User login
Dermoscopic Documentation of a No-see-um Bite
Dermoscopic Documentation of a No-see-um Bite
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00

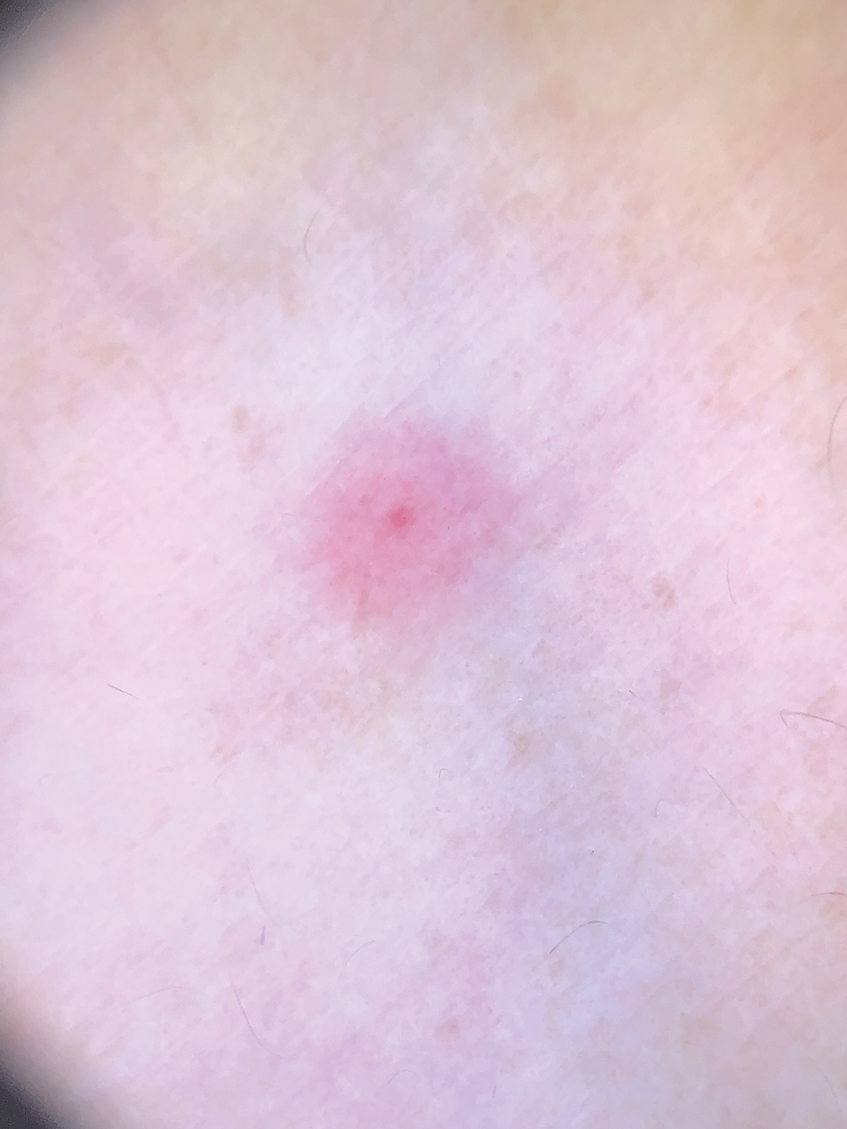
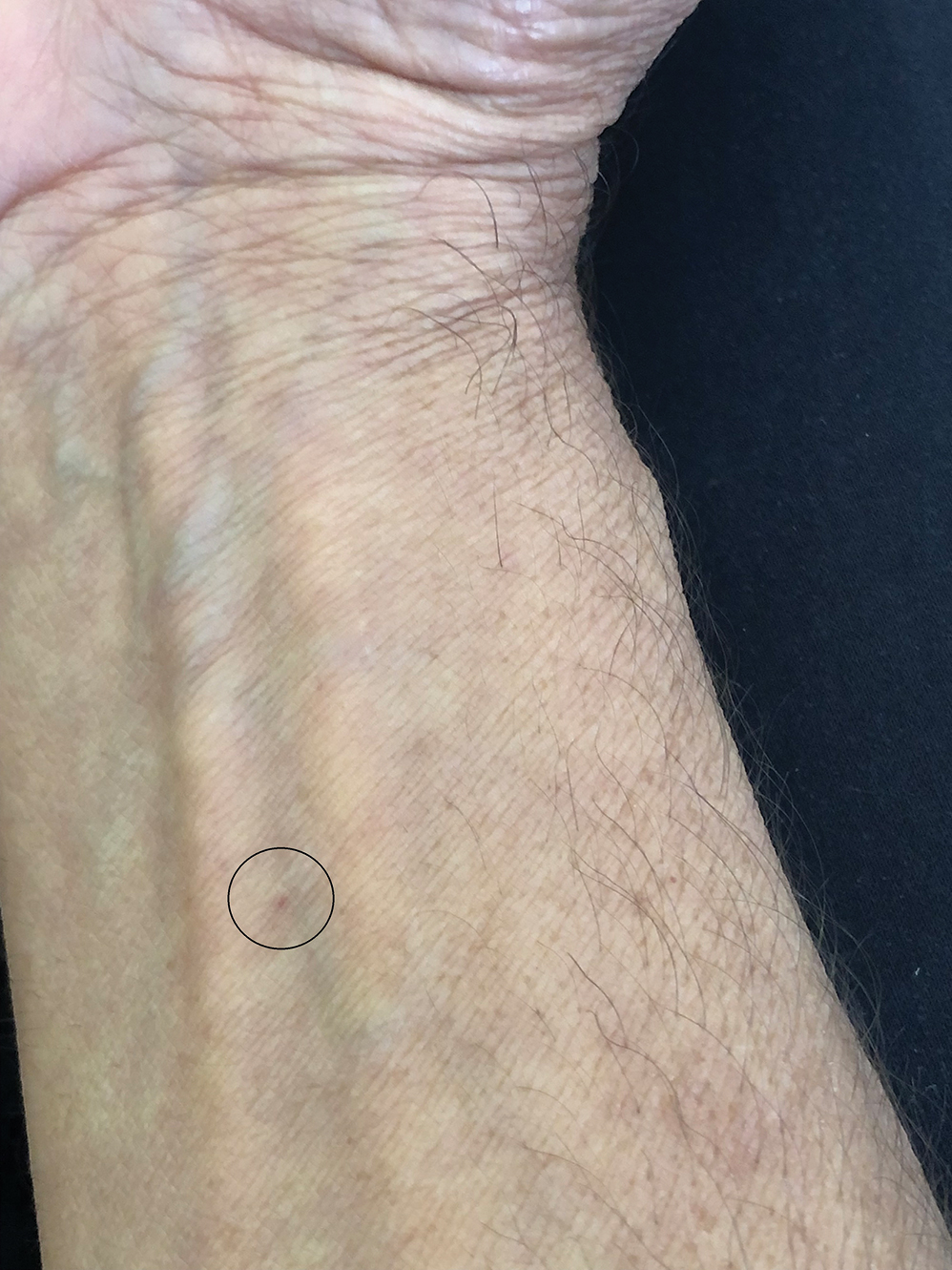
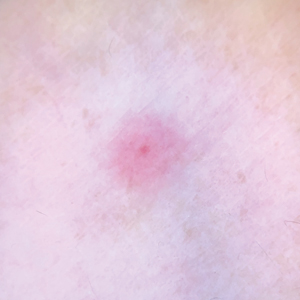
Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00



Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00



Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
Dermoscopic Documentation of a No-see-um Bite
Dermoscopic Documentation of a No-see-um Bite
Practice Points
- Biting midges, commonly known as no-see-ums, are extremely small flies whose bites can cause a burning sensation, mild pain, and reactions ranging from small wheals to intensely pruritic papules.
- Medical management of no-see-um bites is based on the severity of the skin reaction.
Central Line Skin Reactions in Children: Survey Addresses Treatment Protocols in Use
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Barzolvolimab Effective for CSU in Phase 2 Study
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Group Aims to Better Define ‘Extraordinarily Heterogeneous’ Mast Cell Activation Syndrome
Depending on one’s perspective, “mast cell activation syndrome (MCAS)” is either a relatively rare, narrowly defined severe allergic condition or a vastly underrecognized underlying cause of multiple chronic inflammatory conditions that affect roughly 17% of the entire population.
Inappropriate activation of mast cells — now termed mast cell activation disease (MCAD) — has long been known to underlie allergic symptoms and inflammation, and far less commonly, neoplasias such as mastocytosis. The concept of chronic, persistent MCAS associated with aberrant growth and dystrophism is more recent, emerging only in the last couple of decades as a separate entity under the MCAD heading.
Observational studies and clinical experience have linked signs and symptoms of MCAS with other inflammatory chronic conditions such as hypermobile Ehlers-Danlos Syndrome (EDS), postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and recently, long COVID. However, those conditions themselves are diagnostically challenging, and as yet there is no proof of causation.
The idea that MCAS is the entity — or at least, a key one — at the center of “a confoundingly, extraordinarily heterogeneous chronic multisystem polymorbidity” was the theme of a recent 4-day meeting of a professional group informally dubbed “Masterminds.” Since their first meeting in 2018, the group has grown from about 35 to nearly 650 multidisciplinary professionals.
Stephanie L. Grach, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minnesota, gave an introductory talk about the importance of changing “the medical paradigm around complex chronic illness.” Much of the rest of the meeting was devoted to sharing approaches for managing MCAS comorbidities, including dysautonomia, hypermobility, and associated craniocervical dysfunction, and various other multi-system conditions characterized by chronic pain and/or fatigue. Several talks covered the use of agents that block mast cell activity as potential treatment.
In an interview, Grach said “the meeting was an exciting example of how not only research, but also medicine, is moving forward, and it’s really cool to see that people are independently coming to very similar conclusions about shared pathologies, and because of that, the importance of overlap amongst complex medical conditions that historically have really been poorly addressed.”
She added, “mast cell activation, or mast cell hyperactivity, is one part of the greater picture. What’s important about the mast cell component is that of the multiple different targetable pathologies, it’s one that currently has potential available therapies that can be explored, some of them relatively easily.”
But Christopher Chang, MD, PhD, chief of the Pediatric Allergy and Immunology program, Joe DiMaggio Children’s Hospital, Hollywood, Florida, sees it differently. In an interview, he noted that the reason for disagreement over what constitutes MCAS is that “it doesn’t have a lot of objective findings that we can identify. ... We know that mast cells are important immune cells, just like all immune cells are important. It seems like whenever someone has unexplained symptoms, people try to blame it on mast cells. But it’s very hard to prove that.”
Two Definitions Characterize the Illness Differently
One proposed “consensus” MCAS definition was first published in 2011 by a group led by hematologist Peter Valent, MD, of the Medical University of Vienna in Austria. It has been revised since, and similar versions adopted by medical societies, including the American Academy of Allergy, Asthma & Immunology (AAAAI). The most recent versions propose three core MCAS criteria:
- Typical clinical signs of severe, recurrent (episodic) systemic (at least two organ systems) MCA are present (often in the form of anaphylaxis).
- The involvement of mast cells (MCs) is documented by biochemical studies, preferably an increase in serum tryptase levels from the individual’s baseline to plus 20% + 2 ng/mL.
- Response of symptoms to therapy with MC-stabilizing agents, drugs directed against MC mediator production, or drugs blocking mediator release or effects of MC-derived mediators.
The following year, a separate publication authored by Gerhard J. Molderings, MD, University of Bonn in Germany, and colleagues proposed a much broader MCAS definition. Also revised since, the latest “consensus-2” was published in 2020. This definition consists of one major criterion: “A constellation of clinical complaints attributable to pathologically increased MC activity, ie, MC mediator release syndrome.” This “constellation” involves conditions of nearly every organ system that, taken together, are estimated to affect up to 17% of the entire population. These are just a few examples:
- Constitutional: Chronic fatigue, flushing, or sweats
- Dermatologic: Rashes or lesions
- Ophthalmologic: dry eyes
- Oral: Burning or itching in mouth
- Pulmonary: Airway inflammation at any/all levels
- Cardiovascular: Blood pressure lability or codiagnosis of POTS is common
- Gastrointestinal: Reflux, dysphagia, or malabsorption
- Genitourinary: Endometriosis, dysmenorrhea, or dyspareunia
- Musculoskeletal/connective tissue: Fibromyalgia or diagnosis of hypermobile EDS is common
- Neurologic: Headaches or sensory neuropathies
- Psychiatric: Depression or anxiety
- Endocrinologic: Thyroid disease or dyslipidemia
- Hematologic: Polycythemia or anemia (after ruling out other causes)
The diagnosis is made by fulfilling that major criterion, plus at least one objective assessment of pathologically increased release of MC mediators, including infiltrates, abnormal MC morphology, or MC genetic changes shown to increase MC activity. Other alternatives include evidence of above-normal levels of MC mediators, including tryptase, histamine or its metabolites, heparin, or chromatin A, in whole blood, serum, plasma, or urine. Symptomatic response to MC activation inhibitors can also be used but isn’t required as it is in the other definition.
Underdiagnosis vs Overdiagnosis
Lawrence B. Afrin, MD, senior consultant in hematology/oncology at the AIM Center for Personalized Medicine, Westchester, New York, and lead author of the 2020 update of the broader “consensus-2” criteria, said in an interview, “we now know MCAS exists, and it’s prevalent, even though, for understandable and forgivable reasons, we’ve been missing it all along. ... If you see a patient who has this chronic, multisystem unwellness with general themes of inflammation plus or minus allergic issues and you can’t find some other rational explanation that better accounts for what’s going on ... then it’s reasonable to think to include MCAS in the differential diagnosis. If the patient happens not to fit the diagnostic criteria being advanced by one group, that doesn’t necessarily rule out the possibility that this is still going on.”
Afrin, along with his coauthors, faulted the narrower “consensus-1” definition for lacking data to support the “20% + 2” criteria for requiring the difficult determination of a patient’s “baseline” and for requiring evidence of response to treatment prior to making the diagnosis. Not all patients will respond to a given histamine blocker, he noted.
But Lawrence B. Schwartz, MD, PhD, an author on both the Valent and AAAAI criteria, disagreed, noting that the narrower criteria “appear to have a high degree of specificity and sensitivity when the reaction is systemic and involves hypotension. Less severe clinical events, particularly involving the gastrointestinal or central nervous systems, do not have precise clinical or biomarker criteria for identifying mast cell involvement.”
Added Schwartz, who is professor of medicine and chair of the Division of Rheumatology, Allergy, and Immunology and program director of Allergy and Immunology, Virginia Commonwealth University (VCU), Richmond, “when mast cell activation events occur only in the skin, we refer to it as chronic urticaria and in the airways or conjunctiva of allergic individuals as allergic asthma, rhinitis, and/or conjunctivitis. The absence of specific criteria for mast cell activation in the GI [gastrointestinal] tract or CNS [central nervous system] neither rules in mast cell involvement nor does it rule out mast cell involvement. Thus, more research is needed to find better diagnostic criteria.”
Schwartz also pointed to a recent paper reporting the use of artificial intelligence models to “quantify diagnostic precision and specificity” of “alternative” MCAS definitions. The conclusion was a “lack of specificity is pronounced in relation to multiple control criteria, raising the concern that alternative criteria could disproportionately contribute to MCAS overdiagnosis, to the exclusion of more appropriate diagnoses.”
During the meeting, Afrin acknowledged that the broader view risks overdiagnosis of MCAS. However, he also referenced Occam’s razor, the principle that the simplest explanation is probably the best one. “Which scenario is more likely? Multiple diagnoses and problems that are all independent of each other vs one diagnosis that’s biologically capable of causing most or all of the findings, ie, the simplest solution even if it’s not the most immediately obvious solution?”
He said in an interview: “Do we have any proof that MCAS is what’s underlying hypermobile Ehlers-Danlos or POTS or chronic fatigue? No, we don’t have any proof, not because anybody has done studies that have shown there to be no connection but simply because we’re so early in our awareness that the disease even exists that the necessary studies haven’t even been done yet.”
At the meeting, Afrin introduced proposals to turn the “Masterminds” group into a formal professional society and to launch a journal. He also gave an update on progress in developing a symptom assessment tool both for clinical use and to enable clinical trials of new drugs to target mast cells or their mediators. The plan is to field test the tool in 2025 and publish those results in 2026.
Grach, Afrin, and Chang had no disclosures. Schwartz discovered tryptase and invented the Thermo Fisher tryptase assay, for which his institution (VCU) receives royalties that are shared with him. He also invented monoclonal antibodies used for detecting mast cells or basophils, for which VCU receives royalties from several companies, including Millipore, Santa Cruz, BioLegend, and Hycult Biotech, that are also shared with him. He is a paid consultant for Blueprint Medicines, Celldex Therapeutics, Invea, Third Harmonic Bio, HYCOR Biomedical, Jasper, TerSera Therapeutics, and GLG. He also serves on an AstraZeneca data safety monitoring board for a clinical trial involving benralizumab treatment of hypereosinophilic syndrome and receives royalties from UpToDate (biomarkers for anaphylaxis) and Goldman-Cecil Medicine (anaphylaxis).
A version of this article first appeared on Medscape.com.
Depending on one’s perspective, “mast cell activation syndrome (MCAS)” is either a relatively rare, narrowly defined severe allergic condition or a vastly underrecognized underlying cause of multiple chronic inflammatory conditions that affect roughly 17% of the entire population.
Inappropriate activation of mast cells — now termed mast cell activation disease (MCAD) — has long been known to underlie allergic symptoms and inflammation, and far less commonly, neoplasias such as mastocytosis. The concept of chronic, persistent MCAS associated with aberrant growth and dystrophism is more recent, emerging only in the last couple of decades as a separate entity under the MCAD heading.
Observational studies and clinical experience have linked signs and symptoms of MCAS with other inflammatory chronic conditions such as hypermobile Ehlers-Danlos Syndrome (EDS), postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and recently, long COVID. However, those conditions themselves are diagnostically challenging, and as yet there is no proof of causation.
The idea that MCAS is the entity — or at least, a key one — at the center of “a confoundingly, extraordinarily heterogeneous chronic multisystem polymorbidity” was the theme of a recent 4-day meeting of a professional group informally dubbed “Masterminds.” Since their first meeting in 2018, the group has grown from about 35 to nearly 650 multidisciplinary professionals.
Stephanie L. Grach, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minnesota, gave an introductory talk about the importance of changing “the medical paradigm around complex chronic illness.” Much of the rest of the meeting was devoted to sharing approaches for managing MCAS comorbidities, including dysautonomia, hypermobility, and associated craniocervical dysfunction, and various other multi-system conditions characterized by chronic pain and/or fatigue. Several talks covered the use of agents that block mast cell activity as potential treatment.
In an interview, Grach said “the meeting was an exciting example of how not only research, but also medicine, is moving forward, and it’s really cool to see that people are independently coming to very similar conclusions about shared pathologies, and because of that, the importance of overlap amongst complex medical conditions that historically have really been poorly addressed.”
She added, “mast cell activation, or mast cell hyperactivity, is one part of the greater picture. What’s important about the mast cell component is that of the multiple different targetable pathologies, it’s one that currently has potential available therapies that can be explored, some of them relatively easily.”
But Christopher Chang, MD, PhD, chief of the Pediatric Allergy and Immunology program, Joe DiMaggio Children’s Hospital, Hollywood, Florida, sees it differently. In an interview, he noted that the reason for disagreement over what constitutes MCAS is that “it doesn’t have a lot of objective findings that we can identify. ... We know that mast cells are important immune cells, just like all immune cells are important. It seems like whenever someone has unexplained symptoms, people try to blame it on mast cells. But it’s very hard to prove that.”
Two Definitions Characterize the Illness Differently
One proposed “consensus” MCAS definition was first published in 2011 by a group led by hematologist Peter Valent, MD, of the Medical University of Vienna in Austria. It has been revised since, and similar versions adopted by medical societies, including the American Academy of Allergy, Asthma & Immunology (AAAAI). The most recent versions propose three core MCAS criteria:
- Typical clinical signs of severe, recurrent (episodic) systemic (at least two organ systems) MCA are present (often in the form of anaphylaxis).
- The involvement of mast cells (MCs) is documented by biochemical studies, preferably an increase in serum tryptase levels from the individual’s baseline to plus 20% + 2 ng/mL.
- Response of symptoms to therapy with MC-stabilizing agents, drugs directed against MC mediator production, or drugs blocking mediator release or effects of MC-derived mediators.
The following year, a separate publication authored by Gerhard J. Molderings, MD, University of Bonn in Germany, and colleagues proposed a much broader MCAS definition. Also revised since, the latest “consensus-2” was published in 2020. This definition consists of one major criterion: “A constellation of clinical complaints attributable to pathologically increased MC activity, ie, MC mediator release syndrome.” This “constellation” involves conditions of nearly every organ system that, taken together, are estimated to affect up to 17% of the entire population. These are just a few examples:
- Constitutional: Chronic fatigue, flushing, or sweats
- Dermatologic: Rashes or lesions
- Ophthalmologic: dry eyes
- Oral: Burning or itching in mouth
- Pulmonary: Airway inflammation at any/all levels
- Cardiovascular: Blood pressure lability or codiagnosis of POTS is common
- Gastrointestinal: Reflux, dysphagia, or malabsorption
- Genitourinary: Endometriosis, dysmenorrhea, or dyspareunia
- Musculoskeletal/connective tissue: Fibromyalgia or diagnosis of hypermobile EDS is common
- Neurologic: Headaches or sensory neuropathies
- Psychiatric: Depression or anxiety
- Endocrinologic: Thyroid disease or dyslipidemia
- Hematologic: Polycythemia or anemia (after ruling out other causes)
The diagnosis is made by fulfilling that major criterion, plus at least one objective assessment of pathologically increased release of MC mediators, including infiltrates, abnormal MC morphology, or MC genetic changes shown to increase MC activity. Other alternatives include evidence of above-normal levels of MC mediators, including tryptase, histamine or its metabolites, heparin, or chromatin A, in whole blood, serum, plasma, or urine. Symptomatic response to MC activation inhibitors can also be used but isn’t required as it is in the other definition.
Underdiagnosis vs Overdiagnosis
Lawrence B. Afrin, MD, senior consultant in hematology/oncology at the AIM Center for Personalized Medicine, Westchester, New York, and lead author of the 2020 update of the broader “consensus-2” criteria, said in an interview, “we now know MCAS exists, and it’s prevalent, even though, for understandable and forgivable reasons, we’ve been missing it all along. ... If you see a patient who has this chronic, multisystem unwellness with general themes of inflammation plus or minus allergic issues and you can’t find some other rational explanation that better accounts for what’s going on ... then it’s reasonable to think to include MCAS in the differential diagnosis. If the patient happens not to fit the diagnostic criteria being advanced by one group, that doesn’t necessarily rule out the possibility that this is still going on.”
Afrin, along with his coauthors, faulted the narrower “consensus-1” definition for lacking data to support the “20% + 2” criteria for requiring the difficult determination of a patient’s “baseline” and for requiring evidence of response to treatment prior to making the diagnosis. Not all patients will respond to a given histamine blocker, he noted.
But Lawrence B. Schwartz, MD, PhD, an author on both the Valent and AAAAI criteria, disagreed, noting that the narrower criteria “appear to have a high degree of specificity and sensitivity when the reaction is systemic and involves hypotension. Less severe clinical events, particularly involving the gastrointestinal or central nervous systems, do not have precise clinical or biomarker criteria for identifying mast cell involvement.”
Added Schwartz, who is professor of medicine and chair of the Division of Rheumatology, Allergy, and Immunology and program director of Allergy and Immunology, Virginia Commonwealth University (VCU), Richmond, “when mast cell activation events occur only in the skin, we refer to it as chronic urticaria and in the airways or conjunctiva of allergic individuals as allergic asthma, rhinitis, and/or conjunctivitis. The absence of specific criteria for mast cell activation in the GI [gastrointestinal] tract or CNS [central nervous system] neither rules in mast cell involvement nor does it rule out mast cell involvement. Thus, more research is needed to find better diagnostic criteria.”
Schwartz also pointed to a recent paper reporting the use of artificial intelligence models to “quantify diagnostic precision and specificity” of “alternative” MCAS definitions. The conclusion was a “lack of specificity is pronounced in relation to multiple control criteria, raising the concern that alternative criteria could disproportionately contribute to MCAS overdiagnosis, to the exclusion of more appropriate diagnoses.”
During the meeting, Afrin acknowledged that the broader view risks overdiagnosis of MCAS. However, he also referenced Occam’s razor, the principle that the simplest explanation is probably the best one. “Which scenario is more likely? Multiple diagnoses and problems that are all independent of each other vs one diagnosis that’s biologically capable of causing most or all of the findings, ie, the simplest solution even if it’s not the most immediately obvious solution?”
He said in an interview: “Do we have any proof that MCAS is what’s underlying hypermobile Ehlers-Danlos or POTS or chronic fatigue? No, we don’t have any proof, not because anybody has done studies that have shown there to be no connection but simply because we’re so early in our awareness that the disease even exists that the necessary studies haven’t even been done yet.”
At the meeting, Afrin introduced proposals to turn the “Masterminds” group into a formal professional society and to launch a journal. He also gave an update on progress in developing a symptom assessment tool both for clinical use and to enable clinical trials of new drugs to target mast cells or their mediators. The plan is to field test the tool in 2025 and publish those results in 2026.
Grach, Afrin, and Chang had no disclosures. Schwartz discovered tryptase and invented the Thermo Fisher tryptase assay, for which his institution (VCU) receives royalties that are shared with him. He also invented monoclonal antibodies used for detecting mast cells or basophils, for which VCU receives royalties from several companies, including Millipore, Santa Cruz, BioLegend, and Hycult Biotech, that are also shared with him. He is a paid consultant for Blueprint Medicines, Celldex Therapeutics, Invea, Third Harmonic Bio, HYCOR Biomedical, Jasper, TerSera Therapeutics, and GLG. He also serves on an AstraZeneca data safety monitoring board for a clinical trial involving benralizumab treatment of hypereosinophilic syndrome and receives royalties from UpToDate (biomarkers for anaphylaxis) and Goldman-Cecil Medicine (anaphylaxis).
A version of this article first appeared on Medscape.com.
Depending on one’s perspective, “mast cell activation syndrome (MCAS)” is either a relatively rare, narrowly defined severe allergic condition or a vastly underrecognized underlying cause of multiple chronic inflammatory conditions that affect roughly 17% of the entire population.
Inappropriate activation of mast cells — now termed mast cell activation disease (MCAD) — has long been known to underlie allergic symptoms and inflammation, and far less commonly, neoplasias such as mastocytosis. The concept of chronic, persistent MCAS associated with aberrant growth and dystrophism is more recent, emerging only in the last couple of decades as a separate entity under the MCAD heading.
Observational studies and clinical experience have linked signs and symptoms of MCAS with other inflammatory chronic conditions such as hypermobile Ehlers-Danlos Syndrome (EDS), postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and recently, long COVID. However, those conditions themselves are diagnostically challenging, and as yet there is no proof of causation.
The idea that MCAS is the entity — or at least, a key one — at the center of “a confoundingly, extraordinarily heterogeneous chronic multisystem polymorbidity” was the theme of a recent 4-day meeting of a professional group informally dubbed “Masterminds.” Since their first meeting in 2018, the group has grown from about 35 to nearly 650 multidisciplinary professionals.
Stephanie L. Grach, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minnesota, gave an introductory talk about the importance of changing “the medical paradigm around complex chronic illness.” Much of the rest of the meeting was devoted to sharing approaches for managing MCAS comorbidities, including dysautonomia, hypermobility, and associated craniocervical dysfunction, and various other multi-system conditions characterized by chronic pain and/or fatigue. Several talks covered the use of agents that block mast cell activity as potential treatment.
In an interview, Grach said “the meeting was an exciting example of how not only research, but also medicine, is moving forward, and it’s really cool to see that people are independently coming to very similar conclusions about shared pathologies, and because of that, the importance of overlap amongst complex medical conditions that historically have really been poorly addressed.”
She added, “mast cell activation, or mast cell hyperactivity, is one part of the greater picture. What’s important about the mast cell component is that of the multiple different targetable pathologies, it’s one that currently has potential available therapies that can be explored, some of them relatively easily.”
But Christopher Chang, MD, PhD, chief of the Pediatric Allergy and Immunology program, Joe DiMaggio Children’s Hospital, Hollywood, Florida, sees it differently. In an interview, he noted that the reason for disagreement over what constitutes MCAS is that “it doesn’t have a lot of objective findings that we can identify. ... We know that mast cells are important immune cells, just like all immune cells are important. It seems like whenever someone has unexplained symptoms, people try to blame it on mast cells. But it’s very hard to prove that.”
Two Definitions Characterize the Illness Differently
One proposed “consensus” MCAS definition was first published in 2011 by a group led by hematologist Peter Valent, MD, of the Medical University of Vienna in Austria. It has been revised since, and similar versions adopted by medical societies, including the American Academy of Allergy, Asthma & Immunology (AAAAI). The most recent versions propose three core MCAS criteria:
- Typical clinical signs of severe, recurrent (episodic) systemic (at least two organ systems) MCA are present (often in the form of anaphylaxis).
- The involvement of mast cells (MCs) is documented by biochemical studies, preferably an increase in serum tryptase levels from the individual’s baseline to plus 20% + 2 ng/mL.
- Response of symptoms to therapy with MC-stabilizing agents, drugs directed against MC mediator production, or drugs blocking mediator release or effects of MC-derived mediators.
The following year, a separate publication authored by Gerhard J. Molderings, MD, University of Bonn in Germany, and colleagues proposed a much broader MCAS definition. Also revised since, the latest “consensus-2” was published in 2020. This definition consists of one major criterion: “A constellation of clinical complaints attributable to pathologically increased MC activity, ie, MC mediator release syndrome.” This “constellation” involves conditions of nearly every organ system that, taken together, are estimated to affect up to 17% of the entire population. These are just a few examples:
- Constitutional: Chronic fatigue, flushing, or sweats
- Dermatologic: Rashes or lesions
- Ophthalmologic: dry eyes
- Oral: Burning or itching in mouth
- Pulmonary: Airway inflammation at any/all levels
- Cardiovascular: Blood pressure lability or codiagnosis of POTS is common
- Gastrointestinal: Reflux, dysphagia, or malabsorption
- Genitourinary: Endometriosis, dysmenorrhea, or dyspareunia
- Musculoskeletal/connective tissue: Fibromyalgia or diagnosis of hypermobile EDS is common
- Neurologic: Headaches or sensory neuropathies
- Psychiatric: Depression or anxiety
- Endocrinologic: Thyroid disease or dyslipidemia
- Hematologic: Polycythemia or anemia (after ruling out other causes)
The diagnosis is made by fulfilling that major criterion, plus at least one objective assessment of pathologically increased release of MC mediators, including infiltrates, abnormal MC morphology, or MC genetic changes shown to increase MC activity. Other alternatives include evidence of above-normal levels of MC mediators, including tryptase, histamine or its metabolites, heparin, or chromatin A, in whole blood, serum, plasma, or urine. Symptomatic response to MC activation inhibitors can also be used but isn’t required as it is in the other definition.
Underdiagnosis vs Overdiagnosis
Lawrence B. Afrin, MD, senior consultant in hematology/oncology at the AIM Center for Personalized Medicine, Westchester, New York, and lead author of the 2020 update of the broader “consensus-2” criteria, said in an interview, “we now know MCAS exists, and it’s prevalent, even though, for understandable and forgivable reasons, we’ve been missing it all along. ... If you see a patient who has this chronic, multisystem unwellness with general themes of inflammation plus or minus allergic issues and you can’t find some other rational explanation that better accounts for what’s going on ... then it’s reasonable to think to include MCAS in the differential diagnosis. If the patient happens not to fit the diagnostic criteria being advanced by one group, that doesn’t necessarily rule out the possibility that this is still going on.”
Afrin, along with his coauthors, faulted the narrower “consensus-1” definition for lacking data to support the “20% + 2” criteria for requiring the difficult determination of a patient’s “baseline” and for requiring evidence of response to treatment prior to making the diagnosis. Not all patients will respond to a given histamine blocker, he noted.
But Lawrence B. Schwartz, MD, PhD, an author on both the Valent and AAAAI criteria, disagreed, noting that the narrower criteria “appear to have a high degree of specificity and sensitivity when the reaction is systemic and involves hypotension. Less severe clinical events, particularly involving the gastrointestinal or central nervous systems, do not have precise clinical or biomarker criteria for identifying mast cell involvement.”
Added Schwartz, who is professor of medicine and chair of the Division of Rheumatology, Allergy, and Immunology and program director of Allergy and Immunology, Virginia Commonwealth University (VCU), Richmond, “when mast cell activation events occur only in the skin, we refer to it as chronic urticaria and in the airways or conjunctiva of allergic individuals as allergic asthma, rhinitis, and/or conjunctivitis. The absence of specific criteria for mast cell activation in the GI [gastrointestinal] tract or CNS [central nervous system] neither rules in mast cell involvement nor does it rule out mast cell involvement. Thus, more research is needed to find better diagnostic criteria.”
Schwartz also pointed to a recent paper reporting the use of artificial intelligence models to “quantify diagnostic precision and specificity” of “alternative” MCAS definitions. The conclusion was a “lack of specificity is pronounced in relation to multiple control criteria, raising the concern that alternative criteria could disproportionately contribute to MCAS overdiagnosis, to the exclusion of more appropriate diagnoses.”
During the meeting, Afrin acknowledged that the broader view risks overdiagnosis of MCAS. However, he also referenced Occam’s razor, the principle that the simplest explanation is probably the best one. “Which scenario is more likely? Multiple diagnoses and problems that are all independent of each other vs one diagnosis that’s biologically capable of causing most or all of the findings, ie, the simplest solution even if it’s not the most immediately obvious solution?”
He said in an interview: “Do we have any proof that MCAS is what’s underlying hypermobile Ehlers-Danlos or POTS or chronic fatigue? No, we don’t have any proof, not because anybody has done studies that have shown there to be no connection but simply because we’re so early in our awareness that the disease even exists that the necessary studies haven’t even been done yet.”
At the meeting, Afrin introduced proposals to turn the “Masterminds” group into a formal professional society and to launch a journal. He also gave an update on progress in developing a symptom assessment tool both for clinical use and to enable clinical trials of new drugs to target mast cells or their mediators. The plan is to field test the tool in 2025 and publish those results in 2026.
Grach, Afrin, and Chang had no disclosures. Schwartz discovered tryptase and invented the Thermo Fisher tryptase assay, for which his institution (VCU) receives royalties that are shared with him. He also invented monoclonal antibodies used for detecting mast cells or basophils, for which VCU receives royalties from several companies, including Millipore, Santa Cruz, BioLegend, and Hycult Biotech, that are also shared with him. He is a paid consultant for Blueprint Medicines, Celldex Therapeutics, Invea, Third Harmonic Bio, HYCOR Biomedical, Jasper, TerSera Therapeutics, and GLG. He also serves on an AstraZeneca data safety monitoring board for a clinical trial involving benralizumab treatment of hypereosinophilic syndrome and receives royalties from UpToDate (biomarkers for anaphylaxis) and Goldman-Cecil Medicine (anaphylaxis).
A version of this article first appeared on Medscape.com.
Omalizumab for Food Allergies: What PCPs Should Know
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Dupilumab gains off-label uses as clinicians turn to drug for more indications
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
CSU in children: Study identifies biomarkers associated with responses to different treatments
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
AT AAD 2023
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Notalgia paresthetica: Difelikefalin helps upper-back itch, but with side effects
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE