VHA. Meanwhile, data acquired by the algorithm over the decade since its development (FY 2014 to FY 2023) contributed to more accurate epidemiologic information and identification of historic trends. Development of the algorithm represents one of the ways ECoEs have led to improved care for VWEs. ECoEs have been shown to improve health care for veterans in several metrics.15
A strength of this study is the rigorous multitiered validation process to confirm the diagnostic accuracy of ICD-10 codes against the gold standard ILAE definition of epilepsy to identify “definite” epilepsy cases within the VHA. The use of specific ICD codes further enhances the precision of epilepsy diagnoses. The inclusion of ASMs, which are sometimes prescribed for conditions other than epilepsy, could potentially inflate false positive rates.16
This study focused exclusively on the identification and validation of definite epilepsy cases within the VHA VSSC database, employing more stringent diagnostic criteria to ensure the highest level of certainty in ascertaining epilepsy. It is important to note there is a separate category of probable epilepsy, which involves a broader set of diagnostic criteria. While not covered in this study, probable epilepsy would be subject to future research and validation, which could provide insights into a wider spectrum of epilepsy diagnoses. Such future research could help refine the algorithm’s applicability and accuracy and potentially lead to more comprehensive surveillance and management strategies in clinical practice.
This study highlights the inherent challenges in leveraging administrative data for disease identification, particularly for conditions such as epilepsy, where diagnostic clarity can be complex. However, other conditions such as multiple sclerosis have noted similar success with the use of VHA administrative data for categorizing disease.17
Limitations
The algorithm discussed in this article is, in and of itself, generalizable. However, the validation process was unique to the VHA patient population, limiting the generalizability of the findings. Documentation practices and HCP attitudes within the VHA may differ from those in other health care settings. Identifying people with epilepsy can be challenging because of changing definitions of epilepsy over time. In addition to clinical evaluation, EEG and magnetic resonance imaging results, response to ASM treatment, and video-EEG monitoring of habitual events all can help establish the diagnosis. Therefore, studies may vary in how inclusive or exclusive the criteria are. ASMs such as gabapentin, pregabalin, carbamazepine, lamotrigine, topiramate, and valproate are used to treat other conditions, including headaches, generalized pain, and mood disorders. Consequently, including these ASMs in the Tier 1 definition may have increased the false positive rate. Additional research is needed to evaluate whether excluding these ASMs from the algorithm based on specific criteria (eg, dose of ASM used) can further refine the algorithm to identify patients with epilepsy.
Further refinement of this algorithm may also occur as technology changes. Future electronic health records may allow better tracking of different epilepsy factors, the integration of additional diagnostic criteria, and the use of natural language processing or other forms of artificial intelligence.
Conclusions
This study presents a significant step forward in epilepsy surveillance within the VHA. The algorithm offers a robust tool for identifying VWEs with good PPVs, facilitating better resource allocation and targeted care. Despite its limitations, this research lays a foundation for future advancements in the management and understanding of epilepsy within large health care systems. Since this VHA algorithm is based on ASMs and ICD diagnosis codes from patient records, other large managed health care systems also may be able to adapt this algorithm to their data specifications.


- Kobau R, Luncheon C, Greenlund K. Active epilepsy prevalence among U.S. adults is 1.1% and differs by educational level-National Health Interview Survey, United States, 2021. Epilepsy Behav. 2023;142:109180. doi:10.1016/j.yebeh.2023.109180
- GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78:165-176. doi:10.1001/jamaneurol.2020.4152
- Moura LMVR, Karakis I, Zack MM, et al. Drivers of US health care spending for persons with seizures and/or epilepsies, 2010-2018. Epilepsia. 2022;63:2144-2154. doi:10.1111/epi.17305
- Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. The National Academies Press; 2012. Accessed November 11, 2025. www.nap.edu/catalog/13379
- Mbizvo GK, Bennett KH, Schnier C, Simpson CR, Duncan SE, Chin RFM. The accuracy of using administrative healthcare data to identify epilepsy cases: A systematic review of validation studies. Epilepsia. 2020;61:1319-1335. doi:10.1111/epi.16547
- Montouris GD. How will primary care physicians, specialists, and managed care treat epilepsy in the new millennium? Neurology. 2000;55:S42-S44.
- US Department of Veterans Affairs. Veterans Health Administration: About VHA. Accessed November 11, 2025. https://www.va.gov/health/aboutvha.asp
- Veterans’ Mental Health and Other Care Improvements Act of 2008, S 2162, 110th Cong (2008). Accessed November 11, 2025. https://www.congress.gov/bill/110th-congress/senate-bill/2162
- Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of Veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
- Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8:1-14. doi:10.1089/dis.2005.8.1
- Rehman R, Everhart A, Frontera AT, et al. Implementation of an established algorithm and modifications for the identification of epilepsy patients in the Veterans Health Administration. Epilepsy Res. 2016;127:284-290. doi:10.1016/j.eplepsyres.2016.09.012
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi:10.1111/epi.12550
- Smith JR, Jones FJS, Fureman BE, et al. Accuracy of ICD-10-CM claims-based definitions for epilepsy and seizure type. Epilepsy Res. 2020;166:106414. doi:10.1016/j.eplepsyres.2020.106414
- Jetté N, Reid AY, Quan H, et al. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51:62-69. doi:10.1111/j.1528-1167.2009.02201.x
- Kelly P, Chinta R, Privitera G. Do centers of excellence reduce health care costs? Evidence from the US Veterans Health Administration Centers for Epilepsy. Glob Bus Organ Excell. 2015;34:18-29.
- Haneef Z, Rehman R, Husain AM. Association between standardized mortality ratio and utilization of care in US veterans with drug-resistant epilepsy compared with all US veterans and the US general population. JAMA Neurol. 2022;79:879-887. doi:10.1001/jamaneurol.2022.2290
- Culpepper WJ, Marrie RA, Langer-Gould A, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92:e1016-e1028 doi:10.1212/WNL.0000000000007043
- Kobau R, Luncheon C, Greenlund K. Active epilepsy prevalence among U.S. adults is 1.1% and differs by educational level-National Health Interview Survey, United States, 2021. Epilepsy Behav. 2023;142:109180. doi:10.1016/j.yebeh.2023.109180
- GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78:165-176. doi:10.1001/jamaneurol.2020.4152
- Moura LMVR, Karakis I, Zack MM, et al. Drivers of US health care spending for persons with seizures and/or epilepsies, 2010-2018. Epilepsia. 2022;63:2144-2154. doi:10.1111/epi.17305
- Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. The National Academies Press; 2012. Accessed November 11, 2025. www.nap.edu/catalog/13379
- Mbizvo GK, Bennett KH, Schnier C, Simpson CR, Duncan SE, Chin RFM. The accuracy of using administrative healthcare data to identify epilepsy cases: A systematic review of validation studies. Epilepsia. 2020;61:1319-1335. doi:10.1111/epi.16547
- Montouris GD. How will primary care physicians, specialists, and managed care treat epilepsy in the new millennium? Neurology. 2000;55:S42-S44.
- US Department of Veterans Affairs. Veterans Health Administration: About VHA. Accessed November 11, 2025. https://www.va.gov/health/aboutvha.asp
- Veterans’ Mental Health and Other Care Improvements Act of 2008, S 2162, 110th Cong (2008). Accessed November 11, 2025. https://www.congress.gov/bill/110th-congress/senate-bill/2162
- Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of Veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
- Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8:1-14. doi:10.1089/dis.2005.8.1
- Rehman R, Everhart A, Frontera AT, et al. Implementation of an established algorithm and modifications for the identification of epilepsy patients in the Veterans Health Administration. Epilepsy Res. 2016;127:284-290. doi:10.1016/j.eplepsyres.2016.09.012
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi:10.1111/epi.12550
- Smith JR, Jones FJS, Fureman BE, et al. Accuracy of ICD-10-CM claims-based definitions for epilepsy and seizure type. Epilepsy Res. 2020;166:106414. doi:10.1016/j.eplepsyres.2020.106414
- Jetté N, Reid AY, Quan H, et al. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51:62-69. doi:10.1111/j.1528-1167.2009.02201.x
- Kelly P, Chinta R, Privitera G. Do centers of excellence reduce health care costs? Evidence from the US Veterans Health Administration Centers for Epilepsy. Glob Bus Organ Excell. 2015;34:18-29.
- Haneef Z, Rehman R, Husain AM. Association between standardized mortality ratio and utilization of care in US veterans with drug-resistant epilepsy compared with all US veterans and the US general population. JAMA Neurol. 2022;79:879-887. doi:10.1001/jamaneurol.2022.2290
- Culpepper WJ, Marrie RA, Langer-Gould A, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92:e1016-e1028 doi:10.1212/WNL.0000000000007043
Development and Validation of an Administrative Algorithm to Identify Veterans With Epilepsy
Development and Validation of an Administrative Algorithm to Identify Veterans With Epilepsy