User login
New NIH consortium aims to coordinate pediatric research programs
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
RBC transfusions with surgery may increase VTE risk
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
FROM JAMA SURGERY
Key clinical point:
Major finding: Patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3), compared with patients who did not receive transfusions.
Study details: An analysis of prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014.
Disclosures: The study was funded in part by grants from the National Institutes of Health and Cornell University, New York. The researchers reported having no conflicts of interest.
Source: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
Risk of Recurrent ICH Is Higher Among Blacks and Hispanics
The increased severity of hypertension among minorities does not fully account for their increased risk.
Compared with their white peers, black and Hispanic patients with intracerebral hemorrhage (ICH) have a higher risk of recurrence, according to data published online ahead of print June 6 in Neurology. Although black and Hispanic patients have more severe hypertension than whites do, severity of hypertension does not fully account for this increased risk. Future studies should examine whether novel biologic, socioeconomic, or cultural factors play a role, said the researchers.
The scientific literature indicates that blacks and Hispanics have a higher risk of first ICH than whites do. Alessandro Biffi, MD, head of the Aging and Brain Health Research group at Massachusetts General Hospital in Boston, and colleagues hypothesized that hypertension among these populations might contribute toward this increased risk. Because the subject had not been explored previously, Dr. Biffi and colleagues investigated the role of blood pressure and its variability in determining the risk of recurrent ICH among nonwhites.
An Analysis of Two Studies
The authors examined data from a longitudinal study of ICH conducted by Massachusetts General Hospital and from the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. They included patients who were 18 or older with a diagnosis of acute primary ICH in their analysis.
At enrollment, participants reported their race or ethnicity during a structured interview and underwent blood pressure measurement. The investigators performed follow-up through phone calls and reviews of medical records. Every six months, investigators recorded at least one blood-pressure measurement and quantified blood-pressure variability. Dr. Biffi and colleagues used Cox regression survival analysis to identify risk factors for ICH recurrence.
Systolic Blood Pressure Was Associated With Recurrence
Of the 2,291 patients included in the analysis, 1,121 were white, 529 were black, 605 were Hispanic, and 36 were of other race or ethnicity. The median systolic blood pressure during follow-up was 149 mm Hg for black participants, 146 mm Hg for Hispanic participants, and 141 mm Hg for white participants. Systolic blood pressure variability also was higher for black participants (median, 3.5%), compared with white participants (median
In all, 26 (1.7%) white participants had ICH recurrence, compared with 35 (6.6%) black participants and 37 (6.1%) Hispanic participants. In univariable analyses, higher systolic blood pressure and greater systolic blood pressure variability were associated with increased ICH recurrence risk. Diastolic blood pressure and diastolic blood pressure variability, however, were not associated with ICH recurrence risk.
In multivariable analyses, black and Hispanic race or ethnicity remained independently associated with increased ICH recurrence risk in both studies, even after adjustment for systolic blood pressure and systolic blood pressure variability. Exposure to antihypertensive agents during follow-up was not associated with ICH recurrence and did not affect the results significantly. The associations were consistent in both studies.
Suggested Reading
Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018 Jun 6 [Epub ahead of print].
The increased severity of hypertension among minorities does not fully account for their increased risk.
The increased severity of hypertension among minorities does not fully account for their increased risk.
Compared with their white peers, black and Hispanic patients with intracerebral hemorrhage (ICH) have a higher risk of recurrence, according to data published online ahead of print June 6 in Neurology. Although black and Hispanic patients have more severe hypertension than whites do, severity of hypertension does not fully account for this increased risk. Future studies should examine whether novel biologic, socioeconomic, or cultural factors play a role, said the researchers.
The scientific literature indicates that blacks and Hispanics have a higher risk of first ICH than whites do. Alessandro Biffi, MD, head of the Aging and Brain Health Research group at Massachusetts General Hospital in Boston, and colleagues hypothesized that hypertension among these populations might contribute toward this increased risk. Because the subject had not been explored previously, Dr. Biffi and colleagues investigated the role of blood pressure and its variability in determining the risk of recurrent ICH among nonwhites.
An Analysis of Two Studies
The authors examined data from a longitudinal study of ICH conducted by Massachusetts General Hospital and from the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. They included patients who were 18 or older with a diagnosis of acute primary ICH in their analysis.
At enrollment, participants reported their race or ethnicity during a structured interview and underwent blood pressure measurement. The investigators performed follow-up through phone calls and reviews of medical records. Every six months, investigators recorded at least one blood-pressure measurement and quantified blood-pressure variability. Dr. Biffi and colleagues used Cox regression survival analysis to identify risk factors for ICH recurrence.
Systolic Blood Pressure Was Associated With Recurrence
Of the 2,291 patients included in the analysis, 1,121 were white, 529 were black, 605 were Hispanic, and 36 were of other race or ethnicity. The median systolic blood pressure during follow-up was 149 mm Hg for black participants, 146 mm Hg for Hispanic participants, and 141 mm Hg for white participants. Systolic blood pressure variability also was higher for black participants (median, 3.5%), compared with white participants (median
In all, 26 (1.7%) white participants had ICH recurrence, compared with 35 (6.6%) black participants and 37 (6.1%) Hispanic participants. In univariable analyses, higher systolic blood pressure and greater systolic blood pressure variability were associated with increased ICH recurrence risk. Diastolic blood pressure and diastolic blood pressure variability, however, were not associated with ICH recurrence risk.
In multivariable analyses, black and Hispanic race or ethnicity remained independently associated with increased ICH recurrence risk in both studies, even after adjustment for systolic blood pressure and systolic blood pressure variability. Exposure to antihypertensive agents during follow-up was not associated with ICH recurrence and did not affect the results significantly. The associations were consistent in both studies.
Suggested Reading
Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018 Jun 6 [Epub ahead of print].
Compared with their white peers, black and Hispanic patients with intracerebral hemorrhage (ICH) have a higher risk of recurrence, according to data published online ahead of print June 6 in Neurology. Although black and Hispanic patients have more severe hypertension than whites do, severity of hypertension does not fully account for this increased risk. Future studies should examine whether novel biologic, socioeconomic, or cultural factors play a role, said the researchers.
The scientific literature indicates that blacks and Hispanics have a higher risk of first ICH than whites do. Alessandro Biffi, MD, head of the Aging and Brain Health Research group at Massachusetts General Hospital in Boston, and colleagues hypothesized that hypertension among these populations might contribute toward this increased risk. Because the subject had not been explored previously, Dr. Biffi and colleagues investigated the role of blood pressure and its variability in determining the risk of recurrent ICH among nonwhites.
An Analysis of Two Studies
The authors examined data from a longitudinal study of ICH conducted by Massachusetts General Hospital and from the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. They included patients who were 18 or older with a diagnosis of acute primary ICH in their analysis.
At enrollment, participants reported their race or ethnicity during a structured interview and underwent blood pressure measurement. The investigators performed follow-up through phone calls and reviews of medical records. Every six months, investigators recorded at least one blood-pressure measurement and quantified blood-pressure variability. Dr. Biffi and colleagues used Cox regression survival analysis to identify risk factors for ICH recurrence.
Systolic Blood Pressure Was Associated With Recurrence
Of the 2,291 patients included in the analysis, 1,121 were white, 529 were black, 605 were Hispanic, and 36 were of other race or ethnicity. The median systolic blood pressure during follow-up was 149 mm Hg for black participants, 146 mm Hg for Hispanic participants, and 141 mm Hg for white participants. Systolic blood pressure variability also was higher for black participants (median, 3.5%), compared with white participants (median
In all, 26 (1.7%) white participants had ICH recurrence, compared with 35 (6.6%) black participants and 37 (6.1%) Hispanic participants. In univariable analyses, higher systolic blood pressure and greater systolic blood pressure variability were associated with increased ICH recurrence risk. Diastolic blood pressure and diastolic blood pressure variability, however, were not associated with ICH recurrence risk.
In multivariable analyses, black and Hispanic race or ethnicity remained independently associated with increased ICH recurrence risk in both studies, even after adjustment for systolic blood pressure and systolic blood pressure variability. Exposure to antihypertensive agents during follow-up was not associated with ICH recurrence and did not affect the results significantly. The associations were consistent in both studies.
Suggested Reading
Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018 Jun 6 [Epub ahead of print].
Giving hospitalists a larger clinical footprint
Sneak Peek: The Hospital Leader blog
“We are playing the same sport, but a different game,” the wise, thoughtful emergency medicine attending physician once told me. “I am playing speed chess – I need to make a move quickly, or I lose – no matter what. My moves have to be right, but they don’t always necessarily need to be the optimal one. I am not always thinking five moves ahead. You guys [in internal medicine] are playing master chess. You have more time, but that means you are trying to always think about the whole game and make the best move possible.”
The pendulum has swung quickly from, “problem #7, chronic anemia: stable but I am not sure it has been worked up before, so I ordered a smear, retic count, and iron panel,” to “problem #1, acute blood loss anemia: now stable after transfusion, seems safe for discharge and GI follow-up.” (NOTE: “Acute blood loss anemia” is a phrase I learned from our “clinical documentation integrity specialist” – I think it gets me “50 CDI points” or something.)
Our job is not merely to work shifts and stabilize patients – there already is a specialty for that, and it is not the one we chose.
Clearly the correct balance is somewhere between the two extremes of “working up everything” and “deferring (nearly) everything to the outpatient setting.”
There are many forces that are contributing to current hospitalist work styles. As the work continues to become more exhaustingly intense and the average number of patients seen by a hospitalist grows impossibly upward, the duration of on-service stints has shortened.
In most settings, long gone are the days of the month-long teaching attending rotation. By day 12, I feel worn and ragged. For “nonteaching” services, hospitalists seem to increasingly treat each day as a separate shift to be covered, oftentimes handing the service back-and-forth every few days, or a week at most. With this structure, who can possibly think about the “whole patient”? Whose patient is this anyways?
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- How Hospitalists See the Forgotten Victims of Gun Violence by Vineet Arora, MD, MAPP, MHM
- Hospitals, Hospice and SNFs: The Big Deceit by Brad Flansbaum, DO, MPH, MHM
- But He’s a Good Doctor by Leslie Flores, MHA, SFHM
Sneak Peek: The Hospital Leader blog
Sneak Peek: The Hospital Leader blog
“We are playing the same sport, but a different game,” the wise, thoughtful emergency medicine attending physician once told me. “I am playing speed chess – I need to make a move quickly, or I lose – no matter what. My moves have to be right, but they don’t always necessarily need to be the optimal one. I am not always thinking five moves ahead. You guys [in internal medicine] are playing master chess. You have more time, but that means you are trying to always think about the whole game and make the best move possible.”
The pendulum has swung quickly from, “problem #7, chronic anemia: stable but I am not sure it has been worked up before, so I ordered a smear, retic count, and iron panel,” to “problem #1, acute blood loss anemia: now stable after transfusion, seems safe for discharge and GI follow-up.” (NOTE: “Acute blood loss anemia” is a phrase I learned from our “clinical documentation integrity specialist” – I think it gets me “50 CDI points” or something.)
Our job is not merely to work shifts and stabilize patients – there already is a specialty for that, and it is not the one we chose.
Clearly the correct balance is somewhere between the two extremes of “working up everything” and “deferring (nearly) everything to the outpatient setting.”
There are many forces that are contributing to current hospitalist work styles. As the work continues to become more exhaustingly intense and the average number of patients seen by a hospitalist grows impossibly upward, the duration of on-service stints has shortened.
In most settings, long gone are the days of the month-long teaching attending rotation. By day 12, I feel worn and ragged. For “nonteaching” services, hospitalists seem to increasingly treat each day as a separate shift to be covered, oftentimes handing the service back-and-forth every few days, or a week at most. With this structure, who can possibly think about the “whole patient”? Whose patient is this anyways?
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- How Hospitalists See the Forgotten Victims of Gun Violence by Vineet Arora, MD, MAPP, MHM
- Hospitals, Hospice and SNFs: The Big Deceit by Brad Flansbaum, DO, MPH, MHM
- But He’s a Good Doctor by Leslie Flores, MHA, SFHM
“We are playing the same sport, but a different game,” the wise, thoughtful emergency medicine attending physician once told me. “I am playing speed chess – I need to make a move quickly, or I lose – no matter what. My moves have to be right, but they don’t always necessarily need to be the optimal one. I am not always thinking five moves ahead. You guys [in internal medicine] are playing master chess. You have more time, but that means you are trying to always think about the whole game and make the best move possible.”
The pendulum has swung quickly from, “problem #7, chronic anemia: stable but I am not sure it has been worked up before, so I ordered a smear, retic count, and iron panel,” to “problem #1, acute blood loss anemia: now stable after transfusion, seems safe for discharge and GI follow-up.” (NOTE: “Acute blood loss anemia” is a phrase I learned from our “clinical documentation integrity specialist” – I think it gets me “50 CDI points” or something.)
Our job is not merely to work shifts and stabilize patients – there already is a specialty for that, and it is not the one we chose.
Clearly the correct balance is somewhere between the two extremes of “working up everything” and “deferring (nearly) everything to the outpatient setting.”
There are many forces that are contributing to current hospitalist work styles. As the work continues to become more exhaustingly intense and the average number of patients seen by a hospitalist grows impossibly upward, the duration of on-service stints has shortened.
In most settings, long gone are the days of the month-long teaching attending rotation. By day 12, I feel worn and ragged. For “nonteaching” services, hospitalists seem to increasingly treat each day as a separate shift to be covered, oftentimes handing the service back-and-forth every few days, or a week at most. With this structure, who can possibly think about the “whole patient”? Whose patient is this anyways?
Read the full post at hospitalleader.org.
Also on The Hospital Leader …
- How Hospitalists See the Forgotten Victims of Gun Violence by Vineet Arora, MD, MAPP, MHM
- Hospitals, Hospice and SNFs: The Big Deceit by Brad Flansbaum, DO, MPH, MHM
- But He’s a Good Doctor by Leslie Flores, MHA, SFHM
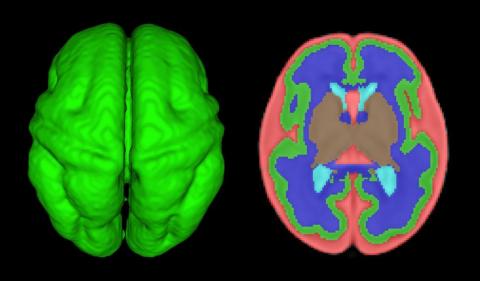
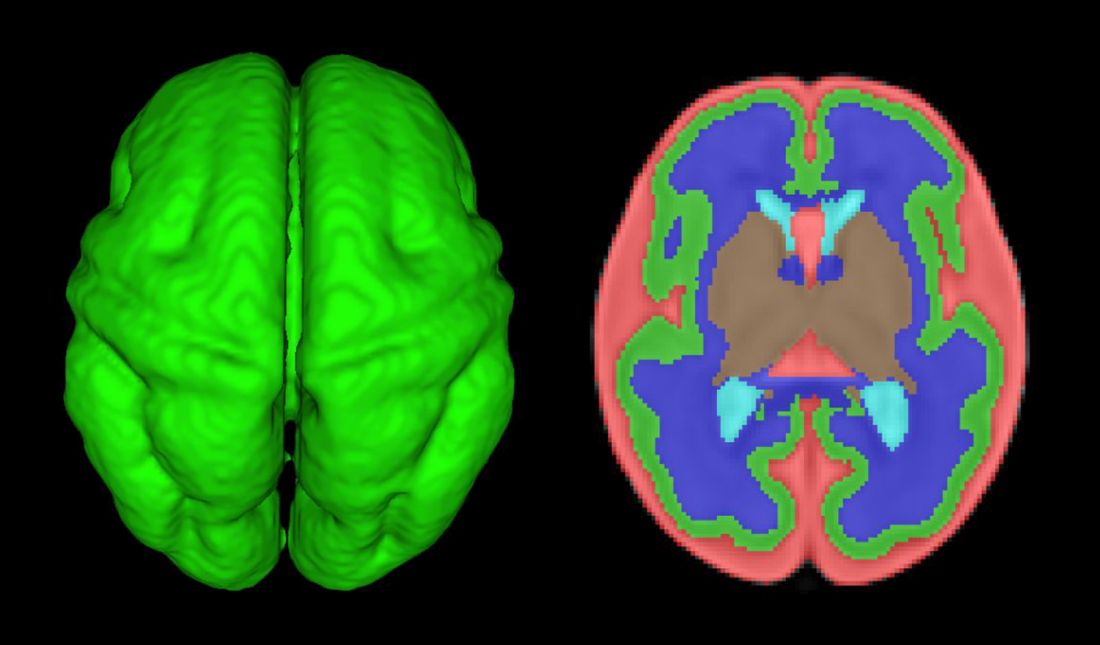
How congenital heart disease affects brain development
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.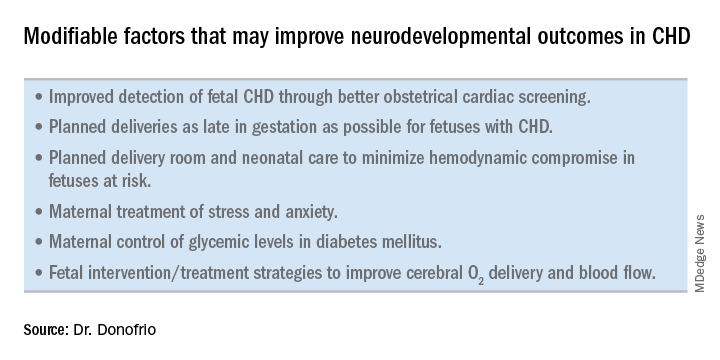
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
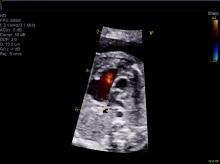
How better imaging technology for prenatal diagnoses can improve outcomes
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Functional disability prevails despite rheumatoid arthritis treatment
AMSTERDAM – Functional disability remains a significant problem for people with rheumatoid arthritis, with the prevalence remaining at least 15% higher over time than in individuals without the disease.
“We found a higher prevalence of functional disability in patients with RA versus non-RA,” the presenting study investigator Elena Myasoedova, MD, PhD, said at the European Congress of Rheumatology.
Dr. Myasoedova, who is a clinical fellow in rheumatology at the Mayo Clinic in Rochester, Minn., added that the increase in prevalence over time was significantly higher in subjects with RA than in those without RA (P = .003), but that there was no difference in the pace of this increase with adjustment for the duration of RA disease (P = .51).
There was also no difference in functional disability between the two groups of patients by about the 8th or 9th decade.
RA remains one of the most common conditions associated with functional disability, Dr. Myasoedova said, with several risk factors for physical impairment identified, including being female, of older age, smoking, and the use of certain medications (glucocorticoids and antidepressants), as well as sociodemographic factors.
A discrepancy between improved RA disease control and persistent impairment in physical function has been noted in prior studies, but there are few data on how this might change over time. Dr. Myasoedova and her associates investigated this by analyzing data from the Rochester Epidemiology Project, which collects medical data on individuals living in Olmsted County, Minnesota. They identified two populations of adults aged 18 and older: one diagnosed with RA according to 1987 American College of Rheumatology criteria between 1999 and 2013, and one without RA but who were of a similar age and sex and enrolled in the project around the same time.
As part of the project, participants completed an annual questionnaire asking about their health and ability to perform six activities of daily living (ADL). These include the ability to wash, dress, feed, and toilet oneself without assistance, as well as perform normal household chores and walk unaided. Over the course of study, 7,466 questionnaires have been completed by the participants and functional disability was defined as having difficulty with at least one of these six ADLs, Dr. Myasoedova explained.
At baseline, subjects with and without RA were aged a mean of 55 and 56 years, respectively, and 70% in both groups were female. Similar percentages were current (about 15%), former (about 30%), or never smokers (about 55%), and about 40% were obese.
Just under two-thirds (64.4%) of patients in the RA cohort were positive for rheumatoid factor (RF) or anti–cyclic citrullinated peptide (CCP) antibodies. While there was a similar prevalence of functional disability in RA patients who were or were not RF or CCP positive (both 25%, P = .67), there was an increasing prevalence noted in those who were positive versus those who were negative over time (P = .027).
Although the investigators did not conduct an objective assessment for functional disability, these findings highlight the need for vigilant management of patients with RA, Dr. Myasoedova proposed.
“Early and aggressive treatment regimens aimed at tight inflammation control can help prevent the disabling effects of high disease activity and joint damage, thereby lowering functional disability,” she said in an interview ahead of the congress.
Future work, she observed, should look at how the pattern of functional disability changes and the use of transition modeling to understand the bidirectional pattern of potential change and accumulation of functional disability in RA. The investigators also plan to look at risk factors for persistent and worsening functional disability and how treatment – including “treat to target” and biologics – might affect this.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases supported the study. Dr. Myasoedova had no conflicts of interest.
SOURCE: Myasoedova E et al. Ann Rheum Dis. 2018;77(Suppl 2):54. Abstract OP0009.
AMSTERDAM – Functional disability remains a significant problem for people with rheumatoid arthritis, with the prevalence remaining at least 15% higher over time than in individuals without the disease.
“We found a higher prevalence of functional disability in patients with RA versus non-RA,” the presenting study investigator Elena Myasoedova, MD, PhD, said at the European Congress of Rheumatology.
Dr. Myasoedova, who is a clinical fellow in rheumatology at the Mayo Clinic in Rochester, Minn., added that the increase in prevalence over time was significantly higher in subjects with RA than in those without RA (P = .003), but that there was no difference in the pace of this increase with adjustment for the duration of RA disease (P = .51).
There was also no difference in functional disability between the two groups of patients by about the 8th or 9th decade.
RA remains one of the most common conditions associated with functional disability, Dr. Myasoedova said, with several risk factors for physical impairment identified, including being female, of older age, smoking, and the use of certain medications (glucocorticoids and antidepressants), as well as sociodemographic factors.
A discrepancy between improved RA disease control and persistent impairment in physical function has been noted in prior studies, but there are few data on how this might change over time. Dr. Myasoedova and her associates investigated this by analyzing data from the Rochester Epidemiology Project, which collects medical data on individuals living in Olmsted County, Minnesota. They identified two populations of adults aged 18 and older: one diagnosed with RA according to 1987 American College of Rheumatology criteria between 1999 and 2013, and one without RA but who were of a similar age and sex and enrolled in the project around the same time.
As part of the project, participants completed an annual questionnaire asking about their health and ability to perform six activities of daily living (ADL). These include the ability to wash, dress, feed, and toilet oneself without assistance, as well as perform normal household chores and walk unaided. Over the course of study, 7,466 questionnaires have been completed by the participants and functional disability was defined as having difficulty with at least one of these six ADLs, Dr. Myasoedova explained.
At baseline, subjects with and without RA were aged a mean of 55 and 56 years, respectively, and 70% in both groups were female. Similar percentages were current (about 15%), former (about 30%), or never smokers (about 55%), and about 40% were obese.
Just under two-thirds (64.4%) of patients in the RA cohort were positive for rheumatoid factor (RF) or anti–cyclic citrullinated peptide (CCP) antibodies. While there was a similar prevalence of functional disability in RA patients who were or were not RF or CCP positive (both 25%, P = .67), there was an increasing prevalence noted in those who were positive versus those who were negative over time (P = .027).
Although the investigators did not conduct an objective assessment for functional disability, these findings highlight the need for vigilant management of patients with RA, Dr. Myasoedova proposed.
“Early and aggressive treatment regimens aimed at tight inflammation control can help prevent the disabling effects of high disease activity and joint damage, thereby lowering functional disability,” she said in an interview ahead of the congress.
Future work, she observed, should look at how the pattern of functional disability changes and the use of transition modeling to understand the bidirectional pattern of potential change and accumulation of functional disability in RA. The investigators also plan to look at risk factors for persistent and worsening functional disability and how treatment – including “treat to target” and biologics – might affect this.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases supported the study. Dr. Myasoedova had no conflicts of interest.
SOURCE: Myasoedova E et al. Ann Rheum Dis. 2018;77(Suppl 2):54. Abstract OP0009.
AMSTERDAM – Functional disability remains a significant problem for people with rheumatoid arthritis, with the prevalence remaining at least 15% higher over time than in individuals without the disease.
“We found a higher prevalence of functional disability in patients with RA versus non-RA,” the presenting study investigator Elena Myasoedova, MD, PhD, said at the European Congress of Rheumatology.
Dr. Myasoedova, who is a clinical fellow in rheumatology at the Mayo Clinic in Rochester, Minn., added that the increase in prevalence over time was significantly higher in subjects with RA than in those without RA (P = .003), but that there was no difference in the pace of this increase with adjustment for the duration of RA disease (P = .51).
There was also no difference in functional disability between the two groups of patients by about the 8th or 9th decade.
RA remains one of the most common conditions associated with functional disability, Dr. Myasoedova said, with several risk factors for physical impairment identified, including being female, of older age, smoking, and the use of certain medications (glucocorticoids and antidepressants), as well as sociodemographic factors.
A discrepancy between improved RA disease control and persistent impairment in physical function has been noted in prior studies, but there are few data on how this might change over time. Dr. Myasoedova and her associates investigated this by analyzing data from the Rochester Epidemiology Project, which collects medical data on individuals living in Olmsted County, Minnesota. They identified two populations of adults aged 18 and older: one diagnosed with RA according to 1987 American College of Rheumatology criteria between 1999 and 2013, and one without RA but who were of a similar age and sex and enrolled in the project around the same time.
As part of the project, participants completed an annual questionnaire asking about their health and ability to perform six activities of daily living (ADL). These include the ability to wash, dress, feed, and toilet oneself without assistance, as well as perform normal household chores and walk unaided. Over the course of study, 7,466 questionnaires have been completed by the participants and functional disability was defined as having difficulty with at least one of these six ADLs, Dr. Myasoedova explained.
At baseline, subjects with and without RA were aged a mean of 55 and 56 years, respectively, and 70% in both groups were female. Similar percentages were current (about 15%), former (about 30%), or never smokers (about 55%), and about 40% were obese.
Just under two-thirds (64.4%) of patients in the RA cohort were positive for rheumatoid factor (RF) or anti–cyclic citrullinated peptide (CCP) antibodies. While there was a similar prevalence of functional disability in RA patients who were or were not RF or CCP positive (both 25%, P = .67), there was an increasing prevalence noted in those who were positive versus those who were negative over time (P = .027).
Although the investigators did not conduct an objective assessment for functional disability, these findings highlight the need for vigilant management of patients with RA, Dr. Myasoedova proposed.
“Early and aggressive treatment regimens aimed at tight inflammation control can help prevent the disabling effects of high disease activity and joint damage, thereby lowering functional disability,” she said in an interview ahead of the congress.
Future work, she observed, should look at how the pattern of functional disability changes and the use of transition modeling to understand the bidirectional pattern of potential change and accumulation of functional disability in RA. The investigators also plan to look at risk factors for persistent and worsening functional disability and how treatment – including “treat to target” and biologics – might affect this.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases supported the study. Dr. Myasoedova had no conflicts of interest.
SOURCE: Myasoedova E et al. Ann Rheum Dis. 2018;77(Suppl 2):54. Abstract OP0009.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Functional disability remains higher over time in patients with rheumatoid arthritis, compared with people without the disease.
Major finding: The prevalence of functional disability was 26% vs. 11% at baseline, a 15% difference that persisted over more than 18 years of follow-up.
Study details: Retrospective, longitudinal, population-based cohort study of 586 individuals with RA, and 531 without.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases supported the study. Dr. Myasoedova had no conflicts of interest.
Source: Myasoedova E et al. Ann Rheum Dis. 2018;77(Suppl 2):54. Abstract OP0009.
Scaly Annular and Concentric Plaques
The Diagnosis: Annular Psoriasis
Because the patient's history was nonconcordant with the clinical appearance, a 4-mm punch biopsy was performed from a lesion on the left hip. Hematoxylin and eosin-stained sections demonstrated mild irregular acanthosis of the epidermis with discrete mounds of parakeratin (Figure 1A). Higher power revealed numerous neutrophils entrapped within focal scale crusts (Figure 1B). Periodic acid-Schiff stain for fungus demonstrated no hyphal elements or yeast forms in the stratum corneum. These histopathology findings were consistent with the diagnosis of annular psoriasis.
The manifestation of psoriasis may take many forms, ranging from classic plaques to pustular eruptions--either annular or generalized--and erythroderma. Primarily annular plaque-type psoriasis without pustules, however, remains an uncommon finding.1 Psoriatic plaques may become annular or arcuate with central clearing from partial treatment with topical medications, though our patient reported annular plaques prior to any treatment. His presentation was notably different than annular pustular psoriasis in that there were no pustules in the leading edge, and there was no trailing scale, which is typical of annular pustular psoriasis.
Topical triamcinolone prescribed at the initial presentation to the dermatology department helped with pruritus, but due to the large body surface area involved, methotrexate later was initiated. After a 10-mg test dose of methotrexate and titration to 15 mg weekly, dramatic improvement in the rash was noted after 8 weeks. As the rash resolved, only faint hyperpigmented patches remained (Figure 2).
Erythema gyratum repens is a rare paraneoplastic syndrome that presents with annular scaly plaques with concentric circles with a wood grain-like appearance. The borders can advance up to 1 cm daily and show nonspecific findings on histopathology.2 Due to the observation that approximately 80% of cases of erythema gyratum repens were associated with an underlying malignancy, most often of the lung,3 this diagnosis was entertained given our patient's clinical presentation.
Erythema annulare centrifugum (EAC) historically has been divided into 2 forms: superficial and deep.4 Both present with slowly expanding, annular, pink plaques. Superficial EAC demonstrates parakeratosis and trailing scale and has not been proven to be associated with other systemic diseases, while deep EAC has infiltrated borders without scale, and many cases of EAC may represent annular forms of tumid lupus.4 Inflammatory cells may cuff vessels tightly, resulting in so-called coat sleeve infiltrate in superficial EAC. Along with trailing scale, this finding suggests the diagnosis. It has been argued that EAC is not an entity on its own and should prompt evaluation for lupus erythematosus, dermatitis, hypersensitivity to tinea pedis, and Lyme disease in appropriate circumstances.5
Tinea corporis always should be considered when evaluating annular scaly plaques with central clearing. Diagnosis and treatment are straightforward when hyphae are found on microscopy of skin scrapings or seen on periodic acid-Schiff stains of formalin-fixed tissue. Tinea imbricata presents with an interesting morphology and appears more ornate or cerebriform than tinea corporis caused by Trichophyton rubrum. It is caused by infection with Trichophyton circumscriptum and occurs in certain regions in the South Pacific, Southeast Asia, and Central and South America, making the diagnosis within the United States unlikely for a patient who has not traveled to these areas.6
Erythema chronicum migrans is diagnostic of Lyme disease infection with Borrelia burgdorferi, and solitary lesions occur surrounding the site of a tick bite in the majority of patients. Only 20% of patients will develop multiple lesions consistent with erythema chronicum migrans due to multiple tick bites, spirochetemia, or lymphatic spread.7 Up to one-third of patients are unaware that they were bitten by a tick. In endemic areas, this diagnosis must be entertained in any patient presenting with an annular rash, as treatment may prevent notable morbidity.
- Guill C, Hoang M, Carder K. Primary annular plaque-type psoriasis. Pediatr Dermatol. 2005;22:15-18.
- Boyd A, Neldner K, Menter A. Erythema gyratum repens: a paraneoplastic eruption. J Am Acad Dermatol. 1992;26:757-762.
- Kawakami T, Saito R. Erythema gyratum repens unassociated with underlying malignancy. J Dermatol. 1995;22:587-589.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Ziemer M, Eisendle K, Zelger B. New concepts on erythema annulare centrifugum: a clinical reaction pattern that does notrepresent a specific clinicopathological entity. Br J Dermatol. 2009;160:119-126.
- Bonifaz A, Vázquez-González D. Tinea imbricata in the Americas. Curr Opin Infect Dis. 2011;24:106-111.
- Müllegger R, Glatz M. Skin manifestations of Lyme borreliosis: diagnosis and management. Am J Clin Dermatol. 2008;9:355-368.
The Diagnosis: Annular Psoriasis
Because the patient's history was nonconcordant with the clinical appearance, a 4-mm punch biopsy was performed from a lesion on the left hip. Hematoxylin and eosin-stained sections demonstrated mild irregular acanthosis of the epidermis with discrete mounds of parakeratin (Figure 1A). Higher power revealed numerous neutrophils entrapped within focal scale crusts (Figure 1B). Periodic acid-Schiff stain for fungus demonstrated no hyphal elements or yeast forms in the stratum corneum. These histopathology findings were consistent with the diagnosis of annular psoriasis.

The manifestation of psoriasis may take many forms, ranging from classic plaques to pustular eruptions--either annular or generalized--and erythroderma. Primarily annular plaque-type psoriasis without pustules, however, remains an uncommon finding.1 Psoriatic plaques may become annular or arcuate with central clearing from partial treatment with topical medications, though our patient reported annular plaques prior to any treatment. His presentation was notably different than annular pustular psoriasis in that there were no pustules in the leading edge, and there was no trailing scale, which is typical of annular pustular psoriasis.
Topical triamcinolone prescribed at the initial presentation to the dermatology department helped with pruritus, but due to the large body surface area involved, methotrexate later was initiated. After a 10-mg test dose of methotrexate and titration to 15 mg weekly, dramatic improvement in the rash was noted after 8 weeks. As the rash resolved, only faint hyperpigmented patches remained (Figure 2).

Erythema gyratum repens is a rare paraneoplastic syndrome that presents with annular scaly plaques with concentric circles with a wood grain-like appearance. The borders can advance up to 1 cm daily and show nonspecific findings on histopathology.2 Due to the observation that approximately 80% of cases of erythema gyratum repens were associated with an underlying malignancy, most often of the lung,3 this diagnosis was entertained given our patient's clinical presentation.
Erythema annulare centrifugum (EAC) historically has been divided into 2 forms: superficial and deep.4 Both present with slowly expanding, annular, pink plaques. Superficial EAC demonstrates parakeratosis and trailing scale and has not been proven to be associated with other systemic diseases, while deep EAC has infiltrated borders without scale, and many cases of EAC may represent annular forms of tumid lupus.4 Inflammatory cells may cuff vessels tightly, resulting in so-called coat sleeve infiltrate in superficial EAC. Along with trailing scale, this finding suggests the diagnosis. It has been argued that EAC is not an entity on its own and should prompt evaluation for lupus erythematosus, dermatitis, hypersensitivity to tinea pedis, and Lyme disease in appropriate circumstances.5
Tinea corporis always should be considered when evaluating annular scaly plaques with central clearing. Diagnosis and treatment are straightforward when hyphae are found on microscopy of skin scrapings or seen on periodic acid-Schiff stains of formalin-fixed tissue. Tinea imbricata presents with an interesting morphology and appears more ornate or cerebriform than tinea corporis caused by Trichophyton rubrum. It is caused by infection with Trichophyton circumscriptum and occurs in certain regions in the South Pacific, Southeast Asia, and Central and South America, making the diagnosis within the United States unlikely for a patient who has not traveled to these areas.6
Erythema chronicum migrans is diagnostic of Lyme disease infection with Borrelia burgdorferi, and solitary lesions occur surrounding the site of a tick bite in the majority of patients. Only 20% of patients will develop multiple lesions consistent with erythema chronicum migrans due to multiple tick bites, spirochetemia, or lymphatic spread.7 Up to one-third of patients are unaware that they were bitten by a tick. In endemic areas, this diagnosis must be entertained in any patient presenting with an annular rash, as treatment may prevent notable morbidity.
The Diagnosis: Annular Psoriasis
Because the patient's history was nonconcordant with the clinical appearance, a 4-mm punch biopsy was performed from a lesion on the left hip. Hematoxylin and eosin-stained sections demonstrated mild irregular acanthosis of the epidermis with discrete mounds of parakeratin (Figure 1A). Higher power revealed numerous neutrophils entrapped within focal scale crusts (Figure 1B). Periodic acid-Schiff stain for fungus demonstrated no hyphal elements or yeast forms in the stratum corneum. These histopathology findings were consistent with the diagnosis of annular psoriasis.

The manifestation of psoriasis may take many forms, ranging from classic plaques to pustular eruptions--either annular or generalized--and erythroderma. Primarily annular plaque-type psoriasis without pustules, however, remains an uncommon finding.1 Psoriatic plaques may become annular or arcuate with central clearing from partial treatment with topical medications, though our patient reported annular plaques prior to any treatment. His presentation was notably different than annular pustular psoriasis in that there were no pustules in the leading edge, and there was no trailing scale, which is typical of annular pustular psoriasis.
Topical triamcinolone prescribed at the initial presentation to the dermatology department helped with pruritus, but due to the large body surface area involved, methotrexate later was initiated. After a 10-mg test dose of methotrexate and titration to 15 mg weekly, dramatic improvement in the rash was noted after 8 weeks. As the rash resolved, only faint hyperpigmented patches remained (Figure 2).

Erythema gyratum repens is a rare paraneoplastic syndrome that presents with annular scaly plaques with concentric circles with a wood grain-like appearance. The borders can advance up to 1 cm daily and show nonspecific findings on histopathology.2 Due to the observation that approximately 80% of cases of erythema gyratum repens were associated with an underlying malignancy, most often of the lung,3 this diagnosis was entertained given our patient's clinical presentation.
Erythema annulare centrifugum (EAC) historically has been divided into 2 forms: superficial and deep.4 Both present with slowly expanding, annular, pink plaques. Superficial EAC demonstrates parakeratosis and trailing scale and has not been proven to be associated with other systemic diseases, while deep EAC has infiltrated borders without scale, and many cases of EAC may represent annular forms of tumid lupus.4 Inflammatory cells may cuff vessels tightly, resulting in so-called coat sleeve infiltrate in superficial EAC. Along with trailing scale, this finding suggests the diagnosis. It has been argued that EAC is not an entity on its own and should prompt evaluation for lupus erythematosus, dermatitis, hypersensitivity to tinea pedis, and Lyme disease in appropriate circumstances.5
Tinea corporis always should be considered when evaluating annular scaly plaques with central clearing. Diagnosis and treatment are straightforward when hyphae are found on microscopy of skin scrapings or seen on periodic acid-Schiff stains of formalin-fixed tissue. Tinea imbricata presents with an interesting morphology and appears more ornate or cerebriform than tinea corporis caused by Trichophyton rubrum. It is caused by infection with Trichophyton circumscriptum and occurs in certain regions in the South Pacific, Southeast Asia, and Central and South America, making the diagnosis within the United States unlikely for a patient who has not traveled to these areas.6
Erythema chronicum migrans is diagnostic of Lyme disease infection with Borrelia burgdorferi, and solitary lesions occur surrounding the site of a tick bite in the majority of patients. Only 20% of patients will develop multiple lesions consistent with erythema chronicum migrans due to multiple tick bites, spirochetemia, or lymphatic spread.7 Up to one-third of patients are unaware that they were bitten by a tick. In endemic areas, this diagnosis must be entertained in any patient presenting with an annular rash, as treatment may prevent notable morbidity.
- Guill C, Hoang M, Carder K. Primary annular plaque-type psoriasis. Pediatr Dermatol. 2005;22:15-18.
- Boyd A, Neldner K, Menter A. Erythema gyratum repens: a paraneoplastic eruption. J Am Acad Dermatol. 1992;26:757-762.
- Kawakami T, Saito R. Erythema gyratum repens unassociated with underlying malignancy. J Dermatol. 1995;22:587-589.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Ziemer M, Eisendle K, Zelger B. New concepts on erythema annulare centrifugum: a clinical reaction pattern that does notrepresent a specific clinicopathological entity. Br J Dermatol. 2009;160:119-126.
- Bonifaz A, Vázquez-González D. Tinea imbricata in the Americas. Curr Opin Infect Dis. 2011;24:106-111.
- Müllegger R, Glatz M. Skin manifestations of Lyme borreliosis: diagnosis and management. Am J Clin Dermatol. 2008;9:355-368.
- Guill C, Hoang M, Carder K. Primary annular plaque-type psoriasis. Pediatr Dermatol. 2005;22:15-18.
- Boyd A, Neldner K, Menter A. Erythema gyratum repens: a paraneoplastic eruption. J Am Acad Dermatol. 1992;26:757-762.
- Kawakami T, Saito R. Erythema gyratum repens unassociated with underlying malignancy. J Dermatol. 1995;22:587-589.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Ziemer M, Eisendle K, Zelger B. New concepts on erythema annulare centrifugum: a clinical reaction pattern that does notrepresent a specific clinicopathological entity. Br J Dermatol. 2009;160:119-126.
- Bonifaz A, Vázquez-González D. Tinea imbricata in the Americas. Curr Opin Infect Dis. 2011;24:106-111.
- Müllegger R, Glatz M. Skin manifestations of Lyme borreliosis: diagnosis and management. Am J Clin Dermatol. 2008;9:355-368.
A healthy 23-year-old man presented for evaluation of an enlarging annular pruritic rash of 1.5 years' duration. Treatment with ciclopirox cream 0.77%, calcipotriene cream 0.005%, tacrolimus ointment 0.1%, fluticasone cream 0.05%, and halobetasol cream 0.05% prescribed by an outside physician provided only modest temporary improvement. The patient reported no history of travel outside of western New York, camping, tick bites, or medications. He denied any joint swelling or morning stiffness. Physical examination revealed multiple 4- to 6-cm pink, annular, scaly plaques with central clearing on the abdomen (top) and thighs. A few 1-cm pink scaly patches were present on the back (bottom), and few 2- to 3-mm pink scaly papules were noted on the extensor aspects of the elbows and forearms. A potassium hydroxide examination revealed no hyphal elements or yeast forms.
Is the suicide story fake – or just misleading?
Recently, a lot has been in the news about the increasing rates of suicide in all communities, including among African American youth, and two high-profile celebrities. Now that we have a CEO in the White House who has made the phrase “fake news” part of the national lexicon (and as a former CEO myself), I feel compelled to take a critical, clinical look at the way the suicide story has been reported.
CEOs tend to be unique people, and many of them are fond of hyperbole – as it promotes “followship” in employees and fosters business deals. I interpret fake news as the kind of information, or maybe spin is a better word, promulgated by CEOs.
While following a research letter published recently in JAMA Pediatrics – “Age-Related Racial Disparity in Suicide Rates Among U.S. Youths From 2001 Through 2015” (2018 May 21. doi: 10.001/jamapediatrics.2018.0399) – it occurred to me that this struck me as fake news. But as I thought about it, I realized that the conclusions in the research letter would be better characterized as perhaps misleading news. My basis for reaching those conclusions is rooted in the lessons I learned as a 2-year member of the Institute of Medicine’s Board on Neuroscience and Behavioral Health, Committee on Pathophysiology & Prevention of Adolescent & Adult Suicide. In fact, the report we produced was the first one referenced in the research letter. Unfortunately, however, the research letter’s authors seemed to miss the IOM report’s major take-away messages.
For example, the research letter authors compared the suicide rates among black children and white children in this way: “However, suicide rates increased from 1993 to 1997 and 2008 to 2012 among black children aged 5 to 11 years (from 1.36 to 2.54 per million) and decreased among white children of the same age (from 1.14 to 0.77 per million).” That sentence supports the conclusions of the IOM’s “Reducing Suicide” report, as it confirms that those are very low base rates. However, because the base rates are so low in most populations, it is difficult to determine scientifically whether a significant rise or decrease in rates occurred.
To quote page 377 of IOM report: “The base rate of completed suicide is sufficiently low to preclude all but the largest of studies. When such studies are performed, resultant comparisons are between extremely small and large groups of individuals (suicide completers versus non–suicide completers, or suicide attempters versus non–suicide attempters). Use of suicidal ideation as an outcome can increase incidence and alleviate the problem to some extent; however, it is unclear whether suicidal ideation is a strong predictor of suicide completion. Using both attempts and completions can confound the analysis since attempters may account for some of the suicides completed within the study period. Because the duration of the prevention studies is frequently too brief to collect sufficient data on the low frequency endpoints of suicide or suicide attempt, proximal measures such as changes in knowledge or attitude are used. Yet the predictive value of these variables is unconfirmed.”
Further, according to page 410 of the report: “As the statistical analysis above points out, at a suicide rate of 10 per 100,000 population, approximately 100,000 participants are needed to achieve statistical significance in an experimental context. In studying suicide among low-risk groups, the numbers needed are even greater.”
Let me break this down a bit. , because the numerator is so small and the dominator is so large. Let me put it this way – if the black female suicide rates are 2/100,000, and those rates quadrupled (sounds impressive, doesn’t it?) then there would be 8/100,000 black female suicides; the difference between 2 and 8 per 100,000 is not really a significant difference because the base-rates are so small. But to say the rates quadrupled sounds scary and impressive. “Figures don’t lie, but liars can figure.”
So, the premise of the research letter is whack.
I am not impressed that the rates of black children aged 5-7 increased from 1.36/1,000,000 to 2.54/1,000,000. I am not even sure those two numbers are significantly different, much less have clinical relevance. I have tried to make this point before, but it always gets lost by the hyperbolic press – which continues to yell about suicides in the United States rising by 30% or doubling, even quadrupling. The low base rates make drawing firm conclusions from this data like spitting into the ocean. I understand that one suicide is one suicide too many. But this is not science.
The characterizations about soaring U.S. suicide rates are not exactly fake news. Instead, I would call these interpretations misleading science and news.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former director of the Institute for Juvenile Research (the birthplace of child psychiatry), and former president/CEO of the Community Mental Health Council, all in Chicago. He also serves as chair of psychiatry at Windsor University, St. Kitts.
Recently, a lot has been in the news about the increasing rates of suicide in all communities, including among African American youth, and two high-profile celebrities. Now that we have a CEO in the White House who has made the phrase “fake news” part of the national lexicon (and as a former CEO myself), I feel compelled to take a critical, clinical look at the way the suicide story has been reported.
CEOs tend to be unique people, and many of them are fond of hyperbole – as it promotes “followship” in employees and fosters business deals. I interpret fake news as the kind of information, or maybe spin is a better word, promulgated by CEOs.
While following a research letter published recently in JAMA Pediatrics – “Age-Related Racial Disparity in Suicide Rates Among U.S. Youths From 2001 Through 2015” (2018 May 21. doi: 10.001/jamapediatrics.2018.0399) – it occurred to me that this struck me as fake news. But as I thought about it, I realized that the conclusions in the research letter would be better characterized as perhaps misleading news. My basis for reaching those conclusions is rooted in the lessons I learned as a 2-year member of the Institute of Medicine’s Board on Neuroscience and Behavioral Health, Committee on Pathophysiology & Prevention of Adolescent & Adult Suicide. In fact, the report we produced was the first one referenced in the research letter. Unfortunately, however, the research letter’s authors seemed to miss the IOM report’s major take-away messages.
For example, the research letter authors compared the suicide rates among black children and white children in this way: “However, suicide rates increased from 1993 to 1997 and 2008 to 2012 among black children aged 5 to 11 years (from 1.36 to 2.54 per million) and decreased among white children of the same age (from 1.14 to 0.77 per million).” That sentence supports the conclusions of the IOM’s “Reducing Suicide” report, as it confirms that those are very low base rates. However, because the base rates are so low in most populations, it is difficult to determine scientifically whether a significant rise or decrease in rates occurred.
To quote page 377 of IOM report: “The base rate of completed suicide is sufficiently low to preclude all but the largest of studies. When such studies are performed, resultant comparisons are between extremely small and large groups of individuals (suicide completers versus non–suicide completers, or suicide attempters versus non–suicide attempters). Use of suicidal ideation as an outcome can increase incidence and alleviate the problem to some extent; however, it is unclear whether suicidal ideation is a strong predictor of suicide completion. Using both attempts and completions can confound the analysis since attempters may account for some of the suicides completed within the study period. Because the duration of the prevention studies is frequently too brief to collect sufficient data on the low frequency endpoints of suicide or suicide attempt, proximal measures such as changes in knowledge or attitude are used. Yet the predictive value of these variables is unconfirmed.”
Further, according to page 410 of the report: “As the statistical analysis above points out, at a suicide rate of 10 per 100,000 population, approximately 100,000 participants are needed to achieve statistical significance in an experimental context. In studying suicide among low-risk groups, the numbers needed are even greater.”
Let me break this down a bit. , because the numerator is so small and the dominator is so large. Let me put it this way – if the black female suicide rates are 2/100,000, and those rates quadrupled (sounds impressive, doesn’t it?) then there would be 8/100,000 black female suicides; the difference between 2 and 8 per 100,000 is not really a significant difference because the base-rates are so small. But to say the rates quadrupled sounds scary and impressive. “Figures don’t lie, but liars can figure.”
So, the premise of the research letter is whack.
I am not impressed that the rates of black children aged 5-7 increased from 1.36/1,000,000 to 2.54/1,000,000. I am not even sure those two numbers are significantly different, much less have clinical relevance. I have tried to make this point before, but it always gets lost by the hyperbolic press – which continues to yell about suicides in the United States rising by 30% or doubling, even quadrupling. The low base rates make drawing firm conclusions from this data like spitting into the ocean. I understand that one suicide is one suicide too many. But this is not science.
The characterizations about soaring U.S. suicide rates are not exactly fake news. Instead, I would call these interpretations misleading science and news.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former director of the Institute for Juvenile Research (the birthplace of child psychiatry), and former president/CEO of the Community Mental Health Council, all in Chicago. He also serves as chair of psychiatry at Windsor University, St. Kitts.
Recently, a lot has been in the news about the increasing rates of suicide in all communities, including among African American youth, and two high-profile celebrities. Now that we have a CEO in the White House who has made the phrase “fake news” part of the national lexicon (and as a former CEO myself), I feel compelled to take a critical, clinical look at the way the suicide story has been reported.
CEOs tend to be unique people, and many of them are fond of hyperbole – as it promotes “followship” in employees and fosters business deals. I interpret fake news as the kind of information, or maybe spin is a better word, promulgated by CEOs.
While following a research letter published recently in JAMA Pediatrics – “Age-Related Racial Disparity in Suicide Rates Among U.S. Youths From 2001 Through 2015” (2018 May 21. doi: 10.001/jamapediatrics.2018.0399) – it occurred to me that this struck me as fake news. But as I thought about it, I realized that the conclusions in the research letter would be better characterized as perhaps misleading news. My basis for reaching those conclusions is rooted in the lessons I learned as a 2-year member of the Institute of Medicine’s Board on Neuroscience and Behavioral Health, Committee on Pathophysiology & Prevention of Adolescent & Adult Suicide. In fact, the report we produced was the first one referenced in the research letter. Unfortunately, however, the research letter’s authors seemed to miss the IOM report’s major take-away messages.
For example, the research letter authors compared the suicide rates among black children and white children in this way: “However, suicide rates increased from 1993 to 1997 and 2008 to 2012 among black children aged 5 to 11 years (from 1.36 to 2.54 per million) and decreased among white children of the same age (from 1.14 to 0.77 per million).” That sentence supports the conclusions of the IOM’s “Reducing Suicide” report, as it confirms that those are very low base rates. However, because the base rates are so low in most populations, it is difficult to determine scientifically whether a significant rise or decrease in rates occurred.
To quote page 377 of IOM report: “The base rate of completed suicide is sufficiently low to preclude all but the largest of studies. When such studies are performed, resultant comparisons are between extremely small and large groups of individuals (suicide completers versus non–suicide completers, or suicide attempters versus non–suicide attempters). Use of suicidal ideation as an outcome can increase incidence and alleviate the problem to some extent; however, it is unclear whether suicidal ideation is a strong predictor of suicide completion. Using both attempts and completions can confound the analysis since attempters may account for some of the suicides completed within the study period. Because the duration of the prevention studies is frequently too brief to collect sufficient data on the low frequency endpoints of suicide or suicide attempt, proximal measures such as changes in knowledge or attitude are used. Yet the predictive value of these variables is unconfirmed.”
Further, according to page 410 of the report: “As the statistical analysis above points out, at a suicide rate of 10 per 100,000 population, approximately 100,000 participants are needed to achieve statistical significance in an experimental context. In studying suicide among low-risk groups, the numbers needed are even greater.”
Let me break this down a bit. , because the numerator is so small and the dominator is so large. Let me put it this way – if the black female suicide rates are 2/100,000, and those rates quadrupled (sounds impressive, doesn’t it?) then there would be 8/100,000 black female suicides; the difference between 2 and 8 per 100,000 is not really a significant difference because the base-rates are so small. But to say the rates quadrupled sounds scary and impressive. “Figures don’t lie, but liars can figure.”
So, the premise of the research letter is whack.
I am not impressed that the rates of black children aged 5-7 increased from 1.36/1,000,000 to 2.54/1,000,000. I am not even sure those two numbers are significantly different, much less have clinical relevance. I have tried to make this point before, but it always gets lost by the hyperbolic press – which continues to yell about suicides in the United States rising by 30% or doubling, even quadrupling. The low base rates make drawing firm conclusions from this data like spitting into the ocean. I understand that one suicide is one suicide too many. But this is not science.
The characterizations about soaring U.S. suicide rates are not exactly fake news. Instead, I would call these interpretations misleading science and news.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former director of the Institute for Juvenile Research (the birthplace of child psychiatry), and former president/CEO of the Community Mental Health Council, all in Chicago. He also serves as chair of psychiatry at Windsor University, St. Kitts.
Most Medicaid enrollees exempt from work requirements
Only 6% of the Medicaid population would be unlikely to qualify for an exemption from work requirements for “able-bodied adults” that states are in the process of being implementing, according to a new report from the Kaiser Family Foundation.
Another 15% were in fair or poor health or didn’t work because of illness or disability, 11% didn’t work because they were providing care for family members, and 6% were attending school, Kaiser wrote in an issue brief.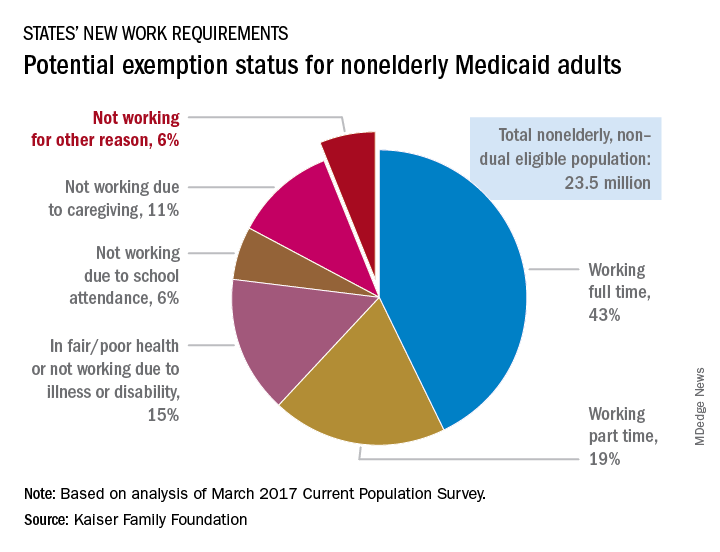
“This target population is much smaller than the groups of enrollees who are already working but would need to comply with new reporting requirements and those who could be exempt and would have to navigate an exemption process,” the Kaiser investigators said.
States will need to set up systems to deal with these issues, but many enrollees face barriers to complying. The waiver program in Arkansas – one of the first four states to receive permission to impose work requirements – “requires beneficiaries to set up an online account and use this account as the sole means of periodic reporting related to work requirements and exemptions,” they noted, but 30% of all nonelderly Medicaid adults say that they have never used a computer, 21% do not use the Internet, and 41% do not use email, based on analysis of 2016 National Health Interview Survey data.
Only 6% of the Medicaid population would be unlikely to qualify for an exemption from work requirements for “able-bodied adults” that states are in the process of being implementing, according to a new report from the Kaiser Family Foundation.
Another 15% were in fair or poor health or didn’t work because of illness or disability, 11% didn’t work because they were providing care for family members, and 6% were attending school, Kaiser wrote in an issue brief.
“This target population is much smaller than the groups of enrollees who are already working but would need to comply with new reporting requirements and those who could be exempt and would have to navigate an exemption process,” the Kaiser investigators said.
States will need to set up systems to deal with these issues, but many enrollees face barriers to complying. The waiver program in Arkansas – one of the first four states to receive permission to impose work requirements – “requires beneficiaries to set up an online account and use this account as the sole means of periodic reporting related to work requirements and exemptions,” they noted, but 30% of all nonelderly Medicaid adults say that they have never used a computer, 21% do not use the Internet, and 41% do not use email, based on analysis of 2016 National Health Interview Survey data.
Only 6% of the Medicaid population would be unlikely to qualify for an exemption from work requirements for “able-bodied adults” that states are in the process of being implementing, according to a new report from the Kaiser Family Foundation.
Another 15% were in fair or poor health or didn’t work because of illness or disability, 11% didn’t work because they were providing care for family members, and 6% were attending school, Kaiser wrote in an issue brief.
“This target population is much smaller than the groups of enrollees who are already working but would need to comply with new reporting requirements and those who could be exempt and would have to navigate an exemption process,” the Kaiser investigators said.
States will need to set up systems to deal with these issues, but many enrollees face barriers to complying. The waiver program in Arkansas – one of the first four states to receive permission to impose work requirements – “requires beneficiaries to set up an online account and use this account as the sole means of periodic reporting related to work requirements and exemptions,” they noted, but 30% of all nonelderly Medicaid adults say that they have never used a computer, 21% do not use the Internet, and 41% do not use email, based on analysis of 2016 National Health Interview Survey data.