User login
FDA proposes broader outcomes for OUD treatment drug approvals
Manufacturers developing the next generation of drugs to help combat opioid use disorder could have a broader set of outcome measures to target when bringing their products before the Food and Drug Administration for approval.
Traditionally, the FDA has used a reduction in drug-taking behavior as the endpoint for approving a medication-assisted treatment to combat opioid use disorder. But a draft guidance issued Aug. 6 could change that.
The guidance, “Opioid Use Disorder: Endpoints for Demonstrating Effectiveness of Drugs for Medication-Assisted Treatment,” proposes numerous clinical endpoints, including reduction in adverse outcomes of opioid use disorder, (for example, mortality, the need for emergency medical interventions, or hepatitis C seroconversion); change in the disease status using diagnostic criteria for opioid use disorder; development of patient-reported outcome measures; or changes in drug use patterns other than the commonly used endpoint of abstinence.
, such as the ability to resume work or school.
“The evidence is clear. Medication-assisted treatment works, and it is a key piece of defeating the drug crisis facing our country,” Department of Health and Human Services Secretary Alex Azar said in a statement. He added that the new guidance has “the potential to bring new medications to market that are more closely tailored to patient needs and help give Americans facing addiction a better change at recovery.”
FDA Commissioner Scott Gottlieb, MD, added in the statement: “We must consider new ways to gauge success beyond simply whether a patient in recovery has stopped using opioids, such as reducing relapse overdoses and infectious disease transmission. Treatments that can impact these aspects of addiction can be important parts of a comprehensive approach to the treatment of opioid use disorder.”
Guidance comments are due Oct. 9 and can be submitted online here.
Manufacturers developing the next generation of drugs to help combat opioid use disorder could have a broader set of outcome measures to target when bringing their products before the Food and Drug Administration for approval.
Traditionally, the FDA has used a reduction in drug-taking behavior as the endpoint for approving a medication-assisted treatment to combat opioid use disorder. But a draft guidance issued Aug. 6 could change that.
The guidance, “Opioid Use Disorder: Endpoints for Demonstrating Effectiveness of Drugs for Medication-Assisted Treatment,” proposes numerous clinical endpoints, including reduction in adverse outcomes of opioid use disorder, (for example, mortality, the need for emergency medical interventions, or hepatitis C seroconversion); change in the disease status using diagnostic criteria for opioid use disorder; development of patient-reported outcome measures; or changes in drug use patterns other than the commonly used endpoint of abstinence.
, such as the ability to resume work or school.
“The evidence is clear. Medication-assisted treatment works, and it is a key piece of defeating the drug crisis facing our country,” Department of Health and Human Services Secretary Alex Azar said in a statement. He added that the new guidance has “the potential to bring new medications to market that are more closely tailored to patient needs and help give Americans facing addiction a better change at recovery.”
FDA Commissioner Scott Gottlieb, MD, added in the statement: “We must consider new ways to gauge success beyond simply whether a patient in recovery has stopped using opioids, such as reducing relapse overdoses and infectious disease transmission. Treatments that can impact these aspects of addiction can be important parts of a comprehensive approach to the treatment of opioid use disorder.”
Guidance comments are due Oct. 9 and can be submitted online here.
Manufacturers developing the next generation of drugs to help combat opioid use disorder could have a broader set of outcome measures to target when bringing their products before the Food and Drug Administration for approval.
Traditionally, the FDA has used a reduction in drug-taking behavior as the endpoint for approving a medication-assisted treatment to combat opioid use disorder. But a draft guidance issued Aug. 6 could change that.
The guidance, “Opioid Use Disorder: Endpoints for Demonstrating Effectiveness of Drugs for Medication-Assisted Treatment,” proposes numerous clinical endpoints, including reduction in adverse outcomes of opioid use disorder, (for example, mortality, the need for emergency medical interventions, or hepatitis C seroconversion); change in the disease status using diagnostic criteria for opioid use disorder; development of patient-reported outcome measures; or changes in drug use patterns other than the commonly used endpoint of abstinence.
, such as the ability to resume work or school.
“The evidence is clear. Medication-assisted treatment works, and it is a key piece of defeating the drug crisis facing our country,” Department of Health and Human Services Secretary Alex Azar said in a statement. He added that the new guidance has “the potential to bring new medications to market that are more closely tailored to patient needs and help give Americans facing addiction a better change at recovery.”
FDA Commissioner Scott Gottlieb, MD, added in the statement: “We must consider new ways to gauge success beyond simply whether a patient in recovery has stopped using opioids, such as reducing relapse overdoses and infectious disease transmission. Treatments that can impact these aspects of addiction can be important parts of a comprehensive approach to the treatment of opioid use disorder.”
Guidance comments are due Oct. 9 and can be submitted online here.
Malpractice reforms reduce invasive cardiac testing
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
FROM JAMA CARDIOLOGY
Key clinical point: Physicians in states with medical malpractice damages caps perform fewer angiographies.
Major finding: In the damages-cap states, doctors ordered 24% fewer angiographies as a first diagnostic test compared with no-cap physicians.
Study details: A study of 36,647 doctors in nine states that have noneconomic damages caps and 39,154 doctors in 20 no-cap states.
Disclosures: No disclosures were reported.
Source: Farmer et al. JAMA Cardiol. 2018 June 6 doi: 10.1001/jamacardio.2018.1360.
Some doctors are warming to single-payer medicine
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Catheter ablation of AF in patients with heart failure decreases mortality and HF admissions
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
MS News From 2018 AAN & CMSC Meetings
Telomere length linked to COPD exacerbations, mortality
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
FROM CHEST
Key clinical point: Shorter telomeres are linked to an increased risk of chronic obstructive pulmonary disease exacerbations.
Major finding: Patients with shorter telomeres had a 800% higher risk of total mortality, compared with individuals with longer telomeres.
Study details: Data from 576 patients with chronic obstructive pulmonary disease who participated in the MACRO study.
Disclosures: MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network and the Canadian Institutes of Health Research Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, and three authors declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
Source: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
Prescribing psychotropics to pediatric patients
Writing about prescribing psychotropics to children for depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD) sometimes brings conspiratorial accusations from readers, pediatrician Perri Klass, MD, writes in her column, “The Checkup” in the New York Times.
Some readers react to these discussions by suggesting that Dr. Klass is beholden to pharmaceutical companies. Others suggest that she wants to medicate young patients for behaviors that are a normal part of childhood. Of course, prescribing those medications to young patients should never be taken lightly, she says.
“It is a big deal, and there are side effects to worry about and doctors should listen to families’ concerns,” writes Dr. Klass, professor of journalism and pediatrics at New York University. “But when a child is suffering and struggling, families need help and medications are often part of the discussion.”
Dr. Klass goes on to interview Doris M. Greenberg, MD, and psychiatrist Timothy Wilens, MD, about the way they approach the treatment of children with psychiatric illness.
Click here to read Dr. Klass’s article in the Times.
Writing about prescribing psychotropics to children for depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD) sometimes brings conspiratorial accusations from readers, pediatrician Perri Klass, MD, writes in her column, “The Checkup” in the New York Times.
Some readers react to these discussions by suggesting that Dr. Klass is beholden to pharmaceutical companies. Others suggest that she wants to medicate young patients for behaviors that are a normal part of childhood. Of course, prescribing those medications to young patients should never be taken lightly, she says.
“It is a big deal, and there are side effects to worry about and doctors should listen to families’ concerns,” writes Dr. Klass, professor of journalism and pediatrics at New York University. “But when a child is suffering and struggling, families need help and medications are often part of the discussion.”
Dr. Klass goes on to interview Doris M. Greenberg, MD, and psychiatrist Timothy Wilens, MD, about the way they approach the treatment of children with psychiatric illness.
Click here to read Dr. Klass’s article in the Times.
Writing about prescribing psychotropics to children for depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD) sometimes brings conspiratorial accusations from readers, pediatrician Perri Klass, MD, writes in her column, “The Checkup” in the New York Times.
Some readers react to these discussions by suggesting that Dr. Klass is beholden to pharmaceutical companies. Others suggest that she wants to medicate young patients for behaviors that are a normal part of childhood. Of course, prescribing those medications to young patients should never be taken lightly, she says.
“It is a big deal, and there are side effects to worry about and doctors should listen to families’ concerns,” writes Dr. Klass, professor of journalism and pediatrics at New York University. “But when a child is suffering and struggling, families need help and medications are often part of the discussion.”
Dr. Klass goes on to interview Doris M. Greenberg, MD, and psychiatrist Timothy Wilens, MD, about the way they approach the treatment of children with psychiatric illness.
Click here to read Dr. Klass’s article in the Times.
Access to Transplant Care and Services Within the Veterans Health Administration
The Veterans Health Administration (VHA) provides health care services to over 9 million eligible and enrolled veterans out of a US veteran population of 18.9 million.1 In 2014, an Office of Inspector General (OIG) investigation identified timely access to health care within the VHA as a serious concern.2 In direct response, Congress enacted the Veterans Access, Choice, and Accountability Act (VACAA) of 2014 to expand access to care options available to veterans through referral to non-VA community care providers when the veteran is waiting longer than 30 days for an outpatient appointment or services, resides a significant distance (≥ 40 miles) from a VA facility, or experiences an undue burden to receive care and services.3 The VHA also responded, implementing several initiatives to improve veteran access to VHA health care generally, including the MyVA transformation and the proliferation of connected health technology; including telehealth capability and the expanded use of secure messaging. 4-6
This study examined veterans’ access to the VA transplant program (VATP) for fiscal year (FY 2014 to FY 2016). Timeliness of services and outcomes in relationship to the distance from a VA transplant center (VATC) were evaluated.
Methods
The VATP comprises the following VATCs: 5 heart (Madison, Wisconsin; Nashville, Tennessee; Palo Alto, California; Richmond, Virginia; and Salt Lake City, Utah); 7 kidney (Birmingham, Alabama; Bronx, New York; Houston, Texas; Iowa City, Iowa; Nashville, Tennessee; Pittsburgh, Pennsylvania; and Portland, Oregon); 6 liver (Houston, Texas; Madison, Wisconsin; Nashville, Tennessee; Pittsburgh, Pennsylvania; Portland, Oregon; and Richmond, Virginia); and 2 lung (Madison, Wisconsin; and Seattle, Washington).
In 2012, the VHA published a policy to establish timeliness standards for a VATC initial review decision and referral evaluation.7 In 2013, the VHA National Surgery Office (NSO) implemented a secure intranet-based application called TRACER to facilitate the referral process and track timeliness of initial review decision, evaluation, United Network of Organ Sharing (UNOS) waitlisting, and transplantation.
The referral process is as follows: The referring VA medical facility submits veteran candidate health information into TRACER, selects a VATC, and then TRACER notifies the VATC. The VATC reviews the information and submits an initial review decision as to whether the clinical information supports further evaluation within 48 hours for an emergency referral and 5 business days for a stable referral. If accepted, the VATC completes an evaluation within 30 calendar days of the referral submission date. On evaluation and acceptance, the VATC accepts handoff for transplant-related care, orders additional testing as needed, and waitlists the veteran with UNOS when the clinical status is deemed appropriate.4
The TRACER data from 3 separate cohorts were analyzed from October 1, 2013, to September 30, 2016, with a follow-up event capture through March 31, 2017: (1) the referral cohort, representing all referrals to the VATP; (2) the waitlist cohort, representing those undergoing initial UNOS waitlisting; and (3) the transplant cohort, representing those receiving a solid organ transplant. The straight-line distance between the referring VA medical facility and the VATC was determined for each referral and categorized as follows: less than 100 miles, 100 to 300 miles, 301 to 500 miles, and greater than 500 miles.
Mortality outcomes in the TRACER database were confirmed using the VHA Vital Status file, which combines the Centers for Medicare & Medicaid Services, Social Security Administration, and VHA internal utilization data to determine a best source, including flagging of records that indicate a death date followed by use of VA services.8,9 Records flagged with VA use after death were not considered deaths in this analysis. The NSO regularly refreshes veteran vital status information in the TRACER database for analysis of long-term outcomes.
The analysis methods for this study included Kruskall-Wallis nonparametric 1-way analysis of variance to compare timeliness metrics by distance group, Fine and Gray competing risks models to compare mortality on the UNOS list by distance group, and log-rank and Wilcoxon-Gehan tests to compare patient survival distributions by distance group.10-14 Analysis was generated using SAS software, version 9.4 (Cary, North Carolina) as well as the R statistical software application (r-project.org).15 Publicly available solid organ transplant survival rates were obtained from the Scientific Registry for Transplant Recipients (SRTR).16
Results
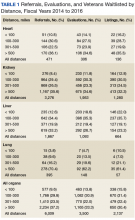
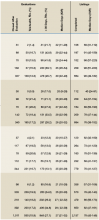
For FY 2014 to FY 2016, the referral cohort identified 6,009 veteran referrals to a VATC for solid organ transplant of which 3,500 underwent an evaluation, and 2,137 were waitlisted for solid organ transplant with UNOS (Table 1).
For the study period, 6,009 referrals resulted in 188 emergency initial review decisions and 3,551 stable initial review decisions with an eligible declaration (Table 2).
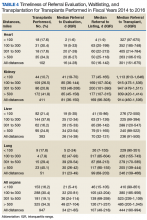
Three thousand five hundred evaluations were performed in a median time of 27 calendar days (IQR 21-32 d) with 948 (27.1%) performed beyond the policy mandated 30 calendar days. Telehealth was used for 555 evaluations (15.9%), primarily for referrals located greater than 100 miles from the VATC. In FY 2016, 13.1% of the 1,321 completed evaluations were performed beyond 30 calendar days, representing an improvement from prior years; 45.7% beyond 30 calendar days in FY 2014 and 26.2% beyond 30 days in FY 2015.
Of the 6,009 referrals submitted in FY 2014 to FY 2016, 2,137 were waitlisted with UNOS. The median time from referral to waitlisting was 78 calendar days (IQR 43-148 d) for the entire study period, decreasing from 90 calendar days in FY 2014 to 70 calendar days in FY 2016.
For all organs and most organ types, the time from referral to initial review decision, evaluation, and waitlisting was statistically less (P < .005) for referrals received from VA medical facilities located less than 100 miles compared with referrals received from VA medical facilities at least 100 miles from the VATC. No statistical difference was found for emergency initial review decision for heart (P = .72) and lung (P = .14), time to evaluation for lung (P = .14), and time to waitlisting for heart (P = .95).
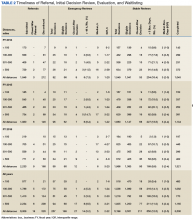
The waitlist cohort data are shown in Table 3.
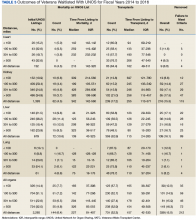
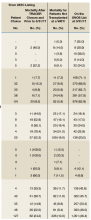
TRACER identified that 339 (15.0%) of the waitlist cohort were removed from the UNOS waitlist of which 212 (62.5%) were removed for failure to meet clinical criteria for transplantation, and 127 (37.5%) were removed for patient choice. Overall, 226 (10.0%) veterans died during the study period without receiving a transplant. Organ-specific mortality rates for veterans waitlisted but not transplanted at a VATC are as follows: heart 6.1%, kidney 5.9%, liver 19.0%, and lung 11.5%. As of March 31, 2017, 1,051 veterans were waitlisted with UNOS of which 876 (83.3%) were waitlisted for a kidney transplant.
The rate of mortality on the UNOS waitlist, the percentage of veterans transplanted, the time from waitlisting to transplantation, and the percentage of patients waitlisted at the end of the study period were not statistically different for referrals less than 100 miles compared with referrals at least 100 miles for all organs or kidney and liver separately (P ≤ .05). The relatively small numbers of veterans waitlisted for heart and lung transplants and nominal mortality events precluded making statements regarding significance for waitlist mortality.
The transplant cohort comprised 947 veterans receiving a solid organ transplant, including 102 (10.8%) heart, 411 (43.4%) kidney, 383 (40.4%) liver, and 51 (5.4%) lung transplants (Table 4).
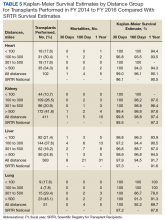
The transplant 30-day, 180-day, and 1-year survival rates are shown in Table 5.
Discussion
This study shows that the VATP delivers timely, high-quality care and services even when the veteran’s referring VA medical facility is located a considerable distance from the VATC. Three separate cohorts of veterans were examined for the FY 2014 to FY 2016 study period: those referred, those waitlisted, and those transplanted. The referral cohort identified 6,009 referral submissions, performed 3,500 evaluations on veterans deemed to be potential candidates for solid organ transplantation, and placed 2,137 of these referrals on the UNOS waitlist. The median time from referral to initial review decision was 5 hours for emergency referrals and 3 business days for stable referrals. The median time from referral to evaluation was 27 calendar days, and the median time from referral to UNOS waitlisting was 78 calendar days. Improvements in timeliness for referral initial review decision, evaluation completion, and waitlisting over the study period were reflective of VHA and NSO efforts to enhance access to services. In FY 2016, 100% of emergency referrals received an initial review decision within 48 hours, 91.4% of stable reviews received an initial review decision within 5 business days, and 86.9% of all referrals underwent evaluation within 30 calendar days.
Distance of less than 100 miles between the referring VA medical facility and the VATC was associated with statistically significant shorter times for initial review decision, evaluation, and UNOS waitlisting. Referrals from less than 100 miles were a minority (9.6%) of referrals and most often represented a direct referral from the VATC to its own program. Timeliness of referral initial review decision, evaluation, or UNOS waitlisting was similar for distance categories greater than 100 miles: 100 to 300 miles, 301 to 500 miles, or greater than 500 miles.
The waitlist cohort identified 2,265 veterans, of which 731 (32.3%) underwent transplantation and 226 (10.0%) died. All-cause mortality for veterans once waitlisted, whether or not maintained on the UNOS waitlist, varied among organs and was found to be 6.1% for heart, 5.9% for kidney, 19.0% for liver, and 11.5% for lung. Waitlist mortality and the time from referral to solid organ transplant was similar for all distance categories.
The transplant cohort identified 947 veterans receiving a solid organ transplant with a median time from referral to transplant that varied considerably by organ type; 301 days (10.0 mo) for heart transplants, 914 days (30.5 mo) for kidney transplants, 236 days (7.9 mo) for liver transplants, and 246 days (8.2 mo) for lung transplants. Time to transplant and posttransplant survival were similar in all distance categories. Moreover, the VATP 1-year survival rates compared favorably with published SRTR data.
Prior studies have shown that distance to a transplant center adversely impacts access to transplant services, mortality on the UNOS waitlist, and transplant outcomes.17-21 Patients living in small towns and isolated rural regions were 8% to 15% less likely to be waitlisted and 10% to 20% less likely to undergo heart, kidney, and liver transplantation than were patients in urban environments.17 This study found that a referral to the VATP from a VA medical facility located less than 100 miles from the VATC received an evaluation 5 to 7 days sooner and be placed on the UNOS waitlist 21 to 29 days sooner than a veteran referred to a VATC located at least 100 miles away. Contrary to prior studies, the distance from the VATC did not have an adverse impact on UNOS waitlist mortality, time to transplantation, or survival outcomes posttransplant.
The VHA offers a number of advantages to the veteran in need of transplant care and services. The VHA is the largest integrated health care system in the US designed specifically for veterans and their complex and specific needs with greater than 1,200 points of care and a single electronic health record optimizing coordinated services.22 In addition, the VHA’s use of telehealth to expedite evaluations and follow-up transplant care closer to home thereby obviating the need for travel. The VHA also has an electronic process to facilitate referral and tracking of timeliness of care (TRACER). Finally, VHA has policies that supports travel benefits, including lodging for the veteran, caregiver, and living donor if applicable for evaluations, transplant procedures, and follow-up care.4,23
The coordination of health care services in a single integrated health care system may be the most significant advantage.24 Multiple studies have examined dual care, representing care and services provided across 2 separate health care systems, showing an association between dual care and an increased risk of hospitalization, duplication of tests, rates for prescribing potentially unsafe medications, and mortality.25-27 Although no study to date is on point, it is reasonable to imply that dual care imposes unnecessary risks to the veteran receiving complex lifelong transplant care when the VATP is shown to provide timely and high-quality care.
Limitations
The retrospective design and limited study period represent limitations. Specifically, survival outcomes for veterans transplanted were limited to 1 year and do not rule out the possibility that distance to a VATC will impact survival rates at 3 and 5 years posttransplant.
Conclusion
A referral distance of less than 100 miles from the VATC most often represents a direct referral and is a factor in timeliness of transplant initial review decision, evaluation, and placement of the veteran on the UNOS waitlist. Distance between the referring VA medical facility and the VATC, including distances of greater than 500 miles, was not found to impact the rate of mortality on the UNOS waitlist, time to transplantation, or posttransplant survival. Overall, the VHA provides timely solid organ transplant care and services with outcomes comparable to that of nationally reported SRTR estimates. Future studies should examine the timeliness of services, outcomes, and costs associated with those veterans authorized by the VHA for non-VA community care and those veterans who independently elect to receive transplant care and services by a non-VA transplant center and return to the VHA for dual care following transplantation.
1. US Department of Veterans Affairs, National Center for VeteransAnalysis and Statistics. Profile of veterans: 2015: data from the American Community Survey. https://www.va.gov/VETDATA/DOCS/SPECIALREPORTS/PROFILE_OF_VETERANS_2015.PDF. Published March 2017. Accessed July 2, 2018.
2. US Department of Veterans Affairs, Office of the Inspector General. Review of alleged patient deaths, patient wait times, and scheduling practices at the Phoenix VA Health Care System. https://www.va.gov/OIG/PUBS/VAOIG-14-02603-267.PDF. Published August 26, 2014. Accessed July 2, 2018.
3. US Department of Veterans Affairs. VHA directive 1700: Veterans Choice Program. https://www.va.gov/VHAPUBLICATIONS/VIEWPUBLICATION.ASP?PUB_ID=3287. Published October 25, 2016. Accessed July 2, 2018.
4. US Department of Veterans Affairs. MyVA. https://www.va.gov/MYVA. Updated November 8, 2016. Accessed July 2, 2018.
5. US Department of Veterans Affairs. Telehealth services. https://www.telehealth.va.gov. Updated March 27, 2017. Accessed July 2, 2018.
6. US Department of Veterans Affairs. Secure messaging. My HealtheVet. https://www.myhealth.va.gov/MHV-PORTAL-WEB/SECURE-MESSAGING-SPOTLIGHT. Updated July 1, 2016. Accessed July 2, 2018.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 2012-018: Solid organ and bone marrow transplantation. Published July 9, 2012.
8. Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6(2):102-109.
9. Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2.
10. Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583-621.
11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509.
12. Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc. 1972;135(2):185-207.
13. Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52(1/2):203-223.
14. Lee ET, Desu MM, Gehan EA. A Monte Carlo study of the power of some two-sample tests. Biometrika. 1975;62(2):425-432.
15. The R Foundation. The R project for statistical computing. https://www.r-project.org. Accessed July 2, 2018.
16. Scientific Registry of Transplant Recipients. https://www.srtr.org. Accessed July 2, 2018.
17. Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202-207.
18. Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12(11):3085-3093.
19. Zorzi D, Rastellini C, Freeman DH, Elias G, Duchini A, Cicalese L. Increase in mortality rate of liver transplant candidates residing in specific geographic areas: analysis of UNOS data. Am J Transplant. 2012;12(8):2188-2197.
20. Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234-1243.
21. Cicalese L, Shirafkan A, Jennings K, Zorzi D, Rastellini C. Increased risk of death for patients on the waitlist for liver transplant residing at greater distance from specialized liver transplant centers in the United States. Transplantation. 2016;100(10):2146-2152.
22. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Updated March 19, 2018. Accessed July 5, 2018.
23. US Department of Veterans Affairs, Veterans Health Administration. Veterans Health Administration handbook 1601B.05: beneficiary travel. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2275. Published July 21, 2010. Accessed July 5, 2018.
24. Gellad WF. The Veterans Choice Act and dual health system use. J Gen Intern Med. 2016;31(2):153-154.
25. Kothari AN, Loy VM, Brownlee SA, et al. Adverse effect of post-discharge care fragmentation on outcomes after readmissions after liver transplantation. J Am Coll Surg. 2017;225(1):62-67.
26. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia. Ann Int Med. 2017;166(3):157-163.
27. Tarlov E, Lee TA, Weichle TW, et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231-2241.
The Veterans Health Administration (VHA) provides health care services to over 9 million eligible and enrolled veterans out of a US veteran population of 18.9 million.1 In 2014, an Office of Inspector General (OIG) investigation identified timely access to health care within the VHA as a serious concern.2 In direct response, Congress enacted the Veterans Access, Choice, and Accountability Act (VACAA) of 2014 to expand access to care options available to veterans through referral to non-VA community care providers when the veteran is waiting longer than 30 days for an outpatient appointment or services, resides a significant distance (≥ 40 miles) from a VA facility, or experiences an undue burden to receive care and services.3 The VHA also responded, implementing several initiatives to improve veteran access to VHA health care generally, including the MyVA transformation and the proliferation of connected health technology; including telehealth capability and the expanded use of secure messaging. 4-6
This study examined veterans’ access to the VA transplant program (VATP) for fiscal year (FY 2014 to FY 2016). Timeliness of services and outcomes in relationship to the distance from a VA transplant center (VATC) were evaluated.
Methods
The VATP comprises the following VATCs: 5 heart (Madison, Wisconsin; Nashville, Tennessee; Palo Alto, California; Richmond, Virginia; and Salt Lake City, Utah); 7 kidney (Birmingham, Alabama; Bronx, New York; Houston, Texas; Iowa City, Iowa; Nashville, Tennessee; Pittsburgh, Pennsylvania; and Portland, Oregon); 6 liver (Houston, Texas; Madison, Wisconsin; Nashville, Tennessee; Pittsburgh, Pennsylvania; Portland, Oregon; and Richmond, Virginia); and 2 lung (Madison, Wisconsin; and Seattle, Washington).
In 2012, the VHA published a policy to establish timeliness standards for a VATC initial review decision and referral evaluation.7 In 2013, the VHA National Surgery Office (NSO) implemented a secure intranet-based application called TRACER to facilitate the referral process and track timeliness of initial review decision, evaluation, United Network of Organ Sharing (UNOS) waitlisting, and transplantation.
The referral process is as follows: The referring VA medical facility submits veteran candidate health information into TRACER, selects a VATC, and then TRACER notifies the VATC. The VATC reviews the information and submits an initial review decision as to whether the clinical information supports further evaluation within 48 hours for an emergency referral and 5 business days for a stable referral. If accepted, the VATC completes an evaluation within 30 calendar days of the referral submission date. On evaluation and acceptance, the VATC accepts handoff for transplant-related care, orders additional testing as needed, and waitlists the veteran with UNOS when the clinical status is deemed appropriate.4
The TRACER data from 3 separate cohorts were analyzed from October 1, 2013, to September 30, 2016, with a follow-up event capture through March 31, 2017: (1) the referral cohort, representing all referrals to the VATP; (2) the waitlist cohort, representing those undergoing initial UNOS waitlisting; and (3) the transplant cohort, representing those receiving a solid organ transplant. The straight-line distance between the referring VA medical facility and the VATC was determined for each referral and categorized as follows: less than 100 miles, 100 to 300 miles, 301 to 500 miles, and greater than 500 miles.
Mortality outcomes in the TRACER database were confirmed using the VHA Vital Status file, which combines the Centers for Medicare & Medicaid Services, Social Security Administration, and VHA internal utilization data to determine a best source, including flagging of records that indicate a death date followed by use of VA services.8,9 Records flagged with VA use after death were not considered deaths in this analysis. The NSO regularly refreshes veteran vital status information in the TRACER database for analysis of long-term outcomes.
The analysis methods for this study included Kruskall-Wallis nonparametric 1-way analysis of variance to compare timeliness metrics by distance group, Fine and Gray competing risks models to compare mortality on the UNOS list by distance group, and log-rank and Wilcoxon-Gehan tests to compare patient survival distributions by distance group.10-14 Analysis was generated using SAS software, version 9.4 (Cary, North Carolina) as well as the R statistical software application (r-project.org).15 Publicly available solid organ transplant survival rates were obtained from the Scientific Registry for Transplant Recipients (SRTR).16
Results
For FY 2014 to FY 2016, the referral cohort identified 6,009 veteran referrals to a VATC for solid organ transplant of which 3,500 underwent an evaluation, and 2,137 were waitlisted for solid organ transplant with UNOS (Table 1).
For the study period, 6,009 referrals resulted in 188 emergency initial review decisions and 3,551 stable initial review decisions with an eligible declaration (Table 2).
Three thousand five hundred evaluations were performed in a median time of 27 calendar days (IQR 21-32 d) with 948 (27.1%) performed beyond the policy mandated 30 calendar days. Telehealth was used for 555 evaluations (15.9%), primarily for referrals located greater than 100 miles from the VATC. In FY 2016, 13.1% of the 1,321 completed evaluations were performed beyond 30 calendar days, representing an improvement from prior years; 45.7% beyond 30 calendar days in FY 2014 and 26.2% beyond 30 days in FY 2015.
Of the 6,009 referrals submitted in FY 2014 to FY 2016, 2,137 were waitlisted with UNOS. The median time from referral to waitlisting was 78 calendar days (IQR 43-148 d) for the entire study period, decreasing from 90 calendar days in FY 2014 to 70 calendar days in FY 2016.
For all organs and most organ types, the time from referral to initial review decision, evaluation, and waitlisting was statistically less (P < .005) for referrals received from VA medical facilities located less than 100 miles compared with referrals received from VA medical facilities at least 100 miles from the VATC. No statistical difference was found for emergency initial review decision for heart (P = .72) and lung (P = .14), time to evaluation for lung (P = .14), and time to waitlisting for heart (P = .95).
The waitlist cohort data are shown in Table 3.
TRACER identified that 339 (15.0%) of the waitlist cohort were removed from the UNOS waitlist of which 212 (62.5%) were removed for failure to meet clinical criteria for transplantation, and 127 (37.5%) were removed for patient choice. Overall, 226 (10.0%) veterans died during the study period without receiving a transplant. Organ-specific mortality rates for veterans waitlisted but not transplanted at a VATC are as follows: heart 6.1%, kidney 5.9%, liver 19.0%, and lung 11.5%. As of March 31, 2017, 1,051 veterans were waitlisted with UNOS of which 876 (83.3%) were waitlisted for a kidney transplant.
The rate of mortality on the UNOS waitlist, the percentage of veterans transplanted, the time from waitlisting to transplantation, and the percentage of patients waitlisted at the end of the study period were not statistically different for referrals less than 100 miles compared with referrals at least 100 miles for all organs or kidney and liver separately (P ≤ .05). The relatively small numbers of veterans waitlisted for heart and lung transplants and nominal mortality events precluded making statements regarding significance for waitlist mortality.
The transplant cohort comprised 947 veterans receiving a solid organ transplant, including 102 (10.8%) heart, 411 (43.4%) kidney, 383 (40.4%) liver, and 51 (5.4%) lung transplants (Table 4).
The transplant 30-day, 180-day, and 1-year survival rates are shown in Table 5.
Discussion
This study shows that the VATP delivers timely, high-quality care and services even when the veteran’s referring VA medical facility is located a considerable distance from the VATC. Three separate cohorts of veterans were examined for the FY 2014 to FY 2016 study period: those referred, those waitlisted, and those transplanted. The referral cohort identified 6,009 referral submissions, performed 3,500 evaluations on veterans deemed to be potential candidates for solid organ transplantation, and placed 2,137 of these referrals on the UNOS waitlist. The median time from referral to initial review decision was 5 hours for emergency referrals and 3 business days for stable referrals. The median time from referral to evaluation was 27 calendar days, and the median time from referral to UNOS waitlisting was 78 calendar days. Improvements in timeliness for referral initial review decision, evaluation completion, and waitlisting over the study period were reflective of VHA and NSO efforts to enhance access to services. In FY 2016, 100% of emergency referrals received an initial review decision within 48 hours, 91.4% of stable reviews received an initial review decision within 5 business days, and 86.9% of all referrals underwent evaluation within 30 calendar days.
Distance of less than 100 miles between the referring VA medical facility and the VATC was associated with statistically significant shorter times for initial review decision, evaluation, and UNOS waitlisting. Referrals from less than 100 miles were a minority (9.6%) of referrals and most often represented a direct referral from the VATC to its own program. Timeliness of referral initial review decision, evaluation, or UNOS waitlisting was similar for distance categories greater than 100 miles: 100 to 300 miles, 301 to 500 miles, or greater than 500 miles.
The waitlist cohort identified 2,265 veterans, of which 731 (32.3%) underwent transplantation and 226 (10.0%) died. All-cause mortality for veterans once waitlisted, whether or not maintained on the UNOS waitlist, varied among organs and was found to be 6.1% for heart, 5.9% for kidney, 19.0% for liver, and 11.5% for lung. Waitlist mortality and the time from referral to solid organ transplant was similar for all distance categories.
The transplant cohort identified 947 veterans receiving a solid organ transplant with a median time from referral to transplant that varied considerably by organ type; 301 days (10.0 mo) for heart transplants, 914 days (30.5 mo) for kidney transplants, 236 days (7.9 mo) for liver transplants, and 246 days (8.2 mo) for lung transplants. Time to transplant and posttransplant survival were similar in all distance categories. Moreover, the VATP 1-year survival rates compared favorably with published SRTR data.
Prior studies have shown that distance to a transplant center adversely impacts access to transplant services, mortality on the UNOS waitlist, and transplant outcomes.17-21 Patients living in small towns and isolated rural regions were 8% to 15% less likely to be waitlisted and 10% to 20% less likely to undergo heart, kidney, and liver transplantation than were patients in urban environments.17 This study found that a referral to the VATP from a VA medical facility located less than 100 miles from the VATC received an evaluation 5 to 7 days sooner and be placed on the UNOS waitlist 21 to 29 days sooner than a veteran referred to a VATC located at least 100 miles away. Contrary to prior studies, the distance from the VATC did not have an adverse impact on UNOS waitlist mortality, time to transplantation, or survival outcomes posttransplant.
The VHA offers a number of advantages to the veteran in need of transplant care and services. The VHA is the largest integrated health care system in the US designed specifically for veterans and their complex and specific needs with greater than 1,200 points of care and a single electronic health record optimizing coordinated services.22 In addition, the VHA’s use of telehealth to expedite evaluations and follow-up transplant care closer to home thereby obviating the need for travel. The VHA also has an electronic process to facilitate referral and tracking of timeliness of care (TRACER). Finally, VHA has policies that supports travel benefits, including lodging for the veteran, caregiver, and living donor if applicable for evaluations, transplant procedures, and follow-up care.4,23
The coordination of health care services in a single integrated health care system may be the most significant advantage.24 Multiple studies have examined dual care, representing care and services provided across 2 separate health care systems, showing an association between dual care and an increased risk of hospitalization, duplication of tests, rates for prescribing potentially unsafe medications, and mortality.25-27 Although no study to date is on point, it is reasonable to imply that dual care imposes unnecessary risks to the veteran receiving complex lifelong transplant care when the VATP is shown to provide timely and high-quality care.
Limitations
The retrospective design and limited study period represent limitations. Specifically, survival outcomes for veterans transplanted were limited to 1 year and do not rule out the possibility that distance to a VATC will impact survival rates at 3 and 5 years posttransplant.
Conclusion
A referral distance of less than 100 miles from the VATC most often represents a direct referral and is a factor in timeliness of transplant initial review decision, evaluation, and placement of the veteran on the UNOS waitlist. Distance between the referring VA medical facility and the VATC, including distances of greater than 500 miles, was not found to impact the rate of mortality on the UNOS waitlist, time to transplantation, or posttransplant survival. Overall, the VHA provides timely solid organ transplant care and services with outcomes comparable to that of nationally reported SRTR estimates. Future studies should examine the timeliness of services, outcomes, and costs associated with those veterans authorized by the VHA for non-VA community care and those veterans who independently elect to receive transplant care and services by a non-VA transplant center and return to the VHA for dual care following transplantation.
The Veterans Health Administration (VHA) provides health care services to over 9 million eligible and enrolled veterans out of a US veteran population of 18.9 million.1 In 2014, an Office of Inspector General (OIG) investigation identified timely access to health care within the VHA as a serious concern.2 In direct response, Congress enacted the Veterans Access, Choice, and Accountability Act (VACAA) of 2014 to expand access to care options available to veterans through referral to non-VA community care providers when the veteran is waiting longer than 30 days for an outpatient appointment or services, resides a significant distance (≥ 40 miles) from a VA facility, or experiences an undue burden to receive care and services.3 The VHA also responded, implementing several initiatives to improve veteran access to VHA health care generally, including the MyVA transformation and the proliferation of connected health technology; including telehealth capability and the expanded use of secure messaging. 4-6
This study examined veterans’ access to the VA transplant program (VATP) for fiscal year (FY 2014 to FY 2016). Timeliness of services and outcomes in relationship to the distance from a VA transplant center (VATC) were evaluated.
Methods
The VATP comprises the following VATCs: 5 heart (Madison, Wisconsin; Nashville, Tennessee; Palo Alto, California; Richmond, Virginia; and Salt Lake City, Utah); 7 kidney (Birmingham, Alabama; Bronx, New York; Houston, Texas; Iowa City, Iowa; Nashville, Tennessee; Pittsburgh, Pennsylvania; and Portland, Oregon); 6 liver (Houston, Texas; Madison, Wisconsin; Nashville, Tennessee; Pittsburgh, Pennsylvania; Portland, Oregon; and Richmond, Virginia); and 2 lung (Madison, Wisconsin; and Seattle, Washington).
In 2012, the VHA published a policy to establish timeliness standards for a VATC initial review decision and referral evaluation.7 In 2013, the VHA National Surgery Office (NSO) implemented a secure intranet-based application called TRACER to facilitate the referral process and track timeliness of initial review decision, evaluation, United Network of Organ Sharing (UNOS) waitlisting, and transplantation.
The referral process is as follows: The referring VA medical facility submits veteran candidate health information into TRACER, selects a VATC, and then TRACER notifies the VATC. The VATC reviews the information and submits an initial review decision as to whether the clinical information supports further evaluation within 48 hours for an emergency referral and 5 business days for a stable referral. If accepted, the VATC completes an evaluation within 30 calendar days of the referral submission date. On evaluation and acceptance, the VATC accepts handoff for transplant-related care, orders additional testing as needed, and waitlists the veteran with UNOS when the clinical status is deemed appropriate.4
The TRACER data from 3 separate cohorts were analyzed from October 1, 2013, to September 30, 2016, with a follow-up event capture through March 31, 2017: (1) the referral cohort, representing all referrals to the VATP; (2) the waitlist cohort, representing those undergoing initial UNOS waitlisting; and (3) the transplant cohort, representing those receiving a solid organ transplant. The straight-line distance between the referring VA medical facility and the VATC was determined for each referral and categorized as follows: less than 100 miles, 100 to 300 miles, 301 to 500 miles, and greater than 500 miles.
Mortality outcomes in the TRACER database were confirmed using the VHA Vital Status file, which combines the Centers for Medicare & Medicaid Services, Social Security Administration, and VHA internal utilization data to determine a best source, including flagging of records that indicate a death date followed by use of VA services.8,9 Records flagged with VA use after death were not considered deaths in this analysis. The NSO regularly refreshes veteran vital status information in the TRACER database for analysis of long-term outcomes.
The analysis methods for this study included Kruskall-Wallis nonparametric 1-way analysis of variance to compare timeliness metrics by distance group, Fine and Gray competing risks models to compare mortality on the UNOS list by distance group, and log-rank and Wilcoxon-Gehan tests to compare patient survival distributions by distance group.10-14 Analysis was generated using SAS software, version 9.4 (Cary, North Carolina) as well as the R statistical software application (r-project.org).15 Publicly available solid organ transplant survival rates were obtained from the Scientific Registry for Transplant Recipients (SRTR).16
Results
For FY 2014 to FY 2016, the referral cohort identified 6,009 veteran referrals to a VATC for solid organ transplant of which 3,500 underwent an evaluation, and 2,137 were waitlisted for solid organ transplant with UNOS (Table 1).
For the study period, 6,009 referrals resulted in 188 emergency initial review decisions and 3,551 stable initial review decisions with an eligible declaration (Table 2).
Three thousand five hundred evaluations were performed in a median time of 27 calendar days (IQR 21-32 d) with 948 (27.1%) performed beyond the policy mandated 30 calendar days. Telehealth was used for 555 evaluations (15.9%), primarily for referrals located greater than 100 miles from the VATC. In FY 2016, 13.1% of the 1,321 completed evaluations were performed beyond 30 calendar days, representing an improvement from prior years; 45.7% beyond 30 calendar days in FY 2014 and 26.2% beyond 30 days in FY 2015.
Of the 6,009 referrals submitted in FY 2014 to FY 2016, 2,137 were waitlisted with UNOS. The median time from referral to waitlisting was 78 calendar days (IQR 43-148 d) for the entire study period, decreasing from 90 calendar days in FY 2014 to 70 calendar days in FY 2016.
For all organs and most organ types, the time from referral to initial review decision, evaluation, and waitlisting was statistically less (P < .005) for referrals received from VA medical facilities located less than 100 miles compared with referrals received from VA medical facilities at least 100 miles from the VATC. No statistical difference was found for emergency initial review decision for heart (P = .72) and lung (P = .14), time to evaluation for lung (P = .14), and time to waitlisting for heart (P = .95).
The waitlist cohort data are shown in Table 3.
TRACER identified that 339 (15.0%) of the waitlist cohort were removed from the UNOS waitlist of which 212 (62.5%) were removed for failure to meet clinical criteria for transplantation, and 127 (37.5%) were removed for patient choice. Overall, 226 (10.0%) veterans died during the study period without receiving a transplant. Organ-specific mortality rates for veterans waitlisted but not transplanted at a VATC are as follows: heart 6.1%, kidney 5.9%, liver 19.0%, and lung 11.5%. As of March 31, 2017, 1,051 veterans were waitlisted with UNOS of which 876 (83.3%) were waitlisted for a kidney transplant.
The rate of mortality on the UNOS waitlist, the percentage of veterans transplanted, the time from waitlisting to transplantation, and the percentage of patients waitlisted at the end of the study period were not statistically different for referrals less than 100 miles compared with referrals at least 100 miles for all organs or kidney and liver separately (P ≤ .05). The relatively small numbers of veterans waitlisted for heart and lung transplants and nominal mortality events precluded making statements regarding significance for waitlist mortality.
The transplant cohort comprised 947 veterans receiving a solid organ transplant, including 102 (10.8%) heart, 411 (43.4%) kidney, 383 (40.4%) liver, and 51 (5.4%) lung transplants (Table 4).
The transplant 30-day, 180-day, and 1-year survival rates are shown in Table 5.
Discussion
This study shows that the VATP delivers timely, high-quality care and services even when the veteran’s referring VA medical facility is located a considerable distance from the VATC. Three separate cohorts of veterans were examined for the FY 2014 to FY 2016 study period: those referred, those waitlisted, and those transplanted. The referral cohort identified 6,009 referral submissions, performed 3,500 evaluations on veterans deemed to be potential candidates for solid organ transplantation, and placed 2,137 of these referrals on the UNOS waitlist. The median time from referral to initial review decision was 5 hours for emergency referrals and 3 business days for stable referrals. The median time from referral to evaluation was 27 calendar days, and the median time from referral to UNOS waitlisting was 78 calendar days. Improvements in timeliness for referral initial review decision, evaluation completion, and waitlisting over the study period were reflective of VHA and NSO efforts to enhance access to services. In FY 2016, 100% of emergency referrals received an initial review decision within 48 hours, 91.4% of stable reviews received an initial review decision within 5 business days, and 86.9% of all referrals underwent evaluation within 30 calendar days.
Distance of less than 100 miles between the referring VA medical facility and the VATC was associated with statistically significant shorter times for initial review decision, evaluation, and UNOS waitlisting. Referrals from less than 100 miles were a minority (9.6%) of referrals and most often represented a direct referral from the VATC to its own program. Timeliness of referral initial review decision, evaluation, or UNOS waitlisting was similar for distance categories greater than 100 miles: 100 to 300 miles, 301 to 500 miles, or greater than 500 miles.
The waitlist cohort identified 2,265 veterans, of which 731 (32.3%) underwent transplantation and 226 (10.0%) died. All-cause mortality for veterans once waitlisted, whether or not maintained on the UNOS waitlist, varied among organs and was found to be 6.1% for heart, 5.9% for kidney, 19.0% for liver, and 11.5% for lung. Waitlist mortality and the time from referral to solid organ transplant was similar for all distance categories.
The transplant cohort identified 947 veterans receiving a solid organ transplant with a median time from referral to transplant that varied considerably by organ type; 301 days (10.0 mo) for heart transplants, 914 days (30.5 mo) for kidney transplants, 236 days (7.9 mo) for liver transplants, and 246 days (8.2 mo) for lung transplants. Time to transplant and posttransplant survival were similar in all distance categories. Moreover, the VATP 1-year survival rates compared favorably with published SRTR data.
Prior studies have shown that distance to a transplant center adversely impacts access to transplant services, mortality on the UNOS waitlist, and transplant outcomes.17-21 Patients living in small towns and isolated rural regions were 8% to 15% less likely to be waitlisted and 10% to 20% less likely to undergo heart, kidney, and liver transplantation than were patients in urban environments.17 This study found that a referral to the VATP from a VA medical facility located less than 100 miles from the VATC received an evaluation 5 to 7 days sooner and be placed on the UNOS waitlist 21 to 29 days sooner than a veteran referred to a VATC located at least 100 miles away. Contrary to prior studies, the distance from the VATC did not have an adverse impact on UNOS waitlist mortality, time to transplantation, or survival outcomes posttransplant.
The VHA offers a number of advantages to the veteran in need of transplant care and services. The VHA is the largest integrated health care system in the US designed specifically for veterans and their complex and specific needs with greater than 1,200 points of care and a single electronic health record optimizing coordinated services.22 In addition, the VHA’s use of telehealth to expedite evaluations and follow-up transplant care closer to home thereby obviating the need for travel. The VHA also has an electronic process to facilitate referral and tracking of timeliness of care (TRACER). Finally, VHA has policies that supports travel benefits, including lodging for the veteran, caregiver, and living donor if applicable for evaluations, transplant procedures, and follow-up care.4,23
The coordination of health care services in a single integrated health care system may be the most significant advantage.24 Multiple studies have examined dual care, representing care and services provided across 2 separate health care systems, showing an association between dual care and an increased risk of hospitalization, duplication of tests, rates for prescribing potentially unsafe medications, and mortality.25-27 Although no study to date is on point, it is reasonable to imply that dual care imposes unnecessary risks to the veteran receiving complex lifelong transplant care when the VATP is shown to provide timely and high-quality care.
Limitations
The retrospective design and limited study period represent limitations. Specifically, survival outcomes for veterans transplanted were limited to 1 year and do not rule out the possibility that distance to a VATC will impact survival rates at 3 and 5 years posttransplant.
Conclusion
A referral distance of less than 100 miles from the VATC most often represents a direct referral and is a factor in timeliness of transplant initial review decision, evaluation, and placement of the veteran on the UNOS waitlist. Distance between the referring VA medical facility and the VATC, including distances of greater than 500 miles, was not found to impact the rate of mortality on the UNOS waitlist, time to transplantation, or posttransplant survival. Overall, the VHA provides timely solid organ transplant care and services with outcomes comparable to that of nationally reported SRTR estimates. Future studies should examine the timeliness of services, outcomes, and costs associated with those veterans authorized by the VHA for non-VA community care and those veterans who independently elect to receive transplant care and services by a non-VA transplant center and return to the VHA for dual care following transplantation.
1. US Department of Veterans Affairs, National Center for VeteransAnalysis and Statistics. Profile of veterans: 2015: data from the American Community Survey. https://www.va.gov/VETDATA/DOCS/SPECIALREPORTS/PROFILE_OF_VETERANS_2015.PDF. Published March 2017. Accessed July 2, 2018.
2. US Department of Veterans Affairs, Office of the Inspector General. Review of alleged patient deaths, patient wait times, and scheduling practices at the Phoenix VA Health Care System. https://www.va.gov/OIG/PUBS/VAOIG-14-02603-267.PDF. Published August 26, 2014. Accessed July 2, 2018.
3. US Department of Veterans Affairs. VHA directive 1700: Veterans Choice Program. https://www.va.gov/VHAPUBLICATIONS/VIEWPUBLICATION.ASP?PUB_ID=3287. Published October 25, 2016. Accessed July 2, 2018.
4. US Department of Veterans Affairs. MyVA. https://www.va.gov/MYVA. Updated November 8, 2016. Accessed July 2, 2018.
5. US Department of Veterans Affairs. Telehealth services. https://www.telehealth.va.gov. Updated March 27, 2017. Accessed July 2, 2018.
6. US Department of Veterans Affairs. Secure messaging. My HealtheVet. https://www.myhealth.va.gov/MHV-PORTAL-WEB/SECURE-MESSAGING-SPOTLIGHT. Updated July 1, 2016. Accessed July 2, 2018.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 2012-018: Solid organ and bone marrow transplantation. Published July 9, 2012.
8. Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6(2):102-109.
9. Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2.
10. Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583-621.
11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509.
12. Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc. 1972;135(2):185-207.
13. Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52(1/2):203-223.
14. Lee ET, Desu MM, Gehan EA. A Monte Carlo study of the power of some two-sample tests. Biometrika. 1975;62(2):425-432.
15. The R Foundation. The R project for statistical computing. https://www.r-project.org. Accessed July 2, 2018.
16. Scientific Registry of Transplant Recipients. https://www.srtr.org. Accessed July 2, 2018.
17. Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202-207.
18. Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12(11):3085-3093.
19. Zorzi D, Rastellini C, Freeman DH, Elias G, Duchini A, Cicalese L. Increase in mortality rate of liver transplant candidates residing in specific geographic areas: analysis of UNOS data. Am J Transplant. 2012;12(8):2188-2197.
20. Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234-1243.
21. Cicalese L, Shirafkan A, Jennings K, Zorzi D, Rastellini C. Increased risk of death for patients on the waitlist for liver transplant residing at greater distance from specialized liver transplant centers in the United States. Transplantation. 2016;100(10):2146-2152.
22. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Updated March 19, 2018. Accessed July 5, 2018.
23. US Department of Veterans Affairs, Veterans Health Administration. Veterans Health Administration handbook 1601B.05: beneficiary travel. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2275. Published July 21, 2010. Accessed July 5, 2018.
24. Gellad WF. The Veterans Choice Act and dual health system use. J Gen Intern Med. 2016;31(2):153-154.
25. Kothari AN, Loy VM, Brownlee SA, et al. Adverse effect of post-discharge care fragmentation on outcomes after readmissions after liver transplantation. J Am Coll Surg. 2017;225(1):62-67.
26. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia. Ann Int Med. 2017;166(3):157-163.
27. Tarlov E, Lee TA, Weichle TW, et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231-2241.
1. US Department of Veterans Affairs, National Center for VeteransAnalysis and Statistics. Profile of veterans: 2015: data from the American Community Survey. https://www.va.gov/VETDATA/DOCS/SPECIALREPORTS/PROFILE_OF_VETERANS_2015.PDF. Published March 2017. Accessed July 2, 2018.
2. US Department of Veterans Affairs, Office of the Inspector General. Review of alleged patient deaths, patient wait times, and scheduling practices at the Phoenix VA Health Care System. https://www.va.gov/OIG/PUBS/VAOIG-14-02603-267.PDF. Published August 26, 2014. Accessed July 2, 2018.
3. US Department of Veterans Affairs. VHA directive 1700: Veterans Choice Program. https://www.va.gov/VHAPUBLICATIONS/VIEWPUBLICATION.ASP?PUB_ID=3287. Published October 25, 2016. Accessed July 2, 2018.
4. US Department of Veterans Affairs. MyVA. https://www.va.gov/MYVA. Updated November 8, 2016. Accessed July 2, 2018.
5. US Department of Veterans Affairs. Telehealth services. https://www.telehealth.va.gov. Updated March 27, 2017. Accessed July 2, 2018.
6. US Department of Veterans Affairs. Secure messaging. My HealtheVet. https://www.myhealth.va.gov/MHV-PORTAL-WEB/SECURE-MESSAGING-SPOTLIGHT. Updated July 1, 2016. Accessed July 2, 2018.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 2012-018: Solid organ and bone marrow transplantation. Published July 9, 2012.
8. Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6(2):102-109.
9. Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2.
10. Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583-621.
11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509.
12. Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc. 1972;135(2):185-207.
13. Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52(1/2):203-223.
14. Lee ET, Desu MM, Gehan EA. A Monte Carlo study of the power of some two-sample tests. Biometrika. 1975;62(2):425-432.
15. The R Foundation. The R project for statistical computing. https://www.r-project.org. Accessed July 2, 2018.
16. Scientific Registry of Transplant Recipients. https://www.srtr.org. Accessed July 2, 2018.
17. Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202-207.
18. Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12(11):3085-3093.
19. Zorzi D, Rastellini C, Freeman DH, Elias G, Duchini A, Cicalese L. Increase in mortality rate of liver transplant candidates residing in specific geographic areas: analysis of UNOS data. Am J Transplant. 2012;12(8):2188-2197.
20. Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234-1243.
21. Cicalese L, Shirafkan A, Jennings K, Zorzi D, Rastellini C. Increased risk of death for patients on the waitlist for liver transplant residing at greater distance from specialized liver transplant centers in the United States. Transplantation. 2016;100(10):2146-2152.
22. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Updated March 19, 2018. Accessed July 5, 2018.
23. US Department of Veterans Affairs, Veterans Health Administration. Veterans Health Administration handbook 1601B.05: beneficiary travel. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2275. Published July 21, 2010. Accessed July 5, 2018.
24. Gellad WF. The Veterans Choice Act and dual health system use. J Gen Intern Med. 2016;31(2):153-154.
25. Kothari AN, Loy VM, Brownlee SA, et al. Adverse effect of post-discharge care fragmentation on outcomes after readmissions after liver transplantation. J Am Coll Surg. 2017;225(1):62-67.
26. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia. Ann Int Med. 2017;166(3):157-163.
27. Tarlov E, Lee TA, Weichle TW, et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231-2241.
Metformin and Long-Acting Insulin Don’t Help Slow Diabetes in Young People
The only 2 medicines currently approved for young people with type 2 diabetes—long-acting insulin and metformin—do not slow the progression of diabetes in young people, according to a study funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases.
A substudy of the Restoring Insulin Secretion (RISE) study, the RISE Pediatric Medication Study looked at the effects of insulin and metformin in 91 patients aged 10 to 19 years. The participants were randomly assigned to 1 of 2 treatment groups. The first received 3 months of glargine, a long-acting insulin, followed by 9 months of metformin. The second group received only metformin for 12 months. The participants were followed for 3 more months after treatment ended. The pediatric study found that beta-cell function declined in both groups during treatment and worsened after treatment ended.
Researchers also compared the pediatric participants with their adult counterparts in 2 other RISE trials and found the young people had more insulin resistance and other signs of disease progression at the same stage in the disease. Moreover, at baseline, the younger patients responded to the severe insulin resistance with a greater insulin response than did the adults, which the researchers say may be a reason for their more rapid loss of beta-cell function.
However, the study also found modest improvement in blood glucose with metformin in both groups. But metformin alone is not a long-term solution for many youth, said Dr. Kristen Nadeau, principal investigator for the pediatric study. Their findings underscore the “urgent and growing need,” she says, for more options.
The only 2 medicines currently approved for young people with type 2 diabetes—long-acting insulin and metformin—do not slow the progression of diabetes in young people, according to a study funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases.
A substudy of the Restoring Insulin Secretion (RISE) study, the RISE Pediatric Medication Study looked at the effects of insulin and metformin in 91 patients aged 10 to 19 years. The participants were randomly assigned to 1 of 2 treatment groups. The first received 3 months of glargine, a long-acting insulin, followed by 9 months of metformin. The second group received only metformin for 12 months. The participants were followed for 3 more months after treatment ended. The pediatric study found that beta-cell function declined in both groups during treatment and worsened after treatment ended.
Researchers also compared the pediatric participants with their adult counterparts in 2 other RISE trials and found the young people had more insulin resistance and other signs of disease progression at the same stage in the disease. Moreover, at baseline, the younger patients responded to the severe insulin resistance with a greater insulin response than did the adults, which the researchers say may be a reason for their more rapid loss of beta-cell function.
However, the study also found modest improvement in blood glucose with metformin in both groups. But metformin alone is not a long-term solution for many youth, said Dr. Kristen Nadeau, principal investigator for the pediatric study. Their findings underscore the “urgent and growing need,” she says, for more options.
The only 2 medicines currently approved for young people with type 2 diabetes—long-acting insulin and metformin—do not slow the progression of diabetes in young people, according to a study funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases.
A substudy of the Restoring Insulin Secretion (RISE) study, the RISE Pediatric Medication Study looked at the effects of insulin and metformin in 91 patients aged 10 to 19 years. The participants were randomly assigned to 1 of 2 treatment groups. The first received 3 months of glargine, a long-acting insulin, followed by 9 months of metformin. The second group received only metformin for 12 months. The participants were followed for 3 more months after treatment ended. The pediatric study found that beta-cell function declined in both groups during treatment and worsened after treatment ended.
Researchers also compared the pediatric participants with their adult counterparts in 2 other RISE trials and found the young people had more insulin resistance and other signs of disease progression at the same stage in the disease. Moreover, at baseline, the younger patients responded to the severe insulin resistance with a greater insulin response than did the adults, which the researchers say may be a reason for their more rapid loss of beta-cell function.
However, the study also found modest improvement in blood glucose with metformin in both groups. But metformin alone is not a long-term solution for many youth, said Dr. Kristen Nadeau, principal investigator for the pediatric study. Their findings underscore the “urgent and growing need,” she says, for more options.
Bendamustine-Based Salvage Regimen Offers Hope
Many patients with primary central nervous system lymphoma (PCNSL) experience rapid, aggressive progression of CNS malignancy. It is “accepted,” say researchers from Chonnam National University Hwasun Hospital in the Republic of Korea, that salvage therapy is beneficial and significantly improves survival in comparison to palliative care, but therapy options remain limited—mainly because few trials have been done. Several case reports have suggested that bendamustine has modest clinical activity against relapsed PCNSL, the researchers note, but its effect as part of combination salvage therapy in these patients has not been established. The study offers some validation of previous findings and new information about the benefits of a bendamustine-based combination regimen.
The researchers enrolled 10 patients, of whom 7 had refractory disease. All had previously been on high-dose methotrexate. Of the 3 relapsed patients, 1 entered the study at second relapse. The patients received either R-B(O)AD or R-BAD (rituximab, vincristine, bendamustine, cytarabine, dexamethasone) every 4 weeks for up to 4 cycles. Vincristine was omitted in 4 regimens, and dosages of bendamustine and cytarabine were reduced for 4 patients who were over 70.
The overall response rate for R-B(O)AD was 50%. One patient achieved complete response and 4 achieved partial response. The researchers observed “remarkable effects” on imaging in patients who responded. They attribute the activity to the anticipated synergy of bendamustine combined with cytarabine—even though disease in the majority of the patients had progressed despite previous treatment with cytarabine.
However, the synergistic effects also led to significant marrow depression; hematologic toxicity with R-B(O)AD was “considerable,” with grade 3 or 4 neutropenia and thrombocytopenia seen in more than 85% of treatment cycles. Moreover, 3 patients developed severe infection, all with involvement of the lungs. The researchers therefore amended the study protocol to reduce cytarabine dosage. While the toxicity is significant, the researchers say, it is manageable with the dose reduction and supportive care.
Bendamustine cerebrospinal fluid levels were minimal, but corresponded to plasma exposure and response to treatment in deep tumor locations.
Although the study is small, it supports the use of the bendamustine-based regimen as an effective salvage option, the researchers conclude, especially for patients who are no longer responding to methotrexate or have developed cumulative renal or neurotoxicity from treatment.
Source:
Kim T, Choi HY, Lee HS, et al. BMC Cancer. 2018;18(1):729
Many patients with primary central nervous system lymphoma (PCNSL) experience rapid, aggressive progression of CNS malignancy. It is “accepted,” say researchers from Chonnam National University Hwasun Hospital in the Republic of Korea, that salvage therapy is beneficial and significantly improves survival in comparison to palliative care, but therapy options remain limited—mainly because few trials have been done. Several case reports have suggested that bendamustine has modest clinical activity against relapsed PCNSL, the researchers note, but its effect as part of combination salvage therapy in these patients has not been established. The study offers some validation of previous findings and new information about the benefits of a bendamustine-based combination regimen.
The researchers enrolled 10 patients, of whom 7 had refractory disease. All had previously been on high-dose methotrexate. Of the 3 relapsed patients, 1 entered the study at second relapse. The patients received either R-B(O)AD or R-BAD (rituximab, vincristine, bendamustine, cytarabine, dexamethasone) every 4 weeks for up to 4 cycles. Vincristine was omitted in 4 regimens, and dosages of bendamustine and cytarabine were reduced for 4 patients who were over 70.
The overall response rate for R-B(O)AD was 50%. One patient achieved complete response and 4 achieved partial response. The researchers observed “remarkable effects” on imaging in patients who responded. They attribute the activity to the anticipated synergy of bendamustine combined with cytarabine—even though disease in the majority of the patients had progressed despite previous treatment with cytarabine.
However, the synergistic effects also led to significant marrow depression; hematologic toxicity with R-B(O)AD was “considerable,” with grade 3 or 4 neutropenia and thrombocytopenia seen in more than 85% of treatment cycles. Moreover, 3 patients developed severe infection, all with involvement of the lungs. The researchers therefore amended the study protocol to reduce cytarabine dosage. While the toxicity is significant, the researchers say, it is manageable with the dose reduction and supportive care.
Bendamustine cerebrospinal fluid levels were minimal, but corresponded to plasma exposure and response to treatment in deep tumor locations.
Although the study is small, it supports the use of the bendamustine-based regimen as an effective salvage option, the researchers conclude, especially for patients who are no longer responding to methotrexate or have developed cumulative renal or neurotoxicity from treatment.
Source:
Kim T, Choi HY, Lee HS, et al. BMC Cancer. 2018;18(1):729
Many patients with primary central nervous system lymphoma (PCNSL) experience rapid, aggressive progression of CNS malignancy. It is “accepted,” say researchers from Chonnam National University Hwasun Hospital in the Republic of Korea, that salvage therapy is beneficial and significantly improves survival in comparison to palliative care, but therapy options remain limited—mainly because few trials have been done. Several case reports have suggested that bendamustine has modest clinical activity against relapsed PCNSL, the researchers note, but its effect as part of combination salvage therapy in these patients has not been established. The study offers some validation of previous findings and new information about the benefits of a bendamustine-based combination regimen.
The researchers enrolled 10 patients, of whom 7 had refractory disease. All had previously been on high-dose methotrexate. Of the 3 relapsed patients, 1 entered the study at second relapse. The patients received either R-B(O)AD or R-BAD (rituximab, vincristine, bendamustine, cytarabine, dexamethasone) every 4 weeks for up to 4 cycles. Vincristine was omitted in 4 regimens, and dosages of bendamustine and cytarabine were reduced for 4 patients who were over 70.
The overall response rate for R-B(O)AD was 50%. One patient achieved complete response and 4 achieved partial response. The researchers observed “remarkable effects” on imaging in patients who responded. They attribute the activity to the anticipated synergy of bendamustine combined with cytarabine—even though disease in the majority of the patients had progressed despite previous treatment with cytarabine.
However, the synergistic effects also led to significant marrow depression; hematologic toxicity with R-B(O)AD was “considerable,” with grade 3 or 4 neutropenia and thrombocytopenia seen in more than 85% of treatment cycles. Moreover, 3 patients developed severe infection, all with involvement of the lungs. The researchers therefore amended the study protocol to reduce cytarabine dosage. While the toxicity is significant, the researchers say, it is manageable with the dose reduction and supportive care.
Bendamustine cerebrospinal fluid levels were minimal, but corresponded to plasma exposure and response to treatment in deep tumor locations.
Although the study is small, it supports the use of the bendamustine-based regimen as an effective salvage option, the researchers conclude, especially for patients who are no longer responding to methotrexate or have developed cumulative renal or neurotoxicity from treatment.
Source:
Kim T, Choi HY, Lee HS, et al. BMC Cancer. 2018;18(1):729