User login
Denosumab better at building bone than zoledronic acid
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
AT THE ACR ANNUAL MEETING
Key clinical point: Denosumab was superior to zoledronic acid in building bone mineral density in postmenopausal women with osteoporosis who had previously been treated with oral bisphosphonates.
Major finding: Denosumab achieved a 2.1% difference in BMD at the lumbar spine and a 1.4% difference at the hip, compared with zoledronic acid.
Data source: Prospective, randomized, double-blind, double-dummy trial that included 643 patients.
Disclosures: Dr. Miller’s financial disclosures include Alexion, Amgen, Merck, Merck Serono, Boehringer Ingelheim, Regeneron, National Bone Health Alliance, Eli Lilly, Pfizer, Radius, and he is owner and director of the Colorado Center for Bone Research. The study was supported by Amgen.
ACR: Study confirms potential genetic basis for poor response to allopurinol in gout
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
AT THE ACR ANNUAL MEETING
Key clinical point: Screening could identify gout patients who require a dose adjustment of allopurinol.
Major finding: Presence of the rs2231142 SNP in the ABCG2 gene was significantly associated with poor response to allopurinol after adjusting for age, sex, body-mass index, and ethnicity (OR, 2.91; 95% CI, 1.71-5.17; P = .00015).
Data source: An analysis of 264 gout patients participating in clinical trials with allopurinol.
Disclosures: Dr. Stamp reported a financial disclosure with AstraZeneca.
ACR: Ozone injections reduce pain and improve function in knee OA
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:Intra-articular ozone injections reduce pain, improve function, and improve quality of life in patients with knee osteoarthritis.
Major finding: On all measures of pain, function, and quality of life, ozone injections were significantly superior to placebo.
Data source: A randomized, double-blind placebo-controlled trial of 98 patients with knee OA.
Disclosures: Dr. Trevisani had no relevant financial disclosures.
The Optum termination thunderbolt
One afternoon, after seeing your last patient, you’re doing the old-school thing, with your feet up, opening and reading your paper mail after a hard day’s work at the dermatology ranch. You see an odd form letter – a green sticker on the outside, certified mail – stating that you have been terminated from a Medicare advantage plan, no reason given.
At first, you don’t care so much. After all, this plan pays you only 95% of Medicare. Then you think about it and realize that this plan represents 50% of all Medicare beneficiaries in your area. You start to freak out, and you immediately go to the American Academy of Dermatology website where you read Rob Portman’s article about how to fight a termination notice and respond expeditiously.
Later that night, your spouse asks why you were singled out. “Are you a bad doctor? What did you do wrong? Can the kids still go to college?
The answer, most often, is that you did nothing wrong. You’ve just been caught up in the insurer’s network management software, Optum 360.
Optum 360 is a large health care information and management subsidiary of UnitedHealthcare. It was created as a joint venture by the Optum insight (health technology) unit of UnitedHealthcare, and Dignity Health (claims processing), forming Optum 360.
Optum claims that its software measures the efficiency of providers, saving insurers money and improving the quality of care – the Valhalla of health care managers everywhere. Unfortunately, Optum doesn’t deliver this vision of heaven on earth, at least not for dermatology.
Optum 360does little more than aggregate and average the costs of individual providers with no recognition of severity of disease or case mix. The physician with the most reimbursement during an episode of care for a given ICD-9 code (now for a group of ICD-10 codes) gets credited with all the expenses under that code. For example, you do two stages of Mohs surgery on a big basal cell on the nose that you send to plastics for repair. If the reimbursement for the Mohs surgery exceeds the plastics reimbursement, the Optum software designates you as the responsible provider. The cost of the plastics repair accrues to you; the hospital OR facility charges accrue to you (facilities are not considered a provider); and the anesthesiologist charges are credited to you. In addition, the superficial basal cell carcinoma on the patient’s back, treated a week later by the referring doctor, is also attributed to you as part of the original episode of care. There are no quality parameters and no subspecialty recognition.
If your patient load regularly includes patients who have Mohs surgery, the dermatologist down the street who does Mohs only once a week looks much better to Optum than you do. The referral-only medical dermatologist in town, who treats very sick patients and routinely prescribes biologics, and (heaven forbid) intravenous immunoglobulin, is similarly tagged for termination from the insurer’s network.
So it looks as though dermatologists who handle the toughest patients lose out. But who really loses the most? The sickest patients! The dermatologist can always fill the schedule with patients with other insurance coverage and reduce the backlog or, if worse comes to worse, he can take the Canadian cure and go on vacation. The sickest patients, however, get eliminated from the system when their doctors get eliminated by UnitedHealthcare’s software. Then patients often cannot find another doctor because most insurer’s physician rosters are 70% inaccurate (JAMA Dermatol. 2014 Dec;150[12]:1290-7).
In some circumstances, the sickest patients have reported either being unable to find other dermatologists willing to provide the special service they need or having to wait up to 6 months for an open appointment. These patients then try to drop back into fee-for-service Medicare, only to find they cannot afford the gap insurance, which costs five times as much. Why? Because those patients now have a preexisting condition. Yes, preexisting conditions still apply in the world of gap insurance.
This is obviously not optimal nor even acceptable. To quote Michael Keaton from the 1982 movie “Night Shift”: “Is this a great country or what?” The answer is a resounding “yes” for medical insurance companies who are booking record profits, but “no” for the sickest patients.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
One afternoon, after seeing your last patient, you’re doing the old-school thing, with your feet up, opening and reading your paper mail after a hard day’s work at the dermatology ranch. You see an odd form letter – a green sticker on the outside, certified mail – stating that you have been terminated from a Medicare advantage plan, no reason given.
At first, you don’t care so much. After all, this plan pays you only 95% of Medicare. Then you think about it and realize that this plan represents 50% of all Medicare beneficiaries in your area. You start to freak out, and you immediately go to the American Academy of Dermatology website where you read Rob Portman’s article about how to fight a termination notice and respond expeditiously.
Later that night, your spouse asks why you were singled out. “Are you a bad doctor? What did you do wrong? Can the kids still go to college?
The answer, most often, is that you did nothing wrong. You’ve just been caught up in the insurer’s network management software, Optum 360.
Optum 360 is a large health care information and management subsidiary of UnitedHealthcare. It was created as a joint venture by the Optum insight (health technology) unit of UnitedHealthcare, and Dignity Health (claims processing), forming Optum 360.
Optum claims that its software measures the efficiency of providers, saving insurers money and improving the quality of care – the Valhalla of health care managers everywhere. Unfortunately, Optum doesn’t deliver this vision of heaven on earth, at least not for dermatology.
Optum 360does little more than aggregate and average the costs of individual providers with no recognition of severity of disease or case mix. The physician with the most reimbursement during an episode of care for a given ICD-9 code (now for a group of ICD-10 codes) gets credited with all the expenses under that code. For example, you do two stages of Mohs surgery on a big basal cell on the nose that you send to plastics for repair. If the reimbursement for the Mohs surgery exceeds the plastics reimbursement, the Optum software designates you as the responsible provider. The cost of the plastics repair accrues to you; the hospital OR facility charges accrue to you (facilities are not considered a provider); and the anesthesiologist charges are credited to you. In addition, the superficial basal cell carcinoma on the patient’s back, treated a week later by the referring doctor, is also attributed to you as part of the original episode of care. There are no quality parameters and no subspecialty recognition.
If your patient load regularly includes patients who have Mohs surgery, the dermatologist down the street who does Mohs only once a week looks much better to Optum than you do. The referral-only medical dermatologist in town, who treats very sick patients and routinely prescribes biologics, and (heaven forbid) intravenous immunoglobulin, is similarly tagged for termination from the insurer’s network.
So it looks as though dermatologists who handle the toughest patients lose out. But who really loses the most? The sickest patients! The dermatologist can always fill the schedule with patients with other insurance coverage and reduce the backlog or, if worse comes to worse, he can take the Canadian cure and go on vacation. The sickest patients, however, get eliminated from the system when their doctors get eliminated by UnitedHealthcare’s software. Then patients often cannot find another doctor because most insurer’s physician rosters are 70% inaccurate (JAMA Dermatol. 2014 Dec;150[12]:1290-7).
In some circumstances, the sickest patients have reported either being unable to find other dermatologists willing to provide the special service they need or having to wait up to 6 months for an open appointment. These patients then try to drop back into fee-for-service Medicare, only to find they cannot afford the gap insurance, which costs five times as much. Why? Because those patients now have a preexisting condition. Yes, preexisting conditions still apply in the world of gap insurance.
This is obviously not optimal nor even acceptable. To quote Michael Keaton from the 1982 movie “Night Shift”: “Is this a great country or what?” The answer is a resounding “yes” for medical insurance companies who are booking record profits, but “no” for the sickest patients.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
One afternoon, after seeing your last patient, you’re doing the old-school thing, with your feet up, opening and reading your paper mail after a hard day’s work at the dermatology ranch. You see an odd form letter – a green sticker on the outside, certified mail – stating that you have been terminated from a Medicare advantage plan, no reason given.
At first, you don’t care so much. After all, this plan pays you only 95% of Medicare. Then you think about it and realize that this plan represents 50% of all Medicare beneficiaries in your area. You start to freak out, and you immediately go to the American Academy of Dermatology website where you read Rob Portman’s article about how to fight a termination notice and respond expeditiously.
Later that night, your spouse asks why you were singled out. “Are you a bad doctor? What did you do wrong? Can the kids still go to college?
The answer, most often, is that you did nothing wrong. You’ve just been caught up in the insurer’s network management software, Optum 360.
Optum 360 is a large health care information and management subsidiary of UnitedHealthcare. It was created as a joint venture by the Optum insight (health technology) unit of UnitedHealthcare, and Dignity Health (claims processing), forming Optum 360.
Optum claims that its software measures the efficiency of providers, saving insurers money and improving the quality of care – the Valhalla of health care managers everywhere. Unfortunately, Optum doesn’t deliver this vision of heaven on earth, at least not for dermatology.
Optum 360does little more than aggregate and average the costs of individual providers with no recognition of severity of disease or case mix. The physician with the most reimbursement during an episode of care for a given ICD-9 code (now for a group of ICD-10 codes) gets credited with all the expenses under that code. For example, you do two stages of Mohs surgery on a big basal cell on the nose that you send to plastics for repair. If the reimbursement for the Mohs surgery exceeds the plastics reimbursement, the Optum software designates you as the responsible provider. The cost of the plastics repair accrues to you; the hospital OR facility charges accrue to you (facilities are not considered a provider); and the anesthesiologist charges are credited to you. In addition, the superficial basal cell carcinoma on the patient’s back, treated a week later by the referring doctor, is also attributed to you as part of the original episode of care. There are no quality parameters and no subspecialty recognition.
If your patient load regularly includes patients who have Mohs surgery, the dermatologist down the street who does Mohs only once a week looks much better to Optum than you do. The referral-only medical dermatologist in town, who treats very sick patients and routinely prescribes biologics, and (heaven forbid) intravenous immunoglobulin, is similarly tagged for termination from the insurer’s network.
So it looks as though dermatologists who handle the toughest patients lose out. But who really loses the most? The sickest patients! The dermatologist can always fill the schedule with patients with other insurance coverage and reduce the backlog or, if worse comes to worse, he can take the Canadian cure and go on vacation. The sickest patients, however, get eliminated from the system when their doctors get eliminated by UnitedHealthcare’s software. Then patients often cannot find another doctor because most insurer’s physician rosters are 70% inaccurate (JAMA Dermatol. 2014 Dec;150[12]:1290-7).
In some circumstances, the sickest patients have reported either being unable to find other dermatologists willing to provide the special service they need or having to wait up to 6 months for an open appointment. These patients then try to drop back into fee-for-service Medicare, only to find they cannot afford the gap insurance, which costs five times as much. Why? Because those patients now have a preexisting condition. Yes, preexisting conditions still apply in the world of gap insurance.
This is obviously not optimal nor even acceptable. To quote Michael Keaton from the 1982 movie “Night Shift”: “Is this a great country or what?” The answer is a resounding “yes” for medical insurance companies who are booking record profits, but “no” for the sickest patients.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
Listen Now: Society of Hospital Medicine's Student Hospitalists Discuss Future of Specialty
Two medical students who participated in SHM's inaugural Student Hospitalist program, Mimi Zander, now a second year student at Touro College of Osteopathic Medicine in New York City, and Frank Zadravecz, a second year student at the University of Illinois College of Medicine at Chicago, share their thoughts about their part in the future of hospital medicine.
Two medical students who participated in SHM's inaugural Student Hospitalist program, Mimi Zander, now a second year student at Touro College of Osteopathic Medicine in New York City, and Frank Zadravecz, a second year student at the University of Illinois College of Medicine at Chicago, share their thoughts about their part in the future of hospital medicine.
Two medical students who participated in SHM's inaugural Student Hospitalist program, Mimi Zander, now a second year student at Touro College of Osteopathic Medicine in New York City, and Frank Zadravecz, a second year student at the University of Illinois College of Medicine at Chicago, share their thoughts about their part in the future of hospital medicine.
Dementia Most Costly Terminal Disease, Study Says
Families may spend almost twice as much caring for dementia patients at the end of life than they might if their loved one suffered from a different disease, a U.S. study suggests.
Costs paid by Medicare, the U.S. health insurance program for the elderly, were similar over the final five years of life for patients with dementia, heart disease, cancer and other conditions, according to the study published in the Annals of Internal Medicine.
But the average out-of-pocket costs absorbed by families of dementia patients totaled $61,522 over those five years, far greater than the typical tab of $34,068 for patients without dementia.
"Many costs related to daily care for patients with dementia are not covered by health insurance, and these care needs, including everything from supervision to bathing and feeding, may span several years," lead author Dr. Amy Kelley of the Icahn School of Medicine at Mount Sinai in New York said by email.
To assess the financial toll dementia takes on families, Dr. Kelley and colleagues analyzed Medicare spending and out-of-pocket costs for about 1,700 people aged 70 and older who died between 2005 and 2010.
Over the five years prior to each patient's date of death, the average total cost, including what Medicare covered as well as what families paid, was about $287,000 for dementia patients. That compares with roughly $175,000 for heart disease, $173,000 for cancer, and $197,000 for people who died of other causes.
Families caring for dementia patients also spent a greater proportion of their wealth than families helping loved ones with other conditions. The financial burden as a proportion of wealth was even more pronounced for patients who were black, had less than a high school education, or were unmarried or widowed women.
Shortcomings of the study include the possibility that insurance payments may have been underestimated as well as the lack of data on wages family members may have lost while caring for their loved ones, the authors acknowledge.
In addition, researchers measured only the probability of dementia and not whether the patients actually had dementia, the authors note. Few death certificates for patients with dementia will list that as the primary cause; instead, they report the problem that actually caused the patient to die, such as pneumonia.
Even so, the study findings highlight a financial burden posed by end-of-life care for elderly dementia patients that care reverberate through multiple generations, noted Carol Levine, director of the Families and Health Care Project at the United Hospital Fund, an independent policy group in New York City.
"There is a cascading effect: the financial drain for the older person's care means fewer resources not only for the caregiver but also for the younger generation's education and future prospects," Levine, who wasn't involved in the study, said by email.
"The immediate need for assistance is so compelling that future needs are often disregarded," Levine added. "The impact is greatest on families with the fewest resources to start with."
Many families also run into financial trouble because they mistakenly believe Medicare will pay for long term care services, said Dr. Mark Lachs, an expert in aging and finances at Weill Cornell Medical College in New York who wasn't involved in the study.
Families may consider long term care insurance to cover this gap in Medicare benefits, Dr. Lachs said by email.
Policy changes that might pay family members to be dementia caregivers would also help ease the financial strain, Dr. Lachs added.
Families may spend almost twice as much caring for dementia patients at the end of life than they might if their loved one suffered from a different disease, a U.S. study suggests.
Costs paid by Medicare, the U.S. health insurance program for the elderly, were similar over the final five years of life for patients with dementia, heart disease, cancer and other conditions, according to the study published in the Annals of Internal Medicine.
But the average out-of-pocket costs absorbed by families of dementia patients totaled $61,522 over those five years, far greater than the typical tab of $34,068 for patients without dementia.
"Many costs related to daily care for patients with dementia are not covered by health insurance, and these care needs, including everything from supervision to bathing and feeding, may span several years," lead author Dr. Amy Kelley of the Icahn School of Medicine at Mount Sinai in New York said by email.
To assess the financial toll dementia takes on families, Dr. Kelley and colleagues analyzed Medicare spending and out-of-pocket costs for about 1,700 people aged 70 and older who died between 2005 and 2010.
Over the five years prior to each patient's date of death, the average total cost, including what Medicare covered as well as what families paid, was about $287,000 for dementia patients. That compares with roughly $175,000 for heart disease, $173,000 for cancer, and $197,000 for people who died of other causes.
Families caring for dementia patients also spent a greater proportion of their wealth than families helping loved ones with other conditions. The financial burden as a proportion of wealth was even more pronounced for patients who were black, had less than a high school education, or were unmarried or widowed women.
Shortcomings of the study include the possibility that insurance payments may have been underestimated as well as the lack of data on wages family members may have lost while caring for their loved ones, the authors acknowledge.
In addition, researchers measured only the probability of dementia and not whether the patients actually had dementia, the authors note. Few death certificates for patients with dementia will list that as the primary cause; instead, they report the problem that actually caused the patient to die, such as pneumonia.
Even so, the study findings highlight a financial burden posed by end-of-life care for elderly dementia patients that care reverberate through multiple generations, noted Carol Levine, director of the Families and Health Care Project at the United Hospital Fund, an independent policy group in New York City.
"There is a cascading effect: the financial drain for the older person's care means fewer resources not only for the caregiver but also for the younger generation's education and future prospects," Levine, who wasn't involved in the study, said by email.
"The immediate need for assistance is so compelling that future needs are often disregarded," Levine added. "The impact is greatest on families with the fewest resources to start with."
Many families also run into financial trouble because they mistakenly believe Medicare will pay for long term care services, said Dr. Mark Lachs, an expert in aging and finances at Weill Cornell Medical College in New York who wasn't involved in the study.
Families may consider long term care insurance to cover this gap in Medicare benefits, Dr. Lachs said by email.
Policy changes that might pay family members to be dementia caregivers would also help ease the financial strain, Dr. Lachs added.
Families may spend almost twice as much caring for dementia patients at the end of life than they might if their loved one suffered from a different disease, a U.S. study suggests.
Costs paid by Medicare, the U.S. health insurance program for the elderly, were similar over the final five years of life for patients with dementia, heart disease, cancer and other conditions, according to the study published in the Annals of Internal Medicine.
But the average out-of-pocket costs absorbed by families of dementia patients totaled $61,522 over those five years, far greater than the typical tab of $34,068 for patients without dementia.
"Many costs related to daily care for patients with dementia are not covered by health insurance, and these care needs, including everything from supervision to bathing and feeding, may span several years," lead author Dr. Amy Kelley of the Icahn School of Medicine at Mount Sinai in New York said by email.
To assess the financial toll dementia takes on families, Dr. Kelley and colleagues analyzed Medicare spending and out-of-pocket costs for about 1,700 people aged 70 and older who died between 2005 and 2010.
Over the five years prior to each patient's date of death, the average total cost, including what Medicare covered as well as what families paid, was about $287,000 for dementia patients. That compares with roughly $175,000 for heart disease, $173,000 for cancer, and $197,000 for people who died of other causes.
Families caring for dementia patients also spent a greater proportion of their wealth than families helping loved ones with other conditions. The financial burden as a proportion of wealth was even more pronounced for patients who were black, had less than a high school education, or were unmarried or widowed women.
Shortcomings of the study include the possibility that insurance payments may have been underestimated as well as the lack of data on wages family members may have lost while caring for their loved ones, the authors acknowledge.
In addition, researchers measured only the probability of dementia and not whether the patients actually had dementia, the authors note. Few death certificates for patients with dementia will list that as the primary cause; instead, they report the problem that actually caused the patient to die, such as pneumonia.
Even so, the study findings highlight a financial burden posed by end-of-life care for elderly dementia patients that care reverberate through multiple generations, noted Carol Levine, director of the Families and Health Care Project at the United Hospital Fund, an independent policy group in New York City.
"There is a cascading effect: the financial drain for the older person's care means fewer resources not only for the caregiver but also for the younger generation's education and future prospects," Levine, who wasn't involved in the study, said by email.
"The immediate need for assistance is so compelling that future needs are often disregarded," Levine added. "The impact is greatest on families with the fewest resources to start with."
Many families also run into financial trouble because they mistakenly believe Medicare will pay for long term care services, said Dr. Mark Lachs, an expert in aging and finances at Weill Cornell Medical College in New York who wasn't involved in the study.
Families may consider long term care insurance to cover this gap in Medicare benefits, Dr. Lachs said by email.
Policy changes that might pay family members to be dementia caregivers would also help ease the financial strain, Dr. Lachs added.
Disseminated Cutaneous Infection with Mycobacterium chelonae in a Renal Transplant Recipient
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
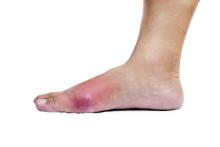
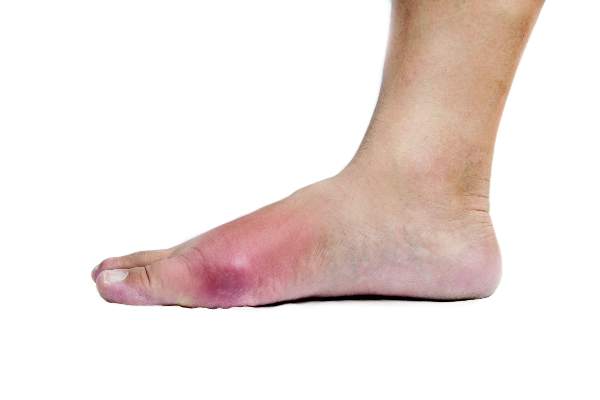
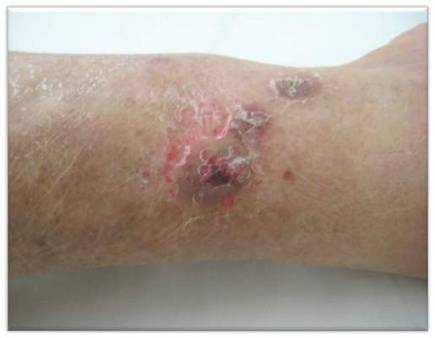
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.
| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
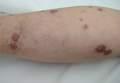
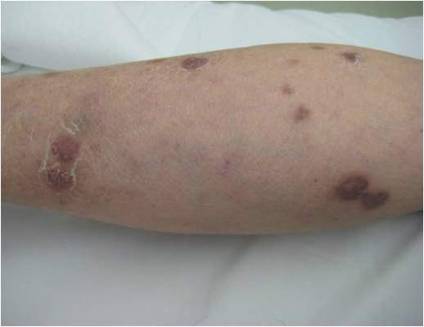
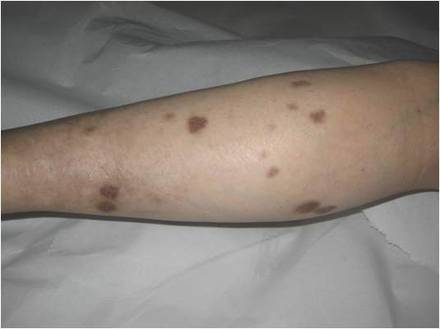
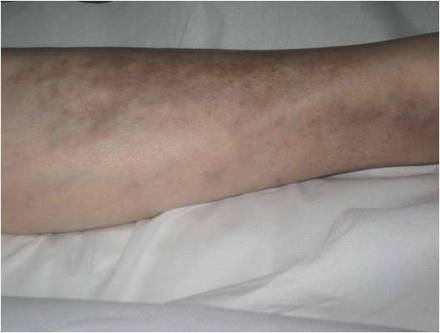
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).
| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Practice Points
- Nontuberculous mycobacteria (NTM) are environmental saprophytes that can cause infection in immunosuppressed individuals as well as immunocompetent individuals with certain predisposing factors.
- It is important for clinicians to consider NTM in the differential diagnosis for patients who present with chronic skin or soft tissue infections.
- Histologic examination and culture of a biopsy specimen followed by polymerase chain reaction assay for genotyping of the specimen are recommended to determine the responsible Mycobacterium species.
- New molecular genetic strip tests can differentiate NTM species more quickly.
ESRD treatments linked to different cancers
Photo by Anna Frodesiak
Patients with end-stage renal disease (ESRD) may have different cancer risks according to the treatment they are receiving, a new study suggests.
Researchers found that patients had a higher risk of developing infection-related and immune-related cancers—including Hodgkin and non-Hodgkin lymphoma (NHL)—after receiving a kidney transplant.
But patients had a higher risk of ESRD-related cancers when they were on dialysis.
Elizabeth Yanik, PhD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues reported these results in the Journal of the American Society of Nephrology.
The researchers theorized that assessing patterns in ESRD patients across periods of dialysis and kidney transplant might inform cancer etiology.
So the team studied registry data on 202,195 kidney transplant candidates and recipients, comparing the incidence of cancers during kidney function intervals (time with a transplant) to the incidence during nonfunction intervals (waitlist or time after transplant failure [dialysis]). The analysis was adjusted for demographic characteristics.
Results showed the incidence of infection-related and immune-related cancers was higher during kidney function intervals than nonfunction intervals.
Cancers with a significantly higher incidence included Kaposi’s sarcoma (hazard ratio [HR]=9.1, P<0.001), NHL (HR=3.2, P<0.001), Hodgkin lymphoma (HR=3.0, P<0.001), lip cancer (HR=3.4, P<0.001), nonepithelial skin cancers (HR=3.8, P<0.001), melanoma (HR=1.9, P<0.001), prostate cancer (HR=1.2, P=0.003), anal cancer (HR=1.8, P=0.01), other genital cancers (HR=1.5, P=0.03), lung cancer (HR=1.3 P<0.001), and pancreatic cancer (HR=1.5, P=0.004).
Dr Yanik and her colleagues noted that, of these cancers, NHL, anal cancer, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer consistently increased in incidence with each transition to a kidney function interval and decreased with each transition to a nonfunction interval.
And the 2 types of transitions were significant for NHL, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer.
The researchers also identified cancers with a significantly lower incidence during kidney function intervals. This included kidney cancer (HR=0.77, P<0.001), thyroid cancer (HR=0.67, P<0.001), breast cancer (HR=0.81, P=0.002), and liver cancer (HR=0.59, P=0.001).
The team noted that, among cancers with a lower incidence during kidney function intervals, kidney cancer, thyroid cancer, and myeloma consistently decreased in incidence with each transition to a kidney function interval and increased in incidence with each transition to a nonfunction interval. But both transitions were only significant for kidney and thyroid cancers.
“Our study indicates that the needs of individuals with end-stage renal disease, in terms of cancer prevention and cancer screening, will likely differ over time,” Dr Yanik said.
“Vigilance for kidney cancer and thyroid cancer may be of particular importance while these individuals are on dialysis. Extra consideration for screening for melanoma or lung cancer may be called for while taking immunosuppressant medications following a kidney transplant.” ![]()

Photo by Anna Frodesiak
Patients with end-stage renal disease (ESRD) may have different cancer risks according to the treatment they are receiving, a new study suggests.
Researchers found that patients had a higher risk of developing infection-related and immune-related cancers—including Hodgkin and non-Hodgkin lymphoma (NHL)—after receiving a kidney transplant.
But patients had a higher risk of ESRD-related cancers when they were on dialysis.
Elizabeth Yanik, PhD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues reported these results in the Journal of the American Society of Nephrology.
The researchers theorized that assessing patterns in ESRD patients across periods of dialysis and kidney transplant might inform cancer etiology.
So the team studied registry data on 202,195 kidney transplant candidates and recipients, comparing the incidence of cancers during kidney function intervals (time with a transplant) to the incidence during nonfunction intervals (waitlist or time after transplant failure [dialysis]). The analysis was adjusted for demographic characteristics.
Results showed the incidence of infection-related and immune-related cancers was higher during kidney function intervals than nonfunction intervals.
Cancers with a significantly higher incidence included Kaposi’s sarcoma (hazard ratio [HR]=9.1, P<0.001), NHL (HR=3.2, P<0.001), Hodgkin lymphoma (HR=3.0, P<0.001), lip cancer (HR=3.4, P<0.001), nonepithelial skin cancers (HR=3.8, P<0.001), melanoma (HR=1.9, P<0.001), prostate cancer (HR=1.2, P=0.003), anal cancer (HR=1.8, P=0.01), other genital cancers (HR=1.5, P=0.03), lung cancer (HR=1.3 P<0.001), and pancreatic cancer (HR=1.5, P=0.004).
Dr Yanik and her colleagues noted that, of these cancers, NHL, anal cancer, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer consistently increased in incidence with each transition to a kidney function interval and decreased with each transition to a nonfunction interval.
And the 2 types of transitions were significant for NHL, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer.
The researchers also identified cancers with a significantly lower incidence during kidney function intervals. This included kidney cancer (HR=0.77, P<0.001), thyroid cancer (HR=0.67, P<0.001), breast cancer (HR=0.81, P=0.002), and liver cancer (HR=0.59, P=0.001).
The team noted that, among cancers with a lower incidence during kidney function intervals, kidney cancer, thyroid cancer, and myeloma consistently decreased in incidence with each transition to a kidney function interval and increased in incidence with each transition to a nonfunction interval. But both transitions were only significant for kidney and thyroid cancers.
“Our study indicates that the needs of individuals with end-stage renal disease, in terms of cancer prevention and cancer screening, will likely differ over time,” Dr Yanik said.
“Vigilance for kidney cancer and thyroid cancer may be of particular importance while these individuals are on dialysis. Extra consideration for screening for melanoma or lung cancer may be called for while taking immunosuppressant medications following a kidney transplant.” ![]()

Photo by Anna Frodesiak
Patients with end-stage renal disease (ESRD) may have different cancer risks according to the treatment they are receiving, a new study suggests.
Researchers found that patients had a higher risk of developing infection-related and immune-related cancers—including Hodgkin and non-Hodgkin lymphoma (NHL)—after receiving a kidney transplant.
But patients had a higher risk of ESRD-related cancers when they were on dialysis.
Elizabeth Yanik, PhD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues reported these results in the Journal of the American Society of Nephrology.
The researchers theorized that assessing patterns in ESRD patients across periods of dialysis and kidney transplant might inform cancer etiology.
So the team studied registry data on 202,195 kidney transplant candidates and recipients, comparing the incidence of cancers during kidney function intervals (time with a transplant) to the incidence during nonfunction intervals (waitlist or time after transplant failure [dialysis]). The analysis was adjusted for demographic characteristics.
Results showed the incidence of infection-related and immune-related cancers was higher during kidney function intervals than nonfunction intervals.
Cancers with a significantly higher incidence included Kaposi’s sarcoma (hazard ratio [HR]=9.1, P<0.001), NHL (HR=3.2, P<0.001), Hodgkin lymphoma (HR=3.0, P<0.001), lip cancer (HR=3.4, P<0.001), nonepithelial skin cancers (HR=3.8, P<0.001), melanoma (HR=1.9, P<0.001), prostate cancer (HR=1.2, P=0.003), anal cancer (HR=1.8, P=0.01), other genital cancers (HR=1.5, P=0.03), lung cancer (HR=1.3 P<0.001), and pancreatic cancer (HR=1.5, P=0.004).
Dr Yanik and her colleagues noted that, of these cancers, NHL, anal cancer, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer consistently increased in incidence with each transition to a kidney function interval and decreased with each transition to a nonfunction interval.
And the 2 types of transitions were significant for NHL, lung cancer, melanoma, nonepithelial skin cancers, and pancreatic cancer.
The researchers also identified cancers with a significantly lower incidence during kidney function intervals. This included kidney cancer (HR=0.77, P<0.001), thyroid cancer (HR=0.67, P<0.001), breast cancer (HR=0.81, P=0.002), and liver cancer (HR=0.59, P=0.001).
The team noted that, among cancers with a lower incidence during kidney function intervals, kidney cancer, thyroid cancer, and myeloma consistently decreased in incidence with each transition to a kidney function interval and increased in incidence with each transition to a nonfunction interval. But both transitions were only significant for kidney and thyroid cancers.
“Our study indicates that the needs of individuals with end-stage renal disease, in terms of cancer prevention and cancer screening, will likely differ over time,” Dr Yanik said.
“Vigilance for kidney cancer and thyroid cancer may be of particular importance while these individuals are on dialysis. Extra consideration for screening for melanoma or lung cancer may be called for while taking immunosuppressant medications following a kidney transplant.” ![]()
Pharma companies fail to disclose trial information

Photo by Esther Dyson
New research indicates that pharmaceutical companies are still failing to disclose trial information publicly, despite efforts made over the last several years to increase transparency.
Investigators examined publicly available information for 15 drugs approved by the US Food and Drug Administration (FDA) in 2012.
They found that two-thirds of clinical trials per drug were disclosed publicly, which is below legal standards.
In an attempt to fix this problem, the investigators developed the “Good Pharma Scorecard,” which ranks companies according to their adherence to transparency practices.
Jennifer Miller, PhD, of NYU Langone Medical Center in New York, New York, and her colleagues described the scorecard and reported their research results in BMJ Open.
“Selectively disclosing trial information can distort the medical evidence and challenge the abilities of physicians, prescription guideline writers, payers, and formulary decision-makers to recommend and provide the right drugs for the right patients,” Dr Miller said.
She also noted that selectively disclosing information violates the rights of human research subjects laid out in the US Common Rule, a rule of ethics that requires that human-subject experiments have the potential to contribute to generalizable knowledge.
Study details
Dr Miller and her colleagues examined publicly available information for all drugs approved by the FDA in 2012 that were sponsored by the 20 pharmaceutical companies with the highest market value.
The information was gathered from a variety of publicly available documents, including Drugs@FDA, ClinicalTrials.gov, and journals indexed in Medline.
From this information, the team identified 15 drugs from 10 companies with more than 318 associated clinical trials involving 99,599 research subjects.
Almost half of all the drugs reviewed had at least 1 undisclosed phase 2 or 3 trial. A median of 57% of trials per drug were properly registered, 20% of final results were reported on ClinicalTrials.gov, and 56% of trials were published in academic journals.
A median of 65% of trial results were publicly available—either on ClinicalTrials.gov or in the medical literature. But the investigators found “considerable variation” between companies.
Per drug, a median of 17% of trials were subject to public disclosure by the 2007 US Food and Drug Administration Amendments Act (FDAAA). Of these trials, a median of 67% were FDAAA-compliant.
A majority of the research subjects—68%—participated in trials subject to the FDAAA, and 51% of these subjects were enrolled in trials that were noncompliant with the act.
Good Pharma Scorecard
Dr Miller and her colleagues believe they have devised a solution to help fix the transparency problem—the Good Pharma Scorecard.
The scorecard ranks drug sponsors according to 5 elements of transparency:
- Trial registration
- Results reporting
- Publication of trial data in a medical journal
- Compliance with legal disclosure requirements
- Adherence with the ethics standards enshrined in the Common Rule.
“The scorecard and rankings have the potential to benefit consumers by helping assure the integrity and completeness of clinical trial information,” Dr Miller said. “Full transparency of clinical trials would also strengthen the protection of human research subjects by avoiding their unknowing recruitment into already failed experiments.”
The pilot rankings on the Good Pharma Scorecard score the largest pharmaceutical companies for drugs approved by the FDA in 2012.
But Dr Miller and her team plan to publish this scorecard annually, ranking each new group of FDA-approved drugs going forward. A ranking of drugs approved in 2015 and their sponsors is scheduled to be released in 2016. ![]()

Photo by Esther Dyson
New research indicates that pharmaceutical companies are still failing to disclose trial information publicly, despite efforts made over the last several years to increase transparency.
Investigators examined publicly available information for 15 drugs approved by the US Food and Drug Administration (FDA) in 2012.
They found that two-thirds of clinical trials per drug were disclosed publicly, which is below legal standards.
In an attempt to fix this problem, the investigators developed the “Good Pharma Scorecard,” which ranks companies according to their adherence to transparency practices.
Jennifer Miller, PhD, of NYU Langone Medical Center in New York, New York, and her colleagues described the scorecard and reported their research results in BMJ Open.
“Selectively disclosing trial information can distort the medical evidence and challenge the abilities of physicians, prescription guideline writers, payers, and formulary decision-makers to recommend and provide the right drugs for the right patients,” Dr Miller said.
She also noted that selectively disclosing information violates the rights of human research subjects laid out in the US Common Rule, a rule of ethics that requires that human-subject experiments have the potential to contribute to generalizable knowledge.
Study details
Dr Miller and her colleagues examined publicly available information for all drugs approved by the FDA in 2012 that were sponsored by the 20 pharmaceutical companies with the highest market value.
The information was gathered from a variety of publicly available documents, including Drugs@FDA, ClinicalTrials.gov, and journals indexed in Medline.
From this information, the team identified 15 drugs from 10 companies with more than 318 associated clinical trials involving 99,599 research subjects.
Almost half of all the drugs reviewed had at least 1 undisclosed phase 2 or 3 trial. A median of 57% of trials per drug were properly registered, 20% of final results were reported on ClinicalTrials.gov, and 56% of trials were published in academic journals.
A median of 65% of trial results were publicly available—either on ClinicalTrials.gov or in the medical literature. But the investigators found “considerable variation” between companies.
Per drug, a median of 17% of trials were subject to public disclosure by the 2007 US Food and Drug Administration Amendments Act (FDAAA). Of these trials, a median of 67% were FDAAA-compliant.
A majority of the research subjects—68%—participated in trials subject to the FDAAA, and 51% of these subjects were enrolled in trials that were noncompliant with the act.
Good Pharma Scorecard
Dr Miller and her colleagues believe they have devised a solution to help fix the transparency problem—the Good Pharma Scorecard.
The scorecard ranks drug sponsors according to 5 elements of transparency:
- Trial registration
- Results reporting
- Publication of trial data in a medical journal
- Compliance with legal disclosure requirements
- Adherence with the ethics standards enshrined in the Common Rule.
“The scorecard and rankings have the potential to benefit consumers by helping assure the integrity and completeness of clinical trial information,” Dr Miller said. “Full transparency of clinical trials would also strengthen the protection of human research subjects by avoiding their unknowing recruitment into already failed experiments.”
The pilot rankings on the Good Pharma Scorecard score the largest pharmaceutical companies for drugs approved by the FDA in 2012.
But Dr Miller and her team plan to publish this scorecard annually, ranking each new group of FDA-approved drugs going forward. A ranking of drugs approved in 2015 and their sponsors is scheduled to be released in 2016. ![]()

Photo by Esther Dyson
New research indicates that pharmaceutical companies are still failing to disclose trial information publicly, despite efforts made over the last several years to increase transparency.
Investigators examined publicly available information for 15 drugs approved by the US Food and Drug Administration (FDA) in 2012.
They found that two-thirds of clinical trials per drug were disclosed publicly, which is below legal standards.
In an attempt to fix this problem, the investigators developed the “Good Pharma Scorecard,” which ranks companies according to their adherence to transparency practices.
Jennifer Miller, PhD, of NYU Langone Medical Center in New York, New York, and her colleagues described the scorecard and reported their research results in BMJ Open.
“Selectively disclosing trial information can distort the medical evidence and challenge the abilities of physicians, prescription guideline writers, payers, and formulary decision-makers to recommend and provide the right drugs for the right patients,” Dr Miller said.
She also noted that selectively disclosing information violates the rights of human research subjects laid out in the US Common Rule, a rule of ethics that requires that human-subject experiments have the potential to contribute to generalizable knowledge.
Study details
Dr Miller and her colleagues examined publicly available information for all drugs approved by the FDA in 2012 that were sponsored by the 20 pharmaceutical companies with the highest market value.
The information was gathered from a variety of publicly available documents, including Drugs@FDA, ClinicalTrials.gov, and journals indexed in Medline.
From this information, the team identified 15 drugs from 10 companies with more than 318 associated clinical trials involving 99,599 research subjects.
Almost half of all the drugs reviewed had at least 1 undisclosed phase 2 or 3 trial. A median of 57% of trials per drug were properly registered, 20% of final results were reported on ClinicalTrials.gov, and 56% of trials were published in academic journals.
A median of 65% of trial results were publicly available—either on ClinicalTrials.gov or in the medical literature. But the investigators found “considerable variation” between companies.
Per drug, a median of 17% of trials were subject to public disclosure by the 2007 US Food and Drug Administration Amendments Act (FDAAA). Of these trials, a median of 67% were FDAAA-compliant.
A majority of the research subjects—68%—participated in trials subject to the FDAAA, and 51% of these subjects were enrolled in trials that were noncompliant with the act.
Good Pharma Scorecard
Dr Miller and her colleagues believe they have devised a solution to help fix the transparency problem—the Good Pharma Scorecard.
The scorecard ranks drug sponsors according to 5 elements of transparency:
- Trial registration
- Results reporting
- Publication of trial data in a medical journal
- Compliance with legal disclosure requirements
- Adherence with the ethics standards enshrined in the Common Rule.
“The scorecard and rankings have the potential to benefit consumers by helping assure the integrity and completeness of clinical trial information,” Dr Miller said. “Full transparency of clinical trials would also strengthen the protection of human research subjects by avoiding their unknowing recruitment into already failed experiments.”
The pilot rankings on the Good Pharma Scorecard score the largest pharmaceutical companies for drugs approved by the FDA in 2012.
But Dr Miller and her team plan to publish this scorecard annually, ranking each new group of FDA-approved drugs going forward. A ranking of drugs approved in 2015 and their sponsors is scheduled to be released in 2016. ![]()
Team evaluates long-term tolerability of ticagrelor

Photo courtesy of AstraZeneca
ORLANDO, FL—A subanalysis of the PEGASUS-TIMI 54 trial has provided a clearer picture of the risks and benefits associated with long-term use of the antiplatelet drug ticagrelor, according to investigators.
The goals of the analysis were to evaluate the long-term efficacy of ticagrelor in patients with a history of myocardial infarction and to determine the rates of, and reasons for, discontinuing ticagrelor in these patients.
The PEGASUS-TIMI 54 trial included 21,162 patients (age 50 and older) who had experienced a heart attack in the previous 1 to 3 years. The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
Earlier results from this study showed that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke.
However, the rates of TIMI major bleeding and dyspnea were higher in patients who received either dose of ticagrelor than in those who received placebo. And premature discontinuation was higher in the ticagrelor arms.
To investigate this further, Marc Bonaca, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted their subanalysis. The results were presented in a late-breaking clinical trials session at the American Heart Association (AHA) Scientific Sessions 2015.
The trial lasted a median of 33 months. During this time, the rate of discontinuation (of ticagrelor or placebo) was 32% in the 90 mg ticagrelor arm, 29% in the 60 mg ticagrelor arm, and 21% in the placebo arm (P<0.001).
The rates of discontinuation due to patient decision or an administrative reason were similar between the arms.
However, patients were more likely to stop taking the study drug due to an adverse event (AE) if they were receiving ticagrelor rather than placebo. Discontinuation due to an AE was 19% in the 90 mg ticagrelor arm, 16.4% in the 60 mg ticagrelor arm, and 8.9% in the placebo arm (P<0.01).
The most frequent AEs leading to discontinuation were bleeding—6.5% in the 90 mg arm, 5.1% in the 60 mg arm, and 1.2% in the placebo arm (P<0.001)—and dyspnea—6.2%, 4.3%, and 0.7%, respectively (P<0.001).
Rates of AEs leading to discontinuation were highest in the first year—16% in the 90 mg arm, 13% in the 60 mg arm, and 6% in the placebo arm.
In those patients who stayed on therapy, discontinuation rates over the subsequent 2 years were 6.5% in the 90 mg arm, 6.0% in the 60 mg arm, 4.6% in the placebo arm.
In patients who stayed on therapy, ticagrelor reduced the rate of the composite efficacy endpoint of cardiovascular death, myocardial infarction, or stroke at 3 years (hazard ratio=0.79), which is consistent with the results of the overall population of the study.
“This analysis pointed to important patterns with regards to common AEs associated with ticagrelor in the context of clinical benefit,” Dr Bonaca said.
“Physicians must consider the overall risks, including higher rates of bleeding and dyspnea, particularly within the first year. For patients at increased risk for recurrent cardiovascular events in the long-term, ticagrelor can provide an important benefit.”
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company developing ticagrelor. Investigators involved in the subanalysis reported relationships with a range of other companies as well. ![]()

Photo courtesy of AstraZeneca
ORLANDO, FL—A subanalysis of the PEGASUS-TIMI 54 trial has provided a clearer picture of the risks and benefits associated with long-term use of the antiplatelet drug ticagrelor, according to investigators.
The goals of the analysis were to evaluate the long-term efficacy of ticagrelor in patients with a history of myocardial infarction and to determine the rates of, and reasons for, discontinuing ticagrelor in these patients.
The PEGASUS-TIMI 54 trial included 21,162 patients (age 50 and older) who had experienced a heart attack in the previous 1 to 3 years. The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
Earlier results from this study showed that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke.
However, the rates of TIMI major bleeding and dyspnea were higher in patients who received either dose of ticagrelor than in those who received placebo. And premature discontinuation was higher in the ticagrelor arms.
To investigate this further, Marc Bonaca, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted their subanalysis. The results were presented in a late-breaking clinical trials session at the American Heart Association (AHA) Scientific Sessions 2015.
The trial lasted a median of 33 months. During this time, the rate of discontinuation (of ticagrelor or placebo) was 32% in the 90 mg ticagrelor arm, 29% in the 60 mg ticagrelor arm, and 21% in the placebo arm (P<0.001).
The rates of discontinuation due to patient decision or an administrative reason were similar between the arms.
However, patients were more likely to stop taking the study drug due to an adverse event (AE) if they were receiving ticagrelor rather than placebo. Discontinuation due to an AE was 19% in the 90 mg ticagrelor arm, 16.4% in the 60 mg ticagrelor arm, and 8.9% in the placebo arm (P<0.01).
The most frequent AEs leading to discontinuation were bleeding—6.5% in the 90 mg arm, 5.1% in the 60 mg arm, and 1.2% in the placebo arm (P<0.001)—and dyspnea—6.2%, 4.3%, and 0.7%, respectively (P<0.001).
Rates of AEs leading to discontinuation were highest in the first year—16% in the 90 mg arm, 13% in the 60 mg arm, and 6% in the placebo arm.
In those patients who stayed on therapy, discontinuation rates over the subsequent 2 years were 6.5% in the 90 mg arm, 6.0% in the 60 mg arm, 4.6% in the placebo arm.
In patients who stayed on therapy, ticagrelor reduced the rate of the composite efficacy endpoint of cardiovascular death, myocardial infarction, or stroke at 3 years (hazard ratio=0.79), which is consistent with the results of the overall population of the study.
“This analysis pointed to important patterns with regards to common AEs associated with ticagrelor in the context of clinical benefit,” Dr Bonaca said.
“Physicians must consider the overall risks, including higher rates of bleeding and dyspnea, particularly within the first year. For patients at increased risk for recurrent cardiovascular events in the long-term, ticagrelor can provide an important benefit.”
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company developing ticagrelor. Investigators involved in the subanalysis reported relationships with a range of other companies as well. ![]()

Photo courtesy of AstraZeneca
ORLANDO, FL—A subanalysis of the PEGASUS-TIMI 54 trial has provided a clearer picture of the risks and benefits associated with long-term use of the antiplatelet drug ticagrelor, according to investigators.
The goals of the analysis were to evaluate the long-term efficacy of ticagrelor in patients with a history of myocardial infarction and to determine the rates of, and reasons for, discontinuing ticagrelor in these patients.
The PEGASUS-TIMI 54 trial included 21,162 patients (age 50 and older) who had experienced a heart attack in the previous 1 to 3 years. The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
Earlier results from this study showed that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke.
However, the rates of TIMI major bleeding and dyspnea were higher in patients who received either dose of ticagrelor than in those who received placebo. And premature discontinuation was higher in the ticagrelor arms.
To investigate this further, Marc Bonaca, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted their subanalysis. The results were presented in a late-breaking clinical trials session at the American Heart Association (AHA) Scientific Sessions 2015.
The trial lasted a median of 33 months. During this time, the rate of discontinuation (of ticagrelor or placebo) was 32% in the 90 mg ticagrelor arm, 29% in the 60 mg ticagrelor arm, and 21% in the placebo arm (P<0.001).
The rates of discontinuation due to patient decision or an administrative reason were similar between the arms.
However, patients were more likely to stop taking the study drug due to an adverse event (AE) if they were receiving ticagrelor rather than placebo. Discontinuation due to an AE was 19% in the 90 mg ticagrelor arm, 16.4% in the 60 mg ticagrelor arm, and 8.9% in the placebo arm (P<0.01).
The most frequent AEs leading to discontinuation were bleeding—6.5% in the 90 mg arm, 5.1% in the 60 mg arm, and 1.2% in the placebo arm (P<0.001)—and dyspnea—6.2%, 4.3%, and 0.7%, respectively (P<0.001).
Rates of AEs leading to discontinuation were highest in the first year—16% in the 90 mg arm, 13% in the 60 mg arm, and 6% in the placebo arm.
In those patients who stayed on therapy, discontinuation rates over the subsequent 2 years were 6.5% in the 90 mg arm, 6.0% in the 60 mg arm, 4.6% in the placebo arm.
In patients who stayed on therapy, ticagrelor reduced the rate of the composite efficacy endpoint of cardiovascular death, myocardial infarction, or stroke at 3 years (hazard ratio=0.79), which is consistent with the results of the overall population of the study.
“This analysis pointed to important patterns with regards to common AEs associated with ticagrelor in the context of clinical benefit,” Dr Bonaca said.
“Physicians must consider the overall risks, including higher rates of bleeding and dyspnea, particularly within the first year. For patients at increased risk for recurrent cardiovascular events in the long-term, ticagrelor can provide an important benefit.”
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company developing ticagrelor. Investigators involved in the subanalysis reported relationships with a range of other companies as well. ![]()