User login
NIH launches HEAL Initiative to combat opioid crisis
The NIH HEAL (Helping to End Addiction Long-term) Initiative aims to bring together agencies across the federal government, as well as academic institutions, private industry, and patient advocates to find new solutions to address the current national health emergency.
“There are 15 initiatives altogether that are being put out that we think are pretty bold and should make a big difference in our understanding of what to do about this national public health crisis,” NIH Director Francis Collins, MD, said in an interview.
HEAL will investigate ways to reformulate existing treatments for opioid use disorder (OUD), to improve efficacy and extend their availability to more patients.
“Although there are effective medications for OUD (methadone, buprenorphine, and naltrexone), only a small percentage of individuals in the United States who would benefit receive these medications,” according to an editorial introducing the NIH HEAL Initiative published in JAMA (doi:10.1001/jama.2018.8826). “Even among those who have initiated these medications, about half will relapse within 6 months.”
The editorial was authored by Dr. Collins, Walter J. Koroshetz, MD, director of the National Institute of Neurological Disorders and Stroke, and Nora Volkow, MD, director of the National Institute on Drug Abuse.
For example, the current formulation of naltrexone lasts about a month within the body, Dr. Collins said in an interview. “If we had a 6-month version of that, I think it would be much more effective because oftentimes the relapses happen after a month or so, before people have fully gotten themselves on the ground.”
Better overdose antidotes are needed as well, he said, particularly for fentanyl overdose. “Narcan may not be strong enough for those long-lasting and very potent opioids like fentanyl,” he said.
HEAL also will seek a better understanding of neonatal opioid withdrawal syndrome (NOWS), also referred to as neonatal abstinence syndrome, which has become alarmingly common as more women of childbearing potential struggle with opioid addiction.
“Innovative methods to identify and treat newborns exposed to opioids, often along with other drugs, have the potential to improve both short- and long-term developmental outcomes in such children,” Dr. Collins and colleagues noted. “To determine better approaches, HEAL will expand Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). This pilot study is designed to assess the prevalence of NOWS, understand current approaches to managing NOWS , and develop common approaches for larger-scale studies that will determine best practices for clinical care of infants with NOWS throughout the country.”
HEAL efforts also seek to find integrated approaches to OUD treatment.
“One particularly bold element is to put together a number of pilot projects that enable bringing together all of the ways in which we are trying to turn this epidemic around by making it possible to assess whether individuals who are addicted can be successfully treated and maintained in abstinence for long periods of time,” Dr. Collins said. “Right now, the success is not so great.
“Suppose we brought together all of the treatment programs – the primary care facilities, the emergency rooms, the fire departments, the social work experts, the health departments in the states, the local communities, the criminal justice system. We brought together all of those players in a research design where we can really see what was working. Could we do a lot more to turn this around than basically doing one of those at a time? There is this multisite idea of a national research effort, still somewhat in development, but to do integration of all of these efforts. I am pretty excited about that one.”
In looking for better ways to treat pain safely and effectively, “we need to understand how it is that people transition from acute pain to chronic pain … and what can we do increase the likelihood of recovery from acute pain without making that transition,” Dr. Collins said. “Then we need to identify additional novel targets for developing pain therapies, both devices and pharmaceuticals. We need better means of testing those ideas.”
In addition to gaining a better understanding of chronic pain, HEAL aims to investigate new nonaddictive pain treatments and find ways to expedite those treatments through the clinical pipeline, according to Dr. Collins and colleagues.
HEAL “lays the foundation for an innovative therapy-development pipeline through a planned new public-private partnership. In collaboration with biopharmaceutical groups, the Food and Drug Administration, and the Foundation for the NIH, the NIH will collect and evaluate treatment assets from academia and biopharmaceutical and device companies to coordinate and accelerate the development of effective treatments for pain and addiction,” they wrote.
SOURCE: Collins F et al, JAMA doi: 10.1001/jama.2018.8826.
The NIH HEAL (Helping to End Addiction Long-term) Initiative aims to bring together agencies across the federal government, as well as academic institutions, private industry, and patient advocates to find new solutions to address the current national health emergency.
“There are 15 initiatives altogether that are being put out that we think are pretty bold and should make a big difference in our understanding of what to do about this national public health crisis,” NIH Director Francis Collins, MD, said in an interview.
HEAL will investigate ways to reformulate existing treatments for opioid use disorder (OUD), to improve efficacy and extend their availability to more patients.
“Although there are effective medications for OUD (methadone, buprenorphine, and naltrexone), only a small percentage of individuals in the United States who would benefit receive these medications,” according to an editorial introducing the NIH HEAL Initiative published in JAMA (doi:10.1001/jama.2018.8826). “Even among those who have initiated these medications, about half will relapse within 6 months.”
The editorial was authored by Dr. Collins, Walter J. Koroshetz, MD, director of the National Institute of Neurological Disorders and Stroke, and Nora Volkow, MD, director of the National Institute on Drug Abuse.
For example, the current formulation of naltrexone lasts about a month within the body, Dr. Collins said in an interview. “If we had a 6-month version of that, I think it would be much more effective because oftentimes the relapses happen after a month or so, before people have fully gotten themselves on the ground.”
Better overdose antidotes are needed as well, he said, particularly for fentanyl overdose. “Narcan may not be strong enough for those long-lasting and very potent opioids like fentanyl,” he said.
HEAL also will seek a better understanding of neonatal opioid withdrawal syndrome (NOWS), also referred to as neonatal abstinence syndrome, which has become alarmingly common as more women of childbearing potential struggle with opioid addiction.
“Innovative methods to identify and treat newborns exposed to opioids, often along with other drugs, have the potential to improve both short- and long-term developmental outcomes in such children,” Dr. Collins and colleagues noted. “To determine better approaches, HEAL will expand Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). This pilot study is designed to assess the prevalence of NOWS, understand current approaches to managing NOWS , and develop common approaches for larger-scale studies that will determine best practices for clinical care of infants with NOWS throughout the country.”
HEAL efforts also seek to find integrated approaches to OUD treatment.
“One particularly bold element is to put together a number of pilot projects that enable bringing together all of the ways in which we are trying to turn this epidemic around by making it possible to assess whether individuals who are addicted can be successfully treated and maintained in abstinence for long periods of time,” Dr. Collins said. “Right now, the success is not so great.
“Suppose we brought together all of the treatment programs – the primary care facilities, the emergency rooms, the fire departments, the social work experts, the health departments in the states, the local communities, the criminal justice system. We brought together all of those players in a research design where we can really see what was working. Could we do a lot more to turn this around than basically doing one of those at a time? There is this multisite idea of a national research effort, still somewhat in development, but to do integration of all of these efforts. I am pretty excited about that one.”
In looking for better ways to treat pain safely and effectively, “we need to understand how it is that people transition from acute pain to chronic pain … and what can we do increase the likelihood of recovery from acute pain without making that transition,” Dr. Collins said. “Then we need to identify additional novel targets for developing pain therapies, both devices and pharmaceuticals. We need better means of testing those ideas.”
In addition to gaining a better understanding of chronic pain, HEAL aims to investigate new nonaddictive pain treatments and find ways to expedite those treatments through the clinical pipeline, according to Dr. Collins and colleagues.
HEAL “lays the foundation for an innovative therapy-development pipeline through a planned new public-private partnership. In collaboration with biopharmaceutical groups, the Food and Drug Administration, and the Foundation for the NIH, the NIH will collect and evaluate treatment assets from academia and biopharmaceutical and device companies to coordinate and accelerate the development of effective treatments for pain and addiction,” they wrote.
SOURCE: Collins F et al, JAMA doi: 10.1001/jama.2018.8826.
The NIH HEAL (Helping to End Addiction Long-term) Initiative aims to bring together agencies across the federal government, as well as academic institutions, private industry, and patient advocates to find new solutions to address the current national health emergency.
“There are 15 initiatives altogether that are being put out that we think are pretty bold and should make a big difference in our understanding of what to do about this national public health crisis,” NIH Director Francis Collins, MD, said in an interview.
HEAL will investigate ways to reformulate existing treatments for opioid use disorder (OUD), to improve efficacy and extend their availability to more patients.
“Although there are effective medications for OUD (methadone, buprenorphine, and naltrexone), only a small percentage of individuals in the United States who would benefit receive these medications,” according to an editorial introducing the NIH HEAL Initiative published in JAMA (doi:10.1001/jama.2018.8826). “Even among those who have initiated these medications, about half will relapse within 6 months.”
The editorial was authored by Dr. Collins, Walter J. Koroshetz, MD, director of the National Institute of Neurological Disorders and Stroke, and Nora Volkow, MD, director of the National Institute on Drug Abuse.
For example, the current formulation of naltrexone lasts about a month within the body, Dr. Collins said in an interview. “If we had a 6-month version of that, I think it would be much more effective because oftentimes the relapses happen after a month or so, before people have fully gotten themselves on the ground.”
Better overdose antidotes are needed as well, he said, particularly for fentanyl overdose. “Narcan may not be strong enough for those long-lasting and very potent opioids like fentanyl,” he said.
HEAL also will seek a better understanding of neonatal opioid withdrawal syndrome (NOWS), also referred to as neonatal abstinence syndrome, which has become alarmingly common as more women of childbearing potential struggle with opioid addiction.
“Innovative methods to identify and treat newborns exposed to opioids, often along with other drugs, have the potential to improve both short- and long-term developmental outcomes in such children,” Dr. Collins and colleagues noted. “To determine better approaches, HEAL will expand Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). This pilot study is designed to assess the prevalence of NOWS, understand current approaches to managing NOWS , and develop common approaches for larger-scale studies that will determine best practices for clinical care of infants with NOWS throughout the country.”
HEAL efforts also seek to find integrated approaches to OUD treatment.
“One particularly bold element is to put together a number of pilot projects that enable bringing together all of the ways in which we are trying to turn this epidemic around by making it possible to assess whether individuals who are addicted can be successfully treated and maintained in abstinence for long periods of time,” Dr. Collins said. “Right now, the success is not so great.
“Suppose we brought together all of the treatment programs – the primary care facilities, the emergency rooms, the fire departments, the social work experts, the health departments in the states, the local communities, the criminal justice system. We brought together all of those players in a research design where we can really see what was working. Could we do a lot more to turn this around than basically doing one of those at a time? There is this multisite idea of a national research effort, still somewhat in development, but to do integration of all of these efforts. I am pretty excited about that one.”
In looking for better ways to treat pain safely and effectively, “we need to understand how it is that people transition from acute pain to chronic pain … and what can we do increase the likelihood of recovery from acute pain without making that transition,” Dr. Collins said. “Then we need to identify additional novel targets for developing pain therapies, both devices and pharmaceuticals. We need better means of testing those ideas.”
In addition to gaining a better understanding of chronic pain, HEAL aims to investigate new nonaddictive pain treatments and find ways to expedite those treatments through the clinical pipeline, according to Dr. Collins and colleagues.
HEAL “lays the foundation for an innovative therapy-development pipeline through a planned new public-private partnership. In collaboration with biopharmaceutical groups, the Food and Drug Administration, and the Foundation for the NIH, the NIH will collect and evaluate treatment assets from academia and biopharmaceutical and device companies to coordinate and accelerate the development of effective treatments for pain and addiction,” they wrote.
SOURCE: Collins F et al, JAMA doi: 10.1001/jama.2018.8826.
FROM JAMA
New JAK-1 inhibitor had high, early efficacy in rheumatoid arthritis trial
LIVERPOOL, ENGLAND – Treatment with the investigational drug upadacitinib resulted in higher percentages of patients with active rheumatoid arthritis achieving good disease control within 12 weeks than did treatment with placebo in a phase 3 trial.
Two-thirds of patients met American College of Rheumatology 20% (ACR 20) response criteria, and almost half (48%) achieved a Disease Activity Score in 28 Joints–C-reactive protein (DAS28-CRP) of 3.2 or less versus 17% of placebo-treated patients (P less than .001).
All patients had been given upadacitinib on top of their existing conventional synthetic disease-modifying antirheumatic drug therapy because they had not been fully responding to csDMARDs alone.
“Onset of action was rapid: By week 1 significantly more patients achieved ACR 20 on upadacitinib at both doses versus placebo,” the study’s investigators noted in a poster presentation given at the British Society for Rheumatology annual conference. Significant improvements in DAS28-CRP and Clinical Disease Activity Index (CDAI) were also seen as early as week 1.
Gerd R. Burmester, MD, of Charité-Universitätsmedizin, Berlin, and associates reported the results of the SELECT-NEXT study, one of six global phase 3 studies testing the efficacy and safety of upadacitinib targeting “a range of different patient populations” with RA; together the trials include more than 4,500 patients.
Upadacitinib (ABT-494) is a selective Janus kinase (JAK)-1 inhibitor and is also in phase 3 trials for the treatment of psoriatic arthritis and Crohn’s disease, as well as being tested as a potential treatment for axial spondyloarthritis, giant cell arteritis, ulcerative colitis, and atopic dermatitis.
SELECT-NEXT consists of two phases, the first of which has been completed and was reported at the meeting. Phase 1 consisted of randomized, double-blind treatment with upadacitinib 15 mg or 30 mg once daily or matching placebo. After the coprimary endpoint assessment of ACR 20 and DAS28-CRP was undertaken at 12 weeks, the trial entered its second phase: This is a blinded-extension phase that will last up to 5 years and during which patients randomized to upadacitinib will continue their treatment, and the patients randomized to placebo will split into two groups and be treated with one or the other dose of upadacitinib.
The study included 661 patients who had been treated with csDMARDs for at least 3 months but still had swollen and tender joint counts of six or higher and high-sensitivity CRP levels of 3 mg/L or higher. At entry, patients were allowed to continue on up to two csDMARDs; stable-dose steroids (less than 10 mg/week), nonsteroidal anti-inflammatory drugs, and acetaminophen were also allowed.
“Significant changes from baseline in several patient-reported outcomes were observed,” with the active treatment versus placebo, the investigators observed. Indeed, by week 12, morning stiffness was reduced by an average of 85 minutes in patients taking upadacitinib versus a decrease of 34 minutes in the placebo group. Significant (P less than .01) improvements were also seen in Short-Form 36–Mental Component scores, they reported.
Furthermore, more patients taking the active treatment than placebo achieved clinical remission by 12 weeks.
“The safety and tolerability profile was consistent with observations in the phase 2 studies,” Dr. Burmester and associates observed. The most frequently reported adverse events in more than 3% of patients were nasopharyngitis, upper respiratory infection, headache, urinary tract infection, cough, nausea, and diarrhea.
Serious adverse events and those leading to discontinuation were both higher in the two upadacitinib groups versus placebo, at 2.7% and 5.9% for the 30-mg dose, 4.1% and 3.2% for the 15-mg dose, and 2.3% and 3.2% for placebo. Of note, infections occurred in a 31.5% of patients on 30 mg, 29.0% on 15 mg, and 21.3% on placebo, and hepatic disorders occurred in 2.7% of patients on 30 mg, 1.8% on 15 mg, and 2.3% on placebo. One (0.5%) patient taking the 30-mg dose had a major cardiovascular event and two (0.9%) patients in the 15-mg group had other adjudicated cardiovascular events.
The study was sponsored and run by Abbvie. The study authors acknowledged receiving research support or consulting fees from Abbvie or being employees of the company.
SOURCE: Burmester GR et al.; BSR 2018 Rheumatology. 2018;57[Suppl. 3]:key075.466.
LIVERPOOL, ENGLAND – Treatment with the investigational drug upadacitinib resulted in higher percentages of patients with active rheumatoid arthritis achieving good disease control within 12 weeks than did treatment with placebo in a phase 3 trial.
Two-thirds of patients met American College of Rheumatology 20% (ACR 20) response criteria, and almost half (48%) achieved a Disease Activity Score in 28 Joints–C-reactive protein (DAS28-CRP) of 3.2 or less versus 17% of placebo-treated patients (P less than .001).
All patients had been given upadacitinib on top of their existing conventional synthetic disease-modifying antirheumatic drug therapy because they had not been fully responding to csDMARDs alone.
“Onset of action was rapid: By week 1 significantly more patients achieved ACR 20 on upadacitinib at both doses versus placebo,” the study’s investigators noted in a poster presentation given at the British Society for Rheumatology annual conference. Significant improvements in DAS28-CRP and Clinical Disease Activity Index (CDAI) were also seen as early as week 1.
Gerd R. Burmester, MD, of Charité-Universitätsmedizin, Berlin, and associates reported the results of the SELECT-NEXT study, one of six global phase 3 studies testing the efficacy and safety of upadacitinib targeting “a range of different patient populations” with RA; together the trials include more than 4,500 patients.
Upadacitinib (ABT-494) is a selective Janus kinase (JAK)-1 inhibitor and is also in phase 3 trials for the treatment of psoriatic arthritis and Crohn’s disease, as well as being tested as a potential treatment for axial spondyloarthritis, giant cell arteritis, ulcerative colitis, and atopic dermatitis.
SELECT-NEXT consists of two phases, the first of which has been completed and was reported at the meeting. Phase 1 consisted of randomized, double-blind treatment with upadacitinib 15 mg or 30 mg once daily or matching placebo. After the coprimary endpoint assessment of ACR 20 and DAS28-CRP was undertaken at 12 weeks, the trial entered its second phase: This is a blinded-extension phase that will last up to 5 years and during which patients randomized to upadacitinib will continue their treatment, and the patients randomized to placebo will split into two groups and be treated with one or the other dose of upadacitinib.
The study included 661 patients who had been treated with csDMARDs for at least 3 months but still had swollen and tender joint counts of six or higher and high-sensitivity CRP levels of 3 mg/L or higher. At entry, patients were allowed to continue on up to two csDMARDs; stable-dose steroids (less than 10 mg/week), nonsteroidal anti-inflammatory drugs, and acetaminophen were also allowed.
“Significant changes from baseline in several patient-reported outcomes were observed,” with the active treatment versus placebo, the investigators observed. Indeed, by week 12, morning stiffness was reduced by an average of 85 minutes in patients taking upadacitinib versus a decrease of 34 minutes in the placebo group. Significant (P less than .01) improvements were also seen in Short-Form 36–Mental Component scores, they reported.
Furthermore, more patients taking the active treatment than placebo achieved clinical remission by 12 weeks.
“The safety and tolerability profile was consistent with observations in the phase 2 studies,” Dr. Burmester and associates observed. The most frequently reported adverse events in more than 3% of patients were nasopharyngitis, upper respiratory infection, headache, urinary tract infection, cough, nausea, and diarrhea.
Serious adverse events and those leading to discontinuation were both higher in the two upadacitinib groups versus placebo, at 2.7% and 5.9% for the 30-mg dose, 4.1% and 3.2% for the 15-mg dose, and 2.3% and 3.2% for placebo. Of note, infections occurred in a 31.5% of patients on 30 mg, 29.0% on 15 mg, and 21.3% on placebo, and hepatic disorders occurred in 2.7% of patients on 30 mg, 1.8% on 15 mg, and 2.3% on placebo. One (0.5%) patient taking the 30-mg dose had a major cardiovascular event and two (0.9%) patients in the 15-mg group had other adjudicated cardiovascular events.
The study was sponsored and run by Abbvie. The study authors acknowledged receiving research support or consulting fees from Abbvie or being employees of the company.
SOURCE: Burmester GR et al.; BSR 2018 Rheumatology. 2018;57[Suppl. 3]:key075.466.
LIVERPOOL, ENGLAND – Treatment with the investigational drug upadacitinib resulted in higher percentages of patients with active rheumatoid arthritis achieving good disease control within 12 weeks than did treatment with placebo in a phase 3 trial.
Two-thirds of patients met American College of Rheumatology 20% (ACR 20) response criteria, and almost half (48%) achieved a Disease Activity Score in 28 Joints–C-reactive protein (DAS28-CRP) of 3.2 or less versus 17% of placebo-treated patients (P less than .001).
All patients had been given upadacitinib on top of their existing conventional synthetic disease-modifying antirheumatic drug therapy because they had not been fully responding to csDMARDs alone.
“Onset of action was rapid: By week 1 significantly more patients achieved ACR 20 on upadacitinib at both doses versus placebo,” the study’s investigators noted in a poster presentation given at the British Society for Rheumatology annual conference. Significant improvements in DAS28-CRP and Clinical Disease Activity Index (CDAI) were also seen as early as week 1.
Gerd R. Burmester, MD, of Charité-Universitätsmedizin, Berlin, and associates reported the results of the SELECT-NEXT study, one of six global phase 3 studies testing the efficacy and safety of upadacitinib targeting “a range of different patient populations” with RA; together the trials include more than 4,500 patients.
Upadacitinib (ABT-494) is a selective Janus kinase (JAK)-1 inhibitor and is also in phase 3 trials for the treatment of psoriatic arthritis and Crohn’s disease, as well as being tested as a potential treatment for axial spondyloarthritis, giant cell arteritis, ulcerative colitis, and atopic dermatitis.
SELECT-NEXT consists of two phases, the first of which has been completed and was reported at the meeting. Phase 1 consisted of randomized, double-blind treatment with upadacitinib 15 mg or 30 mg once daily or matching placebo. After the coprimary endpoint assessment of ACR 20 and DAS28-CRP was undertaken at 12 weeks, the trial entered its second phase: This is a blinded-extension phase that will last up to 5 years and during which patients randomized to upadacitinib will continue their treatment, and the patients randomized to placebo will split into two groups and be treated with one or the other dose of upadacitinib.
The study included 661 patients who had been treated with csDMARDs for at least 3 months but still had swollen and tender joint counts of six or higher and high-sensitivity CRP levels of 3 mg/L or higher. At entry, patients were allowed to continue on up to two csDMARDs; stable-dose steroids (less than 10 mg/week), nonsteroidal anti-inflammatory drugs, and acetaminophen were also allowed.
“Significant changes from baseline in several patient-reported outcomes were observed,” with the active treatment versus placebo, the investigators observed. Indeed, by week 12, morning stiffness was reduced by an average of 85 minutes in patients taking upadacitinib versus a decrease of 34 minutes in the placebo group. Significant (P less than .01) improvements were also seen in Short-Form 36–Mental Component scores, they reported.
Furthermore, more patients taking the active treatment than placebo achieved clinical remission by 12 weeks.
“The safety and tolerability profile was consistent with observations in the phase 2 studies,” Dr. Burmester and associates observed. The most frequently reported adverse events in more than 3% of patients were nasopharyngitis, upper respiratory infection, headache, urinary tract infection, cough, nausea, and diarrhea.
Serious adverse events and those leading to discontinuation were both higher in the two upadacitinib groups versus placebo, at 2.7% and 5.9% for the 30-mg dose, 4.1% and 3.2% for the 15-mg dose, and 2.3% and 3.2% for placebo. Of note, infections occurred in a 31.5% of patients on 30 mg, 29.0% on 15 mg, and 21.3% on placebo, and hepatic disorders occurred in 2.7% of patients on 30 mg, 1.8% on 15 mg, and 2.3% on placebo. One (0.5%) patient taking the 30-mg dose had a major cardiovascular event and two (0.9%) patients in the 15-mg group had other adjudicated cardiovascular events.
The study was sponsored and run by Abbvie. The study authors acknowledged receiving research support or consulting fees from Abbvie or being employees of the company.
SOURCE: Burmester GR et al.; BSR 2018 Rheumatology. 2018;57[Suppl. 3]:key075.466.
REPORTING FROM Rheumatology 2018
Key clinical point: Upadacitinib met both of the coprimary endpoints in the trial – ACR 20 response and low disease activity at 12 weeks.
Major finding: ACR 20 was achieved by 64%/66%/36% of patients taking upadacitinib 15 mg once daily, patients taking upadacitinib 30 mg once daily, and placebo-treated patients, respectively. Low disease activity was achieved by 48%/48%/17% of patients taking upadacitinib 15 mg once daily, patients taking upadacitinib 30 mg once daily, and placebo-treated patients, respectively (P less than .001).
Study details: SELECT-NEXT, a phase 3, randomized, double-blind, placebo-controlled trial of two doses of upadacitinib in 661 patients with rheumatoid arthritis who were not responding to conventional disease-modifying drug treatment.
Disclosures: The study was sponsored by Abbvie. The study authors acknowledged receiving research support or consulting fees from Abbvie or being employees of the company.
Source: Burmester GR et al. BSR 2018; Rheumatology. 2018;57[Suppl. 3]:key075.466.
Insurer denials of DAA therapy for HCV on the rise
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
FROM OPEN FORUM INFECTIOUS DISEASES
Key clinical point: Insurance denials of direct-acting antiviral (DAA) prescriptions have increased over time, contrary to expectations.
Major finding: A total of 35.5% of patients had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Study details: A cohort study including 9,025 patients who had a DAA prescription submitted to a national specialty pharmacy between January 2016 and April 2017.
Disclosures: The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Two coauthors reported grant support and/or advisory board fees from Gilead.
Source: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7.
Tenapanor shows safety, efficacy for irritable bowel syndrome
WASHINGTON –
These data combined with results from an already reported additional phase 3 trial and a phase 2 study will go to the Food and Drug Administration later in 2018 in an application for marketing approval for tenapanor, William D. Chey, MD, said at the annual Digestive Disease Week.®
“Tenapanor may represent a novel, effective treatment option” for patients with constipation-predominant irritable bowel syndrome (IBS-C), said Dr. Chey, a professor of medicine and director of the GI Physiology Laboratory at the University of Michigan in Ann Arbor.
The study results met the trial’s primary endpoint, the percentage of patients with a combined response consisting of at least a 30% drop from baseline in reported abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) per week for 6 of the first 12 weeks of treatment. This combined response occurred in 37% of patients treated with tenapanor at a dosage of 50 mg orally b.i.d., compared with a 24% rate among the placebo-control patients, a statistically significant difference, Dr. Chey reported.
The most common adverse effect seen in the tenapanor-treated patients was diarrhea, which occurred in 16% of the drug-treated patients and in 4% of controls. “I think diarrhea is an expected adverse effect,” Dr. Chey said. Overall, treatment-related adverse effects occurred in 23% of tenapanor-treated patients and in 9% of controls, serious adverse effects occurred in 4% of patients on tenapanor and in 3% of controls, and adverse effects leading to treatment discontinuation occurred in 8% on tenapanor and in 1% of controls. Aside from diarrhea, the other most common adverse effects linked with tenapanor treatment were abdominal distension, in 3%, and flatulence, also in 3%.
Tenapanor is an inhibitor of sodium/hydrogen exchanger isoform 3, the predominant intestinal sodium transporter. Through this inhibition tenapanor reduces sodium uptake in the gut, causing increased intestinal fluid volume and shorter transit time and thereby softening stool consistency and increasing bowel movement frequency. Dr. Chey and his colleagues previously reported results from a phase 2 study of tenapanor (Am J Gastroenterol. 2017 Feb;112[2]:763-74), and from a phase 3 study with 606 patients reported at a meeting in late 2017. Results from these two studies were similar to those from the new study.
The current study, A 26-Week Study to Evaluate the Efficacy and Safety of Tenapanor in IBS-C (T3MPO-2) enrolled 593 patients at 114 U.S. centers. Enrolled patients met the Rome III criteria for IBS-C and had an average CSMB frequency of less than 3/week. The researchers treated and followed patients for 26 weeks, although the primary endpoint occurred after 12 weeks on treatment, and 481 of the enrolled patients remained in the study through 26 weeks. At baseline, patients had an average of 0.12 CSBM/week and an average abdominal pain score of 6.26, indicative of moderate to severe abdominal pain. These characteristics identified the enrolled patients as being “on the more severe spectrum of what we see in clinical practice,” Dr. Chey noted.
Secondary endpoints included the combined endpoint with the target rate of CSBM achieved in at least 9 of the first 12 weeks, 18% on the active drug and 5% on placebo, and in at least 13 of the 26 weeks on treatment, 36% on tenapanor and 24% on placebo. After 26 weeks on treatment, 55% of patients on tenapanor rated themselves as quite satisfied or very satisfied with their treatment, compared with 33% of the placebo-control patients.
T3MPO-2 was funded by Ardelyx, the company developing tenapanor. Dr. Chey has been a consultant to and has received research funding from Ardelyx and from several other companies. A coauthor on the study was an Ardelyx employee.
[email protected]
On Twitter @mitchelzoler
WASHINGTON –
These data combined with results from an already reported additional phase 3 trial and a phase 2 study will go to the Food and Drug Administration later in 2018 in an application for marketing approval for tenapanor, William D. Chey, MD, said at the annual Digestive Disease Week.®
“Tenapanor may represent a novel, effective treatment option” for patients with constipation-predominant irritable bowel syndrome (IBS-C), said Dr. Chey, a professor of medicine and director of the GI Physiology Laboratory at the University of Michigan in Ann Arbor.
The study results met the trial’s primary endpoint, the percentage of patients with a combined response consisting of at least a 30% drop from baseline in reported abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) per week for 6 of the first 12 weeks of treatment. This combined response occurred in 37% of patients treated with tenapanor at a dosage of 50 mg orally b.i.d., compared with a 24% rate among the placebo-control patients, a statistically significant difference, Dr. Chey reported.
The most common adverse effect seen in the tenapanor-treated patients was diarrhea, which occurred in 16% of the drug-treated patients and in 4% of controls. “I think diarrhea is an expected adverse effect,” Dr. Chey said. Overall, treatment-related adverse effects occurred in 23% of tenapanor-treated patients and in 9% of controls, serious adverse effects occurred in 4% of patients on tenapanor and in 3% of controls, and adverse effects leading to treatment discontinuation occurred in 8% on tenapanor and in 1% of controls. Aside from diarrhea, the other most common adverse effects linked with tenapanor treatment were abdominal distension, in 3%, and flatulence, also in 3%.
Tenapanor is an inhibitor of sodium/hydrogen exchanger isoform 3, the predominant intestinal sodium transporter. Through this inhibition tenapanor reduces sodium uptake in the gut, causing increased intestinal fluid volume and shorter transit time and thereby softening stool consistency and increasing bowel movement frequency. Dr. Chey and his colleagues previously reported results from a phase 2 study of tenapanor (Am J Gastroenterol. 2017 Feb;112[2]:763-74), and from a phase 3 study with 606 patients reported at a meeting in late 2017. Results from these two studies were similar to those from the new study.
The current study, A 26-Week Study to Evaluate the Efficacy and Safety of Tenapanor in IBS-C (T3MPO-2) enrolled 593 patients at 114 U.S. centers. Enrolled patients met the Rome III criteria for IBS-C and had an average CSMB frequency of less than 3/week. The researchers treated and followed patients for 26 weeks, although the primary endpoint occurred after 12 weeks on treatment, and 481 of the enrolled patients remained in the study through 26 weeks. At baseline, patients had an average of 0.12 CSBM/week and an average abdominal pain score of 6.26, indicative of moderate to severe abdominal pain. These characteristics identified the enrolled patients as being “on the more severe spectrum of what we see in clinical practice,” Dr. Chey noted.
Secondary endpoints included the combined endpoint with the target rate of CSBM achieved in at least 9 of the first 12 weeks, 18% on the active drug and 5% on placebo, and in at least 13 of the 26 weeks on treatment, 36% on tenapanor and 24% on placebo. After 26 weeks on treatment, 55% of patients on tenapanor rated themselves as quite satisfied or very satisfied with their treatment, compared with 33% of the placebo-control patients.
T3MPO-2 was funded by Ardelyx, the company developing tenapanor. Dr. Chey has been a consultant to and has received research funding from Ardelyx and from several other companies. A coauthor on the study was an Ardelyx employee.
[email protected]
On Twitter @mitchelzoler
WASHINGTON –
These data combined with results from an already reported additional phase 3 trial and a phase 2 study will go to the Food and Drug Administration later in 2018 in an application for marketing approval for tenapanor, William D. Chey, MD, said at the annual Digestive Disease Week.®
“Tenapanor may represent a novel, effective treatment option” for patients with constipation-predominant irritable bowel syndrome (IBS-C), said Dr. Chey, a professor of medicine and director of the GI Physiology Laboratory at the University of Michigan in Ann Arbor.
The study results met the trial’s primary endpoint, the percentage of patients with a combined response consisting of at least a 30% drop from baseline in reported abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) per week for 6 of the first 12 weeks of treatment. This combined response occurred in 37% of patients treated with tenapanor at a dosage of 50 mg orally b.i.d., compared with a 24% rate among the placebo-control patients, a statistically significant difference, Dr. Chey reported.
The most common adverse effect seen in the tenapanor-treated patients was diarrhea, which occurred in 16% of the drug-treated patients and in 4% of controls. “I think diarrhea is an expected adverse effect,” Dr. Chey said. Overall, treatment-related adverse effects occurred in 23% of tenapanor-treated patients and in 9% of controls, serious adverse effects occurred in 4% of patients on tenapanor and in 3% of controls, and adverse effects leading to treatment discontinuation occurred in 8% on tenapanor and in 1% of controls. Aside from diarrhea, the other most common adverse effects linked with tenapanor treatment were abdominal distension, in 3%, and flatulence, also in 3%.
Tenapanor is an inhibitor of sodium/hydrogen exchanger isoform 3, the predominant intestinal sodium transporter. Through this inhibition tenapanor reduces sodium uptake in the gut, causing increased intestinal fluid volume and shorter transit time and thereby softening stool consistency and increasing bowel movement frequency. Dr. Chey and his colleagues previously reported results from a phase 2 study of tenapanor (Am J Gastroenterol. 2017 Feb;112[2]:763-74), and from a phase 3 study with 606 patients reported at a meeting in late 2017. Results from these two studies were similar to those from the new study.
The current study, A 26-Week Study to Evaluate the Efficacy and Safety of Tenapanor in IBS-C (T3MPO-2) enrolled 593 patients at 114 U.S. centers. Enrolled patients met the Rome III criteria for IBS-C and had an average CSMB frequency of less than 3/week. The researchers treated and followed patients for 26 weeks, although the primary endpoint occurred after 12 weeks on treatment, and 481 of the enrolled patients remained in the study through 26 weeks. At baseline, patients had an average of 0.12 CSBM/week and an average abdominal pain score of 6.26, indicative of moderate to severe abdominal pain. These characteristics identified the enrolled patients as being “on the more severe spectrum of what we see in clinical practice,” Dr. Chey noted.
Secondary endpoints included the combined endpoint with the target rate of CSBM achieved in at least 9 of the first 12 weeks, 18% on the active drug and 5% on placebo, and in at least 13 of the 26 weeks on treatment, 36% on tenapanor and 24% on placebo. After 26 weeks on treatment, 55% of patients on tenapanor rated themselves as quite satisfied or very satisfied with their treatment, compared with 33% of the placebo-control patients.
T3MPO-2 was funded by Ardelyx, the company developing tenapanor. Dr. Chey has been a consultant to and has received research funding from Ardelyx and from several other companies. A coauthor on the study was an Ardelyx employee.
[email protected]
On Twitter @mitchelzoler
REPORTING FROM DDW 2018
Key clinical point: New drug shows safety and efficacy for irritable bowel syndrome.
Major finding: The combined primary endpoint occurred in 37% of tenapanor-treated patients and in 24% of patients on placebo.
Study details: T3MPO-2, a multicenter U.S. trial with 593 patients.
Disclosures: T3MPO-2 was funded by Ardelyx, the company developing tenapanor. Dr. Chey has been a consultant to and has received research funding from Ardelyx and from several other companies. A coauthor on the study was an Ardelyx employee.
More Smoke-Free Campuses, Not Enough Screening and Counseling
People with mental and/or substance abuse disorders are more than twice as likely to smoke cigarettes as people without those disorders, and are more likely to die of a smoking-related illness than from a behavioral health condition. Yet many people are not screened for tobacco use in behavioral health facilities. According to the Centers for Disease Control and Prevention (CDC) and Substance Abuse and Mental Health Services Administration (SAMHSA), in 2016, only half of mental health treatment facilities and 64% of substance abuse treatment facilities reported screening patients for tobacco use.
Even fewer facilities provide counseling and treatments. Only 38% of mental health facilities offer tobacco cessation counseling, 25% offer nicotine replacement therapy (NRT), and 22% offer non-nicotine cessation medications. Of substance abuse treatment facilities, 47% offer tobacco cessation counseling, 26% offer NRT, and 20% offer non-nicotine cessation medications. Oklahoma and New York had the highest percentage of programs.
On the other hand, 49% of mental health and 33% of substance abuse treatment facilities now have smoke-free campuses in the 50 states, Washington DC, and Puerto Rico. That number varies by state, ranging from 20% of mental health facilities in Idaho to 78% in Oklahoma, and 10% of substance abuse treatment facilities in Idaho to 83% in New York.
Practical steps can boost the availability of smoking cessation screening and programs. The CDC recommends integrating screening and treatment protocols into workflows and electronic health record systems. The CDC also advises providing outreach to behavioral health providers who reinforce the message that patients can benefit from evidence-based cessation treatment.
The report is based on data from the 2016 National Mental Health Services Survey and the 2016 National Survey of Substance Abuse Treatment Services.
People with mental and/or substance abuse disorders are more than twice as likely to smoke cigarettes as people without those disorders, and are more likely to die of a smoking-related illness than from a behavioral health condition. Yet many people are not screened for tobacco use in behavioral health facilities. According to the Centers for Disease Control and Prevention (CDC) and Substance Abuse and Mental Health Services Administration (SAMHSA), in 2016, only half of mental health treatment facilities and 64% of substance abuse treatment facilities reported screening patients for tobacco use.
Even fewer facilities provide counseling and treatments. Only 38% of mental health facilities offer tobacco cessation counseling, 25% offer nicotine replacement therapy (NRT), and 22% offer non-nicotine cessation medications. Of substance abuse treatment facilities, 47% offer tobacco cessation counseling, 26% offer NRT, and 20% offer non-nicotine cessation medications. Oklahoma and New York had the highest percentage of programs.
On the other hand, 49% of mental health and 33% of substance abuse treatment facilities now have smoke-free campuses in the 50 states, Washington DC, and Puerto Rico. That number varies by state, ranging from 20% of mental health facilities in Idaho to 78% in Oklahoma, and 10% of substance abuse treatment facilities in Idaho to 83% in New York.
Practical steps can boost the availability of smoking cessation screening and programs. The CDC recommends integrating screening and treatment protocols into workflows and electronic health record systems. The CDC also advises providing outreach to behavioral health providers who reinforce the message that patients can benefit from evidence-based cessation treatment.
The report is based on data from the 2016 National Mental Health Services Survey and the 2016 National Survey of Substance Abuse Treatment Services.
People with mental and/or substance abuse disorders are more than twice as likely to smoke cigarettes as people without those disorders, and are more likely to die of a smoking-related illness than from a behavioral health condition. Yet many people are not screened for tobacco use in behavioral health facilities. According to the Centers for Disease Control and Prevention (CDC) and Substance Abuse and Mental Health Services Administration (SAMHSA), in 2016, only half of mental health treatment facilities and 64% of substance abuse treatment facilities reported screening patients for tobacco use.
Even fewer facilities provide counseling and treatments. Only 38% of mental health facilities offer tobacco cessation counseling, 25% offer nicotine replacement therapy (NRT), and 22% offer non-nicotine cessation medications. Of substance abuse treatment facilities, 47% offer tobacco cessation counseling, 26% offer NRT, and 20% offer non-nicotine cessation medications. Oklahoma and New York had the highest percentage of programs.
On the other hand, 49% of mental health and 33% of substance abuse treatment facilities now have smoke-free campuses in the 50 states, Washington DC, and Puerto Rico. That number varies by state, ranging from 20% of mental health facilities in Idaho to 78% in Oklahoma, and 10% of substance abuse treatment facilities in Idaho to 83% in New York.
Practical steps can boost the availability of smoking cessation screening and programs. The CDC recommends integrating screening and treatment protocols into workflows and electronic health record systems. The CDC also advises providing outreach to behavioral health providers who reinforce the message that patients can benefit from evidence-based cessation treatment.
The report is based on data from the 2016 National Mental Health Services Survey and the 2016 National Survey of Substance Abuse Treatment Services.
Depression follows job loss after acute MI
Patients who experienced adverse changes in employment status after acute myocardial infarction (AMI) reported increased depression and lower quality of life, according to results published June 12 in Circulation: Cardiovascular Quality and Outcomes.
At 1-year follow-up, 27.4% of patients with adverse employment change scored high on measures of depression, compared with 16.7% of patients who did not experience a change in status. These patients also reported lower health status and difficulty affording medications, wrote Haider J. Warraich, MD, a cardiologist at Duke University in Durham, N.C., and coauthors.
Baseline data were collected for all patients according to CathPCI Registry standards, and follow-up was conducted via telephone 6 weeks, 6 months, 1 year, and 15 months after discharge. After 1 year, patients who reported working full or part time were defined as working. Adverse change in employment was defined as those who “reported working immediately before the index MI hospitalization but were either no longer working or working fewer hours,” Dr. Warraich and his colleagues reported.
Depression was defined by a Patient Health Questionnaire (PHQ) score greater than 3. Health status was assessed using the EuroQoL-5 Dimensions (EQ-5D) visual analog scale. Medication adherence was assessed using three questions at the follow-up interview, and patients were also asked to rank the financial hardship of their monthly medication costs on a scale of 1-5.
Among patients working at baseline, 492 (10%) reported an adverse change in employment after a year. Of these, 349 (7%) were no longer working, and 143 (3%) were working less than before. Of those with an adverse change in employment, 172 reported involuntary job loss such as being laid off or no longer working due to health concerns. Just 27 patients reported retirement.
The number of readmissions within the first year was the factor most strongly associated with adverse change in employment. Baseline smoking status, hypertension, and postdischarge bleeding were also significantly associated with adverse change in employment, the authors said.
At 1 year follow-up, patients with an adverse change in employment were more likely than those with no change to report depression (27.4% with PHQ score greater than 3, compared with 16.7% in the no-change group). These patients also reported lower health status (mean EuroQoL score of 73 compared with 78) and moderate to extreme financial hardship with medication costs (41.0% compared with 28.4%), though there was no difference in medication adherence, the authors reported.
The results indicate that, although job loss in acute MI patients has dropped in comparison with previous studies, patients who experience an adverse change in employment are still “at increased risk of depression, lower quality of life, and increased financial hardship with medication costs compared with those who continue working,” the authors wrote.
“These results underscore the need for interventions to address this patient-centered outcome and its health impact,” they concluded.
Daiichi Sankyo and Lilly USA funded TRANSLATE-ACS. This analysis was funded in part by a grant from the National Heart, Lung, and Blood Institute.
SOURCE: Warraich H et al. Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.117.004528.
The results of this study are promising, as previous research has shown up to 51% adverse change in employment status at 1-year follow-up.
Nevertheless, the rate of work loss reported in this study requires continued improvements to patient-centered care. Providing such care requires a shift that recognizes that the success of treatments is dependent on patients not only adhering to treatments but also actively engaging in their own self-care.
The U.S. workforce is aging, with a 72% increase since 2000 in the number of workers 55 years and older. Nearly half the workforce is female, and racial and ethnic minority groups make up 25% of it. These same populations experience poorer cardiovascular outcomes after AMI, highlighting the importance of incorporating patient preferences about return to work and continued employment into patient care planning.
Moving forward, future work is required to understand the barriers to successful return to work for these patients. The need for interventions that support successful return to work requires continued attention by researchers and clinicians.
Rachel P. Dreyer, PhD, of Yale University in New Haven, Conn., and Victoria Vaughan Dickson, PhD, of New York University, made these comments in an editorial published with the study (Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.118.004806.)
The results of this study are promising, as previous research has shown up to 51% adverse change in employment status at 1-year follow-up.
Nevertheless, the rate of work loss reported in this study requires continued improvements to patient-centered care. Providing such care requires a shift that recognizes that the success of treatments is dependent on patients not only adhering to treatments but also actively engaging in their own self-care.
The U.S. workforce is aging, with a 72% increase since 2000 in the number of workers 55 years and older. Nearly half the workforce is female, and racial and ethnic minority groups make up 25% of it. These same populations experience poorer cardiovascular outcomes after AMI, highlighting the importance of incorporating patient preferences about return to work and continued employment into patient care planning.
Moving forward, future work is required to understand the barriers to successful return to work for these patients. The need for interventions that support successful return to work requires continued attention by researchers and clinicians.
Rachel P. Dreyer, PhD, of Yale University in New Haven, Conn., and Victoria Vaughan Dickson, PhD, of New York University, made these comments in an editorial published with the study (Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.118.004806.)
The results of this study are promising, as previous research has shown up to 51% adverse change in employment status at 1-year follow-up.
Nevertheless, the rate of work loss reported in this study requires continued improvements to patient-centered care. Providing such care requires a shift that recognizes that the success of treatments is dependent on patients not only adhering to treatments but also actively engaging in their own self-care.
The U.S. workforce is aging, with a 72% increase since 2000 in the number of workers 55 years and older. Nearly half the workforce is female, and racial and ethnic minority groups make up 25% of it. These same populations experience poorer cardiovascular outcomes after AMI, highlighting the importance of incorporating patient preferences about return to work and continued employment into patient care planning.
Moving forward, future work is required to understand the barriers to successful return to work for these patients. The need for interventions that support successful return to work requires continued attention by researchers and clinicians.
Rachel P. Dreyer, PhD, of Yale University in New Haven, Conn., and Victoria Vaughan Dickson, PhD, of New York University, made these comments in an editorial published with the study (Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.118.004806.)
Patients who experienced adverse changes in employment status after acute myocardial infarction (AMI) reported increased depression and lower quality of life, according to results published June 12 in Circulation: Cardiovascular Quality and Outcomes.
At 1-year follow-up, 27.4% of patients with adverse employment change scored high on measures of depression, compared with 16.7% of patients who did not experience a change in status. These patients also reported lower health status and difficulty affording medications, wrote Haider J. Warraich, MD, a cardiologist at Duke University in Durham, N.C., and coauthors.
Baseline data were collected for all patients according to CathPCI Registry standards, and follow-up was conducted via telephone 6 weeks, 6 months, 1 year, and 15 months after discharge. After 1 year, patients who reported working full or part time were defined as working. Adverse change in employment was defined as those who “reported working immediately before the index MI hospitalization but were either no longer working or working fewer hours,” Dr. Warraich and his colleagues reported.
Depression was defined by a Patient Health Questionnaire (PHQ) score greater than 3. Health status was assessed using the EuroQoL-5 Dimensions (EQ-5D) visual analog scale. Medication adherence was assessed using three questions at the follow-up interview, and patients were also asked to rank the financial hardship of their monthly medication costs on a scale of 1-5.
Among patients working at baseline, 492 (10%) reported an adverse change in employment after a year. Of these, 349 (7%) were no longer working, and 143 (3%) were working less than before. Of those with an adverse change in employment, 172 reported involuntary job loss such as being laid off or no longer working due to health concerns. Just 27 patients reported retirement.
The number of readmissions within the first year was the factor most strongly associated with adverse change in employment. Baseline smoking status, hypertension, and postdischarge bleeding were also significantly associated with adverse change in employment, the authors said.
At 1 year follow-up, patients with an adverse change in employment were more likely than those with no change to report depression (27.4% with PHQ score greater than 3, compared with 16.7% in the no-change group). These patients also reported lower health status (mean EuroQoL score of 73 compared with 78) and moderate to extreme financial hardship with medication costs (41.0% compared with 28.4%), though there was no difference in medication adherence, the authors reported.
The results indicate that, although job loss in acute MI patients has dropped in comparison with previous studies, patients who experience an adverse change in employment are still “at increased risk of depression, lower quality of life, and increased financial hardship with medication costs compared with those who continue working,” the authors wrote.
“These results underscore the need for interventions to address this patient-centered outcome and its health impact,” they concluded.
Daiichi Sankyo and Lilly USA funded TRANSLATE-ACS. This analysis was funded in part by a grant from the National Heart, Lung, and Blood Institute.
SOURCE: Warraich H et al. Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.117.004528.
Patients who experienced adverse changes in employment status after acute myocardial infarction (AMI) reported increased depression and lower quality of life, according to results published June 12 in Circulation: Cardiovascular Quality and Outcomes.
At 1-year follow-up, 27.4% of patients with adverse employment change scored high on measures of depression, compared with 16.7% of patients who did not experience a change in status. These patients also reported lower health status and difficulty affording medications, wrote Haider J. Warraich, MD, a cardiologist at Duke University in Durham, N.C., and coauthors.
Baseline data were collected for all patients according to CathPCI Registry standards, and follow-up was conducted via telephone 6 weeks, 6 months, 1 year, and 15 months after discharge. After 1 year, patients who reported working full or part time were defined as working. Adverse change in employment was defined as those who “reported working immediately before the index MI hospitalization but were either no longer working or working fewer hours,” Dr. Warraich and his colleagues reported.
Depression was defined by a Patient Health Questionnaire (PHQ) score greater than 3. Health status was assessed using the EuroQoL-5 Dimensions (EQ-5D) visual analog scale. Medication adherence was assessed using three questions at the follow-up interview, and patients were also asked to rank the financial hardship of their monthly medication costs on a scale of 1-5.
Among patients working at baseline, 492 (10%) reported an adverse change in employment after a year. Of these, 349 (7%) were no longer working, and 143 (3%) were working less than before. Of those with an adverse change in employment, 172 reported involuntary job loss such as being laid off or no longer working due to health concerns. Just 27 patients reported retirement.
The number of readmissions within the first year was the factor most strongly associated with adverse change in employment. Baseline smoking status, hypertension, and postdischarge bleeding were also significantly associated with adverse change in employment, the authors said.
At 1 year follow-up, patients with an adverse change in employment were more likely than those with no change to report depression (27.4% with PHQ score greater than 3, compared with 16.7% in the no-change group). These patients also reported lower health status (mean EuroQoL score of 73 compared with 78) and moderate to extreme financial hardship with medication costs (41.0% compared with 28.4%), though there was no difference in medication adherence, the authors reported.
The results indicate that, although job loss in acute MI patients has dropped in comparison with previous studies, patients who experience an adverse change in employment are still “at increased risk of depression, lower quality of life, and increased financial hardship with medication costs compared with those who continue working,” the authors wrote.
“These results underscore the need for interventions to address this patient-centered outcome and its health impact,” they concluded.
Daiichi Sankyo and Lilly USA funded TRANSLATE-ACS. This analysis was funded in part by a grant from the National Heart, Lung, and Blood Institute.
SOURCE: Warraich H et al. Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.117.004528.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
Key clinical point: Patients who lost their jobs or lost hours of employment after AMI reported increased depression, lower quality of life, and difficulty affording medications.
Major finding: At 1-year follow-up, 27.4% of patients with adverse employment change scored high on measures of depression, compared with 16.7% of patients who did not experience a change in status; these patients also reported lower health status and difficulty affording medications.
Study details: An analysis of 9,319 AMI patients from the (TRANSLATE-ACS) study.
Disclosures: Daiichi Sankyo and Lilly USA funded TRANSLATE-ACS. This analysis was funded in part by a grant from the National Heart, Lung, and Blood Institute.
Source: Warraich H et al. Circ Cardiovasc Qual Outcomes. 2018 Jun 12. doi: 10.1161/circoutcomes.117.004528.
Hepatitis A Outbreak in a Childcare Facility
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
Chemo-free combo provides potential first-line option for FL
CHICAGO—A chemotherapy-free combination of lenalidomide plus rituximab shows similar efficacy and a different safety profile to chemotherapy plus rituximab (R-chemo) followed by rituximab maintenance in patients with previously untreated follicular lymphoma (FL).
According to investigators, the multicenter, international phase 3 RELEVANCE trial is the first to evaluate the chemo-free combination against the standard of care, R-chemo with rituximab maintenance.
“These results show that lenalidomide plus rituximab, a novel immunomodulatory approach, is a potential first-line option for patients with FL requiring treatment,” said investigator Nathan H. Fowler, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fowler presented the results of the study at the 2018 ASCO Annual Meeting (abstract 7500).
The current standard of care in previously untreated symptomatic FL is immunochemotherapy induction followed by rituximab maintenance.
The immunomodulatory agent lenalidomide has complementary mechanisms with rituximab. Phase 2 studies of combined immunotherapy with lenalidomide and rituximab demonstrated 3-year progression-free survival (PFS) of 79%-81% in previously untreated FL, Dr Fowler said.
Phase 3 RELEVANCE trial (NCT01650701)
Investigators evaluated 1030 previously untreated grade 1-3a FL patients who required therapy.
Patients in the lenalidomide-rituximab group (n=513) received lenalidomide doses of 20 mg per day on days 2 to 22 and 28 for 6 to 12 cycles. Responders continued on therapy at 10 mg per day for a total of 18 cycles.
The rituximab dose was 375 mg/m2 weekly in cycle 1 and day 1 in cycles 2 to 6 and continued in responders for 12 additional cycles.
Patients in the R-chemo arm (n=517) received the investigator’s choice of standard rituximab-CHOP, rituximab-bendamustine, or rituximab-CVP, followed by 12 cycles of rituximab.
Most patients (72%) in the R-chemo arm received R-CHOP.
Baseline characteristics were similar in both groups, Dr Fowler said.
Co-primary endpoints were complete remission/complete remission unconfirmed (CR/Cru) at 120 weeks and PFS.
Results
At a median follow-up of 37.9 months, the superiority for lenalidomide and rituximab over rituximab-chemotherapy was not established.
For the lenalidomide-rituximab patients, the CR/Cru was 48% and 3-year PFS was 77% as compared to 53% and 78%, respectively, for rituximab-chemotherapy patients, as assessed by an independent review committee.
Overall survival was 94% in both groups.
Safety
“Important differences in safety profiles were observed between the arms,” Dr Fowler said.
Rituximab-chemotherapy patients had more frequent neutropenia, febrile neutropenia, growth factor usage, nausea, vomiting, neuropathy, and alopecia.
Lenalidomide and rituximab showed more cutaneous reactions, tumor flare, and diarrhea.
Toxicity profiles differed, with higher grade 4 neutropenia (31% vs 8%) and febrile neutropenia (7% vs 2%) with rituximab-chemotherapy compared with lenalidomide-rituximab, respectively.
More patients experienced grade 3/4 cutaneous events (7% vs 1%) with lenalidomide-rituximab.
Second primary malignancies were slightly higher with rituximab-chemotherapy (10%) than with lenalidomide-rituximab (7%). Grade 5 adverse events were 1% in both groups.
About 70% of patients completed treatment in both groups.
“Lenalidomide and rituximab was not superior to rituximab-chemotherapy based on mature CR/Cru at 120 weeks and interim PFS,” Dr Fowler said. “Both treatments showed similar efficacy results. Treatment effects on PFS were consistent across pre-specified subgroups.”
Dr Fowler presented data as of May 31, 2017. Continued follow-up on PFS and OS is ongoing.
The study is sponsored by Celgene Corporation and the Lymphoma Academic Research Organisation (LYSARC).
CHICAGO—A chemotherapy-free combination of lenalidomide plus rituximab shows similar efficacy and a different safety profile to chemotherapy plus rituximab (R-chemo) followed by rituximab maintenance in patients with previously untreated follicular lymphoma (FL).
According to investigators, the multicenter, international phase 3 RELEVANCE trial is the first to evaluate the chemo-free combination against the standard of care, R-chemo with rituximab maintenance.
“These results show that lenalidomide plus rituximab, a novel immunomodulatory approach, is a potential first-line option for patients with FL requiring treatment,” said investigator Nathan H. Fowler, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fowler presented the results of the study at the 2018 ASCO Annual Meeting (abstract 7500).
The current standard of care in previously untreated symptomatic FL is immunochemotherapy induction followed by rituximab maintenance.
The immunomodulatory agent lenalidomide has complementary mechanisms with rituximab. Phase 2 studies of combined immunotherapy with lenalidomide and rituximab demonstrated 3-year progression-free survival (PFS) of 79%-81% in previously untreated FL, Dr Fowler said.
Phase 3 RELEVANCE trial (NCT01650701)
Investigators evaluated 1030 previously untreated grade 1-3a FL patients who required therapy.
Patients in the lenalidomide-rituximab group (n=513) received lenalidomide doses of 20 mg per day on days 2 to 22 and 28 for 6 to 12 cycles. Responders continued on therapy at 10 mg per day for a total of 18 cycles.
The rituximab dose was 375 mg/m2 weekly in cycle 1 and day 1 in cycles 2 to 6 and continued in responders for 12 additional cycles.
Patients in the R-chemo arm (n=517) received the investigator’s choice of standard rituximab-CHOP, rituximab-bendamustine, or rituximab-CVP, followed by 12 cycles of rituximab.
Most patients (72%) in the R-chemo arm received R-CHOP.
Baseline characteristics were similar in both groups, Dr Fowler said.
Co-primary endpoints were complete remission/complete remission unconfirmed (CR/Cru) at 120 weeks and PFS.
Results
At a median follow-up of 37.9 months, the superiority for lenalidomide and rituximab over rituximab-chemotherapy was not established.
For the lenalidomide-rituximab patients, the CR/Cru was 48% and 3-year PFS was 77% as compared to 53% and 78%, respectively, for rituximab-chemotherapy patients, as assessed by an independent review committee.
Overall survival was 94% in both groups.
Safety
“Important differences in safety profiles were observed between the arms,” Dr Fowler said.
Rituximab-chemotherapy patients had more frequent neutropenia, febrile neutropenia, growth factor usage, nausea, vomiting, neuropathy, and alopecia.
Lenalidomide and rituximab showed more cutaneous reactions, tumor flare, and diarrhea.
Toxicity profiles differed, with higher grade 4 neutropenia (31% vs 8%) and febrile neutropenia (7% vs 2%) with rituximab-chemotherapy compared with lenalidomide-rituximab, respectively.
More patients experienced grade 3/4 cutaneous events (7% vs 1%) with lenalidomide-rituximab.
Second primary malignancies were slightly higher with rituximab-chemotherapy (10%) than with lenalidomide-rituximab (7%). Grade 5 adverse events were 1% in both groups.
About 70% of patients completed treatment in both groups.
“Lenalidomide and rituximab was not superior to rituximab-chemotherapy based on mature CR/Cru at 120 weeks and interim PFS,” Dr Fowler said. “Both treatments showed similar efficacy results. Treatment effects on PFS were consistent across pre-specified subgroups.”
Dr Fowler presented data as of May 31, 2017. Continued follow-up on PFS and OS is ongoing.
The study is sponsored by Celgene Corporation and the Lymphoma Academic Research Organisation (LYSARC).
CHICAGO—A chemotherapy-free combination of lenalidomide plus rituximab shows similar efficacy and a different safety profile to chemotherapy plus rituximab (R-chemo) followed by rituximab maintenance in patients with previously untreated follicular lymphoma (FL).
According to investigators, the multicenter, international phase 3 RELEVANCE trial is the first to evaluate the chemo-free combination against the standard of care, R-chemo with rituximab maintenance.
“These results show that lenalidomide plus rituximab, a novel immunomodulatory approach, is a potential first-line option for patients with FL requiring treatment,” said investigator Nathan H. Fowler, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fowler presented the results of the study at the 2018 ASCO Annual Meeting (abstract 7500).
The current standard of care in previously untreated symptomatic FL is immunochemotherapy induction followed by rituximab maintenance.
The immunomodulatory agent lenalidomide has complementary mechanisms with rituximab. Phase 2 studies of combined immunotherapy with lenalidomide and rituximab demonstrated 3-year progression-free survival (PFS) of 79%-81% in previously untreated FL, Dr Fowler said.
Phase 3 RELEVANCE trial (NCT01650701)
Investigators evaluated 1030 previously untreated grade 1-3a FL patients who required therapy.
Patients in the lenalidomide-rituximab group (n=513) received lenalidomide doses of 20 mg per day on days 2 to 22 and 28 for 6 to 12 cycles. Responders continued on therapy at 10 mg per day for a total of 18 cycles.
The rituximab dose was 375 mg/m2 weekly in cycle 1 and day 1 in cycles 2 to 6 and continued in responders for 12 additional cycles.
Patients in the R-chemo arm (n=517) received the investigator’s choice of standard rituximab-CHOP, rituximab-bendamustine, or rituximab-CVP, followed by 12 cycles of rituximab.
Most patients (72%) in the R-chemo arm received R-CHOP.
Baseline characteristics were similar in both groups, Dr Fowler said.
Co-primary endpoints were complete remission/complete remission unconfirmed (CR/Cru) at 120 weeks and PFS.
Results
At a median follow-up of 37.9 months, the superiority for lenalidomide and rituximab over rituximab-chemotherapy was not established.
For the lenalidomide-rituximab patients, the CR/Cru was 48% and 3-year PFS was 77% as compared to 53% and 78%, respectively, for rituximab-chemotherapy patients, as assessed by an independent review committee.
Overall survival was 94% in both groups.
Safety
“Important differences in safety profiles were observed between the arms,” Dr Fowler said.
Rituximab-chemotherapy patients had more frequent neutropenia, febrile neutropenia, growth factor usage, nausea, vomiting, neuropathy, and alopecia.
Lenalidomide and rituximab showed more cutaneous reactions, tumor flare, and diarrhea.
Toxicity profiles differed, with higher grade 4 neutropenia (31% vs 8%) and febrile neutropenia (7% vs 2%) with rituximab-chemotherapy compared with lenalidomide-rituximab, respectively.
More patients experienced grade 3/4 cutaneous events (7% vs 1%) with lenalidomide-rituximab.
Second primary malignancies were slightly higher with rituximab-chemotherapy (10%) than with lenalidomide-rituximab (7%). Grade 5 adverse events were 1% in both groups.
About 70% of patients completed treatment in both groups.
“Lenalidomide and rituximab was not superior to rituximab-chemotherapy based on mature CR/Cru at 120 weeks and interim PFS,” Dr Fowler said. “Both treatments showed similar efficacy results. Treatment effects on PFS were consistent across pre-specified subgroups.”
Dr Fowler presented data as of May 31, 2017. Continued follow-up on PFS and OS is ongoing.
The study is sponsored by Celgene Corporation and the Lymphoma Academic Research Organisation (LYSARC).
Getting Ahead of the Pain
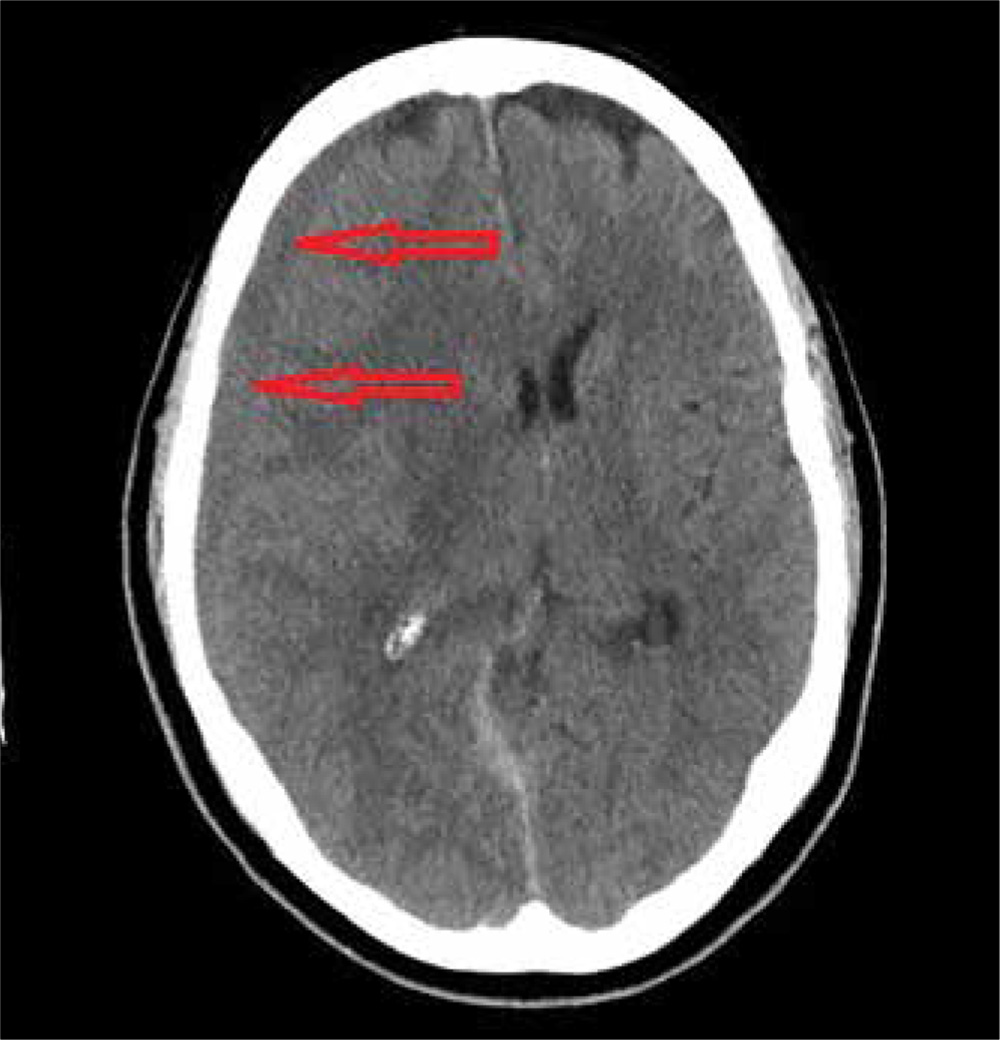
ANSWER
The image reveals a hypodense extra-axial fluid collection in the right frontoparietal region, measuring 8 to 10 mm in diameter. There is some mass effect and evidence of right-to-left shift. These findings are consistent with a subacute subdural hematoma, possibly secondary to the patient’s anticoagulant use. (The patient later recalled bumping his head a couple of months prior—but that may have been incidental.)
Arrangements were made for him at a local hospital where neurosurgical services were available. He underwent successful evacuation and was subsequently symptom free.

ANSWER
The image reveals a hypodense extra-axial fluid collection in the right frontoparietal region, measuring 8 to 10 mm in diameter. There is some mass effect and evidence of right-to-left shift. These findings are consistent with a subacute subdural hematoma, possibly secondary to the patient’s anticoagulant use. (The patient later recalled bumping his head a couple of months prior—but that may have been incidental.)
Arrangements were made for him at a local hospital where neurosurgical services were available. He underwent successful evacuation and was subsequently symptom free.

ANSWER
The image reveals a hypodense extra-axial fluid collection in the right frontoparietal region, measuring 8 to 10 mm in diameter. There is some mass effect and evidence of right-to-left shift. These findings are consistent with a subacute subdural hematoma, possibly secondary to the patient’s anticoagulant use. (The patient later recalled bumping his head a couple of months prior—but that may have been incidental.)
Arrangements were made for him at a local hospital where neurosurgical services were available. He underwent successful evacuation and was subsequently symptom free.
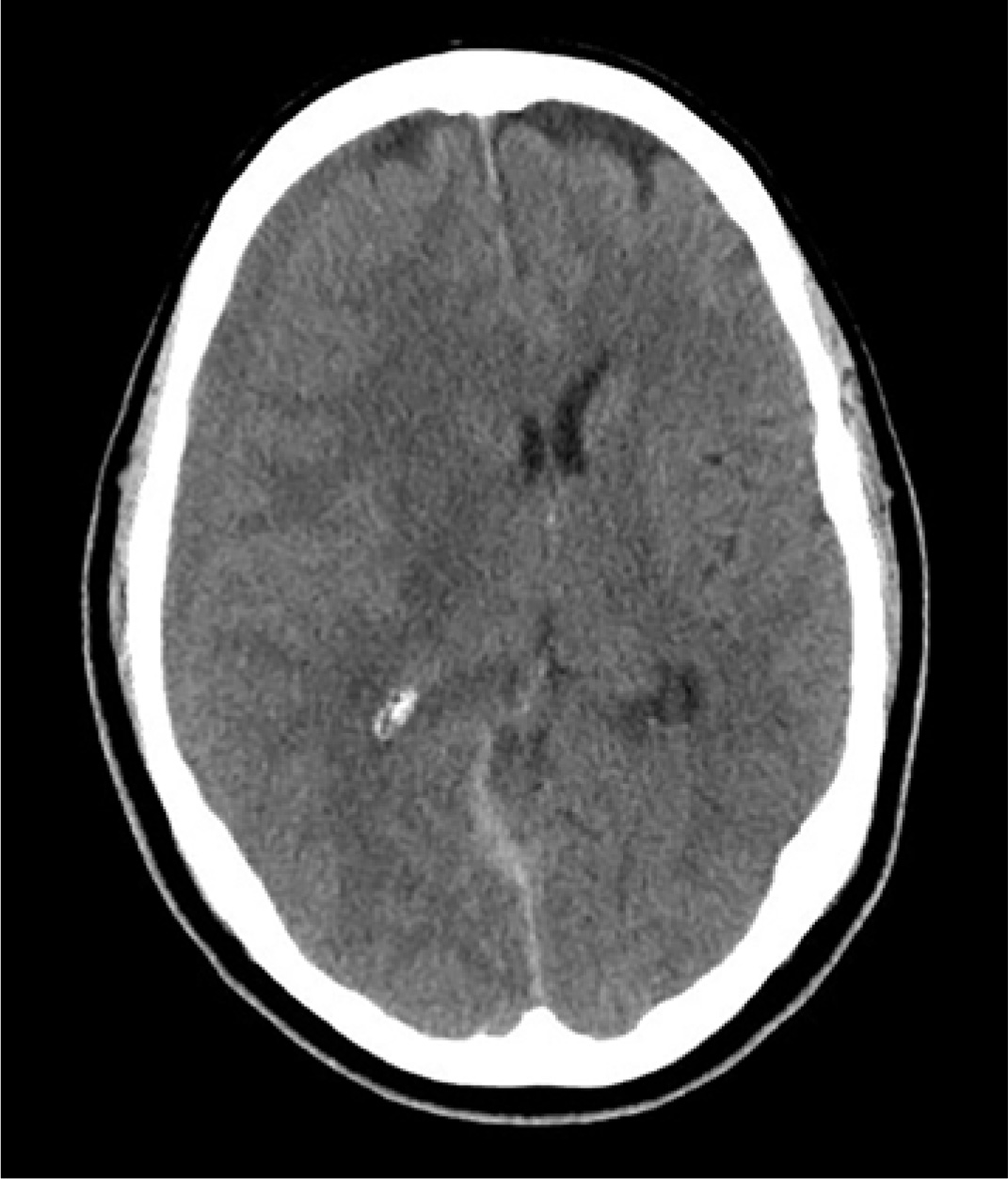
An 80-year-old man presents to urgent care for intermittent severe headaches. The pain is reportedly bifrontal, slightly worse on the right side than the left. He denies any recent injury or trauma, as well as symptoms including fever, chills, nausea, vomiting, and visual disturbance.
His medical history is significant for hypertension and hyperlipidemia. His current medications include prasugrel and aspirin.
On examination, you note an elderly male who is awake, alert, and oriented x 3. His vital signs are normal. His physical exam is overall normal, with no focal findings or neurologic deficits.
Noncontrast CT of the head is obtained at a local hospital. As you review the images, you see the following cut (shown). What is your impression?
Bariatric revision mortality linked to age, comorbidities
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
REPORTING FROM DDW 2018
Key clinical point: Revisional bariatric procedures may carry a greater mortality risk than previous studies have suggested.
Major finding: The mortality rate in the sample was 2.1%.
Study details: The 2014 Nationwide Inpatient Sample database, comprising 14,280 patients who underwent revisional bariatric surgery.
Disclosures: Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
Source: Popov VB et al. DDW 2018, Abstract 324.