User login
LT4 therapy for SCH may improve pregnancy outcomes
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
AT ITC 2015
Key clinical point: LT4 therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores, but not with a decrease in pregnancy loss in a large retrospective cohort study.
Major finding: The frequency of low birth weight was 1.3% vs. 10%, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9% in the treated vs. untreated patients.
Data source: A retrospective cohort study of 364 women.
Disclosures: Dr. Maraka reported having no disclosures.
The Lesion That Grew Unbearable
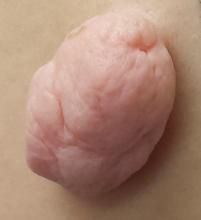
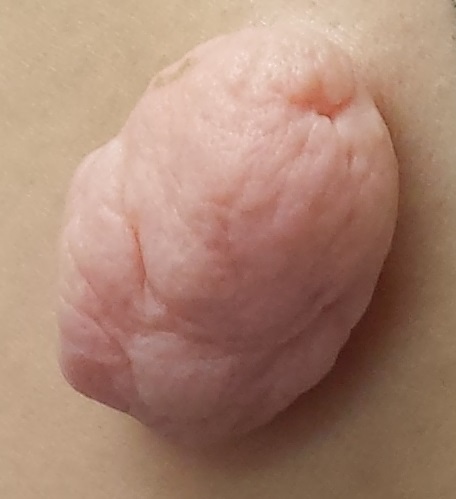
A 49-year-old woman presents to dermatology with a lesion on her back. It’s been there for at least 20 years, slowly growing to its present size; it is now so prominent that it shows through her clothes and is subject to traumatization. The visibility of the lesion, particularly when the patient wears a swimsuit, is a source of considerable embarrassment.
Notable medical history includes polycystic ovarian syndrome and related diabetes and dyslipidemia. Family history reveals that the patient’s mother died of heart disease in her 40s.
EXAMINATION
The lesion, which measures 3 cm x 3 cm, is an impressive, pedunculated, doughy, rubbery, skin-colored mass protruding from her left mid back. No redness or edema is seen on or around the lesion. On palpation, the lesion is found to be uniformly soft and rubbery.
The patient is quite obese and has numerous skin tags in skin folds, under the arms, and around the neck.
What is the diagnosis?
DISCUSSION
The lesion was excised with deep margins and submitted to pathology. The report showed benign histology, consistent with a fibroepitheliomatous polyp.
In one sense, it’s hard to believe this huge lesion was a “skin tag,” and there are other things it might have been. But the microscopic examination of the tissue proved it was simply a large version of the same skin tags we see around necks and in axillae. These lesions are known by various names, including fibroepitheliomatous polyps (FEP) and acrochordons. A more typical FEP would be the size of a grain of rice, but they can take on various forms and sizes.
There is a differential for lesions that look like FEPs; it includes seborrheic keratoses, warts, molluscum contagiosum, neurofibromas, and fibrolipomas. Even melanoma and squamous cell carcinoma can assume a tag-like morphology. So it pays to take a close look at these lesions, checking to see if they’re soft and pliable, as well as skin colored. With any odd feature, including increased size, they need to be removed and sent for pathologic examination.
Heredity appears to play a role in the development of FEPs, as do age and obesity. There are studies showing an association between FEPs and insulin resistance and others identifying FEPs as a marker for increased risk for atherosclerotic vessel disease. In any case, 59% of the population has them by age 70, with men and women affected equally. Of the general population, 46% has some sort of skin tag somewhere.
Treatment may entail cryotherapy, excision, or electrodessication. With lesions as large as the case patient’s, excision is the only reasonable option. Deep margins are required, since a “shave” would leave a gaping, open wound. For the case patient, there was no postoperative sequelae aside from some scarring.
TAKE-HOME LEARNING POINTS
• Skin tags, also known as fibroepitheliomas, fibroepitheliomatous polyps, or acrochordons, are typically the size of a grain of rice, and tend to appear in skin folds (eg, axillae, neck, groin).
• Studies have shown that there may be an association between having skin tags and developing CAD or insulin resistance.
• Obesity, age, and heredity also appear to be factors in developing skin tags.
• Large or odd tags need to be captured and sent for pathologic examination.
• Skin tags are extremely common, affecting 59% of the population older than 70.
A 49-year-old woman presents to dermatology with a lesion on her back. It’s been there for at least 20 years, slowly growing to its present size; it is now so prominent that it shows through her clothes and is subject to traumatization. The visibility of the lesion, particularly when the patient wears a swimsuit, is a source of considerable embarrassment.
Notable medical history includes polycystic ovarian syndrome and related diabetes and dyslipidemia. Family history reveals that the patient’s mother died of heart disease in her 40s.
EXAMINATION
The lesion, which measures 3 cm x 3 cm, is an impressive, pedunculated, doughy, rubbery, skin-colored mass protruding from her left mid back. No redness or edema is seen on or around the lesion. On palpation, the lesion is found to be uniformly soft and rubbery.
The patient is quite obese and has numerous skin tags in skin folds, under the arms, and around the neck.
What is the diagnosis?
DISCUSSION
The lesion was excised with deep margins and submitted to pathology. The report showed benign histology, consistent with a fibroepitheliomatous polyp.
In one sense, it’s hard to believe this huge lesion was a “skin tag,” and there are other things it might have been. But the microscopic examination of the tissue proved it was simply a large version of the same skin tags we see around necks and in axillae. These lesions are known by various names, including fibroepitheliomatous polyps (FEP) and acrochordons. A more typical FEP would be the size of a grain of rice, but they can take on various forms and sizes.
There is a differential for lesions that look like FEPs; it includes seborrheic keratoses, warts, molluscum contagiosum, neurofibromas, and fibrolipomas. Even melanoma and squamous cell carcinoma can assume a tag-like morphology. So it pays to take a close look at these lesions, checking to see if they’re soft and pliable, as well as skin colored. With any odd feature, including increased size, they need to be removed and sent for pathologic examination.
Heredity appears to play a role in the development of FEPs, as do age and obesity. There are studies showing an association between FEPs and insulin resistance and others identifying FEPs as a marker for increased risk for atherosclerotic vessel disease. In any case, 59% of the population has them by age 70, with men and women affected equally. Of the general population, 46% has some sort of skin tag somewhere.
Treatment may entail cryotherapy, excision, or electrodessication. With lesions as large as the case patient’s, excision is the only reasonable option. Deep margins are required, since a “shave” would leave a gaping, open wound. For the case patient, there was no postoperative sequelae aside from some scarring.
TAKE-HOME LEARNING POINTS
• Skin tags, also known as fibroepitheliomas, fibroepitheliomatous polyps, or acrochordons, are typically the size of a grain of rice, and tend to appear in skin folds (eg, axillae, neck, groin).
• Studies have shown that there may be an association between having skin tags and developing CAD or insulin resistance.
• Obesity, age, and heredity also appear to be factors in developing skin tags.
• Large or odd tags need to be captured and sent for pathologic examination.
• Skin tags are extremely common, affecting 59% of the population older than 70.
A 49-year-old woman presents to dermatology with a lesion on her back. It’s been there for at least 20 years, slowly growing to its present size; it is now so prominent that it shows through her clothes and is subject to traumatization. The visibility of the lesion, particularly when the patient wears a swimsuit, is a source of considerable embarrassment.
Notable medical history includes polycystic ovarian syndrome and related diabetes and dyslipidemia. Family history reveals that the patient’s mother died of heart disease in her 40s.
EXAMINATION
The lesion, which measures 3 cm x 3 cm, is an impressive, pedunculated, doughy, rubbery, skin-colored mass protruding from her left mid back. No redness or edema is seen on or around the lesion. On palpation, the lesion is found to be uniformly soft and rubbery.
The patient is quite obese and has numerous skin tags in skin folds, under the arms, and around the neck.
What is the diagnosis?
DISCUSSION
The lesion was excised with deep margins and submitted to pathology. The report showed benign histology, consistent with a fibroepitheliomatous polyp.
In one sense, it’s hard to believe this huge lesion was a “skin tag,” and there are other things it might have been. But the microscopic examination of the tissue proved it was simply a large version of the same skin tags we see around necks and in axillae. These lesions are known by various names, including fibroepitheliomatous polyps (FEP) and acrochordons. A more typical FEP would be the size of a grain of rice, but they can take on various forms and sizes.
There is a differential for lesions that look like FEPs; it includes seborrheic keratoses, warts, molluscum contagiosum, neurofibromas, and fibrolipomas. Even melanoma and squamous cell carcinoma can assume a tag-like morphology. So it pays to take a close look at these lesions, checking to see if they’re soft and pliable, as well as skin colored. With any odd feature, including increased size, they need to be removed and sent for pathologic examination.
Heredity appears to play a role in the development of FEPs, as do age and obesity. There are studies showing an association between FEPs and insulin resistance and others identifying FEPs as a marker for increased risk for atherosclerotic vessel disease. In any case, 59% of the population has them by age 70, with men and women affected equally. Of the general population, 46% has some sort of skin tag somewhere.
Treatment may entail cryotherapy, excision, or electrodessication. With lesions as large as the case patient’s, excision is the only reasonable option. Deep margins are required, since a “shave” would leave a gaping, open wound. For the case patient, there was no postoperative sequelae aside from some scarring.
TAKE-HOME LEARNING POINTS
• Skin tags, also known as fibroepitheliomas, fibroepitheliomatous polyps, or acrochordons, are typically the size of a grain of rice, and tend to appear in skin folds (eg, axillae, neck, groin).
• Studies have shown that there may be an association between having skin tags and developing CAD or insulin resistance.
• Obesity, age, and heredity also appear to be factors in developing skin tags.
• Large or odd tags need to be captured and sent for pathologic examination.
• Skin tags are extremely common, affecting 59% of the population older than 70.
Empagliflozin benefited type 2 diabetes patients with CKD
SAN DIEGO – Empagliflozin used in conjunction with standard of care significantly improved renal outcomes in adults with type 2 diabetes, results from a large randomized trial showed.
“These benefits were consistent among patients with and without chronic kidney disease at baseline,” Dr. Christoph Wanner said at the annual meeting of the American Society of Nephrology.
Previous studies have demonstrated that in patients with type 2 diabetes, empagliflozin, an inhibitor of the sodium glucose cotransporter 2 in the kidney, leads to significant reductions in hemoglobin A1c, weight loss, and reductions in blood pressure without increases in heart rate. Developed by Boehringer Ingelheim and Eli Lilly, the drug was approved in 2014 for the treatment of type 2 diabetes in adults. At the meeting Dr. Wanner presented new finding results from the EMPA-REG OUTCOME trial, which was published in September 2015. That study found that patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, compared with placebo, had a lower rate of the primary composite cardiovascular outcome and death from any cause when the study drug was added to standard care.
For the current analysis, the researchers evaluated renal outcomes in the study participants, which consisted of new-onset or worsening nephropathy, including new onset of macroalbuminuria (defined as a urine albumin to creatinine ratio (UACR) of greater than 300 mg/g); doubling of serum creatinine accompanied by an estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73m2 or less; initiation of renal replacement therapy; or death due to renal disease. There was also a composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease.
The EMPA-REG OUTCOME trial included 2,333 patients in the placebo group and 4,687 in the empagliflozin group who were followed for a median observation time of 3.1 years. The mean age of the study participants was 63 years and 71% were male. Compared with patients in the placebo group, those in the empagliflozin group demonstrated a 39% reduction in new-onset or worsening of nephropathy (hazard ratio, 0.61; P less than .0001). The reduction “started very early,” said Dr. Wanner, who is professor of medicine and head of nephrology at University Hospital of Wurzburg, Germany. “After 3 months you see the curves [between the placebo and treatment groups] separating.”
The impact on empagliflozin on the composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease was even more profound. Compared with patients in the placebo group, those in the empagliflozin demonstrated a 46% reduced risk in the composite outcome (HR, 0.54; P = .0002).
Dr. Wanner went on to present cardiovascular outcomes in patients with chronic kidney disease (CKD). Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, the primary outcome of three-point major adverse cardiac events occurred in 176 of 1,212 patients in the empagliflozin group (15%), compared with 99 of 607 patients in the placebo group (16%; HR, 0.88). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, the primary outcome of three-point major adverse cardiac events occurred in 314 of 3,473 patients in the empagliflozin group (9%), compared with 183 of 1,726 (11%) patients in the placebo group (HR, 0.84). At the same time, the outcome of cardiovascular death occurred in 75 of 1,212 patients in the empagliflozin group (6%), compared with 48 of 607 patients in the placebo group (8%; HR, 0.78). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, cardiovascular death occurred in 97 of 3,473 patients in the empagliflozin group (3%), compared with 89 of 1,726 patients in the placebo group (5%; HR, 0.53). However, there was no detectable benefit for the study medication in reducing the myocardial infarction or stroke in patients with CKD.
Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, hospitalization for heart failure occurred in 51 of 1,212 patients in the empagliflozin group (4%), compared with 43 of 607 patients in the placebo group (7%; HR, 0.59). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 75 of 3,473 patients in the empagliflozin group (2%), compared with 52 of 1,726 patients in the placebo group (3%; HR, 0.70). At the same time, all-cause mortality occurred in 115 of 1,212 patients in the empagliflozin group (9%), compared with 72 of 607 patients in the placebo group (12%; HR, 0.80). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 154 of 3,473 patients in the empagliflozin group (4%), compared with 122 of 1,726 patients in the placebo group (7%; HR, 0.62).
The most common adverse events in patients with CKD related to the study drug were urinary tract infections and genital infections, but there were no detectable signals on acute kidney failure, hyperkalemia, or bone fracture.
“Empagliflozin reduces cardiovascular death, all-cause mortality, and hospitalization for heart failure in patients with type 2 diabetes at high cardiovascular risk,” Dr. Wanner concluded. “Safety and tolerability of empagliflozin in patients with CKD at baseline were similar to the overall trial population and consistent with previous clinical trials.”
The study was supported by Boehringer Ingelheim and Eli Lilly. Dr. Wanner disclosed numerous financial ties to industry.
SAN DIEGO – Empagliflozin used in conjunction with standard of care significantly improved renal outcomes in adults with type 2 diabetes, results from a large randomized trial showed.
“These benefits were consistent among patients with and without chronic kidney disease at baseline,” Dr. Christoph Wanner said at the annual meeting of the American Society of Nephrology.
Previous studies have demonstrated that in patients with type 2 diabetes, empagliflozin, an inhibitor of the sodium glucose cotransporter 2 in the kidney, leads to significant reductions in hemoglobin A1c, weight loss, and reductions in blood pressure without increases in heart rate. Developed by Boehringer Ingelheim and Eli Lilly, the drug was approved in 2014 for the treatment of type 2 diabetes in adults. At the meeting Dr. Wanner presented new finding results from the EMPA-REG OUTCOME trial, which was published in September 2015. That study found that patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, compared with placebo, had a lower rate of the primary composite cardiovascular outcome and death from any cause when the study drug was added to standard care.
For the current analysis, the researchers evaluated renal outcomes in the study participants, which consisted of new-onset or worsening nephropathy, including new onset of macroalbuminuria (defined as a urine albumin to creatinine ratio (UACR) of greater than 300 mg/g); doubling of serum creatinine accompanied by an estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73m2 or less; initiation of renal replacement therapy; or death due to renal disease. There was also a composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease.
The EMPA-REG OUTCOME trial included 2,333 patients in the placebo group and 4,687 in the empagliflozin group who were followed for a median observation time of 3.1 years. The mean age of the study participants was 63 years and 71% were male. Compared with patients in the placebo group, those in the empagliflozin group demonstrated a 39% reduction in new-onset or worsening of nephropathy (hazard ratio, 0.61; P less than .0001). The reduction “started very early,” said Dr. Wanner, who is professor of medicine and head of nephrology at University Hospital of Wurzburg, Germany. “After 3 months you see the curves [between the placebo and treatment groups] separating.”
The impact on empagliflozin on the composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease was even more profound. Compared with patients in the placebo group, those in the empagliflozin demonstrated a 46% reduced risk in the composite outcome (HR, 0.54; P = .0002).
Dr. Wanner went on to present cardiovascular outcomes in patients with chronic kidney disease (CKD). Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, the primary outcome of three-point major adverse cardiac events occurred in 176 of 1,212 patients in the empagliflozin group (15%), compared with 99 of 607 patients in the placebo group (16%; HR, 0.88). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, the primary outcome of three-point major adverse cardiac events occurred in 314 of 3,473 patients in the empagliflozin group (9%), compared with 183 of 1,726 (11%) patients in the placebo group (HR, 0.84). At the same time, the outcome of cardiovascular death occurred in 75 of 1,212 patients in the empagliflozin group (6%), compared with 48 of 607 patients in the placebo group (8%; HR, 0.78). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, cardiovascular death occurred in 97 of 3,473 patients in the empagliflozin group (3%), compared with 89 of 1,726 patients in the placebo group (5%; HR, 0.53). However, there was no detectable benefit for the study medication in reducing the myocardial infarction or stroke in patients with CKD.
Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, hospitalization for heart failure occurred in 51 of 1,212 patients in the empagliflozin group (4%), compared with 43 of 607 patients in the placebo group (7%; HR, 0.59). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 75 of 3,473 patients in the empagliflozin group (2%), compared with 52 of 1,726 patients in the placebo group (3%; HR, 0.70). At the same time, all-cause mortality occurred in 115 of 1,212 patients in the empagliflozin group (9%), compared with 72 of 607 patients in the placebo group (12%; HR, 0.80). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 154 of 3,473 patients in the empagliflozin group (4%), compared with 122 of 1,726 patients in the placebo group (7%; HR, 0.62).
The most common adverse events in patients with CKD related to the study drug were urinary tract infections and genital infections, but there were no detectable signals on acute kidney failure, hyperkalemia, or bone fracture.
“Empagliflozin reduces cardiovascular death, all-cause mortality, and hospitalization for heart failure in patients with type 2 diabetes at high cardiovascular risk,” Dr. Wanner concluded. “Safety and tolerability of empagliflozin in patients with CKD at baseline were similar to the overall trial population and consistent with previous clinical trials.”
The study was supported by Boehringer Ingelheim and Eli Lilly. Dr. Wanner disclosed numerous financial ties to industry.
SAN DIEGO – Empagliflozin used in conjunction with standard of care significantly improved renal outcomes in adults with type 2 diabetes, results from a large randomized trial showed.
“These benefits were consistent among patients with and without chronic kidney disease at baseline,” Dr. Christoph Wanner said at the annual meeting of the American Society of Nephrology.
Previous studies have demonstrated that in patients with type 2 diabetes, empagliflozin, an inhibitor of the sodium glucose cotransporter 2 in the kidney, leads to significant reductions in hemoglobin A1c, weight loss, and reductions in blood pressure without increases in heart rate. Developed by Boehringer Ingelheim and Eli Lilly, the drug was approved in 2014 for the treatment of type 2 diabetes in adults. At the meeting Dr. Wanner presented new finding results from the EMPA-REG OUTCOME trial, which was published in September 2015. That study found that patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, compared with placebo, had a lower rate of the primary composite cardiovascular outcome and death from any cause when the study drug was added to standard care.
For the current analysis, the researchers evaluated renal outcomes in the study participants, which consisted of new-onset or worsening nephropathy, including new onset of macroalbuminuria (defined as a urine albumin to creatinine ratio (UACR) of greater than 300 mg/g); doubling of serum creatinine accompanied by an estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73m2 or less; initiation of renal replacement therapy; or death due to renal disease. There was also a composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease.
The EMPA-REG OUTCOME trial included 2,333 patients in the placebo group and 4,687 in the empagliflozin group who were followed for a median observation time of 3.1 years. The mean age of the study participants was 63 years and 71% were male. Compared with patients in the placebo group, those in the empagliflozin group demonstrated a 39% reduction in new-onset or worsening of nephropathy (hazard ratio, 0.61; P less than .0001). The reduction “started very early,” said Dr. Wanner, who is professor of medicine and head of nephrology at University Hospital of Wurzburg, Germany. “After 3 months you see the curves [between the placebo and treatment groups] separating.”
The impact on empagliflozin on the composite outcome of doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease was even more profound. Compared with patients in the placebo group, those in the empagliflozin demonstrated a 46% reduced risk in the composite outcome (HR, 0.54; P = .0002).
Dr. Wanner went on to present cardiovascular outcomes in patients with chronic kidney disease (CKD). Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, the primary outcome of three-point major adverse cardiac events occurred in 176 of 1,212 patients in the empagliflozin group (15%), compared with 99 of 607 patients in the placebo group (16%; HR, 0.88). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, the primary outcome of three-point major adverse cardiac events occurred in 314 of 3,473 patients in the empagliflozin group (9%), compared with 183 of 1,726 (11%) patients in the placebo group (HR, 0.84). At the same time, the outcome of cardiovascular death occurred in 75 of 1,212 patients in the empagliflozin group (6%), compared with 48 of 607 patients in the placebo group (8%; HR, 0.78). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, cardiovascular death occurred in 97 of 3,473 patients in the empagliflozin group (3%), compared with 89 of 1,726 patients in the placebo group (5%; HR, 0.53). However, there was no detectable benefit for the study medication in reducing the myocardial infarction or stroke in patients with CKD.
Among those with an eGFR of less than 60 mL/min/1.73m2 at baseline, hospitalization for heart failure occurred in 51 of 1,212 patients in the empagliflozin group (4%), compared with 43 of 607 patients in the placebo group (7%; HR, 0.59). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 75 of 3,473 patients in the empagliflozin group (2%), compared with 52 of 1,726 patients in the placebo group (3%; HR, 0.70). At the same time, all-cause mortality occurred in 115 of 1,212 patients in the empagliflozin group (9%), compared with 72 of 607 patients in the placebo group (12%; HR, 0.80). Among those with an eGFR of 60 mL/min/1.73m2 or greater at baseline, hospitalization for heart failure occurred in 154 of 3,473 patients in the empagliflozin group (4%), compared with 122 of 1,726 patients in the placebo group (7%; HR, 0.62).
The most common adverse events in patients with CKD related to the study drug were urinary tract infections and genital infections, but there were no detectable signals on acute kidney failure, hyperkalemia, or bone fracture.
“Empagliflozin reduces cardiovascular death, all-cause mortality, and hospitalization for heart failure in patients with type 2 diabetes at high cardiovascular risk,” Dr. Wanner concluded. “Safety and tolerability of empagliflozin in patients with CKD at baseline were similar to the overall trial population and consistent with previous clinical trials.”
The study was supported by Boehringer Ingelheim and Eli Lilly. Dr. Wanner disclosed numerous financial ties to industry.
AT KIDNEY WEEK 2015
Key clinical point: Empagliflozin was found to improve renal outcomes in patients with type 2 diabetes and chronic kidney disease.
Major finding: Compared with patients in the placebo group, those in the empagliflozin group demonstrated a 39% reduction in new onset or worsening of nephropathy (HR, 0.61; P less than .0001).
Data source: An analysis of data from 7,020 patients enrolled in the EMPA-REG OUTCOME trial, including 2,333 in the placebo group and 4,687 in the empagliflozin group who were followed for a median observation time of 3.1 years.
Disclosures: The study was supported by Boehringer Ingelheim and Eli Lilly. Dr. Wanner disclosed numerous financial ties to industry.
Drug Treatment Key to Fewer Hospitalizations for Schizophrenic Patients
NEW YORK - Initiation of antipsychotic or antidepressant drug treatment is linked to a reduction in hospitalizations for patients with schizophrenia, according to a new study.
"Use of sulpiride, mirtazapine, venlafaxine, and clozapine-aripiprazole and clozapine amisulpride combinations were associated with fewer subsequent admission-days in patients with schizophrenia," Dr. Rudolf N. Cardinal of the Behavioral and Clinical Neuroscience Institute, University of Cambridge, UK, said by email.
"These studies are correlative and do not prove causation," he cautioned.
Dr. Cardinal and colleagues analyzed eight years' of admission records at a secondary mental health care institution in Cambridgeshire. The analysis included nearly 1,500 patients with a diagnosis of schizophrenia and a median follow-up of five years.
In mirror-image analysis covering two years before and after therapy initiation, the researchers found treatment with amisulpride, aripiprazole, clozapine, fluoxetine, mirtazapine, olanzapine, quetiapine, and sulpiride was associated with fewer subsequent admissions in one year.
The association persisted in a "more stringent" two-year analysis for aripiprazole, clozapine, and sulpiride.
Using regression analysis, the researchers found a continued reduction in admissions with sulpiride and mirtazapine (estimated mean change, -20.4 and -11.6 days/year, respectively).
Treatment with clozapine-aripiprazole and clozapine-amisulpride combinations as well as venlafaxine was associated with significantly fewer hospitalized days (-17.7, -13.8, and -12.3 days/year, respectively).
Overall, the mean admission rate was 26.8 days/year.
"This analysis focused on patients with more severe disease, in that they had at least one hospital admission in the pre-drug period," the researchers note in the article online October 21 in NPJ Schizophrenia.
"Larger correlative studies are required to corroborate these effects, followed by randomized controlled trials if appropriate," Dr. Cardinal said. "We are all very keen that these are not misrepresented as causal-grade findings."
The authors reported no funding. One coauthor reported receiving research funding from Genus Pharmaceuticals and consulting fees from Roche/Genentech.
NEW YORK - Initiation of antipsychotic or antidepressant drug treatment is linked to a reduction in hospitalizations for patients with schizophrenia, according to a new study.
"Use of sulpiride, mirtazapine, venlafaxine, and clozapine-aripiprazole and clozapine amisulpride combinations were associated with fewer subsequent admission-days in patients with schizophrenia," Dr. Rudolf N. Cardinal of the Behavioral and Clinical Neuroscience Institute, University of Cambridge, UK, said by email.
"These studies are correlative and do not prove causation," he cautioned.
Dr. Cardinal and colleagues analyzed eight years' of admission records at a secondary mental health care institution in Cambridgeshire. The analysis included nearly 1,500 patients with a diagnosis of schizophrenia and a median follow-up of five years.
In mirror-image analysis covering two years before and after therapy initiation, the researchers found treatment with amisulpride, aripiprazole, clozapine, fluoxetine, mirtazapine, olanzapine, quetiapine, and sulpiride was associated with fewer subsequent admissions in one year.
The association persisted in a "more stringent" two-year analysis for aripiprazole, clozapine, and sulpiride.
Using regression analysis, the researchers found a continued reduction in admissions with sulpiride and mirtazapine (estimated mean change, -20.4 and -11.6 days/year, respectively).
Treatment with clozapine-aripiprazole and clozapine-amisulpride combinations as well as venlafaxine was associated with significantly fewer hospitalized days (-17.7, -13.8, and -12.3 days/year, respectively).
Overall, the mean admission rate was 26.8 days/year.
"This analysis focused on patients with more severe disease, in that they had at least one hospital admission in the pre-drug period," the researchers note in the article online October 21 in NPJ Schizophrenia.
"Larger correlative studies are required to corroborate these effects, followed by randomized controlled trials if appropriate," Dr. Cardinal said. "We are all very keen that these are not misrepresented as causal-grade findings."
The authors reported no funding. One coauthor reported receiving research funding from Genus Pharmaceuticals and consulting fees from Roche/Genentech.
NEW YORK - Initiation of antipsychotic or antidepressant drug treatment is linked to a reduction in hospitalizations for patients with schizophrenia, according to a new study.
"Use of sulpiride, mirtazapine, venlafaxine, and clozapine-aripiprazole and clozapine amisulpride combinations were associated with fewer subsequent admission-days in patients with schizophrenia," Dr. Rudolf N. Cardinal of the Behavioral and Clinical Neuroscience Institute, University of Cambridge, UK, said by email.
"These studies are correlative and do not prove causation," he cautioned.
Dr. Cardinal and colleagues analyzed eight years' of admission records at a secondary mental health care institution in Cambridgeshire. The analysis included nearly 1,500 patients with a diagnosis of schizophrenia and a median follow-up of five years.
In mirror-image analysis covering two years before and after therapy initiation, the researchers found treatment with amisulpride, aripiprazole, clozapine, fluoxetine, mirtazapine, olanzapine, quetiapine, and sulpiride was associated with fewer subsequent admissions in one year.
The association persisted in a "more stringent" two-year analysis for aripiprazole, clozapine, and sulpiride.
Using regression analysis, the researchers found a continued reduction in admissions with sulpiride and mirtazapine (estimated mean change, -20.4 and -11.6 days/year, respectively).
Treatment with clozapine-aripiprazole and clozapine-amisulpride combinations as well as venlafaxine was associated with significantly fewer hospitalized days (-17.7, -13.8, and -12.3 days/year, respectively).
Overall, the mean admission rate was 26.8 days/year.
"This analysis focused on patients with more severe disease, in that they had at least one hospital admission in the pre-drug period," the researchers note in the article online October 21 in NPJ Schizophrenia.
"Larger correlative studies are required to corroborate these effects, followed by randomized controlled trials if appropriate," Dr. Cardinal said. "We are all very keen that these are not misrepresented as causal-grade findings."
The authors reported no funding. One coauthor reported receiving research funding from Genus Pharmaceuticals and consulting fees from Roche/Genentech.
ITC 2015: Review IDs features to aid thyroid lymphoma diagnosis
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
AT ITC 2015
Key clinical point: Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Major finding: Typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%).
Data source: A retrospective review of 75 cases.
Disclosures: Dr. Sharma reported having no disclosures.
VIDEO: Chondroitin tops celecoxib in reducing knee OA structural progression
SAN FRANCISCO – Patients with symptomatic knee osteoarthritis who received pharmaceutical-grade chondroitin sulfate for 2 years lost about 20% less cartilage volume than did patients treated with celecoxib, according to a randomized, double-blind trial.
Improvements were limited to the medial tibiofemoral compartment, but even such modest structural effects can significantly decrease rates of total knee replacement over time, said lead investigator Dr. Jean-Pierre Pelletier, who presented the findings at the annual meeting of the American College of Rheumatology.
The 194 participants in the study received chondroitin sulfate, 1,200 mg a day, or celecoxib, 200 mg daily. Joint effusion and pain and function improved markedly in both groups, and they had similar rates of adverse events, said Dr. Pelletier, who is a rheumatologist at Institut de recherche en rhumatologie de Montréal. He discussed the findings and plans for future research in an exclusive video interview.
Bioibérica sponsored the study and makes the chondroitin sulfate that participants received. Dr. Pelletier and his associates had no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Patients with symptomatic knee osteoarthritis who received pharmaceutical-grade chondroitin sulfate for 2 years lost about 20% less cartilage volume than did patients treated with celecoxib, according to a randomized, double-blind trial.
Improvements were limited to the medial tibiofemoral compartment, but even such modest structural effects can significantly decrease rates of total knee replacement over time, said lead investigator Dr. Jean-Pierre Pelletier, who presented the findings at the annual meeting of the American College of Rheumatology.
The 194 participants in the study received chondroitin sulfate, 1,200 mg a day, or celecoxib, 200 mg daily. Joint effusion and pain and function improved markedly in both groups, and they had similar rates of adverse events, said Dr. Pelletier, who is a rheumatologist at Institut de recherche en rhumatologie de Montréal. He discussed the findings and plans for future research in an exclusive video interview.
Bioibérica sponsored the study and makes the chondroitin sulfate that participants received. Dr. Pelletier and his associates had no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Patients with symptomatic knee osteoarthritis who received pharmaceutical-grade chondroitin sulfate for 2 years lost about 20% less cartilage volume than did patients treated with celecoxib, according to a randomized, double-blind trial.
Improvements were limited to the medial tibiofemoral compartment, but even such modest structural effects can significantly decrease rates of total knee replacement over time, said lead investigator Dr. Jean-Pierre Pelletier, who presented the findings at the annual meeting of the American College of Rheumatology.
The 194 participants in the study received chondroitin sulfate, 1,200 mg a day, or celecoxib, 200 mg daily. Joint effusion and pain and function improved markedly in both groups, and they had similar rates of adverse events, said Dr. Pelletier, who is a rheumatologist at Institut de recherche en rhumatologie de Montréal. He discussed the findings and plans for future research in an exclusive video interview.
Bioibérica sponsored the study and makes the chondroitin sulfate that participants received. Dr. Pelletier and his associates had no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Team describes new way to edit HSPCs

Image by Tom Ellenberger
Researchers say they have discovered a more efficient way to edit the genomes of hematopoietic stem and progenitor cells (HSPCs).
The approach involves adeno-associated virus (AAV) serotype 6 and zinc finger nuclease (ZFN) messenger RNA (mRNA).
Combining these delivery techniques allowed the team to “achieve high levels of precise genome editing” in HSPCs, including the most primitive cell population.
Paula Cannon, PhD, of the University of Southern California in Los Angeles, and her colleagues described this work in Nature Biotechnology.
The researchers have been using ZFNs to cut a cell’s DNA at a precise location or sequence. The cell normally uses a copy of the cut DNA sequence as a template to repair the DNA break.
During this process, there is the opportunity to introduce new DNA sequences or to repair mutations, effectively fooling the cell into making a genetic edit.
To provide the cell with both the targeted nuclease and the new DNA template, researchers can use a variety of delivery vehicles or vectors, including viruses and mRNA.
With this study, Dr Cannon and her colleagues found they could deliver the DNA repair template using AAV6, which can naturally enter HSPCs.
At the same time, they found that delivering the ZFNs as short-lived mRNA molecules allowed the DNA cutting and repair process to occur without disrupting the HSPCs.
By combining these delivery methods, the researchers were able to insert a gene at a precise site in even the most primitive human HSPCs, with efficiency rates ranging from 17% to 43%.
The team then transplanted these edited HSPCs into immune-deficient mice and found the cells thrived and differentiated into many different blood cell types, all of which retained the edits to their DNA.
“Our results provide a strategy for broadening the application of genome editing technologies in HSPCs,” said study author Michael C. Holmes, PhD, vice president of research at Sangamo BioSciences in Richmond, California.
“This significantly advances our progress towards applying genome editing to the treatment of human diseases of the blood and immune systems.” ![]()

Image by Tom Ellenberger
Researchers say they have discovered a more efficient way to edit the genomes of hematopoietic stem and progenitor cells (HSPCs).
The approach involves adeno-associated virus (AAV) serotype 6 and zinc finger nuclease (ZFN) messenger RNA (mRNA).
Combining these delivery techniques allowed the team to “achieve high levels of precise genome editing” in HSPCs, including the most primitive cell population.
Paula Cannon, PhD, of the University of Southern California in Los Angeles, and her colleagues described this work in Nature Biotechnology.
The researchers have been using ZFNs to cut a cell’s DNA at a precise location or sequence. The cell normally uses a copy of the cut DNA sequence as a template to repair the DNA break.
During this process, there is the opportunity to introduce new DNA sequences or to repair mutations, effectively fooling the cell into making a genetic edit.
To provide the cell with both the targeted nuclease and the new DNA template, researchers can use a variety of delivery vehicles or vectors, including viruses and mRNA.
With this study, Dr Cannon and her colleagues found they could deliver the DNA repair template using AAV6, which can naturally enter HSPCs.
At the same time, they found that delivering the ZFNs as short-lived mRNA molecules allowed the DNA cutting and repair process to occur without disrupting the HSPCs.
By combining these delivery methods, the researchers were able to insert a gene at a precise site in even the most primitive human HSPCs, with efficiency rates ranging from 17% to 43%.
The team then transplanted these edited HSPCs into immune-deficient mice and found the cells thrived and differentiated into many different blood cell types, all of which retained the edits to their DNA.
“Our results provide a strategy for broadening the application of genome editing technologies in HSPCs,” said study author Michael C. Holmes, PhD, vice president of research at Sangamo BioSciences in Richmond, California.
“This significantly advances our progress towards applying genome editing to the treatment of human diseases of the blood and immune systems.” ![]()

Image by Tom Ellenberger
Researchers say they have discovered a more efficient way to edit the genomes of hematopoietic stem and progenitor cells (HSPCs).
The approach involves adeno-associated virus (AAV) serotype 6 and zinc finger nuclease (ZFN) messenger RNA (mRNA).
Combining these delivery techniques allowed the team to “achieve high levels of precise genome editing” in HSPCs, including the most primitive cell population.
Paula Cannon, PhD, of the University of Southern California in Los Angeles, and her colleagues described this work in Nature Biotechnology.
The researchers have been using ZFNs to cut a cell’s DNA at a precise location or sequence. The cell normally uses a copy of the cut DNA sequence as a template to repair the DNA break.
During this process, there is the opportunity to introduce new DNA sequences or to repair mutations, effectively fooling the cell into making a genetic edit.
To provide the cell with both the targeted nuclease and the new DNA template, researchers can use a variety of delivery vehicles or vectors, including viruses and mRNA.
With this study, Dr Cannon and her colleagues found they could deliver the DNA repair template using AAV6, which can naturally enter HSPCs.
At the same time, they found that delivering the ZFNs as short-lived mRNA molecules allowed the DNA cutting and repair process to occur without disrupting the HSPCs.
By combining these delivery methods, the researchers were able to insert a gene at a precise site in even the most primitive human HSPCs, with efficiency rates ranging from 17% to 43%.
The team then transplanted these edited HSPCs into immune-deficient mice and found the cells thrived and differentiated into many different blood cell types, all of which retained the edits to their DNA.
“Our results provide a strategy for broadening the application of genome editing technologies in HSPCs,” said study author Michael C. Holmes, PhD, vice president of research at Sangamo BioSciences in Richmond, California.
“This significantly advances our progress towards applying genome editing to the treatment of human diseases of the blood and immune systems.” ![]()
Models predict impact of malaria vaccine candidate

Photo by Caitlin Kleiboer
The malaria vaccine candidate RTS,S/AS01 (Mosquirix) could have a significant impact on public health in a range of settings across sub-Saharan Africa, according to mathematical models.
Researchers found that, over a 15-year time horizon, an average of 116,500 cases of clinical malaria and 484 malaria deaths would be averted for every 100,000 children vaccinated under a 4-dose schedule of immunizations at 6, 7.5, 9, and 27 months of age.
This translates to approximately 1.2 malaria cases averted per vaccinated child and 1 malaria death averted for every 200 children vaccinated.
These data apply to children living in regions of Africa that experience moderate to high malaria transmission—countries where prevalence rates for the malaria parasite Plasmodium falciparum range from 10% to 65%—and assumes a vaccine coverage rate at the fourth dose of approximately 70%.
The findings, published in The Lancet, contribute to the scientific evidence being considered by the World Health Organization, which is assessing the vaccine candidate for use in Africa.
“We took a realistic look at expected coverage of the RTS,S vaccine in a variety of African settings and found it would have significant impact on malaria disease in all but the lowest malaria transmission regions,” said Melissa Penny, PhD, of the Swiss Tropical and Public Health Institute in Basel.
“Our numbers indicate that 6% to 29% of malaria deaths in children younger than age 5 could potentially be averted by the vaccine in the areas in which it is implemented, when used alongside other malaria control interventions.”
This is the first modeling study to use final, site-specific results of the RTS,S phase 3 safety and efficacy trial coordinated by GlaxoSmithKline and conducted at 11 sites in 7 African countries. And it accounts for implementation of the vaccine alongside use of long-lasting, insecticide-treated bed nets.
There was consensus across the predictions from all 4 groups that took part in the study. The participating institutions are Imperial College London in the UK, Swiss Tropical and Public Health Institute, the Institute for Disease Modeling in the US, and GlaxoSmithKline in Belgium.
According to the study authors, public health authorities require these types of impact estimates on malaria disease and deaths to inform vaccine implementation.
Models can account for differences between the trial and real-life settings in transmission levels and healthcare accessibility, as well as predict RTS,S’s impact on malaria mortality, which was not possible to assess in the trial.
Cost-effectiveness
As part of the modeling study, the researchers considered a range of possible prices for RTS,S, from $2 to $10. They found that, compared to current malaria interventions, the vaccine would be cost-effective to implement under an assumed price of USD$5 per dose in areas of moderate and high malaria transmission.
”The cost-effectiveness of RTS,S is similar to what we’ve seen for other recently introduced childhood vaccines,” said Azra Ghani, PhD, of Imperial College London.
“It also overlaps within the ranges of cost-effectiveness of other malaria control interventions like bed nets and indoor residual sprays. However, it is important that the vaccine is introduced in addition to these other highly cost-effective interventions.”
The researchers measured cost-effectiveness in terms of disability-adjusted life years (DALYs), a metric used by health economists to compare the impacts of health interventions in populations over time. One DALY is equivalent to 1 lost year of healthy life. The lower the amount spent per DALY averted, the greater the cost-effectiveness of an intervention.
With the vaccine priced at $5 per dose, the researchers estimated a median cost of $87 per DALY averted for a 4-dose vaccine schedule across the range of transmission settings with parasite prevalence 10% to 65%.
This cost was estimated to vary depending on the level of malaria transmission found in a particular location—with the vaccine being increasingly cost-effective in areas with a higher malaria burden.
The researchers noted that, according to earlier studies, the cost per DALY averted for other malaria interventions indicate averages of $27 for long-lasting, insecticide-treated bed nets; $143 for indoor residual spraying; and $24 for intermittent preventative treatment.
Caveats
The researchers conceded that this study has its limitations. One is the remaining uncertainty regarding the vaccine’s efficacy after the 4 years of follow-up observed in the phase 3 trial.
The team also noted that, since the phase 3 trial of RTS,S was not large enough to test for a reduction in deaths from malaria (versus reduction in incidence of malaria cases) and the quality of care provided to participants was high, the modeling studies’ projection of deaths requires further validation during the implementation phase.
“It will be important to continue to track the long-term impact of this vaccine to ensure that the effectiveness predicted by the models is borne out in practice,” said Caitlin Bever, PhD, of the Institute for Disease Modeling in Bellevue, Washington. ![]()

Photo by Caitlin Kleiboer
The malaria vaccine candidate RTS,S/AS01 (Mosquirix) could have a significant impact on public health in a range of settings across sub-Saharan Africa, according to mathematical models.
Researchers found that, over a 15-year time horizon, an average of 116,500 cases of clinical malaria and 484 malaria deaths would be averted for every 100,000 children vaccinated under a 4-dose schedule of immunizations at 6, 7.5, 9, and 27 months of age.
This translates to approximately 1.2 malaria cases averted per vaccinated child and 1 malaria death averted for every 200 children vaccinated.
These data apply to children living in regions of Africa that experience moderate to high malaria transmission—countries where prevalence rates for the malaria parasite Plasmodium falciparum range from 10% to 65%—and assumes a vaccine coverage rate at the fourth dose of approximately 70%.
The findings, published in The Lancet, contribute to the scientific evidence being considered by the World Health Organization, which is assessing the vaccine candidate for use in Africa.
“We took a realistic look at expected coverage of the RTS,S vaccine in a variety of African settings and found it would have significant impact on malaria disease in all but the lowest malaria transmission regions,” said Melissa Penny, PhD, of the Swiss Tropical and Public Health Institute in Basel.
“Our numbers indicate that 6% to 29% of malaria deaths in children younger than age 5 could potentially be averted by the vaccine in the areas in which it is implemented, when used alongside other malaria control interventions.”
This is the first modeling study to use final, site-specific results of the RTS,S phase 3 safety and efficacy trial coordinated by GlaxoSmithKline and conducted at 11 sites in 7 African countries. And it accounts for implementation of the vaccine alongside use of long-lasting, insecticide-treated bed nets.
There was consensus across the predictions from all 4 groups that took part in the study. The participating institutions are Imperial College London in the UK, Swiss Tropical and Public Health Institute, the Institute for Disease Modeling in the US, and GlaxoSmithKline in Belgium.
According to the study authors, public health authorities require these types of impact estimates on malaria disease and deaths to inform vaccine implementation.
Models can account for differences between the trial and real-life settings in transmission levels and healthcare accessibility, as well as predict RTS,S’s impact on malaria mortality, which was not possible to assess in the trial.
Cost-effectiveness
As part of the modeling study, the researchers considered a range of possible prices for RTS,S, from $2 to $10. They found that, compared to current malaria interventions, the vaccine would be cost-effective to implement under an assumed price of USD$5 per dose in areas of moderate and high malaria transmission.
”The cost-effectiveness of RTS,S is similar to what we’ve seen for other recently introduced childhood vaccines,” said Azra Ghani, PhD, of Imperial College London.
“It also overlaps within the ranges of cost-effectiveness of other malaria control interventions like bed nets and indoor residual sprays. However, it is important that the vaccine is introduced in addition to these other highly cost-effective interventions.”
The researchers measured cost-effectiveness in terms of disability-adjusted life years (DALYs), a metric used by health economists to compare the impacts of health interventions in populations over time. One DALY is equivalent to 1 lost year of healthy life. The lower the amount spent per DALY averted, the greater the cost-effectiveness of an intervention.
With the vaccine priced at $5 per dose, the researchers estimated a median cost of $87 per DALY averted for a 4-dose vaccine schedule across the range of transmission settings with parasite prevalence 10% to 65%.
This cost was estimated to vary depending on the level of malaria transmission found in a particular location—with the vaccine being increasingly cost-effective in areas with a higher malaria burden.
The researchers noted that, according to earlier studies, the cost per DALY averted for other malaria interventions indicate averages of $27 for long-lasting, insecticide-treated bed nets; $143 for indoor residual spraying; and $24 for intermittent preventative treatment.
Caveats
The researchers conceded that this study has its limitations. One is the remaining uncertainty regarding the vaccine’s efficacy after the 4 years of follow-up observed in the phase 3 trial.
The team also noted that, since the phase 3 trial of RTS,S was not large enough to test for a reduction in deaths from malaria (versus reduction in incidence of malaria cases) and the quality of care provided to participants was high, the modeling studies’ projection of deaths requires further validation during the implementation phase.
“It will be important to continue to track the long-term impact of this vaccine to ensure that the effectiveness predicted by the models is borne out in practice,” said Caitlin Bever, PhD, of the Institute for Disease Modeling in Bellevue, Washington. ![]()

Photo by Caitlin Kleiboer
The malaria vaccine candidate RTS,S/AS01 (Mosquirix) could have a significant impact on public health in a range of settings across sub-Saharan Africa, according to mathematical models.
Researchers found that, over a 15-year time horizon, an average of 116,500 cases of clinical malaria and 484 malaria deaths would be averted for every 100,000 children vaccinated under a 4-dose schedule of immunizations at 6, 7.5, 9, and 27 months of age.
This translates to approximately 1.2 malaria cases averted per vaccinated child and 1 malaria death averted for every 200 children vaccinated.
These data apply to children living in regions of Africa that experience moderate to high malaria transmission—countries where prevalence rates for the malaria parasite Plasmodium falciparum range from 10% to 65%—and assumes a vaccine coverage rate at the fourth dose of approximately 70%.
The findings, published in The Lancet, contribute to the scientific evidence being considered by the World Health Organization, which is assessing the vaccine candidate for use in Africa.
“We took a realistic look at expected coverage of the RTS,S vaccine in a variety of African settings and found it would have significant impact on malaria disease in all but the lowest malaria transmission regions,” said Melissa Penny, PhD, of the Swiss Tropical and Public Health Institute in Basel.
“Our numbers indicate that 6% to 29% of malaria deaths in children younger than age 5 could potentially be averted by the vaccine in the areas in which it is implemented, when used alongside other malaria control interventions.”
This is the first modeling study to use final, site-specific results of the RTS,S phase 3 safety and efficacy trial coordinated by GlaxoSmithKline and conducted at 11 sites in 7 African countries. And it accounts for implementation of the vaccine alongside use of long-lasting, insecticide-treated bed nets.
There was consensus across the predictions from all 4 groups that took part in the study. The participating institutions are Imperial College London in the UK, Swiss Tropical and Public Health Institute, the Institute for Disease Modeling in the US, and GlaxoSmithKline in Belgium.
According to the study authors, public health authorities require these types of impact estimates on malaria disease and deaths to inform vaccine implementation.
Models can account for differences between the trial and real-life settings in transmission levels and healthcare accessibility, as well as predict RTS,S’s impact on malaria mortality, which was not possible to assess in the trial.
Cost-effectiveness
As part of the modeling study, the researchers considered a range of possible prices for RTS,S, from $2 to $10. They found that, compared to current malaria interventions, the vaccine would be cost-effective to implement under an assumed price of USD$5 per dose in areas of moderate and high malaria transmission.
”The cost-effectiveness of RTS,S is similar to what we’ve seen for other recently introduced childhood vaccines,” said Azra Ghani, PhD, of Imperial College London.
“It also overlaps within the ranges of cost-effectiveness of other malaria control interventions like bed nets and indoor residual sprays. However, it is important that the vaccine is introduced in addition to these other highly cost-effective interventions.”
The researchers measured cost-effectiveness in terms of disability-adjusted life years (DALYs), a metric used by health economists to compare the impacts of health interventions in populations over time. One DALY is equivalent to 1 lost year of healthy life. The lower the amount spent per DALY averted, the greater the cost-effectiveness of an intervention.
With the vaccine priced at $5 per dose, the researchers estimated a median cost of $87 per DALY averted for a 4-dose vaccine schedule across the range of transmission settings with parasite prevalence 10% to 65%.
This cost was estimated to vary depending on the level of malaria transmission found in a particular location—with the vaccine being increasingly cost-effective in areas with a higher malaria burden.
The researchers noted that, according to earlier studies, the cost per DALY averted for other malaria interventions indicate averages of $27 for long-lasting, insecticide-treated bed nets; $143 for indoor residual spraying; and $24 for intermittent preventative treatment.
Caveats
The researchers conceded that this study has its limitations. One is the remaining uncertainty regarding the vaccine’s efficacy after the 4 years of follow-up observed in the phase 3 trial.
The team also noted that, since the phase 3 trial of RTS,S was not large enough to test for a reduction in deaths from malaria (versus reduction in incidence of malaria cases) and the quality of care provided to participants was high, the modeling studies’ projection of deaths requires further validation during the implementation phase.
“It will be important to continue to track the long-term impact of this vaccine to ensure that the effectiveness predicted by the models is borne out in practice,” said Caitlin Bever, PhD, of the Institute for Disease Modeling in Bellevue, Washington. ![]()
Drug protects fertility in chemo-treated mice

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()
Study raises questions about iron dosing

A new study suggests it may be difficult for the body to absorb iron in the necessary or desired quantities when iron supplements are administered in 24-hour intervals.
Investigators believe this is due to hepcidin. They found that giving subjects iron supplements at doses of 60 mg or higher increased hepcidin levels for up to 24 hours and was associated with lower iron absorption on the following day.
The team postulated that administering low-dose iron on alternate days may overcome this problem, but they said more research is needed to confirm this.
The investigators reported their findings in Blood.
Diego Moretti, PhD, of the Swiss Federal Institute of Technology Zürich, and his colleagues conducted this study in 54 young women.
The women had depleted iron reserves but were not yet anemic (plasma ferritin ≤20 mcg/L). They received a daily dose of at least 40 mg of iron, as is commonly prescribed in cases of iron deficiency.
Afterward, the investigators measured how the hepcidin concentration developed and quantified its effect on the absorption of subsequent doses of iron.
To analyze iron reabsorption, the team used stable iron isotopes as indicator substances. These substances have a modified ratio of stable iron isotopes. Iron-56 is the most frequent naturally occurring stable iron isotope (91.7%), followed by iron-54 (5.8%), and iron-57 (2.1%). Iron-58 occurs only in trace amounts.
The investigators used tablets with increased quantities of iron-57, iron-54, and iron-58. And they were able to measure endogenous iron absorption by observing isotope ratio changes within the body.
The team found that hepcidin reached its peak concentration after 6 to 8 hours, but even 24 hours after the first dose of iron, it was still present in high enough quantities to markedly reduce absorption of the second dose.
The body was only partly able to absorb a second dose of iron, which was given either on the same day or 24 hours after the first dose.
“To improve the percentage of iron absorbed, it would likely be more efficient to wait longer between doses,” Dr Moretti said.
However, he noted that more research is needed to confirm this, particularly because this study had 2 limitations. The first is that participants were all healthy young women without anemia, and the second is that iron absorption was observed over 2 days only.
The investigators are now preparing to conduct a follow-up study to analyze hepcidin concentration over the course of an iron supplementation regimen lasting several weeks. ![]()

A new study suggests it may be difficult for the body to absorb iron in the necessary or desired quantities when iron supplements are administered in 24-hour intervals.
Investigators believe this is due to hepcidin. They found that giving subjects iron supplements at doses of 60 mg or higher increased hepcidin levels for up to 24 hours and was associated with lower iron absorption on the following day.
The team postulated that administering low-dose iron on alternate days may overcome this problem, but they said more research is needed to confirm this.
The investigators reported their findings in Blood.
Diego Moretti, PhD, of the Swiss Federal Institute of Technology Zürich, and his colleagues conducted this study in 54 young women.
The women had depleted iron reserves but were not yet anemic (plasma ferritin ≤20 mcg/L). They received a daily dose of at least 40 mg of iron, as is commonly prescribed in cases of iron deficiency.
Afterward, the investigators measured how the hepcidin concentration developed and quantified its effect on the absorption of subsequent doses of iron.
To analyze iron reabsorption, the team used stable iron isotopes as indicator substances. These substances have a modified ratio of stable iron isotopes. Iron-56 is the most frequent naturally occurring stable iron isotope (91.7%), followed by iron-54 (5.8%), and iron-57 (2.1%). Iron-58 occurs only in trace amounts.
The investigators used tablets with increased quantities of iron-57, iron-54, and iron-58. And they were able to measure endogenous iron absorption by observing isotope ratio changes within the body.
The team found that hepcidin reached its peak concentration after 6 to 8 hours, but even 24 hours after the first dose of iron, it was still present in high enough quantities to markedly reduce absorption of the second dose.
The body was only partly able to absorb a second dose of iron, which was given either on the same day or 24 hours after the first dose.
“To improve the percentage of iron absorbed, it would likely be more efficient to wait longer between doses,” Dr Moretti said.
However, he noted that more research is needed to confirm this, particularly because this study had 2 limitations. The first is that participants were all healthy young women without anemia, and the second is that iron absorption was observed over 2 days only.
The investigators are now preparing to conduct a follow-up study to analyze hepcidin concentration over the course of an iron supplementation regimen lasting several weeks. ![]()

A new study suggests it may be difficult for the body to absorb iron in the necessary or desired quantities when iron supplements are administered in 24-hour intervals.
Investigators believe this is due to hepcidin. They found that giving subjects iron supplements at doses of 60 mg or higher increased hepcidin levels for up to 24 hours and was associated with lower iron absorption on the following day.
The team postulated that administering low-dose iron on alternate days may overcome this problem, but they said more research is needed to confirm this.
The investigators reported their findings in Blood.
Diego Moretti, PhD, of the Swiss Federal Institute of Technology Zürich, and his colleagues conducted this study in 54 young women.
The women had depleted iron reserves but were not yet anemic (plasma ferritin ≤20 mcg/L). They received a daily dose of at least 40 mg of iron, as is commonly prescribed in cases of iron deficiency.
Afterward, the investigators measured how the hepcidin concentration developed and quantified its effect on the absorption of subsequent doses of iron.
To analyze iron reabsorption, the team used stable iron isotopes as indicator substances. These substances have a modified ratio of stable iron isotopes. Iron-56 is the most frequent naturally occurring stable iron isotope (91.7%), followed by iron-54 (5.8%), and iron-57 (2.1%). Iron-58 occurs only in trace amounts.
The investigators used tablets with increased quantities of iron-57, iron-54, and iron-58. And they were able to measure endogenous iron absorption by observing isotope ratio changes within the body.
The team found that hepcidin reached its peak concentration after 6 to 8 hours, but even 24 hours after the first dose of iron, it was still present in high enough quantities to markedly reduce absorption of the second dose.
The body was only partly able to absorb a second dose of iron, which was given either on the same day or 24 hours after the first dose.
“To improve the percentage of iron absorbed, it would likely be more efficient to wait longer between doses,” Dr Moretti said.
However, he noted that more research is needed to confirm this, particularly because this study had 2 limitations. The first is that participants were all healthy young women without anemia, and the second is that iron absorption was observed over 2 days only.
The investigators are now preparing to conduct a follow-up study to analyze hepcidin concentration over the course of an iron supplementation regimen lasting several weeks. ![]()