User login
Cancer Data Trends 2025
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
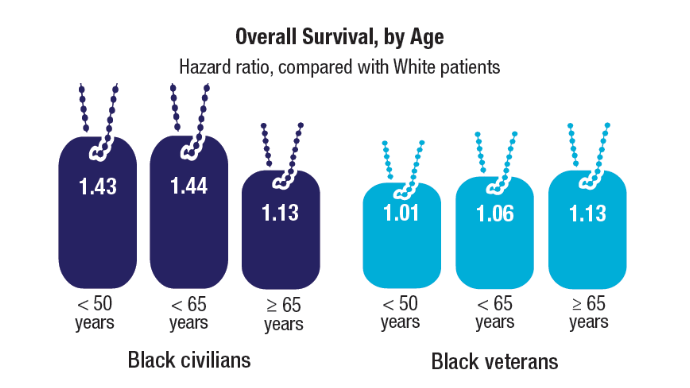
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
Best Practices: Protecting Dry Vulnerable Skin with CeraVe® Healing Ointment
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025
Gastroenterology Data Trends 2025
Gastroenterology Data Trends 2025
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
Gastroenterology Data Trends 2025
Gastroenterology Data Trends 2025
Helping to Protect Our Children From Invasive Pneumococcal Disease
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
Primary Sclerosing Cholangitis (PSC) and Its Importance in Clinical Practice
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
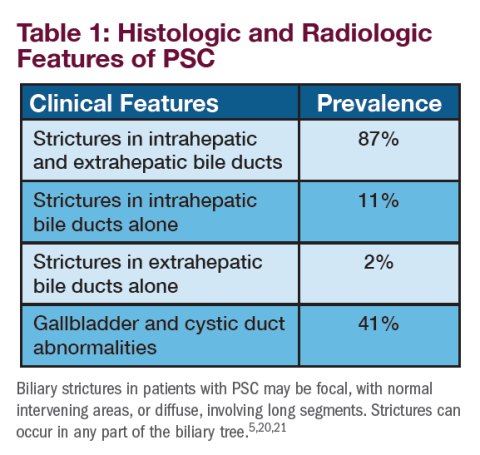
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
A Special Supplement on Hot Topics in Primary Care 2024

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read the 2024 Hot Topics in Primary Care

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read the 2024 Hot Topics in Primary Care

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read the 2024 Hot Topics in Primary Care
2024 Rare Neurological Disease Special Report
Editor’s Note
By Glenn S. Williams
In this year’s Rare Neurological Disease Special Report, we focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust.
A Note From NORD
By Pamela Gavin
Through NORD’s collaboration with Neurology Reviews, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
Genetic Testing for ALS, Now a Standard, Creates a Path Toward Individualized Care
By Ted Bosworth
Overall, there is a sense of progress in ALS. The hope is that clinical research is reaching a tipping point where targeted treatments may offer hope to patients with ALS.
Myasthenia Gravis: Patient Choice, Cultural Change
By John Jesitus
Used appropriately, newer treatments for myasthenia gravis can provide dramatic results faster and more safely than broad immunosuppressants.
Promise for Disease-Modifying Therapies to Tame Huntington’s Disease
By Neil Osterweil
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
Diagnosing and Managing Duchenne Muscular Dystrophy: Tips for Practicing Clinicians
By Batya Swift Yasgur, MA, LSW
Healthcare providers should be familiar enough with Duchenne muscular dystrophy to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers.
Neuromyelitis Optica: Historically Misdiagnosed — Now Demands Prompt Treatment
By Kate Johnson
Rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury.”
Untangling CIDP
By Jennie Smith
Though a preferred biomarker remains elusive, this difficult-to-diagnose neuropathy has seen important recent advances in diagnosis and treatment.
Newborn Screening Programs: What Do Clinicians Need to Know?
By Batya Swift Yasgur, MA, LSW
The goal of newborn screening is to identify babies with genetic disorders who otherwise have no obvious symptoms.
Balancing Act: Weighing the Pros and Cons of Genetic Testing in Rare Diseases
By Frieda Wiley
While genetic testing may offer great potential for providing answers to patients and clinicians seeking insight into a rare disorder, the technology holds some pros and cons that neurologists should be aware of.
Editor’s Note
By Glenn S. Williams
In this year’s Rare Neurological Disease Special Report, we focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust.
A Note From NORD
By Pamela Gavin
Through NORD’s collaboration with Neurology Reviews, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
Genetic Testing for ALS, Now a Standard, Creates a Path Toward Individualized Care
By Ted Bosworth
Overall, there is a sense of progress in ALS. The hope is that clinical research is reaching a tipping point where targeted treatments may offer hope to patients with ALS.
Myasthenia Gravis: Patient Choice, Cultural Change
By John Jesitus
Used appropriately, newer treatments for myasthenia gravis can provide dramatic results faster and more safely than broad immunosuppressants.
Promise for Disease-Modifying Therapies to Tame Huntington’s Disease
By Neil Osterweil
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
Diagnosing and Managing Duchenne Muscular Dystrophy: Tips for Practicing Clinicians
By Batya Swift Yasgur, MA, LSW
Healthcare providers should be familiar enough with Duchenne muscular dystrophy to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers.
Neuromyelitis Optica: Historically Misdiagnosed — Now Demands Prompt Treatment
By Kate Johnson
Rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury.”
Untangling CIDP
By Jennie Smith
Though a preferred biomarker remains elusive, this difficult-to-diagnose neuropathy has seen important recent advances in diagnosis and treatment.
Newborn Screening Programs: What Do Clinicians Need to Know?
By Batya Swift Yasgur, MA, LSW
The goal of newborn screening is to identify babies with genetic disorders who otherwise have no obvious symptoms.
Balancing Act: Weighing the Pros and Cons of Genetic Testing in Rare Diseases
By Frieda Wiley
While genetic testing may offer great potential for providing answers to patients and clinicians seeking insight into a rare disorder, the technology holds some pros and cons that neurologists should be aware of.
Editor’s Note
By Glenn S. Williams
In this year’s Rare Neurological Disease Special Report, we focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust.
A Note From NORD
By Pamela Gavin
Through NORD’s collaboration with Neurology Reviews, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
Genetic Testing for ALS, Now a Standard, Creates a Path Toward Individualized Care
By Ted Bosworth
Overall, there is a sense of progress in ALS. The hope is that clinical research is reaching a tipping point where targeted treatments may offer hope to patients with ALS.
Myasthenia Gravis: Patient Choice, Cultural Change
By John Jesitus
Used appropriately, newer treatments for myasthenia gravis can provide dramatic results faster and more safely than broad immunosuppressants.
Promise for Disease-Modifying Therapies to Tame Huntington’s Disease
By Neil Osterweil
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
Diagnosing and Managing Duchenne Muscular Dystrophy: Tips for Practicing Clinicians
By Batya Swift Yasgur, MA, LSW
Healthcare providers should be familiar enough with Duchenne muscular dystrophy to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers.
Neuromyelitis Optica: Historically Misdiagnosed — Now Demands Prompt Treatment
By Kate Johnson
Rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury.”
Untangling CIDP
By Jennie Smith
Though a preferred biomarker remains elusive, this difficult-to-diagnose neuropathy has seen important recent advances in diagnosis and treatment.
Newborn Screening Programs: What Do Clinicians Need to Know?
By Batya Swift Yasgur, MA, LSW
The goal of newborn screening is to identify babies with genetic disorders who otherwise have no obvious symptoms.
Balancing Act: Weighing the Pros and Cons of Genetic Testing in Rare Diseases
By Frieda Wiley
While genetic testing may offer great potential for providing answers to patients and clinicians seeking insight into a rare disorder, the technology holds some pros and cons that neurologists should be aware of.