User login
Youth with rhabdomyosarcoma see better survival with maintenance chemo
CHICAGO – , finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but very aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the findings were reported. Among pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year overall survival rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr. Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr. Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO Expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with U.S.-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr. Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr. Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs. 48.9%), neutropenia (80.6% vs. 91.6%), and thrombocytopenia (0.6% vs. 26.0%), which translated to less need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs. 56.4%), Dr. Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better disease-free survival (77.6% vs. 69.8%; hazard ratio, 0.68; P = .0613) and had significantly better overall survival (86.5% vs. 73.7%; hazard ratio, 0.52; P = .0111).
Dr. Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. The study received funding from Fondazione Città della Speranza, Italy.
SOURCE: Bisogno et al. ASCO 2018 Abstract LBA2.
CHICAGO – , finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but very aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the findings were reported. Among pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year overall survival rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr. Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr. Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO Expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with U.S.-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr. Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr. Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs. 48.9%), neutropenia (80.6% vs. 91.6%), and thrombocytopenia (0.6% vs. 26.0%), which translated to less need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs. 56.4%), Dr. Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better disease-free survival (77.6% vs. 69.8%; hazard ratio, 0.68; P = .0613) and had significantly better overall survival (86.5% vs. 73.7%; hazard ratio, 0.52; P = .0111).
Dr. Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. The study received funding from Fondazione Città della Speranza, Italy.
SOURCE: Bisogno et al. ASCO 2018 Abstract LBA2.
CHICAGO – , finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but very aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the findings were reported. Among pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year overall survival rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr. Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr. Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO Expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with U.S.-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr. Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr. Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs. 48.9%), neutropenia (80.6% vs. 91.6%), and thrombocytopenia (0.6% vs. 26.0%), which translated to less need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs. 56.4%), Dr. Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better disease-free survival (77.6% vs. 69.8%; hazard ratio, 0.68; P = .0613) and had significantly better overall survival (86.5% vs. 73.7%; hazard ratio, 0.52; P = .0111).
Dr. Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. The study received funding from Fondazione Città della Speranza, Italy.
SOURCE: Bisogno et al. ASCO 2018 Abstract LBA2.
REPORTING FROM ASCO 2018
Key clinical point: Six months of maintenance chemotherapy improves survival in youth with high-risk rhabdomyosarcoma.
Major finding: Compared with counterparts not receiving any additional treatment, patients given maintenance low-dose vinorelbine and cyclophosphamide had better 5-year overall survival (86.5% vs. 73.7%; hazard ratio, 0.52).
Study details: A phase 3 randomized controlled trial among 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy.
Disclosures: Dr. Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. The study received funding from Fondazione Città della Speranza, Italy.
Source: Bisogno et al. ASCO 2018, Abstract LBA2.
CMS sepsis measure a challenge to report
Hospitalists can champion sepsis-improvement efforts
In October 2015, the Centers for Medicare & Medicaid Services implemented its first meaningful policy to attempt for addressing sepsis.
The condition – one of the leading causes of mortality among hospitalized patients – afflicts more than a million people each year in the United States, and between 15% and 30% of them die. Sepsis is one of the leading drivers of hospital readmissions, sending more patients back to the hospital than heart failure, pneumonia, and chronic obstructive pulmonary disease.1
However, while providers seem to agree that time to address sepsis is past due, not everyone has embraced the Sepsis CMS Core Measure program, or SEP-1, as the means to best achieve it. This is, in part, because of discrepancies in how sepsis is defined, the burden of reporting, and what some consider to be arbitrary clinical requirements that may not correlate with better patient outcomes.
“Sepsis is indeed a critical public health problem, and it’s appropriate and valuable that Medicare and other policy makers are focusing on sepsis,” said Jeremy Kahn, MD, professor of critical care medicine and health policy and management at the University of Pittsburgh. “This was really the first approach at that … but at 85-pages long, it really is an enormous effort for hospitals to adhere to this measure.”
This is because of the tension between the “intense desire to improve sepsis outcomes” and the “incredible burden” of keeping up with the necessary documentation while also providing quality care, Dr. Kahn said.
In December 2017, Dr. Kahn helped lead a study published in the Journal of Hospital Medicine aimed at trying to understand hospital perceptions of SEP-1. Over the course of 29 interviews with randomly selected hospital quality leaders across the United States, including physicians and nurses, the results came as a surprise.2
“Generally, hospitals were very supportive of the concept, and there was no pushback on the idea that we should be measuring and reporting sepsis quality to CMS,” he said.
However, the research team found that respondents believed the program’s requirements with respect to treatment and documentation were complex and not always linked to patient-centered outcomes. Meeting the SEP-1 bundles consistently required hospitals to dedicate resources that not all may have, especially those in small, rural communities and those serving as urban safety nets.
Some, like emergency medicine physician Annahieta Kalantari, DO (who did not participate in the survey), feel that SEP-1 forces providers to practice “check-box” medicine and undermines successful efforts that don’t necessarily align with the CMS policy.
She arrived at her institution, Aria-Jefferson Health in Philadelphia, before CMS adopted SEP-1; at that time, she took note of the fact that the rate of sepsis mortalities in her hospital was, in her words, not great when compared with that at similar institutions. And then she helped do something about it.
“I thought, ‘We’re a Premier reporting hospital,’ so we did a gap analysis as to why and put together protocols for the hospital to follow with our sepsis patients, including a sepsis alert and a lot of education,” said Dr. Kalantari, associate program director for the emergency medicine residency program at Aria-Jefferson and a former chair of its sepsis management committee. “Before you knew it, mortalities were below benchmark.”
But once SEP-1 began, she said, the hospital was unable to check all of the boxes all of the time.
“We kept track, but we weren’t hitting all the bundles exactly within the periods of time recommended, but our mortalities were still amazing,” she said. “CMS basically picked definitions [for sepsis], and most of us don’t know what they’re basing them on because no one can agree on a definition anyway. Now they’re penalizing hospitals if they don’t hit the check marks in time, but we’d already demonstrated that our mortality and patient care was exceptional.”
She added: “I am extremely dissatisfied, as someone who provides frontline patient care, with how CMS is choosing to measure us.”
Dr. Kalantari wrote a piece in the Western Journal of Emergency Medicine in July 2017 in which she and coauthors outline the issues they take with SEP-1. They lay out the tension among the varied definitions of what sepsis is – and isn’t – and they also illuminate the apparent conflict between what CMS has officially defined and what evidence-based studies conducted since 2001 have suggested.3
In particular, CMS defines severe sepsis as an initial lactate above 2 mmol/L and septic shock as an initial lactate presentation of greater than 4 mmol/L. However, Dr. Kalantari and here coauthors argue in the paper that there is no standard definition of sepsis and that decades of attempts to achieve one have failed to reach consensus among providers. CMS, she said, fails to acknowledge this.
Defining sepsis
In fact, in 2016, another new definition of sepsis emerged by way of a 19-member task-force of experts: The Third International Consensus Definitions for Sepsis and Septic Shock, also called Sepsis-3.4 In March 2017, the Surviving Sepsis Campaign adopted this definition, which defined sepsis as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.”5
“I think the definition has always been a challenging part of sepsis,” said Kencee Graves, MD, a hospitalist at the University of Utah, Salt Lake City. “The definitions came about for research purposes, so … they are not perfectly sensitive nor specific.”
However, Dr. Graves believes SEP-1 is a step in the right direction in that it brings awareness to sepsis and holds providers accountable. Several years ago, she and her colleague Devin Horton, MD, also a hospitalist at the University of Utah, embarked on a massive undertaking to address sepsis in their hospital. It was, at the time, lacking in “sepsis culture,” Dr. Horton said.
“One of the big things that motivated both of us was that we started doing chart review together and – it’s always easier with 20/20 hindsight – we were noticing that residents were missing the signs of sepsis,” Dr. Horton explained. “The clinical criteria would be there, but no one would say the word.” This is important, he said, because sepsis is time critical.
So the pair set out to create a cultural change by sharing data and collecting input from each service and unit, which relied heavily on nursing staff to perpetuate change. They created an early warning system in the medical record and worked with units to achieve flexibility in their criteria.
While the early warning system seemed helpful on the floor, SEP-1 adherence rates changed little in the emergency department. So Dr. Graves and Dr. Horton worked out an ED-specific process map that started at triage and was modeled after myocardial infarction STEMI protocols. From April through December 2016, the ED achieved between 29.5% adherence to the SEP-1 bundles, they said according to CMS abstractor data. After the change, between January and March 2017, the ED saw 52.2% adherence.
Dr. Kalantari would like to see CMS allow hospitals to evaluate and alter their processes more individually, with the required result being lower sepsis mortality. Hospitalists, said Dr. Kahn, are well poised to champion these sepsis improvement efforts.
“Hospitalists are uniquely positioned to lead in this area because they are a visible presence and a link between providers doing multidisciplinary acute care,” he said. “The other thing hospitalists can do is insist on rolling out approaches that are evidence based and not likely to cause harm by leading to over resuscitation, or ensuring patients are receiving central-line insertions only when needed.”
This is currently a moment for hospitals to innovate and provide meaningful feedback to CMS, which, he said, is listening.
“It’s a myth that CMS rolls out policy without listening to the clinical community, but what they want is constructive criticism, not just to hear ‘We’re not ready and we have to push this down the road,’ ” Dr. Kahn said. “The time is now in the era of accountability in health care.”
References
1. Sepsis. National Institute of General Medical Sciences. https://www.nigms.nih.gov/education/pages/factsheet_sepsis.aspx. Updated Sept 2017. Accessed Jan 4, 2018.
2. Barbash I et al. Hospital perceptions of Medicare’s sepsis quality reporting initiative. J Hosp Med. 2017;12;963-8.
3. Kalantari A et al. Sepsis Definitions: The search for gold and what CMS got wrong. West J Emerg Med. 2017 Aug;18(5):951-6.
4. Singer M et. al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
5. Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304.
Hospitalists can champion sepsis-improvement efforts
Hospitalists can champion sepsis-improvement efforts
In October 2015, the Centers for Medicare & Medicaid Services implemented its first meaningful policy to attempt for addressing sepsis.
The condition – one of the leading causes of mortality among hospitalized patients – afflicts more than a million people each year in the United States, and between 15% and 30% of them die. Sepsis is one of the leading drivers of hospital readmissions, sending more patients back to the hospital than heart failure, pneumonia, and chronic obstructive pulmonary disease.1
However, while providers seem to agree that time to address sepsis is past due, not everyone has embraced the Sepsis CMS Core Measure program, or SEP-1, as the means to best achieve it. This is, in part, because of discrepancies in how sepsis is defined, the burden of reporting, and what some consider to be arbitrary clinical requirements that may not correlate with better patient outcomes.
“Sepsis is indeed a critical public health problem, and it’s appropriate and valuable that Medicare and other policy makers are focusing on sepsis,” said Jeremy Kahn, MD, professor of critical care medicine and health policy and management at the University of Pittsburgh. “This was really the first approach at that … but at 85-pages long, it really is an enormous effort for hospitals to adhere to this measure.”
This is because of the tension between the “intense desire to improve sepsis outcomes” and the “incredible burden” of keeping up with the necessary documentation while also providing quality care, Dr. Kahn said.
In December 2017, Dr. Kahn helped lead a study published in the Journal of Hospital Medicine aimed at trying to understand hospital perceptions of SEP-1. Over the course of 29 interviews with randomly selected hospital quality leaders across the United States, including physicians and nurses, the results came as a surprise.2
“Generally, hospitals were very supportive of the concept, and there was no pushback on the idea that we should be measuring and reporting sepsis quality to CMS,” he said.
However, the research team found that respondents believed the program’s requirements with respect to treatment and documentation were complex and not always linked to patient-centered outcomes. Meeting the SEP-1 bundles consistently required hospitals to dedicate resources that not all may have, especially those in small, rural communities and those serving as urban safety nets.
Some, like emergency medicine physician Annahieta Kalantari, DO (who did not participate in the survey), feel that SEP-1 forces providers to practice “check-box” medicine and undermines successful efforts that don’t necessarily align with the CMS policy.
She arrived at her institution, Aria-Jefferson Health in Philadelphia, before CMS adopted SEP-1; at that time, she took note of the fact that the rate of sepsis mortalities in her hospital was, in her words, not great when compared with that at similar institutions. And then she helped do something about it.
“I thought, ‘We’re a Premier reporting hospital,’ so we did a gap analysis as to why and put together protocols for the hospital to follow with our sepsis patients, including a sepsis alert and a lot of education,” said Dr. Kalantari, associate program director for the emergency medicine residency program at Aria-Jefferson and a former chair of its sepsis management committee. “Before you knew it, mortalities were below benchmark.”
But once SEP-1 began, she said, the hospital was unable to check all of the boxes all of the time.
“We kept track, but we weren’t hitting all the bundles exactly within the periods of time recommended, but our mortalities were still amazing,” she said. “CMS basically picked definitions [for sepsis], and most of us don’t know what they’re basing them on because no one can agree on a definition anyway. Now they’re penalizing hospitals if they don’t hit the check marks in time, but we’d already demonstrated that our mortality and patient care was exceptional.”
She added: “I am extremely dissatisfied, as someone who provides frontline patient care, with how CMS is choosing to measure us.”
Dr. Kalantari wrote a piece in the Western Journal of Emergency Medicine in July 2017 in which she and coauthors outline the issues they take with SEP-1. They lay out the tension among the varied definitions of what sepsis is – and isn’t – and they also illuminate the apparent conflict between what CMS has officially defined and what evidence-based studies conducted since 2001 have suggested.3
In particular, CMS defines severe sepsis as an initial lactate above 2 mmol/L and septic shock as an initial lactate presentation of greater than 4 mmol/L. However, Dr. Kalantari and here coauthors argue in the paper that there is no standard definition of sepsis and that decades of attempts to achieve one have failed to reach consensus among providers. CMS, she said, fails to acknowledge this.
Defining sepsis
In fact, in 2016, another new definition of sepsis emerged by way of a 19-member task-force of experts: The Third International Consensus Definitions for Sepsis and Septic Shock, also called Sepsis-3.4 In March 2017, the Surviving Sepsis Campaign adopted this definition, which defined sepsis as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.”5
“I think the definition has always been a challenging part of sepsis,” said Kencee Graves, MD, a hospitalist at the University of Utah, Salt Lake City. “The definitions came about for research purposes, so … they are not perfectly sensitive nor specific.”
However, Dr. Graves believes SEP-1 is a step in the right direction in that it brings awareness to sepsis and holds providers accountable. Several years ago, she and her colleague Devin Horton, MD, also a hospitalist at the University of Utah, embarked on a massive undertaking to address sepsis in their hospital. It was, at the time, lacking in “sepsis culture,” Dr. Horton said.
“One of the big things that motivated both of us was that we started doing chart review together and – it’s always easier with 20/20 hindsight – we were noticing that residents were missing the signs of sepsis,” Dr. Horton explained. “The clinical criteria would be there, but no one would say the word.” This is important, he said, because sepsis is time critical.
So the pair set out to create a cultural change by sharing data and collecting input from each service and unit, which relied heavily on nursing staff to perpetuate change. They created an early warning system in the medical record and worked with units to achieve flexibility in their criteria.
While the early warning system seemed helpful on the floor, SEP-1 adherence rates changed little in the emergency department. So Dr. Graves and Dr. Horton worked out an ED-specific process map that started at triage and was modeled after myocardial infarction STEMI protocols. From April through December 2016, the ED achieved between 29.5% adherence to the SEP-1 bundles, they said according to CMS abstractor data. After the change, between January and March 2017, the ED saw 52.2% adherence.
Dr. Kalantari would like to see CMS allow hospitals to evaluate and alter their processes more individually, with the required result being lower sepsis mortality. Hospitalists, said Dr. Kahn, are well poised to champion these sepsis improvement efforts.
“Hospitalists are uniquely positioned to lead in this area because they are a visible presence and a link between providers doing multidisciplinary acute care,” he said. “The other thing hospitalists can do is insist on rolling out approaches that are evidence based and not likely to cause harm by leading to over resuscitation, or ensuring patients are receiving central-line insertions only when needed.”
This is currently a moment for hospitals to innovate and provide meaningful feedback to CMS, which, he said, is listening.
“It’s a myth that CMS rolls out policy without listening to the clinical community, but what they want is constructive criticism, not just to hear ‘We’re not ready and we have to push this down the road,’ ” Dr. Kahn said. “The time is now in the era of accountability in health care.”
References
1. Sepsis. National Institute of General Medical Sciences. https://www.nigms.nih.gov/education/pages/factsheet_sepsis.aspx. Updated Sept 2017. Accessed Jan 4, 2018.
2. Barbash I et al. Hospital perceptions of Medicare’s sepsis quality reporting initiative. J Hosp Med. 2017;12;963-8.
3. Kalantari A et al. Sepsis Definitions: The search for gold and what CMS got wrong. West J Emerg Med. 2017 Aug;18(5):951-6.
4. Singer M et. al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
5. Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304.
In October 2015, the Centers for Medicare & Medicaid Services implemented its first meaningful policy to attempt for addressing sepsis.
The condition – one of the leading causes of mortality among hospitalized patients – afflicts more than a million people each year in the United States, and between 15% and 30% of them die. Sepsis is one of the leading drivers of hospital readmissions, sending more patients back to the hospital than heart failure, pneumonia, and chronic obstructive pulmonary disease.1
However, while providers seem to agree that time to address sepsis is past due, not everyone has embraced the Sepsis CMS Core Measure program, or SEP-1, as the means to best achieve it. This is, in part, because of discrepancies in how sepsis is defined, the burden of reporting, and what some consider to be arbitrary clinical requirements that may not correlate with better patient outcomes.
“Sepsis is indeed a critical public health problem, and it’s appropriate and valuable that Medicare and other policy makers are focusing on sepsis,” said Jeremy Kahn, MD, professor of critical care medicine and health policy and management at the University of Pittsburgh. “This was really the first approach at that … but at 85-pages long, it really is an enormous effort for hospitals to adhere to this measure.”
This is because of the tension between the “intense desire to improve sepsis outcomes” and the “incredible burden” of keeping up with the necessary documentation while also providing quality care, Dr. Kahn said.
In December 2017, Dr. Kahn helped lead a study published in the Journal of Hospital Medicine aimed at trying to understand hospital perceptions of SEP-1. Over the course of 29 interviews with randomly selected hospital quality leaders across the United States, including physicians and nurses, the results came as a surprise.2
“Generally, hospitals were very supportive of the concept, and there was no pushback on the idea that we should be measuring and reporting sepsis quality to CMS,” he said.
However, the research team found that respondents believed the program’s requirements with respect to treatment and documentation were complex and not always linked to patient-centered outcomes. Meeting the SEP-1 bundles consistently required hospitals to dedicate resources that not all may have, especially those in small, rural communities and those serving as urban safety nets.
Some, like emergency medicine physician Annahieta Kalantari, DO (who did not participate in the survey), feel that SEP-1 forces providers to practice “check-box” medicine and undermines successful efforts that don’t necessarily align with the CMS policy.
She arrived at her institution, Aria-Jefferson Health in Philadelphia, before CMS adopted SEP-1; at that time, she took note of the fact that the rate of sepsis mortalities in her hospital was, in her words, not great when compared with that at similar institutions. And then she helped do something about it.
“I thought, ‘We’re a Premier reporting hospital,’ so we did a gap analysis as to why and put together protocols for the hospital to follow with our sepsis patients, including a sepsis alert and a lot of education,” said Dr. Kalantari, associate program director for the emergency medicine residency program at Aria-Jefferson and a former chair of its sepsis management committee. “Before you knew it, mortalities were below benchmark.”
But once SEP-1 began, she said, the hospital was unable to check all of the boxes all of the time.
“We kept track, but we weren’t hitting all the bundles exactly within the periods of time recommended, but our mortalities were still amazing,” she said. “CMS basically picked definitions [for sepsis], and most of us don’t know what they’re basing them on because no one can agree on a definition anyway. Now they’re penalizing hospitals if they don’t hit the check marks in time, but we’d already demonstrated that our mortality and patient care was exceptional.”
She added: “I am extremely dissatisfied, as someone who provides frontline patient care, with how CMS is choosing to measure us.”
Dr. Kalantari wrote a piece in the Western Journal of Emergency Medicine in July 2017 in which she and coauthors outline the issues they take with SEP-1. They lay out the tension among the varied definitions of what sepsis is – and isn’t – and they also illuminate the apparent conflict between what CMS has officially defined and what evidence-based studies conducted since 2001 have suggested.3
In particular, CMS defines severe sepsis as an initial lactate above 2 mmol/L and septic shock as an initial lactate presentation of greater than 4 mmol/L. However, Dr. Kalantari and here coauthors argue in the paper that there is no standard definition of sepsis and that decades of attempts to achieve one have failed to reach consensus among providers. CMS, she said, fails to acknowledge this.
Defining sepsis
In fact, in 2016, another new definition of sepsis emerged by way of a 19-member task-force of experts: The Third International Consensus Definitions for Sepsis and Septic Shock, also called Sepsis-3.4 In March 2017, the Surviving Sepsis Campaign adopted this definition, which defined sepsis as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.”5
“I think the definition has always been a challenging part of sepsis,” said Kencee Graves, MD, a hospitalist at the University of Utah, Salt Lake City. “The definitions came about for research purposes, so … they are not perfectly sensitive nor specific.”
However, Dr. Graves believes SEP-1 is a step in the right direction in that it brings awareness to sepsis and holds providers accountable. Several years ago, she and her colleague Devin Horton, MD, also a hospitalist at the University of Utah, embarked on a massive undertaking to address sepsis in their hospital. It was, at the time, lacking in “sepsis culture,” Dr. Horton said.
“One of the big things that motivated both of us was that we started doing chart review together and – it’s always easier with 20/20 hindsight – we were noticing that residents were missing the signs of sepsis,” Dr. Horton explained. “The clinical criteria would be there, but no one would say the word.” This is important, he said, because sepsis is time critical.
So the pair set out to create a cultural change by sharing data and collecting input from each service and unit, which relied heavily on nursing staff to perpetuate change. They created an early warning system in the medical record and worked with units to achieve flexibility in their criteria.
While the early warning system seemed helpful on the floor, SEP-1 adherence rates changed little in the emergency department. So Dr. Graves and Dr. Horton worked out an ED-specific process map that started at triage and was modeled after myocardial infarction STEMI protocols. From April through December 2016, the ED achieved between 29.5% adherence to the SEP-1 bundles, they said according to CMS abstractor data. After the change, between January and March 2017, the ED saw 52.2% adherence.
Dr. Kalantari would like to see CMS allow hospitals to evaluate and alter their processes more individually, with the required result being lower sepsis mortality. Hospitalists, said Dr. Kahn, are well poised to champion these sepsis improvement efforts.
“Hospitalists are uniquely positioned to lead in this area because they are a visible presence and a link between providers doing multidisciplinary acute care,” he said. “The other thing hospitalists can do is insist on rolling out approaches that are evidence based and not likely to cause harm by leading to over resuscitation, or ensuring patients are receiving central-line insertions only when needed.”
This is currently a moment for hospitals to innovate and provide meaningful feedback to CMS, which, he said, is listening.
“It’s a myth that CMS rolls out policy without listening to the clinical community, but what they want is constructive criticism, not just to hear ‘We’re not ready and we have to push this down the road,’ ” Dr. Kahn said. “The time is now in the era of accountability in health care.”
References
1. Sepsis. National Institute of General Medical Sciences. https://www.nigms.nih.gov/education/pages/factsheet_sepsis.aspx. Updated Sept 2017. Accessed Jan 4, 2018.
2. Barbash I et al. Hospital perceptions of Medicare’s sepsis quality reporting initiative. J Hosp Med. 2017;12;963-8.
3. Kalantari A et al. Sepsis Definitions: The search for gold and what CMS got wrong. West J Emerg Med. 2017 Aug;18(5):951-6.
4. Singer M et. al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
5. Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304.
CDC warns of hepatitis A outbreaks in injection drug users
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
EULAR scientific program highlights spectrum of translational research
EULAR 2018’s scientific program in Amsterdam is packed with lectures, clinical and basic science symposia, workshops, and special interest sessions covering the full spectrum of rheumatic diseases, said Dr. Robert Landewé, chair of the Scientific Program Committee.
“More than 5,000 scientific abstracts were submitted, which is an absolute, all-time record,” Dr. Landewé said. Four experts scored each abstract, and only the top 7% were invited for oral presentation during abstract sessions or symposia, he explained in an interview.
Wednesday, June 13
A high point of the 2018 scientific program is Wednesday’s opening plenary session, which will feature abstracts that were handpicked by Dr. Landewé and Dr. Thomas Dörner, professor of rheumatology at Charite Universitätsmedizin, Berlin. “This session includes highly scored abstracts, including late-breakers, on current advances in therapeutics and disease classification,” said Dr. Dörner, who chaired this year’s Abstract Selection Committee.
The plenary abstract session will cover new findings on gout and cardiovascular disease from CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), long-term mortality in patients with early RA from the COBRA (Combinatietherapie Bij Reumatoide Artritis) study, the use of zoledronic acid to treat knee osteoarthritis with bone lesions, and the relationship between bisphosphonate drug holidays and hip fracture risk. Researchers also will discuss baricitinib in systemic lupus erythematosus (SLE), the value of MRI when treating remitted RA to target, the validation of SLE classification criteria, and draft classification criteria for ANCA-associated vasculitides.
A notable clinical science session on Wednesday will cover cancer and inflammation, Dr. Landewé said. “This is a topic of increasing interest because cancer and inflammation share mutual pathways.”
Novel cancer therapies such as immune checkpoint inhibitors have improved outcomes across a range of tumor types, but also can induce rheumatic disease, he added. Accordingly, presenters will discuss inflammation as “friend” versus “foe” in cancer treatment, the role of tumor necrosis factor in cancer, and risk of malignancy among patients with RA.
Also on Wednesday, a session will tackle the relationship between psychological distress and pain in immune-mediated disease. “Pain is the major symptom of rheumatic diseases, and the role of the psyche remains poorly understood,” Dr. Landewé said. “But we know one thing for sure: There is an association, and speakers from outside the field of rheumatology will help explain.”
Attendees at this bench-to-bedside session will learn how distress appears to exacerbate arthritis pain and how managing psychological stress can help optimize outcomes in arthritis pain. Experts also will describe research on integrated brain pathways in pain and distress, as well as risk factors for cognitive impairment in RA.
Thursday, June 14
Topics in this session will include the use of estrogens and other hormonal therapies in patients with rheumatic disease, registry studies of rheumatologic conditions during pregnancy, and how clinicians can best discuss sexual concerns with their rheumatology patients.
Another clinical science session scheduled for Thursday afternoon will delve into structural damage progression in patients with axial spondyloarthritis, Dr. Landewé said. “Can we inhibit this structural progression? Can we show it? Does it make sense? And which drug company will win the battle to have the precedent?”
He hopes that Dr. Désirée van der Heijde of the Netherlands and Dr. Xenofon Baraliakos of Germany will help answer these questions when they discuss the latest evidence on identifying and treating clinically relevant structural progression. Also in this session, researchers will describe the combined effects of tumor necrosis factor inhibitors and NSAIDs on radiographic progression in ankylosing spondylitis, and MRI evidence supporting treating early axial spondyloarthritis to target with the goal of achieving sustained remission of inflammation.
Also on Thursday afternoon, a case-based session will take a deep dive into giant cell arteritis (GCA), Dr. Landewé noted. Attendees will learn about diagnosing and managing vision loss and stroke and the latest on corticosteroid therapy in GCA. The session also will cover biologics. “Giant cell arteritis has entered the field of biologicals!” said Dr. Landewé. “This has major implications for this disease and the clinical choices to be made.”
The past 5 decades have seen marked progress in the diagnosis and treatment of SLE, with corresponding improvements in survival and quality of life. “Still, lupus is awfully difficult,” Dr. Landewé said. “Therefore, we have planned a classical bench-to-bedside symposium to provide an all-inclusive look at current thinking and future developments.”
Talks during this Thursday afternoon session will cover the latest findings on the pathogenesis of SLE, the clinical significance of autoantibodies, distinguishing early SLE from mimics, and the role of blood-brain barrier permeability and neuropsychiatric manifestations of SLE and progressive systemic sclerosis.
Friday, June 15
For the first time, the scientific program also will include a clinical science session held jointly with the European Society of Musculoskeletal Radiology (ESSR). Dr. Joachim Sieper of Germany and ESSR President Dr. Monique Reijnierse of the Netherlands will cochair the Friday afternoon session on the role of MRI in rheumatology. Attendees from both organizations will learn when to use MRI in early and established RA and spondyloarthritis, and how to interpret the results, with abundant time built in for questions and answers. Dr. Landewé called the joint session “a test case” for exciting web-based interactions between EULAR and ESSR.
Another clinical science session on Friday afternoon will dive into the diagnosis of spondyloarthritis, which Dr. Landewé called “a matter of recognizing patterns, not ticking boxes on a list of criteria. This symposium leads you through the art of pattern recognition.”
Later on Friday afternoon, a session will explore advances in biologic therapy of small-vessel vasculitis, he added. “Biologic disease-modifying antirheumatic drugs [bDMARDs] are becoming more and more important in this area of expanding interest.” Experts will address complement inhibition in ANCA-associated vasculitis (AAV), the use of induction and maintenance rituximab in AAV, the evolving role of mepolizumab in eosinophilic granulomatosis with polyangiitis, survival in AAV, and the use of rituximab for treating children with granulomatosis with polyangiitis and microscopic polyangiitis.
Saturday, June 16
On Saturday, a bench-to-bedside session will cover gout and kidney function. “This is an area with important new insights,” Dr. Dörner said. Presenters will discuss the genetics of hyperuricemia, renal urate transporters, and the pros and cons of using xanthine oxidase inhibitors to treat chronic kidney disease. Researchers will also cover studies of impaired neutrophil chemotaxis in patients with chronic kidney disease and hyperuricemia, and the relationship between renal medullar hyperechogenicity and gout severity.
Also on Saturday, a clinical science session titled, “Rheumatoid arthritis: Is it all in your head?” will explore emerging data on the relationship between inflammation and depression. Patients with RA often face both clinical depression and social isolation, and these complex psychosocial conditions can worsen one another. “In addition to proper drug choice, treating RA effectively depends on how concomitant problems, such as nonspecific pain, depression, and social isolation, are coped with in a broad context,” Dr. Landewé said. “When it comes to optimal management, rheumatologists need to communicate and prescribe, not just prescribe.”
Christian Apfelbacher, PhD, of Germany will discuss prevention and treatment strategies and Dr. Jonathan Cavanagh of the United Kingdom will cover neuroimaging in RA. Researchers also will discuss new findings on pain, depression, and anxiety in patients recently diagnosed with RA.
Also on Saturday, a special session will cover EULAR’s initiatives to improve clinical approaches (ESSCA), Dr. Dörner noted. This effort has produced new or updated recommendations on topics such as vaccination, Sjögren’s syndrome, glucocorticoid therapy, and management of hand osteoarthritis, he said. “These recommendations follow a number of others and are expected to impact clinical science as well as clinical practice.”
EULAR 2018’s scientific program in Amsterdam is packed with lectures, clinical and basic science symposia, workshops, and special interest sessions covering the full spectrum of rheumatic diseases, said Dr. Robert Landewé, chair of the Scientific Program Committee.
“More than 5,000 scientific abstracts were submitted, which is an absolute, all-time record,” Dr. Landewé said. Four experts scored each abstract, and only the top 7% were invited for oral presentation during abstract sessions or symposia, he explained in an interview.
Wednesday, June 13
A high point of the 2018 scientific program is Wednesday’s opening plenary session, which will feature abstracts that were handpicked by Dr. Landewé and Dr. Thomas Dörner, professor of rheumatology at Charite Universitätsmedizin, Berlin. “This session includes highly scored abstracts, including late-breakers, on current advances in therapeutics and disease classification,” said Dr. Dörner, who chaired this year’s Abstract Selection Committee.
The plenary abstract session will cover new findings on gout and cardiovascular disease from CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), long-term mortality in patients with early RA from the COBRA (Combinatietherapie Bij Reumatoide Artritis) study, the use of zoledronic acid to treat knee osteoarthritis with bone lesions, and the relationship between bisphosphonate drug holidays and hip fracture risk. Researchers also will discuss baricitinib in systemic lupus erythematosus (SLE), the value of MRI when treating remitted RA to target, the validation of SLE classification criteria, and draft classification criteria for ANCA-associated vasculitides.
A notable clinical science session on Wednesday will cover cancer and inflammation, Dr. Landewé said. “This is a topic of increasing interest because cancer and inflammation share mutual pathways.”
Novel cancer therapies such as immune checkpoint inhibitors have improved outcomes across a range of tumor types, but also can induce rheumatic disease, he added. Accordingly, presenters will discuss inflammation as “friend” versus “foe” in cancer treatment, the role of tumor necrosis factor in cancer, and risk of malignancy among patients with RA.
Also on Wednesday, a session will tackle the relationship between psychological distress and pain in immune-mediated disease. “Pain is the major symptom of rheumatic diseases, and the role of the psyche remains poorly understood,” Dr. Landewé said. “But we know one thing for sure: There is an association, and speakers from outside the field of rheumatology will help explain.”
Attendees at this bench-to-bedside session will learn how distress appears to exacerbate arthritis pain and how managing psychological stress can help optimize outcomes in arthritis pain. Experts also will describe research on integrated brain pathways in pain and distress, as well as risk factors for cognitive impairment in RA.
Thursday, June 14
Topics in this session will include the use of estrogens and other hormonal therapies in patients with rheumatic disease, registry studies of rheumatologic conditions during pregnancy, and how clinicians can best discuss sexual concerns with their rheumatology patients.
Another clinical science session scheduled for Thursday afternoon will delve into structural damage progression in patients with axial spondyloarthritis, Dr. Landewé said. “Can we inhibit this structural progression? Can we show it? Does it make sense? And which drug company will win the battle to have the precedent?”
He hopes that Dr. Désirée van der Heijde of the Netherlands and Dr. Xenofon Baraliakos of Germany will help answer these questions when they discuss the latest evidence on identifying and treating clinically relevant structural progression. Also in this session, researchers will describe the combined effects of tumor necrosis factor inhibitors and NSAIDs on radiographic progression in ankylosing spondylitis, and MRI evidence supporting treating early axial spondyloarthritis to target with the goal of achieving sustained remission of inflammation.
Also on Thursday afternoon, a case-based session will take a deep dive into giant cell arteritis (GCA), Dr. Landewé noted. Attendees will learn about diagnosing and managing vision loss and stroke and the latest on corticosteroid therapy in GCA. The session also will cover biologics. “Giant cell arteritis has entered the field of biologicals!” said Dr. Landewé. “This has major implications for this disease and the clinical choices to be made.”
The past 5 decades have seen marked progress in the diagnosis and treatment of SLE, with corresponding improvements in survival and quality of life. “Still, lupus is awfully difficult,” Dr. Landewé said. “Therefore, we have planned a classical bench-to-bedside symposium to provide an all-inclusive look at current thinking and future developments.”
Talks during this Thursday afternoon session will cover the latest findings on the pathogenesis of SLE, the clinical significance of autoantibodies, distinguishing early SLE from mimics, and the role of blood-brain barrier permeability and neuropsychiatric manifestations of SLE and progressive systemic sclerosis.
Friday, June 15
For the first time, the scientific program also will include a clinical science session held jointly with the European Society of Musculoskeletal Radiology (ESSR). Dr. Joachim Sieper of Germany and ESSR President Dr. Monique Reijnierse of the Netherlands will cochair the Friday afternoon session on the role of MRI in rheumatology. Attendees from both organizations will learn when to use MRI in early and established RA and spondyloarthritis, and how to interpret the results, with abundant time built in for questions and answers. Dr. Landewé called the joint session “a test case” for exciting web-based interactions between EULAR and ESSR.
Another clinical science session on Friday afternoon will dive into the diagnosis of spondyloarthritis, which Dr. Landewé called “a matter of recognizing patterns, not ticking boxes on a list of criteria. This symposium leads you through the art of pattern recognition.”
Later on Friday afternoon, a session will explore advances in biologic therapy of small-vessel vasculitis, he added. “Biologic disease-modifying antirheumatic drugs [bDMARDs] are becoming more and more important in this area of expanding interest.” Experts will address complement inhibition in ANCA-associated vasculitis (AAV), the use of induction and maintenance rituximab in AAV, the evolving role of mepolizumab in eosinophilic granulomatosis with polyangiitis, survival in AAV, and the use of rituximab for treating children with granulomatosis with polyangiitis and microscopic polyangiitis.
Saturday, June 16
On Saturday, a bench-to-bedside session will cover gout and kidney function. “This is an area with important new insights,” Dr. Dörner said. Presenters will discuss the genetics of hyperuricemia, renal urate transporters, and the pros and cons of using xanthine oxidase inhibitors to treat chronic kidney disease. Researchers will also cover studies of impaired neutrophil chemotaxis in patients with chronic kidney disease and hyperuricemia, and the relationship between renal medullar hyperechogenicity and gout severity.
Also on Saturday, a clinical science session titled, “Rheumatoid arthritis: Is it all in your head?” will explore emerging data on the relationship between inflammation and depression. Patients with RA often face both clinical depression and social isolation, and these complex psychosocial conditions can worsen one another. “In addition to proper drug choice, treating RA effectively depends on how concomitant problems, such as nonspecific pain, depression, and social isolation, are coped with in a broad context,” Dr. Landewé said. “When it comes to optimal management, rheumatologists need to communicate and prescribe, not just prescribe.”
Christian Apfelbacher, PhD, of Germany will discuss prevention and treatment strategies and Dr. Jonathan Cavanagh of the United Kingdom will cover neuroimaging in RA. Researchers also will discuss new findings on pain, depression, and anxiety in patients recently diagnosed with RA.
Also on Saturday, a special session will cover EULAR’s initiatives to improve clinical approaches (ESSCA), Dr. Dörner noted. This effort has produced new or updated recommendations on topics such as vaccination, Sjögren’s syndrome, glucocorticoid therapy, and management of hand osteoarthritis, he said. “These recommendations follow a number of others and are expected to impact clinical science as well as clinical practice.”
EULAR 2018’s scientific program in Amsterdam is packed with lectures, clinical and basic science symposia, workshops, and special interest sessions covering the full spectrum of rheumatic diseases, said Dr. Robert Landewé, chair of the Scientific Program Committee.
“More than 5,000 scientific abstracts were submitted, which is an absolute, all-time record,” Dr. Landewé said. Four experts scored each abstract, and only the top 7% were invited for oral presentation during abstract sessions or symposia, he explained in an interview.
Wednesday, June 13
A high point of the 2018 scientific program is Wednesday’s opening plenary session, which will feature abstracts that were handpicked by Dr. Landewé and Dr. Thomas Dörner, professor of rheumatology at Charite Universitätsmedizin, Berlin. “This session includes highly scored abstracts, including late-breakers, on current advances in therapeutics and disease classification,” said Dr. Dörner, who chaired this year’s Abstract Selection Committee.
The plenary abstract session will cover new findings on gout and cardiovascular disease from CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), long-term mortality in patients with early RA from the COBRA (Combinatietherapie Bij Reumatoide Artritis) study, the use of zoledronic acid to treat knee osteoarthritis with bone lesions, and the relationship between bisphosphonate drug holidays and hip fracture risk. Researchers also will discuss baricitinib in systemic lupus erythematosus (SLE), the value of MRI when treating remitted RA to target, the validation of SLE classification criteria, and draft classification criteria for ANCA-associated vasculitides.
A notable clinical science session on Wednesday will cover cancer and inflammation, Dr. Landewé said. “This is a topic of increasing interest because cancer and inflammation share mutual pathways.”
Novel cancer therapies such as immune checkpoint inhibitors have improved outcomes across a range of tumor types, but also can induce rheumatic disease, he added. Accordingly, presenters will discuss inflammation as “friend” versus “foe” in cancer treatment, the role of tumor necrosis factor in cancer, and risk of malignancy among patients with RA.
Also on Wednesday, a session will tackle the relationship between psychological distress and pain in immune-mediated disease. “Pain is the major symptom of rheumatic diseases, and the role of the psyche remains poorly understood,” Dr. Landewé said. “But we know one thing for sure: There is an association, and speakers from outside the field of rheumatology will help explain.”
Attendees at this bench-to-bedside session will learn how distress appears to exacerbate arthritis pain and how managing psychological stress can help optimize outcomes in arthritis pain. Experts also will describe research on integrated brain pathways in pain and distress, as well as risk factors for cognitive impairment in RA.
Thursday, June 14
Topics in this session will include the use of estrogens and other hormonal therapies in patients with rheumatic disease, registry studies of rheumatologic conditions during pregnancy, and how clinicians can best discuss sexual concerns with their rheumatology patients.
Another clinical science session scheduled for Thursday afternoon will delve into structural damage progression in patients with axial spondyloarthritis, Dr. Landewé said. “Can we inhibit this structural progression? Can we show it? Does it make sense? And which drug company will win the battle to have the precedent?”
He hopes that Dr. Désirée van der Heijde of the Netherlands and Dr. Xenofon Baraliakos of Germany will help answer these questions when they discuss the latest evidence on identifying and treating clinically relevant structural progression. Also in this session, researchers will describe the combined effects of tumor necrosis factor inhibitors and NSAIDs on radiographic progression in ankylosing spondylitis, and MRI evidence supporting treating early axial spondyloarthritis to target with the goal of achieving sustained remission of inflammation.
Also on Thursday afternoon, a case-based session will take a deep dive into giant cell arteritis (GCA), Dr. Landewé noted. Attendees will learn about diagnosing and managing vision loss and stroke and the latest on corticosteroid therapy in GCA. The session also will cover biologics. “Giant cell arteritis has entered the field of biologicals!” said Dr. Landewé. “This has major implications for this disease and the clinical choices to be made.”
The past 5 decades have seen marked progress in the diagnosis and treatment of SLE, with corresponding improvements in survival and quality of life. “Still, lupus is awfully difficult,” Dr. Landewé said. “Therefore, we have planned a classical bench-to-bedside symposium to provide an all-inclusive look at current thinking and future developments.”
Talks during this Thursday afternoon session will cover the latest findings on the pathogenesis of SLE, the clinical significance of autoantibodies, distinguishing early SLE from mimics, and the role of blood-brain barrier permeability and neuropsychiatric manifestations of SLE and progressive systemic sclerosis.
Friday, June 15
For the first time, the scientific program also will include a clinical science session held jointly with the European Society of Musculoskeletal Radiology (ESSR). Dr. Joachim Sieper of Germany and ESSR President Dr. Monique Reijnierse of the Netherlands will cochair the Friday afternoon session on the role of MRI in rheumatology. Attendees from both organizations will learn when to use MRI in early and established RA and spondyloarthritis, and how to interpret the results, with abundant time built in for questions and answers. Dr. Landewé called the joint session “a test case” for exciting web-based interactions between EULAR and ESSR.
Another clinical science session on Friday afternoon will dive into the diagnosis of spondyloarthritis, which Dr. Landewé called “a matter of recognizing patterns, not ticking boxes on a list of criteria. This symposium leads you through the art of pattern recognition.”
Later on Friday afternoon, a session will explore advances in biologic therapy of small-vessel vasculitis, he added. “Biologic disease-modifying antirheumatic drugs [bDMARDs] are becoming more and more important in this area of expanding interest.” Experts will address complement inhibition in ANCA-associated vasculitis (AAV), the use of induction and maintenance rituximab in AAV, the evolving role of mepolizumab in eosinophilic granulomatosis with polyangiitis, survival in AAV, and the use of rituximab for treating children with granulomatosis with polyangiitis and microscopic polyangiitis.
Saturday, June 16
On Saturday, a bench-to-bedside session will cover gout and kidney function. “This is an area with important new insights,” Dr. Dörner said. Presenters will discuss the genetics of hyperuricemia, renal urate transporters, and the pros and cons of using xanthine oxidase inhibitors to treat chronic kidney disease. Researchers will also cover studies of impaired neutrophil chemotaxis in patients with chronic kidney disease and hyperuricemia, and the relationship between renal medullar hyperechogenicity and gout severity.
Also on Saturday, a clinical science session titled, “Rheumatoid arthritis: Is it all in your head?” will explore emerging data on the relationship between inflammation and depression. Patients with RA often face both clinical depression and social isolation, and these complex psychosocial conditions can worsen one another. “In addition to proper drug choice, treating RA effectively depends on how concomitant problems, such as nonspecific pain, depression, and social isolation, are coped with in a broad context,” Dr. Landewé said. “When it comes to optimal management, rheumatologists need to communicate and prescribe, not just prescribe.”
Christian Apfelbacher, PhD, of Germany will discuss prevention and treatment strategies and Dr. Jonathan Cavanagh of the United Kingdom will cover neuroimaging in RA. Researchers also will discuss new findings on pain, depression, and anxiety in patients recently diagnosed with RA.
Also on Saturday, a special session will cover EULAR’s initiatives to improve clinical approaches (ESSCA), Dr. Dörner noted. This effort has produced new or updated recommendations on topics such as vaccination, Sjögren’s syndrome, glucocorticoid therapy, and management of hand osteoarthritis, he said. “These recommendations follow a number of others and are expected to impact clinical science as well as clinical practice.”
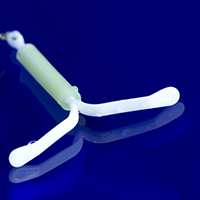
Which IUD is right for me? Answering your patients’ questions about differences in LNG-IUDs
EULAR pediatric sessions to highlight big data, personalized medicine
Personalized medicine, big data, and monogenic inflammatory diseases are just a few of the high points of pediatric rheumatology sessions at this year’s EULAR Congress.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Berent J. Prakken, MD, PhD, said that a bench-to-bedside session on Wednesday afternoon would highlight how EULAR projects are driving advances in pediatric rheumatology.
Attendees will learn from Vicki Seyfert-Margolis, PhD, an internationally recognized expert in personalized medicine, about how digital tools can facilitate cross-border partnerships in pediatric rheumatology, Dr. Prakken said in an interview.
“This talk will be groundbreaking because it’s not just about another useful app,” said Dr. Prakken, professor of pediatric rheumatology and vice dean of education at University Medical Center Utrecht (the Netherlands). “Dr. Seyfert-Margolis will show how the digital revolution will change the way we communicate with patients, monitor disease, and develop novel models for clinical trials.”
The session will also cover work by the Understanding Childhood Arthritis Network (UCAN), created to facilitate international translational research in pediatric rheumatology. Speakers will describe how UCAN is helping to spur personalized medicine and working with the Pediatric Rheumatology International Trials Organization (PRINTO) to align bench and bedside perspectives.
Another program highlight is a Thursday afternoon session on connections between monogenic autoinflammatory and pediatric rheumatic diseases. “Groundbreaking studies of these rare genetic inflammatory diseases have provided important new insights that, in turn, have led to new therapeutic options,” said Dr. Prakken.
During the session, Joost Swart, MD, of Utrecht, the Netherlands, will discuss promising research on the intravenous use of mesenchymal stromal cells derived from bone marrow for the treatment of refractory juvenile idiopathic arthritis (JIA).
Dr. Swart, who helped pioneer the approach, will discuss the first phase I/II trial of its use in children. “This is a truly innovative way to handle refractory inflammation,” Dr. Prakken said.
The session on monogenic autoinflammatory diseases also will cover their clinical presentation in children and adults, their pathogenesis as compared with adult-onset rheumatic diseases, and emerging treatment options, according to Dr. Prakken.
On Friday afternoon, a pediatric session will feature big data science in pediatric rheumatology, a lightning-paced field that is generating new research and treatment paradigms.
Of special note, Salvatore Albani, MD, PhD, will discuss how the human immunome is revolutionizing personalized treatment of paediatric inflammatory diseases, Dr. Prakken said. “This is the first application of big data to develop a completely new, personalized map of the human immune system,” he added. “This technology has the potential to revolutionize human clinical immunology, and it may be the key to true precision medicine in inflammatory diseases.”
Other talks in the session will cover signaling pathways in childhood systemic lupus erythematosus (SLE), galectin-9 as a biomarker in juvenile dermatomyositis, and evidence from the phase 3 PRINTO trial on how best to taper corticosteroids in patients with new-onset juvenile dermatomyositis.
Another crucial topic in pediatric rheumatology is systemic hyperinflammation, a potentially life-threatening situation requiring rapid detection and treatment.
A Saturday morning session will dive deeply into this topic. First, Sebastiaan Vastert, MD, PhD, will share a birds-eye view of systemic inflammation in JIA, setting the stage for a discussion by Angelo Ravelli, MD, of challenges in diagnosing macrophage activation syndrome, which disproportionately affects JIA patients.
Also during the session, Fabrizio de Benedetti, MD, PhD, will review new findings on the pathogenesis of hyperinflammation and how they can guide therapeutic development. Rounding off the session, Rebecca Davies will present research on first-onset uveitis in patients receiving etanercept or methotrexate to treat JIA. “Attendees will learn new insights about diagnosing and treating systemic inflammation in children,” Dr. Prakken said.
Once the dust has settled on the EULAR Congress in Amsterdam, delegates can look forward to the 2019 EULAR Congress in Madrid, which will be held jointly with the Paediatric Rheumatology European Society, further integrating the fields of pediatric and adult rheumatology.
Personalized medicine, big data, and monogenic inflammatory diseases are just a few of the high points of pediatric rheumatology sessions at this year’s EULAR Congress.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Berent J. Prakken, MD, PhD, said that a bench-to-bedside session on Wednesday afternoon would highlight how EULAR projects are driving advances in pediatric rheumatology.
Attendees will learn from Vicki Seyfert-Margolis, PhD, an internationally recognized expert in personalized medicine, about how digital tools can facilitate cross-border partnerships in pediatric rheumatology, Dr. Prakken said in an interview.
“This talk will be groundbreaking because it’s not just about another useful app,” said Dr. Prakken, professor of pediatric rheumatology and vice dean of education at University Medical Center Utrecht (the Netherlands). “Dr. Seyfert-Margolis will show how the digital revolution will change the way we communicate with patients, monitor disease, and develop novel models for clinical trials.”
The session will also cover work by the Understanding Childhood Arthritis Network (UCAN), created to facilitate international translational research in pediatric rheumatology. Speakers will describe how UCAN is helping to spur personalized medicine and working with the Pediatric Rheumatology International Trials Organization (PRINTO) to align bench and bedside perspectives.
Another program highlight is a Thursday afternoon session on connections between monogenic autoinflammatory and pediatric rheumatic diseases. “Groundbreaking studies of these rare genetic inflammatory diseases have provided important new insights that, in turn, have led to new therapeutic options,” said Dr. Prakken.
During the session, Joost Swart, MD, of Utrecht, the Netherlands, will discuss promising research on the intravenous use of mesenchymal stromal cells derived from bone marrow for the treatment of refractory juvenile idiopathic arthritis (JIA).
Dr. Swart, who helped pioneer the approach, will discuss the first phase I/II trial of its use in children. “This is a truly innovative way to handle refractory inflammation,” Dr. Prakken said.
The session on monogenic autoinflammatory diseases also will cover their clinical presentation in children and adults, their pathogenesis as compared with adult-onset rheumatic diseases, and emerging treatment options, according to Dr. Prakken.
On Friday afternoon, a pediatric session will feature big data science in pediatric rheumatology, a lightning-paced field that is generating new research and treatment paradigms.
Of special note, Salvatore Albani, MD, PhD, will discuss how the human immunome is revolutionizing personalized treatment of paediatric inflammatory diseases, Dr. Prakken said. “This is the first application of big data to develop a completely new, personalized map of the human immune system,” he added. “This technology has the potential to revolutionize human clinical immunology, and it may be the key to true precision medicine in inflammatory diseases.”
Other talks in the session will cover signaling pathways in childhood systemic lupus erythematosus (SLE), galectin-9 as a biomarker in juvenile dermatomyositis, and evidence from the phase 3 PRINTO trial on how best to taper corticosteroids in patients with new-onset juvenile dermatomyositis.
Another crucial topic in pediatric rheumatology is systemic hyperinflammation, a potentially life-threatening situation requiring rapid detection and treatment.
A Saturday morning session will dive deeply into this topic. First, Sebastiaan Vastert, MD, PhD, will share a birds-eye view of systemic inflammation in JIA, setting the stage for a discussion by Angelo Ravelli, MD, of challenges in diagnosing macrophage activation syndrome, which disproportionately affects JIA patients.
Also during the session, Fabrizio de Benedetti, MD, PhD, will review new findings on the pathogenesis of hyperinflammation and how they can guide therapeutic development. Rounding off the session, Rebecca Davies will present research on first-onset uveitis in patients receiving etanercept or methotrexate to treat JIA. “Attendees will learn new insights about diagnosing and treating systemic inflammation in children,” Dr. Prakken said.
Once the dust has settled on the EULAR Congress in Amsterdam, delegates can look forward to the 2019 EULAR Congress in Madrid, which will be held jointly with the Paediatric Rheumatology European Society, further integrating the fields of pediatric and adult rheumatology.
Personalized medicine, big data, and monogenic inflammatory diseases are just a few of the high points of pediatric rheumatology sessions at this year’s EULAR Congress.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Berent J. Prakken, MD, PhD, said that a bench-to-bedside session on Wednesday afternoon would highlight how EULAR projects are driving advances in pediatric rheumatology.
Attendees will learn from Vicki Seyfert-Margolis, PhD, an internationally recognized expert in personalized medicine, about how digital tools can facilitate cross-border partnerships in pediatric rheumatology, Dr. Prakken said in an interview.
“This talk will be groundbreaking because it’s not just about another useful app,” said Dr. Prakken, professor of pediatric rheumatology and vice dean of education at University Medical Center Utrecht (the Netherlands). “Dr. Seyfert-Margolis will show how the digital revolution will change the way we communicate with patients, monitor disease, and develop novel models for clinical trials.”
The session will also cover work by the Understanding Childhood Arthritis Network (UCAN), created to facilitate international translational research in pediatric rheumatology. Speakers will describe how UCAN is helping to spur personalized medicine and working with the Pediatric Rheumatology International Trials Organization (PRINTO) to align bench and bedside perspectives.
Another program highlight is a Thursday afternoon session on connections between monogenic autoinflammatory and pediatric rheumatic diseases. “Groundbreaking studies of these rare genetic inflammatory diseases have provided important new insights that, in turn, have led to new therapeutic options,” said Dr. Prakken.
During the session, Joost Swart, MD, of Utrecht, the Netherlands, will discuss promising research on the intravenous use of mesenchymal stromal cells derived from bone marrow for the treatment of refractory juvenile idiopathic arthritis (JIA).
Dr. Swart, who helped pioneer the approach, will discuss the first phase I/II trial of its use in children. “This is a truly innovative way to handle refractory inflammation,” Dr. Prakken said.
The session on monogenic autoinflammatory diseases also will cover their clinical presentation in children and adults, their pathogenesis as compared with adult-onset rheumatic diseases, and emerging treatment options, according to Dr. Prakken.
On Friday afternoon, a pediatric session will feature big data science in pediatric rheumatology, a lightning-paced field that is generating new research and treatment paradigms.
Of special note, Salvatore Albani, MD, PhD, will discuss how the human immunome is revolutionizing personalized treatment of paediatric inflammatory diseases, Dr. Prakken said. “This is the first application of big data to develop a completely new, personalized map of the human immune system,” he added. “This technology has the potential to revolutionize human clinical immunology, and it may be the key to true precision medicine in inflammatory diseases.”
Other talks in the session will cover signaling pathways in childhood systemic lupus erythematosus (SLE), galectin-9 as a biomarker in juvenile dermatomyositis, and evidence from the phase 3 PRINTO trial on how best to taper corticosteroids in patients with new-onset juvenile dermatomyositis.
Another crucial topic in pediatric rheumatology is systemic hyperinflammation, a potentially life-threatening situation requiring rapid detection and treatment.
A Saturday morning session will dive deeply into this topic. First, Sebastiaan Vastert, MD, PhD, will share a birds-eye view of systemic inflammation in JIA, setting the stage for a discussion by Angelo Ravelli, MD, of challenges in diagnosing macrophage activation syndrome, which disproportionately affects JIA patients.
Also during the session, Fabrizio de Benedetti, MD, PhD, will review new findings on the pathogenesis of hyperinflammation and how they can guide therapeutic development. Rounding off the session, Rebecca Davies will present research on first-onset uveitis in patients receiving etanercept or methotrexate to treat JIA. “Attendees will learn new insights about diagnosing and treating systemic inflammation in children,” Dr. Prakken said.
Once the dust has settled on the EULAR Congress in Amsterdam, delegates can look forward to the 2019 EULAR Congress in Madrid, which will be held jointly with the Paediatric Rheumatology European Society, further integrating the fields of pediatric and adult rheumatology.
EMEUNET network of young rheumatologists keeps moving forward
As the largest network of young rheumatologists in the world, the Emerging EULAR Network (EMEUNET) is looking forward to this year’s European Congress of Rheumatology to continue to build on what it set out to do as part of its mission – to give young rheumatologists and researchers an active say in education and research.
The rationale for the birth of EMEUNET in 2009 was to provide a network where European young rheumatologists and researchers, no matter where they were based, could access mentoring programs, research funding, and education initiatives, Alessia Alunno, MD, PhD, chair-elect and EMEUNET steering committee member, said in an interview.
Since its creation, EMEUNET has already achieved its goal several times over, but there’s certainly no intention for the network to rest on its laurels. One of its major achievements has been to secure a voice in the EULAR Strategy for the next 5 years.
“We are now able to sit at the table with the other EULAR pillars so that the educational offering of EULAR is tailored to meet the needs of young rheumatologists and researchers across Europe,” Dr. Alunno said.
And if the success of an organization can be measured by its membership numbers, then it’s clear that EMEUNET is on the right track.
Each year, membership continues to grow, and at 1,909 members, the 2,000 member milestone is tantalizingly close. This makes EMEUNET the largest network of rheumatologists and researchers, which Dr. Alunno said made the steering committee proud because it is a sign that what EMEUNET offers to members is suitable for more and more people.
So what is it that EULAR congress attendees can look forward to, apart from a bike tour around the canals of Amsterdam and an abundance of museums to visit?
“As always, we aim to foster collaboration, encourage educational activities and participation in them, to grow our network and increase our reach,” she said in an interview.
“It’s really an opportunity to attract as many young people as possible through our many educational initiatives, not just during the actual congress but also all year long.”
One of the congress highlights that’s been running successfully for a few years is the EMEUNET mentor/mentee meetings where young and upcoming clinicians and academics are given the opportunity to meet with a mentor, discuss their careers, and get some advice and coaching.
EMEUNET also will be bringing some crowd-pleasing initiatives to Amsterdam, such as its popular networking event that attracted more than 70 attendees in Madrid last year.
“We are very proud of our networking event. Opportunities for us to meet all together are very few, so we have the opportunity at EULAR to provide a platform where people can get together and create their own contacts that can be helpful in their everyday life,” Dr. Alunno said.
The networking event usually involves a relaxed local sightseeing tour followed by a chance to talk, network, and strengthen bonds.
The EULAR/EMEUNET ambassador program is also an important and popular initiative that has received positive feedback from past congress attendees. The program is set up to help first-time attendees who may need some support to get the most out of what can be an overwhelming scientific program.
Fellows are invited to apply for the program (for more information visit www.emeunet.eular.org) and successful applicants will receive congress mentorship from EMEUNET EULAR congress “veterans” on how to make the most of the 4 days.
It goes without saying that EMEUNET is also contributing to the congress’ scientific program.
EMEUNET also invites everyone to check out the Young Rheumatologists sessions in the scientific program, which will take place during the course of the congress.
These will include sessions on basic statistics; understanding the language of basic research, epidemiology, and health services articles; and conducting effective patient-physician communication in routine clinical settings.
“The Young Rheumatologist sessions may be a small grain in the large congress program, but they always gain a lot of interest,” Dr. Nikiphorou said.
As the largest network of young rheumatologists in the world, the Emerging EULAR Network (EMEUNET) is looking forward to this year’s European Congress of Rheumatology to continue to build on what it set out to do as part of its mission – to give young rheumatologists and researchers an active say in education and research.
The rationale for the birth of EMEUNET in 2009 was to provide a network where European young rheumatologists and researchers, no matter where they were based, could access mentoring programs, research funding, and education initiatives, Alessia Alunno, MD, PhD, chair-elect and EMEUNET steering committee member, said in an interview.
Since its creation, EMEUNET has already achieved its goal several times over, but there’s certainly no intention for the network to rest on its laurels. One of its major achievements has been to secure a voice in the EULAR Strategy for the next 5 years.
“We are now able to sit at the table with the other EULAR pillars so that the educational offering of EULAR is tailored to meet the needs of young rheumatologists and researchers across Europe,” Dr. Alunno said.
And if the success of an organization can be measured by its membership numbers, then it’s clear that EMEUNET is on the right track.
Each year, membership continues to grow, and at 1,909 members, the 2,000 member milestone is tantalizingly close. This makes EMEUNET the largest network of rheumatologists and researchers, which Dr. Alunno said made the steering committee proud because it is a sign that what EMEUNET offers to members is suitable for more and more people.
So what is it that EULAR congress attendees can look forward to, apart from a bike tour around the canals of Amsterdam and an abundance of museums to visit?
“As always, we aim to foster collaboration, encourage educational activities and participation in them, to grow our network and increase our reach,” she said in an interview.
“It’s really an opportunity to attract as many young people as possible through our many educational initiatives, not just during the actual congress but also all year long.”
One of the congress highlights that’s been running successfully for a few years is the EMEUNET mentor/mentee meetings where young and upcoming clinicians and academics are given the opportunity to meet with a mentor, discuss their careers, and get some advice and coaching.
EMEUNET also will be bringing some crowd-pleasing initiatives to Amsterdam, such as its popular networking event that attracted more than 70 attendees in Madrid last year.
“We are very proud of our networking event. Opportunities for us to meet all together are very few, so we have the opportunity at EULAR to provide a platform where people can get together and create their own contacts that can be helpful in their everyday life,” Dr. Alunno said.
The networking event usually involves a relaxed local sightseeing tour followed by a chance to talk, network, and strengthen bonds.
The EULAR/EMEUNET ambassador program is also an important and popular initiative that has received positive feedback from past congress attendees. The program is set up to help first-time attendees who may need some support to get the most out of what can be an overwhelming scientific program.
Fellows are invited to apply for the program (for more information visit www.emeunet.eular.org) and successful applicants will receive congress mentorship from EMEUNET EULAR congress “veterans” on how to make the most of the 4 days.
It goes without saying that EMEUNET is also contributing to the congress’ scientific program.
EMEUNET also invites everyone to check out the Young Rheumatologists sessions in the scientific program, which will take place during the course of the congress.
These will include sessions on basic statistics; understanding the language of basic research, epidemiology, and health services articles; and conducting effective patient-physician communication in routine clinical settings.
“The Young Rheumatologist sessions may be a small grain in the large congress program, but they always gain a lot of interest,” Dr. Nikiphorou said.
As the largest network of young rheumatologists in the world, the Emerging EULAR Network (EMEUNET) is looking forward to this year’s European Congress of Rheumatology to continue to build on what it set out to do as part of its mission – to give young rheumatologists and researchers an active say in education and research.
The rationale for the birth of EMEUNET in 2009 was to provide a network where European young rheumatologists and researchers, no matter where they were based, could access mentoring programs, research funding, and education initiatives, Alessia Alunno, MD, PhD, chair-elect and EMEUNET steering committee member, said in an interview.
Since its creation, EMEUNET has already achieved its goal several times over, but there’s certainly no intention for the network to rest on its laurels. One of its major achievements has been to secure a voice in the EULAR Strategy for the next 5 years.
“We are now able to sit at the table with the other EULAR pillars so that the educational offering of EULAR is tailored to meet the needs of young rheumatologists and researchers across Europe,” Dr. Alunno said.
And if the success of an organization can be measured by its membership numbers, then it’s clear that EMEUNET is on the right track.
Each year, membership continues to grow, and at 1,909 members, the 2,000 member milestone is tantalizingly close. This makes EMEUNET the largest network of rheumatologists and researchers, which Dr. Alunno said made the steering committee proud because it is a sign that what EMEUNET offers to members is suitable for more and more people.
So what is it that EULAR congress attendees can look forward to, apart from a bike tour around the canals of Amsterdam and an abundance of museums to visit?
“As always, we aim to foster collaboration, encourage educational activities and participation in them, to grow our network and increase our reach,” she said in an interview.
“It’s really an opportunity to attract as many young people as possible through our many educational initiatives, not just during the actual congress but also all year long.”
One of the congress highlights that’s been running successfully for a few years is the EMEUNET mentor/mentee meetings where young and upcoming clinicians and academics are given the opportunity to meet with a mentor, discuss their careers, and get some advice and coaching.
EMEUNET also will be bringing some crowd-pleasing initiatives to Amsterdam, such as its popular networking event that attracted more than 70 attendees in Madrid last year.
“We are very proud of our networking event. Opportunities for us to meet all together are very few, so we have the opportunity at EULAR to provide a platform where people can get together and create their own contacts that can be helpful in their everyday life,” Dr. Alunno said.
The networking event usually involves a relaxed local sightseeing tour followed by a chance to talk, network, and strengthen bonds.
The EULAR/EMEUNET ambassador program is also an important and popular initiative that has received positive feedback from past congress attendees. The program is set up to help first-time attendees who may need some support to get the most out of what can be an overwhelming scientific program.
Fellows are invited to apply for the program (for more information visit www.emeunet.eular.org) and successful applicants will receive congress mentorship from EMEUNET EULAR congress “veterans” on how to make the most of the 4 days.
It goes without saying that EMEUNET is also contributing to the congress’ scientific program.
EMEUNET also invites everyone to check out the Young Rheumatologists sessions in the scientific program, which will take place during the course of the congress.
These will include sessions on basic statistics; understanding the language of basic research, epidemiology, and health services articles; and conducting effective patient-physician communication in routine clinical settings.
“The Young Rheumatologist sessions may be a small grain in the large congress program, but they always gain a lot of interest,” Dr. Nikiphorou said.
Going to VAM? Download the VAM Mobile App
The mobile app for the 2018 Vascular Annual Meeting will be available Wednesday for both Apple and Android products. The app has been rebuilt from the ground up, for a very user-friendly experience. It’s interactive, comprehensive and searchable and includes many helpful features:
- My Schedule: Mark sessions as favorites on either the Planner or app, then see all of them in the My Schedule section.
- Educational Credits: Take self-assessment exams, via the app, and/or claim Continuing Medical Education credits.
- Scavenger Hunt: Participate in the big game in the Exhibit Hall; using your app, scan QR codes found in the booths of sponsors, then answer the question that pops up. The three people who earn the most points for correct answers win great prizes.
- Social Media – Let all your friends know what you’re up to by linking to social media
Download at Apple’s App Store and at Google Play.
The mobile app for the 2018 Vascular Annual Meeting will be available Wednesday for both Apple and Android products. The app has been rebuilt from the ground up, for a very user-friendly experience. It’s interactive, comprehensive and searchable and includes many helpful features:
- My Schedule: Mark sessions as favorites on either the Planner or app, then see all of them in the My Schedule section.
- Educational Credits: Take self-assessment exams, via the app, and/or claim Continuing Medical Education credits.
- Scavenger Hunt: Participate in the big game in the Exhibit Hall; using your app, scan QR codes found in the booths of sponsors, then answer the question that pops up. The three people who earn the most points for correct answers win great prizes.
- Social Media – Let all your friends know what you’re up to by linking to social media
Download at Apple’s App Store and at Google Play.
The mobile app for the 2018 Vascular Annual Meeting will be available Wednesday for both Apple and Android products. The app has been rebuilt from the ground up, for a very user-friendly experience. It’s interactive, comprehensive and searchable and includes many helpful features:
- My Schedule: Mark sessions as favorites on either the Planner or app, then see all of them in the My Schedule section.
- Educational Credits: Take self-assessment exams, via the app, and/or claim Continuing Medical Education credits.
- Scavenger Hunt: Participate in the big game in the Exhibit Hall; using your app, scan QR codes found in the booths of sponsors, then answer the question that pops up. The three people who earn the most points for correct answers win great prizes.
- Social Media – Let all your friends know what you’re up to by linking to social media
Download at Apple’s App Store and at Google Play.
VAM is Next Week – Are you Registered?
Don’t miss the vascular surgery world’s headline event! Join colleagues and friends in Boston for this year’s Vascular Annual Meeting, June 20 to 23. Scientific sessions are June 21-23 and the Exhibit Hall is open June 21 to 22. Click here to register. To get a full schedule and begin creating your own personal agenda, complete with marking sessions as favorites, see the VAM Planner.
Don’t miss the vascular surgery world’s headline event! Join colleagues and friends in Boston for this year’s Vascular Annual Meeting, June 20 to 23. Scientific sessions are June 21-23 and the Exhibit Hall is open June 21 to 22. Click here to register. To get a full schedule and begin creating your own personal agenda, complete with marking sessions as favorites, see the VAM Planner.
Don’t miss the vascular surgery world’s headline event! Join colleagues and friends in Boston for this year’s Vascular Annual Meeting, June 20 to 23. Scientific sessions are June 21-23 and the Exhibit Hall is open June 21 to 22. Click here to register. To get a full schedule and begin creating your own personal agenda, complete with marking sessions as favorites, see the VAM Planner.
The VA Cannot Be Privatized
As usual, it was a hectic Monday on the psychiatry consult service. All the trainees, from medical student to fellow, were seeing other patients when the call came from the surgery clinic. One of the pleasures of being a VA clinician is the ability to teach and supervise medical students and residents. The attending in that busy clinic said, “There is a patient down here who is refusing care for a gangrenous leg, but he is also talking about his life not being worth living. Could someone evaluate him?” That patient, Mr. S, declined to go to either the emergency department or the urgent care psychiatry clinic, so I went to see him. I realized that I had seen this patient in the hospital several times before.
One of the great clinical benefits of working in the VA, as opposed to in academic or community hospitals, is continuity. In my nearly 20 years at the same medical center, I have had the privilege of following many patients through multiple courses of treatment. This continuity is a huge advantage when there is what Hippocrates called a “critical day,” as on that Monday in the surgery clinic.2 Also, in many cases the continuity allows me to have a reservoir of trust that I can draw on for challenging consultations, like that of Mr. S.
The surgery resident and attending had spent more than an hour talking to Mr. S when I arrived but still they joined me for the conversation. Mr. S was a veteran in his sixties, and after a few minutes of listening to him, it was clear he was talking about ending his life because of its poor quality. He told us that he had acquired the infection in his leg secondary to unsanitary living conditions. The veteran was quite a storyteller, intelligent, and had a wry sense of humor, which only made his point that his living conditions were intolerable more poignant. He apparently had tried to talk to someone about his situation but felt frustrated that he had not obtained more help.
The surgery attending had already told Mr. S that he would respect his right to refuse the amputation but he feared that Mr. S’s refusal was an expression of his depression and hopelessness, hence, the psychiatry consult. Although Mr. S was not acutely suicidal, something about the combination of his despair and deliberation worried me.
The surgery attending offered to admit Mr. S to do a further workup of his leg. I encouraged him to accept this option and added that I would make sure a social worker saw him and the psychiatry service department also would follow him. Mr. S declined even a 24-hour admission, saying that he had just moved to a new apartment and “everything I have in the world is there and I don’t want to lose it.” This comment suggested to me that he was ambivalent about his wish to die and provided an opening to reduce his risk of harming himself either directly or indirectly.
After the discussion, Mr. S seemed to believe we cared about him and was more willing to participate in treatment planning. He agreed to let the surgeons draw blood and to pick up oral antibiotics from the pharmacy. I promised him that if he would come back to clinic that week, I would make sure a social worker met with him and that my team would talk with him more about his depression. Mr. S picked Friday for his return and assured me that now that he knew we were going to try and improve his situation, he would not hurt himself. Obviously, this was a risk on my part—but the show of compassion combined with flexibility had created a therapeutic alliance that I believed was sufficient to protect Mr. S until we met again.
I returned to my office and called the chief of social work: The dedication of career VA employees forges effective working relationships that can be leveraged for the benefit of the patients. At my facility and many others, many of the staff members who are now in positions of leadership rose through the ranks together, giving us a solidarity of purpose and mutual reliance that are rare in community health care settings. The chief of social work looked at the patient’s chart with me on the phone while I explained the circumstances and within a few minutes said, “We can help him. It looks like he is eligible for an increased pension, and I think we can find him better housing.”
I admit to some anxiety on Friday. One of the psychiatry residents on the service had volunteered to see Mr. S after studying his chart in the morning. Most of us are aware that the aging VA electronic health record system is due to be replaced. But having access to more than 20 years of medical history from episodes of inpatient, outpatient, and residential care all over the country is an unrivaled asset that brings a unique breadth that sharpens, deepens, and humanizes diagnosis and treatment planning.
Sure enough at 10
The social worker arranged new housing for Mr. S that day and help to move into his new place. The paperwork was submitted for the pension increase, and help for shopping and meals as well as transportation was either put in place or applied for. As he left to pack, Mr. S told the surgeon he might not want hospice just yet.
The coda to this narrative is equally uplifting. Several weeks after Mr. S was seen in the surgery clinic, I received a call from a midlevel psychiatric practitioner in the urgent care clinic who had been on leave for several weeks. He too had seen Mr. S before and shared my concern about his state of mind and well-being. He thanked me for having the consult service see him and remarked that it was a relief to know Mr. S had been taken care of and was in a better place in every sense of the word.
In response to a rising media tide of concern about the direction VA care is headed, Congress and the VA have issued a strong statements, “debunking” what they called the “myth” of privatization.3 Yet for the first time in my career, many thoughtful people discern a constellation of forces that could eventuate in this reality in our lifetimes. The title and message of this column is that the VA cannot be privatized, not that it will not be privatized. Also, I did not say that it should not be privatized. As I have written in other columns, that is because ethically I do not believe this is even a question.4 Privatization breaks President Abraham Lincoln’s promise to veterans, “to care for him who has borne the battle.” A promise that was kept for Mr. S and is fulfilled for thousands of other veterans every day all over this nation. A promise that far exceeds payments for medical services.
I also do not mean the title to be a rejection of the Veterans Choice Program. The VA has always provided—and should continue to offer—community-based care for veterans that complements VA care. For example, I live in one of the most rural states in the union and recognize that a patient should not have to drive 300 miles to get a routine colonoscopy.
The VA cannot be privatized because of the comprehensive care that it provides: the degree of integration; the wealth of resources; and the level of expertise in caring for the complex medical, psychiatric, and psychosocial problems of veterans cannot be replicated. Nor is this just my opinion—a recent RAND Corporation study documents the evidence.5 There are many medical services in the private sector that may be delivered more efficiently, and Congress has just passed the Mission Act to allocate the funds needed to ensure our veterans have wider and easier access to private care resources.6 Yet someone must coordinate, monitor, and center all these services on the veteran. It is not likely Mr. S’s story would have had this kind of ending in the community. The continuity of care, the access to staff with the knowledge of veterans benefits and health care needs, and the ability to listen and follow up without time or performance constraints is just not possible outside VA.
The other evening in the parking lot of the hospital, I encountered a physician who had left the VA to work in several other large health care organizations. He had some good things to say about their business processes and the volume of patients they saw. He came back to the VA, he said, because “No one else can provide this quality of care for the individual veteran.”
1. Conway E, Batalden P. Like magic? (“Every system is perfectly designed…”). http://www.ihi.org/communities/blogs/o rigin-of-every-system-is-perfectly-designed-quote. Published August 21, 2015. Accessed May 29, 2018.
2. Lloyd GER, ed. Hippocratic Writings . London: Penguin Books ; 1983.
3. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Debunking the VA privatization myth [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4034. Published April 5, 2018. Accessed June 4, 2018.
4. Geppert CMA. Lessons from history: the ethical foundation of VA health care. Fed Pract. 2016;33(4):6-7.
5. Tanielian T, Farmer CM, Burns RM, Duffy EL, Messan Setodji C. Ready or Not? Assessing the Capacity of New York State Health Care Providers to Meet the Needs of Veterans. Santa Monica, CA: RAND Corporation, 2018.
6. VA MISSION Act of 2018, S 2372, 115th Congress, 2nd Sess (2018).
As usual, it was a hectic Monday on the psychiatry consult service. All the trainees, from medical student to fellow, were seeing other patients when the call came from the surgery clinic. One of the pleasures of being a VA clinician is the ability to teach and supervise medical students and residents. The attending in that busy clinic said, “There is a patient down here who is refusing care for a gangrenous leg, but he is also talking about his life not being worth living. Could someone evaluate him?” That patient, Mr. S, declined to go to either the emergency department or the urgent care psychiatry clinic, so I went to see him. I realized that I had seen this patient in the hospital several times before.
One of the great clinical benefits of working in the VA, as opposed to in academic or community hospitals, is continuity. In my nearly 20 years at the same medical center, I have had the privilege of following many patients through multiple courses of treatment. This continuity is a huge advantage when there is what Hippocrates called a “critical day,” as on that Monday in the surgery clinic.2 Also, in many cases the continuity allows me to have a reservoir of trust that I can draw on for challenging consultations, like that of Mr. S.
The surgery resident and attending had spent more than an hour talking to Mr. S when I arrived but still they joined me for the conversation. Mr. S was a veteran in his sixties, and after a few minutes of listening to him, it was clear he was talking about ending his life because of its poor quality. He told us that he had acquired the infection in his leg secondary to unsanitary living conditions. The veteran was quite a storyteller, intelligent, and had a wry sense of humor, which only made his point that his living conditions were intolerable more poignant. He apparently had tried to talk to someone about his situation but felt frustrated that he had not obtained more help.
The surgery attending had already told Mr. S that he would respect his right to refuse the amputation but he feared that Mr. S’s refusal was an expression of his depression and hopelessness, hence, the psychiatry consult. Although Mr. S was not acutely suicidal, something about the combination of his despair and deliberation worried me.
The surgery attending offered to admit Mr. S to do a further workup of his leg. I encouraged him to accept this option and added that I would make sure a social worker saw him and the psychiatry service department also would follow him. Mr. S declined even a 24-hour admission, saying that he had just moved to a new apartment and “everything I have in the world is there and I don’t want to lose it.” This comment suggested to me that he was ambivalent about his wish to die and provided an opening to reduce his risk of harming himself either directly or indirectly.
After the discussion, Mr. S seemed to believe we cared about him and was more willing to participate in treatment planning. He agreed to let the surgeons draw blood and to pick up oral antibiotics from the pharmacy. I promised him that if he would come back to clinic that week, I would make sure a social worker met with him and that my team would talk with him more about his depression. Mr. S picked Friday for his return and assured me that now that he knew we were going to try and improve his situation, he would not hurt himself. Obviously, this was a risk on my part—but the show of compassion combined with flexibility had created a therapeutic alliance that I believed was sufficient to protect Mr. S until we met again.
I returned to my office and called the chief of social work: The dedication of career VA employees forges effective working relationships that can be leveraged for the benefit of the patients. At my facility and many others, many of the staff members who are now in positions of leadership rose through the ranks together, giving us a solidarity of purpose and mutual reliance that are rare in community health care settings. The chief of social work looked at the patient’s chart with me on the phone while I explained the circumstances and within a few minutes said, “We can help him. It looks like he is eligible for an increased pension, and I think we can find him better housing.”
I admit to some anxiety on Friday. One of the psychiatry residents on the service had volunteered to see Mr. S after studying his chart in the morning. Most of us are aware that the aging VA electronic health record system is due to be replaced. But having access to more than 20 years of medical history from episodes of inpatient, outpatient, and residential care all over the country is an unrivaled asset that brings a unique breadth that sharpens, deepens, and humanizes diagnosis and treatment planning.
Sure enough at 10
The social worker arranged new housing for Mr. S that day and help to move into his new place. The paperwork was submitted for the pension increase, and help for shopping and meals as well as transportation was either put in place or applied for. As he left to pack, Mr. S told the surgeon he might not want hospice just yet.
The coda to this narrative is equally uplifting. Several weeks after Mr. S was seen in the surgery clinic, I received a call from a midlevel psychiatric practitioner in the urgent care clinic who had been on leave for several weeks. He too had seen Mr. S before and shared my concern about his state of mind and well-being. He thanked me for having the consult service see him and remarked that it was a relief to know Mr. S had been taken care of and was in a better place in every sense of the word.
In response to a rising media tide of concern about the direction VA care is headed, Congress and the VA have issued a strong statements, “debunking” what they called the “myth” of privatization.3 Yet for the first time in my career, many thoughtful people discern a constellation of forces that could eventuate in this reality in our lifetimes. The title and message of this column is that the VA cannot be privatized, not that it will not be privatized. Also, I did not say that it should not be privatized. As I have written in other columns, that is because ethically I do not believe this is even a question.4 Privatization breaks President Abraham Lincoln’s promise to veterans, “to care for him who has borne the battle.” A promise that was kept for Mr. S and is fulfilled for thousands of other veterans every day all over this nation. A promise that far exceeds payments for medical services.
I also do not mean the title to be a rejection of the Veterans Choice Program. The VA has always provided—and should continue to offer—community-based care for veterans that complements VA care. For example, I live in one of the most rural states in the union and recognize that a patient should not have to drive 300 miles to get a routine colonoscopy.
The VA cannot be privatized because of the comprehensive care that it provides: the degree of integration; the wealth of resources; and the level of expertise in caring for the complex medical, psychiatric, and psychosocial problems of veterans cannot be replicated. Nor is this just my opinion—a recent RAND Corporation study documents the evidence.5 There are many medical services in the private sector that may be delivered more efficiently, and Congress has just passed the Mission Act to allocate the funds needed to ensure our veterans have wider and easier access to private care resources.6 Yet someone must coordinate, monitor, and center all these services on the veteran. It is not likely Mr. S’s story would have had this kind of ending in the community. The continuity of care, the access to staff with the knowledge of veterans benefits and health care needs, and the ability to listen and follow up without time or performance constraints is just not possible outside VA.
The other evening in the parking lot of the hospital, I encountered a physician who had left the VA to work in several other large health care organizations. He had some good things to say about their business processes and the volume of patients they saw. He came back to the VA, he said, because “No one else can provide this quality of care for the individual veteran.”
As usual, it was a hectic Monday on the psychiatry consult service. All the trainees, from medical student to fellow, were seeing other patients when the call came from the surgery clinic. One of the pleasures of being a VA clinician is the ability to teach and supervise medical students and residents. The attending in that busy clinic said, “There is a patient down here who is refusing care for a gangrenous leg, but he is also talking about his life not being worth living. Could someone evaluate him?” That patient, Mr. S, declined to go to either the emergency department or the urgent care psychiatry clinic, so I went to see him. I realized that I had seen this patient in the hospital several times before.
One of the great clinical benefits of working in the VA, as opposed to in academic or community hospitals, is continuity. In my nearly 20 years at the same medical center, I have had the privilege of following many patients through multiple courses of treatment. This continuity is a huge advantage when there is what Hippocrates called a “critical day,” as on that Monday in the surgery clinic.2 Also, in many cases the continuity allows me to have a reservoir of trust that I can draw on for challenging consultations, like that of Mr. S.
The surgery resident and attending had spent more than an hour talking to Mr. S when I arrived but still they joined me for the conversation. Mr. S was a veteran in his sixties, and after a few minutes of listening to him, it was clear he was talking about ending his life because of its poor quality. He told us that he had acquired the infection in his leg secondary to unsanitary living conditions. The veteran was quite a storyteller, intelligent, and had a wry sense of humor, which only made his point that his living conditions were intolerable more poignant. He apparently had tried to talk to someone about his situation but felt frustrated that he had not obtained more help.
The surgery attending had already told Mr. S that he would respect his right to refuse the amputation but he feared that Mr. S’s refusal was an expression of his depression and hopelessness, hence, the psychiatry consult. Although Mr. S was not acutely suicidal, something about the combination of his despair and deliberation worried me.
The surgery attending offered to admit Mr. S to do a further workup of his leg. I encouraged him to accept this option and added that I would make sure a social worker saw him and the psychiatry service department also would follow him. Mr. S declined even a 24-hour admission, saying that he had just moved to a new apartment and “everything I have in the world is there and I don’t want to lose it.” This comment suggested to me that he was ambivalent about his wish to die and provided an opening to reduce his risk of harming himself either directly or indirectly.
After the discussion, Mr. S seemed to believe we cared about him and was more willing to participate in treatment planning. He agreed to let the surgeons draw blood and to pick up oral antibiotics from the pharmacy. I promised him that if he would come back to clinic that week, I would make sure a social worker met with him and that my team would talk with him more about his depression. Mr. S picked Friday for his return and assured me that now that he knew we were going to try and improve his situation, he would not hurt himself. Obviously, this was a risk on my part—but the show of compassion combined with flexibility had created a therapeutic alliance that I believed was sufficient to protect Mr. S until we met again.
I returned to my office and called the chief of social work: The dedication of career VA employees forges effective working relationships that can be leveraged for the benefit of the patients. At my facility and many others, many of the staff members who are now in positions of leadership rose through the ranks together, giving us a solidarity of purpose and mutual reliance that are rare in community health care settings. The chief of social work looked at the patient’s chart with me on the phone while I explained the circumstances and within a few minutes said, “We can help him. It looks like he is eligible for an increased pension, and I think we can find him better housing.”
I admit to some anxiety on Friday. One of the psychiatry residents on the service had volunteered to see Mr. S after studying his chart in the morning. Most of us are aware that the aging VA electronic health record system is due to be replaced. But having access to more than 20 years of medical history from episodes of inpatient, outpatient, and residential care all over the country is an unrivaled asset that brings a unique breadth that sharpens, deepens, and humanizes diagnosis and treatment planning.
Sure enough at 10
The social worker arranged new housing for Mr. S that day and help to move into his new place. The paperwork was submitted for the pension increase, and help for shopping and meals as well as transportation was either put in place or applied for. As he left to pack, Mr. S told the surgeon he might not want hospice just yet.
The coda to this narrative is equally uplifting. Several weeks after Mr. S was seen in the surgery clinic, I received a call from a midlevel psychiatric practitioner in the urgent care clinic who had been on leave for several weeks. He too had seen Mr. S before and shared my concern about his state of mind and well-being. He thanked me for having the consult service see him and remarked that it was a relief to know Mr. S had been taken care of and was in a better place in every sense of the word.
In response to a rising media tide of concern about the direction VA care is headed, Congress and the VA have issued a strong statements, “debunking” what they called the “myth” of privatization.3 Yet for the first time in my career, many thoughtful people discern a constellation of forces that could eventuate in this reality in our lifetimes. The title and message of this column is that the VA cannot be privatized, not that it will not be privatized. Also, I did not say that it should not be privatized. As I have written in other columns, that is because ethically I do not believe this is even a question.4 Privatization breaks President Abraham Lincoln’s promise to veterans, “to care for him who has borne the battle.” A promise that was kept for Mr. S and is fulfilled for thousands of other veterans every day all over this nation. A promise that far exceeds payments for medical services.
I also do not mean the title to be a rejection of the Veterans Choice Program. The VA has always provided—and should continue to offer—community-based care for veterans that complements VA care. For example, I live in one of the most rural states in the union and recognize that a patient should not have to drive 300 miles to get a routine colonoscopy.
The VA cannot be privatized because of the comprehensive care that it provides: the degree of integration; the wealth of resources; and the level of expertise in caring for the complex medical, psychiatric, and psychosocial problems of veterans cannot be replicated. Nor is this just my opinion—a recent RAND Corporation study documents the evidence.5 There are many medical services in the private sector that may be delivered more efficiently, and Congress has just passed the Mission Act to allocate the funds needed to ensure our veterans have wider and easier access to private care resources.6 Yet someone must coordinate, monitor, and center all these services on the veteran. It is not likely Mr. S’s story would have had this kind of ending in the community. The continuity of care, the access to staff with the knowledge of veterans benefits and health care needs, and the ability to listen and follow up without time or performance constraints is just not possible outside VA.
The other evening in the parking lot of the hospital, I encountered a physician who had left the VA to work in several other large health care organizations. He had some good things to say about their business processes and the volume of patients they saw. He came back to the VA, he said, because “No one else can provide this quality of care for the individual veteran.”
1. Conway E, Batalden P. Like magic? (“Every system is perfectly designed…”). http://www.ihi.org/communities/blogs/o rigin-of-every-system-is-perfectly-designed-quote. Published August 21, 2015. Accessed May 29, 2018.
2. Lloyd GER, ed. Hippocratic Writings . London: Penguin Books ; 1983.
3. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Debunking the VA privatization myth [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4034. Published April 5, 2018. Accessed June 4, 2018.
4. Geppert CMA. Lessons from history: the ethical foundation of VA health care. Fed Pract. 2016;33(4):6-7.
5. Tanielian T, Farmer CM, Burns RM, Duffy EL, Messan Setodji C. Ready or Not? Assessing the Capacity of New York State Health Care Providers to Meet the Needs of Veterans. Santa Monica, CA: RAND Corporation, 2018.
6. VA MISSION Act of 2018, S 2372, 115th Congress, 2nd Sess (2018).
1. Conway E, Batalden P. Like magic? (“Every system is perfectly designed…”). http://www.ihi.org/communities/blogs/o rigin-of-every-system-is-perfectly-designed-quote. Published August 21, 2015. Accessed May 29, 2018.
2. Lloyd GER, ed. Hippocratic Writings . London: Penguin Books ; 1983.
3. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Debunking the VA privatization myth [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4034. Published April 5, 2018. Accessed June 4, 2018.
4. Geppert CMA. Lessons from history: the ethical foundation of VA health care. Fed Pract. 2016;33(4):6-7.
5. Tanielian T, Farmer CM, Burns RM, Duffy EL, Messan Setodji C. Ready or Not? Assessing the Capacity of New York State Health Care Providers to Meet the Needs of Veterans. Santa Monica, CA: RAND Corporation, 2018.
6. VA MISSION Act of 2018, S 2372, 115th Congress, 2nd Sess (2018).