User login
Storytelling that heals: Movies and families
Movies are storytelling in high definition. Have you ever used movies to teach your students or residents? Have you ever prescribed therapeutic movie watching to your patients, families, or couples? Movie watching helps us to understand and process the challenges we face. Movie watching helps us to imagine new stories for ourselves.
Stories connect us to our own families. The experiences of our elders and other family members are the story of our family and give coherence to our own life story. We may identify with a family member whose life experience resonates with our life experience; maybe it is the relative who perseveres, or the black sheep, or the one with depression, or the pioneer, or the one who settles for happiness or the alcoholic or workaholic. Maybe we cherish stories that connect us to our generation rather than our family, such as stories about a generational war experience or the stories and camaraderie of Alcoholics Anonymous meetings.
Stories help us understand ourselves. Stories give us scripts, maps, mental models, metaphors, and narratives to help us navigate through our lives. Stories are how we explain our lives to others, reach decisions, understand our place in the world, create our identities, and define and teach social and moral values. Many children come to understand the morals of the world by reading about the lives of animals in Aesop’s Fables. How often do parents have to read the same story over and over to their child? Familiar stories give our lives a narrative structure that is familiar, predictable, and comforting.
Storytelling is fundamental to all cultures. Stories live in our imagination. We can rehearse imagined experiences and imagine our life story in many different ways. Stories help us step out, step forward, see the world and ourselves. Story reading and movie watching help us imagine. Psychotherapy is a therapeutic way to help patients relinquish old stories and begin to construct a new story about themselves.
Movie watching can be used in therapy and has been studied as a therapeutic tool. Cinema therapy, or movie therapy, is considered a form of supplemental therapy and a form of self-help. Cinema therapy was popularized by Gary Solomon, Ph.D., the first to write on using movies as therapy (“The Motion Picture Prescription: Watch This Movie and Call Me in the Morning.” Santa Rosa, Calif.: Aslan Publishing,1995).
Watching movies was used in one arm of the first long-term investigation to compare different types of early marriage intervention programs. In this study, 174 couples in their first 3 years of marriage were enrolled in one of three treatment arms: conflict management, compassion and acceptance training, or relationship awareness through film. Each arm was equally effective in reducing the 3-year divorce/separation rate for newlyweds from 24% to 11%.
The first arm
The conflict management group learned “active listening” or the “speaker-listener technique.” This technique slows down communication and helps individuals focus on what their partner is saying. The practice requires one spouse to listen and then paraphrase back to the partner what the spouse heard to ensure the message has been properly understood. This technique has been shown to be effective at promoting happier and more satisfying relationships for 3-5 years (see the Prevention and Relationship Enhancement Program). This model provides couples with training in communication and problem-solving skills, as well as relationship expectations, friendship, and commitment.
The second arm
The compassion and acceptance training cohort focused on couples working as a team. Through a series of lectures and exercises, couples were taught to approach their relationships with more compassion and empathy by doing things like listening as a friend, practicing random acts of kindness and affection, and using the language of acceptance. Both programs involved weekly lectures, supervised practice sessions, and homework assignments over the course of a month, for a total investment of roughly 20 hours, with 18 hours of therapy time.
The third arm
The movie-and-talk group attended a 10-minute lecture on the importance of relationship awareness and how watching couples in movies could help spouses pay attention to their own behavior, both constructive and destructive. They spent 10 hours on “movie and talk” with 4 hours outside of the home. After each movie, the couple discussed a list of 12 questions about the screen couple’s interactions. Questions included: “How did the movie partners handle arguments? Were they able to open up and tell each other how they really felt, or did they tend to just snap at each other with anger? Did they try using humor to keep things from getting nasty?” The couple was asked to consider in what way the movie relationship was “similar to or different from your own relationship in this area.”
All couples in the third arm met with a therapist to watch “Two for the Road,” a 1967 romantic comedy about the joys and strains of young love, infidelity, and professional pressures across 10 years of a marriage. They were then led by the therapist in a guided discussion. They went home with a list of 47 movies with intimate relationships as a major plot focus, and were asked to watch 1 a week for the next month, followed by a guided discussion for about 45 minutes. For couples interested in trying the film discussions for themselves, the website ofRonald D. Rogge, Ph.D., offers interactive tools to help with the process, including lists of movies and the discussion questions used.
What happened?
The couples invested in their relationship and began to examine their own behavior. Watching a movie together and having a discussion was therapeutic but did not pathologize their relationship, nor did the partners seek to blame each other. The therapist-led marriage intervention programs available usually require trained therapists, and are expensive and intensive. Can couples work on their relationships on their own? The assumption has been that you need to teach couples skills to manage conflict; this might not be true. This study indicates that relationship storytelling can be a step that couples and families can take, on their own.
Movies also can be used in teaching
Family medicine teachers Catherine M. Weber, Ph.D., and Dr. Hugh J. Silk (Fam Med. 2007;39[5]:317-9) developed a movie-watching curriculum to deepen trainees’ understanding of patients and their families. The curriculum was divided into sections. The family and illness section focused on how family members find a will to help their loved ones, even in the midst of a lack of resources or medical futility. They watched “Lorenzo’s Oil” (1992), “The Straight Story” (2006), and “A River Runs Through It” (1992). The family and loss section examined how families become dysfunctional in the presence of the loss of a loved one and what it takes to heal. They watched “Ordinary People” (1980), “The Barbarian Invasions” (2003), and “The Trip to Bountiful” (1985). The section on family and caregiving identifies the strain of illness on family members who provide direct care to ill patients. They watched “Marvin’s Room” (1996) and “Iris” (2001). The section on the family and substance abuse identified how family members got caught in roles and included “When a Man Loves a Woman” (1994) and “The Days of Wine and Roses” (1962). The section on the extended family and illness illustrated how illness brings people together. They watched “Flawless” (1999), “My House in Umbria” (2003), “Beaches” (1988), and “The Barbarian Invasions (2003).
A more specific look at family systems is provided by the curriculum of Dr. Robin O. Winter and Bruce A. Birnberg (Fam Med. 2005;37[2]:96-8) in a series called “Family Systems at the Movies.” Their overarching objective was to promote an understanding of multigenerational issues, the impact of family secrets and family dynamics, the effect of stress on family life, and the attributes of a functional family. Concepts such as homeostasis, boundaries, coalitions, and scapegoating were then applied to the clinical setting. Selected scenes were chosen from three works: “The Joy Luck Club” (1993), “Shine” (1996), and a TV series called “Secrets and Lies” (2015-).
Regarding early marriage, Dr. Salman Ahktar and Dr. Zoe Billinkoff (Am J Psychoanal. 2011; 71[2]:110-20) used movies to discuss how identity, the development of mutuality, and the synthesis of affection and sensuality develop in early marriage. Their goal was to help therapists develop greater empathy with newly married individuals. They focused on three movies: “Barefoot in the Park” (1967), “Raising Arizona” (1987), and “The Quiet Man” (1952). Regarding divorce, Toni Mandelbaum(Am J Psychoanal. 2011;71[2]:121-33) used movies to illustrate the interplay between dependence and independence using “Eat Pray Love” (2010) and “Kramer vs. Kramer” (1979).
Many psychiatry residency programs have journal clubs and film clubs that use movies as a jumping-off point to deepen trainees’ understanding of psychopathology. Implementing these curricula is a simple way of beginning family training in residency, especially when the use of family videos are curtailed by strict confidentiality rules.
Future ‘family and film’ events
Dr. Francis G. Lu, (www.francislumd.com), a regular presenter at many conferences, including the American Psychiatric Association annual meetings, uses film to promote a deeper understanding of the world around us and within us. In 2007, he and Brother David Steindl-Rast, Ph.D., a Benedictine monk, co-led a 7-day seminar entitled, “Families in Film, Now and Forever” at Esalen Institute (go to www.gratefulness.org, then search “films”). From July 10-17, 2016, Dr. Lu and Dr. Steindl-Rast will co-lead “The Resilience of the Family in Film: Epics of Love, Loss, and Recovery” and from July 17-24, 2016, “Through Compassion to Serenity in the Mindful Viewing of Japanese and Western Films.” Registration opens in January 2016 at www.esalen.org. If you have ever attended one of Dr. Lu’s seminars at the APA, then you know you will be in for a rich experience at Esalen.
Dr. Heru is with the department of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013).
Movies are storytelling in high definition. Have you ever used movies to teach your students or residents? Have you ever prescribed therapeutic movie watching to your patients, families, or couples? Movie watching helps us to understand and process the challenges we face. Movie watching helps us to imagine new stories for ourselves.
Stories connect us to our own families. The experiences of our elders and other family members are the story of our family and give coherence to our own life story. We may identify with a family member whose life experience resonates with our life experience; maybe it is the relative who perseveres, or the black sheep, or the one with depression, or the pioneer, or the one who settles for happiness or the alcoholic or workaholic. Maybe we cherish stories that connect us to our generation rather than our family, such as stories about a generational war experience or the stories and camaraderie of Alcoholics Anonymous meetings.
Stories help us understand ourselves. Stories give us scripts, maps, mental models, metaphors, and narratives to help us navigate through our lives. Stories are how we explain our lives to others, reach decisions, understand our place in the world, create our identities, and define and teach social and moral values. Many children come to understand the morals of the world by reading about the lives of animals in Aesop’s Fables. How often do parents have to read the same story over and over to their child? Familiar stories give our lives a narrative structure that is familiar, predictable, and comforting.
Storytelling is fundamental to all cultures. Stories live in our imagination. We can rehearse imagined experiences and imagine our life story in many different ways. Stories help us step out, step forward, see the world and ourselves. Story reading and movie watching help us imagine. Psychotherapy is a therapeutic way to help patients relinquish old stories and begin to construct a new story about themselves.
Movie watching can be used in therapy and has been studied as a therapeutic tool. Cinema therapy, or movie therapy, is considered a form of supplemental therapy and a form of self-help. Cinema therapy was popularized by Gary Solomon, Ph.D., the first to write on using movies as therapy (“The Motion Picture Prescription: Watch This Movie and Call Me in the Morning.” Santa Rosa, Calif.: Aslan Publishing,1995).
Watching movies was used in one arm of the first long-term investigation to compare different types of early marriage intervention programs. In this study, 174 couples in their first 3 years of marriage were enrolled in one of three treatment arms: conflict management, compassion and acceptance training, or relationship awareness through film. Each arm was equally effective in reducing the 3-year divorce/separation rate for newlyweds from 24% to 11%.
The first arm
The conflict management group learned “active listening” or the “speaker-listener technique.” This technique slows down communication and helps individuals focus on what their partner is saying. The practice requires one spouse to listen and then paraphrase back to the partner what the spouse heard to ensure the message has been properly understood. This technique has been shown to be effective at promoting happier and more satisfying relationships for 3-5 years (see the Prevention and Relationship Enhancement Program). This model provides couples with training in communication and problem-solving skills, as well as relationship expectations, friendship, and commitment.
The second arm
The compassion and acceptance training cohort focused on couples working as a team. Through a series of lectures and exercises, couples were taught to approach their relationships with more compassion and empathy by doing things like listening as a friend, practicing random acts of kindness and affection, and using the language of acceptance. Both programs involved weekly lectures, supervised practice sessions, and homework assignments over the course of a month, for a total investment of roughly 20 hours, with 18 hours of therapy time.
The third arm
The movie-and-talk group attended a 10-minute lecture on the importance of relationship awareness and how watching couples in movies could help spouses pay attention to their own behavior, both constructive and destructive. They spent 10 hours on “movie and talk” with 4 hours outside of the home. After each movie, the couple discussed a list of 12 questions about the screen couple’s interactions. Questions included: “How did the movie partners handle arguments? Were they able to open up and tell each other how they really felt, or did they tend to just snap at each other with anger? Did they try using humor to keep things from getting nasty?” The couple was asked to consider in what way the movie relationship was “similar to or different from your own relationship in this area.”
All couples in the third arm met with a therapist to watch “Two for the Road,” a 1967 romantic comedy about the joys and strains of young love, infidelity, and professional pressures across 10 years of a marriage. They were then led by the therapist in a guided discussion. They went home with a list of 47 movies with intimate relationships as a major plot focus, and were asked to watch 1 a week for the next month, followed by a guided discussion for about 45 minutes. For couples interested in trying the film discussions for themselves, the website ofRonald D. Rogge, Ph.D., offers interactive tools to help with the process, including lists of movies and the discussion questions used.
What happened?
The couples invested in their relationship and began to examine their own behavior. Watching a movie together and having a discussion was therapeutic but did not pathologize their relationship, nor did the partners seek to blame each other. The therapist-led marriage intervention programs available usually require trained therapists, and are expensive and intensive. Can couples work on their relationships on their own? The assumption has been that you need to teach couples skills to manage conflict; this might not be true. This study indicates that relationship storytelling can be a step that couples and families can take, on their own.
Movies also can be used in teaching
Family medicine teachers Catherine M. Weber, Ph.D., and Dr. Hugh J. Silk (Fam Med. 2007;39[5]:317-9) developed a movie-watching curriculum to deepen trainees’ understanding of patients and their families. The curriculum was divided into sections. The family and illness section focused on how family members find a will to help their loved ones, even in the midst of a lack of resources or medical futility. They watched “Lorenzo’s Oil” (1992), “The Straight Story” (2006), and “A River Runs Through It” (1992). The family and loss section examined how families become dysfunctional in the presence of the loss of a loved one and what it takes to heal. They watched “Ordinary People” (1980), “The Barbarian Invasions” (2003), and “The Trip to Bountiful” (1985). The section on family and caregiving identifies the strain of illness on family members who provide direct care to ill patients. They watched “Marvin’s Room” (1996) and “Iris” (2001). The section on the family and substance abuse identified how family members got caught in roles and included “When a Man Loves a Woman” (1994) and “The Days of Wine and Roses” (1962). The section on the extended family and illness illustrated how illness brings people together. They watched “Flawless” (1999), “My House in Umbria” (2003), “Beaches” (1988), and “The Barbarian Invasions (2003).
A more specific look at family systems is provided by the curriculum of Dr. Robin O. Winter and Bruce A. Birnberg (Fam Med. 2005;37[2]:96-8) in a series called “Family Systems at the Movies.” Their overarching objective was to promote an understanding of multigenerational issues, the impact of family secrets and family dynamics, the effect of stress on family life, and the attributes of a functional family. Concepts such as homeostasis, boundaries, coalitions, and scapegoating were then applied to the clinical setting. Selected scenes were chosen from three works: “The Joy Luck Club” (1993), “Shine” (1996), and a TV series called “Secrets and Lies” (2015-).
Regarding early marriage, Dr. Salman Ahktar and Dr. Zoe Billinkoff (Am J Psychoanal. 2011; 71[2]:110-20) used movies to discuss how identity, the development of mutuality, and the synthesis of affection and sensuality develop in early marriage. Their goal was to help therapists develop greater empathy with newly married individuals. They focused on three movies: “Barefoot in the Park” (1967), “Raising Arizona” (1987), and “The Quiet Man” (1952). Regarding divorce, Toni Mandelbaum(Am J Psychoanal. 2011;71[2]:121-33) used movies to illustrate the interplay between dependence and independence using “Eat Pray Love” (2010) and “Kramer vs. Kramer” (1979).
Many psychiatry residency programs have journal clubs and film clubs that use movies as a jumping-off point to deepen trainees’ understanding of psychopathology. Implementing these curricula is a simple way of beginning family training in residency, especially when the use of family videos are curtailed by strict confidentiality rules.
Future ‘family and film’ events
Dr. Francis G. Lu, (www.francislumd.com), a regular presenter at many conferences, including the American Psychiatric Association annual meetings, uses film to promote a deeper understanding of the world around us and within us. In 2007, he and Brother David Steindl-Rast, Ph.D., a Benedictine monk, co-led a 7-day seminar entitled, “Families in Film, Now and Forever” at Esalen Institute (go to www.gratefulness.org, then search “films”). From July 10-17, 2016, Dr. Lu and Dr. Steindl-Rast will co-lead “The Resilience of the Family in Film: Epics of Love, Loss, and Recovery” and from July 17-24, 2016, “Through Compassion to Serenity in the Mindful Viewing of Japanese and Western Films.” Registration opens in January 2016 at www.esalen.org. If you have ever attended one of Dr. Lu’s seminars at the APA, then you know you will be in for a rich experience at Esalen.
Dr. Heru is with the department of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013).
Movies are storytelling in high definition. Have you ever used movies to teach your students or residents? Have you ever prescribed therapeutic movie watching to your patients, families, or couples? Movie watching helps us to understand and process the challenges we face. Movie watching helps us to imagine new stories for ourselves.
Stories connect us to our own families. The experiences of our elders and other family members are the story of our family and give coherence to our own life story. We may identify with a family member whose life experience resonates with our life experience; maybe it is the relative who perseveres, or the black sheep, or the one with depression, or the pioneer, or the one who settles for happiness or the alcoholic or workaholic. Maybe we cherish stories that connect us to our generation rather than our family, such as stories about a generational war experience or the stories and camaraderie of Alcoholics Anonymous meetings.
Stories help us understand ourselves. Stories give us scripts, maps, mental models, metaphors, and narratives to help us navigate through our lives. Stories are how we explain our lives to others, reach decisions, understand our place in the world, create our identities, and define and teach social and moral values. Many children come to understand the morals of the world by reading about the lives of animals in Aesop’s Fables. How often do parents have to read the same story over and over to their child? Familiar stories give our lives a narrative structure that is familiar, predictable, and comforting.
Storytelling is fundamental to all cultures. Stories live in our imagination. We can rehearse imagined experiences and imagine our life story in many different ways. Stories help us step out, step forward, see the world and ourselves. Story reading and movie watching help us imagine. Psychotherapy is a therapeutic way to help patients relinquish old stories and begin to construct a new story about themselves.
Movie watching can be used in therapy and has been studied as a therapeutic tool. Cinema therapy, or movie therapy, is considered a form of supplemental therapy and a form of self-help. Cinema therapy was popularized by Gary Solomon, Ph.D., the first to write on using movies as therapy (“The Motion Picture Prescription: Watch This Movie and Call Me in the Morning.” Santa Rosa, Calif.: Aslan Publishing,1995).
Watching movies was used in one arm of the first long-term investigation to compare different types of early marriage intervention programs. In this study, 174 couples in their first 3 years of marriage were enrolled in one of three treatment arms: conflict management, compassion and acceptance training, or relationship awareness through film. Each arm was equally effective in reducing the 3-year divorce/separation rate for newlyweds from 24% to 11%.
The first arm
The conflict management group learned “active listening” or the “speaker-listener technique.” This technique slows down communication and helps individuals focus on what their partner is saying. The practice requires one spouse to listen and then paraphrase back to the partner what the spouse heard to ensure the message has been properly understood. This technique has been shown to be effective at promoting happier and more satisfying relationships for 3-5 years (see the Prevention and Relationship Enhancement Program). This model provides couples with training in communication and problem-solving skills, as well as relationship expectations, friendship, and commitment.
The second arm
The compassion and acceptance training cohort focused on couples working as a team. Through a series of lectures and exercises, couples were taught to approach their relationships with more compassion and empathy by doing things like listening as a friend, practicing random acts of kindness and affection, and using the language of acceptance. Both programs involved weekly lectures, supervised practice sessions, and homework assignments over the course of a month, for a total investment of roughly 20 hours, with 18 hours of therapy time.
The third arm
The movie-and-talk group attended a 10-minute lecture on the importance of relationship awareness and how watching couples in movies could help spouses pay attention to their own behavior, both constructive and destructive. They spent 10 hours on “movie and talk” with 4 hours outside of the home. After each movie, the couple discussed a list of 12 questions about the screen couple’s interactions. Questions included: “How did the movie partners handle arguments? Were they able to open up and tell each other how they really felt, or did they tend to just snap at each other with anger? Did they try using humor to keep things from getting nasty?” The couple was asked to consider in what way the movie relationship was “similar to or different from your own relationship in this area.”
All couples in the third arm met with a therapist to watch “Two for the Road,” a 1967 romantic comedy about the joys and strains of young love, infidelity, and professional pressures across 10 years of a marriage. They were then led by the therapist in a guided discussion. They went home with a list of 47 movies with intimate relationships as a major plot focus, and were asked to watch 1 a week for the next month, followed by a guided discussion for about 45 minutes. For couples interested in trying the film discussions for themselves, the website ofRonald D. Rogge, Ph.D., offers interactive tools to help with the process, including lists of movies and the discussion questions used.
What happened?
The couples invested in their relationship and began to examine their own behavior. Watching a movie together and having a discussion was therapeutic but did not pathologize their relationship, nor did the partners seek to blame each other. The therapist-led marriage intervention programs available usually require trained therapists, and are expensive and intensive. Can couples work on their relationships on their own? The assumption has been that you need to teach couples skills to manage conflict; this might not be true. This study indicates that relationship storytelling can be a step that couples and families can take, on their own.
Movies also can be used in teaching
Family medicine teachers Catherine M. Weber, Ph.D., and Dr. Hugh J. Silk (Fam Med. 2007;39[5]:317-9) developed a movie-watching curriculum to deepen trainees’ understanding of patients and their families. The curriculum was divided into sections. The family and illness section focused on how family members find a will to help their loved ones, even in the midst of a lack of resources or medical futility. They watched “Lorenzo’s Oil” (1992), “The Straight Story” (2006), and “A River Runs Through It” (1992). The family and loss section examined how families become dysfunctional in the presence of the loss of a loved one and what it takes to heal. They watched “Ordinary People” (1980), “The Barbarian Invasions” (2003), and “The Trip to Bountiful” (1985). The section on family and caregiving identifies the strain of illness on family members who provide direct care to ill patients. They watched “Marvin’s Room” (1996) and “Iris” (2001). The section on the family and substance abuse identified how family members got caught in roles and included “When a Man Loves a Woman” (1994) and “The Days of Wine and Roses” (1962). The section on the extended family and illness illustrated how illness brings people together. They watched “Flawless” (1999), “My House in Umbria” (2003), “Beaches” (1988), and “The Barbarian Invasions (2003).
A more specific look at family systems is provided by the curriculum of Dr. Robin O. Winter and Bruce A. Birnberg (Fam Med. 2005;37[2]:96-8) in a series called “Family Systems at the Movies.” Their overarching objective was to promote an understanding of multigenerational issues, the impact of family secrets and family dynamics, the effect of stress on family life, and the attributes of a functional family. Concepts such as homeostasis, boundaries, coalitions, and scapegoating were then applied to the clinical setting. Selected scenes were chosen from three works: “The Joy Luck Club” (1993), “Shine” (1996), and a TV series called “Secrets and Lies” (2015-).
Regarding early marriage, Dr. Salman Ahktar and Dr. Zoe Billinkoff (Am J Psychoanal. 2011; 71[2]:110-20) used movies to discuss how identity, the development of mutuality, and the synthesis of affection and sensuality develop in early marriage. Their goal was to help therapists develop greater empathy with newly married individuals. They focused on three movies: “Barefoot in the Park” (1967), “Raising Arizona” (1987), and “The Quiet Man” (1952). Regarding divorce, Toni Mandelbaum(Am J Psychoanal. 2011;71[2]:121-33) used movies to illustrate the interplay between dependence and independence using “Eat Pray Love” (2010) and “Kramer vs. Kramer” (1979).
Many psychiatry residency programs have journal clubs and film clubs that use movies as a jumping-off point to deepen trainees’ understanding of psychopathology. Implementing these curricula is a simple way of beginning family training in residency, especially when the use of family videos are curtailed by strict confidentiality rules.
Future ‘family and film’ events
Dr. Francis G. Lu, (www.francislumd.com), a regular presenter at many conferences, including the American Psychiatric Association annual meetings, uses film to promote a deeper understanding of the world around us and within us. In 2007, he and Brother David Steindl-Rast, Ph.D., a Benedictine monk, co-led a 7-day seminar entitled, “Families in Film, Now and Forever” at Esalen Institute (go to www.gratefulness.org, then search “films”). From July 10-17, 2016, Dr. Lu and Dr. Steindl-Rast will co-lead “The Resilience of the Family in Film: Epics of Love, Loss, and Recovery” and from July 17-24, 2016, “Through Compassion to Serenity in the Mindful Viewing of Japanese and Western Films.” Registration opens in January 2016 at www.esalen.org. If you have ever attended one of Dr. Lu’s seminars at the APA, then you know you will be in for a rich experience at Esalen.
Dr. Heru is with the department of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013).
Beta-Blockers Increase Mortality in Hypertensive Patients
NEW YORK - Perioperative beta-blocker use in patients with hypertension is associated with increased cardiovascular complications and mortality after noncardiac surgery, researchers from Denmark report.
"The consistency of the findings of increased risks associated with beta-blockers across numerous subgroups was an important finding," Dr. Mads Emil Jorgensen from Gentofte Hospital in Hellerup said by email. "It supports our concern that in a low-risk population, beta-blocker-associated risks may outweigh the advantages of the treatment, in a wide range of low-risk patients."
Several studies have shown that perioperative beta-blocker use may benefit patients at high cardiac risk, but may increase mortality in others. Guidelines from the United States and Europe support continuing beta-blocker use perioperatively in patients already using being treated with them, but literature support for these recommendations is sparse.
Dr. Jorgensen's team used data from Statistics Denmark to investigate whether perioperative beta-blocker use is associated with an increased risk of major adverse cardiovascular events (MACEs) and all-cause mortality in the 30 days after noncardiac surgery in hypertensive patients free of cardiac, renal, and liver disease.
Among 14,644 patients treated with beta-blockers and 40,676 patients treated with other antihypertensives, the incidence of 30-day MACEs was significantly higher among those taking beta-blockers than among those taking other antihypertensives (1.32% vs. 0.84%, p<0.001).
Thirty-day all-cause mortality was also significantly higher in the beta-blocker group than in the other antihypertensives group (1.93% vs. 1.32%, p<0.001), the researchers report in JAMA Internal Medicine, online Oct. 5.
With the exception of combinations that included beta-blockers and two other antihypertensive drugs, all regimens that included a beta-blocker were associated with significantly increased risks of MACE and all-cause mortality, compared with regimens of renin-angiotensin system inhibitors and thiazides.
Results were comparable in subgroup analyses of diabetics versus nondiabetics and patients with low risk versus elevated risk.
The elevated risks were especially notable among patients at least 70 years old (number needed to harm, NNH, 140), men (NNH, 142), and patients undergoing acute surgery (NNH, 97).
"This study, among others, presents the case that the beneficial effects of beta-blockers might be subgroup dependent and that beta-blockers may not be the safe haven that previous perioperative guidelines have suggested," Dr. Jorgensen concluded. "More studies are needed to further the understanding of patient subgroups at risk, preferably in the setting of a randomized trial."
"The use of beta-blockers in 1 out of 4 patients with uncomplicated hypertension was higher than expected, as beta-blockers are no longer first-line drugs for treating hypertension," Dr. Jorgensen noted. "The high prevalence of beta-blocker use in this patient group underlines the clinical importance of these findings."
"Although we cannot conclude on whether the therapy should be changed prior to surgery, clinicians might want to pay special attention to these otherwise low-risk patients in the perioperative setting," he said.
"It is important to keep in mind that our population consisted of hypertensive patients without cardiac, renal or liver disease; thus not all patients with hypertension receiving a beta-blocker are expected to be at increased risk," Dr. Jorgensen cautioned. "Several conditions may necessitate and fully justify the use of beta-blockers, such as congestive heart failure or recent myocardial infarction, as demonstrated in a previous study from our research group."
Dr. Mark L. Friedell from the University of Missouri Kansas City School of Medicine in Kansas City coauthored one of the earlier reports that called into question the safety of perioperative beta-blocker use in low-risk patients. He said by email, "I was surprised at the higher mortality of patients with no cardiac risk factors. But, when taking the POISE trial into consideration, it makes sense that patients with no risk factors might be the ones most harmed by hypotension/stroke since there was no counter balance of cardiac protection such as that given to the patients with 3-4 cardiac risk factors."
His conclusion: "Patients with no or 1-2 cardiac risk factors do not need to be started on a beta-blocker perioperatively to try to mitigate cardiac risk."
"I think patients with perioperative hypertension and tachycardia could probably still be treated with short-acting beta-blockers," Dr. Friedell said. "If starting a beta-blocker in preparation for surgery (3-4 cardiac risk factors), do it at least a week in advance."
Dr. Elisabetta Patorno from Harvard Medical School in Boston recently coauthored a report of patterns of beta-blocker initiation in patients undergoing noncardiac surgery.
"These findings are just one piece of information and it is important not to overreact to only one study," she cautioned in an email. "These findings should be considered in addition to other available research, recommendations from professional societies like the American Heart Association, and patient specific considerations, when deciding regarding the perioperative management of patients with hypertension."
"Doctors should also balance these findings in light of the possible problems associated with this study," Dr. Patorno said. "Beta-blockers are not typically a first-line treatment for hypertension, so it is conceivable that the study participants treated with beta-blockers may have had a longer history and greater severity of hypertension compared with the patients treated with other antihypertensive therapy (the comparison group used in this study). This suggests the groups may be inherently different, with the implication that the underlying differential severity of hypertension and not the specific treatment may be responsible for the post-surgical increased risk of cardiovascular events and mortality observed among patients treated with beta-blocker therapy."
"Finally," she said, "as this study focuses on chronic antihypertensive users only, the question of whether there is real benefit or harm associated with initiating beta-blocker therapy prior to surgery remains unanswered."
Dr. Hermann Blessberger from Johannes Kepler University Linz School of Medicine, Linz, Austria, who has coauthored several papers regarding perioperative beta-blocker use, offered similar comments.
"We learned from our meta-analysis that dosage of beta-blockers (heart rate titrated), time point of start of beta-blockers before surgery (at least 2 weeks prior to surgery to allow the circulatory system to accommodate) as well as type of beta-blocker (issue with metoprolol metabolism - blood level may be heavily increased in poor metabolizers) are crucial," he said. "Except for the type of beta-blocker (mainly metoprolol and atenolol) this information could not be provided in this article as we can read in the discussion section but may be important."
"If a patient suffers from arterial hypertension, do not use a beta-blocker for initial treatment unless a beta-blocker seems desirable for another condition (rhythm disturbances, coronary heart disease, hyperthyroidism, etc.)," he advised.
NEW YORK - Perioperative beta-blocker use in patients with hypertension is associated with increased cardiovascular complications and mortality after noncardiac surgery, researchers from Denmark report.
"The consistency of the findings of increased risks associated with beta-blockers across numerous subgroups was an important finding," Dr. Mads Emil Jorgensen from Gentofte Hospital in Hellerup said by email. "It supports our concern that in a low-risk population, beta-blocker-associated risks may outweigh the advantages of the treatment, in a wide range of low-risk patients."
Several studies have shown that perioperative beta-blocker use may benefit patients at high cardiac risk, but may increase mortality in others. Guidelines from the United States and Europe support continuing beta-blocker use perioperatively in patients already using being treated with them, but literature support for these recommendations is sparse.
Dr. Jorgensen's team used data from Statistics Denmark to investigate whether perioperative beta-blocker use is associated with an increased risk of major adverse cardiovascular events (MACEs) and all-cause mortality in the 30 days after noncardiac surgery in hypertensive patients free of cardiac, renal, and liver disease.
Among 14,644 patients treated with beta-blockers and 40,676 patients treated with other antihypertensives, the incidence of 30-day MACEs was significantly higher among those taking beta-blockers than among those taking other antihypertensives (1.32% vs. 0.84%, p<0.001).
Thirty-day all-cause mortality was also significantly higher in the beta-blocker group than in the other antihypertensives group (1.93% vs. 1.32%, p<0.001), the researchers report in JAMA Internal Medicine, online Oct. 5.
With the exception of combinations that included beta-blockers and two other antihypertensive drugs, all regimens that included a beta-blocker were associated with significantly increased risks of MACE and all-cause mortality, compared with regimens of renin-angiotensin system inhibitors and thiazides.
Results were comparable in subgroup analyses of diabetics versus nondiabetics and patients with low risk versus elevated risk.
The elevated risks were especially notable among patients at least 70 years old (number needed to harm, NNH, 140), men (NNH, 142), and patients undergoing acute surgery (NNH, 97).
"This study, among others, presents the case that the beneficial effects of beta-blockers might be subgroup dependent and that beta-blockers may not be the safe haven that previous perioperative guidelines have suggested," Dr. Jorgensen concluded. "More studies are needed to further the understanding of patient subgroups at risk, preferably in the setting of a randomized trial."
"The use of beta-blockers in 1 out of 4 patients with uncomplicated hypertension was higher than expected, as beta-blockers are no longer first-line drugs for treating hypertension," Dr. Jorgensen noted. "The high prevalence of beta-blocker use in this patient group underlines the clinical importance of these findings."
"Although we cannot conclude on whether the therapy should be changed prior to surgery, clinicians might want to pay special attention to these otherwise low-risk patients in the perioperative setting," he said.
"It is important to keep in mind that our population consisted of hypertensive patients without cardiac, renal or liver disease; thus not all patients with hypertension receiving a beta-blocker are expected to be at increased risk," Dr. Jorgensen cautioned. "Several conditions may necessitate and fully justify the use of beta-blockers, such as congestive heart failure or recent myocardial infarction, as demonstrated in a previous study from our research group."
Dr. Mark L. Friedell from the University of Missouri Kansas City School of Medicine in Kansas City coauthored one of the earlier reports that called into question the safety of perioperative beta-blocker use in low-risk patients. He said by email, "I was surprised at the higher mortality of patients with no cardiac risk factors. But, when taking the POISE trial into consideration, it makes sense that patients with no risk factors might be the ones most harmed by hypotension/stroke since there was no counter balance of cardiac protection such as that given to the patients with 3-4 cardiac risk factors."
His conclusion: "Patients with no or 1-2 cardiac risk factors do not need to be started on a beta-blocker perioperatively to try to mitigate cardiac risk."
"I think patients with perioperative hypertension and tachycardia could probably still be treated with short-acting beta-blockers," Dr. Friedell said. "If starting a beta-blocker in preparation for surgery (3-4 cardiac risk factors), do it at least a week in advance."
Dr. Elisabetta Patorno from Harvard Medical School in Boston recently coauthored a report of patterns of beta-blocker initiation in patients undergoing noncardiac surgery.
"These findings are just one piece of information and it is important not to overreact to only one study," she cautioned in an email. "These findings should be considered in addition to other available research, recommendations from professional societies like the American Heart Association, and patient specific considerations, when deciding regarding the perioperative management of patients with hypertension."
"Doctors should also balance these findings in light of the possible problems associated with this study," Dr. Patorno said. "Beta-blockers are not typically a first-line treatment for hypertension, so it is conceivable that the study participants treated with beta-blockers may have had a longer history and greater severity of hypertension compared with the patients treated with other antihypertensive therapy (the comparison group used in this study). This suggests the groups may be inherently different, with the implication that the underlying differential severity of hypertension and not the specific treatment may be responsible for the post-surgical increased risk of cardiovascular events and mortality observed among patients treated with beta-blocker therapy."
"Finally," she said, "as this study focuses on chronic antihypertensive users only, the question of whether there is real benefit or harm associated with initiating beta-blocker therapy prior to surgery remains unanswered."
Dr. Hermann Blessberger from Johannes Kepler University Linz School of Medicine, Linz, Austria, who has coauthored several papers regarding perioperative beta-blocker use, offered similar comments.
"We learned from our meta-analysis that dosage of beta-blockers (heart rate titrated), time point of start of beta-blockers before surgery (at least 2 weeks prior to surgery to allow the circulatory system to accommodate) as well as type of beta-blocker (issue with metoprolol metabolism - blood level may be heavily increased in poor metabolizers) are crucial," he said. "Except for the type of beta-blocker (mainly metoprolol and atenolol) this information could not be provided in this article as we can read in the discussion section but may be important."
"If a patient suffers from arterial hypertension, do not use a beta-blocker for initial treatment unless a beta-blocker seems desirable for another condition (rhythm disturbances, coronary heart disease, hyperthyroidism, etc.)," he advised.
NEW YORK - Perioperative beta-blocker use in patients with hypertension is associated with increased cardiovascular complications and mortality after noncardiac surgery, researchers from Denmark report.
"The consistency of the findings of increased risks associated with beta-blockers across numerous subgroups was an important finding," Dr. Mads Emil Jorgensen from Gentofte Hospital in Hellerup said by email. "It supports our concern that in a low-risk population, beta-blocker-associated risks may outweigh the advantages of the treatment, in a wide range of low-risk patients."
Several studies have shown that perioperative beta-blocker use may benefit patients at high cardiac risk, but may increase mortality in others. Guidelines from the United States and Europe support continuing beta-blocker use perioperatively in patients already using being treated with them, but literature support for these recommendations is sparse.
Dr. Jorgensen's team used data from Statistics Denmark to investigate whether perioperative beta-blocker use is associated with an increased risk of major adverse cardiovascular events (MACEs) and all-cause mortality in the 30 days after noncardiac surgery in hypertensive patients free of cardiac, renal, and liver disease.
Among 14,644 patients treated with beta-blockers and 40,676 patients treated with other antihypertensives, the incidence of 30-day MACEs was significantly higher among those taking beta-blockers than among those taking other antihypertensives (1.32% vs. 0.84%, p<0.001).
Thirty-day all-cause mortality was also significantly higher in the beta-blocker group than in the other antihypertensives group (1.93% vs. 1.32%, p<0.001), the researchers report in JAMA Internal Medicine, online Oct. 5.
With the exception of combinations that included beta-blockers and two other antihypertensive drugs, all regimens that included a beta-blocker were associated with significantly increased risks of MACE and all-cause mortality, compared with regimens of renin-angiotensin system inhibitors and thiazides.
Results were comparable in subgroup analyses of diabetics versus nondiabetics and patients with low risk versus elevated risk.
The elevated risks were especially notable among patients at least 70 years old (number needed to harm, NNH, 140), men (NNH, 142), and patients undergoing acute surgery (NNH, 97).
"This study, among others, presents the case that the beneficial effects of beta-blockers might be subgroup dependent and that beta-blockers may not be the safe haven that previous perioperative guidelines have suggested," Dr. Jorgensen concluded. "More studies are needed to further the understanding of patient subgroups at risk, preferably in the setting of a randomized trial."
"The use of beta-blockers in 1 out of 4 patients with uncomplicated hypertension was higher than expected, as beta-blockers are no longer first-line drugs for treating hypertension," Dr. Jorgensen noted. "The high prevalence of beta-blocker use in this patient group underlines the clinical importance of these findings."
"Although we cannot conclude on whether the therapy should be changed prior to surgery, clinicians might want to pay special attention to these otherwise low-risk patients in the perioperative setting," he said.
"It is important to keep in mind that our population consisted of hypertensive patients without cardiac, renal or liver disease; thus not all patients with hypertension receiving a beta-blocker are expected to be at increased risk," Dr. Jorgensen cautioned. "Several conditions may necessitate and fully justify the use of beta-blockers, such as congestive heart failure or recent myocardial infarction, as demonstrated in a previous study from our research group."
Dr. Mark L. Friedell from the University of Missouri Kansas City School of Medicine in Kansas City coauthored one of the earlier reports that called into question the safety of perioperative beta-blocker use in low-risk patients. He said by email, "I was surprised at the higher mortality of patients with no cardiac risk factors. But, when taking the POISE trial into consideration, it makes sense that patients with no risk factors might be the ones most harmed by hypotension/stroke since there was no counter balance of cardiac protection such as that given to the patients with 3-4 cardiac risk factors."
His conclusion: "Patients with no or 1-2 cardiac risk factors do not need to be started on a beta-blocker perioperatively to try to mitigate cardiac risk."
"I think patients with perioperative hypertension and tachycardia could probably still be treated with short-acting beta-blockers," Dr. Friedell said. "If starting a beta-blocker in preparation for surgery (3-4 cardiac risk factors), do it at least a week in advance."
Dr. Elisabetta Patorno from Harvard Medical School in Boston recently coauthored a report of patterns of beta-blocker initiation in patients undergoing noncardiac surgery.
"These findings are just one piece of information and it is important not to overreact to only one study," she cautioned in an email. "These findings should be considered in addition to other available research, recommendations from professional societies like the American Heart Association, and patient specific considerations, when deciding regarding the perioperative management of patients with hypertension."
"Doctors should also balance these findings in light of the possible problems associated with this study," Dr. Patorno said. "Beta-blockers are not typically a first-line treatment for hypertension, so it is conceivable that the study participants treated with beta-blockers may have had a longer history and greater severity of hypertension compared with the patients treated with other antihypertensive therapy (the comparison group used in this study). This suggests the groups may be inherently different, with the implication that the underlying differential severity of hypertension and not the specific treatment may be responsible for the post-surgical increased risk of cardiovascular events and mortality observed among patients treated with beta-blocker therapy."
"Finally," she said, "as this study focuses on chronic antihypertensive users only, the question of whether there is real benefit or harm associated with initiating beta-blocker therapy prior to surgery remains unanswered."
Dr. Hermann Blessberger from Johannes Kepler University Linz School of Medicine, Linz, Austria, who has coauthored several papers regarding perioperative beta-blocker use, offered similar comments.
"We learned from our meta-analysis that dosage of beta-blockers (heart rate titrated), time point of start of beta-blockers before surgery (at least 2 weeks prior to surgery to allow the circulatory system to accommodate) as well as type of beta-blocker (issue with metoprolol metabolism - blood level may be heavily increased in poor metabolizers) are crucial," he said. "Except for the type of beta-blocker (mainly metoprolol and atenolol) this information could not be provided in this article as we can read in the discussion section but may be important."
"If a patient suffers from arterial hypertension, do not use a beta-blocker for initial treatment unless a beta-blocker seems desirable for another condition (rhythm disturbances, coronary heart disease, hyperthyroidism, etc.)," he advised.
PTSD in Patients With Cancer
Patients with PTSD already face difficulties, but the diagnosis of a serious illness, such as cancer, can have an effect on their symptoms in a treatment-hindering way. Matthew Cordova, PhD, a staff psychologist at the VA Northern California Martinez Outpatient Clinic, explained how PTSD can create difficulties for patients when they are diagnosed with a life-threating illness like cancer.
“Clinically what we see is that these patients are triggered to experience anxiety and avoidance specifically because of their cancer experience,” explained Dr. Cordova. Patients’ symptoms also worsen with “button pushers” such as the uncertainties of a treatment setting, diagnosis, and of people who will be around them.
Dr. Cordova also spoke about the challenge in creating trust between the patient and practitioner, which requires extra effort by the practitioner. Some best practices, he suggested, were focusing on the emotional safety of the patient, being emotionally and physically present during their time together, and creating a sense of predictability.
Another important way to make treatment transitions easier for patients is having a mental health team always present at the oncology and hematology clinics. In the video below Dr. Cordova elaborated on other best practices caregivers should be aware of when treating a patient with cancer and preexisting PTSD.
Patients with PTSD already face difficulties, but the diagnosis of a serious illness, such as cancer, can have an effect on their symptoms in a treatment-hindering way. Matthew Cordova, PhD, a staff psychologist at the VA Northern California Martinez Outpatient Clinic, explained how PTSD can create difficulties for patients when they are diagnosed with a life-threating illness like cancer.
“Clinically what we see is that these patients are triggered to experience anxiety and avoidance specifically because of their cancer experience,” explained Dr. Cordova. Patients’ symptoms also worsen with “button pushers” such as the uncertainties of a treatment setting, diagnosis, and of people who will be around them.
Dr. Cordova also spoke about the challenge in creating trust between the patient and practitioner, which requires extra effort by the practitioner. Some best practices, he suggested, were focusing on the emotional safety of the patient, being emotionally and physically present during their time together, and creating a sense of predictability.
Another important way to make treatment transitions easier for patients is having a mental health team always present at the oncology and hematology clinics. In the video below Dr. Cordova elaborated on other best practices caregivers should be aware of when treating a patient with cancer and preexisting PTSD.
Patients with PTSD already face difficulties, but the diagnosis of a serious illness, such as cancer, can have an effect on their symptoms in a treatment-hindering way. Matthew Cordova, PhD, a staff psychologist at the VA Northern California Martinez Outpatient Clinic, explained how PTSD can create difficulties for patients when they are diagnosed with a life-threating illness like cancer.
“Clinically what we see is that these patients are triggered to experience anxiety and avoidance specifically because of their cancer experience,” explained Dr. Cordova. Patients’ symptoms also worsen with “button pushers” such as the uncertainties of a treatment setting, diagnosis, and of people who will be around them.
Dr. Cordova also spoke about the challenge in creating trust between the patient and practitioner, which requires extra effort by the practitioner. Some best practices, he suggested, were focusing on the emotional safety of the patient, being emotionally and physically present during their time together, and creating a sense of predictability.
Another important way to make treatment transitions easier for patients is having a mental health team always present at the oncology and hematology clinics. In the video below Dr. Cordova elaborated on other best practices caregivers should be aware of when treating a patient with cancer and preexisting PTSD.
COMMENTARY—Facilitating International Cooperation
Two years ago, I was approached when I was president of the International Headache Society (IHS) to help arrange for the Iranian Headache Association (IHA) to become a member society in the IHS. This was not hard to do, but had to be arranged correctly. After it was done, Dr. Togha, the leading headache specialist from Iran, got in touch with me to ask me to be the featured speaker at their first meeting under the auspices of the IHS in Tehran. I was a bit concerned because of what I read in the papers and saw on TV about Iran and what it thinks of America and Israel. Many friends were shocked that I was willing to go, but I thought I should go to Iran and forge the way for a good relationship, at least on the headache level, between Iran and the Western world. The Iranians helped me with my visa and travel plans and arranged a wonderful scientific program in which I and other invited guests participated.
I was treated very well and was impressed by the turnout and the high level of talks and discussion. On my first day, we made rounds at the oldest hospital in Tehran, Sina Hospital, enjoyed high-level presentations of unusual cases, and were taken on tours of several neurologic departments. The research going on was impressive, and results are published in major journals. The three-day headache meeting covered a large number of headache issues, and the presentations were detailed and accurate. The hands-on clinics were overflowing their rooms. The Iranians’ headache knowledge is on a par with that in Western societies, and I found myself learning facts I did not know. One lecture by a PhD-level pharmacologist about headache drug–drug interactions was the best I have seen on that topic.
I never felt worried about my safety and, in fact, had a wonderful trip. I enjoyed the food and the long ride to the ancient city of Isfahan with its magnificent architecture and mosaics. The Iranian neurologists and the people I met on the street seem to love Americans and Western ways. The Iranians are nice and friendly people, and I strongly encourage many Americans to visit Iran on a tour.
I look forward to many contributions of the Iranian headache doctors over the next few years and expect to see their papers in Cephalalgia and Headache and to welcome them at international conferences. Maybe Iran will sponsor a regional headache conference for members of their neighboring countries in the future. I expect neurologic and headache medicine diplomacy will advance more quickly than international diplomacy.
—Alan M. Rapoport, MD
Clinical Professor of Neurology, David Geffen School of Medicine at UCLA
and Immediate Past President of the International Headache Society.
Two years ago, I was approached when I was president of the International Headache Society (IHS) to help arrange for the Iranian Headache Association (IHA) to become a member society in the IHS. This was not hard to do, but had to be arranged correctly. After it was done, Dr. Togha, the leading headache specialist from Iran, got in touch with me to ask me to be the featured speaker at their first meeting under the auspices of the IHS in Tehran. I was a bit concerned because of what I read in the papers and saw on TV about Iran and what it thinks of America and Israel. Many friends were shocked that I was willing to go, but I thought I should go to Iran and forge the way for a good relationship, at least on the headache level, between Iran and the Western world. The Iranians helped me with my visa and travel plans and arranged a wonderful scientific program in which I and other invited guests participated.
I was treated very well and was impressed by the turnout and the high level of talks and discussion. On my first day, we made rounds at the oldest hospital in Tehran, Sina Hospital, enjoyed high-level presentations of unusual cases, and were taken on tours of several neurologic departments. The research going on was impressive, and results are published in major journals. The three-day headache meeting covered a large number of headache issues, and the presentations were detailed and accurate. The hands-on clinics were overflowing their rooms. The Iranians’ headache knowledge is on a par with that in Western societies, and I found myself learning facts I did not know. One lecture by a PhD-level pharmacologist about headache drug–drug interactions was the best I have seen on that topic.
I never felt worried about my safety and, in fact, had a wonderful trip. I enjoyed the food and the long ride to the ancient city of Isfahan with its magnificent architecture and mosaics. The Iranian neurologists and the people I met on the street seem to love Americans and Western ways. The Iranians are nice and friendly people, and I strongly encourage many Americans to visit Iran on a tour.
I look forward to many contributions of the Iranian headache doctors over the next few years and expect to see their papers in Cephalalgia and Headache and to welcome them at international conferences. Maybe Iran will sponsor a regional headache conference for members of their neighboring countries in the future. I expect neurologic and headache medicine diplomacy will advance more quickly than international diplomacy.
—Alan M. Rapoport, MD
Clinical Professor of Neurology, David Geffen School of Medicine at UCLA
and Immediate Past President of the International Headache Society.
Two years ago, I was approached when I was president of the International Headache Society (IHS) to help arrange for the Iranian Headache Association (IHA) to become a member society in the IHS. This was not hard to do, but had to be arranged correctly. After it was done, Dr. Togha, the leading headache specialist from Iran, got in touch with me to ask me to be the featured speaker at their first meeting under the auspices of the IHS in Tehran. I was a bit concerned because of what I read in the papers and saw on TV about Iran and what it thinks of America and Israel. Many friends were shocked that I was willing to go, but I thought I should go to Iran and forge the way for a good relationship, at least on the headache level, between Iran and the Western world. The Iranians helped me with my visa and travel plans and arranged a wonderful scientific program in which I and other invited guests participated.
I was treated very well and was impressed by the turnout and the high level of talks and discussion. On my first day, we made rounds at the oldest hospital in Tehran, Sina Hospital, enjoyed high-level presentations of unusual cases, and were taken on tours of several neurologic departments. The research going on was impressive, and results are published in major journals. The three-day headache meeting covered a large number of headache issues, and the presentations were detailed and accurate. The hands-on clinics were overflowing their rooms. The Iranians’ headache knowledge is on a par with that in Western societies, and I found myself learning facts I did not know. One lecture by a PhD-level pharmacologist about headache drug–drug interactions was the best I have seen on that topic.
I never felt worried about my safety and, in fact, had a wonderful trip. I enjoyed the food and the long ride to the ancient city of Isfahan with its magnificent architecture and mosaics. The Iranian neurologists and the people I met on the street seem to love Americans and Western ways. The Iranians are nice and friendly people, and I strongly encourage many Americans to visit Iran on a tour.
I look forward to many contributions of the Iranian headache doctors over the next few years and expect to see their papers in Cephalalgia and Headache and to welcome them at international conferences. Maybe Iran will sponsor a regional headache conference for members of their neighboring countries in the future. I expect neurologic and headache medicine diplomacy will advance more quickly than international diplomacy.
—Alan M. Rapoport, MD
Clinical Professor of Neurology, David Geffen School of Medicine at UCLA
and Immediate Past President of the International Headache Society.
Recurrent Omphalitis Secondary to a Hair-Containing Umbilical Foreign Body
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.
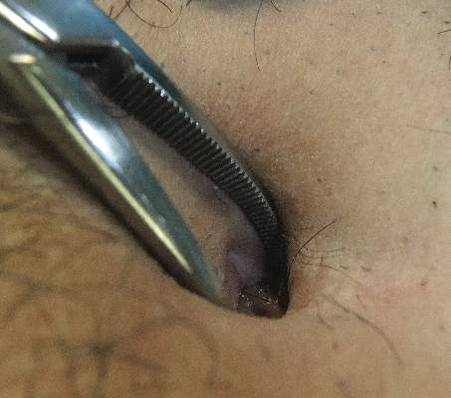
| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.

| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.

| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
Antidepressants for functional dyspepsia
Functional, a.k.a. “nonulcer,” dyspepsia is a challenging diagnosis and likely afflicts many more patients than we have identified in our practices. Functional dyspepsia (FD) is defined by the presence of postprandial fullness, early satiety, epigastric pain or burning, and no evidence of structural disease. These are the patients who do not get better with proton pump inhibitors or feel better after a bowel movement.
After a negative upper endoscopy and Helicobacter pylori stool antigen test, the task turns to symptom control. But what’s the best treatment?
Dr. Nicholas J. Talley of the University of Newcastle in Callaghan, Australia, and colleagues conducted a multicenter, randomized trial evaluating the comparative efficacy of amitriptyline or escitalopram for symptom control, gastric emptying, and meal-induced satiety in patients with FD (Gastroenterology. 2015;149(2):340-9.e2).
Participants were enrolled if they met Rome II criteria for FD requiring that folks in the preceding 12 months have at least 12 weeks of dyspepsia, absence of organic disease, and no relationship to defecation. Patients were randomized to placebo, amitriptyline 50 mg (titrated), or escitalopram 10 mg. Medication was given for 10 weeks. The primary endpoint was adequate relief of symptoms for at least 5 weeks.
A total of 292 patients (most of whom [75%] were female) with an average age of 44 years were randomized. Seventy percent had dysmotility-like FD and 30% had ulcer-like FD.
Patients with ulcer-like FD receiving amitriptyline were more likely to report adequate relief (odds ratio, 3.1; 95% confidence interval, 1.1-9.0). Neither medication affected gastric emptying or meal-induced satiety. Both medications improved overall quality of life.
The data support the use of amitriptyline for ulcer-like FD. Some of these patients may have comorbid psychiatric illness that may be improved with escitalopram. Perhaps this is what is impacting the quality-of-life metric that taps into dimensions above and beyond relief of symptoms (such as sleep disturbance or work/study).
Proton pump inhibitors tend to be overused, and many of our patients take them indefinitely without trying to see how they do off of them. Some patients for whom we have not considered a diagnosis of FD may be on PPIs because we have had nothing else to offer them. Maybe they felt better because of a PPI placebo effect and we have continued them.
If we can, we should review the diagnosis of dyspepsia, consider FD as a possibility etiology for gastrointestinal distress, stop the PPIs, and try amitriptyline.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Functional, a.k.a. “nonulcer,” dyspepsia is a challenging diagnosis and likely afflicts many more patients than we have identified in our practices. Functional dyspepsia (FD) is defined by the presence of postprandial fullness, early satiety, epigastric pain or burning, and no evidence of structural disease. These are the patients who do not get better with proton pump inhibitors or feel better after a bowel movement.
After a negative upper endoscopy and Helicobacter pylori stool antigen test, the task turns to symptom control. But what’s the best treatment?
Dr. Nicholas J. Talley of the University of Newcastle in Callaghan, Australia, and colleagues conducted a multicenter, randomized trial evaluating the comparative efficacy of amitriptyline or escitalopram for symptom control, gastric emptying, and meal-induced satiety in patients with FD (Gastroenterology. 2015;149(2):340-9.e2).
Participants were enrolled if they met Rome II criteria for FD requiring that folks in the preceding 12 months have at least 12 weeks of dyspepsia, absence of organic disease, and no relationship to defecation. Patients were randomized to placebo, amitriptyline 50 mg (titrated), or escitalopram 10 mg. Medication was given for 10 weeks. The primary endpoint was adequate relief of symptoms for at least 5 weeks.
A total of 292 patients (most of whom [75%] were female) with an average age of 44 years were randomized. Seventy percent had dysmotility-like FD and 30% had ulcer-like FD.
Patients with ulcer-like FD receiving amitriptyline were more likely to report adequate relief (odds ratio, 3.1; 95% confidence interval, 1.1-9.0). Neither medication affected gastric emptying or meal-induced satiety. Both medications improved overall quality of life.
The data support the use of amitriptyline for ulcer-like FD. Some of these patients may have comorbid psychiatric illness that may be improved with escitalopram. Perhaps this is what is impacting the quality-of-life metric that taps into dimensions above and beyond relief of symptoms (such as sleep disturbance or work/study).
Proton pump inhibitors tend to be overused, and many of our patients take them indefinitely without trying to see how they do off of them. Some patients for whom we have not considered a diagnosis of FD may be on PPIs because we have had nothing else to offer them. Maybe they felt better because of a PPI placebo effect and we have continued them.
If we can, we should review the diagnosis of dyspepsia, consider FD as a possibility etiology for gastrointestinal distress, stop the PPIs, and try amitriptyline.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Functional, a.k.a. “nonulcer,” dyspepsia is a challenging diagnosis and likely afflicts many more patients than we have identified in our practices. Functional dyspepsia (FD) is defined by the presence of postprandial fullness, early satiety, epigastric pain or burning, and no evidence of structural disease. These are the patients who do not get better with proton pump inhibitors or feel better after a bowel movement.
After a negative upper endoscopy and Helicobacter pylori stool antigen test, the task turns to symptom control. But what’s the best treatment?
Dr. Nicholas J. Talley of the University of Newcastle in Callaghan, Australia, and colleagues conducted a multicenter, randomized trial evaluating the comparative efficacy of amitriptyline or escitalopram for symptom control, gastric emptying, and meal-induced satiety in patients with FD (Gastroenterology. 2015;149(2):340-9.e2).
Participants were enrolled if they met Rome II criteria for FD requiring that folks in the preceding 12 months have at least 12 weeks of dyspepsia, absence of organic disease, and no relationship to defecation. Patients were randomized to placebo, amitriptyline 50 mg (titrated), or escitalopram 10 mg. Medication was given for 10 weeks. The primary endpoint was adequate relief of symptoms for at least 5 weeks.
A total of 292 patients (most of whom [75%] were female) with an average age of 44 years were randomized. Seventy percent had dysmotility-like FD and 30% had ulcer-like FD.
Patients with ulcer-like FD receiving amitriptyline were more likely to report adequate relief (odds ratio, 3.1; 95% confidence interval, 1.1-9.0). Neither medication affected gastric emptying or meal-induced satiety. Both medications improved overall quality of life.
The data support the use of amitriptyline for ulcer-like FD. Some of these patients may have comorbid psychiatric illness that may be improved with escitalopram. Perhaps this is what is impacting the quality-of-life metric that taps into dimensions above and beyond relief of symptoms (such as sleep disturbance or work/study).
Proton pump inhibitors tend to be overused, and many of our patients take them indefinitely without trying to see how they do off of them. Some patients for whom we have not considered a diagnosis of FD may be on PPIs because we have had nothing else to offer them. Maybe they felt better because of a PPI placebo effect and we have continued them.
If we can, we should review the diagnosis of dyspepsia, consider FD as a possibility etiology for gastrointestinal distress, stop the PPIs, and try amitriptyline.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
International Cooperation Facilitates Iranian Headache Congress
TEHRAN—For the first time, the International Headache Society (IHS) and the Iranian Headache Association (IHA) collaborated to host the Fourth Iranian International Headache Congress. IHS helped facilitate the conference, which took place from September 16 to 18, 2015, as part of its visiting professor program. The gathering marked the first such meeting since the IHA became affiliated with IHS in January 2015.
The IHA organized the congress, which was sponsored by Sanofi (Paris) and Dr. Abidi Pharmaceuticals (Tehran). A total of 340 physicians and researchers participated in the congress. Guest speakers included Robert Cowan, MD, Clinical Professor of Neurology and Neurological Sciences at Stanford School of Medicine in California; Hossein Ansari, MD, Director of the Headache Clinic at the University of California, San Diego; Hayrunnisa Bolay, MD, PhD, Professor of Neurology and Algology at Gazi University in Ankara, Turkey; and Farooq Maniyar, MD, Lead for Headache Medicine at the Royal London Hospital.
The congress took place in the Olympic Hotel, one of the largest conference centers in Tehran. Its main aims were to familiarize neurologists and other specialists with the latest scientific achievements in the field of headache and to provide guidance about diagnosing and treating various headache types. During the opening ceremony, Mansoureh Togha, MD, Chair of the IHA, and Hossein Pakdaman, MD, President of the Iranian Neurological Association and Professor of Neurology at Beheshtee University School of Medicine in Tehran, welcomed attendees.
Alan M. Rapoport, MD, Clinical Professor of Neurology at David Geffen School of Medicine at the University of California, Los Angeles, was an honorary guest speaker. During his term as president of IHS, Dr. Rapoport helped IHA to become an affiliate of IHS. He briefly reviewed the benefits of IHA’s new affiliation and the services now available to IHA members. He welcomed the IHA into the international community of the IHS and discussed ways that Iranians could join the IHS and access Cephalalgia. In addition, Dr. Rapoport discussed the various travel grants, scholarships, and fellowships that the IHS makes available to its members. He expressed regret that several young Iranians had received travel grants to attend the IHS Congress in Valencia, Spain, in May 2015, but could not attend because they were unable to obtain visas. He expressed hope that the neurologists would be able to attend the next IHS Congress in Vancouver in 2017.
Abstracts and most full texts of presented lectures at the Iranian International Headache Congress were available online on the IHA website on the first day of the congress. In addition, printed copies of the abstracts were offered to all participants upon registration.
Main Lectures
Headache specialists and physicians who were considered experts in a specific field related to primary or secondary headaches presented a total of 35 lectures. Some of the highlights included Dr. Bolay’s lecture on how neurologists can use animal models and Dr. Rapoport’s lecture on the pathophysiology of migraine, which emphasized the role of calcitonin gene-related peptide (CGRP).
In addition, various lectures presenting updates in the diagnosis and treatment of primary headaches were presented, including Dr. Cowan’s talk about noncephalic migraine equivalents, Dr. Maniyar’s presentation about optimizing the treatment of acute migraine attacks, and Dr. Ansari’s review of cluster headache.
Several neurologists gave presentations about migraine variants, secondary headaches, and related subjects, such as metabolic headache and ictal epileptic headache. Dr. Togha spoke about migraine and vertigo, and Dr. Ansari provided an overview of cervicogenic headache. In addition, Leila Kuti, PhD, talked about drug interactions in headache treatment.
In his first lecture, Dr. Rapoport discussed the importance of an organized, widely used hierarchical classification for headache. He described the new International Classification of Headache Disorders III beta and indicated its changes from the previous version, as well as its potentially problematic areas.
In his final talk, Dr. Cowan used case presentations to discuss how technology can help neurologists bring expert diagnosis and treatment to patients with headache. He reviewed recent advances in technology that promise to make headache diagnosis and treatment more accurate and shorten the time that physicians spend collecting detailed histories.
In one of her three presentations, Dr. Bolay discussed the challenges in pediatric headache. She used case presentations to illustrate key points and clarify diagnostic and management challenges.
Case Presentations
Seyed Masood Nabavi, MD, Professor of Neurology at Beheshtee University School of Medicine, moderated a session dedicated to case presentations, which was one of the most popular portions of the congress. During this time, neurologists presented five challenging cases and invited responses from the audience. Attendees discussed differential diagnoses and management strategies for each case.
Workshops
Another part of the congress was devoted to workshops. Dr. Ansari moderated two workshops, the first of which was intended for younger neurologists and provided training on techniques of onabotulinumtoxinA injection for chronic migraine. The second workshop focused on peripheral nerve block techniques for headache, including sphenopalatine ganglion blocks. All participants had the opportunity to acquire hands-on experience in injection techniques and were given time to ask questions.
Panel Discussions
The congress also included two panel discussions. The first discussion examined various aspects of headache in idiopathic intracranial hypertension and idiopathic intracranial hypotension (ie, low CSF pressure headache). The panel spoke about challenging aspects of the diagnosis and management of these two secondary headache disorders. Drs. Rapoport, Cowan, and Bolay oversaw the panel and answered attendees’ questions.
The second panel, presented by Dr. Ansari and colleagues, reviewed several unapproved but potentially effective treatment modalities for headache, including migraine surgery (ie, trigger-point deactivation), nutritional treatments, exercise, and the techniques of Iranian traditional medicine.
Residents Scientific Competition
Neurology residents from 13 Iranian universities participated in a competition. First-, second-, and third-place winners were awarded valuable prizes.
In addition, 21 exhibitors presented their products and books in the exhibit hall.
The Future of Iranian Headache Medicine
The Fourth Iranian International Headache Congress was intended to advance the status of headache medicine in Iran. Positive feedback from attendees suggested that the congress had achieved its primary goal, which was to present high-quality programs and inspire neurologists and headache doctors to continue their already advanced headache work and care.
The attendance of distinguished international headache specialists, the cooperation of the International Headache Association, the participation of esteemed Iranian neurologists as lecturers, and the attendance of participants from various parts of Iran have all contributed to the establishment of a positive working relationship between the Iranian Headache Association and IHS.
The next step in the advancement of headache medicine in Iran could be a regional headache congress with the participation of the country’s neighbors. The conference’s organizers hope to replicate their successful planning, organization, and execution by hosting such a regional event in the future.
—Hossein Ansari, MD, and Mansoureh Togha, MD
TEHRAN—For the first time, the International Headache Society (IHS) and the Iranian Headache Association (IHA) collaborated to host the Fourth Iranian International Headache Congress. IHS helped facilitate the conference, which took place from September 16 to 18, 2015, as part of its visiting professor program. The gathering marked the first such meeting since the IHA became affiliated with IHS in January 2015.
The IHA organized the congress, which was sponsored by Sanofi (Paris) and Dr. Abidi Pharmaceuticals (Tehran). A total of 340 physicians and researchers participated in the congress. Guest speakers included Robert Cowan, MD, Clinical Professor of Neurology and Neurological Sciences at Stanford School of Medicine in California; Hossein Ansari, MD, Director of the Headache Clinic at the University of California, San Diego; Hayrunnisa Bolay, MD, PhD, Professor of Neurology and Algology at Gazi University in Ankara, Turkey; and Farooq Maniyar, MD, Lead for Headache Medicine at the Royal London Hospital.
The congress took place in the Olympic Hotel, one of the largest conference centers in Tehran. Its main aims were to familiarize neurologists and other specialists with the latest scientific achievements in the field of headache and to provide guidance about diagnosing and treating various headache types. During the opening ceremony, Mansoureh Togha, MD, Chair of the IHA, and Hossein Pakdaman, MD, President of the Iranian Neurological Association and Professor of Neurology at Beheshtee University School of Medicine in Tehran, welcomed attendees.
Alan M. Rapoport, MD, Clinical Professor of Neurology at David Geffen School of Medicine at the University of California, Los Angeles, was an honorary guest speaker. During his term as president of IHS, Dr. Rapoport helped IHA to become an affiliate of IHS. He briefly reviewed the benefits of IHA’s new affiliation and the services now available to IHA members. He welcomed the IHA into the international community of the IHS and discussed ways that Iranians could join the IHS and access Cephalalgia. In addition, Dr. Rapoport discussed the various travel grants, scholarships, and fellowships that the IHS makes available to its members. He expressed regret that several young Iranians had received travel grants to attend the IHS Congress in Valencia, Spain, in May 2015, but could not attend because they were unable to obtain visas. He expressed hope that the neurologists would be able to attend the next IHS Congress in Vancouver in 2017.
Abstracts and most full texts of presented lectures at the Iranian International Headache Congress were available online on the IHA website on the first day of the congress. In addition, printed copies of the abstracts were offered to all participants upon registration.
Main Lectures
Headache specialists and physicians who were considered experts in a specific field related to primary or secondary headaches presented a total of 35 lectures. Some of the highlights included Dr. Bolay’s lecture on how neurologists can use animal models and Dr. Rapoport’s lecture on the pathophysiology of migraine, which emphasized the role of calcitonin gene-related peptide (CGRP).
In addition, various lectures presenting updates in the diagnosis and treatment of primary headaches were presented, including Dr. Cowan’s talk about noncephalic migraine equivalents, Dr. Maniyar’s presentation about optimizing the treatment of acute migraine attacks, and Dr. Ansari’s review of cluster headache.
Several neurologists gave presentations about migraine variants, secondary headaches, and related subjects, such as metabolic headache and ictal epileptic headache. Dr. Togha spoke about migraine and vertigo, and Dr. Ansari provided an overview of cervicogenic headache. In addition, Leila Kuti, PhD, talked about drug interactions in headache treatment.
In his first lecture, Dr. Rapoport discussed the importance of an organized, widely used hierarchical classification for headache. He described the new International Classification of Headache Disorders III beta and indicated its changes from the previous version, as well as its potentially problematic areas.
In his final talk, Dr. Cowan used case presentations to discuss how technology can help neurologists bring expert diagnosis and treatment to patients with headache. He reviewed recent advances in technology that promise to make headache diagnosis and treatment more accurate and shorten the time that physicians spend collecting detailed histories.
In one of her three presentations, Dr. Bolay discussed the challenges in pediatric headache. She used case presentations to illustrate key points and clarify diagnostic and management challenges.
Case Presentations
Seyed Masood Nabavi, MD, Professor of Neurology at Beheshtee University School of Medicine, moderated a session dedicated to case presentations, which was one of the most popular portions of the congress. During this time, neurologists presented five challenging cases and invited responses from the audience. Attendees discussed differential diagnoses and management strategies for each case.
Workshops
Another part of the congress was devoted to workshops. Dr. Ansari moderated two workshops, the first of which was intended for younger neurologists and provided training on techniques of onabotulinumtoxinA injection for chronic migraine. The second workshop focused on peripheral nerve block techniques for headache, including sphenopalatine ganglion blocks. All participants had the opportunity to acquire hands-on experience in injection techniques and were given time to ask questions.
Panel Discussions
The congress also included two panel discussions. The first discussion examined various aspects of headache in idiopathic intracranial hypertension and idiopathic intracranial hypotension (ie, low CSF pressure headache). The panel spoke about challenging aspects of the diagnosis and management of these two secondary headache disorders. Drs. Rapoport, Cowan, and Bolay oversaw the panel and answered attendees’ questions.
The second panel, presented by Dr. Ansari and colleagues, reviewed several unapproved but potentially effective treatment modalities for headache, including migraine surgery (ie, trigger-point deactivation), nutritional treatments, exercise, and the techniques of Iranian traditional medicine.
Residents Scientific Competition
Neurology residents from 13 Iranian universities participated in a competition. First-, second-, and third-place winners were awarded valuable prizes.
In addition, 21 exhibitors presented their products and books in the exhibit hall.
The Future of Iranian Headache Medicine
The Fourth Iranian International Headache Congress was intended to advance the status of headache medicine in Iran. Positive feedback from attendees suggested that the congress had achieved its primary goal, which was to present high-quality programs and inspire neurologists and headache doctors to continue their already advanced headache work and care.
The attendance of distinguished international headache specialists, the cooperation of the International Headache Association, the participation of esteemed Iranian neurologists as lecturers, and the attendance of participants from various parts of Iran have all contributed to the establishment of a positive working relationship between the Iranian Headache Association and IHS.
The next step in the advancement of headache medicine in Iran could be a regional headache congress with the participation of the country’s neighbors. The conference’s organizers hope to replicate their successful planning, organization, and execution by hosting such a regional event in the future.
—Hossein Ansari, MD, and Mansoureh Togha, MD
TEHRAN—For the first time, the International Headache Society (IHS) and the Iranian Headache Association (IHA) collaborated to host the Fourth Iranian International Headache Congress. IHS helped facilitate the conference, which took place from September 16 to 18, 2015, as part of its visiting professor program. The gathering marked the first such meeting since the IHA became affiliated with IHS in January 2015.
The IHA organized the congress, which was sponsored by Sanofi (Paris) and Dr. Abidi Pharmaceuticals (Tehran). A total of 340 physicians and researchers participated in the congress. Guest speakers included Robert Cowan, MD, Clinical Professor of Neurology and Neurological Sciences at Stanford School of Medicine in California; Hossein Ansari, MD, Director of the Headache Clinic at the University of California, San Diego; Hayrunnisa Bolay, MD, PhD, Professor of Neurology and Algology at Gazi University in Ankara, Turkey; and Farooq Maniyar, MD, Lead for Headache Medicine at the Royal London Hospital.
The congress took place in the Olympic Hotel, one of the largest conference centers in Tehran. Its main aims were to familiarize neurologists and other specialists with the latest scientific achievements in the field of headache and to provide guidance about diagnosing and treating various headache types. During the opening ceremony, Mansoureh Togha, MD, Chair of the IHA, and Hossein Pakdaman, MD, President of the Iranian Neurological Association and Professor of Neurology at Beheshtee University School of Medicine in Tehran, welcomed attendees.
Alan M. Rapoport, MD, Clinical Professor of Neurology at David Geffen School of Medicine at the University of California, Los Angeles, was an honorary guest speaker. During his term as president of IHS, Dr. Rapoport helped IHA to become an affiliate of IHS. He briefly reviewed the benefits of IHA’s new affiliation and the services now available to IHA members. He welcomed the IHA into the international community of the IHS and discussed ways that Iranians could join the IHS and access Cephalalgia. In addition, Dr. Rapoport discussed the various travel grants, scholarships, and fellowships that the IHS makes available to its members. He expressed regret that several young Iranians had received travel grants to attend the IHS Congress in Valencia, Spain, in May 2015, but could not attend because they were unable to obtain visas. He expressed hope that the neurologists would be able to attend the next IHS Congress in Vancouver in 2017.
Abstracts and most full texts of presented lectures at the Iranian International Headache Congress were available online on the IHA website on the first day of the congress. In addition, printed copies of the abstracts were offered to all participants upon registration.
Main Lectures
Headache specialists and physicians who were considered experts in a specific field related to primary or secondary headaches presented a total of 35 lectures. Some of the highlights included Dr. Bolay’s lecture on how neurologists can use animal models and Dr. Rapoport’s lecture on the pathophysiology of migraine, which emphasized the role of calcitonin gene-related peptide (CGRP).
In addition, various lectures presenting updates in the diagnosis and treatment of primary headaches were presented, including Dr. Cowan’s talk about noncephalic migraine equivalents, Dr. Maniyar’s presentation about optimizing the treatment of acute migraine attacks, and Dr. Ansari’s review of cluster headache.
Several neurologists gave presentations about migraine variants, secondary headaches, and related subjects, such as metabolic headache and ictal epileptic headache. Dr. Togha spoke about migraine and vertigo, and Dr. Ansari provided an overview of cervicogenic headache. In addition, Leila Kuti, PhD, talked about drug interactions in headache treatment.
In his first lecture, Dr. Rapoport discussed the importance of an organized, widely used hierarchical classification for headache. He described the new International Classification of Headache Disorders III beta and indicated its changes from the previous version, as well as its potentially problematic areas.
In his final talk, Dr. Cowan used case presentations to discuss how technology can help neurologists bring expert diagnosis and treatment to patients with headache. He reviewed recent advances in technology that promise to make headache diagnosis and treatment more accurate and shorten the time that physicians spend collecting detailed histories.
In one of her three presentations, Dr. Bolay discussed the challenges in pediatric headache. She used case presentations to illustrate key points and clarify diagnostic and management challenges.
Case Presentations
Seyed Masood Nabavi, MD, Professor of Neurology at Beheshtee University School of Medicine, moderated a session dedicated to case presentations, which was one of the most popular portions of the congress. During this time, neurologists presented five challenging cases and invited responses from the audience. Attendees discussed differential diagnoses and management strategies for each case.
Workshops
Another part of the congress was devoted to workshops. Dr. Ansari moderated two workshops, the first of which was intended for younger neurologists and provided training on techniques of onabotulinumtoxinA injection for chronic migraine. The second workshop focused on peripheral nerve block techniques for headache, including sphenopalatine ganglion blocks. All participants had the opportunity to acquire hands-on experience in injection techniques and were given time to ask questions.
Panel Discussions
The congress also included two panel discussions. The first discussion examined various aspects of headache in idiopathic intracranial hypertension and idiopathic intracranial hypotension (ie, low CSF pressure headache). The panel spoke about challenging aspects of the diagnosis and management of these two secondary headache disorders. Drs. Rapoport, Cowan, and Bolay oversaw the panel and answered attendees’ questions.
The second panel, presented by Dr. Ansari and colleagues, reviewed several unapproved but potentially effective treatment modalities for headache, including migraine surgery (ie, trigger-point deactivation), nutritional treatments, exercise, and the techniques of Iranian traditional medicine.
Residents Scientific Competition
Neurology residents from 13 Iranian universities participated in a competition. First-, second-, and third-place winners were awarded valuable prizes.
In addition, 21 exhibitors presented their products and books in the exhibit hall.
The Future of Iranian Headache Medicine
The Fourth Iranian International Headache Congress was intended to advance the status of headache medicine in Iran. Positive feedback from attendees suggested that the congress had achieved its primary goal, which was to present high-quality programs and inspire neurologists and headache doctors to continue their already advanced headache work and care.
The attendance of distinguished international headache specialists, the cooperation of the International Headache Association, the participation of esteemed Iranian neurologists as lecturers, and the attendance of participants from various parts of Iran have all contributed to the establishment of a positive working relationship between the Iranian Headache Association and IHS.
The next step in the advancement of headache medicine in Iran could be a regional headache congress with the participation of the country’s neighbors. The conference’s organizers hope to replicate their successful planning, organization, and execution by hosting such a regional event in the future.
—Hossein Ansari, MD, and Mansoureh Togha, MD
EADV: Venous leg ulcers herald increased cancer risk
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.
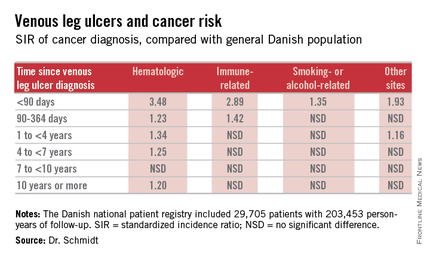
It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
AT THE EADV CONGRESS
Key clinical point: Venous leg ulceration appears to be a red flag for occult malignancy.
Major finding: During the first 89 days following diagnosis of a venous leg ulcer, affected patients are at a 3.48-fold increased risk of being diagnosed with a hematologic malignancy and a 2.89-fold greater risk of immune-related cancer compared with the general population.
Data source: This Danish nationwide cohort study compared standardized incidence ratios for various types of cancer during more than 200,000 person-years of follow-up in 29,705 patients with a venous leg ulcer vs. the general population.
Disclosures: The presenter reported having no financial conflicts of interest regarding her study, which was supported by Danish institutional funds.
New Medicare Rule Will Reimburse Physicians for Advance Care Planning
Hospitalists care for patients with the most serious, chronic, and complex illnesses. As a result, they are often faced with the daunting task of counseling their patients to help them clearly define their end-of-life wishes. The mere subject of death is met with apprehension and avoidance, but its inevitability warrants an early discussion.
End-of-life care, also known as Advance Care Planning (ACP), enables patients to formulate advanced directives: a living will, the designation of a healthcare proxy, Medical Orders for Life-Sustaining Treatment (MOLST), and the preparation for hospice care, among others. Patients should start thinking about their healthcare options and share such important decisions with their physicians and family before the need for hospitalization.
On October 30, 2015, the Centers for Medicare and Medicaid Services (CMS) released the final payment rules for Medicare reimbursement of physicians who consult with their patients on advance care planning. This separate payment system under the 2016 Physician Fee Schedule will impact the almost 55 million Medicare beneficiaries and their healthcare providers.
Effective January 1, 2016, Medicare will pay $86 for 30 minutes of ACP in a physician’s office and will pay $80 for the same service in a hospital (CPT billing code 99497). In both settings, Medicare will pay up to $75 for 30 additional minutes of consultation (add-on CPT billing code 99498). Such counseling can take place during a senior’s annual wellness visit or during a routine office visit and at various stages of health, always “at the discretion of the beneficiary.”
Six years ago, proposed legislation on Medicare reimbursement for ACP under the Accountable Care Act (ACA) sparked political debate over fears that the implementation of so-called “death panels” could influence decisions to avoid medical care. The goal was to reduce healthcare costs, but these controversial provisions were dropped with the passage of the ACA. This time, there was less resistance.
Proponents of this new legislation, such as the American Medical Association and the American Academy of Palliative and Hospice Medicine, say that this rule will encourage physicians to make time for these lengthy discussions and facilitate patient choices while improving quality of care for seniors. Opponents, including the Association of American Physicians and Surgeons, contend that such payments will “create financial incentives to persuade patients to consent to the denial of care.”
Patrick Conway, MD, CMS' chief medical officer, told the New York Times, "We received overwhelmingly positive comments about the importance of these conversations between physicians and patients. We know that many patients and families want to have these discussions."
Future endeavors should focus on efforts to improve the quality of delivering end-of-life care that honors and upholds a patient’s wishes. Strengthening the clinical training of physicians in palliative care, developing quality metrics and standards, and educating the public should remain a top priority.
Will tying a financial incentive to these services have an impact on the cost and quality of care delivered? Hospitalists can begin billing for valuable services they are already providing on a daily basis, and can better coordinate inpatient medical care when more seniors have clear advanced directives. TH
Dr. Zeitoun is a member of Team Hospitalist.
Hospitalists care for patients with the most serious, chronic, and complex illnesses. As a result, they are often faced with the daunting task of counseling their patients to help them clearly define their end-of-life wishes. The mere subject of death is met with apprehension and avoidance, but its inevitability warrants an early discussion.
End-of-life care, also known as Advance Care Planning (ACP), enables patients to formulate advanced directives: a living will, the designation of a healthcare proxy, Medical Orders for Life-Sustaining Treatment (MOLST), and the preparation for hospice care, among others. Patients should start thinking about their healthcare options and share such important decisions with their physicians and family before the need for hospitalization.
On October 30, 2015, the Centers for Medicare and Medicaid Services (CMS) released the final payment rules for Medicare reimbursement of physicians who consult with their patients on advance care planning. This separate payment system under the 2016 Physician Fee Schedule will impact the almost 55 million Medicare beneficiaries and their healthcare providers.
Effective January 1, 2016, Medicare will pay $86 for 30 minutes of ACP in a physician’s office and will pay $80 for the same service in a hospital (CPT billing code 99497). In both settings, Medicare will pay up to $75 for 30 additional minutes of consultation (add-on CPT billing code 99498). Such counseling can take place during a senior’s annual wellness visit or during a routine office visit and at various stages of health, always “at the discretion of the beneficiary.”
Six years ago, proposed legislation on Medicare reimbursement for ACP under the Accountable Care Act (ACA) sparked political debate over fears that the implementation of so-called “death panels” could influence decisions to avoid medical care. The goal was to reduce healthcare costs, but these controversial provisions were dropped with the passage of the ACA. This time, there was less resistance.
Proponents of this new legislation, such as the American Medical Association and the American Academy of Palliative and Hospice Medicine, say that this rule will encourage physicians to make time for these lengthy discussions and facilitate patient choices while improving quality of care for seniors. Opponents, including the Association of American Physicians and Surgeons, contend that such payments will “create financial incentives to persuade patients to consent to the denial of care.”
Patrick Conway, MD, CMS' chief medical officer, told the New York Times, "We received overwhelmingly positive comments about the importance of these conversations between physicians and patients. We know that many patients and families want to have these discussions."
Future endeavors should focus on efforts to improve the quality of delivering end-of-life care that honors and upholds a patient’s wishes. Strengthening the clinical training of physicians in palliative care, developing quality metrics and standards, and educating the public should remain a top priority.
Will tying a financial incentive to these services have an impact on the cost and quality of care delivered? Hospitalists can begin billing for valuable services they are already providing on a daily basis, and can better coordinate inpatient medical care when more seniors have clear advanced directives. TH
Dr. Zeitoun is a member of Team Hospitalist.
Hospitalists care for patients with the most serious, chronic, and complex illnesses. As a result, they are often faced with the daunting task of counseling their patients to help them clearly define their end-of-life wishes. The mere subject of death is met with apprehension and avoidance, but its inevitability warrants an early discussion.
End-of-life care, also known as Advance Care Planning (ACP), enables patients to formulate advanced directives: a living will, the designation of a healthcare proxy, Medical Orders for Life-Sustaining Treatment (MOLST), and the preparation for hospice care, among others. Patients should start thinking about their healthcare options and share such important decisions with their physicians and family before the need for hospitalization.
On October 30, 2015, the Centers for Medicare and Medicaid Services (CMS) released the final payment rules for Medicare reimbursement of physicians who consult with their patients on advance care planning. This separate payment system under the 2016 Physician Fee Schedule will impact the almost 55 million Medicare beneficiaries and their healthcare providers.
Effective January 1, 2016, Medicare will pay $86 for 30 minutes of ACP in a physician’s office and will pay $80 for the same service in a hospital (CPT billing code 99497). In both settings, Medicare will pay up to $75 for 30 additional minutes of consultation (add-on CPT billing code 99498). Such counseling can take place during a senior’s annual wellness visit or during a routine office visit and at various stages of health, always “at the discretion of the beneficiary.”
Six years ago, proposed legislation on Medicare reimbursement for ACP under the Accountable Care Act (ACA) sparked political debate over fears that the implementation of so-called “death panels” could influence decisions to avoid medical care. The goal was to reduce healthcare costs, but these controversial provisions were dropped with the passage of the ACA. This time, there was less resistance.
Proponents of this new legislation, such as the American Medical Association and the American Academy of Palliative and Hospice Medicine, say that this rule will encourage physicians to make time for these lengthy discussions and facilitate patient choices while improving quality of care for seniors. Opponents, including the Association of American Physicians and Surgeons, contend that such payments will “create financial incentives to persuade patients to consent to the denial of care.”
Patrick Conway, MD, CMS' chief medical officer, told the New York Times, "We received overwhelmingly positive comments about the importance of these conversations between physicians and patients. We know that many patients and families want to have these discussions."
Future endeavors should focus on efforts to improve the quality of delivering end-of-life care that honors and upholds a patient’s wishes. Strengthening the clinical training of physicians in palliative care, developing quality metrics and standards, and educating the public should remain a top priority.
Will tying a financial incentive to these services have an impact on the cost and quality of care delivered? Hospitalists can begin billing for valuable services they are already providing on a daily basis, and can better coordinate inpatient medical care when more seniors have clear advanced directives. TH
Dr. Zeitoun is a member of Team Hospitalist.
AAP: Creating safe environment aids recovery from trauma disorders
WASHINGTON – Maintaining safety and helping to ensure caregiver well-being are critical interventions for children with posttraumatic stress disorder (PTSD) and other trauma syndromes, Dr. Mary Margaret Gleason said at the annual meeting of the American Academy of Pediatrics.
“Talk a lot [with caregivers] about safety, meaning the [importance of a] lack of violence in the home and the lack of corporal punishment,” said Dr. Gleason, a pediatrician and child and adolescent psychiatrist at Tulane University in New Orleans. “And equally important, make sure that parents are taking care of themselves and are getting treatment [if they’ve also experienced trauma].”
PTSD must be treated with evidence-based therapy or it will become more entrenched with time, she said. Both child-parent psychotherapy in toddlers and preschool-aged children, and cognitive behavioral therapy (CBT) in children of preschool age and up, lead to short-term and sustained reductions in children’s symptoms.
In communities without treatment specialists and resources, stressing safety becomes even more important. Help children with PTSD and other trauma disorders to learn to recognize and label their feelings, manage emotional dysregulation, learn the principles of CBT, and achieve deep relaxation. “You can put a pulse oximeter on a child and teach them how to do deep relaxation,” she said. “You can challenge them to bring down the numbers.”
Books on CBT for general anxiety – and, increasingly, online CBT tools – can be helpful for some families, as can the National Child Traumatic Stress Network’s online resources (www.nctsn.org).
Dr. Gleason advised looking for a broad range of disorders in children who have experienced trauma – whether the trauma is acute or chronic, and whether it involves personal or community events. “You need to be thinking about PTSD, certainly, but also attention-deficit/hyperactivity disorder (ADHD), disruptive behaviors, mood disorders, separation anxiety disorders, and sleep disorders,” she said. Conversely, “if a child develops another disorder after a traumatic event, look for signs of PTSD.”
A study of children living in and around New Orleans when Hurricane Katrina struck in 2005 found a wide range of disorders as well as high prevalence of PTSD. “There were [few] children who developed new diagnoses who didn’t also have some symptoms of PTSD,” Dr. Gleason said.
The Diagnostic and Statistical Manual of Mental Disorders–5 placed PTSD in the diagnostic category of trauma- and stressor-related disorders and included separate criteria for PTSD in preschool children. PTSD broadly involves symptoms of at least 1-month duration from four symptom clusters: Intrusive thoughts (e.g. distressing dreams), avoidance, negative alterations in cognition and mood, and changed arousal and reactivity.
Preschool PTSD applies to children ages 6 years and younger, and requires fewer symptoms to be present. The arousal/reactivity symptoms cluster includes irritability and extreme tantrums, she noted.
For children of any age, it is important to note that distressing dreams do not have to involve content specific to the traumatic event to meet diagnostic criteria, Dr. Gleason said.
Compared with PTSD, less is known about the presentation in school-age children and adolescents of reactive attachment disorder (RAD) and disinhibited social engagement disorder (DSED) – two other trauma-related disorders in DSM-5. Both are related to the attachment system and require a developmental age of at least 9 months, and both involve “pathogenic care,” which can mean emotional neglect and/or persistent disregard of the child’s basic physical needs.
The child with RAD “rarely seeks comfort” when distressed, has a limited response to comfort, and has limited positive affect, Dr. Gleason explained.
“When they fall and hurt their knee, or when they build a tower and it falls over, they don’t look for anyone to help them organize their feelings. And when someone tries to help them, this doesn’t reduce their distress,” she said.
There is some overlap with the clinical presentation of autism spectrum disorder, so it is important to rule out ASD in diagnosing RAD.
Treatment, she said, entails placement in an adequate caregiving environment. “What’s really important to know about RAD is that it’s really a reflection of the current caregiving environment,” Dr. Gleason said. “It’s not about the history. It’s about the [trauma] of what’s happening now.”
DSED is quite different in its clinical construct and course. “These children are indiscriminately social,” she said. “They’ll go off with a stranger … off into new situations without ever looking back to see if their caregiver is there.”
Unlike RAD, this disorder is not associated with current caregiving quality. “It’s important for caregivers to know that this syndrome is a reflection of what happened earlier and not what’s happening now,” Dr. Gleason said. “And it does respond to quality caregiving, but very, very slowly. It can take years.”
Dr. Gleason reported that she has no relevant financial disclosures.
WASHINGTON – Maintaining safety and helping to ensure caregiver well-being are critical interventions for children with posttraumatic stress disorder (PTSD) and other trauma syndromes, Dr. Mary Margaret Gleason said at the annual meeting of the American Academy of Pediatrics.
“Talk a lot [with caregivers] about safety, meaning the [importance of a] lack of violence in the home and the lack of corporal punishment,” said Dr. Gleason, a pediatrician and child and adolescent psychiatrist at Tulane University in New Orleans. “And equally important, make sure that parents are taking care of themselves and are getting treatment [if they’ve also experienced trauma].”
PTSD must be treated with evidence-based therapy or it will become more entrenched with time, she said. Both child-parent psychotherapy in toddlers and preschool-aged children, and cognitive behavioral therapy (CBT) in children of preschool age and up, lead to short-term and sustained reductions in children’s symptoms.
In communities without treatment specialists and resources, stressing safety becomes even more important. Help children with PTSD and other trauma disorders to learn to recognize and label their feelings, manage emotional dysregulation, learn the principles of CBT, and achieve deep relaxation. “You can put a pulse oximeter on a child and teach them how to do deep relaxation,” she said. “You can challenge them to bring down the numbers.”
Books on CBT for general anxiety – and, increasingly, online CBT tools – can be helpful for some families, as can the National Child Traumatic Stress Network’s online resources (www.nctsn.org).
Dr. Gleason advised looking for a broad range of disorders in children who have experienced trauma – whether the trauma is acute or chronic, and whether it involves personal or community events. “You need to be thinking about PTSD, certainly, but also attention-deficit/hyperactivity disorder (ADHD), disruptive behaviors, mood disorders, separation anxiety disorders, and sleep disorders,” she said. Conversely, “if a child develops another disorder after a traumatic event, look for signs of PTSD.”
A study of children living in and around New Orleans when Hurricane Katrina struck in 2005 found a wide range of disorders as well as high prevalence of PTSD. “There were [few] children who developed new diagnoses who didn’t also have some symptoms of PTSD,” Dr. Gleason said.
The Diagnostic and Statistical Manual of Mental Disorders–5 placed PTSD in the diagnostic category of trauma- and stressor-related disorders and included separate criteria for PTSD in preschool children. PTSD broadly involves symptoms of at least 1-month duration from four symptom clusters: Intrusive thoughts (e.g. distressing dreams), avoidance, negative alterations in cognition and mood, and changed arousal and reactivity.
Preschool PTSD applies to children ages 6 years and younger, and requires fewer symptoms to be present. The arousal/reactivity symptoms cluster includes irritability and extreme tantrums, she noted.
For children of any age, it is important to note that distressing dreams do not have to involve content specific to the traumatic event to meet diagnostic criteria, Dr. Gleason said.
Compared with PTSD, less is known about the presentation in school-age children and adolescents of reactive attachment disorder (RAD) and disinhibited social engagement disorder (DSED) – two other trauma-related disorders in DSM-5. Both are related to the attachment system and require a developmental age of at least 9 months, and both involve “pathogenic care,” which can mean emotional neglect and/or persistent disregard of the child’s basic physical needs.
The child with RAD “rarely seeks comfort” when distressed, has a limited response to comfort, and has limited positive affect, Dr. Gleason explained.
“When they fall and hurt their knee, or when they build a tower and it falls over, they don’t look for anyone to help them organize their feelings. And when someone tries to help them, this doesn’t reduce their distress,” she said.
There is some overlap with the clinical presentation of autism spectrum disorder, so it is important to rule out ASD in diagnosing RAD.
Treatment, she said, entails placement in an adequate caregiving environment. “What’s really important to know about RAD is that it’s really a reflection of the current caregiving environment,” Dr. Gleason said. “It’s not about the history. It’s about the [trauma] of what’s happening now.”
DSED is quite different in its clinical construct and course. “These children are indiscriminately social,” she said. “They’ll go off with a stranger … off into new situations without ever looking back to see if their caregiver is there.”
Unlike RAD, this disorder is not associated with current caregiving quality. “It’s important for caregivers to know that this syndrome is a reflection of what happened earlier and not what’s happening now,” Dr. Gleason said. “And it does respond to quality caregiving, but very, very slowly. It can take years.”
Dr. Gleason reported that she has no relevant financial disclosures.
WASHINGTON – Maintaining safety and helping to ensure caregiver well-being are critical interventions for children with posttraumatic stress disorder (PTSD) and other trauma syndromes, Dr. Mary Margaret Gleason said at the annual meeting of the American Academy of Pediatrics.
“Talk a lot [with caregivers] about safety, meaning the [importance of a] lack of violence in the home and the lack of corporal punishment,” said Dr. Gleason, a pediatrician and child and adolescent psychiatrist at Tulane University in New Orleans. “And equally important, make sure that parents are taking care of themselves and are getting treatment [if they’ve also experienced trauma].”
PTSD must be treated with evidence-based therapy or it will become more entrenched with time, she said. Both child-parent psychotherapy in toddlers and preschool-aged children, and cognitive behavioral therapy (CBT) in children of preschool age and up, lead to short-term and sustained reductions in children’s symptoms.
In communities without treatment specialists and resources, stressing safety becomes even more important. Help children with PTSD and other trauma disorders to learn to recognize and label their feelings, manage emotional dysregulation, learn the principles of CBT, and achieve deep relaxation. “You can put a pulse oximeter on a child and teach them how to do deep relaxation,” she said. “You can challenge them to bring down the numbers.”
Books on CBT for general anxiety – and, increasingly, online CBT tools – can be helpful for some families, as can the National Child Traumatic Stress Network’s online resources (www.nctsn.org).
Dr. Gleason advised looking for a broad range of disorders in children who have experienced trauma – whether the trauma is acute or chronic, and whether it involves personal or community events. “You need to be thinking about PTSD, certainly, but also attention-deficit/hyperactivity disorder (ADHD), disruptive behaviors, mood disorders, separation anxiety disorders, and sleep disorders,” she said. Conversely, “if a child develops another disorder after a traumatic event, look for signs of PTSD.”
A study of children living in and around New Orleans when Hurricane Katrina struck in 2005 found a wide range of disorders as well as high prevalence of PTSD. “There were [few] children who developed new diagnoses who didn’t also have some symptoms of PTSD,” Dr. Gleason said.
The Diagnostic and Statistical Manual of Mental Disorders–5 placed PTSD in the diagnostic category of trauma- and stressor-related disorders and included separate criteria for PTSD in preschool children. PTSD broadly involves symptoms of at least 1-month duration from four symptom clusters: Intrusive thoughts (e.g. distressing dreams), avoidance, negative alterations in cognition and mood, and changed arousal and reactivity.
Preschool PTSD applies to children ages 6 years and younger, and requires fewer symptoms to be present. The arousal/reactivity symptoms cluster includes irritability and extreme tantrums, she noted.
For children of any age, it is important to note that distressing dreams do not have to involve content specific to the traumatic event to meet diagnostic criteria, Dr. Gleason said.
Compared with PTSD, less is known about the presentation in school-age children and adolescents of reactive attachment disorder (RAD) and disinhibited social engagement disorder (DSED) – two other trauma-related disorders in DSM-5. Both are related to the attachment system and require a developmental age of at least 9 months, and both involve “pathogenic care,” which can mean emotional neglect and/or persistent disregard of the child’s basic physical needs.
The child with RAD “rarely seeks comfort” when distressed, has a limited response to comfort, and has limited positive affect, Dr. Gleason explained.
“When they fall and hurt their knee, or when they build a tower and it falls over, they don’t look for anyone to help them organize their feelings. And when someone tries to help them, this doesn’t reduce their distress,” she said.
There is some overlap with the clinical presentation of autism spectrum disorder, so it is important to rule out ASD in diagnosing RAD.
Treatment, she said, entails placement in an adequate caregiving environment. “What’s really important to know about RAD is that it’s really a reflection of the current caregiving environment,” Dr. Gleason said. “It’s not about the history. It’s about the [trauma] of what’s happening now.”
DSED is quite different in its clinical construct and course. “These children are indiscriminately social,” she said. “They’ll go off with a stranger … off into new situations without ever looking back to see if their caregiver is there.”
Unlike RAD, this disorder is not associated with current caregiving quality. “It’s important for caregivers to know that this syndrome is a reflection of what happened earlier and not what’s happening now,” Dr. Gleason said. “And it does respond to quality caregiving, but very, very slowly. It can take years.”
Dr. Gleason reported that she has no relevant financial disclosures.
EXPERT ANALYSIS FROM THE AAP NATIONAL CONFERENCE