User login
Polypoid Melanoma: An Aggressive Variant of Nodular Melanoma
To the Editor:
An 81-year-old man presented with a nodular polypoid lesion that developed on a flat lesion on the back of 2 years’ duration. The lesion grew progressively over the course of 3 months prior to presentation. The patient had a history of melanoma in situ on the forehead that was treated with conventional surgery with clear surgical margins 6 years prior to the current presentation.
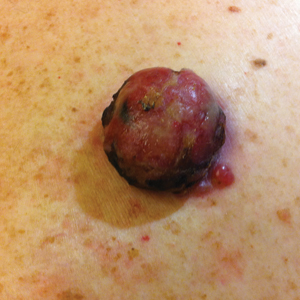
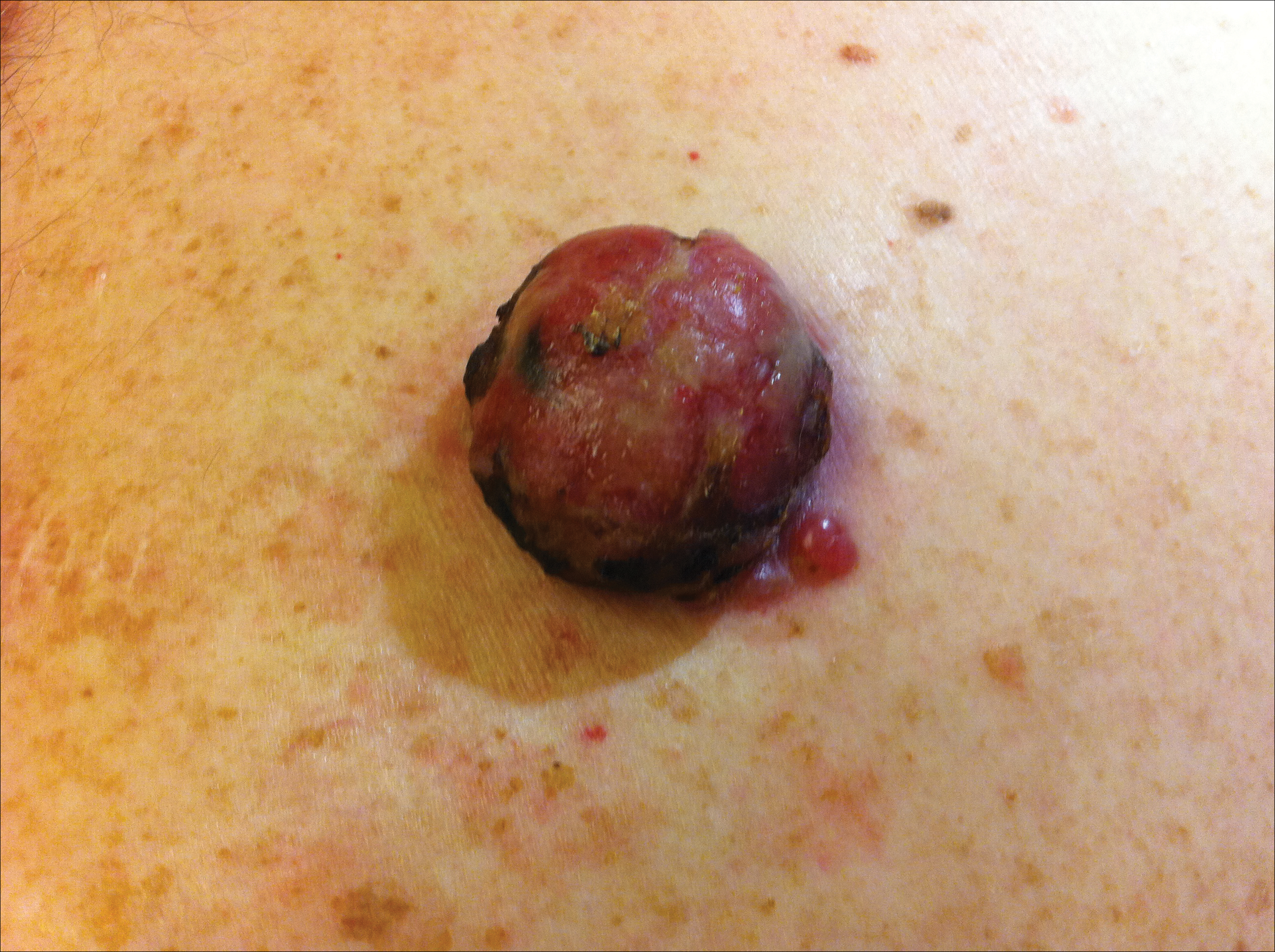
On physical examination the patient had a 4×2-cm ulcerated polypoid lesion on the back. The lesion was pink with a pigmented base. Additionally, 2 pink papules with superficial telangiectases were observed around the main lesion (Figure 1).
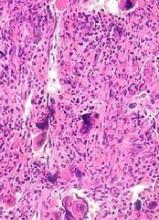
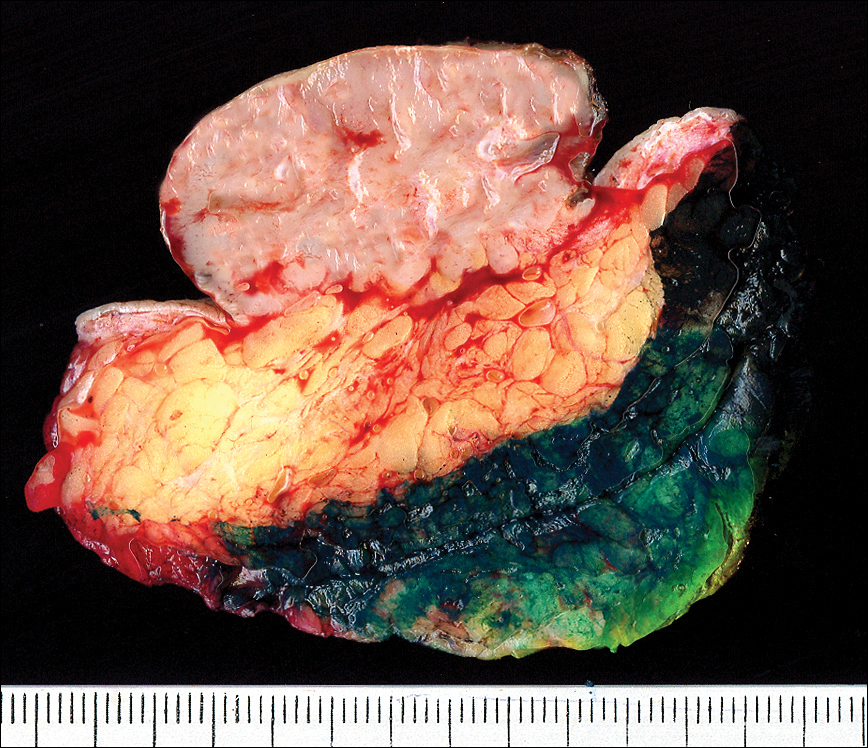
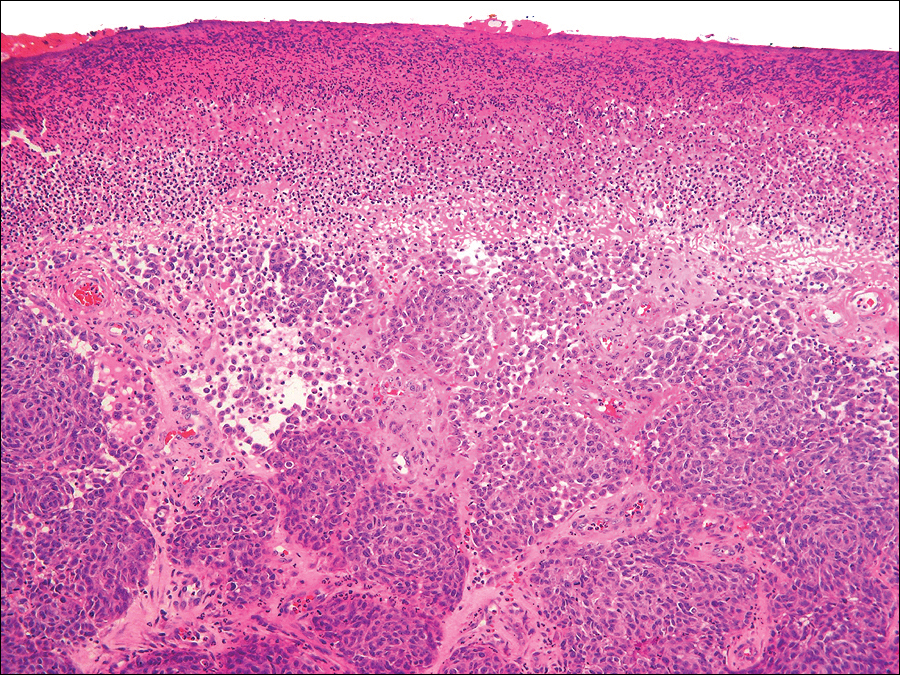
The gross section showed an exophytic tumor largely growing above the skin surface (Figure 2). Histopathologic analysis revealed an ulcerated lesion consisting of confluent nest and sheets of epithelioid and spindle atypical cells with numerous mitotic figures and necrotic foci (Figure 3). The thickness of the lesion was 2200 µm, and the mitotic count was 28 mitoses/mm2. There also was peritumoral vascular invasion and satellite metastasis within the perilesional hypodermis measuring 0.4 mm. Immunohistochemistry staining for S-100, human melanoma black 45 (HMB-45)(Figure 4), and Melan-A was positive in neoplastic cells.

The dissemination study revealed multiple mediastinal and axillary lymphadenopathies and lesions with metastatic appearance in the brain, liver, pancreas, and muscle, together with peritoneal carcinomatosis. The patient was lost to follow-up and did not follow coadjuvant therapy with interferon alfa.
Polypoid melanoma initially was described as a type of melanoma characterized by an exophytic growth in which most of the tumor is located on the cutaneous surface, together with ulceration.1 It usually occurs in patients aged 20 to 39 years,2 and the reported incidence ranges from 1.9% to 43.3%.1 It more commonly affects mucosae, including the upper respiratory tract, esophagus, and vagina. Polypoid melanoma has a rapid progression and a poor prognosis.3 Polypoid melanoma involving the skin primarily affects the back and has a 5-year survival rate of 32% to 42%.4 Poor prognosis has been attributed to the high risk for vascular embolization under the lesion.5 Histologically, there is marked cell atypia with nuclear and cellular pleomorphism and a high mitotic count. The tumor rarely involves the reticular dermis.1,2
Polypoid melanomas are rare; however, reported frequency rates cover a wide range. These frequency rates may be due to the definition of polypoid melanoma used by the pathologist issuing the report. One of the most accepted definitions at present is a pigmented macule that progresses in months with a rapid vertical growth, invading the epidermis and the papillary dermis.2 The differential diagnosis includes pyogenic granuloma, squamous cell carcinoma, basal cell carcinoma, soft tissue sarcomas, and hemangioma.
Although our patient had a history of melanoma and the polypoid lesion developed from a flat lesion, he was late to seek medical care. The diagnosis of melanoma is made on increasingly smaller lesions with better prognosis, but there still are reports of larger melanomas. This case highlights the role dermatologists serve in the education of patients on their diagnoses and risk factors so that we may be able to diagnose non–life-threatening small lesions. It is important to remember this morphologic variety of melanoma and highlight its rapid progression and poor prognosis.
- Knezević F, Duancić V, Sitić S, et al. Histological types of polypoid cutaneous melanoma II. Coll Antropol. 2007;31:1049-1053.
- Dini M, Quercioli F, Caldarella V, et al. Head and neck polypoid melanoma. J Craniofac Surg. 2012;23:E23-E25.
- Plotnick H, Rachmaninoff N, VandenBerg HJ Jr. Polypoid melanoma: a virulent variant of nodular melanoma. report of three cases and literature review. J Am Acad Dermatol. 1990;23(5, pt 1):880-884.
- Manci EA, Balch CM, Murad TM, et al. Polypoid melanoma, a virulent variant of the nodular growth pattern. Am J Clin Pathol. 1981;75:810-815.
- De Giorgi V, Massi D, Gerlini G, et al. Immediate local and regional recurrence after the excision of a polypoid melanoma: tumor dormancy or tumor activation? Dermatol Surg. 2003;29:664-667.
To the Editor:
An 81-year-old man presented with a nodular polypoid lesion that developed on a flat lesion on the back of 2 years’ duration. The lesion grew progressively over the course of 3 months prior to presentation. The patient had a history of melanoma in situ on the forehead that was treated with conventional surgery with clear surgical margins 6 years prior to the current presentation.
On physical examination the patient had a 4×2-cm ulcerated polypoid lesion on the back. The lesion was pink with a pigmented base. Additionally, 2 pink papules with superficial telangiectases were observed around the main lesion (Figure 1).
The gross section showed an exophytic tumor largely growing above the skin surface (Figure 2). Histopathologic analysis revealed an ulcerated lesion consisting of confluent nest and sheets of epithelioid and spindle atypical cells with numerous mitotic figures and necrotic foci (Figure 3). The thickness of the lesion was 2200 µm, and the mitotic count was 28 mitoses/mm2. There also was peritumoral vascular invasion and satellite metastasis within the perilesional hypodermis measuring 0.4 mm. Immunohistochemistry staining for S-100, human melanoma black 45 (HMB-45)(Figure 4), and Melan-A was positive in neoplastic cells.




The dissemination study revealed multiple mediastinal and axillary lymphadenopathies and lesions with metastatic appearance in the brain, liver, pancreas, and muscle, together with peritoneal carcinomatosis. The patient was lost to follow-up and did not follow coadjuvant therapy with interferon alfa.
Polypoid melanoma initially was described as a type of melanoma characterized by an exophytic growth in which most of the tumor is located on the cutaneous surface, together with ulceration.1 It usually occurs in patients aged 20 to 39 years,2 and the reported incidence ranges from 1.9% to 43.3%.1 It more commonly affects mucosae, including the upper respiratory tract, esophagus, and vagina. Polypoid melanoma has a rapid progression and a poor prognosis.3 Polypoid melanoma involving the skin primarily affects the back and has a 5-year survival rate of 32% to 42%.4 Poor prognosis has been attributed to the high risk for vascular embolization under the lesion.5 Histologically, there is marked cell atypia with nuclear and cellular pleomorphism and a high mitotic count. The tumor rarely involves the reticular dermis.1,2
Polypoid melanomas are rare; however, reported frequency rates cover a wide range. These frequency rates may be due to the definition of polypoid melanoma used by the pathologist issuing the report. One of the most accepted definitions at present is a pigmented macule that progresses in months with a rapid vertical growth, invading the epidermis and the papillary dermis.2 The differential diagnosis includes pyogenic granuloma, squamous cell carcinoma, basal cell carcinoma, soft tissue sarcomas, and hemangioma.
Although our patient had a history of melanoma and the polypoid lesion developed from a flat lesion, he was late to seek medical care. The diagnosis of melanoma is made on increasingly smaller lesions with better prognosis, but there still are reports of larger melanomas. This case highlights the role dermatologists serve in the education of patients on their diagnoses and risk factors so that we may be able to diagnose non–life-threatening small lesions. It is important to remember this morphologic variety of melanoma and highlight its rapid progression and poor prognosis.
To the Editor:
An 81-year-old man presented with a nodular polypoid lesion that developed on a flat lesion on the back of 2 years’ duration. The lesion grew progressively over the course of 3 months prior to presentation. The patient had a history of melanoma in situ on the forehead that was treated with conventional surgery with clear surgical margins 6 years prior to the current presentation.
On physical examination the patient had a 4×2-cm ulcerated polypoid lesion on the back. The lesion was pink with a pigmented base. Additionally, 2 pink papules with superficial telangiectases were observed around the main lesion (Figure 1).
The gross section showed an exophytic tumor largely growing above the skin surface (Figure 2). Histopathologic analysis revealed an ulcerated lesion consisting of confluent nest and sheets of epithelioid and spindle atypical cells with numerous mitotic figures and necrotic foci (Figure 3). The thickness of the lesion was 2200 µm, and the mitotic count was 28 mitoses/mm2. There also was peritumoral vascular invasion and satellite metastasis within the perilesional hypodermis measuring 0.4 mm. Immunohistochemistry staining for S-100, human melanoma black 45 (HMB-45)(Figure 4), and Melan-A was positive in neoplastic cells.




The dissemination study revealed multiple mediastinal and axillary lymphadenopathies and lesions with metastatic appearance in the brain, liver, pancreas, and muscle, together with peritoneal carcinomatosis. The patient was lost to follow-up and did not follow coadjuvant therapy with interferon alfa.
Polypoid melanoma initially was described as a type of melanoma characterized by an exophytic growth in which most of the tumor is located on the cutaneous surface, together with ulceration.1 It usually occurs in patients aged 20 to 39 years,2 and the reported incidence ranges from 1.9% to 43.3%.1 It more commonly affects mucosae, including the upper respiratory tract, esophagus, and vagina. Polypoid melanoma has a rapid progression and a poor prognosis.3 Polypoid melanoma involving the skin primarily affects the back and has a 5-year survival rate of 32% to 42%.4 Poor prognosis has been attributed to the high risk for vascular embolization under the lesion.5 Histologically, there is marked cell atypia with nuclear and cellular pleomorphism and a high mitotic count. The tumor rarely involves the reticular dermis.1,2
Polypoid melanomas are rare; however, reported frequency rates cover a wide range. These frequency rates may be due to the definition of polypoid melanoma used by the pathologist issuing the report. One of the most accepted definitions at present is a pigmented macule that progresses in months with a rapid vertical growth, invading the epidermis and the papillary dermis.2 The differential diagnosis includes pyogenic granuloma, squamous cell carcinoma, basal cell carcinoma, soft tissue sarcomas, and hemangioma.
Although our patient had a history of melanoma and the polypoid lesion developed from a flat lesion, he was late to seek medical care. The diagnosis of melanoma is made on increasingly smaller lesions with better prognosis, but there still are reports of larger melanomas. This case highlights the role dermatologists serve in the education of patients on their diagnoses and risk factors so that we may be able to diagnose non–life-threatening small lesions. It is important to remember this morphologic variety of melanoma and highlight its rapid progression and poor prognosis.
- Knezević F, Duancić V, Sitić S, et al. Histological types of polypoid cutaneous melanoma II. Coll Antropol. 2007;31:1049-1053.
- Dini M, Quercioli F, Caldarella V, et al. Head and neck polypoid melanoma. J Craniofac Surg. 2012;23:E23-E25.
- Plotnick H, Rachmaninoff N, VandenBerg HJ Jr. Polypoid melanoma: a virulent variant of nodular melanoma. report of three cases and literature review. J Am Acad Dermatol. 1990;23(5, pt 1):880-884.
- Manci EA, Balch CM, Murad TM, et al. Polypoid melanoma, a virulent variant of the nodular growth pattern. Am J Clin Pathol. 1981;75:810-815.
- De Giorgi V, Massi D, Gerlini G, et al. Immediate local and regional recurrence after the excision of a polypoid melanoma: tumor dormancy or tumor activation? Dermatol Surg. 2003;29:664-667.
- Knezević F, Duancić V, Sitić S, et al. Histological types of polypoid cutaneous melanoma II. Coll Antropol. 2007;31:1049-1053.
- Dini M, Quercioli F, Caldarella V, et al. Head and neck polypoid melanoma. J Craniofac Surg. 2012;23:E23-E25.
- Plotnick H, Rachmaninoff N, VandenBerg HJ Jr. Polypoid melanoma: a virulent variant of nodular melanoma. report of three cases and literature review. J Am Acad Dermatol. 1990;23(5, pt 1):880-884.
- Manci EA, Balch CM, Murad TM, et al. Polypoid melanoma, a virulent variant of the nodular growth pattern. Am J Clin Pathol. 1981;75:810-815.
- De Giorgi V, Massi D, Gerlini G, et al. Immediate local and regional recurrence after the excision of a polypoid melanoma: tumor dormancy or tumor activation? Dermatol Surg. 2003;29:664-667.
Practice Points
- The differential diagnosis of polypoid melanoma includes pyogenic granuloma and squamous cell carcinoma.
- Polypoid melanoma has a poor prognosis because of its thickness and ulceration at the time of diagnosis and the risk of vascular embolization.
Baricitinib shows potential as lupus treatment
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Baricitinib at 4 mg was associated with significant clinical improvements versus placebo and had an acceptable safety and tolerability profile.
Major finding: A higher percentage of patients receiving 4 mg of baricitinib than those receiving placebo achieved the primary endpoint of arthritis and/or rash remission as defined by the Systemic Lupus Erythematosus Disease Activity Index 2000 at week 24 (P less than .05).
Study details: A phase 2, multinational, double-blind, placebo-controlled, parallel group study of once-daily, oral baricitinib (2 mg and 4 mg) in 314 patients with SLE receiving standard therapy.
Disclosures: The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
Source: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
Honors Committee Accepting Nominations for Prestigious ACS Awards
The American College of Surgeons (ACS) Honors Committee is soliciting nominations for several prestigious awards and honors. These include the Distinguished Service Award; the Rodman E. and Thomas G. Sheen Award; the Jacobson Innovation Award; the Lifetime Achievement Award; candidates for Honorary Fellowship (from countries outside of the U.S. and Canada); and potential innovative speakers for the Martin Memorial Lecture, delivered at the Opening Ceremony of the annual ACS Clinical Congress.
Nominations are accepted all year long; however, Honorary Fellowship nominees are selected each October for induction at the following year’s Clinical Congress.
Visit the Honors Committee web page at www.facs.org/about-acs/governance/acs-committees/honors-committee for additional details about the criteria for nominations. Specific questions may be directed to Donna Coulombe, Honors Committee Staff Liaison, at [email protected] or 312-202-5203.
The American College of Surgeons (ACS) Honors Committee is soliciting nominations for several prestigious awards and honors. These include the Distinguished Service Award; the Rodman E. and Thomas G. Sheen Award; the Jacobson Innovation Award; the Lifetime Achievement Award; candidates for Honorary Fellowship (from countries outside of the U.S. and Canada); and potential innovative speakers for the Martin Memorial Lecture, delivered at the Opening Ceremony of the annual ACS Clinical Congress.
Nominations are accepted all year long; however, Honorary Fellowship nominees are selected each October for induction at the following year’s Clinical Congress.
Visit the Honors Committee web page at www.facs.org/about-acs/governance/acs-committees/honors-committee for additional details about the criteria for nominations. Specific questions may be directed to Donna Coulombe, Honors Committee Staff Liaison, at [email protected] or 312-202-5203.
The American College of Surgeons (ACS) Honors Committee is soliciting nominations for several prestigious awards and honors. These include the Distinguished Service Award; the Rodman E. and Thomas G. Sheen Award; the Jacobson Innovation Award; the Lifetime Achievement Award; candidates for Honorary Fellowship (from countries outside of the U.S. and Canada); and potential innovative speakers for the Martin Memorial Lecture, delivered at the Opening Ceremony of the annual ACS Clinical Congress.
Nominations are accepted all year long; however, Honorary Fellowship nominees are selected each October for induction at the following year’s Clinical Congress.
Visit the Honors Committee web page at www.facs.org/about-acs/governance/acs-committees/honors-committee for additional details about the criteria for nominations. Specific questions may be directed to Donna Coulombe, Honors Committee Staff Liaison, at [email protected] or 312-202-5203.
PU-H71 receives orphan drug designation for myelofibrosis
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
NAPRC Awards accredited John Muir Health Rectal Cancer Program
The National Accreditation Program for Rectal Cancer (NAPRC), recently launched by the American College of Surgeons (ACS), has awarded its first accreditation to the John Muir Health Rectal Cancer Program, Walnut Creek and Concord, CA. To earn the voluntary accreditation, the John Muir Health Rectal Cancer Program met 19 standards, including the establishment of a rectal cancer multidisciplinary team (RC-MDT) with clinical representatives from surgery, pathology, radiology, radiation oncology, and medical oncology.
Thirteen of those standards address clinical services that the program was required to provide, including carcinoembryonic antigen testing and magnetic resonance and computed tomography imaging for cancer staging, and ensuring a process whereby the patient starts treatment within a defined time frame. One of the most important clinical standards requires all rectal cancer patients to be present at both pre- and post-treatment RC-MDT meetings.
“When a cancer center achieves this type of specialized accreditation, it means that their rectal cancer patients will receive streamlined, modern evaluation and treatment for the disease. Compliance with our standards will assure optimal care for these patients,” said David P. Winchester, MD, FACS, Medical Director of ACS Cancer Programs.
The NAPRC was developed through a collaboration between the Optimizing the Surgical Treatment of Rectal Cancer Consortium and the ACS Commission on Cancer. It is based on successful international models that emphasize program structure, patient care processes, performance improvement, and performance measures. Its goal is to ensure that rectal cancer patients receive appropriate care using a multidisciplinary approach.
Read more in the ACS press release at www.facs.org/media/press-releases/2018/naprc052218. For more information about the program and instructions on how to apply for accreditation, visit the NAPRC website at www.facs.org/quality-programs/cancer/naprc, or contact [email protected].
The National Accreditation Program for Rectal Cancer (NAPRC), recently launched by the American College of Surgeons (ACS), has awarded its first accreditation to the John Muir Health Rectal Cancer Program, Walnut Creek and Concord, CA. To earn the voluntary accreditation, the John Muir Health Rectal Cancer Program met 19 standards, including the establishment of a rectal cancer multidisciplinary team (RC-MDT) with clinical representatives from surgery, pathology, radiology, radiation oncology, and medical oncology.
Thirteen of those standards address clinical services that the program was required to provide, including carcinoembryonic antigen testing and magnetic resonance and computed tomography imaging for cancer staging, and ensuring a process whereby the patient starts treatment within a defined time frame. One of the most important clinical standards requires all rectal cancer patients to be present at both pre- and post-treatment RC-MDT meetings.
“When a cancer center achieves this type of specialized accreditation, it means that their rectal cancer patients will receive streamlined, modern evaluation and treatment for the disease. Compliance with our standards will assure optimal care for these patients,” said David P. Winchester, MD, FACS, Medical Director of ACS Cancer Programs.
The NAPRC was developed through a collaboration between the Optimizing the Surgical Treatment of Rectal Cancer Consortium and the ACS Commission on Cancer. It is based on successful international models that emphasize program structure, patient care processes, performance improvement, and performance measures. Its goal is to ensure that rectal cancer patients receive appropriate care using a multidisciplinary approach.
Read more in the ACS press release at www.facs.org/media/press-releases/2018/naprc052218. For more information about the program and instructions on how to apply for accreditation, visit the NAPRC website at www.facs.org/quality-programs/cancer/naprc, or contact [email protected].
The National Accreditation Program for Rectal Cancer (NAPRC), recently launched by the American College of Surgeons (ACS), has awarded its first accreditation to the John Muir Health Rectal Cancer Program, Walnut Creek and Concord, CA. To earn the voluntary accreditation, the John Muir Health Rectal Cancer Program met 19 standards, including the establishment of a rectal cancer multidisciplinary team (RC-MDT) with clinical representatives from surgery, pathology, radiology, radiation oncology, and medical oncology.
Thirteen of those standards address clinical services that the program was required to provide, including carcinoembryonic antigen testing and magnetic resonance and computed tomography imaging for cancer staging, and ensuring a process whereby the patient starts treatment within a defined time frame. One of the most important clinical standards requires all rectal cancer patients to be present at both pre- and post-treatment RC-MDT meetings.
“When a cancer center achieves this type of specialized accreditation, it means that their rectal cancer patients will receive streamlined, modern evaluation and treatment for the disease. Compliance with our standards will assure optimal care for these patients,” said David P. Winchester, MD, FACS, Medical Director of ACS Cancer Programs.
The NAPRC was developed through a collaboration between the Optimizing the Surgical Treatment of Rectal Cancer Consortium and the ACS Commission on Cancer. It is based on successful international models that emphasize program structure, patient care processes, performance improvement, and performance measures. Its goal is to ensure that rectal cancer patients receive appropriate care using a multidisciplinary approach.
Read more in the ACS press release at www.facs.org/media/press-releases/2018/naprc052218. For more information about the program and instructions on how to apply for accreditation, visit the NAPRC website at www.facs.org/quality-programs/cancer/naprc, or contact [email protected].
NCDB-Sourced Study Focuses on Post-Treatment Surveillance for Colorectal Cancer Patients
The first findings from a collaborative study within the American College of Surgeons (ACS) Cancer Programs—and published earlier this week in the Journal of the American Medical Association (JAMA)—showed no significant association between frequency of surveillance testing and the time to detection of recurrence for colorectal cancer patients.
The study is an effort of the ACS Clinical Research Program and the Commission on Cancer and uses data from the National Cancer Database (NCDB), which is jointly sponsored by the ACS and the American Cancer Society. It focuses on post-treatment surveillance for breast, colon, and lung cancers and was funded by the Patient-Centered Outcomes Research Institute.
This portion of the study included more than 8,500 patients and was the first of eight manuscripts accepted for publication from a larger study conducted in 2015. The corresponding author is George J. Chang, MD, FACS, chief, section of colon and rectal surgery; professor of surgical oncology and health services research; and director of clinical operations, minimally invasive and new technologies in oncologic surgery program, University of Texas MD Anderson Cancer Center, Houston.
View the full text article on the JAMA website at https://jamanetwork.com/journals/jama/fullarticle/2681746/.
The first findings from a collaborative study within the American College of Surgeons (ACS) Cancer Programs—and published earlier this week in the Journal of the American Medical Association (JAMA)—showed no significant association between frequency of surveillance testing and the time to detection of recurrence for colorectal cancer patients.
The study is an effort of the ACS Clinical Research Program and the Commission on Cancer and uses data from the National Cancer Database (NCDB), which is jointly sponsored by the ACS and the American Cancer Society. It focuses on post-treatment surveillance for breast, colon, and lung cancers and was funded by the Patient-Centered Outcomes Research Institute.
This portion of the study included more than 8,500 patients and was the first of eight manuscripts accepted for publication from a larger study conducted in 2015. The corresponding author is George J. Chang, MD, FACS, chief, section of colon and rectal surgery; professor of surgical oncology and health services research; and director of clinical operations, minimally invasive and new technologies in oncologic surgery program, University of Texas MD Anderson Cancer Center, Houston.
View the full text article on the JAMA website at https://jamanetwork.com/journals/jama/fullarticle/2681746/.
The first findings from a collaborative study within the American College of Surgeons (ACS) Cancer Programs—and published earlier this week in the Journal of the American Medical Association (JAMA)—showed no significant association between frequency of surveillance testing and the time to detection of recurrence for colorectal cancer patients.
The study is an effort of the ACS Clinical Research Program and the Commission on Cancer and uses data from the National Cancer Database (NCDB), which is jointly sponsored by the ACS and the American Cancer Society. It focuses on post-treatment surveillance for breast, colon, and lung cancers and was funded by the Patient-Centered Outcomes Research Institute.
This portion of the study included more than 8,500 patients and was the first of eight manuscripts accepted for publication from a larger study conducted in 2015. The corresponding author is George J. Chang, MD, FACS, chief, section of colon and rectal surgery; professor of surgical oncology and health services research; and director of clinical operations, minimally invasive and new technologies in oncologic surgery program, University of Texas MD Anderson Cancer Center, Houston.
View the full text article on the JAMA website at https://jamanetwork.com/journals/jama/fullarticle/2681746/.
Routine screening for AAA in older men may harm more than help
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
FROM THE LANCET
Key clinical point: Screening for abdominal aortic aneurysms in men saved few lives, but significantly increased the risk of overdiagnosis and unnecessary surgery.
Major finding: Screening saved two lives per 10,000 men, but showed an increased risk of overtreatment of 19%.
Study details: The population-based longitudinal cohort study comprised 131,352 men.
Disclosures: The authors had no financial disclosures.
Source: Johansson et al. Lancet 2018;391:2441-7.
Breastfeeding with the FDA’s novel drugs approved in 2017, and others
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.
‘Captain of the ship’ doctrine
Question: The “Captain of the Ship” doctrine:
A. Is a legal principle used mostly in maritime law.
B. Is applicable only to surgeons in the operating room.
C. Is good law in all jurisdictions.
D. May be used by plaintiffs in emergency department triage litigation.
E. Originated when hospitals lost their charitable immunity.
Answer: D. Historically, the Captain of the Ship doctrine imputes liability to the surgeon who has the authority and right to control the actions of his assistants in the operating room.
Pennsylvania famously saw the use of the phrase in a 1949 case: “In the course of an operation in the operating room of a hospital, and until the surgeon leaves that room at the conclusion of the operation … he is in the same complete charge of those who are present and assisting him as is the captain of a ship over all on board.”1
Public hospitals in the 1940s were immune from liability because they were charitable organizations, so the Captain of the Ship doctrine emerged as a means for injured patients to recover damages against the surgeon instead. Courts have used various legal theories to justify this doctrine, which is basically grounded in vicarious liability, e.g., master-servant relationship (respondeat superior), borrowed servant, a nondelegable duty, or more broadly, principles of agency.
Use of the doctrine to shift liability to the surgeon in the operating room is well exemplified in litigation over retained sponges, left-behind instruments, burns in the operating room, administration of the wrong blood type, and allergic reaction to penicillin. Actual control of the surgeon’s assistants is not essential, but the right to merely supervise is insufficient. What is dispositive is the right and authority to determine an assistant’s actions.
However, what constitutes an “operating room” has been in dispute. It may simply mean a circumscribed and controlled area for medical procedures and/or treatment. Thus, the term has been extended to a room where only local anesthesia was used for esophageal dilation. Reasoning by analogy, the modern-day heart catheterization lab or interventional radiology suite would arguably count as “operating rooms” where the procedurist-doctor, usually a nonsurgeon, may be deemed to function as the captain of the ship.
Another place where a nonsurgeon may be involved is the hospital ED. It has been stated that emergency physicians have been held liable for adverse outcomes resulting from the patients under triage, based on the Captain of the Ship doctrine.2 Once a patient arrives in the ED, a legal duty to provide care arises, even if the physician has yet to see the patient. The federal Emergency Medical Treatment and Labor Act, which regulates much of what happens in the nation’s emergency departments, covers “any individual ... [who] comes to the emergency department and a request is made on the individual’s behalf for examination or treatment for a medical condition.”
Still, the doctrine is less likely to be invoked in a more spread-out area such as a general medical ward, where a physician’s control cannot be reasonably expected.
For example, courts have held that ward nurses giving injections into the buttock causing permanent neuropathy to a patient’s leg were not the agents of the prescribing physician, but just of the hospital employing them. The doctrine also was rejected in Collins v. Hand by the Pennsylvania Supreme Court, which reversed a judgment against a psychiatrist defendant.3 In the Collins case, notwithstanding the fact that the psychiatrist, Dr. Hand, had personally arranged for the patient’s transfer to another hospital and wrote orders for electroconvulsive therapy (which was complicated by fractures), Dr. Hand did not choose the doctor who was to administer the therapy, nor did he hire, compensate, or control any of the team members.
In the 1960s, hospitals began losing their charitable immunity status and assumed direct as well as vicarious liability for injuries to patients from the negligent acts of their employees, such as nurses. The key policy reason for having the Captain of the Ship doctrine then no longer existed. Besides, operating rooms became increasingly complex, and the senior surgeon was thought to be incapable of being in charge of all activities there.
Wisconsin is typical: A retained sponge following a laparoscopic cholecystectomy led to complications, and the patient sued the hospital and surgeon, claiming each was responsible for the nurses’ sponge-count error. The lower court had found that “as a matter of law [the surgeon] is in fact responsible and liable for the actions of the parties that were in the operating room with him and working under his supervision ... [the] doctor is the captain of the ship. That doctor is responsible for everything.”
Upon appeal, the Wisconsin Supreme Court reversed the decision of the lower court by rejecting the doctrine altogether, finding that it failed to reflect the emergence of hospitals as modern health care facilities.5
Still, the doctrine is by no means obsolete. In a Colorado case, the court wrote that, even if the nurse were an employee of the hospital and her negligence caused the death of plaintiff’s husband, the Captain of the Ship doctrine would preclude recovery against the hospital.6 It relied on a precedent-setting case that held that once the operating surgeon assumed control in the operating room, the surgeon is liable for the negligence of all persons working there.
Likewise, California has recently breathed new life into the doctrine.7 A case in 2006 involved a patient who underwent arterial bypass surgery in his right leg. A case in which a nurse’s counting error led to a retained sponge ended up with the patient losing his leg. The surgeon initially escaped liability by virtue of the court’s refusal to include Captain of the Ship instructions to the jury, which found the doctor not negligent. The state court of appeals reversed, however, concluding that it was reasonably probable that the jury might have reached a different result had it been so instructed.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. McConnell v. Williams, 361 Pa. 355, 65 A.2d 243 (1949).
2. ED Legal Letter, Feb 1, 2018.
3. Collins v. Hand, 246 A.2d 398 (Pa 1968).
4. AORN J. 2001 Oct;74(4):525-8.
5. Lewis v. Physicians Insurance Company et al., 627 NW2d 484 (Wis 2001).
6. Krane v. St. Anthony Hospital Systems, 738 P.2d 75 (Co 1987).
7. Fields v. Yusuf, 144 Cal.App.4th 1381 (2006).
Question: The “Captain of the Ship” doctrine:
A. Is a legal principle used mostly in maritime law.
B. Is applicable only to surgeons in the operating room.
C. Is good law in all jurisdictions.
D. May be used by plaintiffs in emergency department triage litigation.
E. Originated when hospitals lost their charitable immunity.
Answer: D. Historically, the Captain of the Ship doctrine imputes liability to the surgeon who has the authority and right to control the actions of his assistants in the operating room.
Pennsylvania famously saw the use of the phrase in a 1949 case: “In the course of an operation in the operating room of a hospital, and until the surgeon leaves that room at the conclusion of the operation … he is in the same complete charge of those who are present and assisting him as is the captain of a ship over all on board.”1
Public hospitals in the 1940s were immune from liability because they were charitable organizations, so the Captain of the Ship doctrine emerged as a means for injured patients to recover damages against the surgeon instead. Courts have used various legal theories to justify this doctrine, which is basically grounded in vicarious liability, e.g., master-servant relationship (respondeat superior), borrowed servant, a nondelegable duty, or more broadly, principles of agency.
Use of the doctrine to shift liability to the surgeon in the operating room is well exemplified in litigation over retained sponges, left-behind instruments, burns in the operating room, administration of the wrong blood type, and allergic reaction to penicillin. Actual control of the surgeon’s assistants is not essential, but the right to merely supervise is insufficient. What is dispositive is the right and authority to determine an assistant’s actions.
However, what constitutes an “operating room” has been in dispute. It may simply mean a circumscribed and controlled area for medical procedures and/or treatment. Thus, the term has been extended to a room where only local anesthesia was used for esophageal dilation. Reasoning by analogy, the modern-day heart catheterization lab or interventional radiology suite would arguably count as “operating rooms” where the procedurist-doctor, usually a nonsurgeon, may be deemed to function as the captain of the ship.
Another place where a nonsurgeon may be involved is the hospital ED. It has been stated that emergency physicians have been held liable for adverse outcomes resulting from the patients under triage, based on the Captain of the Ship doctrine.2 Once a patient arrives in the ED, a legal duty to provide care arises, even if the physician has yet to see the patient. The federal Emergency Medical Treatment and Labor Act, which regulates much of what happens in the nation’s emergency departments, covers “any individual ... [who] comes to the emergency department and a request is made on the individual’s behalf for examination or treatment for a medical condition.”
Still, the doctrine is less likely to be invoked in a more spread-out area such as a general medical ward, where a physician’s control cannot be reasonably expected.
For example, courts have held that ward nurses giving injections into the buttock causing permanent neuropathy to a patient’s leg were not the agents of the prescribing physician, but just of the hospital employing them. The doctrine also was rejected in Collins v. Hand by the Pennsylvania Supreme Court, which reversed a judgment against a psychiatrist defendant.3 In the Collins case, notwithstanding the fact that the psychiatrist, Dr. Hand, had personally arranged for the patient’s transfer to another hospital and wrote orders for electroconvulsive therapy (which was complicated by fractures), Dr. Hand did not choose the doctor who was to administer the therapy, nor did he hire, compensate, or control any of the team members.
In the 1960s, hospitals began losing their charitable immunity status and assumed direct as well as vicarious liability for injuries to patients from the negligent acts of their employees, such as nurses. The key policy reason for having the Captain of the Ship doctrine then no longer existed. Besides, operating rooms became increasingly complex, and the senior surgeon was thought to be incapable of being in charge of all activities there.
Wisconsin is typical: A retained sponge following a laparoscopic cholecystectomy led to complications, and the patient sued the hospital and surgeon, claiming each was responsible for the nurses’ sponge-count error. The lower court had found that “as a matter of law [the surgeon] is in fact responsible and liable for the actions of the parties that were in the operating room with him and working under his supervision ... [the] doctor is the captain of the ship. That doctor is responsible for everything.”
Upon appeal, the Wisconsin Supreme Court reversed the decision of the lower court by rejecting the doctrine altogether, finding that it failed to reflect the emergence of hospitals as modern health care facilities.5
Still, the doctrine is by no means obsolete. In a Colorado case, the court wrote that, even if the nurse were an employee of the hospital and her negligence caused the death of plaintiff’s husband, the Captain of the Ship doctrine would preclude recovery against the hospital.6 It relied on a precedent-setting case that held that once the operating surgeon assumed control in the operating room, the surgeon is liable for the negligence of all persons working there.
Likewise, California has recently breathed new life into the doctrine.7 A case in 2006 involved a patient who underwent arterial bypass surgery in his right leg. A case in which a nurse’s counting error led to a retained sponge ended up with the patient losing his leg. The surgeon initially escaped liability by virtue of the court’s refusal to include Captain of the Ship instructions to the jury, which found the doctor not negligent. The state court of appeals reversed, however, concluding that it was reasonably probable that the jury might have reached a different result had it been so instructed.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. McConnell v. Williams, 361 Pa. 355, 65 A.2d 243 (1949).
2. ED Legal Letter, Feb 1, 2018.
3. Collins v. Hand, 246 A.2d 398 (Pa 1968).
4. AORN J. 2001 Oct;74(4):525-8.
5. Lewis v. Physicians Insurance Company et al., 627 NW2d 484 (Wis 2001).
6. Krane v. St. Anthony Hospital Systems, 738 P.2d 75 (Co 1987).
7. Fields v. Yusuf, 144 Cal.App.4th 1381 (2006).
Question: The “Captain of the Ship” doctrine:
A. Is a legal principle used mostly in maritime law.
B. Is applicable only to surgeons in the operating room.
C. Is good law in all jurisdictions.
D. May be used by plaintiffs in emergency department triage litigation.
E. Originated when hospitals lost their charitable immunity.
Answer: D. Historically, the Captain of the Ship doctrine imputes liability to the surgeon who has the authority and right to control the actions of his assistants in the operating room.
Pennsylvania famously saw the use of the phrase in a 1949 case: “In the course of an operation in the operating room of a hospital, and until the surgeon leaves that room at the conclusion of the operation … he is in the same complete charge of those who are present and assisting him as is the captain of a ship over all on board.”1
Public hospitals in the 1940s were immune from liability because they were charitable organizations, so the Captain of the Ship doctrine emerged as a means for injured patients to recover damages against the surgeon instead. Courts have used various legal theories to justify this doctrine, which is basically grounded in vicarious liability, e.g., master-servant relationship (respondeat superior), borrowed servant, a nondelegable duty, or more broadly, principles of agency.
Use of the doctrine to shift liability to the surgeon in the operating room is well exemplified in litigation over retained sponges, left-behind instruments, burns in the operating room, administration of the wrong blood type, and allergic reaction to penicillin. Actual control of the surgeon’s assistants is not essential, but the right to merely supervise is insufficient. What is dispositive is the right and authority to determine an assistant’s actions.
However, what constitutes an “operating room” has been in dispute. It may simply mean a circumscribed and controlled area for medical procedures and/or treatment. Thus, the term has been extended to a room where only local anesthesia was used for esophageal dilation. Reasoning by analogy, the modern-day heart catheterization lab or interventional radiology suite would arguably count as “operating rooms” where the procedurist-doctor, usually a nonsurgeon, may be deemed to function as the captain of the ship.
Another place where a nonsurgeon may be involved is the hospital ED. It has been stated that emergency physicians have been held liable for adverse outcomes resulting from the patients under triage, based on the Captain of the Ship doctrine.2 Once a patient arrives in the ED, a legal duty to provide care arises, even if the physician has yet to see the patient. The federal Emergency Medical Treatment and Labor Act, which regulates much of what happens in the nation’s emergency departments, covers “any individual ... [who] comes to the emergency department and a request is made on the individual’s behalf for examination or treatment for a medical condition.”
Still, the doctrine is less likely to be invoked in a more spread-out area such as a general medical ward, where a physician’s control cannot be reasonably expected.
For example, courts have held that ward nurses giving injections into the buttock causing permanent neuropathy to a patient’s leg were not the agents of the prescribing physician, but just of the hospital employing them. The doctrine also was rejected in Collins v. Hand by the Pennsylvania Supreme Court, which reversed a judgment against a psychiatrist defendant.3 In the Collins case, notwithstanding the fact that the psychiatrist, Dr. Hand, had personally arranged for the patient’s transfer to another hospital and wrote orders for electroconvulsive therapy (which was complicated by fractures), Dr. Hand did not choose the doctor who was to administer the therapy, nor did he hire, compensate, or control any of the team members.
In the 1960s, hospitals began losing their charitable immunity status and assumed direct as well as vicarious liability for injuries to patients from the negligent acts of their employees, such as nurses. The key policy reason for having the Captain of the Ship doctrine then no longer existed. Besides, operating rooms became increasingly complex, and the senior surgeon was thought to be incapable of being in charge of all activities there.
Wisconsin is typical: A retained sponge following a laparoscopic cholecystectomy led to complications, and the patient sued the hospital and surgeon, claiming each was responsible for the nurses’ sponge-count error. The lower court had found that “as a matter of law [the surgeon] is in fact responsible and liable for the actions of the parties that were in the operating room with him and working under his supervision ... [the] doctor is the captain of the ship. That doctor is responsible for everything.”
Upon appeal, the Wisconsin Supreme Court reversed the decision of the lower court by rejecting the doctrine altogether, finding that it failed to reflect the emergence of hospitals as modern health care facilities.5
Still, the doctrine is by no means obsolete. In a Colorado case, the court wrote that, even if the nurse were an employee of the hospital and her negligence caused the death of plaintiff’s husband, the Captain of the Ship doctrine would preclude recovery against the hospital.6 It relied on a precedent-setting case that held that once the operating surgeon assumed control in the operating room, the surgeon is liable for the negligence of all persons working there.
Likewise, California has recently breathed new life into the doctrine.7 A case in 2006 involved a patient who underwent arterial bypass surgery in his right leg. A case in which a nurse’s counting error led to a retained sponge ended up with the patient losing his leg. The surgeon initially escaped liability by virtue of the court’s refusal to include Captain of the Ship instructions to the jury, which found the doctor not negligent. The state court of appeals reversed, however, concluding that it was reasonably probable that the jury might have reached a different result had it been so instructed.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. McConnell v. Williams, 361 Pa. 355, 65 A.2d 243 (1949).
2. ED Legal Letter, Feb 1, 2018.
3. Collins v. Hand, 246 A.2d 398 (Pa 1968).
4. AORN J. 2001 Oct;74(4):525-8.
5. Lewis v. Physicians Insurance Company et al., 627 NW2d 484 (Wis 2001).
6. Krane v. St. Anthony Hospital Systems, 738 P.2d 75 (Co 1987).
7. Fields v. Yusuf, 144 Cal.App.4th 1381 (2006).
A combination hormone capsule for vasomotor symptoms
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: A 17-beta-estradiol–progesterone capsule significantly decreased the frequency and severity of vasomotor symptoms.
Major finding: The compound reduced vasomotor symptoms, with no cases of endometrial hyperplasia.
Study details: The study randomized 1,845 women to placebo or one of four active hormone doses.
Disclosures: TherapeuticsMD sponsored the study; Dr. Lobo is a consultant for the company.
Source: Lobo RA et al. Obstet Gynecol. 2018 Jan;132:161-70.