User login
OxyContin marketing push still exacting a deadly toll, study says
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
FROM HEALTH AFFAIRS
Cigna accused of using AI, not doctors, to deny claims: Lawsuit
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
Black women weigh emerging risks of ‘creamy crack’ hair straighteners
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
FDA approves first pill for postpartum depression
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
Rising viral load on dolutegravir? Investigators try fix before switch
Brisbane, Australia – , according to new data from the ADVANCE trial.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” said Andrew Hill, MD, from the department of pharmacology and therapeutics at the University of Liverpool, England.
The new data was presented here at the International AIDS Society conference on HIV Science. The ADVANCE trial is a three-arm randomized study involving 1,053 treatment-naive individuals comparing two triple-therapy combinations — dolutegravir, emtricitabine, and one of two tenofovir prodrugs – with a standard care regimen of tenofovir disoproxil fumarate, emtricitabine, and efavirenz.
Although the usual approach for someone in a clinical trial who experiences elevations in HIV RNA levels while on a dolutegravir-based treatment is to switch them to another therapy, the ADVANCE investigators opted for a different strategy.
“We actually continued treatment despite high viral load, and we didn’t have standard discontinuation preferences,” Dr. Hill said at the meeting. They instead provided counseling about adherence, which gave an opportunity to examine viral resuppression rates in participants in both the dolutegravir and efavirenz arms.
This revealed that 95% of patients in the two dolutegravir arms of the study were able to achieve resuppression of their viral load – defined as below 50 viral RNA copies per mL – without any emergence of resistance.
The guidelines
Current World Health Organization guidelines recommend that anyone whose viral load goes above 1,000 copies per mL who is on a non-nucleoside reverse transcriptase inhibitor such as efavirenz should be switched to an appropriate regimen.
Those who experience viremia on an integrase inhibitor such as dolutegravir should receive adherence counseling, have a repeat viral load test done in 3 months, and – if their viral load is still elevated – be switched to another regimen.
Dr. Hill and his team were examining how this might play out in a clinical trial setting and they found that there were a similar number of episodes of initial virologic failure in both the dolutegravir and efavirenz groups.
But after adherence counseling, testing for resistance and – if no resistance was evident – continuation with treatment, they saw differences emerge between the two groups.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” he said.
Time to viral resuppression
At 24 weeks after restarting treatment, 88% of people in the dolutegravir group had resuppressed their viral RNA, compared with 46% of people in the efavirenz group. At 48 weeks, those figures were 95% and 66%, respectively.
Dr. Hill pointed out that a significant number of people were lost to follow-up after virologic failure, and genotyping was not performed at baseline.
We addressed the question of how much adherence counseling should be undertaken in people who experienced viremia while on dolutegravir therapy, Dr. Hill said, particularly as there were often very good reasons for lack of adherence, such as homelessness.
“If you can get through those difficult phases, people can go back on their meds,” he said in an interview. “It’s almost a sociological problem rather than a clinical issue.”
And with efavirenz and the lower rates of resuppression observed in the study, Dr. Hill said it was a more fragile drug, so viremia therefore provided the opportunity for resistance to emerge, “and then once the resistant virus is there, you can’t get virus undetectable.”
Laura Waters, MD, a genitourinary and HIV medicine consultant at the Mortimer Market Centre in London, who was not involved in the study, said the results support recommendations to give people on drugs such as dolutegravir, which have a high genetic barrier to resistance, more time to improve their adherence before switching to another therapy.
“Although it provides that reassuring proof of concept, it doesn’t negate the importance of having to continue to monitor, because nothing is infallible,” she told this news organization. “We’ve talked about high-barrier drugs in the past, and you do start seeing resistance emerge.”
A version of this article first appeared on Medscape.com.
Brisbane, Australia – , according to new data from the ADVANCE trial.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” said Andrew Hill, MD, from the department of pharmacology and therapeutics at the University of Liverpool, England.
The new data was presented here at the International AIDS Society conference on HIV Science. The ADVANCE trial is a three-arm randomized study involving 1,053 treatment-naive individuals comparing two triple-therapy combinations — dolutegravir, emtricitabine, and one of two tenofovir prodrugs – with a standard care regimen of tenofovir disoproxil fumarate, emtricitabine, and efavirenz.
Although the usual approach for someone in a clinical trial who experiences elevations in HIV RNA levels while on a dolutegravir-based treatment is to switch them to another therapy, the ADVANCE investigators opted for a different strategy.
“We actually continued treatment despite high viral load, and we didn’t have standard discontinuation preferences,” Dr. Hill said at the meeting. They instead provided counseling about adherence, which gave an opportunity to examine viral resuppression rates in participants in both the dolutegravir and efavirenz arms.
This revealed that 95% of patients in the two dolutegravir arms of the study were able to achieve resuppression of their viral load – defined as below 50 viral RNA copies per mL – without any emergence of resistance.
The guidelines
Current World Health Organization guidelines recommend that anyone whose viral load goes above 1,000 copies per mL who is on a non-nucleoside reverse transcriptase inhibitor such as efavirenz should be switched to an appropriate regimen.
Those who experience viremia on an integrase inhibitor such as dolutegravir should receive adherence counseling, have a repeat viral load test done in 3 months, and – if their viral load is still elevated – be switched to another regimen.
Dr. Hill and his team were examining how this might play out in a clinical trial setting and they found that there were a similar number of episodes of initial virologic failure in both the dolutegravir and efavirenz groups.
But after adherence counseling, testing for resistance and – if no resistance was evident – continuation with treatment, they saw differences emerge between the two groups.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” he said.
Time to viral resuppression
At 24 weeks after restarting treatment, 88% of people in the dolutegravir group had resuppressed their viral RNA, compared with 46% of people in the efavirenz group. At 48 weeks, those figures were 95% and 66%, respectively.
Dr. Hill pointed out that a significant number of people were lost to follow-up after virologic failure, and genotyping was not performed at baseline.
We addressed the question of how much adherence counseling should be undertaken in people who experienced viremia while on dolutegravir therapy, Dr. Hill said, particularly as there were often very good reasons for lack of adherence, such as homelessness.
“If you can get through those difficult phases, people can go back on their meds,” he said in an interview. “It’s almost a sociological problem rather than a clinical issue.”
And with efavirenz and the lower rates of resuppression observed in the study, Dr. Hill said it was a more fragile drug, so viremia therefore provided the opportunity for resistance to emerge, “and then once the resistant virus is there, you can’t get virus undetectable.”
Laura Waters, MD, a genitourinary and HIV medicine consultant at the Mortimer Market Centre in London, who was not involved in the study, said the results support recommendations to give people on drugs such as dolutegravir, which have a high genetic barrier to resistance, more time to improve their adherence before switching to another therapy.
“Although it provides that reassuring proof of concept, it doesn’t negate the importance of having to continue to monitor, because nothing is infallible,” she told this news organization. “We’ve talked about high-barrier drugs in the past, and you do start seeing resistance emerge.”
A version of this article first appeared on Medscape.com.
Brisbane, Australia – , according to new data from the ADVANCE trial.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” said Andrew Hill, MD, from the department of pharmacology and therapeutics at the University of Liverpool, England.
The new data was presented here at the International AIDS Society conference on HIV Science. The ADVANCE trial is a three-arm randomized study involving 1,053 treatment-naive individuals comparing two triple-therapy combinations — dolutegravir, emtricitabine, and one of two tenofovir prodrugs – with a standard care regimen of tenofovir disoproxil fumarate, emtricitabine, and efavirenz.
Although the usual approach for someone in a clinical trial who experiences elevations in HIV RNA levels while on a dolutegravir-based treatment is to switch them to another therapy, the ADVANCE investigators opted for a different strategy.
“We actually continued treatment despite high viral load, and we didn’t have standard discontinuation preferences,” Dr. Hill said at the meeting. They instead provided counseling about adherence, which gave an opportunity to examine viral resuppression rates in participants in both the dolutegravir and efavirenz arms.
This revealed that 95% of patients in the two dolutegravir arms of the study were able to achieve resuppression of their viral load – defined as below 50 viral RNA copies per mL – without any emergence of resistance.
The guidelines
Current World Health Organization guidelines recommend that anyone whose viral load goes above 1,000 copies per mL who is on a non-nucleoside reverse transcriptase inhibitor such as efavirenz should be switched to an appropriate regimen.
Those who experience viremia on an integrase inhibitor such as dolutegravir should receive adherence counseling, have a repeat viral load test done in 3 months, and – if their viral load is still elevated – be switched to another regimen.
Dr. Hill and his team were examining how this might play out in a clinical trial setting and they found that there were a similar number of episodes of initial virologic failure in both the dolutegravir and efavirenz groups.
But after adherence counseling, testing for resistance and – if no resistance was evident – continuation with treatment, they saw differences emerge between the two groups.
“What we saw was a faster time to resuppression in the people followed-up long term on dolutegravir and also a higher percentage of people becoming suppressed when they remained on dolutegravir,” he said.
Time to viral resuppression
At 24 weeks after restarting treatment, 88% of people in the dolutegravir group had resuppressed their viral RNA, compared with 46% of people in the efavirenz group. At 48 weeks, those figures were 95% and 66%, respectively.
Dr. Hill pointed out that a significant number of people were lost to follow-up after virologic failure, and genotyping was not performed at baseline.
We addressed the question of how much adherence counseling should be undertaken in people who experienced viremia while on dolutegravir therapy, Dr. Hill said, particularly as there were often very good reasons for lack of adherence, such as homelessness.
“If you can get through those difficult phases, people can go back on their meds,” he said in an interview. “It’s almost a sociological problem rather than a clinical issue.”
And with efavirenz and the lower rates of resuppression observed in the study, Dr. Hill said it was a more fragile drug, so viremia therefore provided the opportunity for resistance to emerge, “and then once the resistant virus is there, you can’t get virus undetectable.”
Laura Waters, MD, a genitourinary and HIV medicine consultant at the Mortimer Market Centre in London, who was not involved in the study, said the results support recommendations to give people on drugs such as dolutegravir, which have a high genetic barrier to resistance, more time to improve their adherence before switching to another therapy.
“Although it provides that reassuring proof of concept, it doesn’t negate the importance of having to continue to monitor, because nothing is infallible,” she told this news organization. “We’ve talked about high-barrier drugs in the past, and you do start seeing resistance emerge.”
A version of this article first appeared on Medscape.com.
AT IAS 2023
Many users of skin-lightening product unaware of risks
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Trends in prepregnancy diabetes rates in the United States, 2016 -2021
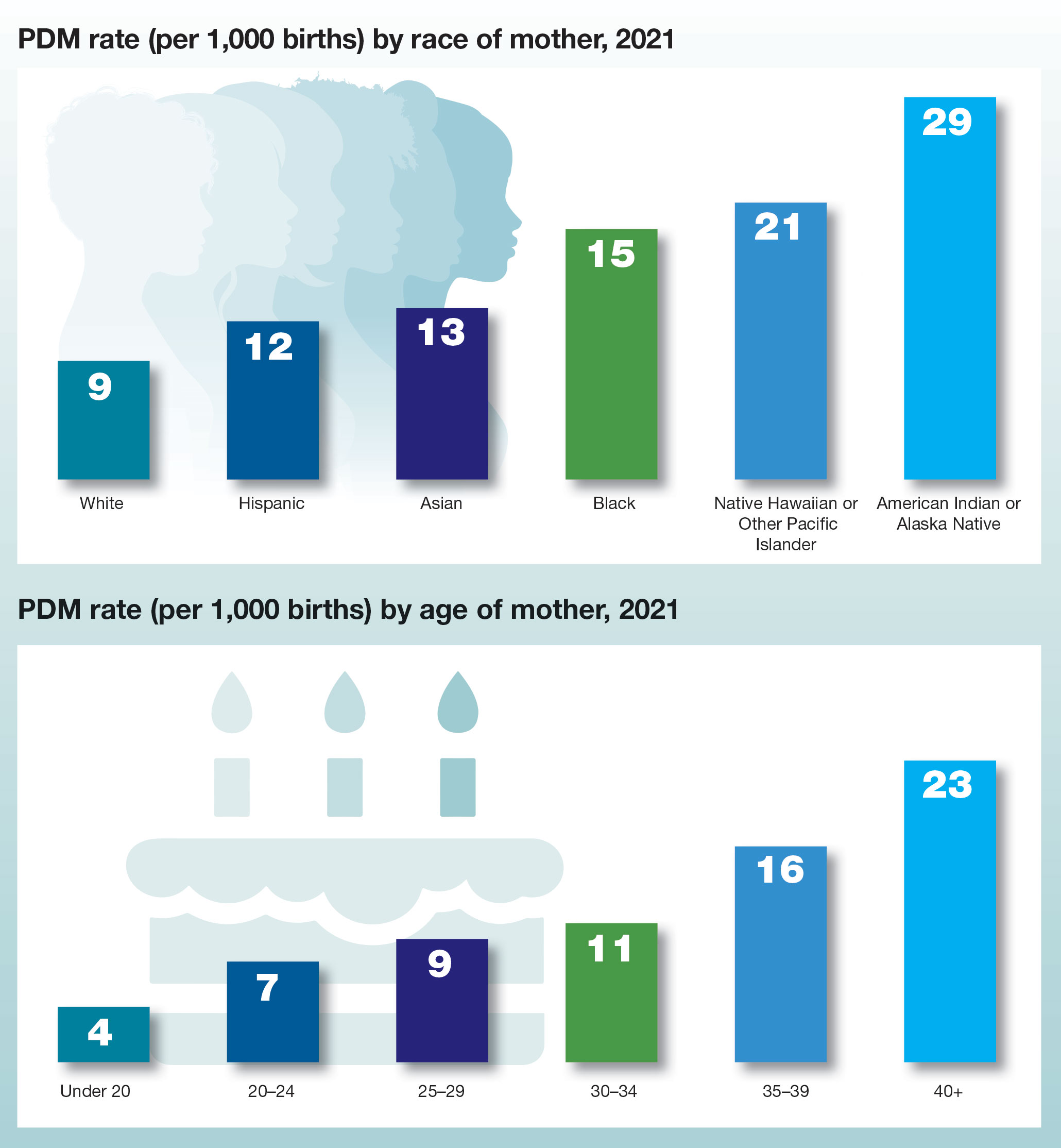


Managing clinician burnout: Challenges and opportunities
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.
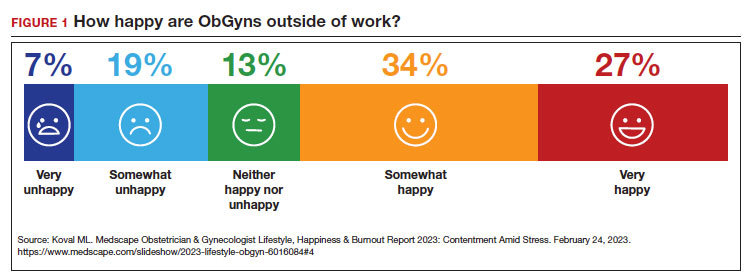
How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.
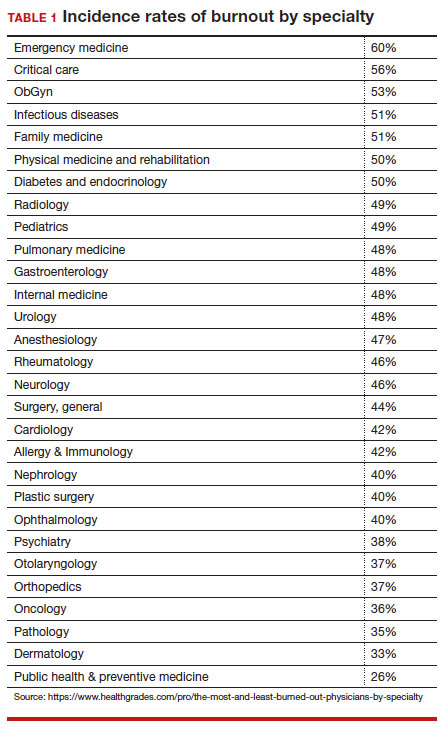
Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).
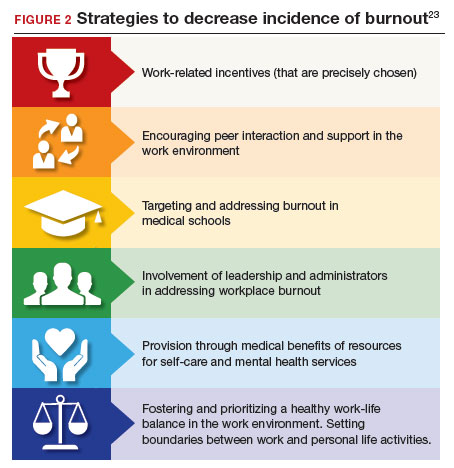
Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.
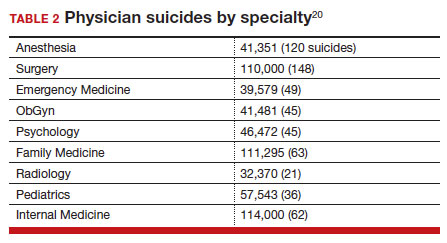
Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.

How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.

Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).

Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.

Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.

How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.

Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).

Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.

Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
Recurrent pregnancy loss and inherited thrombophilias: Does low molecular weight heparin improve the live birth rate?
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
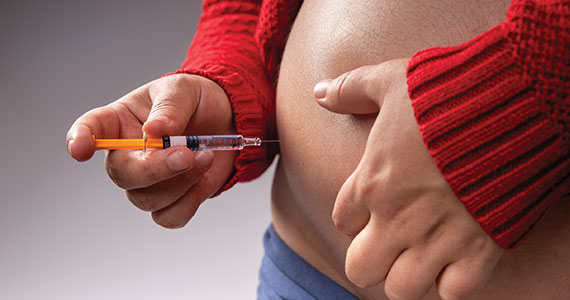 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
Results from halted islatravir antiretroviral trial presented
Brisbane, Australia – , according to investigators.
The nucleoside reverse transcriptase translocation inhibitor hit a roadblock in December 2021 when the U.S. Food and Drug Administration put a hold on investigational new drug applications for both the oral and implant formulations of islatravir after some patients in clinical trials showed decreases in total lymphocyte and CD4+ cell counts.
A phase 3, double-blind, randomized, controlled trial was underway at the time of once-daily islatravir (0.75 mg) in combination with 100 mg doravirine, compared with bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) as initial therapy for HIV infection in treatment-naïve individuals. Recruitment was stopped, 83 participants short of the planned 680, but the trial could continue the full 48 weeks.
Jürgen Rockstroh, MD, professor of medicine and head of the HIV Outpatient Clinic at the University of Bonn, Germany, presented the latest results from that trial of 597 patients at the International AIDS Society conference on HIV Science.
At week 48 after starting therapy, 88.9% of participants in the islatravir and doravirine arm and 88.3% of patients in the B/F/TAF arm achieved the primary outcome of HIV-1 RNA levels below 50 copies/mL.
One patient treated with islatravir and doravirine and four patients taking B/F/TAF experienced virologic failure.
Dr. Rockstroh told the conference the patient who developed viremia with the new islatravir combination had very high viral load at baseline but showed a rapid response to treatment, which reduced his viral load down to around 1,200 copies/mL by week 4.
However, by week 24, his islatravir concentration had reduced below detectable levels, suggesting a problem with adherence. His viral load increased again, and three treatment-resistant mutations were detected.
While both arms of the study showed a significant increase in CD4+ T cell counts, Dr. Rockstroh pointed out that some individuals in the islatravir and doravirine arm had a lower absolute increase in lymphocyte counts at week 48.
Overall, the mean change in CD4+ T cell counts was 182 cells/mL in the islatravir and doravirine group, compared with 234 cells/mL in the B/F/TAF group.
More patients in the islatravir combination arm discontinued treatment due to a decrease in CD4+ T cell or total lymphocyte counts – 5.4%, compared with 2% in the B/F/TAF arm, however.
“These changes in lymphocyte counts did not lead to any difference in the amount of infection-related adverse events, which happened in both arms in relatively comparable percentages,” Dr. Rockstroh said at the conference.
New lower dose
Because of persistent concerns about the impact on CD4+ T cells and total lymphocytes, Dr. Rockstroh said another phase 3 trial is now underway using a lower 0.25 mg dose of islatravir, again combined with 100 mg doravirine, in people who are treatment-naïve and virologically suppressed.
The study also examined the impact of both treatments on weight gain and found the mean change in weight was similar between both arms – 3.45 kg gain in those on islatravir with doravirine and 3.32 kg gain in those on B/F/TAF, which was not significantly different.
There are several important reasons it is a good idea to have more treatment options available for people with HIV, Dr. Rockstroh said in an interview.
With integrase inhibitors potentially interfering with metabolic health, weight gain, hypertension, insulin resistance, and possible diabetes, “I think it’s wise that we at least have alternative strategies,” he said.
James McMahon, MD, PhD, an infectious diseases physician and head of the Infectious Diseases Clinical Research Unit at the Alfred Hospital and Monash University in Melbourne, said there is always a need for new HIV treatments, particularly ones that are more powerful.
“Whenever you get a drug that’s potent at low dose, it means you can have smaller pills, [and] you can then consider giving more of it in long-acting formulations,” Dr. McMahon said.
He pointed out that the study did not show any signal of increased infections with the lower CD4+ T cell counts in the islatravir and doravirine arm, “but that difference is enough to raise that concern that it’s not ideal, and it should be moved forward with a lower dose, which is what they’ve done.”
A version of this article first appeared on Medscape.com.
Brisbane, Australia – , according to investigators.
The nucleoside reverse transcriptase translocation inhibitor hit a roadblock in December 2021 when the U.S. Food and Drug Administration put a hold on investigational new drug applications for both the oral and implant formulations of islatravir after some patients in clinical trials showed decreases in total lymphocyte and CD4+ cell counts.
A phase 3, double-blind, randomized, controlled trial was underway at the time of once-daily islatravir (0.75 mg) in combination with 100 mg doravirine, compared with bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) as initial therapy for HIV infection in treatment-naïve individuals. Recruitment was stopped, 83 participants short of the planned 680, but the trial could continue the full 48 weeks.
Jürgen Rockstroh, MD, professor of medicine and head of the HIV Outpatient Clinic at the University of Bonn, Germany, presented the latest results from that trial of 597 patients at the International AIDS Society conference on HIV Science.
At week 48 after starting therapy, 88.9% of participants in the islatravir and doravirine arm and 88.3% of patients in the B/F/TAF arm achieved the primary outcome of HIV-1 RNA levels below 50 copies/mL.
One patient treated with islatravir and doravirine and four patients taking B/F/TAF experienced virologic failure.
Dr. Rockstroh told the conference the patient who developed viremia with the new islatravir combination had very high viral load at baseline but showed a rapid response to treatment, which reduced his viral load down to around 1,200 copies/mL by week 4.
However, by week 24, his islatravir concentration had reduced below detectable levels, suggesting a problem with adherence. His viral load increased again, and three treatment-resistant mutations were detected.
While both arms of the study showed a significant increase in CD4+ T cell counts, Dr. Rockstroh pointed out that some individuals in the islatravir and doravirine arm had a lower absolute increase in lymphocyte counts at week 48.
Overall, the mean change in CD4+ T cell counts was 182 cells/mL in the islatravir and doravirine group, compared with 234 cells/mL in the B/F/TAF group.
More patients in the islatravir combination arm discontinued treatment due to a decrease in CD4+ T cell or total lymphocyte counts – 5.4%, compared with 2% in the B/F/TAF arm, however.
“These changes in lymphocyte counts did not lead to any difference in the amount of infection-related adverse events, which happened in both arms in relatively comparable percentages,” Dr. Rockstroh said at the conference.
New lower dose
Because of persistent concerns about the impact on CD4+ T cells and total lymphocytes, Dr. Rockstroh said another phase 3 trial is now underway using a lower 0.25 mg dose of islatravir, again combined with 100 mg doravirine, in people who are treatment-naïve and virologically suppressed.
The study also examined the impact of both treatments on weight gain and found the mean change in weight was similar between both arms – 3.45 kg gain in those on islatravir with doravirine and 3.32 kg gain in those on B/F/TAF, which was not significantly different.
There are several important reasons it is a good idea to have more treatment options available for people with HIV, Dr. Rockstroh said in an interview.
With integrase inhibitors potentially interfering with metabolic health, weight gain, hypertension, insulin resistance, and possible diabetes, “I think it’s wise that we at least have alternative strategies,” he said.
James McMahon, MD, PhD, an infectious diseases physician and head of the Infectious Diseases Clinical Research Unit at the Alfred Hospital and Monash University in Melbourne, said there is always a need for new HIV treatments, particularly ones that are more powerful.
“Whenever you get a drug that’s potent at low dose, it means you can have smaller pills, [and] you can then consider giving more of it in long-acting formulations,” Dr. McMahon said.
He pointed out that the study did not show any signal of increased infections with the lower CD4+ T cell counts in the islatravir and doravirine arm, “but that difference is enough to raise that concern that it’s not ideal, and it should be moved forward with a lower dose, which is what they’ve done.”
A version of this article first appeared on Medscape.com.
Brisbane, Australia – , according to investigators.
The nucleoside reverse transcriptase translocation inhibitor hit a roadblock in December 2021 when the U.S. Food and Drug Administration put a hold on investigational new drug applications for both the oral and implant formulations of islatravir after some patients in clinical trials showed decreases in total lymphocyte and CD4+ cell counts.
A phase 3, double-blind, randomized, controlled trial was underway at the time of once-daily islatravir (0.75 mg) in combination with 100 mg doravirine, compared with bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) as initial therapy for HIV infection in treatment-naïve individuals. Recruitment was stopped, 83 participants short of the planned 680, but the trial could continue the full 48 weeks.
Jürgen Rockstroh, MD, professor of medicine and head of the HIV Outpatient Clinic at the University of Bonn, Germany, presented the latest results from that trial of 597 patients at the International AIDS Society conference on HIV Science.
At week 48 after starting therapy, 88.9% of participants in the islatravir and doravirine arm and 88.3% of patients in the B/F/TAF arm achieved the primary outcome of HIV-1 RNA levels below 50 copies/mL.
One patient treated with islatravir and doravirine and four patients taking B/F/TAF experienced virologic failure.
Dr. Rockstroh told the conference the patient who developed viremia with the new islatravir combination had very high viral load at baseline but showed a rapid response to treatment, which reduced his viral load down to around 1,200 copies/mL by week 4.
However, by week 24, his islatravir concentration had reduced below detectable levels, suggesting a problem with adherence. His viral load increased again, and three treatment-resistant mutations were detected.
While both arms of the study showed a significant increase in CD4+ T cell counts, Dr. Rockstroh pointed out that some individuals in the islatravir and doravirine arm had a lower absolute increase in lymphocyte counts at week 48.
Overall, the mean change in CD4+ T cell counts was 182 cells/mL in the islatravir and doravirine group, compared with 234 cells/mL in the B/F/TAF group.
More patients in the islatravir combination arm discontinued treatment due to a decrease in CD4+ T cell or total lymphocyte counts – 5.4%, compared with 2% in the B/F/TAF arm, however.
“These changes in lymphocyte counts did not lead to any difference in the amount of infection-related adverse events, which happened in both arms in relatively comparable percentages,” Dr. Rockstroh said at the conference.
New lower dose
Because of persistent concerns about the impact on CD4+ T cells and total lymphocytes, Dr. Rockstroh said another phase 3 trial is now underway using a lower 0.25 mg dose of islatravir, again combined with 100 mg doravirine, in people who are treatment-naïve and virologically suppressed.
The study also examined the impact of both treatments on weight gain and found the mean change in weight was similar between both arms – 3.45 kg gain in those on islatravir with doravirine and 3.32 kg gain in those on B/F/TAF, which was not significantly different.
There are several important reasons it is a good idea to have more treatment options available for people with HIV, Dr. Rockstroh said in an interview.
With integrase inhibitors potentially interfering with metabolic health, weight gain, hypertension, insulin resistance, and possible diabetes, “I think it’s wise that we at least have alternative strategies,” he said.
James McMahon, MD, PhD, an infectious diseases physician and head of the Infectious Diseases Clinical Research Unit at the Alfred Hospital and Monash University in Melbourne, said there is always a need for new HIV treatments, particularly ones that are more powerful.
“Whenever you get a drug that’s potent at low dose, it means you can have smaller pills, [and] you can then consider giving more of it in long-acting formulations,” Dr. McMahon said.
He pointed out that the study did not show any signal of increased infections with the lower CD4+ T cell counts in the islatravir and doravirine arm, “but that difference is enough to raise that concern that it’s not ideal, and it should be moved forward with a lower dose, which is what they’ve done.”
A version of this article first appeared on Medscape.com.