User login
Prescribing lifestyle changes: When medicine isn’t enough
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
Family physicians get lowest net return for HPV vaccine
Family physicians receive less private insurer reimbursement for the human papillomavirus (HPV) vaccine than do pediatricians, according to a new analysis in Family Medicine.
HPV is the most expensive of all routine pediatric vaccines and the reimbursement by third-party payers varies widely. The concerns about HPV reimbursement often appear on clinician surveys.
This study, led by Yenan Zhu, PhD, who was with the department of public health sciences, college of medicine, Medical University of South Carolina, Charleston, at the time of the research, found that, on average, pediatricians received higher reimbursement ($216.07) for HPV vaccine cost when compared with family physicians ($211.33), internists ($212.97), nurse practitioners ($212.91), and “other” clinicians who administer the vaccine ($213.29) (P values for all comparisons were < .001).
The final sample for this study included 34,247 clinicians.
The net return from vaccine cost reimbursements was lowest for family physicians ($0.34 per HPV vaccine dose administered) and highest for pediatricians ($5.08 per HPV vaccine dose administered).
“Adequate cost reimbursement by third-party payers is a critical enabling factor for clinicians to continue offering vaccines,” the authors wrote.
The authors concluded that “reimbursement for HPV vaccine costs by private payers is adequate; however, return margins are small for nonpediatric specialties.”
CDC, AAP differ in recommendations
In the United States, private insurers use the Centers for Disease Control and Prevention vaccine list price as a benchmark.
Overall in this study, HPV vaccine cost reimbursement by private payers was at or above the CDC list price of $210.99 but below the American Academy of Pediatrics recommendations ($263.74).
The study found that every $1 increment in return was associated with an increase in HPV vaccine doses administered. That was highest for family physicians at 0.08% per dollar.
The modeling showed that changing the HPV vaccine reimbursement to the AAP-recommended level could translate to “an estimated 18,643 additional HPV vaccine doses administrated by pediatricians, 4,041 additional doses by family physicians, and 433 doses by ‘other’ specialties in 2017-2018.”
The authors noted that U.S. vaccination coverage has improved in recent years but initiation and completion rates are lower among privately insured adolescents (4.6% lower for initiation and 2.0% points lower for completion in 2021), compared with adolescents covered under public insurance.
Why the difference among specialties?
Variation in reimbursements might be tied to the ability to negotiate reimbursements for adolescent vaccines, the authors said.
“For instance, pediatricians may be able to negotiate higher cost reimbursement, compared with nonpediatric specialties, given that adolescents constitute a large fraction of their patient volume,” they wrote.
Dr. Zhu and colleagues wrote that it should be noted that HPV vaccine cost reimbursement to family practitioners was considerably less than other specialties and they are barely breaking even though they have the second-highest volume of HPV vaccinations (after pediatricians).
The authors acknowledged that it may not be possible to raise reimbursement to the AAP level, but added that “a reasonable increase that can cover direct and indirect expenses (acquisition cost, storage cost, personnel cost for monitoring inventory, insurance, waste, and lost opportunity costs) will reduce the financial strain on nonpediatric clinicians.” That may encourage clinicians to stock and offer the vaccine.
Limitations
The researchers acknowledged several limitations. The models did not account for factors such as vaccination bundling, physicians’ recommendation style or differences in knowledge of the vaccination schedule.
The models were also not able to adjust for whether a clinic had reminder prompts in the electronic health records, the overhead costs of vaccines, or vaccine knowledge or hesitancy on the part of the adolescents’ parents.
Additionally, they used data from one private payer, which limits generalizability.
Researchers identified a sample of adolescents eligible for the HPV vaccine (9-14 years old) enrolled in a large private health insurance plan during 2017-2018. Data from states with universal or universal select vaccine purchasing were excluded. These states included Alaska, Hawaii, Idaho, Maine, Massachusetts, South Dakota, New Hampshire, New Mexico, Rhode Island, Vermont, Washington, Wisconsin, and Wyoming.
One coauthor reported receiving a consulting fee from Merck on unrelated projects. Another coauthor has provided consultancy to Value Analytics Labs on unrelated projects. All other authors declared no competing interests.
Family physicians receive less private insurer reimbursement for the human papillomavirus (HPV) vaccine than do pediatricians, according to a new analysis in Family Medicine.
HPV is the most expensive of all routine pediatric vaccines and the reimbursement by third-party payers varies widely. The concerns about HPV reimbursement often appear on clinician surveys.
This study, led by Yenan Zhu, PhD, who was with the department of public health sciences, college of medicine, Medical University of South Carolina, Charleston, at the time of the research, found that, on average, pediatricians received higher reimbursement ($216.07) for HPV vaccine cost when compared with family physicians ($211.33), internists ($212.97), nurse practitioners ($212.91), and “other” clinicians who administer the vaccine ($213.29) (P values for all comparisons were < .001).
The final sample for this study included 34,247 clinicians.
The net return from vaccine cost reimbursements was lowest for family physicians ($0.34 per HPV vaccine dose administered) and highest for pediatricians ($5.08 per HPV vaccine dose administered).
“Adequate cost reimbursement by third-party payers is a critical enabling factor for clinicians to continue offering vaccines,” the authors wrote.
The authors concluded that “reimbursement for HPV vaccine costs by private payers is adequate; however, return margins are small for nonpediatric specialties.”
CDC, AAP differ in recommendations
In the United States, private insurers use the Centers for Disease Control and Prevention vaccine list price as a benchmark.
Overall in this study, HPV vaccine cost reimbursement by private payers was at or above the CDC list price of $210.99 but below the American Academy of Pediatrics recommendations ($263.74).
The study found that every $1 increment in return was associated with an increase in HPV vaccine doses administered. That was highest for family physicians at 0.08% per dollar.
The modeling showed that changing the HPV vaccine reimbursement to the AAP-recommended level could translate to “an estimated 18,643 additional HPV vaccine doses administrated by pediatricians, 4,041 additional doses by family physicians, and 433 doses by ‘other’ specialties in 2017-2018.”
The authors noted that U.S. vaccination coverage has improved in recent years but initiation and completion rates are lower among privately insured adolescents (4.6% lower for initiation and 2.0% points lower for completion in 2021), compared with adolescents covered under public insurance.
Why the difference among specialties?
Variation in reimbursements might be tied to the ability to negotiate reimbursements for adolescent vaccines, the authors said.
“For instance, pediatricians may be able to negotiate higher cost reimbursement, compared with nonpediatric specialties, given that adolescents constitute a large fraction of their patient volume,” they wrote.
Dr. Zhu and colleagues wrote that it should be noted that HPV vaccine cost reimbursement to family practitioners was considerably less than other specialties and they are barely breaking even though they have the second-highest volume of HPV vaccinations (after pediatricians).
The authors acknowledged that it may not be possible to raise reimbursement to the AAP level, but added that “a reasonable increase that can cover direct and indirect expenses (acquisition cost, storage cost, personnel cost for monitoring inventory, insurance, waste, and lost opportunity costs) will reduce the financial strain on nonpediatric clinicians.” That may encourage clinicians to stock and offer the vaccine.
Limitations
The researchers acknowledged several limitations. The models did not account for factors such as vaccination bundling, physicians’ recommendation style or differences in knowledge of the vaccination schedule.
The models were also not able to adjust for whether a clinic had reminder prompts in the electronic health records, the overhead costs of vaccines, or vaccine knowledge or hesitancy on the part of the adolescents’ parents.
Additionally, they used data from one private payer, which limits generalizability.
Researchers identified a sample of adolescents eligible for the HPV vaccine (9-14 years old) enrolled in a large private health insurance plan during 2017-2018. Data from states with universal or universal select vaccine purchasing were excluded. These states included Alaska, Hawaii, Idaho, Maine, Massachusetts, South Dakota, New Hampshire, New Mexico, Rhode Island, Vermont, Washington, Wisconsin, and Wyoming.
One coauthor reported receiving a consulting fee from Merck on unrelated projects. Another coauthor has provided consultancy to Value Analytics Labs on unrelated projects. All other authors declared no competing interests.
Family physicians receive less private insurer reimbursement for the human papillomavirus (HPV) vaccine than do pediatricians, according to a new analysis in Family Medicine.
HPV is the most expensive of all routine pediatric vaccines and the reimbursement by third-party payers varies widely. The concerns about HPV reimbursement often appear on clinician surveys.
This study, led by Yenan Zhu, PhD, who was with the department of public health sciences, college of medicine, Medical University of South Carolina, Charleston, at the time of the research, found that, on average, pediatricians received higher reimbursement ($216.07) for HPV vaccine cost when compared with family physicians ($211.33), internists ($212.97), nurse practitioners ($212.91), and “other” clinicians who administer the vaccine ($213.29) (P values for all comparisons were < .001).
The final sample for this study included 34,247 clinicians.
The net return from vaccine cost reimbursements was lowest for family physicians ($0.34 per HPV vaccine dose administered) and highest for pediatricians ($5.08 per HPV vaccine dose administered).
“Adequate cost reimbursement by third-party payers is a critical enabling factor for clinicians to continue offering vaccines,” the authors wrote.
The authors concluded that “reimbursement for HPV vaccine costs by private payers is adequate; however, return margins are small for nonpediatric specialties.”
CDC, AAP differ in recommendations
In the United States, private insurers use the Centers for Disease Control and Prevention vaccine list price as a benchmark.
Overall in this study, HPV vaccine cost reimbursement by private payers was at or above the CDC list price of $210.99 but below the American Academy of Pediatrics recommendations ($263.74).
The study found that every $1 increment in return was associated with an increase in HPV vaccine doses administered. That was highest for family physicians at 0.08% per dollar.
The modeling showed that changing the HPV vaccine reimbursement to the AAP-recommended level could translate to “an estimated 18,643 additional HPV vaccine doses administrated by pediatricians, 4,041 additional doses by family physicians, and 433 doses by ‘other’ specialties in 2017-2018.”
The authors noted that U.S. vaccination coverage has improved in recent years but initiation and completion rates are lower among privately insured adolescents (4.6% lower for initiation and 2.0% points lower for completion in 2021), compared with adolescents covered under public insurance.
Why the difference among specialties?
Variation in reimbursements might be tied to the ability to negotiate reimbursements for adolescent vaccines, the authors said.
“For instance, pediatricians may be able to negotiate higher cost reimbursement, compared with nonpediatric specialties, given that adolescents constitute a large fraction of their patient volume,” they wrote.
Dr. Zhu and colleagues wrote that it should be noted that HPV vaccine cost reimbursement to family practitioners was considerably less than other specialties and they are barely breaking even though they have the second-highest volume of HPV vaccinations (after pediatricians).
The authors acknowledged that it may not be possible to raise reimbursement to the AAP level, but added that “a reasonable increase that can cover direct and indirect expenses (acquisition cost, storage cost, personnel cost for monitoring inventory, insurance, waste, and lost opportunity costs) will reduce the financial strain on nonpediatric clinicians.” That may encourage clinicians to stock and offer the vaccine.
Limitations
The researchers acknowledged several limitations. The models did not account for factors such as vaccination bundling, physicians’ recommendation style or differences in knowledge of the vaccination schedule.
The models were also not able to adjust for whether a clinic had reminder prompts in the electronic health records, the overhead costs of vaccines, or vaccine knowledge or hesitancy on the part of the adolescents’ parents.
Additionally, they used data from one private payer, which limits generalizability.
Researchers identified a sample of adolescents eligible for the HPV vaccine (9-14 years old) enrolled in a large private health insurance plan during 2017-2018. Data from states with universal or universal select vaccine purchasing were excluded. These states included Alaska, Hawaii, Idaho, Maine, Massachusetts, South Dakota, New Hampshire, New Mexico, Rhode Island, Vermont, Washington, Wisconsin, and Wyoming.
One coauthor reported receiving a consulting fee from Merck on unrelated projects. Another coauthor has provided consultancy to Value Analytics Labs on unrelated projects. All other authors declared no competing interests.
FROM FAMILY MEDICINE
Rosacea look-alike
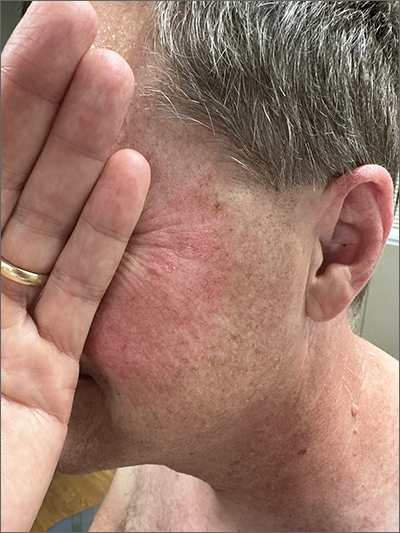
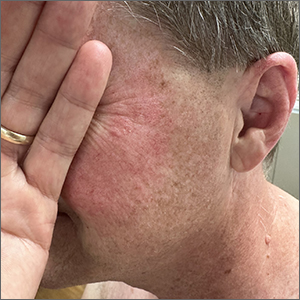
Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
Two-pronged approach needed in alcohol-associated hepatitis
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Skin reactions common at insulin pump infusion sites
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Advanced Breast Cancer Practice Essentials
Tools may predict inflammatory arthritis in at-risk patients
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Commentary: BTK inhibition in CLL and MCL, August 2023
The treatment landscape of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has been transformed over the past decade with the advent of targeted therapies, including Bruton tyrosine kinase (BTK) inhibitors. Covalent BTK inhibitors, which are available in both the frontline and relapsed/refractory settings, include ibrutinib, acalabrutinib, and zanubrutinib.1-3
BTK inhibitors have also demonstrated activity in higher-risk subgroups, including patients harboring TP53 aberrations. Clinical trials have shown encouraging outcomes of BTK inhibitors in these patients, and real-world studies have demonstrated similar findings.4-6 Recently, a real-world Italian registry study similarly showed favorable outcomes in 747 patients with CLL carrying 17p- or TP53 or both mutations treated with first-line ibrutinib. in what appears to be the largest real-world analysis of this patient population (Rigolin et al). At 24 months, the median overall survival was not reached; the estimated treatment persistence and survival rates were 63.4% (95% CI 60.0%-67.0%) and 82.6% (95% CI 79.9%-85.4%), respectively. The median time to treatment discontinuation was 37.4 months (95% CI 34.8-42.2 months). Disease progression or death was the reason for discontinuation in 45.8% of patients. Although ibrutinib is not generally the favored BTK inhibitor given an improved safety profile with next-generation options, these data provide real-world estimates for outcomes with BTK inhibitors in this large dataset of high-risk patients.
Although outcomes have improved for patients with CLL or SLL, it is common for resistance to targeted therapy to eventually occur. Noncovalent BTK inhibitors, such as pirtobrutinib, offer a promising approach for this population. The BRUIN trial was a phase 1/2 trial of pirtobrutinib in patients with relapsed/refractory CLL or SLL (Mato et al). A total of 317 patients were treated, including 247 who had previously received a BTK inhibitor. Patients had been treated with a median of three prior lines of therapy and over 40% had been treated with a BCL-2 inhibitor. The overall response rate was 73.3% (95% CI 67.3%-78.7%) and increased to 82.2% (95% CI 76.8%-86.7%) when partial response with lymphocytosis was included. At a 19.4-month median follow-up, the median progression-free survival was 19.6 (95% CI 16.9-22.1) months. The drug was also well-tolerated, with 2.8% of patients discontinuing therapy owing to treatment-related adverse events. Adverse events that can be seen with BTK inhibition, including hypertension, atrial fibrillation or flutter, and bleeding, were rare. This trial demonstrates that CLL/SLL cells can maintain dependency on the B-cell receptor pathway following treatment with a covalent inhibitor and that ongoing BTK inhibition using a novel mechanism is a feasible strategy. The optimal sequencing of pirtobrutinib with other available therapies, including BCL-2 inhibitors, remains unknown.
BTK inhibitors are also active in mantle cell lymphoma (MCL). They are approved for relapsed/refractory disease and are being studied in earlier lines of therapy. Whereas early progression of disease (POD) has been shown to be an important prognostic marker in MCL, the impact of early relapse on outcome specifically after BTK inhibitor initiation is less clear.7 A recent multicente retrospective observational study aimed to determine the impact on time-to-POD between rituximab-containing front-line therapy and second-line BTK inhibitor and overall outcomes (Villa et al). This study included 360 adult patients with relapsed or refractory MCL treated with second-line BTK inhibitor. Not surprisingly, the authors found that patients with POD within 24 months of first-line therapy had significantly shorter median progression-free survival (0.45 year vs 2.3 years; P < .001) and overall survival (0.9 year vs 5.5 years P < .001) compared with patients with relapse beyond 24 months. Furthermore, they found that Ki-67 ≥ 30% and Mantle Cell Lymphoma International Prognostic Index (MIPI) were also associated with progression‐free survival and overall survival from the start of a second-line BTK inhibitor, though to a lesser extent than time-to-POD. These variables were subsequently used to determine a second-line BTK MIPI, which can help inform which patients are most likely to benefit from a BTK inhibitor compared with other available options, such as chimeric antigen receptor (CAR) T-cell therapy or a clinical trial strategy.
BTK inhibitors are an important drug class for the treatment of lymphoid cancers and have changed the treatment paradigms in CLL/SLL and MCL. Additional studies evaluating combination strategies, sequencing approaches, time-limited options, and predictors of response are likely to further refine optimization of use in these diseases.
Additional References
1. Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440-3450. doi:10.1182/bloodadvances.2021006434
2. Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naive chronic lymphocytic leukemia. Leukemia. 2022;36:1171-1175. doi:10.1038/s41375-021-01485-x
3. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23:1031-1043. doi:10.1016/S1470-2045(22)00293-5
4. Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383:498-500. doi:10.1056/NEJMc2005943
5. Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196:947-953. doi:10.1111/bjh.17984
6. Mato AR, Tang B, Azmi S, et al. A clinical practice comparison of patients with chronic lymphocytic leukemia with and without deletion 17p receiving first-line treatment with ibrutinib. Haematologica. 2022;107:2630-2640. doi:10.3324/haematol.2021.280376
7. Bond DA, Switchenko JM, Villa D, et al. Early relapse identifies MCL patients with inferior survival after intensive or less intensive frontline therapy. Blood Adv. 2021;5:5179-5189. doi:10.1182/bloodadvances.2021004765
The treatment landscape of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has been transformed over the past decade with the advent of targeted therapies, including Bruton tyrosine kinase (BTK) inhibitors. Covalent BTK inhibitors, which are available in both the frontline and relapsed/refractory settings, include ibrutinib, acalabrutinib, and zanubrutinib.1-3
BTK inhibitors have also demonstrated activity in higher-risk subgroups, including patients harboring TP53 aberrations. Clinical trials have shown encouraging outcomes of BTK inhibitors in these patients, and real-world studies have demonstrated similar findings.4-6 Recently, a real-world Italian registry study similarly showed favorable outcomes in 747 patients with CLL carrying 17p- or TP53 or both mutations treated with first-line ibrutinib. in what appears to be the largest real-world analysis of this patient population (Rigolin et al). At 24 months, the median overall survival was not reached; the estimated treatment persistence and survival rates were 63.4% (95% CI 60.0%-67.0%) and 82.6% (95% CI 79.9%-85.4%), respectively. The median time to treatment discontinuation was 37.4 months (95% CI 34.8-42.2 months). Disease progression or death was the reason for discontinuation in 45.8% of patients. Although ibrutinib is not generally the favored BTK inhibitor given an improved safety profile with next-generation options, these data provide real-world estimates for outcomes with BTK inhibitors in this large dataset of high-risk patients.
Although outcomes have improved for patients with CLL or SLL, it is common for resistance to targeted therapy to eventually occur. Noncovalent BTK inhibitors, such as pirtobrutinib, offer a promising approach for this population. The BRUIN trial was a phase 1/2 trial of pirtobrutinib in patients with relapsed/refractory CLL or SLL (Mato et al). A total of 317 patients were treated, including 247 who had previously received a BTK inhibitor. Patients had been treated with a median of three prior lines of therapy and over 40% had been treated with a BCL-2 inhibitor. The overall response rate was 73.3% (95% CI 67.3%-78.7%) and increased to 82.2% (95% CI 76.8%-86.7%) when partial response with lymphocytosis was included. At a 19.4-month median follow-up, the median progression-free survival was 19.6 (95% CI 16.9-22.1) months. The drug was also well-tolerated, with 2.8% of patients discontinuing therapy owing to treatment-related adverse events. Adverse events that can be seen with BTK inhibition, including hypertension, atrial fibrillation or flutter, and bleeding, were rare. This trial demonstrates that CLL/SLL cells can maintain dependency on the B-cell receptor pathway following treatment with a covalent inhibitor and that ongoing BTK inhibition using a novel mechanism is a feasible strategy. The optimal sequencing of pirtobrutinib with other available therapies, including BCL-2 inhibitors, remains unknown.
BTK inhibitors are also active in mantle cell lymphoma (MCL). They are approved for relapsed/refractory disease and are being studied in earlier lines of therapy. Whereas early progression of disease (POD) has been shown to be an important prognostic marker in MCL, the impact of early relapse on outcome specifically after BTK inhibitor initiation is less clear.7 A recent multicente retrospective observational study aimed to determine the impact on time-to-POD between rituximab-containing front-line therapy and second-line BTK inhibitor and overall outcomes (Villa et al). This study included 360 adult patients with relapsed or refractory MCL treated with second-line BTK inhibitor. Not surprisingly, the authors found that patients with POD within 24 months of first-line therapy had significantly shorter median progression-free survival (0.45 year vs 2.3 years; P < .001) and overall survival (0.9 year vs 5.5 years P < .001) compared with patients with relapse beyond 24 months. Furthermore, they found that Ki-67 ≥ 30% and Mantle Cell Lymphoma International Prognostic Index (MIPI) were also associated with progression‐free survival and overall survival from the start of a second-line BTK inhibitor, though to a lesser extent than time-to-POD. These variables were subsequently used to determine a second-line BTK MIPI, which can help inform which patients are most likely to benefit from a BTK inhibitor compared with other available options, such as chimeric antigen receptor (CAR) T-cell therapy or a clinical trial strategy.
BTK inhibitors are an important drug class for the treatment of lymphoid cancers and have changed the treatment paradigms in CLL/SLL and MCL. Additional studies evaluating combination strategies, sequencing approaches, time-limited options, and predictors of response are likely to further refine optimization of use in these diseases.
Additional References
1. Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440-3450. doi:10.1182/bloodadvances.2021006434
2. Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naive chronic lymphocytic leukemia. Leukemia. 2022;36:1171-1175. doi:10.1038/s41375-021-01485-x
3. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23:1031-1043. doi:10.1016/S1470-2045(22)00293-5
4. Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383:498-500. doi:10.1056/NEJMc2005943
5. Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196:947-953. doi:10.1111/bjh.17984
6. Mato AR, Tang B, Azmi S, et al. A clinical practice comparison of patients with chronic lymphocytic leukemia with and without deletion 17p receiving first-line treatment with ibrutinib. Haematologica. 2022;107:2630-2640. doi:10.3324/haematol.2021.280376
7. Bond DA, Switchenko JM, Villa D, et al. Early relapse identifies MCL patients with inferior survival after intensive or less intensive frontline therapy. Blood Adv. 2021;5:5179-5189. doi:10.1182/bloodadvances.2021004765
The treatment landscape of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has been transformed over the past decade with the advent of targeted therapies, including Bruton tyrosine kinase (BTK) inhibitors. Covalent BTK inhibitors, which are available in both the frontline and relapsed/refractory settings, include ibrutinib, acalabrutinib, and zanubrutinib.1-3
BTK inhibitors have also demonstrated activity in higher-risk subgroups, including patients harboring TP53 aberrations. Clinical trials have shown encouraging outcomes of BTK inhibitors in these patients, and real-world studies have demonstrated similar findings.4-6 Recently, a real-world Italian registry study similarly showed favorable outcomes in 747 patients with CLL carrying 17p- or TP53 or both mutations treated with first-line ibrutinib. in what appears to be the largest real-world analysis of this patient population (Rigolin et al). At 24 months, the median overall survival was not reached; the estimated treatment persistence and survival rates were 63.4% (95% CI 60.0%-67.0%) and 82.6% (95% CI 79.9%-85.4%), respectively. The median time to treatment discontinuation was 37.4 months (95% CI 34.8-42.2 months). Disease progression or death was the reason for discontinuation in 45.8% of patients. Although ibrutinib is not generally the favored BTK inhibitor given an improved safety profile with next-generation options, these data provide real-world estimates for outcomes with BTK inhibitors in this large dataset of high-risk patients.
Although outcomes have improved for patients with CLL or SLL, it is common for resistance to targeted therapy to eventually occur. Noncovalent BTK inhibitors, such as pirtobrutinib, offer a promising approach for this population. The BRUIN trial was a phase 1/2 trial of pirtobrutinib in patients with relapsed/refractory CLL or SLL (Mato et al). A total of 317 patients were treated, including 247 who had previously received a BTK inhibitor. Patients had been treated with a median of three prior lines of therapy and over 40% had been treated with a BCL-2 inhibitor. The overall response rate was 73.3% (95% CI 67.3%-78.7%) and increased to 82.2% (95% CI 76.8%-86.7%) when partial response with lymphocytosis was included. At a 19.4-month median follow-up, the median progression-free survival was 19.6 (95% CI 16.9-22.1) months. The drug was also well-tolerated, with 2.8% of patients discontinuing therapy owing to treatment-related adverse events. Adverse events that can be seen with BTK inhibition, including hypertension, atrial fibrillation or flutter, and bleeding, were rare. This trial demonstrates that CLL/SLL cells can maintain dependency on the B-cell receptor pathway following treatment with a covalent inhibitor and that ongoing BTK inhibition using a novel mechanism is a feasible strategy. The optimal sequencing of pirtobrutinib with other available therapies, including BCL-2 inhibitors, remains unknown.
BTK inhibitors are also active in mantle cell lymphoma (MCL). They are approved for relapsed/refractory disease and are being studied in earlier lines of therapy. Whereas early progression of disease (POD) has been shown to be an important prognostic marker in MCL, the impact of early relapse on outcome specifically after BTK inhibitor initiation is less clear.7 A recent multicente retrospective observational study aimed to determine the impact on time-to-POD between rituximab-containing front-line therapy and second-line BTK inhibitor and overall outcomes (Villa et al). This study included 360 adult patients with relapsed or refractory MCL treated with second-line BTK inhibitor. Not surprisingly, the authors found that patients with POD within 24 months of first-line therapy had significantly shorter median progression-free survival (0.45 year vs 2.3 years; P < .001) and overall survival (0.9 year vs 5.5 years P < .001) compared with patients with relapse beyond 24 months. Furthermore, they found that Ki-67 ≥ 30% and Mantle Cell Lymphoma International Prognostic Index (MIPI) were also associated with progression‐free survival and overall survival from the start of a second-line BTK inhibitor, though to a lesser extent than time-to-POD. These variables were subsequently used to determine a second-line BTK MIPI, which can help inform which patients are most likely to benefit from a BTK inhibitor compared with other available options, such as chimeric antigen receptor (CAR) T-cell therapy or a clinical trial strategy.
BTK inhibitors are an important drug class for the treatment of lymphoid cancers and have changed the treatment paradigms in CLL/SLL and MCL. Additional studies evaluating combination strategies, sequencing approaches, time-limited options, and predictors of response are likely to further refine optimization of use in these diseases.
Additional References
1. Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440-3450. doi:10.1182/bloodadvances.2021006434
2. Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naive chronic lymphocytic leukemia. Leukemia. 2022;36:1171-1175. doi:10.1038/s41375-021-01485-x
3. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23:1031-1043. doi:10.1016/S1470-2045(22)00293-5
4. Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383:498-500. doi:10.1056/NEJMc2005943
5. Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196:947-953. doi:10.1111/bjh.17984
6. Mato AR, Tang B, Azmi S, et al. A clinical practice comparison of patients with chronic lymphocytic leukemia with and without deletion 17p receiving first-line treatment with ibrutinib. Haematologica. 2022;107:2630-2640. doi:10.3324/haematol.2021.280376
7. Bond DA, Switchenko JM, Villa D, et al. Early relapse identifies MCL patients with inferior survival after intensive or less intensive frontline therapy. Blood Adv. 2021;5:5179-5189. doi:10.1182/bloodadvances.2021004765
Johns Hopkins retains title as best hospital for rheumatology
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
Roflumilast cream appears safe, effective for children with psoriasis, researchers report
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
FROM SPD 2023