User login
Ebola virus, social media, opioid crisis, gender in pulmonary disease
Disaster Response
Ebola virus outbreak preparedness
The 2014-2016 Ebola virus disease (EVD) outbreak in West Africa highlighted the global reach of emerging infectious diseases and shattered a sense of complacency in an increasingly interconnected world. Consequently, a subsequent outbreak of EVD in the Democratic Republic of the Congo (DRC) in early May 2018 triggered a swift response. International agencies and workers benefited from increased experience with the disease, new investigational vaccines, including the rVSV-ZEBOV vaccine, and novel therapies, including ZMapp, favipiravir, and remdesivir (GS-5734).
However, are health-care providers and facilities outside of outbreak areas truly more prepared to handle high-risk pathogens today than they were in 2014? The answer, at least in the United States, seems to be “yes,” due to a regional concentration of funding and resources. The US Department of Health and Human Services (HHS) has identified treatment centers for Ebola and other special pathogens nationwide.1 The National Ebola Training and Education Center (NETEC) trains health systems to implement disease management plans.2 The Centers for Disease Control and Prevention (CDC) has prepared recommendations for public health planners.3
In nonreferral centers, providers should always obtain a travel history, remain cognizant of emerging diseases,4 and optimize supportive care. Early collaboration with public health authorities and appropriate infection control precautions are necessary for rapid confirmation of a suspected high-risk pathogen and for ensuring patient and staff safety. Most centers will not need to care for a patient with EVD for an extended period, but the ability to recognize, contain, and refer is essential for good outcomes.
Ryan Maves, MD, FCCP
Cristian Madar, MD
Steering Committee Members
References
1. www.hhs.gov/about/news/2016/06/14/hhs-selects-regional-ebola-treatment-center-southwestern-us.html. Accessed July 18, 2018.
2. www.netec.org. Accessed July 18, 2018.
3. www.cdc.gov/vhf/ebola/public-health-planners. Accessed July 18, 2018.
4. www.cdc.gov/travel/notices. Accessed July 18, 2018.
Practice Operations
Current impact of social media on health care
In an age of connectivity, social media websites pose many challenges. Not immune to this are the physicians and their health-care practices, particularly their online presence to their patients. Many of these sites publish user-submitted patient appreciation or complaints. These postings are generally viewable to the public and often not moderated or restricted in content. With value-based care at the front lines, these posts may be detrimental to the success of the practice. Public postings exist regardless of providers’ awareness or management of them.
There is limited training on social media presence, handling negative reviews, addressing patient-specific posts online, or mediating conflicts. This includes legal issues related to licensing, privacy, litigation, and fraud. Compliance to ethical requirements and protecting patient privacy online still remains crucial in the heavily regulated health-care industry. The burden of social media remains a widely unacknowledged impediment to growing physicians’ practice. While several organizations have published guidelines to help ensure success and to better inform physicians, these are not widely practiced or well known.
However, significant potential benefits to social media include marketing opportunities, education, and connection with patients. Social media has been key for support group networks amongst patients. Similar to professionals in other fields, it is recommended that providers separate their public and private social media accounts or use alternate names. For more information about social media and answers to many legal questions, attend the Practice Operations NetWork Featured Lecture at the CHEST Annual Meeting on Monday, October 8, at 1:30 PM.
Megan Sisk, DO
Fellow-in-Training Member
Humayun Anjum, MD
Steering Committee Member
Transplant
Implications of the opioid crisis on organ donation for lung transplantation
The opioid epidemic in the United States claims a substantial number of lives annually, with overdose-related deaths increasing five times between 2000 and2016.1 In the midst of this national crisis, perhaps one solace is an increase in organ donation for thoracic transplantation. In fact, data show that patients dying of overdose have the highest donation rates,2 and a staggering 10 times increase in the proportion of eligible donors dying of overdose has been witnessed over this period (1.2% of donors in 2000, 13.7% in 2016),3 with a parallel increase in transplants performed.4
Despite this, transplant program organ utilization in overdose deaths falls well short of expected, in part due to disease transmission concerns, supported by the observation that these donors are two to five times more likely designated as “PHS-Increased-Risk” Criteria for transmission of HBV, HCV, and HIV.2,5 In lung transplantation, additional concerns over donor quality often exist, including aspiration, edema, or other opioid-induced injuries. Although a disturbing premise, as the health-care community and lawmakers attempt to curtail the opioid epidemic, it is important to recognize opportunities for improvement in organ utilization, which offers potential to help many patients with cardiopulmonary disease. In addition to community-wide organ donation campaigns, this may stem from dissemination of knowledge of the low infectious risks in PHS-increased-risk donors,5 as well as analyses showing similar survival among recipients of allografts from overdose-death donors compared with donors from other causes.3 Use of HCV-positive organs, particularly in the modern era of infectious testing and therapies, offers additional potential,6 as does fine-tuning technologies such as ex-vivo lung perfusion, which may enhance organ quality making lungs suitable for transplant.
Anupam Kumar, MD
Fellow-in-Training Member
Siddhartha G. Kapnadak, MD
Steering Committee Member
References
1. Rudd RA, et al. MMWR Morb Mortal Wkly Rep. 2016;Dec 30;65(5051):1445.
2. Goldberg DS, et al. Am J Transplant. 2016 Oct; 16(10): 2836.
3. Mehra MR, et al. N Engl J Med. 2018 May 17;378(20):1943.
4. Durand CM, et al. Ann Intern Med. 2018 May 15;168(10):702.
5. Sibulesky L, et al. Clin Transplant. 2015 Sep;29(9):724.
6. Abdelbasit A, et al. Am J Respir Crit Care Med. 2018 Jun 1;197(11):1492.
Women’s Health
Sex and gender in pulmonary disease
On September 18-19, 2017, the National Heart, Lung, and Blood Institute convened a workshop of investigators with the National Institutes of Health, the Office of Research on Women’s Health, and the Office of Rare Diseases Research to discuss the role of sex and gender in pulmonary disease. The findings of this workshop, published online ahead of print (Han MK, et al. Am J Respir Crit Care Med. 2018 May 10. doi: 10.1164/rccm.201801-0168WS. [Epub ahead of print]), outline important future directions for research in pulmonary medicine.
The group identified several areas in which there are substantial sex-specific differences in clinical presentation and treatment outcomes in pulmonary diseases, including tobacco cessation, circadian rhythms and sleep-disordered breathing, COPD, asthma, cystic fibrosis, and interstitial lung disease.
In addition to defining the terms sex and gender, the committee called for standardization of the reporting of sex as a variable in animal and cellular models. Given the observed relationship between sex hormones and the development of lung disease, a collaboration across disciplines, including endocrinology, would be useful to understand this relationship at a basic and clinical science level. Furthermore, in the era of big data research, sex and gender should be included as co-variates when possible to better clarify the contributions of these variables in pulmonary disease.
The workshop also highlighted the need to educate clinicians about these differences. Just as trainees are taught that women can present with atypical symptoms for a heart attack, so should they be taught about the differences in management of chronic lung disease and tobacco dependence between men and women.
Nikita Desai, MD
Fellow-in-Training Member
Disaster Response
Ebola virus outbreak preparedness
The 2014-2016 Ebola virus disease (EVD) outbreak in West Africa highlighted the global reach of emerging infectious diseases and shattered a sense of complacency in an increasingly interconnected world. Consequently, a subsequent outbreak of EVD in the Democratic Republic of the Congo (DRC) in early May 2018 triggered a swift response. International agencies and workers benefited from increased experience with the disease, new investigational vaccines, including the rVSV-ZEBOV vaccine, and novel therapies, including ZMapp, favipiravir, and remdesivir (GS-5734).
However, are health-care providers and facilities outside of outbreak areas truly more prepared to handle high-risk pathogens today than they were in 2014? The answer, at least in the United States, seems to be “yes,” due to a regional concentration of funding and resources. The US Department of Health and Human Services (HHS) has identified treatment centers for Ebola and other special pathogens nationwide.1 The National Ebola Training and Education Center (NETEC) trains health systems to implement disease management plans.2 The Centers for Disease Control and Prevention (CDC) has prepared recommendations for public health planners.3
In nonreferral centers, providers should always obtain a travel history, remain cognizant of emerging diseases,4 and optimize supportive care. Early collaboration with public health authorities and appropriate infection control precautions are necessary for rapid confirmation of a suspected high-risk pathogen and for ensuring patient and staff safety. Most centers will not need to care for a patient with EVD for an extended period, but the ability to recognize, contain, and refer is essential for good outcomes.
Ryan Maves, MD, FCCP
Cristian Madar, MD
Steering Committee Members
References
1. www.hhs.gov/about/news/2016/06/14/hhs-selects-regional-ebola-treatment-center-southwestern-us.html. Accessed July 18, 2018.
2. www.netec.org. Accessed July 18, 2018.
3. www.cdc.gov/vhf/ebola/public-health-planners. Accessed July 18, 2018.
4. www.cdc.gov/travel/notices. Accessed July 18, 2018.
Practice Operations
Current impact of social media on health care
In an age of connectivity, social media websites pose many challenges. Not immune to this are the physicians and their health-care practices, particularly their online presence to their patients. Many of these sites publish user-submitted patient appreciation or complaints. These postings are generally viewable to the public and often not moderated or restricted in content. With value-based care at the front lines, these posts may be detrimental to the success of the practice. Public postings exist regardless of providers’ awareness or management of them.
There is limited training on social media presence, handling negative reviews, addressing patient-specific posts online, or mediating conflicts. This includes legal issues related to licensing, privacy, litigation, and fraud. Compliance to ethical requirements and protecting patient privacy online still remains crucial in the heavily regulated health-care industry. The burden of social media remains a widely unacknowledged impediment to growing physicians’ practice. While several organizations have published guidelines to help ensure success and to better inform physicians, these are not widely practiced or well known.
However, significant potential benefits to social media include marketing opportunities, education, and connection with patients. Social media has been key for support group networks amongst patients. Similar to professionals in other fields, it is recommended that providers separate their public and private social media accounts or use alternate names. For more information about social media and answers to many legal questions, attend the Practice Operations NetWork Featured Lecture at the CHEST Annual Meeting on Monday, October 8, at 1:30 PM.
Megan Sisk, DO
Fellow-in-Training Member
Humayun Anjum, MD
Steering Committee Member
Transplant
Implications of the opioid crisis on organ donation for lung transplantation
The opioid epidemic in the United States claims a substantial number of lives annually, with overdose-related deaths increasing five times between 2000 and2016.1 In the midst of this national crisis, perhaps one solace is an increase in organ donation for thoracic transplantation. In fact, data show that patients dying of overdose have the highest donation rates,2 and a staggering 10 times increase in the proportion of eligible donors dying of overdose has been witnessed over this period (1.2% of donors in 2000, 13.7% in 2016),3 with a parallel increase in transplants performed.4
Despite this, transplant program organ utilization in overdose deaths falls well short of expected, in part due to disease transmission concerns, supported by the observation that these donors are two to five times more likely designated as “PHS-Increased-Risk” Criteria for transmission of HBV, HCV, and HIV.2,5 In lung transplantation, additional concerns over donor quality often exist, including aspiration, edema, or other opioid-induced injuries. Although a disturbing premise, as the health-care community and lawmakers attempt to curtail the opioid epidemic, it is important to recognize opportunities for improvement in organ utilization, which offers potential to help many patients with cardiopulmonary disease. In addition to community-wide organ donation campaigns, this may stem from dissemination of knowledge of the low infectious risks in PHS-increased-risk donors,5 as well as analyses showing similar survival among recipients of allografts from overdose-death donors compared with donors from other causes.3 Use of HCV-positive organs, particularly in the modern era of infectious testing and therapies, offers additional potential,6 as does fine-tuning technologies such as ex-vivo lung perfusion, which may enhance organ quality making lungs suitable for transplant.
Anupam Kumar, MD
Fellow-in-Training Member
Siddhartha G. Kapnadak, MD
Steering Committee Member
References
1. Rudd RA, et al. MMWR Morb Mortal Wkly Rep. 2016;Dec 30;65(5051):1445.
2. Goldberg DS, et al. Am J Transplant. 2016 Oct; 16(10): 2836.
3. Mehra MR, et al. N Engl J Med. 2018 May 17;378(20):1943.
4. Durand CM, et al. Ann Intern Med. 2018 May 15;168(10):702.
5. Sibulesky L, et al. Clin Transplant. 2015 Sep;29(9):724.
6. Abdelbasit A, et al. Am J Respir Crit Care Med. 2018 Jun 1;197(11):1492.
Women’s Health
Sex and gender in pulmonary disease
On September 18-19, 2017, the National Heart, Lung, and Blood Institute convened a workshop of investigators with the National Institutes of Health, the Office of Research on Women’s Health, and the Office of Rare Diseases Research to discuss the role of sex and gender in pulmonary disease. The findings of this workshop, published online ahead of print (Han MK, et al. Am J Respir Crit Care Med. 2018 May 10. doi: 10.1164/rccm.201801-0168WS. [Epub ahead of print]), outline important future directions for research in pulmonary medicine.
The group identified several areas in which there are substantial sex-specific differences in clinical presentation and treatment outcomes in pulmonary diseases, including tobacco cessation, circadian rhythms and sleep-disordered breathing, COPD, asthma, cystic fibrosis, and interstitial lung disease.
In addition to defining the terms sex and gender, the committee called for standardization of the reporting of sex as a variable in animal and cellular models. Given the observed relationship between sex hormones and the development of lung disease, a collaboration across disciplines, including endocrinology, would be useful to understand this relationship at a basic and clinical science level. Furthermore, in the era of big data research, sex and gender should be included as co-variates when possible to better clarify the contributions of these variables in pulmonary disease.
The workshop also highlighted the need to educate clinicians about these differences. Just as trainees are taught that women can present with atypical symptoms for a heart attack, so should they be taught about the differences in management of chronic lung disease and tobacco dependence between men and women.
Nikita Desai, MD
Fellow-in-Training Member
Disaster Response
Ebola virus outbreak preparedness
The 2014-2016 Ebola virus disease (EVD) outbreak in West Africa highlighted the global reach of emerging infectious diseases and shattered a sense of complacency in an increasingly interconnected world. Consequently, a subsequent outbreak of EVD in the Democratic Republic of the Congo (DRC) in early May 2018 triggered a swift response. International agencies and workers benefited from increased experience with the disease, new investigational vaccines, including the rVSV-ZEBOV vaccine, and novel therapies, including ZMapp, favipiravir, and remdesivir (GS-5734).
However, are health-care providers and facilities outside of outbreak areas truly more prepared to handle high-risk pathogens today than they were in 2014? The answer, at least in the United States, seems to be “yes,” due to a regional concentration of funding and resources. The US Department of Health and Human Services (HHS) has identified treatment centers for Ebola and other special pathogens nationwide.1 The National Ebola Training and Education Center (NETEC) trains health systems to implement disease management plans.2 The Centers for Disease Control and Prevention (CDC) has prepared recommendations for public health planners.3
In nonreferral centers, providers should always obtain a travel history, remain cognizant of emerging diseases,4 and optimize supportive care. Early collaboration with public health authorities and appropriate infection control precautions are necessary for rapid confirmation of a suspected high-risk pathogen and for ensuring patient and staff safety. Most centers will not need to care for a patient with EVD for an extended period, but the ability to recognize, contain, and refer is essential for good outcomes.
Ryan Maves, MD, FCCP
Cristian Madar, MD
Steering Committee Members
References
1. www.hhs.gov/about/news/2016/06/14/hhs-selects-regional-ebola-treatment-center-southwestern-us.html. Accessed July 18, 2018.
2. www.netec.org. Accessed July 18, 2018.
3. www.cdc.gov/vhf/ebola/public-health-planners. Accessed July 18, 2018.
4. www.cdc.gov/travel/notices. Accessed July 18, 2018.
Practice Operations
Current impact of social media on health care
In an age of connectivity, social media websites pose many challenges. Not immune to this are the physicians and their health-care practices, particularly their online presence to their patients. Many of these sites publish user-submitted patient appreciation or complaints. These postings are generally viewable to the public and often not moderated or restricted in content. With value-based care at the front lines, these posts may be detrimental to the success of the practice. Public postings exist regardless of providers’ awareness or management of them.
There is limited training on social media presence, handling negative reviews, addressing patient-specific posts online, or mediating conflicts. This includes legal issues related to licensing, privacy, litigation, and fraud. Compliance to ethical requirements and protecting patient privacy online still remains crucial in the heavily regulated health-care industry. The burden of social media remains a widely unacknowledged impediment to growing physicians’ practice. While several organizations have published guidelines to help ensure success and to better inform physicians, these are not widely practiced or well known.
However, significant potential benefits to social media include marketing opportunities, education, and connection with patients. Social media has been key for support group networks amongst patients. Similar to professionals in other fields, it is recommended that providers separate their public and private social media accounts or use alternate names. For more information about social media and answers to many legal questions, attend the Practice Operations NetWork Featured Lecture at the CHEST Annual Meeting on Monday, October 8, at 1:30 PM.
Megan Sisk, DO
Fellow-in-Training Member
Humayun Anjum, MD
Steering Committee Member
Transplant
Implications of the opioid crisis on organ donation for lung transplantation
The opioid epidemic in the United States claims a substantial number of lives annually, with overdose-related deaths increasing five times between 2000 and2016.1 In the midst of this national crisis, perhaps one solace is an increase in organ donation for thoracic transplantation. In fact, data show that patients dying of overdose have the highest donation rates,2 and a staggering 10 times increase in the proportion of eligible donors dying of overdose has been witnessed over this period (1.2% of donors in 2000, 13.7% in 2016),3 with a parallel increase in transplants performed.4
Despite this, transplant program organ utilization in overdose deaths falls well short of expected, in part due to disease transmission concerns, supported by the observation that these donors are two to five times more likely designated as “PHS-Increased-Risk” Criteria for transmission of HBV, HCV, and HIV.2,5 In lung transplantation, additional concerns over donor quality often exist, including aspiration, edema, or other opioid-induced injuries. Although a disturbing premise, as the health-care community and lawmakers attempt to curtail the opioid epidemic, it is important to recognize opportunities for improvement in organ utilization, which offers potential to help many patients with cardiopulmonary disease. In addition to community-wide organ donation campaigns, this may stem from dissemination of knowledge of the low infectious risks in PHS-increased-risk donors,5 as well as analyses showing similar survival among recipients of allografts from overdose-death donors compared with donors from other causes.3 Use of HCV-positive organs, particularly in the modern era of infectious testing and therapies, offers additional potential,6 as does fine-tuning technologies such as ex-vivo lung perfusion, which may enhance organ quality making lungs suitable for transplant.
Anupam Kumar, MD
Fellow-in-Training Member
Siddhartha G. Kapnadak, MD
Steering Committee Member
References
1. Rudd RA, et al. MMWR Morb Mortal Wkly Rep. 2016;Dec 30;65(5051):1445.
2. Goldberg DS, et al. Am J Transplant. 2016 Oct; 16(10): 2836.
3. Mehra MR, et al. N Engl J Med. 2018 May 17;378(20):1943.
4. Durand CM, et al. Ann Intern Med. 2018 May 15;168(10):702.
5. Sibulesky L, et al. Clin Transplant. 2015 Sep;29(9):724.
6. Abdelbasit A, et al. Am J Respir Crit Care Med. 2018 Jun 1;197(11):1492.
Women’s Health
Sex and gender in pulmonary disease
On September 18-19, 2017, the National Heart, Lung, and Blood Institute convened a workshop of investigators with the National Institutes of Health, the Office of Research on Women’s Health, and the Office of Rare Diseases Research to discuss the role of sex and gender in pulmonary disease. The findings of this workshop, published online ahead of print (Han MK, et al. Am J Respir Crit Care Med. 2018 May 10. doi: 10.1164/rccm.201801-0168WS. [Epub ahead of print]), outline important future directions for research in pulmonary medicine.
The group identified several areas in which there are substantial sex-specific differences in clinical presentation and treatment outcomes in pulmonary diseases, including tobacco cessation, circadian rhythms and sleep-disordered breathing, COPD, asthma, cystic fibrosis, and interstitial lung disease.
In addition to defining the terms sex and gender, the committee called for standardization of the reporting of sex as a variable in animal and cellular models. Given the observed relationship between sex hormones and the development of lung disease, a collaboration across disciplines, including endocrinology, would be useful to understand this relationship at a basic and clinical science level. Furthermore, in the era of big data research, sex and gender should be included as co-variates when possible to better clarify the contributions of these variables in pulmonary disease.
The workshop also highlighted the need to educate clinicians about these differences. Just as trainees are taught that women can present with atypical symptoms for a heart attack, so should they be taught about the differences in management of chronic lung disease and tobacco dependence between men and women.
Nikita Desai, MD
Fellow-in-Training Member
Methylphenidate deemed best first-line option for ADHD in children
Methylphenidate appears to be the safest and most effective treatment option for attention-deficit/hyperactivity disorder in children and adolescents, while amphetamines are the preferred first-line choice in adults, a systematic review and meta-analysis have found.
Researchers reported the results of a network meta-analysis of 133 double-blind randomized controlled trials – 81 in children and adolescents, 51 in adults, and 1 in both – involving a total of 10,068 children and adolescents, and 8,131 adults. The included studies all compared a range of medications to placebo or in head-to-head trials. The meta-analysis was published online Aug. 7 in The Lancet Psychiatry.
At 12 weeks, all the medications, which included amphetamines, atomoxetine, bupropion, clonidine, guanfacine, methylphenidate, and modafinil, were found to be better than placebo in reducing core ADHD symptoms in children and adolescents, according to clinicians’ ratings. However, when teachers’ ratings were used, only methylphenidate and modafinil were better than placebo.
In adults, clinicians’ ratings found that amphetamines, methylphenidate, bupropion, and atomoxetine – but not modafinil – were better than placebo.
In head-to-head trials, clinicians’ ratings favored amphetamines over modafinil, atomoxetine, and methylphenidate in children, adolescents, and adults.
But in adults, amphetamines, bupropion, and methylphenidate all beat placebo.
When it came to tolerability in children and adolescents, guanfacine and amphetamines were the only two treatments that were less well tolerated than placebo. However, a post hoc analysis suggested lisdexamfetamine had a lower tolerability relative to other amphetamines, at least in children and adolescents. In adults, modafinil, amphetamines, methylphenidate, and atomoxetine were beaten by placebo for tolerability.
“Overall, all medications, except modafinil in adults, were more efficacious than placebo for the short-term treatment of ADHD, and they were less efficacious and less well tolerated in adults than in children and adolescents,” wrote Samuele Cortese, MD, PhD, of the University of Southampton (England), and his coauthors. “However, the included medications were not equivalent in relation to their mean effect size, which ranged from moderate to high and varied according to the type of rater.”
For example, while atomoxetine had the lowest mean effect size in children and adolescents based on clinicians’ ratings, in adults, it was on par with methylphenidate. Amphetamines increased diastolic blood pressure in children but not in adults.
“Taking into account both efficacy and safety, evidence from this meta-analysis supports methylphenidate in children and adolescents, and amphetamines in adults, as preferred first-choice medications for the short-term treatment of ADHD,” the authors wrote.
Dr. Cortese and his coauthors cited a few limitations. One is that the most recent study included in their meta-analysis was published in April 2017. When the researchers conducted a PubMed search in May 2018, they found three additional studies that met their criteria. “Since we already had 133 included studies, we decided that adding these three studies would not have changed the final results materially,” they wrote.
The study was supported by the Stichting Eunethydis (European Network for Hyperkinetic Disorders), and the U.K. National Institute for Health Research Oxford Health Biomedical Research Centre. Nine authors declared support, funding, or advisory roles with a range of organizations or the pharmaceutical industry.
SOURCE: Cortese S et al. Lancet Psychiatry. 2018 Aug 7. doi: 10.1016/S2215-0366(18)30269-4.
Methylphenidate appears to be the safest and most effective treatment option for attention-deficit/hyperactivity disorder in children and adolescents, while amphetamines are the preferred first-line choice in adults, a systematic review and meta-analysis have found.
Researchers reported the results of a network meta-analysis of 133 double-blind randomized controlled trials – 81 in children and adolescents, 51 in adults, and 1 in both – involving a total of 10,068 children and adolescents, and 8,131 adults. The included studies all compared a range of medications to placebo or in head-to-head trials. The meta-analysis was published online Aug. 7 in The Lancet Psychiatry.
At 12 weeks, all the medications, which included amphetamines, atomoxetine, bupropion, clonidine, guanfacine, methylphenidate, and modafinil, were found to be better than placebo in reducing core ADHD symptoms in children and adolescents, according to clinicians’ ratings. However, when teachers’ ratings were used, only methylphenidate and modafinil were better than placebo.
In adults, clinicians’ ratings found that amphetamines, methylphenidate, bupropion, and atomoxetine – but not modafinil – were better than placebo.
In head-to-head trials, clinicians’ ratings favored amphetamines over modafinil, atomoxetine, and methylphenidate in children, adolescents, and adults.
But in adults, amphetamines, bupropion, and methylphenidate all beat placebo.
When it came to tolerability in children and adolescents, guanfacine and amphetamines were the only two treatments that were less well tolerated than placebo. However, a post hoc analysis suggested lisdexamfetamine had a lower tolerability relative to other amphetamines, at least in children and adolescents. In adults, modafinil, amphetamines, methylphenidate, and atomoxetine were beaten by placebo for tolerability.
“Overall, all medications, except modafinil in adults, were more efficacious than placebo for the short-term treatment of ADHD, and they were less efficacious and less well tolerated in adults than in children and adolescents,” wrote Samuele Cortese, MD, PhD, of the University of Southampton (England), and his coauthors. “However, the included medications were not equivalent in relation to their mean effect size, which ranged from moderate to high and varied according to the type of rater.”
For example, while atomoxetine had the lowest mean effect size in children and adolescents based on clinicians’ ratings, in adults, it was on par with methylphenidate. Amphetamines increased diastolic blood pressure in children but not in adults.
“Taking into account both efficacy and safety, evidence from this meta-analysis supports methylphenidate in children and adolescents, and amphetamines in adults, as preferred first-choice medications for the short-term treatment of ADHD,” the authors wrote.
Dr. Cortese and his coauthors cited a few limitations. One is that the most recent study included in their meta-analysis was published in April 2017. When the researchers conducted a PubMed search in May 2018, they found three additional studies that met their criteria. “Since we already had 133 included studies, we decided that adding these three studies would not have changed the final results materially,” they wrote.
The study was supported by the Stichting Eunethydis (European Network for Hyperkinetic Disorders), and the U.K. National Institute for Health Research Oxford Health Biomedical Research Centre. Nine authors declared support, funding, or advisory roles with a range of organizations or the pharmaceutical industry.
SOURCE: Cortese S et al. Lancet Psychiatry. 2018 Aug 7. doi: 10.1016/S2215-0366(18)30269-4.
Methylphenidate appears to be the safest and most effective treatment option for attention-deficit/hyperactivity disorder in children and adolescents, while amphetamines are the preferred first-line choice in adults, a systematic review and meta-analysis have found.
Researchers reported the results of a network meta-analysis of 133 double-blind randomized controlled trials – 81 in children and adolescents, 51 in adults, and 1 in both – involving a total of 10,068 children and adolescents, and 8,131 adults. The included studies all compared a range of medications to placebo or in head-to-head trials. The meta-analysis was published online Aug. 7 in The Lancet Psychiatry.
At 12 weeks, all the medications, which included amphetamines, atomoxetine, bupropion, clonidine, guanfacine, methylphenidate, and modafinil, were found to be better than placebo in reducing core ADHD symptoms in children and adolescents, according to clinicians’ ratings. However, when teachers’ ratings were used, only methylphenidate and modafinil were better than placebo.
In adults, clinicians’ ratings found that amphetamines, methylphenidate, bupropion, and atomoxetine – but not modafinil – were better than placebo.
In head-to-head trials, clinicians’ ratings favored amphetamines over modafinil, atomoxetine, and methylphenidate in children, adolescents, and adults.
But in adults, amphetamines, bupropion, and methylphenidate all beat placebo.
When it came to tolerability in children and adolescents, guanfacine and amphetamines were the only two treatments that were less well tolerated than placebo. However, a post hoc analysis suggested lisdexamfetamine had a lower tolerability relative to other amphetamines, at least in children and adolescents. In adults, modafinil, amphetamines, methylphenidate, and atomoxetine were beaten by placebo for tolerability.
“Overall, all medications, except modafinil in adults, were more efficacious than placebo for the short-term treatment of ADHD, and they were less efficacious and less well tolerated in adults than in children and adolescents,” wrote Samuele Cortese, MD, PhD, of the University of Southampton (England), and his coauthors. “However, the included medications were not equivalent in relation to their mean effect size, which ranged from moderate to high and varied according to the type of rater.”
For example, while atomoxetine had the lowest mean effect size in children and adolescents based on clinicians’ ratings, in adults, it was on par with methylphenidate. Amphetamines increased diastolic blood pressure in children but not in adults.
“Taking into account both efficacy and safety, evidence from this meta-analysis supports methylphenidate in children and adolescents, and amphetamines in adults, as preferred first-choice medications for the short-term treatment of ADHD,” the authors wrote.
Dr. Cortese and his coauthors cited a few limitations. One is that the most recent study included in their meta-analysis was published in April 2017. When the researchers conducted a PubMed search in May 2018, they found three additional studies that met their criteria. “Since we already had 133 included studies, we decided that adding these three studies would not have changed the final results materially,” they wrote.
The study was supported by the Stichting Eunethydis (European Network for Hyperkinetic Disorders), and the U.K. National Institute for Health Research Oxford Health Biomedical Research Centre. Nine authors declared support, funding, or advisory roles with a range of organizations or the pharmaceutical industry.
SOURCE: Cortese S et al. Lancet Psychiatry. 2018 Aug 7. doi: 10.1016/S2215-0366(18)30269-4.
FROM THE LANCET PSYCHIATRY
Key clinical point: “All medications, except modafinil in adults, were more efficacious than placebo for the short-term treatment of ADHD.”
Major finding: Methylphenidate showed the greatest tolerability and efficacy of ADHD treatments for children and adolescents.
Study details: Systematic review and meta-analysis of 133 double-blind randomized controlled trials.
Disclosures: The study was supported by the Stichting Eunethydis (European Network for Hyperkinetic Disorders), and the U.K. National Institute for Health Research Oxford Health Biomedical Research Centre. Nine authors declared support, funding, or advisory roles with a range of organizations or the pharmaceutical industry.
Source: Cortese S et al. Lancet Psychiatry. 2018 Aug 7. doi: 10.1016/S2215-0366(18)30269-4.
Hip fracture outcomes are the next ERAS improvement goal
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
REPORTING FROM ACSQSC 2018
Key clinical point: Fractured hip patients managed with the ERAS protocol had improved outcomes.
Major finding: After implementation of the ERAS protocol, 43% of fractured hip patients were discharged to home, which is up from 20% before the project.
Study details: More than 200 patients treated for hip fracture during 2016-2017 at the Langley (B.C.) Memorial Hospital.
Disclosures: The investigator had no disclosures. .
1 in 7 Zika-exposed babies have at least one health problem related to the virus
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
FROM MMWR
Key clinical point: Zika-related health problems are present in a substantial number of prenatally exposed babies.
Major finding: Problems occurred in 14% of 4,800 included in a national registry.
Study details: USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. Disclosures: No relevant conflicts of interest were disclosed.
Source: Honein, MA et al. MMWR 2018; 67: 1-10.
Advise parents on validity of AD-associated conditions
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Percutaneous drainage upped morbidity risk in hepatobiliary cancer patients
ORLANDO – In patients with was associated with an increased risk of death or serious morbidity versus endoscopic drainage, results of a recent retrospective study show.
Patients undergoing percutaneous transhepatic biliary drainage did have more preoperative comorbidities, compared with those undergoing endoscopic biliary stenting, according researcher Q. Lina Hu, MD, an American College of Surgeons Clinical Scholar-in-Residence.
“Nevertheless, compared to endoscopic drainage, percutaneous drainage was associated with a significantly increased morbidity and mortality, even after adjustment for measured confounders,” Dr. Hu said a presentation at the American College of Surgeons Quality and Safety Conference.
Patients with resectable hepatobiliary malignancies often present with biliary obstruction, which may increase risk of perioperative morbidity and mortality, said Dr. Hu, a general surgery resident at University of California, Los Angeles.
“Preoperative biliary drainage is thought to reduce this risk by resolving cholestasis and preserving liver function,” she said.
However, the preferred drainage technique is not established, she added.
The endoscopic approach approximates normal physiologic drainage, she said, but is associated with complications including pancreatitis and cholangitis. By contrast, percutaneous drainage has a lower contamination risk and higher rate of success, but involves external catheters and has catheter-related complications.
To evaluate associations between preoperative drainage technique and postoperative outcomes, Dr. Hu and her colleagues queried the ACS National Surgical Quality Improvement Program (NSQIP) Procedure-Targeted Hepatectomy Database. They identified 527 patients who underwent preoperative biliary drainage prior to resection between 2014 and 2017, of whom about 80% underwent endoscopic drainage and 20% underwent percutaneous drainage. The primary outcome of their analysis was 30-day death or serious morbidity.
Patients who were selected for percutaneous drainage had significantly more preoperative comorbidities, including higher American Society of Anesthesiologists class, recent weight loss, and lower albumin levels, Dr. Hu said.
Death or serious morbidity occurred in 250 of the patients, or approximately 48% of the cohort.
In unadjusted analysis, the incidence of death or serious morbidity was significantly more frequent in the percutaneous group, compared with endoscopic group. The percutaneous group also had greater odds of surgical site infection, liver failure, bile leakage, and prolonged length of stay.
Those associations remained significant for death or serious morbidity and surgical site infection in both multivariable– and propensity score–adjusted models, Dr. Hu said.
In a propensity score–matched model, 93 patients who received percutaneous drainage were matched one-to-one to 93 patients who received endoscopic drainage based on relevant baseline characteristics. In that rigorous analysis, the odds ratio for death or serious morbidity was 2.17 (95% confidence interval, 1.16-4.09), according to the report.
“Death and serious morbidity was significantly associated with percutaneous drainage across all models, suggesting that patients receiving percutaneous drainage were more likely to experience an adverse event, compared to patients receiving endoscopic drainage,” Dr. Hu said.
However, Dr. Hu acknowledged the limitations of the retrospective study, noting that propensity score adjustment and matching accounts for measured confounders. “It obviously cannot account for any unmeasured confounders,” she said.
Dr. Hu reported funding from the Agency for Healthcare Research and Quality related to her position. She had no disclosures related to her presentation.
ORLANDO – In patients with was associated with an increased risk of death or serious morbidity versus endoscopic drainage, results of a recent retrospective study show.
Patients undergoing percutaneous transhepatic biliary drainage did have more preoperative comorbidities, compared with those undergoing endoscopic biliary stenting, according researcher Q. Lina Hu, MD, an American College of Surgeons Clinical Scholar-in-Residence.
“Nevertheless, compared to endoscopic drainage, percutaneous drainage was associated with a significantly increased morbidity and mortality, even after adjustment for measured confounders,” Dr. Hu said a presentation at the American College of Surgeons Quality and Safety Conference.
Patients with resectable hepatobiliary malignancies often present with biliary obstruction, which may increase risk of perioperative morbidity and mortality, said Dr. Hu, a general surgery resident at University of California, Los Angeles.
“Preoperative biliary drainage is thought to reduce this risk by resolving cholestasis and preserving liver function,” she said.
However, the preferred drainage technique is not established, she added.
The endoscopic approach approximates normal physiologic drainage, she said, but is associated with complications including pancreatitis and cholangitis. By contrast, percutaneous drainage has a lower contamination risk and higher rate of success, but involves external catheters and has catheter-related complications.
To evaluate associations between preoperative drainage technique and postoperative outcomes, Dr. Hu and her colleagues queried the ACS National Surgical Quality Improvement Program (NSQIP) Procedure-Targeted Hepatectomy Database. They identified 527 patients who underwent preoperative biliary drainage prior to resection between 2014 and 2017, of whom about 80% underwent endoscopic drainage and 20% underwent percutaneous drainage. The primary outcome of their analysis was 30-day death or serious morbidity.
Patients who were selected for percutaneous drainage had significantly more preoperative comorbidities, including higher American Society of Anesthesiologists class, recent weight loss, and lower albumin levels, Dr. Hu said.
Death or serious morbidity occurred in 250 of the patients, or approximately 48% of the cohort.
In unadjusted analysis, the incidence of death or serious morbidity was significantly more frequent in the percutaneous group, compared with endoscopic group. The percutaneous group also had greater odds of surgical site infection, liver failure, bile leakage, and prolonged length of stay.
Those associations remained significant for death or serious morbidity and surgical site infection in both multivariable– and propensity score–adjusted models, Dr. Hu said.
In a propensity score–matched model, 93 patients who received percutaneous drainage were matched one-to-one to 93 patients who received endoscopic drainage based on relevant baseline characteristics. In that rigorous analysis, the odds ratio for death or serious morbidity was 2.17 (95% confidence interval, 1.16-4.09), according to the report.
“Death and serious morbidity was significantly associated with percutaneous drainage across all models, suggesting that patients receiving percutaneous drainage were more likely to experience an adverse event, compared to patients receiving endoscopic drainage,” Dr. Hu said.
However, Dr. Hu acknowledged the limitations of the retrospective study, noting that propensity score adjustment and matching accounts for measured confounders. “It obviously cannot account for any unmeasured confounders,” she said.
Dr. Hu reported funding from the Agency for Healthcare Research and Quality related to her position. She had no disclosures related to her presentation.
ORLANDO – In patients with was associated with an increased risk of death or serious morbidity versus endoscopic drainage, results of a recent retrospective study show.
Patients undergoing percutaneous transhepatic biliary drainage did have more preoperative comorbidities, compared with those undergoing endoscopic biliary stenting, according researcher Q. Lina Hu, MD, an American College of Surgeons Clinical Scholar-in-Residence.
“Nevertheless, compared to endoscopic drainage, percutaneous drainage was associated with a significantly increased morbidity and mortality, even after adjustment for measured confounders,” Dr. Hu said a presentation at the American College of Surgeons Quality and Safety Conference.
Patients with resectable hepatobiliary malignancies often present with biliary obstruction, which may increase risk of perioperative morbidity and mortality, said Dr. Hu, a general surgery resident at University of California, Los Angeles.
“Preoperative biliary drainage is thought to reduce this risk by resolving cholestasis and preserving liver function,” she said.
However, the preferred drainage technique is not established, she added.
The endoscopic approach approximates normal physiologic drainage, she said, but is associated with complications including pancreatitis and cholangitis. By contrast, percutaneous drainage has a lower contamination risk and higher rate of success, but involves external catheters and has catheter-related complications.
To evaluate associations between preoperative drainage technique and postoperative outcomes, Dr. Hu and her colleagues queried the ACS National Surgical Quality Improvement Program (NSQIP) Procedure-Targeted Hepatectomy Database. They identified 527 patients who underwent preoperative biliary drainage prior to resection between 2014 and 2017, of whom about 80% underwent endoscopic drainage and 20% underwent percutaneous drainage. The primary outcome of their analysis was 30-day death or serious morbidity.
Patients who were selected for percutaneous drainage had significantly more preoperative comorbidities, including higher American Society of Anesthesiologists class, recent weight loss, and lower albumin levels, Dr. Hu said.
Death or serious morbidity occurred in 250 of the patients, or approximately 48% of the cohort.
In unadjusted analysis, the incidence of death or serious morbidity was significantly more frequent in the percutaneous group, compared with endoscopic group. The percutaneous group also had greater odds of surgical site infection, liver failure, bile leakage, and prolonged length of stay.
Those associations remained significant for death or serious morbidity and surgical site infection in both multivariable– and propensity score–adjusted models, Dr. Hu said.
In a propensity score–matched model, 93 patients who received percutaneous drainage were matched one-to-one to 93 patients who received endoscopic drainage based on relevant baseline characteristics. In that rigorous analysis, the odds ratio for death or serious morbidity was 2.17 (95% confidence interval, 1.16-4.09), according to the report.
“Death and serious morbidity was significantly associated with percutaneous drainage across all models, suggesting that patients receiving percutaneous drainage were more likely to experience an adverse event, compared to patients receiving endoscopic drainage,” Dr. Hu said.
However, Dr. Hu acknowledged the limitations of the retrospective study, noting that propensity score adjustment and matching accounts for measured confounders. “It obviously cannot account for any unmeasured confounders,” she said.
Dr. Hu reported funding from the Agency for Healthcare Research and Quality related to her position. She had no disclosures related to her presentation.
REPORTING FROM ACSQSC 2018
Key clinical point: Percutaneous biliary drainage for resectable hepatobiliary cancer was associated with an increased risk of death or serious morbidity, compared with endoscopic drainage.
Major finding: For patients having percutaneous biliary drainage, the odds ratio for death or serious morbidity was 2.17 (95% confidence interval, 1.16-4.09).
Study details: Cohort of 327 patients in the ACS NSQIP database who underwent preoperative biliary drainage.
Disclosures: The investigators had no disclosures.
Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome Management
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
Ranked: State of the states’ health care
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
Genetic composition of HCV changes with HIV coinfection
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
FROM INFECTION, GENETICS, AND EVOLUTION
Sapien 3 performs well (mostly) in bicuspid aortic stenosis
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.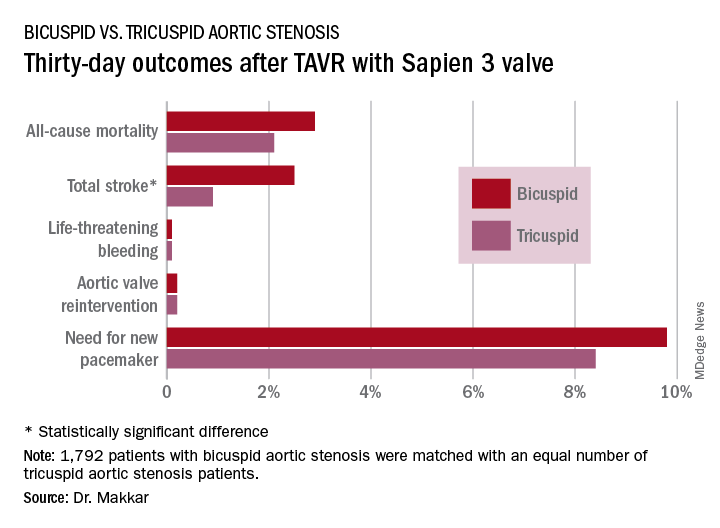
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
REPORTING FROM EUROPCR 2018
Key clinical point: Overall, outcomes were similarly favorable between patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis.
Major finding: The 1-year all-cause mortality and total stroke rates in 1,792 TAVR patients who got the Sapien 3 valve for bicuspid aortic stenosis were 10.4% and 3.4%.
Study details: This was a propensity-matched comparison of TAVR outcomes using the Sapien 3 valve in 1,792 patients with bicuspid aortic stenosis and in an equal number with tricuspid aortic stenosis in the STS/ACC TVT Registry.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.