User login
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
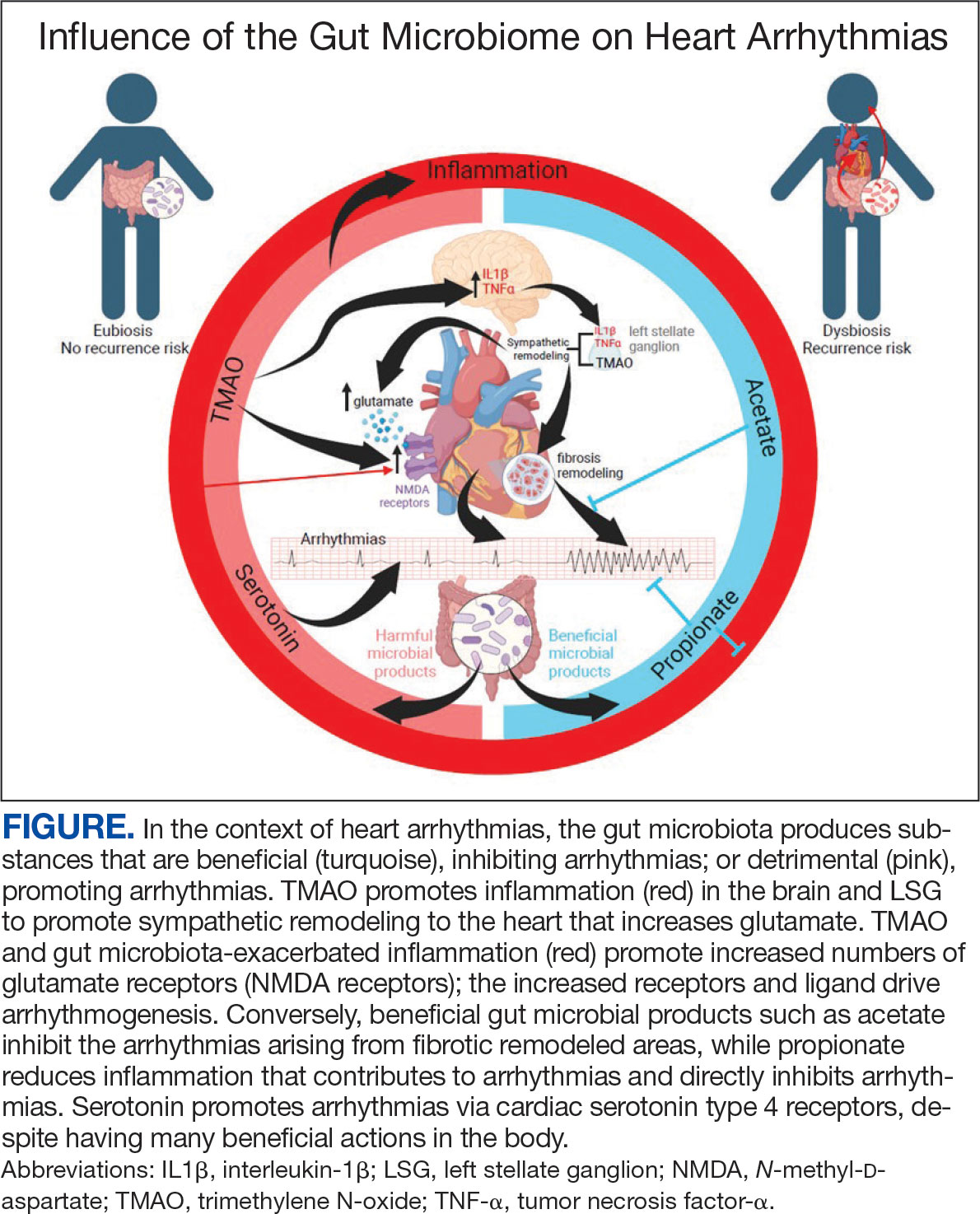
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12
Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
Zebrafish Provide the Keys to the Heart’s ‘Mini-Brain’
The heart’s “mini-brain” is independent and highly localized, according to researchers at the Karolinska Institutet in Stockholm, Sweden. The findings could lead to new research into arrhythmia, dementia, and Parkinson’s disease.
Although controlled by the brain, the heart has a separate, smaller intracardiac nervous system (IcNS) embedded within the superficial layers of the heart wall. Nicknamed the mini-brain by researchers decades ago, the IcNS was assumed to be a simple structure capable only of relaying simple information from the brain to the heart.
The neurons in the mini-brain, however, have been under-researched, said Konstantinos Ampatzis, principal researcher and assistant professor of neuroscience at the Karolinska Institutet. “Cardiologists know that neurons exist but never study them because their first concern is the cardiac muscle cells, or cardiomyocytes, that are responsible for the heartbeat,” he explained. “Neuroscientists understand and decode neurons but don’t know about neurons in the heart.”
Ampatzis’s team mapped the exact composition, organization, and function of neurons in the IcNS using zebrafish as an animal model. “The heart of the zebrafish is closer to that of humans than the mouse heart is,” he explained. “The heart rate of a zebrafish is exactly the same.”
Several techniques were used to characterize these neurons. Electrophysiology determined their function, and researchers at Columbia University in New York City helped identify their molecular signatures using single-cell RNA sequencing. Ampatzis and his team also analyzed neurotransmitters that the neurons release to communicate with each other. Researchers in Sweden and New York worked on this project in their spare time because they had no additional funding.
Ampatzis expected to see ganglions or relay neurons capable only of receiving or sending information. “But we found a very diverse set of neurons in a small network,” he said. Their findings included sympathetic, parasympathetic, and sensory neurons with apparent neurochemical and functional diversity. Most surprising was a subset of pacemaker neurons. “You cannot have a network that produces a rhythm without these neurons, and we didn’t expect exactly that, to be honest,” he said.
Pacemaker neurons are usually associated with so-called central pattern generator networks within the central nervous system. These independent, highly localized neuronal networks generate and control complex rhythmic behaviors such as respiration, mastication, urination, and ejaculation. “Most importantly, we found that this neuronal network works in an isolated heart, without brain information, and can change the rhythm of the heart and the regularity by itself,” said Ampatzis.
Further studies confirmed that neurons do not produce the rhythm, which is controlled by the cardiomyocytes. The neurons’ main function is to regulate the speed of the heartbeat. In other words, this smaller localized network acts as a kind of insurance system to safeguard the brain’s control of the heartbeat. “From an evolutionary perspective, I think that the system is like this because the heartbeat defines life,” Ampatzis added.
With the neurons of the heart mapped, medical researchers now have a toolbox of molecular markers, neurotransmitters, and other information on how such neurons function. These findings could become the basis of new research. It might be possible to investigate heart arrhythmia by modulating pacemaker neurons, Ampatzis suggested. “You could even repurpose or find specific drugs that can interfere with this local network of the heart,” he said, adding that this might be a less invasive option than is possible today.
Arrhythmia affects millions of people, said Oliver Guttmann, MD, a consultant cardiologist at The Wellington Hospital and honorary associate professor of cardiology at University College London, both in London, England. Beta-blockers remain the drug of choice for arrhythmia, but other options can be invasive. “We do ablations to try and burn or freeze certain areas of the heart to get rid of a rhythm because often this comes from hyperactive cells somewhere,” he said. Pacemakers and defibrillators are also needed to modulate dangerous rhythms. Innovation is focusing on making interventions far less invasive than they are today by creating smaller and smaller pacemakers, for example.
Moving from zebrafish to more complex mammalian systems will be the next big step, said David Paterson, DPhil, head of the Department of Physiology, Anatomy, and Genetics and honorary director of Burdon Sanderson Cardiac Science Centre at the University of Oxford in England. “If you can find the molecular road map of dysregulation, then that could be a potential target for a gene therapy or cell therapy or for neuromodulation therapy,” he explained. Interest in this field, which is sometimes called bioelectronic medicine, is mounting. “It’s like pharmaceutics, but there’s no drug. You’re tapping into the wiring of the nervous system,” he added.
More radical research pathways might look at ways to tackle neurodegenerative disorders from dementia to Parkinson’s disease. “If neurons die in the brain, then they die in the heart and can affect the rhythm of the heart,” said Ampatzis. But zebrafish neurons are now known to produce substances that induce a proliferation of stem cells in bones, skin, and even the nervous system. “We think those neurons of the heart could perhaps contribute to the regeneration of the heart,” he said.
Ampatzis, Guttmann, and Paterson reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
The heart’s “mini-brain” is independent and highly localized, according to researchers at the Karolinska Institutet in Stockholm, Sweden. The findings could lead to new research into arrhythmia, dementia, and Parkinson’s disease.
Although controlled by the brain, the heart has a separate, smaller intracardiac nervous system (IcNS) embedded within the superficial layers of the heart wall. Nicknamed the mini-brain by researchers decades ago, the IcNS was assumed to be a simple structure capable only of relaying simple information from the brain to the heart.
The neurons in the mini-brain, however, have been under-researched, said Konstantinos Ampatzis, principal researcher and assistant professor of neuroscience at the Karolinska Institutet. “Cardiologists know that neurons exist but never study them because their first concern is the cardiac muscle cells, or cardiomyocytes, that are responsible for the heartbeat,” he explained. “Neuroscientists understand and decode neurons but don’t know about neurons in the heart.”
Ampatzis’s team mapped the exact composition, organization, and function of neurons in the IcNS using zebrafish as an animal model. “The heart of the zebrafish is closer to that of humans than the mouse heart is,” he explained. “The heart rate of a zebrafish is exactly the same.”
Several techniques were used to characterize these neurons. Electrophysiology determined their function, and researchers at Columbia University in New York City helped identify their molecular signatures using single-cell RNA sequencing. Ampatzis and his team also analyzed neurotransmitters that the neurons release to communicate with each other. Researchers in Sweden and New York worked on this project in their spare time because they had no additional funding.
Ampatzis expected to see ganglions or relay neurons capable only of receiving or sending information. “But we found a very diverse set of neurons in a small network,” he said. Their findings included sympathetic, parasympathetic, and sensory neurons with apparent neurochemical and functional diversity. Most surprising was a subset of pacemaker neurons. “You cannot have a network that produces a rhythm without these neurons, and we didn’t expect exactly that, to be honest,” he said.
Pacemaker neurons are usually associated with so-called central pattern generator networks within the central nervous system. These independent, highly localized neuronal networks generate and control complex rhythmic behaviors such as respiration, mastication, urination, and ejaculation. “Most importantly, we found that this neuronal network works in an isolated heart, without brain information, and can change the rhythm of the heart and the regularity by itself,” said Ampatzis.
Further studies confirmed that neurons do not produce the rhythm, which is controlled by the cardiomyocytes. The neurons’ main function is to regulate the speed of the heartbeat. In other words, this smaller localized network acts as a kind of insurance system to safeguard the brain’s control of the heartbeat. “From an evolutionary perspective, I think that the system is like this because the heartbeat defines life,” Ampatzis added.
With the neurons of the heart mapped, medical researchers now have a toolbox of molecular markers, neurotransmitters, and other information on how such neurons function. These findings could become the basis of new research. It might be possible to investigate heart arrhythmia by modulating pacemaker neurons, Ampatzis suggested. “You could even repurpose or find specific drugs that can interfere with this local network of the heart,” he said, adding that this might be a less invasive option than is possible today.
Arrhythmia affects millions of people, said Oliver Guttmann, MD, a consultant cardiologist at The Wellington Hospital and honorary associate professor of cardiology at University College London, both in London, England. Beta-blockers remain the drug of choice for arrhythmia, but other options can be invasive. “We do ablations to try and burn or freeze certain areas of the heart to get rid of a rhythm because often this comes from hyperactive cells somewhere,” he said. Pacemakers and defibrillators are also needed to modulate dangerous rhythms. Innovation is focusing on making interventions far less invasive than they are today by creating smaller and smaller pacemakers, for example.
Moving from zebrafish to more complex mammalian systems will be the next big step, said David Paterson, DPhil, head of the Department of Physiology, Anatomy, and Genetics and honorary director of Burdon Sanderson Cardiac Science Centre at the University of Oxford in England. “If you can find the molecular road map of dysregulation, then that could be a potential target for a gene therapy or cell therapy or for neuromodulation therapy,” he explained. Interest in this field, which is sometimes called bioelectronic medicine, is mounting. “It’s like pharmaceutics, but there’s no drug. You’re tapping into the wiring of the nervous system,” he added.
More radical research pathways might look at ways to tackle neurodegenerative disorders from dementia to Parkinson’s disease. “If neurons die in the brain, then they die in the heart and can affect the rhythm of the heart,” said Ampatzis. But zebrafish neurons are now known to produce substances that induce a proliferation of stem cells in bones, skin, and even the nervous system. “We think those neurons of the heart could perhaps contribute to the regeneration of the heart,” he said.
Ampatzis, Guttmann, and Paterson reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
The heart’s “mini-brain” is independent and highly localized, according to researchers at the Karolinska Institutet in Stockholm, Sweden. The findings could lead to new research into arrhythmia, dementia, and Parkinson’s disease.
Although controlled by the brain, the heart has a separate, smaller intracardiac nervous system (IcNS) embedded within the superficial layers of the heart wall. Nicknamed the mini-brain by researchers decades ago, the IcNS was assumed to be a simple structure capable only of relaying simple information from the brain to the heart.
The neurons in the mini-brain, however, have been under-researched, said Konstantinos Ampatzis, principal researcher and assistant professor of neuroscience at the Karolinska Institutet. “Cardiologists know that neurons exist but never study them because their first concern is the cardiac muscle cells, or cardiomyocytes, that are responsible for the heartbeat,” he explained. “Neuroscientists understand and decode neurons but don’t know about neurons in the heart.”
Ampatzis’s team mapped the exact composition, organization, and function of neurons in the IcNS using zebrafish as an animal model. “The heart of the zebrafish is closer to that of humans than the mouse heart is,” he explained. “The heart rate of a zebrafish is exactly the same.”
Several techniques were used to characterize these neurons. Electrophysiology determined their function, and researchers at Columbia University in New York City helped identify their molecular signatures using single-cell RNA sequencing. Ampatzis and his team also analyzed neurotransmitters that the neurons release to communicate with each other. Researchers in Sweden and New York worked on this project in their spare time because they had no additional funding.
Ampatzis expected to see ganglions or relay neurons capable only of receiving or sending information. “But we found a very diverse set of neurons in a small network,” he said. Their findings included sympathetic, parasympathetic, and sensory neurons with apparent neurochemical and functional diversity. Most surprising was a subset of pacemaker neurons. “You cannot have a network that produces a rhythm without these neurons, and we didn’t expect exactly that, to be honest,” he said.
Pacemaker neurons are usually associated with so-called central pattern generator networks within the central nervous system. These independent, highly localized neuronal networks generate and control complex rhythmic behaviors such as respiration, mastication, urination, and ejaculation. “Most importantly, we found that this neuronal network works in an isolated heart, without brain information, and can change the rhythm of the heart and the regularity by itself,” said Ampatzis.
Further studies confirmed that neurons do not produce the rhythm, which is controlled by the cardiomyocytes. The neurons’ main function is to regulate the speed of the heartbeat. In other words, this smaller localized network acts as a kind of insurance system to safeguard the brain’s control of the heartbeat. “From an evolutionary perspective, I think that the system is like this because the heartbeat defines life,” Ampatzis added.
With the neurons of the heart mapped, medical researchers now have a toolbox of molecular markers, neurotransmitters, and other information on how such neurons function. These findings could become the basis of new research. It might be possible to investigate heart arrhythmia by modulating pacemaker neurons, Ampatzis suggested. “You could even repurpose or find specific drugs that can interfere with this local network of the heart,” he said, adding that this might be a less invasive option than is possible today.
Arrhythmia affects millions of people, said Oliver Guttmann, MD, a consultant cardiologist at The Wellington Hospital and honorary associate professor of cardiology at University College London, both in London, England. Beta-blockers remain the drug of choice for arrhythmia, but other options can be invasive. “We do ablations to try and burn or freeze certain areas of the heart to get rid of a rhythm because often this comes from hyperactive cells somewhere,” he said. Pacemakers and defibrillators are also needed to modulate dangerous rhythms. Innovation is focusing on making interventions far less invasive than they are today by creating smaller and smaller pacemakers, for example.
Moving from zebrafish to more complex mammalian systems will be the next big step, said David Paterson, DPhil, head of the Department of Physiology, Anatomy, and Genetics and honorary director of Burdon Sanderson Cardiac Science Centre at the University of Oxford in England. “If you can find the molecular road map of dysregulation, then that could be a potential target for a gene therapy or cell therapy or for neuromodulation therapy,” he explained. Interest in this field, which is sometimes called bioelectronic medicine, is mounting. “It’s like pharmaceutics, but there’s no drug. You’re tapping into the wiring of the nervous system,” he added.
More radical research pathways might look at ways to tackle neurodegenerative disorders from dementia to Parkinson’s disease. “If neurons die in the brain, then they die in the heart and can affect the rhythm of the heart,” said Ampatzis. But zebrafish neurons are now known to produce substances that induce a proliferation of stem cells in bones, skin, and even the nervous system. “We think those neurons of the heart could perhaps contribute to the regeneration of the heart,” he said.
Ampatzis, Guttmann, and Paterson reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
NT-proBNP May Predict Atrial Fibrillation Risk Early
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
REALIZE-K: A New Potassium Binder to Help Keep Spiro on Board
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
Goodbye CHADSVASc: Sex Complicates Stroke Risk Scoring in AF
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
SCD: Can Atrial Arrhythmias Predict Strokes?
TOPLINE:
METHODOLOGY:
- A total of 130 adult patients with SCD were included in the DREPACOEUR prospective registry from November 2018 to November 2022.
- The patients underwent a comprehensive cardiac evaluation, including 24-hour electrocardiogram monitoring, echocardiography, and laboratory tests.
- The primary endpoint was the occurrence of atrial arrhythmias, defined by excessive supraventricular ectopic activity or any recent history of atrial fibrillation.
- Patients with a history of stroke or transient ischemic attack were also included in the PCDREP prospective registry for further assessment.
- Written informed consent was collected from all participating patients, and the study was approved by the ethics committee.
TAKEAWAY:
- Atrial arrhythmias were found in 26% of patients with SCD, with a significant association with stroke history (P = .001).
- Age and left atrial volume were independently associated with atrial arrhythmias, with optimal cutoffs of 47 years and 55 mL/m2, respectively.
- Patients with atrial arrhythmias had higher diastolic blood pressure, worse kidney function, and higher NT pro-BNP levels than those without arrhythmias.
- Atrial arrhythmias were associated with an increased risk for stroke unrelated to cerebral vasculopathy or other defined causes (odds ratio, 6.6; P = .009).
“Atrial arrhythmias were found in 26% of patients with sickle cell anemia, with a significant association with stroke history,” wrote the authors of the study. In a commentary published concurrently, Jonathan Uniat, MD, of Children’s Hospital Los Angeles in California, wrote, “Early detection and treatment of atrial arrhythmias may help prevent strokes in this population.”
SOURCE:
The study was led by Thomas d’Humières, Henri Mondor Hospital in Créteil, France. It was published online on November 12 in Blood Advances.
LIMITATIONS:
This study was a pilot prospective study and was underpowered with atrial arrhythmias occurring in only 34 patients. The population was relatively old for sickle cell anemia (45 years), and the study was biased because patients were selected based on clinical criteria indicative of underlying cardiovascular abnormalities. The population was heterogeneous in terms of antiarrhythmic therapy, and overall, at an advanced stage of the disease with frequent organ complications.
DISCLOSURES:
The study was supported by grants from FHU-SENEC. Pablo Bartolucci received grants from Addmedica, the Fabre Foundation, Novartis, and Bluebird in the past 36 months; received consulting fees from Addmedica, Novartis, Roche, GBT, Bluebird, Emmaus, Hemanext, and Agios; received honoraria for lectures from Novartis, Addmedica, and Jazz Pharmaceuticals; and reported being a member of the Novartis steering committee and cofounder of Innovhem. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A total of 130 adult patients with SCD were included in the DREPACOEUR prospective registry from November 2018 to November 2022.
- The patients underwent a comprehensive cardiac evaluation, including 24-hour electrocardiogram monitoring, echocardiography, and laboratory tests.
- The primary endpoint was the occurrence of atrial arrhythmias, defined by excessive supraventricular ectopic activity or any recent history of atrial fibrillation.
- Patients with a history of stroke or transient ischemic attack were also included in the PCDREP prospective registry for further assessment.
- Written informed consent was collected from all participating patients, and the study was approved by the ethics committee.
TAKEAWAY:
- Atrial arrhythmias were found in 26% of patients with SCD, with a significant association with stroke history (P = .001).
- Age and left atrial volume were independently associated with atrial arrhythmias, with optimal cutoffs of 47 years and 55 mL/m2, respectively.
- Patients with atrial arrhythmias had higher diastolic blood pressure, worse kidney function, and higher NT pro-BNP levels than those without arrhythmias.
- Atrial arrhythmias were associated with an increased risk for stroke unrelated to cerebral vasculopathy or other defined causes (odds ratio, 6.6; P = .009).
“Atrial arrhythmias were found in 26% of patients with sickle cell anemia, with a significant association with stroke history,” wrote the authors of the study. In a commentary published concurrently, Jonathan Uniat, MD, of Children’s Hospital Los Angeles in California, wrote, “Early detection and treatment of atrial arrhythmias may help prevent strokes in this population.”
SOURCE:
The study was led by Thomas d’Humières, Henri Mondor Hospital in Créteil, France. It was published online on November 12 in Blood Advances.
LIMITATIONS:
This study was a pilot prospective study and was underpowered with atrial arrhythmias occurring in only 34 patients. The population was relatively old for sickle cell anemia (45 years), and the study was biased because patients were selected based on clinical criteria indicative of underlying cardiovascular abnormalities. The population was heterogeneous in terms of antiarrhythmic therapy, and overall, at an advanced stage of the disease with frequent organ complications.
DISCLOSURES:
The study was supported by grants from FHU-SENEC. Pablo Bartolucci received grants from Addmedica, the Fabre Foundation, Novartis, and Bluebird in the past 36 months; received consulting fees from Addmedica, Novartis, Roche, GBT, Bluebird, Emmaus, Hemanext, and Agios; received honoraria for lectures from Novartis, Addmedica, and Jazz Pharmaceuticals; and reported being a member of the Novartis steering committee and cofounder of Innovhem. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A total of 130 adult patients with SCD were included in the DREPACOEUR prospective registry from November 2018 to November 2022.
- The patients underwent a comprehensive cardiac evaluation, including 24-hour electrocardiogram monitoring, echocardiography, and laboratory tests.
- The primary endpoint was the occurrence of atrial arrhythmias, defined by excessive supraventricular ectopic activity or any recent history of atrial fibrillation.
- Patients with a history of stroke or transient ischemic attack were also included in the PCDREP prospective registry for further assessment.
- Written informed consent was collected from all participating patients, and the study was approved by the ethics committee.
TAKEAWAY:
- Atrial arrhythmias were found in 26% of patients with SCD, with a significant association with stroke history (P = .001).
- Age and left atrial volume were independently associated with atrial arrhythmias, with optimal cutoffs of 47 years and 55 mL/m2, respectively.
- Patients with atrial arrhythmias had higher diastolic blood pressure, worse kidney function, and higher NT pro-BNP levels than those without arrhythmias.
- Atrial arrhythmias were associated with an increased risk for stroke unrelated to cerebral vasculopathy or other defined causes (odds ratio, 6.6; P = .009).
“Atrial arrhythmias were found in 26% of patients with sickle cell anemia, with a significant association with stroke history,” wrote the authors of the study. In a commentary published concurrently, Jonathan Uniat, MD, of Children’s Hospital Los Angeles in California, wrote, “Early detection and treatment of atrial arrhythmias may help prevent strokes in this population.”
SOURCE:
The study was led by Thomas d’Humières, Henri Mondor Hospital in Créteil, France. It was published online on November 12 in Blood Advances.
LIMITATIONS:
This study was a pilot prospective study and was underpowered with atrial arrhythmias occurring in only 34 patients. The population was relatively old for sickle cell anemia (45 years), and the study was biased because patients were selected based on clinical criteria indicative of underlying cardiovascular abnormalities. The population was heterogeneous in terms of antiarrhythmic therapy, and overall, at an advanced stage of the disease with frequent organ complications.
DISCLOSURES:
The study was supported by grants from FHU-SENEC. Pablo Bartolucci received grants from Addmedica, the Fabre Foundation, Novartis, and Bluebird in the past 36 months; received consulting fees from Addmedica, Novartis, Roche, GBT, Bluebird, Emmaus, Hemanext, and Agios; received honoraria for lectures from Novartis, Addmedica, and Jazz Pharmaceuticals; and reported being a member of the Novartis steering committee and cofounder of Innovhem. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
AF Burden Increases Around Time of COPD Hospitalizations
BOSTON — Patients with COPD who have exacerbations requiring hospitalization should be monitored for cardiac arrhythmias, investigators said.
This recommendation is based on results of a study of medical records showing that among more than 20,000 hospitalizations for patients with COPD without concurrent heart failure (HF), 40% patients had at least 6 minutes of daily atrial fibrillation (AF) burden, and nearly half of these patients had at least an hour of daily AF burden; patients with COPD and concurrent HF had similar daily AF burdens, reported Trent Fischer, MD, MS, senior principal scientist at Medtronic in Minneapolis.
“We can conclude that AF burden increases in the weeks after a hospitalization for COPD if they don’t have a concurrent diagnosis of heart failure. Also, having concurrent heart failure increases the risk of atrial fibrillation and increases the atrial fibrillation burden around the time of COPD hospitalization,” he said in a rapid-fire oral abstract session at the CHEST Annual Meeting.
The findings indicated a need for increased vigilance for AF around the time of a serious COPD exacerbation and may explain at least some of the increased risks for stroke observed in patients who are hospitalized for COPD exacerbations, he said.
Retrospective Study
They drew data from 2007 through 2021 on patients with implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, and implantable cardiac monitors, using the Optum de-identified electronic health record dataset linked with Medtronic’s CareLink database to conduct a retrospective analysis.
They looked at admissions for COPD linked to available device diagnostic parameters between 30 days prior to and 60 days after admission for COPD.
They identified a total of 20,056 COPD hospitalizations for patients with concurrent HF and 3877 for those without HF.
Among patients with HF, 43% had a daily AF burden of at least 6 minutes, and 22% had at least 1 hour of irregular rhythms. Among patients without HF, 40% had at least 6 minutes of irregular rhythms daily, and 18% had at least 1 hour.
Among patients with HF, the daily average AF burden increased from a baseline of 158 min/d 30 days before an admission to 170 min/d at admission, returning to baseline by 20 days after hospitalization.
For patients without HF, the AF burden increased from 107 min/d at baseline to 113 min/d during hospitalization and returned to baseline by 20 days after hospitalization.
Confounding Factor?
In the Q&A, session moderator Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, said that when patients with HF get admitted for COPD exacerbations, their HF typically worsens and asked Dr. Fischer how he could tell the difference.
“I know there’s a lot of interaction between heart failure and COPD. They’re well-know comorbidities, and the exacerbation of one can bring on worsening of the other. At least with this database, we can’t really tease out any sort of differences,” Dr. Fischer replied.
“I think that a diagnosis of COPD exacerbation is pretty well laid out, but it’s sometimes difficult to separate worsening of heart failure in these patients, and often these patients get treated for both problems. It’s clear that it’s the heart failure patients who are having more atrial fibrillation episodes, which is not surprising, but the question is how much is the COPD exacerbation contributing to the atrial fibrillation?” said Dr. Sundar.
The study was supported by Medtronic. Dr. Fischer is employed by the company. Dr. Sundar reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with COPD who have exacerbations requiring hospitalization should be monitored for cardiac arrhythmias, investigators said.
This recommendation is based on results of a study of medical records showing that among more than 20,000 hospitalizations for patients with COPD without concurrent heart failure (HF), 40% patients had at least 6 minutes of daily atrial fibrillation (AF) burden, and nearly half of these patients had at least an hour of daily AF burden; patients with COPD and concurrent HF had similar daily AF burdens, reported Trent Fischer, MD, MS, senior principal scientist at Medtronic in Minneapolis.
“We can conclude that AF burden increases in the weeks after a hospitalization for COPD if they don’t have a concurrent diagnosis of heart failure. Also, having concurrent heart failure increases the risk of atrial fibrillation and increases the atrial fibrillation burden around the time of COPD hospitalization,” he said in a rapid-fire oral abstract session at the CHEST Annual Meeting.
The findings indicated a need for increased vigilance for AF around the time of a serious COPD exacerbation and may explain at least some of the increased risks for stroke observed in patients who are hospitalized for COPD exacerbations, he said.
Retrospective Study
They drew data from 2007 through 2021 on patients with implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, and implantable cardiac monitors, using the Optum de-identified electronic health record dataset linked with Medtronic’s CareLink database to conduct a retrospective analysis.
They looked at admissions for COPD linked to available device diagnostic parameters between 30 days prior to and 60 days after admission for COPD.
They identified a total of 20,056 COPD hospitalizations for patients with concurrent HF and 3877 for those without HF.
Among patients with HF, 43% had a daily AF burden of at least 6 minutes, and 22% had at least 1 hour of irregular rhythms. Among patients without HF, 40% had at least 6 minutes of irregular rhythms daily, and 18% had at least 1 hour.
Among patients with HF, the daily average AF burden increased from a baseline of 158 min/d 30 days before an admission to 170 min/d at admission, returning to baseline by 20 days after hospitalization.
For patients without HF, the AF burden increased from 107 min/d at baseline to 113 min/d during hospitalization and returned to baseline by 20 days after hospitalization.
Confounding Factor?
In the Q&A, session moderator Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, said that when patients with HF get admitted for COPD exacerbations, their HF typically worsens and asked Dr. Fischer how he could tell the difference.
“I know there’s a lot of interaction between heart failure and COPD. They’re well-know comorbidities, and the exacerbation of one can bring on worsening of the other. At least with this database, we can’t really tease out any sort of differences,” Dr. Fischer replied.
“I think that a diagnosis of COPD exacerbation is pretty well laid out, but it’s sometimes difficult to separate worsening of heart failure in these patients, and often these patients get treated for both problems. It’s clear that it’s the heart failure patients who are having more atrial fibrillation episodes, which is not surprising, but the question is how much is the COPD exacerbation contributing to the atrial fibrillation?” said Dr. Sundar.
The study was supported by Medtronic. Dr. Fischer is employed by the company. Dr. Sundar reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with COPD who have exacerbations requiring hospitalization should be monitored for cardiac arrhythmias, investigators said.
This recommendation is based on results of a study of medical records showing that among more than 20,000 hospitalizations for patients with COPD without concurrent heart failure (HF), 40% patients had at least 6 minutes of daily atrial fibrillation (AF) burden, and nearly half of these patients had at least an hour of daily AF burden; patients with COPD and concurrent HF had similar daily AF burdens, reported Trent Fischer, MD, MS, senior principal scientist at Medtronic in Minneapolis.
“We can conclude that AF burden increases in the weeks after a hospitalization for COPD if they don’t have a concurrent diagnosis of heart failure. Also, having concurrent heart failure increases the risk of atrial fibrillation and increases the atrial fibrillation burden around the time of COPD hospitalization,” he said in a rapid-fire oral abstract session at the CHEST Annual Meeting.
The findings indicated a need for increased vigilance for AF around the time of a serious COPD exacerbation and may explain at least some of the increased risks for stroke observed in patients who are hospitalized for COPD exacerbations, he said.
Retrospective Study
They drew data from 2007 through 2021 on patients with implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, and implantable cardiac monitors, using the Optum de-identified electronic health record dataset linked with Medtronic’s CareLink database to conduct a retrospective analysis.
They looked at admissions for COPD linked to available device diagnostic parameters between 30 days prior to and 60 days after admission for COPD.
They identified a total of 20,056 COPD hospitalizations for patients with concurrent HF and 3877 for those without HF.
Among patients with HF, 43% had a daily AF burden of at least 6 minutes, and 22% had at least 1 hour of irregular rhythms. Among patients without HF, 40% had at least 6 minutes of irregular rhythms daily, and 18% had at least 1 hour.
Among patients with HF, the daily average AF burden increased from a baseline of 158 min/d 30 days before an admission to 170 min/d at admission, returning to baseline by 20 days after hospitalization.
For patients without HF, the AF burden increased from 107 min/d at baseline to 113 min/d during hospitalization and returned to baseline by 20 days after hospitalization.
Confounding Factor?
In the Q&A, session moderator Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, said that when patients with HF get admitted for COPD exacerbations, their HF typically worsens and asked Dr. Fischer how he could tell the difference.
“I know there’s a lot of interaction between heart failure and COPD. They’re well-know comorbidities, and the exacerbation of one can bring on worsening of the other. At least with this database, we can’t really tease out any sort of differences,” Dr. Fischer replied.
“I think that a diagnosis of COPD exacerbation is pretty well laid out, but it’s sometimes difficult to separate worsening of heart failure in these patients, and often these patients get treated for both problems. It’s clear that it’s the heart failure patients who are having more atrial fibrillation episodes, which is not surprising, but the question is how much is the COPD exacerbation contributing to the atrial fibrillation?” said Dr. Sundar.
The study was supported by Medtronic. Dr. Fischer is employed by the company. Dr. Sundar reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CHEST 2024
The Rising Tide of Atrial Fibrillation: Is Primary Care Ready?
The incidence of atrial fibrillation (AF) is on the rise, and recent joint guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) stress the role of primary care clinicians in prevention and management.
One in three White and one in five Black Americans will develop AF in their lifetime, and the projected number of individuals diagnosed with AF in the United States is expected to double by 2050.
Cardiologists who spoke to Medscape Medical News said primary care clinicians can help control AF by focusing on diabetes and hypertension, along with lifestyle factors such as diet, exercise, and alcohol intake.
“It’s not just a rhythm abnormality, but a complex disease that needs to be addressed in a multidisciplinary, holistic way,” said Jose Joglar, MD, a professor in the Department of Internal Medicine at the UT Southwestern Medical Center in Dallas and lead author of the guidelines.
Joglar said primary care clinicians can play an important role in counseling on lifestyle changes for patients with the most common etiologies such as poorly controlled hypertension, diabetes, and obesity.
The Primary Care Physicians ABCs: Risk Factors and Comorbidities
“As a primary care physician or as a cardiologist, I often think that if I do these things, I’m going to help with a lot of conditions, not just atrial fibrillation,” said Manesh Patel, MD, chief of the Divisions of Cardiology and Clinical Pharmacology at the Duke University School of Medicine in Durham, North Carolina.
Lifestyle choices such as sleeping habits can play a big part in AF outcomes. Although the guidelines specifically address obstructive sleep apnea as a risk factor, he said more data are needed on the effect of sleep hygiene — getting 8 hours of sleep a night — a goal few people attain.
“What we do know is people that can routinely try to go to sleep and sleep with some regularity seem to have less cardiovascular risk,” Patel said.
Although existing data are limited, literature reviews have found evidence that sleep disruptions, sleep duration, circadian rhythm, and insomnia are associated with heart disease, independent of obstructive sleep apnea.
Use of alcohol should also be discussed with patients, as many are unaware of the effects of the drug on cardiovascular disease, said Joglar, who is also the program director of the Clinical Cardiac Electrophysiology Fellowship program at the UT Southwestern Medical Center.
“Doctors can inform the patient that this is not a judgment call but simple medical fact,” he said.
Joglar also said many physicians need to become educated on a common misconception.
“Every time a patient develops palpitations or atrial fibrillation, the first thing every patient tells me is, I quit drinking coffee,” Joglar said.
However, as the guidelines point out, the link between caffeine and AF is uncertain at best.
Preventing AF
A newer class of drugs may help clinicians manage comorbidities that contribute to AF, such as hypertension, sleep apnea, and obesity, said John Mandrola, MD, an electrophysiologist in Louisville, Kentucky, who hosts This Week in Cardiology on Medscape.
Although originally approved for treatment of diabetes, sodium-glucose cotransporter-2 inhibitors are also approved for management of heart failure. Mandrola started prescribing these drugs 2 years ago for patients, given the links of both conditions with AF.
“I think the next frontier for us in cardiology and AF management will be the GLP-1 agonists,” Mandrola said. He hasn’t started prescribing these drugs for his patients yet but said they will likely play a role in the management of patients with AF with the common constellation of comorbidities such as obesity, hypertension, and sleep apnea.
“The GLP-1 agonists have a really good chance of competing with AF ablation for rhythm control over the long term,” he said.
Decisions, Decisions: Stroke Risk Scoring Systems
The risk for stroke varies widely among patients with AF, so primary care clinicians can pick among several scoring systems to estimate the risk for stroke and guide the decision on whether to initiate anticoagulation therapy.
The ACC/AHA guidelines do not state a preference for a particular instrument. The Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke, Vascular disease, Sex (CHA2DS2-VASc) score is the most widely used and validated instrument, Joglar said. He usually recommends anticoagulation if the CHA2DS2-VASc score is > 2, dependent on individual patient factors.
“If you have a CHA2DS2-VASc score of 1, and you only had one episode of AF for a few hours a year ago, then your risk of stroke is not as high as somebody who has a score of 1 but has more frequent or persistent AF,” Joglar said.
None of the systems is perfect at predicting risk for stroke, so clinicians should discuss options with patients.
“The real message is, are you talking about the risk of stroke and systemic embolism to your patient, so that the patient understands that risk?” he said.
Patel also said measuring creatine clearance can be analogous to using an instrument like CHA2DS2-VASc.
“I often think about renal disease as a very good risk marker and something that does elevate your risk,” he said.
Which Anticoagulant?
Although the ACC/AHA guidelines still recommend warfarin for patients with AF with mechanical heart valves or moderate to severe rheumatic fever, direct oral anticoagulants (DOACs) are the first-line therapy for all other patients with AF.
In terms of which DOACs to use, the differences are subtle, according to Patel.
“I don’t know that they’re that different from each other,” he said. “All of the new drugs are better than warfarin by far.”
Patel pointed out that dabigatran at 150 mg is the only DOAC shown to reduce the incidence of ischemic stroke. For patients with renal dysfunction, he has a slight preference for a 15-mg dose of rivaroxaban.
Mandrola said he mainly prescribes apixaban and rivaroxaban, the latter of which requires only once a day dosing.
“We stopped using dabigatran because 10% of people get gastrointestinal upset,” he said.
Although studies suggest aspirin is less effective than either warfarin or DOACs for the prevention of stroke, Joglar said he still sees patients who come to him after being prescribed low-dose aspirin from primary care clinicians.
“We made it very clear that it should not be recommended just for mitigating stroke risk in atrial fibrillation,” Joglar said. “You could use it if the patient has another indication, such as a prior heart attack.”
Does My Patient Have to Be in Normal Sinus Rhythm?
The new guidelines present evidence maintaining sinus rhythm should be favored over controlling heart rate for managing AF.
“We’ve focused on rhythm control as a better strategy, especially catheter ablation, which seems to be particularly effective in parallel to lifestyle interventions and management of comorbidities,” Joglar said. Rhythm control is of particular benefit for patients with AF triggered by heart failure. Control of rhythm in these patients has been shown to improve multiple outcomes such as ejection fraction, symptoms, and survival.
Patel said as a patient’s symptoms increase, the more likely a clinician will be able to control sinus rhythm. Some patients do not notice their arrhythmia, but others feel dizzy or have chest pain.
“The less symptomatic the patient is, the more likely they’re going to tolerate it, especially if they’re older, and it’s hard to get them into sinus rhythm,” Patel said.
When to Refer for Catheter Ablation?
The new guidelines upgraded the recommendation for catheter ablation to class I (strong recommendation) for patients with symptomatic AF in whom anti-arrhythmic therapy is unsuccessful, not tolerated, or contraindicated; patients with symptomatic paroxysmal AF (typically younger patients with few comorbidities); and patients with symptomatic or clinically significant atrial flutter. The previous iteration recommended trying drug therapy first.
Multiple randomized clinical trials have demonstrated the effectiveness of catheter ablation.
“In somebody who is younger, with a healthy heart, the 1-year success rate of the procedure might be about 70%,” Joglar said. While 70% of patients receiving a catheter have no AF episodes in the following year, Joglar said 20%-25% of those who do have recurrences will experience fewer or shorter episodes.
Conversations about rate vs rhythm control and whether to pursue catheter ablation often come down to preference, Patel said. He would tend to intervene earlier using ablation in patients with heart failure or those experiencing symptoms of AF who cannot be controlled with a heart rate < 100 beats/min.
But he said he prefers using medication for rate control in many of his patients who are older, have chronic AF, and do not have heart failure.
Mandrola takes a more conservative approach, reserving catheter ablation for patients in whom risk factor management and anti-arrhythmic drugs have not been successful.
“In my hospital, it’s done for patients who have symptomatic AF that’s really impacting their quality of life,” he said. But for those with fewer symptoms, his advice is to provide education, reassurance, and time because AF can resolve on its own.
What About Data From Implantables and Wearables?
The guidelines provide an algorithm for when to treat non-symptomatic atrial high-rate episodes detected by a cardiovascular implantable electronic device such as a pacemaker or defibrillator. Episodes less than 5 minutes can be ignored, while treatment could be considered for those with episodes lasting 5 minutes up to 24 hours with a CHA2DS2-VASc score ≥ 3, or lasting longer than 24 hours with a CHA2DS2-VASc score ≥ 2.
But whether anticoagulation improves outcomes is unclear.
“That is a $64,000 question,” Mandrola said. “I would bet every day I get a notification in the electronic health record that says Mr. Smith had 2 hours of AFib 2 weeks ago.”
He also hears from patients who report their Apple Watch has detected an episode of AF.
Mandrola cited evidence from two recent studies of patients who had an atrial high-rate episode longer than 6 minutes detected by implantable devices. The NOAH-AFNET 6 trial randomized patients over 65 years with one or more risk factors for stroke to receive a DOAC or placebo, while the ARTESIA trial used similar inclusion criteria to assign patients to receive either DOAC or aspirin. Both studies reported modest reductions in stroke that were outweighed by a higher incidence of major bleeding in the group receiving anticoagulation.
Shared decision-making should play a role in deciding how aggressively to treat episodes of AF detected by implantable or wearable devices.
He said some patients fear having a stroke, while others are adamantly opposed to taking an anticoagulant.
For patients who present with a documented episode of AF but who otherwise have no symptoms, Patel said clinicians should consider risk for stroke and frequency and duration of episodes.
“One way clinicians should be thinking about it is, the more risk factors they have, the lower burden of AF I need to treat,” Patel said. Even for patients who are having only short episodes of AF, he has a low threshold for recommending an anticoagulation drug if the patient’s CHA2DS2-VASc score is high.
Patel reported research grants from Bayer, Novartis, Idorsia, NHLBI, and Janssen Pharmaceuticals and served as a consultant on the advisory boards of Bayer, Janssen Pharmaceuticals, and Esperion Therapeutics.
Joglar and Mandrola had no disclosures.
A version of this article appeared on Medscape.com.
The incidence of atrial fibrillation (AF) is on the rise, and recent joint guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) stress the role of primary care clinicians in prevention and management.
One in three White and one in five Black Americans will develop AF in their lifetime, and the projected number of individuals diagnosed with AF in the United States is expected to double by 2050.
Cardiologists who spoke to Medscape Medical News said primary care clinicians can help control AF by focusing on diabetes and hypertension, along with lifestyle factors such as diet, exercise, and alcohol intake.
“It’s not just a rhythm abnormality, but a complex disease that needs to be addressed in a multidisciplinary, holistic way,” said Jose Joglar, MD, a professor in the Department of Internal Medicine at the UT Southwestern Medical Center in Dallas and lead author of the guidelines.
Joglar said primary care clinicians can play an important role in counseling on lifestyle changes for patients with the most common etiologies such as poorly controlled hypertension, diabetes, and obesity.
The Primary Care Physicians ABCs: Risk Factors and Comorbidities
“As a primary care physician or as a cardiologist, I often think that if I do these things, I’m going to help with a lot of conditions, not just atrial fibrillation,” said Manesh Patel, MD, chief of the Divisions of Cardiology and Clinical Pharmacology at the Duke University School of Medicine in Durham, North Carolina.
Lifestyle choices such as sleeping habits can play a big part in AF outcomes. Although the guidelines specifically address obstructive sleep apnea as a risk factor, he said more data are needed on the effect of sleep hygiene — getting 8 hours of sleep a night — a goal few people attain.
“What we do know is people that can routinely try to go to sleep and sleep with some regularity seem to have less cardiovascular risk,” Patel said.
Although existing data are limited, literature reviews have found evidence that sleep disruptions, sleep duration, circadian rhythm, and insomnia are associated with heart disease, independent of obstructive sleep apnea.
Use of alcohol should also be discussed with patients, as many are unaware of the effects of the drug on cardiovascular disease, said Joglar, who is also the program director of the Clinical Cardiac Electrophysiology Fellowship program at the UT Southwestern Medical Center.
“Doctors can inform the patient that this is not a judgment call but simple medical fact,” he said.
Joglar also said many physicians need to become educated on a common misconception.
“Every time a patient develops palpitations or atrial fibrillation, the first thing every patient tells me is, I quit drinking coffee,” Joglar said.
However, as the guidelines point out, the link between caffeine and AF is uncertain at best.
Preventing AF
A newer class of drugs may help clinicians manage comorbidities that contribute to AF, such as hypertension, sleep apnea, and obesity, said John Mandrola, MD, an electrophysiologist in Louisville, Kentucky, who hosts This Week in Cardiology on Medscape.
Although originally approved for treatment of diabetes, sodium-glucose cotransporter-2 inhibitors are also approved for management of heart failure. Mandrola started prescribing these drugs 2 years ago for patients, given the links of both conditions with AF.
“I think the next frontier for us in cardiology and AF management will be the GLP-1 agonists,” Mandrola said. He hasn’t started prescribing these drugs for his patients yet but said they will likely play a role in the management of patients with AF with the common constellation of comorbidities such as obesity, hypertension, and sleep apnea.
“The GLP-1 agonists have a really good chance of competing with AF ablation for rhythm control over the long term,” he said.
Decisions, Decisions: Stroke Risk Scoring Systems
The risk for stroke varies widely among patients with AF, so primary care clinicians can pick among several scoring systems to estimate the risk for stroke and guide the decision on whether to initiate anticoagulation therapy.
The ACC/AHA guidelines do not state a preference for a particular instrument. The Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke, Vascular disease, Sex (CHA2DS2-VASc) score is the most widely used and validated instrument, Joglar said. He usually recommends anticoagulation if the CHA2DS2-VASc score is > 2, dependent on individual patient factors.
“If you have a CHA2DS2-VASc score of 1, and you only had one episode of AF for a few hours a year ago, then your risk of stroke is not as high as somebody who has a score of 1 but has more frequent or persistent AF,” Joglar said.
None of the systems is perfect at predicting risk for stroke, so clinicians should discuss options with patients.
“The real message is, are you talking about the risk of stroke and systemic embolism to your patient, so that the patient understands that risk?” he said.
Patel also said measuring creatine clearance can be analogous to using an instrument like CHA2DS2-VASc.
“I often think about renal disease as a very good risk marker and something that does elevate your risk,” he said.
Which Anticoagulant?
Although the ACC/AHA guidelines still recommend warfarin for patients with AF with mechanical heart valves or moderate to severe rheumatic fever, direct oral anticoagulants (DOACs) are the first-line therapy for all other patients with AF.
In terms of which DOACs to use, the differences are subtle, according to Patel.
“I don’t know that they’re that different from each other,” he said. “All of the new drugs are better than warfarin by far.”
Patel pointed out that dabigatran at 150 mg is the only DOAC shown to reduce the incidence of ischemic stroke. For patients with renal dysfunction, he has a slight preference for a 15-mg dose of rivaroxaban.
Mandrola said he mainly prescribes apixaban and rivaroxaban, the latter of which requires only once a day dosing.
“We stopped using dabigatran because 10% of people get gastrointestinal upset,” he said.
Although studies suggest aspirin is less effective than either warfarin or DOACs for the prevention of stroke, Joglar said he still sees patients who come to him after being prescribed low-dose aspirin from primary care clinicians.
“We made it very clear that it should not be recommended just for mitigating stroke risk in atrial fibrillation,” Joglar said. “You could use it if the patient has another indication, such as a prior heart attack.”
Does My Patient Have to Be in Normal Sinus Rhythm?
The new guidelines present evidence maintaining sinus rhythm should be favored over controlling heart rate for managing AF.
“We’ve focused on rhythm control as a better strategy, especially catheter ablation, which seems to be particularly effective in parallel to lifestyle interventions and management of comorbidities,” Joglar said. Rhythm control is of particular benefit for patients with AF triggered by heart failure. Control of rhythm in these patients has been shown to improve multiple outcomes such as ejection fraction, symptoms, and survival.
Patel said as a patient’s symptoms increase, the more likely a clinician will be able to control sinus rhythm. Some patients do not notice their arrhythmia, but others feel dizzy or have chest pain.
“The less symptomatic the patient is, the more likely they’re going to tolerate it, especially if they’re older, and it’s hard to get them into sinus rhythm,” Patel said.
When to Refer for Catheter Ablation?
The new guidelines upgraded the recommendation for catheter ablation to class I (strong recommendation) for patients with symptomatic AF in whom anti-arrhythmic therapy is unsuccessful, not tolerated, or contraindicated; patients with symptomatic paroxysmal AF (typically younger patients with few comorbidities); and patients with symptomatic or clinically significant atrial flutter. The previous iteration recommended trying drug therapy first.
Multiple randomized clinical trials have demonstrated the effectiveness of catheter ablation.
“In somebody who is younger, with a healthy heart, the 1-year success rate of the procedure might be about 70%,” Joglar said. While 70% of patients receiving a catheter have no AF episodes in the following year, Joglar said 20%-25% of those who do have recurrences will experience fewer or shorter episodes.
Conversations about rate vs rhythm control and whether to pursue catheter ablation often come down to preference, Patel said. He would tend to intervene earlier using ablation in patients with heart failure or those experiencing symptoms of AF who cannot be controlled with a heart rate < 100 beats/min.
But he said he prefers using medication for rate control in many of his patients who are older, have chronic AF, and do not have heart failure.
Mandrola takes a more conservative approach, reserving catheter ablation for patients in whom risk factor management and anti-arrhythmic drugs have not been successful.
“In my hospital, it’s done for patients who have symptomatic AF that’s really impacting their quality of life,” he said. But for those with fewer symptoms, his advice is to provide education, reassurance, and time because AF can resolve on its own.
What About Data From Implantables and Wearables?
The guidelines provide an algorithm for when to treat non-symptomatic atrial high-rate episodes detected by a cardiovascular implantable electronic device such as a pacemaker or defibrillator. Episodes less than 5 minutes can be ignored, while treatment could be considered for those with episodes lasting 5 minutes up to 24 hours with a CHA2DS2-VASc score ≥ 3, or lasting longer than 24 hours with a CHA2DS2-VASc score ≥ 2.
But whether anticoagulation improves outcomes is unclear.
“That is a $64,000 question,” Mandrola said. “I would bet every day I get a notification in the electronic health record that says Mr. Smith had 2 hours of AFib 2 weeks ago.”
He also hears from patients who report their Apple Watch has detected an episode of AF.
Mandrola cited evidence from two recent studies of patients who had an atrial high-rate episode longer than 6 minutes detected by implantable devices. The NOAH-AFNET 6 trial randomized patients over 65 years with one or more risk factors for stroke to receive a DOAC or placebo, while the ARTESIA trial used similar inclusion criteria to assign patients to receive either DOAC or aspirin. Both studies reported modest reductions in stroke that were outweighed by a higher incidence of major bleeding in the group receiving anticoagulation.
Shared decision-making should play a role in deciding how aggressively to treat episodes of AF detected by implantable or wearable devices.
He said some patients fear having a stroke, while others are adamantly opposed to taking an anticoagulant.
For patients who present with a documented episode of AF but who otherwise have no symptoms, Patel said clinicians should consider risk for stroke and frequency and duration of episodes.
“One way clinicians should be thinking about it is, the more risk factors they have, the lower burden of AF I need to treat,” Patel said. Even for patients who are having only short episodes of AF, he has a low threshold for recommending an anticoagulation drug if the patient’s CHA2DS2-VASc score is high.
Patel reported research grants from Bayer, Novartis, Idorsia, NHLBI, and Janssen Pharmaceuticals and served as a consultant on the advisory boards of Bayer, Janssen Pharmaceuticals, and Esperion Therapeutics.
Joglar and Mandrola had no disclosures.
A version of this article appeared on Medscape.com.
The incidence of atrial fibrillation (AF) is on the rise, and recent joint guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) stress the role of primary care clinicians in prevention and management.
One in three White and one in five Black Americans will develop AF in their lifetime, and the projected number of individuals diagnosed with AF in the United States is expected to double by 2050.
Cardiologists who spoke to Medscape Medical News said primary care clinicians can help control AF by focusing on diabetes and hypertension, along with lifestyle factors such as diet, exercise, and alcohol intake.
“It’s not just a rhythm abnormality, but a complex disease that needs to be addressed in a multidisciplinary, holistic way,” said Jose Joglar, MD, a professor in the Department of Internal Medicine at the UT Southwestern Medical Center in Dallas and lead author of the guidelines.
Joglar said primary care clinicians can play an important role in counseling on lifestyle changes for patients with the most common etiologies such as poorly controlled hypertension, diabetes, and obesity.
The Primary Care Physicians ABCs: Risk Factors and Comorbidities
“As a primary care physician or as a cardiologist, I often think that if I do these things, I’m going to help with a lot of conditions, not just atrial fibrillation,” said Manesh Patel, MD, chief of the Divisions of Cardiology and Clinical Pharmacology at the Duke University School of Medicine in Durham, North Carolina.
Lifestyle choices such as sleeping habits can play a big part in AF outcomes. Although the guidelines specifically address obstructive sleep apnea as a risk factor, he said more data are needed on the effect of sleep hygiene — getting 8 hours of sleep a night — a goal few people attain.
“What we do know is people that can routinely try to go to sleep and sleep with some regularity seem to have less cardiovascular risk,” Patel said.
Although existing data are limited, literature reviews have found evidence that sleep disruptions, sleep duration, circadian rhythm, and insomnia are associated with heart disease, independent of obstructive sleep apnea.
Use of alcohol should also be discussed with patients, as many are unaware of the effects of the drug on cardiovascular disease, said Joglar, who is also the program director of the Clinical Cardiac Electrophysiology Fellowship program at the UT Southwestern Medical Center.
“Doctors can inform the patient that this is not a judgment call but simple medical fact,” he said.
Joglar also said many physicians need to become educated on a common misconception.
“Every time a patient develops palpitations or atrial fibrillation, the first thing every patient tells me is, I quit drinking coffee,” Joglar said.
However, as the guidelines point out, the link between caffeine and AF is uncertain at best.
Preventing AF
A newer class of drugs may help clinicians manage comorbidities that contribute to AF, such as hypertension, sleep apnea, and obesity, said John Mandrola, MD, an electrophysiologist in Louisville, Kentucky, who hosts This Week in Cardiology on Medscape.
Although originally approved for treatment of diabetes, sodium-glucose cotransporter-2 inhibitors are also approved for management of heart failure. Mandrola started prescribing these drugs 2 years ago for patients, given the links of both conditions with AF.
“I think the next frontier for us in cardiology and AF management will be the GLP-1 agonists,” Mandrola said. He hasn’t started prescribing these drugs for his patients yet but said they will likely play a role in the management of patients with AF with the common constellation of comorbidities such as obesity, hypertension, and sleep apnea.
“The GLP-1 agonists have a really good chance of competing with AF ablation for rhythm control over the long term,” he said.
Decisions, Decisions: Stroke Risk Scoring Systems
The risk for stroke varies widely among patients with AF, so primary care clinicians can pick among several scoring systems to estimate the risk for stroke and guide the decision on whether to initiate anticoagulation therapy.
The ACC/AHA guidelines do not state a preference for a particular instrument. The Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke, Vascular disease, Sex (CHA2DS2-VASc) score is the most widely used and validated instrument, Joglar said. He usually recommends anticoagulation if the CHA2DS2-VASc score is > 2, dependent on individual patient factors.
“If you have a CHA2DS2-VASc score of 1, and you only had one episode of AF for a few hours a year ago, then your risk of stroke is not as high as somebody who has a score of 1 but has more frequent or persistent AF,” Joglar said.
None of the systems is perfect at predicting risk for stroke, so clinicians should discuss options with patients.
“The real message is, are you talking about the risk of stroke and systemic embolism to your patient, so that the patient understands that risk?” he said.
Patel also said measuring creatine clearance can be analogous to using an instrument like CHA2DS2-VASc.
“I often think about renal disease as a very good risk marker and something that does elevate your risk,” he said.
Which Anticoagulant?
Although the ACC/AHA guidelines still recommend warfarin for patients with AF with mechanical heart valves or moderate to severe rheumatic fever, direct oral anticoagulants (DOACs) are the first-line therapy for all other patients with AF.
In terms of which DOACs to use, the differences are subtle, according to Patel.
“I don’t know that they’re that different from each other,” he said. “All of the new drugs are better than warfarin by far.”
Patel pointed out that dabigatran at 150 mg is the only DOAC shown to reduce the incidence of ischemic stroke. For patients with renal dysfunction, he has a slight preference for a 15-mg dose of rivaroxaban.
Mandrola said he mainly prescribes apixaban and rivaroxaban, the latter of which requires only once a day dosing.
“We stopped using dabigatran because 10% of people get gastrointestinal upset,” he said.
Although studies suggest aspirin is less effective than either warfarin or DOACs for the prevention of stroke, Joglar said he still sees patients who come to him after being prescribed low-dose aspirin from primary care clinicians.
“We made it very clear that it should not be recommended just for mitigating stroke risk in atrial fibrillation,” Joglar said. “You could use it if the patient has another indication, such as a prior heart attack.”
Does My Patient Have to Be in Normal Sinus Rhythm?
The new guidelines present evidence maintaining sinus rhythm should be favored over controlling heart rate for managing AF.
“We’ve focused on rhythm control as a better strategy, especially catheter ablation, which seems to be particularly effective in parallel to lifestyle interventions and management of comorbidities,” Joglar said. Rhythm control is of particular benefit for patients with AF triggered by heart failure. Control of rhythm in these patients has been shown to improve multiple outcomes such as ejection fraction, symptoms, and survival.
Patel said as a patient’s symptoms increase, the more likely a clinician will be able to control sinus rhythm. Some patients do not notice their arrhythmia, but others feel dizzy or have chest pain.
“The less symptomatic the patient is, the more likely they’re going to tolerate it, especially if they’re older, and it’s hard to get them into sinus rhythm,” Patel said.
When to Refer for Catheter Ablation?
The new guidelines upgraded the recommendation for catheter ablation to class I (strong recommendation) for patients with symptomatic AF in whom anti-arrhythmic therapy is unsuccessful, not tolerated, or contraindicated; patients with symptomatic paroxysmal AF (typically younger patients with few comorbidities); and patients with symptomatic or clinically significant atrial flutter. The previous iteration recommended trying drug therapy first.
Multiple randomized clinical trials have demonstrated the effectiveness of catheter ablation.
“In somebody who is younger, with a healthy heart, the 1-year success rate of the procedure might be about 70%,” Joglar said. While 70% of patients receiving a catheter have no AF episodes in the following year, Joglar said 20%-25% of those who do have recurrences will experience fewer or shorter episodes.
Conversations about rate vs rhythm control and whether to pursue catheter ablation often come down to preference, Patel said. He would tend to intervene earlier using ablation in patients with heart failure or those experiencing symptoms of AF who cannot be controlled with a heart rate < 100 beats/min.
But he said he prefers using medication for rate control in many of his patients who are older, have chronic AF, and do not have heart failure.
Mandrola takes a more conservative approach, reserving catheter ablation for patients in whom risk factor management and anti-arrhythmic drugs have not been successful.
“In my hospital, it’s done for patients who have symptomatic AF that’s really impacting their quality of life,” he said. But for those with fewer symptoms, his advice is to provide education, reassurance, and time because AF can resolve on its own.
What About Data From Implantables and Wearables?
The guidelines provide an algorithm for when to treat non-symptomatic atrial high-rate episodes detected by a cardiovascular implantable electronic device such as a pacemaker or defibrillator. Episodes less than 5 minutes can be ignored, while treatment could be considered for those with episodes lasting 5 minutes up to 24 hours with a CHA2DS2-VASc score ≥ 3, or lasting longer than 24 hours with a CHA2DS2-VASc score ≥ 2.
But whether anticoagulation improves outcomes is unclear.
“That is a $64,000 question,” Mandrola said. “I would bet every day I get a notification in the electronic health record that says Mr. Smith had 2 hours of AFib 2 weeks ago.”
He also hears from patients who report their Apple Watch has detected an episode of AF.
Mandrola cited evidence from two recent studies of patients who had an atrial high-rate episode longer than 6 minutes detected by implantable devices. The NOAH-AFNET 6 trial randomized patients over 65 years with one or more risk factors for stroke to receive a DOAC or placebo, while the ARTESIA trial used similar inclusion criteria to assign patients to receive either DOAC or aspirin. Both studies reported modest reductions in stroke that were outweighed by a higher incidence of major bleeding in the group receiving anticoagulation.
Shared decision-making should play a role in deciding how aggressively to treat episodes of AF detected by implantable or wearable devices.
He said some patients fear having a stroke, while others are adamantly opposed to taking an anticoagulant.
For patients who present with a documented episode of AF but who otherwise have no symptoms, Patel said clinicians should consider risk for stroke and frequency and duration of episodes.
“One way clinicians should be thinking about it is, the more risk factors they have, the lower burden of AF I need to treat,” Patel said. Even for patients who are having only short episodes of AF, he has a low threshold for recommending an anticoagulation drug if the patient’s CHA2DS2-VASc score is high.
Patel reported research grants from Bayer, Novartis, Idorsia, NHLBI, and Janssen Pharmaceuticals and served as a consultant on the advisory boards of Bayer, Janssen Pharmaceuticals, and Esperion Therapeutics.
Joglar and Mandrola had no disclosures.
A version of this article appeared on Medscape.com.
Skip Potassium After Cardiac Surgery
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2024
Stroke Risk from Atrial Fibrillation Rises in Presence of Rheumatoid Arthritis
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.