User login
The Lone Hospitalist in the Nuba Mountains of Sudan
The lives of academic hospitalists are fraught with pitfalls and perils that can lead the unsuspecting far afield of their original intentions. It was in the murky depths of such professional perplexity that I recently found myself, lying in bed and attempting repose in vain. Thoughts of living a life underpaid, underresourced, and overworked buzzed in my psyche. After some tossing and turning, I slipped out of bed and into my home office and began watching cooking videos, which tend to have a calming effect on me, on The New York Times website.
The title of the first video in the queue, however, caught my eye: “The Worst Atrocity You’ve Never Heard Of,” by Times columnist Nicholas Kristof. To be honest, I try to avoid reading about the multitude of human disasters in Africa—the sheer numbers, size, scope, and hopelessness of human suffering make reading about them an exercise in despair and futility. Yet I clicked the link and began to see and hear about the plight of the people living in the Nuba Mountains of Sudan.
The Nuba Mountains, in the southern region of Sudan, lie north of a new border recently established between Sudan and the new country of South Sudan, for whose independence the Sudan People’s Liberation Army rebels fought in a war that ended in 2005. The rebels, however, continue to fight the Sudanese government in the Nuba Mountain region, with the Sudanese Air Force escalating a bombing campaign against the rebels in 2011. Since 2012, 3,740 bombs have been dropped on civilian targets in the Nuba Mountain region, resulting in countless deaths, shrapnel injuries, and burns.
In the midst of this mayhem, few medical providers have stood their ground. On January 20, a fighter jet dropped a cluster of 13 bombs into a hospital operated by Médecins Sans Frontières (MSF) in the Nuba Mountains village of Frandala—the second time the hospital had been bombed. MSF suspended its operations in Sudan soon thereafter. That left exactly one hospital operating in an area of the Nuba Mountains the size of Switzerland, with a population of 750,000. And, since 2008, there has been only one physician, Tom Catena, MD, at Mother of Mercy Hospital.
Dr. Catena, who received his medical degree from Duke University after being an all-Ivy League nose guard and Rhodes Scholar candidate at Brown, came to Mother of Mercy Hospital after missionary medicine work elsewhere in Africa and Latin America. Since his arrival at the 435-bed hospital, he has been the only physician there other than the occasional visitor. He is on call 24-7 and leaves only rarely. The hospital runs off the electrical grid, has no running water, subsists on scarce medical supplies, and has nary an X-ray machine to aid in diagnosing more trauma in one year than an average ED physician would see in an entire career. Trained in family medicine, he performs more than 1,000 operations yearly on patients suffering from the most egregious trauma and burns imaginable, with only the most basic support staff.
“He is Jesus Christ,” asserted a local Muslim chief in The New York Times video, with any difference in religious background—Dr. Catena is a devout Catholic—fading into irrelevance in the face of such heroic care. From repairing orthopedic injuries, delivering babies, and treating horrific trauma and burns, to handling measles and malaria outbreaks, Dr. Catena ministers to the ill and dying without regard to religion or reimbursement.
And, yet, why? Why would Dr. Catena forgo a comfortable life anywhere in the United States, give up the possibility of practicing in any number of lucrative professional settings (based on the diverse skill set he displays in the video), to earn $350 monthly, with no retirement plan, no disability, and no health insurance? As I watched the video, any concern I had regarding my own future earnings and career evaporated, at least momentarily, in the glow of Dr. Catena’s selfless devotion to his patients. One could understand volunteering in this fashion for a month, maybe a year, but seven years? What could keep him going?
I later referred to an article written by Pat Cawley, MD, and others last year and published in the Journal of Hospital Medicine, outlining the characteristics of an effective hospital medicine group, because, in effect, Dr. Catena is a one-man HMG—a solo practice if you will. It is tempting to ascribe his ability to persevere and even thrive in the most inhospitable work environment possible to religious fervor alone. But although he is clearly a devout Catholic, his laconic, “aw shucks” manner suggests the demeanor of an old-time country doctor more than a zealot. As I reread Dr. Cawley’s article, I found that, not surprisingly, Dr. Catena’s “group” fails on many counts. A small sampling:
- 1.2: The HMG has an active leadership development plan that is supported with appropriate budget, time, and other resources. Underresourced seems an understatement here.
- 4.5: The HMG is supported by appropriate practice management information technology, clinical information technology, and data analytics. The last HMG on earth without an EMR?
- 10.1: Hospitalist compensation is market competitive. And the market is…?
On other characteristics, however, Dr. Catena does surprisingly well:
- 3.2: All HMG team members (including physicians, nurse practitioners, physician assistants, and ancillary staff) have clearly defined, meaningful roles. Dr. Catena spends extensive time training undereducated but well-meaning local volunteers in providing ancillary services.
- 5.4: The HMG periodically solicits satisfaction feedback from key stakeholder groups, which is shared with all hospitalists and used to develop and implement improvement plans. The local population is so thankful for his care, they have tried to introduce him to local eligible women in the hope that he will marry and never leave.
- 9.1: The HMG’s hospitalists provide care that respects and responds to patient and family preferences, needs, and values. Dr. Catena’s humility and respect for the Nubian people and culture is what keeps him at Mother of Mercy, despite 11 bombings, grueling work, and negligible pay.
But the one characteristic missing from Dr. Cawley’s list is likely what keeps Dr. Catena in the Nuba Mountains: He practices at the limit of his skill set on a daily basis and spends the majority of his time in doing what he loves most, patient care (when he is not in his self-dug bomb shelter hole), not being chained behind a computer.
It is the failure of Dr. Catena’s group on one of the last characteristics, however, that likely is its greatest:
- 10.3: The HMG’s hospitalists are actively engaged in sourcing and recruiting new members. Somehow, I think finding someone to take Dr. Catena’s place will be difficult.

The lives of academic hospitalists are fraught with pitfalls and perils that can lead the unsuspecting far afield of their original intentions. It was in the murky depths of such professional perplexity that I recently found myself, lying in bed and attempting repose in vain. Thoughts of living a life underpaid, underresourced, and overworked buzzed in my psyche. After some tossing and turning, I slipped out of bed and into my home office and began watching cooking videos, which tend to have a calming effect on me, on The New York Times website.
The title of the first video in the queue, however, caught my eye: “The Worst Atrocity You’ve Never Heard Of,” by Times columnist Nicholas Kristof. To be honest, I try to avoid reading about the multitude of human disasters in Africa—the sheer numbers, size, scope, and hopelessness of human suffering make reading about them an exercise in despair and futility. Yet I clicked the link and began to see and hear about the plight of the people living in the Nuba Mountains of Sudan.
The Nuba Mountains, in the southern region of Sudan, lie north of a new border recently established between Sudan and the new country of South Sudan, for whose independence the Sudan People’s Liberation Army rebels fought in a war that ended in 2005. The rebels, however, continue to fight the Sudanese government in the Nuba Mountain region, with the Sudanese Air Force escalating a bombing campaign against the rebels in 2011. Since 2012, 3,740 bombs have been dropped on civilian targets in the Nuba Mountain region, resulting in countless deaths, shrapnel injuries, and burns.
In the midst of this mayhem, few medical providers have stood their ground. On January 20, a fighter jet dropped a cluster of 13 bombs into a hospital operated by Médecins Sans Frontières (MSF) in the Nuba Mountains village of Frandala—the second time the hospital had been bombed. MSF suspended its operations in Sudan soon thereafter. That left exactly one hospital operating in an area of the Nuba Mountains the size of Switzerland, with a population of 750,000. And, since 2008, there has been only one physician, Tom Catena, MD, at Mother of Mercy Hospital.
Dr. Catena, who received his medical degree from Duke University after being an all-Ivy League nose guard and Rhodes Scholar candidate at Brown, came to Mother of Mercy Hospital after missionary medicine work elsewhere in Africa and Latin America. Since his arrival at the 435-bed hospital, he has been the only physician there other than the occasional visitor. He is on call 24-7 and leaves only rarely. The hospital runs off the electrical grid, has no running water, subsists on scarce medical supplies, and has nary an X-ray machine to aid in diagnosing more trauma in one year than an average ED physician would see in an entire career. Trained in family medicine, he performs more than 1,000 operations yearly on patients suffering from the most egregious trauma and burns imaginable, with only the most basic support staff.
“He is Jesus Christ,” asserted a local Muslim chief in The New York Times video, with any difference in religious background—Dr. Catena is a devout Catholic—fading into irrelevance in the face of such heroic care. From repairing orthopedic injuries, delivering babies, and treating horrific trauma and burns, to handling measles and malaria outbreaks, Dr. Catena ministers to the ill and dying without regard to religion or reimbursement.
And, yet, why? Why would Dr. Catena forgo a comfortable life anywhere in the United States, give up the possibility of practicing in any number of lucrative professional settings (based on the diverse skill set he displays in the video), to earn $350 monthly, with no retirement plan, no disability, and no health insurance? As I watched the video, any concern I had regarding my own future earnings and career evaporated, at least momentarily, in the glow of Dr. Catena’s selfless devotion to his patients. One could understand volunteering in this fashion for a month, maybe a year, but seven years? What could keep him going?
I later referred to an article written by Pat Cawley, MD, and others last year and published in the Journal of Hospital Medicine, outlining the characteristics of an effective hospital medicine group, because, in effect, Dr. Catena is a one-man HMG—a solo practice if you will. It is tempting to ascribe his ability to persevere and even thrive in the most inhospitable work environment possible to religious fervor alone. But although he is clearly a devout Catholic, his laconic, “aw shucks” manner suggests the demeanor of an old-time country doctor more than a zealot. As I reread Dr. Cawley’s article, I found that, not surprisingly, Dr. Catena’s “group” fails on many counts. A small sampling:
- 1.2: The HMG has an active leadership development plan that is supported with appropriate budget, time, and other resources. Underresourced seems an understatement here.
- 4.5: The HMG is supported by appropriate practice management information technology, clinical information technology, and data analytics. The last HMG on earth without an EMR?
- 10.1: Hospitalist compensation is market competitive. And the market is…?
On other characteristics, however, Dr. Catena does surprisingly well:
- 3.2: All HMG team members (including physicians, nurse practitioners, physician assistants, and ancillary staff) have clearly defined, meaningful roles. Dr. Catena spends extensive time training undereducated but well-meaning local volunteers in providing ancillary services.
- 5.4: The HMG periodically solicits satisfaction feedback from key stakeholder groups, which is shared with all hospitalists and used to develop and implement improvement plans. The local population is so thankful for his care, they have tried to introduce him to local eligible women in the hope that he will marry and never leave.
- 9.1: The HMG’s hospitalists provide care that respects and responds to patient and family preferences, needs, and values. Dr. Catena’s humility and respect for the Nubian people and culture is what keeps him at Mother of Mercy, despite 11 bombings, grueling work, and negligible pay.
But the one characteristic missing from Dr. Cawley’s list is likely what keeps Dr. Catena in the Nuba Mountains: He practices at the limit of his skill set on a daily basis and spends the majority of his time in doing what he loves most, patient care (when he is not in his self-dug bomb shelter hole), not being chained behind a computer.
It is the failure of Dr. Catena’s group on one of the last characteristics, however, that likely is its greatest:
- 10.3: The HMG’s hospitalists are actively engaged in sourcing and recruiting new members. Somehow, I think finding someone to take Dr. Catena’s place will be difficult.

The lives of academic hospitalists are fraught with pitfalls and perils that can lead the unsuspecting far afield of their original intentions. It was in the murky depths of such professional perplexity that I recently found myself, lying in bed and attempting repose in vain. Thoughts of living a life underpaid, underresourced, and overworked buzzed in my psyche. After some tossing and turning, I slipped out of bed and into my home office and began watching cooking videos, which tend to have a calming effect on me, on The New York Times website.
The title of the first video in the queue, however, caught my eye: “The Worst Atrocity You’ve Never Heard Of,” by Times columnist Nicholas Kristof. To be honest, I try to avoid reading about the multitude of human disasters in Africa—the sheer numbers, size, scope, and hopelessness of human suffering make reading about them an exercise in despair and futility. Yet I clicked the link and began to see and hear about the plight of the people living in the Nuba Mountains of Sudan.
The Nuba Mountains, in the southern region of Sudan, lie north of a new border recently established between Sudan and the new country of South Sudan, for whose independence the Sudan People’s Liberation Army rebels fought in a war that ended in 2005. The rebels, however, continue to fight the Sudanese government in the Nuba Mountain region, with the Sudanese Air Force escalating a bombing campaign against the rebels in 2011. Since 2012, 3,740 bombs have been dropped on civilian targets in the Nuba Mountain region, resulting in countless deaths, shrapnel injuries, and burns.
In the midst of this mayhem, few medical providers have stood their ground. On January 20, a fighter jet dropped a cluster of 13 bombs into a hospital operated by Médecins Sans Frontières (MSF) in the Nuba Mountains village of Frandala—the second time the hospital had been bombed. MSF suspended its operations in Sudan soon thereafter. That left exactly one hospital operating in an area of the Nuba Mountains the size of Switzerland, with a population of 750,000. And, since 2008, there has been only one physician, Tom Catena, MD, at Mother of Mercy Hospital.
Dr. Catena, who received his medical degree from Duke University after being an all-Ivy League nose guard and Rhodes Scholar candidate at Brown, came to Mother of Mercy Hospital after missionary medicine work elsewhere in Africa and Latin America. Since his arrival at the 435-bed hospital, he has been the only physician there other than the occasional visitor. He is on call 24-7 and leaves only rarely. The hospital runs off the electrical grid, has no running water, subsists on scarce medical supplies, and has nary an X-ray machine to aid in diagnosing more trauma in one year than an average ED physician would see in an entire career. Trained in family medicine, he performs more than 1,000 operations yearly on patients suffering from the most egregious trauma and burns imaginable, with only the most basic support staff.
“He is Jesus Christ,” asserted a local Muslim chief in The New York Times video, with any difference in religious background—Dr. Catena is a devout Catholic—fading into irrelevance in the face of such heroic care. From repairing orthopedic injuries, delivering babies, and treating horrific trauma and burns, to handling measles and malaria outbreaks, Dr. Catena ministers to the ill and dying without regard to religion or reimbursement.
And, yet, why? Why would Dr. Catena forgo a comfortable life anywhere in the United States, give up the possibility of practicing in any number of lucrative professional settings (based on the diverse skill set he displays in the video), to earn $350 monthly, with no retirement plan, no disability, and no health insurance? As I watched the video, any concern I had regarding my own future earnings and career evaporated, at least momentarily, in the glow of Dr. Catena’s selfless devotion to his patients. One could understand volunteering in this fashion for a month, maybe a year, but seven years? What could keep him going?
I later referred to an article written by Pat Cawley, MD, and others last year and published in the Journal of Hospital Medicine, outlining the characteristics of an effective hospital medicine group, because, in effect, Dr. Catena is a one-man HMG—a solo practice if you will. It is tempting to ascribe his ability to persevere and even thrive in the most inhospitable work environment possible to religious fervor alone. But although he is clearly a devout Catholic, his laconic, “aw shucks” manner suggests the demeanor of an old-time country doctor more than a zealot. As I reread Dr. Cawley’s article, I found that, not surprisingly, Dr. Catena’s “group” fails on many counts. A small sampling:
- 1.2: The HMG has an active leadership development plan that is supported with appropriate budget, time, and other resources. Underresourced seems an understatement here.
- 4.5: The HMG is supported by appropriate practice management information technology, clinical information technology, and data analytics. The last HMG on earth without an EMR?
- 10.1: Hospitalist compensation is market competitive. And the market is…?
On other characteristics, however, Dr. Catena does surprisingly well:
- 3.2: All HMG team members (including physicians, nurse practitioners, physician assistants, and ancillary staff) have clearly defined, meaningful roles. Dr. Catena spends extensive time training undereducated but well-meaning local volunteers in providing ancillary services.
- 5.4: The HMG periodically solicits satisfaction feedback from key stakeholder groups, which is shared with all hospitalists and used to develop and implement improvement plans. The local population is so thankful for his care, they have tried to introduce him to local eligible women in the hope that he will marry and never leave.
- 9.1: The HMG’s hospitalists provide care that respects and responds to patient and family preferences, needs, and values. Dr. Catena’s humility and respect for the Nubian people and culture is what keeps him at Mother of Mercy, despite 11 bombings, grueling work, and negligible pay.
But the one characteristic missing from Dr. Cawley’s list is likely what keeps Dr. Catena in the Nuba Mountains: He practices at the limit of his skill set on a daily basis and spends the majority of his time in doing what he loves most, patient care (when he is not in his self-dug bomb shelter hole), not being chained behind a computer.
It is the failure of Dr. Catena’s group on one of the last characteristics, however, that likely is its greatest:
- 10.3: The HMG’s hospitalists are actively engaged in sourcing and recruiting new members. Somehow, I think finding someone to take Dr. Catena’s place will be difficult.

Policy Changes Hospitalists May See in 2016
The year 2015 brought the repeal of the sustainable growth rate (SGR) and new rules for advanced care planning reimbursement. It saw hospitalists take the lead on improving the two-midnight rule and respond to a global infectious disease scare.
The Hospitalist caught up with Dr. Greeno, chief strategy officer at North Hollywood, Calif.-based IPC Healthcare, to ask him about what he sees for the year ahead in policy.
Question: What are the biggest changes in store for 2016 that stand to impact hospitalists?
Answer: Much of it is just a magnification of the things that most hospitalists are already feeling or sensing. Clearly, there is a very solid movement toward alternative payment methodologies. BPCI (the Bundled Payments for Care Improvement initiative) has been embraced by hospitalists and other physicians all over the country at a scale that has surprised everybody.
There is also more consolidation in the healthcare industry as a whole. Hospital organizations are getting bigger, and we’re seeing consolidation of hospitalist groups. We will see cross-integration in the healthcare system that occurs at a rapid pace: hospitals buying physician groups, health systems and providers starting health plans, health plans acquiring hospital systems. In the not-too-distant future, we are all going to be in the population health business. This is a complete realignment of the healthcare system, and we haven’t seen the half of it yet. We have to be prepared to do it all, or a very big piece of it. The good news is, we are an absolute necessity for success in the future.
Q: It’s a presidential election year. How much weight should physicians put on claims made by candidates?
A: I encourage people to be politically engaged, but I don’t think the majority of what’s happening in healthcare is being driven by politics. It’s being driven by dispassionate economic forces that aren’t going to go away, no matter who is president. We have to figure out how to care for our population more cost-effectively. The ACA (Affordable Care Act) has driven a lot of the political environment in D.C. since its passage, including a big divide between the two parties, but it’s about three things: insurance reform, expanded access, and, particularly, delivery system reform. That’s the part we really care about and can influence the most, I think. Both parties feel like the delivery system needs to be reformed. I don’t think the election will have a major impact on hospitalists and what we do.
The ACA created an environment where things moved faster, created the (CMS) Innovation Center that drives alternative payment methodologies. It created a burning platform for things that already needed to happen.
Q: Is there anything new for meaningful use/EHR in 2016?
A: There are implications of meaningful use for hospitalists. Last year was the first that meaningful use penalties for physician groups came into effect. The way it was written, there was an exception to meaningful use requirements for hospital-based physicians, but a majority of SHM’s membership does not qualify for exemption and are subject to penalties. It’s not small: $2,500 to $5,000 per doctor. The Public Policy Committee at SHM has been working in Washington the last couple of years. We were able to get a one-year exemption, and now they’ve given us a second year, but we can only do five years according to law, and we have to apply every year. We have applied to CMS for a specialty code for hospitalists, and if that gets approved, it will be used to identify who is a hospitalist and who is not. If we submit under that code, then we’re not subject to penalty.

–Dr. Greeno
Q: What is the future of the two-midnight rule?
A: The committee and SHM took that on several years ago at my urging because it didn’t seem like other specialties were leading that issue. It doesn’t affect hospitalists in terms of how we’re paid, but it does affect the patients we care for. I think we’ll have a better solution in the coming years.
Q: What should hospitalists be thinking about heading into 2016?
A: They should be starting to prepare for a world where they no longer get paid with fee-for-service. Hospitalists are in the post-acute setting, where a lot of the action takes place, and it’s the high-cost action. My lesson is to embrace the changes; don’t fight it. As a hospitalist, your job is going to be different a year from now. We might as well get ready for the change, because there’s going to be a lot of change in the system.
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The year 2015 brought the repeal of the sustainable growth rate (SGR) and new rules for advanced care planning reimbursement. It saw hospitalists take the lead on improving the two-midnight rule and respond to a global infectious disease scare.
The Hospitalist caught up with Dr. Greeno, chief strategy officer at North Hollywood, Calif.-based IPC Healthcare, to ask him about what he sees for the year ahead in policy.
Question: What are the biggest changes in store for 2016 that stand to impact hospitalists?
Answer: Much of it is just a magnification of the things that most hospitalists are already feeling or sensing. Clearly, there is a very solid movement toward alternative payment methodologies. BPCI (the Bundled Payments for Care Improvement initiative) has been embraced by hospitalists and other physicians all over the country at a scale that has surprised everybody.
There is also more consolidation in the healthcare industry as a whole. Hospital organizations are getting bigger, and we’re seeing consolidation of hospitalist groups. We will see cross-integration in the healthcare system that occurs at a rapid pace: hospitals buying physician groups, health systems and providers starting health plans, health plans acquiring hospital systems. In the not-too-distant future, we are all going to be in the population health business. This is a complete realignment of the healthcare system, and we haven’t seen the half of it yet. We have to be prepared to do it all, or a very big piece of it. The good news is, we are an absolute necessity for success in the future.
Q: It’s a presidential election year. How much weight should physicians put on claims made by candidates?
A: I encourage people to be politically engaged, but I don’t think the majority of what’s happening in healthcare is being driven by politics. It’s being driven by dispassionate economic forces that aren’t going to go away, no matter who is president. We have to figure out how to care for our population more cost-effectively. The ACA (Affordable Care Act) has driven a lot of the political environment in D.C. since its passage, including a big divide between the two parties, but it’s about three things: insurance reform, expanded access, and, particularly, delivery system reform. That’s the part we really care about and can influence the most, I think. Both parties feel like the delivery system needs to be reformed. I don’t think the election will have a major impact on hospitalists and what we do.
The ACA created an environment where things moved faster, created the (CMS) Innovation Center that drives alternative payment methodologies. It created a burning platform for things that already needed to happen.
Q: Is there anything new for meaningful use/EHR in 2016?
A: There are implications of meaningful use for hospitalists. Last year was the first that meaningful use penalties for physician groups came into effect. The way it was written, there was an exception to meaningful use requirements for hospital-based physicians, but a majority of SHM’s membership does not qualify for exemption and are subject to penalties. It’s not small: $2,500 to $5,000 per doctor. The Public Policy Committee at SHM has been working in Washington the last couple of years. We were able to get a one-year exemption, and now they’ve given us a second year, but we can only do five years according to law, and we have to apply every year. We have applied to CMS for a specialty code for hospitalists, and if that gets approved, it will be used to identify who is a hospitalist and who is not. If we submit under that code, then we’re not subject to penalty.

–Dr. Greeno
Q: What is the future of the two-midnight rule?
A: The committee and SHM took that on several years ago at my urging because it didn’t seem like other specialties were leading that issue. It doesn’t affect hospitalists in terms of how we’re paid, but it does affect the patients we care for. I think we’ll have a better solution in the coming years.
Q: What should hospitalists be thinking about heading into 2016?
A: They should be starting to prepare for a world where they no longer get paid with fee-for-service. Hospitalists are in the post-acute setting, where a lot of the action takes place, and it’s the high-cost action. My lesson is to embrace the changes; don’t fight it. As a hospitalist, your job is going to be different a year from now. We might as well get ready for the change, because there’s going to be a lot of change in the system.
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The year 2015 brought the repeal of the sustainable growth rate (SGR) and new rules for advanced care planning reimbursement. It saw hospitalists take the lead on improving the two-midnight rule and respond to a global infectious disease scare.
The Hospitalist caught up with Dr. Greeno, chief strategy officer at North Hollywood, Calif.-based IPC Healthcare, to ask him about what he sees for the year ahead in policy.
Question: What are the biggest changes in store for 2016 that stand to impact hospitalists?
Answer: Much of it is just a magnification of the things that most hospitalists are already feeling or sensing. Clearly, there is a very solid movement toward alternative payment methodologies. BPCI (the Bundled Payments for Care Improvement initiative) has been embraced by hospitalists and other physicians all over the country at a scale that has surprised everybody.
There is also more consolidation in the healthcare industry as a whole. Hospital organizations are getting bigger, and we’re seeing consolidation of hospitalist groups. We will see cross-integration in the healthcare system that occurs at a rapid pace: hospitals buying physician groups, health systems and providers starting health plans, health plans acquiring hospital systems. In the not-too-distant future, we are all going to be in the population health business. This is a complete realignment of the healthcare system, and we haven’t seen the half of it yet. We have to be prepared to do it all, or a very big piece of it. The good news is, we are an absolute necessity for success in the future.
Q: It’s a presidential election year. How much weight should physicians put on claims made by candidates?
A: I encourage people to be politically engaged, but I don’t think the majority of what’s happening in healthcare is being driven by politics. It’s being driven by dispassionate economic forces that aren’t going to go away, no matter who is president. We have to figure out how to care for our population more cost-effectively. The ACA (Affordable Care Act) has driven a lot of the political environment in D.C. since its passage, including a big divide between the two parties, but it’s about three things: insurance reform, expanded access, and, particularly, delivery system reform. That’s the part we really care about and can influence the most, I think. Both parties feel like the delivery system needs to be reformed. I don’t think the election will have a major impact on hospitalists and what we do.
The ACA created an environment where things moved faster, created the (CMS) Innovation Center that drives alternative payment methodologies. It created a burning platform for things that already needed to happen.
Q: Is there anything new for meaningful use/EHR in 2016?
A: There are implications of meaningful use for hospitalists. Last year was the first that meaningful use penalties for physician groups came into effect. The way it was written, there was an exception to meaningful use requirements for hospital-based physicians, but a majority of SHM’s membership does not qualify for exemption and are subject to penalties. It’s not small: $2,500 to $5,000 per doctor. The Public Policy Committee at SHM has been working in Washington the last couple of years. We were able to get a one-year exemption, and now they’ve given us a second year, but we can only do five years according to law, and we have to apply every year. We have applied to CMS for a specialty code for hospitalists, and if that gets approved, it will be used to identify who is a hospitalist and who is not. If we submit under that code, then we’re not subject to penalty.

–Dr. Greeno
Q: What is the future of the two-midnight rule?
A: The committee and SHM took that on several years ago at my urging because it didn’t seem like other specialties were leading that issue. It doesn’t affect hospitalists in terms of how we’re paid, but it does affect the patients we care for. I think we’ll have a better solution in the coming years.
Q: What should hospitalists be thinking about heading into 2016?
A: They should be starting to prepare for a world where they no longer get paid with fee-for-service. Hospitalists are in the post-acute setting, where a lot of the action takes place, and it’s the high-cost action. My lesson is to embrace the changes; don’t fight it. As a hospitalist, your job is going to be different a year from now. We might as well get ready for the change, because there’s going to be a lot of change in the system.
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Hospitalist David Weidig, MD, Witnessed the Field Grow During His Decades-long Career
David Weidig, MD, was there at the beginning. He was one of the first internal medicine-trained physicians to adopt hospital-based practice. He was one of the first to proudly call himself a hospitalist. And, he was one of the first hospitalists to adapt and prosper as a hospitalist group director.
He graduated from the University of Wisconsin in 1987, completed medical school at Northwestern University in Chicago in 1991, and trained at Cleveland Clinic and Mercy Hospital in San Diego before taking a position with a “traditional” practice in 1995. It wasn’t long before the gravitational forces of hospital medicine pulled him in. He joined a hospitalist group a year later and assumed his first leadership position in 1998, as director of hospital medicine at Pacific Medical Group in Seattle.
“Hospital medicine was a new concept at the time,” he says. “There were only a few of us in the entire city.”
In 2007, he returned to the Midwest, to the HM group at Aurora Health Care, based in Milwaukee, Wis. As system director of hospital medicine at Aurora, he managed academic and community programs throughout the state, many of which are affiliated with his alma mater. When he joined Aurora, it was made up of one HM group and six full-time physicians. Today, it boasts 13 programs and more than 150 providers.
After eight years at Aurora, Dr. Weidig recently joined Tacoma, Wash.-based Sound Physicians. He will serve as director of their Evergreen Region.
During his two decades as a hospitalist, Dr. Weidig has seen massive changes in both the field of hospital medicine and healthcare. He’s witnessed firsthand the growth in the field, the shift to value and performance in healthcare, and the challenges faced by HM groups large and small, urban and rural, academic and community.

—Dr. Weidig
“We have had successes and failures, and we have learned from our efforts,” he says, adding that his biggest professional reward is “having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.”
Dr. Weidig is a longtime SHM member, serving as the SHM Northwest Chapter president from 2005-2007, and currently as a member of SHM’s Multisite Hospitalist Leader Subcommittee. He also is one of seven new members of Team Hospitalist, the volunteer editorial board for The Hospitalist. We chatted with him recently about his interests in hospital medicine and beyond.
Question: Why did you choose a career in medicine?
Answer: Being able to help someone during a serious time of need was a strong initial draw. I was also fascinated by physiology in my undergrad studies, which fit well with the study of medicine.
Q: Was there a single moment you knew “I can do this hospital medicine thing?”
Answer: Honestly, I was a bit put off by the way medical school was taught and some of the attitudes I encountered. It was a much different feel than my undergrad experience, in biochemistry, which I very much enjoyed. I believe it was my fourth-year rotations where I hit a milestone, and a significant increase in confidence about caring for a patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the acute intervention to rapidly [hopefully] effect an improvement in the patient’s symptoms. I also enjoy the systematic, best-practice approach that the field has developed.
Q: What do you dislike most?
A: Poorly managed end-of-life care. I am a strong proponent of proper utilization of palliative care to maximize quality of life and minimize suffering, both for the patient and family members.
Q: What’s the best advice you ever received?
A: Focus and respond with thoughtfulness and empathy. Do not react with emotion.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: I had a biochemistry instructor in undergrad who was an all-around high quality individual. I had a great deal of respect for how he treated and interacted with others. I have always tried to emulate it.
Q: What’s the biggest change you’ve seen in HM in your career?
A: I started in the infancy of the field, so everything has changed. Acceptance and understanding of a team-based inpatient approach is possibly one of the biggest.
Q: What’s the biggest change you would like to see in HM?
A: Further understanding and promotion of the benefits of focused management of the inpatient population, as well as the benefits of more intensive transitional care after hospitalization.
Q: Why is it important for you, as a hospitalist group leader, to continue seeing patients?
A: To stay in touch with what patients experience.
Q: What aspect of patient care is most rewarding?
A: An acutely ill patient who responds well to medical intervention and within hours to days is feeling dramatically better.
Q: What aspect of teaching medicine in the 21st century is most difficult? And what is most enjoyable?
A: Most difficult is managing the time to teach around other duties of the day. Most rewarding is teaching the concept of not just medicine, but team organization and leadership within the hospital setting.
Q: What is your biggest professional reward?
A: Having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.
Q: What SHM event or meeting has made the most lasting impression on you?
A: I always feel that the annual Society of Hospital Medicine meeting has an amazing wealth of knowledge and experience to gain from.
Q: Where do you see yourself in 10 years?
A: Hopefully, working with quality outcomes and patient experience on a large scale.
Q: If you weren’t a doctor, what would you be doing right now?
A: Likely something in the area of biochemistry/genetics.
Q: When you aren’t working, what is important to you?
A: Family, friends, music, travel.
Q: PC or tablet?
A: Tablet.
Q: What’s the best book you’ve read recently?
A: Thinking Fast and Slow by Daniel Kahneman. It’s very deep and insightful in how we react to situations and make decisions. There are factors that have major effects on decisions that we can be completely unaware of unless we are cognizant of them. It significantly changed my approach to many situations and also applies well to the decision-making process in medicine.
Q: Apple or Android?
A: Apple.
David Weidig, MD, was there at the beginning. He was one of the first internal medicine-trained physicians to adopt hospital-based practice. He was one of the first to proudly call himself a hospitalist. And, he was one of the first hospitalists to adapt and prosper as a hospitalist group director.
He graduated from the University of Wisconsin in 1987, completed medical school at Northwestern University in Chicago in 1991, and trained at Cleveland Clinic and Mercy Hospital in San Diego before taking a position with a “traditional” practice in 1995. It wasn’t long before the gravitational forces of hospital medicine pulled him in. He joined a hospitalist group a year later and assumed his first leadership position in 1998, as director of hospital medicine at Pacific Medical Group in Seattle.
“Hospital medicine was a new concept at the time,” he says. “There were only a few of us in the entire city.”
In 2007, he returned to the Midwest, to the HM group at Aurora Health Care, based in Milwaukee, Wis. As system director of hospital medicine at Aurora, he managed academic and community programs throughout the state, many of which are affiliated with his alma mater. When he joined Aurora, it was made up of one HM group and six full-time physicians. Today, it boasts 13 programs and more than 150 providers.
After eight years at Aurora, Dr. Weidig recently joined Tacoma, Wash.-based Sound Physicians. He will serve as director of their Evergreen Region.
During his two decades as a hospitalist, Dr. Weidig has seen massive changes in both the field of hospital medicine and healthcare. He’s witnessed firsthand the growth in the field, the shift to value and performance in healthcare, and the challenges faced by HM groups large and small, urban and rural, academic and community.

—Dr. Weidig
“We have had successes and failures, and we have learned from our efforts,” he says, adding that his biggest professional reward is “having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.”
Dr. Weidig is a longtime SHM member, serving as the SHM Northwest Chapter president from 2005-2007, and currently as a member of SHM’s Multisite Hospitalist Leader Subcommittee. He also is one of seven new members of Team Hospitalist, the volunteer editorial board for The Hospitalist. We chatted with him recently about his interests in hospital medicine and beyond.
Question: Why did you choose a career in medicine?
Answer: Being able to help someone during a serious time of need was a strong initial draw. I was also fascinated by physiology in my undergrad studies, which fit well with the study of medicine.
Q: Was there a single moment you knew “I can do this hospital medicine thing?”
Answer: Honestly, I was a bit put off by the way medical school was taught and some of the attitudes I encountered. It was a much different feel than my undergrad experience, in biochemistry, which I very much enjoyed. I believe it was my fourth-year rotations where I hit a milestone, and a significant increase in confidence about caring for a patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the acute intervention to rapidly [hopefully] effect an improvement in the patient’s symptoms. I also enjoy the systematic, best-practice approach that the field has developed.
Q: What do you dislike most?
A: Poorly managed end-of-life care. I am a strong proponent of proper utilization of palliative care to maximize quality of life and minimize suffering, both for the patient and family members.
Q: What’s the best advice you ever received?
A: Focus and respond with thoughtfulness and empathy. Do not react with emotion.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: I had a biochemistry instructor in undergrad who was an all-around high quality individual. I had a great deal of respect for how he treated and interacted with others. I have always tried to emulate it.
Q: What’s the biggest change you’ve seen in HM in your career?
A: I started in the infancy of the field, so everything has changed. Acceptance and understanding of a team-based inpatient approach is possibly one of the biggest.
Q: What’s the biggest change you would like to see in HM?
A: Further understanding and promotion of the benefits of focused management of the inpatient population, as well as the benefits of more intensive transitional care after hospitalization.
Q: Why is it important for you, as a hospitalist group leader, to continue seeing patients?
A: To stay in touch with what patients experience.
Q: What aspect of patient care is most rewarding?
A: An acutely ill patient who responds well to medical intervention and within hours to days is feeling dramatically better.
Q: What aspect of teaching medicine in the 21st century is most difficult? And what is most enjoyable?
A: Most difficult is managing the time to teach around other duties of the day. Most rewarding is teaching the concept of not just medicine, but team organization and leadership within the hospital setting.
Q: What is your biggest professional reward?
A: Having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.
Q: What SHM event or meeting has made the most lasting impression on you?
A: I always feel that the annual Society of Hospital Medicine meeting has an amazing wealth of knowledge and experience to gain from.
Q: Where do you see yourself in 10 years?
A: Hopefully, working with quality outcomes and patient experience on a large scale.
Q: If you weren’t a doctor, what would you be doing right now?
A: Likely something in the area of biochemistry/genetics.
Q: When you aren’t working, what is important to you?
A: Family, friends, music, travel.
Q: PC or tablet?
A: Tablet.
Q: What’s the best book you’ve read recently?
A: Thinking Fast and Slow by Daniel Kahneman. It’s very deep and insightful in how we react to situations and make decisions. There are factors that have major effects on decisions that we can be completely unaware of unless we are cognizant of them. It significantly changed my approach to many situations and also applies well to the decision-making process in medicine.
Q: Apple or Android?
A: Apple.
David Weidig, MD, was there at the beginning. He was one of the first internal medicine-trained physicians to adopt hospital-based practice. He was one of the first to proudly call himself a hospitalist. And, he was one of the first hospitalists to adapt and prosper as a hospitalist group director.
He graduated from the University of Wisconsin in 1987, completed medical school at Northwestern University in Chicago in 1991, and trained at Cleveland Clinic and Mercy Hospital in San Diego before taking a position with a “traditional” practice in 1995. It wasn’t long before the gravitational forces of hospital medicine pulled him in. He joined a hospitalist group a year later and assumed his first leadership position in 1998, as director of hospital medicine at Pacific Medical Group in Seattle.
“Hospital medicine was a new concept at the time,” he says. “There were only a few of us in the entire city.”
In 2007, he returned to the Midwest, to the HM group at Aurora Health Care, based in Milwaukee, Wis. As system director of hospital medicine at Aurora, he managed academic and community programs throughout the state, many of which are affiliated with his alma mater. When he joined Aurora, it was made up of one HM group and six full-time physicians. Today, it boasts 13 programs and more than 150 providers.
After eight years at Aurora, Dr. Weidig recently joined Tacoma, Wash.-based Sound Physicians. He will serve as director of their Evergreen Region.
During his two decades as a hospitalist, Dr. Weidig has seen massive changes in both the field of hospital medicine and healthcare. He’s witnessed firsthand the growth in the field, the shift to value and performance in healthcare, and the challenges faced by HM groups large and small, urban and rural, academic and community.

—Dr. Weidig
“We have had successes and failures, and we have learned from our efforts,” he says, adding that his biggest professional reward is “having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.”
Dr. Weidig is a longtime SHM member, serving as the SHM Northwest Chapter president from 2005-2007, and currently as a member of SHM’s Multisite Hospitalist Leader Subcommittee. He also is one of seven new members of Team Hospitalist, the volunteer editorial board for The Hospitalist. We chatted with him recently about his interests in hospital medicine and beyond.
Question: Why did you choose a career in medicine?
Answer: Being able to help someone during a serious time of need was a strong initial draw. I was also fascinated by physiology in my undergrad studies, which fit well with the study of medicine.
Q: Was there a single moment you knew “I can do this hospital medicine thing?”
Answer: Honestly, I was a bit put off by the way medical school was taught and some of the attitudes I encountered. It was a much different feel than my undergrad experience, in biochemistry, which I very much enjoyed. I believe it was my fourth-year rotations where I hit a milestone, and a significant increase in confidence about caring for a patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the acute intervention to rapidly [hopefully] effect an improvement in the patient’s symptoms. I also enjoy the systematic, best-practice approach that the field has developed.
Q: What do you dislike most?
A: Poorly managed end-of-life care. I am a strong proponent of proper utilization of palliative care to maximize quality of life and minimize suffering, both for the patient and family members.
Q: What’s the best advice you ever received?
A: Focus and respond with thoughtfulness and empathy. Do not react with emotion.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: I had a biochemistry instructor in undergrad who was an all-around high quality individual. I had a great deal of respect for how he treated and interacted with others. I have always tried to emulate it.
Q: What’s the biggest change you’ve seen in HM in your career?
A: I started in the infancy of the field, so everything has changed. Acceptance and understanding of a team-based inpatient approach is possibly one of the biggest.
Q: What’s the biggest change you would like to see in HM?
A: Further understanding and promotion of the benefits of focused management of the inpatient population, as well as the benefits of more intensive transitional care after hospitalization.
Q: Why is it important for you, as a hospitalist group leader, to continue seeing patients?
A: To stay in touch with what patients experience.
Q: What aspect of patient care is most rewarding?
A: An acutely ill patient who responds well to medical intervention and within hours to days is feeling dramatically better.
Q: What aspect of teaching medicine in the 21st century is most difficult? And what is most enjoyable?
A: Most difficult is managing the time to teach around other duties of the day. Most rewarding is teaching the concept of not just medicine, but team organization and leadership within the hospital setting.
Q: What is your biggest professional reward?
A: Having built a large hospital medicine system, along with the camaraderie and respect for the people who I worked with to do it.
Q: What SHM event or meeting has made the most lasting impression on you?
A: I always feel that the annual Society of Hospital Medicine meeting has an amazing wealth of knowledge and experience to gain from.
Q: Where do you see yourself in 10 years?
A: Hopefully, working with quality outcomes and patient experience on a large scale.
Q: If you weren’t a doctor, what would you be doing right now?
A: Likely something in the area of biochemistry/genetics.
Q: When you aren’t working, what is important to you?
A: Family, friends, music, travel.
Q: PC or tablet?
A: Tablet.
Q: What’s the best book you’ve read recently?
A: Thinking Fast and Slow by Daniel Kahneman. It’s very deep and insightful in how we react to situations and make decisions. There are factors that have major effects on decisions that we can be completely unaware of unless we are cognizant of them. It significantly changed my approach to many situations and also applies well to the decision-making process in medicine.
Q: Apple or Android?
A: Apple.
Oral Steroids Not Inferior to Intravenous Steroids in Multiple Sclerosis Relapses
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Differences in Care, Outcomes for In-Hospital Versus Community-Onset Stroke
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
Tips for Hospitalists on Improving Diagnostic Skills
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
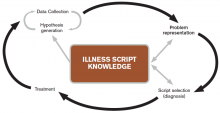
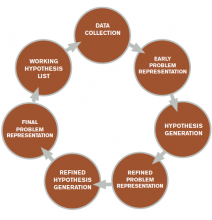
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
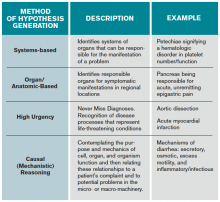
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Hospitalists Can Join SHM's New 'Fight the Resistance' Campaign
In June, Eric Howell, MD, SFHM, SHM’s senior physician advisor, attended a White House forum on antibiotic resistance. That day, SHM committed to empowering hospitalists to fight antibiotic resistance through better prescribing behaviors.
In a blog post on The Hospital Leader, Dr. Howell wrote, “This isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of this fight against antibiotic resistance.”
Now, hospitalists everywhere can join Dr. Howell at the front line of protecting patients from the many harms of overprescribing antibiotics, with SHM’s new
“Fight the Resistance” campaign.
At the heart of the campaign are three posters intended to remind all hospital-based staff about the need for antibiotic stewardship. The posters are available for download now, and a large-format version of one of the posters is included in the November issue of The Hospitalist.
SHM encourages hospitalists to post these posters in their hospitals as a reminder to use antibiotics only when necessary—and for only as long as necessary.
SHM will be hosting a social media-driven contest encouraging hospitalists to share pictures of the posters on the wall in their hospital, using the #FightTheResistance hashtag.
For more information, visit www.fighttheresistance.org.
In June, Eric Howell, MD, SFHM, SHM’s senior physician advisor, attended a White House forum on antibiotic resistance. That day, SHM committed to empowering hospitalists to fight antibiotic resistance through better prescribing behaviors.
In a blog post on The Hospital Leader, Dr. Howell wrote, “This isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of this fight against antibiotic resistance.”
Now, hospitalists everywhere can join Dr. Howell at the front line of protecting patients from the many harms of overprescribing antibiotics, with SHM’s new
“Fight the Resistance” campaign.
At the heart of the campaign are three posters intended to remind all hospital-based staff about the need for antibiotic stewardship. The posters are available for download now, and a large-format version of one of the posters is included in the November issue of The Hospitalist.
SHM encourages hospitalists to post these posters in their hospitals as a reminder to use antibiotics only when necessary—and for only as long as necessary.
SHM will be hosting a social media-driven contest encouraging hospitalists to share pictures of the posters on the wall in their hospital, using the #FightTheResistance hashtag.
For more information, visit www.fighttheresistance.org.
In June, Eric Howell, MD, SFHM, SHM’s senior physician advisor, attended a White House forum on antibiotic resistance. That day, SHM committed to empowering hospitalists to fight antibiotic resistance through better prescribing behaviors.
In a blog post on The Hospital Leader, Dr. Howell wrote, “This isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of this fight against antibiotic resistance.”
Now, hospitalists everywhere can join Dr. Howell at the front line of protecting patients from the many harms of overprescribing antibiotics, with SHM’s new
“Fight the Resistance” campaign.
At the heart of the campaign are three posters intended to remind all hospital-based staff about the need for antibiotic stewardship. The posters are available for download now, and a large-format version of one of the posters is included in the November issue of The Hospitalist.
SHM encourages hospitalists to post these posters in their hospitals as a reminder to use antibiotics only when necessary—and for only as long as necessary.
SHM will be hosting a social media-driven contest encouraging hospitalists to share pictures of the posters on the wall in their hospital, using the #FightTheResistance hashtag.
For more information, visit www.fighttheresistance.org.
Society of Hospital Medicine Resources Provide Quality Improvement Tips
Whether you’ve been tasked with leading a major quality improvement program or simply want to make a few things better for your patients, SHM has resources for every scale of quality improvement initiative, all created by national experts in their fields.
1. Attend an SHM Quality Improvement Webinar
The last of SHM’s live quality improvement webinars, “Elevating Provider Experience to Improve Patient Experience,” will be presented at 2 p.m. EST on Nov. 11, by Mark Rudolph, MD, SFHM. You can visit the archives of our other quality improvement webinars on topics like improving patient discharge, managing diabetes in the hospital, and general principles of quality improvement.
2. Tune in to RADEO: SHM’s New Opioid Stewardship Mentored Implementation Program
SHM has introduced RADEO: Reducing Adverse Drug Events related to Opioids, a new implementation guide and mentored implementation program designed to empower hospitalists to make opioid prescribing safer, with fewer adverse events, and much less likely to result in dangerous sedation, respiratory depression, and death.
SHM is recruiting now for participants in the mentored implementation program, and the guide is available online.
3. Download a New QI Resource
When you download a quality improvement toolkit or resource from SHM, you can be confident you are getting the best guidance from the national authorities on improving care for hospitalized patients.
SHM provides materials on the most pressing issues in hospital medicine, including:
- End-of-life care;
- Opioid monitoring;
- Antibiotic stewardship;
- Post-acute care;
- Veinous thromboembolism (VTE);
- Pain management; and
- Congestive heart failure (CHF).
To download, visit www.hospitalmedicine.org/qi.
Whether you’ve been tasked with leading a major quality improvement program or simply want to make a few things better for your patients, SHM has resources for every scale of quality improvement initiative, all created by national experts in their fields.
1. Attend an SHM Quality Improvement Webinar
The last of SHM’s live quality improvement webinars, “Elevating Provider Experience to Improve Patient Experience,” will be presented at 2 p.m. EST on Nov. 11, by Mark Rudolph, MD, SFHM. You can visit the archives of our other quality improvement webinars on topics like improving patient discharge, managing diabetes in the hospital, and general principles of quality improvement.
2. Tune in to RADEO: SHM’s New Opioid Stewardship Mentored Implementation Program
SHM has introduced RADEO: Reducing Adverse Drug Events related to Opioids, a new implementation guide and mentored implementation program designed to empower hospitalists to make opioid prescribing safer, with fewer adverse events, and much less likely to result in dangerous sedation, respiratory depression, and death.
SHM is recruiting now for participants in the mentored implementation program, and the guide is available online.
3. Download a New QI Resource
When you download a quality improvement toolkit or resource from SHM, you can be confident you are getting the best guidance from the national authorities on improving care for hospitalized patients.
SHM provides materials on the most pressing issues in hospital medicine, including:
- End-of-life care;
- Opioid monitoring;
- Antibiotic stewardship;
- Post-acute care;
- Veinous thromboembolism (VTE);
- Pain management; and
- Congestive heart failure (CHF).
To download, visit www.hospitalmedicine.org/qi.
Whether you’ve been tasked with leading a major quality improvement program or simply want to make a few things better for your patients, SHM has resources for every scale of quality improvement initiative, all created by national experts in their fields.
1. Attend an SHM Quality Improvement Webinar
The last of SHM’s live quality improvement webinars, “Elevating Provider Experience to Improve Patient Experience,” will be presented at 2 p.m. EST on Nov. 11, by Mark Rudolph, MD, SFHM. You can visit the archives of our other quality improvement webinars on topics like improving patient discharge, managing diabetes in the hospital, and general principles of quality improvement.
2. Tune in to RADEO: SHM’s New Opioid Stewardship Mentored Implementation Program
SHM has introduced RADEO: Reducing Adverse Drug Events related to Opioids, a new implementation guide and mentored implementation program designed to empower hospitalists to make opioid prescribing safer, with fewer adverse events, and much less likely to result in dangerous sedation, respiratory depression, and death.
SHM is recruiting now for participants in the mentored implementation program, and the guide is available online.
3. Download a New QI Resource
When you download a quality improvement toolkit or resource from SHM, you can be confident you are getting the best guidance from the national authorities on improving care for hospitalized patients.
SHM provides materials on the most pressing issues in hospital medicine, including:
- End-of-life care;
- Opioid monitoring;
- Antibiotic stewardship;
- Post-acute care;
- Veinous thromboembolism (VTE);
- Pain management; and
- Congestive heart failure (CHF).
To download, visit www.hospitalmedicine.org/qi.
Start Planning for Hospital Medicine 2016
At the end of the year, many hospitalists still have funds left in their CME stipends. Now is the time to plan for 2016.
SHM’s annual meeting, Hospital Medicine 2016 (HM16), is the premier destination for hospitalists looking to brush up on skills, learn the latest clinical and management concepts, and grow their network in a growing field. Last year, more than one in 10 meeting attendees said they would recommend SHM’s annual meeting to a colleague.
The meeting is divided into two sections: a day of in-depth pre-courses and the official program. This year’s pre-courses include:
- ABIM Maintenance of Certification and board preparation;
- Bedside procedures;
- Point-of-care ultrasound for the hospitalist;
- Perioperative Medicine: Spy into the world of perioperative medicine;
- The Highly Effective Hospital Medicine Group: Using SHM’s key characteristics to drive performance; and
- Advanced interactive critical care.
The official meeting’s content is broken up into separate tracks, making it easier for hospitalists to schedule their sessions based on their roles and interests in the hospital medicine movement. HM16 tracks feature many favorites, with updated content and three entirely new tracks.
- Academic/Research: This combined track is for hospitalists who practice in academic medical centers, as well as for those in any setting who are interested in research.
- Clinical: The clinical track focuses on essential topics in adult clinical medicine, emphasizing recent advances that should be incorporated into the hospitalist’s approach to clinical care delivery.
- NEW! Co-Management/Perioperative Medicine: This hospitalist core competency increases in complexity, yet many physicians were not even taught the basics in residency. This new track explores the perioperative and consultative medicine questions that challenge hospitalists on a daily basis.
- Doctor-Patient Relationship: As service is a quality outcome, this track is dedicated to giving frontline hospitalists practical skills for success in enhancing the doctor-patient relationship and information on what to do when the therapeutic relationship fails.
- NEW! Health IT for Hospitalists: Health information technology has changed the practice of medicine. While the potential is great, the result is often inefficiency and frustration, coupled with a lack of improvement in quality and safety. All hospitalists need to properly utilize the available technology and engage in improving these systems.
- Pediatric: The 2016 pediatric track focuses on “The Edges of the Practice” and will highlight hot topics and issues for pediatric hospitalists who take care of newborns and/or adolescents.
- NEW! Post-Acute Care: The post-acute care track targets two audiences. The first—mainstream hospitalists—are increasingly being asked to assume responsibility for the full episode of care, including the post-acute care services after discharge.
- Potpourri: The potpourri track offers presentations on a variety of nonclinical topics of interest to the practicing hospitalist. Topics include medical-legal aspects of hospital medicine, best practices and strategies to improve sleep in the hospital, and personal professional optimization.
- Practice Management: The importance of organizational infrastructure related to the practice of hospital medicine is well recognized, and information in this area continues to accumulate.
- Quality: Given the importance of quality and patient safety in the delivery of healthcare, the quality track is offered to address the imperatives around development and implementation of improvement efforts in the hospital.
- Rapid Fire: The rapid fire track is designed to provide participants with “rapid bursts” of content and to address specific questions framed by the Annual Meeting Committee.
- Workshops: Workshop track topics were submitted by SHM members, peer reviewed, and selected based on their relevancy to hospitalists, designed engagement and interaction with participants, presenter experience, and clarity of submission.
- Young Hospitalists: Inspired by SHM Past President Eric Howell’s vision to increase the hospital medicine “pipeline,” this track is dedicated to new hospitalists, residents, and medical students—the future of SHM.
For details, visit www.hospitalmedicine2016.org.
Call for HM16 RIV Abstracts
Seize the opportunity to present your research, innovative ideas, and clinical stories to a national audience at HM16.
Visit the submission site for full details. To ensure success, SHM strongly recommends that you complete your submission well ahead of the deadline of Wednesday, Dec. 1, 2015, at 11:30 p.m. EST.
For details, visit www.hospitalmedicine2016.org.
At the end of the year, many hospitalists still have funds left in their CME stipends. Now is the time to plan for 2016.
SHM’s annual meeting, Hospital Medicine 2016 (HM16), is the premier destination for hospitalists looking to brush up on skills, learn the latest clinical and management concepts, and grow their network in a growing field. Last year, more than one in 10 meeting attendees said they would recommend SHM’s annual meeting to a colleague.
The meeting is divided into two sections: a day of in-depth pre-courses and the official program. This year’s pre-courses include:
- ABIM Maintenance of Certification and board preparation;
- Bedside procedures;
- Point-of-care ultrasound for the hospitalist;
- Perioperative Medicine: Spy into the world of perioperative medicine;
- The Highly Effective Hospital Medicine Group: Using SHM’s key characteristics to drive performance; and
- Advanced interactive critical care.
The official meeting’s content is broken up into separate tracks, making it easier for hospitalists to schedule their sessions based on their roles and interests in the hospital medicine movement. HM16 tracks feature many favorites, with updated content and three entirely new tracks.
- Academic/Research: This combined track is for hospitalists who practice in academic medical centers, as well as for those in any setting who are interested in research.
- Clinical: The clinical track focuses on essential topics in adult clinical medicine, emphasizing recent advances that should be incorporated into the hospitalist’s approach to clinical care delivery.
- NEW! Co-Management/Perioperative Medicine: This hospitalist core competency increases in complexity, yet many physicians were not even taught the basics in residency. This new track explores the perioperative and consultative medicine questions that challenge hospitalists on a daily basis.
- Doctor-Patient Relationship: As service is a quality outcome, this track is dedicated to giving frontline hospitalists practical skills for success in enhancing the doctor-patient relationship and information on what to do when the therapeutic relationship fails.
- NEW! Health IT for Hospitalists: Health information technology has changed the practice of medicine. While the potential is great, the result is often inefficiency and frustration, coupled with a lack of improvement in quality and safety. All hospitalists need to properly utilize the available technology and engage in improving these systems.
- Pediatric: The 2016 pediatric track focuses on “The Edges of the Practice” and will highlight hot topics and issues for pediatric hospitalists who take care of newborns and/or adolescents.
- NEW! Post-Acute Care: The post-acute care track targets two audiences. The first—mainstream hospitalists—are increasingly being asked to assume responsibility for the full episode of care, including the post-acute care services after discharge.
- Potpourri: The potpourri track offers presentations on a variety of nonclinical topics of interest to the practicing hospitalist. Topics include medical-legal aspects of hospital medicine, best practices and strategies to improve sleep in the hospital, and personal professional optimization.
- Practice Management: The importance of organizational infrastructure related to the practice of hospital medicine is well recognized, and information in this area continues to accumulate.
- Quality: Given the importance of quality and patient safety in the delivery of healthcare, the quality track is offered to address the imperatives around development and implementation of improvement efforts in the hospital.
- Rapid Fire: The rapid fire track is designed to provide participants with “rapid bursts” of content and to address specific questions framed by the Annual Meeting Committee.
- Workshops: Workshop track topics were submitted by SHM members, peer reviewed, and selected based on their relevancy to hospitalists, designed engagement and interaction with participants, presenter experience, and clarity of submission.
- Young Hospitalists: Inspired by SHM Past President Eric Howell’s vision to increase the hospital medicine “pipeline,” this track is dedicated to new hospitalists, residents, and medical students—the future of SHM.
For details, visit www.hospitalmedicine2016.org.
Call for HM16 RIV Abstracts
Seize the opportunity to present your research, innovative ideas, and clinical stories to a national audience at HM16.
Visit the submission site for full details. To ensure success, SHM strongly recommends that you complete your submission well ahead of the deadline of Wednesday, Dec. 1, 2015, at 11:30 p.m. EST.
For details, visit www.hospitalmedicine2016.org.
At the end of the year, many hospitalists still have funds left in their CME stipends. Now is the time to plan for 2016.
SHM’s annual meeting, Hospital Medicine 2016 (HM16), is the premier destination for hospitalists looking to brush up on skills, learn the latest clinical and management concepts, and grow their network in a growing field. Last year, more than one in 10 meeting attendees said they would recommend SHM’s annual meeting to a colleague.
The meeting is divided into two sections: a day of in-depth pre-courses and the official program. This year’s pre-courses include:
- ABIM Maintenance of Certification and board preparation;
- Bedside procedures;
- Point-of-care ultrasound for the hospitalist;
- Perioperative Medicine: Spy into the world of perioperative medicine;
- The Highly Effective Hospital Medicine Group: Using SHM’s key characteristics to drive performance; and
- Advanced interactive critical care.
The official meeting’s content is broken up into separate tracks, making it easier for hospitalists to schedule their sessions based on their roles and interests in the hospital medicine movement. HM16 tracks feature many favorites, with updated content and three entirely new tracks.
- Academic/Research: This combined track is for hospitalists who practice in academic medical centers, as well as for those in any setting who are interested in research.
- Clinical: The clinical track focuses on essential topics in adult clinical medicine, emphasizing recent advances that should be incorporated into the hospitalist’s approach to clinical care delivery.
- NEW! Co-Management/Perioperative Medicine: This hospitalist core competency increases in complexity, yet many physicians were not even taught the basics in residency. This new track explores the perioperative and consultative medicine questions that challenge hospitalists on a daily basis.
- Doctor-Patient Relationship: As service is a quality outcome, this track is dedicated to giving frontline hospitalists practical skills for success in enhancing the doctor-patient relationship and information on what to do when the therapeutic relationship fails.
- NEW! Health IT for Hospitalists: Health information technology has changed the practice of medicine. While the potential is great, the result is often inefficiency and frustration, coupled with a lack of improvement in quality and safety. All hospitalists need to properly utilize the available technology and engage in improving these systems.
- Pediatric: The 2016 pediatric track focuses on “The Edges of the Practice” and will highlight hot topics and issues for pediatric hospitalists who take care of newborns and/or adolescents.
- NEW! Post-Acute Care: The post-acute care track targets two audiences. The first—mainstream hospitalists—are increasingly being asked to assume responsibility for the full episode of care, including the post-acute care services after discharge.
- Potpourri: The potpourri track offers presentations on a variety of nonclinical topics of interest to the practicing hospitalist. Topics include medical-legal aspects of hospital medicine, best practices and strategies to improve sleep in the hospital, and personal professional optimization.
- Practice Management: The importance of organizational infrastructure related to the practice of hospital medicine is well recognized, and information in this area continues to accumulate.
- Quality: Given the importance of quality and patient safety in the delivery of healthcare, the quality track is offered to address the imperatives around development and implementation of improvement efforts in the hospital.
- Rapid Fire: The rapid fire track is designed to provide participants with “rapid bursts” of content and to address specific questions framed by the Annual Meeting Committee.
- Workshops: Workshop track topics were submitted by SHM members, peer reviewed, and selected based on their relevancy to hospitalists, designed engagement and interaction with participants, presenter experience, and clarity of submission.
- Young Hospitalists: Inspired by SHM Past President Eric Howell’s vision to increase the hospital medicine “pipeline,” this track is dedicated to new hospitalists, residents, and medical students—the future of SHM.
For details, visit www.hospitalmedicine2016.org.
Call for HM16 RIV Abstracts
Seize the opportunity to present your research, innovative ideas, and clinical stories to a national audience at HM16.
Visit the submission site for full details. To ensure success, SHM strongly recommends that you complete your submission well ahead of the deadline of Wednesday, Dec. 1, 2015, at 11:30 p.m. EST.
For details, visit www.hospitalmedicine2016.org.
Mark Your Calendar: Dates for Hospitalists
There are always ways to get more involved in the hospital medicine movement, and deadlines for some of them are approaching:
- November 22 – Fellows and Senior Fellows Applications: Interested in earning the only designations specifically for hospitalists? Start your application today; it can take some time to assemble and submit.
- December 31 – Membership Ambassadors: Through the end of the year, all active SHM members can earn 2016-2017 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
- February 15, 2016 – Student Hospitalist Scholarship: Are you an aspiring medical student who’s joined the hospitalist movement? Apply for the Student Hospitalist Scholarship and receive a stipend to support your research over the summer of 2016.
There are always ways to get more involved in the hospital medicine movement, and deadlines for some of them are approaching:
- November 22 – Fellows and Senior Fellows Applications: Interested in earning the only designations specifically for hospitalists? Start your application today; it can take some time to assemble and submit.
- December 31 – Membership Ambassadors: Through the end of the year, all active SHM members can earn 2016-2017 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
- February 15, 2016 – Student Hospitalist Scholarship: Are you an aspiring medical student who’s joined the hospitalist movement? Apply for the Student Hospitalist Scholarship and receive a stipend to support your research over the summer of 2016.
There are always ways to get more involved in the hospital medicine movement, and deadlines for some of them are approaching:
- November 22 – Fellows and Senior Fellows Applications: Interested in earning the only designations specifically for hospitalists? Start your application today; it can take some time to assemble and submit.
- December 31 – Membership Ambassadors: Through the end of the year, all active SHM members can earn 2016-2017 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
- February 15, 2016 – Student Hospitalist Scholarship: Are you an aspiring medical student who’s joined the hospitalist movement? Apply for the Student Hospitalist Scholarship and receive a stipend to support your research over the summer of 2016.