User login
Risks of tobacco use in the LGBT community
Tobacco addiction and smoke exposure are among the leading causes of preventable and premature death and disability in the United States and elsewhere in the world. The landmark 2012 Surgeon General’s report, Preventing Tobacco Use Among Youth and Young Adults, stated, “The vast majority of Americans who begin daily smoking during adolescence are addicted to nicotine by young adulthood.” All youth are at risk for experimenting and using “standard” tobacco products, as well as relatively new merchandise such as e-cigarettes, hookas, bidis (small, hand-rolled cigarettes), and little cigars (Pediatrics. 2013 Aug 5;132:e578-86). In a 2015 article, data show more than twice as many youth use two or more types of tobacco products than use cigarettes alone (Pediatrics. 2015 March;135:409-15).
In a 2015 American Academy of Pediatrics policy statement, the academy stated that all children, adolescents, and young adults must be safeguarded from using all of the various tobacco products (Pediatrics. 2015 Oct 26. doi: 10.1542/peds.2015-3108). Therefore, a working knowledge of the various types of products is essential when speaking with youth on this subject.
The AAP recommended that all children and youth, without exception, must be considered to be at risk for using tobacco products. Therefore, all youth should be counseled, as the AAP made no exceptions with regard to race, national origin, ethnic group, socioeconomic status, or membership in the LGBT community.
Tobacco use by sexual identity
Much more needs to be known with regard to tobacco use for all children, youth, and young adults. National surveillance data are needed for the LGBT community, whose members have unique health care needs. A 2013 article demonstrated disparities in the use of tobacco products in young adults in the LGBT community (Nicotine Tob Res. 2013;15[11]:1822-31).
This study used data from the American Legacy Foundation’s Young Adult Study. The survey compared the use of tobacco by the LGBT community versus the heterosexual community during the previous 30-day period. The prevalence of use of tobacco products for young adults who self-identified as sexual minorities was statistically higher than for their heterosexual counterparts. For example, current use of tobacco products was 22% in heterosexual young adults, compared with 35% in young adults who identified as homosexual and 31% in young adults who identified as bisexual.
However, this same publication stated that there are very few studies on this subject, including why there are these disparities. This information is important to know so better approaches can be developed to address these issues. In addition, health care providers must address the issue of tobacco use by youth and young adults, and develop specific approaches that can specifically target at-risk populations that are culturally competent. The authors concluded that it remains unclear why the tobacco use rate among the LGBT community is significantly higher than in their peers who are heterosexual. Risk factors that have been posited include social stigma, the role of bars in this community, and alcohol and drug use. Also, social acceptance issues, inclusion issues, alienation and depression, and marketing by tobacco manufacturers may be risk factors.
Much work remains to be done to address these risk factors and, therefore, the use of tobacco and similar drugs. The 2012 surgeon general’s report emphasized that health care providers of young people must address these issues directly and consistently with their patients.
Practical next steps for your practice
One approach to addressing tobacco use with your patients is to implement the “5 A’s”:
1. Anticipate/Ask. Ask young people if they or their friends are interested in tobacco products and/or if they use tobacco products of any type. Do this at every visit.
2. Advise. In clear, strong, personalized language, urge the tobacco user to quit.
3. Assess. Assess the willingness of the tobacco user to quit, and urge the youth to quit smoking.
4. Assist. For the youth willing to quit, use counseling yourself and/or refer for counseling to individuals with expertise in tobacco cessation or support groups, and consider pharmacotherapy if necessary.
5. Arrange. Schedule a follow-up contact in person within the first week and then on a regularly scheduled basis.
The 5 A’s have been recognized by the Agency for Healthcare Research and Quality as an evidence-based practice for both adult and pediatric patients. In a 2014 article, Dr. Jonathan Klein reported on a study of youth who had seen a clinician for a preventive visit within the past year. However, the youth also reported that the rate of their receiving counseling on tobacco use was relatively low (Pediatrics. 2014 Sep;134[3]:600-1) Most recently, Howard University completed a project funded by the District of Columbia Department of Health to develop and teach a curriculum for medical and nursing students, residents, and physician and nursing staff on this important issue. The youth whom we interviewed in developing the curriculum reported that they had never received counseling by health professionals during their preventive health care visits. This important issue needs to be addressed because it truly is a key to future health for our children and youth.
Dr. Tierney is a Washington-based pediatrician who is a member of the Institute for Public Health Innovation and the D.C. Primary Care Association Medicaid Financing Task Force on Medicaid payment for community health workers. She also works with Howard University Hospital’s Cancer Center on tobacco use avoidance and/or cessation by African American youth. She is on the board of Whitman-Walker Health to advise on Medicaid payment and sits on the quality assurance committee.
Tobacco addiction and smoke exposure are among the leading causes of preventable and premature death and disability in the United States and elsewhere in the world. The landmark 2012 Surgeon General’s report, Preventing Tobacco Use Among Youth and Young Adults, stated, “The vast majority of Americans who begin daily smoking during adolescence are addicted to nicotine by young adulthood.” All youth are at risk for experimenting and using “standard” tobacco products, as well as relatively new merchandise such as e-cigarettes, hookas, bidis (small, hand-rolled cigarettes), and little cigars (Pediatrics. 2013 Aug 5;132:e578-86). In a 2015 article, data show more than twice as many youth use two or more types of tobacco products than use cigarettes alone (Pediatrics. 2015 March;135:409-15).
In a 2015 American Academy of Pediatrics policy statement, the academy stated that all children, adolescents, and young adults must be safeguarded from using all of the various tobacco products (Pediatrics. 2015 Oct 26. doi: 10.1542/peds.2015-3108). Therefore, a working knowledge of the various types of products is essential when speaking with youth on this subject.
The AAP recommended that all children and youth, without exception, must be considered to be at risk for using tobacco products. Therefore, all youth should be counseled, as the AAP made no exceptions with regard to race, national origin, ethnic group, socioeconomic status, or membership in the LGBT community.
Tobacco use by sexual identity
Much more needs to be known with regard to tobacco use for all children, youth, and young adults. National surveillance data are needed for the LGBT community, whose members have unique health care needs. A 2013 article demonstrated disparities in the use of tobacco products in young adults in the LGBT community (Nicotine Tob Res. 2013;15[11]:1822-31).
This study used data from the American Legacy Foundation’s Young Adult Study. The survey compared the use of tobacco by the LGBT community versus the heterosexual community during the previous 30-day period. The prevalence of use of tobacco products for young adults who self-identified as sexual minorities was statistically higher than for their heterosexual counterparts. For example, current use of tobacco products was 22% in heterosexual young adults, compared with 35% in young adults who identified as homosexual and 31% in young adults who identified as bisexual.
However, this same publication stated that there are very few studies on this subject, including why there are these disparities. This information is important to know so better approaches can be developed to address these issues. In addition, health care providers must address the issue of tobacco use by youth and young adults, and develop specific approaches that can specifically target at-risk populations that are culturally competent. The authors concluded that it remains unclear why the tobacco use rate among the LGBT community is significantly higher than in their peers who are heterosexual. Risk factors that have been posited include social stigma, the role of bars in this community, and alcohol and drug use. Also, social acceptance issues, inclusion issues, alienation and depression, and marketing by tobacco manufacturers may be risk factors.
Much work remains to be done to address these risk factors and, therefore, the use of tobacco and similar drugs. The 2012 surgeon general’s report emphasized that health care providers of young people must address these issues directly and consistently with their patients.
Practical next steps for your practice
One approach to addressing tobacco use with your patients is to implement the “5 A’s”:
1. Anticipate/Ask. Ask young people if they or their friends are interested in tobacco products and/or if they use tobacco products of any type. Do this at every visit.
2. Advise. In clear, strong, personalized language, urge the tobacco user to quit.
3. Assess. Assess the willingness of the tobacco user to quit, and urge the youth to quit smoking.
4. Assist. For the youth willing to quit, use counseling yourself and/or refer for counseling to individuals with expertise in tobacco cessation or support groups, and consider pharmacotherapy if necessary.
5. Arrange. Schedule a follow-up contact in person within the first week and then on a regularly scheduled basis.
The 5 A’s have been recognized by the Agency for Healthcare Research and Quality as an evidence-based practice for both adult and pediatric patients. In a 2014 article, Dr. Jonathan Klein reported on a study of youth who had seen a clinician for a preventive visit within the past year. However, the youth also reported that the rate of their receiving counseling on tobacco use was relatively low (Pediatrics. 2014 Sep;134[3]:600-1) Most recently, Howard University completed a project funded by the District of Columbia Department of Health to develop and teach a curriculum for medical and nursing students, residents, and physician and nursing staff on this important issue. The youth whom we interviewed in developing the curriculum reported that they had never received counseling by health professionals during their preventive health care visits. This important issue needs to be addressed because it truly is a key to future health for our children and youth.
Dr. Tierney is a Washington-based pediatrician who is a member of the Institute for Public Health Innovation and the D.C. Primary Care Association Medicaid Financing Task Force on Medicaid payment for community health workers. She also works with Howard University Hospital’s Cancer Center on tobacco use avoidance and/or cessation by African American youth. She is on the board of Whitman-Walker Health to advise on Medicaid payment and sits on the quality assurance committee.
Tobacco addiction and smoke exposure are among the leading causes of preventable and premature death and disability in the United States and elsewhere in the world. The landmark 2012 Surgeon General’s report, Preventing Tobacco Use Among Youth and Young Adults, stated, “The vast majority of Americans who begin daily smoking during adolescence are addicted to nicotine by young adulthood.” All youth are at risk for experimenting and using “standard” tobacco products, as well as relatively new merchandise such as e-cigarettes, hookas, bidis (small, hand-rolled cigarettes), and little cigars (Pediatrics. 2013 Aug 5;132:e578-86). In a 2015 article, data show more than twice as many youth use two or more types of tobacco products than use cigarettes alone (Pediatrics. 2015 March;135:409-15).
In a 2015 American Academy of Pediatrics policy statement, the academy stated that all children, adolescents, and young adults must be safeguarded from using all of the various tobacco products (Pediatrics. 2015 Oct 26. doi: 10.1542/peds.2015-3108). Therefore, a working knowledge of the various types of products is essential when speaking with youth on this subject.
The AAP recommended that all children and youth, without exception, must be considered to be at risk for using tobacco products. Therefore, all youth should be counseled, as the AAP made no exceptions with regard to race, national origin, ethnic group, socioeconomic status, or membership in the LGBT community.
Tobacco use by sexual identity
Much more needs to be known with regard to tobacco use for all children, youth, and young adults. National surveillance data are needed for the LGBT community, whose members have unique health care needs. A 2013 article demonstrated disparities in the use of tobacco products in young adults in the LGBT community (Nicotine Tob Res. 2013;15[11]:1822-31).
This study used data from the American Legacy Foundation’s Young Adult Study. The survey compared the use of tobacco by the LGBT community versus the heterosexual community during the previous 30-day period. The prevalence of use of tobacco products for young adults who self-identified as sexual minorities was statistically higher than for their heterosexual counterparts. For example, current use of tobacco products was 22% in heterosexual young adults, compared with 35% in young adults who identified as homosexual and 31% in young adults who identified as bisexual.
However, this same publication stated that there are very few studies on this subject, including why there are these disparities. This information is important to know so better approaches can be developed to address these issues. In addition, health care providers must address the issue of tobacco use by youth and young adults, and develop specific approaches that can specifically target at-risk populations that are culturally competent. The authors concluded that it remains unclear why the tobacco use rate among the LGBT community is significantly higher than in their peers who are heterosexual. Risk factors that have been posited include social stigma, the role of bars in this community, and alcohol and drug use. Also, social acceptance issues, inclusion issues, alienation and depression, and marketing by tobacco manufacturers may be risk factors.
Much work remains to be done to address these risk factors and, therefore, the use of tobacco and similar drugs. The 2012 surgeon general’s report emphasized that health care providers of young people must address these issues directly and consistently with their patients.
Practical next steps for your practice
One approach to addressing tobacco use with your patients is to implement the “5 A’s”:
1. Anticipate/Ask. Ask young people if they or their friends are interested in tobacco products and/or if they use tobacco products of any type. Do this at every visit.
2. Advise. In clear, strong, personalized language, urge the tobacco user to quit.
3. Assess. Assess the willingness of the tobacco user to quit, and urge the youth to quit smoking.
4. Assist. For the youth willing to quit, use counseling yourself and/or refer for counseling to individuals with expertise in tobacco cessation or support groups, and consider pharmacotherapy if necessary.
5. Arrange. Schedule a follow-up contact in person within the first week and then on a regularly scheduled basis.
The 5 A’s have been recognized by the Agency for Healthcare Research and Quality as an evidence-based practice for both adult and pediatric patients. In a 2014 article, Dr. Jonathan Klein reported on a study of youth who had seen a clinician for a preventive visit within the past year. However, the youth also reported that the rate of their receiving counseling on tobacco use was relatively low (Pediatrics. 2014 Sep;134[3]:600-1) Most recently, Howard University completed a project funded by the District of Columbia Department of Health to develop and teach a curriculum for medical and nursing students, residents, and physician and nursing staff on this important issue. The youth whom we interviewed in developing the curriculum reported that they had never received counseling by health professionals during their preventive health care visits. This important issue needs to be addressed because it truly is a key to future health for our children and youth.
Dr. Tierney is a Washington-based pediatrician who is a member of the Institute for Public Health Innovation and the D.C. Primary Care Association Medicaid Financing Task Force on Medicaid payment for community health workers. She also works with Howard University Hospital’s Cancer Center on tobacco use avoidance and/or cessation by African American youth. She is on the board of Whitman-Walker Health to advise on Medicaid payment and sits on the quality assurance committee.
Supporting siblings of children with special needs
Even the name “special needs” gives a clue about what it can be like to be a sibling of a child with a chronic condition. When a sibling has special needs, from a child’s perspective, that sibling can seem to be regarded as more special than me!
Whether parents are caring for a child with a physical or mental condition, the amount of attention required almost inevitably consumes some of the time the siblings might otherwise access. There are more visits to doctors and other professionals, hospitalizations with one parent staying overnight, more tests, and sometimes even therapists coming into the family’s home for monitoring or care giving. If the primary issue is mental health that takes attention, too! Sometimes even routine health care for the sibling falls behind.
When the special needs child is acutely ill, or a time-sensitive need or crisis occurs, everything planned for the family that day can go out the window. There is hardly an adult who would not resent always being second fiddle, much less a child with less maturity.
There are more subtle reasons for potential resentment that come from having a special sibling. Although federal regulations now mandate accessibility, often the kinds of outings typical families enjoy are too much hassle to arrange when there is equipment to haul, there are medicines to refrigerate, or there are toileting requirements such as for catheter care. Travel team soccer is out. So these events just don’t happen for the family as a whole.
Even with greater community awareness of different special needs conditions such as autism, stigma still exists. Siblings already are embarrassed about behaviors of typically developing children who pick their noses, laugh too loudly, or slurp their milkshake! It is not hard to see how being in public with a sibling who is shouting, rocking, or having tantrums could be mortifying. Children often choose playdates at someone else’s house to avoid having to explain or deal with their special sibling. This creates a kind of uneven relationship with peers and also deprives parents from being involved.
Siblings of children with special needs are often asked to help out. They have to answer the door when the parent is suctioning, babysit that child or siblings, feed them, or even provide direct care for the child’s medical needs. Household chores that would have been shared with a typically developing child may fall on the sibling.
Finances often add another constraint on the opportunities for a sibling of a child with special needs. Even really good insurance does not cover all the extra costs associated with chronic conditions. Babysitters may need extra skills; vehicles may need lifts or extra space; the home may need structural changes. Paying all these costs means other things don’t fit the budget. Even for a family of means, for the sibling this may result in public school rather than an elite private school, no music lessons, or no overnight camp. For families of lesser means, even life’s basics are hard to afford.
As children get older, they begin to worry about their future options. Will there be enough money for college? Will I have to live at home to help out? What if my parents die and I am the only one left to care for my special sibling? How will I ever get a date or get married?
While all of these factors are stresses, studies have shown being a sibling of a special needs child promotes greater compassion and maturity. Being of real help in the family and to a beloved sibling gives meaning to a child’s life that may be hard to come by during more typical circumstances of growing up. Certainly you have seen in your practice how siblings of special needs children disproportionately aim for careers in health care, psychology, social work, or other helping professions. This may even have been a factor in your own life.
But some siblings of children with special needs become depressed, develop hardened resentment, or take a defiant, acting-out stance. How can you support siblings so that this is not the outcome?
The first step is to ask about their well-being at every visit for the child with special needs. When parents respond with a balanced expression of the positive and the problematic, you can be reassured. If they seem immune to the difficulties the sibling must be experiencing or interpret jealous or attention-seeking behaviors as being solely “bad,” then more discussion or even referral for counseling may be needed. A parent who ordinarily might have understood may not when overburdened or depressed. The siblings with greater maturity and compassion were likely raised by parents who made sure that the point of view and positive attributes of every child in the family were voiced, and that each had opportunities to contribute to the family in meaningful ways as well as express themselves.
“Strong empathic talk” about the sibling’s feelings has been shown to be one of the best ways to build all positive sibling relationships starting in infancy. Acknowledging jealousy and providing individual attention come next. When things aren’t “fair” (which they often cannot be), token fairness helps. For the sake of the sibling, a child with special needs should still have “chores” (such as pulling sheets out of the dryer) and consequences for misbehavior (such as time out), whether the child understands or not.
At their own health supervision visit, I ask siblings about their relationship with the child with special needs, what is fun for them together and what is the hardest part about living with them. The aim is to assess their attitudes and also give them permission to complain. I almost always do this interview with the parents present, giving them a chance to hear those views (although private time may be needed as well). It often is hard for parents and the child himself or herself to express negative feelings because the special needs situation was no one’s fault and everyone has to work hard to be optimistic and to cope. You can then model a sympathetic ear plus assist in coming up with solutions, when possible. The interventions I suggest are often “tokens” to address the family dynamics rather than solutions to the problem. For example, a teen sibling of a child with autism under my care showed depression. Although for fun he played video games with his brother with autism, the hardest part was the lack of an age-appropriate social life. He had to make breakfast and get the special needs sibling off to school every morning as well as care for him after school while their single mother worked. We discussed this, and their mother determined to pay the teen for child care plus loosen her restrictions on his bedtime so that he could go out with friends.
Simply allowing the sibling expression that it can be hard is helpful. Many times stress is denied. To open up discussion, ask such questions as, “How is it having friends over?” or “Do you get enough time with your mom, just you and her?” or “Are there things you miss out on because you have a sister with special needs?”. These same topics are relevant for the parents themselves, too. Protecting the sibling’s privacy during playdates, having 10 minutes daily alone with the sibling to talk or play cards, or setting some family savings goals for the missing privileges are possible solutions that parents could implement. I suggest regular family meetings at home to ensure that these discussions occur and that the solutions are maintained or refined over time.
There are services available for some special needs conditions that are helpful in supporting siblings. Wraparound services may include respite care to allow family vacations, babysitters with needed skills, or payment for summer camp for children with special needs. Summer camps specific to siblings of children with special needs exist as well.
When free services are not available or finances are tight, you can encourage families to think outside the box. Maybe they can trade child care with another family with a child with special needs – each taking both kids for weekly breaks for the other family. Both condition-specific and general parent support groups often are helpful for coming up with ideas and making friendships that can lead to this kind of exchange.
When parents do not have a sitter they trust to manage care for the child with special needs, I often suggest getting a less-skilled (and less expensive) sitter (an 11-year old-works) to mind the child, but then the parent can stay in the house or yard to allow for individual time with the sibling without having to worry. (This is a good tip for time alone for spouses, too!)
The long-term questions that older siblings will have are best addressed by making a plan with a financial adviser. Organizations such as the Arc of the United States (www.thearc.org) and lawyers specializing in disabilities can provide this kind of advice. A trust fund, an up-to-date will, and a written plan for care giving long term are important to reduce worries parents have and can be communicated to the siblings when the time is right.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline.
Even the name “special needs” gives a clue about what it can be like to be a sibling of a child with a chronic condition. When a sibling has special needs, from a child’s perspective, that sibling can seem to be regarded as more special than me!
Whether parents are caring for a child with a physical or mental condition, the amount of attention required almost inevitably consumes some of the time the siblings might otherwise access. There are more visits to doctors and other professionals, hospitalizations with one parent staying overnight, more tests, and sometimes even therapists coming into the family’s home for monitoring or care giving. If the primary issue is mental health that takes attention, too! Sometimes even routine health care for the sibling falls behind.
When the special needs child is acutely ill, or a time-sensitive need or crisis occurs, everything planned for the family that day can go out the window. There is hardly an adult who would not resent always being second fiddle, much less a child with less maturity.
There are more subtle reasons for potential resentment that come from having a special sibling. Although federal regulations now mandate accessibility, often the kinds of outings typical families enjoy are too much hassle to arrange when there is equipment to haul, there are medicines to refrigerate, or there are toileting requirements such as for catheter care. Travel team soccer is out. So these events just don’t happen for the family as a whole.
Even with greater community awareness of different special needs conditions such as autism, stigma still exists. Siblings already are embarrassed about behaviors of typically developing children who pick their noses, laugh too loudly, or slurp their milkshake! It is not hard to see how being in public with a sibling who is shouting, rocking, or having tantrums could be mortifying. Children often choose playdates at someone else’s house to avoid having to explain or deal with their special sibling. This creates a kind of uneven relationship with peers and also deprives parents from being involved.
Siblings of children with special needs are often asked to help out. They have to answer the door when the parent is suctioning, babysit that child or siblings, feed them, or even provide direct care for the child’s medical needs. Household chores that would have been shared with a typically developing child may fall on the sibling.
Finances often add another constraint on the opportunities for a sibling of a child with special needs. Even really good insurance does not cover all the extra costs associated with chronic conditions. Babysitters may need extra skills; vehicles may need lifts or extra space; the home may need structural changes. Paying all these costs means other things don’t fit the budget. Even for a family of means, for the sibling this may result in public school rather than an elite private school, no music lessons, or no overnight camp. For families of lesser means, even life’s basics are hard to afford.
As children get older, they begin to worry about their future options. Will there be enough money for college? Will I have to live at home to help out? What if my parents die and I am the only one left to care for my special sibling? How will I ever get a date or get married?
While all of these factors are stresses, studies have shown being a sibling of a special needs child promotes greater compassion and maturity. Being of real help in the family and to a beloved sibling gives meaning to a child’s life that may be hard to come by during more typical circumstances of growing up. Certainly you have seen in your practice how siblings of special needs children disproportionately aim for careers in health care, psychology, social work, or other helping professions. This may even have been a factor in your own life.
But some siblings of children with special needs become depressed, develop hardened resentment, or take a defiant, acting-out stance. How can you support siblings so that this is not the outcome?
The first step is to ask about their well-being at every visit for the child with special needs. When parents respond with a balanced expression of the positive and the problematic, you can be reassured. If they seem immune to the difficulties the sibling must be experiencing or interpret jealous or attention-seeking behaviors as being solely “bad,” then more discussion or even referral for counseling may be needed. A parent who ordinarily might have understood may not when overburdened or depressed. The siblings with greater maturity and compassion were likely raised by parents who made sure that the point of view and positive attributes of every child in the family were voiced, and that each had opportunities to contribute to the family in meaningful ways as well as express themselves.
“Strong empathic talk” about the sibling’s feelings has been shown to be one of the best ways to build all positive sibling relationships starting in infancy. Acknowledging jealousy and providing individual attention come next. When things aren’t “fair” (which they often cannot be), token fairness helps. For the sake of the sibling, a child with special needs should still have “chores” (such as pulling sheets out of the dryer) and consequences for misbehavior (such as time out), whether the child understands or not.
At their own health supervision visit, I ask siblings about their relationship with the child with special needs, what is fun for them together and what is the hardest part about living with them. The aim is to assess their attitudes and also give them permission to complain. I almost always do this interview with the parents present, giving them a chance to hear those views (although private time may be needed as well). It often is hard for parents and the child himself or herself to express negative feelings because the special needs situation was no one’s fault and everyone has to work hard to be optimistic and to cope. You can then model a sympathetic ear plus assist in coming up with solutions, when possible. The interventions I suggest are often “tokens” to address the family dynamics rather than solutions to the problem. For example, a teen sibling of a child with autism under my care showed depression. Although for fun he played video games with his brother with autism, the hardest part was the lack of an age-appropriate social life. He had to make breakfast and get the special needs sibling off to school every morning as well as care for him after school while their single mother worked. We discussed this, and their mother determined to pay the teen for child care plus loosen her restrictions on his bedtime so that he could go out with friends.
Simply allowing the sibling expression that it can be hard is helpful. Many times stress is denied. To open up discussion, ask such questions as, “How is it having friends over?” or “Do you get enough time with your mom, just you and her?” or “Are there things you miss out on because you have a sister with special needs?”. These same topics are relevant for the parents themselves, too. Protecting the sibling’s privacy during playdates, having 10 minutes daily alone with the sibling to talk or play cards, or setting some family savings goals for the missing privileges are possible solutions that parents could implement. I suggest regular family meetings at home to ensure that these discussions occur and that the solutions are maintained or refined over time.
There are services available for some special needs conditions that are helpful in supporting siblings. Wraparound services may include respite care to allow family vacations, babysitters with needed skills, or payment for summer camp for children with special needs. Summer camps specific to siblings of children with special needs exist as well.
When free services are not available or finances are tight, you can encourage families to think outside the box. Maybe they can trade child care with another family with a child with special needs – each taking both kids for weekly breaks for the other family. Both condition-specific and general parent support groups often are helpful for coming up with ideas and making friendships that can lead to this kind of exchange.
When parents do not have a sitter they trust to manage care for the child with special needs, I often suggest getting a less-skilled (and less expensive) sitter (an 11-year old-works) to mind the child, but then the parent can stay in the house or yard to allow for individual time with the sibling without having to worry. (This is a good tip for time alone for spouses, too!)
The long-term questions that older siblings will have are best addressed by making a plan with a financial adviser. Organizations such as the Arc of the United States (www.thearc.org) and lawyers specializing in disabilities can provide this kind of advice. A trust fund, an up-to-date will, and a written plan for care giving long term are important to reduce worries parents have and can be communicated to the siblings when the time is right.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline.
Even the name “special needs” gives a clue about what it can be like to be a sibling of a child with a chronic condition. When a sibling has special needs, from a child’s perspective, that sibling can seem to be regarded as more special than me!
Whether parents are caring for a child with a physical or mental condition, the amount of attention required almost inevitably consumes some of the time the siblings might otherwise access. There are more visits to doctors and other professionals, hospitalizations with one parent staying overnight, more tests, and sometimes even therapists coming into the family’s home for monitoring or care giving. If the primary issue is mental health that takes attention, too! Sometimes even routine health care for the sibling falls behind.
When the special needs child is acutely ill, or a time-sensitive need or crisis occurs, everything planned for the family that day can go out the window. There is hardly an adult who would not resent always being second fiddle, much less a child with less maturity.
There are more subtle reasons for potential resentment that come from having a special sibling. Although federal regulations now mandate accessibility, often the kinds of outings typical families enjoy are too much hassle to arrange when there is equipment to haul, there are medicines to refrigerate, or there are toileting requirements such as for catheter care. Travel team soccer is out. So these events just don’t happen for the family as a whole.
Even with greater community awareness of different special needs conditions such as autism, stigma still exists. Siblings already are embarrassed about behaviors of typically developing children who pick their noses, laugh too loudly, or slurp their milkshake! It is not hard to see how being in public with a sibling who is shouting, rocking, or having tantrums could be mortifying. Children often choose playdates at someone else’s house to avoid having to explain or deal with their special sibling. This creates a kind of uneven relationship with peers and also deprives parents from being involved.
Siblings of children with special needs are often asked to help out. They have to answer the door when the parent is suctioning, babysit that child or siblings, feed them, or even provide direct care for the child’s medical needs. Household chores that would have been shared with a typically developing child may fall on the sibling.
Finances often add another constraint on the opportunities for a sibling of a child with special needs. Even really good insurance does not cover all the extra costs associated with chronic conditions. Babysitters may need extra skills; vehicles may need lifts or extra space; the home may need structural changes. Paying all these costs means other things don’t fit the budget. Even for a family of means, for the sibling this may result in public school rather than an elite private school, no music lessons, or no overnight camp. For families of lesser means, even life’s basics are hard to afford.
As children get older, they begin to worry about their future options. Will there be enough money for college? Will I have to live at home to help out? What if my parents die and I am the only one left to care for my special sibling? How will I ever get a date or get married?
While all of these factors are stresses, studies have shown being a sibling of a special needs child promotes greater compassion and maturity. Being of real help in the family and to a beloved sibling gives meaning to a child’s life that may be hard to come by during more typical circumstances of growing up. Certainly you have seen in your practice how siblings of special needs children disproportionately aim for careers in health care, psychology, social work, or other helping professions. This may even have been a factor in your own life.
But some siblings of children with special needs become depressed, develop hardened resentment, or take a defiant, acting-out stance. How can you support siblings so that this is not the outcome?
The first step is to ask about their well-being at every visit for the child with special needs. When parents respond with a balanced expression of the positive and the problematic, you can be reassured. If they seem immune to the difficulties the sibling must be experiencing or interpret jealous or attention-seeking behaviors as being solely “bad,” then more discussion or even referral for counseling may be needed. A parent who ordinarily might have understood may not when overburdened or depressed. The siblings with greater maturity and compassion were likely raised by parents who made sure that the point of view and positive attributes of every child in the family were voiced, and that each had opportunities to contribute to the family in meaningful ways as well as express themselves.
“Strong empathic talk” about the sibling’s feelings has been shown to be one of the best ways to build all positive sibling relationships starting in infancy. Acknowledging jealousy and providing individual attention come next. When things aren’t “fair” (which they often cannot be), token fairness helps. For the sake of the sibling, a child with special needs should still have “chores” (such as pulling sheets out of the dryer) and consequences for misbehavior (such as time out), whether the child understands or not.
At their own health supervision visit, I ask siblings about their relationship with the child with special needs, what is fun for them together and what is the hardest part about living with them. The aim is to assess their attitudes and also give them permission to complain. I almost always do this interview with the parents present, giving them a chance to hear those views (although private time may be needed as well). It often is hard for parents and the child himself or herself to express negative feelings because the special needs situation was no one’s fault and everyone has to work hard to be optimistic and to cope. You can then model a sympathetic ear plus assist in coming up with solutions, when possible. The interventions I suggest are often “tokens” to address the family dynamics rather than solutions to the problem. For example, a teen sibling of a child with autism under my care showed depression. Although for fun he played video games with his brother with autism, the hardest part was the lack of an age-appropriate social life. He had to make breakfast and get the special needs sibling off to school every morning as well as care for him after school while their single mother worked. We discussed this, and their mother determined to pay the teen for child care plus loosen her restrictions on his bedtime so that he could go out with friends.
Simply allowing the sibling expression that it can be hard is helpful. Many times stress is denied. To open up discussion, ask such questions as, “How is it having friends over?” or “Do you get enough time with your mom, just you and her?” or “Are there things you miss out on because you have a sister with special needs?”. These same topics are relevant for the parents themselves, too. Protecting the sibling’s privacy during playdates, having 10 minutes daily alone with the sibling to talk or play cards, or setting some family savings goals for the missing privileges are possible solutions that parents could implement. I suggest regular family meetings at home to ensure that these discussions occur and that the solutions are maintained or refined over time.
There are services available for some special needs conditions that are helpful in supporting siblings. Wraparound services may include respite care to allow family vacations, babysitters with needed skills, or payment for summer camp for children with special needs. Summer camps specific to siblings of children with special needs exist as well.
When free services are not available or finances are tight, you can encourage families to think outside the box. Maybe they can trade child care with another family with a child with special needs – each taking both kids for weekly breaks for the other family. Both condition-specific and general parent support groups often are helpful for coming up with ideas and making friendships that can lead to this kind of exchange.
When parents do not have a sitter they trust to manage care for the child with special needs, I often suggest getting a less-skilled (and less expensive) sitter (an 11-year old-works) to mind the child, but then the parent can stay in the house or yard to allow for individual time with the sibling without having to worry. (This is a good tip for time alone for spouses, too!)
The long-term questions that older siblings will have are best addressed by making a plan with a financial adviser. Organizations such as the Arc of the United States (www.thearc.org) and lawyers specializing in disabilities can provide this kind of advice. A trust fund, an up-to-date will, and a written plan for care giving long term are important to reduce worries parents have and can be communicated to the siblings when the time is right.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline.
IDH1 mutant inhibitor targets gliomas, chondrosarcomas
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
AT THE AACR–NCI–EORTC
Key clinical point: AG-120 is a first-in-class inhibitor of IDH1 mutations that may drive oncogenesis.
Major finding: 10 of 20 patients with gliomas and 7 of 11 with chondrosarcomas had stable disease on AG-120.
Data source: Phase I dose-escalation trial in 62 patients (55 available for efficacy analysis).
Disclosures: The trial is supported by Agios Pharmaceuticals. Dr. Burris and Dr. Helman reported no conflicts of interest.
VIDEO: Survival benefits, relapse risks in liver transplant for alcoholic hepatitis
SAN FRANCISCO – Are policies requiring 6 months of abstinence before liver transplantation in severe alcoholic hepatitis justified, given the potential survival advantage that earlier transplantation offers?
Alcoholic hepatitis has an “exceptionally high mortality rate,” noted Dr. Brian Lee of Johns Hopkins University, Baltimore. But a policy requiring 6 months of abstinence before liver transplantation in patients with severe alcoholic hepatitis “is possibly even causing a precondition that’s death for these patients.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lee discussed a study in which early liver transplantation in 40 patients with severe alcoholic hepatitis achieved a 100% survival rate at 1 year, with a 22% alcohol relapse rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Are policies requiring 6 months of abstinence before liver transplantation in severe alcoholic hepatitis justified, given the potential survival advantage that earlier transplantation offers?
Alcoholic hepatitis has an “exceptionally high mortality rate,” noted Dr. Brian Lee of Johns Hopkins University, Baltimore. But a policy requiring 6 months of abstinence before liver transplantation in patients with severe alcoholic hepatitis “is possibly even causing a precondition that’s death for these patients.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lee discussed a study in which early liver transplantation in 40 patients with severe alcoholic hepatitis achieved a 100% survival rate at 1 year, with a 22% alcohol relapse rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Are policies requiring 6 months of abstinence before liver transplantation in severe alcoholic hepatitis justified, given the potential survival advantage that earlier transplantation offers?
Alcoholic hepatitis has an “exceptionally high mortality rate,” noted Dr. Brian Lee of Johns Hopkins University, Baltimore. But a policy requiring 6 months of abstinence before liver transplantation in patients with severe alcoholic hepatitis “is possibly even causing a precondition that’s death for these patients.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lee discussed a study in which early liver transplantation in 40 patients with severe alcoholic hepatitis achieved a 100% survival rate at 1 year, with a 22% alcohol relapse rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2015
Andexanet reverses anticoagulant effects of factor Xa inhibitors
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the Nov. 11 issue of the New England Journal of Medicine.
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2-5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced anti-factor Xa activity by 92%, compared with 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth, and the effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMoa1510991).
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr. Deborah M. Siegal of McMaster University, Hamilton, Ont., and coauthors wrote.
The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters.
Factor Xa inhibitors represent an important advance in anticoagulation therapy, but concern over the lack of antidotes has tempered enthusiasm for their use among patients and physicians. Warfarin is perceived as being safer as a result of the availability of effective reversal strategies.
Although additional studies will be needed to optimize the use of andexanet and to determine its true efficacy and safety, it represents a giant step forward in our ability to control anticoagulation therapy.
Dr. Jean M. Connors is with the hematology division at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMe1513258). Dr. Connors declared personal fees from Boehringer Ingelheim and Bristol-Myers Squibb outside the submitted work.
Factor Xa inhibitors represent an important advance in anticoagulation therapy, but concern over the lack of antidotes has tempered enthusiasm for their use among patients and physicians. Warfarin is perceived as being safer as a result of the availability of effective reversal strategies.
Although additional studies will be needed to optimize the use of andexanet and to determine its true efficacy and safety, it represents a giant step forward in our ability to control anticoagulation therapy.
Dr. Jean M. Connors is with the hematology division at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMe1513258). Dr. Connors declared personal fees from Boehringer Ingelheim and Bristol-Myers Squibb outside the submitted work.
Factor Xa inhibitors represent an important advance in anticoagulation therapy, but concern over the lack of antidotes has tempered enthusiasm for their use among patients and physicians. Warfarin is perceived as being safer as a result of the availability of effective reversal strategies.
Although additional studies will be needed to optimize the use of andexanet and to determine its true efficacy and safety, it represents a giant step forward in our ability to control anticoagulation therapy.
Dr. Jean M. Connors is with the hematology division at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMe1513258). Dr. Connors declared personal fees from Boehringer Ingelheim and Bristol-Myers Squibb outside the submitted work.
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the Nov. 11 issue of the New England Journal of Medicine.
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2-5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced anti-factor Xa activity by 92%, compared with 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth, and the effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMoa1510991).
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr. Deborah M. Siegal of McMaster University, Hamilton, Ont., and coauthors wrote.
The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters.
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the Nov. 11 issue of the New England Journal of Medicine.
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2-5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced anti-factor Xa activity by 92%, compared with 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth, and the effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus (N Engl J Med. 2015 Nov 11. doi: 10.1056/NEJMoa1510991).
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr. Deborah M. Siegal of McMaster University, Hamilton, Ont., and coauthors wrote.
The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:Andexanet reverses the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban in healthy older adults.
Major finding: Andexanet achieved a 92%-94% reduction in anti-factor Xa activity, compared with an 18%-21% reduction with placebo.
Data source: A two-part randomized, placebo-controlled study in 145 healthy individuals.
Disclosures: The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and a related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters.
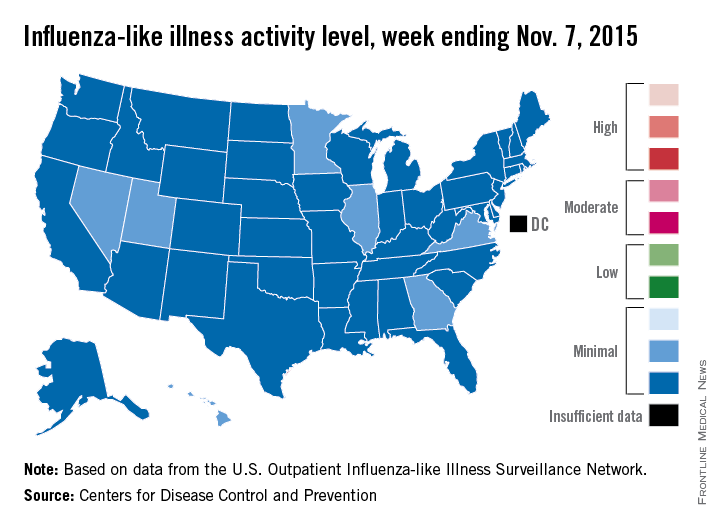
U.S. influenza activity minimal so far
Four weeks into the 2015-2016 flu season, activity levels of influenza-like illness (ILI) are minimal in all 50 states, the Centers for Disease Control and Prevention reported Nov. 13.
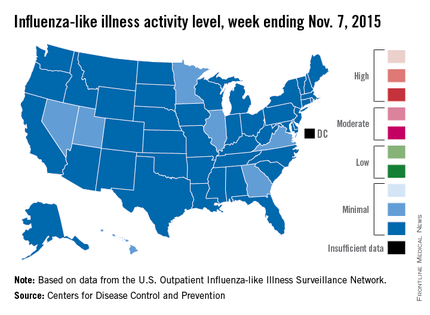
Seven states – Georgia, Hawaii, Illinois, Minnesota, Nevada, Utah, and Virginia – had level 2 activity for the week ending Nov. 7, 2015, which, although still minimal, was higher than the level 1 activity in the other 43 states. There was a moderate level of influenza-like illness activity (level 7) in Puerto Rico, which was up from level 6 the week before, and there were insufficient data to determine flu activity in the District of Columbia, according to the CDC.
ILI is defined as fever (temperature of 100° F or greater) and cough and/or sore throat. Activity level within a state is the proportion of outpatient visits to health care providers for influenza-like illness.
For the country overall, the proportion of outpatient visits for ILI was 1.4%, which is below the national baseline of 2.1% for week 4 of the flu season, the CDC noted.
Four weeks into the 2015-2016 flu season, activity levels of influenza-like illness (ILI) are minimal in all 50 states, the Centers for Disease Control and Prevention reported Nov. 13.

Seven states – Georgia, Hawaii, Illinois, Minnesota, Nevada, Utah, and Virginia – had level 2 activity for the week ending Nov. 7, 2015, which, although still minimal, was higher than the level 1 activity in the other 43 states. There was a moderate level of influenza-like illness activity (level 7) in Puerto Rico, which was up from level 6 the week before, and there were insufficient data to determine flu activity in the District of Columbia, according to the CDC.
ILI is defined as fever (temperature of 100° F or greater) and cough and/or sore throat. Activity level within a state is the proportion of outpatient visits to health care providers for influenza-like illness.
For the country overall, the proportion of outpatient visits for ILI was 1.4%, which is below the national baseline of 2.1% for week 4 of the flu season, the CDC noted.
Four weeks into the 2015-2016 flu season, activity levels of influenza-like illness (ILI) are minimal in all 50 states, the Centers for Disease Control and Prevention reported Nov. 13.

Seven states – Georgia, Hawaii, Illinois, Minnesota, Nevada, Utah, and Virginia – had level 2 activity for the week ending Nov. 7, 2015, which, although still minimal, was higher than the level 1 activity in the other 43 states. There was a moderate level of influenza-like illness activity (level 7) in Puerto Rico, which was up from level 6 the week before, and there were insufficient data to determine flu activity in the District of Columbia, according to the CDC.
ILI is defined as fever (temperature of 100° F or greater) and cough and/or sore throat. Activity level within a state is the proportion of outpatient visits to health care providers for influenza-like illness.
For the country overall, the proportion of outpatient visits for ILI was 1.4%, which is below the national baseline of 2.1% for week 4 of the flu season, the CDC noted.
Desmoplastic Melanoma
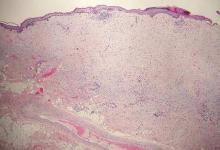
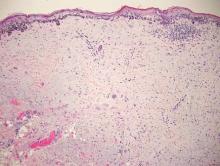
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
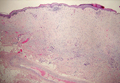
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6
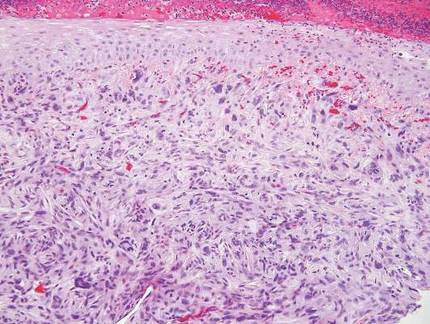
| 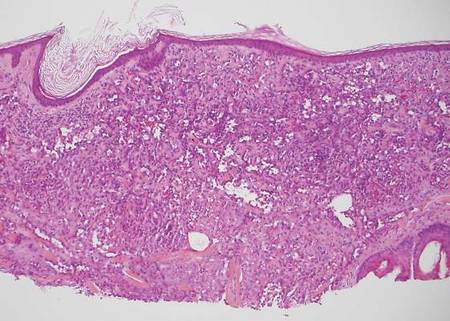
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.
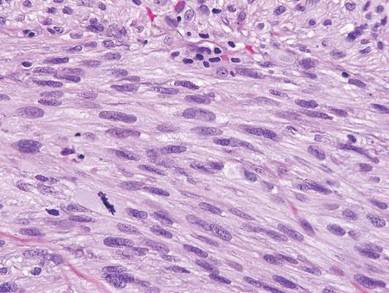
| 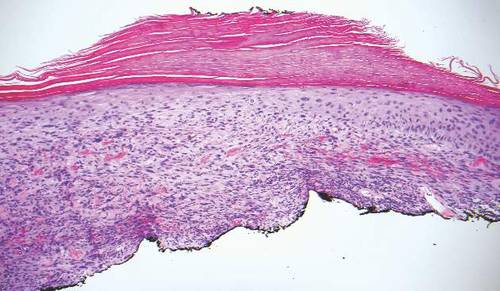
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6

| 
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.

| 
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6

| 
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.

| 
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.
The high price of drugs
As I listened to Dr. Jackson T. Wright, one of the investigators of the SPRINT Trial, emphasize the importance of treating systolic blood pressure to the target of 120 mm Hg, I thought about the difficulty in treating blood pressure in the elderly.
Successfully lowering blood pressure even to the current target of 140 mm Hg systolic is one of the most difficult therapeutic tasks that I face. But, to my mind, the worldwide success achieved by hypertension therapy has had the most profound effect on cardiovascular medicine in the last half century. I am no epidemiologist, but the striking decrease in cardiovascular mortality in the United States associated with the introduction of the hypertension therapy is undeniable.
The pharmaceutical companies that developed of these drugs, some of which are still around and some that have merged with other entities, never priced these drugs at the potential benefit of decreasing the cost of hypertension to society. If they had, their price would be so exorbitant that their universal benefit to mankind would never have been seen. They were doing their job and returning significant benefit both to humanity and their investors. They priced the drugs so that we could afford to pay for them. All of these drugs – ACE inhibitors, calcium- and beta-blockers, and diuretics – are now available as generics and as a class have had a lasting and continuing impact on the societal cost and benefit.
Yet somehow, the world has changed. I am not the first to notice it. We now have drugs that can cost thousands of dollars a month and none of us can afford to pay for them. Because we now all share in the cost of drug therapy in one way or another, we all pay the cost. The justification for the high price is not based solely on their development cost, which in many instances were developed decades if not centuries ago, but on the presumed net expense of the untreated disease to society or to the achievement of entrepreneurial profits. Hepatitis C was one of the first in which the cost of new drug therapy was based on the net savings associated with the prevention of the chronic liver disease and its subsequent long-term societal expense. Since I am not a hepatologist, it took me a little leap of faith to accept the potential economic benefit of this therapy, but at least the current data suggest that indeed this therapy works. I remain dubious about the calculations of their societal net benefit cost.
Now move forward. We now have two drugs that can lower cholesterol, potentially to levels beyond our imagination, that are now on the high-expense list. According to recent rumors, evolocumab (Repatha, Amgen) and alirocumab (Praluent, Sanofi/Regeneron) will cost someone $12,000-$14,000 a year to lower serum cholesterol almost to immeasurable levels without any evidence of benefit in decreasing cardiovascular disease. (For those of you with short-term memory deficits, we just tried to pump up HDL cholesterol only to find that when successful, it increased mortality.) Now, if these drugs are successful in preventing the development and progression of atherosclerotic disease, as in the example of hepatitis C, I may change my mind and write the script for it.
Meanwhile, drug prices have escalated as a result of speculation in the pharmaceutical market by Wall Street entrepreneurs who buy up the drug patents that have been available for years like isoproterenol and digitalis, for the sole purpose of a creating a monopoly to inflate the price and return large profits to their investors. When questioned about the 20-fold increase in the cost of isoproterenol, a representative of one of the pharmaceutical companies stated, “that the price was based on many factors, including clinical benefits and the value they bring to the patients, physicians, payers, and society” (New York Times 2015 Oct 4 p. BU1, “Side Effects of Hijacking Drug Prices”). The audacity of drug company executives to presume that they are in a position to make that judgment is outrageous. And yet the U.S. Congress makes it illegal for Medicare and Medicaid to bargain for the best drug prices, while European and Asian pay a fraction of what we pay here.
These ruminations only suggest that madness in the pharmaceutical world is not limited to Wall Street but reaches even the higher levels of government. Get your checkbooks out everyone; the best is yet to come.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
As I listened to Dr. Jackson T. Wright, one of the investigators of the SPRINT Trial, emphasize the importance of treating systolic blood pressure to the target of 120 mm Hg, I thought about the difficulty in treating blood pressure in the elderly.
Successfully lowering blood pressure even to the current target of 140 mm Hg systolic is one of the most difficult therapeutic tasks that I face. But, to my mind, the worldwide success achieved by hypertension therapy has had the most profound effect on cardiovascular medicine in the last half century. I am no epidemiologist, but the striking decrease in cardiovascular mortality in the United States associated with the introduction of the hypertension therapy is undeniable.
The pharmaceutical companies that developed of these drugs, some of which are still around and some that have merged with other entities, never priced these drugs at the potential benefit of decreasing the cost of hypertension to society. If they had, their price would be so exorbitant that their universal benefit to mankind would never have been seen. They were doing their job and returning significant benefit both to humanity and their investors. They priced the drugs so that we could afford to pay for them. All of these drugs – ACE inhibitors, calcium- and beta-blockers, and diuretics – are now available as generics and as a class have had a lasting and continuing impact on the societal cost and benefit.
Yet somehow, the world has changed. I am not the first to notice it. We now have drugs that can cost thousands of dollars a month and none of us can afford to pay for them. Because we now all share in the cost of drug therapy in one way or another, we all pay the cost. The justification for the high price is not based solely on their development cost, which in many instances were developed decades if not centuries ago, but on the presumed net expense of the untreated disease to society or to the achievement of entrepreneurial profits. Hepatitis C was one of the first in which the cost of new drug therapy was based on the net savings associated with the prevention of the chronic liver disease and its subsequent long-term societal expense. Since I am not a hepatologist, it took me a little leap of faith to accept the potential economic benefit of this therapy, but at least the current data suggest that indeed this therapy works. I remain dubious about the calculations of their societal net benefit cost.
Now move forward. We now have two drugs that can lower cholesterol, potentially to levels beyond our imagination, that are now on the high-expense list. According to recent rumors, evolocumab (Repatha, Amgen) and alirocumab (Praluent, Sanofi/Regeneron) will cost someone $12,000-$14,000 a year to lower serum cholesterol almost to immeasurable levels without any evidence of benefit in decreasing cardiovascular disease. (For those of you with short-term memory deficits, we just tried to pump up HDL cholesterol only to find that when successful, it increased mortality.) Now, if these drugs are successful in preventing the development and progression of atherosclerotic disease, as in the example of hepatitis C, I may change my mind and write the script for it.
Meanwhile, drug prices have escalated as a result of speculation in the pharmaceutical market by Wall Street entrepreneurs who buy up the drug patents that have been available for years like isoproterenol and digitalis, for the sole purpose of a creating a monopoly to inflate the price and return large profits to their investors. When questioned about the 20-fold increase in the cost of isoproterenol, a representative of one of the pharmaceutical companies stated, “that the price was based on many factors, including clinical benefits and the value they bring to the patients, physicians, payers, and society” (New York Times 2015 Oct 4 p. BU1, “Side Effects of Hijacking Drug Prices”). The audacity of drug company executives to presume that they are in a position to make that judgment is outrageous. And yet the U.S. Congress makes it illegal for Medicare and Medicaid to bargain for the best drug prices, while European and Asian pay a fraction of what we pay here.
These ruminations only suggest that madness in the pharmaceutical world is not limited to Wall Street but reaches even the higher levels of government. Get your checkbooks out everyone; the best is yet to come.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
As I listened to Dr. Jackson T. Wright, one of the investigators of the SPRINT Trial, emphasize the importance of treating systolic blood pressure to the target of 120 mm Hg, I thought about the difficulty in treating blood pressure in the elderly.
Successfully lowering blood pressure even to the current target of 140 mm Hg systolic is one of the most difficult therapeutic tasks that I face. But, to my mind, the worldwide success achieved by hypertension therapy has had the most profound effect on cardiovascular medicine in the last half century. I am no epidemiologist, but the striking decrease in cardiovascular mortality in the United States associated with the introduction of the hypertension therapy is undeniable.
The pharmaceutical companies that developed of these drugs, some of which are still around and some that have merged with other entities, never priced these drugs at the potential benefit of decreasing the cost of hypertension to society. If they had, their price would be so exorbitant that their universal benefit to mankind would never have been seen. They were doing their job and returning significant benefit both to humanity and their investors. They priced the drugs so that we could afford to pay for them. All of these drugs – ACE inhibitors, calcium- and beta-blockers, and diuretics – are now available as generics and as a class have had a lasting and continuing impact on the societal cost and benefit.
Yet somehow, the world has changed. I am not the first to notice it. We now have drugs that can cost thousands of dollars a month and none of us can afford to pay for them. Because we now all share in the cost of drug therapy in one way or another, we all pay the cost. The justification for the high price is not based solely on their development cost, which in many instances were developed decades if not centuries ago, but on the presumed net expense of the untreated disease to society or to the achievement of entrepreneurial profits. Hepatitis C was one of the first in which the cost of new drug therapy was based on the net savings associated with the prevention of the chronic liver disease and its subsequent long-term societal expense. Since I am not a hepatologist, it took me a little leap of faith to accept the potential economic benefit of this therapy, but at least the current data suggest that indeed this therapy works. I remain dubious about the calculations of their societal net benefit cost.
Now move forward. We now have two drugs that can lower cholesterol, potentially to levels beyond our imagination, that are now on the high-expense list. According to recent rumors, evolocumab (Repatha, Amgen) and alirocumab (Praluent, Sanofi/Regeneron) will cost someone $12,000-$14,000 a year to lower serum cholesterol almost to immeasurable levels without any evidence of benefit in decreasing cardiovascular disease. (For those of you with short-term memory deficits, we just tried to pump up HDL cholesterol only to find that when successful, it increased mortality.) Now, if these drugs are successful in preventing the development and progression of atherosclerotic disease, as in the example of hepatitis C, I may change my mind and write the script for it.
Meanwhile, drug prices have escalated as a result of speculation in the pharmaceutical market by Wall Street entrepreneurs who buy up the drug patents that have been available for years like isoproterenol and digitalis, for the sole purpose of a creating a monopoly to inflate the price and return large profits to their investors. When questioned about the 20-fold increase in the cost of isoproterenol, a representative of one of the pharmaceutical companies stated, “that the price was based on many factors, including clinical benefits and the value they bring to the patients, physicians, payers, and society” (New York Times 2015 Oct 4 p. BU1, “Side Effects of Hijacking Drug Prices”). The audacity of drug company executives to presume that they are in a position to make that judgment is outrageous. And yet the U.S. Congress makes it illegal for Medicare and Medicaid to bargain for the best drug prices, while European and Asian pay a fraction of what we pay here.
These ruminations only suggest that madness in the pharmaceutical world is not limited to Wall Street but reaches even the higher levels of government. Get your checkbooks out everyone; the best is yet to come.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Poor Surgical Outcomes for Safety-Net Hospitals
NEW YORK - Hospital resources, and not necessarily patient characteristics, may be causing safety-net hospitals to deliver inferior surgical outcomes at increased cost in elective surgical procedures, according to a new study.
"Analysis of Medicare Hospital Compare data revealed that safety-net hospitals perform worse on Surgical Care Improvement Project (SCIP) measures and have less efficient emergency departments throughput," first author Dr. Richard S. Hoehn from the University of Cincinnati, Ohio, said by email. "This last category indicates that these hospitals have insufficient staffing and/or resources to handle their patient burden."
"Safety-net hospitals care for a vulnerable population and maintain an open door to all patients, regardless of their ability to pay. Our study of nine major surgical procedures at academic medical centers in the United States found that hospitals with the highest safety net burden have the most patients of low socioeconomic status, extreme severity of illness, and in need of urgent surgery," Dr. Hoehn said.
"These hospitals also had the worst mortality and readmission rates and highest costs for most procedures. After controlling for patient age, race, severity of illness, and socioeconomic status, safety-net hospitals still had worse outcomes. Their inferior mortality and readmission rates were somewhat reduced, but the increased costs observed at these centers persisted, implying that other characteristics intrinsic to safety-net hospitals are associated with increased costs," he added.
Dr. Hoehn and colleagues analyzed outcomes for nine surgical procedures at 231 hospitals in the University HealthSystem Consortium over a four-year period ending in 2012, accounting for more than 12.6 million patient encounters. They sorted the hospitals into high safety-net burden (HBH) versus hospitals with low (LBH) or medium (MBH) safety-net burden.
They found the HBHs overall to have the most patients likely to be young, black, have the lowest socioeconomic status, and have the highest severity of illness and the highest cost for surgical care (p<0.01 for all). They also had the highest proportion of patient-emergent cases and the longest lengths of stay (p<0.01 for all).
After the researchers adjusted for patient characteristics and hospital volume, the HBHs still had higher odds ratios of mortality for three procedures (OR, 1.81 to 2.08, p<0.05), readmission for two procedures (OR, 1.19 to 1.30, p<0.05), and the highest cost of care for seven procedures (risk ratios, 1.23 to 1.35, p<0.05).
Postoperative mortality was worse for colectomy, esophagectomy, pancreaticoduodenectomy, and ventral hernia repair. Readmission odds were higher for coronary artery bypass, colectomy, kidney transplant, and ventral hernia repair.
"[W]hen assessing markers of emergency department throughput, HBHs were inferior to LBHs in all measures, including time from arrival to evaluation, admission decision time, times from arrival to departure for discharged and admitted patients, time for pain medicine administration to patients with long-bone fractures, and patients who left without being seen (p <= 0.002 for all)," the researchers write in JAMA Surgery, online October 14.
In an accompanying editorial, Dr. Larry R. Kaiser of Temple University School of Medicine, Philadelphia, and colleagues note, "At best, emergency department throughput as a surrogate for staffing adequacy and systems efficiency is an indirect estimate of these important hospital factors. Deficiencies in documentation and coding may or may not be influenced by overall hospital performance but could have a significant influence on expected rates of death and complications."
Dr. Kaiser said by email, "Physicians and surgeons should be very careful in drawing substantive conclusions from the study because of factors we don't know about these patients. Let the message be greater resources should be provided to those hospitals bearing the burden of caring for this underserved population of patients who, if anything, are more complex than commercially insured patients with similar problems."
"It's important to acknowledge that this study is not trying to criticize safety-net hospitals," Dr. Hoehn emphasized. "We are trying to highlight the unique situation these providers face, and also show that current policy changes that financially penalize these hospitals may adversely impact surgical care and further exacerbate the disparities in health care that already exist in our country. Safety-net hospitals in America have always been important institutions that train doctors and care for indigent patients, and our goal is to find a way to preserve this model in the face of changing healthcare policy."
Dr. Hoehn continued, "There are two options to improve the care at these centers: either close these hospitals and send their patients elsewhere or invest in initiatives that will allow these hospitals to improve not only their outcomes but also their efficiency. To do this, we must better understand their needs."
Dr. Kaiser said, "Continued scrutiny of outcomes with transparency and sharing of data with all those involved in the care of patients will result in continued quality improvement. Participation in [universal health care] that allows institutions to benchmark their data with similar institutions will tend to push those who need improvement to continue to improve."
"There must be a concerted effort among all involved in caring for patients at an institution to continue to improve quality. The designation of a chief quality officer working in concert with the chief medical officer also is critically important in working toward improved quality. But all of this depends on accurate recording and reporting of quality metrics," he concluded.
NEW YORK - Hospital resources, and not necessarily patient characteristics, may be causing safety-net hospitals to deliver inferior surgical outcomes at increased cost in elective surgical procedures, according to a new study.
"Analysis of Medicare Hospital Compare data revealed that safety-net hospitals perform worse on Surgical Care Improvement Project (SCIP) measures and have less efficient emergency departments throughput," first author Dr. Richard S. Hoehn from the University of Cincinnati, Ohio, said by email. "This last category indicates that these hospitals have insufficient staffing and/or resources to handle their patient burden."
"Safety-net hospitals care for a vulnerable population and maintain an open door to all patients, regardless of their ability to pay. Our study of nine major surgical procedures at academic medical centers in the United States found that hospitals with the highest safety net burden have the most patients of low socioeconomic status, extreme severity of illness, and in need of urgent surgery," Dr. Hoehn said.
"These hospitals also had the worst mortality and readmission rates and highest costs for most procedures. After controlling for patient age, race, severity of illness, and socioeconomic status, safety-net hospitals still had worse outcomes. Their inferior mortality and readmission rates were somewhat reduced, but the increased costs observed at these centers persisted, implying that other characteristics intrinsic to safety-net hospitals are associated with increased costs," he added.
Dr. Hoehn and colleagues analyzed outcomes for nine surgical procedures at 231 hospitals in the University HealthSystem Consortium over a four-year period ending in 2012, accounting for more than 12.6 million patient encounters. They sorted the hospitals into high safety-net burden (HBH) versus hospitals with low (LBH) or medium (MBH) safety-net burden.
They found the HBHs overall to have the most patients likely to be young, black, have the lowest socioeconomic status, and have the highest severity of illness and the highest cost for surgical care (p<0.01 for all). They also had the highest proportion of patient-emergent cases and the longest lengths of stay (p<0.01 for all).
After the researchers adjusted for patient characteristics and hospital volume, the HBHs still had higher odds ratios of mortality for three procedures (OR, 1.81 to 2.08, p<0.05), readmission for two procedures (OR, 1.19 to 1.30, p<0.05), and the highest cost of care for seven procedures (risk ratios, 1.23 to 1.35, p<0.05).
Postoperative mortality was worse for colectomy, esophagectomy, pancreaticoduodenectomy, and ventral hernia repair. Readmission odds were higher for coronary artery bypass, colectomy, kidney transplant, and ventral hernia repair.
"[W]hen assessing markers of emergency department throughput, HBHs were inferior to LBHs in all measures, including time from arrival to evaluation, admission decision time, times from arrival to departure for discharged and admitted patients, time for pain medicine administration to patients with long-bone fractures, and patients who left without being seen (p <= 0.002 for all)," the researchers write in JAMA Surgery, online October 14.
In an accompanying editorial, Dr. Larry R. Kaiser of Temple University School of Medicine, Philadelphia, and colleagues note, "At best, emergency department throughput as a surrogate for staffing adequacy and systems efficiency is an indirect estimate of these important hospital factors. Deficiencies in documentation and coding may or may not be influenced by overall hospital performance but could have a significant influence on expected rates of death and complications."
Dr. Kaiser said by email, "Physicians and surgeons should be very careful in drawing substantive conclusions from the study because of factors we don't know about these patients. Let the message be greater resources should be provided to those hospitals bearing the burden of caring for this underserved population of patients who, if anything, are more complex than commercially insured patients with similar problems."
"It's important to acknowledge that this study is not trying to criticize safety-net hospitals," Dr. Hoehn emphasized. "We are trying to highlight the unique situation these providers face, and also show that current policy changes that financially penalize these hospitals may adversely impact surgical care and further exacerbate the disparities in health care that already exist in our country. Safety-net hospitals in America have always been important institutions that train doctors and care for indigent patients, and our goal is to find a way to preserve this model in the face of changing healthcare policy."
Dr. Hoehn continued, "There are two options to improve the care at these centers: either close these hospitals and send their patients elsewhere or invest in initiatives that will allow these hospitals to improve not only their outcomes but also their efficiency. To do this, we must better understand their needs."
Dr. Kaiser said, "Continued scrutiny of outcomes with transparency and sharing of data with all those involved in the care of patients will result in continued quality improvement. Participation in [universal health care] that allows institutions to benchmark their data with similar institutions will tend to push those who need improvement to continue to improve."
"There must be a concerted effort among all involved in caring for patients at an institution to continue to improve quality. The designation of a chief quality officer working in concert with the chief medical officer also is critically important in working toward improved quality. But all of this depends on accurate recording and reporting of quality metrics," he concluded.
NEW YORK - Hospital resources, and not necessarily patient characteristics, may be causing safety-net hospitals to deliver inferior surgical outcomes at increased cost in elective surgical procedures, according to a new study.
"Analysis of Medicare Hospital Compare data revealed that safety-net hospitals perform worse on Surgical Care Improvement Project (SCIP) measures and have less efficient emergency departments throughput," first author Dr. Richard S. Hoehn from the University of Cincinnati, Ohio, said by email. "This last category indicates that these hospitals have insufficient staffing and/or resources to handle their patient burden."
"Safety-net hospitals care for a vulnerable population and maintain an open door to all patients, regardless of their ability to pay. Our study of nine major surgical procedures at academic medical centers in the United States found that hospitals with the highest safety net burden have the most patients of low socioeconomic status, extreme severity of illness, and in need of urgent surgery," Dr. Hoehn said.
"These hospitals also had the worst mortality and readmission rates and highest costs for most procedures. After controlling for patient age, race, severity of illness, and socioeconomic status, safety-net hospitals still had worse outcomes. Their inferior mortality and readmission rates were somewhat reduced, but the increased costs observed at these centers persisted, implying that other characteristics intrinsic to safety-net hospitals are associated with increased costs," he added.
Dr. Hoehn and colleagues analyzed outcomes for nine surgical procedures at 231 hospitals in the University HealthSystem Consortium over a four-year period ending in 2012, accounting for more than 12.6 million patient encounters. They sorted the hospitals into high safety-net burden (HBH) versus hospitals with low (LBH) or medium (MBH) safety-net burden.
They found the HBHs overall to have the most patients likely to be young, black, have the lowest socioeconomic status, and have the highest severity of illness and the highest cost for surgical care (p<0.01 for all). They also had the highest proportion of patient-emergent cases and the longest lengths of stay (p<0.01 for all).
After the researchers adjusted for patient characteristics and hospital volume, the HBHs still had higher odds ratios of mortality for three procedures (OR, 1.81 to 2.08, p<0.05), readmission for two procedures (OR, 1.19 to 1.30, p<0.05), and the highest cost of care for seven procedures (risk ratios, 1.23 to 1.35, p<0.05).
Postoperative mortality was worse for colectomy, esophagectomy, pancreaticoduodenectomy, and ventral hernia repair. Readmission odds were higher for coronary artery bypass, colectomy, kidney transplant, and ventral hernia repair.
"[W]hen assessing markers of emergency department throughput, HBHs were inferior to LBHs in all measures, including time from arrival to evaluation, admission decision time, times from arrival to departure for discharged and admitted patients, time for pain medicine administration to patients with long-bone fractures, and patients who left without being seen (p <= 0.002 for all)," the researchers write in JAMA Surgery, online October 14.
In an accompanying editorial, Dr. Larry R. Kaiser of Temple University School of Medicine, Philadelphia, and colleagues note, "At best, emergency department throughput as a surrogate for staffing adequacy and systems efficiency is an indirect estimate of these important hospital factors. Deficiencies in documentation and coding may or may not be influenced by overall hospital performance but could have a significant influence on expected rates of death and complications."
Dr. Kaiser said by email, "Physicians and surgeons should be very careful in drawing substantive conclusions from the study because of factors we don't know about these patients. Let the message be greater resources should be provided to those hospitals bearing the burden of caring for this underserved population of patients who, if anything, are more complex than commercially insured patients with similar problems."
"It's important to acknowledge that this study is not trying to criticize safety-net hospitals," Dr. Hoehn emphasized. "We are trying to highlight the unique situation these providers face, and also show that current policy changes that financially penalize these hospitals may adversely impact surgical care and further exacerbate the disparities in health care that already exist in our country. Safety-net hospitals in America have always been important institutions that train doctors and care for indigent patients, and our goal is to find a way to preserve this model in the face of changing healthcare policy."
Dr. Hoehn continued, "There are two options to improve the care at these centers: either close these hospitals and send their patients elsewhere or invest in initiatives that will allow these hospitals to improve not only their outcomes but also their efficiency. To do this, we must better understand their needs."
Dr. Kaiser said, "Continued scrutiny of outcomes with transparency and sharing of data with all those involved in the care of patients will result in continued quality improvement. Participation in [universal health care] that allows institutions to benchmark their data with similar institutions will tend to push those who need improvement to continue to improve."
"There must be a concerted effort among all involved in caring for patients at an institution to continue to improve quality. The designation of a chief quality officer working in concert with the chief medical officer also is critically important in working toward improved quality. But all of this depends on accurate recording and reporting of quality metrics," he concluded.
What Is Your Diagnosis? Tinea Corporis
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.
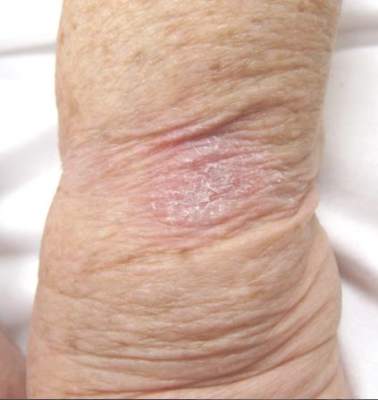
We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
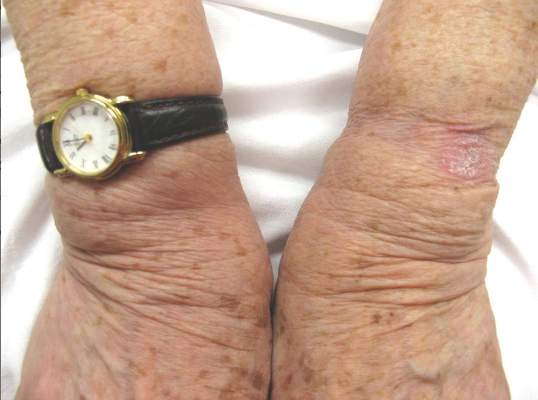
An 81-year-old woman presented with a 2-cm erythematous, scaly, pruritic rash on the left dorsal wrist localized to the skin under her watch. The patient first noticed the lesion 2 months prior. She moved the watch to the right wrist a few days prior to presentation and no symptoms developed in that location. No other areas of the skin were affected. She had no known allergies and was otherwise in good health.