User login
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
THE DIAGNOSIS: Folliculitis Decalvans
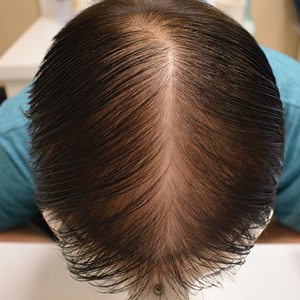
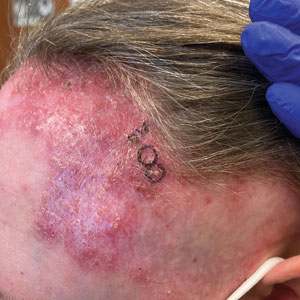
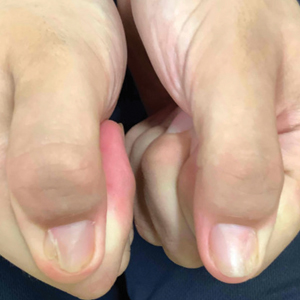
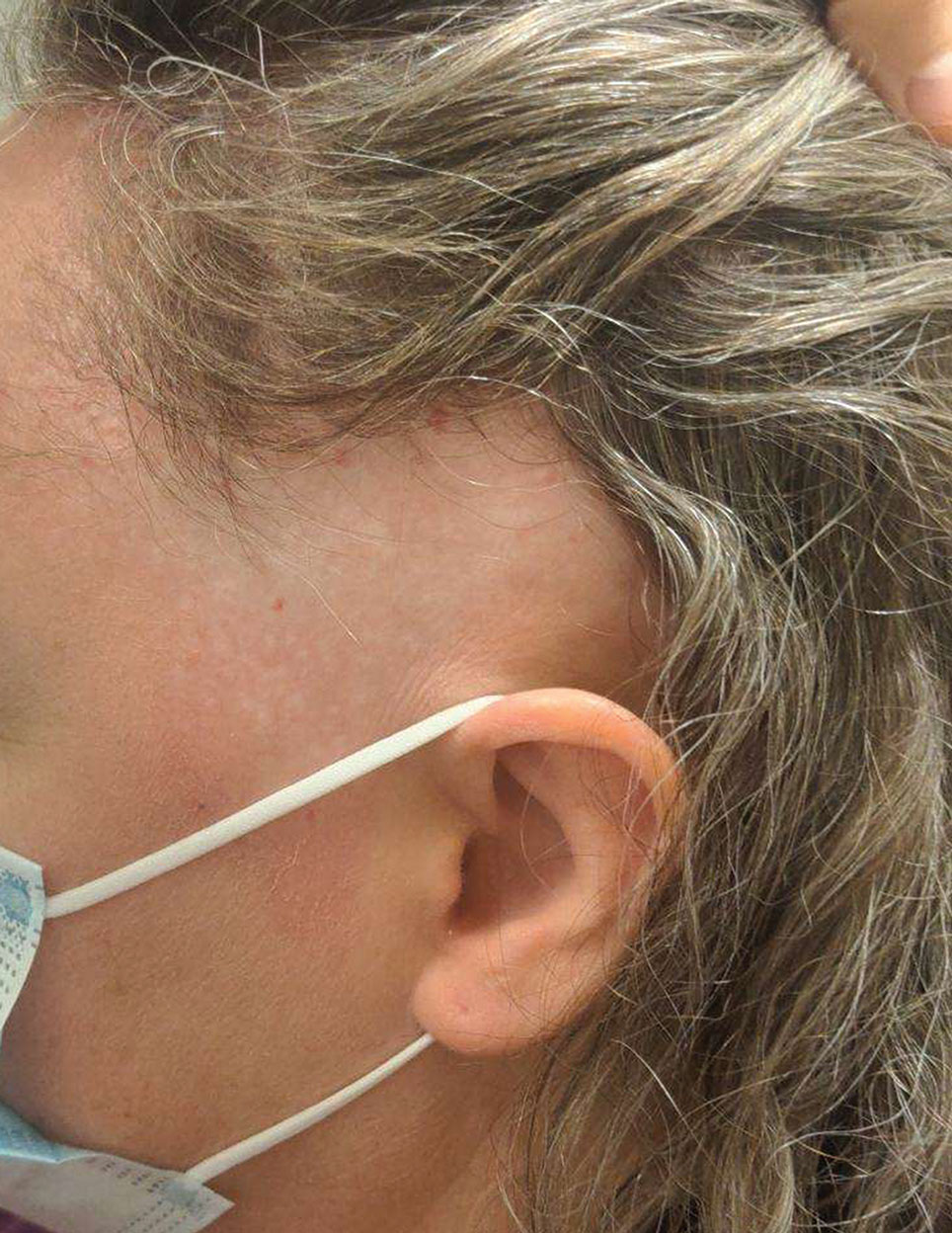
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.
Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
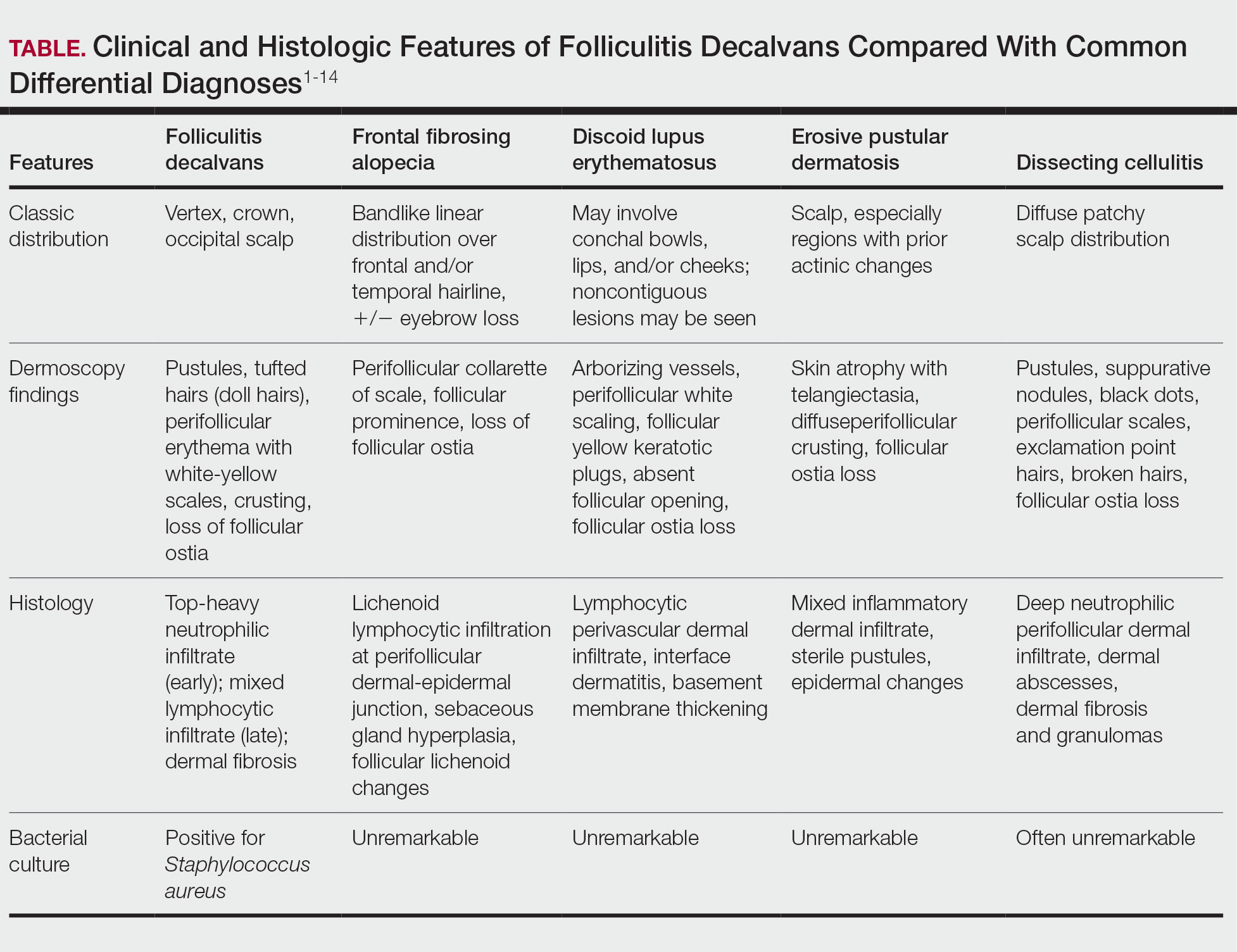
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10
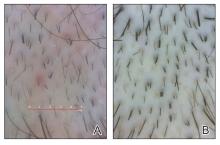
While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
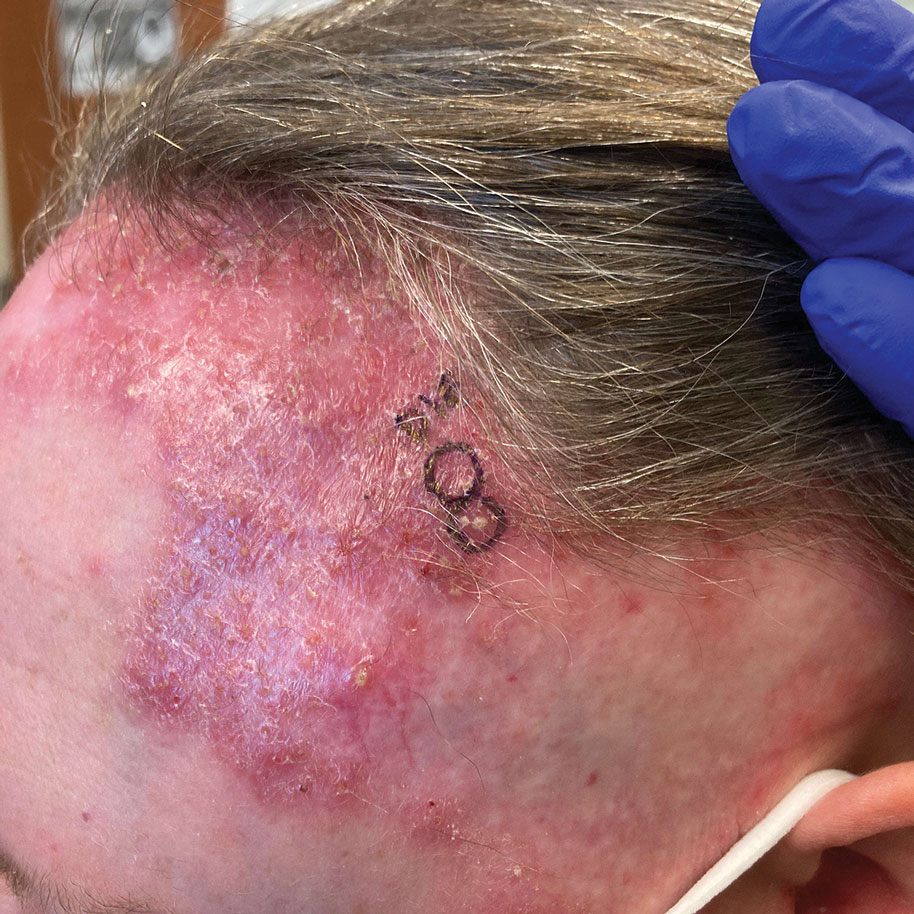
A 52-year-old woman presented to the dermatology department with an intermittently pruritic rash in a bandlike distribution on the left upper forehead and the frontal and temporal scalp of 4 years’ duration. The rash initially was diagnosed as psoriasis at an outside facility. Treatment over the year prior to presentation included tildrakizumab-asmn; topical crisaborole 2%; and excimer laser, which was complicated by blistering. The patient reported no history of topical or injected steroid use in the involved areas. Physical examination at the current presentation revealed arcuate erythematous plaques with follicular prominence, perifollicular scaling, pustules, and lone hairs. There also were porcelain-white atrophic plaques with loss of follicular ostia that were most prominent over the temporal scalp. A biopsy of the left lateral forehead was performed.
Management of Facial Hair in Women
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).
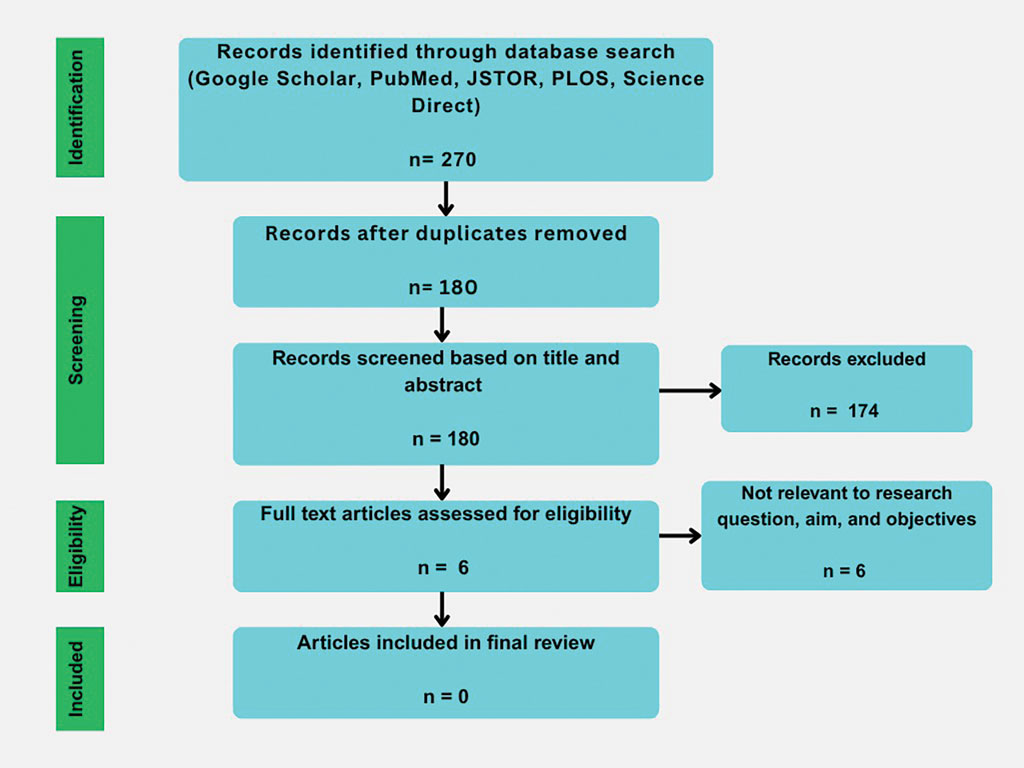
Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Practice Points
- Synthetic hair extensions are made from materials that can expose patients to high levels of carcinogens beginning in early childhood.
- The apple cider vinegar rinse method is an anecdotal remedy lacking data validating its ability to mitigate adverse reactions and complications associated with synthetic hair extensions, including carcinogenic exposure to materials they comprise.
- Dermatologists should inform patients of the potential exposure risks when using synthetic hair extensions to help patients make informed decisions regarding future styling habits and hair care choices.
Longitudinal Erythronychia Manifesting With Pain and Cold Sensitivity
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7
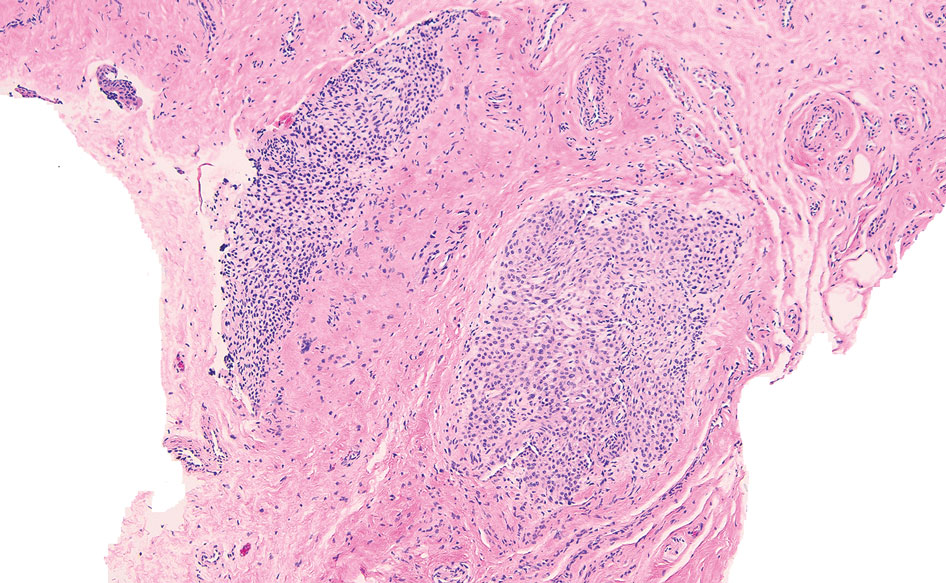
Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20
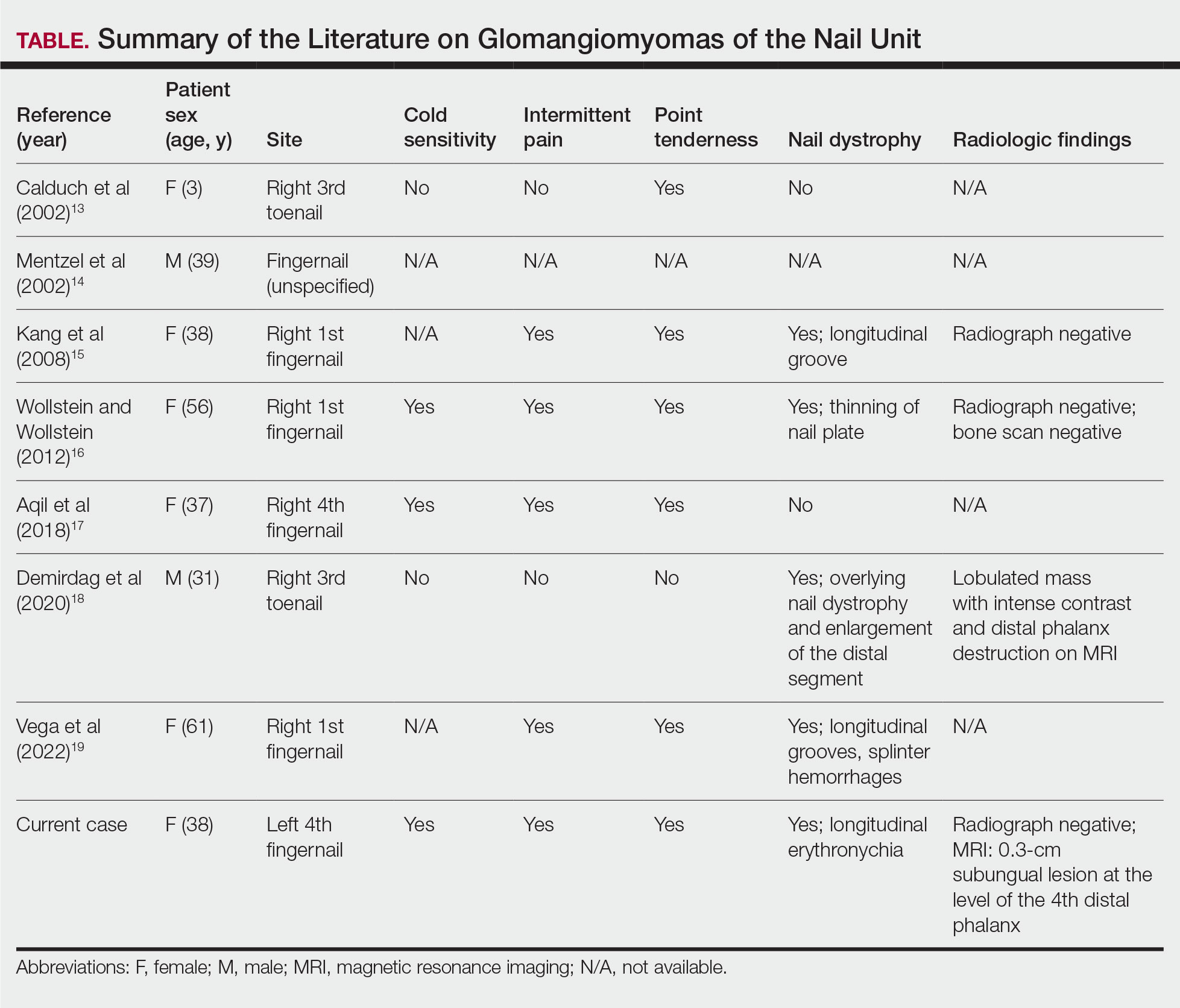
A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
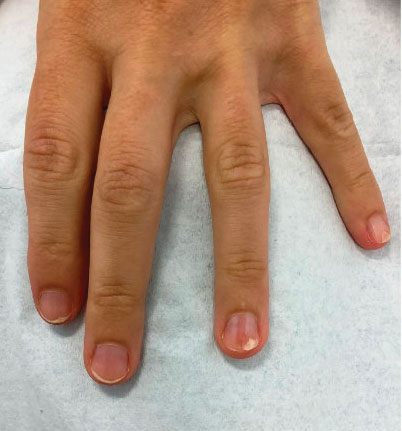
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
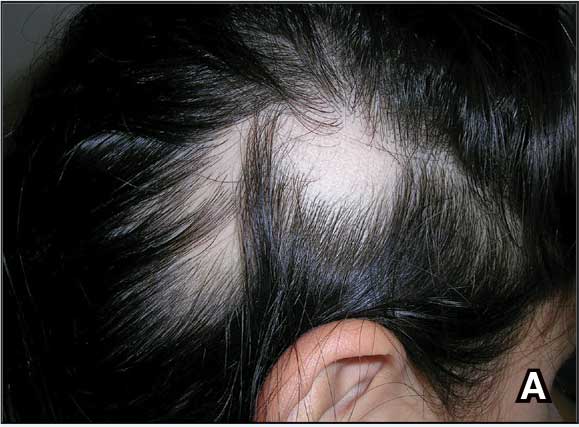
young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.
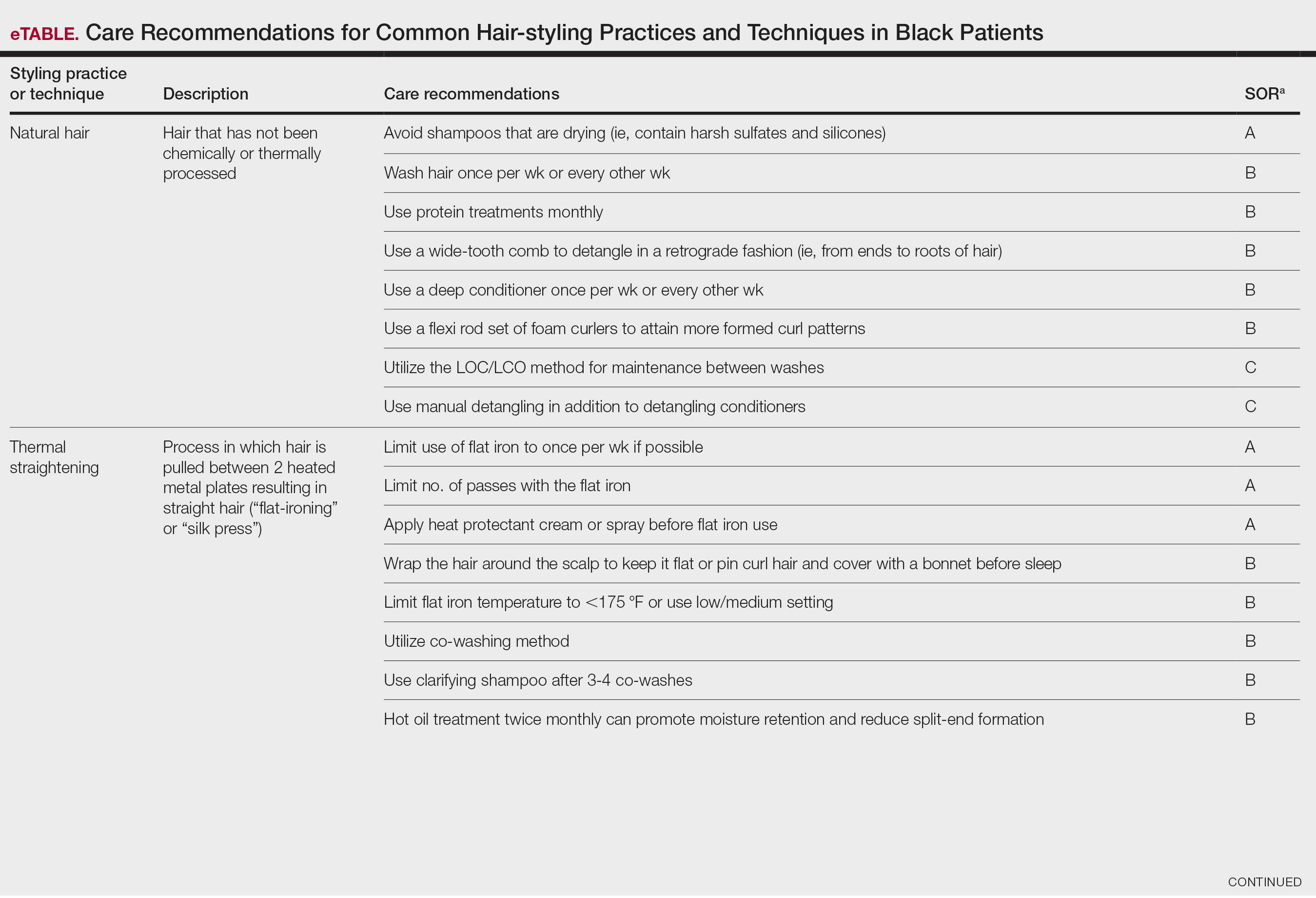
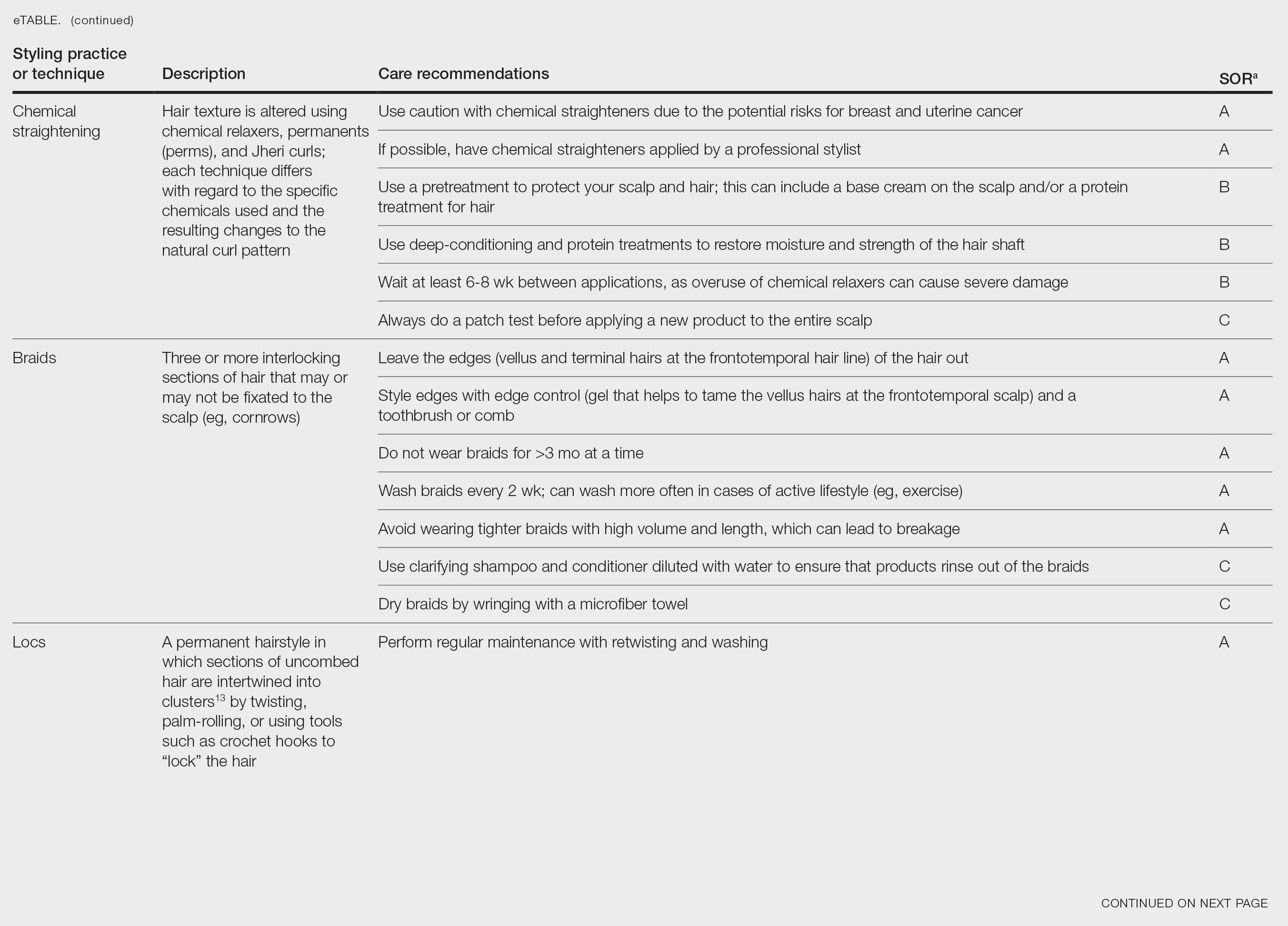
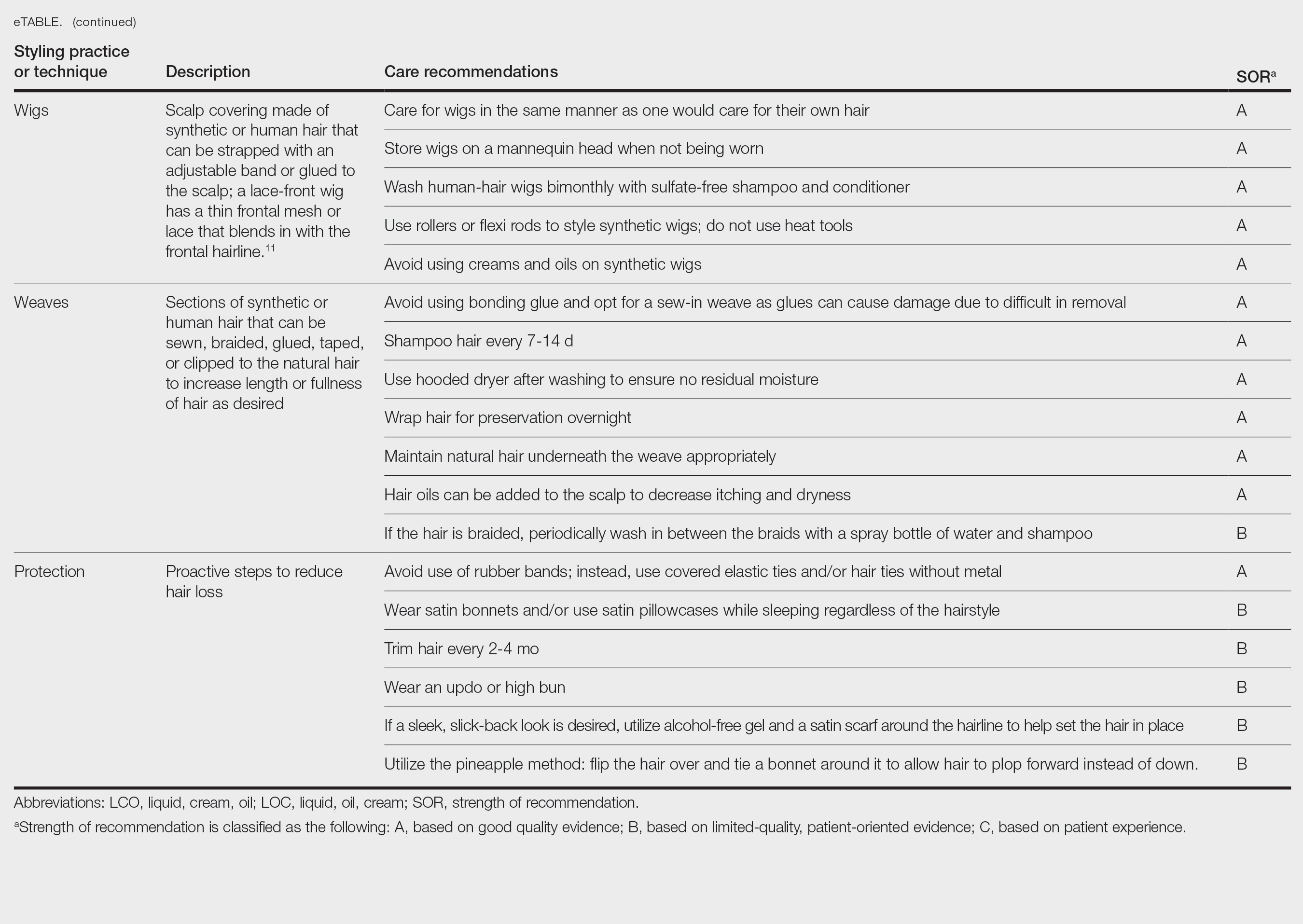
Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
PRACTICE POINTS
- There is a dearth in understanding of hair care practices in Black women among health care professionals.
- Increased knowledge and cultural understanding of past and present hair care practices in Black women enhances patient care.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8
Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
A 14-year-old boy with a history of toe walking, attention-deficit/hyperactivity disorder, and mixed receptive expressive language disorder presented to our pediatric dermatology clinic with fingernail abnormalities that had been present since birth. Physical examination revealed narrowing and longitudinal splitting of the nail plates with triangular lacunae and progressive improvement appreciated toward the fifth digits. The nail changes were most prominent on the first digits. A review of the patient’s medical record revealed incidental bilateral iliac horns of the pelvis on radiographs taken at age 18 months. The patient reported waxing and waning knee pain that worsened with prolonged activity and when climbing stairs. Urinalysis demonstrated mild hematuria without proteinuria. The patient was normotensive. There was no evidence of glaucoma, cataracts, or hyperpigmentation of the pupillary margin (Lester iris) on ophthalmologic examination. Genetic testing was performed.
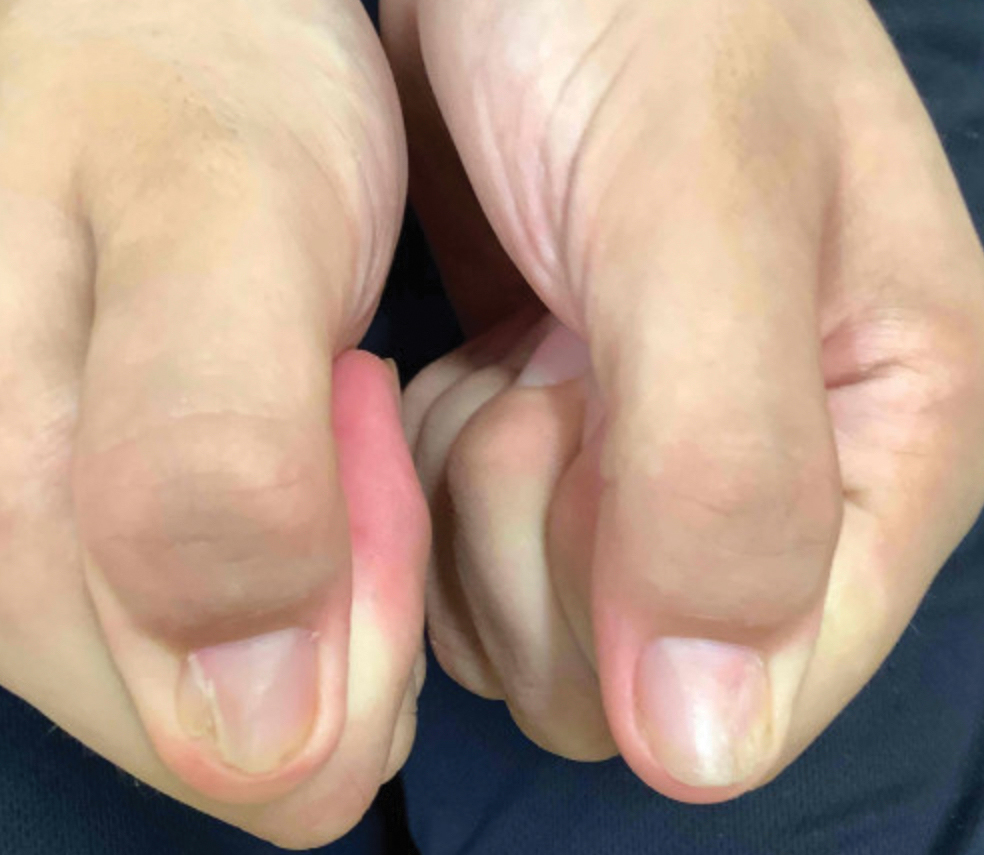
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products

Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
PRACTICE POINTS
- Early identification of androgenetic alopecia (AGA) is key in pediatric patients, especially in those with a family history of AGA and comorbidities such as seborrheic dermatitis, acne, or sleep disturbances.
- It is important to evaluate pediatric patients with AGA for hormonal imbalances and deficiencies in vitamin D and iron to guide treatment.
- Use targeted therapies such as topical minoxidil to treat pediatric AGA while also monitoring for adverse effects. For optimal outcomes, encourage consistent medication use and regular follow-up.