User login
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
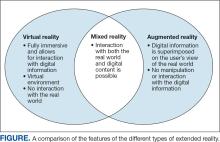
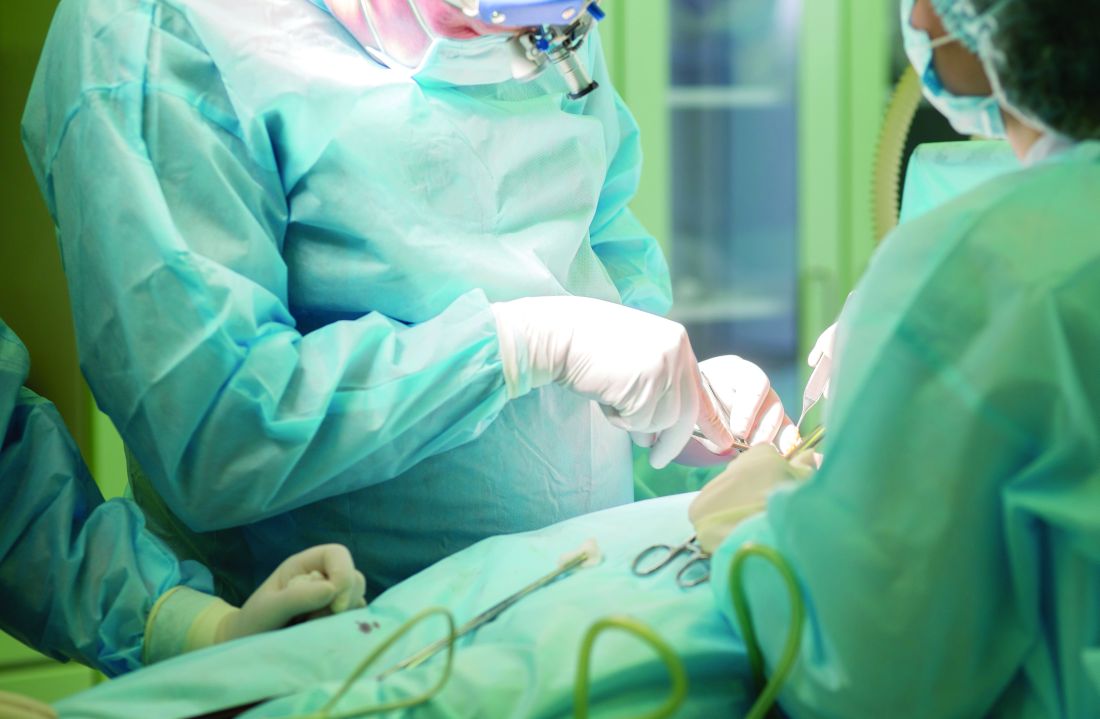
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
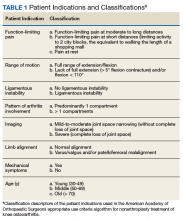
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
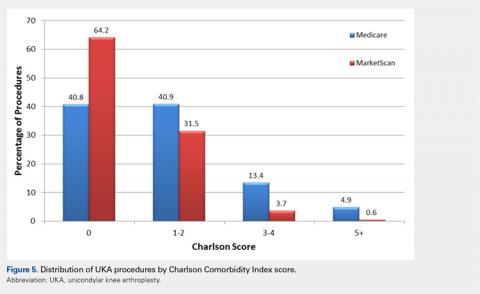
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).
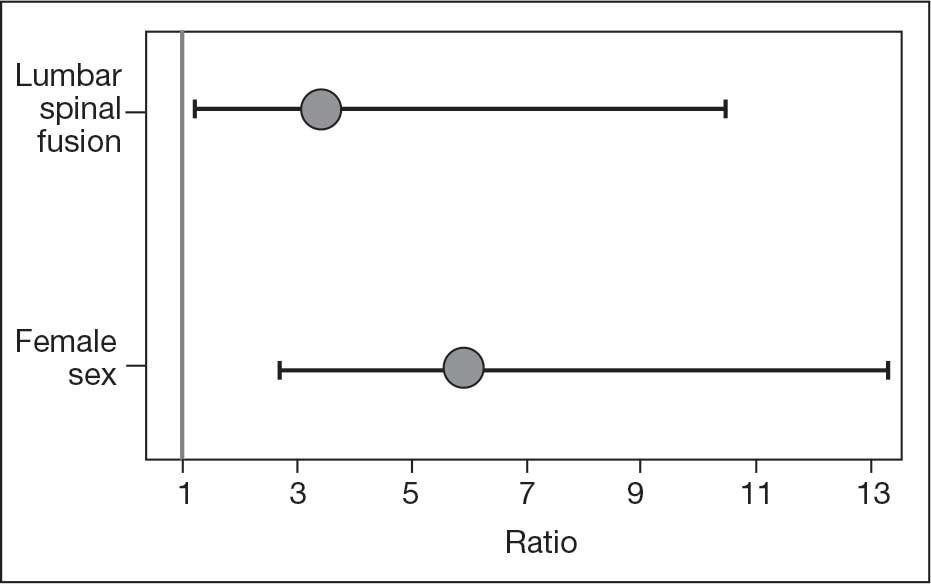
The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.
Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).

The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.

Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).

The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.

Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Bone Infections Increase After S. aureus Bacteremia in Patients With Rheumatoid Arthritis
TOPLINE:
After Staphylococcus aureus bacteremia, patients with rheumatoid arthritis (RA) face nearly double the risk for osteoarticular infections compared with those without RA, with similar mortality risks in both groups.
METHODOLOGY:
- The contraction of S aureus bacteremia is linked to poor clinical outcomes in patients with RA; however, no well-sized studies have evaluated the risk for osteoarticular infections and mortality outcomes in patients with RA following S aureus bacteremia.
- This Danish nationwide cohort study aimed to explore whether the cumulative incidence of osteoarticular infections and death would be higher in patients with RA than in those without RA after contracting S aureus bacteremia.
- The study cohort included 18,274 patients with a first episode of S aureus bacteremia between 2006 and 2018, of whom 367 had been diagnosed with RA before contracting S aureus bacteremia.
- The RA cohort had more women (62%) and a higher median age of participants (73 years) than the non-RA cohort (37% women; median age of participants, 70 years).
TAKEAWAY:
- The 90-day cumulative incidence of osteoarticular infections (septic arthritis, spondylitis, osteomyelitis, psoas muscle abscess, or prosthetic joint infection) was nearly double in patients with RA compared with in those without RA (23.1% vs 12.5%; hazard ratio [HR], 1.93; 95% CI, 1.54-2.41).
- In patients with RA, the risk for osteoarticular infections increased with tumor necrosis factor inhibitor use (HR, 2.27; 95% CI, 1.29-3.98) and orthopedic implants (HR, 1.75; 95% CI, 1.08-2.85).
- Moreover, 90-day all-cause mortality was comparable in the RA (35.4%) and non-RA cohorts (33.9%).
IN PRACTICE:
“Our findings stress the need for vigilance in patients with RA who present with S aureus bacteremia to ensure timely identification and treatment of osteoarticular infections, especially in current TNFi [tumor necrosis factor inhibitor] users and patients with orthopedic implants,” the authors wrote.
SOURCE:
This study, led by Sabine S. Dieperink, MD, of the Centre of Head and Orthopaedics, Copenhagen University Rigshospitalet Glostrup, Denmark, was published online March 9 in Rheumatology (Oxford).
LIMITATIONS:
There might have been chances of misclassification of metastatic S aureus infections owing to the lack of specificity in diagnoses or procedure codes. This study relied on administrative data to record osteoarticular infections, which might have led investigators to underestimate the true cumulative incidence of osteoarticular infections. Also, some patients might have passed away before being diagnosed with osteoarticular infection owing to the high mortality.
DISCLOSURES:
This work was supported by grants from The Danish Rheumatism Association and Beckett Fonden. Some of the authors, including the lead author, declared receiving grants from various funding agencies and other sources, including pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
After Staphylococcus aureus bacteremia, patients with rheumatoid arthritis (RA) face nearly double the risk for osteoarticular infections compared with those without RA, with similar mortality risks in both groups.
METHODOLOGY:
- The contraction of S aureus bacteremia is linked to poor clinical outcomes in patients with RA; however, no well-sized studies have evaluated the risk for osteoarticular infections and mortality outcomes in patients with RA following S aureus bacteremia.
- This Danish nationwide cohort study aimed to explore whether the cumulative incidence of osteoarticular infections and death would be higher in patients with RA than in those without RA after contracting S aureus bacteremia.
- The study cohort included 18,274 patients with a first episode of S aureus bacteremia between 2006 and 2018, of whom 367 had been diagnosed with RA before contracting S aureus bacteremia.
- The RA cohort had more women (62%) and a higher median age of participants (73 years) than the non-RA cohort (37% women; median age of participants, 70 years).
TAKEAWAY:
- The 90-day cumulative incidence of osteoarticular infections (septic arthritis, spondylitis, osteomyelitis, psoas muscle abscess, or prosthetic joint infection) was nearly double in patients with RA compared with in those without RA (23.1% vs 12.5%; hazard ratio [HR], 1.93; 95% CI, 1.54-2.41).
- In patients with RA, the risk for osteoarticular infections increased with tumor necrosis factor inhibitor use (HR, 2.27; 95% CI, 1.29-3.98) and orthopedic implants (HR, 1.75; 95% CI, 1.08-2.85).
- Moreover, 90-day all-cause mortality was comparable in the RA (35.4%) and non-RA cohorts (33.9%).
IN PRACTICE:
“Our findings stress the need for vigilance in patients with RA who present with S aureus bacteremia to ensure timely identification and treatment of osteoarticular infections, especially in current TNFi [tumor necrosis factor inhibitor] users and patients with orthopedic implants,” the authors wrote.
SOURCE:
This study, led by Sabine S. Dieperink, MD, of the Centre of Head and Orthopaedics, Copenhagen University Rigshospitalet Glostrup, Denmark, was published online March 9 in Rheumatology (Oxford).
LIMITATIONS:
There might have been chances of misclassification of metastatic S aureus infections owing to the lack of specificity in diagnoses or procedure codes. This study relied on administrative data to record osteoarticular infections, which might have led investigators to underestimate the true cumulative incidence of osteoarticular infections. Also, some patients might have passed away before being diagnosed with osteoarticular infection owing to the high mortality.
DISCLOSURES:
This work was supported by grants from The Danish Rheumatism Association and Beckett Fonden. Some of the authors, including the lead author, declared receiving grants from various funding agencies and other sources, including pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
After Staphylococcus aureus bacteremia, patients with rheumatoid arthritis (RA) face nearly double the risk for osteoarticular infections compared with those without RA, with similar mortality risks in both groups.
METHODOLOGY:
- The contraction of S aureus bacteremia is linked to poor clinical outcomes in patients with RA; however, no well-sized studies have evaluated the risk for osteoarticular infections and mortality outcomes in patients with RA following S aureus bacteremia.
- This Danish nationwide cohort study aimed to explore whether the cumulative incidence of osteoarticular infections and death would be higher in patients with RA than in those without RA after contracting S aureus bacteremia.
- The study cohort included 18,274 patients with a first episode of S aureus bacteremia between 2006 and 2018, of whom 367 had been diagnosed with RA before contracting S aureus bacteremia.
- The RA cohort had more women (62%) and a higher median age of participants (73 years) than the non-RA cohort (37% women; median age of participants, 70 years).
TAKEAWAY:
- The 90-day cumulative incidence of osteoarticular infections (septic arthritis, spondylitis, osteomyelitis, psoas muscle abscess, or prosthetic joint infection) was nearly double in patients with RA compared with in those without RA (23.1% vs 12.5%; hazard ratio [HR], 1.93; 95% CI, 1.54-2.41).
- In patients with RA, the risk for osteoarticular infections increased with tumor necrosis factor inhibitor use (HR, 2.27; 95% CI, 1.29-3.98) and orthopedic implants (HR, 1.75; 95% CI, 1.08-2.85).
- Moreover, 90-day all-cause mortality was comparable in the RA (35.4%) and non-RA cohorts (33.9%).
IN PRACTICE:
“Our findings stress the need for vigilance in patients with RA who present with S aureus bacteremia to ensure timely identification and treatment of osteoarticular infections, especially in current TNFi [tumor necrosis factor inhibitor] users and patients with orthopedic implants,” the authors wrote.
SOURCE:
This study, led by Sabine S. Dieperink, MD, of the Centre of Head and Orthopaedics, Copenhagen University Rigshospitalet Glostrup, Denmark, was published online March 9 in Rheumatology (Oxford).
LIMITATIONS:
There might have been chances of misclassification of metastatic S aureus infections owing to the lack of specificity in diagnoses or procedure codes. This study relied on administrative data to record osteoarticular infections, which might have led investigators to underestimate the true cumulative incidence of osteoarticular infections. Also, some patients might have passed away before being diagnosed with osteoarticular infection owing to the high mortality.
DISCLOSURES:
This work was supported by grants from The Danish Rheumatism Association and Beckett Fonden. Some of the authors, including the lead author, declared receiving grants from various funding agencies and other sources, including pharmaceutical companies.
A version of this article appeared on Medscape.com.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is aspirin the best way to prevent blood clots after THA/TKA?
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAOS 2022
Simple prevention strategies can lessen postoperative delirium after orthopedic surgery
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
FROM BMC GERIATRICS
Minor surgeries appear safe for hemophilia patients on emicizumab
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
REPORTING FROM 2019 ISTH CONGRESS
Evaluation of the American Academy of Orthopaedic Surgeons Appropriate Use Criteria for the Nonarthroplasty Treatment of Knee Osteoarthritis in Veterans
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Unicondylar Knee Arthroplasty in the U.S. Patient Population: Prevalence and Epidemiology
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
TAKE-HOME POINTS
- Prior publications on prevalence of unicondylar knee arthroplasty (UKA) in the United States using a single database may have underestimated the “true” number of cases performed.
- For the time periods analyzed, a total of 5,235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively.
- Rates of UKA generally increased until 2008, after which there was a decline through 2012.
- Gender and year of operation were found to be significantly associated with UKA rate.
- Males ages 55-64, 65-69, and 70-74 were the only age-gender groups whose UKA rates appear to be trending upward.