User login
Uncommon Presentation of Chromoblastomycosis
Case Report
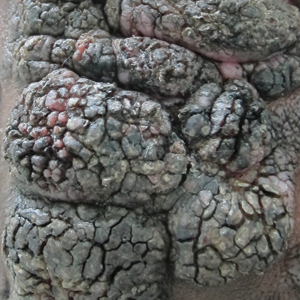
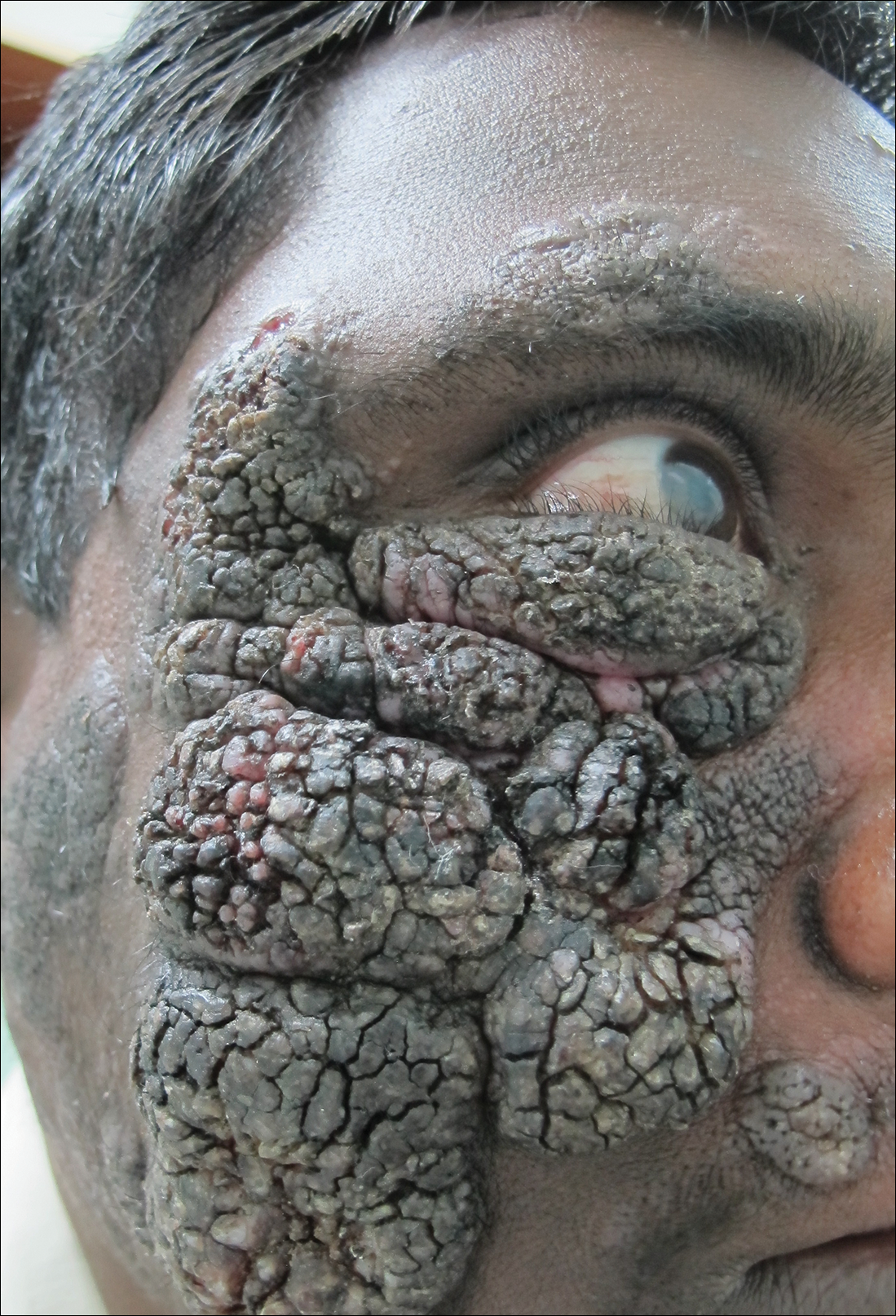
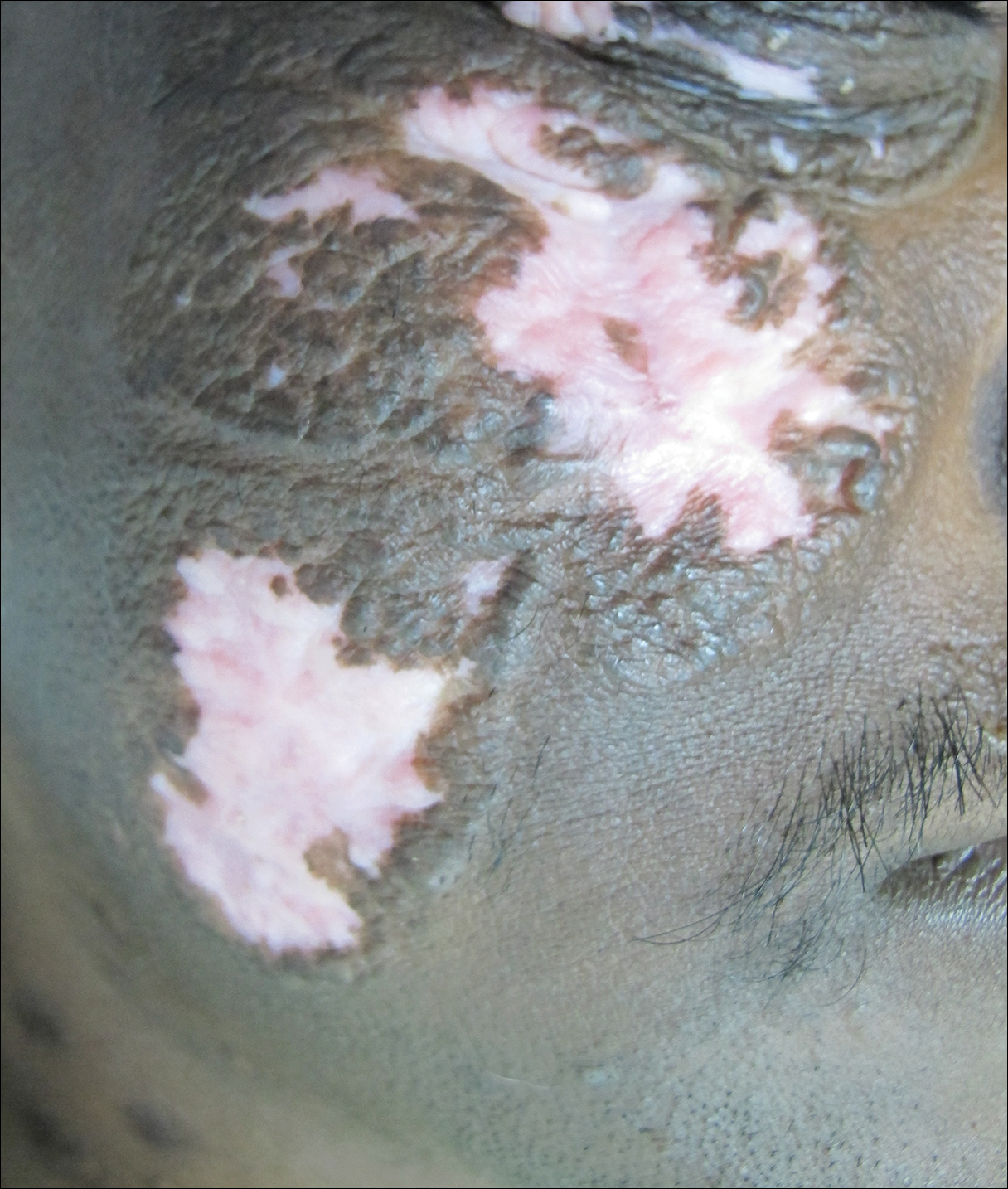
A 25-year-old man who was a dairy farmer in Ahmednagar, Maharashtra, India, presented with a history of slowly growing, occasionally itchy lesions on both cheeks of 20 years’ duration. Most of the right cheek was covered by a well-defined, lobulated, gray-brown verrucous mass with a cerebriform surface (Figure 1). The left cheek was covered with a gray-brown infiltrated plaque surrounded by brown-tinged monomorphic papules.
Routine investigations were normal at presentation. Tests for purified protein derivative (tuberculin) and antibodies to human immunodeficiency virus were negative. Magnetic resonance imaging of the head showed soft tissue thickening with ulcerations involving the skin, subcutaneous tissue, and underlying facial muscles of the right cheek.
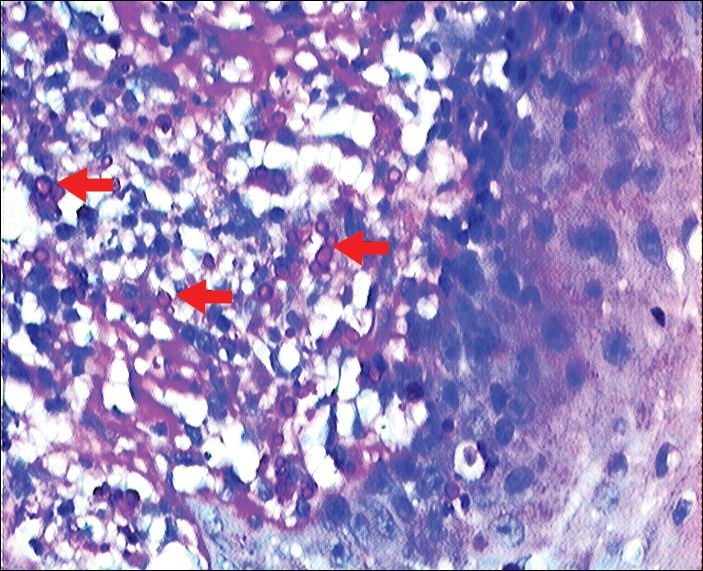
On histopathology, a hematoxylin and eosin–stained section showed hyperkeratosis, parakeratosis, pseudoepitheliomatous hyperplasia, and follicular plugs in the epidermis, as well as a mixed cellular infiltrate with Langhans giant cells and sclerotic bodies in the dermis (Figure 2). Periodic acid–Schiff and methenamine silver special stains revealed sclerotic bodies.
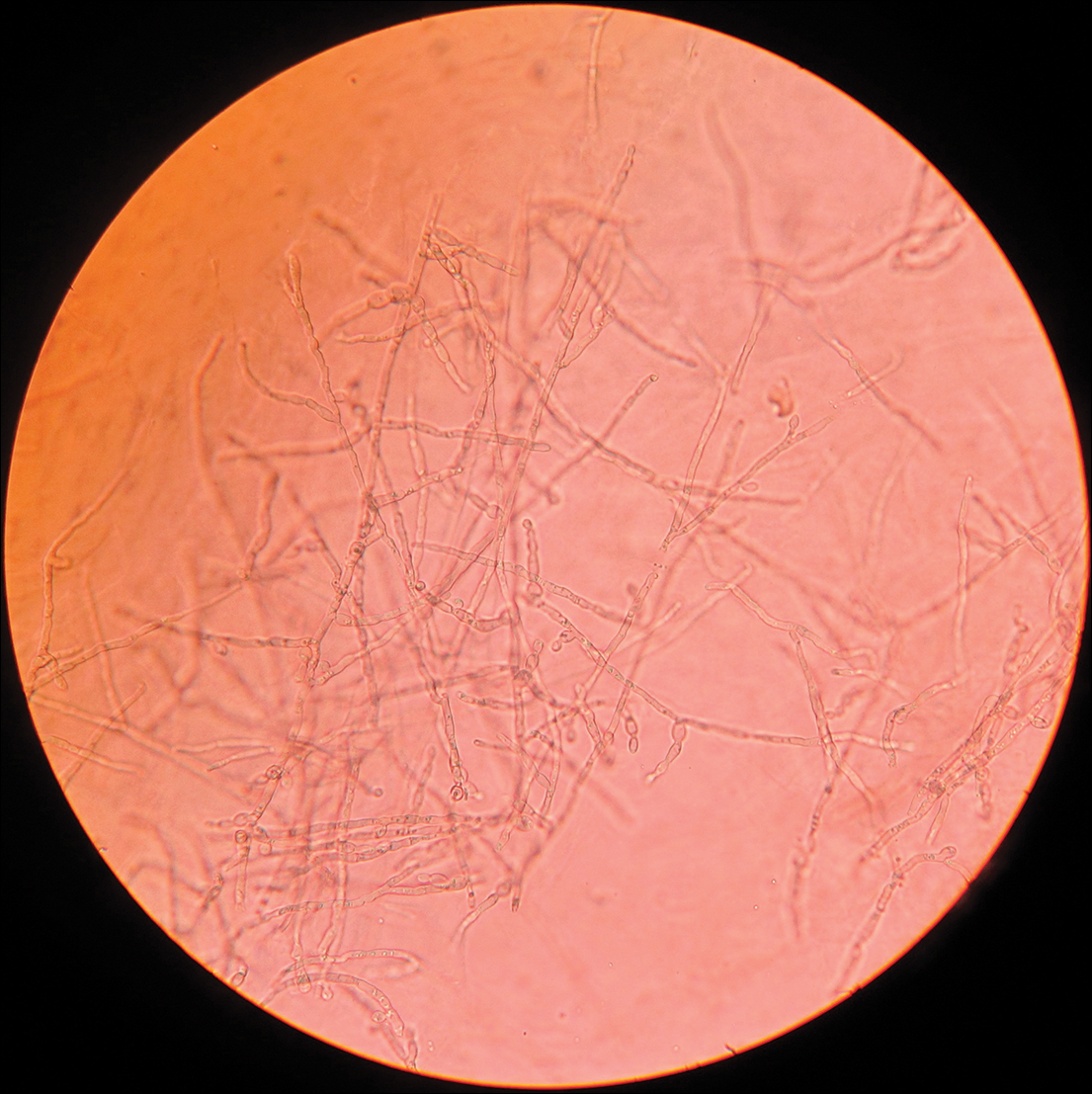
Fungal culture on Sabouraud dextrose agar at 25°C and 37°C grew olive green, rugose, velvety, leathery colonies within 48 hours, with pigmentation front and reverse (Figure 3). A panfungal polymerase chain reaction assay was positive. Direct microscopic examination of a 10% potassium hydroxide mount of the colonies showed mycelia with dematiaceous septate hyphae (Figure 4), apical branching, branching conidiophores, elliptical conidia in long chains, and pathognomonic round yeastlike bodies resembling copper pennies known as sclerotic cells (also called muriform cells and medlar bodies).1,2 The causative organism was identified as Cladosporium carrionii. A final diagnosis of chromoblastomycosis was made.

After 2 months of treatment with oral itraconazole 400 mg daily, there was no notable clinical improvement and fungal elements were still seen on culture. Four treatment cycles of intravenous liposomal amphotericin B 50 mg daily (1 mg/kg daily) for 15 days followed by itraconazole 200 mg daily for another 15 days caused substantial reduction and flattening of the lesion on the right side and resolution of the lesions on the left side. Healing was accompanied by central erythema and depigmentation (Figure 5). With a suspicion of continuing C carrionii activity on the right cheek, intralesional liposomal amphotericin B 0.2 mL (in a dilution of 5 mg in 1 mL) was given weekly in the peripheral hyperpigmented raised margin, which resulted in further flattening and reduction in tissue resistance. Fungal elements were absent on repeat biopsy and culture after 4 weeks.
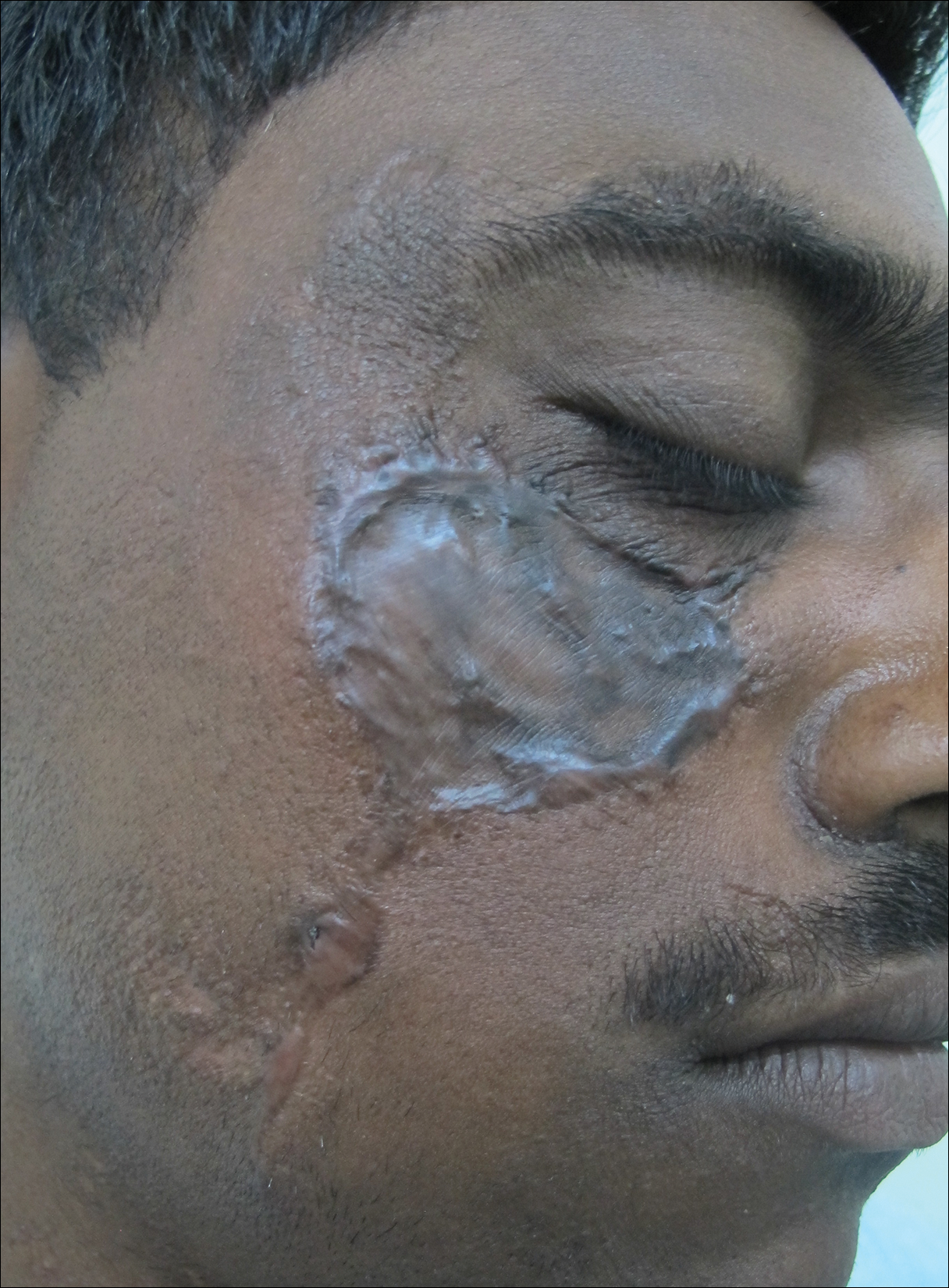
Six months after negative culture, further cosmetic correction of the scar on the right cheek was performed with a patterned full-thickness graft for the upper half and excision with approximation of the edges for the lower half (Figure 6). Cultures have been negative for the last 20 months; as of this writing, there has been no recurrence of lesions.
Comment
Distribution
Chromoblastomycosis, also known as chromomycosis and verrucous dermatitis,3 is a chronic subcutaneous mycosis found in tropical and subtropical regions.3,4 It is caused by traumatic inoculation of any of several members of a specific group of dematiaceous fungi through the skin.2,3 Common causative organisms include Fonsecaea pedrosoi, C carrionii, Fonsecaea compacta, and Phialophora verrucosa, all of which are saprophytes in soil and plants. Fonsecaea pedrosoi is the most common causative agent worldwide (70%–90% of cases).2Cladosporium carrionii tends to be the predominant pathogen isolated in patients who present in drier climates, with F pedrosoi in humid forests.1-4
In India, chromoblastomycosis has been reported from the sub-Himalayan belt and western and eastern coasts.1,5 Our patient resided in Ahmednagar, Maharashtra, India, which has a predominantly hot and dry climate. The history might include vegetational trauma, such as a thorn prick. Time between inoculation and development of disease is believed to be years.
Clinical Presentation
Chromoblastomycosis is characterized by a slowly enlarging lesion at the site of inoculation. Five morphological variants are known: nodular, tumoral, verrucous, plaque, and cicatricial; verrucous and nodular types are most common.3,4
The disease is limited to the skin and subcutaneous tissue, growing in extent rather than in depth and not directly invading muscle or bone.4 Lymphatic and hematogenous dissemination can occur.3,4 Secondary bacterial infection is common. The most common affected site is the lower limb, especially the foot.1,3 The upper limb and rarely the ear, trunk, face, and breast can be affected.
Diagnosis
Routine laboratory investigations are usually within reference range. Diagnosis is made by histopathological and mycological studies. Preferably, scrapings or biopsy material are taken from lesions that are covered with what is described as “black dots” (an area of transepidermal elimination of the fungus) where there is a better diagnostic yield.2-4 Routine histopathology shows hyperkeratosis, pseudoepitheliomatous hyperplasia of the epidermis, a mixed granulomatous neutrophil response with multinucleated giant cells and neutrophil abscesses, refractile fungal spores, typical sclerotic cells around abscesses or granulomas, and a dense fibrous response in the dermis and subcutaneous tissue.
Extensive fibrosis, coupled with a chronic inflammatory infiltrate and increased susceptibility to secondary infection, leads to obstruction of lymphatic flow and lymphedema below the affected site.2-4 Periodic acid–Schiff and Gomori methenamine silver stains confirm the presence of fungus. Direct microscopic examination of a 10% potassium hydroxide mount of scrapings reveals spherical, thick-walled, darkly pigmented, multiseptate sclerotic cells known as medlar bodies, copper pennies, and muriform cells that are pathognomonic for chromoblastomycosis.1-4Cladosporium carrionii culture on Sabouraud dextrose agar at 37°C shows olive green, dark, rugose, smooth, hairy, leathery or velvety colonies with pigmentation front and reverse. Direct microscopic examination of the colonies shows dematiaceous septate hyphae and sparsely branching conidiophores bearing ellipsoidal, smooth-walled conidia in long acropetal chains.1,4
Treatment
Treatment options for chromoblastomycosis can be divided into antifungal agents and physical methods.Antifungal agents include itraconazole (200–400 mg daily),3 terbinafine (250–500 mg daily),3 5-fluorocytosine (100–150 mg/kg daily),3 amphotericin B (intravenous/intralesional), and others (eg, fluconazole, ketoconazole, posaconazole [800 mg daily],6,7 potassium iodide, voriconazole). Physical methods include CO2 laser, cryosurgery, local heat therapy, Mohs micrographic surgery, and standard surgery.3 There is no evidence-based treatment protocol. Itraconazole and terbinafine are considered drugs of first choice1,8; however, combination therapy is the best option.9
- Ajanta S, Naba KH, Deepak G. Chromoblastomycosis in sub-tropical regions of India. Mycopathologia. 2010;169:381-386.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Flavio QT, Phillippe E, Maigualida PB, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clin Dermatol. 2007;25:188-194.
- Pradhan SV, Talwar OP, Ghosh A, et al. Chromoblastomycosis in Nepal: a study of 13 cases. Indian J Dermatol Venereol Leprol. 2007;73:176-178.
- Krzys´ciak PM, Pindycka-Piaszczys´ska M, Piaszczys´ski M. Chromoblastomycosis [published online October 22, 2014]. Postepy Dermatol Alergol. 2014;31:310-321.
- Negroni R, Tobón A, Bustamante B, et al. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339-346.
- Mohanty L, Mohanty P, Padhi T, et al. Verrucous growth on leg. Indian J Dermatol Venereol Leprol. 2006;72:399-400.
- Najafzadeh MJ, Rezusta A, Cameo MI, et al. Successful treatment of chromoblastomycosis of 36 years duration caused by Fonsecaea monophora. Med Mycol. 2010;48:390-393.
Case Report
A 25-year-old man who was a dairy farmer in Ahmednagar, Maharashtra, India, presented with a history of slowly growing, occasionally itchy lesions on both cheeks of 20 years’ duration. Most of the right cheek was covered by a well-defined, lobulated, gray-brown verrucous mass with a cerebriform surface (Figure 1). The left cheek was covered with a gray-brown infiltrated plaque surrounded by brown-tinged monomorphic papules.

Routine investigations were normal at presentation. Tests for purified protein derivative (tuberculin) and antibodies to human immunodeficiency virus were negative. Magnetic resonance imaging of the head showed soft tissue thickening with ulcerations involving the skin, subcutaneous tissue, and underlying facial muscles of the right cheek.
On histopathology, a hematoxylin and eosin–stained section showed hyperkeratosis, parakeratosis, pseudoepitheliomatous hyperplasia, and follicular plugs in the epidermis, as well as a mixed cellular infiltrate with Langhans giant cells and sclerotic bodies in the dermis (Figure 2). Periodic acid–Schiff and methenamine silver special stains revealed sclerotic bodies.

Fungal culture on Sabouraud dextrose agar at 25°C and 37°C grew olive green, rugose, velvety, leathery colonies within 48 hours, with pigmentation front and reverse (Figure 3). A panfungal polymerase chain reaction assay was positive. Direct microscopic examination of a 10% potassium hydroxide mount of the colonies showed mycelia with dematiaceous septate hyphae (Figure 4), apical branching, branching conidiophores, elliptical conidia in long chains, and pathognomonic round yeastlike bodies resembling copper pennies known as sclerotic cells (also called muriform cells and medlar bodies).1,2 The causative organism was identified as Cladosporium carrionii. A final diagnosis of chromoblastomycosis was made.


After 2 months of treatment with oral itraconazole 400 mg daily, there was no notable clinical improvement and fungal elements were still seen on culture. Four treatment cycles of intravenous liposomal amphotericin B 50 mg daily (1 mg/kg daily) for 15 days followed by itraconazole 200 mg daily for another 15 days caused substantial reduction and flattening of the lesion on the right side and resolution of the lesions on the left side. Healing was accompanied by central erythema and depigmentation (Figure 5). With a suspicion of continuing C carrionii activity on the right cheek, intralesional liposomal amphotericin B 0.2 mL (in a dilution of 5 mg in 1 mL) was given weekly in the peripheral hyperpigmented raised margin, which resulted in further flattening and reduction in tissue resistance. Fungal elements were absent on repeat biopsy and culture after 4 weeks.

Six months after negative culture, further cosmetic correction of the scar on the right cheek was performed with a patterned full-thickness graft for the upper half and excision with approximation of the edges for the lower half (Figure 6). Cultures have been negative for the last 20 months; as of this writing, there has been no recurrence of lesions.

Comment
Distribution
Chromoblastomycosis, also known as chromomycosis and verrucous dermatitis,3 is a chronic subcutaneous mycosis found in tropical and subtropical regions.3,4 It is caused by traumatic inoculation of any of several members of a specific group of dematiaceous fungi through the skin.2,3 Common causative organisms include Fonsecaea pedrosoi, C carrionii, Fonsecaea compacta, and Phialophora verrucosa, all of which are saprophytes in soil and plants. Fonsecaea pedrosoi is the most common causative agent worldwide (70%–90% of cases).2Cladosporium carrionii tends to be the predominant pathogen isolated in patients who present in drier climates, with F pedrosoi in humid forests.1-4
In India, chromoblastomycosis has been reported from the sub-Himalayan belt and western and eastern coasts.1,5 Our patient resided in Ahmednagar, Maharashtra, India, which has a predominantly hot and dry climate. The history might include vegetational trauma, such as a thorn prick. Time between inoculation and development of disease is believed to be years.
Clinical Presentation
Chromoblastomycosis is characterized by a slowly enlarging lesion at the site of inoculation. Five morphological variants are known: nodular, tumoral, verrucous, plaque, and cicatricial; verrucous and nodular types are most common.3,4
The disease is limited to the skin and subcutaneous tissue, growing in extent rather than in depth and not directly invading muscle or bone.4 Lymphatic and hematogenous dissemination can occur.3,4 Secondary bacterial infection is common. The most common affected site is the lower limb, especially the foot.1,3 The upper limb and rarely the ear, trunk, face, and breast can be affected.
Diagnosis
Routine laboratory investigations are usually within reference range. Diagnosis is made by histopathological and mycological studies. Preferably, scrapings or biopsy material are taken from lesions that are covered with what is described as “black dots” (an area of transepidermal elimination of the fungus) where there is a better diagnostic yield.2-4 Routine histopathology shows hyperkeratosis, pseudoepitheliomatous hyperplasia of the epidermis, a mixed granulomatous neutrophil response with multinucleated giant cells and neutrophil abscesses, refractile fungal spores, typical sclerotic cells around abscesses or granulomas, and a dense fibrous response in the dermis and subcutaneous tissue.
Extensive fibrosis, coupled with a chronic inflammatory infiltrate and increased susceptibility to secondary infection, leads to obstruction of lymphatic flow and lymphedema below the affected site.2-4 Periodic acid–Schiff and Gomori methenamine silver stains confirm the presence of fungus. Direct microscopic examination of a 10% potassium hydroxide mount of scrapings reveals spherical, thick-walled, darkly pigmented, multiseptate sclerotic cells known as medlar bodies, copper pennies, and muriform cells that are pathognomonic for chromoblastomycosis.1-4Cladosporium carrionii culture on Sabouraud dextrose agar at 37°C shows olive green, dark, rugose, smooth, hairy, leathery or velvety colonies with pigmentation front and reverse. Direct microscopic examination of the colonies shows dematiaceous septate hyphae and sparsely branching conidiophores bearing ellipsoidal, smooth-walled conidia in long acropetal chains.1,4
Treatment
Treatment options for chromoblastomycosis can be divided into antifungal agents and physical methods.Antifungal agents include itraconazole (200–400 mg daily),3 terbinafine (250–500 mg daily),3 5-fluorocytosine (100–150 mg/kg daily),3 amphotericin B (intravenous/intralesional), and others (eg, fluconazole, ketoconazole, posaconazole [800 mg daily],6,7 potassium iodide, voriconazole). Physical methods include CO2 laser, cryosurgery, local heat therapy, Mohs micrographic surgery, and standard surgery.3 There is no evidence-based treatment protocol. Itraconazole and terbinafine are considered drugs of first choice1,8; however, combination therapy is the best option.9
Case Report
A 25-year-old man who was a dairy farmer in Ahmednagar, Maharashtra, India, presented with a history of slowly growing, occasionally itchy lesions on both cheeks of 20 years’ duration. Most of the right cheek was covered by a well-defined, lobulated, gray-brown verrucous mass with a cerebriform surface (Figure 1). The left cheek was covered with a gray-brown infiltrated plaque surrounded by brown-tinged monomorphic papules.

Routine investigations were normal at presentation. Tests for purified protein derivative (tuberculin) and antibodies to human immunodeficiency virus were negative. Magnetic resonance imaging of the head showed soft tissue thickening with ulcerations involving the skin, subcutaneous tissue, and underlying facial muscles of the right cheek.
On histopathology, a hematoxylin and eosin–stained section showed hyperkeratosis, parakeratosis, pseudoepitheliomatous hyperplasia, and follicular plugs in the epidermis, as well as a mixed cellular infiltrate with Langhans giant cells and sclerotic bodies in the dermis (Figure 2). Periodic acid–Schiff and methenamine silver special stains revealed sclerotic bodies.

Fungal culture on Sabouraud dextrose agar at 25°C and 37°C grew olive green, rugose, velvety, leathery colonies within 48 hours, with pigmentation front and reverse (Figure 3). A panfungal polymerase chain reaction assay was positive. Direct microscopic examination of a 10% potassium hydroxide mount of the colonies showed mycelia with dematiaceous septate hyphae (Figure 4), apical branching, branching conidiophores, elliptical conidia in long chains, and pathognomonic round yeastlike bodies resembling copper pennies known as sclerotic cells (also called muriform cells and medlar bodies).1,2 The causative organism was identified as Cladosporium carrionii. A final diagnosis of chromoblastomycosis was made.


After 2 months of treatment with oral itraconazole 400 mg daily, there was no notable clinical improvement and fungal elements were still seen on culture. Four treatment cycles of intravenous liposomal amphotericin B 50 mg daily (1 mg/kg daily) for 15 days followed by itraconazole 200 mg daily for another 15 days caused substantial reduction and flattening of the lesion on the right side and resolution of the lesions on the left side. Healing was accompanied by central erythema and depigmentation (Figure 5). With a suspicion of continuing C carrionii activity on the right cheek, intralesional liposomal amphotericin B 0.2 mL (in a dilution of 5 mg in 1 mL) was given weekly in the peripheral hyperpigmented raised margin, which resulted in further flattening and reduction in tissue resistance. Fungal elements were absent on repeat biopsy and culture after 4 weeks.

Six months after negative culture, further cosmetic correction of the scar on the right cheek was performed with a patterned full-thickness graft for the upper half and excision with approximation of the edges for the lower half (Figure 6). Cultures have been negative for the last 20 months; as of this writing, there has been no recurrence of lesions.

Comment
Distribution
Chromoblastomycosis, also known as chromomycosis and verrucous dermatitis,3 is a chronic subcutaneous mycosis found in tropical and subtropical regions.3,4 It is caused by traumatic inoculation of any of several members of a specific group of dematiaceous fungi through the skin.2,3 Common causative organisms include Fonsecaea pedrosoi, C carrionii, Fonsecaea compacta, and Phialophora verrucosa, all of which are saprophytes in soil and plants. Fonsecaea pedrosoi is the most common causative agent worldwide (70%–90% of cases).2Cladosporium carrionii tends to be the predominant pathogen isolated in patients who present in drier climates, with F pedrosoi in humid forests.1-4
In India, chromoblastomycosis has been reported from the sub-Himalayan belt and western and eastern coasts.1,5 Our patient resided in Ahmednagar, Maharashtra, India, which has a predominantly hot and dry climate. The history might include vegetational trauma, such as a thorn prick. Time between inoculation and development of disease is believed to be years.
Clinical Presentation
Chromoblastomycosis is characterized by a slowly enlarging lesion at the site of inoculation. Five morphological variants are known: nodular, tumoral, verrucous, plaque, and cicatricial; verrucous and nodular types are most common.3,4
The disease is limited to the skin and subcutaneous tissue, growing in extent rather than in depth and not directly invading muscle or bone.4 Lymphatic and hematogenous dissemination can occur.3,4 Secondary bacterial infection is common. The most common affected site is the lower limb, especially the foot.1,3 The upper limb and rarely the ear, trunk, face, and breast can be affected.
Diagnosis
Routine laboratory investigations are usually within reference range. Diagnosis is made by histopathological and mycological studies. Preferably, scrapings or biopsy material are taken from lesions that are covered with what is described as “black dots” (an area of transepidermal elimination of the fungus) where there is a better diagnostic yield.2-4 Routine histopathology shows hyperkeratosis, pseudoepitheliomatous hyperplasia of the epidermis, a mixed granulomatous neutrophil response with multinucleated giant cells and neutrophil abscesses, refractile fungal spores, typical sclerotic cells around abscesses or granulomas, and a dense fibrous response in the dermis and subcutaneous tissue.
Extensive fibrosis, coupled with a chronic inflammatory infiltrate and increased susceptibility to secondary infection, leads to obstruction of lymphatic flow and lymphedema below the affected site.2-4 Periodic acid–Schiff and Gomori methenamine silver stains confirm the presence of fungus. Direct microscopic examination of a 10% potassium hydroxide mount of scrapings reveals spherical, thick-walled, darkly pigmented, multiseptate sclerotic cells known as medlar bodies, copper pennies, and muriform cells that are pathognomonic for chromoblastomycosis.1-4Cladosporium carrionii culture on Sabouraud dextrose agar at 37°C shows olive green, dark, rugose, smooth, hairy, leathery or velvety colonies with pigmentation front and reverse. Direct microscopic examination of the colonies shows dematiaceous septate hyphae and sparsely branching conidiophores bearing ellipsoidal, smooth-walled conidia in long acropetal chains.1,4
Treatment
Treatment options for chromoblastomycosis can be divided into antifungal agents and physical methods.Antifungal agents include itraconazole (200–400 mg daily),3 terbinafine (250–500 mg daily),3 5-fluorocytosine (100–150 mg/kg daily),3 amphotericin B (intravenous/intralesional), and others (eg, fluconazole, ketoconazole, posaconazole [800 mg daily],6,7 potassium iodide, voriconazole). Physical methods include CO2 laser, cryosurgery, local heat therapy, Mohs micrographic surgery, and standard surgery.3 There is no evidence-based treatment protocol. Itraconazole and terbinafine are considered drugs of first choice1,8; however, combination therapy is the best option.9
- Ajanta S, Naba KH, Deepak G. Chromoblastomycosis in sub-tropical regions of India. Mycopathologia. 2010;169:381-386.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Flavio QT, Phillippe E, Maigualida PB, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clin Dermatol. 2007;25:188-194.
- Pradhan SV, Talwar OP, Ghosh A, et al. Chromoblastomycosis in Nepal: a study of 13 cases. Indian J Dermatol Venereol Leprol. 2007;73:176-178.
- Krzys´ciak PM, Pindycka-Piaszczys´ska M, Piaszczys´ski M. Chromoblastomycosis [published online October 22, 2014]. Postepy Dermatol Alergol. 2014;31:310-321.
- Negroni R, Tobón A, Bustamante B, et al. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339-346.
- Mohanty L, Mohanty P, Padhi T, et al. Verrucous growth on leg. Indian J Dermatol Venereol Leprol. 2006;72:399-400.
- Najafzadeh MJ, Rezusta A, Cameo MI, et al. Successful treatment of chromoblastomycosis of 36 years duration caused by Fonsecaea monophora. Med Mycol. 2010;48:390-393.
- Ajanta S, Naba KH, Deepak G. Chromoblastomycosis in sub-tropical regions of India. Mycopathologia. 2010;169:381-386.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Flavio QT, Phillippe E, Maigualida PB, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clin Dermatol. 2007;25:188-194.
- Pradhan SV, Talwar OP, Ghosh A, et al. Chromoblastomycosis in Nepal: a study of 13 cases. Indian J Dermatol Venereol Leprol. 2007;73:176-178.
- Krzys´ciak PM, Pindycka-Piaszczys´ska M, Piaszczys´ski M. Chromoblastomycosis [published online October 22, 2014]. Postepy Dermatol Alergol. 2014;31:310-321.
- Negroni R, Tobón A, Bustamante B, et al. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339-346.
- Mohanty L, Mohanty P, Padhi T, et al. Verrucous growth on leg. Indian J Dermatol Venereol Leprol. 2006;72:399-400.
- Najafzadeh MJ, Rezusta A, Cameo MI, et al. Successful treatment of chromoblastomycosis of 36 years duration caused by Fonsecaea monophora. Med Mycol. 2010;48:390-393.
Practice Points
- Chromoblastomycosis is limited to skin and subcutaneous tissue, most commonly of the lower limb, especially the foot; it does not directly invade muscle or bone. Secondary bacterial infection is common.
- Chromoblastomycosis is a therapeutic challenge due to its recalcitrant nature. Itraconazole and terbinafine are considered drugs of first choice, but consensus and evidence are lacking on a standard of care.