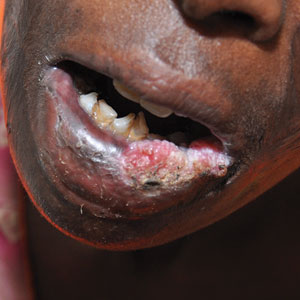
User login
Top U.S. hospitals for psychiatric care ranked
McLean Hospital claimed the top spot in the 2022-2023 ranking as well.
Massachusetts General Hospital in Boston holds the No. 2 spot in the 2023-2024 U.S. News ranking for best psychiatry hospitals, up from No. 3 in 2022-2023.
New York–Presbyterian Hospital–Columbia and Cornell sits at No. 3 in 2023-2024, up from No. 4 in 2022-2023, while Johns Hopkins Hospital, Baltimore is ranked No. 4, down from No. 2.
Resnick Neuropsychiatric Hospital at the University of California, Los Angeles, is ranked No. 5 in 2023-2024 (up from No. 6 in 2022-2023), while UCSF Health–UCSF Medical Center, San Francisco, dropped to No. 6 in 2023-2024 (from No. 5 in 2022-2023).
No. 7 in 2023-2024 is Menninger Clinic, Houston, which held the No. 10 spot in 2022-2023.
According to U.S. News, the psychiatry rating is based on the expert opinion of surveyed psychiatrists. The seven ranked hospitals in psychiatry or psychiatric care were recommended by at least 5% of the psychiatric specialists responding to the magazine’s surveys in 2021, 2022, and 2023 as a facility where they would refer their patients.
“Consumers want useful resources to help them assess which hospital can best meet their specific care needs,” Ben Harder, chief of health analysis and managing editor at U.S. News, said in a statement.
“The 2023-2024 Best Hospitals rankings offer patients and the physicians with whom they consult a data-driven source for comparing performance in outcomes, patient satisfaction, and other metrics that matter to them,” Mr. Harder said.
Honor roll
This year, as in prior years, U.S. News also recognized “honor roll” hospitals that have excelled across multiple areas of care. However, in 2023-2024, for the first time, there is no ordinal ranking of hospitals making the honor roll. Instead, they are listed in alphabetical order.
In a letter to hospital leaders, U.S. News explained that the major change in format came after months of deliberation, feedback from health care organizations and professionals, and an analysis of how consumers navigate the magazine’s website.
Ordinal ranking of hospitals that make the honor roll “obscures the fact that all of the Honor Roll hospitals have attained the highest standard of care in the nation,” the letter reads.
With the new format, honor roll hospitals are listed in alphabetical order. In 2023-2024 there are 22.
2023-2024 Honor Roll Hospitals
- Barnes-Jewish Hospital, St. Louis
- Brigham and Women’s Hospital, Boston
- Cedars-Sinai Medical Center, Los Angeles
- Cleveland Clinic
- Hospitals of the University of Pennsylvania–Penn Medicine, Philadelphia
- Houston Methodist Hospital
- Johns Hopkins Hospital, Baltimore
- Massachusetts General Hospital, Boston
- Mayo Clinic, Rochester, Minn.
- Mount Sinai Hospital, New York
- New York–Presbyterian Hospital–Columbia and Cornell
- North Shore University Hospital at Northwell Health, Manhasset, N.Y.
- Northwestern Memorial Hospital, Chicago
- NYU Langone Hospitals, New York
- Rush University Medical Center, Chicago
- Stanford (Calif.) Health Care–Stanford Hospital
- UC San Diego Health–La Jolla and Hillcrest Hospitals
- UCLA Medical Center, Los Angeles
- UCSF Health–UCSF Medical Center, San Francisco
- University of Michigan Health–Ann Arbor
- UT Southwestern Medical Center, Dallas
- Vanderbilt University Medical Center, Nashville, Tenn.
According to U.S. News, to keep pace with consumers’ needs and the ever-evolving landscape of health care, “several refinements” are reflected in the latest best hospitals rankings.
These include the introduction of outpatient outcomes in key specialty rankings and surgical ratings, the expanded inclusion of other outpatient data, an increased weight on objective quality measures, and a reduced weight on expert opinion.
In addition, hospital profiles on USNews.com feature refined health equity measures, including a new measure of racial disparities in outcomes.
The full report for best hospitals, best specialty hospitals, and methodology is available online.
A version of this article first appeared on Medscape.com.
McLean Hospital claimed the top spot in the 2022-2023 ranking as well.
Massachusetts General Hospital in Boston holds the No. 2 spot in the 2023-2024 U.S. News ranking for best psychiatry hospitals, up from No. 3 in 2022-2023.
New York–Presbyterian Hospital–Columbia and Cornell sits at No. 3 in 2023-2024, up from No. 4 in 2022-2023, while Johns Hopkins Hospital, Baltimore is ranked No. 4, down from No. 2.
Resnick Neuropsychiatric Hospital at the University of California, Los Angeles, is ranked No. 5 in 2023-2024 (up from No. 6 in 2022-2023), while UCSF Health–UCSF Medical Center, San Francisco, dropped to No. 6 in 2023-2024 (from No. 5 in 2022-2023).
No. 7 in 2023-2024 is Menninger Clinic, Houston, which held the No. 10 spot in 2022-2023.
According to U.S. News, the psychiatry rating is based on the expert opinion of surveyed psychiatrists. The seven ranked hospitals in psychiatry or psychiatric care were recommended by at least 5% of the psychiatric specialists responding to the magazine’s surveys in 2021, 2022, and 2023 as a facility where they would refer their patients.
“Consumers want useful resources to help them assess which hospital can best meet their specific care needs,” Ben Harder, chief of health analysis and managing editor at U.S. News, said in a statement.
“The 2023-2024 Best Hospitals rankings offer patients and the physicians with whom they consult a data-driven source for comparing performance in outcomes, patient satisfaction, and other metrics that matter to them,” Mr. Harder said.
Honor roll
This year, as in prior years, U.S. News also recognized “honor roll” hospitals that have excelled across multiple areas of care. However, in 2023-2024, for the first time, there is no ordinal ranking of hospitals making the honor roll. Instead, they are listed in alphabetical order.
In a letter to hospital leaders, U.S. News explained that the major change in format came after months of deliberation, feedback from health care organizations and professionals, and an analysis of how consumers navigate the magazine’s website.
Ordinal ranking of hospitals that make the honor roll “obscures the fact that all of the Honor Roll hospitals have attained the highest standard of care in the nation,” the letter reads.
With the new format, honor roll hospitals are listed in alphabetical order. In 2023-2024 there are 22.
2023-2024 Honor Roll Hospitals
- Barnes-Jewish Hospital, St. Louis
- Brigham and Women’s Hospital, Boston
- Cedars-Sinai Medical Center, Los Angeles
- Cleveland Clinic
- Hospitals of the University of Pennsylvania–Penn Medicine, Philadelphia
- Houston Methodist Hospital
- Johns Hopkins Hospital, Baltimore
- Massachusetts General Hospital, Boston
- Mayo Clinic, Rochester, Minn.
- Mount Sinai Hospital, New York
- New York–Presbyterian Hospital–Columbia and Cornell
- North Shore University Hospital at Northwell Health, Manhasset, N.Y.
- Northwestern Memorial Hospital, Chicago
- NYU Langone Hospitals, New York
- Rush University Medical Center, Chicago
- Stanford (Calif.) Health Care–Stanford Hospital
- UC San Diego Health–La Jolla and Hillcrest Hospitals
- UCLA Medical Center, Los Angeles
- UCSF Health–UCSF Medical Center, San Francisco
- University of Michigan Health–Ann Arbor
- UT Southwestern Medical Center, Dallas
- Vanderbilt University Medical Center, Nashville, Tenn.
According to U.S. News, to keep pace with consumers’ needs and the ever-evolving landscape of health care, “several refinements” are reflected in the latest best hospitals rankings.
These include the introduction of outpatient outcomes in key specialty rankings and surgical ratings, the expanded inclusion of other outpatient data, an increased weight on objective quality measures, and a reduced weight on expert opinion.
In addition, hospital profiles on USNews.com feature refined health equity measures, including a new measure of racial disparities in outcomes.
The full report for best hospitals, best specialty hospitals, and methodology is available online.
A version of this article first appeared on Medscape.com.
McLean Hospital claimed the top spot in the 2022-2023 ranking as well.
Massachusetts General Hospital in Boston holds the No. 2 spot in the 2023-2024 U.S. News ranking for best psychiatry hospitals, up from No. 3 in 2022-2023.
New York–Presbyterian Hospital–Columbia and Cornell sits at No. 3 in 2023-2024, up from No. 4 in 2022-2023, while Johns Hopkins Hospital, Baltimore is ranked No. 4, down from No. 2.
Resnick Neuropsychiatric Hospital at the University of California, Los Angeles, is ranked No. 5 in 2023-2024 (up from No. 6 in 2022-2023), while UCSF Health–UCSF Medical Center, San Francisco, dropped to No. 6 in 2023-2024 (from No. 5 in 2022-2023).
No. 7 in 2023-2024 is Menninger Clinic, Houston, which held the No. 10 spot in 2022-2023.
According to U.S. News, the psychiatry rating is based on the expert opinion of surveyed psychiatrists. The seven ranked hospitals in psychiatry or psychiatric care were recommended by at least 5% of the psychiatric specialists responding to the magazine’s surveys in 2021, 2022, and 2023 as a facility where they would refer their patients.
“Consumers want useful resources to help them assess which hospital can best meet their specific care needs,” Ben Harder, chief of health analysis and managing editor at U.S. News, said in a statement.
“The 2023-2024 Best Hospitals rankings offer patients and the physicians with whom they consult a data-driven source for comparing performance in outcomes, patient satisfaction, and other metrics that matter to them,” Mr. Harder said.
Honor roll
This year, as in prior years, U.S. News also recognized “honor roll” hospitals that have excelled across multiple areas of care. However, in 2023-2024, for the first time, there is no ordinal ranking of hospitals making the honor roll. Instead, they are listed in alphabetical order.
In a letter to hospital leaders, U.S. News explained that the major change in format came after months of deliberation, feedback from health care organizations and professionals, and an analysis of how consumers navigate the magazine’s website.
Ordinal ranking of hospitals that make the honor roll “obscures the fact that all of the Honor Roll hospitals have attained the highest standard of care in the nation,” the letter reads.
With the new format, honor roll hospitals are listed in alphabetical order. In 2023-2024 there are 22.
2023-2024 Honor Roll Hospitals
- Barnes-Jewish Hospital, St. Louis
- Brigham and Women’s Hospital, Boston
- Cedars-Sinai Medical Center, Los Angeles
- Cleveland Clinic
- Hospitals of the University of Pennsylvania–Penn Medicine, Philadelphia
- Houston Methodist Hospital
- Johns Hopkins Hospital, Baltimore
- Massachusetts General Hospital, Boston
- Mayo Clinic, Rochester, Minn.
- Mount Sinai Hospital, New York
- New York–Presbyterian Hospital–Columbia and Cornell
- North Shore University Hospital at Northwell Health, Manhasset, N.Y.
- Northwestern Memorial Hospital, Chicago
- NYU Langone Hospitals, New York
- Rush University Medical Center, Chicago
- Stanford (Calif.) Health Care–Stanford Hospital
- UC San Diego Health–La Jolla and Hillcrest Hospitals
- UCLA Medical Center, Los Angeles
- UCSF Health–UCSF Medical Center, San Francisco
- University of Michigan Health–Ann Arbor
- UT Southwestern Medical Center, Dallas
- Vanderbilt University Medical Center, Nashville, Tenn.
According to U.S. News, to keep pace with consumers’ needs and the ever-evolving landscape of health care, “several refinements” are reflected in the latest best hospitals rankings.
These include the introduction of outpatient outcomes in key specialty rankings and surgical ratings, the expanded inclusion of other outpatient data, an increased weight on objective quality measures, and a reduced weight on expert opinion.
In addition, hospital profiles on USNews.com feature refined health equity measures, including a new measure of racial disparities in outcomes.
The full report for best hospitals, best specialty hospitals, and methodology is available online.
A version of this article first appeared on Medscape.com.
Antibody shows promise in preventing GVHD
Early, intriguing research suggests that preventing acute graft-versus-host disease (GVHD) in the gut – a potentially life-threatening complication of allogeneic hematopoietic cell transplantation (allo-HCT) – could be accomplished by the administration of a single antibody that targets the anti-DLL4 Notch signaling pathway, without compromising the stem cell transplant.
“The major surprise was that none of the anti–DLL4-treated animals developed acute gastrointestinal GVHD for the entire duration of the study. This was a remarkable finding, given that intestinal GVHD is otherwise seen in the vast majority of nonhuman primate transplant recipients that receive either no prophylaxis, or prophylaxis with agents other than anti-DLL4 antibodies,” co–senior author Ivan Maillard, MD, PhD, a professor of medicine and vice chief for research in hematology-oncology at the University of Pennsylvania, Philadelphia, said in an interview.
“The timing was critical,” the authors noted in the study, recently published in Science Translational Medicine. “Intervening before any symptoms of GvHD appear made the long-term protection possible.”
While GVHD may be mild to moderate in chronic forms, acute cases can be serious, if not fatal, and nearly all severe acute GVHD prominently involves the gastrointestinal tract, which can drive activation of pathogenic T cells and potentially lead to tissue damage following allo-HCT.
Systemic corticosteroids are standard first-line treatment for acute GVHD. However, response rates generally range only from 40% to 60%, and there are concerns of side effects. Meanwhile, second-line treatments are of inconsistent benefit.
With previous studies on mice showing benefits of targeting Notch pathway inhibition, particularly DLL4, Dr. Maillard and colleagues further investigated the effects in nonhuman primates that were allo-HCT recipients, using the anti-DLL4 antibody REGN421, which has pharmacokinetic and toxicity information available from previous studies.
The nonhuman primates were treated with one of two dosing regimens: a single dose of REGN421 3 mg/kg at baseline, post HCT, (n = 7) or three weekly doses at days 0, 7 and 14, post transplant (n = 4). Those primates were compared with 11 primates receiving allo-HCT transplants that received supportive care only.
Primates receiving three weekly doses of REGN421 showed antibody concentrations of greater than 2 mcg/mL for more than 30 days post HCT. A single dose of REGN421 was associated with protection from acute GVHD at day 0, while three weekly doses showed protection at day 0, 7, and 14, consistent with an impact of REGN421 during the early phases of T-cell activation.
Compared with animals receiving only supportive care, prophylaxis with REGN421 was associated with delayed acute GVHD onset and lengthened survival.
Of the 11 primates treated with REGN421, none developed clinical signs of gastrointestinal acute GVHD, whereas the majority of those receiving standard care or other preventive interventions did.
“Detailed analysis of acute GVHD clinical presentations in REGN421-treated animals in comparison to no treatment controls revealed near complete protection from GI-acute GvHD with REGN421,” the authors reported.
Furthermore, pathology scores in the gastrointestinal tract were lower with REGN421 treatment, compared with the no-treatment cohort, and the scores matched those of healthy nontransplanted nonhuman primates.
The primates treated with REGN421 did ultimately develop other clinical and pathologic signs of skin, hepatic or pulmonary acute GVHD, but without gastrointestinal disease.
The treatment was not associated with any adverse effects on the allo-HCT, with primates receiving either a single dose or three weekly doses of REGN421 showing rapid donor engraftment after allo-HCT, including high bone marrow, whole blood, and T-cell donor chimerism.
“Reassuringly, short-term systemic DLL4 blockade with REGN421 did not trigger unexpected side effects in our nonhuman primate model, while preserving rapid engraftment as well hematopoietic and immune reconstitution.”
The mechanism preserving the engraftment, described as a “major surprise,” specifically involved DLL4 inhibition blocking the homing of pathogenic T cells to the gut while preserving homing of regulatory T cells that dampen the immune response, Dr. Maillard explained.
“This effect turned out to be at least in part through a posttranslational effect of DLL4/Notch blockade on integrin pairing at the T-cell surface,” he explained. “This was a novel and quite unexpected mechanism of action conserved from mice to nonhuman primates.”
The results are encouraging in terms of translating to humans because of their closer similarities in various physiological factors, Dr. Maillard said.
“The nonhuman primate model of transplantation [offers] a transplantation model very close to what is being performed in humans, as well as the opportunity to study an immune system very similar to that of humans in nonhuman primates,” he said.
Dr. Maillard noted that, while trials in humans are not underway yet, “we are in active discussions about it,” and the team is indeed interested in testing REGN421 itself, with the effects likely to be as a prophylactic strategy.
There are currently no approved anti-DLL4 antibody drugs for use in humans.
“Our approach is mostly promising as a preventive treatment, rather than as a secondary treatment for GVHD, because DLL4/Notch blockade seems most active when applied early after transplantation during the time of initial seeding of the gut by T cells (in mice, we had observed the critical time window for a successful intervention to be within 48 hours of transplantation),” Dr. Maillard said.“There remain questions about which other prophylactic treatments we should ideally combine anti-DLL4 antibodies with.”
Dr. Maillard has received research funding from Regeneron and Genentech and is a member of Garuda Therapeutics’s scientific advisory board.
Early, intriguing research suggests that preventing acute graft-versus-host disease (GVHD) in the gut – a potentially life-threatening complication of allogeneic hematopoietic cell transplantation (allo-HCT) – could be accomplished by the administration of a single antibody that targets the anti-DLL4 Notch signaling pathway, without compromising the stem cell transplant.
“The major surprise was that none of the anti–DLL4-treated animals developed acute gastrointestinal GVHD for the entire duration of the study. This was a remarkable finding, given that intestinal GVHD is otherwise seen in the vast majority of nonhuman primate transplant recipients that receive either no prophylaxis, or prophylaxis with agents other than anti-DLL4 antibodies,” co–senior author Ivan Maillard, MD, PhD, a professor of medicine and vice chief for research in hematology-oncology at the University of Pennsylvania, Philadelphia, said in an interview.
“The timing was critical,” the authors noted in the study, recently published in Science Translational Medicine. “Intervening before any symptoms of GvHD appear made the long-term protection possible.”
While GVHD may be mild to moderate in chronic forms, acute cases can be serious, if not fatal, and nearly all severe acute GVHD prominently involves the gastrointestinal tract, which can drive activation of pathogenic T cells and potentially lead to tissue damage following allo-HCT.
Systemic corticosteroids are standard first-line treatment for acute GVHD. However, response rates generally range only from 40% to 60%, and there are concerns of side effects. Meanwhile, second-line treatments are of inconsistent benefit.
With previous studies on mice showing benefits of targeting Notch pathway inhibition, particularly DLL4, Dr. Maillard and colleagues further investigated the effects in nonhuman primates that were allo-HCT recipients, using the anti-DLL4 antibody REGN421, which has pharmacokinetic and toxicity information available from previous studies.
The nonhuman primates were treated with one of two dosing regimens: a single dose of REGN421 3 mg/kg at baseline, post HCT, (n = 7) or three weekly doses at days 0, 7 and 14, post transplant (n = 4). Those primates were compared with 11 primates receiving allo-HCT transplants that received supportive care only.
Primates receiving three weekly doses of REGN421 showed antibody concentrations of greater than 2 mcg/mL for more than 30 days post HCT. A single dose of REGN421 was associated with protection from acute GVHD at day 0, while three weekly doses showed protection at day 0, 7, and 14, consistent with an impact of REGN421 during the early phases of T-cell activation.
Compared with animals receiving only supportive care, prophylaxis with REGN421 was associated with delayed acute GVHD onset and lengthened survival.
Of the 11 primates treated with REGN421, none developed clinical signs of gastrointestinal acute GVHD, whereas the majority of those receiving standard care or other preventive interventions did.
“Detailed analysis of acute GVHD clinical presentations in REGN421-treated animals in comparison to no treatment controls revealed near complete protection from GI-acute GvHD with REGN421,” the authors reported.
Furthermore, pathology scores in the gastrointestinal tract were lower with REGN421 treatment, compared with the no-treatment cohort, and the scores matched those of healthy nontransplanted nonhuman primates.
The primates treated with REGN421 did ultimately develop other clinical and pathologic signs of skin, hepatic or pulmonary acute GVHD, but without gastrointestinal disease.
The treatment was not associated with any adverse effects on the allo-HCT, with primates receiving either a single dose or three weekly doses of REGN421 showing rapid donor engraftment after allo-HCT, including high bone marrow, whole blood, and T-cell donor chimerism.
“Reassuringly, short-term systemic DLL4 blockade with REGN421 did not trigger unexpected side effects in our nonhuman primate model, while preserving rapid engraftment as well hematopoietic and immune reconstitution.”
The mechanism preserving the engraftment, described as a “major surprise,” specifically involved DLL4 inhibition blocking the homing of pathogenic T cells to the gut while preserving homing of regulatory T cells that dampen the immune response, Dr. Maillard explained.
“This effect turned out to be at least in part through a posttranslational effect of DLL4/Notch blockade on integrin pairing at the T-cell surface,” he explained. “This was a novel and quite unexpected mechanism of action conserved from mice to nonhuman primates.”
The results are encouraging in terms of translating to humans because of their closer similarities in various physiological factors, Dr. Maillard said.
“The nonhuman primate model of transplantation [offers] a transplantation model very close to what is being performed in humans, as well as the opportunity to study an immune system very similar to that of humans in nonhuman primates,” he said.
Dr. Maillard noted that, while trials in humans are not underway yet, “we are in active discussions about it,” and the team is indeed interested in testing REGN421 itself, with the effects likely to be as a prophylactic strategy.
There are currently no approved anti-DLL4 antibody drugs for use in humans.
“Our approach is mostly promising as a preventive treatment, rather than as a secondary treatment for GVHD, because DLL4/Notch blockade seems most active when applied early after transplantation during the time of initial seeding of the gut by T cells (in mice, we had observed the critical time window for a successful intervention to be within 48 hours of transplantation),” Dr. Maillard said.“There remain questions about which other prophylactic treatments we should ideally combine anti-DLL4 antibodies with.”
Dr. Maillard has received research funding from Regeneron and Genentech and is a member of Garuda Therapeutics’s scientific advisory board.
Early, intriguing research suggests that preventing acute graft-versus-host disease (GVHD) in the gut – a potentially life-threatening complication of allogeneic hematopoietic cell transplantation (allo-HCT) – could be accomplished by the administration of a single antibody that targets the anti-DLL4 Notch signaling pathway, without compromising the stem cell transplant.
“The major surprise was that none of the anti–DLL4-treated animals developed acute gastrointestinal GVHD for the entire duration of the study. This was a remarkable finding, given that intestinal GVHD is otherwise seen in the vast majority of nonhuman primate transplant recipients that receive either no prophylaxis, or prophylaxis with agents other than anti-DLL4 antibodies,” co–senior author Ivan Maillard, MD, PhD, a professor of medicine and vice chief for research in hematology-oncology at the University of Pennsylvania, Philadelphia, said in an interview.
“The timing was critical,” the authors noted in the study, recently published in Science Translational Medicine. “Intervening before any symptoms of GvHD appear made the long-term protection possible.”
While GVHD may be mild to moderate in chronic forms, acute cases can be serious, if not fatal, and nearly all severe acute GVHD prominently involves the gastrointestinal tract, which can drive activation of pathogenic T cells and potentially lead to tissue damage following allo-HCT.
Systemic corticosteroids are standard first-line treatment for acute GVHD. However, response rates generally range only from 40% to 60%, and there are concerns of side effects. Meanwhile, second-line treatments are of inconsistent benefit.
With previous studies on mice showing benefits of targeting Notch pathway inhibition, particularly DLL4, Dr. Maillard and colleagues further investigated the effects in nonhuman primates that were allo-HCT recipients, using the anti-DLL4 antibody REGN421, which has pharmacokinetic and toxicity information available from previous studies.
The nonhuman primates were treated with one of two dosing regimens: a single dose of REGN421 3 mg/kg at baseline, post HCT, (n = 7) or three weekly doses at days 0, 7 and 14, post transplant (n = 4). Those primates were compared with 11 primates receiving allo-HCT transplants that received supportive care only.
Primates receiving three weekly doses of REGN421 showed antibody concentrations of greater than 2 mcg/mL for more than 30 days post HCT. A single dose of REGN421 was associated with protection from acute GVHD at day 0, while three weekly doses showed protection at day 0, 7, and 14, consistent with an impact of REGN421 during the early phases of T-cell activation.
Compared with animals receiving only supportive care, prophylaxis with REGN421 was associated with delayed acute GVHD onset and lengthened survival.
Of the 11 primates treated with REGN421, none developed clinical signs of gastrointestinal acute GVHD, whereas the majority of those receiving standard care or other preventive interventions did.
“Detailed analysis of acute GVHD clinical presentations in REGN421-treated animals in comparison to no treatment controls revealed near complete protection from GI-acute GvHD with REGN421,” the authors reported.
Furthermore, pathology scores in the gastrointestinal tract were lower with REGN421 treatment, compared with the no-treatment cohort, and the scores matched those of healthy nontransplanted nonhuman primates.
The primates treated with REGN421 did ultimately develop other clinical and pathologic signs of skin, hepatic or pulmonary acute GVHD, but without gastrointestinal disease.
The treatment was not associated with any adverse effects on the allo-HCT, with primates receiving either a single dose or three weekly doses of REGN421 showing rapid donor engraftment after allo-HCT, including high bone marrow, whole blood, and T-cell donor chimerism.
“Reassuringly, short-term systemic DLL4 blockade with REGN421 did not trigger unexpected side effects in our nonhuman primate model, while preserving rapid engraftment as well hematopoietic and immune reconstitution.”
The mechanism preserving the engraftment, described as a “major surprise,” specifically involved DLL4 inhibition blocking the homing of pathogenic T cells to the gut while preserving homing of regulatory T cells that dampen the immune response, Dr. Maillard explained.
“This effect turned out to be at least in part through a posttranslational effect of DLL4/Notch blockade on integrin pairing at the T-cell surface,” he explained. “This was a novel and quite unexpected mechanism of action conserved from mice to nonhuman primates.”
The results are encouraging in terms of translating to humans because of their closer similarities in various physiological factors, Dr. Maillard said.
“The nonhuman primate model of transplantation [offers] a transplantation model very close to what is being performed in humans, as well as the opportunity to study an immune system very similar to that of humans in nonhuman primates,” he said.
Dr. Maillard noted that, while trials in humans are not underway yet, “we are in active discussions about it,” and the team is indeed interested in testing REGN421 itself, with the effects likely to be as a prophylactic strategy.
There are currently no approved anti-DLL4 antibody drugs for use in humans.
“Our approach is mostly promising as a preventive treatment, rather than as a secondary treatment for GVHD, because DLL4/Notch blockade seems most active when applied early after transplantation during the time of initial seeding of the gut by T cells (in mice, we had observed the critical time window for a successful intervention to be within 48 hours of transplantation),” Dr. Maillard said.“There remain questions about which other prophylactic treatments we should ideally combine anti-DLL4 antibodies with.”
Dr. Maillard has received research funding from Regeneron and Genentech and is a member of Garuda Therapeutics’s scientific advisory board.
FROM SCIENCE TRANSLATIONAL MEDICINE
PPIs may curb benefits of palbociclib in breast cancer
TOPLINE:
and lead to worse progression-free survival (PFS) and overall survival, new data suggest.
METHODOLOGY:
- The study retrospectively identified 1,310 women with advanced breast cancer receiving palbociclib using South Korean nationwide claims data.
- Overall, 344 women in the concomitant group, those who were coadministered a PPI for more than one-third of their palbociclib treatment duration, were propensity-score matched to 966 women who did not have PPI exposure: the nonconcomitant group.
- Main outcomes were time to progression and death, presented as PFS and overall survival.
TAKEAWAY:
- Median clinical PFS was significantly shorter by about 15 months in the concomitant PPI group vs. the nonconcomitant group (25.3 vs. 39.8 months; adjusted hazard ratio, 1.76).
- Concomitant PPI use was also associated with shorter overall survival (HR, 2.71).
- Overall, 83.1% of patients in the concomitant group were alive at 1 year vs. 94.0% in the nonconcomitant group (P < .001), and 69.5% vs. 89.3%, respectively, were alive at 2 years (P < .001), though the median overall survival was not reached in either group.
- In a subgroup analysis, concomitant PPI use was associated with shorter clinical PFS (HR, 1.75 for those receiving endocrine-sensitive treatment and 1.82 for those receiving endocrine-resistant treatment), and shorter overall survival (HR, 2.68 in the endocrine-sensitive subgroup and 2.98 in the endocrine-resistant subgroup).
IN PRACTICE:
“The findings suggest that taking PPIs with palbociclib may interrupt the full therapeutic benefits of palbociclib,” the authors conclude. “Physicians should be cautious when prescribing PPIs to patients who are receiving palbociclib.”
SOURCE:
The study, led by Ju-Eun Lee, MS, PharmD, School of Pharmacy, Sungkyunkwan University, South Korea, was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by its retrospective design and use of claims data as well as the inability to confirm whether patients actually took the PPI medication.
DISCLOSURES:
The authors report no relevant financial relationships. The study reported no commercial funding.
A version of this article first appeared on Medscape.com.
TOPLINE:
and lead to worse progression-free survival (PFS) and overall survival, new data suggest.
METHODOLOGY:
- The study retrospectively identified 1,310 women with advanced breast cancer receiving palbociclib using South Korean nationwide claims data.
- Overall, 344 women in the concomitant group, those who were coadministered a PPI for more than one-third of their palbociclib treatment duration, were propensity-score matched to 966 women who did not have PPI exposure: the nonconcomitant group.
- Main outcomes were time to progression and death, presented as PFS and overall survival.
TAKEAWAY:
- Median clinical PFS was significantly shorter by about 15 months in the concomitant PPI group vs. the nonconcomitant group (25.3 vs. 39.8 months; adjusted hazard ratio, 1.76).
- Concomitant PPI use was also associated with shorter overall survival (HR, 2.71).
- Overall, 83.1% of patients in the concomitant group were alive at 1 year vs. 94.0% in the nonconcomitant group (P < .001), and 69.5% vs. 89.3%, respectively, were alive at 2 years (P < .001), though the median overall survival was not reached in either group.
- In a subgroup analysis, concomitant PPI use was associated with shorter clinical PFS (HR, 1.75 for those receiving endocrine-sensitive treatment and 1.82 for those receiving endocrine-resistant treatment), and shorter overall survival (HR, 2.68 in the endocrine-sensitive subgroup and 2.98 in the endocrine-resistant subgroup).
IN PRACTICE:
“The findings suggest that taking PPIs with palbociclib may interrupt the full therapeutic benefits of palbociclib,” the authors conclude. “Physicians should be cautious when prescribing PPIs to patients who are receiving palbociclib.”
SOURCE:
The study, led by Ju-Eun Lee, MS, PharmD, School of Pharmacy, Sungkyunkwan University, South Korea, was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by its retrospective design and use of claims data as well as the inability to confirm whether patients actually took the PPI medication.
DISCLOSURES:
The authors report no relevant financial relationships. The study reported no commercial funding.
A version of this article first appeared on Medscape.com.
TOPLINE:
and lead to worse progression-free survival (PFS) and overall survival, new data suggest.
METHODOLOGY:
- The study retrospectively identified 1,310 women with advanced breast cancer receiving palbociclib using South Korean nationwide claims data.
- Overall, 344 women in the concomitant group, those who were coadministered a PPI for more than one-third of their palbociclib treatment duration, were propensity-score matched to 966 women who did not have PPI exposure: the nonconcomitant group.
- Main outcomes were time to progression and death, presented as PFS and overall survival.
TAKEAWAY:
- Median clinical PFS was significantly shorter by about 15 months in the concomitant PPI group vs. the nonconcomitant group (25.3 vs. 39.8 months; adjusted hazard ratio, 1.76).
- Concomitant PPI use was also associated with shorter overall survival (HR, 2.71).
- Overall, 83.1% of patients in the concomitant group were alive at 1 year vs. 94.0% in the nonconcomitant group (P < .001), and 69.5% vs. 89.3%, respectively, were alive at 2 years (P < .001), though the median overall survival was not reached in either group.
- In a subgroup analysis, concomitant PPI use was associated with shorter clinical PFS (HR, 1.75 for those receiving endocrine-sensitive treatment and 1.82 for those receiving endocrine-resistant treatment), and shorter overall survival (HR, 2.68 in the endocrine-sensitive subgroup and 2.98 in the endocrine-resistant subgroup).
IN PRACTICE:
“The findings suggest that taking PPIs with palbociclib may interrupt the full therapeutic benefits of palbociclib,” the authors conclude. “Physicians should be cautious when prescribing PPIs to patients who are receiving palbociclib.”
SOURCE:
The study, led by Ju-Eun Lee, MS, PharmD, School of Pharmacy, Sungkyunkwan University, South Korea, was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by its retrospective design and use of claims data as well as the inability to confirm whether patients actually took the PPI medication.
DISCLOSURES:
The authors report no relevant financial relationships. The study reported no commercial funding.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Low-dose steroids may not increase cardiovascular risk in rheumatoid arthritis
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Social needs case management cuts acute care usage
Hospitalizations fell by 11% in patients assigned to integrated social needs case management, a randomized controlled study conducted in California found.
The reduction in acute care use was likely because of the 3% increase in primary care visits with this approach, according to lead study author Mark D. Fleming, PhD, MS, assistant professor of health and social behavior at the University of California, Berkeley. The study was published in Annals of Internal Medicine.
The findings provide evidence for the theory that social needs case management can decrease acute care use by facilitating access to primary care, Dr. Fleming said in an interview. “While an increasing number of studies have measured the effects of social needs case management on hospital use, the findings have been inconsistent, with some studies showing a decrease in hospital use and others showing no change.” There was no strong evidence of an effect on acute care.
A 2018 study, however, found that liaising with community care workers substantially reduced hospital days in disadvantaged patients.
Case management, a complex approach linking medical and social needs, can overcome barriers to care by facilitating access to transportation and helping patients navigate the health care system, the authors noted. It can also streamline patient access to insurance coverage and social benefits.
The study
The current data came from a secondary analysis of a randomized encouragement study in Costa County, Calif., during 2017 and 2018. That study allocated adult California Medicaid beneficiaries of diverse race and ethnicity, relatively high social needs, and high risk for acute care use to two arms: social needs case management (n = 21,422) or administrative observation (22,389 weighted). Chronic health issues ranged from arthritis, diabetes, and back conditions to heart or lung disease, and psychological disorders. About 50% in both groups were younger than age 40 and 60% were women.
Case managers assessed patient needs, created a patient-centered care plan, and facilitated community resource referrals, primary care visits, and applications for public benefits.
The professionally diverse managers included public health nurses, social workers, substance misuse counselors, and mental health clinicians, as well as homeless service specialists and community health workers. Case management was offered as in-person or remote telephonic services for 1 year.
While rates of primary care visits were significantly higher in the case management group – incidence rate 1.03 (95% confidence interval [CI],1.00-1.07) – no intergroup differences emerged in visits for specialty care, behavioral health, psychiatric emergency visits, or jail intakes.
Although the analysis could not measure a direct effect of primary care use on hospitalizations, the results suggested it would take 6.6 primary care visits to avert one hospitalization. As a limitation, the outcomes were studied for only 1 year, but further effects of case management on health and service use could take longer to appear.
Commenting on the analysis but not involved in it, Laura Gottlieb, MD, MPH, professor in the department of family and community medicine at the University of California, San Francisco, said a few studies have suggested several pathways through which case management might influence health and health care utilization – and not solely through access to social services.
“The current findings underscore that one of those pathways is likely via connection to health care services,” she said.
As to the cost effectiveness of social needs case management given the necessary increase in personnel costs, she added, that it is a matter of society’s priorities. “If we want to achieve equity, we need to invest dollars differently. That is not a hospital-level issue. It is a society-level issue. Hospitals need to be able to stay afloat, so health care policies need to enable them to make different decisions,” she added. Broadly implementing such an approach will obviously take investment, Dr. Gottlieb continued.
“California Medicaid is trying to enable this shift in investments, but it is hard to move existing structures.” She added that more data are needed on the interaction between social services, patient experiences of care, and self-efficacy to understand a wider array of mechanisms through which case management might affect outcomes.
This analysis was supported by the Agency for Healthcare Research and Quality and Contra Costa Health Services. The authors disclosed no relevant conflicts of interest.
Hospitalizations fell by 11% in patients assigned to integrated social needs case management, a randomized controlled study conducted in California found.
The reduction in acute care use was likely because of the 3% increase in primary care visits with this approach, according to lead study author Mark D. Fleming, PhD, MS, assistant professor of health and social behavior at the University of California, Berkeley. The study was published in Annals of Internal Medicine.
The findings provide evidence for the theory that social needs case management can decrease acute care use by facilitating access to primary care, Dr. Fleming said in an interview. “While an increasing number of studies have measured the effects of social needs case management on hospital use, the findings have been inconsistent, with some studies showing a decrease in hospital use and others showing no change.” There was no strong evidence of an effect on acute care.
A 2018 study, however, found that liaising with community care workers substantially reduced hospital days in disadvantaged patients.
Case management, a complex approach linking medical and social needs, can overcome barriers to care by facilitating access to transportation and helping patients navigate the health care system, the authors noted. It can also streamline patient access to insurance coverage and social benefits.
The study
The current data came from a secondary analysis of a randomized encouragement study in Costa County, Calif., during 2017 and 2018. That study allocated adult California Medicaid beneficiaries of diverse race and ethnicity, relatively high social needs, and high risk for acute care use to two arms: social needs case management (n = 21,422) or administrative observation (22,389 weighted). Chronic health issues ranged from arthritis, diabetes, and back conditions to heart or lung disease, and psychological disorders. About 50% in both groups were younger than age 40 and 60% were women.
Case managers assessed patient needs, created a patient-centered care plan, and facilitated community resource referrals, primary care visits, and applications for public benefits.
The professionally diverse managers included public health nurses, social workers, substance misuse counselors, and mental health clinicians, as well as homeless service specialists and community health workers. Case management was offered as in-person or remote telephonic services for 1 year.
While rates of primary care visits were significantly higher in the case management group – incidence rate 1.03 (95% confidence interval [CI],1.00-1.07) – no intergroup differences emerged in visits for specialty care, behavioral health, psychiatric emergency visits, or jail intakes.
Although the analysis could not measure a direct effect of primary care use on hospitalizations, the results suggested it would take 6.6 primary care visits to avert one hospitalization. As a limitation, the outcomes were studied for only 1 year, but further effects of case management on health and service use could take longer to appear.
Commenting on the analysis but not involved in it, Laura Gottlieb, MD, MPH, professor in the department of family and community medicine at the University of California, San Francisco, said a few studies have suggested several pathways through which case management might influence health and health care utilization – and not solely through access to social services.
“The current findings underscore that one of those pathways is likely via connection to health care services,” she said.
As to the cost effectiveness of social needs case management given the necessary increase in personnel costs, she added, that it is a matter of society’s priorities. “If we want to achieve equity, we need to invest dollars differently. That is not a hospital-level issue. It is a society-level issue. Hospitals need to be able to stay afloat, so health care policies need to enable them to make different decisions,” she added. Broadly implementing such an approach will obviously take investment, Dr. Gottlieb continued.
“California Medicaid is trying to enable this shift in investments, but it is hard to move existing structures.” She added that more data are needed on the interaction between social services, patient experiences of care, and self-efficacy to understand a wider array of mechanisms through which case management might affect outcomes.
This analysis was supported by the Agency for Healthcare Research and Quality and Contra Costa Health Services. The authors disclosed no relevant conflicts of interest.
Hospitalizations fell by 11% in patients assigned to integrated social needs case management, a randomized controlled study conducted in California found.
The reduction in acute care use was likely because of the 3% increase in primary care visits with this approach, according to lead study author Mark D. Fleming, PhD, MS, assistant professor of health and social behavior at the University of California, Berkeley. The study was published in Annals of Internal Medicine.
The findings provide evidence for the theory that social needs case management can decrease acute care use by facilitating access to primary care, Dr. Fleming said in an interview. “While an increasing number of studies have measured the effects of social needs case management on hospital use, the findings have been inconsistent, with some studies showing a decrease in hospital use and others showing no change.” There was no strong evidence of an effect on acute care.
A 2018 study, however, found that liaising with community care workers substantially reduced hospital days in disadvantaged patients.
Case management, a complex approach linking medical and social needs, can overcome barriers to care by facilitating access to transportation and helping patients navigate the health care system, the authors noted. It can also streamline patient access to insurance coverage and social benefits.
The study
The current data came from a secondary analysis of a randomized encouragement study in Costa County, Calif., during 2017 and 2018. That study allocated adult California Medicaid beneficiaries of diverse race and ethnicity, relatively high social needs, and high risk for acute care use to two arms: social needs case management (n = 21,422) or administrative observation (22,389 weighted). Chronic health issues ranged from arthritis, diabetes, and back conditions to heart or lung disease, and psychological disorders. About 50% in both groups were younger than age 40 and 60% were women.
Case managers assessed patient needs, created a patient-centered care plan, and facilitated community resource referrals, primary care visits, and applications for public benefits.
The professionally diverse managers included public health nurses, social workers, substance misuse counselors, and mental health clinicians, as well as homeless service specialists and community health workers. Case management was offered as in-person or remote telephonic services for 1 year.
While rates of primary care visits were significantly higher in the case management group – incidence rate 1.03 (95% confidence interval [CI],1.00-1.07) – no intergroup differences emerged in visits for specialty care, behavioral health, psychiatric emergency visits, or jail intakes.
Although the analysis could not measure a direct effect of primary care use on hospitalizations, the results suggested it would take 6.6 primary care visits to avert one hospitalization. As a limitation, the outcomes were studied for only 1 year, but further effects of case management on health and service use could take longer to appear.
Commenting on the analysis but not involved in it, Laura Gottlieb, MD, MPH, professor in the department of family and community medicine at the University of California, San Francisco, said a few studies have suggested several pathways through which case management might influence health and health care utilization – and not solely through access to social services.
“The current findings underscore that one of those pathways is likely via connection to health care services,” she said.
As to the cost effectiveness of social needs case management given the necessary increase in personnel costs, she added, that it is a matter of society’s priorities. “If we want to achieve equity, we need to invest dollars differently. That is not a hospital-level issue. It is a society-level issue. Hospitals need to be able to stay afloat, so health care policies need to enable them to make different decisions,” she added. Broadly implementing such an approach will obviously take investment, Dr. Gottlieb continued.
“California Medicaid is trying to enable this shift in investments, but it is hard to move existing structures.” She added that more data are needed on the interaction between social services, patient experiences of care, and self-efficacy to understand a wider array of mechanisms through which case management might affect outcomes.
This analysis was supported by the Agency for Healthcare Research and Quality and Contra Costa Health Services. The authors disclosed no relevant conflicts of interest.
FROM ANNALS of INTERNAL MEDICINE
Should we rename obesity?
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Dural-puncture epidural drives faster conversion to cesarean anesthesia
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Just 1 in 10 with overweight/obesity lose 5% of body weight
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Squamous Cell Carcinoma
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3
Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Lanolin: The 2023 American Contact Dermatitis Society Allergen of the Year
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Practice Points
- Lanolin is a common ingredient in personal care products (PCPs), cosmetics, topical medicaments, and industrial materials.
- Allergic contact dermatitis to lanolin appears to be most common in patients with stasis dermatitis, chronic leg ulcers, atopic dermatitis, and perianal/genital dermatitis.
- There is no single best lanolin patch test formulation. Patch testing and repeat open application testing to PCPs containing lanolin also may be of benefit.