User login
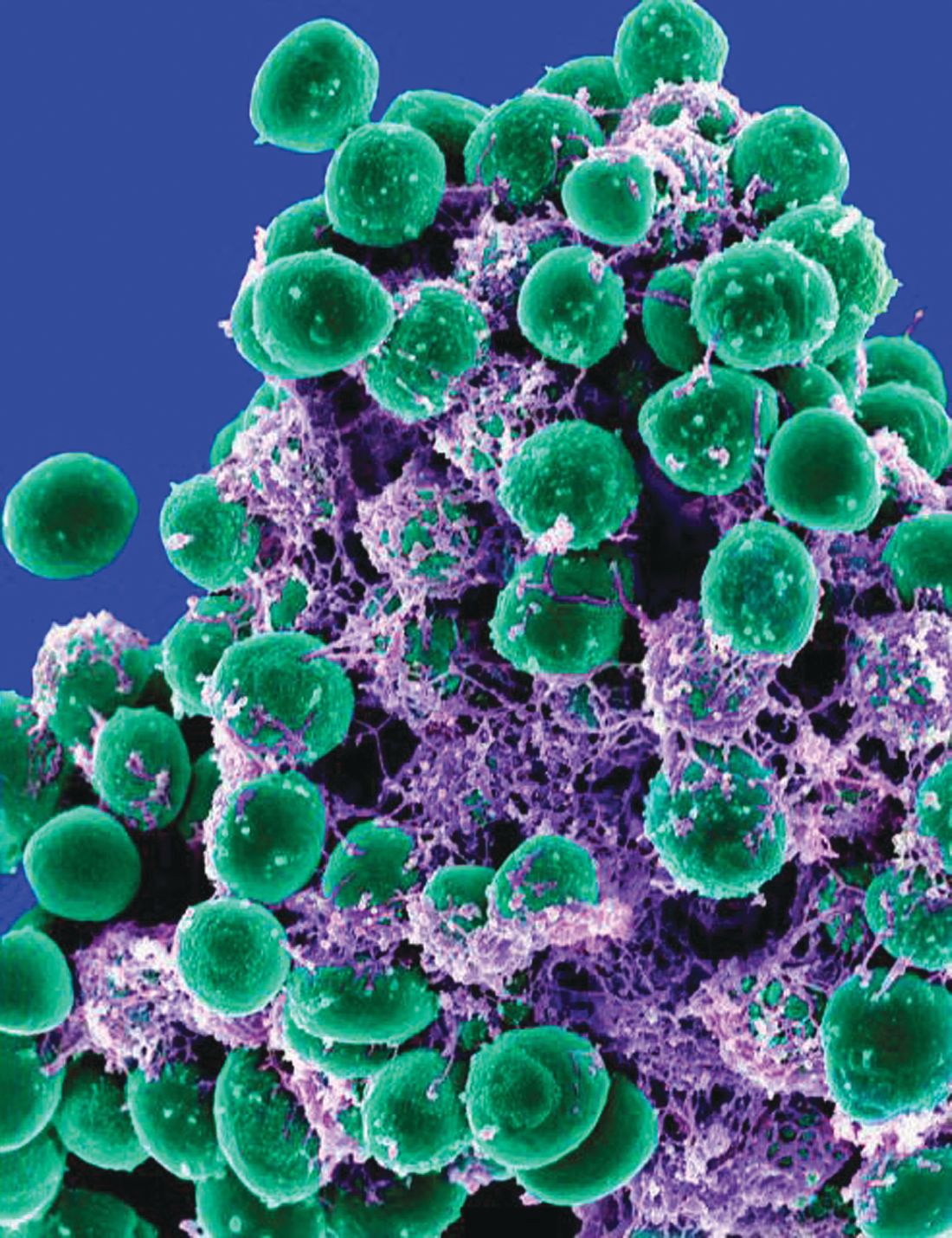
as researchers found all reprocessed bronchoscopes they observed had residual contamination and over half showed microbial growth, according to results from a recent study in CHEST.
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Cori L. Ofstead, MSPH, president and CEO of Ofstead & Associates, and her colleagues wrote in their study. “Shifting toward using sterilized or single-use bronchoscopes could reduce the risk of infection transmission among vulnerable pulmonary patient populations.”
The researchers inspected 24 reprocessed bronchoscopes used clinically (9 pediatric, 9 therapeutic, 6 endobronchial ultrasound) at three U.S. tertiary care centers in 2017 and compared them with two bronchoscopes that had not been used. Of the bronchoscopes observed, all had residual contamination after manual cleaning and high-level disinfection (HLD). Manually cleaned bronchoscopes had microbial growth in 11 of 20 (55%) samples, while 14 of 24 (58%) of HLD samples contained microbial growth. Upon inspection, the researchers said they discovered “oily residue; dried fluid spots; brown, red, and white residue; scratches; insertion tube buckling; and damaged distal ends,” while internal inspections yielded “fluid, discoloration, scratches, filamentous debris, and dented channels.”
Ms. Ofstead and colleagues noted that, while the first site exceeded national guidelines, sites B and C contained technicians who did not wear personal protective equipment and the sites did not follow national or manufacturer use guidelines, such as passing bronchoscopes “through a window to a clean room for automated cleaning and HLD with peracetic acid in automated endoscope reprocessor,” flushing the bronchoscopes with alcohol, drying them with medical-grade forced air pressure, and storing them in a dedicated clean and dry area. Site A had microbial growth in 20% and 50% of manually cleaned and HLD bronchoscopes, respectively, site B had microbial growth in 100% and 75% of manually cleaned and HLD bronchoscopes, respectively, and site C had microbial growth in 83% and 50% of manually cleaned and HLD bronchoscopes, respectively. Among the microbial species identified were “environmental bacteria and normal flora” such as Bacillus spp and Staphylococcus epidermidis, molds such as Lecanicillium lecanii and Verticillium dahliae, and pathogens such as Stenotrophomonas maltophilia and Escherichia coli/Shigella spp.
“The clinical implications for patients are unknown as the study did not involve assessing patients or reviewing medical records,” Ms. Ofstead and her colleagues wrote. “However, the results are worrisome as patients undergoing bronchoscopy are commonly at high risk for infection due to transplant status, critical illness, or immune suppression due to malignancy or chronic disease.”
Ms. Ofstead and three authors are employees of Ofstead & Associates, which received research funding and speaking honoraria from 3M, Advanced Sterilization Products (Johnson & Johnson), Ambu, Auris Health, Boston Scientific, Cogentix, ConvergAscent, Healthmark Industries, Invendo Medical, Medivators, Nanosonics, and STERIS. Dr. Ferguson has received fees from NeuWave Medical and PPD, research grants and personal fees from OncoCyte, and research grants from Concordia and PneumRx. Dr. Sonetti reported no relevant financial disclosures.
SOURCE: Ofstead CL et al. Chest. 2018 May 30. doi: 10.1016/j.chest.2018.04.045.
as researchers found all reprocessed bronchoscopes they observed had residual contamination and over half showed microbial growth, according to results from a recent study in CHEST.
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Cori L. Ofstead, MSPH, president and CEO of Ofstead & Associates, and her colleagues wrote in their study. “Shifting toward using sterilized or single-use bronchoscopes could reduce the risk of infection transmission among vulnerable pulmonary patient populations.”
The researchers inspected 24 reprocessed bronchoscopes used clinically (9 pediatric, 9 therapeutic, 6 endobronchial ultrasound) at three U.S. tertiary care centers in 2017 and compared them with two bronchoscopes that had not been used. Of the bronchoscopes observed, all had residual contamination after manual cleaning and high-level disinfection (HLD). Manually cleaned bronchoscopes had microbial growth in 11 of 20 (55%) samples, while 14 of 24 (58%) of HLD samples contained microbial growth. Upon inspection, the researchers said they discovered “oily residue; dried fluid spots; brown, red, and white residue; scratches; insertion tube buckling; and damaged distal ends,” while internal inspections yielded “fluid, discoloration, scratches, filamentous debris, and dented channels.”
Ms. Ofstead and colleagues noted that, while the first site exceeded national guidelines, sites B and C contained technicians who did not wear personal protective equipment and the sites did not follow national or manufacturer use guidelines, such as passing bronchoscopes “through a window to a clean room for automated cleaning and HLD with peracetic acid in automated endoscope reprocessor,” flushing the bronchoscopes with alcohol, drying them with medical-grade forced air pressure, and storing them in a dedicated clean and dry area. Site A had microbial growth in 20% and 50% of manually cleaned and HLD bronchoscopes, respectively, site B had microbial growth in 100% and 75% of manually cleaned and HLD bronchoscopes, respectively, and site C had microbial growth in 83% and 50% of manually cleaned and HLD bronchoscopes, respectively. Among the microbial species identified were “environmental bacteria and normal flora” such as Bacillus spp and Staphylococcus epidermidis, molds such as Lecanicillium lecanii and Verticillium dahliae, and pathogens such as Stenotrophomonas maltophilia and Escherichia coli/Shigella spp.
“The clinical implications for patients are unknown as the study did not involve assessing patients or reviewing medical records,” Ms. Ofstead and her colleagues wrote. “However, the results are worrisome as patients undergoing bronchoscopy are commonly at high risk for infection due to transplant status, critical illness, or immune suppression due to malignancy or chronic disease.”
Ms. Ofstead and three authors are employees of Ofstead & Associates, which received research funding and speaking honoraria from 3M, Advanced Sterilization Products (Johnson & Johnson), Ambu, Auris Health, Boston Scientific, Cogentix, ConvergAscent, Healthmark Industries, Invendo Medical, Medivators, Nanosonics, and STERIS. Dr. Ferguson has received fees from NeuWave Medical and PPD, research grants and personal fees from OncoCyte, and research grants from Concordia and PneumRx. Dr. Sonetti reported no relevant financial disclosures.
SOURCE: Ofstead CL et al. Chest. 2018 May 30. doi: 10.1016/j.chest.2018.04.045.
as researchers found all reprocessed bronchoscopes they observed had residual contamination and over half showed microbial growth, according to results from a recent study in CHEST.
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Cori L. Ofstead, MSPH, president and CEO of Ofstead & Associates, and her colleagues wrote in their study. “Shifting toward using sterilized or single-use bronchoscopes could reduce the risk of infection transmission among vulnerable pulmonary patient populations.”
The researchers inspected 24 reprocessed bronchoscopes used clinically (9 pediatric, 9 therapeutic, 6 endobronchial ultrasound) at three U.S. tertiary care centers in 2017 and compared them with two bronchoscopes that had not been used. Of the bronchoscopes observed, all had residual contamination after manual cleaning and high-level disinfection (HLD). Manually cleaned bronchoscopes had microbial growth in 11 of 20 (55%) samples, while 14 of 24 (58%) of HLD samples contained microbial growth. Upon inspection, the researchers said they discovered “oily residue; dried fluid spots; brown, red, and white residue; scratches; insertion tube buckling; and damaged distal ends,” while internal inspections yielded “fluid, discoloration, scratches, filamentous debris, and dented channels.”
Ms. Ofstead and colleagues noted that, while the first site exceeded national guidelines, sites B and C contained technicians who did not wear personal protective equipment and the sites did not follow national or manufacturer use guidelines, such as passing bronchoscopes “through a window to a clean room for automated cleaning and HLD with peracetic acid in automated endoscope reprocessor,” flushing the bronchoscopes with alcohol, drying them with medical-grade forced air pressure, and storing them in a dedicated clean and dry area. Site A had microbial growth in 20% and 50% of manually cleaned and HLD bronchoscopes, respectively, site B had microbial growth in 100% and 75% of manually cleaned and HLD bronchoscopes, respectively, and site C had microbial growth in 83% and 50% of manually cleaned and HLD bronchoscopes, respectively. Among the microbial species identified were “environmental bacteria and normal flora” such as Bacillus spp and Staphylococcus epidermidis, molds such as Lecanicillium lecanii and Verticillium dahliae, and pathogens such as Stenotrophomonas maltophilia and Escherichia coli/Shigella spp.
“The clinical implications for patients are unknown as the study did not involve assessing patients or reviewing medical records,” Ms. Ofstead and her colleagues wrote. “However, the results are worrisome as patients undergoing bronchoscopy are commonly at high risk for infection due to transplant status, critical illness, or immune suppression due to malignancy or chronic disease.”
Ms. Ofstead and three authors are employees of Ofstead & Associates, which received research funding and speaking honoraria from 3M, Advanced Sterilization Products (Johnson & Johnson), Ambu, Auris Health, Boston Scientific, Cogentix, ConvergAscent, Healthmark Industries, Invendo Medical, Medivators, Nanosonics, and STERIS. Dr. Ferguson has received fees from NeuWave Medical and PPD, research grants and personal fees from OncoCyte, and research grants from Concordia and PneumRx. Dr. Sonetti reported no relevant financial disclosures.
SOURCE: Ofstead CL et al. Chest. 2018 May 30. doi: 10.1016/j.chest.2018.04.045.
FROM CHEST
Key clinical point: Despite following disinfection guidelines, damaged or contaminated reprocessed bronchoscopes were being used at all study sites.
Major finding: All reprocessed bronchoscopes observed had residual contamination, while 58% that underwent high-level disinfection contained microbial growth.
Data source: A prospective study of 24 bronchoscopes from three large U.S. tertiary care hospitals in 2017.
Disclosures: Ms. Ofstead and three authors are employees of Ofstead & Associates, which received research funding and speaking honoraria from 3M, Advanced Sterilization Products (Johnson & Johnson), Ambu, Auris Health, Boston Scientific, Cogentix, ConvergAscent, Healthmark Industries, Invendo Medical, Medivators, Nanosonics, and STERIS. Dr. Ferguson has received fees from NeuWave Medical and PPD, research grants and personal fees from OncoCyte, and research grants from Concordia and PneumRx. Dr. Sonetti reported no relevant financial disclosures.
Source: Ofstead CL et al. Chest. 2018 May 30. doi: 10.1016/j.chest.2018.04.045.