User login
FDA warns against azithromycin in blood or lymph node cancers
The Food and Drug Administration has issued a in patients with blood or lymph node cancers who have received donor stem cell transplants.
This use of azithromycin can lead to increased risk of cancer relapse and death in this population. The FDA is continuing to review data and is expected to issue further recommendations.
Patients with blood or lymph node cancers are at an increased risk of bronchiolitis obliterans syndrome after donor stem cell transplant; although azithromycin is not approved for prevention of this condition, the antibiotic is sometimes prescribed for that purpose.
A French study of 480 patients was undertaken to assess the effectiveness of this prophylaxis but revealed the increased risk of relapse and death and was halted 13 months after completing enrollment. The rate of cancer relapse was 32.9% in the azithromycin group and just 20.8% in the placebo group; the 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (JAMA 2017;318[6]:557-66).
Bronchiolitis obliterans syndrome is marked by inflammation and scarring of the airways that leads to severe shortness of breath and dry cough. There are no known effective antibiotic treatments for prophylaxis of the condition, according to the FDA.
FDA officials are advising physicians not to prescribe long-term azithromycin in this population. Patients who have had a stem cell transplant and are already taking the antibiotic, should consult a doctor before discontinuing.
The manufacturer of brand name azithromycin (Zithromax) has issued a Dear Healthcare Provider letter about the safety issue, and more information can be found in the FDA’s safety announcement.
The Food and Drug Administration has issued a in patients with blood or lymph node cancers who have received donor stem cell transplants.
This use of azithromycin can lead to increased risk of cancer relapse and death in this population. The FDA is continuing to review data and is expected to issue further recommendations.
Patients with blood or lymph node cancers are at an increased risk of bronchiolitis obliterans syndrome after donor stem cell transplant; although azithromycin is not approved for prevention of this condition, the antibiotic is sometimes prescribed for that purpose.
A French study of 480 patients was undertaken to assess the effectiveness of this prophylaxis but revealed the increased risk of relapse and death and was halted 13 months after completing enrollment. The rate of cancer relapse was 32.9% in the azithromycin group and just 20.8% in the placebo group; the 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (JAMA 2017;318[6]:557-66).
Bronchiolitis obliterans syndrome is marked by inflammation and scarring of the airways that leads to severe shortness of breath and dry cough. There are no known effective antibiotic treatments for prophylaxis of the condition, according to the FDA.
FDA officials are advising physicians not to prescribe long-term azithromycin in this population. Patients who have had a stem cell transplant and are already taking the antibiotic, should consult a doctor before discontinuing.
The manufacturer of brand name azithromycin (Zithromax) has issued a Dear Healthcare Provider letter about the safety issue, and more information can be found in the FDA’s safety announcement.
The Food and Drug Administration has issued a in patients with blood or lymph node cancers who have received donor stem cell transplants.
This use of azithromycin can lead to increased risk of cancer relapse and death in this population. The FDA is continuing to review data and is expected to issue further recommendations.
Patients with blood or lymph node cancers are at an increased risk of bronchiolitis obliterans syndrome after donor stem cell transplant; although azithromycin is not approved for prevention of this condition, the antibiotic is sometimes prescribed for that purpose.
A French study of 480 patients was undertaken to assess the effectiveness of this prophylaxis but revealed the increased risk of relapse and death and was halted 13 months after completing enrollment. The rate of cancer relapse was 32.9% in the azithromycin group and just 20.8% in the placebo group; the 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (JAMA 2017;318[6]:557-66).
Bronchiolitis obliterans syndrome is marked by inflammation and scarring of the airways that leads to severe shortness of breath and dry cough. There are no known effective antibiotic treatments for prophylaxis of the condition, according to the FDA.
FDA officials are advising physicians not to prescribe long-term azithromycin in this population. Patients who have had a stem cell transplant and are already taking the antibiotic, should consult a doctor before discontinuing.
The manufacturer of brand name azithromycin (Zithromax) has issued a Dear Healthcare Provider letter about the safety issue, and more information can be found in the FDA’s safety announcement.
Documentation and billing: Tips for hospitalists
Is it AMS, Delirium, or Encephalopathy?
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)
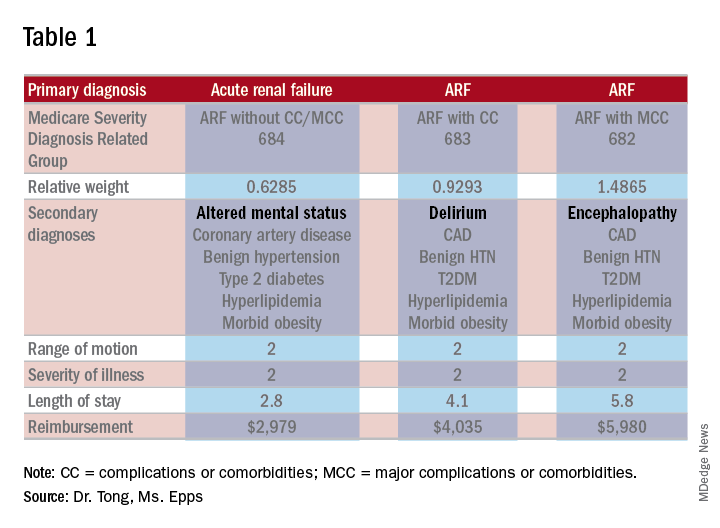
As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
Is it AMS, Delirium, or Encephalopathy?
Is it AMS, Delirium, or Encephalopathy?
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)

As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)

As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
Positive change through advocacy
SHM seen as an ‘honest broker’ on Capitol Hill
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
SHM seen as an ‘honest broker’ on Capitol Hill
SHM seen as an ‘honest broker’ on Capitol Hill
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
Conference News Roundup—European Academy of Neurology
Thrombectomy Is Feasible for the Elderly, but Entails Risks
Mechanical thrombectomy is an increasingly important therapy for acute stroke that can benefit the very old, assuming a careful selection of patients and risk assessment, according to a Portuguese study.
For several years, endovascular thrombectomy has been a way of removing larger vascular obstructions. In this procedure, the thrombus is extracted from the cerebral vessel via a catheter inserted in the groin. Numerous international studies have shown that endovascular treatment is a substantial improvement over purely drug-based therapy. The procedure is especially effective in dealing with extremely long blood clots and large obstructions of the cerebral arteries and often yields positive results. Thanks to this procedure, more than 60% of patients treated survive the stroke with no or minor subsequent impairment.
“More and more study results show the high effectiveness of mechanical removal of blood clots after a stroke. But researchers are still trying to determine the type of patient for whom this relatively new procedure is the best treatment option,” said Ary Lopes de Sousa, MD, a neurology resident at Central Lisbon Hospital Center.
Dr. de Sousa and his colleagues reviewed the treatment success of thrombectomy in more than 200 patients with anterior acute ischemic stroke and no or slight disability prior to this event. The researchers separated patients into two groups: one with individuals younger than 80 and one with individuals age 80 and older.
In the group of patients age 80 and older, hypertension and transient ischemic attacks were more frequent. The treatment did not differ between the two groups (eg, in terms of the time frame of the revascularization). But in the older group, two-thirds of the patients exhibited a poor functional outcome at three months after the treatment (ie, they were moderately or severely limited in their ability to handle their daily tasks). The number of impaired individuals in that group was substantially larger than in the younger group, where 46% faced limitations in their everyday lives. On the other hand, one-third of the patients age 80 and older were able to handle their everyday lives three months after the treatment with no or mild impairments from the stroke. No difference in mortality was observed between the two age groups.
“For patients over 80, thrombectomy appears to be riskier than for younger patients,” said Dr. de Sousa. “But one third of the patients over 80 can be fully functional in their everyday lives after the procedure, so we must identify the factors associated with this favorable outcome. This [step] will support us applying this modern procedure efficiently to those individuals among the very old who can benefit from it.”
Studies Gauge the Cost of Migraine
A pair of studies have evaluated the cost of migraine to individuals, society, and businesses. A French study looked at the socioeconomic impact of the condition. In a survey of more than 7,700 people, a representative sample of the general population, 3.8% indicated that they experienced severe migraines on at least eight days per month. “Two-thirds of those [patients] were women, and the average age of those affected was 41, meaning that migraines significantly affect people at the peak of their careers, and who have families to provide for. These regular attacks represent a serious problem as far as keeping their jobs is concerned,” said Dr. Guillaume Leiba, Pricing and Market Access Manager at Novartis in Paris. In the current study, patients with severe migraine reported missing 33 working days per year because of their condition. This absence translates into a cost to society of approximately EUR 3.8 billion. Migraine also has an impact on patients’ social environment: 14% of respondents indicated that family members had to adjust their working hours because of patients’ migraine headaches. The study also quantified the financial burden placed on migraineurs: 58% reported an average monthly cost of more than EUR 30 per month for nonreimbursed medicines. Approximately 43% spent more than EUR 50 each month on other, nonpharmaceutical therapies. Despite the high level of public and private spending associated with the condition, quality of life for migraineurs remains far from satisfactory. More than three-quarters have sleep disorders and benefit less from their free time than healthy controls.
A Swiss study obtained more detailed results regarding absenteeism in the workplace. A group of 700 working migraineurs reported losing an average of 32 days per year because of migraine. This rate is similar to that reported in the French study. But there were significant differences depending on the specific type of headache, according to study author François Cadiou, CEO of Healint in Singapore. “With an average of more than 56 working days missed per year, patients with chronic migraine had the highest rate of absenteeism. People with episodic migraine were unable to go to work on 33 days of the year, while those with low-frequency episodic migraine took an average of 15 days off because of their condition.” Another finding has implications for preventive measures: the number of sick days was not always constant. In fact, the total steadily increased, and with it the amount of medication taken if patients indicated anxiety or depression as a symptom or trigger at least once within the 28-day observation period. In light of the outcomes presented, experts at the EAN Congress have issued a call for increased investment in migraine research and prevention, citing the advantages to society.
Both studies were funded by Novartis Pharma.
Parkinson’s Disease Progression Varies by Gender
A current study has now furnished the first neurophysiologic evidence that Parkinson’s disease progresses differently in women than in men. “Numerous demographic studies have provided evidence that men contract Parkinson’s disease nearly twice as often as women. What was unclear, however, was whether a gender-specific pathophysiology exists as soon as the first symptoms appear,” said Maja Kojovic, MD, PhD, a consultant neurologist at Ljubljana University Medical Center in Slovenia.
The international research team proceeded from the concept that in early Parkinson’s disease, functional changes can be detected in the primary motor cortex (M1) using transcranial magnetic stimulation (TMS). If pathophysiology differs between genders in Parkinson’s disease, they hypothesized, it will be reflected in differences of M1 TMS measurements.
Thirty-nine newly diagnosed and untreated patients with Parkinson’s disease (23 males) were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS). Then the patients and a group of healthy controls underwent TMS measurements of motor thresholds of the brain, input–output curve, short interval intracortical inhibition, cortical silent period, and intracortical facilitation. Brain plasticity was also measured using paired associative stimulation.
The UPDRS tests did not yield any differences in motor scores between the genders. However, the female patients had a less steep input–output curve than the male patients on the side of the brain more affected by Parkinson’s disease.
The women with Parkinson’s disease also exhibited better preserved short interval intracortical inhibition in both hemispheres, compared with affected men, and tended to have a better response to the paired associative stimulation protocol on the side less affected by symptoms. No gender-specific differences were determined, however, in the motor thresholds, intracortical facilitation, and the cortical silent period. The healthy control group did not show any gender or interhemispheric differences for any of the TMS parameters measured. “The detected gender differences in corticospinal and intracortical excitability in patients with early untreated Parkinson’s disease represent differences in disease pathophysiology. Gender may also prove to be a relevant factor when choosing appropriate treatment,” said Dr. Kojovic.
EAN Develops Guideline on Palliative Care of Patients With Severe MS
A cohort of 934 individuals affected by multiple sclerosis (MS) from seven European countries played an instrumental part in developing the European Academy of Neurology’s (EAN) new guideline on palliative care for people with severe MS. “There were 751 MS patients and 183 caregiver relatives involved,” said Sascha Köpke, PhD, Professor of Nursing Research at the University of Lübeck in Germany.
With the involvement of patients and their families in a new guideline, the EAN is emphasizing shared decision-making as an increasingly important concept that underscores patient autonomy and promotes the individualization of diagnosis and therapy. According to this approach, patients and physicians undergo a detailed consultation and then choose the medical treatment. The EAN has supported this patient-centered approach for a long time, and it is becoming increasingly established in other medical areas as well.
“It was resource- and time-intensive to include consumers in the guideline process, but also highly rewarding,” said Prof. Köpke. “Patients and caregivers really helped us to formulate the guideline in a way that was in line with actual practice and their own needs. We were able to see clearly which of our ideas met with approval or rejection.” The comments were also instructive for the group of EAN experts. They raised new aspects as well as sensitive issues that had been left out of the first draft.
Two approaches were chosen to ensure that consumers would participate. “First, there was an international online survey launched by national MS societies following a trial run involving 20 patients and 18 caregivers. Second, we invited MS patients and caregiver relatives to focus group meetings,” said Prof. Köpke. The majority of participants approved the topics proposed by the EAN group of experts. About 98% agreed to incorporate the subject of multidisciplinary rehabilitation in the guideline. There were 569 free comments, of which 182 (32%) pertained to the specified topics. A further 227 comments (40%) addressed additional topics, of which 16 were pertinent to the guideline. Five of the focus group meetings corroborated the results of the online survey and helped to work out important issues for the individuals affected. “The involvement of patients and caregivers increases the reliability and relevance of the guideline for clinical practice,” said Prof. Köpke.
Thrombectomy Is Feasible for the Elderly, but Entails Risks
Mechanical thrombectomy is an increasingly important therapy for acute stroke that can benefit the very old, assuming a careful selection of patients and risk assessment, according to a Portuguese study.
For several years, endovascular thrombectomy has been a way of removing larger vascular obstructions. In this procedure, the thrombus is extracted from the cerebral vessel via a catheter inserted in the groin. Numerous international studies have shown that endovascular treatment is a substantial improvement over purely drug-based therapy. The procedure is especially effective in dealing with extremely long blood clots and large obstructions of the cerebral arteries and often yields positive results. Thanks to this procedure, more than 60% of patients treated survive the stroke with no or minor subsequent impairment.
“More and more study results show the high effectiveness of mechanical removal of blood clots after a stroke. But researchers are still trying to determine the type of patient for whom this relatively new procedure is the best treatment option,” said Ary Lopes de Sousa, MD, a neurology resident at Central Lisbon Hospital Center.
Dr. de Sousa and his colleagues reviewed the treatment success of thrombectomy in more than 200 patients with anterior acute ischemic stroke and no or slight disability prior to this event. The researchers separated patients into two groups: one with individuals younger than 80 and one with individuals age 80 and older.
In the group of patients age 80 and older, hypertension and transient ischemic attacks were more frequent. The treatment did not differ between the two groups (eg, in terms of the time frame of the revascularization). But in the older group, two-thirds of the patients exhibited a poor functional outcome at three months after the treatment (ie, they were moderately or severely limited in their ability to handle their daily tasks). The number of impaired individuals in that group was substantially larger than in the younger group, where 46% faced limitations in their everyday lives. On the other hand, one-third of the patients age 80 and older were able to handle their everyday lives three months after the treatment with no or mild impairments from the stroke. No difference in mortality was observed between the two age groups.
“For patients over 80, thrombectomy appears to be riskier than for younger patients,” said Dr. de Sousa. “But one third of the patients over 80 can be fully functional in their everyday lives after the procedure, so we must identify the factors associated with this favorable outcome. This [step] will support us applying this modern procedure efficiently to those individuals among the very old who can benefit from it.”
Studies Gauge the Cost of Migraine
A pair of studies have evaluated the cost of migraine to individuals, society, and businesses. A French study looked at the socioeconomic impact of the condition. In a survey of more than 7,700 people, a representative sample of the general population, 3.8% indicated that they experienced severe migraines on at least eight days per month. “Two-thirds of those [patients] were women, and the average age of those affected was 41, meaning that migraines significantly affect people at the peak of their careers, and who have families to provide for. These regular attacks represent a serious problem as far as keeping their jobs is concerned,” said Dr. Guillaume Leiba, Pricing and Market Access Manager at Novartis in Paris. In the current study, patients with severe migraine reported missing 33 working days per year because of their condition. This absence translates into a cost to society of approximately EUR 3.8 billion. Migraine also has an impact on patients’ social environment: 14% of respondents indicated that family members had to adjust their working hours because of patients’ migraine headaches. The study also quantified the financial burden placed on migraineurs: 58% reported an average monthly cost of more than EUR 30 per month for nonreimbursed medicines. Approximately 43% spent more than EUR 50 each month on other, nonpharmaceutical therapies. Despite the high level of public and private spending associated with the condition, quality of life for migraineurs remains far from satisfactory. More than three-quarters have sleep disorders and benefit less from their free time than healthy controls.
A Swiss study obtained more detailed results regarding absenteeism in the workplace. A group of 700 working migraineurs reported losing an average of 32 days per year because of migraine. This rate is similar to that reported in the French study. But there were significant differences depending on the specific type of headache, according to study author François Cadiou, CEO of Healint in Singapore. “With an average of more than 56 working days missed per year, patients with chronic migraine had the highest rate of absenteeism. People with episodic migraine were unable to go to work on 33 days of the year, while those with low-frequency episodic migraine took an average of 15 days off because of their condition.” Another finding has implications for preventive measures: the number of sick days was not always constant. In fact, the total steadily increased, and with it the amount of medication taken if patients indicated anxiety or depression as a symptom or trigger at least once within the 28-day observation period. In light of the outcomes presented, experts at the EAN Congress have issued a call for increased investment in migraine research and prevention, citing the advantages to society.
Both studies were funded by Novartis Pharma.
Parkinson’s Disease Progression Varies by Gender
A current study has now furnished the first neurophysiologic evidence that Parkinson’s disease progresses differently in women than in men. “Numerous demographic studies have provided evidence that men contract Parkinson’s disease nearly twice as often as women. What was unclear, however, was whether a gender-specific pathophysiology exists as soon as the first symptoms appear,” said Maja Kojovic, MD, PhD, a consultant neurologist at Ljubljana University Medical Center in Slovenia.
The international research team proceeded from the concept that in early Parkinson’s disease, functional changes can be detected in the primary motor cortex (M1) using transcranial magnetic stimulation (TMS). If pathophysiology differs between genders in Parkinson’s disease, they hypothesized, it will be reflected in differences of M1 TMS measurements.
Thirty-nine newly diagnosed and untreated patients with Parkinson’s disease (23 males) were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS). Then the patients and a group of healthy controls underwent TMS measurements of motor thresholds of the brain, input–output curve, short interval intracortical inhibition, cortical silent period, and intracortical facilitation. Brain plasticity was also measured using paired associative stimulation.
The UPDRS tests did not yield any differences in motor scores between the genders. However, the female patients had a less steep input–output curve than the male patients on the side of the brain more affected by Parkinson’s disease.
The women with Parkinson’s disease also exhibited better preserved short interval intracortical inhibition in both hemispheres, compared with affected men, and tended to have a better response to the paired associative stimulation protocol on the side less affected by symptoms. No gender-specific differences were determined, however, in the motor thresholds, intracortical facilitation, and the cortical silent period. The healthy control group did not show any gender or interhemispheric differences for any of the TMS parameters measured. “The detected gender differences in corticospinal and intracortical excitability in patients with early untreated Parkinson’s disease represent differences in disease pathophysiology. Gender may also prove to be a relevant factor when choosing appropriate treatment,” said Dr. Kojovic.
EAN Develops Guideline on Palliative Care of Patients With Severe MS
A cohort of 934 individuals affected by multiple sclerosis (MS) from seven European countries played an instrumental part in developing the European Academy of Neurology’s (EAN) new guideline on palliative care for people with severe MS. “There were 751 MS patients and 183 caregiver relatives involved,” said Sascha Köpke, PhD, Professor of Nursing Research at the University of Lübeck in Germany.
With the involvement of patients and their families in a new guideline, the EAN is emphasizing shared decision-making as an increasingly important concept that underscores patient autonomy and promotes the individualization of diagnosis and therapy. According to this approach, patients and physicians undergo a detailed consultation and then choose the medical treatment. The EAN has supported this patient-centered approach for a long time, and it is becoming increasingly established in other medical areas as well.
“It was resource- and time-intensive to include consumers in the guideline process, but also highly rewarding,” said Prof. Köpke. “Patients and caregivers really helped us to formulate the guideline in a way that was in line with actual practice and their own needs. We were able to see clearly which of our ideas met with approval or rejection.” The comments were also instructive for the group of EAN experts. They raised new aspects as well as sensitive issues that had been left out of the first draft.
Two approaches were chosen to ensure that consumers would participate. “First, there was an international online survey launched by national MS societies following a trial run involving 20 patients and 18 caregivers. Second, we invited MS patients and caregiver relatives to focus group meetings,” said Prof. Köpke. The majority of participants approved the topics proposed by the EAN group of experts. About 98% agreed to incorporate the subject of multidisciplinary rehabilitation in the guideline. There were 569 free comments, of which 182 (32%) pertained to the specified topics. A further 227 comments (40%) addressed additional topics, of which 16 were pertinent to the guideline. Five of the focus group meetings corroborated the results of the online survey and helped to work out important issues for the individuals affected. “The involvement of patients and caregivers increases the reliability and relevance of the guideline for clinical practice,” said Prof. Köpke.
Thrombectomy Is Feasible for the Elderly, but Entails Risks
Mechanical thrombectomy is an increasingly important therapy for acute stroke that can benefit the very old, assuming a careful selection of patients and risk assessment, according to a Portuguese study.
For several years, endovascular thrombectomy has been a way of removing larger vascular obstructions. In this procedure, the thrombus is extracted from the cerebral vessel via a catheter inserted in the groin. Numerous international studies have shown that endovascular treatment is a substantial improvement over purely drug-based therapy. The procedure is especially effective in dealing with extremely long blood clots and large obstructions of the cerebral arteries and often yields positive results. Thanks to this procedure, more than 60% of patients treated survive the stroke with no or minor subsequent impairment.
“More and more study results show the high effectiveness of mechanical removal of blood clots after a stroke. But researchers are still trying to determine the type of patient for whom this relatively new procedure is the best treatment option,” said Ary Lopes de Sousa, MD, a neurology resident at Central Lisbon Hospital Center.
Dr. de Sousa and his colleagues reviewed the treatment success of thrombectomy in more than 200 patients with anterior acute ischemic stroke and no or slight disability prior to this event. The researchers separated patients into two groups: one with individuals younger than 80 and one with individuals age 80 and older.
In the group of patients age 80 and older, hypertension and transient ischemic attacks were more frequent. The treatment did not differ between the two groups (eg, in terms of the time frame of the revascularization). But in the older group, two-thirds of the patients exhibited a poor functional outcome at three months after the treatment (ie, they were moderately or severely limited in their ability to handle their daily tasks). The number of impaired individuals in that group was substantially larger than in the younger group, where 46% faced limitations in their everyday lives. On the other hand, one-third of the patients age 80 and older were able to handle their everyday lives three months after the treatment with no or mild impairments from the stroke. No difference in mortality was observed between the two age groups.
“For patients over 80, thrombectomy appears to be riskier than for younger patients,” said Dr. de Sousa. “But one third of the patients over 80 can be fully functional in their everyday lives after the procedure, so we must identify the factors associated with this favorable outcome. This [step] will support us applying this modern procedure efficiently to those individuals among the very old who can benefit from it.”
Studies Gauge the Cost of Migraine
A pair of studies have evaluated the cost of migraine to individuals, society, and businesses. A French study looked at the socioeconomic impact of the condition. In a survey of more than 7,700 people, a representative sample of the general population, 3.8% indicated that they experienced severe migraines on at least eight days per month. “Two-thirds of those [patients] were women, and the average age of those affected was 41, meaning that migraines significantly affect people at the peak of their careers, and who have families to provide for. These regular attacks represent a serious problem as far as keeping their jobs is concerned,” said Dr. Guillaume Leiba, Pricing and Market Access Manager at Novartis in Paris. In the current study, patients with severe migraine reported missing 33 working days per year because of their condition. This absence translates into a cost to society of approximately EUR 3.8 billion. Migraine also has an impact on patients’ social environment: 14% of respondents indicated that family members had to adjust their working hours because of patients’ migraine headaches. The study also quantified the financial burden placed on migraineurs: 58% reported an average monthly cost of more than EUR 30 per month for nonreimbursed medicines. Approximately 43% spent more than EUR 50 each month on other, nonpharmaceutical therapies. Despite the high level of public and private spending associated with the condition, quality of life for migraineurs remains far from satisfactory. More than three-quarters have sleep disorders and benefit less from their free time than healthy controls.
A Swiss study obtained more detailed results regarding absenteeism in the workplace. A group of 700 working migraineurs reported losing an average of 32 days per year because of migraine. This rate is similar to that reported in the French study. But there were significant differences depending on the specific type of headache, according to study author François Cadiou, CEO of Healint in Singapore. “With an average of more than 56 working days missed per year, patients with chronic migraine had the highest rate of absenteeism. People with episodic migraine were unable to go to work on 33 days of the year, while those with low-frequency episodic migraine took an average of 15 days off because of their condition.” Another finding has implications for preventive measures: the number of sick days was not always constant. In fact, the total steadily increased, and with it the amount of medication taken if patients indicated anxiety or depression as a symptom or trigger at least once within the 28-day observation period. In light of the outcomes presented, experts at the EAN Congress have issued a call for increased investment in migraine research and prevention, citing the advantages to society.
Both studies were funded by Novartis Pharma.
Parkinson’s Disease Progression Varies by Gender
A current study has now furnished the first neurophysiologic evidence that Parkinson’s disease progresses differently in women than in men. “Numerous demographic studies have provided evidence that men contract Parkinson’s disease nearly twice as often as women. What was unclear, however, was whether a gender-specific pathophysiology exists as soon as the first symptoms appear,” said Maja Kojovic, MD, PhD, a consultant neurologist at Ljubljana University Medical Center in Slovenia.
The international research team proceeded from the concept that in early Parkinson’s disease, functional changes can be detected in the primary motor cortex (M1) using transcranial magnetic stimulation (TMS). If pathophysiology differs between genders in Parkinson’s disease, they hypothesized, it will be reflected in differences of M1 TMS measurements.
Thirty-nine newly diagnosed and untreated patients with Parkinson’s disease (23 males) were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS). Then the patients and a group of healthy controls underwent TMS measurements of motor thresholds of the brain, input–output curve, short interval intracortical inhibition, cortical silent period, and intracortical facilitation. Brain plasticity was also measured using paired associative stimulation.
The UPDRS tests did not yield any differences in motor scores between the genders. However, the female patients had a less steep input–output curve than the male patients on the side of the brain more affected by Parkinson’s disease.
The women with Parkinson’s disease also exhibited better preserved short interval intracortical inhibition in both hemispheres, compared with affected men, and tended to have a better response to the paired associative stimulation protocol on the side less affected by symptoms. No gender-specific differences were determined, however, in the motor thresholds, intracortical facilitation, and the cortical silent period. The healthy control group did not show any gender or interhemispheric differences for any of the TMS parameters measured. “The detected gender differences in corticospinal and intracortical excitability in patients with early untreated Parkinson’s disease represent differences in disease pathophysiology. Gender may also prove to be a relevant factor when choosing appropriate treatment,” said Dr. Kojovic.
EAN Develops Guideline on Palliative Care of Patients With Severe MS
A cohort of 934 individuals affected by multiple sclerosis (MS) from seven European countries played an instrumental part in developing the European Academy of Neurology’s (EAN) new guideline on palliative care for people with severe MS. “There were 751 MS patients and 183 caregiver relatives involved,” said Sascha Köpke, PhD, Professor of Nursing Research at the University of Lübeck in Germany.
With the involvement of patients and their families in a new guideline, the EAN is emphasizing shared decision-making as an increasingly important concept that underscores patient autonomy and promotes the individualization of diagnosis and therapy. According to this approach, patients and physicians undergo a detailed consultation and then choose the medical treatment. The EAN has supported this patient-centered approach for a long time, and it is becoming increasingly established in other medical areas as well.
“It was resource- and time-intensive to include consumers in the guideline process, but also highly rewarding,” said Prof. Köpke. “Patients and caregivers really helped us to formulate the guideline in a way that was in line with actual practice and their own needs. We were able to see clearly which of our ideas met with approval or rejection.” The comments were also instructive for the group of EAN experts. They raised new aspects as well as sensitive issues that had been left out of the first draft.
Two approaches were chosen to ensure that consumers would participate. “First, there was an international online survey launched by national MS societies following a trial run involving 20 patients and 18 caregivers. Second, we invited MS patients and caregiver relatives to focus group meetings,” said Prof. Köpke. The majority of participants approved the topics proposed by the EAN group of experts. About 98% agreed to incorporate the subject of multidisciplinary rehabilitation in the guideline. There were 569 free comments, of which 182 (32%) pertained to the specified topics. A further 227 comments (40%) addressed additional topics, of which 16 were pertinent to the guideline. Five of the focus group meetings corroborated the results of the online survey and helped to work out important issues for the individuals affected. “The involvement of patients and caregivers increases the reliability and relevance of the guideline for clinical practice,” said Prof. Köpke.
Childhood Neuromuscular Disease as a Metaphor for the Scientific Advances in Child Neurology of the Last Quarter Century
John B. Bodensteiner, MD
Dr. Bodensteiner is Director of the American Board of Psychiatry and Neurology, Founding Editor and Senior Associate Editor of Seminars in Pediatric Neurology, and Senior Associate Editor of the Journal of Child Neurology.
The impetus for this article is the celebration of 25 years of publication of Neurology Reviews. Child neurology has advanced so much in these years that it is hardly recognizable from the previous state of our understanding. Many components of the discipline include, but are not limited to, epilepsy, headache, demyelinating diseases, autoimmune diseases, neoplastic, neonatal, neuromuscular disease, and developmental conditions. My interest has been related to the neuromuscular diseases of childhood. Thus, I have chosen to describe some of the ways the advances in basic medical science and our understanding of the genetic and molecular mechanisms of disease have altered the way we think about these conditions. For the first time, this knowledge has allowed us to identify targets and techniques for effective intervention and disease modification.
Myotonic Muscular Dystrophy
One example of how the advancement in the discipline of genetics has changed our understanding of disease would be myotonic muscular dystrophy (MyoD1). When I started seeing patients in the mid 1960s, MyoD1, particularly as seen in children, was recognized as an example of autosomal dominant inheritance. The tendency for subsequent generations to be more severely affected than their parents is a phenomenon known as anticipation. Anticipation was recognized, though not explained, with the understanding of the genetic mechanisms of the time. The fact that when the disease affected an infant or newborn it was almost always inherited from the mother was also well established, though the reason for this was also not understood. Actually, during the late 1970s and early 1980s the existence of the phenomenon of anticipation was widely questioned and some publications proposed that anticipation was just an artifact of observation due to the fact that we (medical clinicians) were getting better and thus identifying the disease earlier than in past years. The recognition of the importance of repeated segments of DNA in the pathogenesis of human disease and the subsequent development of the technology to study the phenomenon allowed recognition of this previously unappreciated mechanism of genetic disease causation. This new genetic mechanism explained how anticipation could occur and confirmed it as a real phenomenon. Expanded numbers of tandem repeats probably allowed the explanation of the difference in gene transmission related to the parental gender as well.1
Genetic Insights
Advancements in the understanding of genetic mechanisms of disease have made it possible to begin to identify the molecular mechanisms operating in many previously incomprehensible diseases. The development of laboratory techniques necessary for the identification of these genetic abnormalities has brought the recognition of these disease mechanisms into clinical practice. Twenty-five years ago, the ability to identify alterations in DNA sequences, the presence of deletions/duplications and expansion of trinucleotide repeats, as well as the determination (and recognition of the significance) of gene copy number, was not available to the clinician. This advancement in genetic understanding has, in turn, allowed the identification of therapeutic targets in the hope of being able to moderate or eliminate the consequence of the defective gene.
Spinal Muscular Atrophy
In the last few years, the use of relatively small molecules such as oligonucleotides to alter the transcription of mutated RNA to produce a functioning protein has been developed. Because they are small molecules, they can be delivered with relative ease to the desired site. This technology has been applied to the treatment of spinal muscular atrophy (SMA) with considerable success.
Survival motor neuron gene product is essential for the health of the anterior horn cells of the spinal cord of the central nervous system. In the human genome there are two copies of the survival motor neuron gene, labeled SMN1 and SMN2. SMN1 normally produces a functional protein that is stable and necessary for the anterior horn survival. All patients with SMA have mutations in SMN1 that result in the gene being inactive. The protein product of SMN2 is usually truncated and not very stable, though it has some function. The severity of the resulting disease is influenced by the number of copies of the SMN2 gene present, and thus the available amount of partially functional SMN2 product. The oligonucleotide, in this case delivered via lumbar puncture, serves to alter the splicing of the protein product from SMN2, allowing the production of the more effective and stable protein with properties more similar to the SMN1 protein, thus ameliorating the effect of the SMN1 mutation. The patient, however, requires the administration of the therapeutic oligonucleotide indefinitely.
Gene Editing
The Gold Ring of therapeutic intervention, being able to actually correct the gene defect in any given disease, is still a goal of medical science. For the first time, this is a realistic aspiration due to the identification, development, and application of the gene editing tool Cas9 and CRISPR-Cas9 techniques.2 This new technology allows the production of a custom piece of DNA (cassette) that can repair the defective gene if incorporated into the DNA sequence of the host. The techniques necessary to introduce the therapeutic cassette to the affected cell and encourage the incorporation of the new material into the DNA have largely been developed already. The hurdle has always been the difficulty producing the therapeutic cassette for the given defect. The application of the CRISPR-Cas9 technology offers the promise of being able to produce a custom cassette specific for the given mutation, and thus potentially correcting the mutation involved in a wide variety of diseases.
The last quarter century has seen the expansion of the genetic techniques to allow the recognition and diagnosis of diseases that had resisted definitive diagnosis up to this time. These techniques have also led to the uncovering of disease mechanisms previously opaque to our understanding. The next quarter century promises to produce an explosion of therapeutic possibilities on a scale previously unimaginable. Despite the considerable initial expense of these new therapies, I believe they will be of incalculable value going forward.
References
1. de Koning AP, Gu W, Castoe TA, et al. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7(12):e1002384.
2. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
John B. Bodensteiner, MD
Dr. Bodensteiner is Director of the American Board of Psychiatry and Neurology, Founding Editor and Senior Associate Editor of Seminars in Pediatric Neurology, and Senior Associate Editor of the Journal of Child Neurology.
The impetus for this article is the celebration of 25 years of publication of Neurology Reviews. Child neurology has advanced so much in these years that it is hardly recognizable from the previous state of our understanding. Many components of the discipline include, but are not limited to, epilepsy, headache, demyelinating diseases, autoimmune diseases, neoplastic, neonatal, neuromuscular disease, and developmental conditions. My interest has been related to the neuromuscular diseases of childhood. Thus, I have chosen to describe some of the ways the advances in basic medical science and our understanding of the genetic and molecular mechanisms of disease have altered the way we think about these conditions. For the first time, this knowledge has allowed us to identify targets and techniques for effective intervention and disease modification.
Myotonic Muscular Dystrophy
One example of how the advancement in the discipline of genetics has changed our understanding of disease would be myotonic muscular dystrophy (MyoD1). When I started seeing patients in the mid 1960s, MyoD1, particularly as seen in children, was recognized as an example of autosomal dominant inheritance. The tendency for subsequent generations to be more severely affected than their parents is a phenomenon known as anticipation. Anticipation was recognized, though not explained, with the understanding of the genetic mechanisms of the time. The fact that when the disease affected an infant or newborn it was almost always inherited from the mother was also well established, though the reason for this was also not understood. Actually, during the late 1970s and early 1980s the existence of the phenomenon of anticipation was widely questioned and some publications proposed that anticipation was just an artifact of observation due to the fact that we (medical clinicians) were getting better and thus identifying the disease earlier than in past years. The recognition of the importance of repeated segments of DNA in the pathogenesis of human disease and the subsequent development of the technology to study the phenomenon allowed recognition of this previously unappreciated mechanism of genetic disease causation. This new genetic mechanism explained how anticipation could occur and confirmed it as a real phenomenon. Expanded numbers of tandem repeats probably allowed the explanation of the difference in gene transmission related to the parental gender as well.1
Genetic Insights
Advancements in the understanding of genetic mechanisms of disease have made it possible to begin to identify the molecular mechanisms operating in many previously incomprehensible diseases. The development of laboratory techniques necessary for the identification of these genetic abnormalities has brought the recognition of these disease mechanisms into clinical practice. Twenty-five years ago, the ability to identify alterations in DNA sequences, the presence of deletions/duplications and expansion of trinucleotide repeats, as well as the determination (and recognition of the significance) of gene copy number, was not available to the clinician. This advancement in genetic understanding has, in turn, allowed the identification of therapeutic targets in the hope of being able to moderate or eliminate the consequence of the defective gene.
Spinal Muscular Atrophy
In the last few years, the use of relatively small molecules such as oligonucleotides to alter the transcription of mutated RNA to produce a functioning protein has been developed. Because they are small molecules, they can be delivered with relative ease to the desired site. This technology has been applied to the treatment of spinal muscular atrophy (SMA) with considerable success.
Survival motor neuron gene product is essential for the health of the anterior horn cells of the spinal cord of the central nervous system. In the human genome there are two copies of the survival motor neuron gene, labeled SMN1 and SMN2. SMN1 normally produces a functional protein that is stable and necessary for the anterior horn survival. All patients with SMA have mutations in SMN1 that result in the gene being inactive. The protein product of SMN2 is usually truncated and not very stable, though it has some function. The severity of the resulting disease is influenced by the number of copies of the SMN2 gene present, and thus the available amount of partially functional SMN2 product. The oligonucleotide, in this case delivered via lumbar puncture, serves to alter the splicing of the protein product from SMN2, allowing the production of the more effective and stable protein with properties more similar to the SMN1 protein, thus ameliorating the effect of the SMN1 mutation. The patient, however, requires the administration of the therapeutic oligonucleotide indefinitely.
Gene Editing
The Gold Ring of therapeutic intervention, being able to actually correct the gene defect in any given disease, is still a goal of medical science. For the first time, this is a realistic aspiration due to the identification, development, and application of the gene editing tool Cas9 and CRISPR-Cas9 techniques.2 This new technology allows the production of a custom piece of DNA (cassette) that can repair the defective gene if incorporated into the DNA sequence of the host. The techniques necessary to introduce the therapeutic cassette to the affected cell and encourage the incorporation of the new material into the DNA have largely been developed already. The hurdle has always been the difficulty producing the therapeutic cassette for the given defect. The application of the CRISPR-Cas9 technology offers the promise of being able to produce a custom cassette specific for the given mutation, and thus potentially correcting the mutation involved in a wide variety of diseases.
The last quarter century has seen the expansion of the genetic techniques to allow the recognition and diagnosis of diseases that had resisted definitive diagnosis up to this time. These techniques have also led to the uncovering of disease mechanisms previously opaque to our understanding. The next quarter century promises to produce an explosion of therapeutic possibilities on a scale previously unimaginable. Despite the considerable initial expense of these new therapies, I believe they will be of incalculable value going forward.
References
1. de Koning AP, Gu W, Castoe TA, et al. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7(12):e1002384.
2. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
John B. Bodensteiner, MD
Dr. Bodensteiner is Director of the American Board of Psychiatry and Neurology, Founding Editor and Senior Associate Editor of Seminars in Pediatric Neurology, and Senior Associate Editor of the Journal of Child Neurology.
The impetus for this article is the celebration of 25 years of publication of Neurology Reviews. Child neurology has advanced so much in these years that it is hardly recognizable from the previous state of our understanding. Many components of the discipline include, but are not limited to, epilepsy, headache, demyelinating diseases, autoimmune diseases, neoplastic, neonatal, neuromuscular disease, and developmental conditions. My interest has been related to the neuromuscular diseases of childhood. Thus, I have chosen to describe some of the ways the advances in basic medical science and our understanding of the genetic and molecular mechanisms of disease have altered the way we think about these conditions. For the first time, this knowledge has allowed us to identify targets and techniques for effective intervention and disease modification.
Myotonic Muscular Dystrophy
One example of how the advancement in the discipline of genetics has changed our understanding of disease would be myotonic muscular dystrophy (MyoD1). When I started seeing patients in the mid 1960s, MyoD1, particularly as seen in children, was recognized as an example of autosomal dominant inheritance. The tendency for subsequent generations to be more severely affected than their parents is a phenomenon known as anticipation. Anticipation was recognized, though not explained, with the understanding of the genetic mechanisms of the time. The fact that when the disease affected an infant or newborn it was almost always inherited from the mother was also well established, though the reason for this was also not understood. Actually, during the late 1970s and early 1980s the existence of the phenomenon of anticipation was widely questioned and some publications proposed that anticipation was just an artifact of observation due to the fact that we (medical clinicians) were getting better and thus identifying the disease earlier than in past years. The recognition of the importance of repeated segments of DNA in the pathogenesis of human disease and the subsequent development of the technology to study the phenomenon allowed recognition of this previously unappreciated mechanism of genetic disease causation. This new genetic mechanism explained how anticipation could occur and confirmed it as a real phenomenon. Expanded numbers of tandem repeats probably allowed the explanation of the difference in gene transmission related to the parental gender as well.1
Genetic Insights
Advancements in the understanding of genetic mechanisms of disease have made it possible to begin to identify the molecular mechanisms operating in many previously incomprehensible diseases. The development of laboratory techniques necessary for the identification of these genetic abnormalities has brought the recognition of these disease mechanisms into clinical practice. Twenty-five years ago, the ability to identify alterations in DNA sequences, the presence of deletions/duplications and expansion of trinucleotide repeats, as well as the determination (and recognition of the significance) of gene copy number, was not available to the clinician. This advancement in genetic understanding has, in turn, allowed the identification of therapeutic targets in the hope of being able to moderate or eliminate the consequence of the defective gene.
Spinal Muscular Atrophy
In the last few years, the use of relatively small molecules such as oligonucleotides to alter the transcription of mutated RNA to produce a functioning protein has been developed. Because they are small molecules, they can be delivered with relative ease to the desired site. This technology has been applied to the treatment of spinal muscular atrophy (SMA) with considerable success.
Survival motor neuron gene product is essential for the health of the anterior horn cells of the spinal cord of the central nervous system. In the human genome there are two copies of the survival motor neuron gene, labeled SMN1 and SMN2. SMN1 normally produces a functional protein that is stable and necessary for the anterior horn survival. All patients with SMA have mutations in SMN1 that result in the gene being inactive. The protein product of SMN2 is usually truncated and not very stable, though it has some function. The severity of the resulting disease is influenced by the number of copies of the SMN2 gene present, and thus the available amount of partially functional SMN2 product. The oligonucleotide, in this case delivered via lumbar puncture, serves to alter the splicing of the protein product from SMN2, allowing the production of the more effective and stable protein with properties more similar to the SMN1 protein, thus ameliorating the effect of the SMN1 mutation. The patient, however, requires the administration of the therapeutic oligonucleotide indefinitely.
Gene Editing
The Gold Ring of therapeutic intervention, being able to actually correct the gene defect in any given disease, is still a goal of medical science. For the first time, this is a realistic aspiration due to the identification, development, and application of the gene editing tool Cas9 and CRISPR-Cas9 techniques.2 This new technology allows the production of a custom piece of DNA (cassette) that can repair the defective gene if incorporated into the DNA sequence of the host. The techniques necessary to introduce the therapeutic cassette to the affected cell and encourage the incorporation of the new material into the DNA have largely been developed already. The hurdle has always been the difficulty producing the therapeutic cassette for the given defect. The application of the CRISPR-Cas9 technology offers the promise of being able to produce a custom cassette specific for the given mutation, and thus potentially correcting the mutation involved in a wide variety of diseases.
The last quarter century has seen the expansion of the genetic techniques to allow the recognition and diagnosis of diseases that had resisted definitive diagnosis up to this time. These techniques have also led to the uncovering of disease mechanisms previously opaque to our understanding. The next quarter century promises to produce an explosion of therapeutic possibilities on a scale previously unimaginable. Despite the considerable initial expense of these new therapies, I believe they will be of incalculable value going forward.
References
1. de Koning AP, Gu W, Castoe TA, et al. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7(12):e1002384.
2. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
Tasimelteon May Be Effective for Jet Lag Disorder
The drug increased total sleep time by about an hour and a half.
BALTIMORE—Tasimelteon, a drug approved for non–24-hour sleep-wake disorder, has been shown to increase sleep times in travelers with jet lag, according to results from a phase III trial. “Tasimelteon demonstrated an increase in total sleep time of 85 minutes versus placebo and also demonstrated improvement in next-day alertness versus placebo,” said Christoph Polymeropoulos, MD, Medical Director of Vanda Pharmaceuticals, and colleagues at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Tasimelteon, sold under the trade name Hetlioz, is a melatonin receptor agonist that is FDA-approved for non–24-hour sleep-wake disorder but not for the treatment of
“Jet lag disorder is a circadian disorder frequently observed in millions of travelers who cross multiple time zones,” Dr. Polymeropoulos said. “Jet lag disorder is characterized by nighttime sleep disruption, decrease in daytime alertness, and impairment in social and occupational function.”
The JET8 trial induced the circadian challenge equivalent to crossing eight time zones. The study involved 318 individuals randomized evenly to 20 mg tasimelteon or placebo 30 minutes before bedtime. The primary end point of the study was total sleep time in the first two-thirds of sleep time, as measured by polysomnography.
Those on tasimelteon averaged 216.4 minutes of total sleep time in the first two-thirds of their sleep time versus 156.1 minutes for those on placebo, Dr. Polymeropoulos said. Total sleep times were 315.8 minutes versus 230.3 minutes, respectively. “For total sleep time, the tasimelteon subjects gained about an hour and a half, as measured by polysomnography,” Dr. Polymeropoulos said.
Other key markers the trial measured were latency to persistent sleep and wakefulness after sleep onset. They measured 15 minutes less and 74.6 minutes less, respectively, in the tasimelteon arm.
Dr. Polymeropoulos also disclosed early results of a second trial of tasimelteon in jet lag disorder: the JET Study, a two-phase transatlantic travel study of 25 patients. The subjects flew from four US cities to London for three nights, receiving tasimelteon or placebo each night in London. The study was terminated before reaching its enrollment goal of 90 patients because of its complexity, Vanda said in a separate press release. Over three nights of study, participants in the tasimelteon arm gained a total of about 130 minutes of sleep versus 40 minutes for those in the placebo arm, Dr. Polymeropoulos said.
Vanda plans to file a supplemental new drug application for tasimelteon for the treatment of jet lag disorder in the second half of this year.
Suggested Reading
Herxheimer A. Jet lag. BMJ Clin Evid. 2014 Apr 29;pii2303.
Srinivasan V, Singh J, Pandi-Perumal SR, et al. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27(11):796-813.
The drug increased total sleep time by about an hour and a half.
The drug increased total sleep time by about an hour and a half.
BALTIMORE—Tasimelteon, a drug approved for non–24-hour sleep-wake disorder, has been shown to increase sleep times in travelers with jet lag, according to results from a phase III trial. “Tasimelteon demonstrated an increase in total sleep time of 85 minutes versus placebo and also demonstrated improvement in next-day alertness versus placebo,” said Christoph Polymeropoulos, MD, Medical Director of Vanda Pharmaceuticals, and colleagues at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Tasimelteon, sold under the trade name Hetlioz, is a melatonin receptor agonist that is FDA-approved for non–24-hour sleep-wake disorder but not for the treatment of
“Jet lag disorder is a circadian disorder frequently observed in millions of travelers who cross multiple time zones,” Dr. Polymeropoulos said. “Jet lag disorder is characterized by nighttime sleep disruption, decrease in daytime alertness, and impairment in social and occupational function.”
The JET8 trial induced the circadian challenge equivalent to crossing eight time zones. The study involved 318 individuals randomized evenly to 20 mg tasimelteon or placebo 30 minutes before bedtime. The primary end point of the study was total sleep time in the first two-thirds of sleep time, as measured by polysomnography.
Those on tasimelteon averaged 216.4 minutes of total sleep time in the first two-thirds of their sleep time versus 156.1 minutes for those on placebo, Dr. Polymeropoulos said. Total sleep times were 315.8 minutes versus 230.3 minutes, respectively. “For total sleep time, the tasimelteon subjects gained about an hour and a half, as measured by polysomnography,” Dr. Polymeropoulos said.
Other key markers the trial measured were latency to persistent sleep and wakefulness after sleep onset. They measured 15 minutes less and 74.6 minutes less, respectively, in the tasimelteon arm.
Dr. Polymeropoulos also disclosed early results of a second trial of tasimelteon in jet lag disorder: the JET Study, a two-phase transatlantic travel study of 25 patients. The subjects flew from four US cities to London for three nights, receiving tasimelteon or placebo each night in London. The study was terminated before reaching its enrollment goal of 90 patients because of its complexity, Vanda said in a separate press release. Over three nights of study, participants in the tasimelteon arm gained a total of about 130 minutes of sleep versus 40 minutes for those in the placebo arm, Dr. Polymeropoulos said.
Vanda plans to file a supplemental new drug application for tasimelteon for the treatment of jet lag disorder in the second half of this year.
Suggested Reading
Herxheimer A. Jet lag. BMJ Clin Evid. 2014 Apr 29;pii2303.
Srinivasan V, Singh J, Pandi-Perumal SR, et al. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27(11):796-813.
BALTIMORE—Tasimelteon, a drug approved for non–24-hour sleep-wake disorder, has been shown to increase sleep times in travelers with jet lag, according to results from a phase III trial. “Tasimelteon demonstrated an increase in total sleep time of 85 minutes versus placebo and also demonstrated improvement in next-day alertness versus placebo,” said Christoph Polymeropoulos, MD, Medical Director of Vanda Pharmaceuticals, and colleagues at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Tasimelteon, sold under the trade name Hetlioz, is a melatonin receptor agonist that is FDA-approved for non–24-hour sleep-wake disorder but not for the treatment of
“Jet lag disorder is a circadian disorder frequently observed in millions of travelers who cross multiple time zones,” Dr. Polymeropoulos said. “Jet lag disorder is characterized by nighttime sleep disruption, decrease in daytime alertness, and impairment in social and occupational function.”
The JET8 trial induced the circadian challenge equivalent to crossing eight time zones. The study involved 318 individuals randomized evenly to 20 mg tasimelteon or placebo 30 minutes before bedtime. The primary end point of the study was total sleep time in the first two-thirds of sleep time, as measured by polysomnography.
Those on tasimelteon averaged 216.4 minutes of total sleep time in the first two-thirds of their sleep time versus 156.1 minutes for those on placebo, Dr. Polymeropoulos said. Total sleep times were 315.8 minutes versus 230.3 minutes, respectively. “For total sleep time, the tasimelteon subjects gained about an hour and a half, as measured by polysomnography,” Dr. Polymeropoulos said.
Other key markers the trial measured were latency to persistent sleep and wakefulness after sleep onset. They measured 15 minutes less and 74.6 minutes less, respectively, in the tasimelteon arm.
Dr. Polymeropoulos also disclosed early results of a second trial of tasimelteon in jet lag disorder: the JET Study, a two-phase transatlantic travel study of 25 patients. The subjects flew from four US cities to London for three nights, receiving tasimelteon or placebo each night in London. The study was terminated before reaching its enrollment goal of 90 patients because of its complexity, Vanda said in a separate press release. Over three nights of study, participants in the tasimelteon arm gained a total of about 130 minutes of sleep versus 40 minutes for those in the placebo arm, Dr. Polymeropoulos said.
Vanda plans to file a supplemental new drug application for tasimelteon for the treatment of jet lag disorder in the second half of this year.
Suggested Reading
Herxheimer A. Jet lag. BMJ Clin Evid. 2014 Apr 29;pii2303.
Srinivasan V, Singh J, Pandi-Perumal SR, et al. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27(11):796-813.
Slow-Wave Activity May Affect Depression
Disruption of slow-wave activity decreases waking theta activity in patients with depression.
BALTIMORE—Disruption of slow-wave activity (SWA) may explain the positive influence that sleep deprivation appears to have on major depressive disorder, according to results of a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Jennifer Goldschmied, PhD, a postdoctoral fellow at the University of Pennsylvania in Philadelphia, reported preliminary results of a study of SWA disruption in 26 subjects (12 healthy controls and 14 people with major depressive disorder) that found a significant decrease of about 20% in waking theta activity, as measured with EEG, among patients with depression. In the three-night sleep study, conducted at the University of Michigan in Ann Arbor, an adaptation night was followed by baseline and SWA disruption nights. Investigators performed EEGs each night. After the baseline night, patients also had a morning and afternoon EEG.
Across the baseline day, patients with depression showed “no modulation of theta activity whatsoever,” said Dr. Goldschmied. “Then we see, following slow-wave disruption, a significant decrease in theta activity,” whereas healthy controls showed no change in waking theta following SWA disruption. “What this means is that the presence of SWA may actually be facilitating the reduction of theta or sleep propensity during typical sleep in healthy individuals,” she added. In patients with depression, theta power declined from around 5.4 Hz to 4.3 Hz following SWA disruption.
This finding supports the synaptic homeostasis hypothesis proposed by Giulio Tononi, MD, PhD, and Chiara Cirelli, MD, PhD, of the University of Wisconsin, said Dr. Goldschmied. This hypothesis holds that SWA is a marker of synaptic strength and promotes the downscaling of synaptic strength during sleep. No method for measuring synaptic strength in humans exists, Dr. Goldschmied added, but waking theta can be considered a proxy for net synaptic strength across the cortex.
Other research that has found SWA disruption improves mood, but Dr. Goldschmied and colleagues found no role of decreased theta activity in that change. “To go even further,” she said, “we looked at the entire data set and found no relationship between the decrease in theta and any of the measures of sleep architecture, so there’s really no way to predict this decrease in our sample of people with depression.”
SWA plays a significant role in depression and merits more study, said Dr. Goldschmied. Future research should examine the effects of SWA disruption in a larger sample, investigate theta findings with other proxy measures of synaptic strength such as brain-derived neurotrophic factor and transcranial magnetic stimulation, explore differences in SWA between sexes, and explore how SWA enhancement influences mood and theta activity, she concluded.
—Richard Mark Kirkner
Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143-150.
Disruption of slow-wave activity decreases waking theta activity in patients with depression.
Disruption of slow-wave activity decreases waking theta activity in patients with depression.
BALTIMORE—Disruption of slow-wave activity (SWA) may explain the positive influence that sleep deprivation appears to have on major depressive disorder, according to results of a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Jennifer Goldschmied, PhD, a postdoctoral fellow at the University of Pennsylvania in Philadelphia, reported preliminary results of a study of SWA disruption in 26 subjects (12 healthy controls and 14 people with major depressive disorder) that found a significant decrease of about 20% in waking theta activity, as measured with EEG, among patients with depression. In the three-night sleep study, conducted at the University of Michigan in Ann Arbor, an adaptation night was followed by baseline and SWA disruption nights. Investigators performed EEGs each night. After the baseline night, patients also had a morning and afternoon EEG.
Across the baseline day, patients with depression showed “no modulation of theta activity whatsoever,” said Dr. Goldschmied. “Then we see, following slow-wave disruption, a significant decrease in theta activity,” whereas healthy controls showed no change in waking theta following SWA disruption. “What this means is that the presence of SWA may actually be facilitating the reduction of theta or sleep propensity during typical sleep in healthy individuals,” she added. In patients with depression, theta power declined from around 5.4 Hz to 4.3 Hz following SWA disruption.
This finding supports the synaptic homeostasis hypothesis proposed by Giulio Tononi, MD, PhD, and Chiara Cirelli, MD, PhD, of the University of Wisconsin, said Dr. Goldschmied. This hypothesis holds that SWA is a marker of synaptic strength and promotes the downscaling of synaptic strength during sleep. No method for measuring synaptic strength in humans exists, Dr. Goldschmied added, but waking theta can be considered a proxy for net synaptic strength across the cortex.
Other research that has found SWA disruption improves mood, but Dr. Goldschmied and colleagues found no role of decreased theta activity in that change. “To go even further,” she said, “we looked at the entire data set and found no relationship between the decrease in theta and any of the measures of sleep architecture, so there’s really no way to predict this decrease in our sample of people with depression.”
SWA plays a significant role in depression and merits more study, said Dr. Goldschmied. Future research should examine the effects of SWA disruption in a larger sample, investigate theta findings with other proxy measures of synaptic strength such as brain-derived neurotrophic factor and transcranial magnetic stimulation, explore differences in SWA between sexes, and explore how SWA enhancement influences mood and theta activity, she concluded.
—Richard Mark Kirkner
Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143-150.
BALTIMORE—Disruption of slow-wave activity (SWA) may explain the positive influence that sleep deprivation appears to have on major depressive disorder, according to results of a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
Jennifer Goldschmied, PhD, a postdoctoral fellow at the University of Pennsylvania in Philadelphia, reported preliminary results of a study of SWA disruption in 26 subjects (12 healthy controls and 14 people with major depressive disorder) that found a significant decrease of about 20% in waking theta activity, as measured with EEG, among patients with depression. In the three-night sleep study, conducted at the University of Michigan in Ann Arbor, an adaptation night was followed by baseline and SWA disruption nights. Investigators performed EEGs each night. After the baseline night, patients also had a morning and afternoon EEG.
Across the baseline day, patients with depression showed “no modulation of theta activity whatsoever,” said Dr. Goldschmied. “Then we see, following slow-wave disruption, a significant decrease in theta activity,” whereas healthy controls showed no change in waking theta following SWA disruption. “What this means is that the presence of SWA may actually be facilitating the reduction of theta or sleep propensity during typical sleep in healthy individuals,” she added. In patients with depression, theta power declined from around 5.4 Hz to 4.3 Hz following SWA disruption.
This finding supports the synaptic homeostasis hypothesis proposed by Giulio Tononi, MD, PhD, and Chiara Cirelli, MD, PhD, of the University of Wisconsin, said Dr. Goldschmied. This hypothesis holds that SWA is a marker of synaptic strength and promotes the downscaling of synaptic strength during sleep. No method for measuring synaptic strength in humans exists, Dr. Goldschmied added, but waking theta can be considered a proxy for net synaptic strength across the cortex.
Other research that has found SWA disruption improves mood, but Dr. Goldschmied and colleagues found no role of decreased theta activity in that change. “To go even further,” she said, “we looked at the entire data set and found no relationship between the decrease in theta and any of the measures of sleep architecture, so there’s really no way to predict this decrease in our sample of people with depression.”
SWA plays a significant role in depression and merits more study, said Dr. Goldschmied. Future research should examine the effects of SWA disruption in a larger sample, investigate theta findings with other proxy measures of synaptic strength such as brain-derived neurotrophic factor and transcranial magnetic stimulation, explore differences in SWA between sexes, and explore how SWA enhancement influences mood and theta activity, she concluded.
—Richard Mark Kirkner
Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143-150.
OSA With Worsening Hypoxemia Raises Risk of Metabolic Syndrome
Older age and increased BMI also heighten the risk of incident metabolic syndrome.
BALTIMORE—Patients with obstructive sleep apnea (OSA) who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, according to an eight-year cohort study described at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
“Considering that we have a very high prevalence of moderate to severe OSA in the general population, this is a very important finding because it indicates that we need some clinical options for treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events,” said Camila Hirotsu, PhD, of the Federal University of São Paulo, Brazil.
Dr. Hirotsu presented eight-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate how OSA can affect the risk of developing metabolic syndrome in the general population. Metabolic syndrome is defined as a cluster of three or more of the following cardiovascular metabolic components: low HDL levels, high glucose and triglyceride levels, hypertension, and abdominal obesity. Between 50% and 60% of patients with metabolic syndrome have OSA, said Dr. Hirotsu.
The study enrolled 1,074 patients at baseline, and enrollment ended in 2008. The researchers obtained follow-up on 712 participants, who were evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of metabolic syndrome at baseline. Of this sample, 44% subsequently developed metabolic syndrome.
The median age of patients who developed metabolic syndrome was 40.8 versus 36.1 for those who did not. Patients who developed metabolic syndrome also had a higher BMI, but were not obese, compared with the other participants (26.9 kg/m2 vs 23.8 kg/m2). Patients completed questionnaires and underwent full polysomnography and clinical assessments.
For patients with moderate to severe OSA, the odds ratio (OR) of developing incident metabolic syndrome was 2.47, said Dr. Hirotsu. Rates of moderate to severe OSA were 21.3% for the group that developed metabolic syndrome versus 9% for the group that did not.
Apnea–hypopnea index (OR, 1.16), 3% oxygen desaturation index (OR, 1.24), and time with oxygen saturation by pulse oximeter less than 90% (OR, 1.42) were associated with incident metabolic syndrome, according to the researchers.
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of metabolic syndrome in the general population,” said Dr. Hirotsu.
A secondary aim of the study was to evaluate the impact of metabolic syndrome on the risk of developing OSA in the general population. “It seems that metabolic syndrome is not really an independent risk factor for OSA,” Dr. Hirotsu said.
—Richard Mark Kirkner
Suggested Reading
Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065.
Older age and increased BMI also heighten the risk of incident metabolic syndrome.
Older age and increased BMI also heighten the risk of incident metabolic syndrome.
BALTIMORE—Patients with obstructive sleep apnea (OSA) who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, according to an eight-year cohort study described at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
“Considering that we have a very high prevalence of moderate to severe OSA in the general population, this is a very important finding because it indicates that we need some clinical options for treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events,” said Camila Hirotsu, PhD, of the Federal University of São Paulo, Brazil.
Dr. Hirotsu presented eight-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate how OSA can affect the risk of developing metabolic syndrome in the general population. Metabolic syndrome is defined as a cluster of three or more of the following cardiovascular metabolic components: low HDL levels, high glucose and triglyceride levels, hypertension, and abdominal obesity. Between 50% and 60% of patients with metabolic syndrome have OSA, said Dr. Hirotsu.
The study enrolled 1,074 patients at baseline, and enrollment ended in 2008. The researchers obtained follow-up on 712 participants, who were evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of metabolic syndrome at baseline. Of this sample, 44% subsequently developed metabolic syndrome.
The median age of patients who developed metabolic syndrome was 40.8 versus 36.1 for those who did not. Patients who developed metabolic syndrome also had a higher BMI, but were not obese, compared with the other participants (26.9 kg/m2 vs 23.8 kg/m2). Patients completed questionnaires and underwent full polysomnography and clinical assessments.
For patients with moderate to severe OSA, the odds ratio (OR) of developing incident metabolic syndrome was 2.47, said Dr. Hirotsu. Rates of moderate to severe OSA were 21.3% for the group that developed metabolic syndrome versus 9% for the group that did not.
Apnea–hypopnea index (OR, 1.16), 3% oxygen desaturation index (OR, 1.24), and time with oxygen saturation by pulse oximeter less than 90% (OR, 1.42) were associated with incident metabolic syndrome, according to the researchers.
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of metabolic syndrome in the general population,” said Dr. Hirotsu.
A secondary aim of the study was to evaluate the impact of metabolic syndrome on the risk of developing OSA in the general population. “It seems that metabolic syndrome is not really an independent risk factor for OSA,” Dr. Hirotsu said.
—Richard Mark Kirkner
Suggested Reading
Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065.
BALTIMORE—Patients with obstructive sleep apnea (OSA) who are prone to worsening of hypoxemia at night have a heightened risk of developing metabolic syndrome, according to an eight-year cohort study described at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
“Considering that we have a very high prevalence of moderate to severe OSA in the general population, this is a very important finding because it indicates that we need some clinical options for treating OSA in those that have metabolic syndrome to decrease the risk of morbidity and mortality and cardiac events,” said Camila Hirotsu, PhD, of the Federal University of São Paulo, Brazil.
Dr. Hirotsu presented eight-year follow-up results of the EPISONO cohort, an observational prospective study conducted in Brazil, the goal of which was to evaluate how OSA can affect the risk of developing metabolic syndrome in the general population. Metabolic syndrome is defined as a cluster of three or more of the following cardiovascular metabolic components: low HDL levels, high glucose and triglyceride levels, hypertension, and abdominal obesity. Between 50% and 60% of patients with metabolic syndrome have OSA, said Dr. Hirotsu.
The study enrolled 1,074 patients at baseline, and enrollment ended in 2008. The researchers obtained follow-up on 712 participants, who were evaluated from July 2015 to April 2016. After exclusions, the study evaluated 476 patients who were free of metabolic syndrome at baseline. Of this sample, 44% subsequently developed metabolic syndrome.
The median age of patients who developed metabolic syndrome was 40.8 versus 36.1 for those who did not. Patients who developed metabolic syndrome also had a higher BMI, but were not obese, compared with the other participants (26.9 kg/m2 vs 23.8 kg/m2). Patients completed questionnaires and underwent full polysomnography and clinical assessments.
For patients with moderate to severe OSA, the odds ratio (OR) of developing incident metabolic syndrome was 2.47, said Dr. Hirotsu. Rates of moderate to severe OSA were 21.3% for the group that developed metabolic syndrome versus 9% for the group that did not.
Apnea–hypopnea index (OR, 1.16), 3% oxygen desaturation index (OR, 1.24), and time with oxygen saturation by pulse oximeter less than 90% (OR, 1.42) were associated with incident metabolic syndrome, according to the researchers.
“Moderate to severe OSA at baseline and worsening of nocturnal hyperemia from baseline to follow-up are really independent risk factors to increase the incidence of metabolic syndrome in the general population,” said Dr. Hirotsu.
A secondary aim of the study was to evaluate the impact of metabolic syndrome on the risk of developing OSA in the general population. “It seems that metabolic syndrome is not really an independent risk factor for OSA,” Dr. Hirotsu said.
—Richard Mark Kirkner
Suggested Reading
Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065.
HPV positivity associated with good esophageal adenocarcinoma outcomes
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
FROM JAMA NETWORK OPEN
Key clinical point: Human papillomavirus infection is associated with better outcomes for patients with esophageal adenocarcinoma and other head and neck cancers.
Major finding: Mean disease-free survival was 40.3 months for HPV-positive patients versus 24.1 months for HPV-negative patients (P = .003).
Study details: A retrospective case-control study of 142 patients with Barrett high-grade dysplasia or esophageal adenocarcinoma.
Disclosures: The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
Source: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi: 10.1001/jamanetworkopen.2018.1054.
A Rational Approach to Starting, Stopping, and Switching DMTs
Dr. Patricia Coyle’s Donald Paty Memorial Lecture covered current controversies in DMT use.
NASHVILLE—What guides decisions to start, stop, switch, or restart disease-modifying therapy (DMT) in patients with multiple sclerosis (MS)—particularly in the absence of extensive robust data? Because multiple treatment options exist, there is a higher bar for a DMT to be considered successful. Clinicians need to base individualized decisions on the best available evidence, according to a presentation at the 2018 CMSC Annual Meeting.
“We have practice guidelines, but unfortunately they are almost always out of date,” said Patricia K. Coyle, MD, Professor and Vice Chair of Clinical Affairs and Director of the MS Comprehensive Care Center at Stony Brook University Hospital in New York. “Guidelines have a lot of good things to say and several things that I would not quite agree with, but at least they are a helpful start. In the future, as we use cooperative studies, databases, and meaningful observations on large numbers of patients with MS to maximize our information and data, we will ultimately resolve all of these DMT debates.”
Starting Treatment
Controversies surrounding initiation of DMTs include whether to treat patients with radiologically isolated syndrome, when to start treating relapsing forms of MS that are not active, and, in patients with primary progressive MS, when the monoclonal agent ocrelizumab is appropriate. Current American Academy of Neurology (AAN) practice guidelines recommend both prescribing a DMT to patients with clinically isolated syndrome (CIS), patients with a first attack who have at least two MRI lesions characteristic of MS, and offering DMTs to patients with relapsing forms of MS and recent attacks or MRI activity.
The AAN recommendations stem from the benefits seen with early treatment, Dr. Coyle emphasized. “There may be a critical window of opportunity in treating early,” she said. “If MS involves accumulating permanent damage to the CNS, wouldn’t you want to stop or minimize that as quickly as possible—not wait until there has already been significant injury?” Virtually all studies comparing early versus delayed treatment support early treatment—and “age probably matters.”
In a 2017 meta-analysis of 38 clinical trials that assessed disability worsening and disease progression in a total population of 28,000 subjects with MS, the authors found that DMT efficacy and impact on disability fell with advancing age. The model created from this study predicted that none of the DMTs would be efficacious on disability in patients older than 53. Evidence also suggested that, after patients reached age 40.5, high-efficacy DMTs (eg, ocrelizumab, natalizumab) no longer outperformed lower-efficacy oral agents (fingolimod, teriflunomide, dimethyl fumarate) or injectables (interferon betas and glatiramer acetates).
While skeptical of this latter finding, Dr. Coyle termed it “provocative” because it demonstrates that patients should be treated at a younger age. “When MS declares itself, the disease has probably existed for years, damaging CNS tissue,” she said. Older age at onset probably means that the disease has been present but silent for a long period of time. In addition, CNS and brain reserve become increasingly impaired with age. “The older you are, in theory, the less CNS reserve you have.”
Stopping Treatment
Potential reasons for stopping DMT include relapsing MS or CIS that has been inactive for years; secondary progressive MS that is distant from the relapsing phase and now in a purely neurodegenerative one; age older than 60; and pregnancy.
Citing just a handful of studies in this area, Dr. Coyle pointed to the MSBase Registry of patients with relapsing MS who, on injectables, had no relapse for five years or more. Among 485 patients who stopped DMT versus 854 who continued treatment, time to relapse was similar but time to confirmed disability was shorter in those who stopped treatment. Those who stopped treatment also had an increased risk for entering the progressive stage. In another study of two cohorts who stopped DMT, 11.7% of patients with secondary progressive MS who were stable for two to 20 years, versus 58.8% of patients with relapsing MS, had acute disease within one to two years. The authors noted the secondary progressive MS cohort was older with higher EDSS scores. A retrospective review of patients with MS older than 60 who had been on DMT for more than two years showed that, of the 29.7% who stopped DMT, only one relapsed and just 10.7% reinitiated DMT.
“I would not stop a well-tolerated DMT in relapsing MS,” said Dr. Coyle. “I would continue DMT in CIS if patients were at high risk, even if they had had no disease activity for years. I would discuss stopping therapy in patients with secondary progressive MS who were older than 60, and in very debilitated patients with progressive MS.”
Switching DMTs
In terms of switching, a suboptimal response is likely magnified by delaying therapy, not matching patients well to their initial DMT, and not identifying poor response quickly,” said Dr. Coyle.
Reasons to switch include breakthrough activity—ie, the minimal evidence of disease activity (MEDA) or no evidence of disease activity (NEDA) target not met; MRI activity; poor tolerability; and abnormal laboratory data or clinical complications. Clinicians should consult the AAN practice guidelines for recommendations on when and which DMTs to switch.
Dr. Coyle again cited the MSBase Registry, in which three studies were conducted involving patients with MS who were on interferon beta or glatiramer acetate who had breakthrough attacks or disability. “In one study they switched them to another injectable or fingolimod,” she said. “They did better if they got to fingolimod. Another study involved switching to another injectable or natalizumab. They did better if they were on natalizumab. A third study looked at switching to fingolimod or natalizumab. They did better with natalizumab.”
Dr. Coyle observed that the first one to two years after initiating DMT may be the most important for determining whether a patient is doing well. “A DMT takes a few months to be fully operational,” she said. “If you are switching for breakthrough activity, you need to go to a higher-efficacy DMT. My treat-to target is not NEDA, it is MEDA.”
—Fred Balzac
Suggested Reading
Berkovich R. Clinical and MRI outcomes after stopping or switching disease-modifying therapy in stable MS patients: a case series report. Mult Scler Relat Disord. 2017;17:123-127.
Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
Coyle PK. Switching therapies in multiple sclerosis. CNS Drugs. 2013;27(4):239-247.
Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler. 2018 Mar 1 [Epub ahead of print].
Kappos L, Radue EW, Comi G, et al, for the TOFINGO study group. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology. 2015;85(1):29-39.
Kister I, Spelman T, Alroughani R, et al, for the MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
Maillart E, Vidal JS, Brassat D, et al. Natalizumab-PML survivors with subsequent MS treatment: clinico-radiologic outcome. Neurol Neuroimmunol Neuroinflamm. 2017;4(3):e346.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
Dr. Patricia Coyle’s Donald Paty Memorial Lecture covered current controversies in DMT use.
Dr. Patricia Coyle’s Donald Paty Memorial Lecture covered current controversies in DMT use.
NASHVILLE—What guides decisions to start, stop, switch, or restart disease-modifying therapy (DMT) in patients with multiple sclerosis (MS)—particularly in the absence of extensive robust data? Because multiple treatment options exist, there is a higher bar for a DMT to be considered successful. Clinicians need to base individualized decisions on the best available evidence, according to a presentation at the 2018 CMSC Annual Meeting.
“We have practice guidelines, but unfortunately they are almost always out of date,” said Patricia K. Coyle, MD, Professor and Vice Chair of Clinical Affairs and Director of the MS Comprehensive Care Center at Stony Brook University Hospital in New York. “Guidelines have a lot of good things to say and several things that I would not quite agree with, but at least they are a helpful start. In the future, as we use cooperative studies, databases, and meaningful observations on large numbers of patients with MS to maximize our information and data, we will ultimately resolve all of these DMT debates.”
Starting Treatment
Controversies surrounding initiation of DMTs include whether to treat patients with radiologically isolated syndrome, when to start treating relapsing forms of MS that are not active, and, in patients with primary progressive MS, when the monoclonal agent ocrelizumab is appropriate. Current American Academy of Neurology (AAN) practice guidelines recommend both prescribing a DMT to patients with clinically isolated syndrome (CIS), patients with a first attack who have at least two MRI lesions characteristic of MS, and offering DMTs to patients with relapsing forms of MS and recent attacks or MRI activity.
The AAN recommendations stem from the benefits seen with early treatment, Dr. Coyle emphasized. “There may be a critical window of opportunity in treating early,” she said. “If MS involves accumulating permanent damage to the CNS, wouldn’t you want to stop or minimize that as quickly as possible—not wait until there has already been significant injury?” Virtually all studies comparing early versus delayed treatment support early treatment—and “age probably matters.”
In a 2017 meta-analysis of 38 clinical trials that assessed disability worsening and disease progression in a total population of 28,000 subjects with MS, the authors found that DMT efficacy and impact on disability fell with advancing age. The model created from this study predicted that none of the DMTs would be efficacious on disability in patients older than 53. Evidence also suggested that, after patients reached age 40.5, high-efficacy DMTs (eg, ocrelizumab, natalizumab) no longer outperformed lower-efficacy oral agents (fingolimod, teriflunomide, dimethyl fumarate) or injectables (interferon betas and glatiramer acetates).
While skeptical of this latter finding, Dr. Coyle termed it “provocative” because it demonstrates that patients should be treated at a younger age. “When MS declares itself, the disease has probably existed for years, damaging CNS tissue,” she said. Older age at onset probably means that the disease has been present but silent for a long period of time. In addition, CNS and brain reserve become increasingly impaired with age. “The older you are, in theory, the less CNS reserve you have.”
Stopping Treatment
Potential reasons for stopping DMT include relapsing MS or CIS that has been inactive for years; secondary progressive MS that is distant from the relapsing phase and now in a purely neurodegenerative one; age older than 60; and pregnancy.
Citing just a handful of studies in this area, Dr. Coyle pointed to the MSBase Registry of patients with relapsing MS who, on injectables, had no relapse for five years or more. Among 485 patients who stopped DMT versus 854 who continued treatment, time to relapse was similar but time to confirmed disability was shorter in those who stopped treatment. Those who stopped treatment also had an increased risk for entering the progressive stage. In another study of two cohorts who stopped DMT, 11.7% of patients with secondary progressive MS who were stable for two to 20 years, versus 58.8% of patients with relapsing MS, had acute disease within one to two years. The authors noted the secondary progressive MS cohort was older with higher EDSS scores. A retrospective review of patients with MS older than 60 who had been on DMT for more than two years showed that, of the 29.7% who stopped DMT, only one relapsed and just 10.7% reinitiated DMT.
“I would not stop a well-tolerated DMT in relapsing MS,” said Dr. Coyle. “I would continue DMT in CIS if patients were at high risk, even if they had had no disease activity for years. I would discuss stopping therapy in patients with secondary progressive MS who were older than 60, and in very debilitated patients with progressive MS.”
Switching DMTs
In terms of switching, a suboptimal response is likely magnified by delaying therapy, not matching patients well to their initial DMT, and not identifying poor response quickly,” said Dr. Coyle.
Reasons to switch include breakthrough activity—ie, the minimal evidence of disease activity (MEDA) or no evidence of disease activity (NEDA) target not met; MRI activity; poor tolerability; and abnormal laboratory data or clinical complications. Clinicians should consult the AAN practice guidelines for recommendations on when and which DMTs to switch.
Dr. Coyle again cited the MSBase Registry, in which three studies were conducted involving patients with MS who were on interferon beta or glatiramer acetate who had breakthrough attacks or disability. “In one study they switched them to another injectable or fingolimod,” she said. “They did better if they got to fingolimod. Another study involved switching to another injectable or natalizumab. They did better if they were on natalizumab. A third study looked at switching to fingolimod or natalizumab. They did better with natalizumab.”
Dr. Coyle observed that the first one to two years after initiating DMT may be the most important for determining whether a patient is doing well. “A DMT takes a few months to be fully operational,” she said. “If you are switching for breakthrough activity, you need to go to a higher-efficacy DMT. My treat-to target is not NEDA, it is MEDA.”
—Fred Balzac
Suggested Reading
Berkovich R. Clinical and MRI outcomes after stopping or switching disease-modifying therapy in stable MS patients: a case series report. Mult Scler Relat Disord. 2017;17:123-127.
Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
Coyle PK. Switching therapies in multiple sclerosis. CNS Drugs. 2013;27(4):239-247.
Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler. 2018 Mar 1 [Epub ahead of print].
Kappos L, Radue EW, Comi G, et al, for the TOFINGO study group. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology. 2015;85(1):29-39.
Kister I, Spelman T, Alroughani R, et al, for the MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
Maillart E, Vidal JS, Brassat D, et al. Natalizumab-PML survivors with subsequent MS treatment: clinico-radiologic outcome. Neurol Neuroimmunol Neuroinflamm. 2017;4(3):e346.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
NASHVILLE—What guides decisions to start, stop, switch, or restart disease-modifying therapy (DMT) in patients with multiple sclerosis (MS)—particularly in the absence of extensive robust data? Because multiple treatment options exist, there is a higher bar for a DMT to be considered successful. Clinicians need to base individualized decisions on the best available evidence, according to a presentation at the 2018 CMSC Annual Meeting.
“We have practice guidelines, but unfortunately they are almost always out of date,” said Patricia K. Coyle, MD, Professor and Vice Chair of Clinical Affairs and Director of the MS Comprehensive Care Center at Stony Brook University Hospital in New York. “Guidelines have a lot of good things to say and several things that I would not quite agree with, but at least they are a helpful start. In the future, as we use cooperative studies, databases, and meaningful observations on large numbers of patients with MS to maximize our information and data, we will ultimately resolve all of these DMT debates.”
Starting Treatment
Controversies surrounding initiation of DMTs include whether to treat patients with radiologically isolated syndrome, when to start treating relapsing forms of MS that are not active, and, in patients with primary progressive MS, when the monoclonal agent ocrelizumab is appropriate. Current American Academy of Neurology (AAN) practice guidelines recommend both prescribing a DMT to patients with clinically isolated syndrome (CIS), patients with a first attack who have at least two MRI lesions characteristic of MS, and offering DMTs to patients with relapsing forms of MS and recent attacks or MRI activity.
The AAN recommendations stem from the benefits seen with early treatment, Dr. Coyle emphasized. “There may be a critical window of opportunity in treating early,” she said. “If MS involves accumulating permanent damage to the CNS, wouldn’t you want to stop or minimize that as quickly as possible—not wait until there has already been significant injury?” Virtually all studies comparing early versus delayed treatment support early treatment—and “age probably matters.”
In a 2017 meta-analysis of 38 clinical trials that assessed disability worsening and disease progression in a total population of 28,000 subjects with MS, the authors found that DMT efficacy and impact on disability fell with advancing age. The model created from this study predicted that none of the DMTs would be efficacious on disability in patients older than 53. Evidence also suggested that, after patients reached age 40.5, high-efficacy DMTs (eg, ocrelizumab, natalizumab) no longer outperformed lower-efficacy oral agents (fingolimod, teriflunomide, dimethyl fumarate) or injectables (interferon betas and glatiramer acetates).
While skeptical of this latter finding, Dr. Coyle termed it “provocative” because it demonstrates that patients should be treated at a younger age. “When MS declares itself, the disease has probably existed for years, damaging CNS tissue,” she said. Older age at onset probably means that the disease has been present but silent for a long period of time. In addition, CNS and brain reserve become increasingly impaired with age. “The older you are, in theory, the less CNS reserve you have.”
Stopping Treatment
Potential reasons for stopping DMT include relapsing MS or CIS that has been inactive for years; secondary progressive MS that is distant from the relapsing phase and now in a purely neurodegenerative one; age older than 60; and pregnancy.
Citing just a handful of studies in this area, Dr. Coyle pointed to the MSBase Registry of patients with relapsing MS who, on injectables, had no relapse for five years or more. Among 485 patients who stopped DMT versus 854 who continued treatment, time to relapse was similar but time to confirmed disability was shorter in those who stopped treatment. Those who stopped treatment also had an increased risk for entering the progressive stage. In another study of two cohorts who stopped DMT, 11.7% of patients with secondary progressive MS who were stable for two to 20 years, versus 58.8% of patients with relapsing MS, had acute disease within one to two years. The authors noted the secondary progressive MS cohort was older with higher EDSS scores. A retrospective review of patients with MS older than 60 who had been on DMT for more than two years showed that, of the 29.7% who stopped DMT, only one relapsed and just 10.7% reinitiated DMT.
“I would not stop a well-tolerated DMT in relapsing MS,” said Dr. Coyle. “I would continue DMT in CIS if patients were at high risk, even if they had had no disease activity for years. I would discuss stopping therapy in patients with secondary progressive MS who were older than 60, and in very debilitated patients with progressive MS.”
Switching DMTs
In terms of switching, a suboptimal response is likely magnified by delaying therapy, not matching patients well to their initial DMT, and not identifying poor response quickly,” said Dr. Coyle.
Reasons to switch include breakthrough activity—ie, the minimal evidence of disease activity (MEDA) or no evidence of disease activity (NEDA) target not met; MRI activity; poor tolerability; and abnormal laboratory data or clinical complications. Clinicians should consult the AAN practice guidelines for recommendations on when and which DMTs to switch.
Dr. Coyle again cited the MSBase Registry, in which three studies were conducted involving patients with MS who were on interferon beta or glatiramer acetate who had breakthrough attacks or disability. “In one study they switched them to another injectable or fingolimod,” she said. “They did better if they got to fingolimod. Another study involved switching to another injectable or natalizumab. They did better if they were on natalizumab. A third study looked at switching to fingolimod or natalizumab. They did better with natalizumab.”
Dr. Coyle observed that the first one to two years after initiating DMT may be the most important for determining whether a patient is doing well. “A DMT takes a few months to be fully operational,” she said. “If you are switching for breakthrough activity, you need to go to a higher-efficacy DMT. My treat-to target is not NEDA, it is MEDA.”
—Fred Balzac
Suggested Reading
Berkovich R. Clinical and MRI outcomes after stopping or switching disease-modifying therapy in stable MS patients: a case series report. Mult Scler Relat Disord. 2017;17:123-127.
Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
Coyle PK. Switching therapies in multiple sclerosis. CNS Drugs. 2013;27(4):239-247.
Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler. 2018 Mar 1 [Epub ahead of print].
Kappos L, Radue EW, Comi G, et al, for the TOFINGO study group. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology. 2015;85(1):29-39.
Kister I, Spelman T, Alroughani R, et al, for the MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
Maillart E, Vidal JS, Brassat D, et al. Natalizumab-PML survivors with subsequent MS treatment: clinico-radiologic outcome. Neurol Neuroimmunol Neuroinflamm. 2017;4(3):e346.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.












