User login
OSA with worsening hypoxemia raises metabolic syndrome risk
BALTIMORE – An 8-year cohort study has found that patients with who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, an investigator reported at the annual meeting of the Associated Professional Sleep Societies
“Considering that we have a very high prevalence of moderate to severe obstructive sleep apnea (OSA) in the general population, this is a very important finding because it indicates that we need some clinical options of treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events in these patients,” said Camila Hirotsu, PhD, of the Federal University of São Paulo (Brazil).
Dr. Hirotsu presented 8-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate the how OSA can impact the risk of developing metabolic syndrome (MetS) the general population. MetS is defined as a cluster of three or more cardiovascular metabolic components: low HDL levels, high glucose and triglycerides, hypertension and abdominal obesity. Dr. Hirotsu said that 50%-60% of MetS patients have OSA (PLoS One 2010;5:e12065).
The study enrolled 1,074 patients at baseline, closing enrollment in 2008, and obtained follow-up on 712, evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of MetS at baseline. Of those 476, 44% went on to develop MetS.
Median age of patients who developed MetS was 40.8 years vs. 36.1 for those who did not. Patients who developed MetS also had a higher body mass index, but were not obese: 26.9 kg/m2 vs. 23.8 kg/m2. Patients were evaluated by completing questionnaires, undergoing full polysomnography, and having clinical assessments.
Patients with moderate to severe OSA were found to have an odds ratio of 2.47 (P = .016) of developing incident MetS, Dr. Hirotsu said. Rates of moderate to severe OSA were 21.3% for the group that developed MetS vs. 9% for the non-MetS group, said Dr. Hirotsu.
The study determined that the following sleep changes were associated with incident MetS: apnea-hypopnea index (AHI) (OR 1.16); 3% oxygen desaturation index (ODI) (OR 1.24); and time with oxygen saturation by pulse oximeter (SpO2) less than 90% (OR 1.42).
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of MetS in the general population,” Dr. Hirotsu said.
A secondary aim of the study was to evaluate the impact of MetS on the risk of developing OSA in the general population. “It seems that MetS is not really an independent risk factor for OSA.”
Dr. Hirotsu reported having no conflicts of interest.
BALTIMORE – An 8-year cohort study has found that patients with who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, an investigator reported at the annual meeting of the Associated Professional Sleep Societies
“Considering that we have a very high prevalence of moderate to severe obstructive sleep apnea (OSA) in the general population, this is a very important finding because it indicates that we need some clinical options of treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events in these patients,” said Camila Hirotsu, PhD, of the Federal University of São Paulo (Brazil).
Dr. Hirotsu presented 8-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate the how OSA can impact the risk of developing metabolic syndrome (MetS) the general population. MetS is defined as a cluster of three or more cardiovascular metabolic components: low HDL levels, high glucose and triglycerides, hypertension and abdominal obesity. Dr. Hirotsu said that 50%-60% of MetS patients have OSA (PLoS One 2010;5:e12065).
The study enrolled 1,074 patients at baseline, closing enrollment in 2008, and obtained follow-up on 712, evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of MetS at baseline. Of those 476, 44% went on to develop MetS.
Median age of patients who developed MetS was 40.8 years vs. 36.1 for those who did not. Patients who developed MetS also had a higher body mass index, but were not obese: 26.9 kg/m2 vs. 23.8 kg/m2. Patients were evaluated by completing questionnaires, undergoing full polysomnography, and having clinical assessments.
Patients with moderate to severe OSA were found to have an odds ratio of 2.47 (P = .016) of developing incident MetS, Dr. Hirotsu said. Rates of moderate to severe OSA were 21.3% for the group that developed MetS vs. 9% for the non-MetS group, said Dr. Hirotsu.
The study determined that the following sleep changes were associated with incident MetS: apnea-hypopnea index (AHI) (OR 1.16); 3% oxygen desaturation index (ODI) (OR 1.24); and time with oxygen saturation by pulse oximeter (SpO2) less than 90% (OR 1.42).
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of MetS in the general population,” Dr. Hirotsu said.
A secondary aim of the study was to evaluate the impact of MetS on the risk of developing OSA in the general population. “It seems that MetS is not really an independent risk factor for OSA.”
Dr. Hirotsu reported having no conflicts of interest.
BALTIMORE – An 8-year cohort study has found that patients with who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, an investigator reported at the annual meeting of the Associated Professional Sleep Societies
“Considering that we have a very high prevalence of moderate to severe obstructive sleep apnea (OSA) in the general population, this is a very important finding because it indicates that we need some clinical options of treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events in these patients,” said Camila Hirotsu, PhD, of the Federal University of São Paulo (Brazil).
Dr. Hirotsu presented 8-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate the how OSA can impact the risk of developing metabolic syndrome (MetS) the general population. MetS is defined as a cluster of three or more cardiovascular metabolic components: low HDL levels, high glucose and triglycerides, hypertension and abdominal obesity. Dr. Hirotsu said that 50%-60% of MetS patients have OSA (PLoS One 2010;5:e12065).
The study enrolled 1,074 patients at baseline, closing enrollment in 2008, and obtained follow-up on 712, evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of MetS at baseline. Of those 476, 44% went on to develop MetS.
Median age of patients who developed MetS was 40.8 years vs. 36.1 for those who did not. Patients who developed MetS also had a higher body mass index, but were not obese: 26.9 kg/m2 vs. 23.8 kg/m2. Patients were evaluated by completing questionnaires, undergoing full polysomnography, and having clinical assessments.
Patients with moderate to severe OSA were found to have an odds ratio of 2.47 (P = .016) of developing incident MetS, Dr. Hirotsu said. Rates of moderate to severe OSA were 21.3% for the group that developed MetS vs. 9% for the non-MetS group, said Dr. Hirotsu.
The study determined that the following sleep changes were associated with incident MetS: apnea-hypopnea index (AHI) (OR 1.16); 3% oxygen desaturation index (ODI) (OR 1.24); and time with oxygen saturation by pulse oximeter (SpO2) less than 90% (OR 1.42).
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of MetS in the general population,” Dr. Hirotsu said.
A secondary aim of the study was to evaluate the impact of MetS on the risk of developing OSA in the general population. “It seems that MetS is not really an independent risk factor for OSA.”
Dr. Hirotsu reported having no conflicts of interest.
REPORTING FROM SLEEP 2018
Key clinical point: Obstructive sleep apnea may raise the risk of metabolic syndrome.
Major finding: Rates of moderate to severe OSA were 21.3% in the MetS group vs. 9% in the non-MetS group.
Study details: Observational, prospective cohort study of 476 patients with OSA who were free of MetS at enrollment in 2008.
Disclosures: Dr. Hirotsu and coauthors reported no financial relationships. The São Paulo Research Foundation, Association Research Incentive Fund and Brazilian National Council for Scientific and Technological Development funded the study.
Sleep problems may point to health disparity for black women
BALTIMORE – Analysis of data from a national multicenter study of women’s health has found that according to a presentation at the annual meeting of the Associated Sleep Societies.
“Race is emerging as a significant moderator in the relationship between sleep and health outcomes,” said Marissa Bowman, a doctoral student at the University of Pittsburgh. The study was based on 10- to 15-year follow-up data from the SWAN (Study of Women’s Health Across the Nation) sleep research. The researchers evaluated sleep in 265 midlife women, 45% of whom were black.
At baseline, the study assessed sleep health using both actigraphy and a daily diary, along with body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHpR), then collected data on the anthropometric factors 10-15 years later.
Cross-sectional and prospective analyses found that sleep health was correlated with lower BMI but was not significantly associated with WC or WHpR. A prospective analysis found no overall significant correlation between sleep health and any of the three factors. But in a separate the analysis of the study group by race, all three anthropometric factors had a stronger link to sleep health in black women than in those of European descent, respectively, with beta coefficients of –0.14 vs. 0.1 for BMI, –0.17 and 0.1 for WC and –0.17 and 0.07 for WHpR.
“We need to explain this association and conceptualize how sleep health might be more strongly related with weight in African Americans,” Ms. Bowman said. “One possibility might be that sleep health reflects a health disparity. We can see how race is related to other health disparities, and this might be one of them.”
During questions, Ms. Bowman acknowledged that SWAN did not have data on what kind of access to health care the black women in the study had. “That might be a possible reason they’re not getting their sleep treated; they’re not getting other health factors treated,” she said.
Ms. Bowman and her coauthors reported no financial relationships. The study was funded by the National Institute on Aging, National Institutes of Health, and the National Institutes of Health Office of Research on Women’s Health.
BALTIMORE – Analysis of data from a national multicenter study of women’s health has found that according to a presentation at the annual meeting of the Associated Sleep Societies.
“Race is emerging as a significant moderator in the relationship between sleep and health outcomes,” said Marissa Bowman, a doctoral student at the University of Pittsburgh. The study was based on 10- to 15-year follow-up data from the SWAN (Study of Women’s Health Across the Nation) sleep research. The researchers evaluated sleep in 265 midlife women, 45% of whom were black.
At baseline, the study assessed sleep health using both actigraphy and a daily diary, along with body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHpR), then collected data on the anthropometric factors 10-15 years later.
Cross-sectional and prospective analyses found that sleep health was correlated with lower BMI but was not significantly associated with WC or WHpR. A prospective analysis found no overall significant correlation between sleep health and any of the three factors. But in a separate the analysis of the study group by race, all three anthropometric factors had a stronger link to sleep health in black women than in those of European descent, respectively, with beta coefficients of –0.14 vs. 0.1 for BMI, –0.17 and 0.1 for WC and –0.17 and 0.07 for WHpR.
“We need to explain this association and conceptualize how sleep health might be more strongly related with weight in African Americans,” Ms. Bowman said. “One possibility might be that sleep health reflects a health disparity. We can see how race is related to other health disparities, and this might be one of them.”
During questions, Ms. Bowman acknowledged that SWAN did not have data on what kind of access to health care the black women in the study had. “That might be a possible reason they’re not getting their sleep treated; they’re not getting other health factors treated,” she said.
Ms. Bowman and her coauthors reported no financial relationships. The study was funded by the National Institute on Aging, National Institutes of Health, and the National Institutes of Health Office of Research on Women’s Health.
BALTIMORE – Analysis of data from a national multicenter study of women’s health has found that according to a presentation at the annual meeting of the Associated Sleep Societies.
“Race is emerging as a significant moderator in the relationship between sleep and health outcomes,” said Marissa Bowman, a doctoral student at the University of Pittsburgh. The study was based on 10- to 15-year follow-up data from the SWAN (Study of Women’s Health Across the Nation) sleep research. The researchers evaluated sleep in 265 midlife women, 45% of whom were black.
At baseline, the study assessed sleep health using both actigraphy and a daily diary, along with body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHpR), then collected data on the anthropometric factors 10-15 years later.
Cross-sectional and prospective analyses found that sleep health was correlated with lower BMI but was not significantly associated with WC or WHpR. A prospective analysis found no overall significant correlation between sleep health and any of the three factors. But in a separate the analysis of the study group by race, all three anthropometric factors had a stronger link to sleep health in black women than in those of European descent, respectively, with beta coefficients of –0.14 vs. 0.1 for BMI, –0.17 and 0.1 for WC and –0.17 and 0.07 for WHpR.
“We need to explain this association and conceptualize how sleep health might be more strongly related with weight in African Americans,” Ms. Bowman said. “One possibility might be that sleep health reflects a health disparity. We can see how race is related to other health disparities, and this might be one of them.”
During questions, Ms. Bowman acknowledged that SWAN did not have data on what kind of access to health care the black women in the study had. “That might be a possible reason they’re not getting their sleep treated; they’re not getting other health factors treated,” she said.
Ms. Bowman and her coauthors reported no financial relationships. The study was funded by the National Institute on Aging, National Institutes of Health, and the National Institutes of Health Office of Research on Women’s Health.
REPORTING FROM SLEEP 2018
Key clinical point: Black women are at greater risk for poor sleep help than white women.
Major finding: Beta coefficient for BMI and sleep health was –0.14 for black women and 0.1 for white women.
Study details: The SWAN sleep study, a multicenter, longitudinal, epidemiologic study of 265 midlife women.
Disclosures: Ms. Bowman and her coauthors reported no financial relationships. The study was funded by the National Institute on Aging, National Institutes of Health, and the National Institutes of Health Office of Research on Women’s Health.
Impact of marijuana on sleep not well understood
BALTIMORE – Although the national trend of legalization of marijuana for medical and recreational uses has accelerated, physicians should be cautious about prescribing , a sleep specialist told attendees at the annual meeting of the Associated Professional Sleep Societies.
“Increased legalization of medical marijuana may cause reduction in perception of the risk of potential harm” said Ashima Sahni, MD, of Northwestern University, Chicago.
“Overall the consensus is that the short-term use of medical marijuana causes an increase in slow-wave sleep (SWS), a decrease in sleep onset latency, a decrease in wake after sleep onset (WASO) and a decrease in REM sleep,” Dr. Sahni said. But chronic use decreases SWS and results in inconsistencies in REM sleep patterns and sleep fragmentation. These changes lead to a self-perpetuating negative cycle that causes chronic users to progressively increase their intake, furthering sleep disruption, she noted.
Marijuana withdrawal also can cause significant disturbances in sleep patterns, including reduced total sleep time and SWS, increased WASO, increased REM sleep associated with strange dreams, and increased limb movements during sleep, Dr. Sahni said. “The effects can be seen as early as 24 hours after discontinuation and can last as long as 6 weeks,” she said. In addition, poor sleep quality prior to a withdrawal attempt has been linked to relapse (Am J Psychiatry. 2004;161:1967-77).
The use of medical marijuana in the management of sleep disorders is fraught with controversy, Dr. Sahni said. She reviewed studies investigating the use of dronabinol for obstructive sleep apnea (OSA). “This is not medical marijuana,” Dr. Sahni said. “It’s a synthetic tetrahydrocannabinol (THC) cannabinoid, which acts on the nonselective CB1 and CB2 agonists,” she said. THC is the euphoria-inducing compound in marijuana. While the mechanism of action of dronabinol is similar to marijuana, the pharmacokinetics may differ. Dronabinol has been approved by the Food and Drug Administration for cancer-related nausea and appetite stimulation in AIDS patients. She referred to a proof-of-concept study of 17 patients with OSA in which dronabinol reduced the apnea-hypopnea index (AHI) with no degradation of sleep architecture or serious adverse events (Front Psychiatry. 2013 Jan 22;4:1-5). Dr. Sahni also noted a randomized, placebo-controlled trial of 73 patients that reported an average reduction in AHI of 12.9 (Sleep. 2018 Jan 1;41[1]; doi: 10.1093/sleep/zsx184). But she pointed out that the American Academy of Sleep Medicine does not recommend medical cannabis or its synthetic extracts for treatment of OSA (J Clin Sleep Med. 2018 Apr 15;14:679-81).
Insomnia, on the other hand, represents the most common use of medical marijuana for sleep. “Studies have shown mixed results because of differences in the ratios of THC to CBD [corticobasal degeneration] in the forms of marijuana examined,” she said. “In the short term, subjective sleepiness is reported to be better, but then the self-perpetuating negative cycle initiates with chronic long-term use.”
In treatment of nightmares and posttraumatic stress syndrome, Dr. Sahni cited studies that found “good effects” of medical marijuana use (CNS Neurosci Ther. 2009;15:84-8; J Clin Psychopharmacol. 2014 Oct;34:559-64). For REM behavior disorder, medical marijuana was found to be beneficial in four patients with Parkinson disease (J Clin Pharm Ther. 2014 Oct;39:564-6). In poorly treated restless leg syndrome, medical marijuana was reported to be beneficial (Sleep Med. 2017 Aug;36:182-3).
“It should be noted that these were very small studies and therefore more research is needed before we change our medical practices toward various sleep disorders,” Dr. Sahni said.
Dr. Sahni and her coauthors reported having no financial relationships to disclose.
BALTIMORE – Although the national trend of legalization of marijuana for medical and recreational uses has accelerated, physicians should be cautious about prescribing , a sleep specialist told attendees at the annual meeting of the Associated Professional Sleep Societies.
“Increased legalization of medical marijuana may cause reduction in perception of the risk of potential harm” said Ashima Sahni, MD, of Northwestern University, Chicago.
“Overall the consensus is that the short-term use of medical marijuana causes an increase in slow-wave sleep (SWS), a decrease in sleep onset latency, a decrease in wake after sleep onset (WASO) and a decrease in REM sleep,” Dr. Sahni said. But chronic use decreases SWS and results in inconsistencies in REM sleep patterns and sleep fragmentation. These changes lead to a self-perpetuating negative cycle that causes chronic users to progressively increase their intake, furthering sleep disruption, she noted.
Marijuana withdrawal also can cause significant disturbances in sleep patterns, including reduced total sleep time and SWS, increased WASO, increased REM sleep associated with strange dreams, and increased limb movements during sleep, Dr. Sahni said. “The effects can be seen as early as 24 hours after discontinuation and can last as long as 6 weeks,” she said. In addition, poor sleep quality prior to a withdrawal attempt has been linked to relapse (Am J Psychiatry. 2004;161:1967-77).
The use of medical marijuana in the management of sleep disorders is fraught with controversy, Dr. Sahni said. She reviewed studies investigating the use of dronabinol for obstructive sleep apnea (OSA). “This is not medical marijuana,” Dr. Sahni said. “It’s a synthetic tetrahydrocannabinol (THC) cannabinoid, which acts on the nonselective CB1 and CB2 agonists,” she said. THC is the euphoria-inducing compound in marijuana. While the mechanism of action of dronabinol is similar to marijuana, the pharmacokinetics may differ. Dronabinol has been approved by the Food and Drug Administration for cancer-related nausea and appetite stimulation in AIDS patients. She referred to a proof-of-concept study of 17 patients with OSA in which dronabinol reduced the apnea-hypopnea index (AHI) with no degradation of sleep architecture or serious adverse events (Front Psychiatry. 2013 Jan 22;4:1-5). Dr. Sahni also noted a randomized, placebo-controlled trial of 73 patients that reported an average reduction in AHI of 12.9 (Sleep. 2018 Jan 1;41[1]; doi: 10.1093/sleep/zsx184). But she pointed out that the American Academy of Sleep Medicine does not recommend medical cannabis or its synthetic extracts for treatment of OSA (J Clin Sleep Med. 2018 Apr 15;14:679-81).
Insomnia, on the other hand, represents the most common use of medical marijuana for sleep. “Studies have shown mixed results because of differences in the ratios of THC to CBD [corticobasal degeneration] in the forms of marijuana examined,” she said. “In the short term, subjective sleepiness is reported to be better, but then the self-perpetuating negative cycle initiates with chronic long-term use.”
In treatment of nightmares and posttraumatic stress syndrome, Dr. Sahni cited studies that found “good effects” of medical marijuana use (CNS Neurosci Ther. 2009;15:84-8; J Clin Psychopharmacol. 2014 Oct;34:559-64). For REM behavior disorder, medical marijuana was found to be beneficial in four patients with Parkinson disease (J Clin Pharm Ther. 2014 Oct;39:564-6). In poorly treated restless leg syndrome, medical marijuana was reported to be beneficial (Sleep Med. 2017 Aug;36:182-3).
“It should be noted that these were very small studies and therefore more research is needed before we change our medical practices toward various sleep disorders,” Dr. Sahni said.
Dr. Sahni and her coauthors reported having no financial relationships to disclose.
BALTIMORE – Although the national trend of legalization of marijuana for medical and recreational uses has accelerated, physicians should be cautious about prescribing , a sleep specialist told attendees at the annual meeting of the Associated Professional Sleep Societies.
“Increased legalization of medical marijuana may cause reduction in perception of the risk of potential harm” said Ashima Sahni, MD, of Northwestern University, Chicago.
“Overall the consensus is that the short-term use of medical marijuana causes an increase in slow-wave sleep (SWS), a decrease in sleep onset latency, a decrease in wake after sleep onset (WASO) and a decrease in REM sleep,” Dr. Sahni said. But chronic use decreases SWS and results in inconsistencies in REM sleep patterns and sleep fragmentation. These changes lead to a self-perpetuating negative cycle that causes chronic users to progressively increase their intake, furthering sleep disruption, she noted.
Marijuana withdrawal also can cause significant disturbances in sleep patterns, including reduced total sleep time and SWS, increased WASO, increased REM sleep associated with strange dreams, and increased limb movements during sleep, Dr. Sahni said. “The effects can be seen as early as 24 hours after discontinuation and can last as long as 6 weeks,” she said. In addition, poor sleep quality prior to a withdrawal attempt has been linked to relapse (Am J Psychiatry. 2004;161:1967-77).
The use of medical marijuana in the management of sleep disorders is fraught with controversy, Dr. Sahni said. She reviewed studies investigating the use of dronabinol for obstructive sleep apnea (OSA). “This is not medical marijuana,” Dr. Sahni said. “It’s a synthetic tetrahydrocannabinol (THC) cannabinoid, which acts on the nonselective CB1 and CB2 agonists,” she said. THC is the euphoria-inducing compound in marijuana. While the mechanism of action of dronabinol is similar to marijuana, the pharmacokinetics may differ. Dronabinol has been approved by the Food and Drug Administration for cancer-related nausea and appetite stimulation in AIDS patients. She referred to a proof-of-concept study of 17 patients with OSA in which dronabinol reduced the apnea-hypopnea index (AHI) with no degradation of sleep architecture or serious adverse events (Front Psychiatry. 2013 Jan 22;4:1-5). Dr. Sahni also noted a randomized, placebo-controlled trial of 73 patients that reported an average reduction in AHI of 12.9 (Sleep. 2018 Jan 1;41[1]; doi: 10.1093/sleep/zsx184). But she pointed out that the American Academy of Sleep Medicine does not recommend medical cannabis or its synthetic extracts for treatment of OSA (J Clin Sleep Med. 2018 Apr 15;14:679-81).
Insomnia, on the other hand, represents the most common use of medical marijuana for sleep. “Studies have shown mixed results because of differences in the ratios of THC to CBD [corticobasal degeneration] in the forms of marijuana examined,” she said. “In the short term, subjective sleepiness is reported to be better, but then the self-perpetuating negative cycle initiates with chronic long-term use.”
In treatment of nightmares and posttraumatic stress syndrome, Dr. Sahni cited studies that found “good effects” of medical marijuana use (CNS Neurosci Ther. 2009;15:84-8; J Clin Psychopharmacol. 2014 Oct;34:559-64). For REM behavior disorder, medical marijuana was found to be beneficial in four patients with Parkinson disease (J Clin Pharm Ther. 2014 Oct;39:564-6). In poorly treated restless leg syndrome, medical marijuana was reported to be beneficial (Sleep Med. 2017 Aug;36:182-3).
“It should be noted that these were very small studies and therefore more research is needed before we change our medical practices toward various sleep disorders,” Dr. Sahni said.
Dr. Sahni and her coauthors reported having no financial relationships to disclose.
EXPERT ANALYSIS FROM SLEEP 2018
FDA grants regular approval to venetoclax for CLL/SLL
Venetoclax (Venclexta) has received regular approval from the Food and Drug Administration for the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy.
The approval was based results from the MURANO trial of 389 patients, which was a randomized, multicenter, open-label trial of venetoclax plus rituximab versus bendamustine plus rituximab.
Neutropenia, diarrhea, upper respiratory tract infection, fatigue, cough, and nausea were the most common adverse events seen in the venetoclax arm. Grade 3 or 4 neutropenia developed in 64% of those patients, and grade 4 in 31%. The most common infection in venetoclax patients was pneumonia, but overall, 21% of patients in that arm experienced some kind of infection.
Because of the rapid reduction in tumor size, tumor lysis syndrome is possible with venetoclax treatment, the FDA noted.
In 2016, the FDA granted accelerated approval to venetoclax for treatment of patients with CLL with 17d deletion who had received at least one prior line of therapy.
Venetoclax (Venclexta) has received regular approval from the Food and Drug Administration for the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy.
The approval was based results from the MURANO trial of 389 patients, which was a randomized, multicenter, open-label trial of venetoclax plus rituximab versus bendamustine plus rituximab.
Neutropenia, diarrhea, upper respiratory tract infection, fatigue, cough, and nausea were the most common adverse events seen in the venetoclax arm. Grade 3 or 4 neutropenia developed in 64% of those patients, and grade 4 in 31%. The most common infection in venetoclax patients was pneumonia, but overall, 21% of patients in that arm experienced some kind of infection.
Because of the rapid reduction in tumor size, tumor lysis syndrome is possible with venetoclax treatment, the FDA noted.
In 2016, the FDA granted accelerated approval to venetoclax for treatment of patients with CLL with 17d deletion who had received at least one prior line of therapy.
Venetoclax (Venclexta) has received regular approval from the Food and Drug Administration for the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy.
The approval was based results from the MURANO trial of 389 patients, which was a randomized, multicenter, open-label trial of venetoclax plus rituximab versus bendamustine plus rituximab.
Neutropenia, diarrhea, upper respiratory tract infection, fatigue, cough, and nausea were the most common adverse events seen in the venetoclax arm. Grade 3 or 4 neutropenia developed in 64% of those patients, and grade 4 in 31%. The most common infection in venetoclax patients was pneumonia, but overall, 21% of patients in that arm experienced some kind of infection.
Because of the rapid reduction in tumor size, tumor lysis syndrome is possible with venetoclax treatment, the FDA noted.
In 2016, the FDA granted accelerated approval to venetoclax for treatment of patients with CLL with 17d deletion who had received at least one prior line of therapy.
Reducing the Risk of Overt Hepatic Encephalopathy (HE) Recurrence and HE-Related Hospitalizations
In this supplement to The Hospitalist, Dr. Hameed Ali discusses HE and the importance of identifying and properly managing this common complication of cirrhosis.
Topics include:
- The various stages of HE
- The burden of HE and hospital readmission rates
- A medication for overt HE management
About the Author:
Hameed Q. Ali, DO, FHM
Clinical Assistant Professor
Department of Internal Medicine
Texas A&M Health Science Center
Temple, TX
XIF.0097.USA.18
In this supplement to The Hospitalist, Dr. Hameed Ali discusses HE and the importance of identifying and properly managing this common complication of cirrhosis.
Topics include:
- The various stages of HE
- The burden of HE and hospital readmission rates
- A medication for overt HE management
About the Author:
Hameed Q. Ali, DO, FHM
Clinical Assistant Professor
Department of Internal Medicine
Texas A&M Health Science Center
Temple, TX
XIF.0097.USA.18
In this supplement to The Hospitalist, Dr. Hameed Ali discusses HE and the importance of identifying and properly managing this common complication of cirrhosis.
Topics include:
- The various stages of HE
- The burden of HE and hospital readmission rates
- A medication for overt HE management
About the Author:
Hameed Q. Ali, DO, FHM
Clinical Assistant Professor
Department of Internal Medicine
Texas A&M Health Science Center
Temple, TX
XIF.0097.USA.18
Venetoclax plus ibrutinib yields encouraging MRD results in first-line CLL
CHICAGO – The combination of ibrutinib plus venetoclax yielded a high rate of undetectable minimal residual disease (MRD) when used as first-line treatment for chronic lymphocytic leukemia (CLL), according to preliminary results of the CAPTIVATE trial.
Of the first 30 patients in the trial, 23 (77%) had undetectable blood MRD after just six cycles of combined treatment, said investigator William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston.
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” Dr. Wierda said in a presentation of the CAPTIVATE results at the annual meeting of the American Society of Clinical Oncology.
Those MRD results are “at least as good as we can achieve with chemoimmunotherapy,” Bruce D. Cheson, MD, head of hematology at Georgetown University, Washington, said during a discussion of the CAPTIVATE study results.
Dr. Cheson referenced MRD results from a 2016 analysis of the CLL8 and CLL10 trials, which included patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) and bendamustine plus rituximab (BR). In that analysis, 33.6% of patients achieved MRD-negative complete response and 29.1% achieved MRD-negative partial response.
In CAPTIVATE, by contrast, all of the complete remissions were MRD negative, as were a majority of the partial responders, Dr. Cheson noted.
Venetoclax and ibrutinib have “clinically complimentary activity” that provided a rationale for combining the two, Dr. Wierda said at ASCO. Ibrutinib is a BTK inhibitor that has a high rate of response and durable disease control, though continuous treatment is indicated, he said, because most patients achieve partial remissions as best response and continue to have residual disease in blood or bone marrow. Venetoclax, he added, is a BCL-2 inhibitor that produces durable partial remissions, though “residual disease is typically present in the form of persistently enlarged lymph nodes,” he said. “Venetoclax is highly effective at clearing disease from blood and bone marrow.”
The phase 2 CAPTIVATE trial includes a total of 164 patients younger than 70 years of age who receive a 3-cycle ibrutinib lead-in, followed by ibrutinib plus venetoclax for 12 cycles. At that point, patients are randomized according to MRD status. Patients with confirmed undetectable MRD are randomized to further treatment with ibrutinib or placebo, and those with undetectable MRD not confirmed are randomized to ibrutinib versus ibrutinib plus venetoclax.
In addition to early efficacy data, Dr. Wierda also reported some safety data. Compared with the single-agent ibrutinib lead-in period, combined ibrutinib plus venetoclax treatment had more gastrointestinal-associated events and neutropenia. Almost half of patients (45%) have had a treatment-related grade 3-4 adverse event, though just 18 (11%) have had treatment-related adverse events classified as serious, and there have been no adverse event-related deaths on study.
The high activity of ibrutinib plus venetoclax in CAPTIVATE supports further study of the combination, Dr. Wierda said. A randomized, open-label phase 3 trial of ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab as first-line treatment for CLL is currently recruiting.
The study was sponsored by Pharmacyclics, an AbbVie company. Dr. Wierda reported consulting and research funding from Pharmacyclics, AbbVie, and several other companies.
SOURCE: Wierda WG et al. ASCO 2018, Abstract 7502.
CHICAGO – The combination of ibrutinib plus venetoclax yielded a high rate of undetectable minimal residual disease (MRD) when used as first-line treatment for chronic lymphocytic leukemia (CLL), according to preliminary results of the CAPTIVATE trial.
Of the first 30 patients in the trial, 23 (77%) had undetectable blood MRD after just six cycles of combined treatment, said investigator William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston.
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” Dr. Wierda said in a presentation of the CAPTIVATE results at the annual meeting of the American Society of Clinical Oncology.
Those MRD results are “at least as good as we can achieve with chemoimmunotherapy,” Bruce D. Cheson, MD, head of hematology at Georgetown University, Washington, said during a discussion of the CAPTIVATE study results.
Dr. Cheson referenced MRD results from a 2016 analysis of the CLL8 and CLL10 trials, which included patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) and bendamustine plus rituximab (BR). In that analysis, 33.6% of patients achieved MRD-negative complete response and 29.1% achieved MRD-negative partial response.
In CAPTIVATE, by contrast, all of the complete remissions were MRD negative, as were a majority of the partial responders, Dr. Cheson noted.
Venetoclax and ibrutinib have “clinically complimentary activity” that provided a rationale for combining the two, Dr. Wierda said at ASCO. Ibrutinib is a BTK inhibitor that has a high rate of response and durable disease control, though continuous treatment is indicated, he said, because most patients achieve partial remissions as best response and continue to have residual disease in blood or bone marrow. Venetoclax, he added, is a BCL-2 inhibitor that produces durable partial remissions, though “residual disease is typically present in the form of persistently enlarged lymph nodes,” he said. “Venetoclax is highly effective at clearing disease from blood and bone marrow.”
The phase 2 CAPTIVATE trial includes a total of 164 patients younger than 70 years of age who receive a 3-cycle ibrutinib lead-in, followed by ibrutinib plus venetoclax for 12 cycles. At that point, patients are randomized according to MRD status. Patients with confirmed undetectable MRD are randomized to further treatment with ibrutinib or placebo, and those with undetectable MRD not confirmed are randomized to ibrutinib versus ibrutinib plus venetoclax.
In addition to early efficacy data, Dr. Wierda also reported some safety data. Compared with the single-agent ibrutinib lead-in period, combined ibrutinib plus venetoclax treatment had more gastrointestinal-associated events and neutropenia. Almost half of patients (45%) have had a treatment-related grade 3-4 adverse event, though just 18 (11%) have had treatment-related adverse events classified as serious, and there have been no adverse event-related deaths on study.
The high activity of ibrutinib plus venetoclax in CAPTIVATE supports further study of the combination, Dr. Wierda said. A randomized, open-label phase 3 trial of ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab as first-line treatment for CLL is currently recruiting.
The study was sponsored by Pharmacyclics, an AbbVie company. Dr. Wierda reported consulting and research funding from Pharmacyclics, AbbVie, and several other companies.
SOURCE: Wierda WG et al. ASCO 2018, Abstract 7502.
CHICAGO – The combination of ibrutinib plus venetoclax yielded a high rate of undetectable minimal residual disease (MRD) when used as first-line treatment for chronic lymphocytic leukemia (CLL), according to preliminary results of the CAPTIVATE trial.
Of the first 30 patients in the trial, 23 (77%) had undetectable blood MRD after just six cycles of combined treatment, said investigator William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston.
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” Dr. Wierda said in a presentation of the CAPTIVATE results at the annual meeting of the American Society of Clinical Oncology.
Those MRD results are “at least as good as we can achieve with chemoimmunotherapy,” Bruce D. Cheson, MD, head of hematology at Georgetown University, Washington, said during a discussion of the CAPTIVATE study results.
Dr. Cheson referenced MRD results from a 2016 analysis of the CLL8 and CLL10 trials, which included patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) and bendamustine plus rituximab (BR). In that analysis, 33.6% of patients achieved MRD-negative complete response and 29.1% achieved MRD-negative partial response.
In CAPTIVATE, by contrast, all of the complete remissions were MRD negative, as were a majority of the partial responders, Dr. Cheson noted.
Venetoclax and ibrutinib have “clinically complimentary activity” that provided a rationale for combining the two, Dr. Wierda said at ASCO. Ibrutinib is a BTK inhibitor that has a high rate of response and durable disease control, though continuous treatment is indicated, he said, because most patients achieve partial remissions as best response and continue to have residual disease in blood or bone marrow. Venetoclax, he added, is a BCL-2 inhibitor that produces durable partial remissions, though “residual disease is typically present in the form of persistently enlarged lymph nodes,” he said. “Venetoclax is highly effective at clearing disease from blood and bone marrow.”
The phase 2 CAPTIVATE trial includes a total of 164 patients younger than 70 years of age who receive a 3-cycle ibrutinib lead-in, followed by ibrutinib plus venetoclax for 12 cycles. At that point, patients are randomized according to MRD status. Patients with confirmed undetectable MRD are randomized to further treatment with ibrutinib or placebo, and those with undetectable MRD not confirmed are randomized to ibrutinib versus ibrutinib plus venetoclax.
In addition to early efficacy data, Dr. Wierda also reported some safety data. Compared with the single-agent ibrutinib lead-in period, combined ibrutinib plus venetoclax treatment had more gastrointestinal-associated events and neutropenia. Almost half of patients (45%) have had a treatment-related grade 3-4 adverse event, though just 18 (11%) have had treatment-related adverse events classified as serious, and there have been no adverse event-related deaths on study.
The high activity of ibrutinib plus venetoclax in CAPTIVATE supports further study of the combination, Dr. Wierda said. A randomized, open-label phase 3 trial of ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab as first-line treatment for CLL is currently recruiting.
The study was sponsored by Pharmacyclics, an AbbVie company. Dr. Wierda reported consulting and research funding from Pharmacyclics, AbbVie, and several other companies.
SOURCE: Wierda WG et al. ASCO 2018, Abstract 7502.
REPORTING FROM ASCO 2018
Key clinical point:
Major finding: Of 14 patients, 12 (86%) who completed 12 cycles of treatment had undetectable bone marrow MRD.
Study details: Early results of the phase 2 CAPTIVATE trial including 164 patients younger than 70 years of age with previously untreated CLL.
Disclosures: The study was sponsored by Pharmacyclics, an Abbvie company. Dr. Wierda reported consulting and research funding from Pharmacyclics, AbbVie, and several other companies.
Source: Wierda WG et al. ASCO 2018, Abstract 7502.
Sleep apnea treatment may not prevent sleepiness
Physicians who treat obstructive sleep apnea reported that patients with OSA can experience excessive sleepiness despite being on optimized airway treatment, findings from an online surgery of clinicians show.
Jazz Pharmaceuticals and the social media network Sermo conducted an online questionnaire on topics in excessive sleepiness and obstructive sleep apnea. The study was conducted March 30-31, 2018.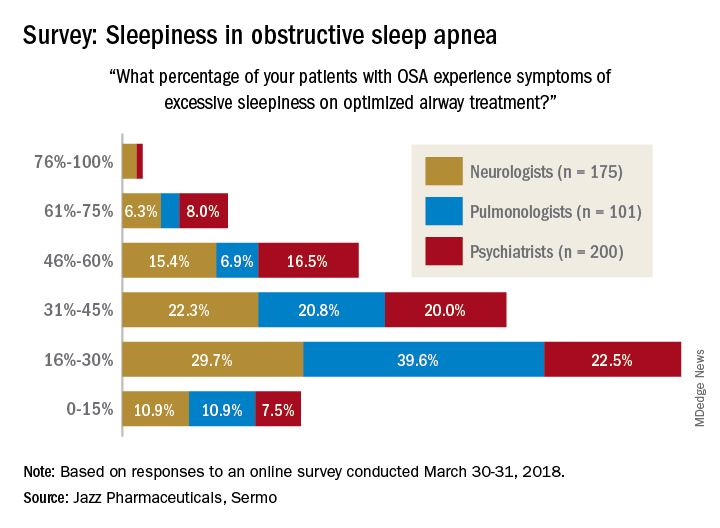
When asked how often they assess their OSA patients’ sleepiness, 46% of respondents said every 3 months, 28% said every 6 months, 19% said once a month, 6% said once a year, and 0.4% (one neurologist and one pulmonologist) said never. The method of evaluation varied by specialty: 82% of pulmonologists most often use the Epworth Sleepiness Scale and 76% of psychiatrists primarily use an informal set of questions, with neurologists in between but leaning toward informal questions, Jazz reported.
“As more scientific evidence emerges around the neuronal injury occurring due to OSA and the potential neurocognitive effects of excessive sleepiness, it’s imperative that pulmonologists, neurologists and psychiatrists understand the impact ES [excessive sleepiness] can have on patients’ lives,” Richard K. Bogan, MD, of the University of South Carolina, Columbia, a paid consultant to Jazz, said in a written statement.
Physicians who treat obstructive sleep apnea reported that patients with OSA can experience excessive sleepiness despite being on optimized airway treatment, findings from an online surgery of clinicians show.
Jazz Pharmaceuticals and the social media network Sermo conducted an online questionnaire on topics in excessive sleepiness and obstructive sleep apnea. The study was conducted March 30-31, 2018.
When asked how often they assess their OSA patients’ sleepiness, 46% of respondents said every 3 months, 28% said every 6 months, 19% said once a month, 6% said once a year, and 0.4% (one neurologist and one pulmonologist) said never. The method of evaluation varied by specialty: 82% of pulmonologists most often use the Epworth Sleepiness Scale and 76% of psychiatrists primarily use an informal set of questions, with neurologists in between but leaning toward informal questions, Jazz reported.
“As more scientific evidence emerges around the neuronal injury occurring due to OSA and the potential neurocognitive effects of excessive sleepiness, it’s imperative that pulmonologists, neurologists and psychiatrists understand the impact ES [excessive sleepiness] can have on patients’ lives,” Richard K. Bogan, MD, of the University of South Carolina, Columbia, a paid consultant to Jazz, said in a written statement.
Physicians who treat obstructive sleep apnea reported that patients with OSA can experience excessive sleepiness despite being on optimized airway treatment, findings from an online surgery of clinicians show.
Jazz Pharmaceuticals and the social media network Sermo conducted an online questionnaire on topics in excessive sleepiness and obstructive sleep apnea. The study was conducted March 30-31, 2018.
When asked how often they assess their OSA patients’ sleepiness, 46% of respondents said every 3 months, 28% said every 6 months, 19% said once a month, 6% said once a year, and 0.4% (one neurologist and one pulmonologist) said never. The method of evaluation varied by specialty: 82% of pulmonologists most often use the Epworth Sleepiness Scale and 76% of psychiatrists primarily use an informal set of questions, with neurologists in between but leaning toward informal questions, Jazz reported.
“As more scientific evidence emerges around the neuronal injury occurring due to OSA and the potential neurocognitive effects of excessive sleepiness, it’s imperative that pulmonologists, neurologists and psychiatrists understand the impact ES [excessive sleepiness] can have on patients’ lives,” Richard K. Bogan, MD, of the University of South Carolina, Columbia, a paid consultant to Jazz, said in a written statement.
Customized airway stents show promise in feasibility trial
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
REPORTING FROM ATS 2018
Key clinical point: Customized, 3-D airway stents have the potential for improving tolerance and decreasing the complication rate.
Major finding: Congruence and outcomes tended to be better in stenoses involving the bronchial level (three of three, no complications).
Study details: A feasibility study of eight patients with nonmalignant, anatomically complex, and symptomatic stenosis for which conventional stents were not suitable.
Disclosures: Dr. Guibert reported having no financial disclosures.
Source: Guibert N et al. ATS 2018, Abstract 4433.
New cell-free DNA assays hold promise for lung cancer screening
CHICAGO – according to the first interim report from a substudy of the large, ongoing Circulating Cell-free Genome Atlas (CCGA).
“Lung cancer screening with low-dose CT is known to improve outcomes. And yet, CT-based lung cancer screening is not widely adopted,” said lead study author Geoffrey R. Oxnard, MD, associate professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, Boston, in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the study was reported. “Criticisms of low-dose CT include the risk of false positives and overdiagnosis. We proposed to investigate an untapped opportunity for cancer detection, which is using cell-free DNA.”
Main substudy results among 164 patients with lung cancer and 923 comparable individuals without known cancer showed that at a specificity of 98%, the three assays evaluated detected up to 51% of early-stage (stage I-IIIA) lung cancers and up to 91% of late-stage (stage IIIB-IV) lung cancers. And among the healthy participants with false-positive results for lung cancer, several were ultimately found to have cancers of other types.
“This first interim analysis of the CCGA study demonstrates that comprehensive sequencing of the plasma cell-free DNA can generate high-quality data across the entire genome, and it permits noninvasive cancer detection. The assays can detect lung cancer across stages, across histologies, across populations,” Dr. Oxnard said.
“Together, these results support the promise of using cell-free, DNA-based assays to develop an early cancer detection test with high specificity. Further assay and clinical development is ongoing: There is a separate prospective trial enrolling, the STRIVE study, and there remain thousands of patients still on this CCGA study to be analyzed for further optimization and focusing of this assay towards an eventual cancer diagnostic.”
The cohort studied was not a screening population, so the assays’ performance cannot be compared with that of low-dose CT at this point, he said. But the hypothesis going forward is that the assays will have comparatively higher specificity, sparing some patients an unnecessary diagnostic work-up.
The population in which the final blood test might be used will depend on its diagnostic performance once the assays are fully refined and clinic ready, which will take some time, according to Dr. Oxnard. However, “2 years ago, this was a pipe dream. Two years ago, it was completely just a brainstorm that had no data to support it, and I didn’t believe that this could be done. Today, we actually have data to show that this is really feasible to find early-stage cancer in the blood. So this is a huge step forward and actually means that this is going to be a reality.”
“This is an important first step towards an easier way to detect lung cancer at earlier and hopefully more curable stages,” agreed ASCO Expert David Graham, MD, who is also medical director at the Levine Cancer Institute in Charlotte, N.C. “If the promise of this report holds, we could easily see a day when a person could be screened for lung cancer and possibly other cancers simply by going into their regular doctor’s office for a blood draw.”
Study details
The CCGA study has enrolled more than 12,000 of its planned 15,000 participants (70% with cancer, 30% without) across 142 U.S. and Canadian sites.
The substudy reported had a development cohort (118 patients with lung cancer, 561 individuals without cancer) and a validation cohort (46 patients with lung cancer, 362 individuals without cancer), with the lung cancer and noncancer groups matched on age, race, and body mass index. “Having a comparable control cohort is very important in developing such a diagnostic for accurate analysis of the potential false-positive rate,” Dr. Oxnard noted.
Three prototype assays were tested: A targeted sequencing assay entailing very deep sequencing across 507 genes for somatic mutations such as single-nucleotide variants and small insertions and/or deletions; a novel, whole-genome sequencing assay to detect somatic gene copy number changes; and a novel, whole-genome methylation sequencing assay to detect abnormal epigenetic changes.
Sequencing was also performed on DNA from white blood cells. “That’s very important. The white blood cells are rich with mutations that can pollute the DNA and make you think that there is cancer present in the cell-free DNA,” Dr. Oxnard explained. “You screen out this interference from the white blood cells and other biologic noise, and you are left with the final features: mutations, copy number variations, and methylation signatures that then go into the final assays being studied.”
Results showed that when assay specificity was 98%, sensitivity for early-stage (stage I-IIIA) lung cancer ranged from 38% to 51%, and sensitivity for late-stage (stage IIIB-IV) lung cancer ranged from 87% to 91%.
Among five presumed cancer-free individuals having positive results on all three assays, two subsequently received a cancer diagnosis (one with stage III ovarian cancer, one with stage II endometrial cancer).
An additional 19 cancer types across all stages were tested in the CCGA substudy. Early results for breast, gastrointestinal, gynecologic, blood, and other cancers were also reported at the meeting (abstracts 536, 12021, and 12003).
Dr. Oxnard disclosed that he has a consulting or advisory role with AstraZeneca, Inivata, Boehringer Ingelheim, Takeda, Genentech/Roche, Novartis, Loxo Oncology, Ignyta, DropWorks, and GRAIL, and that he has patents, royalties, and/or other intellectual property with Chugai Pharmaceutical, Bio-Rad, Sysmex, and Guardant Health. The study was funded by GRAIL.
SOURCE: Oxnard GR et al. ASCO 2018. Abstract LBA8501.
CHICAGO – according to the first interim report from a substudy of the large, ongoing Circulating Cell-free Genome Atlas (CCGA).
“Lung cancer screening with low-dose CT is known to improve outcomes. And yet, CT-based lung cancer screening is not widely adopted,” said lead study author Geoffrey R. Oxnard, MD, associate professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, Boston, in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the study was reported. “Criticisms of low-dose CT include the risk of false positives and overdiagnosis. We proposed to investigate an untapped opportunity for cancer detection, which is using cell-free DNA.”
Main substudy results among 164 patients with lung cancer and 923 comparable individuals without known cancer showed that at a specificity of 98%, the three assays evaluated detected up to 51% of early-stage (stage I-IIIA) lung cancers and up to 91% of late-stage (stage IIIB-IV) lung cancers. And among the healthy participants with false-positive results for lung cancer, several were ultimately found to have cancers of other types.
“This first interim analysis of the CCGA study demonstrates that comprehensive sequencing of the plasma cell-free DNA can generate high-quality data across the entire genome, and it permits noninvasive cancer detection. The assays can detect lung cancer across stages, across histologies, across populations,” Dr. Oxnard said.
“Together, these results support the promise of using cell-free, DNA-based assays to develop an early cancer detection test with high specificity. Further assay and clinical development is ongoing: There is a separate prospective trial enrolling, the STRIVE study, and there remain thousands of patients still on this CCGA study to be analyzed for further optimization and focusing of this assay towards an eventual cancer diagnostic.”
The cohort studied was not a screening population, so the assays’ performance cannot be compared with that of low-dose CT at this point, he said. But the hypothesis going forward is that the assays will have comparatively higher specificity, sparing some patients an unnecessary diagnostic work-up.
The population in which the final blood test might be used will depend on its diagnostic performance once the assays are fully refined and clinic ready, which will take some time, according to Dr. Oxnard. However, “2 years ago, this was a pipe dream. Two years ago, it was completely just a brainstorm that had no data to support it, and I didn’t believe that this could be done. Today, we actually have data to show that this is really feasible to find early-stage cancer in the blood. So this is a huge step forward and actually means that this is going to be a reality.”
“This is an important first step towards an easier way to detect lung cancer at earlier and hopefully more curable stages,” agreed ASCO Expert David Graham, MD, who is also medical director at the Levine Cancer Institute in Charlotte, N.C. “If the promise of this report holds, we could easily see a day when a person could be screened for lung cancer and possibly other cancers simply by going into their regular doctor’s office for a blood draw.”
Study details
The CCGA study has enrolled more than 12,000 of its planned 15,000 participants (70% with cancer, 30% without) across 142 U.S. and Canadian sites.
The substudy reported had a development cohort (118 patients with lung cancer, 561 individuals without cancer) and a validation cohort (46 patients with lung cancer, 362 individuals without cancer), with the lung cancer and noncancer groups matched on age, race, and body mass index. “Having a comparable control cohort is very important in developing such a diagnostic for accurate analysis of the potential false-positive rate,” Dr. Oxnard noted.
Three prototype assays were tested: A targeted sequencing assay entailing very deep sequencing across 507 genes for somatic mutations such as single-nucleotide variants and small insertions and/or deletions; a novel, whole-genome sequencing assay to detect somatic gene copy number changes; and a novel, whole-genome methylation sequencing assay to detect abnormal epigenetic changes.
Sequencing was also performed on DNA from white blood cells. “That’s very important. The white blood cells are rich with mutations that can pollute the DNA and make you think that there is cancer present in the cell-free DNA,” Dr. Oxnard explained. “You screen out this interference from the white blood cells and other biologic noise, and you are left with the final features: mutations, copy number variations, and methylation signatures that then go into the final assays being studied.”
Results showed that when assay specificity was 98%, sensitivity for early-stage (stage I-IIIA) lung cancer ranged from 38% to 51%, and sensitivity for late-stage (stage IIIB-IV) lung cancer ranged from 87% to 91%.
Among five presumed cancer-free individuals having positive results on all three assays, two subsequently received a cancer diagnosis (one with stage III ovarian cancer, one with stage II endometrial cancer).
An additional 19 cancer types across all stages were tested in the CCGA substudy. Early results for breast, gastrointestinal, gynecologic, blood, and other cancers were also reported at the meeting (abstracts 536, 12021, and 12003).
Dr. Oxnard disclosed that he has a consulting or advisory role with AstraZeneca, Inivata, Boehringer Ingelheim, Takeda, Genentech/Roche, Novartis, Loxo Oncology, Ignyta, DropWorks, and GRAIL, and that he has patents, royalties, and/or other intellectual property with Chugai Pharmaceutical, Bio-Rad, Sysmex, and Guardant Health. The study was funded by GRAIL.
SOURCE: Oxnard GR et al. ASCO 2018. Abstract LBA8501.
CHICAGO – according to the first interim report from a substudy of the large, ongoing Circulating Cell-free Genome Atlas (CCGA).
“Lung cancer screening with low-dose CT is known to improve outcomes. And yet, CT-based lung cancer screening is not widely adopted,” said lead study author Geoffrey R. Oxnard, MD, associate professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, Boston, in a press briefing at the annual meeting of the American Society of Clinical Oncology, where the study was reported. “Criticisms of low-dose CT include the risk of false positives and overdiagnosis. We proposed to investigate an untapped opportunity for cancer detection, which is using cell-free DNA.”
Main substudy results among 164 patients with lung cancer and 923 comparable individuals without known cancer showed that at a specificity of 98%, the three assays evaluated detected up to 51% of early-stage (stage I-IIIA) lung cancers and up to 91% of late-stage (stage IIIB-IV) lung cancers. And among the healthy participants with false-positive results for lung cancer, several were ultimately found to have cancers of other types.
“This first interim analysis of the CCGA study demonstrates that comprehensive sequencing of the plasma cell-free DNA can generate high-quality data across the entire genome, and it permits noninvasive cancer detection. The assays can detect lung cancer across stages, across histologies, across populations,” Dr. Oxnard said.
“Together, these results support the promise of using cell-free, DNA-based assays to develop an early cancer detection test with high specificity. Further assay and clinical development is ongoing: There is a separate prospective trial enrolling, the STRIVE study, and there remain thousands of patients still on this CCGA study to be analyzed for further optimization and focusing of this assay towards an eventual cancer diagnostic.”
The cohort studied was not a screening population, so the assays’ performance cannot be compared with that of low-dose CT at this point, he said. But the hypothesis going forward is that the assays will have comparatively higher specificity, sparing some patients an unnecessary diagnostic work-up.
The population in which the final blood test might be used will depend on its diagnostic performance once the assays are fully refined and clinic ready, which will take some time, according to Dr. Oxnard. However, “2 years ago, this was a pipe dream. Two years ago, it was completely just a brainstorm that had no data to support it, and I didn’t believe that this could be done. Today, we actually have data to show that this is really feasible to find early-stage cancer in the blood. So this is a huge step forward and actually means that this is going to be a reality.”
“This is an important first step towards an easier way to detect lung cancer at earlier and hopefully more curable stages,” agreed ASCO Expert David Graham, MD, who is also medical director at the Levine Cancer Institute in Charlotte, N.C. “If the promise of this report holds, we could easily see a day when a person could be screened for lung cancer and possibly other cancers simply by going into their regular doctor’s office for a blood draw.”
Study details
The CCGA study has enrolled more than 12,000 of its planned 15,000 participants (70% with cancer, 30% without) across 142 U.S. and Canadian sites.
The substudy reported had a development cohort (118 patients with lung cancer, 561 individuals without cancer) and a validation cohort (46 patients with lung cancer, 362 individuals without cancer), with the lung cancer and noncancer groups matched on age, race, and body mass index. “Having a comparable control cohort is very important in developing such a diagnostic for accurate analysis of the potential false-positive rate,” Dr. Oxnard noted.
Three prototype assays were tested: A targeted sequencing assay entailing very deep sequencing across 507 genes for somatic mutations such as single-nucleotide variants and small insertions and/or deletions; a novel, whole-genome sequencing assay to detect somatic gene copy number changes; and a novel, whole-genome methylation sequencing assay to detect abnormal epigenetic changes.
Sequencing was also performed on DNA from white blood cells. “That’s very important. The white blood cells are rich with mutations that can pollute the DNA and make you think that there is cancer present in the cell-free DNA,” Dr. Oxnard explained. “You screen out this interference from the white blood cells and other biologic noise, and you are left with the final features: mutations, copy number variations, and methylation signatures that then go into the final assays being studied.”
Results showed that when assay specificity was 98%, sensitivity for early-stage (stage I-IIIA) lung cancer ranged from 38% to 51%, and sensitivity for late-stage (stage IIIB-IV) lung cancer ranged from 87% to 91%.
Among five presumed cancer-free individuals having positive results on all three assays, two subsequently received a cancer diagnosis (one with stage III ovarian cancer, one with stage II endometrial cancer).
An additional 19 cancer types across all stages were tested in the CCGA substudy. Early results for breast, gastrointestinal, gynecologic, blood, and other cancers were also reported at the meeting (abstracts 536, 12021, and 12003).
Dr. Oxnard disclosed that he has a consulting or advisory role with AstraZeneca, Inivata, Boehringer Ingelheim, Takeda, Genentech/Roche, Novartis, Loxo Oncology, Ignyta, DropWorks, and GRAIL, and that he has patents, royalties, and/or other intellectual property with Chugai Pharmaceutical, Bio-Rad, Sysmex, and Guardant Health. The study was funded by GRAIL.
SOURCE: Oxnard GR et al. ASCO 2018. Abstract LBA8501.
REPORTING FROM ASCO 2018
Key clinical point: Three blood-based assays performed moderately well for identifying lung cancer in early, potentially curable stages.
Major finding: At 98% specificity, the assays had sensitivities of 38%-51% for detecting lung cancers of stage I-IIIA.
Study details: A case-control study of circulating cell-free DNA assays among 164 patients with lung cancer and 923 comparable individuals without cancer.
Disclosures: Dr. Oxnard disclosed that he has a consulting or advisory role with AstraZeneca, Inivata, Boehringer Ingelheim, Takeda, Genentech/Roche, Novartis, Loxo Oncology, Ignyta, DropWorks, and GRAIL, and that he has patents, royalties, and/or other intellectual property with Chugai Pharmaceutical, Bio-Rad, Sysmex, and Guardant Health. The study was funded by GRAIL.
Source: Oxnard GR et al. ASCO 2018. Abstract LBA8501.
What Are Predictors of Mortality in Veterans With MS?
A retrospective study finds that 39% of patients who died during follow-up were never on a disease-modifying therapy, compared with 22% of those who were still alive.
NASHVILLE—Among veterans with multiple sclerosis (MS), excess MS-related mortality is mainly influenced by initial presentation with progressive MS, sensory and cerebellar complaints, and higher levels of disability. Excess mortality also may be influenced by motor complaints and low BMI, according to research presented at the 2018 CMSC Annual Meeting. Main causes of death include MS itself, infection, respiratory disease, and cancer.
These findings suggest a need to pay more attention “to preventive strategies such as yearly influenza immunization, aggressively treating MS-related complications, and comorbidities, especially vascular risk factors,” said the researchers.
Studying mortality in chronic neurologic diseases may help identify treatable risk factors, and population-based studies of English death records have found that about 64% of patients with MS have a neurologic cause of death.
To identify the predictors of mortality in veterans with MS attending outpatient clinics, Meheroz H. Rabadi, MD, Clinical Professor of Neurology at the University of Oklahoma College of Medicine and Medical Director of the Oklahoma City Veterans Affairs Medical Center MS Program, and Christopher E. Aston, PhD, Associate Research Professor of Pediatrics and a biostatistician at the University of Oklahoma Sciences Center, conducted a retrospective study.
Data From a Veterans Affairs Medical Center
The researchers conducted a retrospective electronic chart review of data from 229 veterans with MS diagnosed based on the McDonald criteria who were registered in the MS program at the Oklahoma City Veterans Affairs Medical Center between January 1, 2000, and December 31, 2014. Participants were followed up every four months in the clinic.
Data included age at initial clinic visit, gender, ethnicity and race, age at MS diagnosis, clinical MS subtype (ie, relapsing-remitting, secondary progressive, primary progressive, clinically isolated syndrome, or radiologically isolated syndrome), and initial presenting features (eg, visual complaints, motor weakness, balance disorder, and sensory complaints).
Drs. Rabadi and Aston determined an impairment index based on the presence or absence of motor and nonmotor signs on initial examination. MS severity was measured by initial Expanded Disability Status Scale and total Functional Independence Measure scores.
The researchers recorded the presence of pre-existing and new-onset comorbidities that are commonly encountered in veterans and are the most common causes of disability and death in the United States, such as hypertension, hyperlipidemia, diabetes mellitus, BMI, and history of smoking. They also recorded the presence of MS-related complications such as pressure ulcers, neurogenic bowel, and neurogenic bladder.
Main Causes of Death
A total of 226 participants were included in the analysis; 17% were female. Most participants were white (81%), 13% were black, 1% were Hispanic, and 2% were Native American.
The mortality rate at the end of the 15-year study period was 14%. Among the 33 patients who died, the main causes of death documented were MS disease itself (57% of cases), infection (43%), cancer (18%), and respiratory failure (18%). Cox regression analysis using the whole cohort found that significant predictors of mortality were progressive MS type; older age at entry into the study; the presence of sensory, cerebellar, or motor complaints on presentation; more disability on presentation; lower BMI; diabetes; not having been on disease-modifying therapy; and the presence of pressure ulcers or neurogenic bladder.
Among patients who died during follow up, 36% had primary progressive MS, compared with 17% of patients who were alive; 42% of patients who died had secondary progressive MS, compared with 21% of those who were alive.
In addition, patients who died had an average age of 56 at study entry, compared with an average age of 48 among those who were alive. BMI among those who died was 23.7, compared with 28 among patients who were alive. Thirty-nine percent of patients who died were never on a disease-modifying therapy, compared with 22% of patients who were alive. Thirty-three percent of patients who died had diabetes mellitus, compared with 16% of those who were alive. Finally, 12% of patients who died had pressure ulcers, compared with 2% of patients who were alive, and 82% of patients who died had neurogenic bladder, compared with 53% of patients who were alive.
—Erica Tricarico
A retrospective study finds that 39% of patients who died during follow-up were never on a disease-modifying therapy, compared with 22% of those who were still alive.
A retrospective study finds that 39% of patients who died during follow-up were never on a disease-modifying therapy, compared with 22% of those who were still alive.
NASHVILLE—Among veterans with multiple sclerosis (MS), excess MS-related mortality is mainly influenced by initial presentation with progressive MS, sensory and cerebellar complaints, and higher levels of disability. Excess mortality also may be influenced by motor complaints and low BMI, according to research presented at the 2018 CMSC Annual Meeting. Main causes of death include MS itself, infection, respiratory disease, and cancer.
These findings suggest a need to pay more attention “to preventive strategies such as yearly influenza immunization, aggressively treating MS-related complications, and comorbidities, especially vascular risk factors,” said the researchers.
Studying mortality in chronic neurologic diseases may help identify treatable risk factors, and population-based studies of English death records have found that about 64% of patients with MS have a neurologic cause of death.
To identify the predictors of mortality in veterans with MS attending outpatient clinics, Meheroz H. Rabadi, MD, Clinical Professor of Neurology at the University of Oklahoma College of Medicine and Medical Director of the Oklahoma City Veterans Affairs Medical Center MS Program, and Christopher E. Aston, PhD, Associate Research Professor of Pediatrics and a biostatistician at the University of Oklahoma Sciences Center, conducted a retrospective study.
Data From a Veterans Affairs Medical Center
The researchers conducted a retrospective electronic chart review of data from 229 veterans with MS diagnosed based on the McDonald criteria who were registered in the MS program at the Oklahoma City Veterans Affairs Medical Center between January 1, 2000, and December 31, 2014. Participants were followed up every four months in the clinic.
Data included age at initial clinic visit, gender, ethnicity and race, age at MS diagnosis, clinical MS subtype (ie, relapsing-remitting, secondary progressive, primary progressive, clinically isolated syndrome, or radiologically isolated syndrome), and initial presenting features (eg, visual complaints, motor weakness, balance disorder, and sensory complaints).
Drs. Rabadi and Aston determined an impairment index based on the presence or absence of motor and nonmotor signs on initial examination. MS severity was measured by initial Expanded Disability Status Scale and total Functional Independence Measure scores.
The researchers recorded the presence of pre-existing and new-onset comorbidities that are commonly encountered in veterans and are the most common causes of disability and death in the United States, such as hypertension, hyperlipidemia, diabetes mellitus, BMI, and history of smoking. They also recorded the presence of MS-related complications such as pressure ulcers, neurogenic bowel, and neurogenic bladder.
Main Causes of Death
A total of 226 participants were included in the analysis; 17% were female. Most participants were white (81%), 13% were black, 1% were Hispanic, and 2% were Native American.
The mortality rate at the end of the 15-year study period was 14%. Among the 33 patients who died, the main causes of death documented were MS disease itself (57% of cases), infection (43%), cancer (18%), and respiratory failure (18%). Cox regression analysis using the whole cohort found that significant predictors of mortality were progressive MS type; older age at entry into the study; the presence of sensory, cerebellar, or motor complaints on presentation; more disability on presentation; lower BMI; diabetes; not having been on disease-modifying therapy; and the presence of pressure ulcers or neurogenic bladder.
Among patients who died during follow up, 36% had primary progressive MS, compared with 17% of patients who were alive; 42% of patients who died had secondary progressive MS, compared with 21% of those who were alive.
In addition, patients who died had an average age of 56 at study entry, compared with an average age of 48 among those who were alive. BMI among those who died was 23.7, compared with 28 among patients who were alive. Thirty-nine percent of patients who died were never on a disease-modifying therapy, compared with 22% of patients who were alive. Thirty-three percent of patients who died had diabetes mellitus, compared with 16% of those who were alive. Finally, 12% of patients who died had pressure ulcers, compared with 2% of patients who were alive, and 82% of patients who died had neurogenic bladder, compared with 53% of patients who were alive.
—Erica Tricarico
NASHVILLE—Among veterans with multiple sclerosis (MS), excess MS-related mortality is mainly influenced by initial presentation with progressive MS, sensory and cerebellar complaints, and higher levels of disability. Excess mortality also may be influenced by motor complaints and low BMI, according to research presented at the 2018 CMSC Annual Meeting. Main causes of death include MS itself, infection, respiratory disease, and cancer.
These findings suggest a need to pay more attention “to preventive strategies such as yearly influenza immunization, aggressively treating MS-related complications, and comorbidities, especially vascular risk factors,” said the researchers.
Studying mortality in chronic neurologic diseases may help identify treatable risk factors, and population-based studies of English death records have found that about 64% of patients with MS have a neurologic cause of death.
To identify the predictors of mortality in veterans with MS attending outpatient clinics, Meheroz H. Rabadi, MD, Clinical Professor of Neurology at the University of Oklahoma College of Medicine and Medical Director of the Oklahoma City Veterans Affairs Medical Center MS Program, and Christopher E. Aston, PhD, Associate Research Professor of Pediatrics and a biostatistician at the University of Oklahoma Sciences Center, conducted a retrospective study.
Data From a Veterans Affairs Medical Center
The researchers conducted a retrospective electronic chart review of data from 229 veterans with MS diagnosed based on the McDonald criteria who were registered in the MS program at the Oklahoma City Veterans Affairs Medical Center between January 1, 2000, and December 31, 2014. Participants were followed up every four months in the clinic.
Data included age at initial clinic visit, gender, ethnicity and race, age at MS diagnosis, clinical MS subtype (ie, relapsing-remitting, secondary progressive, primary progressive, clinically isolated syndrome, or radiologically isolated syndrome), and initial presenting features (eg, visual complaints, motor weakness, balance disorder, and sensory complaints).
Drs. Rabadi and Aston determined an impairment index based on the presence or absence of motor and nonmotor signs on initial examination. MS severity was measured by initial Expanded Disability Status Scale and total Functional Independence Measure scores.
The researchers recorded the presence of pre-existing and new-onset comorbidities that are commonly encountered in veterans and are the most common causes of disability and death in the United States, such as hypertension, hyperlipidemia, diabetes mellitus, BMI, and history of smoking. They also recorded the presence of MS-related complications such as pressure ulcers, neurogenic bowel, and neurogenic bladder.
Main Causes of Death
A total of 226 participants were included in the analysis; 17% were female. Most participants were white (81%), 13% were black, 1% were Hispanic, and 2% were Native American.
The mortality rate at the end of the 15-year study period was 14%. Among the 33 patients who died, the main causes of death documented were MS disease itself (57% of cases), infection (43%), cancer (18%), and respiratory failure (18%). Cox regression analysis using the whole cohort found that significant predictors of mortality were progressive MS type; older age at entry into the study; the presence of sensory, cerebellar, or motor complaints on presentation; more disability on presentation; lower BMI; diabetes; not having been on disease-modifying therapy; and the presence of pressure ulcers or neurogenic bladder.
Among patients who died during follow up, 36% had primary progressive MS, compared with 17% of patients who were alive; 42% of patients who died had secondary progressive MS, compared with 21% of those who were alive.
In addition, patients who died had an average age of 56 at study entry, compared with an average age of 48 among those who were alive. BMI among those who died was 23.7, compared with 28 among patients who were alive. Thirty-nine percent of patients who died were never on a disease-modifying therapy, compared with 22% of patients who were alive. Thirty-three percent of patients who died had diabetes mellitus, compared with 16% of those who were alive. Finally, 12% of patients who died had pressure ulcers, compared with 2% of patients who were alive, and 82% of patients who died had neurogenic bladder, compared with 53% of patients who were alive.
—Erica Tricarico












