User login
Latest COVID-19 Shot May Cut Severe Outcomes in Veterans
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID Linked to Eye Issues, But Vaccine Offers Protection
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
RSV Vaccine Effective in Older Veterans, But Protection Declines Over Time
RSV Vaccine Effective in Older Veterans, But Protection Declines Over Time
TOPLINE:
A single dose of respiratory syncytial virus (RSV) vaccine provided protection against RSV illness and associated health care use in nearly 290,000 older US veterans over 2 respiratory illness seasons compared with unvaccinated individuals; however, protection declined over time, particularly among immunocompromised individuals.
METHODOLOGY:
- Researchers emulated a target trial to assess the long-term effectiveness of a single does of RSV vaccine, administered between September 2023 and March 2024, to prevent RSV infection and associated health care use among older US veterans.
- The primary outcome was any positive RSV test from 14 days after vaccination; secondary outcomes included RSV-associated emergency department or urgent care visits, hospitalizations, and ICU admissions.
- The median follow-up duration, measured from 14 days after vaccination, was 15.8 months, with a maximum of 19.0 months.
TAKEAWAY:
- The estimated vaccines effectiveness against RSV infection decreased from 82.5% (95% CI, 77.5%-86.9%) over 0 to 1 month to 59.4% (95% CI, 55.6%-63.5%) over 0 to 18 months of follow-up.
- Protection against RSV-associated emergency department and urgent care visits fell from 84.9% over 0 to 1 month to 60.6% over 0 to 18 months, and the estimated effectiveness against hospitalizations decreased from 88.9% to 57.3% over the same interval.
- The estimated effectiveness against RSV-associated ICU admissions reduced from 92.5% (95% CI, 61.1%-100%) over 0 to 1 month to 71.9% (95% CI, 42.8%-90.0%) over 0 to 18 months.
- Among immunocompromised individuals, protection against RSV infection showed the largest decline from 75.2% at 0 to 1 month to 39.7% over 18 months.
IN PRACTICE:
"Boosters may be needed, but for now, our efforts should be focused on saving lives and decreasing disease by encouraging vaccination of persons 75 years and older and those 60 years and older with underlying health issues," experts wrote in an accompanying editorial.
SOURCE:
The study was led by Kristina L. Bajema, MD, Veterans Affairs Portland Health Care System, Portland, Oregon. It was published online on November 24, 2025, in JAMA Internal Medicine.
LIMITATIONS:
RSV documentation may have been incomplete for veterans who sought care outside the Veterans Health Administration. The cohort was predominantly White men, limiting generalizability. Residual confounding could not be excluded. Estimates of long-term effectiveness should be interpreted cautiously because they reflect patients who remained in care and may differ from the original matched cohort.
DISCLOSURES:
The study was supported by the US Department of Veterans Affairs Cooperative Studies Program, US Department of Health and Human Services, Biomedical Advanced Research and Development Authority, and FDA. Two authors reported receiving grants from the study funder and/or the Patient-Centered Outcomes Research Institute; one of these authors also reported co-ownership of van Breemen & Hynes, LLC, unrelated to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A single dose of respiratory syncytial virus (RSV) vaccine provided protection against RSV illness and associated health care use in nearly 290,000 older US veterans over 2 respiratory illness seasons compared with unvaccinated individuals; however, protection declined over time, particularly among immunocompromised individuals.
METHODOLOGY:
- Researchers emulated a target trial to assess the long-term effectiveness of a single does of RSV vaccine, administered between September 2023 and March 2024, to prevent RSV infection and associated health care use among older US veterans.
- The primary outcome was any positive RSV test from 14 days after vaccination; secondary outcomes included RSV-associated emergency department or urgent care visits, hospitalizations, and ICU admissions.
- The median follow-up duration, measured from 14 days after vaccination, was 15.8 months, with a maximum of 19.0 months.
TAKEAWAY:
- The estimated vaccines effectiveness against RSV infection decreased from 82.5% (95% CI, 77.5%-86.9%) over 0 to 1 month to 59.4% (95% CI, 55.6%-63.5%) over 0 to 18 months of follow-up.
- Protection against RSV-associated emergency department and urgent care visits fell from 84.9% over 0 to 1 month to 60.6% over 0 to 18 months, and the estimated effectiveness against hospitalizations decreased from 88.9% to 57.3% over the same interval.
- The estimated effectiveness against RSV-associated ICU admissions reduced from 92.5% (95% CI, 61.1%-100%) over 0 to 1 month to 71.9% (95% CI, 42.8%-90.0%) over 0 to 18 months.
- Among immunocompromised individuals, protection against RSV infection showed the largest decline from 75.2% at 0 to 1 month to 39.7% over 18 months.
IN PRACTICE:
"Boosters may be needed, but for now, our efforts should be focused on saving lives and decreasing disease by encouraging vaccination of persons 75 years and older and those 60 years and older with underlying health issues," experts wrote in an accompanying editorial.
SOURCE:
The study was led by Kristina L. Bajema, MD, Veterans Affairs Portland Health Care System, Portland, Oregon. It was published online on November 24, 2025, in JAMA Internal Medicine.
LIMITATIONS:
RSV documentation may have been incomplete for veterans who sought care outside the Veterans Health Administration. The cohort was predominantly White men, limiting generalizability. Residual confounding could not be excluded. Estimates of long-term effectiveness should be interpreted cautiously because they reflect patients who remained in care and may differ from the original matched cohort.
DISCLOSURES:
The study was supported by the US Department of Veterans Affairs Cooperative Studies Program, US Department of Health and Human Services, Biomedical Advanced Research and Development Authority, and FDA. Two authors reported receiving grants from the study funder and/or the Patient-Centered Outcomes Research Institute; one of these authors also reported co-ownership of van Breemen & Hynes, LLC, unrelated to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A single dose of respiratory syncytial virus (RSV) vaccine provided protection against RSV illness and associated health care use in nearly 290,000 older US veterans over 2 respiratory illness seasons compared with unvaccinated individuals; however, protection declined over time, particularly among immunocompromised individuals.
METHODOLOGY:
- Researchers emulated a target trial to assess the long-term effectiveness of a single does of RSV vaccine, administered between September 2023 and March 2024, to prevent RSV infection and associated health care use among older US veterans.
- The primary outcome was any positive RSV test from 14 days after vaccination; secondary outcomes included RSV-associated emergency department or urgent care visits, hospitalizations, and ICU admissions.
- The median follow-up duration, measured from 14 days after vaccination, was 15.8 months, with a maximum of 19.0 months.
TAKEAWAY:
- The estimated vaccines effectiveness against RSV infection decreased from 82.5% (95% CI, 77.5%-86.9%) over 0 to 1 month to 59.4% (95% CI, 55.6%-63.5%) over 0 to 18 months of follow-up.
- Protection against RSV-associated emergency department and urgent care visits fell from 84.9% over 0 to 1 month to 60.6% over 0 to 18 months, and the estimated effectiveness against hospitalizations decreased from 88.9% to 57.3% over the same interval.
- The estimated effectiveness against RSV-associated ICU admissions reduced from 92.5% (95% CI, 61.1%-100%) over 0 to 1 month to 71.9% (95% CI, 42.8%-90.0%) over 0 to 18 months.
- Among immunocompromised individuals, protection against RSV infection showed the largest decline from 75.2% at 0 to 1 month to 39.7% over 18 months.
IN PRACTICE:
"Boosters may be needed, but for now, our efforts should be focused on saving lives and decreasing disease by encouraging vaccination of persons 75 years and older and those 60 years and older with underlying health issues," experts wrote in an accompanying editorial.
SOURCE:
The study was led by Kristina L. Bajema, MD, Veterans Affairs Portland Health Care System, Portland, Oregon. It was published online on November 24, 2025, in JAMA Internal Medicine.
LIMITATIONS:
RSV documentation may have been incomplete for veterans who sought care outside the Veterans Health Administration. The cohort was predominantly White men, limiting generalizability. Residual confounding could not be excluded. Estimates of long-term effectiveness should be interpreted cautiously because they reflect patients who remained in care and may differ from the original matched cohort.
DISCLOSURES:
The study was supported by the US Department of Veterans Affairs Cooperative Studies Program, US Department of Health and Human Services, Biomedical Advanced Research and Development Authority, and FDA. Two authors reported receiving grants from the study funder and/or the Patient-Centered Outcomes Research Institute; one of these authors also reported co-ownership of van Breemen & Hynes, LLC, unrelated to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
RSV Vaccine Effective in Older Veterans, But Protection Declines Over Time
RSV Vaccine Effective in Older Veterans, But Protection Declines Over Time
COVID-19 Vaccines: Navigating the Chaos of Conflicting Guidance
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
mRNA Cancer Vaccines: Pipeline Insights for Clinicians
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
US Health Official Calls for Separating Measles Combination Shots, Pulls Broad COVID Vaccine Support
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
HPV Vaccine Reduces Immune Disease Risk in Women
TOPLINE: Human Papillomavirus (HPV) vaccination is associated with reduced risks of rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes among females aged 9 to 45 years. The analysis of 208,638 vaccinated individuals shows particularly strong protective effects in those aged 9 to 26 years and recipients of 9-valent HPV vaccines.
METHODOLOGY:
Researchers analyzed data from the US Collaborative Network in TriNetX spanning January 1, 2018, to December 20, 2022, enrolling 208,638 females aged 9 to 45 years who received HPV vaccination and matching them with 208,638 unvaccinated individuals using propensity scores.
Analysis included Cox proportional hazard regression to estimate hazard ratios and 95% CIs for immune-mediated diseases, with subgroup analyses stratified by age, race, smoking, obesity, asthma, and HPV vaccine types.
Participants were monitored from 31 days up to 365 days following their respective index dates, with sensitivity analyses conducted to evaluate short-term outcomes and compare results with influenza virus vaccine recipients.
TAKEAWAY:
HPV vaccination demonstrated reduced risks for rheumatoid arthritis (hazard ratio [HR], 0.487; 95% confidence interval [CI], 0.311-0.762), systemic lupus erythematosus (HR, 0.287; 95% CI, 0.179-0.460), and dermatomyositis (HR, 0.299; 95% CI, 0.098-0.908).
Recipients showed lower risks for inflammatory bowel disease (HR, 0.876; 95% CI, 0.811-0.946), celiac disease (HR, 0.400; 95% CI, 0.304-0.526), and type 1 diabetes (HR, 0.242; 95% CI, 0.184-0.318).
Subgroup analyses revealed significant risk reductions among females aged 9 to 26 years and those receiving 9-valent HPV vaccines compared to unvaccinated populations.
White and Black/African American individuals demonstrated reduced risks for various immune-mediated diseases, while Asians showed lower risks only for inflammatory bowel disease and overall immune-mediated diseases.
SOURCE: The study was led by Qianru Zhang, MD, Beijing Tsinghua Changgung Hospital in Beijing, China, James Cheng-Chung Wei, and Shiow-Ing Wang who contributed equally as first authors. It was published online in QJM: An International Journal of Medicine.
LIMITATIONS: According to the authors, research relying on Electronic Health Records (EHR) faced several constraints, including the absence of serial data on HPV antibody titers in vaccinated individuals and limited data regarding vaccination dosing numbers. Additionally, the current functionality of TriNetX prevented performing interaction terms in the statistical model for comprehensive subgroup analysis stratified by age, race, and vaccine types.
DISCLOSURES: The study received support from Chung Shan Medical University Hospital (Grant No. CSH-2023-E-001-Y2), Kaohsiung Veterans General Hospital (KSVGH 113-117), National Science and Technology Council (NSTC 112-2314-B-075B-020), and KSVNSU112-008. The funders had no role in the study's design, conduct, data analysis, or manuscript approval.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Human Papillomavirus (HPV) vaccination is associated with reduced risks of rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes among females aged 9 to 45 years. The analysis of 208,638 vaccinated individuals shows particularly strong protective effects in those aged 9 to 26 years and recipients of 9-valent HPV vaccines.
METHODOLOGY:
Researchers analyzed data from the US Collaborative Network in TriNetX spanning January 1, 2018, to December 20, 2022, enrolling 208,638 females aged 9 to 45 years who received HPV vaccination and matching them with 208,638 unvaccinated individuals using propensity scores.
Analysis included Cox proportional hazard regression to estimate hazard ratios and 95% CIs for immune-mediated diseases, with subgroup analyses stratified by age, race, smoking, obesity, asthma, and HPV vaccine types.
Participants were monitored from 31 days up to 365 days following their respective index dates, with sensitivity analyses conducted to evaluate short-term outcomes and compare results with influenza virus vaccine recipients.
TAKEAWAY:
HPV vaccination demonstrated reduced risks for rheumatoid arthritis (hazard ratio [HR], 0.487; 95% confidence interval [CI], 0.311-0.762), systemic lupus erythematosus (HR, 0.287; 95% CI, 0.179-0.460), and dermatomyositis (HR, 0.299; 95% CI, 0.098-0.908).
Recipients showed lower risks for inflammatory bowel disease (HR, 0.876; 95% CI, 0.811-0.946), celiac disease (HR, 0.400; 95% CI, 0.304-0.526), and type 1 diabetes (HR, 0.242; 95% CI, 0.184-0.318).
Subgroup analyses revealed significant risk reductions among females aged 9 to 26 years and those receiving 9-valent HPV vaccines compared to unvaccinated populations.
White and Black/African American individuals demonstrated reduced risks for various immune-mediated diseases, while Asians showed lower risks only for inflammatory bowel disease and overall immune-mediated diseases.
SOURCE: The study was led by Qianru Zhang, MD, Beijing Tsinghua Changgung Hospital in Beijing, China, James Cheng-Chung Wei, and Shiow-Ing Wang who contributed equally as first authors. It was published online in QJM: An International Journal of Medicine.
LIMITATIONS: According to the authors, research relying on Electronic Health Records (EHR) faced several constraints, including the absence of serial data on HPV antibody titers in vaccinated individuals and limited data regarding vaccination dosing numbers. Additionally, the current functionality of TriNetX prevented performing interaction terms in the statistical model for comprehensive subgroup analysis stratified by age, race, and vaccine types.
DISCLOSURES: The study received support from Chung Shan Medical University Hospital (Grant No. CSH-2023-E-001-Y2), Kaohsiung Veterans General Hospital (KSVGH 113-117), National Science and Technology Council (NSTC 112-2314-B-075B-020), and KSVNSU112-008. The funders had no role in the study's design, conduct, data analysis, or manuscript approval.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Human Papillomavirus (HPV) vaccination is associated with reduced risks of rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes among females aged 9 to 45 years. The analysis of 208,638 vaccinated individuals shows particularly strong protective effects in those aged 9 to 26 years and recipients of 9-valent HPV vaccines.
METHODOLOGY:
Researchers analyzed data from the US Collaborative Network in TriNetX spanning January 1, 2018, to December 20, 2022, enrolling 208,638 females aged 9 to 45 years who received HPV vaccination and matching them with 208,638 unvaccinated individuals using propensity scores.
Analysis included Cox proportional hazard regression to estimate hazard ratios and 95% CIs for immune-mediated diseases, with subgroup analyses stratified by age, race, smoking, obesity, asthma, and HPV vaccine types.
Participants were monitored from 31 days up to 365 days following their respective index dates, with sensitivity analyses conducted to evaluate short-term outcomes and compare results with influenza virus vaccine recipients.
TAKEAWAY:
HPV vaccination demonstrated reduced risks for rheumatoid arthritis (hazard ratio [HR], 0.487; 95% confidence interval [CI], 0.311-0.762), systemic lupus erythematosus (HR, 0.287; 95% CI, 0.179-0.460), and dermatomyositis (HR, 0.299; 95% CI, 0.098-0.908).
Recipients showed lower risks for inflammatory bowel disease (HR, 0.876; 95% CI, 0.811-0.946), celiac disease (HR, 0.400; 95% CI, 0.304-0.526), and type 1 diabetes (HR, 0.242; 95% CI, 0.184-0.318).
Subgroup analyses revealed significant risk reductions among females aged 9 to 26 years and those receiving 9-valent HPV vaccines compared to unvaccinated populations.
White and Black/African American individuals demonstrated reduced risks for various immune-mediated diseases, while Asians showed lower risks only for inflammatory bowel disease and overall immune-mediated diseases.
SOURCE: The study was led by Qianru Zhang, MD, Beijing Tsinghua Changgung Hospital in Beijing, China, James Cheng-Chung Wei, and Shiow-Ing Wang who contributed equally as first authors. It was published online in QJM: An International Journal of Medicine.
LIMITATIONS: According to the authors, research relying on Electronic Health Records (EHR) faced several constraints, including the absence of serial data on HPV antibody titers in vaccinated individuals and limited data regarding vaccination dosing numbers. Additionally, the current functionality of TriNetX prevented performing interaction terms in the statistical model for comprehensive subgroup analysis stratified by age, race, and vaccine types.
DISCLOSURES: The study received support from Chung Shan Medical University Hospital (Grant No. CSH-2023-E-001-Y2), Kaohsiung Veterans General Hospital (KSVGH 113-117), National Science and Technology Council (NSTC 112-2314-B-075B-020), and KSVNSU112-008. The funders had no role in the study's design, conduct, data analysis, or manuscript approval.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
DoD Surveillance: Low to Moderate Effectiveness for Flu Vaccine
A mid-season analysis of the influenza vaccine by the US Department of Defensive (DoD) Global Respiratory Pathogen Surveillance Program (DoDGRPSP) has reported low to moderate vaccine effectiveness (VE).
The study included 295 Military Health System (MHS) beneficiaries (adults and children) who tested positive for influenza and 965 controls who tested negative. Vaccinated patients had received the 2024-2025 influenza vaccine at least 14 days prior to symptom onset. The study conducted VE analyses for influenza A (any subtype), influenza A(H1N1)pdm09, and influenza A(H3N2).
Overall, moderate effectiveness against influenza A(H1N1)pdm09 was reported in all beneficiaries and children aged 6 months to 17 years. In adults aged 18 to 64 years—and all beneficiaries—there was moderate effectiveness against influenza A(H3N2). VE estimates against influenza A (any subtype) for all beneficiaries, children, and adults were not significant; VE estimates were also not effective among children for influenza A(H3N2) and in adults for influenza A(H1N1)pdm09.
Adjusted VE estimates among all participants for influenza A (any subtypes), influenza A(H1N1)pdm09, and influenza A(H3N2) were 25%, 58%, and 42%, respectively. VE for influenza B was not calculated due to a low number of cases.
Flu vaccination rates for adults are usually in the 30% to 60% range despite the recommended target of 70%. Flu vaccination rates were rising by around 1% to 2% annually before 2020, but began dropping after the COVID-19 pandemic, especially in higher-risk groups. In adults aged ≥ 65 years, flu vaccination rates dropped from 52% in 2019-2020 to 43% in 2024-2025.
According to the Centers for Disease Control and Prevention (CDC), at the end of the 2023-2024 flu season, 9.2 million fewer doses were administered in pharmacies and doctors offices compared with the baseline before the COVID-19 pandemic. Since 2022, private manufacturers have distributed significantly fewer influenza vaccine doses.
Each March, the US Food and Drug Association (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) meets to analyze the current influenza season and forecast the next. The committee reviews and discusses data on influenza strain circulation and VE, which come from DoDGRPSP analyses. In February, US Department of Health and Human Services officials indefinitely postponed a public meeting of the CDC Advisory Committee on Immunization Practice (ACIP), at which members were also expected to discuss, among other things, VE and vaccine recommendations. The FDA canceled a March 13 VRBPAC meeting and provided no reason for the cancelation to members. That day, however, the FDA issued new recommendations for the influenza vaccine for the 2025-2026 season without the input of VRBPAC. Instead, experts from the FDA, CDC, and DoD made recommendations after reviewing surveillance data from the US and globally.
For the 2025-2026 influenza season, the FDA recommends the vaccines be trivalent and target 2 strains of influenza A and 1 strain of influenza B. The FDA anticipates there will be an “adequate and diverse supply” of approved trivalent seasonal influenza vaccines. Trivalent flu vaccines are formulated to protect against 3 influenza viruses: an A(H1N1) virus, an A(H3N2) virus, and a B/Victoria virus. All influenza vaccines for the 2025-2026 season are anticipated to be trivalent in the US.
A mid-season analysis of the influenza vaccine by the US Department of Defensive (DoD) Global Respiratory Pathogen Surveillance Program (DoDGRPSP) has reported low to moderate vaccine effectiveness (VE).
The study included 295 Military Health System (MHS) beneficiaries (adults and children) who tested positive for influenza and 965 controls who tested negative. Vaccinated patients had received the 2024-2025 influenza vaccine at least 14 days prior to symptom onset. The study conducted VE analyses for influenza A (any subtype), influenza A(H1N1)pdm09, and influenza A(H3N2).
Overall, moderate effectiveness against influenza A(H1N1)pdm09 was reported in all beneficiaries and children aged 6 months to 17 years. In adults aged 18 to 64 years—and all beneficiaries—there was moderate effectiveness against influenza A(H3N2). VE estimates against influenza A (any subtype) for all beneficiaries, children, and adults were not significant; VE estimates were also not effective among children for influenza A(H3N2) and in adults for influenza A(H1N1)pdm09.
Adjusted VE estimates among all participants for influenza A (any subtypes), influenza A(H1N1)pdm09, and influenza A(H3N2) were 25%, 58%, and 42%, respectively. VE for influenza B was not calculated due to a low number of cases.
Flu vaccination rates for adults are usually in the 30% to 60% range despite the recommended target of 70%. Flu vaccination rates were rising by around 1% to 2% annually before 2020, but began dropping after the COVID-19 pandemic, especially in higher-risk groups. In adults aged ≥ 65 years, flu vaccination rates dropped from 52% in 2019-2020 to 43% in 2024-2025.
According to the Centers for Disease Control and Prevention (CDC), at the end of the 2023-2024 flu season, 9.2 million fewer doses were administered in pharmacies and doctors offices compared with the baseline before the COVID-19 pandemic. Since 2022, private manufacturers have distributed significantly fewer influenza vaccine doses.
Each March, the US Food and Drug Association (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) meets to analyze the current influenza season and forecast the next. The committee reviews and discusses data on influenza strain circulation and VE, which come from DoDGRPSP analyses. In February, US Department of Health and Human Services officials indefinitely postponed a public meeting of the CDC Advisory Committee on Immunization Practice (ACIP), at which members were also expected to discuss, among other things, VE and vaccine recommendations. The FDA canceled a March 13 VRBPAC meeting and provided no reason for the cancelation to members. That day, however, the FDA issued new recommendations for the influenza vaccine for the 2025-2026 season without the input of VRBPAC. Instead, experts from the FDA, CDC, and DoD made recommendations after reviewing surveillance data from the US and globally.
For the 2025-2026 influenza season, the FDA recommends the vaccines be trivalent and target 2 strains of influenza A and 1 strain of influenza B. The FDA anticipates there will be an “adequate and diverse supply” of approved trivalent seasonal influenza vaccines. Trivalent flu vaccines are formulated to protect against 3 influenza viruses: an A(H1N1) virus, an A(H3N2) virus, and a B/Victoria virus. All influenza vaccines for the 2025-2026 season are anticipated to be trivalent in the US.
A mid-season analysis of the influenza vaccine by the US Department of Defensive (DoD) Global Respiratory Pathogen Surveillance Program (DoDGRPSP) has reported low to moderate vaccine effectiveness (VE).
The study included 295 Military Health System (MHS) beneficiaries (adults and children) who tested positive for influenza and 965 controls who tested negative. Vaccinated patients had received the 2024-2025 influenza vaccine at least 14 days prior to symptom onset. The study conducted VE analyses for influenza A (any subtype), influenza A(H1N1)pdm09, and influenza A(H3N2).
Overall, moderate effectiveness against influenza A(H1N1)pdm09 was reported in all beneficiaries and children aged 6 months to 17 years. In adults aged 18 to 64 years—and all beneficiaries—there was moderate effectiveness against influenza A(H3N2). VE estimates against influenza A (any subtype) for all beneficiaries, children, and adults were not significant; VE estimates were also not effective among children for influenza A(H3N2) and in adults for influenza A(H1N1)pdm09.
Adjusted VE estimates among all participants for influenza A (any subtypes), influenza A(H1N1)pdm09, and influenza A(H3N2) were 25%, 58%, and 42%, respectively. VE for influenza B was not calculated due to a low number of cases.
Flu vaccination rates for adults are usually in the 30% to 60% range despite the recommended target of 70%. Flu vaccination rates were rising by around 1% to 2% annually before 2020, but began dropping after the COVID-19 pandemic, especially in higher-risk groups. In adults aged ≥ 65 years, flu vaccination rates dropped from 52% in 2019-2020 to 43% in 2024-2025.
According to the Centers for Disease Control and Prevention (CDC), at the end of the 2023-2024 flu season, 9.2 million fewer doses were administered in pharmacies and doctors offices compared with the baseline before the COVID-19 pandemic. Since 2022, private manufacturers have distributed significantly fewer influenza vaccine doses.
Each March, the US Food and Drug Association (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) meets to analyze the current influenza season and forecast the next. The committee reviews and discusses data on influenza strain circulation and VE, which come from DoDGRPSP analyses. In February, US Department of Health and Human Services officials indefinitely postponed a public meeting of the CDC Advisory Committee on Immunization Practice (ACIP), at which members were also expected to discuss, among other things, VE and vaccine recommendations. The FDA canceled a March 13 VRBPAC meeting and provided no reason for the cancelation to members. That day, however, the FDA issued new recommendations for the influenza vaccine for the 2025-2026 season without the input of VRBPAC. Instead, experts from the FDA, CDC, and DoD made recommendations after reviewing surveillance data from the US and globally.
For the 2025-2026 influenza season, the FDA recommends the vaccines be trivalent and target 2 strains of influenza A and 1 strain of influenza B. The FDA anticipates there will be an “adequate and diverse supply” of approved trivalent seasonal influenza vaccines. Trivalent flu vaccines are formulated to protect against 3 influenza viruses: an A(H1N1) virus, an A(H3N2) virus, and a B/Victoria virus. All influenza vaccines for the 2025-2026 season are anticipated to be trivalent in the US.
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).
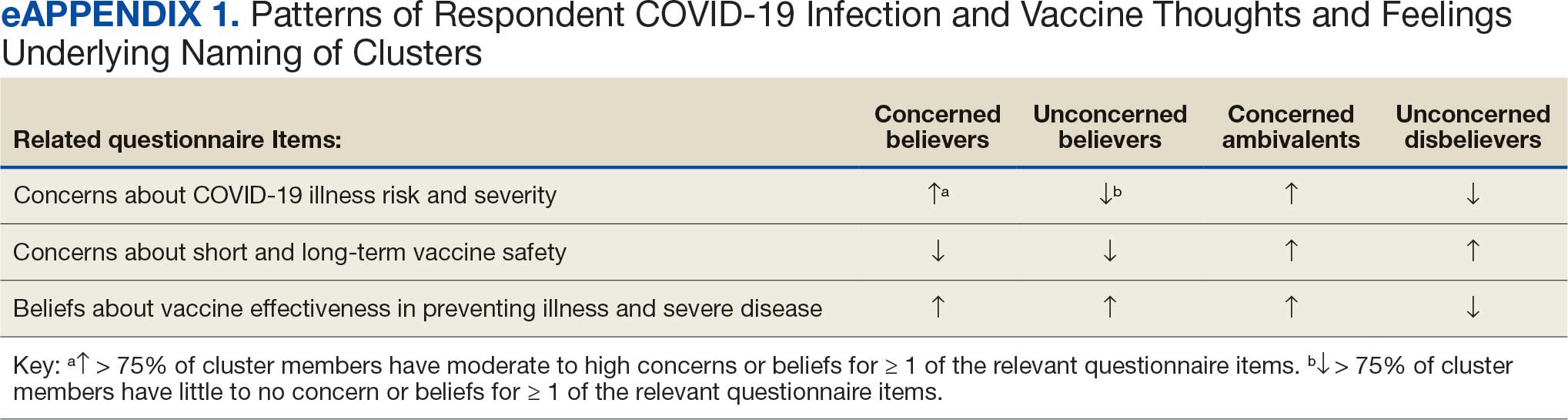
Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).
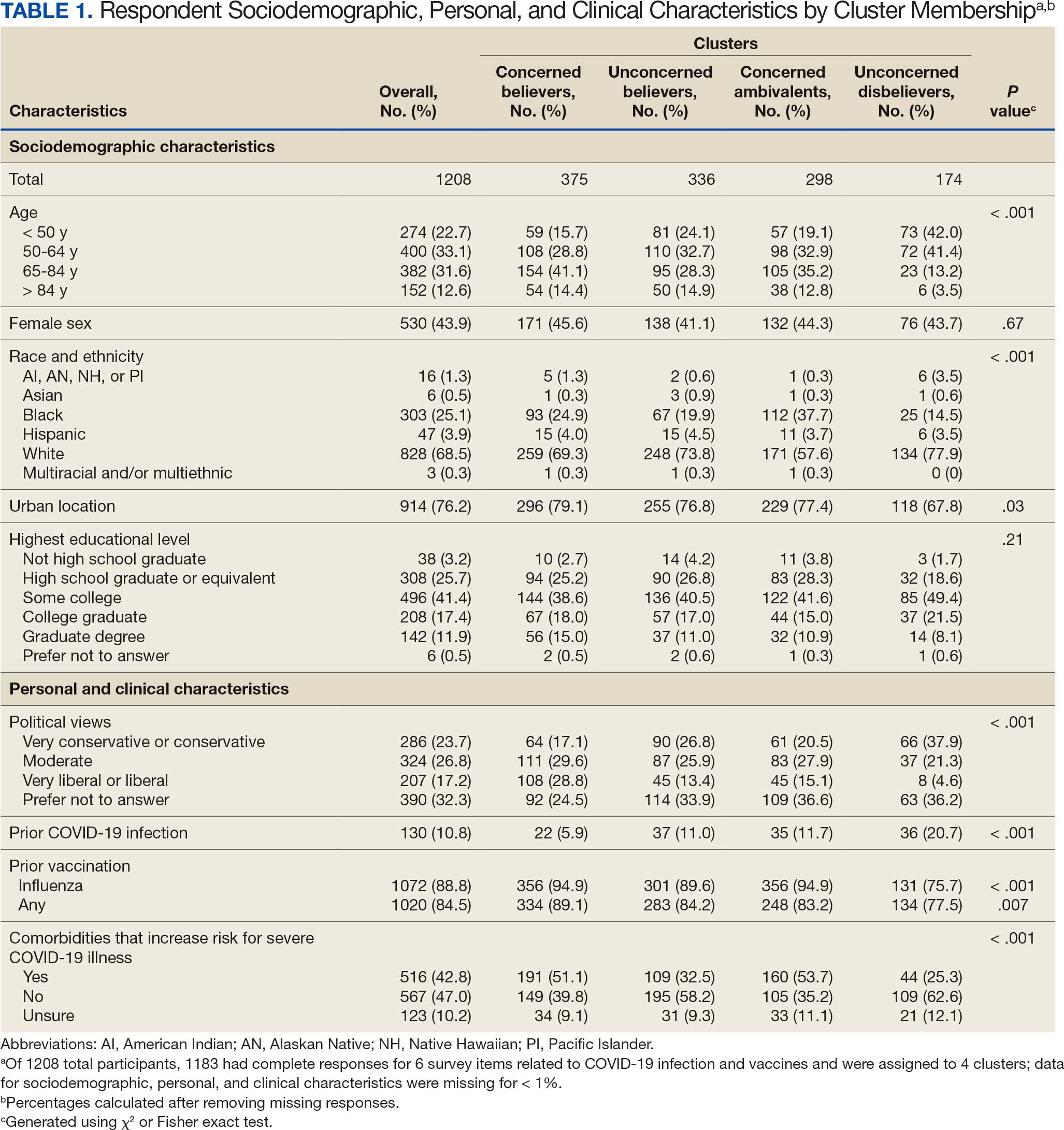
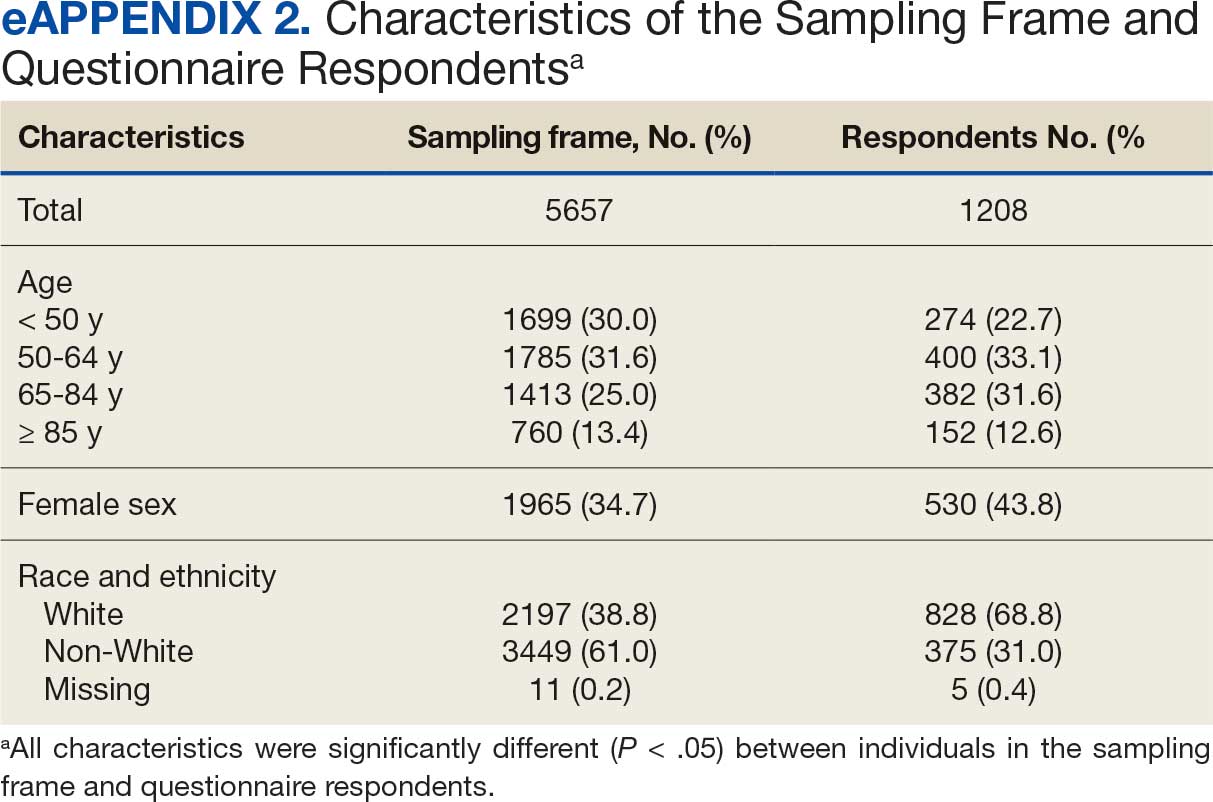
Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.
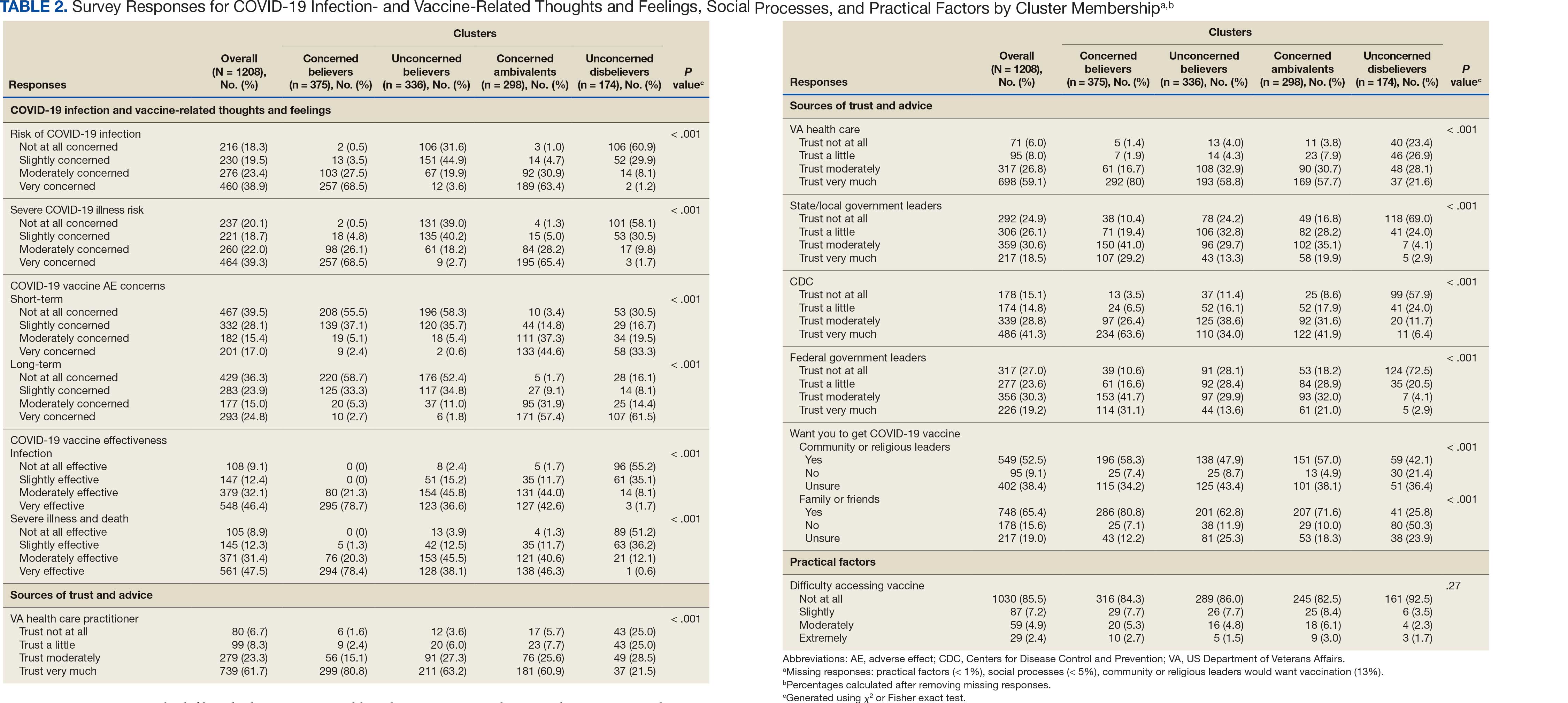
Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.
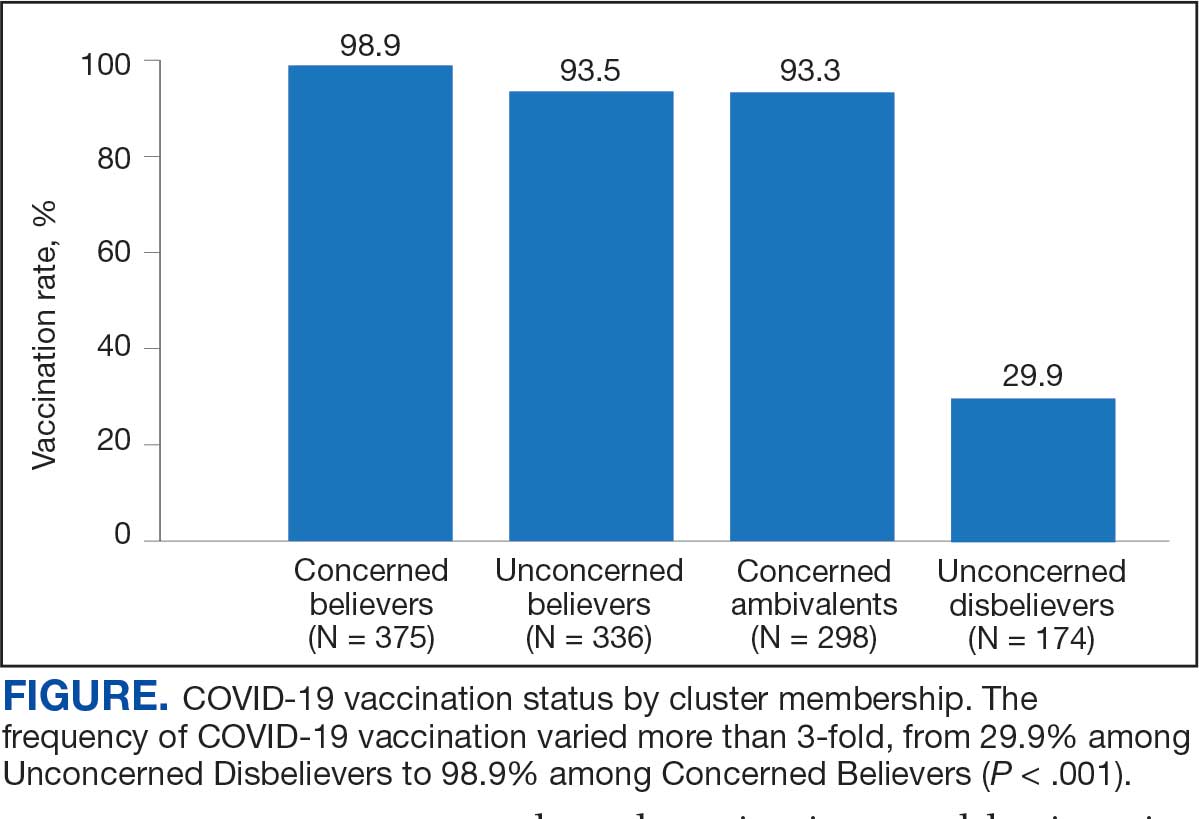
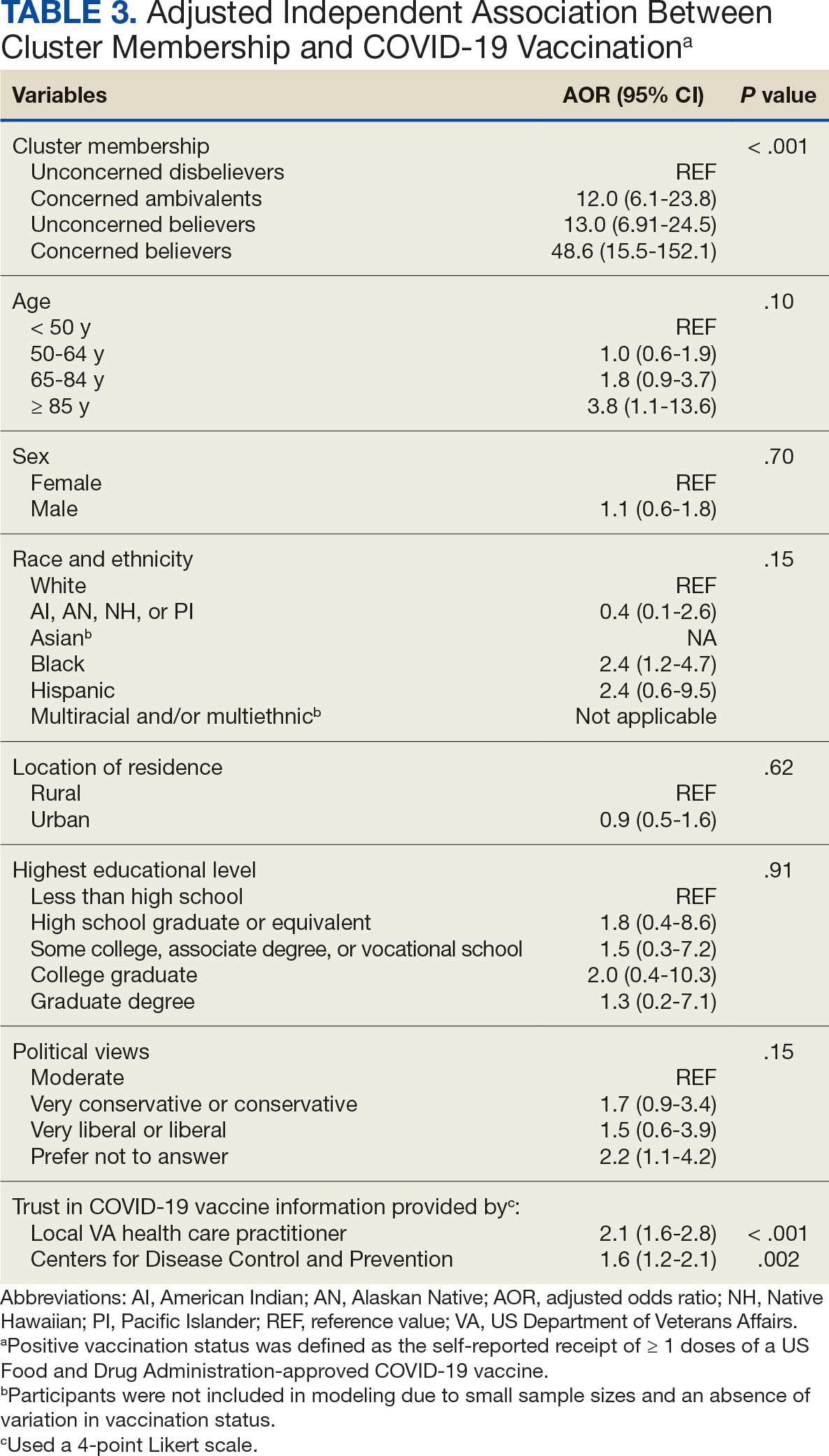
Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10
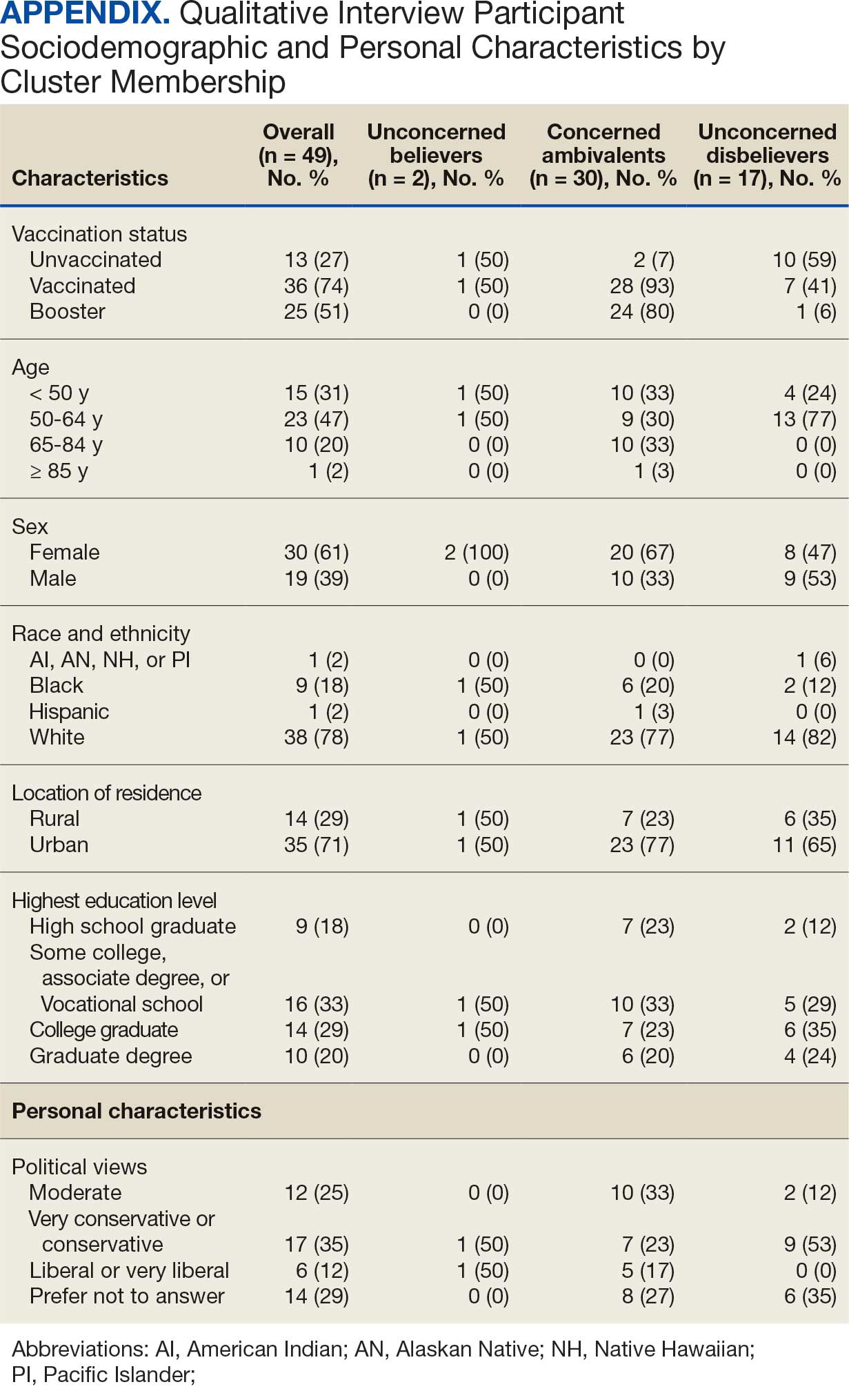
This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).

Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).


Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.

Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.


Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10

This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).

Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).


Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.

Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.


Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10

This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
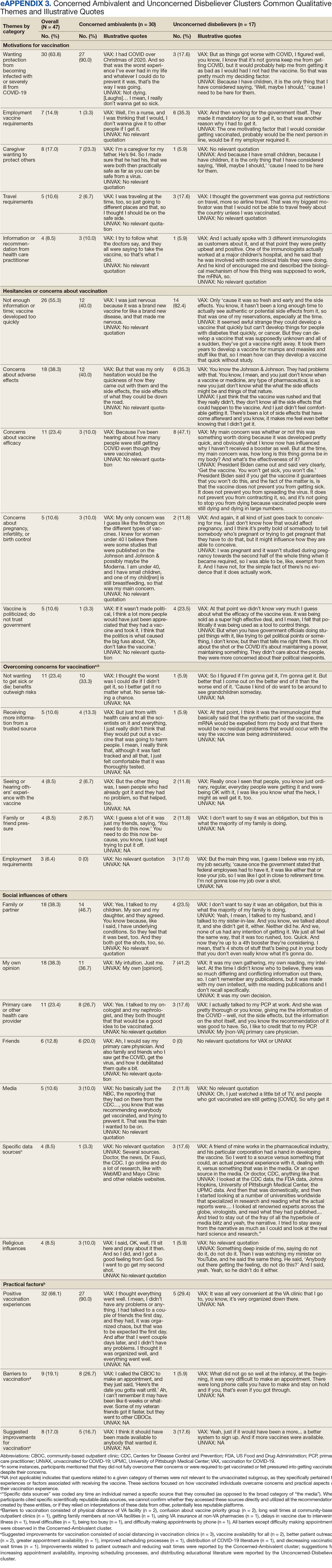
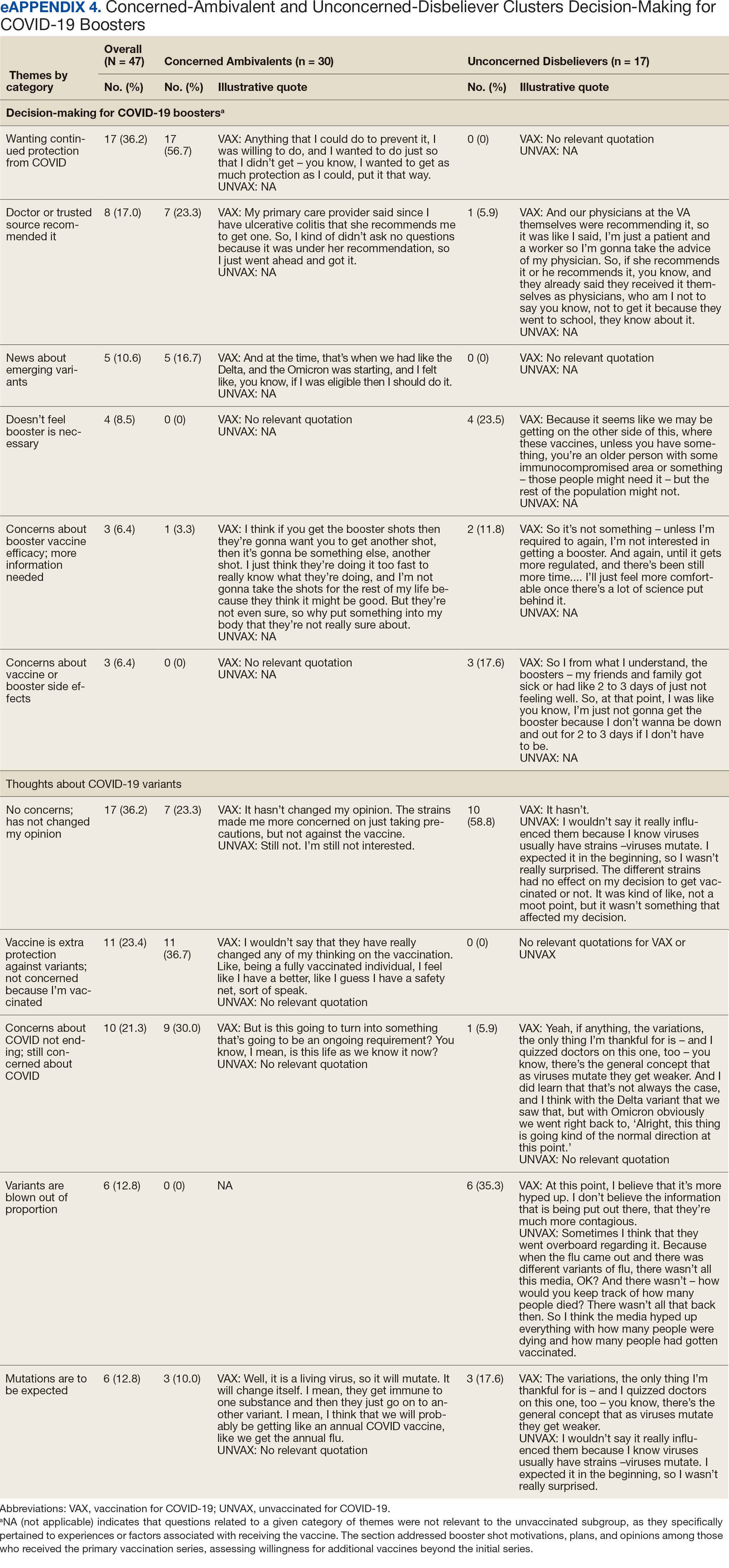
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak