User login
Deer Ked: A Lyme-Carrying Ectoparasite on the Move
How to Prevent Mosquito and Tick-Borne Disease
Case Report
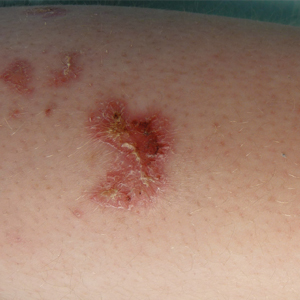
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
Practice Points
- There are many more disease-carrying arthropods than are routinely studied by scientists and physicians.
- Even if the insect cannot be identified, it is important to monitor patients who have experienced arthropod assault for signs of clinical diseases.
Melanoma diagnosis does not deter pregnancy
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Pregnancy after melanoma does not appear to increase risk for melanoma recurrence.
Major finding: The rate of pregnancy for women with melanoma was 15.8%, compared with 13.6% for controls (P less than .001).
Study details: A retrospective study of claims database records on 11,801 women with melanoma and an equal number of matched controls.
Disclosures: The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
Source: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Chronic Migraine Is Associated With Changes in Age-Related Cortical Thickness
Some brain areas are thicker, and others thinner, in patients with chronic migraine, compared with controls.
SAN FRANCISCO—Chronic migraine is associated with increased age-related cortical thinning of some brain areas and decreased age-related cortical thinning in other areas, according to research presented at the 60th Annual Scientific Meeting of the American Headache Society. It remains uncertain whether these cortical changes are a cause or an effect of chronic migraine.
Age-related cortical integrity had not been explored in chronic migraine previously. To investigate this topic, Yohannes Woubishet Woldeamanuel, MD, Instructor in Neurology and Neurologic Sciences at Stanford University in California, and colleagues enrolled 30 patients with chronic migraine and 30 age- and sex-matched healthy controls into a study. All participants were right-handed. The mean age in the chronic migraine group was 40.5, and the male-to-female ratio was 1:4. Investigators obtained the duration of chronic migraine and lifetime migraine from participants with chronic migraine.
Dr. Woldeamanuel and colleagues acquired T1-weighted brain images on a 3T MRI from all participants. They analyzed whole-brain cortical thickness on unmasked images. The investigators used linear regression to examine group differences on age by cortical thickness between people with chronic migraine and controls. Multiple regression enabled the researchers to control for the confounding effect of duration of chronic migraine and lifetime migraine on age-related cortical thickness changes.
Compared with controls, patients with chronic migraine had significant age-related thinning of the lateral orbitofrontal and supramarginal cortex of the left hemisphere. Patients with chronic migraine had a lack of age-related thinning of the pars orbitalis, superior and inferior parietal, superior temporal, pars opercularis, posterior cingulate, precuneus, superior frontal of the left hemisphere, and the left hemisphere mean cortical thickness, however. In the right hemisphere, the chronic migraine group had significant age-related thinning of the banks of the superior temporal sulcus, caudal anterior cingulate, inferior parietal, precuneus, and supramarginal cortex. The chronic migraine group lacked age-related thinning of the caudal middle frontal, isthmus cingulate, lateral orbitofrontal, paracentral, pars orbitalis, posterior cingulate, rostral middle frontal, superior frontal, and temporal pole of the right hemisphere. These results were not influenced by duration of chronic migraine and lifetime migraine.
The absence of normative age-related cortical thinning in implicated brain areas possibly indicates a perpetually enhanced headache response, head pain cognition, visual and auditory processing, affective behavior, interaction with internal and external cues, and multisensory integration, said the researchers. Accelerated age-related thinning in brain areas involved in sensory pain pathways could represent reduced habituation in chronic migraine, they added. In addition, age-related cortical thinning might indicate progressive loss of migraine modulation. The investigators hypothesized that repetitive migraine attacks might increase allostatic load from headache and nonheadache migraine symptoms.
“We are following these cohorts to determine whether these age-related cortical thickness changes signify cause or effect of chronic migraine,” said Dr. Woldeamanuel.
Suggested Reading
Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014;34(14):1115-1124.
Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015 Feb 13;10(2):e0116687.
Some brain areas are thicker, and others thinner, in patients with chronic migraine, compared with controls.
Some brain areas are thicker, and others thinner, in patients with chronic migraine, compared with controls.
SAN FRANCISCO—Chronic migraine is associated with increased age-related cortical thinning of some brain areas and decreased age-related cortical thinning in other areas, according to research presented at the 60th Annual Scientific Meeting of the American Headache Society. It remains uncertain whether these cortical changes are a cause or an effect of chronic migraine.
Age-related cortical integrity had not been explored in chronic migraine previously. To investigate this topic, Yohannes Woubishet Woldeamanuel, MD, Instructor in Neurology and Neurologic Sciences at Stanford University in California, and colleagues enrolled 30 patients with chronic migraine and 30 age- and sex-matched healthy controls into a study. All participants were right-handed. The mean age in the chronic migraine group was 40.5, and the male-to-female ratio was 1:4. Investigators obtained the duration of chronic migraine and lifetime migraine from participants with chronic migraine.
Dr. Woldeamanuel and colleagues acquired T1-weighted brain images on a 3T MRI from all participants. They analyzed whole-brain cortical thickness on unmasked images. The investigators used linear regression to examine group differences on age by cortical thickness between people with chronic migraine and controls. Multiple regression enabled the researchers to control for the confounding effect of duration of chronic migraine and lifetime migraine on age-related cortical thickness changes.
Compared with controls, patients with chronic migraine had significant age-related thinning of the lateral orbitofrontal and supramarginal cortex of the left hemisphere. Patients with chronic migraine had a lack of age-related thinning of the pars orbitalis, superior and inferior parietal, superior temporal, pars opercularis, posterior cingulate, precuneus, superior frontal of the left hemisphere, and the left hemisphere mean cortical thickness, however. In the right hemisphere, the chronic migraine group had significant age-related thinning of the banks of the superior temporal sulcus, caudal anterior cingulate, inferior parietal, precuneus, and supramarginal cortex. The chronic migraine group lacked age-related thinning of the caudal middle frontal, isthmus cingulate, lateral orbitofrontal, paracentral, pars orbitalis, posterior cingulate, rostral middle frontal, superior frontal, and temporal pole of the right hemisphere. These results were not influenced by duration of chronic migraine and lifetime migraine.
The absence of normative age-related cortical thinning in implicated brain areas possibly indicates a perpetually enhanced headache response, head pain cognition, visual and auditory processing, affective behavior, interaction with internal and external cues, and multisensory integration, said the researchers. Accelerated age-related thinning in brain areas involved in sensory pain pathways could represent reduced habituation in chronic migraine, they added. In addition, age-related cortical thinning might indicate progressive loss of migraine modulation. The investigators hypothesized that repetitive migraine attacks might increase allostatic load from headache and nonheadache migraine symptoms.
“We are following these cohorts to determine whether these age-related cortical thickness changes signify cause or effect of chronic migraine,” said Dr. Woldeamanuel.
Suggested Reading
Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014;34(14):1115-1124.
Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015 Feb 13;10(2):e0116687.
SAN FRANCISCO—Chronic migraine is associated with increased age-related cortical thinning of some brain areas and decreased age-related cortical thinning in other areas, according to research presented at the 60th Annual Scientific Meeting of the American Headache Society. It remains uncertain whether these cortical changes are a cause or an effect of chronic migraine.
Age-related cortical integrity had not been explored in chronic migraine previously. To investigate this topic, Yohannes Woubishet Woldeamanuel, MD, Instructor in Neurology and Neurologic Sciences at Stanford University in California, and colleagues enrolled 30 patients with chronic migraine and 30 age- and sex-matched healthy controls into a study. All participants were right-handed. The mean age in the chronic migraine group was 40.5, and the male-to-female ratio was 1:4. Investigators obtained the duration of chronic migraine and lifetime migraine from participants with chronic migraine.
Dr. Woldeamanuel and colleagues acquired T1-weighted brain images on a 3T MRI from all participants. They analyzed whole-brain cortical thickness on unmasked images. The investigators used linear regression to examine group differences on age by cortical thickness between people with chronic migraine and controls. Multiple regression enabled the researchers to control for the confounding effect of duration of chronic migraine and lifetime migraine on age-related cortical thickness changes.
Compared with controls, patients with chronic migraine had significant age-related thinning of the lateral orbitofrontal and supramarginal cortex of the left hemisphere. Patients with chronic migraine had a lack of age-related thinning of the pars orbitalis, superior and inferior parietal, superior temporal, pars opercularis, posterior cingulate, precuneus, superior frontal of the left hemisphere, and the left hemisphere mean cortical thickness, however. In the right hemisphere, the chronic migraine group had significant age-related thinning of the banks of the superior temporal sulcus, caudal anterior cingulate, inferior parietal, precuneus, and supramarginal cortex. The chronic migraine group lacked age-related thinning of the caudal middle frontal, isthmus cingulate, lateral orbitofrontal, paracentral, pars orbitalis, posterior cingulate, rostral middle frontal, superior frontal, and temporal pole of the right hemisphere. These results were not influenced by duration of chronic migraine and lifetime migraine.
The absence of normative age-related cortical thinning in implicated brain areas possibly indicates a perpetually enhanced headache response, head pain cognition, visual and auditory processing, affective behavior, interaction with internal and external cues, and multisensory integration, said the researchers. Accelerated age-related thinning in brain areas involved in sensory pain pathways could represent reduced habituation in chronic migraine, they added. In addition, age-related cortical thinning might indicate progressive loss of migraine modulation. The investigators hypothesized that repetitive migraine attacks might increase allostatic load from headache and nonheadache migraine symptoms.
“We are following these cohorts to determine whether these age-related cortical thickness changes signify cause or effect of chronic migraine,” said Dr. Woldeamanuel.
Suggested Reading
Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014;34(14):1115-1124.
Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015 Feb 13;10(2):e0116687.
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
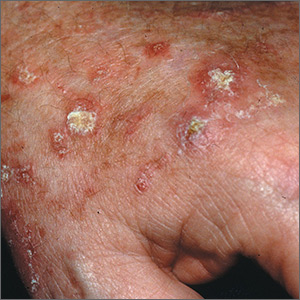
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
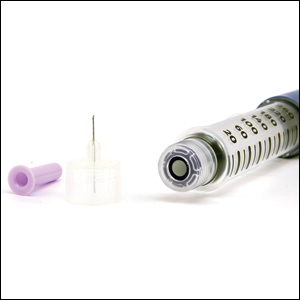
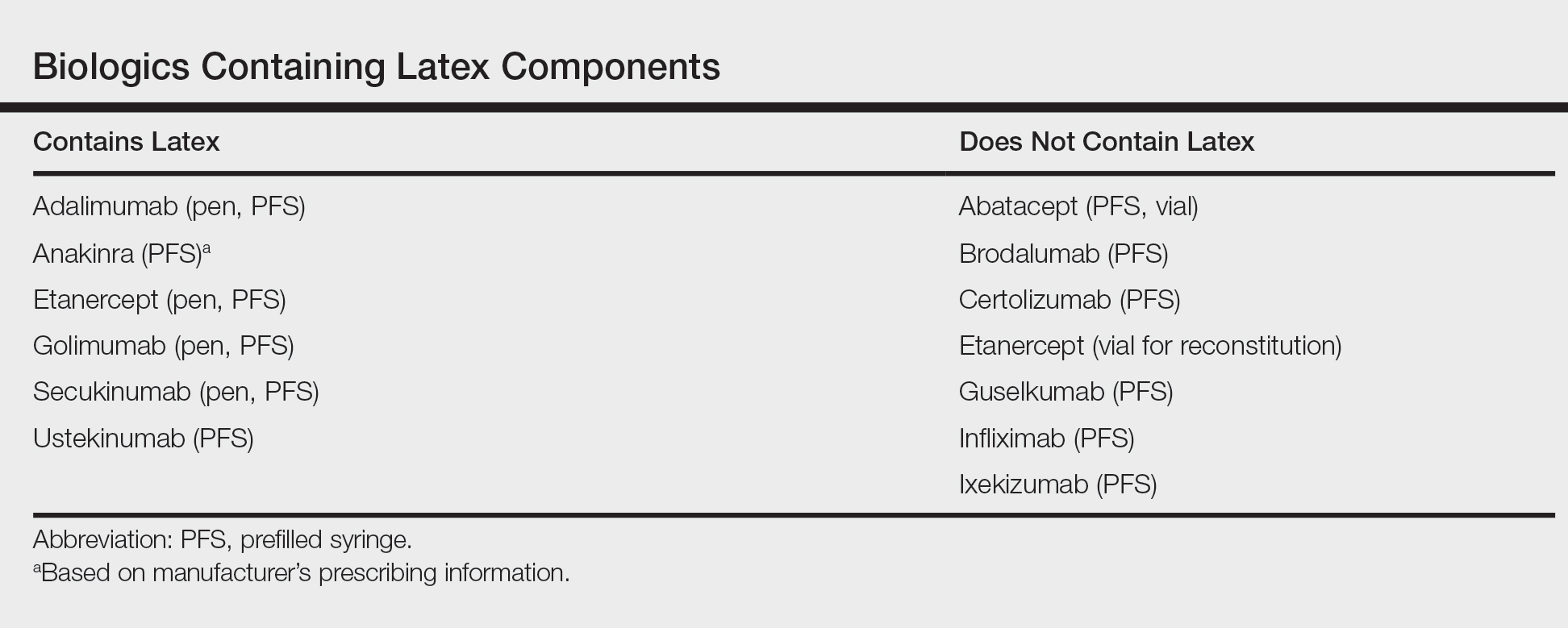
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Researchers Identify Unmet Treatment Needs Among Migraineurs
An analysis of survey data indicates that migraineurs do not receive adequate treatment for nausea.
SAN FRANCISCO—Unmet treatment needs among migraineurs receiving oral prescription medications include therapies to reduce nausea and disturbed sleep, according to a study described at the 60th Annual Scientific Meeting of the American Headache Society. The population also has unmet needs for therapies that provide pain freedom and rapid onset of action.
In 2017, investigators conducted the Migraine in America Symptoms and Treatment (MAST) study, which focused on migraine symptoms, current treatment patterns, and the assessment of unmet treatment needs. Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues examined MAST data to empirically characterize the patterns of poorly controlled migraine and assess corresponding rates of migraine-related disability.
A Survey of American Migraineurs
The MAST survey data were obtained from a general US population sample of migraineurs age 18 and older. Migraineurs were identified using a validated migraine symptom screen based on modified ICHD-3b criteria. Eligible participants had an average of at least one headache day per month during the previous three months.
Respondents provided information about sociodemographics and medication use and responded to a 13-item battery evaluating headache burden and response to medication. The items on the battery were derived from a review of relevant literature, clinician input, and interviews with migraine patients. Participants provided frequency data for each item on a five-point scale ranging from “1) Never” to “5) All or Nearly All of the Time.” To measure construct validity for the scale, the investigators assessed moderate to severe disability for each respondent using the Migraine Disability Assessment Scale (MIDAS).
To determine the underlying structure of unmet treatment needs, Dr. Lipton and colleagues conducted Exploratory Factor Analysis (EFA) among the subset of respondents currently using oral acute prescription medications. They assessed the internal consistency for the derived factors using Cronbach’s alpha. Mean factor scores (range, 1 to 5) were calculated by summing item responses for each factor and dividing by the number of items loading on that factor. The researchers used Mantel-Haenszel Chi Square Test for Trend to evaluate the relationship between mean factor score and rates of moderate to severe migraine-related disability.
Treatment May Act Too Slowly
Among 15,133 respondents meeting inclusion criteria, 3,930 reported current use of acute oral prescription headache medication (mean age, 45.0, 73.6% women, 81.6% Caucasian). Injection and nasal spray medication users were eliminated from the sample. The most commonly endorsed needs were “severe headache attacks come on very rapidly” (52.8%), “attacks reach peak intensity in less than 30 minutes” (50.4%), “severe headache presents upon awakening” (40.9%), and “the return of pain within 24 hours after initial pain relief” (38.6%). EFA identified the following four unmet needs: nausea interference, disturbed sleep, rapid onset, and lack of pain freedom. Internal consistency exceeded acceptable levels for all factors except disturbed sleep. MIDAS disability increased in a clear linear pattern with increasing mean factor scores.
Suggested Reading
Blumenfeld AM, Aurora SK, Laranjo K, Papapetropoulos S. Unmet clinical needs in chronic migraine: Rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxinA for headache prophylaxis in adults with chronic migraine. BMC Neurol. 2015;15:1
Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300-1311.
An analysis of survey data indicates that migraineurs do not receive adequate treatment for nausea.
An analysis of survey data indicates that migraineurs do not receive adequate treatment for nausea.
SAN FRANCISCO—Unmet treatment needs among migraineurs receiving oral prescription medications include therapies to reduce nausea and disturbed sleep, according to a study described at the 60th Annual Scientific Meeting of the American Headache Society. The population also has unmet needs for therapies that provide pain freedom and rapid onset of action.
In 2017, investigators conducted the Migraine in America Symptoms and Treatment (MAST) study, which focused on migraine symptoms, current treatment patterns, and the assessment of unmet treatment needs. Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues examined MAST data to empirically characterize the patterns of poorly controlled migraine and assess corresponding rates of migraine-related disability.
A Survey of American Migraineurs
The MAST survey data were obtained from a general US population sample of migraineurs age 18 and older. Migraineurs were identified using a validated migraine symptom screen based on modified ICHD-3b criteria. Eligible participants had an average of at least one headache day per month during the previous three months.
Respondents provided information about sociodemographics and medication use and responded to a 13-item battery evaluating headache burden and response to medication. The items on the battery were derived from a review of relevant literature, clinician input, and interviews with migraine patients. Participants provided frequency data for each item on a five-point scale ranging from “1) Never” to “5) All or Nearly All of the Time.” To measure construct validity for the scale, the investigators assessed moderate to severe disability for each respondent using the Migraine Disability Assessment Scale (MIDAS).
To determine the underlying structure of unmet treatment needs, Dr. Lipton and colleagues conducted Exploratory Factor Analysis (EFA) among the subset of respondents currently using oral acute prescription medications. They assessed the internal consistency for the derived factors using Cronbach’s alpha. Mean factor scores (range, 1 to 5) were calculated by summing item responses for each factor and dividing by the number of items loading on that factor. The researchers used Mantel-Haenszel Chi Square Test for Trend to evaluate the relationship between mean factor score and rates of moderate to severe migraine-related disability.
Treatment May Act Too Slowly
Among 15,133 respondents meeting inclusion criteria, 3,930 reported current use of acute oral prescription headache medication (mean age, 45.0, 73.6% women, 81.6% Caucasian). Injection and nasal spray medication users were eliminated from the sample. The most commonly endorsed needs were “severe headache attacks come on very rapidly” (52.8%), “attacks reach peak intensity in less than 30 minutes” (50.4%), “severe headache presents upon awakening” (40.9%), and “the return of pain within 24 hours after initial pain relief” (38.6%). EFA identified the following four unmet needs: nausea interference, disturbed sleep, rapid onset, and lack of pain freedom. Internal consistency exceeded acceptable levels for all factors except disturbed sleep. MIDAS disability increased in a clear linear pattern with increasing mean factor scores.
Suggested Reading
Blumenfeld AM, Aurora SK, Laranjo K, Papapetropoulos S. Unmet clinical needs in chronic migraine: Rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxinA for headache prophylaxis in adults with chronic migraine. BMC Neurol. 2015;15:1
Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300-1311.
SAN FRANCISCO—Unmet treatment needs among migraineurs receiving oral prescription medications include therapies to reduce nausea and disturbed sleep, according to a study described at the 60th Annual Scientific Meeting of the American Headache Society. The population also has unmet needs for therapies that provide pain freedom and rapid onset of action.
In 2017, investigators conducted the Migraine in America Symptoms and Treatment (MAST) study, which focused on migraine symptoms, current treatment patterns, and the assessment of unmet treatment needs. Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in the Bronx, New York, and colleagues examined MAST data to empirically characterize the patterns of poorly controlled migraine and assess corresponding rates of migraine-related disability.
A Survey of American Migraineurs
The MAST survey data were obtained from a general US population sample of migraineurs age 18 and older. Migraineurs were identified using a validated migraine symptom screen based on modified ICHD-3b criteria. Eligible participants had an average of at least one headache day per month during the previous three months.
Respondents provided information about sociodemographics and medication use and responded to a 13-item battery evaluating headache burden and response to medication. The items on the battery were derived from a review of relevant literature, clinician input, and interviews with migraine patients. Participants provided frequency data for each item on a five-point scale ranging from “1) Never” to “5) All or Nearly All of the Time.” To measure construct validity for the scale, the investigators assessed moderate to severe disability for each respondent using the Migraine Disability Assessment Scale (MIDAS).
To determine the underlying structure of unmet treatment needs, Dr. Lipton and colleagues conducted Exploratory Factor Analysis (EFA) among the subset of respondents currently using oral acute prescription medications. They assessed the internal consistency for the derived factors using Cronbach’s alpha. Mean factor scores (range, 1 to 5) were calculated by summing item responses for each factor and dividing by the number of items loading on that factor. The researchers used Mantel-Haenszel Chi Square Test for Trend to evaluate the relationship between mean factor score and rates of moderate to severe migraine-related disability.
Treatment May Act Too Slowly
Among 15,133 respondents meeting inclusion criteria, 3,930 reported current use of acute oral prescription headache medication (mean age, 45.0, 73.6% women, 81.6% Caucasian). Injection and nasal spray medication users were eliminated from the sample. The most commonly endorsed needs were “severe headache attacks come on very rapidly” (52.8%), “attacks reach peak intensity in less than 30 minutes” (50.4%), “severe headache presents upon awakening” (40.9%), and “the return of pain within 24 hours after initial pain relief” (38.6%). EFA identified the following four unmet needs: nausea interference, disturbed sleep, rapid onset, and lack of pain freedom. Internal consistency exceeded acceptable levels for all factors except disturbed sleep. MIDAS disability increased in a clear linear pattern with increasing mean factor scores.
Suggested Reading
Blumenfeld AM, Aurora SK, Laranjo K, Papapetropoulos S. Unmet clinical needs in chronic migraine: Rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxinA for headache prophylaxis in adults with chronic migraine. BMC Neurol. 2015;15:1
Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300-1311.
Lenalidomide becomes standard of care for multiple myeloma in the maintenance setting
The treatment of multiple myeloma has been revolutionized in the past few decades, with the introduction of numerous novel drug classes that have more than doubled median survival times. The immunomodulatory drug (IMiD), lenalidomide, forms the backbone of the majority of treatment paradigms, first receiving US Food and Drug Administration approval in 2006 for use in combination with dexamethasone in previously treated patients with multiple myeloma. Since then, approved indications for lenalidomide in multiple myeloma have continued to expand.
Most recently, on February 22, 2017, lenalidomide was approved for use as maintenance therapy following autologous stem cell transplant (ASCT), making it the first and only treatment available in this setting. This approval was based on 2 randomized, controlled trials that evaluated the efficacy and safety of lenalidomide in more than 1,000 patients in this setting and demonstrated a significant advantage in progression-free survival (PFS) compared with patients receiving placebo.
CALGB 1001041 and IFM 2005-022 were randomized, double-blind phase 3 trials conducted at 47 locations across the United States and 78 centers in France, Belgium, and Switzerland, respectively. In the CALGB trial, eligible patients were 18-70 years of age, with a European Cooperative Oncology Group (ECOG) performance status of 0 or 1, symptomatic disease requiring treatment (Durie-Salmon stage ≥1), and who received any induction therapy of 2-12 months duration. In the IFM trial, eligible patients were younger than 65 years, with multiple myeloma that had not progressed in the interval between first-line ASCT, performed within the previous 6 months, and randomization, and who had normal liver function tests and blood cell counts.
In CALGB 100104, after undergoing ASCT, 460 patients were randomly assigned to lenalidomide (starting at a dose of 10 mg/day) or placebo between day 100 and day 110 after transplantation. In IFM 2005-02, after undergoing ASCT, 614 patients were randomized 1:1 to receive either consolidation treatment with lenalidomide (at a dose of 25 mg/day on days 1-21 of each 28-day cycle for 2 cycles) followed by maintenance with lenalidomide (10 mg/day for the first 3 months, increasing to 15 mg if tolerated), or the same consolidation treatment followed by maintenance therapy with placebo.
The primary endpoint of CALGB 100104 was time to progression (TTP) and lenalidomide was associated with a significantly longer TTP. Median PFS was also improved by around 15 months (hazard ratio [HR], 0.38; P < .001). In a more recent long-term PFS analysis, median PFS was 5.7 years in the lenalidomide arm compared with 1.9 years with placebo, a difference of 3.8 years (HR, 0.38).3
The primary endpoint for IFM 2005-02 was PFS and lenalidomide maintenance therapy resulted in a significant improvement in PFS in both the originally published study (18-month PFS advantage) and long-term follow-up. The most recent PFS analysis demonstrated a PFS of 3.9 years for lenalidomide, compared with 2 years for no maintenance, a difference of 1.9 years (HR, 0.53). Although the studies were not powered for an overall survival (OS) endpoint, a descriptive analysis showed a median OS of 9.3 years, compared with 7 years in CALGB 100104, and 8.8 years compared with 7.3 years in IFM 2005-02.
In a meta-analysis of data pooled from these 2 studies and a third randomized trial (GIMEMA-RVMM-PI-209),4 which was presented at the 2016 annual meeting of the American Society of Clinical Oncology, maintenance therapy with lenalidomide following frontline treatment with high-dose melphalan and ASCT reduced the risk of death by 26% compared with placebo or no maintenance therapy, prompting suggestions that lenalidomide become standard of care in this setting.
The safety profile of lenalidomide in this setting was similar to that previously described in other studies. The most frequently reported adverse events (AEs), across both studies, were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, asthenia, muscle spasm, and pyrexia. The most common grade 3/4 AEs included neutropenia, thrombocytopenia, and leukopenia. AEs were generally most common in the first 6 months of treatment and subsequently declined in frequency over time or remained stable.
The prescribing information carries warnings and precautions about embryo-fetal toxicity, hematologic toxicity, venous/arterial thromboembolic events, secondary primary malignancies, hepatotoxicity, allergic reactions, tumor lysis syndrome, and thyroid disorders.5 Given its teratogenic effects, lenalidomide is only available through a restricted program under a risk evaluation mitigation strategy.
Patients with neutropenia should be monitored for signs of infections, patients advised to look for signs of bleeding or bruising, and weekly complete blood count performed for the first 2 cycles, on days 1 and 15 of cycle 3 and every 4 weeks thereafter.
Action should be taken to try to reduce the risk of venous and arterial thromboembolic events where possible and thrombophylaxis is recommended, based on the assessment of the underlying risk. Since lenalidomide can increase the risk of secondary primary malignancies, each case should be evaluated for risk-to-benefit ratio.
Liver enzymes should be monitored periodically and treatment interrupted upon their elevation, resuming at a lower dose if levels return to baseline values. Patients who have a history of grade 4 rash following thalidomide treatment should not receive lenalidomide. If grade 2-3 skin rash occurs, treatment interruption or discontinuation should be considered and lenalidomide should be discontinued in the event of angioedema, grade 4 rash, exfoliative or bullous rash, or if Stevens-Johnson s
Patients with high tumor burden prior to treatment are at highest risk of tumor lysis syndrome and should be monitored closely and appropriate precautions taken, and thyroid function should be measured before and during lenalidomide treatment to address potential thyroid disorders. Lenalidomide is marketed as Revlimid by Celgene Corporation.
The treatment of multiple myeloma has been revolutionized in the past few decades, with the introduction of numerous novel drug classes that have more than doubled median survival times. The immunomodulatory drug (IMiD), lenalidomide, forms the backbone of the majority of treatment paradigms, first receiving US Food and Drug Administration approval in 2006 for use in combination with dexamethasone in previously treated patients with multiple myeloma. Since then, approved indications for lenalidomide in multiple myeloma have continued to expand.
Most recently, on February 22, 2017, lenalidomide was approved for use as maintenance therapy following autologous stem cell transplant (ASCT), making it the first and only treatment available in this setting. This approval was based on 2 randomized, controlled trials that evaluated the efficacy and safety of lenalidomide in more than 1,000 patients in this setting and demonstrated a significant advantage in progression-free survival (PFS) compared with patients receiving placebo.
CALGB 1001041 and IFM 2005-022 were randomized, double-blind phase 3 trials conducted at 47 locations across the United States and 78 centers in France, Belgium, and Switzerland, respectively. In the CALGB trial, eligible patients were 18-70 years of age, with a European Cooperative Oncology Group (ECOG) performance status of 0 or 1, symptomatic disease requiring treatment (Durie-Salmon stage ≥1), and who received any induction therapy of 2-12 months duration. In the IFM trial, eligible patients were younger than 65 years, with multiple myeloma that had not progressed in the interval between first-line ASCT, performed within the previous 6 months, and randomization, and who had normal liver function tests and blood cell counts.
In CALGB 100104, after undergoing ASCT, 460 patients were randomly assigned to lenalidomide (starting at a dose of 10 mg/day) or placebo between day 100 and day 110 after transplantation. In IFM 2005-02, after undergoing ASCT, 614 patients were randomized 1:1 to receive either consolidation treatment with lenalidomide (at a dose of 25 mg/day on days 1-21 of each 28-day cycle for 2 cycles) followed by maintenance with lenalidomide (10 mg/day for the first 3 months, increasing to 15 mg if tolerated), or the same consolidation treatment followed by maintenance therapy with placebo.
The primary endpoint of CALGB 100104 was time to progression (TTP) and lenalidomide was associated with a significantly longer TTP. Median PFS was also improved by around 15 months (hazard ratio [HR], 0.38; P < .001). In a more recent long-term PFS analysis, median PFS was 5.7 years in the lenalidomide arm compared with 1.9 years with placebo, a difference of 3.8 years (HR, 0.38).3
The primary endpoint for IFM 2005-02 was PFS and lenalidomide maintenance therapy resulted in a significant improvement in PFS in both the originally published study (18-month PFS advantage) and long-term follow-up. The most recent PFS analysis demonstrated a PFS of 3.9 years for lenalidomide, compared with 2 years for no maintenance, a difference of 1.9 years (HR, 0.53). Although the studies were not powered for an overall survival (OS) endpoint, a descriptive analysis showed a median OS of 9.3 years, compared with 7 years in CALGB 100104, and 8.8 years compared with 7.3 years in IFM 2005-02.
In a meta-analysis of data pooled from these 2 studies and a third randomized trial (GIMEMA-RVMM-PI-209),4 which was presented at the 2016 annual meeting of the American Society of Clinical Oncology, maintenance therapy with lenalidomide following frontline treatment with high-dose melphalan and ASCT reduced the risk of death by 26% compared with placebo or no maintenance therapy, prompting suggestions that lenalidomide become standard of care in this setting.
The safety profile of lenalidomide in this setting was similar to that previously described in other studies. The most frequently reported adverse events (AEs), across both studies, were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, asthenia, muscle spasm, and pyrexia. The most common grade 3/4 AEs included neutropenia, thrombocytopenia, and leukopenia. AEs were generally most common in the first 6 months of treatment and subsequently declined in frequency over time or remained stable.
The prescribing information carries warnings and precautions about embryo-fetal toxicity, hematologic toxicity, venous/arterial thromboembolic events, secondary primary malignancies, hepatotoxicity, allergic reactions, tumor lysis syndrome, and thyroid disorders.5 Given its teratogenic effects, lenalidomide is only available through a restricted program under a risk evaluation mitigation strategy.
Patients with neutropenia should be monitored for signs of infections, patients advised to look for signs of bleeding or bruising, and weekly complete blood count performed for the first 2 cycles, on days 1 and 15 of cycle 3 and every 4 weeks thereafter.
Action should be taken to try to reduce the risk of venous and arterial thromboembolic events where possible and thrombophylaxis is recommended, based on the assessment of the underlying risk. Since lenalidomide can increase the risk of secondary primary malignancies, each case should be evaluated for risk-to-benefit ratio.
Liver enzymes should be monitored periodically and treatment interrupted upon their elevation, resuming at a lower dose if levels return to baseline values. Patients who have a history of grade 4 rash following thalidomide treatment should not receive lenalidomide. If grade 2-3 skin rash occurs, treatment interruption or discontinuation should be considered and lenalidomide should be discontinued in the event of angioedema, grade 4 rash, exfoliative or bullous rash, or if Stevens-Johnson s
Patients with high tumor burden prior to treatment are at highest risk of tumor lysis syndrome and should be monitored closely and appropriate precautions taken, and thyroid function should be measured before and during lenalidomide treatment to address potential thyroid disorders. Lenalidomide is marketed as Revlimid by Celgene Corporation.
The treatment of multiple myeloma has been revolutionized in the past few decades, with the introduction of numerous novel drug classes that have more than doubled median survival times. The immunomodulatory drug (IMiD), lenalidomide, forms the backbone of the majority of treatment paradigms, first receiving US Food and Drug Administration approval in 2006 for use in combination with dexamethasone in previously treated patients with multiple myeloma. Since then, approved indications for lenalidomide in multiple myeloma have continued to expand.
Most recently, on February 22, 2017, lenalidomide was approved for use as maintenance therapy following autologous stem cell transplant (ASCT), making it the first and only treatment available in this setting. This approval was based on 2 randomized, controlled trials that evaluated the efficacy and safety of lenalidomide in more than 1,000 patients in this setting and demonstrated a significant advantage in progression-free survival (PFS) compared with patients receiving placebo.
CALGB 1001041 and IFM 2005-022 were randomized, double-blind phase 3 trials conducted at 47 locations across the United States and 78 centers in France, Belgium, and Switzerland, respectively. In the CALGB trial, eligible patients were 18-70 years of age, with a European Cooperative Oncology Group (ECOG) performance status of 0 or 1, symptomatic disease requiring treatment (Durie-Salmon stage ≥1), and who received any induction therapy of 2-12 months duration. In the IFM trial, eligible patients were younger than 65 years, with multiple myeloma that had not progressed in the interval between first-line ASCT, performed within the previous 6 months, and randomization, and who had normal liver function tests and blood cell counts.
In CALGB 100104, after undergoing ASCT, 460 patients were randomly assigned to lenalidomide (starting at a dose of 10 mg/day) or placebo between day 100 and day 110 after transplantation. In IFM 2005-02, after undergoing ASCT, 614 patients were randomized 1:1 to receive either consolidation treatment with lenalidomide (at a dose of 25 mg/day on days 1-21 of each 28-day cycle for 2 cycles) followed by maintenance with lenalidomide (10 mg/day for the first 3 months, increasing to 15 mg if tolerated), or the same consolidation treatment followed by maintenance therapy with placebo.
The primary endpoint of CALGB 100104 was time to progression (TTP) and lenalidomide was associated with a significantly longer TTP. Median PFS was also improved by around 15 months (hazard ratio [HR], 0.38; P < .001). In a more recent long-term PFS analysis, median PFS was 5.7 years in the lenalidomide arm compared with 1.9 years with placebo, a difference of 3.8 years (HR, 0.38).3
The primary endpoint for IFM 2005-02 was PFS and lenalidomide maintenance therapy resulted in a significant improvement in PFS in both the originally published study (18-month PFS advantage) and long-term follow-up. The most recent PFS analysis demonstrated a PFS of 3.9 years for lenalidomide, compared with 2 years for no maintenance, a difference of 1.9 years (HR, 0.53). Although the studies were not powered for an overall survival (OS) endpoint, a descriptive analysis showed a median OS of 9.3 years, compared with 7 years in CALGB 100104, and 8.8 years compared with 7.3 years in IFM 2005-02.
In a meta-analysis of data pooled from these 2 studies and a third randomized trial (GIMEMA-RVMM-PI-209),4 which was presented at the 2016 annual meeting of the American Society of Clinical Oncology, maintenance therapy with lenalidomide following frontline treatment with high-dose melphalan and ASCT reduced the risk of death by 26% compared with placebo or no maintenance therapy, prompting suggestions that lenalidomide become standard of care in this setting.
The safety profile of lenalidomide in this setting was similar to that previously described in other studies. The most frequently reported adverse events (AEs), across both studies, were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, asthenia, muscle spasm, and pyrexia. The most common grade 3/4 AEs included neutropenia, thrombocytopenia, and leukopenia. AEs were generally most common in the first 6 months of treatment and subsequently declined in frequency over time or remained stable.
The prescribing information carries warnings and precautions about embryo-fetal toxicity, hematologic toxicity, venous/arterial thromboembolic events, secondary primary malignancies, hepatotoxicity, allergic reactions, tumor lysis syndrome, and thyroid disorders.5 Given its teratogenic effects, lenalidomide is only available through a restricted program under a risk evaluation mitigation strategy.
Patients with neutropenia should be monitored for signs of infections, patients advised to look for signs of bleeding or bruising, and weekly complete blood count performed for the first 2 cycles, on days 1 and 15 of cycle 3 and every 4 weeks thereafter.
Action should be taken to try to reduce the risk of venous and arterial thromboembolic events where possible and thrombophylaxis is recommended, based on the assessment of the underlying risk. Since lenalidomide can increase the risk of secondary primary malignancies, each case should be evaluated for risk-to-benefit ratio.
Liver enzymes should be monitored periodically and treatment interrupted upon their elevation, resuming at a lower dose if levels return to baseline values. Patients who have a history of grade 4 rash following thalidomide treatment should not receive lenalidomide. If grade 2-3 skin rash occurs, treatment interruption or discontinuation should be considered and lenalidomide should be discontinued in the event of angioedema, grade 4 rash, exfoliative or bullous rash, or if Stevens-Johnson s
Patients with high tumor burden prior to treatment are at highest risk of tumor lysis syndrome and should be monitored closely and appropriate precautions taken, and thyroid function should be measured before and during lenalidomide treatment to address potential thyroid disorders. Lenalidomide is marketed as Revlimid by Celgene Corporation.
Sublingual Apomorphine Effectively Manages Off Episodes in Parkinson’s Disease
The treatment significantly improves motor function at 30 minutes after dosing, compared with placebo.
MIAMI—The sublingual apomorphine film APL-130277 (APL) is effective and well-tolerated for the acute management of off episodes in patients with Parkinson’s disease, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress.
APL is in development for the acute, intermittent treatment of off episodes associated with Parkinson’s disease, including end-of-dose wearing off (including early morning off); partial, delayed, or no on; and unpredictable off.
Evaluating the Efficacy and Safety of APL
To evaluate the efficacy and safety of APL, C. Warren Olanow, MD, Professor Emeritus and Chair Emeritus of Neurology and Professor of Neuroscience at the Mount Sinai School of Medicine in New York City, and colleagues conducted a double-blind, placebo-controlled trial.
Eligible participants were 18 or older, had idiopathic Parkinson’s disease according to UK Brain Bank criteria, were stage I–III according to the modified Hoehn and Yahr scale when on, and had a clinically meaningful response to levodopa with well-defined, early-morning off episodes. They had one or more off episodes per day and a total daily off time of two or more hours when receiving stable doses of levodopa qid or carbidopa–levodopa extended-release capsules tid for four or more weeks, or monoamine oxidase B inhibitors for eight or more weeks.
Patients with atypical or secondary parkinsonism, a major psychiatric disorder, or mouth cankers or sores were excluded. Patients who had undergone a neurosurgical procedure for Parkinson’s disease, received continuous subcutaneous apomorphine infusion, or received Duopa also were excluded. Finally, patients currently taking 5-HT3 antagonists, selective dopamine antagonists (excluding quetiapine or clozapine), or dopamine-depleting agents were excluded.
The APL dose (10 mg to 35 mg) to produce a full on was determined during the open-label titration phase. During the double-blind treatment phase, researchers randomized patients to the dose of APL identified during the titration phase or placebo that could be self-administered as many as five times per day for 12 weeks. Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Part III (MDS-UPDRS-III) scores were determined monthly before dosing and at 15, 30, 45, 60, and 90 minutes post dose. The primary end point was the change in MDS-UPDRS-III score at 30 minutes post dose at 12 weeks. The key secondary end point was the percentage of patients with a patient-determined full on response within 30 minutes at 12 weeks. Safety assessments were also performed.
Treated Patients Were More Likely to Be On
A total of 109 patients were randomized to the double-blind treatment phase, and had a mean of 3.9 off episodes per day. Participants’ mean age was 62.7, and 37.6% of participants were female. More than 90% of participants were white.
In all, 80 patients completed the study. The least squares mean change from predose to 30 minutes post dose for the MDS-UPDRS-III score at 12 weeks was –11.1 and –3.5 for the APL and placebo groups, respectively (mean difference, –7.6). Similar results were observed at day 1 and weeks 4 and 8.
The difference between treatment arms in motor score became significant at 15 minutes and remained significant until 90 minutes. There was a significant difference favoring APL over placebo in the percentage of patients achieving a self-rated full on response at 30 minutes post dose at week 12. A home dosing diary showed that a larger percentage of patients receiving APL were on within 30 minutes post dose (least squares mean, 78.70%), compared with controls (least squares mean, 31.10%).
In the double-blind treatment phase, the overall discontinuation rate was 16.4% for placebo and 37.0% for APL. The discontinuation rate due to adverse events was 9.1% and 27.8% for placebo and APL, respectively. Discontinuation due to adverse events was the most common reason. During the double-blind treatment phase of APL, the most frequent adverse events were nausea (27.8%), somnolence (13%), and dizziness (9.3%). Most treatment-emergent adverse events were mild to moderate. Six patients experienced severe adverse events in the placebo and APL groups combined. Three patients experienced serious adverse events combined. One patient in the APL group died from cardiac arrest considered possibly related to treatment by the investigator. Oral adverse events occurred in 31.5% of patients in the APL group versus 7.3% of controls. These events were generally mild and reversible, said the researchers.
—Erica Tricarico
The treatment significantly improves motor function at 30 minutes after dosing, compared with placebo.
The treatment significantly improves motor function at 30 minutes after dosing, compared with placebo.
MIAMI—The sublingual apomorphine film APL-130277 (APL) is effective and well-tolerated for the acute management of off episodes in patients with Parkinson’s disease, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress.
APL is in development for the acute, intermittent treatment of off episodes associated with Parkinson’s disease, including end-of-dose wearing off (including early morning off); partial, delayed, or no on; and unpredictable off.
Evaluating the Efficacy and Safety of APL
To evaluate the efficacy and safety of APL, C. Warren Olanow, MD, Professor Emeritus and Chair Emeritus of Neurology and Professor of Neuroscience at the Mount Sinai School of Medicine in New York City, and colleagues conducted a double-blind, placebo-controlled trial.
Eligible participants were 18 or older, had idiopathic Parkinson’s disease according to UK Brain Bank criteria, were stage I–III according to the modified Hoehn and Yahr scale when on, and had a clinically meaningful response to levodopa with well-defined, early-morning off episodes. They had one or more off episodes per day and a total daily off time of two or more hours when receiving stable doses of levodopa qid or carbidopa–levodopa extended-release capsules tid for four or more weeks, or monoamine oxidase B inhibitors for eight or more weeks.
Patients with atypical or secondary parkinsonism, a major psychiatric disorder, or mouth cankers or sores were excluded. Patients who had undergone a neurosurgical procedure for Parkinson’s disease, received continuous subcutaneous apomorphine infusion, or received Duopa also were excluded. Finally, patients currently taking 5-HT3 antagonists, selective dopamine antagonists (excluding quetiapine or clozapine), or dopamine-depleting agents were excluded.
The APL dose (10 mg to 35 mg) to produce a full on was determined during the open-label titration phase. During the double-blind treatment phase, researchers randomized patients to the dose of APL identified during the titration phase or placebo that could be self-administered as many as five times per day for 12 weeks. Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Part III (MDS-UPDRS-III) scores were determined monthly before dosing and at 15, 30, 45, 60, and 90 minutes post dose. The primary end point was the change in MDS-UPDRS-III score at 30 minutes post dose at 12 weeks. The key secondary end point was the percentage of patients with a patient-determined full on response within 30 minutes at 12 weeks. Safety assessments were also performed.
Treated Patients Were More Likely to Be On
A total of 109 patients were randomized to the double-blind treatment phase, and had a mean of 3.9 off episodes per day. Participants’ mean age was 62.7, and 37.6% of participants were female. More than 90% of participants were white.
In all, 80 patients completed the study. The least squares mean change from predose to 30 minutes post dose for the MDS-UPDRS-III score at 12 weeks was –11.1 and –3.5 for the APL and placebo groups, respectively (mean difference, –7.6). Similar results were observed at day 1 and weeks 4 and 8.
The difference between treatment arms in motor score became significant at 15 minutes and remained significant until 90 minutes. There was a significant difference favoring APL over placebo in the percentage of patients achieving a self-rated full on response at 30 minutes post dose at week 12. A home dosing diary showed that a larger percentage of patients receiving APL were on within 30 minutes post dose (least squares mean, 78.70%), compared with controls (least squares mean, 31.10%).
In the double-blind treatment phase, the overall discontinuation rate was 16.4% for placebo and 37.0% for APL. The discontinuation rate due to adverse events was 9.1% and 27.8% for placebo and APL, respectively. Discontinuation due to adverse events was the most common reason. During the double-blind treatment phase of APL, the most frequent adverse events were nausea (27.8%), somnolence (13%), and dizziness (9.3%). Most treatment-emergent adverse events were mild to moderate. Six patients experienced severe adverse events in the placebo and APL groups combined. Three patients experienced serious adverse events combined. One patient in the APL group died from cardiac arrest considered possibly related to treatment by the investigator. Oral adverse events occurred in 31.5% of patients in the APL group versus 7.3% of controls. These events were generally mild and reversible, said the researchers.
—Erica Tricarico
MIAMI—The sublingual apomorphine film APL-130277 (APL) is effective and well-tolerated for the acute management of off episodes in patients with Parkinson’s disease, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress.
APL is in development for the acute, intermittent treatment of off episodes associated with Parkinson’s disease, including end-of-dose wearing off (including early morning off); partial, delayed, or no on; and unpredictable off.
Evaluating the Efficacy and Safety of APL
To evaluate the efficacy and safety of APL, C. Warren Olanow, MD, Professor Emeritus and Chair Emeritus of Neurology and Professor of Neuroscience at the Mount Sinai School of Medicine in New York City, and colleagues conducted a double-blind, placebo-controlled trial.
Eligible participants were 18 or older, had idiopathic Parkinson’s disease according to UK Brain Bank criteria, were stage I–III according to the modified Hoehn and Yahr scale when on, and had a clinically meaningful response to levodopa with well-defined, early-morning off episodes. They had one or more off episodes per day and a total daily off time of two or more hours when receiving stable doses of levodopa qid or carbidopa–levodopa extended-release capsules tid for four or more weeks, or monoamine oxidase B inhibitors for eight or more weeks.
Patients with atypical or secondary parkinsonism, a major psychiatric disorder, or mouth cankers or sores were excluded. Patients who had undergone a neurosurgical procedure for Parkinson’s disease, received continuous subcutaneous apomorphine infusion, or received Duopa also were excluded. Finally, patients currently taking 5-HT3 antagonists, selective dopamine antagonists (excluding quetiapine or clozapine), or dopamine-depleting agents were excluded.
The APL dose (10 mg to 35 mg) to produce a full on was determined during the open-label titration phase. During the double-blind treatment phase, researchers randomized patients to the dose of APL identified during the titration phase or placebo that could be self-administered as many as five times per day for 12 weeks. Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Part III (MDS-UPDRS-III) scores were determined monthly before dosing and at 15, 30, 45, 60, and 90 minutes post dose. The primary end point was the change in MDS-UPDRS-III score at 30 minutes post dose at 12 weeks. The key secondary end point was the percentage of patients with a patient-determined full on response within 30 minutes at 12 weeks. Safety assessments were also performed.
Treated Patients Were More Likely to Be On
A total of 109 patients were randomized to the double-blind treatment phase, and had a mean of 3.9 off episodes per day. Participants’ mean age was 62.7, and 37.6% of participants were female. More than 90% of participants were white.
In all, 80 patients completed the study. The least squares mean change from predose to 30 minutes post dose for the MDS-UPDRS-III score at 12 weeks was –11.1 and –3.5 for the APL and placebo groups, respectively (mean difference, –7.6). Similar results were observed at day 1 and weeks 4 and 8.
The difference between treatment arms in motor score became significant at 15 minutes and remained significant until 90 minutes. There was a significant difference favoring APL over placebo in the percentage of patients achieving a self-rated full on response at 30 minutes post dose at week 12. A home dosing diary showed that a larger percentage of patients receiving APL were on within 30 minutes post dose (least squares mean, 78.70%), compared with controls (least squares mean, 31.10%).
In the double-blind treatment phase, the overall discontinuation rate was 16.4% for placebo and 37.0% for APL. The discontinuation rate due to adverse events was 9.1% and 27.8% for placebo and APL, respectively. Discontinuation due to adverse events was the most common reason. During the double-blind treatment phase of APL, the most frequent adverse events were nausea (27.8%), somnolence (13%), and dizziness (9.3%). Most treatment-emergent adverse events were mild to moderate. Six patients experienced severe adverse events in the placebo and APL groups combined. Three patients experienced serious adverse events combined. One patient in the APL group died from cardiac arrest considered possibly related to treatment by the investigator. Oral adverse events occurred in 31.5% of patients in the APL group versus 7.3% of controls. These events were generally mild and reversible, said the researchers.
—Erica Tricarico
Inflammatory Linear Verrucous Epidermal Nevus Responsive to 308-nm Excimer Laser Treatment
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Four syndromes suggest life-threatening PVL-positive S. aureus infection
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
EXPERT ANALYSIS FROM ESPID 2018
Rough skin on hands
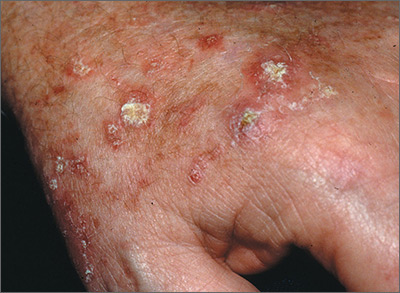
The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.