User login
Parameters of Scratch Pleasurability in the Management of Pruritic Conditions
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
Practice Points
- In individuals with pruritic skin conditions, the itch-scratch cycle can have damaging consequences such as anxiety, infection, and secondary skin changes.
- Understanding the pleasurability of scratching in pruritic skin conditions allows providers to help patients break the itch-scratch cycle and improve quality of life.
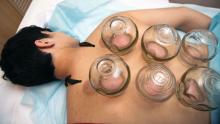
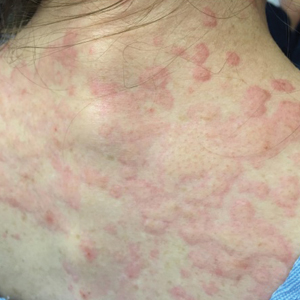
Cupping in dermatology
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
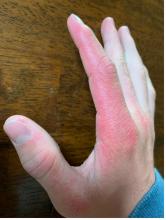
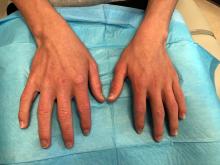
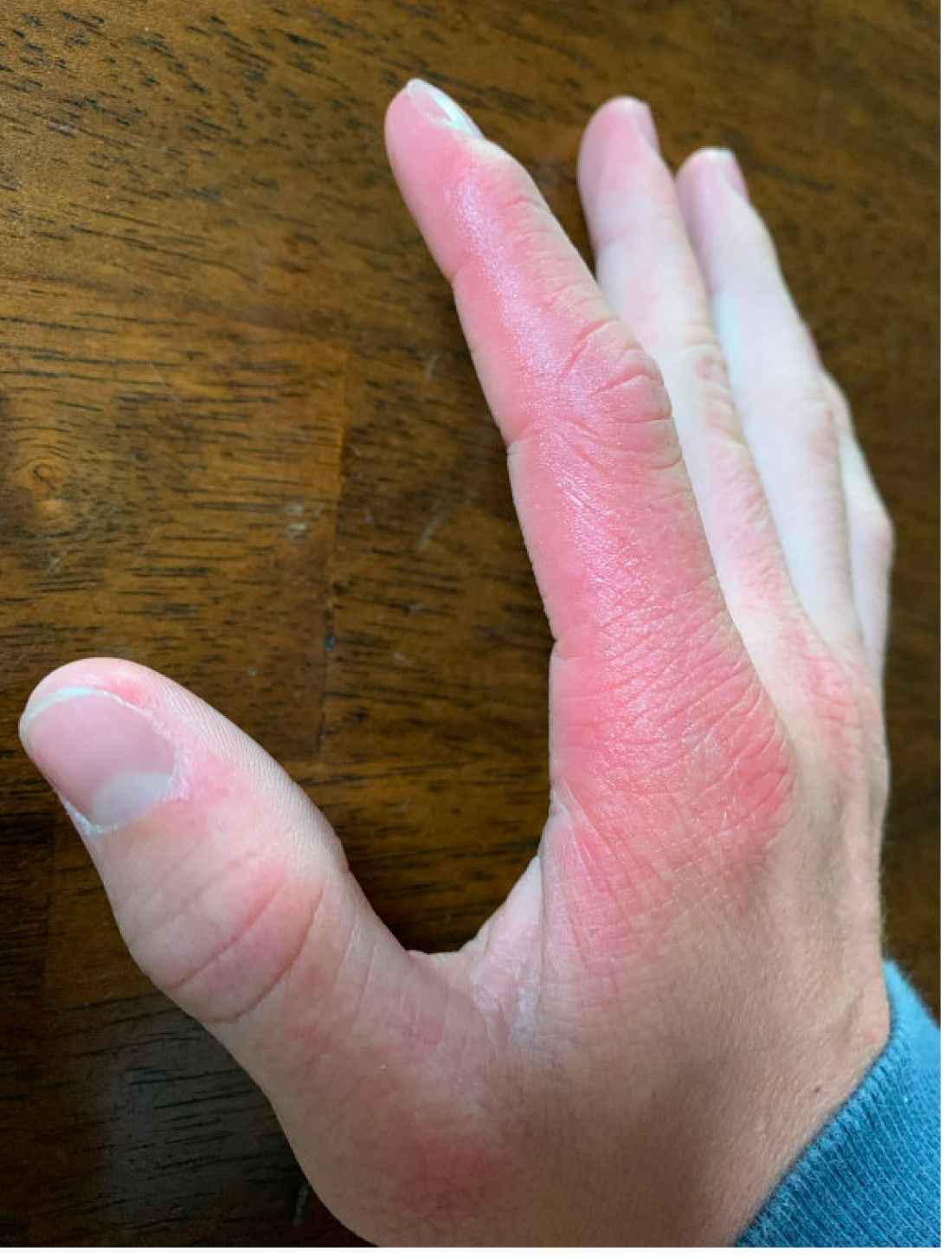
A 14-year-old male presents to clinic with a new-onset rash of the hands
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
He reported no hiking or gardening, no new topical products such as new sunscreens or lotions, and no new medications. The patient had a history of acne, for which he used over-the-counter benzoyl peroxide wash, adapalene gel, and an oral antibiotic for 3 months. His review of systems was negative for fevers, chills, muscle weakness, mouth sores, or joint pain and no prior rashes following sun exposure.
On physical exam he presented with pink plaques with thin vesicles on the dorsum of the hands that were more noticeable on the lateral aspect of both the first and second fingers (Figures 1 and 2). His nails also had a yellow discoloration.
Angioimmunoblastic T-cell Lymphoma Mimicking DRESS Syndrome
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
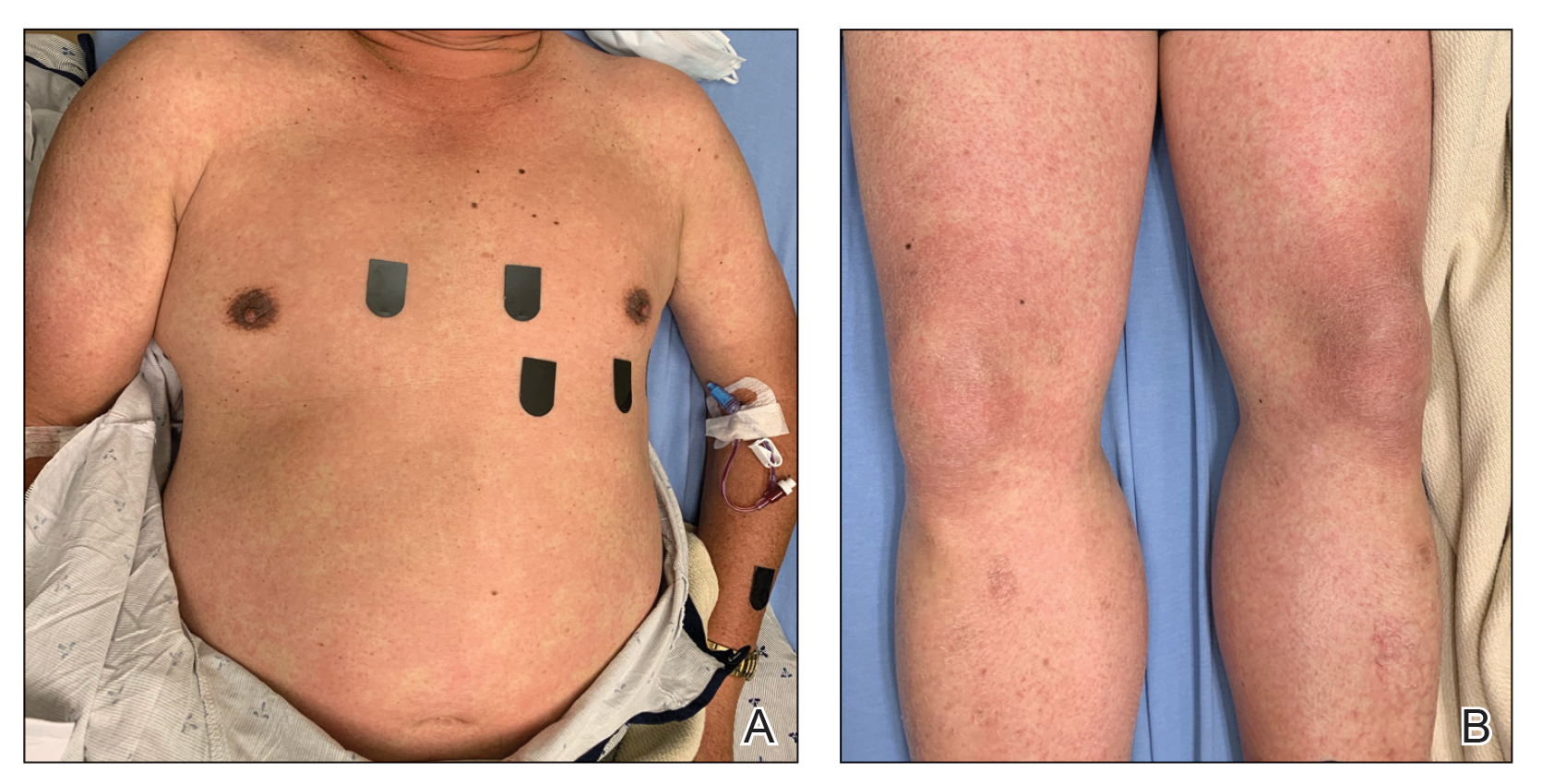
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.
What’s Eating You? Mosquitoes (Culicidae)
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
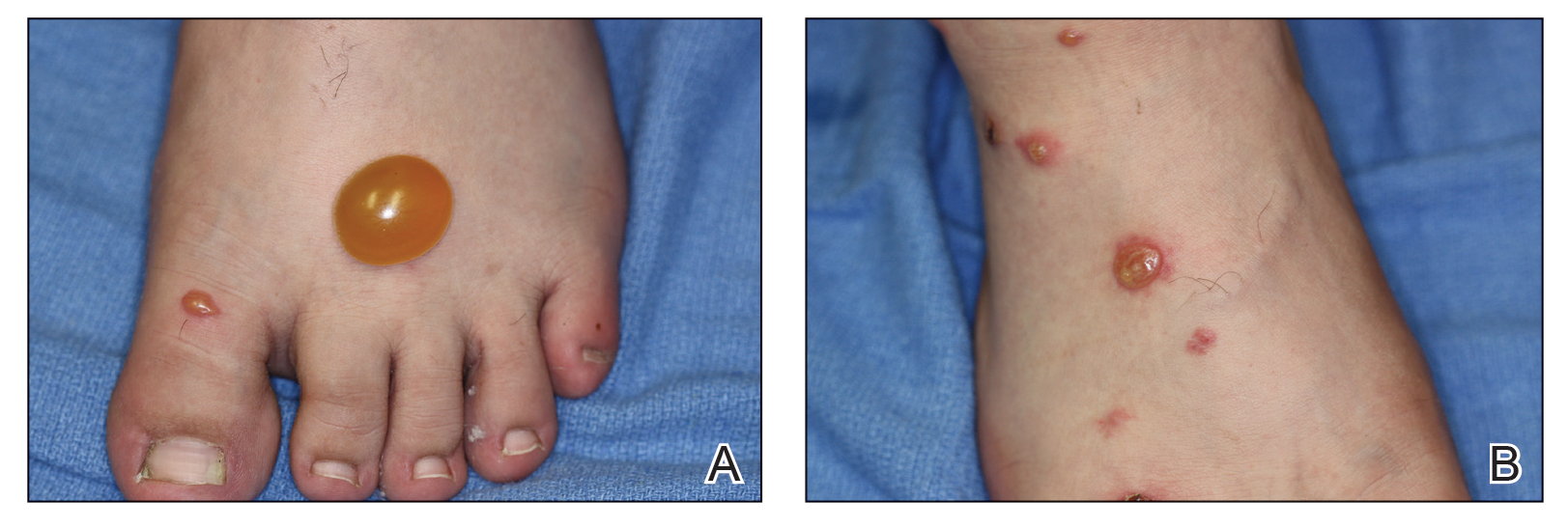
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Practice Points
- Common local reactions to mosquito bites include immediate and delayed hypersensitivity reactions. With repeated exposure, reactions can increase in severity.
- Hypersensitivity to mosquito bites is a severe systemic reaction to mosquito salivary gland components characterized by bullous necrotic skin lesions associated with systemic manifestations such as high fever, malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement.
- Hypersensitivity to mosquito bites is closely associated with chronic Epstein-Barr virus infection and lymphoproliferative disorders.
Urticaria: An elusive disorder with ‘a high unmet need for treatment’
In the clinical experience of Adam Friedman, MD, when patients present with acute urticaria, the culprit is usually food, a drug, or a bug.
But in some cases, the trigger remains elusive. “We don’t always find the source, but don’t beat yourself up about it,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said at the ODAC Dermatology, Aesthetic, and Surgical Conference. “The basic rule is to treat patients to clearance and keep them clear.”
Chronic urticaria is characterized by plaques with a burning/itch sensation that often “move” to different locations on the body over minutes to hours, and they typically last for less than 24 hours. The plaques are often oval, round, or irregular in shape and they typically leave no postinflammatory pigmentary alteration or scarring other than from scratching.
Urticaria affects an estimated 20% of the population, Dr. Friedman said, and is more common in females than males. More than two-thirds of cases are self-limiting but 10% can persist longer than 5 years. Acute episodes are more likely to have an identifiable trigger, while chronic episodes, which last more than six weeks, typically do not. The longer the duration, the lower the chance of identifying the root cause. The foods/food products most commonly affecting children with acute urticaria include milk, egg, peanut, wheat, and soy, while the common culprits in adults are tree nuts, peanuts, and shellfish. Other triggers include the yellow food dye annatto, the red food dye carmine, contact with raw fruits or vegetables, animal saliva, and certain detergents or perfume.
“When you have no idea what the cause is for acute urticaria, I think about viral or bacterial infections, especially in children,” Dr. Friedman said, particularly mycoplasma, adenovirus, enterovirus, rotavirus, respiratory syncytial virus, Epstein-Barr virus, and cytomegalovirus. COVID-19 has also been a new etiologic source for a recent rise in acute urticaria cases.
Other causes include certain medications such as antibiotics, opiates, muscle relaxants, salicylates, and NSAIDs; stinging insects; and exposure to latex products, which can cross react with passion fruit, banana, avocado, chestnut, and kiwi. Alcohol consumption can also trigger urticaria.
“Ask patients if they have joint discomfort or pain,” Dr. Friedman advised, referring to urticaria arthritis syndrome that is typically seen more often in women than in men. “It’s rare but important, because that may distinguish for you what is needed to get those patients under control.”
which spontaneously occurs after an exposure to an external force. “The distinguishing feature here is that it doesn’t last long – 30 minutes or so – and is typically unresponsive to corticosteroids,” Dr. Friedman said. “It comes on quickly but disappears quickly whereas with chronic spontaneous urticaria, someone might be getting those wheals of flare for hours and hours.”
The most common form of physical urticaria is dermatographism, while other examples include physical urticaria resulting from exposure to cholinergic agents, heat, exercise, cold, water, sunlight, and pressure on the skin.
About half of patients with chronic urticaria are disease free within 1 year, but 20% continue to experience episodes for more than 10 years. One study found that patients with chronic spontaneous urticaria who were diagnosed at a younger age trended toward a longer disease course, and rates were higher in women, compared with men. “Perceived stress can make this worse,” Dr. Friedman added.
According to Dr. Friedman, it’s more important to ask patients targeted questions during office visits than it is to do a full workup. “I ask patients to keep a diary, which can help them identify triggers if there are any,” he said. “I also ask them to take a picture of the papules with their smartphone. There can be a genetic association, so it’s important to ask if anyone else in the family has urticaria. No routine lab tests are required unless there’s something in the history that suggests it’s worthwhile. Let the patient guide the diagnostic workup; don’t just order a million tests.”
That said, known comorbidities associated with urticaria include autoimmune disease, atopy, infections, metabolic conditions, and neoplastic disorders. “Biopsies are typically useless because this is an invisible dermatosis,” he said. “They’re useful when it’s urticarial, not urticaria, when you’re trying to figure out what it is.”
According to recently published international guidelines on urticaria, published in September 2021, the recommended first line of treatment for urticaria is with second-generation nonsedating antihistamines such as cetirizine and loratadine, up to four times the recommended dose.
Second-generation derivatives include desloratadine, levocetirizine, and fexofenadine. “I like using fexofenadine in the morning for folks who don’t tolerate cetirizine, then I’ll recommend something a little more sedating at night,” Dr. Friedman said. “We max out [the dose] by week 4. If it works, great. If not, we move on to something else.”
In late 2021, the British Association of Dermatologists also published guidelines on the treatment of chronic urticaria.
As for markers of treatment success, a study of 240 children with chronic spontaneous urticaria found that risk factors for a poor response included longer duration of disease, higher treatment step until initial disease control, and food sensitization.
Vitamin D supplementation may also add some benefit. One study of 42 adults with urticaria found that low and high doses of vitamin D added to antihistamine therapy can boost effectiveness. “This may be because vitamin D could be a marker of severity,” Dr. Friedman said. “The reality is, however, that a lot of patients don’t do well.”
Data from the large, prospective study known as AWARE (A World-Wide Antihistamine-Refractory Chronic Urticaria Patient Evaluation) found that 23% patients treated with nonsedating H1-antihistamines and 42% patients treated with up-dosed nonsedating H1-antihistamines had uncontrolled chronic spontaneous urticaria at month 24.
A second-line treatment option for patients aged 6 and older is the anti-IgE antibody omalizumab, 150-300 mg by subcutaneous injection every 4 weeks. Dr. Friedman typically uses only the 300-mg dose. “You do not need to take pretreatment serum IgE levels,” he said. “The most significant adverse event is anaphylaxis, which only affects 0.2% of patients.”
A third-line option is cyclosporine A. A dose of 3-5mg/kg per day appears to benefit about two-thirds of patients with antihistamine recalcitrant chronic urticaria. “It works fast but you can’t keep patients on it for very long,” he said.
Another third-line option is mycophenolate mofetil, which may work by inhibiting the production of autoantibodies to the high-affinity IgE receptor and/or IgE. “It does work well, especially in conjunction with antihistamines; it’s kind of a softer immunosuppressant,” he said. Methotrexate can also be used as an add-on therapy to H1 antihistamine therapy in difficult-to-treat cases.
“It’s great we have [a Food and Drug Administration]–approved biologic therapy in omalizumab and access to over-the-counter antihistamines, but there is a high unmet need for treatment,” and a need for new therapies, Dr. Friedman said. “Only about 39% achieve symptomatic control with conventional dosing of antihistamines, and 63% of nonresponders achieve symptom control with a fourfold increased dosing of antihistamines.” In addition, about 20% of patients will not respond to either standard or increased doses of antihistamines and are eligible for treatment with omalizumab. However, more than 50% of such patients experience a delay or lack of response to omalizumab. “We need innovation; we need to understand the disease better,” he said.
Dr. Friedman disclosed that he serves as a consultant and/or adviser for Loreal, La Roche Posay, Cerave, Galderma, Aveeno, Microcures, Pfizer, Novartis, Dermira, Brickell Biotech, Incyte, UCB, Janssen, Pfizer, Bristol-Myers Squibb, Almirall, Zylo Therapeutics, Hoth Therapeutics, Corbus, Greenway Therapeutics, TruPotency, and Dermavant. He is a speaker for Regeneron/Sanofi, AbbVie, Janssen, Brickell Biotech, and Incyte, and has received grants from Pfizer, the Dermatology Foundation, Incyte, and Galderma.
In the clinical experience of Adam Friedman, MD, when patients present with acute urticaria, the culprit is usually food, a drug, or a bug.
But in some cases, the trigger remains elusive. “We don’t always find the source, but don’t beat yourself up about it,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said at the ODAC Dermatology, Aesthetic, and Surgical Conference. “The basic rule is to treat patients to clearance and keep them clear.”
Chronic urticaria is characterized by plaques with a burning/itch sensation that often “move” to different locations on the body over minutes to hours, and they typically last for less than 24 hours. The plaques are often oval, round, or irregular in shape and they typically leave no postinflammatory pigmentary alteration or scarring other than from scratching.
Urticaria affects an estimated 20% of the population, Dr. Friedman said, and is more common in females than males. More than two-thirds of cases are self-limiting but 10% can persist longer than 5 years. Acute episodes are more likely to have an identifiable trigger, while chronic episodes, which last more than six weeks, typically do not. The longer the duration, the lower the chance of identifying the root cause. The foods/food products most commonly affecting children with acute urticaria include milk, egg, peanut, wheat, and soy, while the common culprits in adults are tree nuts, peanuts, and shellfish. Other triggers include the yellow food dye annatto, the red food dye carmine, contact with raw fruits or vegetables, animal saliva, and certain detergents or perfume.
“When you have no idea what the cause is for acute urticaria, I think about viral or bacterial infections, especially in children,” Dr. Friedman said, particularly mycoplasma, adenovirus, enterovirus, rotavirus, respiratory syncytial virus, Epstein-Barr virus, and cytomegalovirus. COVID-19 has also been a new etiologic source for a recent rise in acute urticaria cases.
Other causes include certain medications such as antibiotics, opiates, muscle relaxants, salicylates, and NSAIDs; stinging insects; and exposure to latex products, which can cross react with passion fruit, banana, avocado, chestnut, and kiwi. Alcohol consumption can also trigger urticaria.
“Ask patients if they have joint discomfort or pain,” Dr. Friedman advised, referring to urticaria arthritis syndrome that is typically seen more often in women than in men. “It’s rare but important, because that may distinguish for you what is needed to get those patients under control.”
which spontaneously occurs after an exposure to an external force. “The distinguishing feature here is that it doesn’t last long – 30 minutes or so – and is typically unresponsive to corticosteroids,” Dr. Friedman said. “It comes on quickly but disappears quickly whereas with chronic spontaneous urticaria, someone might be getting those wheals of flare for hours and hours.”
The most common form of physical urticaria is dermatographism, while other examples include physical urticaria resulting from exposure to cholinergic agents, heat, exercise, cold, water, sunlight, and pressure on the skin.
About half of patients with chronic urticaria are disease free within 1 year, but 20% continue to experience episodes for more than 10 years. One study found that patients with chronic spontaneous urticaria who were diagnosed at a younger age trended toward a longer disease course, and rates were higher in women, compared with men. “Perceived stress can make this worse,” Dr. Friedman added.
According to Dr. Friedman, it’s more important to ask patients targeted questions during office visits than it is to do a full workup. “I ask patients to keep a diary, which can help them identify triggers if there are any,” he said. “I also ask them to take a picture of the papules with their smartphone. There can be a genetic association, so it’s important to ask if anyone else in the family has urticaria. No routine lab tests are required unless there’s something in the history that suggests it’s worthwhile. Let the patient guide the diagnostic workup; don’t just order a million tests.”
That said, known comorbidities associated with urticaria include autoimmune disease, atopy, infections, metabolic conditions, and neoplastic disorders. “Biopsies are typically useless because this is an invisible dermatosis,” he said. “They’re useful when it’s urticarial, not urticaria, when you’re trying to figure out what it is.”
According to recently published international guidelines on urticaria, published in September 2021, the recommended first line of treatment for urticaria is with second-generation nonsedating antihistamines such as cetirizine and loratadine, up to four times the recommended dose.
Second-generation derivatives include desloratadine, levocetirizine, and fexofenadine. “I like using fexofenadine in the morning for folks who don’t tolerate cetirizine, then I’ll recommend something a little more sedating at night,” Dr. Friedman said. “We max out [the dose] by week 4. If it works, great. If not, we move on to something else.”
In late 2021, the British Association of Dermatologists also published guidelines on the treatment of chronic urticaria.
As for markers of treatment success, a study of 240 children with chronic spontaneous urticaria found that risk factors for a poor response included longer duration of disease, higher treatment step until initial disease control, and food sensitization.
Vitamin D supplementation may also add some benefit. One study of 42 adults with urticaria found that low and high doses of vitamin D added to antihistamine therapy can boost effectiveness. “This may be because vitamin D could be a marker of severity,” Dr. Friedman said. “The reality is, however, that a lot of patients don’t do well.”
Data from the large, prospective study known as AWARE (A World-Wide Antihistamine-Refractory Chronic Urticaria Patient Evaluation) found that 23% patients treated with nonsedating H1-antihistamines and 42% patients treated with up-dosed nonsedating H1-antihistamines had uncontrolled chronic spontaneous urticaria at month 24.
A second-line treatment option for patients aged 6 and older is the anti-IgE antibody omalizumab, 150-300 mg by subcutaneous injection every 4 weeks. Dr. Friedman typically uses only the 300-mg dose. “You do not need to take pretreatment serum IgE levels,” he said. “The most significant adverse event is anaphylaxis, which only affects 0.2% of patients.”
A third-line option is cyclosporine A. A dose of 3-5mg/kg per day appears to benefit about two-thirds of patients with antihistamine recalcitrant chronic urticaria. “It works fast but you can’t keep patients on it for very long,” he said.
Another third-line option is mycophenolate mofetil, which may work by inhibiting the production of autoantibodies to the high-affinity IgE receptor and/or IgE. “It does work well, especially in conjunction with antihistamines; it’s kind of a softer immunosuppressant,” he said. Methotrexate can also be used as an add-on therapy to H1 antihistamine therapy in difficult-to-treat cases.
“It’s great we have [a Food and Drug Administration]–approved biologic therapy in omalizumab and access to over-the-counter antihistamines, but there is a high unmet need for treatment,” and a need for new therapies, Dr. Friedman said. “Only about 39% achieve symptomatic control with conventional dosing of antihistamines, and 63% of nonresponders achieve symptom control with a fourfold increased dosing of antihistamines.” In addition, about 20% of patients will not respond to either standard or increased doses of antihistamines and are eligible for treatment with omalizumab. However, more than 50% of such patients experience a delay or lack of response to omalizumab. “We need innovation; we need to understand the disease better,” he said.
Dr. Friedman disclosed that he serves as a consultant and/or adviser for Loreal, La Roche Posay, Cerave, Galderma, Aveeno, Microcures, Pfizer, Novartis, Dermira, Brickell Biotech, Incyte, UCB, Janssen, Pfizer, Bristol-Myers Squibb, Almirall, Zylo Therapeutics, Hoth Therapeutics, Corbus, Greenway Therapeutics, TruPotency, and Dermavant. He is a speaker for Regeneron/Sanofi, AbbVie, Janssen, Brickell Biotech, and Incyte, and has received grants from Pfizer, the Dermatology Foundation, Incyte, and Galderma.
In the clinical experience of Adam Friedman, MD, when patients present with acute urticaria, the culprit is usually food, a drug, or a bug.
But in some cases, the trigger remains elusive. “We don’t always find the source, but don’t beat yourself up about it,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said at the ODAC Dermatology, Aesthetic, and Surgical Conference. “The basic rule is to treat patients to clearance and keep them clear.”
Chronic urticaria is characterized by plaques with a burning/itch sensation that often “move” to different locations on the body over minutes to hours, and they typically last for less than 24 hours. The plaques are often oval, round, or irregular in shape and they typically leave no postinflammatory pigmentary alteration or scarring other than from scratching.
Urticaria affects an estimated 20% of the population, Dr. Friedman said, and is more common in females than males. More than two-thirds of cases are self-limiting but 10% can persist longer than 5 years. Acute episodes are more likely to have an identifiable trigger, while chronic episodes, which last more than six weeks, typically do not. The longer the duration, the lower the chance of identifying the root cause. The foods/food products most commonly affecting children with acute urticaria include milk, egg, peanut, wheat, and soy, while the common culprits in adults are tree nuts, peanuts, and shellfish. Other triggers include the yellow food dye annatto, the red food dye carmine, contact with raw fruits or vegetables, animal saliva, and certain detergents or perfume.
“When you have no idea what the cause is for acute urticaria, I think about viral or bacterial infections, especially in children,” Dr. Friedman said, particularly mycoplasma, adenovirus, enterovirus, rotavirus, respiratory syncytial virus, Epstein-Barr virus, and cytomegalovirus. COVID-19 has also been a new etiologic source for a recent rise in acute urticaria cases.
Other causes include certain medications such as antibiotics, opiates, muscle relaxants, salicylates, and NSAIDs; stinging insects; and exposure to latex products, which can cross react with passion fruit, banana, avocado, chestnut, and kiwi. Alcohol consumption can also trigger urticaria.
“Ask patients if they have joint discomfort or pain,” Dr. Friedman advised, referring to urticaria arthritis syndrome that is typically seen more often in women than in men. “It’s rare but important, because that may distinguish for you what is needed to get those patients under control.”
which spontaneously occurs after an exposure to an external force. “The distinguishing feature here is that it doesn’t last long – 30 minutes or so – and is typically unresponsive to corticosteroids,” Dr. Friedman said. “It comes on quickly but disappears quickly whereas with chronic spontaneous urticaria, someone might be getting those wheals of flare for hours and hours.”
The most common form of physical urticaria is dermatographism, while other examples include physical urticaria resulting from exposure to cholinergic agents, heat, exercise, cold, water, sunlight, and pressure on the skin.
About half of patients with chronic urticaria are disease free within 1 year, but 20% continue to experience episodes for more than 10 years. One study found that patients with chronic spontaneous urticaria who were diagnosed at a younger age trended toward a longer disease course, and rates were higher in women, compared with men. “Perceived stress can make this worse,” Dr. Friedman added.
According to Dr. Friedman, it’s more important to ask patients targeted questions during office visits than it is to do a full workup. “I ask patients to keep a diary, which can help them identify triggers if there are any,” he said. “I also ask them to take a picture of the papules with their smartphone. There can be a genetic association, so it’s important to ask if anyone else in the family has urticaria. No routine lab tests are required unless there’s something in the history that suggests it’s worthwhile. Let the patient guide the diagnostic workup; don’t just order a million tests.”
That said, known comorbidities associated with urticaria include autoimmune disease, atopy, infections, metabolic conditions, and neoplastic disorders. “Biopsies are typically useless because this is an invisible dermatosis,” he said. “They’re useful when it’s urticarial, not urticaria, when you’re trying to figure out what it is.”
According to recently published international guidelines on urticaria, published in September 2021, the recommended first line of treatment for urticaria is with second-generation nonsedating antihistamines such as cetirizine and loratadine, up to four times the recommended dose.
Second-generation derivatives include desloratadine, levocetirizine, and fexofenadine. “I like using fexofenadine in the morning for folks who don’t tolerate cetirizine, then I’ll recommend something a little more sedating at night,” Dr. Friedman said. “We max out [the dose] by week 4. If it works, great. If not, we move on to something else.”
In late 2021, the British Association of Dermatologists also published guidelines on the treatment of chronic urticaria.
As for markers of treatment success, a study of 240 children with chronic spontaneous urticaria found that risk factors for a poor response included longer duration of disease, higher treatment step until initial disease control, and food sensitization.
Vitamin D supplementation may also add some benefit. One study of 42 adults with urticaria found that low and high doses of vitamin D added to antihistamine therapy can boost effectiveness. “This may be because vitamin D could be a marker of severity,” Dr. Friedman said. “The reality is, however, that a lot of patients don’t do well.”
Data from the large, prospective study known as AWARE (A World-Wide Antihistamine-Refractory Chronic Urticaria Patient Evaluation) found that 23% patients treated with nonsedating H1-antihistamines and 42% patients treated with up-dosed nonsedating H1-antihistamines had uncontrolled chronic spontaneous urticaria at month 24.
A second-line treatment option for patients aged 6 and older is the anti-IgE antibody omalizumab, 150-300 mg by subcutaneous injection every 4 weeks. Dr. Friedman typically uses only the 300-mg dose. “You do not need to take pretreatment serum IgE levels,” he said. “The most significant adverse event is anaphylaxis, which only affects 0.2% of patients.”
A third-line option is cyclosporine A. A dose of 3-5mg/kg per day appears to benefit about two-thirds of patients with antihistamine recalcitrant chronic urticaria. “It works fast but you can’t keep patients on it for very long,” he said.
Another third-line option is mycophenolate mofetil, which may work by inhibiting the production of autoantibodies to the high-affinity IgE receptor and/or IgE. “It does work well, especially in conjunction with antihistamines; it’s kind of a softer immunosuppressant,” he said. Methotrexate can also be used as an add-on therapy to H1 antihistamine therapy in difficult-to-treat cases.
“It’s great we have [a Food and Drug Administration]–approved biologic therapy in omalizumab and access to over-the-counter antihistamines, but there is a high unmet need for treatment,” and a need for new therapies, Dr. Friedman said. “Only about 39% achieve symptomatic control with conventional dosing of antihistamines, and 63% of nonresponders achieve symptom control with a fourfold increased dosing of antihistamines.” In addition, about 20% of patients will not respond to either standard or increased doses of antihistamines and are eligible for treatment with omalizumab. However, more than 50% of such patients experience a delay or lack of response to omalizumab. “We need innovation; we need to understand the disease better,” he said.
Dr. Friedman disclosed that he serves as a consultant and/or adviser for Loreal, La Roche Posay, Cerave, Galderma, Aveeno, Microcures, Pfizer, Novartis, Dermira, Brickell Biotech, Incyte, UCB, Janssen, Pfizer, Bristol-Myers Squibb, Almirall, Zylo Therapeutics, Hoth Therapeutics, Corbus, Greenway Therapeutics, TruPotency, and Dermavant. He is a speaker for Regeneron/Sanofi, AbbVie, Janssen, Brickell Biotech, and Incyte, and has received grants from Pfizer, the Dermatology Foundation, Incyte, and Galderma.
FROM ODAC 2022
Diffuse Urticarial Rash in a Pregnant Patient
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
A 29-year-old pregnant woman at 18 weeks and 5 days of gestation presented with a diffuse, pruritic, blistering rash of 5 weeks’ duration that started on the forearms and generalized to affect the trunk, legs, palms, and soles. Physical examination showed diffuse urticarial papules and plaques with small tense vesicles with an annular configuration on the abdomen and marked periumbilical involvement.
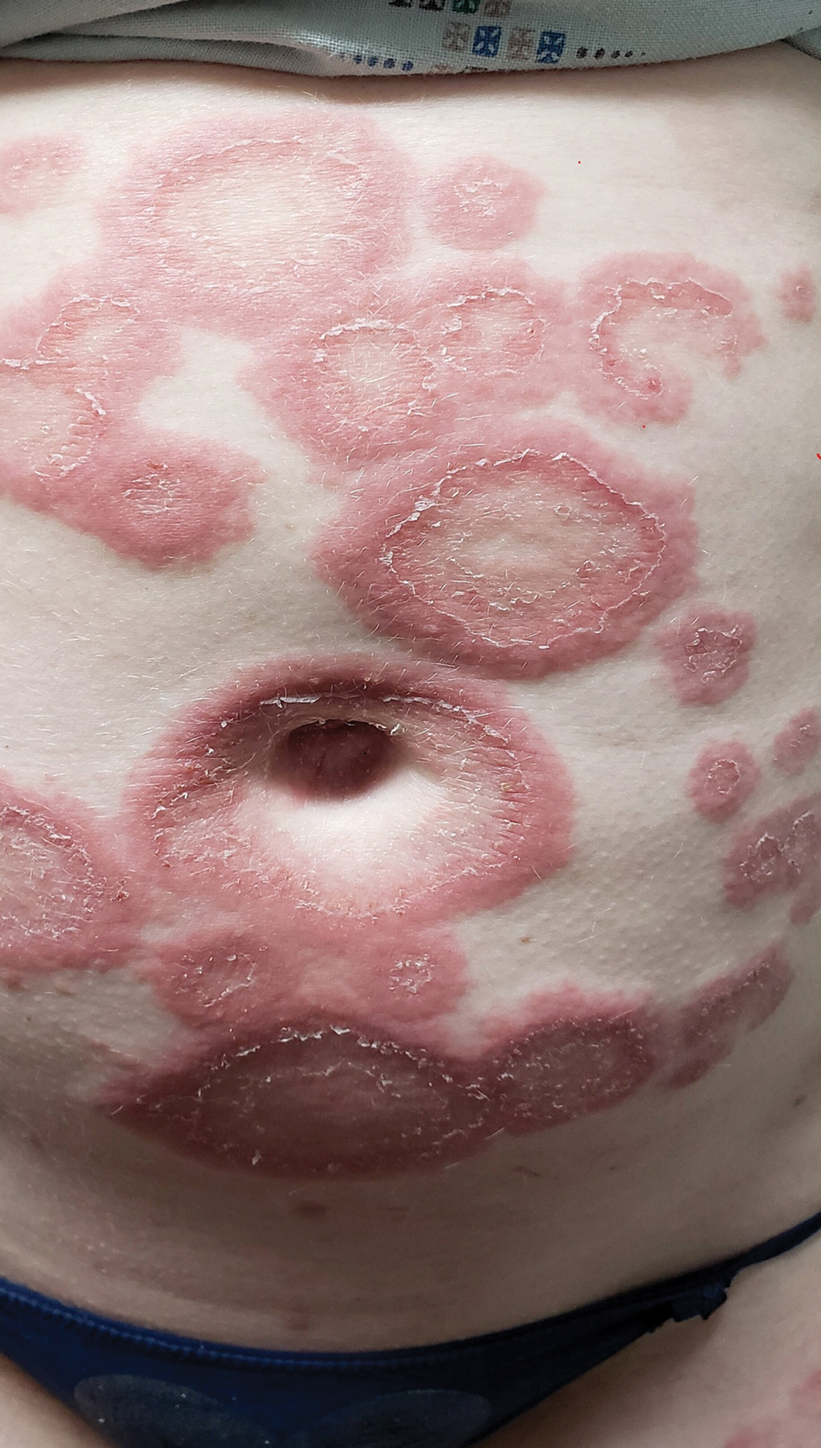
Pruritic Papules on the Trunk, Extremities, and Face
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
A 69-year-old woman presented in early summer in southeastern Michigan with several itchy bumps (top) of 4 to 5 weeks’ duration that erupted and remitted over the trunk, extremities, and face. She had taken no new medications. She had an asymptomatic cat and no exposure to anyone else who had been itching. Physical examination revealed approximately a dozen 2- to 5-mm edematous papules on the trunk, arms, shins, thighs, and left cheek, as well as one 3-mm vesicle on the forearm. No burrows could be identified on physical examination. Lesions treated with betamethasone dipropionate cream 0.05% improved, but new lesions continued to arise. An exterminator was contacted but found no signs of bedbugs or other infestations. Later, the patient reported seeing 3 tiny black dots crawl across the screen of her cell phone as she read in bed. She was able to capture them on tape and bring them to her appointment. The specimens were approximately 1 mm in length (bottom).
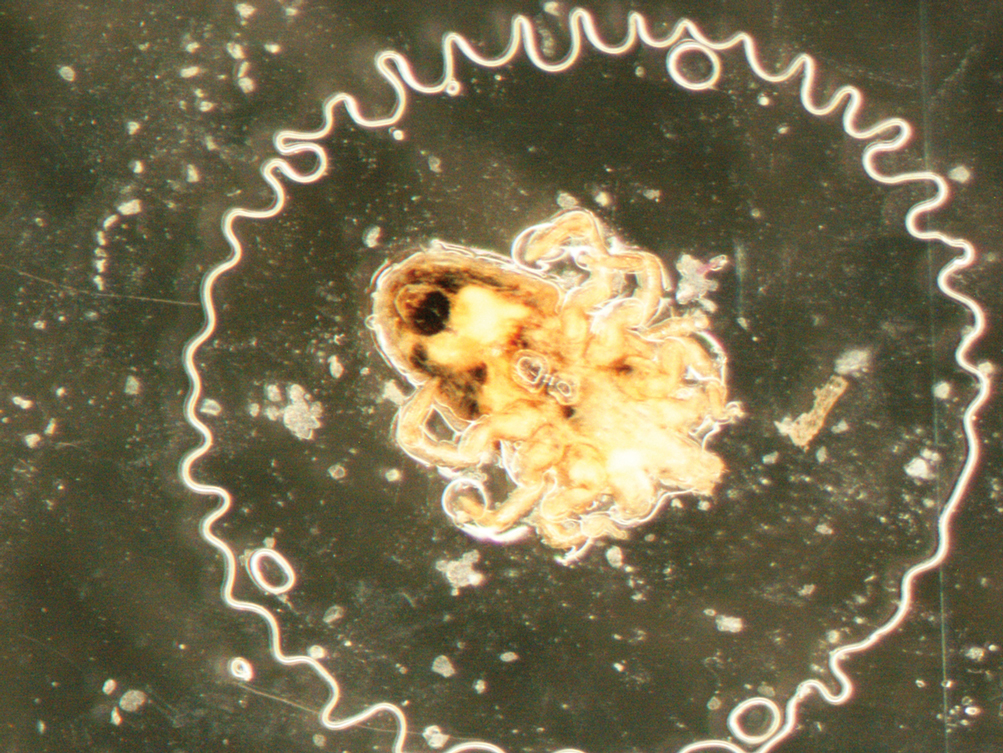
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.
Erratum (Cutis. 2021;108:181-184, 202)
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD