User login
A new way to classify endometrial cancer
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
Marijuana use is affecting the job market
I have a friend who owns a large paving and excavating company. He currently is turning away large contracts because he can’t find employees to drive his dump trucks and operate his heavy machinery. The situation is so dire that he has begun to explore the possibility of recruiting employees out of the corrections system.
Like much of the country, Maine is experiencing a low level of unemployment that few of us over the age of 50 years can recall. Coupled with a confused and unwelcoming immigration policy at the federal level many small and large companies are struggling to find employees. The employment opportunities my friend’s company is offering are well above minimum wage, paying in the $30,000-$70,000 range with benefits. While the jobs require some special skills, his company is large enough that it can provide in-house training.
Maine residents recently have voted to decriminalize the possession of small amounts of marijuana. It is unclear exactly how this change in the official position of the state government will translate into a distribution network and a system of local codes. However, it does reflect a more tolerant attitude toward marijuana use. It also suggests that job seekers who are avoiding positions that require drug testing are not worried about the stigma of being identified as a user. They understand enough pharmacology to know that marijuana is detectable days and even weeks after it was last ingested or inhaled. Even the recreational users realize that the chances of being able to pass a drug test before employment and at any subsequent random testing are slim.
The problem is that these good-paying jobs are going unfilled because of the pharmacologic properties of a drug, and our current inability to devise a test that can accurately and consistently correlate a person’s blood level and his or her ability to safely operate a motor vehicle or piece of heavy equipment (“Establishing legal limit for driving under the influence of marijuana,” Inj Epidemiol. 2014 Dec.;1[1]: 26). There is some correlation between blood levels and whether a person is a heavy or infrequent user. Laws that rely on a zero tolerance philosophy are not bringing us any closer to a solution. And it is probably unrealistic to hope that in the near future scientists will develop a single, simply administered test that can provide a clear yes or no to the issue of impairment in the workplace.
I can envision a two-tier system in which all employees are blood or urine tested on a 3-month schedule. Those with a positive test must then take a 10-minute test on a laptop computer simulator with a joy stick each morning that they arrive on the job to demonstrate that, despite a history of marijuana use, they are not impaired.
Even if such a test is developed, we still owe our patients the reminder that, despite its decriminalization, marijuana is a drug and like any drug has side effects. One of them is that it can put limits on your employment opportunities.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
I have a friend who owns a large paving and excavating company. He currently is turning away large contracts because he can’t find employees to drive his dump trucks and operate his heavy machinery. The situation is so dire that he has begun to explore the possibility of recruiting employees out of the corrections system.
Like much of the country, Maine is experiencing a low level of unemployment that few of us over the age of 50 years can recall. Coupled with a confused and unwelcoming immigration policy at the federal level many small and large companies are struggling to find employees. The employment opportunities my friend’s company is offering are well above minimum wage, paying in the $30,000-$70,000 range with benefits. While the jobs require some special skills, his company is large enough that it can provide in-house training.
Maine residents recently have voted to decriminalize the possession of small amounts of marijuana. It is unclear exactly how this change in the official position of the state government will translate into a distribution network and a system of local codes. However, it does reflect a more tolerant attitude toward marijuana use. It also suggests that job seekers who are avoiding positions that require drug testing are not worried about the stigma of being identified as a user. They understand enough pharmacology to know that marijuana is detectable days and even weeks after it was last ingested or inhaled. Even the recreational users realize that the chances of being able to pass a drug test before employment and at any subsequent random testing are slim.
The problem is that these good-paying jobs are going unfilled because of the pharmacologic properties of a drug, and our current inability to devise a test that can accurately and consistently correlate a person’s blood level and his or her ability to safely operate a motor vehicle or piece of heavy equipment (“Establishing legal limit for driving under the influence of marijuana,” Inj Epidemiol. 2014 Dec.;1[1]: 26). There is some correlation between blood levels and whether a person is a heavy or infrequent user. Laws that rely on a zero tolerance philosophy are not bringing us any closer to a solution. And it is probably unrealistic to hope that in the near future scientists will develop a single, simply administered test that can provide a clear yes or no to the issue of impairment in the workplace.
I can envision a two-tier system in which all employees are blood or urine tested on a 3-month schedule. Those with a positive test must then take a 10-minute test on a laptop computer simulator with a joy stick each morning that they arrive on the job to demonstrate that, despite a history of marijuana use, they are not impaired.
Even if such a test is developed, we still owe our patients the reminder that, despite its decriminalization, marijuana is a drug and like any drug has side effects. One of them is that it can put limits on your employment opportunities.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
I have a friend who owns a large paving and excavating company. He currently is turning away large contracts because he can’t find employees to drive his dump trucks and operate his heavy machinery. The situation is so dire that he has begun to explore the possibility of recruiting employees out of the corrections system.
Like much of the country, Maine is experiencing a low level of unemployment that few of us over the age of 50 years can recall. Coupled with a confused and unwelcoming immigration policy at the federal level many small and large companies are struggling to find employees. The employment opportunities my friend’s company is offering are well above minimum wage, paying in the $30,000-$70,000 range with benefits. While the jobs require some special skills, his company is large enough that it can provide in-house training.
Maine residents recently have voted to decriminalize the possession of small amounts of marijuana. It is unclear exactly how this change in the official position of the state government will translate into a distribution network and a system of local codes. However, it does reflect a more tolerant attitude toward marijuana use. It also suggests that job seekers who are avoiding positions that require drug testing are not worried about the stigma of being identified as a user. They understand enough pharmacology to know that marijuana is detectable days and even weeks after it was last ingested or inhaled. Even the recreational users realize that the chances of being able to pass a drug test before employment and at any subsequent random testing are slim.
The problem is that these good-paying jobs are going unfilled because of the pharmacologic properties of a drug, and our current inability to devise a test that can accurately and consistently correlate a person’s blood level and his or her ability to safely operate a motor vehicle or piece of heavy equipment (“Establishing legal limit for driving under the influence of marijuana,” Inj Epidemiol. 2014 Dec.;1[1]: 26). There is some correlation between blood levels and whether a person is a heavy or infrequent user. Laws that rely on a zero tolerance philosophy are not bringing us any closer to a solution. And it is probably unrealistic to hope that in the near future scientists will develop a single, simply administered test that can provide a clear yes or no to the issue of impairment in the workplace.
I can envision a two-tier system in which all employees are blood or urine tested on a 3-month schedule. Those with a positive test must then take a 10-minute test on a laptop computer simulator with a joy stick each morning that they arrive on the job to demonstrate that, despite a history of marijuana use, they are not impaired.
Even if such a test is developed, we still owe our patients the reminder that, despite its decriminalization, marijuana is a drug and like any drug has side effects. One of them is that it can put limits on your employment opportunities.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Accuracy of Distal Femoral Valgus Deformity Correction: Fixator-Assisted Nailing vs Fixator-Assisted Locked Plating
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
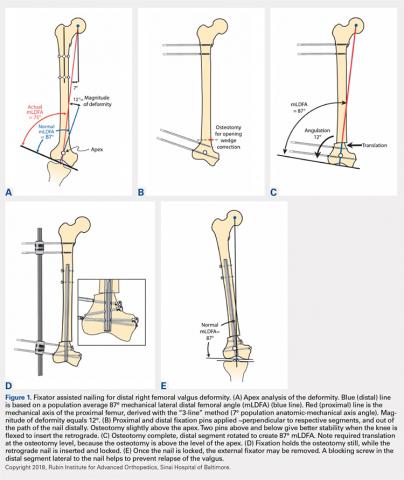
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
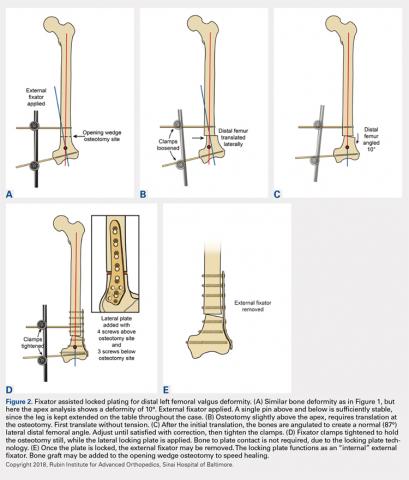
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
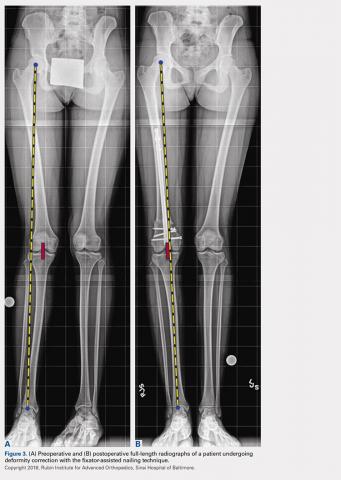
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
TAKE-HOME POINTS
- FAN and FALP are methods to improve the accuracy of long bone deformity correction.
- Both methods include temporary stabilization of the osteotomy with an external fixator.
- FALP is technically easier, since the external fixation pins do not have to be positioned out of the path of the nail, as in FAN.
- Acute corrections in the distal femur from valgus to varus can stretch the peroneal nerve.
- FAN and FALP are equivalent techniques for improving accuracy of deformity correction.
Most U.S. buprenorphine prescribers assess patients for diversion
Most prescribers of buprenorphine view diversion or misappropriation of the drug as a concern, and most assess their patients for diversion, according to Lewei (Allison) Lin, MD, and her associates.
In a survey of 1,174 buprenorphine prescribers conducted from July 2014 to January 2017, 79% of the prescribers reported assessing all their patients for diversion of the drug, and 79.1% reported conducting urine tests for the presence of buprenorphine. In addition, 85% of prescribers viewed diversion as a problem in their community, and 80.3% of prescribers were willing to terminate treatment because of diversion, reported Dr. Lin of the department of psychiatry at the University of Michigan, Ann Arbor, and her associates in Drug and Alcohol Dependence.
Diversion was defined as “unauthorized rerouting or misappropriation of prescribed buprenorphine to someone other than the person for whom it was intended.”
Dr. Lin and her associates found that prescribers were more likely to assess all of their patients for diversion if they viewed diversion as a problem in their community, had fewer years of experience, or were white. Urine testing was more likely if prescribers viewed diversion as a problem in their community, had more patients, had less experience, had a 100-patient waiver, or were nonpsychiatrists. Treatment termination was more likely for prescribers who had a greater percentages of patients with medication counts, viewed diversion as a problem in their community, or practiced in nonaddiction/nonpsychiatric specialties.
“Although we did not include detailed questions about termination, the high proportion of prescribers who would terminate patients for diversion is important to note,” wrote Dr. Lin, also a research investigator at the VA Center for Clinical Management Research, Ann Arbor, and her associates. – which suggests that additional training in addiction might make clinicians more comfortable managing patients with high-risk behaviors, the investigators said.
They cited several limitations. For example, the survey’s cross-sectional design makes causal inferences difficult. In addition, when the survey was conducted, physicians were the only buprenorphine prescribers. It is therefore unclear whether the study results are generalizable to physician assistants or nurse practitioners, who now are able to prescribe buprenorphine after meeting certain requirements.
Still, the study uncovered new information about the steps that prescribers take to mitigate diversion. “Addressing diversion is a complex clinical dilemma and contextual factors, including availability of services, may need to be explored in the future alongside provider practices addressing diversion,” the investigators wrote.
SOURCE: LA Lin et al. Drug Alcohol Depend. 2018 May 1. doi: 10.1016/j.drugalcdep.2018.01.015.
Most prescribers of buprenorphine view diversion or misappropriation of the drug as a concern, and most assess their patients for diversion, according to Lewei (Allison) Lin, MD, and her associates.
In a survey of 1,174 buprenorphine prescribers conducted from July 2014 to January 2017, 79% of the prescribers reported assessing all their patients for diversion of the drug, and 79.1% reported conducting urine tests for the presence of buprenorphine. In addition, 85% of prescribers viewed diversion as a problem in their community, and 80.3% of prescribers were willing to terminate treatment because of diversion, reported Dr. Lin of the department of psychiatry at the University of Michigan, Ann Arbor, and her associates in Drug and Alcohol Dependence.
Diversion was defined as “unauthorized rerouting or misappropriation of prescribed buprenorphine to someone other than the person for whom it was intended.”
Dr. Lin and her associates found that prescribers were more likely to assess all of their patients for diversion if they viewed diversion as a problem in their community, had fewer years of experience, or were white. Urine testing was more likely if prescribers viewed diversion as a problem in their community, had more patients, had less experience, had a 100-patient waiver, or were nonpsychiatrists. Treatment termination was more likely for prescribers who had a greater percentages of patients with medication counts, viewed diversion as a problem in their community, or practiced in nonaddiction/nonpsychiatric specialties.
“Although we did not include detailed questions about termination, the high proportion of prescribers who would terminate patients for diversion is important to note,” wrote Dr. Lin, also a research investigator at the VA Center for Clinical Management Research, Ann Arbor, and her associates. – which suggests that additional training in addiction might make clinicians more comfortable managing patients with high-risk behaviors, the investigators said.
They cited several limitations. For example, the survey’s cross-sectional design makes causal inferences difficult. In addition, when the survey was conducted, physicians were the only buprenorphine prescribers. It is therefore unclear whether the study results are generalizable to physician assistants or nurse practitioners, who now are able to prescribe buprenorphine after meeting certain requirements.
Still, the study uncovered new information about the steps that prescribers take to mitigate diversion. “Addressing diversion is a complex clinical dilemma and contextual factors, including availability of services, may need to be explored in the future alongside provider practices addressing diversion,” the investigators wrote.
SOURCE: LA Lin et al. Drug Alcohol Depend. 2018 May 1. doi: 10.1016/j.drugalcdep.2018.01.015.
Most prescribers of buprenorphine view diversion or misappropriation of the drug as a concern, and most assess their patients for diversion, according to Lewei (Allison) Lin, MD, and her associates.
In a survey of 1,174 buprenorphine prescribers conducted from July 2014 to January 2017, 79% of the prescribers reported assessing all their patients for diversion of the drug, and 79.1% reported conducting urine tests for the presence of buprenorphine. In addition, 85% of prescribers viewed diversion as a problem in their community, and 80.3% of prescribers were willing to terminate treatment because of diversion, reported Dr. Lin of the department of psychiatry at the University of Michigan, Ann Arbor, and her associates in Drug and Alcohol Dependence.
Diversion was defined as “unauthorized rerouting or misappropriation of prescribed buprenorphine to someone other than the person for whom it was intended.”
Dr. Lin and her associates found that prescribers were more likely to assess all of their patients for diversion if they viewed diversion as a problem in their community, had fewer years of experience, or were white. Urine testing was more likely if prescribers viewed diversion as a problem in their community, had more patients, had less experience, had a 100-patient waiver, or were nonpsychiatrists. Treatment termination was more likely for prescribers who had a greater percentages of patients with medication counts, viewed diversion as a problem in their community, or practiced in nonaddiction/nonpsychiatric specialties.
“Although we did not include detailed questions about termination, the high proportion of prescribers who would terminate patients for diversion is important to note,” wrote Dr. Lin, also a research investigator at the VA Center for Clinical Management Research, Ann Arbor, and her associates. – which suggests that additional training in addiction might make clinicians more comfortable managing patients with high-risk behaviors, the investigators said.
They cited several limitations. For example, the survey’s cross-sectional design makes causal inferences difficult. In addition, when the survey was conducted, physicians were the only buprenorphine prescribers. It is therefore unclear whether the study results are generalizable to physician assistants or nurse practitioners, who now are able to prescribe buprenorphine after meeting certain requirements.
Still, the study uncovered new information about the steps that prescribers take to mitigate diversion. “Addressing diversion is a complex clinical dilemma and contextual factors, including availability of services, may need to be explored in the future alongside provider practices addressing diversion,” the investigators wrote.
SOURCE: LA Lin et al. Drug Alcohol Depend. 2018 May 1. doi: 10.1016/j.drugalcdep.2018.01.015.
FROM DRUG AND ALCOHOL DEPENDENCE
Idiopathic Eruptive Macular Pigmentation With Papillomatosis
To the Editor:
A 13-year-old white adolescent girl presented with asymptomatic discrete hyperpigmented papules on the chest, back, arms, and upper legs of 7 months’ duration. The patient otherwise was in good health; her weight and height were on the 40th percentile on growth curves and she had no history of any medications. Treatments for the skin condition prescribed by outside dermatologists included minocycline 75 mg twice daily for 2 months, lactic acid lotion 12% daily, and ketoconazole 400 mg administered twice 1 week apart.
Physical examination revealed more than 50 scattered hyperpigmented papules on the chest, back, arms, and upper legs ranging in size from 2 to 3.5 cm (Figure 1). Stroking of lesions failed to elicit Darier sign. A potassium hydroxide preparation and fungal culture were negative for pathogenic fungal organisms. The plasma insulin level was within reference range. A punch biopsy from the abdomen was obtained and sent for histopathologic examination. Histopathology showed mild hyperkeratosis, subtle papillomatosis, and interanastomosing acanthosis comprising squamoid cells with mild basilar hyperpigmentation (Figure 2). Sparse superficial perivascular lymphocytic infiltrate and increased pigmentation was seen in the basal layer. The dermis showed a few scattered dermal melanophages. A periodic acid–Schiff with diastase stain was negative. Giemsa and Leder stains highlighted a normal number and distribution of mast cells. Based on the histologic findings, the patient was diagnosed with idiopathic eruptive macular pigmentation (IEMP).
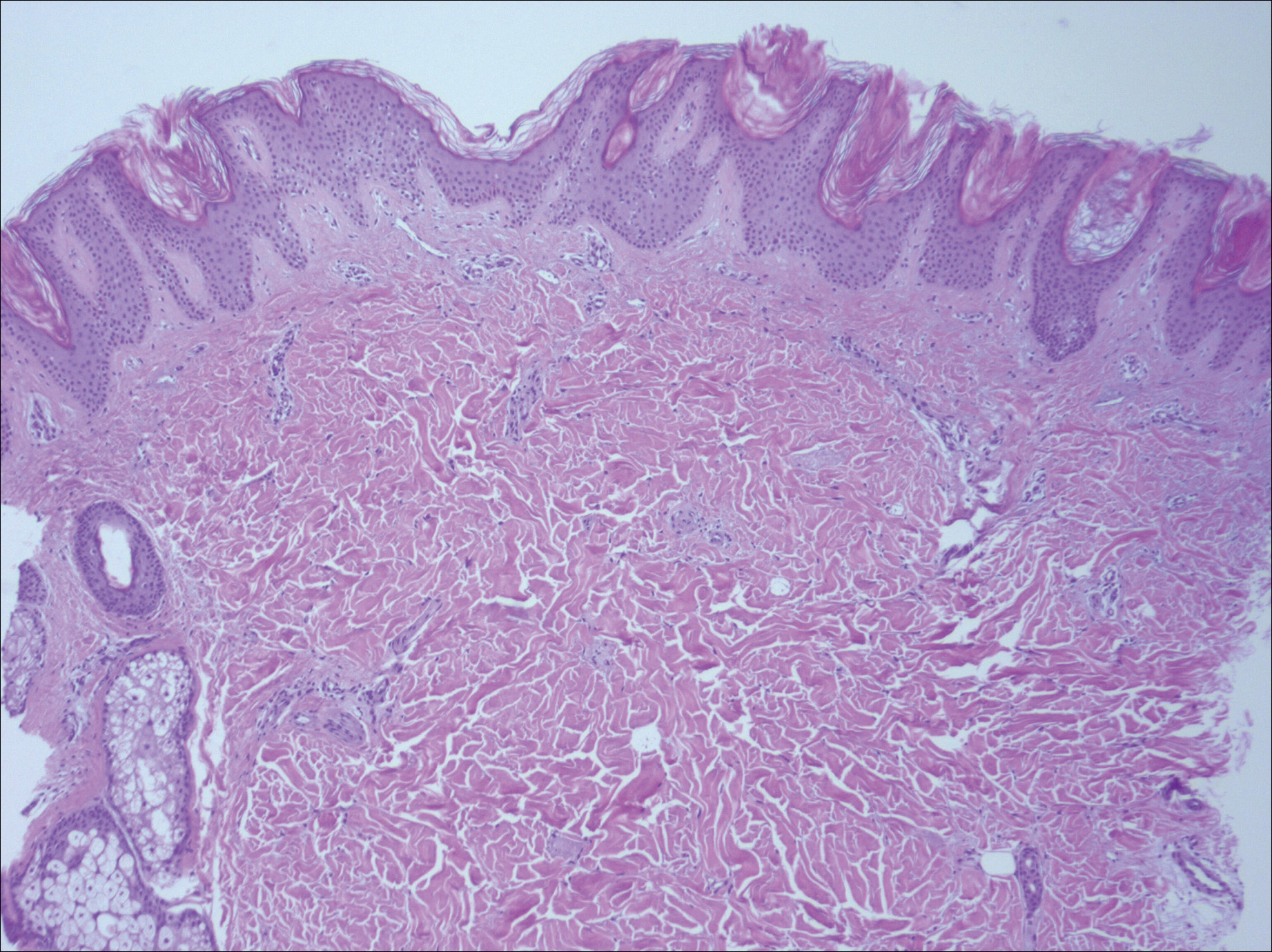
Idiopathic eruptive macular pigmentation is a rare condition that was described in 1978 by Degos et al.1 Sanz de Galdeano et al2 established the following diagnostic criteria: (1) eruption of brownish black, nonconfluent, asymptomatic macules involving the trunk, neck, and proximal arms and legs in children or adolescents; (2) absence of preceding inflammatory lesions; (3) no prior drug exposure; (4) basal cell layer hyperpigmentation of the epidermis and prominent dermal melanophages without visible basal layer damage or lichenoid inflammatory infiltrate; and (5) normal mast cell count.
Idiopathic eruptive macular pigmentation with papillomatosis (IEMPwP) is a variant of IEMP.3 It is undecided if IEMP and IEMPwP are variants of the same entity or distinct conditions. Until a clear etiology of these entities is established, we prefer to separate them on purely morphologic grounds. Marcoux et al4 labeled IEMPwP as a variant of acanthosis nigricans. Although morphologically the 2 conditions appear similar, our patient’s plasma insulin level essentially ruled out acanthosis nigricans.
Idiopathic eruptive macular pigmentation is a rare condition with the majority of cases reported in the Asian population with some reports in white, Hispanic, and black individuals.5 Idiopathic eruptive macular pigmentation with papillomatosis was reported by Joshi3 in 2007 in 9 Indian children with the classic findings of IEMP along with a velvety rash that correlated with papillomatosis. Diagnosis of IEMPwP is important, as the disease generally is self-limited and resolves over the course of a few weeks to a few years.
- Degos R, Civatte J, Belaïch S. Idiopathic eruptive macular pigmentation (author’s transl)[in French]. Ann Dermatol Venereol. 1978;105:177-182.
- Sanz de Galdeano C, Léauté-Labrèze C, Bioulac-Sage P, et al. Idiopathic eruptive macular pigmentation: report of five patients. Pediatr Dermatol. 1996;13:274-277.
- Joshi R. Idiopathic eruptive macular pigmentation with papillomatosis: report of nine cases. Indian J Dermatol Venereol Leprol. 2007;73:402-405.
- Marcoux DA, Durán-McKinster C, Baselga E. Pigmentary abnormalities. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. Philadelphia, PA: Mosby; 2011:700-746.
- Torres-Romero LF, Lisle A, Waxman L. Asymptomatic hyperpigmented macules and patches on the trunk. Am J Dermatopathol. 2015;37:546, 586.
To the Editor:
A 13-year-old white adolescent girl presented with asymptomatic discrete hyperpigmented papules on the chest, back, arms, and upper legs of 7 months’ duration. The patient otherwise was in good health; her weight and height were on the 40th percentile on growth curves and she had no history of any medications. Treatments for the skin condition prescribed by outside dermatologists included minocycline 75 mg twice daily for 2 months, lactic acid lotion 12% daily, and ketoconazole 400 mg administered twice 1 week apart.
Physical examination revealed more than 50 scattered hyperpigmented papules on the chest, back, arms, and upper legs ranging in size from 2 to 3.5 cm (Figure 1). Stroking of lesions failed to elicit Darier sign. A potassium hydroxide preparation and fungal culture were negative for pathogenic fungal organisms. The plasma insulin level was within reference range. A punch biopsy from the abdomen was obtained and sent for histopathologic examination. Histopathology showed mild hyperkeratosis, subtle papillomatosis, and interanastomosing acanthosis comprising squamoid cells with mild basilar hyperpigmentation (Figure 2). Sparse superficial perivascular lymphocytic infiltrate and increased pigmentation was seen in the basal layer. The dermis showed a few scattered dermal melanophages. A periodic acid–Schiff with diastase stain was negative. Giemsa and Leder stains highlighted a normal number and distribution of mast cells. Based on the histologic findings, the patient was diagnosed with idiopathic eruptive macular pigmentation (IEMP).


Idiopathic eruptive macular pigmentation is a rare condition that was described in 1978 by Degos et al.1 Sanz de Galdeano et al2 established the following diagnostic criteria: (1) eruption of brownish black, nonconfluent, asymptomatic macules involving the trunk, neck, and proximal arms and legs in children or adolescents; (2) absence of preceding inflammatory lesions; (3) no prior drug exposure; (4) basal cell layer hyperpigmentation of the epidermis and prominent dermal melanophages without visible basal layer damage or lichenoid inflammatory infiltrate; and (5) normal mast cell count.
Idiopathic eruptive macular pigmentation with papillomatosis (IEMPwP) is a variant of IEMP.3 It is undecided if IEMP and IEMPwP are variants of the same entity or distinct conditions. Until a clear etiology of these entities is established, we prefer to separate them on purely morphologic grounds. Marcoux et al4 labeled IEMPwP as a variant of acanthosis nigricans. Although morphologically the 2 conditions appear similar, our patient’s plasma insulin level essentially ruled out acanthosis nigricans.
Idiopathic eruptive macular pigmentation is a rare condition with the majority of cases reported in the Asian population with some reports in white, Hispanic, and black individuals.5 Idiopathic eruptive macular pigmentation with papillomatosis was reported by Joshi3 in 2007 in 9 Indian children with the classic findings of IEMP along with a velvety rash that correlated with papillomatosis. Diagnosis of IEMPwP is important, as the disease generally is self-limited and resolves over the course of a few weeks to a few years.
To the Editor:
A 13-year-old white adolescent girl presented with asymptomatic discrete hyperpigmented papules on the chest, back, arms, and upper legs of 7 months’ duration. The patient otherwise was in good health; her weight and height were on the 40th percentile on growth curves and she had no history of any medications. Treatments for the skin condition prescribed by outside dermatologists included minocycline 75 mg twice daily for 2 months, lactic acid lotion 12% daily, and ketoconazole 400 mg administered twice 1 week apart.
Physical examination revealed more than 50 scattered hyperpigmented papules on the chest, back, arms, and upper legs ranging in size from 2 to 3.5 cm (Figure 1). Stroking of lesions failed to elicit Darier sign. A potassium hydroxide preparation and fungal culture were negative for pathogenic fungal organisms. The plasma insulin level was within reference range. A punch biopsy from the abdomen was obtained and sent for histopathologic examination. Histopathology showed mild hyperkeratosis, subtle papillomatosis, and interanastomosing acanthosis comprising squamoid cells with mild basilar hyperpigmentation (Figure 2). Sparse superficial perivascular lymphocytic infiltrate and increased pigmentation was seen in the basal layer. The dermis showed a few scattered dermal melanophages. A periodic acid–Schiff with diastase stain was negative. Giemsa and Leder stains highlighted a normal number and distribution of mast cells. Based on the histologic findings, the patient was diagnosed with idiopathic eruptive macular pigmentation (IEMP).


Idiopathic eruptive macular pigmentation is a rare condition that was described in 1978 by Degos et al.1 Sanz de Galdeano et al2 established the following diagnostic criteria: (1) eruption of brownish black, nonconfluent, asymptomatic macules involving the trunk, neck, and proximal arms and legs in children or adolescents; (2) absence of preceding inflammatory lesions; (3) no prior drug exposure; (4) basal cell layer hyperpigmentation of the epidermis and prominent dermal melanophages without visible basal layer damage or lichenoid inflammatory infiltrate; and (5) normal mast cell count.
Idiopathic eruptive macular pigmentation with papillomatosis (IEMPwP) is a variant of IEMP.3 It is undecided if IEMP and IEMPwP are variants of the same entity or distinct conditions. Until a clear etiology of these entities is established, we prefer to separate them on purely morphologic grounds. Marcoux et al4 labeled IEMPwP as a variant of acanthosis nigricans. Although morphologically the 2 conditions appear similar, our patient’s plasma insulin level essentially ruled out acanthosis nigricans.
Idiopathic eruptive macular pigmentation is a rare condition with the majority of cases reported in the Asian population with some reports in white, Hispanic, and black individuals.5 Idiopathic eruptive macular pigmentation with papillomatosis was reported by Joshi3 in 2007 in 9 Indian children with the classic findings of IEMP along with a velvety rash that correlated with papillomatosis. Diagnosis of IEMPwP is important, as the disease generally is self-limited and resolves over the course of a few weeks to a few years.
- Degos R, Civatte J, Belaïch S. Idiopathic eruptive macular pigmentation (author’s transl)[in French]. Ann Dermatol Venereol. 1978;105:177-182.
- Sanz de Galdeano C, Léauté-Labrèze C, Bioulac-Sage P, et al. Idiopathic eruptive macular pigmentation: report of five patients. Pediatr Dermatol. 1996;13:274-277.
- Joshi R. Idiopathic eruptive macular pigmentation with papillomatosis: report of nine cases. Indian J Dermatol Venereol Leprol. 2007;73:402-405.
- Marcoux DA, Durán-McKinster C, Baselga E. Pigmentary abnormalities. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. Philadelphia, PA: Mosby; 2011:700-746.
- Torres-Romero LF, Lisle A, Waxman L. Asymptomatic hyperpigmented macules and patches on the trunk. Am J Dermatopathol. 2015;37:546, 586.
- Degos R, Civatte J, Belaïch S. Idiopathic eruptive macular pigmentation (author’s transl)[in French]. Ann Dermatol Venereol. 1978;105:177-182.
- Sanz de Galdeano C, Léauté-Labrèze C, Bioulac-Sage P, et al. Idiopathic eruptive macular pigmentation: report of five patients. Pediatr Dermatol. 1996;13:274-277.
- Joshi R. Idiopathic eruptive macular pigmentation with papillomatosis: report of nine cases. Indian J Dermatol Venereol Leprol. 2007;73:402-405.
- Marcoux DA, Durán-McKinster C, Baselga E. Pigmentary abnormalities. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. Philadelphia, PA: Mosby; 2011:700-746.
- Torres-Romero LF, Lisle A, Waxman L. Asymptomatic hyperpigmented macules and patches on the trunk. Am J Dermatopathol. 2015;37:546, 586.
Practice Points
- Idiopathic eruptive macular pigmentation with papillomatosis is a rare disorder that most frequently affects children and young adults.
- Idiopathic eruptive macular pigmentation with papillomatosis is characterized by asymptomatic, brownish, hyperpigmented macules involving the neck, trunk, arms, and legs.
- The disorder is important to consider in the differential diagnosis of asymptomatic pigmentary disorders to avoid unnecessary treatment because the disease is self-limiting and resolves over weeks to years.
DOAC’s edge over warfarin fades with low adherence
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Good adherence is needed for direct-acting oral anticoagulants to outperform warfarin.
Major finding: Thromboembolic events in low-adherence patients were 4.05/100 patient years with a DOAC and 3.37/100 patient years with warfarin.
Study details: Analysis of 83,168 insured U.S. atrial fibrillation patients treated with an oral anticoagulant in 2014-2015.
Disclosures: Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
Source: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
Four phase 3 studies highlighted at ASCO mark progress in GI cancers
CHICAGO – Findings from four recent, phase 3 gastrointestinal cancer studies mark a step forward toward “the answers we need” for patients with pancreatic, colorectal, or esophageal cancer, according to Andrew S. Epstein, MD.
In this video interview, Dr. Epstein summarizes and provides context for the findings, which were presented at the annual meeting of the American Society of Clinical Oncology and highlighted during a press briefing there. Dr. Epstein, an ASCO Expert and a medical oncologist at Memorial Sloan Kettering Cancer Center, New York, who was invited to discuss each of the studies at the briefing, said the UNICANCER-sponsored Prodige 7 trial addressed an important, long-unanswered question about the value of hyperthermic intraperitoneal chemotherapy (HIPEC) with surgery for colorectal peritoneal carcinomatosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This randomized study, very importantly, answered that longstanding question and showed us in a less-is-more type of way that the addition of the chemotherapy during surgery actually did not improve the overall survival of these patients,” he said, adding that, at 60 days, HIPEC actually had done more harm than good.
The findings are helpful, as HIPEC has been widely used without a solid data foundation, and now the use of an “additional toxic nonbeneficial treatment” can be avoided in a subset of patients.
Two studies regarding chemotherapy in patients with pancreatic cancer also provided important information about treatment. Preliminary data from one, the PREOPANC-1 trial, suggested that perioperative chemoradiotherapy significantly improves outcomes in resectable and borderline resectable patients, compared with immediate surgery; the other – the Prodige 24/CCTG PA.6 trial – demonstrated that adjuvant mFOLFIRINOX, a four-agent regimen, improved disease-free, metastasis-free, and overall survival, with treated patients living a median of 20 months longer and being cancer free for a median of 9 months longer than those who received gemcitabine therapy.
“We saw a very impressive, encouraging, statistically and clinically significant improvement,” he said regarding survival outcomes in Prodige 24. In patients with good performance status who can tolerate the regimen, mFOLFIRINOX “seems to be the way to go now,” he added, noting that patients receiving the regimen require close monitoring by a medical oncologist.
The fourth study, a prevention trial known as the ASPECT trial, showed that high-dose esomeprazole and low-dose aspirin taken for at least 7 years moderately reduces the risk of high-grade dysplasia and esophageal cancer, and may delay death from any cause in patients with Barrett’s esophagus.
“[It is] obviously of huge importance to be able to prevent a cancer before its onset. ... So with esophagus cancer, which also is a very difficult disease to treat in whatever stage it is, it would be a huge benefit to have a way in which to effectively prevent it,” Dr. Epstein said.
However, more information is needed about the actual benefits in terms of all-cause mortality and the contributors from aspirin versus the proton pump inhibitor versus both, he noted, adding that it is important for the public to know that the findings only apply to those with Barrett’s esophagus and shouldn’t be attempted with over-the-counter treatments as some treatments are associated with complications, and the proton pump inhibitor dose used in this study is not available over the counter.
“So I think it is an intriguing study which needs more clarity and more follow-up, as the author himself said,” he added.
In summing up the findings presented at the briefing, Dr. Epstein said that “collectively we see that the challenge of cancer remains significant and we need high-quality studies like the ones presented today in order to best present ...what the best therapies are for [patients].
“With good sound science like this we continue to inch closer to the answers we need,” he concluded.
Dr. Epstein reported having no disclosures.
CHICAGO – Findings from four recent, phase 3 gastrointestinal cancer studies mark a step forward toward “the answers we need” for patients with pancreatic, colorectal, or esophageal cancer, according to Andrew S. Epstein, MD.
In this video interview, Dr. Epstein summarizes and provides context for the findings, which were presented at the annual meeting of the American Society of Clinical Oncology and highlighted during a press briefing there. Dr. Epstein, an ASCO Expert and a medical oncologist at Memorial Sloan Kettering Cancer Center, New York, who was invited to discuss each of the studies at the briefing, said the UNICANCER-sponsored Prodige 7 trial addressed an important, long-unanswered question about the value of hyperthermic intraperitoneal chemotherapy (HIPEC) with surgery for colorectal peritoneal carcinomatosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This randomized study, very importantly, answered that longstanding question and showed us in a less-is-more type of way that the addition of the chemotherapy during surgery actually did not improve the overall survival of these patients,” he said, adding that, at 60 days, HIPEC actually had done more harm than good.
The findings are helpful, as HIPEC has been widely used without a solid data foundation, and now the use of an “additional toxic nonbeneficial treatment” can be avoided in a subset of patients.
Two studies regarding chemotherapy in patients with pancreatic cancer also provided important information about treatment. Preliminary data from one, the PREOPANC-1 trial, suggested that perioperative chemoradiotherapy significantly improves outcomes in resectable and borderline resectable patients, compared with immediate surgery; the other – the Prodige 24/CCTG PA.6 trial – demonstrated that adjuvant mFOLFIRINOX, a four-agent regimen, improved disease-free, metastasis-free, and overall survival, with treated patients living a median of 20 months longer and being cancer free for a median of 9 months longer than those who received gemcitabine therapy.
“We saw a very impressive, encouraging, statistically and clinically significant improvement,” he said regarding survival outcomes in Prodige 24. In patients with good performance status who can tolerate the regimen, mFOLFIRINOX “seems to be the way to go now,” he added, noting that patients receiving the regimen require close monitoring by a medical oncologist.
The fourth study, a prevention trial known as the ASPECT trial, showed that high-dose esomeprazole and low-dose aspirin taken for at least 7 years moderately reduces the risk of high-grade dysplasia and esophageal cancer, and may delay death from any cause in patients with Barrett’s esophagus.
“[It is] obviously of huge importance to be able to prevent a cancer before its onset. ... So with esophagus cancer, which also is a very difficult disease to treat in whatever stage it is, it would be a huge benefit to have a way in which to effectively prevent it,” Dr. Epstein said.
However, more information is needed about the actual benefits in terms of all-cause mortality and the contributors from aspirin versus the proton pump inhibitor versus both, he noted, adding that it is important for the public to know that the findings only apply to those with Barrett’s esophagus and shouldn’t be attempted with over-the-counter treatments as some treatments are associated with complications, and the proton pump inhibitor dose used in this study is not available over the counter.
“So I think it is an intriguing study which needs more clarity and more follow-up, as the author himself said,” he added.
In summing up the findings presented at the briefing, Dr. Epstein said that “collectively we see that the challenge of cancer remains significant and we need high-quality studies like the ones presented today in order to best present ...what the best therapies are for [patients].
“With good sound science like this we continue to inch closer to the answers we need,” he concluded.
Dr. Epstein reported having no disclosures.
CHICAGO – Findings from four recent, phase 3 gastrointestinal cancer studies mark a step forward toward “the answers we need” for patients with pancreatic, colorectal, or esophageal cancer, according to Andrew S. Epstein, MD.
In this video interview, Dr. Epstein summarizes and provides context for the findings, which were presented at the annual meeting of the American Society of Clinical Oncology and highlighted during a press briefing there. Dr. Epstein, an ASCO Expert and a medical oncologist at Memorial Sloan Kettering Cancer Center, New York, who was invited to discuss each of the studies at the briefing, said the UNICANCER-sponsored Prodige 7 trial addressed an important, long-unanswered question about the value of hyperthermic intraperitoneal chemotherapy (HIPEC) with surgery for colorectal peritoneal carcinomatosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“This randomized study, very importantly, answered that longstanding question and showed us in a less-is-more type of way that the addition of the chemotherapy during surgery actually did not improve the overall survival of these patients,” he said, adding that, at 60 days, HIPEC actually had done more harm than good.
The findings are helpful, as HIPEC has been widely used without a solid data foundation, and now the use of an “additional toxic nonbeneficial treatment” can be avoided in a subset of patients.
Two studies regarding chemotherapy in patients with pancreatic cancer also provided important information about treatment. Preliminary data from one, the PREOPANC-1 trial, suggested that perioperative chemoradiotherapy significantly improves outcomes in resectable and borderline resectable patients, compared with immediate surgery; the other – the Prodige 24/CCTG PA.6 trial – demonstrated that adjuvant mFOLFIRINOX, a four-agent regimen, improved disease-free, metastasis-free, and overall survival, with treated patients living a median of 20 months longer and being cancer free for a median of 9 months longer than those who received gemcitabine therapy.
“We saw a very impressive, encouraging, statistically and clinically significant improvement,” he said regarding survival outcomes in Prodige 24. In patients with good performance status who can tolerate the regimen, mFOLFIRINOX “seems to be the way to go now,” he added, noting that patients receiving the regimen require close monitoring by a medical oncologist.
The fourth study, a prevention trial known as the ASPECT trial, showed that high-dose esomeprazole and low-dose aspirin taken for at least 7 years moderately reduces the risk of high-grade dysplasia and esophageal cancer, and may delay death from any cause in patients with Barrett’s esophagus.
“[It is] obviously of huge importance to be able to prevent a cancer before its onset. ... So with esophagus cancer, which also is a very difficult disease to treat in whatever stage it is, it would be a huge benefit to have a way in which to effectively prevent it,” Dr. Epstein said.
However, more information is needed about the actual benefits in terms of all-cause mortality and the contributors from aspirin versus the proton pump inhibitor versus both, he noted, adding that it is important for the public to know that the findings only apply to those with Barrett’s esophagus and shouldn’t be attempted with over-the-counter treatments as some treatments are associated with complications, and the proton pump inhibitor dose used in this study is not available over the counter.
“So I think it is an intriguing study which needs more clarity and more follow-up, as the author himself said,” he added.
In summing up the findings presented at the briefing, Dr. Epstein said that “collectively we see that the challenge of cancer remains significant and we need high-quality studies like the ones presented today in order to best present ...what the best therapies are for [patients].
“With good sound science like this we continue to inch closer to the answers we need,” he concluded.
Dr. Epstein reported having no disclosures.
EXPERT ANALYSIS FROM ASCO 2018
Vaccine nonmedical exemptions creating metro ‘hotspots’
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
FROM PLOS MEDICINE
Cognitive Behavioral Therapy for Pediatric Migraine
Mind and body cognitive behavioral therapy (CBT‐HA) relaxation skills emerged as popular and effective for pediatric migraine sufferers, based on patient and parent reports in a recent study. Qualitative interviews were conducted with 10 patients and 9 of their parents who had undergone CBT‐HA. Interviews were analyzed using an inductive thematic analysis approach based upon modified grounded theory. Patients were ranged in age from 13 to 17.5 years (median=15.4, standard deviation=1.63) and had undergone CBT‐HA about 1 to 2 years prior to participating in the study. Researchers found:
- Overall, patients and their parents reported that CBT‐HA was helpful in reducing headache frequency and related disability.
- Although patients provided mixed reports on the effectiveness of different CBT‐HA skills, the majority of patients indicated that the mind and body relaxation skills of CBT‐HA (deep breathing, progressive muscle relaxation, and activity pacing in particular) were the most helpful and most frequently used skills.
- Patients and parents also generally reported that treatment was easy to learn, and noted at least some aspect of treatment was enjoyable.
CBT for pediatric migraine: A qualitative study of patient and parent experience. Headache. 2018;58(5):661-675. doi:10.1111/head.13285.
Mind and body cognitive behavioral therapy (CBT‐HA) relaxation skills emerged as popular and effective for pediatric migraine sufferers, based on patient and parent reports in a recent study. Qualitative interviews were conducted with 10 patients and 9 of their parents who had undergone CBT‐HA. Interviews were analyzed using an inductive thematic analysis approach based upon modified grounded theory. Patients were ranged in age from 13 to 17.5 years (median=15.4, standard deviation=1.63) and had undergone CBT‐HA about 1 to 2 years prior to participating in the study. Researchers found:
- Overall, patients and their parents reported that CBT‐HA was helpful in reducing headache frequency and related disability.
- Although patients provided mixed reports on the effectiveness of different CBT‐HA skills, the majority of patients indicated that the mind and body relaxation skills of CBT‐HA (deep breathing, progressive muscle relaxation, and activity pacing in particular) were the most helpful and most frequently used skills.
- Patients and parents also generally reported that treatment was easy to learn, and noted at least some aspect of treatment was enjoyable.
CBT for pediatric migraine: A qualitative study of patient and parent experience. Headache. 2018;58(5):661-675. doi:10.1111/head.13285.
Mind and body cognitive behavioral therapy (CBT‐HA) relaxation skills emerged as popular and effective for pediatric migraine sufferers, based on patient and parent reports in a recent study. Qualitative interviews were conducted with 10 patients and 9 of their parents who had undergone CBT‐HA. Interviews were analyzed using an inductive thematic analysis approach based upon modified grounded theory. Patients were ranged in age from 13 to 17.5 years (median=15.4, standard deviation=1.63) and had undergone CBT‐HA about 1 to 2 years prior to participating in the study. Researchers found:
- Overall, patients and their parents reported that CBT‐HA was helpful in reducing headache frequency and related disability.
- Although patients provided mixed reports on the effectiveness of different CBT‐HA skills, the majority of patients indicated that the mind and body relaxation skills of CBT‐HA (deep breathing, progressive muscle relaxation, and activity pacing in particular) were the most helpful and most frequently used skills.
- Patients and parents also generally reported that treatment was easy to learn, and noted at least some aspect of treatment was enjoyable.
CBT for pediatric migraine: A qualitative study of patient and parent experience. Headache. 2018;58(5):661-675. doi:10.1111/head.13285.
Migraineurs Have Reduced Visual Quality of Life
Visual quality of life (QOL) is significantly adversely affected in migraine sufferers, according to a recent study. In fact, patients with chronic migraine may have visual QOL impacts that are as significant as those associated with other common neuro‐ophthalmic disorders. In this cross‐sectional quantitative survey, visual QOL in individuals with chronic and episodic migraine was assessed using the National Eye Institute Visual Function Questionnaire‐25, and the 10‐item National Eye Institute Visual Function Questionnaire‐25 Neuro‐Ophthalmic Supplement. Overall headache severity and impact was assessed using the Migraine‐specific Quality of Life Questionnaire and the Headache Impact Test‐6. Researchers found:
- Among 29 participants with chronic migraine, vision‐specific QOL scores were all statistically significantly decreased compared to disease‐free controls.
- Among 37 participants with episodic migraine, vision‐specific QOL scores were also decreased compared to disease‐free controls.
- Chronic migraineurs had decreased visual QOL scores compared to those with episodic migraines.
- Participants with chronic migraine had visual QOL scores that were as poor as those previously published for patients with other neuro‐ophthalmic disorders, such as multiple sclerosis, myasthenia gravis, and ischemic optic neuropathy.
Patients with migraine have substantial reductions in measures of visual quality of life. [Published online ahead of print June 7, 2018]. Headache. doi:10.1111/head.13330.
Visual quality of life (QOL) is significantly adversely affected in migraine sufferers, according to a recent study. In fact, patients with chronic migraine may have visual QOL impacts that are as significant as those associated with other common neuro‐ophthalmic disorders. In this cross‐sectional quantitative survey, visual QOL in individuals with chronic and episodic migraine was assessed using the National Eye Institute Visual Function Questionnaire‐25, and the 10‐item National Eye Institute Visual Function Questionnaire‐25 Neuro‐Ophthalmic Supplement. Overall headache severity and impact was assessed using the Migraine‐specific Quality of Life Questionnaire and the Headache Impact Test‐6. Researchers found:
- Among 29 participants with chronic migraine, vision‐specific QOL scores were all statistically significantly decreased compared to disease‐free controls.
- Among 37 participants with episodic migraine, vision‐specific QOL scores were also decreased compared to disease‐free controls.
- Chronic migraineurs had decreased visual QOL scores compared to those with episodic migraines.
- Participants with chronic migraine had visual QOL scores that were as poor as those previously published for patients with other neuro‐ophthalmic disorders, such as multiple sclerosis, myasthenia gravis, and ischemic optic neuropathy.
Patients with migraine have substantial reductions in measures of visual quality of life. [Published online ahead of print June 7, 2018]. Headache. doi:10.1111/head.13330.
Visual quality of life (QOL) is significantly adversely affected in migraine sufferers, according to a recent study. In fact, patients with chronic migraine may have visual QOL impacts that are as significant as those associated with other common neuro‐ophthalmic disorders. In this cross‐sectional quantitative survey, visual QOL in individuals with chronic and episodic migraine was assessed using the National Eye Institute Visual Function Questionnaire‐25, and the 10‐item National Eye Institute Visual Function Questionnaire‐25 Neuro‐Ophthalmic Supplement. Overall headache severity and impact was assessed using the Migraine‐specific Quality of Life Questionnaire and the Headache Impact Test‐6. Researchers found:
- Among 29 participants with chronic migraine, vision‐specific QOL scores were all statistically significantly decreased compared to disease‐free controls.
- Among 37 participants with episodic migraine, vision‐specific QOL scores were also decreased compared to disease‐free controls.
- Chronic migraineurs had decreased visual QOL scores compared to those with episodic migraines.
- Participants with chronic migraine had visual QOL scores that were as poor as those previously published for patients with other neuro‐ophthalmic disorders, such as multiple sclerosis, myasthenia gravis, and ischemic optic neuropathy.
Patients with migraine have substantial reductions in measures of visual quality of life. [Published online ahead of print June 7, 2018]. Headache. doi:10.1111/head.13330.