User login
hs-cTnT test tied to fewer reinfarctions but no survival benefit
The introduction of the new high-sensitivity cardiac troponin T (hs-cTnT) test was linked with an 11% decrease in reinfarctions but showed no evidence of improving survival in a large longitudinal cohort study.
Over an average of 3.9 years of follow-up, the adjusted risk of all-cause mortality was identical whether patients’ initial MI was diagnosed with the new troponin test or the conventional one, reported Maria Odqvist, MD, of South Alvsborg Hospital, Boras, Sweden, and her associates. “As hs-cTn assays become widely available and clinicians gain experience interpreting the results, more work is needed to enhance clinical reasoning and implementation to improve patient outcomes,” the researchers wrote in the Journal of the American College of Cardiology.
High-sensitivity cardiac troponin T assays detect acute coronary syndrome more rapidly than their conventional predecessors. However, hs-cTnT’s higher sensitivity comes with lost specificity and hence has raised concerns about potentially wasting health care resources. Some clinicians have asked whether the new test is worthwhile and how to maximize its benefits.
The study, which included nearly 88,000 patients with initial MI from the Swedish National Patient Registry, spanned 2009-2013, when 73% of Swedish acute care hospitals transitioned from conventional troponin tests (cTn) to hs-cTnT. After adjustment for factors such as age, sex, location, chronic kidney disease, cardiovascular history, and prescriptions, Dr. Odqvist and her associates compared each test types’ risks of death, reinfarction, coronary angiography, and revascularization among patients diagnosed.
In all, 47,133 (54%) patients’ initial MI was diagnosed with cTn while 46% were diagnosed with hs-cTnT. Overall, the rate of MI rose by 5% during the 90 days after hospitals implemented hs-cTnT, compared with the 90 days before. But hospitals used varying hs-cTnT thresholds for MI, which might explain why some hospitals initially observed a marked initial decrease in MI while others saw a relatively large increase, the investigators wrote.
There were 15,766 reinfarctions over an average of 3.1 years of follow-up. Although there was no difference in all-cause mortality, risk of reinfarction was 11% lower among patients diagnosed using hs-cTnT, compared with those diagnosed by cTn.
At the hospital level, coronary angiography and revascularization became only slightly more common during the 3 months after hospitals switched to hs-cTnT, the researchers wrote. However, patients whose MIs were diagnosed with hs-cTnT were 16% more likely to undergo coronary angiography and 13% more likely to undergo revascularization within the next 30 days, compared with patients diagnosed with cTn. Patients diagnosed with hs-cTnT also were more likely to receive statins, but trends in other prescriptions did not change.
The Swedish Heart-Lung Foundation funded one investigator. Dr. Odqvist and one coinvestigator reported having no relevant conflicts of interest. Senior author Martin J. Holzmann, MD, PhD, disclosed ties to Actelion and Pfizer. Two other coinvestigators disclosed ties to Roche, Gilead, Janssen, Abbvie, CTI Bipharma, GlaxoSmithKline, Abbott Laboratories, and AstraZeneca, and Fiomi Diagnostics.
SOURCE: Odqvist M et al. J Am Coll Cardiol. 2018 Jun 12;71(23):2616-24
It’s unclear why reinfarctions were 11% lower after MI was diagnosed with high-sensitivity cardiac troponin (hs-cTn) assays instead of conventional troponin tests in this study.
Use of evidence-based medicines changed only slightly after hospitals implemented the switch. It is tempting to associate the reduction in reinfarction with increased use of angiography and revascularization, but this may be too simplistic. Intervention might have most benefited patients whom conventional troponin assay would have missed. Shifts in medication compliance or rehabilitation might also have been factors.
Used appropriately, hs-cTnT assays can more rapidly rule MI in or out, shorten emergency department stays, and cut costs. Based on the Swedish experience, the increase in MI diagnoses will be modest and manageable. Nonetheless, we will need to develop strategies to manage patients with myonecrosis from etiologies other than MI that will be detected using hs-cTn assays.
L. Kristin Newby, MD, MHS , and Angela Lowenstern, MD , of Duke University in Durham, N.C., made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;71:625-7). Dr. Newby has received consulting honoraria from Roche Diagnostics, Ortho-Clinical Diagnostics, and Philips Healthcare. Dr. Lowenstern is supported by a National Institutes of Health training grant to Duke University .
It’s unclear why reinfarctions were 11% lower after MI was diagnosed with high-sensitivity cardiac troponin (hs-cTn) assays instead of conventional troponin tests in this study.
Use of evidence-based medicines changed only slightly after hospitals implemented the switch. It is tempting to associate the reduction in reinfarction with increased use of angiography and revascularization, but this may be too simplistic. Intervention might have most benefited patients whom conventional troponin assay would have missed. Shifts in medication compliance or rehabilitation might also have been factors.
Used appropriately, hs-cTnT assays can more rapidly rule MI in or out, shorten emergency department stays, and cut costs. Based on the Swedish experience, the increase in MI diagnoses will be modest and manageable. Nonetheless, we will need to develop strategies to manage patients with myonecrosis from etiologies other than MI that will be detected using hs-cTn assays.
L. Kristin Newby, MD, MHS , and Angela Lowenstern, MD , of Duke University in Durham, N.C., made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;71:625-7). Dr. Newby has received consulting honoraria from Roche Diagnostics, Ortho-Clinical Diagnostics, and Philips Healthcare. Dr. Lowenstern is supported by a National Institutes of Health training grant to Duke University .
It’s unclear why reinfarctions were 11% lower after MI was diagnosed with high-sensitivity cardiac troponin (hs-cTn) assays instead of conventional troponin tests in this study.
Use of evidence-based medicines changed only slightly after hospitals implemented the switch. It is tempting to associate the reduction in reinfarction with increased use of angiography and revascularization, but this may be too simplistic. Intervention might have most benefited patients whom conventional troponin assay would have missed. Shifts in medication compliance or rehabilitation might also have been factors.
Used appropriately, hs-cTnT assays can more rapidly rule MI in or out, shorten emergency department stays, and cut costs. Based on the Swedish experience, the increase in MI diagnoses will be modest and manageable. Nonetheless, we will need to develop strategies to manage patients with myonecrosis from etiologies other than MI that will be detected using hs-cTn assays.
L. Kristin Newby, MD, MHS , and Angela Lowenstern, MD , of Duke University in Durham, N.C., made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;71:625-7). Dr. Newby has received consulting honoraria from Roche Diagnostics, Ortho-Clinical Diagnostics, and Philips Healthcare. Dr. Lowenstern is supported by a National Institutes of Health training grant to Duke University .
The introduction of the new high-sensitivity cardiac troponin T (hs-cTnT) test was linked with an 11% decrease in reinfarctions but showed no evidence of improving survival in a large longitudinal cohort study.
Over an average of 3.9 years of follow-up, the adjusted risk of all-cause mortality was identical whether patients’ initial MI was diagnosed with the new troponin test or the conventional one, reported Maria Odqvist, MD, of South Alvsborg Hospital, Boras, Sweden, and her associates. “As hs-cTn assays become widely available and clinicians gain experience interpreting the results, more work is needed to enhance clinical reasoning and implementation to improve patient outcomes,” the researchers wrote in the Journal of the American College of Cardiology.
High-sensitivity cardiac troponin T assays detect acute coronary syndrome more rapidly than their conventional predecessors. However, hs-cTnT’s higher sensitivity comes with lost specificity and hence has raised concerns about potentially wasting health care resources. Some clinicians have asked whether the new test is worthwhile and how to maximize its benefits.
The study, which included nearly 88,000 patients with initial MI from the Swedish National Patient Registry, spanned 2009-2013, when 73% of Swedish acute care hospitals transitioned from conventional troponin tests (cTn) to hs-cTnT. After adjustment for factors such as age, sex, location, chronic kidney disease, cardiovascular history, and prescriptions, Dr. Odqvist and her associates compared each test types’ risks of death, reinfarction, coronary angiography, and revascularization among patients diagnosed.
In all, 47,133 (54%) patients’ initial MI was diagnosed with cTn while 46% were diagnosed with hs-cTnT. Overall, the rate of MI rose by 5% during the 90 days after hospitals implemented hs-cTnT, compared with the 90 days before. But hospitals used varying hs-cTnT thresholds for MI, which might explain why some hospitals initially observed a marked initial decrease in MI while others saw a relatively large increase, the investigators wrote.
There were 15,766 reinfarctions over an average of 3.1 years of follow-up. Although there was no difference in all-cause mortality, risk of reinfarction was 11% lower among patients diagnosed using hs-cTnT, compared with those diagnosed by cTn.
At the hospital level, coronary angiography and revascularization became only slightly more common during the 3 months after hospitals switched to hs-cTnT, the researchers wrote. However, patients whose MIs were diagnosed with hs-cTnT were 16% more likely to undergo coronary angiography and 13% more likely to undergo revascularization within the next 30 days, compared with patients diagnosed with cTn. Patients diagnosed with hs-cTnT also were more likely to receive statins, but trends in other prescriptions did not change.
The Swedish Heart-Lung Foundation funded one investigator. Dr. Odqvist and one coinvestigator reported having no relevant conflicts of interest. Senior author Martin J. Holzmann, MD, PhD, disclosed ties to Actelion and Pfizer. Two other coinvestigators disclosed ties to Roche, Gilead, Janssen, Abbvie, CTI Bipharma, GlaxoSmithKline, Abbott Laboratories, and AstraZeneca, and Fiomi Diagnostics.
SOURCE: Odqvist M et al. J Am Coll Cardiol. 2018 Jun 12;71(23):2616-24
The introduction of the new high-sensitivity cardiac troponin T (hs-cTnT) test was linked with an 11% decrease in reinfarctions but showed no evidence of improving survival in a large longitudinal cohort study.
Over an average of 3.9 years of follow-up, the adjusted risk of all-cause mortality was identical whether patients’ initial MI was diagnosed with the new troponin test or the conventional one, reported Maria Odqvist, MD, of South Alvsborg Hospital, Boras, Sweden, and her associates. “As hs-cTn assays become widely available and clinicians gain experience interpreting the results, more work is needed to enhance clinical reasoning and implementation to improve patient outcomes,” the researchers wrote in the Journal of the American College of Cardiology.
High-sensitivity cardiac troponin T assays detect acute coronary syndrome more rapidly than their conventional predecessors. However, hs-cTnT’s higher sensitivity comes with lost specificity and hence has raised concerns about potentially wasting health care resources. Some clinicians have asked whether the new test is worthwhile and how to maximize its benefits.
The study, which included nearly 88,000 patients with initial MI from the Swedish National Patient Registry, spanned 2009-2013, when 73% of Swedish acute care hospitals transitioned from conventional troponin tests (cTn) to hs-cTnT. After adjustment for factors such as age, sex, location, chronic kidney disease, cardiovascular history, and prescriptions, Dr. Odqvist and her associates compared each test types’ risks of death, reinfarction, coronary angiography, and revascularization among patients diagnosed.
In all, 47,133 (54%) patients’ initial MI was diagnosed with cTn while 46% were diagnosed with hs-cTnT. Overall, the rate of MI rose by 5% during the 90 days after hospitals implemented hs-cTnT, compared with the 90 days before. But hospitals used varying hs-cTnT thresholds for MI, which might explain why some hospitals initially observed a marked initial decrease in MI while others saw a relatively large increase, the investigators wrote.
There were 15,766 reinfarctions over an average of 3.1 years of follow-up. Although there was no difference in all-cause mortality, risk of reinfarction was 11% lower among patients diagnosed using hs-cTnT, compared with those diagnosed by cTn.
At the hospital level, coronary angiography and revascularization became only slightly more common during the 3 months after hospitals switched to hs-cTnT, the researchers wrote. However, patients whose MIs were diagnosed with hs-cTnT were 16% more likely to undergo coronary angiography and 13% more likely to undergo revascularization within the next 30 days, compared with patients diagnosed with cTn. Patients diagnosed with hs-cTnT also were more likely to receive statins, but trends in other prescriptions did not change.
The Swedish Heart-Lung Foundation funded one investigator. Dr. Odqvist and one coinvestigator reported having no relevant conflicts of interest. Senior author Martin J. Holzmann, MD, PhD, disclosed ties to Actelion and Pfizer. Two other coinvestigators disclosed ties to Roche, Gilead, Janssen, Abbvie, CTI Bipharma, GlaxoSmithKline, Abbott Laboratories, and AstraZeneca, and Fiomi Diagnostics.
SOURCE: Odqvist M et al. J Am Coll Cardiol. 2018 Jun 12;71(23):2616-24
FROM JACC
Key clinical point: The introduction of the new high-sensitivity cardiac troponin T test was associated with an 11% decrease in reinfarctions but showed no evidence of improving survival.
Major finding: Over an average of 3.9 years of follow-up, the adjusted risk of all-cause mortality was identical whether patients’ initial MI was diagnosed with the new troponin test or with the conventional one.
Study details: National cohort study of 87,879 patients with first myocardial infarction, 2009-2013.
Disclosures: Dr. Odqvist and one coinvestigator had no relevant disclosures. Senior author Martin J. Holzmann, MD, PhD, disclosed ties to Actelion and Pfizer. Two other coinvestigators disclosed ties to Roche, Gilead, Janssen, AbbVie, CTI Biopharma, GlaxoSmithKline, Abbott Laboratories, and AstraZeneca, and Fiomi Diagnostics.
Source: Odqvist M et al. J Am Coll Cardiol. 2018 Jun 12;71(23):2616-24.
Russell Rosenberg, PhD
Stop extending credit
For as long as I have been writing this column, I have stressed that ; and yet, all these years later, AR is still the subject that generates the most questions.
Okay; let’s go over it one more time: Basically, physicians extend more credit than any business except banks. Despite what you may have read recently, banks are good at it, and they charge interest (and a myriad of fees) to do it. Doctors do it for free. Are we crazy? No business owner in his or her right mind allows customers to take away goods or services without paying for them; but doctors do it every day.
What to do? Common sense tells you to collect everything you can at the time of service; but some patients inevitably brandish the old “I forgot my checkbook” excuse and escape without paying. And the patient-owed portion of most insurance charges is often unknown, and unknowable, at the time of service.
That means you’ll need to send a bill; and every bill you send (or hire somebody to send) costs you a bundle. And when it arrives, it goes right to the bottom of your patient’s payment priority list. That is, each month your patients will pay their electric, water, gas, and telephone bills … and just about any other bill ... before getting around to yours. If there is no more money when your bill finally surfaces, that’s just too bad. The electric company can shut off their power; what can you do?
What we do is what every hotel, rental car agency, and many other businesses have done for years: We ask for a credit card number, keep it on file, and bill balances to it as they come in. Plastic runs the show everywhere you go – except in most medical offices.
New patients in my office receive a letter at their first visit explaining our policy. At the bottom is a brief consent for the patient to sign, and a place to write the credit card number and expiration date. (See below for a copy of our letter; feel free to use it as a template for creating your own.)
Do patients object? Some do – mostly older people, and fewer each year. But when we explain that we’re doing nothing different than a hotel does at each check-in, and that it will work to their advantage as well by decreasing the bills they will receive and the checks they must write, most come around. Make it an option at first if you wish; then, when everyone is accustomed to it, you can make it mandatory.
Do patients worry about confidentiality, or unauthorized use? They don’t anywhere else. They think nothing of handing a card to a waiter or waitress in a restaurant with no thought of what he or she might do with it in the kitchen. They hand cards over to hotel clerks, and never think to ask how long they keep the information or who has access to it. They blithely shoot their numbers into black holes on the Internet. We explain that we guard our patients’ financial information as carefully as we do their medical information. (If you have EHR, it can go in the chart with everything else; if not, I suggest a separate portable Rolodex-type file that can be locked up each night.)
Does it work? In only a year, our accounts receivable totals dropped by nearly 50%; after another year, they stabilized at 30%-35% of previous levels and have remained there ever since. When my accountant retired a few years ago, I hired a new one. Something must be wrong, he said nervously, after his first look at our books; AR totals are “never” that low in a practice with our level of volume. His eyes widened as I explained our system. “Why doesn’t every medical office do that?” he asked.
Why indeed? The business of health care delivery is being rocked to its very foundations as we speak. In my humble opinion, private practice will only survive those changes if physicians learn to do more of what we do best – treating patients – and leave the business of extending credit to the banks.
Patient consent form
This generic letter is intended to be used as an example for a letter you might draft for a similar purpose. We take no responsibility for your use of its content, either verbatim or altered, or for any inappropriate usage. Click on the attachment below for a printable copy of the letter.
To Our Patients:
As you know if you have ever checked into a hotel or rented a car, the first thing you are asked for is a credit card, which is imprinted and later used to pay your bill. This is an advantage for both you and the hotel or rental company, since it makes checkout easier, faster, and more efficient.
We have implemented a similar policy. You will be asked for a credit card number at the time you check in and the information will be held securely until your insurances have paid their portion and notified us of the amount of your share. At that time, any remaining balance owed by you will be charged to your credit card, and a copy of the charge will be mailed to you.
This will be an advantage to you, since you will no longer have to write out and mail us checks. It will be an advantage to us as well, since it will greatly decrease the number of statements that we have to generate and send out. The combination will benefit everybody in helping to keep the cost of health care down.
This in no way will compromise your ability to dispute a charge or question your insurance company’s determination of payment.
Copays due at the time of the visit will, of course, still be due at the time of the visit.
If you have any questions about this payment method, do not hesitate to ask.
Sincerely yours,
I authorize ********************, PA to charge outstanding balances on my account to the following credit card:
Visa Mastercard American Express Other: ____________________________
Account number _______________________Expiration Date ____________CVV_____
Name on card (please print) _________________________________________________
Signature _____________________________________ Date ______________________
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]
For as long as I have been writing this column, I have stressed that ; and yet, all these years later, AR is still the subject that generates the most questions.
Okay; let’s go over it one more time: Basically, physicians extend more credit than any business except banks. Despite what you may have read recently, banks are good at it, and they charge interest (and a myriad of fees) to do it. Doctors do it for free. Are we crazy? No business owner in his or her right mind allows customers to take away goods or services without paying for them; but doctors do it every day.
What to do? Common sense tells you to collect everything you can at the time of service; but some patients inevitably brandish the old “I forgot my checkbook” excuse and escape without paying. And the patient-owed portion of most insurance charges is often unknown, and unknowable, at the time of service.
That means you’ll need to send a bill; and every bill you send (or hire somebody to send) costs you a bundle. And when it arrives, it goes right to the bottom of your patient’s payment priority list. That is, each month your patients will pay their electric, water, gas, and telephone bills … and just about any other bill ... before getting around to yours. If there is no more money when your bill finally surfaces, that’s just too bad. The electric company can shut off their power; what can you do?
What we do is what every hotel, rental car agency, and many other businesses have done for years: We ask for a credit card number, keep it on file, and bill balances to it as they come in. Plastic runs the show everywhere you go – except in most medical offices.
New patients in my office receive a letter at their first visit explaining our policy. At the bottom is a brief consent for the patient to sign, and a place to write the credit card number and expiration date. (See below for a copy of our letter; feel free to use it as a template for creating your own.)
Do patients object? Some do – mostly older people, and fewer each year. But when we explain that we’re doing nothing different than a hotel does at each check-in, and that it will work to their advantage as well by decreasing the bills they will receive and the checks they must write, most come around. Make it an option at first if you wish; then, when everyone is accustomed to it, you can make it mandatory.
Do patients worry about confidentiality, or unauthorized use? They don’t anywhere else. They think nothing of handing a card to a waiter or waitress in a restaurant with no thought of what he or she might do with it in the kitchen. They hand cards over to hotel clerks, and never think to ask how long they keep the information or who has access to it. They blithely shoot their numbers into black holes on the Internet. We explain that we guard our patients’ financial information as carefully as we do their medical information. (If you have EHR, it can go in the chart with everything else; if not, I suggest a separate portable Rolodex-type file that can be locked up each night.)
Does it work? In only a year, our accounts receivable totals dropped by nearly 50%; after another year, they stabilized at 30%-35% of previous levels and have remained there ever since. When my accountant retired a few years ago, I hired a new one. Something must be wrong, he said nervously, after his first look at our books; AR totals are “never” that low in a practice with our level of volume. His eyes widened as I explained our system. “Why doesn’t every medical office do that?” he asked.
Why indeed? The business of health care delivery is being rocked to its very foundations as we speak. In my humble opinion, private practice will only survive those changes if physicians learn to do more of what we do best – treating patients – and leave the business of extending credit to the banks.
Patient consent form
This generic letter is intended to be used as an example for a letter you might draft for a similar purpose. We take no responsibility for your use of its content, either verbatim or altered, or for any inappropriate usage. Click on the attachment below for a printable copy of the letter.
To Our Patients:
As you know if you have ever checked into a hotel or rented a car, the first thing you are asked for is a credit card, which is imprinted and later used to pay your bill. This is an advantage for both you and the hotel or rental company, since it makes checkout easier, faster, and more efficient.
We have implemented a similar policy. You will be asked for a credit card number at the time you check in and the information will be held securely until your insurances have paid their portion and notified us of the amount of your share. At that time, any remaining balance owed by you will be charged to your credit card, and a copy of the charge will be mailed to you.
This will be an advantage to you, since you will no longer have to write out and mail us checks. It will be an advantage to us as well, since it will greatly decrease the number of statements that we have to generate and send out. The combination will benefit everybody in helping to keep the cost of health care down.
This in no way will compromise your ability to dispute a charge or question your insurance company’s determination of payment.
Copays due at the time of the visit will, of course, still be due at the time of the visit.
If you have any questions about this payment method, do not hesitate to ask.
Sincerely yours,
I authorize ********************, PA to charge outstanding balances on my account to the following credit card:
Visa Mastercard American Express Other: ____________________________
Account number _______________________Expiration Date ____________CVV_____
Name on card (please print) _________________________________________________
Signature _____________________________________ Date ______________________
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]
For as long as I have been writing this column, I have stressed that ; and yet, all these years later, AR is still the subject that generates the most questions.
Okay; let’s go over it one more time: Basically, physicians extend more credit than any business except banks. Despite what you may have read recently, banks are good at it, and they charge interest (and a myriad of fees) to do it. Doctors do it for free. Are we crazy? No business owner in his or her right mind allows customers to take away goods or services without paying for them; but doctors do it every day.
What to do? Common sense tells you to collect everything you can at the time of service; but some patients inevitably brandish the old “I forgot my checkbook” excuse and escape without paying. And the patient-owed portion of most insurance charges is often unknown, and unknowable, at the time of service.
That means you’ll need to send a bill; and every bill you send (or hire somebody to send) costs you a bundle. And when it arrives, it goes right to the bottom of your patient’s payment priority list. That is, each month your patients will pay their electric, water, gas, and telephone bills … and just about any other bill ... before getting around to yours. If there is no more money when your bill finally surfaces, that’s just too bad. The electric company can shut off their power; what can you do?
What we do is what every hotel, rental car agency, and many other businesses have done for years: We ask for a credit card number, keep it on file, and bill balances to it as they come in. Plastic runs the show everywhere you go – except in most medical offices.
New patients in my office receive a letter at their first visit explaining our policy. At the bottom is a brief consent for the patient to sign, and a place to write the credit card number and expiration date. (See below for a copy of our letter; feel free to use it as a template for creating your own.)
Do patients object? Some do – mostly older people, and fewer each year. But when we explain that we’re doing nothing different than a hotel does at each check-in, and that it will work to their advantage as well by decreasing the bills they will receive and the checks they must write, most come around. Make it an option at first if you wish; then, when everyone is accustomed to it, you can make it mandatory.
Do patients worry about confidentiality, or unauthorized use? They don’t anywhere else. They think nothing of handing a card to a waiter or waitress in a restaurant with no thought of what he or she might do with it in the kitchen. They hand cards over to hotel clerks, and never think to ask how long they keep the information or who has access to it. They blithely shoot their numbers into black holes on the Internet. We explain that we guard our patients’ financial information as carefully as we do their medical information. (If you have EHR, it can go in the chart with everything else; if not, I suggest a separate portable Rolodex-type file that can be locked up each night.)
Does it work? In only a year, our accounts receivable totals dropped by nearly 50%; after another year, they stabilized at 30%-35% of previous levels and have remained there ever since. When my accountant retired a few years ago, I hired a new one. Something must be wrong, he said nervously, after his first look at our books; AR totals are “never” that low in a practice with our level of volume. His eyes widened as I explained our system. “Why doesn’t every medical office do that?” he asked.
Why indeed? The business of health care delivery is being rocked to its very foundations as we speak. In my humble opinion, private practice will only survive those changes if physicians learn to do more of what we do best – treating patients – and leave the business of extending credit to the banks.
Patient consent form
This generic letter is intended to be used as an example for a letter you might draft for a similar purpose. We take no responsibility for your use of its content, either verbatim or altered, or for any inappropriate usage. Click on the attachment below for a printable copy of the letter.
To Our Patients:
As you know if you have ever checked into a hotel or rented a car, the first thing you are asked for is a credit card, which is imprinted and later used to pay your bill. This is an advantage for both you and the hotel or rental company, since it makes checkout easier, faster, and more efficient.
We have implemented a similar policy. You will be asked for a credit card number at the time you check in and the information will be held securely until your insurances have paid their portion and notified us of the amount of your share. At that time, any remaining balance owed by you will be charged to your credit card, and a copy of the charge will be mailed to you.
This will be an advantage to you, since you will no longer have to write out and mail us checks. It will be an advantage to us as well, since it will greatly decrease the number of statements that we have to generate and send out. The combination will benefit everybody in helping to keep the cost of health care down.
This in no way will compromise your ability to dispute a charge or question your insurance company’s determination of payment.
Copays due at the time of the visit will, of course, still be due at the time of the visit.
If you have any questions about this payment method, do not hesitate to ask.
Sincerely yours,
I authorize ********************, PA to charge outstanding balances on my account to the following credit card:
Visa Mastercard American Express Other: ____________________________
Account number _______________________Expiration Date ____________CVV_____
Name on card (please print) _________________________________________________
Signature _____________________________________ Date ______________________
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]
Planner, Mobile App Work Together to Simplify VAM Navigation
Something old, something new, something improved.
The old is providing a schedule of VAM’s myriad events. This year, find that schedule on “something new,” our interactive VAM Planner (vsweb.org/VAMPlanner), which not only includes the lineup but also lets attendees create their own VAM schedules from beginning to end.
And something improved is the VAM Mobile App, which has been redesigned to be more intuitive, more useful, and to more easily integrate with the VAM Planner.
As its name suggests, the VAM Planner permits users to plan their own VAM experience. See a session you want to attend? Mark it as a favorite. The Planner will add it to your calendar and remind you of what you planned to attend. Then, when you install the app and fire it up, those favorites, and other details you entered in the Planner, will be right there on your phone or other mobile device.
The Planner lets attendees seek out information easily: a presenter, a topic, the kind of session, an area of interest, intended audience. Perhaps someone wants to view all the sessions of interest to academics. Done. Want to know which sessions offer Continuing Medical Education credits? That information is easily found as well.
Pop-ups provide pertinent information on a given program with date and time, location, moderators, learning objectives, and more. People can read presenter biographies and mark various events as favorites, “like” them, post them to Facebook, or tweet about them.
Single sign-on adds convenience: To save information on the Planner, users must create a log-in. That “single sign-on” log-in, with just one password, is used for registration and syncing with the mobile app and Planner. Only one log-in and one password, during any VAM process, will open the door to navigating all platforms. SVS nonmembers or VQI attendees will need to create a guest account in order to take advantage of this functionality.
Online integration: If you’ve already registered for VAM, your ticketed courses such as breakfast sessions, workshops, or postgraduate courses as well as special events, will automatically transfer to the Planner after you log in.
It all adds up to an improved and easier VAM experience.
Something old, something new, something improved.
The old is providing a schedule of VAM’s myriad events. This year, find that schedule on “something new,” our interactive VAM Planner (vsweb.org/VAMPlanner), which not only includes the lineup but also lets attendees create their own VAM schedules from beginning to end.
And something improved is the VAM Mobile App, which has been redesigned to be more intuitive, more useful, and to more easily integrate with the VAM Planner.
As its name suggests, the VAM Planner permits users to plan their own VAM experience. See a session you want to attend? Mark it as a favorite. The Planner will add it to your calendar and remind you of what you planned to attend. Then, when you install the app and fire it up, those favorites, and other details you entered in the Planner, will be right there on your phone or other mobile device.
The Planner lets attendees seek out information easily: a presenter, a topic, the kind of session, an area of interest, intended audience. Perhaps someone wants to view all the sessions of interest to academics. Done. Want to know which sessions offer Continuing Medical Education credits? That information is easily found as well.
Pop-ups provide pertinent information on a given program with date and time, location, moderators, learning objectives, and more. People can read presenter biographies and mark various events as favorites, “like” them, post them to Facebook, or tweet about them.
Single sign-on adds convenience: To save information on the Planner, users must create a log-in. That “single sign-on” log-in, with just one password, is used for registration and syncing with the mobile app and Planner. Only one log-in and one password, during any VAM process, will open the door to navigating all platforms. SVS nonmembers or VQI attendees will need to create a guest account in order to take advantage of this functionality.
Online integration: If you’ve already registered for VAM, your ticketed courses such as breakfast sessions, workshops, or postgraduate courses as well as special events, will automatically transfer to the Planner after you log in.
It all adds up to an improved and easier VAM experience.
Something old, something new, something improved.
The old is providing a schedule of VAM’s myriad events. This year, find that schedule on “something new,” our interactive VAM Planner (vsweb.org/VAMPlanner), which not only includes the lineup but also lets attendees create their own VAM schedules from beginning to end.
And something improved is the VAM Mobile App, which has been redesigned to be more intuitive, more useful, and to more easily integrate with the VAM Planner.
As its name suggests, the VAM Planner permits users to plan their own VAM experience. See a session you want to attend? Mark it as a favorite. The Planner will add it to your calendar and remind you of what you planned to attend. Then, when you install the app and fire it up, those favorites, and other details you entered in the Planner, will be right there on your phone or other mobile device.
The Planner lets attendees seek out information easily: a presenter, a topic, the kind of session, an area of interest, intended audience. Perhaps someone wants to view all the sessions of interest to academics. Done. Want to know which sessions offer Continuing Medical Education credits? That information is easily found as well.
Pop-ups provide pertinent information on a given program with date and time, location, moderators, learning objectives, and more. People can read presenter biographies and mark various events as favorites, “like” them, post them to Facebook, or tweet about them.
Single sign-on adds convenience: To save information on the Planner, users must create a log-in. That “single sign-on” log-in, with just one password, is used for registration and syncing with the mobile app and Planner. Only one log-in and one password, during any VAM process, will open the door to navigating all platforms. SVS nonmembers or VQI attendees will need to create a guest account in order to take advantage of this functionality.
Online integration: If you’ve already registered for VAM, your ticketed courses such as breakfast sessions, workshops, or postgraduate courses as well as special events, will automatically transfer to the Planner after you log in.
It all adds up to an improved and easier VAM experience.
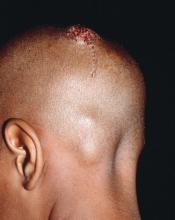
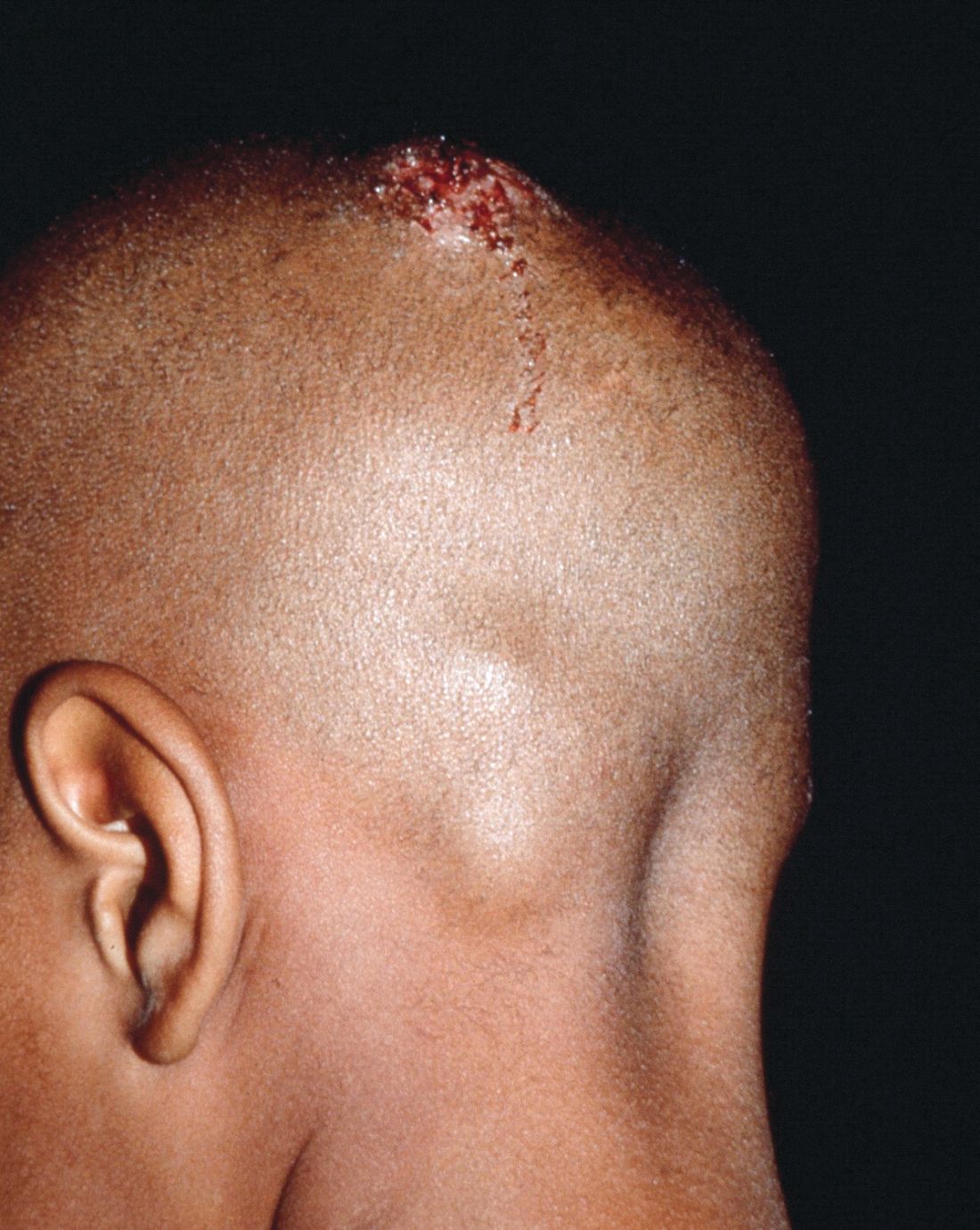
Early diagnosis, treatment key to prevent permanent baldness in tinea capitis
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The psychosocial impact and overall lower quality of health associated with tinea capitis is significant.
Major finding: Wood’s light should not be the only method of organism identification.
Study details: A systematic literature review of 4,190 studies.
Disclosures: The researchers had no relevant financial disclosures.
Source: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
Yale meeting draws cadre of physician-scientists
E. Albert Reece, MD, PhD, MBA, the dean of the University of Maryland School of Medicine, Baltimore, and medical editor of Ob.Gyn. News, has spoken often in the newspaper’s pages about how the fetus has become a visible and intimate patient – one who, “like the mother, can be interrogated, monitored, and sometimes treated before birth.”
Physician-scientists have been instrumental in lifting the cloud of mystery that surrounded the fetus and fetal outcomes. Yet today, in a trend that Dr. Reece and his colleagues call deeply concerning, the number of physician-scientists is declining. “We’re missing out on a workforce that is dedicated to exploring the biologic basis of disease – knowledge that enables the development of targeted therapeutic interventions,” he said in an interview.
Notable Yale physician-scientist alumni have been honored over the years as part of the YOGS meetings, including John C. Hobbins, MD, a former division head of maternal-fetal medicine and a pioneer of ultrasound imaging in the field of obstetrics and gynecology; Roberto Romero, MD, DMedSci, chief of the Perinatology Research Branch at the National Institute of Child Health and Human Development, and editor-in-chief of the American Journal of Obstetrics and Gynecology; and Charles J. Lockwood, MD, dean of the University of South Florida’s Morsani College of Medicine, Tampa, and a former chair of Yale’s ob.gyn. department.
As this year’s honoree, Dr. Reece spoke about the importance of inspiring a new generation of physician-scientists not only within colleges and universities, but also by reaching out to younger students to spark interest in science and research. He recalled being a postdoctoral fellow in perinatology at Yale in the 1980s and being inspired by Dr. Hobbins, whom he credits as his mentor, as well as Dr. Romero, who was finishing his fellowship at Yale while Dr. Reece was beginning his fellowship.
Yale’s department of ob.gyn. and its division of maternal-fetal medicine have had a long history of “firsts” and seminal contributions, including the first ultrasound-guided fetal blood sampling and transfusions in the United States, invention of the fetal heart monitor, the first karyotype in amniotic fluid, the development of postcoital contraception and of methods for early detection of ectopic pregnancies, the discovery of endometrial stem cells and the role that endocrine-disrupting chemicals play in the developmental programming of the uterus, and discovery of the role of cytokines in premature labor and fetal injury.
According to current department chair, Hugh S. Taylor, MD, the 1980s and 1990s were a particularly “exciting time.” Under the tutelage of Dr. Hobbins, who directed both obstetrics and maternal-fetal medicine, obstetrical ultrasound was fast advancing, for instance, and fetoscopy was drawing patients and other physician-scientists from around the world.
“It was an unbelievable time – a magnetic period when many of the things we now take for granted were first being introduced,” said Dr. Reece, who went on after his fellowship to serve as an instructor in ob.gyn. (1982-4), assistant professor (1984-7), and then associate professor (1987-91) at Yale. “It was like going to the symphony and getting to choose the best seat in the house to see the rehearsals all the way through the concert.”
After leaving the Yale faculty and prior to joining the University of Maryland School of Medicine, Dr. Reece served as the chair of obstetrics and gynecology at Temple University School of Medicine, Philadelphia, and then vice chancellor and dean of the University of Arkansas College of Medicine, Little Rock. “Dr. Reece is an incredible bulldog,” said Dr. Hobbins, speaking of the honor given to Dr. Reece at the YOGS meeting. “We could see this right at the beginning at Yale. He latches into something and won’t let it go. He has a work ethic that’s remarkable ... He’s always thinking, ‘How can this be done better?’ ”
Dr. Hobbins, who went on after Yale to a tenure at the University of Colorado at Denver, Aurora, told Ob.Gyn. News that what he remembers “more than anything else, is that we would sit down in a room and just kind of spitball – just brainstorm.”
It is this intellectual curiosity and scientific drive that seems increasingly at risk of being lost, Dr. Hobbins said. “There’s not the same impetus to do a fellowship or to become a physician-scientist or pursue an MD-PhD,” he said. “There just doesn’t seem to be the same oomph to get into the nuts and bolts of how things work, to explore and understand the science. Yes, it has to do with funding. But there’s more to it: We have to somehow stimulate more fire in the belly.”
Dr. Lockwood, who served his fellowship in maternal-fetal medicine at Yale under the guidance of Dr. Hobbins, Dr. Reece, and other faculty, and who later chaired the Yale department of ob.gyn. for 9 years, said that research-rich environments that are “full of inquiry” drive better clinical care.
“The same rigor [gets] applied to the clinical enterprise. Where evidence-based medicine is applicable, it’s done ... and where there are gaps in knowledge, there’s a real spirit of research and inquiry to try to improve care,” Dr. Lockwood said in an interview. “All the great stuff in our health care system is really a direct correlate with the fact that we’ve had this extraordinary research enterprise for so long – most of it funded by the National Institutes of Health, either directly or indirectly.”
The University of Maryland requires all its medical students to take a course in research and critical thinking and to complete a research project. It also runs programs for young students such as a “mini medical school” for underprivileged children who live in nearby neighborhoods. “If you get them excited about science early, and you keep the research continuum going, we believe you’ll have a better chance of recruiting committed physician-scientists into the field,” Dr. Reece said.
The infection has “become an important issue because 10%-20% of women who receive an epidural develop a fever and many of these babies have to have a septic workup and antibiotic treatment,” he said in an e-mail after the meeting. ”Our data indicate that antibiotic administration is not indicated in 40% of cases and the antibiotics currently used do not cover frequent organisms causing infection.”
Dr. Hobbins, who has been using sophisticated imaging techniques to assess subtle changes in fetuses with growth restriction, spoke about the potential value of cardiac size as an indicator of cardiac dysfunction. In utero cardiac dysfunction “sets the tone” for later cardiovascular and neurologic function, he told Ob.Gyn. News. “We think that you can use cardiac size in small babies as a screening tool to tell you whether you need to delve a little further into cardiac function ... Let’s get away from old protocols and rethink other things that are going on in the [small] fetus. Let’s cast a wider net.”
Dr. Lockwood has long been investigating the prevention of recurrent pregnancy loss and preterm delivery, and at the meeting he presented March of Dimes–funded research aimed at identifying mechanisms for dysfunction of the progesterone receptor in premature birth.
Dr. Reece spoke about his research on diabetes in pregnancy and birth defects, and how years of research on diabetes-induced birth defects has shown that maternal hyperglycemia is a teratogen that can trigger a series of developmental fetal defects. “We now have enough information such that we truly have a biomolecular map regarding the precise steps and cascading events which lead to the induction of diabetes-induced birth defects,” said Dr. Reece, who holds a PhD in biochemistry and directs a multimillion-dollar NIH-funded research laboratory at the University of Maryland.
This research began when Dr. Reece asked a question during his fellowship at Yale. “I was struck by the number of birth defects I saw in women with diabetes. I asked Jerry Mahoney, one of the geneticists: Do we know the cause of this? Why is this happening?” he recalled in the interview. “Dr. Mahoney took me to his office, opened his file cabinet and showed me some papers of an [in-vitro rat embryo model], where the rats were made diabetic and the serum seemed to have a way of inducing these birth defects in the embryo. That intrigued me immensely and I thought: I can do this!”
Dr. Reece got his feet wet in an embryology laboratory. As he moved on after his fellowship to join the faculty at Yale, he began directing his own research team – the Diabetes-in-Pregnancy Study Unit.
Dr. Romero said this was the start of “many important contributions to optimize the care of pregnant women with diabetes.” Dr. Reece, he said, has been “able to dissect the role of oxidative stress, program cell death, and lipid metabolism in the genesis of congenital anomalies” in babies of mothers with diabetes.
In other talks at the YOGS meeting, Yale alumnus Ray Bahado-Singh, MD, of Oakwood University, Rochester, Mich., addressed the epigenetics of cardiac dysfunction and the “new frontier” of using epigenetic markers to assess fetal cardiac function. Frank A. Chervenak, MD, of Cornell University, New York, rounded out the meeting by addressing the issue of professionalism and putting the patient first, as well as the professional virtues of self-sacrifice, compassion, and integrity – themes that Dr. Reece frequently cites as integral to both practice and research in ob.gyn.
Clinical care and “the research we’re all doing to assess fetal health both directly and indirectly has to be sitting on a platform of moral, ethical, and solid principles,” said Dr. Reece, who authored a special feature for Ob.Gyn. News – “Obstetrics Moonshots: 50 Years of Discoveries,” on the recent history of obstetrics.
Mary Jane Minkin, MD, a Yale alumna of many levels (medical school through residency) and a longtime Yale faculty member and private-practice ob.gyn. in New Haven, Conn., noted that the YOGS meeting was attended by the 94-year-old Virginia Stuermer, MD, who joined Yale’s ob.gyn. department in 1954 and who is “celebrated within the department” for defying legal barriers to provide patients with contraception and services. “She wanted to come see Dr. Reece,” said Dr. Minkin, who has served as director of YOGS since its inception.
Dr. Stuermer was running the Planned Parenthood clinic in New Haven the day in 1961 when then-department chair Charles Lee Buxton, MD, and Connecticut Planned Parenthood League executive director Estelle Griswold were arrested and jailed. “Everyone knows about the Supreme Court decision, Griswold v. Connecticut [1965], that legalized contraception in the U.S.,” said Dr. Minkin. “But most don’t realize that the doctor who was actually fitting the diaphragms that day was Dr. Stuermer.”
The YOGS reunion preceded a symposium held early in June commemorating the 100-year anniversary of women at Yale Medical School.
E. Albert Reece, MD, PhD, MBA, the dean of the University of Maryland School of Medicine, Baltimore, and medical editor of Ob.Gyn. News, has spoken often in the newspaper’s pages about how the fetus has become a visible and intimate patient – one who, “like the mother, can be interrogated, monitored, and sometimes treated before birth.”
Physician-scientists have been instrumental in lifting the cloud of mystery that surrounded the fetus and fetal outcomes. Yet today, in a trend that Dr. Reece and his colleagues call deeply concerning, the number of physician-scientists is declining. “We’re missing out on a workforce that is dedicated to exploring the biologic basis of disease – knowledge that enables the development of targeted therapeutic interventions,” he said in an interview.
Notable Yale physician-scientist alumni have been honored over the years as part of the YOGS meetings, including John C. Hobbins, MD, a former division head of maternal-fetal medicine and a pioneer of ultrasound imaging in the field of obstetrics and gynecology; Roberto Romero, MD, DMedSci, chief of the Perinatology Research Branch at the National Institute of Child Health and Human Development, and editor-in-chief of the American Journal of Obstetrics and Gynecology; and Charles J. Lockwood, MD, dean of the University of South Florida’s Morsani College of Medicine, Tampa, and a former chair of Yale’s ob.gyn. department.
As this year’s honoree, Dr. Reece spoke about the importance of inspiring a new generation of physician-scientists not only within colleges and universities, but also by reaching out to younger students to spark interest in science and research. He recalled being a postdoctoral fellow in perinatology at Yale in the 1980s and being inspired by Dr. Hobbins, whom he credits as his mentor, as well as Dr. Romero, who was finishing his fellowship at Yale while Dr. Reece was beginning his fellowship.
Yale’s department of ob.gyn. and its division of maternal-fetal medicine have had a long history of “firsts” and seminal contributions, including the first ultrasound-guided fetal blood sampling and transfusions in the United States, invention of the fetal heart monitor, the first karyotype in amniotic fluid, the development of postcoital contraception and of methods for early detection of ectopic pregnancies, the discovery of endometrial stem cells and the role that endocrine-disrupting chemicals play in the developmental programming of the uterus, and discovery of the role of cytokines in premature labor and fetal injury.
According to current department chair, Hugh S. Taylor, MD, the 1980s and 1990s were a particularly “exciting time.” Under the tutelage of Dr. Hobbins, who directed both obstetrics and maternal-fetal medicine, obstetrical ultrasound was fast advancing, for instance, and fetoscopy was drawing patients and other physician-scientists from around the world.
“It was an unbelievable time – a magnetic period when many of the things we now take for granted were first being introduced,” said Dr. Reece, who went on after his fellowship to serve as an instructor in ob.gyn. (1982-4), assistant professor (1984-7), and then associate professor (1987-91) at Yale. “It was like going to the symphony and getting to choose the best seat in the house to see the rehearsals all the way through the concert.”
After leaving the Yale faculty and prior to joining the University of Maryland School of Medicine, Dr. Reece served as the chair of obstetrics and gynecology at Temple University School of Medicine, Philadelphia, and then vice chancellor and dean of the University of Arkansas College of Medicine, Little Rock. “Dr. Reece is an incredible bulldog,” said Dr. Hobbins, speaking of the honor given to Dr. Reece at the YOGS meeting. “We could see this right at the beginning at Yale. He latches into something and won’t let it go. He has a work ethic that’s remarkable ... He’s always thinking, ‘How can this be done better?’ ”
Dr. Hobbins, who went on after Yale to a tenure at the University of Colorado at Denver, Aurora, told Ob.Gyn. News that what he remembers “more than anything else, is that we would sit down in a room and just kind of spitball – just brainstorm.”
It is this intellectual curiosity and scientific drive that seems increasingly at risk of being lost, Dr. Hobbins said. “There’s not the same impetus to do a fellowship or to become a physician-scientist or pursue an MD-PhD,” he said. “There just doesn’t seem to be the same oomph to get into the nuts and bolts of how things work, to explore and understand the science. Yes, it has to do with funding. But there’s more to it: We have to somehow stimulate more fire in the belly.”
Dr. Lockwood, who served his fellowship in maternal-fetal medicine at Yale under the guidance of Dr. Hobbins, Dr. Reece, and other faculty, and who later chaired the Yale department of ob.gyn. for 9 years, said that research-rich environments that are “full of inquiry” drive better clinical care.
“The same rigor [gets] applied to the clinical enterprise. Where evidence-based medicine is applicable, it’s done ... and where there are gaps in knowledge, there’s a real spirit of research and inquiry to try to improve care,” Dr. Lockwood said in an interview. “All the great stuff in our health care system is really a direct correlate with the fact that we’ve had this extraordinary research enterprise for so long – most of it funded by the National Institutes of Health, either directly or indirectly.”
The University of Maryland requires all its medical students to take a course in research and critical thinking and to complete a research project. It also runs programs for young students such as a “mini medical school” for underprivileged children who live in nearby neighborhoods. “If you get them excited about science early, and you keep the research continuum going, we believe you’ll have a better chance of recruiting committed physician-scientists into the field,” Dr. Reece said.
The infection has “become an important issue because 10%-20% of women who receive an epidural develop a fever and many of these babies have to have a septic workup and antibiotic treatment,” he said in an e-mail after the meeting. ”Our data indicate that antibiotic administration is not indicated in 40% of cases and the antibiotics currently used do not cover frequent organisms causing infection.”
Dr. Hobbins, who has been using sophisticated imaging techniques to assess subtle changes in fetuses with growth restriction, spoke about the potential value of cardiac size as an indicator of cardiac dysfunction. In utero cardiac dysfunction “sets the tone” for later cardiovascular and neurologic function, he told Ob.Gyn. News. “We think that you can use cardiac size in small babies as a screening tool to tell you whether you need to delve a little further into cardiac function ... Let’s get away from old protocols and rethink other things that are going on in the [small] fetus. Let’s cast a wider net.”
Dr. Lockwood has long been investigating the prevention of recurrent pregnancy loss and preterm delivery, and at the meeting he presented March of Dimes–funded research aimed at identifying mechanisms for dysfunction of the progesterone receptor in premature birth.
Dr. Reece spoke about his research on diabetes in pregnancy and birth defects, and how years of research on diabetes-induced birth defects has shown that maternal hyperglycemia is a teratogen that can trigger a series of developmental fetal defects. “We now have enough information such that we truly have a biomolecular map regarding the precise steps and cascading events which lead to the induction of diabetes-induced birth defects,” said Dr. Reece, who holds a PhD in biochemistry and directs a multimillion-dollar NIH-funded research laboratory at the University of Maryland.
This research began when Dr. Reece asked a question during his fellowship at Yale. “I was struck by the number of birth defects I saw in women with diabetes. I asked Jerry Mahoney, one of the geneticists: Do we know the cause of this? Why is this happening?” he recalled in the interview. “Dr. Mahoney took me to his office, opened his file cabinet and showed me some papers of an [in-vitro rat embryo model], where the rats were made diabetic and the serum seemed to have a way of inducing these birth defects in the embryo. That intrigued me immensely and I thought: I can do this!”
Dr. Reece got his feet wet in an embryology laboratory. As he moved on after his fellowship to join the faculty at Yale, he began directing his own research team – the Diabetes-in-Pregnancy Study Unit.
Dr. Romero said this was the start of “many important contributions to optimize the care of pregnant women with diabetes.” Dr. Reece, he said, has been “able to dissect the role of oxidative stress, program cell death, and lipid metabolism in the genesis of congenital anomalies” in babies of mothers with diabetes.
In other talks at the YOGS meeting, Yale alumnus Ray Bahado-Singh, MD, of Oakwood University, Rochester, Mich., addressed the epigenetics of cardiac dysfunction and the “new frontier” of using epigenetic markers to assess fetal cardiac function. Frank A. Chervenak, MD, of Cornell University, New York, rounded out the meeting by addressing the issue of professionalism and putting the patient first, as well as the professional virtues of self-sacrifice, compassion, and integrity – themes that Dr. Reece frequently cites as integral to both practice and research in ob.gyn.
Clinical care and “the research we’re all doing to assess fetal health both directly and indirectly has to be sitting on a platform of moral, ethical, and solid principles,” said Dr. Reece, who authored a special feature for Ob.Gyn. News – “Obstetrics Moonshots: 50 Years of Discoveries,” on the recent history of obstetrics.
Mary Jane Minkin, MD, a Yale alumna of many levels (medical school through residency) and a longtime Yale faculty member and private-practice ob.gyn. in New Haven, Conn., noted that the YOGS meeting was attended by the 94-year-old Virginia Stuermer, MD, who joined Yale’s ob.gyn. department in 1954 and who is “celebrated within the department” for defying legal barriers to provide patients with contraception and services. “She wanted to come see Dr. Reece,” said Dr. Minkin, who has served as director of YOGS since its inception.
Dr. Stuermer was running the Planned Parenthood clinic in New Haven the day in 1961 when then-department chair Charles Lee Buxton, MD, and Connecticut Planned Parenthood League executive director Estelle Griswold were arrested and jailed. “Everyone knows about the Supreme Court decision, Griswold v. Connecticut [1965], that legalized contraception in the U.S.,” said Dr. Minkin. “But most don’t realize that the doctor who was actually fitting the diaphragms that day was Dr. Stuermer.”
The YOGS reunion preceded a symposium held early in June commemorating the 100-year anniversary of women at Yale Medical School.
E. Albert Reece, MD, PhD, MBA, the dean of the University of Maryland School of Medicine, Baltimore, and medical editor of Ob.Gyn. News, has spoken often in the newspaper’s pages about how the fetus has become a visible and intimate patient – one who, “like the mother, can be interrogated, monitored, and sometimes treated before birth.”
Physician-scientists have been instrumental in lifting the cloud of mystery that surrounded the fetus and fetal outcomes. Yet today, in a trend that Dr. Reece and his colleagues call deeply concerning, the number of physician-scientists is declining. “We’re missing out on a workforce that is dedicated to exploring the biologic basis of disease – knowledge that enables the development of targeted therapeutic interventions,” he said in an interview.
Notable Yale physician-scientist alumni have been honored over the years as part of the YOGS meetings, including John C. Hobbins, MD, a former division head of maternal-fetal medicine and a pioneer of ultrasound imaging in the field of obstetrics and gynecology; Roberto Romero, MD, DMedSci, chief of the Perinatology Research Branch at the National Institute of Child Health and Human Development, and editor-in-chief of the American Journal of Obstetrics and Gynecology; and Charles J. Lockwood, MD, dean of the University of South Florida’s Morsani College of Medicine, Tampa, and a former chair of Yale’s ob.gyn. department.
As this year’s honoree, Dr. Reece spoke about the importance of inspiring a new generation of physician-scientists not only within colleges and universities, but also by reaching out to younger students to spark interest in science and research. He recalled being a postdoctoral fellow in perinatology at Yale in the 1980s and being inspired by Dr. Hobbins, whom he credits as his mentor, as well as Dr. Romero, who was finishing his fellowship at Yale while Dr. Reece was beginning his fellowship.
Yale’s department of ob.gyn. and its division of maternal-fetal medicine have had a long history of “firsts” and seminal contributions, including the first ultrasound-guided fetal blood sampling and transfusions in the United States, invention of the fetal heart monitor, the first karyotype in amniotic fluid, the development of postcoital contraception and of methods for early detection of ectopic pregnancies, the discovery of endometrial stem cells and the role that endocrine-disrupting chemicals play in the developmental programming of the uterus, and discovery of the role of cytokines in premature labor and fetal injury.
According to current department chair, Hugh S. Taylor, MD, the 1980s and 1990s were a particularly “exciting time.” Under the tutelage of Dr. Hobbins, who directed both obstetrics and maternal-fetal medicine, obstetrical ultrasound was fast advancing, for instance, and fetoscopy was drawing patients and other physician-scientists from around the world.
“It was an unbelievable time – a magnetic period when many of the things we now take for granted were first being introduced,” said Dr. Reece, who went on after his fellowship to serve as an instructor in ob.gyn. (1982-4), assistant professor (1984-7), and then associate professor (1987-91) at Yale. “It was like going to the symphony and getting to choose the best seat in the house to see the rehearsals all the way through the concert.”
After leaving the Yale faculty and prior to joining the University of Maryland School of Medicine, Dr. Reece served as the chair of obstetrics and gynecology at Temple University School of Medicine, Philadelphia, and then vice chancellor and dean of the University of Arkansas College of Medicine, Little Rock. “Dr. Reece is an incredible bulldog,” said Dr. Hobbins, speaking of the honor given to Dr. Reece at the YOGS meeting. “We could see this right at the beginning at Yale. He latches into something and won’t let it go. He has a work ethic that’s remarkable ... He’s always thinking, ‘How can this be done better?’ ”
Dr. Hobbins, who went on after Yale to a tenure at the University of Colorado at Denver, Aurora, told Ob.Gyn. News that what he remembers “more than anything else, is that we would sit down in a room and just kind of spitball – just brainstorm.”
It is this intellectual curiosity and scientific drive that seems increasingly at risk of being lost, Dr. Hobbins said. “There’s not the same impetus to do a fellowship or to become a physician-scientist or pursue an MD-PhD,” he said. “There just doesn’t seem to be the same oomph to get into the nuts and bolts of how things work, to explore and understand the science. Yes, it has to do with funding. But there’s more to it: We have to somehow stimulate more fire in the belly.”
Dr. Lockwood, who served his fellowship in maternal-fetal medicine at Yale under the guidance of Dr. Hobbins, Dr. Reece, and other faculty, and who later chaired the Yale department of ob.gyn. for 9 years, said that research-rich environments that are “full of inquiry” drive better clinical care.
“The same rigor [gets] applied to the clinical enterprise. Where evidence-based medicine is applicable, it’s done ... and where there are gaps in knowledge, there’s a real spirit of research and inquiry to try to improve care,” Dr. Lockwood said in an interview. “All the great stuff in our health care system is really a direct correlate with the fact that we’ve had this extraordinary research enterprise for so long – most of it funded by the National Institutes of Health, either directly or indirectly.”
The University of Maryland requires all its medical students to take a course in research and critical thinking and to complete a research project. It also runs programs for young students such as a “mini medical school” for underprivileged children who live in nearby neighborhoods. “If you get them excited about science early, and you keep the research continuum going, we believe you’ll have a better chance of recruiting committed physician-scientists into the field,” Dr. Reece said.
The infection has “become an important issue because 10%-20% of women who receive an epidural develop a fever and many of these babies have to have a septic workup and antibiotic treatment,” he said in an e-mail after the meeting. ”Our data indicate that antibiotic administration is not indicated in 40% of cases and the antibiotics currently used do not cover frequent organisms causing infection.”
Dr. Hobbins, who has been using sophisticated imaging techniques to assess subtle changes in fetuses with growth restriction, spoke about the potential value of cardiac size as an indicator of cardiac dysfunction. In utero cardiac dysfunction “sets the tone” for later cardiovascular and neurologic function, he told Ob.Gyn. News. “We think that you can use cardiac size in small babies as a screening tool to tell you whether you need to delve a little further into cardiac function ... Let’s get away from old protocols and rethink other things that are going on in the [small] fetus. Let’s cast a wider net.”
Dr. Lockwood has long been investigating the prevention of recurrent pregnancy loss and preterm delivery, and at the meeting he presented March of Dimes–funded research aimed at identifying mechanisms for dysfunction of the progesterone receptor in premature birth.
Dr. Reece spoke about his research on diabetes in pregnancy and birth defects, and how years of research on diabetes-induced birth defects has shown that maternal hyperglycemia is a teratogen that can trigger a series of developmental fetal defects. “We now have enough information such that we truly have a biomolecular map regarding the precise steps and cascading events which lead to the induction of diabetes-induced birth defects,” said Dr. Reece, who holds a PhD in biochemistry and directs a multimillion-dollar NIH-funded research laboratory at the University of Maryland.
This research began when Dr. Reece asked a question during his fellowship at Yale. “I was struck by the number of birth defects I saw in women with diabetes. I asked Jerry Mahoney, one of the geneticists: Do we know the cause of this? Why is this happening?” he recalled in the interview. “Dr. Mahoney took me to his office, opened his file cabinet and showed me some papers of an [in-vitro rat embryo model], where the rats were made diabetic and the serum seemed to have a way of inducing these birth defects in the embryo. That intrigued me immensely and I thought: I can do this!”
Dr. Reece got his feet wet in an embryology laboratory. As he moved on after his fellowship to join the faculty at Yale, he began directing his own research team – the Diabetes-in-Pregnancy Study Unit.
Dr. Romero said this was the start of “many important contributions to optimize the care of pregnant women with diabetes.” Dr. Reece, he said, has been “able to dissect the role of oxidative stress, program cell death, and lipid metabolism in the genesis of congenital anomalies” in babies of mothers with diabetes.
In other talks at the YOGS meeting, Yale alumnus Ray Bahado-Singh, MD, of Oakwood University, Rochester, Mich., addressed the epigenetics of cardiac dysfunction and the “new frontier” of using epigenetic markers to assess fetal cardiac function. Frank A. Chervenak, MD, of Cornell University, New York, rounded out the meeting by addressing the issue of professionalism and putting the patient first, as well as the professional virtues of self-sacrifice, compassion, and integrity – themes that Dr. Reece frequently cites as integral to both practice and research in ob.gyn.
Clinical care and “the research we’re all doing to assess fetal health both directly and indirectly has to be sitting on a platform of moral, ethical, and solid principles,” said Dr. Reece, who authored a special feature for Ob.Gyn. News – “Obstetrics Moonshots: 50 Years of Discoveries,” on the recent history of obstetrics.
Mary Jane Minkin, MD, a Yale alumna of many levels (medical school through residency) and a longtime Yale faculty member and private-practice ob.gyn. in New Haven, Conn., noted that the YOGS meeting was attended by the 94-year-old Virginia Stuermer, MD, who joined Yale’s ob.gyn. department in 1954 and who is “celebrated within the department” for defying legal barriers to provide patients with contraception and services. “She wanted to come see Dr. Reece,” said Dr. Minkin, who has served as director of YOGS since its inception.
Dr. Stuermer was running the Planned Parenthood clinic in New Haven the day in 1961 when then-department chair Charles Lee Buxton, MD, and Connecticut Planned Parenthood League executive director Estelle Griswold were arrested and jailed. “Everyone knows about the Supreme Court decision, Griswold v. Connecticut [1965], that legalized contraception in the U.S.,” said Dr. Minkin. “But most don’t realize that the doctor who was actually fitting the diaphragms that day was Dr. Stuermer.”
The YOGS reunion preceded a symposium held early in June commemorating the 100-year anniversary of women at Yale Medical School.
John DeLuca, PhD
High-dose opioid prescribing linked to heroin use risk among U.S. veterans
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
REPORTING CPDD 2018
Key clinical point: Screen patients who receive high-dose opioids and meet several other criteria for heroin use.
Major finding:. On multivariate Cox regression, prior prescription of high-dose opioids was an independent predictor of recent heroin use (adjusted hazard ratio, 2.54).
Study details: A prospective analysis of 3,833 participants in the Veterans Aging Cohort Study.
Disclosures: The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
FDA approves pembrolizumab for cervical cancer
with disease progression on or after chemotherapy whose tumors express programmed cell death ligand 1, as determined by an FDA approved test.
“This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” the FDA said in updated labeling.
Two patients (2.6%) had a complete response, and nine (11.7%) had a partial response. Of these 11 patients, 10 had response durations of 6 months or longer. Patients were treated with 200 mg every 3 weeks until unacceptable toxicity or documented disease progression. Over a third had serious adverse reactions, most frequently anemia, fistula, hemorrhage, and infection.
“Keytruda is now the first anti-PD-1 [anti–programmed cell death 1] therapy approved for the treatment of advanced cervical cancer, providing an important new second-line option for certain patients with this disease,” Roy Baynes, MD, a Merck executive, said in a company press release.
with disease progression on or after chemotherapy whose tumors express programmed cell death ligand 1, as determined by an FDA approved test.
“This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” the FDA said in updated labeling.
Two patients (2.6%) had a complete response, and nine (11.7%) had a partial response. Of these 11 patients, 10 had response durations of 6 months or longer. Patients were treated with 200 mg every 3 weeks until unacceptable toxicity or documented disease progression. Over a third had serious adverse reactions, most frequently anemia, fistula, hemorrhage, and infection.
“Keytruda is now the first anti-PD-1 [anti–programmed cell death 1] therapy approved for the treatment of advanced cervical cancer, providing an important new second-line option for certain patients with this disease,” Roy Baynes, MD, a Merck executive, said in a company press release.
with disease progression on or after chemotherapy whose tumors express programmed cell death ligand 1, as determined by an FDA approved test.
“This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” the FDA said in updated labeling.
Two patients (2.6%) had a complete response, and nine (11.7%) had a partial response. Of these 11 patients, 10 had response durations of 6 months or longer. Patients were treated with 200 mg every 3 weeks until unacceptable toxicity or documented disease progression. Over a third had serious adverse reactions, most frequently anemia, fistula, hemorrhage, and infection.
“Keytruda is now the first anti-PD-1 [anti–programmed cell death 1] therapy approved for the treatment of advanced cervical cancer, providing an important new second-line option for certain patients with this disease,” Roy Baynes, MD, a Merck executive, said in a company press release.
Serum uromodulin independently predicts mortality in CAD
ORLANDO – Low serum uromodulin proved to be an independent predictor of all-cause mortality in a prospective study of 529 patients with stable coronary artery disease followed for up to 8 years, according to Christoph Saely, MD, vice president of the Vorarlberg Institute for Vascular Investigation and Treatment in Feldkirch, Austria.
“Baseline serum uromodulin is a valuable biomarker to predict overall mortality in coronary patients independent from kidney disease and the presence of type 2 diabetes. The lower the serum uromodulin, the higher the mortality,” he noted in an interview at the annual meeting of the American College of Cardiology.
In addition to presenting new evidence at ACC 2018 of serum uromodulin’s merits as a predictor of increased mortality risk in patients with CAD, Dr. Saely and his coinvestigators also showed that low serum uromodulin is associated with chronic kidney disease, type 2 diabetes, and prediabetes. Moreover, those with normal glucose metabolism and kidney function but a low serum uromodulin at baseline were at increased risk of developing abnormal glucose metabolism and impaired renal function during 4 years of follow-up.
Of the 529 patients with stable CAD, 95 died during follow-up. Among those with a low baseline serum uromodulin, defined bimodally as a level below 123.3 ng/mL, the mortality rate was 27.6%, roughly twice the 13.7% figure in patients with a baseline uromodulin above that threshold.
In a multivariate analysis adjusted extensively for age, sex, smoking, LDL cholesterol, HDL cholesterol, C-reactive protein, diabetes, estimated glomerular filtration rate, and N-terminal pro b-type natriuretic peptide level, a high baseline serum uromodulin was independently associated with a 43% reduction in the risk of mortality, according to Dr. Saely, who is both a cardiologist and an endocrinologist.
The overall mortality rate was 41% in patients with diabetes and a low baseline uromodulin, 20% in those with low uromodulin but not diabetes, 18% in diabetic patients with high uromodulin, and less than 13% with high uromodulin and no diabetes.
Enzyme-linked immunosorbent assay tests for uromodulin are marketed in Europe but remain investigational for now in the United States.
Dr. Saely reported having no financial conflicts of interest; the study was conducted without commercial support.
SOURCE: Saely C. ACC 2018, Abstract 1212-418/418.
ORLANDO – Low serum uromodulin proved to be an independent predictor of all-cause mortality in a prospective study of 529 patients with stable coronary artery disease followed for up to 8 years, according to Christoph Saely, MD, vice president of the Vorarlberg Institute for Vascular Investigation and Treatment in Feldkirch, Austria.
“Baseline serum uromodulin is a valuable biomarker to predict overall mortality in coronary patients independent from kidney disease and the presence of type 2 diabetes. The lower the serum uromodulin, the higher the mortality,” he noted in an interview at the annual meeting of the American College of Cardiology.
In addition to presenting new evidence at ACC 2018 of serum uromodulin’s merits as a predictor of increased mortality risk in patients with CAD, Dr. Saely and his coinvestigators also showed that low serum uromodulin is associated with chronic kidney disease, type 2 diabetes, and prediabetes. Moreover, those with normal glucose metabolism and kidney function but a low serum uromodulin at baseline were at increased risk of developing abnormal glucose metabolism and impaired renal function during 4 years of follow-up.
Of the 529 patients with stable CAD, 95 died during follow-up. Among those with a low baseline serum uromodulin, defined bimodally as a level below 123.3 ng/mL, the mortality rate was 27.6%, roughly twice the 13.7% figure in patients with a baseline uromodulin above that threshold.
In a multivariate analysis adjusted extensively for age, sex, smoking, LDL cholesterol, HDL cholesterol, C-reactive protein, diabetes, estimated glomerular filtration rate, and N-terminal pro b-type natriuretic peptide level, a high baseline serum uromodulin was independently associated with a 43% reduction in the risk of mortality, according to Dr. Saely, who is both a cardiologist and an endocrinologist.
The overall mortality rate was 41% in patients with diabetes and a low baseline uromodulin, 20% in those with low uromodulin but not diabetes, 18% in diabetic patients with high uromodulin, and less than 13% with high uromodulin and no diabetes.
Enzyme-linked immunosorbent assay tests for uromodulin are marketed in Europe but remain investigational for now in the United States.
Dr. Saely reported having no financial conflicts of interest; the study was conducted without commercial support.
SOURCE: Saely C. ACC 2018, Abstract 1212-418/418.
ORLANDO – Low serum uromodulin proved to be an independent predictor of all-cause mortality in a prospective study of 529 patients with stable coronary artery disease followed for up to 8 years, according to Christoph Saely, MD, vice president of the Vorarlberg Institute for Vascular Investigation and Treatment in Feldkirch, Austria.
“Baseline serum uromodulin is a valuable biomarker to predict overall mortality in coronary patients independent from kidney disease and the presence of type 2 diabetes. The lower the serum uromodulin, the higher the mortality,” he noted in an interview at the annual meeting of the American College of Cardiology.
In addition to presenting new evidence at ACC 2018 of serum uromodulin’s merits as a predictor of increased mortality risk in patients with CAD, Dr. Saely and his coinvestigators also showed that low serum uromodulin is associated with chronic kidney disease, type 2 diabetes, and prediabetes. Moreover, those with normal glucose metabolism and kidney function but a low serum uromodulin at baseline were at increased risk of developing abnormal glucose metabolism and impaired renal function during 4 years of follow-up.
Of the 529 patients with stable CAD, 95 died during follow-up. Among those with a low baseline serum uromodulin, defined bimodally as a level below 123.3 ng/mL, the mortality rate was 27.6%, roughly twice the 13.7% figure in patients with a baseline uromodulin above that threshold.
In a multivariate analysis adjusted extensively for age, sex, smoking, LDL cholesterol, HDL cholesterol, C-reactive protein, diabetes, estimated glomerular filtration rate, and N-terminal pro b-type natriuretic peptide level, a high baseline serum uromodulin was independently associated with a 43% reduction in the risk of mortality, according to Dr. Saely, who is both a cardiologist and an endocrinologist.
The overall mortality rate was 41% in patients with diabetes and a low baseline uromodulin, 20% in those with low uromodulin but not diabetes, 18% in diabetic patients with high uromodulin, and less than 13% with high uromodulin and no diabetes.
Enzyme-linked immunosorbent assay tests for uromodulin are marketed in Europe but remain investigational for now in the United States.
Dr. Saely reported having no financial conflicts of interest; the study was conducted without commercial support.
SOURCE: Saely C. ACC 2018, Abstract 1212-418/418.
REPORTING FROM ACC 2018
Key clinical point: The lower the baseline serum uromodulin in patients with CAD, the higher the subsequent mortality.
Major finding: A high baseline serum uromodulin was independently associated with a 43% reduction in all-cause mortality in patients with stable coronary artery disease.
Study details: This was a prospective study of 529 patients with stable CAD followed for up to 8 years.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted without commercial support.
Source: Saely C. ACC 2018, Abstract 1212-418/418.