User login
Orphan designation recommended for PCM-075
The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has recommended that PCM-075 receive orphan drug designation as a treatment for acute myeloid leukemia (AML).
PCM-075 is an oral adenosine triphosphate competitive inhibitor of the serine/threonine Polo-like kinase 1 (PLK1) enzyme, which is overexpressed in hematologic and solid tumor malignancies.
The COMP’s recommendation for PCM-075 is expected to be adopted by the European Commission at the end of this month.
Orphan drug designation in Europe is available to companies developing products intended to treat a life-threatening or chronically debilitating condition that affects fewer than 5 in 10,000 people in the European Union (EU).
The designation allows for financial and regulatory incentives that include 10 years of marketing exclusivity in the EU after product approval, eligibility for conditional marketing authorization, protocol assistance from the European Medicines Agency at reduced fees during the product development phase, and direct access to centralized marketing authorization in the EU.
PCM-075 research
PCM-075 only targets the PLK1 isoform (not PLK2 or PLK3) and has a 24-hour drug half-life with reversible, on-target hematologic toxicities, according to Trovagene, Inc., the company developing PCM-075.
Trovagene believes that PCM-075’s reversible, on-target activity, combined with an improved dose/scheduling protocol, could mean that PCM-075 will improve upon long-term outcomes observed in previous studies with a PLK inhibitor in AML.
This includes a phase 2 study in which AML patients who received a PLK inhibitor plus low-dose cytarabine (LDAC) had a higher response rate than patients who received LDAC alone—31% and 13.3%, respectively.
Trovagene said preclinical studies have shown that PCM-075 synergizes with more than 10 drugs used to treat hematologic and solid tumor malignancies. This includes FLT3 and HDAC inhibitors, taxanes, and cytotoxins.
Trovagene is now conducting a phase 1b/2 trial of PCM-075 in combination with standard care (LDAC or decitabine) in patients with AML (NCT03303339).
The company has already completed a phase 1 dose-escalation study of PCM-075 in patients with advanced metastatic solid tumor malignancies. Results from this study were published in Investigational New Drugs.
The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has recommended that PCM-075 receive orphan drug designation as a treatment for acute myeloid leukemia (AML).
PCM-075 is an oral adenosine triphosphate competitive inhibitor of the serine/threonine Polo-like kinase 1 (PLK1) enzyme, which is overexpressed in hematologic and solid tumor malignancies.
The COMP’s recommendation for PCM-075 is expected to be adopted by the European Commission at the end of this month.
Orphan drug designation in Europe is available to companies developing products intended to treat a life-threatening or chronically debilitating condition that affects fewer than 5 in 10,000 people in the European Union (EU).
The designation allows for financial and regulatory incentives that include 10 years of marketing exclusivity in the EU after product approval, eligibility for conditional marketing authorization, protocol assistance from the European Medicines Agency at reduced fees during the product development phase, and direct access to centralized marketing authorization in the EU.
PCM-075 research
PCM-075 only targets the PLK1 isoform (not PLK2 or PLK3) and has a 24-hour drug half-life with reversible, on-target hematologic toxicities, according to Trovagene, Inc., the company developing PCM-075.
Trovagene believes that PCM-075’s reversible, on-target activity, combined with an improved dose/scheduling protocol, could mean that PCM-075 will improve upon long-term outcomes observed in previous studies with a PLK inhibitor in AML.
This includes a phase 2 study in which AML patients who received a PLK inhibitor plus low-dose cytarabine (LDAC) had a higher response rate than patients who received LDAC alone—31% and 13.3%, respectively.
Trovagene said preclinical studies have shown that PCM-075 synergizes with more than 10 drugs used to treat hematologic and solid tumor malignancies. This includes FLT3 and HDAC inhibitors, taxanes, and cytotoxins.
Trovagene is now conducting a phase 1b/2 trial of PCM-075 in combination with standard care (LDAC or decitabine) in patients with AML (NCT03303339).
The company has already completed a phase 1 dose-escalation study of PCM-075 in patients with advanced metastatic solid tumor malignancies. Results from this study were published in Investigational New Drugs.
The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has recommended that PCM-075 receive orphan drug designation as a treatment for acute myeloid leukemia (AML).
PCM-075 is an oral adenosine triphosphate competitive inhibitor of the serine/threonine Polo-like kinase 1 (PLK1) enzyme, which is overexpressed in hematologic and solid tumor malignancies.
The COMP’s recommendation for PCM-075 is expected to be adopted by the European Commission at the end of this month.
Orphan drug designation in Europe is available to companies developing products intended to treat a life-threatening or chronically debilitating condition that affects fewer than 5 in 10,000 people in the European Union (EU).
The designation allows for financial and regulatory incentives that include 10 years of marketing exclusivity in the EU after product approval, eligibility for conditional marketing authorization, protocol assistance from the European Medicines Agency at reduced fees during the product development phase, and direct access to centralized marketing authorization in the EU.
PCM-075 research
PCM-075 only targets the PLK1 isoform (not PLK2 or PLK3) and has a 24-hour drug half-life with reversible, on-target hematologic toxicities, according to Trovagene, Inc., the company developing PCM-075.
Trovagene believes that PCM-075’s reversible, on-target activity, combined with an improved dose/scheduling protocol, could mean that PCM-075 will improve upon long-term outcomes observed in previous studies with a PLK inhibitor in AML.
This includes a phase 2 study in which AML patients who received a PLK inhibitor plus low-dose cytarabine (LDAC) had a higher response rate than patients who received LDAC alone—31% and 13.3%, respectively.
Trovagene said preclinical studies have shown that PCM-075 synergizes with more than 10 drugs used to treat hematologic and solid tumor malignancies. This includes FLT3 and HDAC inhibitors, taxanes, and cytotoxins.
Trovagene is now conducting a phase 1b/2 trial of PCM-075 in combination with standard care (LDAC or decitabine) in patients with AML (NCT03303339).
The company has already completed a phase 1 dose-escalation study of PCM-075 in patients with advanced metastatic solid tumor malignancies. Results from this study were published in Investigational New Drugs.
Conditional OS estimates show upfront TKI benefit in mRCC
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Conditional overall survival estimates may help clinicians adjust surveillance planning in patients with mRCC.
Major finding: At all survival times after the start of TKI therapy, conditional overall survival gradually increased when compared with baseline survival estimates.
Study details: Retrospective review of records on 1,131 patients with mRCC.
Disclosures: The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
Source: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
How Does Migraine Affect a Patient’s Relationships?
Compared with episodic migraine, chronic migraine is more likely to have detrimental effects on family life.
SAN FRANCISCO—Patients with chronic migraine are significantly more likely than those with episodic migraine to report that headaches contribute to relationship problems and have a detrimental effect on family life, researchers said at the 60th Annual Scientific Meeting of the American Headache Society. Negative effects on family life include a delay in having children and having fewer children.
Migraine can detract from many aspects of family life and affect all members of the patient’s family. Dawn C. Buse, PhD, Clinical Professor of Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to understand the effects of episodic and chronic migraine on a person’s relationships and family life.
An Analysis of CaMEO Data
The CaMEO study is a prospective, longitudinal, web-based survey designed to characterize the impact of migraine, among other objectives, in a systematic US sample of people meeting modified ICHD-2R criteria. A total of 19,891 respondents met study criteria, including criteria for migraine, and were invited to complete the Family Burden Module (FBM), which assessed the impact, perception, and emotions related to living with migraine among people with migraine and their family members.
Dr. Buse and colleagues presented the results of migraineurs’ responses to the FBM regarding relationships with spouses or significant others and relationships with children living at home. The investigators stratified migraineurs by episodic migraine (ie, those with fewer than 14 headache days per month) and chronic migraine (ie, those with 15 or more headache days per month). Migraineurs were asked about their current relationship status (ie, in a current relationship or not, living together or not). Questions evaluated how headaches had affected past or current relationships with Likert-type response options that corresponded to degrees of disagreement or agreement. Dr. Buse’s group analyzed items by relationship status, episodic or chronic migraine status, and gender.
Women and Men Were Affected Similarly
In all, 13,064 respondents provided valid data. Of this population, 11,938 (91.4%) had episodic migraine and 1,126 (8.6%) had chronic migraine. Of those not currently in a relationship (n = 3,189), respondents with chronic migraine were significantly more likely to indicate that headaches had contributed to relationship problems (37.0%), compared with patients with episodic migraine (15.0%). Of those in a relationship but not living together (n = 1,323), 43.9% of respondents with chronic migraine indicated that headaches were causing relationship concerns or were preventing a closer relationship, such as moving in together or getting married, compared with 15.8% of patients with episodic migraine. The responses were similar among men (18.0%) and women (17.8%).
About 47% of respondents with chronic migraine reported that headaches have caused one or more previous relationship to end or have other problems, compared with 18.2% of respondents with episodic migraine. Headache contributions to relationship problems (ie, breakup or other difficulties) were similar among men (20.6%) and women (19.9%).
Of those in a relationship and living together (n = 8,127), 78.2% of respondents with chronic migraine agreed that they would be a better partner if they did not have headaches, compared with 46.2% of respondents with episodic migraine. Furthermore, 9.6% of patients with chronic migraine had delayed having children or had fewer children, compared with 2.6% of patients with episodic migraine. The researchers observed no differences between men and women.
Compared with episodic migraine, chronic migraine is more likely to have detrimental effects on family life.
Compared with episodic migraine, chronic migraine is more likely to have detrimental effects on family life.
SAN FRANCISCO—Patients with chronic migraine are significantly more likely than those with episodic migraine to report that headaches contribute to relationship problems and have a detrimental effect on family life, researchers said at the 60th Annual Scientific Meeting of the American Headache Society. Negative effects on family life include a delay in having children and having fewer children.
Migraine can detract from many aspects of family life and affect all members of the patient’s family. Dawn C. Buse, PhD, Clinical Professor of Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to understand the effects of episodic and chronic migraine on a person’s relationships and family life.
An Analysis of CaMEO Data
The CaMEO study is a prospective, longitudinal, web-based survey designed to characterize the impact of migraine, among other objectives, in a systematic US sample of people meeting modified ICHD-2R criteria. A total of 19,891 respondents met study criteria, including criteria for migraine, and were invited to complete the Family Burden Module (FBM), which assessed the impact, perception, and emotions related to living with migraine among people with migraine and their family members.
Dr. Buse and colleagues presented the results of migraineurs’ responses to the FBM regarding relationships with spouses or significant others and relationships with children living at home. The investigators stratified migraineurs by episodic migraine (ie, those with fewer than 14 headache days per month) and chronic migraine (ie, those with 15 or more headache days per month). Migraineurs were asked about their current relationship status (ie, in a current relationship or not, living together or not). Questions evaluated how headaches had affected past or current relationships with Likert-type response options that corresponded to degrees of disagreement or agreement. Dr. Buse’s group analyzed items by relationship status, episodic or chronic migraine status, and gender.
Women and Men Were Affected Similarly
In all, 13,064 respondents provided valid data. Of this population, 11,938 (91.4%) had episodic migraine and 1,126 (8.6%) had chronic migraine. Of those not currently in a relationship (n = 3,189), respondents with chronic migraine were significantly more likely to indicate that headaches had contributed to relationship problems (37.0%), compared with patients with episodic migraine (15.0%). Of those in a relationship but not living together (n = 1,323), 43.9% of respondents with chronic migraine indicated that headaches were causing relationship concerns or were preventing a closer relationship, such as moving in together or getting married, compared with 15.8% of patients with episodic migraine. The responses were similar among men (18.0%) and women (17.8%).
About 47% of respondents with chronic migraine reported that headaches have caused one or more previous relationship to end or have other problems, compared with 18.2% of respondents with episodic migraine. Headache contributions to relationship problems (ie, breakup or other difficulties) were similar among men (20.6%) and women (19.9%).
Of those in a relationship and living together (n = 8,127), 78.2% of respondents with chronic migraine agreed that they would be a better partner if they did not have headaches, compared with 46.2% of respondents with episodic migraine. Furthermore, 9.6% of patients with chronic migraine had delayed having children or had fewer children, compared with 2.6% of patients with episodic migraine. The researchers observed no differences between men and women.
SAN FRANCISCO—Patients with chronic migraine are significantly more likely than those with episodic migraine to report that headaches contribute to relationship problems and have a detrimental effect on family life, researchers said at the 60th Annual Scientific Meeting of the American Headache Society. Negative effects on family life include a delay in having children and having fewer children.
Migraine can detract from many aspects of family life and affect all members of the patient’s family. Dawn C. Buse, PhD, Clinical Professor of Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to understand the effects of episodic and chronic migraine on a person’s relationships and family life.
An Analysis of CaMEO Data
The CaMEO study is a prospective, longitudinal, web-based survey designed to characterize the impact of migraine, among other objectives, in a systematic US sample of people meeting modified ICHD-2R criteria. A total of 19,891 respondents met study criteria, including criteria for migraine, and were invited to complete the Family Burden Module (FBM), which assessed the impact, perception, and emotions related to living with migraine among people with migraine and their family members.
Dr. Buse and colleagues presented the results of migraineurs’ responses to the FBM regarding relationships with spouses or significant others and relationships with children living at home. The investigators stratified migraineurs by episodic migraine (ie, those with fewer than 14 headache days per month) and chronic migraine (ie, those with 15 or more headache days per month). Migraineurs were asked about their current relationship status (ie, in a current relationship or not, living together or not). Questions evaluated how headaches had affected past or current relationships with Likert-type response options that corresponded to degrees of disagreement or agreement. Dr. Buse’s group analyzed items by relationship status, episodic or chronic migraine status, and gender.
Women and Men Were Affected Similarly
In all, 13,064 respondents provided valid data. Of this population, 11,938 (91.4%) had episodic migraine and 1,126 (8.6%) had chronic migraine. Of those not currently in a relationship (n = 3,189), respondents with chronic migraine were significantly more likely to indicate that headaches had contributed to relationship problems (37.0%), compared with patients with episodic migraine (15.0%). Of those in a relationship but not living together (n = 1,323), 43.9% of respondents with chronic migraine indicated that headaches were causing relationship concerns or were preventing a closer relationship, such as moving in together or getting married, compared with 15.8% of patients with episodic migraine. The responses were similar among men (18.0%) and women (17.8%).
About 47% of respondents with chronic migraine reported that headaches have caused one or more previous relationship to end or have other problems, compared with 18.2% of respondents with episodic migraine. Headache contributions to relationship problems (ie, breakup or other difficulties) were similar among men (20.6%) and women (19.9%).
Of those in a relationship and living together (n = 8,127), 78.2% of respondents with chronic migraine agreed that they would be a better partner if they did not have headaches, compared with 46.2% of respondents with episodic migraine. Furthermore, 9.6% of patients with chronic migraine had delayed having children or had fewer children, compared with 2.6% of patients with episodic migraine. The researchers observed no differences between men and women.
Are Posttraumatic Headaches Different Than Nontraumatic Headaches?
Over time, headache frequency diminished in those with mild traumatic brain injury and posttraumatic headache.
SAN FRANCISCO—Among a cohort of recently deployed soldiers, headaches were frequent but were more severe, frequent, and migrainous if associated with concussion, according to a report presented at the 60th Annual Scientific Meeting of the American Headache Society. At one-year follow-up, headache frequency had decreased in soldiers with posttraumatic headache (PTH) but remained higher in this group than in those whose headaches were presumed to be unrelated to head injury, said Ann I. Scher, PhD, Director and Professor of Preventive Medicine and Biostatistics at the Uniformed Services University of the Health Sciences in Bethesda, Maryland, and colleagues.
“There are limited data on the phenotypic differences between headaches related to mild traumatic brain injury and ‘regular’ headaches,” Dr. Scher said. “A better understanding of the posttraumatic headache phenotype will inform the design of interventional studies for this difficult to treat population.”
Dr. Scher and colleagues designed a study to compare headache features and one-year prognosis in a cohort of recently deployed soldiers with and without a recent history of a deployment-related mild traumatic brain injury (ie, concussion).
In all, 1,567 soldiers were randomly recruited at Fort Bragg, North Carolina, and Fort Carson, Colorado, within a few days of return from Iraq or Afghanistan. Soldiers with mild traumatic brain injury (ie, cases) and controls were identified based on whether they reported sustaining a mild traumatic brain injury during their most recent deployment. Participants completed a detailed self-administered headache questionnaire. Cases who reported having headaches that started or worsened after a head injury were defined as cases with PTH, and all other cases were defined as cases without PTH. Headache and migraine features assessed were unilateral location, photophobia, phonophobia, nausea, exacerbation, pulsatility, visual aura, sensory aura, pain level, frequency, and allodynia. Headaches were assessed again at three months and 12 months.
Soldiers were primarily young men (mean age, 27; 92% male). Most controls (64%) and mild traumatic brain injury cases (80%) reported having headaches in the past year. Among those with headaches, daily or continuous headache was reported by 5% of controls, 7% of cases without PTH, and 24% of cases with PTH. All headache and migraine features were less common in controls than in cases and less common in cases without PTH than in cases with PTH. Finally, cases without PTH and controls had a similar prevalence of most headache and migraine features, with the exceptions of sensory aura and headache frequency.
At three months, mean annualized headache frequency decreased by about 20 days among cases with PTH but remained unchanged in the other groups. Results were similar at 12 months. Baseline visual or sensory aura and pulsatility were positive prognostic factors associated with reduced headache frequency at 12 months. Baseline headache pain was a negative prognostic factor.
Over time, headache frequency diminished in those with mild traumatic brain injury and posttraumatic headache.
Over time, headache frequency diminished in those with mild traumatic brain injury and posttraumatic headache.
SAN FRANCISCO—Among a cohort of recently deployed soldiers, headaches were frequent but were more severe, frequent, and migrainous if associated with concussion, according to a report presented at the 60th Annual Scientific Meeting of the American Headache Society. At one-year follow-up, headache frequency had decreased in soldiers with posttraumatic headache (PTH) but remained higher in this group than in those whose headaches were presumed to be unrelated to head injury, said Ann I. Scher, PhD, Director and Professor of Preventive Medicine and Biostatistics at the Uniformed Services University of the Health Sciences in Bethesda, Maryland, and colleagues.
“There are limited data on the phenotypic differences between headaches related to mild traumatic brain injury and ‘regular’ headaches,” Dr. Scher said. “A better understanding of the posttraumatic headache phenotype will inform the design of interventional studies for this difficult to treat population.”
Dr. Scher and colleagues designed a study to compare headache features and one-year prognosis in a cohort of recently deployed soldiers with and without a recent history of a deployment-related mild traumatic brain injury (ie, concussion).
In all, 1,567 soldiers were randomly recruited at Fort Bragg, North Carolina, and Fort Carson, Colorado, within a few days of return from Iraq or Afghanistan. Soldiers with mild traumatic brain injury (ie, cases) and controls were identified based on whether they reported sustaining a mild traumatic brain injury during their most recent deployment. Participants completed a detailed self-administered headache questionnaire. Cases who reported having headaches that started or worsened after a head injury were defined as cases with PTH, and all other cases were defined as cases without PTH. Headache and migraine features assessed were unilateral location, photophobia, phonophobia, nausea, exacerbation, pulsatility, visual aura, sensory aura, pain level, frequency, and allodynia. Headaches were assessed again at three months and 12 months.
Soldiers were primarily young men (mean age, 27; 92% male). Most controls (64%) and mild traumatic brain injury cases (80%) reported having headaches in the past year. Among those with headaches, daily or continuous headache was reported by 5% of controls, 7% of cases without PTH, and 24% of cases with PTH. All headache and migraine features were less common in controls than in cases and less common in cases without PTH than in cases with PTH. Finally, cases without PTH and controls had a similar prevalence of most headache and migraine features, with the exceptions of sensory aura and headache frequency.
At three months, mean annualized headache frequency decreased by about 20 days among cases with PTH but remained unchanged in the other groups. Results were similar at 12 months. Baseline visual or sensory aura and pulsatility were positive prognostic factors associated with reduced headache frequency at 12 months. Baseline headache pain was a negative prognostic factor.
SAN FRANCISCO—Among a cohort of recently deployed soldiers, headaches were frequent but were more severe, frequent, and migrainous if associated with concussion, according to a report presented at the 60th Annual Scientific Meeting of the American Headache Society. At one-year follow-up, headache frequency had decreased in soldiers with posttraumatic headache (PTH) but remained higher in this group than in those whose headaches were presumed to be unrelated to head injury, said Ann I. Scher, PhD, Director and Professor of Preventive Medicine and Biostatistics at the Uniformed Services University of the Health Sciences in Bethesda, Maryland, and colleagues.
“There are limited data on the phenotypic differences between headaches related to mild traumatic brain injury and ‘regular’ headaches,” Dr. Scher said. “A better understanding of the posttraumatic headache phenotype will inform the design of interventional studies for this difficult to treat population.”
Dr. Scher and colleagues designed a study to compare headache features and one-year prognosis in a cohort of recently deployed soldiers with and without a recent history of a deployment-related mild traumatic brain injury (ie, concussion).
In all, 1,567 soldiers were randomly recruited at Fort Bragg, North Carolina, and Fort Carson, Colorado, within a few days of return from Iraq or Afghanistan. Soldiers with mild traumatic brain injury (ie, cases) and controls were identified based on whether they reported sustaining a mild traumatic brain injury during their most recent deployment. Participants completed a detailed self-administered headache questionnaire. Cases who reported having headaches that started or worsened after a head injury were defined as cases with PTH, and all other cases were defined as cases without PTH. Headache and migraine features assessed were unilateral location, photophobia, phonophobia, nausea, exacerbation, pulsatility, visual aura, sensory aura, pain level, frequency, and allodynia. Headaches were assessed again at three months and 12 months.
Soldiers were primarily young men (mean age, 27; 92% male). Most controls (64%) and mild traumatic brain injury cases (80%) reported having headaches in the past year. Among those with headaches, daily or continuous headache was reported by 5% of controls, 7% of cases without PTH, and 24% of cases with PTH. All headache and migraine features were less common in controls than in cases and less common in cases without PTH than in cases with PTH. Finally, cases without PTH and controls had a similar prevalence of most headache and migraine features, with the exceptions of sensory aura and headache frequency.
At three months, mean annualized headache frequency decreased by about 20 days among cases with PTH but remained unchanged in the other groups. Results were similar at 12 months. Baseline visual or sensory aura and pulsatility were positive prognostic factors associated with reduced headache frequency at 12 months. Baseline headache pain was a negative prognostic factor.
Osteochondritis Dissecans Lesion of the Radial Head
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
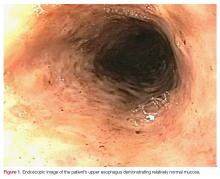
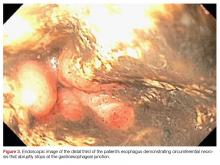
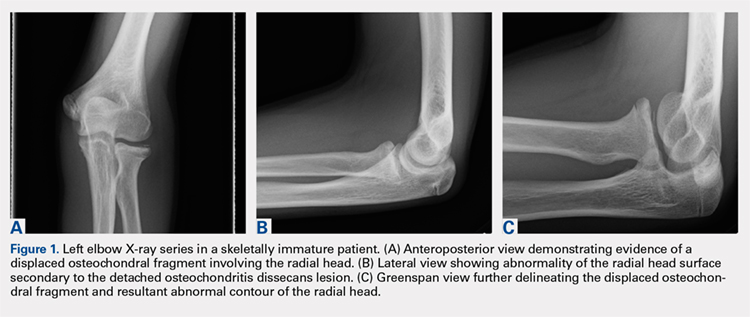
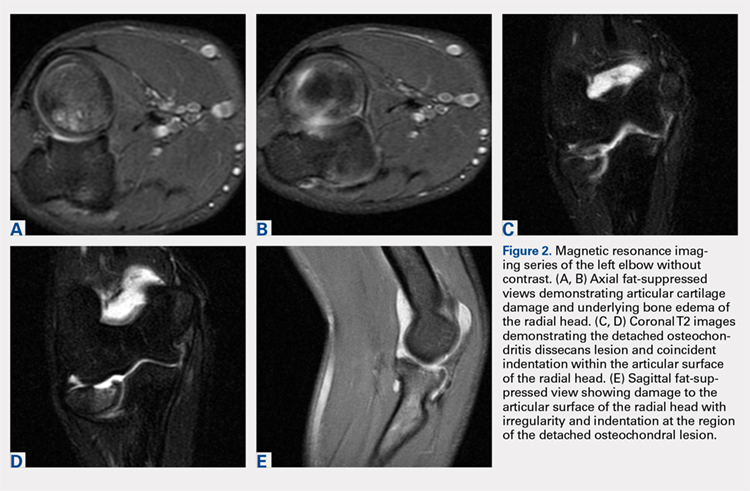
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).
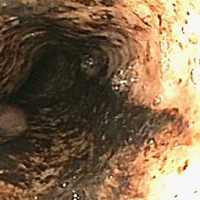
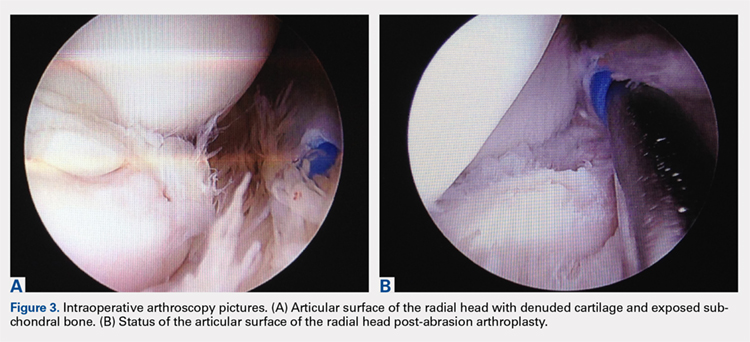
Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).
The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
TAKE-HOME POINTS
- Radial Head OCD lesions are uncommon.
- Typically present in athletes that engage in repetitive trauma to elbow (throwers, gymnasts).
- MRI is the best modality for making diagnosis.
- Attempt nonsurgical treatment initially, especially in skeletally immature patients.
- If nonsurgical fails or there is an unstable lesion, consider operative intervention.
Are Psychiatric Symptoms a Prodrome of Parkinson’s Disease?
When present, anxiety and depressive disorders preceded Parkinson’s disease diagnosis by approximately two decades, on average.
MIAMI—Anxiety and depressive disorders may precede Parkinson’s disease diagnosis in more than half of patients—anxiety by 25 years and depression by 17 years, on average, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The data suggest
“Anxiety, depression, impulse control disorders, irritability, mania, psychosis, and cognitive deficits are the most common psychiatric features of Parkinson’s disease,” said Andreea L. Seritan, MD, Professor of Psychiatry at the University of California, San Francisco (UCSF), and colleagues. Previous research has indicated that anxiety and depression increase the risk of Parkinson’s disease.
A Retrospective Chart Review
The researchers analyzed data from all 108 patients with Parkinson’s disease seen between October 2015 and January 2018.
Psychiatric diagnoses and onset ages were established through clinical interview, using DSM-V criteria. Researchers used neurologists’ notes to identify age of Parkinson’s disease diagnosis. When exact onset ages were not available, the researchers imputed missing onset ages by decade of life.
Most Patients Had a Lifetime Prevalence of Depressive Disorders
Of the 108 participants, 33.3% were women, and the mean age was 63.7. In all, 67% of patients had a lifetime prevalence of anxiety disorders, and 87% of participants had a lifetime prevalence of depressive disorders.
Psychiatric symptoms preceded Parkinson’s disease diagnosis in 48 (72%) patients with anxiety disorders and in 58 (68%) of those with depressive disorders. “Both anxiety and depressive disorders had onset in the fourth decade of life, on average, preceding Parkinson’s disease diagnosis by approximately two decades,” Dr. Seritan and colleagues said.
—Erica Tricarico
When present, anxiety and depressive disorders preceded Parkinson’s disease diagnosis by approximately two decades, on average.
When present, anxiety and depressive disorders preceded Parkinson’s disease diagnosis by approximately two decades, on average.
MIAMI—Anxiety and depressive disorders may precede Parkinson’s disease diagnosis in more than half of patients—anxiety by 25 years and depression by 17 years, on average, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The data suggest
“Anxiety, depression, impulse control disorders, irritability, mania, psychosis, and cognitive deficits are the most common psychiatric features of Parkinson’s disease,” said Andreea L. Seritan, MD, Professor of Psychiatry at the University of California, San Francisco (UCSF), and colleagues. Previous research has indicated that anxiety and depression increase the risk of Parkinson’s disease.
A Retrospective Chart Review
The researchers analyzed data from all 108 patients with Parkinson’s disease seen between October 2015 and January 2018.
Psychiatric diagnoses and onset ages were established through clinical interview, using DSM-V criteria. Researchers used neurologists’ notes to identify age of Parkinson’s disease diagnosis. When exact onset ages were not available, the researchers imputed missing onset ages by decade of life.
Most Patients Had a Lifetime Prevalence of Depressive Disorders
Of the 108 participants, 33.3% were women, and the mean age was 63.7. In all, 67% of patients had a lifetime prevalence of anxiety disorders, and 87% of participants had a lifetime prevalence of depressive disorders.
Psychiatric symptoms preceded Parkinson’s disease diagnosis in 48 (72%) patients with anxiety disorders and in 58 (68%) of those with depressive disorders. “Both anxiety and depressive disorders had onset in the fourth decade of life, on average, preceding Parkinson’s disease diagnosis by approximately two decades,” Dr. Seritan and colleagues said.
—Erica Tricarico
MIAMI—Anxiety and depressive disorders may precede Parkinson’s disease diagnosis in more than half of patients—anxiety by 25 years and depression by 17 years, on average, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The data suggest
“Anxiety, depression, impulse control disorders, irritability, mania, psychosis, and cognitive deficits are the most common psychiatric features of Parkinson’s disease,” said Andreea L. Seritan, MD, Professor of Psychiatry at the University of California, San Francisco (UCSF), and colleagues. Previous research has indicated that anxiety and depression increase the risk of Parkinson’s disease.
A Retrospective Chart Review
The researchers analyzed data from all 108 patients with Parkinson’s disease seen between October 2015 and January 2018.
Psychiatric diagnoses and onset ages were established through clinical interview, using DSM-V criteria. Researchers used neurologists’ notes to identify age of Parkinson’s disease diagnosis. When exact onset ages were not available, the researchers imputed missing onset ages by decade of life.
Most Patients Had a Lifetime Prevalence of Depressive Disorders
Of the 108 participants, 33.3% were women, and the mean age was 63.7. In all, 67% of patients had a lifetime prevalence of anxiety disorders, and 87% of participants had a lifetime prevalence of depressive disorders.
Psychiatric symptoms preceded Parkinson’s disease diagnosis in 48 (72%) patients with anxiety disorders and in 58 (68%) of those with depressive disorders. “Both anxiety and depressive disorders had onset in the fourth decade of life, on average, preceding Parkinson’s disease diagnosis by approximately two decades,” Dr. Seritan and colleagues said.
—Erica Tricarico
Risk of ALS May Increase With Greater Exposure to Diesel Exhaust
In men, an association exists between risk of ALS and occupational exposure to diesel exhaust.
LOS ANGELES—People with consistently high occupational exposures to diesel exhaust may have a higher risk of amyotrophic lateral sclerosis (ALS), and that risk may increase with greater exposure, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting.
“There is some suggestion from previous studies of occupation that workers in jobs with higher exposure to diesel exhaust may have a higher risk of ALS. However, no studies have directly looked at the relationship between diesel exhaust exposure during different time points in life and ALS,” said lead study author Aisha Dickerson, PhD, a postdoctoral fellow in the Departments of Environmental Health and Epidemiology at the Harvard T.H. Chan School of Public Health in Boston. “The overall risk of developing ALS is low, but our findings suggest that the greater the exposure to diesel exhaust, the greater the risk of developing ALS.”
For this study, researchers identified 1,639 people with an average age of 56 from the Danish National Patient Registry who were diagnosed with ALS between 1982 and 2013. For each case, 100 birth year- and sex-matched controls were selected using the Danish Central Person Registry. Employment history since 1964 was acquired from the nationwide Danish Pension Fund.
Cumulative diesel exhaust exposures prior to index dates (the date of ALS diagnosis in the ALS case) were estimated using a job exposure matrix. The estimated exposure was based on potential hazards for specific jobs, including service station attendants, bus drivers, and construction workers. Cumulative exposure was calculated for up to five and 10 years before the diagnosis time period, allowing for the time it may take diesel exhaust to have an effect on the body. Subjects who were older than age 25 in 1964 were excluded to diminish exposure misclassification.
The participants were divided into quartiles based on the amount of exposure to diesel exhaust. Men with any exposure to diesel exhaust at jobs held at least 10 years prior to their date of inclusion in the study were 20% more likely to have ALS than men with no exposure to exhaust during the same time period. For men who had a greater than 50% likelihood of being exposed to exhaust based on their occupation, the link was stronger. That group was 45% more likely to develop ALS than those with no exhaust exposure at both five and 10 years prior to study inclusion. No associations were observed among women, although the types of jobs and even tasks performed in the same job can differ substantially for men and women.
The results were adjusted for other factors that could affect risk of ALS including socioeconomic status and the region of Denmark where a participant lived.
“This type of exposure deserves more attention and study as we work to develop a better understanding of what causes ALS,” Dr. Dickerson said. “Importantly, the general population can be exposed to diesel exhaust from traffic pollution. Understanding whether that exposure increases ALS risk is also an important question to pursue.”
This study does not show that diesel exhaust causes ALS; it only shows an association, the researchers said. One study limitation was that investigators used a job exposure matrix to estimate occupational diesel exhaust levels and could not directly measure personal exposures. However, any potential misclassification caused by this would likely have diminished the observed associations, the researchers noted.
The study was supported by the National Institute of Environmental Health Sciences and the National Institutes of Health.
In men, an association exists between risk of ALS and occupational exposure to diesel exhaust.
In men, an association exists between risk of ALS and occupational exposure to diesel exhaust.
LOS ANGELES—People with consistently high occupational exposures to diesel exhaust may have a higher risk of amyotrophic lateral sclerosis (ALS), and that risk may increase with greater exposure, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting.
“There is some suggestion from previous studies of occupation that workers in jobs with higher exposure to diesel exhaust may have a higher risk of ALS. However, no studies have directly looked at the relationship between diesel exhaust exposure during different time points in life and ALS,” said lead study author Aisha Dickerson, PhD, a postdoctoral fellow in the Departments of Environmental Health and Epidemiology at the Harvard T.H. Chan School of Public Health in Boston. “The overall risk of developing ALS is low, but our findings suggest that the greater the exposure to diesel exhaust, the greater the risk of developing ALS.”
For this study, researchers identified 1,639 people with an average age of 56 from the Danish National Patient Registry who were diagnosed with ALS between 1982 and 2013. For each case, 100 birth year- and sex-matched controls were selected using the Danish Central Person Registry. Employment history since 1964 was acquired from the nationwide Danish Pension Fund.
Cumulative diesel exhaust exposures prior to index dates (the date of ALS diagnosis in the ALS case) were estimated using a job exposure matrix. The estimated exposure was based on potential hazards for specific jobs, including service station attendants, bus drivers, and construction workers. Cumulative exposure was calculated for up to five and 10 years before the diagnosis time period, allowing for the time it may take diesel exhaust to have an effect on the body. Subjects who were older than age 25 in 1964 were excluded to diminish exposure misclassification.
The participants were divided into quartiles based on the amount of exposure to diesel exhaust. Men with any exposure to diesel exhaust at jobs held at least 10 years prior to their date of inclusion in the study were 20% more likely to have ALS than men with no exposure to exhaust during the same time period. For men who had a greater than 50% likelihood of being exposed to exhaust based on their occupation, the link was stronger. That group was 45% more likely to develop ALS than those with no exhaust exposure at both five and 10 years prior to study inclusion. No associations were observed among women, although the types of jobs and even tasks performed in the same job can differ substantially for men and women.
The results were adjusted for other factors that could affect risk of ALS including socioeconomic status and the region of Denmark where a participant lived.
“This type of exposure deserves more attention and study as we work to develop a better understanding of what causes ALS,” Dr. Dickerson said. “Importantly, the general population can be exposed to diesel exhaust from traffic pollution. Understanding whether that exposure increases ALS risk is also an important question to pursue.”
This study does not show that diesel exhaust causes ALS; it only shows an association, the researchers said. One study limitation was that investigators used a job exposure matrix to estimate occupational diesel exhaust levels and could not directly measure personal exposures. However, any potential misclassification caused by this would likely have diminished the observed associations, the researchers noted.
The study was supported by the National Institute of Environmental Health Sciences and the National Institutes of Health.
LOS ANGELES—People with consistently high occupational exposures to diesel exhaust may have a higher risk of amyotrophic lateral sclerosis (ALS), and that risk may increase with greater exposure, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting.
“There is some suggestion from previous studies of occupation that workers in jobs with higher exposure to diesel exhaust may have a higher risk of ALS. However, no studies have directly looked at the relationship between diesel exhaust exposure during different time points in life and ALS,” said lead study author Aisha Dickerson, PhD, a postdoctoral fellow in the Departments of Environmental Health and Epidemiology at the Harvard T.H. Chan School of Public Health in Boston. “The overall risk of developing ALS is low, but our findings suggest that the greater the exposure to diesel exhaust, the greater the risk of developing ALS.”
For this study, researchers identified 1,639 people with an average age of 56 from the Danish National Patient Registry who were diagnosed with ALS between 1982 and 2013. For each case, 100 birth year- and sex-matched controls were selected using the Danish Central Person Registry. Employment history since 1964 was acquired from the nationwide Danish Pension Fund.
Cumulative diesel exhaust exposures prior to index dates (the date of ALS diagnosis in the ALS case) were estimated using a job exposure matrix. The estimated exposure was based on potential hazards for specific jobs, including service station attendants, bus drivers, and construction workers. Cumulative exposure was calculated for up to five and 10 years before the diagnosis time period, allowing for the time it may take diesel exhaust to have an effect on the body. Subjects who were older than age 25 in 1964 were excluded to diminish exposure misclassification.
The participants were divided into quartiles based on the amount of exposure to diesel exhaust. Men with any exposure to diesel exhaust at jobs held at least 10 years prior to their date of inclusion in the study were 20% more likely to have ALS than men with no exposure to exhaust during the same time period. For men who had a greater than 50% likelihood of being exposed to exhaust based on their occupation, the link was stronger. That group was 45% more likely to develop ALS than those with no exhaust exposure at both five and 10 years prior to study inclusion. No associations were observed among women, although the types of jobs and even tasks performed in the same job can differ substantially for men and women.
The results were adjusted for other factors that could affect risk of ALS including socioeconomic status and the region of Denmark where a participant lived.
“This type of exposure deserves more attention and study as we work to develop a better understanding of what causes ALS,” Dr. Dickerson said. “Importantly, the general population can be exposed to diesel exhaust from traffic pollution. Understanding whether that exposure increases ALS risk is also an important question to pursue.”
This study does not show that diesel exhaust causes ALS; it only shows an association, the researchers said. One study limitation was that investigators used a job exposure matrix to estimate occupational diesel exhaust levels and could not directly measure personal exposures. However, any potential misclassification caused by this would likely have diminished the observed associations, the researchers noted.
The study was supported by the National Institute of Environmental Health Sciences and the National Institutes of Health.
PhRMA spending leads health-sector lobbying efforts
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.
PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.

PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.

PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
New and Noteworthy Information—August 2018
Practice Effects May Influence MCI Detection
Failing to account for practice effects may lead to underdiagnosis of mild cognitive impairment (MCI), according to a study published May 14 in Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. In an approximately six-year follow-up of the Vietnam Era Twin Study of Aging, researchers retested the cognitive function of 995 late-middle-aged men. In addition, the researchers tested 170 age-matched study replacements for the first time. The investigators used group differences to calculate practice effects after controlling for attrition effects and generated MCI diagnoses from practice-adjusted scores. There were significant practice effects on most cognitive domains, even though participants’ uncorrected scores may have declined. Conversion to MCI doubled after correcting for practice effects, from 4.5% to 9%.
Elman JA, Jak AJ, Panizzon MS, et al. Underdiagnosis of mild cognitive impairment: a consequence of ignoring practice effects. Alzheimers Dement (Amst). 2018;10:372-381.
Brain Iron Levels Predict Disability in Patients With MS
Iron levels in the brains of patients with multiple sclerosis (MS) measured using quantitative susceptibility mapping (QSM) may help identify people at a higher risk of physical disability, according to a study published online ahead of print July 17 in Radiology. In this prospective study, 600 participants with MS and 250 age- and sex-matched healthy controls were imaged with 3.0-T MRI. The researchers assessed QSM and MRI volumetric differences between study groups and associations with clinical outcomes using analysis of covariance, multivariable linear regression, and voxelwise analyses, controlling for age and sex. Compared with controls, participants with MS had lower thalamic susceptibility and higher susceptibility of basal ganglia. Lower thalamic susceptibility was associated with longer disease duration, greater disability, and secondary-progressive disease course.
Zivadinov R, Tavazzi E, Bergsland N, et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology. 2018 Jul 17 [Epub ahead of print].
Concussion and ADHD May Increase Depression and Anxiety
Athletes with attention deficit hyperactivity disorder (ADHD) may be at greater risk of persistent anxiety and depression after a concussion, compared with athletes without ADHD, according to a study presented at the American Academy of Neurology’s Sports Concussion Conference. The study included 979 NCAA Division I college athletes. Researchers gathered information on ADHD diagnosis and history of concussion, and athletes completed questionnaires measuring anxiety and depression symptoms before the start of their sporting seasons. The investigators divided athletes into four groups—those with ADHD who had had a concussion, those with ADHD who had not had a concussion, those without ADHD who had had a concussion, and those without a history of concussion or ADHD. Athletes with ADHD and concussion had significantly higher anxiety and depression scores, compared with the other groups.
Late-Life Blood Pressure Is Associated With Brain Lesions
Higher average late-life systolic blood pressure and diastolic blood pressure are associated with an increasing number of brain infarcts, including gross and microinfarcts, according to a study published online ahead of print July 11 in Neurology. In addition, faster decline in systolic blood pressure increases the likelihood of an infarct. This clinical-pathologic study included data from 1,288 people who participated in prospective, community-based cohort studies of aging with similar designs and data collection. Blood pressure measurements were obtained annually. Participants were followed for an average of eight years, and the average age at death was 89. The mean standardized person-specific systolic blood pressure was 134 mm Hg and diastolic blood pressure was 71 mm Hg. Alzheimer’s disease pathology analyses found that systolic blood pressure was associated with the number of tangles but not plaques or other pathology.
Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018 Jul 11 [Epub ahead of print].
Is t-PA Beneficial for Minor Stroke?
Among patients with mild acute ischemic stroke, treatment with alteplase does not increase the likelihood of favorable functional outcome at 90 days, compared with treatment with aspirin, according to a study published July 10 in JAMA. The PRISMS trial, a phase IIIb, double-blind, double-placebo, randomized clinical trial, compared alteplase with aspirin for the treatment of emergent stroke. The study enrolled patients with NIH Stroke Scale scores of 0 to 5 and deficits that were not clearly disabling. Eligible patients were able to receive treatment within three hours of onset. Participants were randomized to receive IV alteplase (0.9 mg/kg) with oral placebo (n = 156) or oral aspirin (325 mg) with IV placebo (n = 157). At 90 days, 78.2% of patients in the alteplase group and 81.5% of patients in the aspirin group had a favorable outcome (ie, a modified Rankin Scale score of 0 or 1). The trial originally was designed to enroll 948 patients but was ended early because of slow enrollment. The early study termination precludes definitive conclusions, the investigators said.
Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018 Jul 10;320(2):156-166.
FDA Approves Xeomin for Adults With Sialorrhea
The FDA has approved the supplemental Biologics License Application for Xeomin (incobotulinumtoxinA) for the treatment of chronic sialorrhea in adult patients. The approval was based on a phase III, randomized, double-blind, placebo-controlled, multicenter trial that included 184 patients. The coprimary end points of change in unstimulated salivary flow rate and Global Impression of Change Scale at week four as compared with baseline significantly improved for participants administered 100 U incobotulinumtoxinA versus placebo. The overall frequency of adverse events was similar between placebo and treatment groups with no new or unexpected adverse events reported. Participants in the study received placebo, incobotulinumtoxinA 75 U, or incobotulinumtoxinA 100 U. Merz North America, which markets Xeomin, is headquartered in Raleigh, North Carolina.
Does Exposure to Organic Solvents Heighten the Risk of MS?
People who are exposed to organic solvents and carry genes that make them more susceptible to developing multiple sclerosis (MS) may be at greater risk of the disease than people who have only the exposure to solvents or the MS risk genes, according to a study published online ahead of print July 3 in Neurology. Using a Swedish population-based case–control study of 2,042 incident cases of MS and 2,947 controls, investigators compared the occurrence of MS in participants with different genotypes, smoking habits, and exposures to organic solvents such as paint and varnish. A potential interaction between exposure to organic solvents and MS risk human leukocyte antigen genes was evaluated by calculating the attributable proportion due to interaction. The MS genes and exposure to solvents combined were responsible for an estimated 60% of the risk of developing MS.
Hedström AK, Hössjer O, Katsoulis M, et al. Organic solvents and MS susceptibility: interaction with MS risk HLA genes. Neurology. 2018 Jul 3 [Epub ahead of print].
Epidiolex Approved for the Treatment of Seizures
The FDA approved Epidiolex (cannabidiol) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana. It also is the first FDA approval of a drug for the treatment of patients with Dravet syndrome. The drug’s effectiveness was studied in three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Taken with other medications, cannabidiol reduced the frequency of seizures, compared with placebo. Epidiolex must be dispensed with a patient medication guide that describes the drug’s uses and risks. GW Pharmaceuticals, located in the United Kingdom, markets Epidiolex.
FDA Approves Nuplazid Capsule Formulation and 10-mg Tablet
The FDA approved a new capsule dose formulation and tablet strength of Nuplazid (pimavanserin), a treatment for hallucinations and delusions associated with Parkinson’s disease psychosis. The 34-mg capsule formulation provides the recommended once daily dose in one capsule, versus the current administration of two 17-mg tablets. The 10-mg tablet provides a lower dosage strength for patients who are concomitantly receiving strong cytochrome 3A4 inhibitors, which can inhibit the metabolism of Nuplazid. The drug is a nondopaminergic, selective serotonin inverse agonist preferentially targeting 5-HT2A receptors. Acadia Pharmaceuticals, which markets the therapy, is headquartered in San Diego.
Mild Sleep Problems May Elevate Blood Pressure in Women
Mild sleep problems such as trouble falling asleep are associated with increased blood pressure and vascular inflammation in women, according to a study published June 9 in the Journal of the American Heart Association. Researchers examined blood pressure and sleep habits in 323 women in the ongoing American Heart Association Go Red for Women Strategically Focused Research Network. Investigators assessed participant’s sleep quality, obstructive sleep apnea risk, and insomnia severity. In a subset of women, sleep duration was assessed using actigraphy, and endothelial inflammation was assessed directly in harvested endothelial cells. Systolic blood pressure was associated with poor sleep quality. Poor sleep quality, insomnia, and longer sleep onset latency were associated with endothelial inflammation.
Aggarwal B, Makarem N, Shah R, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12):e008590.
DBS May Slow Tremor Progression in Early Parkinson’s Disease
Deep brain stimulation (DBS) in the early stage of Parkinson’s disease may slow tremor progression, according to a study published online ahead of print June 29 in Neurology. The prospective pilot trial enrolled patients with Parkinson’s disease who were ages 50 to 75, had been treated with Parkinson’s disease medications for six months to four years, and had no history of dyskinesia or other motor fluctuations. Participants were randomized to receive optimal drug therapy (ODT) or DBS and ODT. At baseline and six, 12, 18, and 24 months, all patients stopped all Parkinson’s disease therapy for one week. Unified Parkinson’s Disease Rating Scale-III scores were compared between the ODT and DBS and ODT groups (n = 28). Rest tremor slopes from baseline to 24 months favored DBS plus ODT off and on therapy, compared with ODT alone.
Hacker ML, DeLong MR, Turchan M, et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology. 2018 Jun 29 [Epub ahead of print].
—Kimberly Williams
Practice Effects May Influence MCI Detection
Failing to account for practice effects may lead to underdiagnosis of mild cognitive impairment (MCI), according to a study published May 14 in Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. In an approximately six-year follow-up of the Vietnam Era Twin Study of Aging, researchers retested the cognitive function of 995 late-middle-aged men. In addition, the researchers tested 170 age-matched study replacements for the first time. The investigators used group differences to calculate practice effects after controlling for attrition effects and generated MCI diagnoses from practice-adjusted scores. There were significant practice effects on most cognitive domains, even though participants’ uncorrected scores may have declined. Conversion to MCI doubled after correcting for practice effects, from 4.5% to 9%.
Elman JA, Jak AJ, Panizzon MS, et al. Underdiagnosis of mild cognitive impairment: a consequence of ignoring practice effects. Alzheimers Dement (Amst). 2018;10:372-381.
Brain Iron Levels Predict Disability in Patients With MS
Iron levels in the brains of patients with multiple sclerosis (MS) measured using quantitative susceptibility mapping (QSM) may help identify people at a higher risk of physical disability, according to a study published online ahead of print July 17 in Radiology. In this prospective study, 600 participants with MS and 250 age- and sex-matched healthy controls were imaged with 3.0-T MRI. The researchers assessed QSM and MRI volumetric differences between study groups and associations with clinical outcomes using analysis of covariance, multivariable linear regression, and voxelwise analyses, controlling for age and sex. Compared with controls, participants with MS had lower thalamic susceptibility and higher susceptibility of basal ganglia. Lower thalamic susceptibility was associated with longer disease duration, greater disability, and secondary-progressive disease course.
Zivadinov R, Tavazzi E, Bergsland N, et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology. 2018 Jul 17 [Epub ahead of print].
Concussion and ADHD May Increase Depression and Anxiety
Athletes with attention deficit hyperactivity disorder (ADHD) may be at greater risk of persistent anxiety and depression after a concussion, compared with athletes without ADHD, according to a study presented at the American Academy of Neurology’s Sports Concussion Conference. The study included 979 NCAA Division I college athletes. Researchers gathered information on ADHD diagnosis and history of concussion, and athletes completed questionnaires measuring anxiety and depression symptoms before the start of their sporting seasons. The investigators divided athletes into four groups—those with ADHD who had had a concussion, those with ADHD who had not had a concussion, those without ADHD who had had a concussion, and those without a history of concussion or ADHD. Athletes with ADHD and concussion had significantly higher anxiety and depression scores, compared with the other groups.
Late-Life Blood Pressure Is Associated With Brain Lesions
Higher average late-life systolic blood pressure and diastolic blood pressure are associated with an increasing number of brain infarcts, including gross and microinfarcts, according to a study published online ahead of print July 11 in Neurology. In addition, faster decline in systolic blood pressure increases the likelihood of an infarct. This clinical-pathologic study included data from 1,288 people who participated in prospective, community-based cohort studies of aging with similar designs and data collection. Blood pressure measurements were obtained annually. Participants were followed for an average of eight years, and the average age at death was 89. The mean standardized person-specific systolic blood pressure was 134 mm Hg and diastolic blood pressure was 71 mm Hg. Alzheimer’s disease pathology analyses found that systolic blood pressure was associated with the number of tangles but not plaques or other pathology.
Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018 Jul 11 [Epub ahead of print].
Is t-PA Beneficial for Minor Stroke?
Among patients with mild acute ischemic stroke, treatment with alteplase does not increase the likelihood of favorable functional outcome at 90 days, compared with treatment with aspirin, according to a study published July 10 in JAMA. The PRISMS trial, a phase IIIb, double-blind, double-placebo, randomized clinical trial, compared alteplase with aspirin for the treatment of emergent stroke. The study enrolled patients with NIH Stroke Scale scores of 0 to 5 and deficits that were not clearly disabling. Eligible patients were able to receive treatment within three hours of onset. Participants were randomized to receive IV alteplase (0.9 mg/kg) with oral placebo (n = 156) or oral aspirin (325 mg) with IV placebo (n = 157). At 90 days, 78.2% of patients in the alteplase group and 81.5% of patients in the aspirin group had a favorable outcome (ie, a modified Rankin Scale score of 0 or 1). The trial originally was designed to enroll 948 patients but was ended early because of slow enrollment. The early study termination precludes definitive conclusions, the investigators said.
Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018 Jul 10;320(2):156-166.
FDA Approves Xeomin for Adults With Sialorrhea
The FDA has approved the supplemental Biologics License Application for Xeomin (incobotulinumtoxinA) for the treatment of chronic sialorrhea in adult patients. The approval was based on a phase III, randomized, double-blind, placebo-controlled, multicenter trial that included 184 patients. The coprimary end points of change in unstimulated salivary flow rate and Global Impression of Change Scale at week four as compared with baseline significantly improved for participants administered 100 U incobotulinumtoxinA versus placebo. The overall frequency of adverse events was similar between placebo and treatment groups with no new or unexpected adverse events reported. Participants in the study received placebo, incobotulinumtoxinA 75 U, or incobotulinumtoxinA 100 U. Merz North America, which markets Xeomin, is headquartered in Raleigh, North Carolina.
Does Exposure to Organic Solvents Heighten the Risk of MS?
People who are exposed to organic solvents and carry genes that make them more susceptible to developing multiple sclerosis (MS) may be at greater risk of the disease than people who have only the exposure to solvents or the MS risk genes, according to a study published online ahead of print July 3 in Neurology. Using a Swedish population-based case–control study of 2,042 incident cases of MS and 2,947 controls, investigators compared the occurrence of MS in participants with different genotypes, smoking habits, and exposures to organic solvents such as paint and varnish. A potential interaction between exposure to organic solvents and MS risk human leukocyte antigen genes was evaluated by calculating the attributable proportion due to interaction. The MS genes and exposure to solvents combined were responsible for an estimated 60% of the risk of developing MS.
Hedström AK, Hössjer O, Katsoulis M, et al. Organic solvents and MS susceptibility: interaction with MS risk HLA genes. Neurology. 2018 Jul 3 [Epub ahead of print].
Epidiolex Approved for the Treatment of Seizures
The FDA approved Epidiolex (cannabidiol) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana. It also is the first FDA approval of a drug for the treatment of patients with Dravet syndrome. The drug’s effectiveness was studied in three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Taken with other medications, cannabidiol reduced the frequency of seizures, compared with placebo. Epidiolex must be dispensed with a patient medication guide that describes the drug’s uses and risks. GW Pharmaceuticals, located in the United Kingdom, markets Epidiolex.
FDA Approves Nuplazid Capsule Formulation and 10-mg Tablet
The FDA approved a new capsule dose formulation and tablet strength of Nuplazid (pimavanserin), a treatment for hallucinations and delusions associated with Parkinson’s disease psychosis. The 34-mg capsule formulation provides the recommended once daily dose in one capsule, versus the current administration of two 17-mg tablets. The 10-mg tablet provides a lower dosage strength for patients who are concomitantly receiving strong cytochrome 3A4 inhibitors, which can inhibit the metabolism of Nuplazid. The drug is a nondopaminergic, selective serotonin inverse agonist preferentially targeting 5-HT2A receptors. Acadia Pharmaceuticals, which markets the therapy, is headquartered in San Diego.
Mild Sleep Problems May Elevate Blood Pressure in Women
Mild sleep problems such as trouble falling asleep are associated with increased blood pressure and vascular inflammation in women, according to a study published June 9 in the Journal of the American Heart Association. Researchers examined blood pressure and sleep habits in 323 women in the ongoing American Heart Association Go Red for Women Strategically Focused Research Network. Investigators assessed participant’s sleep quality, obstructive sleep apnea risk, and insomnia severity. In a subset of women, sleep duration was assessed using actigraphy, and endothelial inflammation was assessed directly in harvested endothelial cells. Systolic blood pressure was associated with poor sleep quality. Poor sleep quality, insomnia, and longer sleep onset latency were associated with endothelial inflammation.
Aggarwal B, Makarem N, Shah R, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12):e008590.
DBS May Slow Tremor Progression in Early Parkinson’s Disease
Deep brain stimulation (DBS) in the early stage of Parkinson’s disease may slow tremor progression, according to a study published online ahead of print June 29 in Neurology. The prospective pilot trial enrolled patients with Parkinson’s disease who were ages 50 to 75, had been treated with Parkinson’s disease medications for six months to four years, and had no history of dyskinesia or other motor fluctuations. Participants were randomized to receive optimal drug therapy (ODT) or DBS and ODT. At baseline and six, 12, 18, and 24 months, all patients stopped all Parkinson’s disease therapy for one week. Unified Parkinson’s Disease Rating Scale-III scores were compared between the ODT and DBS and ODT groups (n = 28). Rest tremor slopes from baseline to 24 months favored DBS plus ODT off and on therapy, compared with ODT alone.
Hacker ML, DeLong MR, Turchan M, et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology. 2018 Jun 29 [Epub ahead of print].
—Kimberly Williams
Practice Effects May Influence MCI Detection
Failing to account for practice effects may lead to underdiagnosis of mild cognitive impairment (MCI), according to a study published May 14 in Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. In an approximately six-year follow-up of the Vietnam Era Twin Study of Aging, researchers retested the cognitive function of 995 late-middle-aged men. In addition, the researchers tested 170 age-matched study replacements for the first time. The investigators used group differences to calculate practice effects after controlling for attrition effects and generated MCI diagnoses from practice-adjusted scores. There were significant practice effects on most cognitive domains, even though participants’ uncorrected scores may have declined. Conversion to MCI doubled after correcting for practice effects, from 4.5% to 9%.
Elman JA, Jak AJ, Panizzon MS, et al. Underdiagnosis of mild cognitive impairment: a consequence of ignoring practice effects. Alzheimers Dement (Amst). 2018;10:372-381.
Brain Iron Levels Predict Disability in Patients With MS
Iron levels in the brains of patients with multiple sclerosis (MS) measured using quantitative susceptibility mapping (QSM) may help identify people at a higher risk of physical disability, according to a study published online ahead of print July 17 in Radiology. In this prospective study, 600 participants with MS and 250 age- and sex-matched healthy controls were imaged with 3.0-T MRI. The researchers assessed QSM and MRI volumetric differences between study groups and associations with clinical outcomes using analysis of covariance, multivariable linear regression, and voxelwise analyses, controlling for age and sex. Compared with controls, participants with MS had lower thalamic susceptibility and higher susceptibility of basal ganglia. Lower thalamic susceptibility was associated with longer disease duration, greater disability, and secondary-progressive disease course.
Zivadinov R, Tavazzi E, Bergsland N, et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology. 2018 Jul 17 [Epub ahead of print].
Concussion and ADHD May Increase Depression and Anxiety
Athletes with attention deficit hyperactivity disorder (ADHD) may be at greater risk of persistent anxiety and depression after a concussion, compared with athletes without ADHD, according to a study presented at the American Academy of Neurology’s Sports Concussion Conference. The study included 979 NCAA Division I college athletes. Researchers gathered information on ADHD diagnosis and history of concussion, and athletes completed questionnaires measuring anxiety and depression symptoms before the start of their sporting seasons. The investigators divided athletes into four groups—those with ADHD who had had a concussion, those with ADHD who had not had a concussion, those without ADHD who had had a concussion, and those without a history of concussion or ADHD. Athletes with ADHD and concussion had significantly higher anxiety and depression scores, compared with the other groups.
Late-Life Blood Pressure Is Associated With Brain Lesions
Higher average late-life systolic blood pressure and diastolic blood pressure are associated with an increasing number of brain infarcts, including gross and microinfarcts, according to a study published online ahead of print July 11 in Neurology. In addition, faster decline in systolic blood pressure increases the likelihood of an infarct. This clinical-pathologic study included data from 1,288 people who participated in prospective, community-based cohort studies of aging with similar designs and data collection. Blood pressure measurements were obtained annually. Participants were followed for an average of eight years, and the average age at death was 89. The mean standardized person-specific systolic blood pressure was 134 mm Hg and diastolic blood pressure was 71 mm Hg. Alzheimer’s disease pathology analyses found that systolic blood pressure was associated with the number of tangles but not plaques or other pathology.
Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018 Jul 11 [Epub ahead of print].
Is t-PA Beneficial for Minor Stroke?
Among patients with mild acute ischemic stroke, treatment with alteplase does not increase the likelihood of favorable functional outcome at 90 days, compared with treatment with aspirin, according to a study published July 10 in JAMA. The PRISMS trial, a phase IIIb, double-blind, double-placebo, randomized clinical trial, compared alteplase with aspirin for the treatment of emergent stroke. The study enrolled patients with NIH Stroke Scale scores of 0 to 5 and deficits that were not clearly disabling. Eligible patients were able to receive treatment within three hours of onset. Participants were randomized to receive IV alteplase (0.9 mg/kg) with oral placebo (n = 156) or oral aspirin (325 mg) with IV placebo (n = 157). At 90 days, 78.2% of patients in the alteplase group and 81.5% of patients in the aspirin group had a favorable outcome (ie, a modified Rankin Scale score of 0 or 1). The trial originally was designed to enroll 948 patients but was ended early because of slow enrollment. The early study termination precludes definitive conclusions, the investigators said.
Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018 Jul 10;320(2):156-166.
FDA Approves Xeomin for Adults With Sialorrhea
The FDA has approved the supplemental Biologics License Application for Xeomin (incobotulinumtoxinA) for the treatment of chronic sialorrhea in adult patients. The approval was based on a phase III, randomized, double-blind, placebo-controlled, multicenter trial that included 184 patients. The coprimary end points of change in unstimulated salivary flow rate and Global Impression of Change Scale at week four as compared with baseline significantly improved for participants administered 100 U incobotulinumtoxinA versus placebo. The overall frequency of adverse events was similar between placebo and treatment groups with no new or unexpected adverse events reported. Participants in the study received placebo, incobotulinumtoxinA 75 U, or incobotulinumtoxinA 100 U. Merz North America, which markets Xeomin, is headquartered in Raleigh, North Carolina.
Does Exposure to Organic Solvents Heighten the Risk of MS?
People who are exposed to organic solvents and carry genes that make them more susceptible to developing multiple sclerosis (MS) may be at greater risk of the disease than people who have only the exposure to solvents or the MS risk genes, according to a study published online ahead of print July 3 in Neurology. Using a Swedish population-based case–control study of 2,042 incident cases of MS and 2,947 controls, investigators compared the occurrence of MS in participants with different genotypes, smoking habits, and exposures to organic solvents such as paint and varnish. A potential interaction between exposure to organic solvents and MS risk human leukocyte antigen genes was evaluated by calculating the attributable proportion due to interaction. The MS genes and exposure to solvents combined were responsible for an estimated 60% of the risk of developing MS.
Hedström AK, Hössjer O, Katsoulis M, et al. Organic solvents and MS susceptibility: interaction with MS risk HLA genes. Neurology. 2018 Jul 3 [Epub ahead of print].
Epidiolex Approved for the Treatment of Seizures
The FDA approved Epidiolex (cannabidiol) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana. It also is the first FDA approval of a drug for the treatment of patients with Dravet syndrome. The drug’s effectiveness was studied in three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Taken with other medications, cannabidiol reduced the frequency of seizures, compared with placebo. Epidiolex must be dispensed with a patient medication guide that describes the drug’s uses and risks. GW Pharmaceuticals, located in the United Kingdom, markets Epidiolex.
FDA Approves Nuplazid Capsule Formulation and 10-mg Tablet
The FDA approved a new capsule dose formulation and tablet strength of Nuplazid (pimavanserin), a treatment for hallucinations and delusions associated with Parkinson’s disease psychosis. The 34-mg capsule formulation provides the recommended once daily dose in one capsule, versus the current administration of two 17-mg tablets. The 10-mg tablet provides a lower dosage strength for patients who are concomitantly receiving strong cytochrome 3A4 inhibitors, which can inhibit the metabolism of Nuplazid. The drug is a nondopaminergic, selective serotonin inverse agonist preferentially targeting 5-HT2A receptors. Acadia Pharmaceuticals, which markets the therapy, is headquartered in San Diego.
Mild Sleep Problems May Elevate Blood Pressure in Women
Mild sleep problems such as trouble falling asleep are associated with increased blood pressure and vascular inflammation in women, according to a study published June 9 in the Journal of the American Heart Association. Researchers examined blood pressure and sleep habits in 323 women in the ongoing American Heart Association Go Red for Women Strategically Focused Research Network. Investigators assessed participant’s sleep quality, obstructive sleep apnea risk, and insomnia severity. In a subset of women, sleep duration was assessed using actigraphy, and endothelial inflammation was assessed directly in harvested endothelial cells. Systolic blood pressure was associated with poor sleep quality. Poor sleep quality, insomnia, and longer sleep onset latency were associated with endothelial inflammation.
Aggarwal B, Makarem N, Shah R, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12):e008590.
DBS May Slow Tremor Progression in Early Parkinson’s Disease
Deep brain stimulation (DBS) in the early stage of Parkinson’s disease may slow tremor progression, according to a study published online ahead of print June 29 in Neurology. The prospective pilot trial enrolled patients with Parkinson’s disease who were ages 50 to 75, had been treated with Parkinson’s disease medications for six months to four years, and had no history of dyskinesia or other motor fluctuations. Participants were randomized to receive optimal drug therapy (ODT) or DBS and ODT. At baseline and six, 12, 18, and 24 months, all patients stopped all Parkinson’s disease therapy for one week. Unified Parkinson’s Disease Rating Scale-III scores were compared between the ODT and DBS and ODT groups (n = 28). Rest tremor slopes from baseline to 24 months favored DBS plus ODT off and on therapy, compared with ODT alone.
Hacker ML, DeLong MR, Turchan M, et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology. 2018 Jun 29 [Epub ahead of print].
—Kimberly Williams
Black Esophagus: A Rare Cause of Gastrointestinal Hemorrhage in the Emergency Department
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.