User login
Lay health workers improve end-of-life care
Lay health workers (LHWs) can improve documentation of cancer patients’ care preferences in the aftermath of their diagnosis, investigators report.
Physicians, palliative care workers, and other health professionals can help cancer patients understand their prognosis and establish end-of-life care preferences, but busy schedules and professional reluctance often prevent this. Nonclinical, nonprofessional LHWs often are employed to assist with screening and adherence, but little work has been done to use them in end-of-life care, Manali I. Patel, MD, MPH, and her associates wrote in JAMA Oncology.
To investigate their potential in this role, the researchers randomized 213 patients with stage 3 or 4 recurrent cancer to usual care or the LHW intervention, which included a 6-month structured program delivered by a single LHW who was enrolled in a part-time graduate health education program. The LHWs received an 80-hour online skills-based seminar and 4 weeks’ observation training with a palliative care team.
The LHWs helped patients with advanced care planning, including: education about goals of care, establishment of care preferences, establishment of a surrogate decision maker, creation of an advanced directive, and encouragement to discuss the patient’s care with the clinician.
In the intervention arm, 92.4% of patients successfully had their goals of care documented in the electronic health record within 6 months of randomization, compared with 17.6% of the usual care group (P less than .001). They also were more likely to have created an advanced directive (67.6% vs. 25.9%; P less than .001), Dr. Patel, of Stanford (Calif.) University, and her associates reported.
Patient satisfaction was also greater in the intervention arm, as measured by the Consumer Assessment of Health Care Providers & Systems “satisfaction with provider” item (mean score, 9.16 vs. 7.83; P less than .001). At 6 months, with respect to their oncology provider, patients in the intervention arm registered a mean increase in satisfaction score of 1.53 (P less than .001).
Patients who died were more likely to receive hospice care if they were in the LHW group (76.7% vs. 48.3%; P =.002). Those in the LHW group also had lower health care costs during the final 30 days of their lives ($1,048 vs. $23,482; P less than .001).
Overall, the study shows that LHWs may be one mechanism for delivering high-value care and avoiding unnecessary, burdensome late-life treatments, at least in cancer patients.
SOURCE: Patel MI et al. JAMA Oncol. 2018 Jul 26doi:10.1001/jamaoncol.2018.2446.
Lay health workers (LHWs) can improve documentation of cancer patients’ care preferences in the aftermath of their diagnosis, investigators report.
Physicians, palliative care workers, and other health professionals can help cancer patients understand their prognosis and establish end-of-life care preferences, but busy schedules and professional reluctance often prevent this. Nonclinical, nonprofessional LHWs often are employed to assist with screening and adherence, but little work has been done to use them in end-of-life care, Manali I. Patel, MD, MPH, and her associates wrote in JAMA Oncology.
To investigate their potential in this role, the researchers randomized 213 patients with stage 3 or 4 recurrent cancer to usual care or the LHW intervention, which included a 6-month structured program delivered by a single LHW who was enrolled in a part-time graduate health education program. The LHWs received an 80-hour online skills-based seminar and 4 weeks’ observation training with a palliative care team.
The LHWs helped patients with advanced care planning, including: education about goals of care, establishment of care preferences, establishment of a surrogate decision maker, creation of an advanced directive, and encouragement to discuss the patient’s care with the clinician.
In the intervention arm, 92.4% of patients successfully had their goals of care documented in the electronic health record within 6 months of randomization, compared with 17.6% of the usual care group (P less than .001). They also were more likely to have created an advanced directive (67.6% vs. 25.9%; P less than .001), Dr. Patel, of Stanford (Calif.) University, and her associates reported.
Patient satisfaction was also greater in the intervention arm, as measured by the Consumer Assessment of Health Care Providers & Systems “satisfaction with provider” item (mean score, 9.16 vs. 7.83; P less than .001). At 6 months, with respect to their oncology provider, patients in the intervention arm registered a mean increase in satisfaction score of 1.53 (P less than .001).
Patients who died were more likely to receive hospice care if they were in the LHW group (76.7% vs. 48.3%; P =.002). Those in the LHW group also had lower health care costs during the final 30 days of their lives ($1,048 vs. $23,482; P less than .001).
Overall, the study shows that LHWs may be one mechanism for delivering high-value care and avoiding unnecessary, burdensome late-life treatments, at least in cancer patients.
SOURCE: Patel MI et al. JAMA Oncol. 2018 Jul 26doi:10.1001/jamaoncol.2018.2446.
Lay health workers (LHWs) can improve documentation of cancer patients’ care preferences in the aftermath of their diagnosis, investigators report.
Physicians, palliative care workers, and other health professionals can help cancer patients understand their prognosis and establish end-of-life care preferences, but busy schedules and professional reluctance often prevent this. Nonclinical, nonprofessional LHWs often are employed to assist with screening and adherence, but little work has been done to use them in end-of-life care, Manali I. Patel, MD, MPH, and her associates wrote in JAMA Oncology.
To investigate their potential in this role, the researchers randomized 213 patients with stage 3 or 4 recurrent cancer to usual care or the LHW intervention, which included a 6-month structured program delivered by a single LHW who was enrolled in a part-time graduate health education program. The LHWs received an 80-hour online skills-based seminar and 4 weeks’ observation training with a palliative care team.
The LHWs helped patients with advanced care planning, including: education about goals of care, establishment of care preferences, establishment of a surrogate decision maker, creation of an advanced directive, and encouragement to discuss the patient’s care with the clinician.
In the intervention arm, 92.4% of patients successfully had their goals of care documented in the electronic health record within 6 months of randomization, compared with 17.6% of the usual care group (P less than .001). They also were more likely to have created an advanced directive (67.6% vs. 25.9%; P less than .001), Dr. Patel, of Stanford (Calif.) University, and her associates reported.
Patient satisfaction was also greater in the intervention arm, as measured by the Consumer Assessment of Health Care Providers & Systems “satisfaction with provider” item (mean score, 9.16 vs. 7.83; P less than .001). At 6 months, with respect to their oncology provider, patients in the intervention arm registered a mean increase in satisfaction score of 1.53 (P less than .001).
Patients who died were more likely to receive hospice care if they were in the LHW group (76.7% vs. 48.3%; P =.002). Those in the LHW group also had lower health care costs during the final 30 days of their lives ($1,048 vs. $23,482; P less than .001).
Overall, the study shows that LHWs may be one mechanism for delivering high-value care and avoiding unnecessary, burdensome late-life treatments, at least in cancer patients.
SOURCE: Patel MI et al. JAMA Oncol. 2018 Jul 26doi:10.1001/jamaoncol.2018.2446.
FROM JAMA ONCOLOGY
Key clinical point: Lay health workers helped advanced cancer patients document their treatment preferences and reduced end of life costs.
Major finding: 92.4% of patients working with LHWs had their preferences recorded at 6 months, compared with 17.6% of controls.
Study details: Randomized, controlled trial of 213 patients with stage 3 or 4 recurrent cancer.
Disclosures: The study was funded by the Department of Veterans Affairs, the National Institutes of Health, and the California Healthcare Foundation.
Source: Patel MI et al. JAMA Oncology. 2018 Jul 26. doi: 10.1001/jamaoncol.2018.2446.
Asystole Following Nitroglycerin: A Review of Two Cases
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
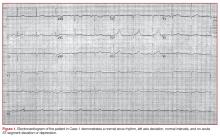
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
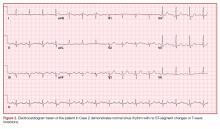
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
Neoadjuvant-treated N2 rectal cancer linked to PCR failure
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
REPORTING FROM ACSQSC 2018
Key clinical point: N2 disease may be a negative predictor of pathological complete response after neoadjuvant chemoradiotherapy for rectal cancer.
Major finding: Pretreatment N2 stage was a negative predictor of complete pathological response, with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026).
Study details: A study of 369 elective cases of cT2-4 N0-2 rectal cancer treated with neoadjuvant chemoradiotherapy from 2016 in the ACS NSQIP proctectomy-specific database.
Disclosures: Dr. Salama had no conflicts of interest to report for his presentation.
PET/CT accurately predicts MCL stage
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
REPORTING FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: If greater than 38% of the voxels demonstrated an standard uptake value of less than 0.95, there was a sensitivity of 100% and a specificity of 80%.
Study details: A retrospective cohort study of 23 patients with mantle cell leukemia and 5 controls.
Disclosures: There was no external funding for the study and the researchers reported having no financial disclosures.
Source: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Plantar Ulcerative Lichen Planus: Rapid Improvement With a Novel Triple-Therapy Approach
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
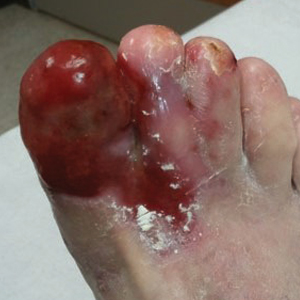
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.
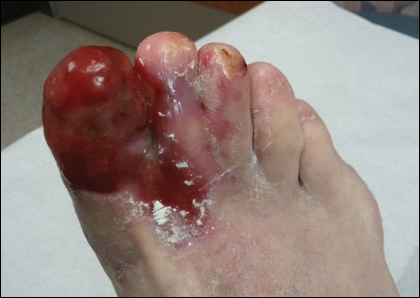

Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.
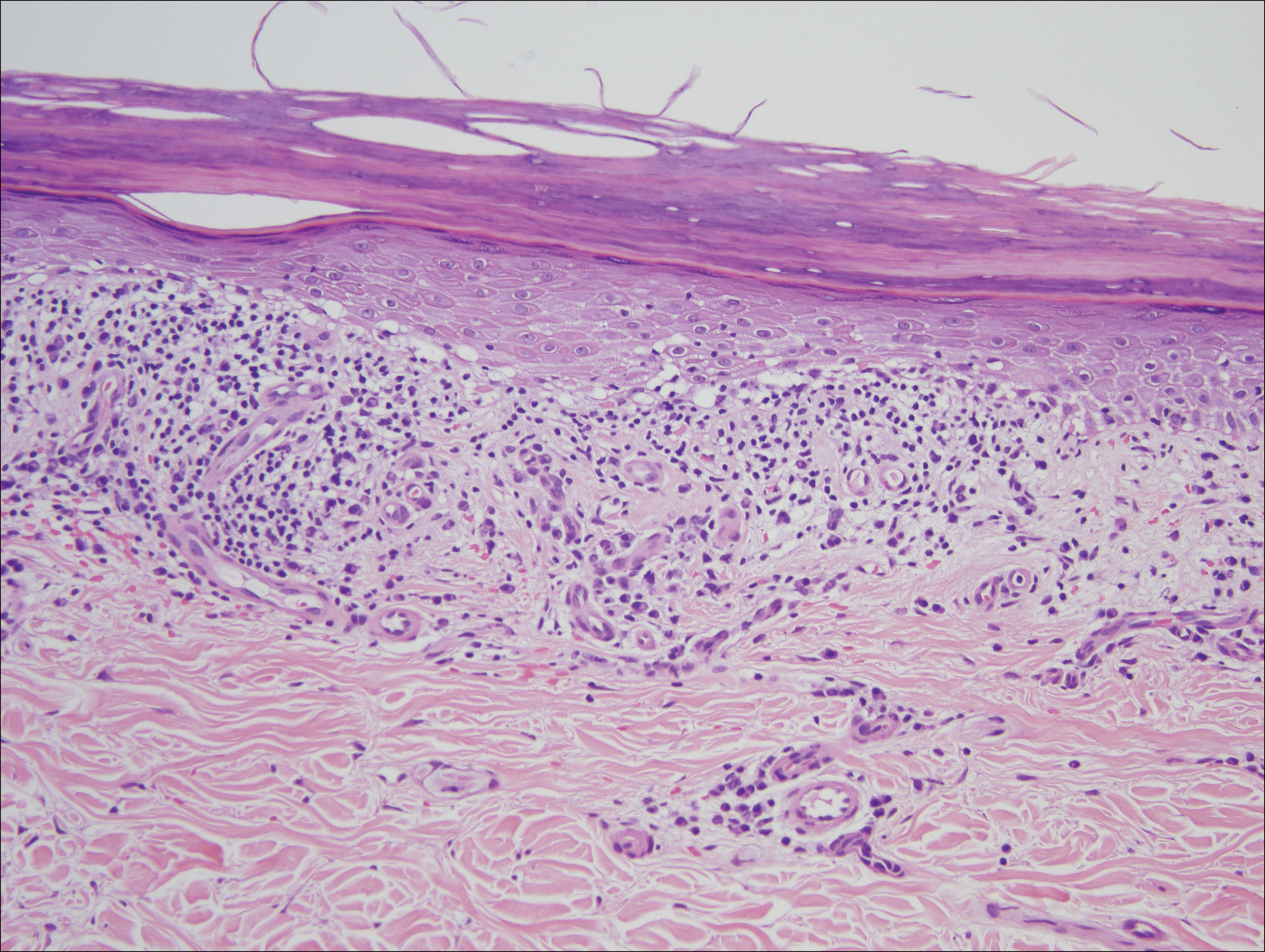
Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.
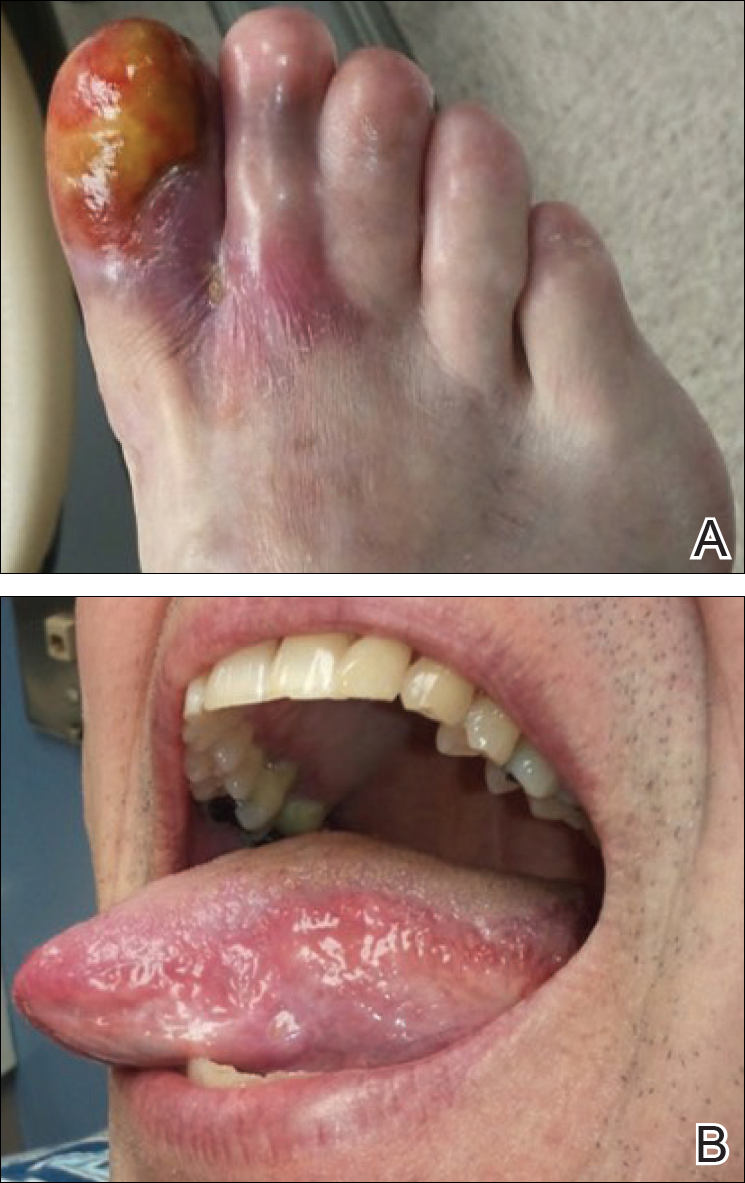
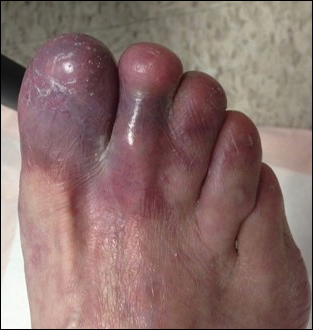
Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
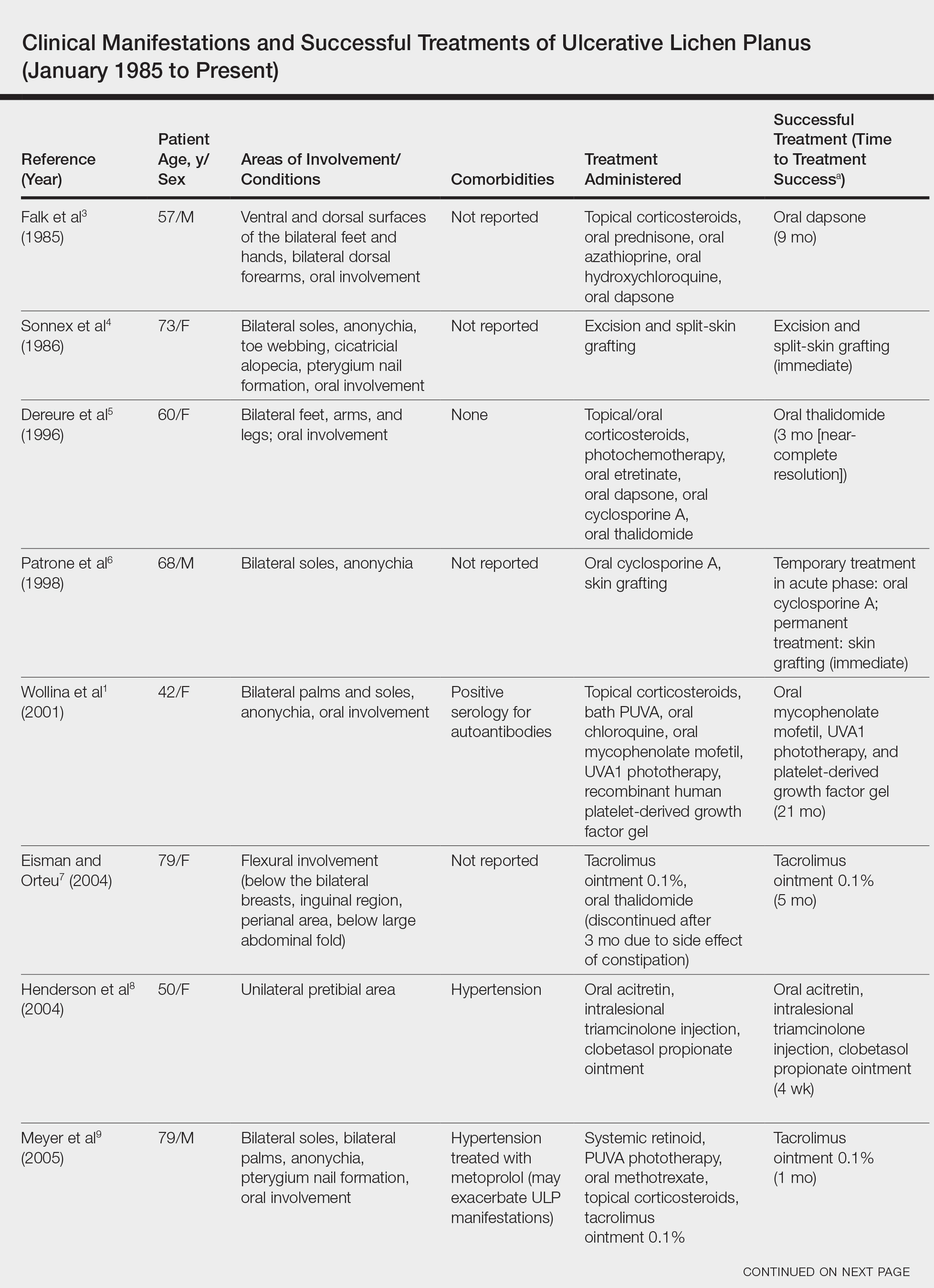
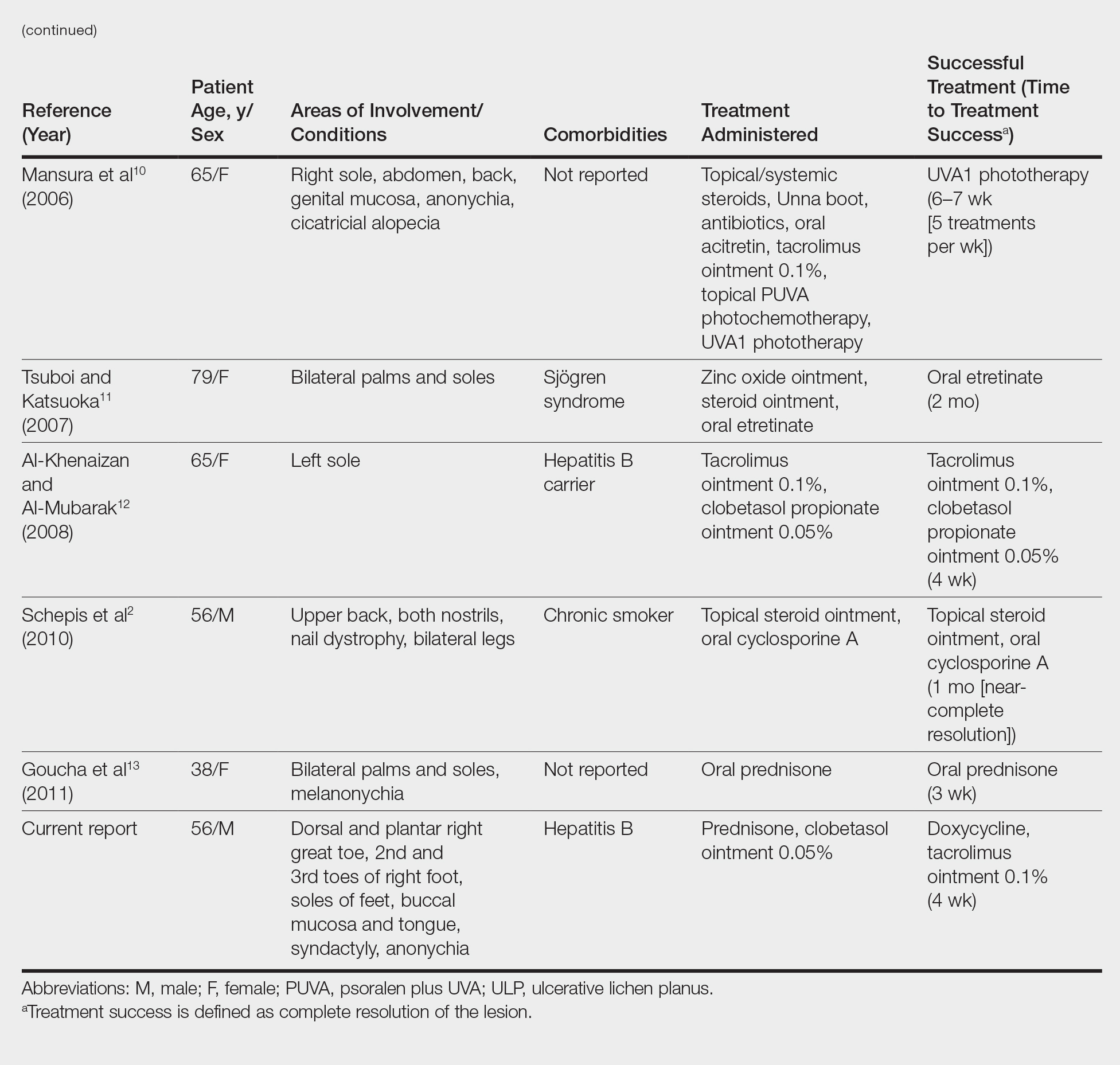
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Practice Points
- Consider ulcerative lichen planus (ULP) for chronic wounds on the soles.
- Topical therapeutic options may present a rapidly effective and relatively safe alternative to conventional immunosuppressive agents for long-term management of plantar ULP.
Impact of Provider Attire on Patient Satisfaction in an Outpatient Dermatology Clinic
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.
The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.
There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.


The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.

There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.


The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.

There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
Practice Points
- Provider attire is known to harbor disease-causing microorganisms, potentially serving as a vector and contributing to hospital-acquired infections.
- A change from formal provider attire, including white coats, to fitted scrubs had no measurable impact on patient satisfaction in an outpatient dermatology clinic.
- Patient satisfaction is most strongly linked to other provider characteristics, such as communication skills, concern for patient well-being, ability to empathize, and timeliness.
ED visits up for acute pancreatitis linked to younger age, alcohol, chronic disease
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: The number of U.S. emergency visits for acute pancreatitis associated with alcohol abuse, chronic pancreatitis, and younger age has risen in recent years.
Major finding: From 2006 to 2012, visits were up about 9% for patients under 65 years of age, 15.9% for acute pancreatitis associated with alcohol abuse, and 59.5% for acute on chronic pancreatitis.
Study details: Retrospective analysis of ED visits during 2006-2012 for nearly 2.2 million adults.
Disclosures: The authors had no disclosures.
Source: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
Sudden hearing loss
A 46-year-old man presents to your clinic with hearing loss and ear fullness. He awoke with sudden and unexplained right-sided hearing loss with aural fullness 6 days prior to presentation and reports associated unilateral tinnitus and vertigo. He denies otorrhea and otalgia. Hearing on the left is otherwise preserved, and he has no prior otologic history. He denies inciting events. He has never experienced this before.
On examination, the auricles are normal, and there are no vesicular lesions. His ear canals are patent and without occluding cerumen. Tympanic membranes are translucent and intact without effusion or retraction. On tuning fork examination, Weber lateralizes to the left, and air conduction is greater than bone conduction bilaterally.
What is the most likely diagnosis?
A. Otitis media with effusion.
B. Ménière’s disease.
C. Ramsay Hunt syndrome.
D. Sudden sensorineural hearing loss.
E. Bell’s palsy.
F. Otosclerosis.
G. Benign paroxysmal positional vertigo.
This patient is presenting with sudden sensorineural hearing loss (SSNHL). There are approximately 4,000 new cases of SSNHL reported each year in the United States, and many primary care physicians will encounter this disorder.1
Idiopathic SSNHL is an otolaryngology emergency, and recognition and prompt treatment is imperative to potentially salvage hearing and improve quality of life.2 The rates of spontaneous recovery within the literature vary widely from 32% to 65%. However, this constitutes a significant portion of patients who still require the use of hearing aids. Recovery at lower frequencies occurs more commonly, and the rates of true spontaneous recovery are likely approximately one-third of all cases, with only 15% of patients who recover fully.3
Patients with SSNHL present to an otolaryngologist on average 55 days after symptom onset. Yet it is believed that the earlier the intervention, the more improved the recovery, especially if instituted within 1-2 weeks.4 Most patients, however, initially present to their primary care physicians.
The history and physical are particularly important to rule out other etiologies of hearing loss, including otitis media with effusion, acute otitis media, and cerumen impaction.
The onset and progression of SSNHL are unique, in that patients will experience near immediate unilateral hearing loss, typically with a normal ear examination. Aural pressure and tinnitus are frequently reported in SSNHL. In fact, ear fullness is the most common presenting symptom, and tinnitus is reported nearly universally. Dizziness or vertigo does not refute the diagnosis of SSNHL, as this occurs in 30%-40% of cases and is a negative prognostic factor, along with profound sensorineural loss, contralateral hearing impairment, and delayed treatment.3,5
In 9 out of 10 patients with SSNHL, a precipitating factor or cause will never be identified, and approximately one-third of patients will spontaneously recover hearing.6
This has created controversial treatment options. Regardless, specialty guidelines advocate for early medical treatment with systemic corticosteroids within the first 14 days of symptom onset. Clinicians must be prepared to empirically and rapidly treat patients with suspected SSNHL without understanding the etiology and without conclusive audiologic or imaging data.
Other causes indeterminate of sudden hearing loss include autoimmune and Ménière’s disease; however, diagnosis and management of these disorders does not have the same urgency as that of SSNHL.
Otolaryngology follow-up for SSNHL is recommended to provide treatment options and counseling, monitor hearing thresholds, provide ongoing evaluation for hearing augmentation, and establish expert consultation to ensure there are no underlying causes.
Primary care providers need to institute the initial treatment recommendations, which include systemic steroids, potentially in combination with alternative treatment options. There is conflicting evidence on the efficacy of early high-dose steroid therapy in SSNHL. But, unfortunately, the alternative may include permanent unilateral deafness.7 The earlier the treatment, the better the outcomes, although oral steroids may contribute to significant improvement even at late presentation.4
Dosing recommendations vary, but include prednisone 1 mg/kg per day (maximum of 60 mg), methylprednisolone 48 mg/day, and dexamethasone 10 mg/day for 7-14 days prior to tapering. Examination and audiometry are ideally obtained at the time of symptom onset and immediately after treatment for full evaluation.
Evidence supports the use of intratympanic steroid injections after primary treatment failure, although there is increasing interest in combining transtympanic perfusion with oral steroids for primary treatment.7,8 In a survey of otolaryngologists, 86% of respondents reported the concomitant use of intratympanic steroid injections for primary treatment.9 Of these, greater than 50% and 32% used steroid injections up to 1 month, and up to 3 months following the onset of symptoms, respectively.
There may be benefit with hyperbaric oxygen therapy, which is rarely used in the United States.6 There may be benefit with a low side effect profile in combining systemic steroids with antioxidants, such as vitamins A, C, and E.10
In summary, SSNHL is not uncommon; ear fullness and tinnitus are common and may represent the chief complaint. A high index of suspicion is necessary, and decisions are often made without full knowledge of the etiology. Conductive hearing loss should be ruled out on history and examination. Initial diagnostic workup includes formal audiometry with initiation of high-dose corticosteroid therapy, ideally within 2 weeks of symptom onset.
Consultation to otolaryngology or neurotology is important for ongoing discussions regarding potential recovery, hearing augmentation, and to ensure there is no underlying condition.
Dr. Shinn is with the department of otolaryngology–head and neck surgery at Vanderbilt University Medical Center in Nashville, Tenn. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1-35.
2. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.1072.
3. Braz J Otorhinolaryngol. 2015 Oct-Sep;81(5):520-6.
4. Cureus. 2017 Dec;9(12):e1945.
5. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.0648.
6. Otolaryngol Clin North Am. 2008;41(3):633-49.
7. Curr Opin Otolaryngol Head Neck Surg. 2016 Oct;24(5):413-9.
8. Eur Arch Otorhinolaryngol. 2015 Oct;272(10):2777-82.
9. Ann Otol Rhinol Laryngol. 2018 Jul;127(7):481-2.
10. Audiol Neurotol. 2018 Jun 22;23(1):1-7.
A 46-year-old man presents to your clinic with hearing loss and ear fullness. He awoke with sudden and unexplained right-sided hearing loss with aural fullness 6 days prior to presentation and reports associated unilateral tinnitus and vertigo. He denies otorrhea and otalgia. Hearing on the left is otherwise preserved, and he has no prior otologic history. He denies inciting events. He has never experienced this before.
On examination, the auricles are normal, and there are no vesicular lesions. His ear canals are patent and without occluding cerumen. Tympanic membranes are translucent and intact without effusion or retraction. On tuning fork examination, Weber lateralizes to the left, and air conduction is greater than bone conduction bilaterally.
What is the most likely diagnosis?
A. Otitis media with effusion.
B. Ménière’s disease.
C. Ramsay Hunt syndrome.
D. Sudden sensorineural hearing loss.
E. Bell’s palsy.
F. Otosclerosis.
G. Benign paroxysmal positional vertigo.
This patient is presenting with sudden sensorineural hearing loss (SSNHL). There are approximately 4,000 new cases of SSNHL reported each year in the United States, and many primary care physicians will encounter this disorder.1
Idiopathic SSNHL is an otolaryngology emergency, and recognition and prompt treatment is imperative to potentially salvage hearing and improve quality of life.2 The rates of spontaneous recovery within the literature vary widely from 32% to 65%. However, this constitutes a significant portion of patients who still require the use of hearing aids. Recovery at lower frequencies occurs more commonly, and the rates of true spontaneous recovery are likely approximately one-third of all cases, with only 15% of patients who recover fully.3
Patients with SSNHL present to an otolaryngologist on average 55 days after symptom onset. Yet it is believed that the earlier the intervention, the more improved the recovery, especially if instituted within 1-2 weeks.4 Most patients, however, initially present to their primary care physicians.
The history and physical are particularly important to rule out other etiologies of hearing loss, including otitis media with effusion, acute otitis media, and cerumen impaction.
The onset and progression of SSNHL are unique, in that patients will experience near immediate unilateral hearing loss, typically with a normal ear examination. Aural pressure and tinnitus are frequently reported in SSNHL. In fact, ear fullness is the most common presenting symptom, and tinnitus is reported nearly universally. Dizziness or vertigo does not refute the diagnosis of SSNHL, as this occurs in 30%-40% of cases and is a negative prognostic factor, along with profound sensorineural loss, contralateral hearing impairment, and delayed treatment.3,5
In 9 out of 10 patients with SSNHL, a precipitating factor or cause will never be identified, and approximately one-third of patients will spontaneously recover hearing.6
This has created controversial treatment options. Regardless, specialty guidelines advocate for early medical treatment with systemic corticosteroids within the first 14 days of symptom onset. Clinicians must be prepared to empirically and rapidly treat patients with suspected SSNHL without understanding the etiology and without conclusive audiologic or imaging data.
Other causes indeterminate of sudden hearing loss include autoimmune and Ménière’s disease; however, diagnosis and management of these disorders does not have the same urgency as that of SSNHL.
Otolaryngology follow-up for SSNHL is recommended to provide treatment options and counseling, monitor hearing thresholds, provide ongoing evaluation for hearing augmentation, and establish expert consultation to ensure there are no underlying causes.
Primary care providers need to institute the initial treatment recommendations, which include systemic steroids, potentially in combination with alternative treatment options. There is conflicting evidence on the efficacy of early high-dose steroid therapy in SSNHL. But, unfortunately, the alternative may include permanent unilateral deafness.7 The earlier the treatment, the better the outcomes, although oral steroids may contribute to significant improvement even at late presentation.4
Dosing recommendations vary, but include prednisone 1 mg/kg per day (maximum of 60 mg), methylprednisolone 48 mg/day, and dexamethasone 10 mg/day for 7-14 days prior to tapering. Examination and audiometry are ideally obtained at the time of symptom onset and immediately after treatment for full evaluation.
Evidence supports the use of intratympanic steroid injections after primary treatment failure, although there is increasing interest in combining transtympanic perfusion with oral steroids for primary treatment.7,8 In a survey of otolaryngologists, 86% of respondents reported the concomitant use of intratympanic steroid injections for primary treatment.9 Of these, greater than 50% and 32% used steroid injections up to 1 month, and up to 3 months following the onset of symptoms, respectively.
There may be benefit with hyperbaric oxygen therapy, which is rarely used in the United States.6 There may be benefit with a low side effect profile in combining systemic steroids with antioxidants, such as vitamins A, C, and E.10
In summary, SSNHL is not uncommon; ear fullness and tinnitus are common and may represent the chief complaint. A high index of suspicion is necessary, and decisions are often made without full knowledge of the etiology. Conductive hearing loss should be ruled out on history and examination. Initial diagnostic workup includes formal audiometry with initiation of high-dose corticosteroid therapy, ideally within 2 weeks of symptom onset.
Consultation to otolaryngology or neurotology is important for ongoing discussions regarding potential recovery, hearing augmentation, and to ensure there is no underlying condition.
Dr. Shinn is with the department of otolaryngology–head and neck surgery at Vanderbilt University Medical Center in Nashville, Tenn. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1-35.
2. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.1072.
3. Braz J Otorhinolaryngol. 2015 Oct-Sep;81(5):520-6.
4. Cureus. 2017 Dec;9(12):e1945.
5. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.0648.
6. Otolaryngol Clin North Am. 2008;41(3):633-49.
7. Curr Opin Otolaryngol Head Neck Surg. 2016 Oct;24(5):413-9.
8. Eur Arch Otorhinolaryngol. 2015 Oct;272(10):2777-82.
9. Ann Otol Rhinol Laryngol. 2018 Jul;127(7):481-2.
10. Audiol Neurotol. 2018 Jun 22;23(1):1-7.
A 46-year-old man presents to your clinic with hearing loss and ear fullness. He awoke with sudden and unexplained right-sided hearing loss with aural fullness 6 days prior to presentation and reports associated unilateral tinnitus and vertigo. He denies otorrhea and otalgia. Hearing on the left is otherwise preserved, and he has no prior otologic history. He denies inciting events. He has never experienced this before.
On examination, the auricles are normal, and there are no vesicular lesions. His ear canals are patent and without occluding cerumen. Tympanic membranes are translucent and intact without effusion or retraction. On tuning fork examination, Weber lateralizes to the left, and air conduction is greater than bone conduction bilaterally.
What is the most likely diagnosis?
A. Otitis media with effusion.
B. Ménière’s disease.
C. Ramsay Hunt syndrome.
D. Sudden sensorineural hearing loss.
E. Bell’s palsy.
F. Otosclerosis.
G. Benign paroxysmal positional vertigo.
This patient is presenting with sudden sensorineural hearing loss (SSNHL). There are approximately 4,000 new cases of SSNHL reported each year in the United States, and many primary care physicians will encounter this disorder.1
Idiopathic SSNHL is an otolaryngology emergency, and recognition and prompt treatment is imperative to potentially salvage hearing and improve quality of life.2 The rates of spontaneous recovery within the literature vary widely from 32% to 65%. However, this constitutes a significant portion of patients who still require the use of hearing aids. Recovery at lower frequencies occurs more commonly, and the rates of true spontaneous recovery are likely approximately one-third of all cases, with only 15% of patients who recover fully.3
Patients with SSNHL present to an otolaryngologist on average 55 days after symptom onset. Yet it is believed that the earlier the intervention, the more improved the recovery, especially if instituted within 1-2 weeks.4 Most patients, however, initially present to their primary care physicians.
The history and physical are particularly important to rule out other etiologies of hearing loss, including otitis media with effusion, acute otitis media, and cerumen impaction.
The onset and progression of SSNHL are unique, in that patients will experience near immediate unilateral hearing loss, typically with a normal ear examination. Aural pressure and tinnitus are frequently reported in SSNHL. In fact, ear fullness is the most common presenting symptom, and tinnitus is reported nearly universally. Dizziness or vertigo does not refute the diagnosis of SSNHL, as this occurs in 30%-40% of cases and is a negative prognostic factor, along with profound sensorineural loss, contralateral hearing impairment, and delayed treatment.3,5
In 9 out of 10 patients with SSNHL, a precipitating factor or cause will never be identified, and approximately one-third of patients will spontaneously recover hearing.6
This has created controversial treatment options. Regardless, specialty guidelines advocate for early medical treatment with systemic corticosteroids within the first 14 days of symptom onset. Clinicians must be prepared to empirically and rapidly treat patients with suspected SSNHL without understanding the etiology and without conclusive audiologic or imaging data.
Other causes indeterminate of sudden hearing loss include autoimmune and Ménière’s disease; however, diagnosis and management of these disorders does not have the same urgency as that of SSNHL.
Otolaryngology follow-up for SSNHL is recommended to provide treatment options and counseling, monitor hearing thresholds, provide ongoing evaluation for hearing augmentation, and establish expert consultation to ensure there are no underlying causes.
Primary care providers need to institute the initial treatment recommendations, which include systemic steroids, potentially in combination with alternative treatment options. There is conflicting evidence on the efficacy of early high-dose steroid therapy in SSNHL. But, unfortunately, the alternative may include permanent unilateral deafness.7 The earlier the treatment, the better the outcomes, although oral steroids may contribute to significant improvement even at late presentation.4
Dosing recommendations vary, but include prednisone 1 mg/kg per day (maximum of 60 mg), methylprednisolone 48 mg/day, and dexamethasone 10 mg/day for 7-14 days prior to tapering. Examination and audiometry are ideally obtained at the time of symptom onset and immediately after treatment for full evaluation.
Evidence supports the use of intratympanic steroid injections after primary treatment failure, although there is increasing interest in combining transtympanic perfusion with oral steroids for primary treatment.7,8 In a survey of otolaryngologists, 86% of respondents reported the concomitant use of intratympanic steroid injections for primary treatment.9 Of these, greater than 50% and 32% used steroid injections up to 1 month, and up to 3 months following the onset of symptoms, respectively.
There may be benefit with hyperbaric oxygen therapy, which is rarely used in the United States.6 There may be benefit with a low side effect profile in combining systemic steroids with antioxidants, such as vitamins A, C, and E.10
In summary, SSNHL is not uncommon; ear fullness and tinnitus are common and may represent the chief complaint. A high index of suspicion is necessary, and decisions are often made without full knowledge of the etiology. Conductive hearing loss should be ruled out on history and examination. Initial diagnostic workup includes formal audiometry with initiation of high-dose corticosteroid therapy, ideally within 2 weeks of symptom onset.
Consultation to otolaryngology or neurotology is important for ongoing discussions regarding potential recovery, hearing augmentation, and to ensure there is no underlying condition.
Dr. Shinn is with the department of otolaryngology–head and neck surgery at Vanderbilt University Medical Center in Nashville, Tenn. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1-35.
2. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.1072.
3. Braz J Otorhinolaryngol. 2015 Oct-Sep;81(5):520-6.
4. Cureus. 2017 Dec;9(12):e1945.
5. JAMA Otolaryngol Head Neck Surg. 2018 Jun 21. doi: 10.1001/jamaoto.2018.0648.
6. Otolaryngol Clin North Am. 2008;41(3):633-49.
7. Curr Opin Otolaryngol Head Neck Surg. 2016 Oct;24(5):413-9.
8. Eur Arch Otorhinolaryngol. 2015 Oct;272(10):2777-82.
9. Ann Otol Rhinol Laryngol. 2018 Jul;127(7):481-2.
10. Audiol Neurotol. 2018 Jun 22;23(1):1-7.
Status Epilepticus in Pregnancy
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing
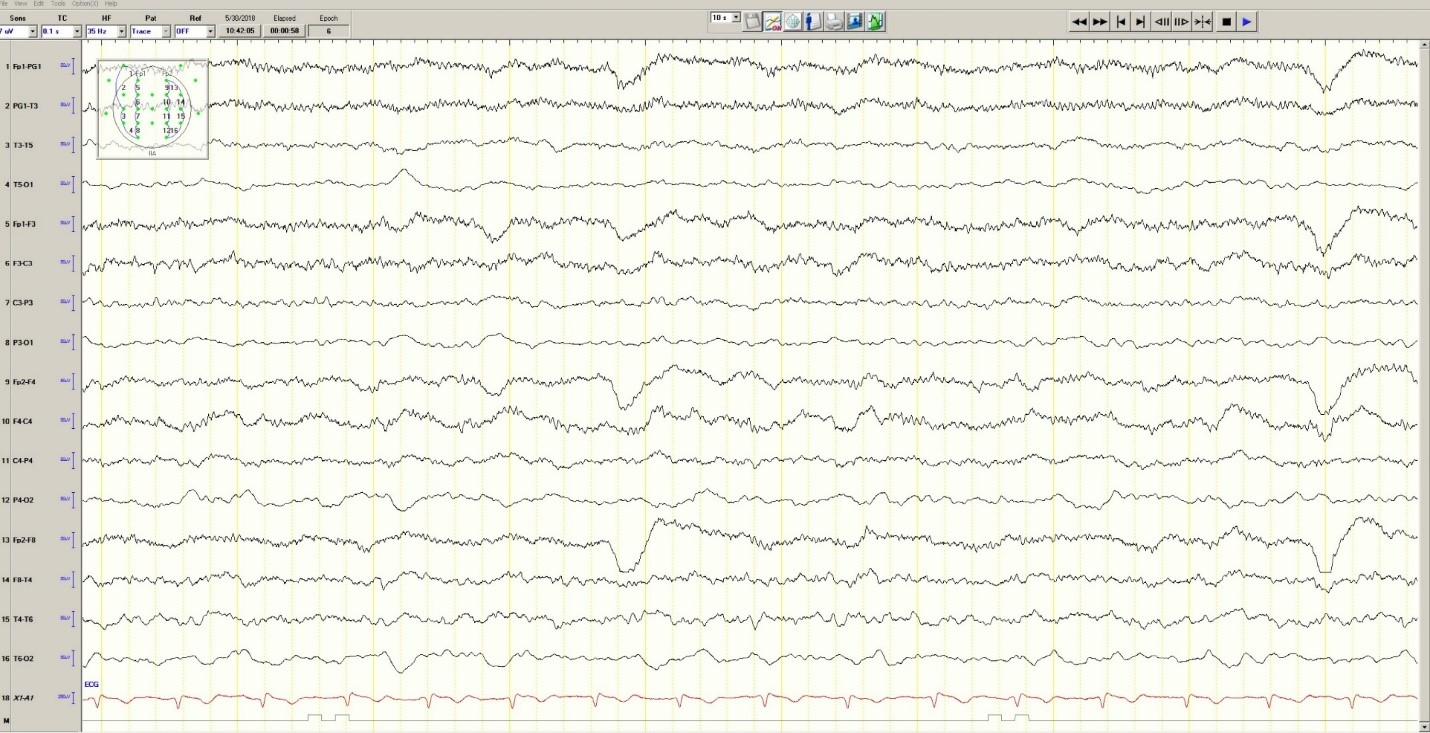
During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region
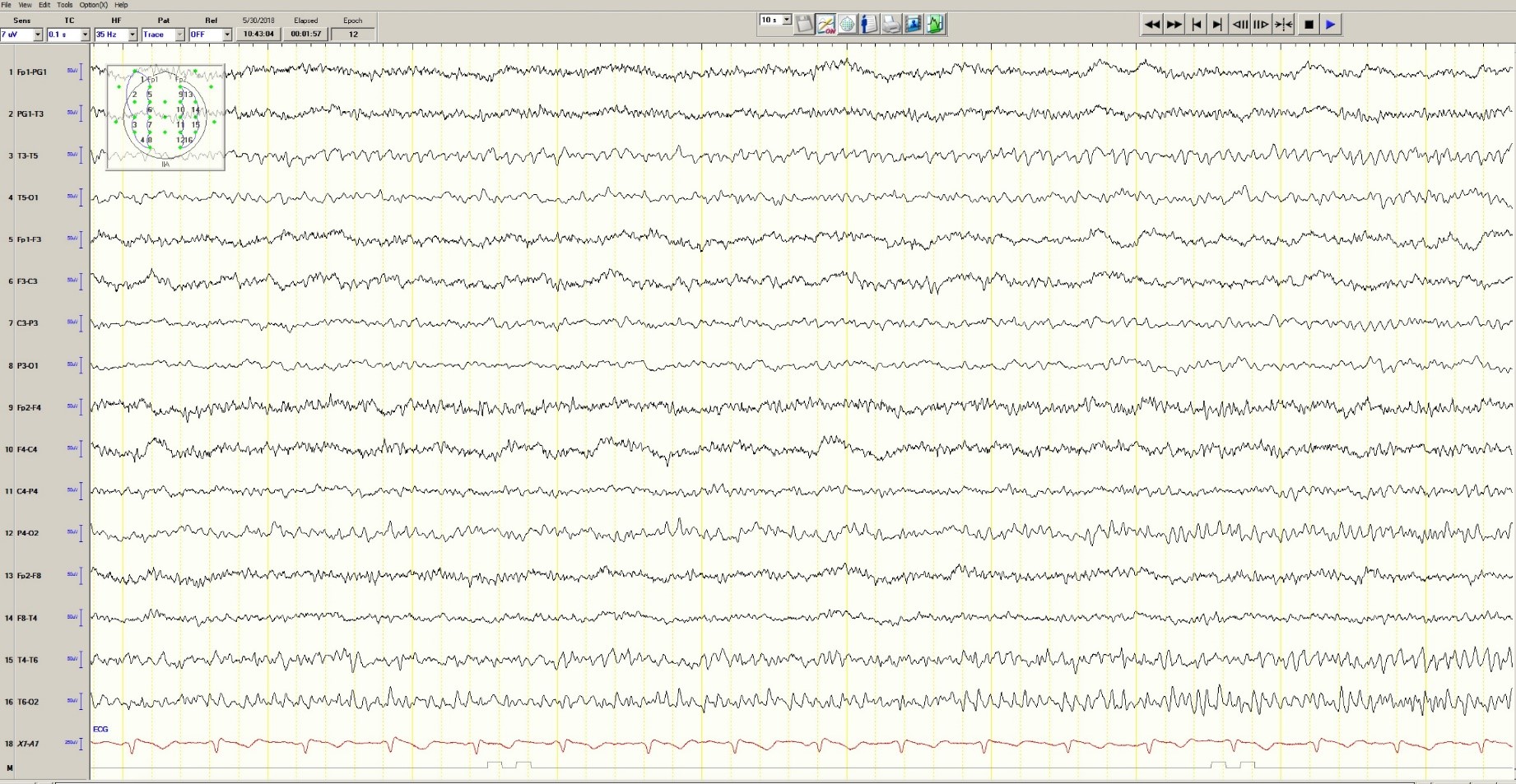
Figure 3. Mild generalized arophy, greater in right hemisphere
The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing
Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
E-cigarettes: Prices down, sales up
Any economist could have predicted it: As the .
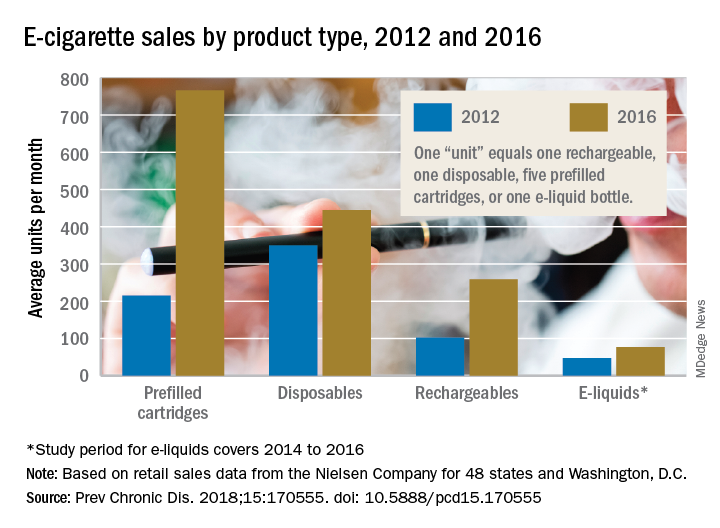
The average prices of three mutually exclusive e-cigarette products – rechargeable devices, disposable devices, and disposable cartridges filled with e-liquid – all dropped from 2012 to 2016, as did that of a fourth product – e-liquid bottles for filling reusable cartridges – not available nationwide until 2014. At the same time, average monthly sales for e-cigarette products overall rose by a statistically significant 132%, Teresa W. Wang, PhD, of the Centers for Disease Control and Prevention, Atlanta, and her associates reported in Preventing Chronic Disease.
Sales of prefilled cartridges, the most popular product by the end of the study period, increased 256%, going from 215 units per 100,000 people each month in 2012 to 766 units. [For the study, a unit was defined as one rechargeable, one disposable, one pack of five prefilled cartridges, or one bottle of e-liquid.] Disposables were the most popular product at the start of the study period but had the smallest relative increase (27%), while monthly sales of rechargeables jumped by 154% and e-liquids saw a 64% rise, the investigators said.
Price decreases for the three products available in 2012 were all significant: The average price per unit was down 48% for rechargeables by 2016, 14% for disposables, and 12% for prefilled cartridges. E-liquids were 9% cheaper by 2016, but that change did not reach significance, they noted.
“Overall, the increase in e-cigarette sales and decrease in price is consistent with previous studies demonstrating that e-cigarette sales are responsive to their own price changes. These trends suggest that, if e-cigarette prices continue to decrease, their sales may also continue to rise,” Dr. Wang and her associates wrote.
The data for the study came from the Nielsen Company and were based on retail sales at convenience stores; supermarkets; drug, dollar, and club stores; and military commissaries in the 48 contiguous states and Washington, D.C. One study limitation was the lack of data from tobacco/vape shops and the Internet.
SOURCE: Wang TW et al. Prev Chronic Dis. 2018;15:170555. doi: 10.5888/pcd15.170555.
Any economist could have predicted it: As the .

The average prices of three mutually exclusive e-cigarette products – rechargeable devices, disposable devices, and disposable cartridges filled with e-liquid – all dropped from 2012 to 2016, as did that of a fourth product – e-liquid bottles for filling reusable cartridges – not available nationwide until 2014. At the same time, average monthly sales for e-cigarette products overall rose by a statistically significant 132%, Teresa W. Wang, PhD, of the Centers for Disease Control and Prevention, Atlanta, and her associates reported in Preventing Chronic Disease.
Sales of prefilled cartridges, the most popular product by the end of the study period, increased 256%, going from 215 units per 100,000 people each month in 2012 to 766 units. [For the study, a unit was defined as one rechargeable, one disposable, one pack of five prefilled cartridges, or one bottle of e-liquid.] Disposables were the most popular product at the start of the study period but had the smallest relative increase (27%), while monthly sales of rechargeables jumped by 154% and e-liquids saw a 64% rise, the investigators said.
Price decreases for the three products available in 2012 were all significant: The average price per unit was down 48% for rechargeables by 2016, 14% for disposables, and 12% for prefilled cartridges. E-liquids were 9% cheaper by 2016, but that change did not reach significance, they noted.
“Overall, the increase in e-cigarette sales and decrease in price is consistent with previous studies demonstrating that e-cigarette sales are responsive to their own price changes. These trends suggest that, if e-cigarette prices continue to decrease, their sales may also continue to rise,” Dr. Wang and her associates wrote.
The data for the study came from the Nielsen Company and were based on retail sales at convenience stores; supermarkets; drug, dollar, and club stores; and military commissaries in the 48 contiguous states and Washington, D.C. One study limitation was the lack of data from tobacco/vape shops and the Internet.
SOURCE: Wang TW et al. Prev Chronic Dis. 2018;15:170555. doi: 10.5888/pcd15.170555.
Any economist could have predicted it: As the .

The average prices of three mutually exclusive e-cigarette products – rechargeable devices, disposable devices, and disposable cartridges filled with e-liquid – all dropped from 2012 to 2016, as did that of a fourth product – e-liquid bottles for filling reusable cartridges – not available nationwide until 2014. At the same time, average monthly sales for e-cigarette products overall rose by a statistically significant 132%, Teresa W. Wang, PhD, of the Centers for Disease Control and Prevention, Atlanta, and her associates reported in Preventing Chronic Disease.
Sales of prefilled cartridges, the most popular product by the end of the study period, increased 256%, going from 215 units per 100,000 people each month in 2012 to 766 units. [For the study, a unit was defined as one rechargeable, one disposable, one pack of five prefilled cartridges, or one bottle of e-liquid.] Disposables were the most popular product at the start of the study period but had the smallest relative increase (27%), while monthly sales of rechargeables jumped by 154% and e-liquids saw a 64% rise, the investigators said.
Price decreases for the three products available in 2012 were all significant: The average price per unit was down 48% for rechargeables by 2016, 14% for disposables, and 12% for prefilled cartridges. E-liquids were 9% cheaper by 2016, but that change did not reach significance, they noted.
“Overall, the increase in e-cigarette sales and decrease in price is consistent with previous studies demonstrating that e-cigarette sales are responsive to their own price changes. These trends suggest that, if e-cigarette prices continue to decrease, their sales may also continue to rise,” Dr. Wang and her associates wrote.
The data for the study came from the Nielsen Company and were based on retail sales at convenience stores; supermarkets; drug, dollar, and club stores; and military commissaries in the 48 contiguous states and Washington, D.C. One study limitation was the lack of data from tobacco/vape shops and the Internet.
SOURCE: Wang TW et al. Prev Chronic Dis. 2018;15:170555. doi: 10.5888/pcd15.170555.
FROM PREVENTING CHRONIC DISEASE