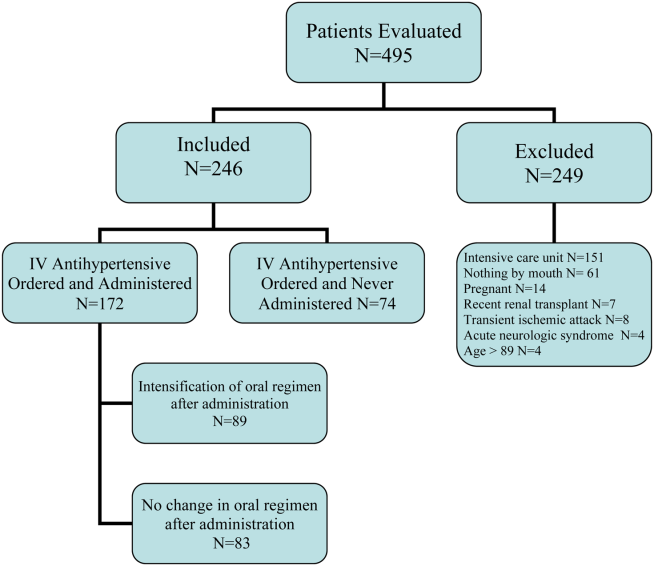
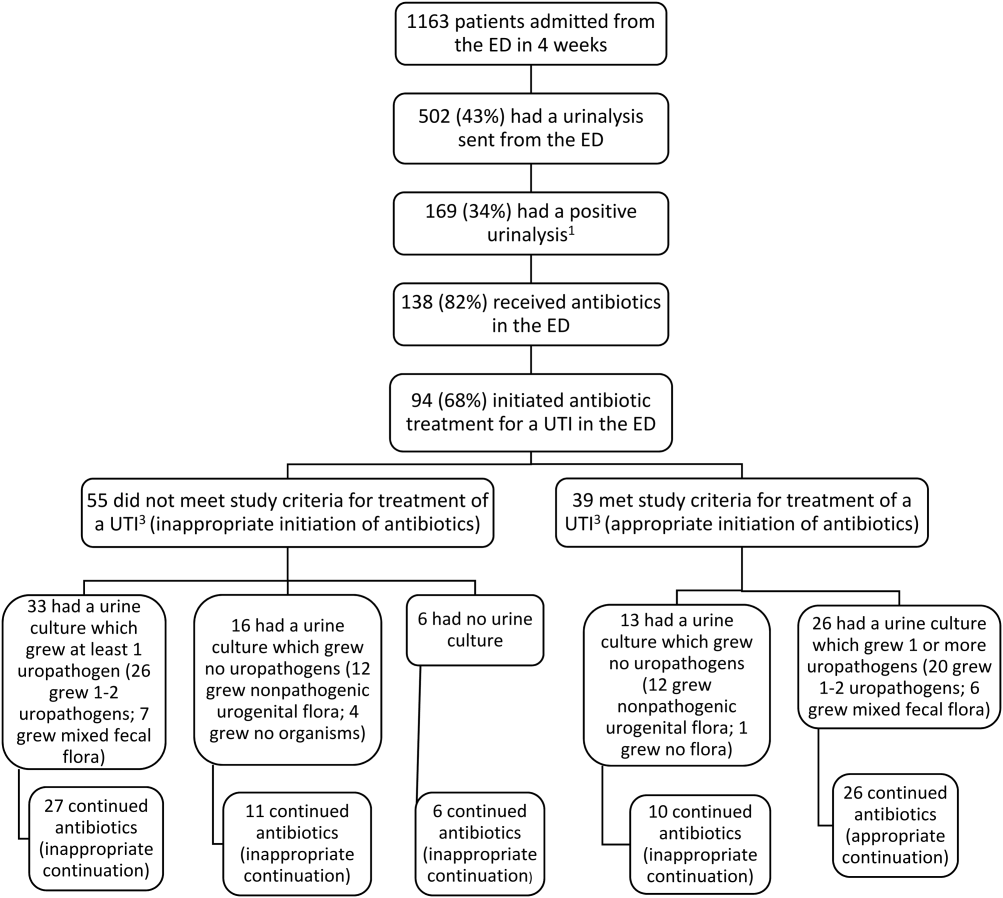
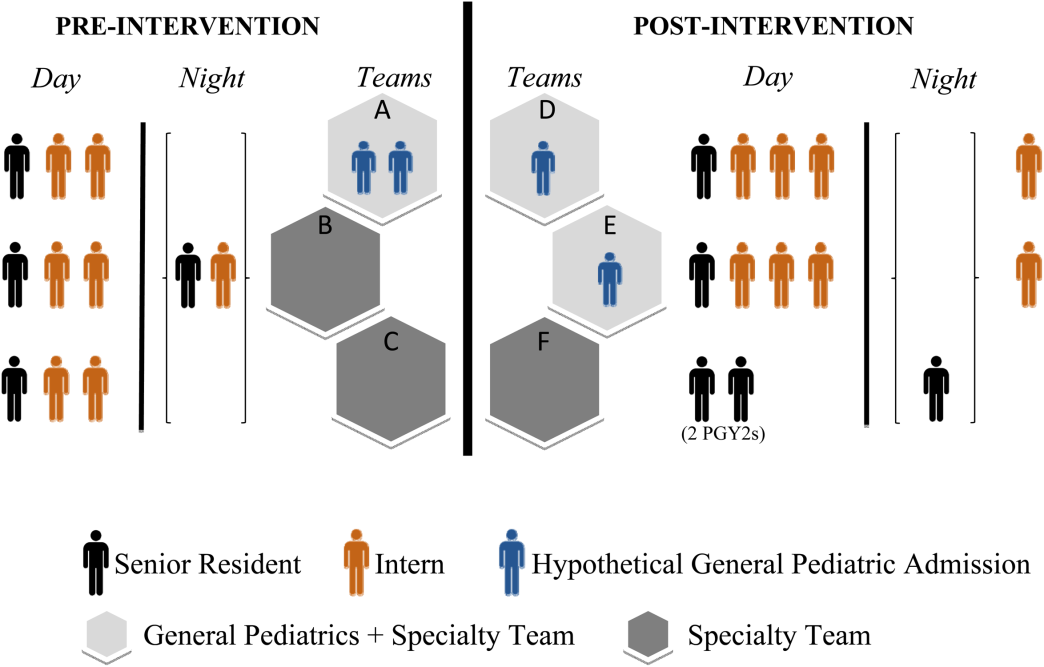
User login
Judicious antibiotic use key in ambulatory settings
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”
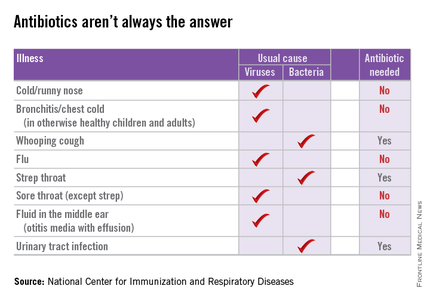
Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
Inhibitor exhibits ‘modest’ activity in lymphoma

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()
Drug ‘life-changing’ for CLL patients in phase 1 trial

Photo courtesy of NIH
A novel Bruton’s tyrosine kinase inhibitor has proven life-changing for patients with chronic lymphocytic leukemia (CLL) who received the drug as part of a phase 1 trial, according to the study’s lead author.
The inhibitor, ONO/GS-4059, produced a response in 96% of evaluable CLL patients.
Most CLL patients are still on the study after 3 years, although a handful withdrew due to adverse events (AEs) or disease progression.
“These patients were confronted with a cruel reality: they had failed multiple chemotherapy lines, and there were no other treatment options available for them,” said lead study author Harriet Walter, MBChB, of the University of Leicester in the UK.
“This drug has changed their lives. From desperate and tired, they are now leading a normal and really active life. This is hugely rewarding and encouraging.”
Dr Walter and her colleagues reported these results in Blood. The trial was funded by ONO Pharmaceuticals, the company developing ONO/GS-4059.
This study opened in January 2012, and 90 patients were enrolled at centers in the UK and France. There were 28 patients with CLL and 62 with non-Hodgkin lymphoma (NHL), including 16 with mantle cell lymphoma (MCL) and 35 with diffuse large B-cell lymphoma (DLBCL).
The study also included patients with follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia, but patient numbers were small for these groups, so the results were not discussed in detail.
There were 9 dose-escalation cohorts in this study. ONO/GS-4059 was given once-daily at doses ranging from 20 mg to 600 mg. Or the drug was given twice daily at doses of 240 mg or 300 mg.
Results
The maximum tolerated dose was not reached in the CLL cohort, but it was 480 mg once-daily in the NHL cohort. Four NHL patients had a dose-limiting toxicity.
In the CLL cohort, 2 patients went off study due to progression and 5 due to AEs.
In the NHL cohort, 49 patients discontinued treatment, 32 due to progression and 5 due to dose-limiting toxicities or AEs. The other 12 NHL patients discontinued due to patient or investigator decision, proceeding to transplant (n=1), or death due to progressive disease.
The median duration of follow-up was 560 days for CLL patients, 309 days for MCL patients, and 60 days for DLBCL patients.
The overall estimated mean progression-free survival was 874 days for CLL patients, 341 days for MCL patients, and 54 days for DLBCL patients.
CLL patients
Of all 28 CLL patients, 16 had relapsed CLL, 11 had refractory disease, and 1 had unknown status. The median number of prior therapies was 3.5 (range, 2-7).
Twenty-five patients were evaluable. Of the 3 who were not evaluable, 1 had not reached cycle 3 disease assessment at the time of data analysis, 1 progressed during cycle 1, and 1 was withdrawn due to an AE (idiopathic thrombocytopenia).
Of the 25 evaluable patients, 24 (96%) responded to ONO/GS-4059. The researchers said they observed rapid resolution of bulky lymphadenopathy within the first 3 months of treatment, but improvement in lymphadenopathy continued for up to 18 months in most patients.
The median treatment duration for these patients is 80 weeks, and 21 patients are still on treatment. Two of the evaluable patients progressed during therapy, one at cycle 3 and one at cycle 12.
MCL patients
Of the 16 MCL patients enrolled, 7 were refractory to their last course of immuno-chemotherapy. The median number of prior therapies was 3 (range, 2-7).
Eleven of 12 (92%) evaluable patients with MCL responded to ONO/GS-4059. Six patients had a partial response, and 5 had a complete response (CR) or unconfirmed CR.
Three patients progressed after an initial response. Four patients were not evaluable because they progressed.
The median treatment duration for MCL patients is 40 weeks, and 8 patients remain on study.
DLBCL patients
All 35 DLBCL patients had relapsed or refractory disease. The median number of prior treatments was 3
(range, 2-10), and 30 patients were refractory to their last line of chemotherapy.
Eleven of 31 (35%) patients with non-germinal center B-cell (non-GCB) DLBCL responded to ONO/GS-4059. Two non-GCB DLBCL patients had a confirmed CR, 1 had an unconfirmed CR, and the rest had partial responses.
The median duration of response was 54 days. And, among responders, the median treatment duration was 12 weeks.
The majority of non-GCB DLBCL patients progressed. There were no responses among the 2 patients with GCB DLBCL, and there were no responses among patients with primary mediastinal B-cell lymphoma or plasmablastic DLBCL.
Toxicity
AEs in this study were mostly grade 1/2—75% in the CLL cohort and 50% in the NHL cohort. However, treatment-related grade 3/4 AEs occurred in 14.3% of CLL patients and 16.1% of NHL patients.
Grade 3/4 events were mainly hematologic in nature and included neutropenia (10%), anemia (13.3%), and thrombocytopenia (13.3%).
There was a grade 3 episode of drug-related hemorrhage in a CLL patient, which resulted in a psoas hematoma (with concomitant CLL progression) in the presence of a normal platelet count. This patient was among those taken off the study.
“The next step is now to see how best we can improve on these outstanding results,” said study author Martin Dyer, DPhil, of the University of Leicester.
“A further study using this drug in combination with additional targeted agents is shortly to open in Leicester with the aim of achieving cure. In parallel with the clinical development of the drug, our team of scientists at the Haematological Research Institute are studying how this drug is working and how to overcome potential resistance.” ![]()

Photo courtesy of NIH
A novel Bruton’s tyrosine kinase inhibitor has proven life-changing for patients with chronic lymphocytic leukemia (CLL) who received the drug as part of a phase 1 trial, according to the study’s lead author.
The inhibitor, ONO/GS-4059, produced a response in 96% of evaluable CLL patients.
Most CLL patients are still on the study after 3 years, although a handful withdrew due to adverse events (AEs) or disease progression.
“These patients were confronted with a cruel reality: they had failed multiple chemotherapy lines, and there were no other treatment options available for them,” said lead study author Harriet Walter, MBChB, of the University of Leicester in the UK.
“This drug has changed their lives. From desperate and tired, they are now leading a normal and really active life. This is hugely rewarding and encouraging.”
Dr Walter and her colleagues reported these results in Blood. The trial was funded by ONO Pharmaceuticals, the company developing ONO/GS-4059.
This study opened in January 2012, and 90 patients were enrolled at centers in the UK and France. There were 28 patients with CLL and 62 with non-Hodgkin lymphoma (NHL), including 16 with mantle cell lymphoma (MCL) and 35 with diffuse large B-cell lymphoma (DLBCL).
The study also included patients with follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia, but patient numbers were small for these groups, so the results were not discussed in detail.
There were 9 dose-escalation cohorts in this study. ONO/GS-4059 was given once-daily at doses ranging from 20 mg to 600 mg. Or the drug was given twice daily at doses of 240 mg or 300 mg.
Results
The maximum tolerated dose was not reached in the CLL cohort, but it was 480 mg once-daily in the NHL cohort. Four NHL patients had a dose-limiting toxicity.
In the CLL cohort, 2 patients went off study due to progression and 5 due to AEs.
In the NHL cohort, 49 patients discontinued treatment, 32 due to progression and 5 due to dose-limiting toxicities or AEs. The other 12 NHL patients discontinued due to patient or investigator decision, proceeding to transplant (n=1), or death due to progressive disease.
The median duration of follow-up was 560 days for CLL patients, 309 days for MCL patients, and 60 days for DLBCL patients.
The overall estimated mean progression-free survival was 874 days for CLL patients, 341 days for MCL patients, and 54 days for DLBCL patients.
CLL patients
Of all 28 CLL patients, 16 had relapsed CLL, 11 had refractory disease, and 1 had unknown status. The median number of prior therapies was 3.5 (range, 2-7).
Twenty-five patients were evaluable. Of the 3 who were not evaluable, 1 had not reached cycle 3 disease assessment at the time of data analysis, 1 progressed during cycle 1, and 1 was withdrawn due to an AE (idiopathic thrombocytopenia).
Of the 25 evaluable patients, 24 (96%) responded to ONO/GS-4059. The researchers said they observed rapid resolution of bulky lymphadenopathy within the first 3 months of treatment, but improvement in lymphadenopathy continued for up to 18 months in most patients.
The median treatment duration for these patients is 80 weeks, and 21 patients are still on treatment. Two of the evaluable patients progressed during therapy, one at cycle 3 and one at cycle 12.
MCL patients
Of the 16 MCL patients enrolled, 7 were refractory to their last course of immuno-chemotherapy. The median number of prior therapies was 3 (range, 2-7).
Eleven of 12 (92%) evaluable patients with MCL responded to ONO/GS-4059. Six patients had a partial response, and 5 had a complete response (CR) or unconfirmed CR.
Three patients progressed after an initial response. Four patients were not evaluable because they progressed.
The median treatment duration for MCL patients is 40 weeks, and 8 patients remain on study.
DLBCL patients
All 35 DLBCL patients had relapsed or refractory disease. The median number of prior treatments was 3
(range, 2-10), and 30 patients were refractory to their last line of chemotherapy.
Eleven of 31 (35%) patients with non-germinal center B-cell (non-GCB) DLBCL responded to ONO/GS-4059. Two non-GCB DLBCL patients had a confirmed CR, 1 had an unconfirmed CR, and the rest had partial responses.
The median duration of response was 54 days. And, among responders, the median treatment duration was 12 weeks.
The majority of non-GCB DLBCL patients progressed. There were no responses among the 2 patients with GCB DLBCL, and there were no responses among patients with primary mediastinal B-cell lymphoma or plasmablastic DLBCL.
Toxicity
AEs in this study were mostly grade 1/2—75% in the CLL cohort and 50% in the NHL cohort. However, treatment-related grade 3/4 AEs occurred in 14.3% of CLL patients and 16.1% of NHL patients.
Grade 3/4 events were mainly hematologic in nature and included neutropenia (10%), anemia (13.3%), and thrombocytopenia (13.3%).
There was a grade 3 episode of drug-related hemorrhage in a CLL patient, which resulted in a psoas hematoma (with concomitant CLL progression) in the presence of a normal platelet count. This patient was among those taken off the study.
“The next step is now to see how best we can improve on these outstanding results,” said study author Martin Dyer, DPhil, of the University of Leicester.
“A further study using this drug in combination with additional targeted agents is shortly to open in Leicester with the aim of achieving cure. In parallel with the clinical development of the drug, our team of scientists at the Haematological Research Institute are studying how this drug is working and how to overcome potential resistance.” ![]()

Photo courtesy of NIH
A novel Bruton’s tyrosine kinase inhibitor has proven life-changing for patients with chronic lymphocytic leukemia (CLL) who received the drug as part of a phase 1 trial, according to the study’s lead author.
The inhibitor, ONO/GS-4059, produced a response in 96% of evaluable CLL patients.
Most CLL patients are still on the study after 3 years, although a handful withdrew due to adverse events (AEs) or disease progression.
“These patients were confronted with a cruel reality: they had failed multiple chemotherapy lines, and there were no other treatment options available for them,” said lead study author Harriet Walter, MBChB, of the University of Leicester in the UK.
“This drug has changed their lives. From desperate and tired, they are now leading a normal and really active life. This is hugely rewarding and encouraging.”
Dr Walter and her colleagues reported these results in Blood. The trial was funded by ONO Pharmaceuticals, the company developing ONO/GS-4059.
This study opened in January 2012, and 90 patients were enrolled at centers in the UK and France. There were 28 patients with CLL and 62 with non-Hodgkin lymphoma (NHL), including 16 with mantle cell lymphoma (MCL) and 35 with diffuse large B-cell lymphoma (DLBCL).
The study also included patients with follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia, but patient numbers were small for these groups, so the results were not discussed in detail.
There were 9 dose-escalation cohorts in this study. ONO/GS-4059 was given once-daily at doses ranging from 20 mg to 600 mg. Or the drug was given twice daily at doses of 240 mg or 300 mg.
Results
The maximum tolerated dose was not reached in the CLL cohort, but it was 480 mg once-daily in the NHL cohort. Four NHL patients had a dose-limiting toxicity.
In the CLL cohort, 2 patients went off study due to progression and 5 due to AEs.
In the NHL cohort, 49 patients discontinued treatment, 32 due to progression and 5 due to dose-limiting toxicities or AEs. The other 12 NHL patients discontinued due to patient or investigator decision, proceeding to transplant (n=1), or death due to progressive disease.
The median duration of follow-up was 560 days for CLL patients, 309 days for MCL patients, and 60 days for DLBCL patients.
The overall estimated mean progression-free survival was 874 days for CLL patients, 341 days for MCL patients, and 54 days for DLBCL patients.
CLL patients
Of all 28 CLL patients, 16 had relapsed CLL, 11 had refractory disease, and 1 had unknown status. The median number of prior therapies was 3.5 (range, 2-7).
Twenty-five patients were evaluable. Of the 3 who were not evaluable, 1 had not reached cycle 3 disease assessment at the time of data analysis, 1 progressed during cycle 1, and 1 was withdrawn due to an AE (idiopathic thrombocytopenia).
Of the 25 evaluable patients, 24 (96%) responded to ONO/GS-4059. The researchers said they observed rapid resolution of bulky lymphadenopathy within the first 3 months of treatment, but improvement in lymphadenopathy continued for up to 18 months in most patients.
The median treatment duration for these patients is 80 weeks, and 21 patients are still on treatment. Two of the evaluable patients progressed during therapy, one at cycle 3 and one at cycle 12.
MCL patients
Of the 16 MCL patients enrolled, 7 were refractory to their last course of immuno-chemotherapy. The median number of prior therapies was 3 (range, 2-7).
Eleven of 12 (92%) evaluable patients with MCL responded to ONO/GS-4059. Six patients had a partial response, and 5 had a complete response (CR) or unconfirmed CR.
Three patients progressed after an initial response. Four patients were not evaluable because they progressed.
The median treatment duration for MCL patients is 40 weeks, and 8 patients remain on study.
DLBCL patients
All 35 DLBCL patients had relapsed or refractory disease. The median number of prior treatments was 3
(range, 2-10), and 30 patients were refractory to their last line of chemotherapy.
Eleven of 31 (35%) patients with non-germinal center B-cell (non-GCB) DLBCL responded to ONO/GS-4059. Two non-GCB DLBCL patients had a confirmed CR, 1 had an unconfirmed CR, and the rest had partial responses.
The median duration of response was 54 days. And, among responders, the median treatment duration was 12 weeks.
The majority of non-GCB DLBCL patients progressed. There were no responses among the 2 patients with GCB DLBCL, and there were no responses among patients with primary mediastinal B-cell lymphoma or plasmablastic DLBCL.
Toxicity
AEs in this study were mostly grade 1/2—75% in the CLL cohort and 50% in the NHL cohort. However, treatment-related grade 3/4 AEs occurred in 14.3% of CLL patients and 16.1% of NHL patients.
Grade 3/4 events were mainly hematologic in nature and included neutropenia (10%), anemia (13.3%), and thrombocytopenia (13.3%).
There was a grade 3 episode of drug-related hemorrhage in a CLL patient, which resulted in a psoas hematoma (with concomitant CLL progression) in the presence of a normal platelet count. This patient was among those taken off the study.
“The next step is now to see how best we can improve on these outstanding results,” said study author Martin Dyer, DPhil, of the University of Leicester.
“A further study using this drug in combination with additional targeted agents is shortly to open in Leicester with the aim of achieving cure. In parallel with the clinical development of the drug, our team of scientists at the Haematological Research Institute are studying how this drug is working and how to overcome potential resistance.” ![]()
Antidote reverses effects of apixaban, rivaroxaban in healthy subjects

An investigational antidote to factor Xa inhibitors has proven succesful in reversing the effects of apixaban and rivaroxaban in a pair of phase 3 studies of healthy
volunteers.
In the ANNEXA-R and ANNEXA-A studies, researchers evaluated the safety and efficacy of the antidote, andexanet alfa, in volunteers receiving rivaroxaban and apixaban, respectively.
In both studies, andexanet alfa met all efficacy endpoints.
There were no serious or severe adverse events and no thrombotic events in either study.
“The findings of [these studies] are an advance towards resolving major bleeding complications effectively within minutes,” said Deborah Siegal, MD, of McMaster University in Hamilton, Ontario, Canada.
Dr Siegal and her colleagues reported the findings in NEJM. The studies were funded by Portola Pharmaceuticals, the company developing andexanet alfa.
The randomized, double-blind, placebo-controlled ANNEXA-R and ANNEXA-A studies were conducted to evaluate the safety and efficacy of andexanet alfa in reversing the anticoagulant effect of rivaroxaban and apixaban, respectively, in healthy volunteers ages 50 to 68.
The primary endpoint was reduction in anti-factor Xa levels. Secondary endpoints included reduction in plasma levels of unbound rivaroxaban or apixaban and restoration of the endogenous thrombin potential, a measure of thrombin generation.
ANNEXA-R efficacy
In part 1 of this study, 41 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg intravenous (IV) bolus (n=27) or placebo (n=14).
Within 2 to 5 minutes of bolus completion, andexanet alfa significantly reduced the anti-factor Xa activity of rivaroxaban compared with placebo—92% and 18%, respectively (P<0.001).
And andexanet alfa significantly reduced the level of unbound rivaroxaban in the plasma compared with placebo—23.4 ng/mL and 4.2 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 96% of subjects who received andexanet alfa and 7% of placebo-treated subjects (P<0.001).
In part 2 of the study, 39 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus followed by a continuous infusion of 8 mg/min for 120 minutes (n=26) or placebo (n=13).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—97% and 45%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound rivaroxaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound rivaroxaban compared with placebo—30.3 ng/mL and 12.1 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 0% of placebo-treated subjects (P<0.001).
ANNEXA-A efficacy
In part 1 of this study, 33 subjects received apixaban at 5 mg twice daily for 3.5 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus (n=24) or placebo (n=9).
Within 2 to 5 minutes of bolus completion, andexanet alfa reduced the anti-factor Xa activity of apixaban compared with placebo—94% and 21%, respectively (P<0.001).
Andexanet alfa significantly reduced the level of unbound apixaban in the plasma compared with placebo—9.3 ng/mL and 1.9 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 11% of placebo-treated subjects (P<0.001).
In part 2, 31 healthy volunteers received apixaban at 5 mg twice daily for 4 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus followed by a continuous infusion of 4 mg/min for 120 minutes (n=24) or placebo (n=8).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—92% and 33%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound apixaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound apixaban compared with placebo—6.5 ng/mL and 3.0 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 25% of placebo-treated subjects (P<0.001).
ANNEXA safety results
There were no serious or severe adverse events and no thrombotic events in either study. All adverse events related to andexanet alfa were considered mild.
Among subjects who received andexanet alfa in the ANNEXA-A study, there were 4 cases of gastrointestinal disorders—2 cases of constipation and 2 cases of dysgeusia. There were 3 cases in which subjects felt hot after administration of the drug, 4 cases of flushing, and 1 case of urticaria.
Among subjects who received andexanet alfa in the ANNEXA-R study, there were 2 cases of flushing and 1 case of urticaria.
Among subjects who received placebo in either study, there was 1 case of flushing.
One subject with a history of hives developed erythematous hives after receiving andexanet alfa. The infusion was stopped after 35 minutes, the subject received a single dose of diphenhydramine, and the hives resolved.
None of the subjects developed antibodies to factor X or factor Xa, and there were no neutralizing antibodies against andexanet alfa.
However, 1 subject who received placebo (2%) and 17 subjects who received andexanet alfa (17%) had non-neutralizing antibodies against andexanet alfa. Two of the subjects had non-neutralizing antibodies before andexanet alfa administration.
The antibodies tended to appear within 15 to 30 days of andexanet administration, and the titers were generally low (at or below 1:640). The exception was 1 subject who had a titer of 1:2560.
About andexanet alfa
Andexanet alfa is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Portola Pharmaceuticals is currently evaluating andexanet alfa in ANNEXA-4, a phase 4, single-arm, confirmatory study in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed.
Data from a small number of patients from ANNEXA-4, as well as data from ANNEXA-A and ANNEXA-R, will serve as the clinical basis for the biologics license application to the US Food and Drug Administration.
A rolling submission of the application has been initiated under accelerated approval, and the submission package is expected to be complete by the end of this year. The Food and Drug Administration has already granted andexanet alfa orphan drug designation and breakthrough therapy designation. ![]()

An investigational antidote to factor Xa inhibitors has proven succesful in reversing the effects of apixaban and rivaroxaban in a pair of phase 3 studies of healthy
volunteers.
In the ANNEXA-R and ANNEXA-A studies, researchers evaluated the safety and efficacy of the antidote, andexanet alfa, in volunteers receiving rivaroxaban and apixaban, respectively.
In both studies, andexanet alfa met all efficacy endpoints.
There were no serious or severe adverse events and no thrombotic events in either study.
“The findings of [these studies] are an advance towards resolving major bleeding complications effectively within minutes,” said Deborah Siegal, MD, of McMaster University in Hamilton, Ontario, Canada.
Dr Siegal and her colleagues reported the findings in NEJM. The studies were funded by Portola Pharmaceuticals, the company developing andexanet alfa.
The randomized, double-blind, placebo-controlled ANNEXA-R and ANNEXA-A studies were conducted to evaluate the safety and efficacy of andexanet alfa in reversing the anticoagulant effect of rivaroxaban and apixaban, respectively, in healthy volunteers ages 50 to 68.
The primary endpoint was reduction in anti-factor Xa levels. Secondary endpoints included reduction in plasma levels of unbound rivaroxaban or apixaban and restoration of the endogenous thrombin potential, a measure of thrombin generation.
ANNEXA-R efficacy
In part 1 of this study, 41 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg intravenous (IV) bolus (n=27) or placebo (n=14).
Within 2 to 5 minutes of bolus completion, andexanet alfa significantly reduced the anti-factor Xa activity of rivaroxaban compared with placebo—92% and 18%, respectively (P<0.001).
And andexanet alfa significantly reduced the level of unbound rivaroxaban in the plasma compared with placebo—23.4 ng/mL and 4.2 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 96% of subjects who received andexanet alfa and 7% of placebo-treated subjects (P<0.001).
In part 2 of the study, 39 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus followed by a continuous infusion of 8 mg/min for 120 minutes (n=26) or placebo (n=13).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—97% and 45%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound rivaroxaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound rivaroxaban compared with placebo—30.3 ng/mL and 12.1 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 0% of placebo-treated subjects (P<0.001).
ANNEXA-A efficacy
In part 1 of this study, 33 subjects received apixaban at 5 mg twice daily for 3.5 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus (n=24) or placebo (n=9).
Within 2 to 5 minutes of bolus completion, andexanet alfa reduced the anti-factor Xa activity of apixaban compared with placebo—94% and 21%, respectively (P<0.001).
Andexanet alfa significantly reduced the level of unbound apixaban in the plasma compared with placebo—9.3 ng/mL and 1.9 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 11% of placebo-treated subjects (P<0.001).
In part 2, 31 healthy volunteers received apixaban at 5 mg twice daily for 4 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus followed by a continuous infusion of 4 mg/min for 120 minutes (n=24) or placebo (n=8).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—92% and 33%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound apixaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound apixaban compared with placebo—6.5 ng/mL and 3.0 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 25% of placebo-treated subjects (P<0.001).
ANNEXA safety results
There were no serious or severe adverse events and no thrombotic events in either study. All adverse events related to andexanet alfa were considered mild.
Among subjects who received andexanet alfa in the ANNEXA-A study, there were 4 cases of gastrointestinal disorders—2 cases of constipation and 2 cases of dysgeusia. There were 3 cases in which subjects felt hot after administration of the drug, 4 cases of flushing, and 1 case of urticaria.
Among subjects who received andexanet alfa in the ANNEXA-R study, there were 2 cases of flushing and 1 case of urticaria.
Among subjects who received placebo in either study, there was 1 case of flushing.
One subject with a history of hives developed erythematous hives after receiving andexanet alfa. The infusion was stopped after 35 minutes, the subject received a single dose of diphenhydramine, and the hives resolved.
None of the subjects developed antibodies to factor X or factor Xa, and there were no neutralizing antibodies against andexanet alfa.
However, 1 subject who received placebo (2%) and 17 subjects who received andexanet alfa (17%) had non-neutralizing antibodies against andexanet alfa. Two of the subjects had non-neutralizing antibodies before andexanet alfa administration.
The antibodies tended to appear within 15 to 30 days of andexanet administration, and the titers were generally low (at or below 1:640). The exception was 1 subject who had a titer of 1:2560.
About andexanet alfa
Andexanet alfa is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Portola Pharmaceuticals is currently evaluating andexanet alfa in ANNEXA-4, a phase 4, single-arm, confirmatory study in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed.
Data from a small number of patients from ANNEXA-4, as well as data from ANNEXA-A and ANNEXA-R, will serve as the clinical basis for the biologics license application to the US Food and Drug Administration.
A rolling submission of the application has been initiated under accelerated approval, and the submission package is expected to be complete by the end of this year. The Food and Drug Administration has already granted andexanet alfa orphan drug designation and breakthrough therapy designation. ![]()

An investigational antidote to factor Xa inhibitors has proven succesful in reversing the effects of apixaban and rivaroxaban in a pair of phase 3 studies of healthy
volunteers.
In the ANNEXA-R and ANNEXA-A studies, researchers evaluated the safety and efficacy of the antidote, andexanet alfa, in volunteers receiving rivaroxaban and apixaban, respectively.
In both studies, andexanet alfa met all efficacy endpoints.
There were no serious or severe adverse events and no thrombotic events in either study.
“The findings of [these studies] are an advance towards resolving major bleeding complications effectively within minutes,” said Deborah Siegal, MD, of McMaster University in Hamilton, Ontario, Canada.
Dr Siegal and her colleagues reported the findings in NEJM. The studies were funded by Portola Pharmaceuticals, the company developing andexanet alfa.
The randomized, double-blind, placebo-controlled ANNEXA-R and ANNEXA-A studies were conducted to evaluate the safety and efficacy of andexanet alfa in reversing the anticoagulant effect of rivaroxaban and apixaban, respectively, in healthy volunteers ages 50 to 68.
The primary endpoint was reduction in anti-factor Xa levels. Secondary endpoints included reduction in plasma levels of unbound rivaroxaban or apixaban and restoration of the endogenous thrombin potential, a measure of thrombin generation.
ANNEXA-R efficacy
In part 1 of this study, 41 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg intravenous (IV) bolus (n=27) or placebo (n=14).
Within 2 to 5 minutes of bolus completion, andexanet alfa significantly reduced the anti-factor Xa activity of rivaroxaban compared with placebo—92% and 18%, respectively (P<0.001).
And andexanet alfa significantly reduced the level of unbound rivaroxaban in the plasma compared with placebo—23.4 ng/mL and 4.2 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 96% of subjects who received andexanet alfa and 7% of placebo-treated subjects (P<0.001).
In part 2 of the study, 39 healthy volunteers received rivaroxaban at 20 mg once daily for 4 days. They were then randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus followed by a continuous infusion of 8 mg/min for 120 minutes (n=26) or placebo (n=13).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—97% and 45%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound rivaroxaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound rivaroxaban compared with placebo—30.3 ng/mL and 12.1 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 0% of placebo-treated subjects (P<0.001).
ANNEXA-A efficacy
In part 1 of this study, 33 subjects received apixaban at 5 mg twice daily for 3.5 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus (n=24) or placebo (n=9).
Within 2 to 5 minutes of bolus completion, andexanet alfa reduced the anti-factor Xa activity of apixaban compared with placebo—94% and 21%, respectively (P<0.001).
Andexanet alfa significantly reduced the level of unbound apixaban in the plasma compared with placebo—9.3 ng/mL and 1.9 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 11% of placebo-treated subjects (P<0.001).
In part 2, 31 healthy volunteers received apixaban at 5 mg twice daily for 4 days. They were then randomized in a 3:1 ratio to receive either andexanet alfa administered as a 400 mg IV bolus followed by a continuous infusion of 4 mg/min for 120 minutes (n=24) or placebo (n=8).
Andexanet alfa significantly reduced anti-factor Xa activity compared with placebo—92% and 33%, respectively (P<0.001). And reversal persisted in andexanet alfa-treated subjects for 1 to 2 hours after the infusion was complete.
The reduction in unbound apixaban was sustained with the bolus plus infusion, which significantly reduced the mean plasma concentration of unbound apixaban compared with placebo—6.5 ng/mL and 3.0 ng/mL, respectively (P<0.001).
Thrombin generation was fully restored in 100% of subjects who received andexanet alfa and 25% of placebo-treated subjects (P<0.001).
ANNEXA safety results
There were no serious or severe adverse events and no thrombotic events in either study. All adverse events related to andexanet alfa were considered mild.
Among subjects who received andexanet alfa in the ANNEXA-A study, there were 4 cases of gastrointestinal disorders—2 cases of constipation and 2 cases of dysgeusia. There were 3 cases in which subjects felt hot after administration of the drug, 4 cases of flushing, and 1 case of urticaria.
Among subjects who received andexanet alfa in the ANNEXA-R study, there were 2 cases of flushing and 1 case of urticaria.
Among subjects who received placebo in either study, there was 1 case of flushing.
One subject with a history of hives developed erythematous hives after receiving andexanet alfa. The infusion was stopped after 35 minutes, the subject received a single dose of diphenhydramine, and the hives resolved.
None of the subjects developed antibodies to factor X or factor Xa, and there were no neutralizing antibodies against andexanet alfa.
However, 1 subject who received placebo (2%) and 17 subjects who received andexanet alfa (17%) had non-neutralizing antibodies against andexanet alfa. Two of the subjects had non-neutralizing antibodies before andexanet alfa administration.
The antibodies tended to appear within 15 to 30 days of andexanet administration, and the titers were generally low (at or below 1:640). The exception was 1 subject who had a titer of 1:2560.
About andexanet alfa
Andexanet alfa is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Portola Pharmaceuticals is currently evaluating andexanet alfa in ANNEXA-4, a phase 4, single-arm, confirmatory study in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed.
Data from a small number of patients from ANNEXA-4, as well as data from ANNEXA-A and ANNEXA-R, will serve as the clinical basis for the biologics license application to the US Food and Drug Administration.
A rolling submission of the application has been initiated under accelerated approval, and the submission package is expected to be complete by the end of this year. The Food and Drug Administration has already granted andexanet alfa orphan drug designation and breakthrough therapy designation. ![]()
Analysis suggests dabigatran is safer than warfarin

ORLANDO, FL—Interim results from a long-term, “real world” study suggest dabigatran etexilate mesylate (Pradaxa) may be safer than warfarin in routine
clinical practice.
The data, which come from a pooled analysis of 2 large US commercial health insurance databases, showed that patients with non-valvular atrial fibrillation (NVAF) who received dabigatran had fewer strokes and major bleeding events than NVAF patients who received warfarin.
The results were presented at the American Heart Association (AHA) Scientific Sessions 2015 (abstract M 2129*).
The research was supported by Boehringer Ingelheim, the company developing dabigatran, and employees from the company were involved in the study.
“Beyond clinical trials, there is a wealth of available health insurance data that provides an excellent opportunity to grow our knowledge of oral anticoagulant use and outcomes for patients,” said study investigator John Seeger, PharmD, DrPH, of Brigham and Women’s Hospital in Boston, Massachusetts.
“These real-world data further define the safety and effectiveness of dabigatran for patients and its use in routine care, and are consistent with the results of the pivotal RE-LY clinical trial.”
Dr Seeger and his colleagues analyzed data collected over 32 months and encompassing 44,672 NVAF patients—22,336 propensity-score-matched patients receiving dabigatran or warfarin for the first time.
The data were collected from 2 insurance databases—Truven MarketScan (18,276 patients per group) and Optum Clinformatics (4060 patients per group).
Patients were followed until they switched or discontinued anticoagulant treatment, experienced an outcome event, or discontinued enrollment. The average follow-up period was 5 months for dabigatran-treated patients and 4 months for warfarin-treated patients.
The primary outcomes of the study were stroke and major bleeding rates.
There were 65 strokes among dabigatran-treated patients (0.73 incidence rate per 100 patient-years) and 78 strokes among warfarin-treated patients (1.08 incidence rate per 100 patient-years).
The investigators said this represents a 28% reduction in stroke risk for dabigatran compared to warfarin. (The hazard ratio was 0.72.)
There were 395 major bleeding events among dabigatran-treated patients (4.47 incidence rate per 100 patient-years) and 459 events for warfarin-treated patients (6.42 incidence rate per 100 patient-years).
This represents a 26% reduction in the risk of major bleeding events with dabigatran compared to warfarin. (The hazard ratio was 0.74.)
The investigators said effectiveness assessments beyond the first 6 months of therapy were limited by the short average follow-up time. But future analyses from this long-term study program will yield more stable estimates.
This study is part of an ongoing research program to evaluate prescribing patterns and real-world safety and effectiveness of novel oral anticoagulants, including dabigatran, for reducing stroke risk. ![]()
*Data in the abstract differ from data presented at the meeting.

ORLANDO, FL—Interim results from a long-term, “real world” study suggest dabigatran etexilate mesylate (Pradaxa) may be safer than warfarin in routine
clinical practice.
The data, which come from a pooled analysis of 2 large US commercial health insurance databases, showed that patients with non-valvular atrial fibrillation (NVAF) who received dabigatran had fewer strokes and major bleeding events than NVAF patients who received warfarin.
The results were presented at the American Heart Association (AHA) Scientific Sessions 2015 (abstract M 2129*).
The research was supported by Boehringer Ingelheim, the company developing dabigatran, and employees from the company were involved in the study.
“Beyond clinical trials, there is a wealth of available health insurance data that provides an excellent opportunity to grow our knowledge of oral anticoagulant use and outcomes for patients,” said study investigator John Seeger, PharmD, DrPH, of Brigham and Women’s Hospital in Boston, Massachusetts.
“These real-world data further define the safety and effectiveness of dabigatran for patients and its use in routine care, and are consistent with the results of the pivotal RE-LY clinical trial.”
Dr Seeger and his colleagues analyzed data collected over 32 months and encompassing 44,672 NVAF patients—22,336 propensity-score-matched patients receiving dabigatran or warfarin for the first time.
The data were collected from 2 insurance databases—Truven MarketScan (18,276 patients per group) and Optum Clinformatics (4060 patients per group).
Patients were followed until they switched or discontinued anticoagulant treatment, experienced an outcome event, or discontinued enrollment. The average follow-up period was 5 months for dabigatran-treated patients and 4 months for warfarin-treated patients.
The primary outcomes of the study were stroke and major bleeding rates.
There were 65 strokes among dabigatran-treated patients (0.73 incidence rate per 100 patient-years) and 78 strokes among warfarin-treated patients (1.08 incidence rate per 100 patient-years).
The investigators said this represents a 28% reduction in stroke risk for dabigatran compared to warfarin. (The hazard ratio was 0.72.)
There were 395 major bleeding events among dabigatran-treated patients (4.47 incidence rate per 100 patient-years) and 459 events for warfarin-treated patients (6.42 incidence rate per 100 patient-years).
This represents a 26% reduction in the risk of major bleeding events with dabigatran compared to warfarin. (The hazard ratio was 0.74.)
The investigators said effectiveness assessments beyond the first 6 months of therapy were limited by the short average follow-up time. But future analyses from this long-term study program will yield more stable estimates.
This study is part of an ongoing research program to evaluate prescribing patterns and real-world safety and effectiveness of novel oral anticoagulants, including dabigatran, for reducing stroke risk. ![]()
*Data in the abstract differ from data presented at the meeting.

ORLANDO, FL—Interim results from a long-term, “real world” study suggest dabigatran etexilate mesylate (Pradaxa) may be safer than warfarin in routine
clinical practice.
The data, which come from a pooled analysis of 2 large US commercial health insurance databases, showed that patients with non-valvular atrial fibrillation (NVAF) who received dabigatran had fewer strokes and major bleeding events than NVAF patients who received warfarin.
The results were presented at the American Heart Association (AHA) Scientific Sessions 2015 (abstract M 2129*).
The research was supported by Boehringer Ingelheim, the company developing dabigatran, and employees from the company were involved in the study.
“Beyond clinical trials, there is a wealth of available health insurance data that provides an excellent opportunity to grow our knowledge of oral anticoagulant use and outcomes for patients,” said study investigator John Seeger, PharmD, DrPH, of Brigham and Women’s Hospital in Boston, Massachusetts.
“These real-world data further define the safety and effectiveness of dabigatran for patients and its use in routine care, and are consistent with the results of the pivotal RE-LY clinical trial.”
Dr Seeger and his colleagues analyzed data collected over 32 months and encompassing 44,672 NVAF patients—22,336 propensity-score-matched patients receiving dabigatran or warfarin for the first time.
The data were collected from 2 insurance databases—Truven MarketScan (18,276 patients per group) and Optum Clinformatics (4060 patients per group).
Patients were followed until they switched or discontinued anticoagulant treatment, experienced an outcome event, or discontinued enrollment. The average follow-up period was 5 months for dabigatran-treated patients and 4 months for warfarin-treated patients.
The primary outcomes of the study were stroke and major bleeding rates.
There were 65 strokes among dabigatran-treated patients (0.73 incidence rate per 100 patient-years) and 78 strokes among warfarin-treated patients (1.08 incidence rate per 100 patient-years).
The investigators said this represents a 28% reduction in stroke risk for dabigatran compared to warfarin. (The hazard ratio was 0.72.)
There were 395 major bleeding events among dabigatran-treated patients (4.47 incidence rate per 100 patient-years) and 459 events for warfarin-treated patients (6.42 incidence rate per 100 patient-years).
This represents a 26% reduction in the risk of major bleeding events with dabigatran compared to warfarin. (The hazard ratio was 0.74.)
The investigators said effectiveness assessments beyond the first 6 months of therapy were limited by the short average follow-up time. But future analyses from this long-term study program will yield more stable estimates.
This study is part of an ongoing research program to evaluate prescribing patterns and real-world safety and effectiveness of novel oral anticoagulants, including dabigatran, for reducing stroke risk. ![]()
*Data in the abstract differ from data presented at the meeting.
Antihypertensive Therapy and BP Control
Current recommendations for blood pressure (BP) control focus on chronic management of ambulatory patients; however, treatment guidelines for hospitalized patients who have acute increases in BP or simply uncontrolled BP lack clarity regarding appropriate therapeutic options and short‐term treatment goals.[1, 2] For patients with a history of hypertension, management in the hospital setting typically involves continuation of home therapies. In the inpatient setting, uncontrolled hypertension can be categorized as hypertensive emergency, hypertensive urgency, or asymptomatic poor BP control.[3] Asymptomatic BP elevations occur when the BP is not at goal (but not inordinately high) and the patient has no signs of new or worsening end‐organ damage.[4, 5, 6]
Published data have not demonstrated that aggressive treatment of asymptomatic hypertension in the inpatient setting improves short‐ or long‐term outcomes; however, such aggressive treatment may be associated with iatrogenic adverse effects.[5, 7, 8] Despite the lack of evidence of patient benefit, there is a tendency to treat hospitalized patients with asymptomatic BP elevations aggressively by prescribing IV antihypertensive agents on an as‐needed basis.[9] Intravenous hydralazine and labetalol are frequently used, although these agents are not recommended as initial therapy in consensus recommendations for asymptomatic uncontrolled hypertension in either the inpatient or outpatient setting.[10]
We therefore undertook the present study to determine the type and frequency of ordered and administered episodic intravenous (IV) antihypertensive drug therapy, the BP thresholds triggering such administration, and subsequent in‐hospital clinical outcomes after administration of IV antihypertensive drugs. Accordingly, we evaluated a series of hospitalized patients, in noncritical care settings with no evidence of new or worsening target‐organ injury, who were treated with episodic (either as needed or 1 time only) IV antihypertensive therapy.
METHODS
This study is a retrospective review. Between November 1, 2010 and January 31, 2011 we reviewed the charts of all patients who had at least 1 dose of IV hydralazine, enalaprilat, labetalol, or metoprolol ordered, regardless of previous oral antihypertensive treatment or hypertension diagnosis. Other IV antihypertensive agents were not evaluated in this study, as they are only available in critical care units at our institution. This study took place at an 806‐bed urban hospital that utilizes 100% computer prescriber order entry and bar code technology to document medication administration. The institutional review boards of the Detroit Medical Center and Wayne State University, Detroit, Michigan approved this study.
Patient Identification
Patients were identified through a list of all 1‐time‐only and as‐needed orders for IV hydralazine, enalaprilat, labetolol, or metoprolol. The list was generated daily through the hospital electronic medical record system (Cerner Powerchart, North Kansas City, MO). Patients were excluded if they were younger than 18 or older than 89 years of age, admitted to the intensive care or coronary care unit, were receiving nothing by mouth, pregnant, received a renal transplant in the past 3 months, or if there was any clinical manifestation of new or worsening target‐organ injury consistent with the diagnosis of hypertensive emergency.
Data Collection
The following data were collected for all patients: basic demographic information including factors that have been specifically associated with differences in hypertension risk (ie, age, sex, race, weight, and renal function), antihypertensive regimen (if any) prior to admission, changes to oral antihypertensive therapy during admission, order for sodium‐restricted diet, baseline and discharge laboratory values and vital signs. In addition, the details of their antihypertensive therapy order and administration were collected, including prescriber type (attending, resident, or physician extender), service of prescriber, criteria for use, and date and time of drug administration categorized by shift (morning shift, 7 am to 3 pm; afternoon shift, 3 pm to 11 pm; and night shift, 11 pm to 7 am). To analyze the outcomes of administering episodic IV antihypertensive therapy, the following data were collected: changes in average BP within 30 minutes to 6 hours after drug administration and occurrence of antihypertensive therapy‐related adverse events, including any interventions required after administration and adjustments to oral antihypertensive therapy during admission or upon discharge. In cases where BP data were not available (either just prior to or within 6 hours following administration of an IV antihypertensive), the data were not included in the analysis. To determine whether an antihypertensive drug regimen had been intensified, a therapeutic intensity score (TIS) was calculated for the oral antihypertensive regimen on admission and again at discharge. The antihypertensive TIS was calculated by dividing the total daily dose of each antihypertensive medication by the maximum US Food and Drug Administrationapproved daily dose.[11]
Adverse Outcomes Definition
We defined an adverse outcome as a 25% decrease in systolic or diastolic BP within 6 hours and/or intervention to treat symptoms of hypotension. This definition is consistent with Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommendations to assure safety when lowering BP in the setting of hypertensive emergency.[6] Although the patients in this study were not experiencing hypertensive emergency, this definition is supported by reports of negative sequelae from overzealous lowering of BP,[12, 13, 14] and it reflects criteria used in other trials.[10] Hypotension was deemed to have occurred if any of the following were documented: as IV fluid administration; scheduled BP medication held (at either the nurses discretion or per physician order); change in level of care; change in mental status; or transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration. Heart rate changes were also considered to be adverse outcomes, including tachycardia (heart rate >100 beats per minute [bpm] or increase 20 bpm from baseline) or bradycardia (heart rate <50 bpm).
Analysis
Descriptive statistics were performed for all variables. Continuous data were summarized using means and standard deviations. Categorical variables were summarized as counts and percentages. Paired t tests were used to contrast changes from baseline for continuous variables pre‐ and post‐BP, and heart rate changes were evaluated only for the first episode of IV antihypertensive drug administration in patients receiving multiple doses of antihypertensive medication to avoid the bias created by repeated or clustered measures in a given patient. 2 tests were used to test differences in categorical variables. All statistical testing was considered significant when 2‐tailed P values were <0.05. Analyses were generated using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patients
During the study period, there were 6133 inpatient adult admissions. Of 495 patients who had at least 1 order for IV hydralazine, enalaprilat, labetolol, or metoprolol, 246 were included in the analysis after applying the exclusion criteria (Figure 1). Patients were divided into 2 groups. One group had an order for an IV antihypertensive that was not administered (n = 74), and the other had an order for an IV antihypertensive and received at least 1 dose (n = 172). The demographic characteristics of the 2 groups are compared in Table 1. Patients who had their chronic oral antihypertensive regimens intensified after receiving IV antihypertensive medications were more often African American, leaner, more intensively treated, and had higher baseline BP.
| Did Not Receive IV Antihypertensive (n = 74) | Did Receive IV Antihypertensive (n = 172) | P | |
|---|---|---|---|
| |||
| Age, y | 61.6 13.9 | 60.6 13.7 | |
| Male sex | 51% | 47% | |
| African American | 74% | 87% | 0.008 |
| Weight, kg | 94.6 33.2 | 88.5 27.7 | |
| Admit systolic BP | 148 23 | 163 32 | <0.0001 |
| Admit diastolic BP | 82 13 | 87 18 | 0.009 |
| Admit heart rate | 87 18 | 82 20 | 0.069 |
| Admit TIS | 0.84 0.72 | 1.08 0.88 | 0.026 |
| Baseline SCr | 1.78 2.00 | 2.74 3.30 | 0.006 |
| Baseline AST | 26.5 12.5 | 65 126.2 | 0.046 |
| Low‐sodium diet order | 65% | 83% | 0.002 |
| Ordering service | |||
| Cardiology | 14% | 19% | |
| Internal medicine | 49% | 47% | |
| Nephrology | 0% | 6% | |
| Other services | 37% | 28% | |
| Prescriber type | |||
| Resident | 30% | 49% | |
| Physician extender | 53% | 35% | |
| Attending | 17% | 16% | |
| 1‐time‐only order | 5% | 19% | |
| As‐needed order | 95% | 81% | |
Prescribing Patterns
Medical residents prescribed nearly half (49%) of the orders for episodic IV antihypertensives. Attending physicians were responsible for 16% of episodic antihypertensive orders and physician extenders (physician's assistants and nurse practitioners) for 35%. A total of 321 orders were prescribed for the 246 patients in the study. Hydralazine was the preferred antihypertensive agent (80.1%), with IV ‐blockers prescribed less frequently (labetalol 15.6% and metoprolol 4.4%). There were no orders for IV enalaprilat. BP parameters were included in 181 (56%) of the episodic IV antihypertensive orders. Of the IV antihypertensive orders containing criteria, 153 (84.5%) had systolic BP threshold for administration <180 mm Hg (Table 2).
| BP Criteria for Administration of IV Antihypertensive Contained in Order, mm Hg | Did Not Receive IV Antihypertensive, n (%), n = 71* | Did Receive IV Antihypertensive, n (%), n = 133* |
|---|---|---|
| ||
| SBP >120 | 2 (2.8) | 1 (0.7) |
| SBP >130 | 2 (2.8) | 9 (6.8) |
| SBP >140 | 2 (2.8) | 5 (3.8) |
| SBP >150 | 4 (5.6) | 8 (6) |
| SBP >160 | 27 (38) | 58 (43.7) |
| SBP >170 | 26 (36.6) | 29 (21.8) |
| SBP >180 | 8 (11.4) | 18 (13.5) |
| SBP >200 | 4 (3) | |
| DBP >100 | 1 (0.7) | |
Drug Administration and Short‐term Data
Table 2 indicates the BP criteria specified in the episodic IV antihypertensive orders. For the 74 patients who did not receive an episodic IV antihypertensive agent, despite having an order, the nurses caring for the patients determined that their BPs never met the criteria for administration of the IV antihypertensive agent. The remainder of the results apply only to the 172 patients who actually received episodic IV antihypertensive therapy. Two of these patients did not have BP data available and were not included in the short‐term BP analysis. Almost half (48%) of the patients received 1 dose of episodic IV antihypertensive, 26% received 2 doses, and 11% received 3 doses. One patient received 10 doses. Hydralazine significantly lowered BP, whereas metoprolol did not (Figure 2).
The number of IV antihypertensive doses (for which BP data are available) administered during the night shift (n = 75) was numerically higher than the morning (n = 54) and the afternoon (n = 41) shifts. The mean BPs that triggered administration of IV antihypertensives did not differ among shifts (night shift 183/93, morning shift 184/99, afternoon shift 182/97).
Changes to Oral Antihypertensive Regimen After Administration of IV Antihypertensive Drugs
After administration of an episodic IV antihypertensive, the inpatient oral medication regimen was intensified in only 89 patients (52%). The BP reduction from admission to discharge in patients who had their inpatient oral medication regimen adjusted versus those who did not have an inpatient oral regimen adjustment after receiving IV antihypertensive medication is shown in Figure 3. Patients with intensification of their oral medications had a greater reduction in systolic BP from admission to discharge, compared to patients who received episodic IV antihypertensives but had no subsequent change to their inpatient oral antihypertensive regimen (Figure 3).
Adverse Events
Fifty‐six patients (32.6%) demonstrated BP reductions of more than 25% within 6 hours of antihypertensive administration. Of these patients, 2 received IV fluids, and 6 (3.5%) had a scheduled oral BP medication held. Of the patients who received IV hydralazine, 13 (4.4%) had an increase in heart rate >20 bpm, with 7 having a heart rate >100 bpm. One patient who received labetolol experienced bradycardia. No patient required a higher level of care (transfer to an intensive care unit) because of hemodynamic instability. In addition, no patient experienced a change in mental status, transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration.
DISCUSSION
The overwhelming majority of administrations of costly episodic IV antihypertensive drugs among this low‐risk population were in patients with modest BP elevations who may have merited no more, at most, than intensification of their oral antihypertensive drug regimen or observation. Such administration was infrequently followed by intensification of the oral antihypertensive drug regimen, and a significant number of patients experienced a potentially adverse clinical event. Excessive reduction of BP resulting in withholding of oral agents or administering IV fluids (as seen in 8 patients) is clinically relevant, especially in a setting where rapid lowering of BP with IV antihypertensives have no proven clinical benefit. There were differences between patients who did and did not received IV antihypertensive drug therapy, as those receiving therapy were higher‐risk patients. Of the patients initially evaluated for inclusion in this analysis, approximately half had a clear indication for IV antihypertensive therapy and were not included in this analysis. It should also be noted that one‐third of the patients included in the study did not subsequently receive an IV antihypertensive agent.
Recently updated hypertensive guidelines do not address the treatment of hypertensive urgency and emergency, whereas the JNC 7 addressed hypertensive urgency but did not provide a specific BP definition or goals because of concerns about overly aggressive management of severe asymptomatic hypertension.[2, 6] For patients with chronically elevated BP, its rapid reduction, even to levels that remain in the frankly hypertensive range, can be associated with negative clinical sequelae, attributable to decreased target organ perfusion causing clinically manifest ischemia.[3] Accordingly, there have been reports of ischemic events related to unwarranted and overzealous BP lowering.[12, 13, 14] In such patients, resistance vessel remodeling causes a rightward shift of the entire pressure/flow auto regulatory curve in critical arterial beds (eg, cerebral, coronary, and renal). Higher systemic pressure is necessary to maintain adequate perfusion in the target organ, at least over the short‐term. Thus, rapid, aggressive BP reduction can result in the aforementioned negative sequelae because remodeled resistance arterioles are not capable of vasodilating enough to ensure adequate blood flow when systemic pressure falls precipitously.
The patients in this study had no evidence of new or worsening pressure‐related end‐organ damage; therefore, there appeared to be no medical justification for emergent BP lowering via the IV route (a very small minority may have had BP high enough to have justified being diagnosed with hypertensive urgency in which fast‐acting oral therapy would be used). Despite the paucity of data to support this practice, it does, however, appear to be relatively common.[9] The high prevalence of IV hydralazine use in this inpatient study is consistent with the retrospective study reported by Weder and Erickson at the University of Michigan.[9]
Even among those with hypertensive urgencies, oral medication is the preferred route (assuming the patient can eat and swallow without difficulty and does not manifest an altered sensorium). Furthermore, the risks associated with overzealous BP lowering can be devastating. The likelihood of target‐organ ischemia (eg, angina pectoris, myocardial infarction, azotemia, stroke, transient ischemic attack) is most strongly correlated to the rapidity of the BP reduction, even to levels within the hypertensive range, in patients with persistent poor BP control.[4, 15, 16] Thus, the justification for considering a >25% drop in systolic BP within 6 hours of the administration of the IV antihypertensive agent as a potential adverse event, especially because there was only a very small immediate risk for adverse cardiovascular sequelae at the BP levels triggering administration of IV antihypertensive drug therapy.
Although we found that residents and physician assistants prescribed most IV antihypertensives, the practice of prescribing IV antihypertensive therapy appears to be common among all prescriber types. A recent survey assessing the attitudes and practices of resident physicians regarding hypertension in the inpatient setting found that 44% of respondents would treat acute asymptomatic, moderately elevated BP (182/100 mm Hg) with either an oral or intravenous agent.[17]
In addition to there being no proven clinical benefit in this setting, the use of unnecessary IV antihypertensives is associated with unnecessary risks and excess cost. Another report of IV hydralazine in asymptomatic patients found that 17 of 94 patients experienced an adverse effect after administration.[18] Not only is the drug acquisition cost for IV antihypertensives greater than their oral counterparts, often by a factor of 10 to 100, the intravenous route requires additional care to monitor their effects, adding to the human resource expense. Finally, the onset of action of intravenous agents is generally more rapid, which increases the risk of inducing hypotension and therefore target‐organ ischemia.
This study does, however, have limitations. This is a single‐center study, so the findings may not be generalizable to different hospital settings. The findings of this study depend on the accuracy and completeness of the medical record as recorded during routine clinical care; therefore, errors and omissions of data input and documentation may affect the quality of the data. Omissions and errors in the medication history can affect inpatient management as well as appropriateness of discharge medications. BP values before and after administration of an IV antihypertensive were not always available, limiting some of the short‐term outcomes data that were available. The impact of acuity of illness and concomitant disease states of patients were not assessed, which could also affect outcomes. The outcomes measured in this investigation were all short‐term outcomes and did not include important clinical outcomes (long‐term BP control, rehospitalization rates, or patient morbidity or mortality).
We speculate that the practice of episodic IV antihypertensive therapy has developed out of convenience for the practitioner and is likely commonplace across the country.[17] Healthcare systems should examine practices locally and address them as appropriate. To assist in promoting evidence‐based practice that is safe, prudent, and clinically appropriate, we propose that national BP organizations and consensus development groups consider placing priority on developing recommendations for inpatient hypertension treatment algorithms beyond those for hypertensive emergencies. In many cases, adjustments to a patient's oral regimen or observation of the patient are the only interventions that are needed. In addition, appropriate coordination of ambulatory follow‐up care upon discharge is prudent. Finally, individual healthcare systems might need to identify formal programs to modify institutional behavior of both medical and nursing staff to eliminate or limit this practice that is not supported by clinical evidence and potentially places the patient at risk.
CONCLUSIONS
Our study found that the practice of prescribing episodic IV antihypertensive agents at our institution occurred across all prescriber types. Hydralazine was the most frequently ordered agent. The majority of orders containing systolic BP criteria for administration of an episodic IV antihypertensive agent were well below the BP level associated with immediate or near‐immediate cardiovascular risk. Administration of episodic IV antihypertensive agents, without subsequent intensification of the patient's chronic oral antihypertensive regimen was nearly as likely to occur as subsequent intensification of the oral regimen in our study. The absence of evidence‐based guidelines, combined with the results of this evaluation, provide a rationale for implementing hospital‐ and health systembased policies limiting the use of episodic IV antihypertensive agents in asymptomatic patients with uncontrolled BP in noncritical care settings in the absence of new or worsening target‐organ injury.
Disclosure: Nothing to report.
- , , , et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115(21):2761–2788.
- , , , et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520.
- , . Hypertensive crises: challenges and management. Chest. 2007;131(6):1949–1962.
- , . Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4):513–529.
- , , , , ; American College of Emergency Physicians Clinical Policies Subcommittee on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med.2006;47(3):237–249.
- , , , et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- , . Best Evidence on management of asymptomatic hypertension in ED patients. J Emerg Nurs. 2011;37(2):174–178.
- , , , et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150–160.
- , . Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29–33.
- , , , et al. Patterns of antihypertensive treatment in patients with acute severe hypertension from a nonneurologic cause: Studying the Treatment of Acute Hypertension (STAT) registry. Pharmacotherapy. 2010;30(11):1087–1096.
- , , , , . Does earlier attainment of blood pressure goal translate into fewer cardiovascular events? Curr Hypertens Rep. 2008;10(5):398–404.
- . Symptomatic hypotension induced by nifedipine in the acute treatment of severe hypertension. Arch Intern Med. 1987;147(3):556–558.
- , , . Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186–2189.
- , , . Nifedipine‐associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185–186.
- , , , , . Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;9(4):339–346.
- , , , . Adverse events associated with aggressive treatment of increased blood pressure. Int J Clin Prac. 2004;58(5):517–519.
- , , , et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens. 2010;12(9):698–705.
- , , , . Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473–477.
Current recommendations for blood pressure (BP) control focus on chronic management of ambulatory patients; however, treatment guidelines for hospitalized patients who have acute increases in BP or simply uncontrolled BP lack clarity regarding appropriate therapeutic options and short‐term treatment goals.[1, 2] For patients with a history of hypertension, management in the hospital setting typically involves continuation of home therapies. In the inpatient setting, uncontrolled hypertension can be categorized as hypertensive emergency, hypertensive urgency, or asymptomatic poor BP control.[3] Asymptomatic BP elevations occur when the BP is not at goal (but not inordinately high) and the patient has no signs of new or worsening end‐organ damage.[4, 5, 6]
Published data have not demonstrated that aggressive treatment of asymptomatic hypertension in the inpatient setting improves short‐ or long‐term outcomes; however, such aggressive treatment may be associated with iatrogenic adverse effects.[5, 7, 8] Despite the lack of evidence of patient benefit, there is a tendency to treat hospitalized patients with asymptomatic BP elevations aggressively by prescribing IV antihypertensive agents on an as‐needed basis.[9] Intravenous hydralazine and labetalol are frequently used, although these agents are not recommended as initial therapy in consensus recommendations for asymptomatic uncontrolled hypertension in either the inpatient or outpatient setting.[10]
We therefore undertook the present study to determine the type and frequency of ordered and administered episodic intravenous (IV) antihypertensive drug therapy, the BP thresholds triggering such administration, and subsequent in‐hospital clinical outcomes after administration of IV antihypertensive drugs. Accordingly, we evaluated a series of hospitalized patients, in noncritical care settings with no evidence of new or worsening target‐organ injury, who were treated with episodic (either as needed or 1 time only) IV antihypertensive therapy.
METHODS
This study is a retrospective review. Between November 1, 2010 and January 31, 2011 we reviewed the charts of all patients who had at least 1 dose of IV hydralazine, enalaprilat, labetalol, or metoprolol ordered, regardless of previous oral antihypertensive treatment or hypertension diagnosis. Other IV antihypertensive agents were not evaluated in this study, as they are only available in critical care units at our institution. This study took place at an 806‐bed urban hospital that utilizes 100% computer prescriber order entry and bar code technology to document medication administration. The institutional review boards of the Detroit Medical Center and Wayne State University, Detroit, Michigan approved this study.
Patient Identification
Patients were identified through a list of all 1‐time‐only and as‐needed orders for IV hydralazine, enalaprilat, labetolol, or metoprolol. The list was generated daily through the hospital electronic medical record system (Cerner Powerchart, North Kansas City, MO). Patients were excluded if they were younger than 18 or older than 89 years of age, admitted to the intensive care or coronary care unit, were receiving nothing by mouth, pregnant, received a renal transplant in the past 3 months, or if there was any clinical manifestation of new or worsening target‐organ injury consistent with the diagnosis of hypertensive emergency.
Data Collection
The following data were collected for all patients: basic demographic information including factors that have been specifically associated with differences in hypertension risk (ie, age, sex, race, weight, and renal function), antihypertensive regimen (if any) prior to admission, changes to oral antihypertensive therapy during admission, order for sodium‐restricted diet, baseline and discharge laboratory values and vital signs. In addition, the details of their antihypertensive therapy order and administration were collected, including prescriber type (attending, resident, or physician extender), service of prescriber, criteria for use, and date and time of drug administration categorized by shift (morning shift, 7 am to 3 pm; afternoon shift, 3 pm to 11 pm; and night shift, 11 pm to 7 am). To analyze the outcomes of administering episodic IV antihypertensive therapy, the following data were collected: changes in average BP within 30 minutes to 6 hours after drug administration and occurrence of antihypertensive therapy‐related adverse events, including any interventions required after administration and adjustments to oral antihypertensive therapy during admission or upon discharge. In cases where BP data were not available (either just prior to or within 6 hours following administration of an IV antihypertensive), the data were not included in the analysis. To determine whether an antihypertensive drug regimen had been intensified, a therapeutic intensity score (TIS) was calculated for the oral antihypertensive regimen on admission and again at discharge. The antihypertensive TIS was calculated by dividing the total daily dose of each antihypertensive medication by the maximum US Food and Drug Administrationapproved daily dose.[11]
Adverse Outcomes Definition
We defined an adverse outcome as a 25% decrease in systolic or diastolic BP within 6 hours and/or intervention to treat symptoms of hypotension. This definition is consistent with Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommendations to assure safety when lowering BP in the setting of hypertensive emergency.[6] Although the patients in this study were not experiencing hypertensive emergency, this definition is supported by reports of negative sequelae from overzealous lowering of BP,[12, 13, 14] and it reflects criteria used in other trials.[10] Hypotension was deemed to have occurred if any of the following were documented: as IV fluid administration; scheduled BP medication held (at either the nurses discretion or per physician order); change in level of care; change in mental status; or transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration. Heart rate changes were also considered to be adverse outcomes, including tachycardia (heart rate >100 beats per minute [bpm] or increase 20 bpm from baseline) or bradycardia (heart rate <50 bpm).
Analysis
Descriptive statistics were performed for all variables. Continuous data were summarized using means and standard deviations. Categorical variables were summarized as counts and percentages. Paired t tests were used to contrast changes from baseline for continuous variables pre‐ and post‐BP, and heart rate changes were evaluated only for the first episode of IV antihypertensive drug administration in patients receiving multiple doses of antihypertensive medication to avoid the bias created by repeated or clustered measures in a given patient. 2 tests were used to test differences in categorical variables. All statistical testing was considered significant when 2‐tailed P values were <0.05. Analyses were generated using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patients
During the study period, there were 6133 inpatient adult admissions. Of 495 patients who had at least 1 order for IV hydralazine, enalaprilat, labetolol, or metoprolol, 246 were included in the analysis after applying the exclusion criteria (Figure 1). Patients were divided into 2 groups. One group had an order for an IV antihypertensive that was not administered (n = 74), and the other had an order for an IV antihypertensive and received at least 1 dose (n = 172). The demographic characteristics of the 2 groups are compared in Table 1. Patients who had their chronic oral antihypertensive regimens intensified after receiving IV antihypertensive medications were more often African American, leaner, more intensively treated, and had higher baseline BP.
| Did Not Receive IV Antihypertensive (n = 74) | Did Receive IV Antihypertensive (n = 172) | P | |
|---|---|---|---|
| |||
| Age, y | 61.6 13.9 | 60.6 13.7 | |
| Male sex | 51% | 47% | |
| African American | 74% | 87% | 0.008 |
| Weight, kg | 94.6 33.2 | 88.5 27.7 | |
| Admit systolic BP | 148 23 | 163 32 | <0.0001 |
| Admit diastolic BP | 82 13 | 87 18 | 0.009 |
| Admit heart rate | 87 18 | 82 20 | 0.069 |
| Admit TIS | 0.84 0.72 | 1.08 0.88 | 0.026 |
| Baseline SCr | 1.78 2.00 | 2.74 3.30 | 0.006 |
| Baseline AST | 26.5 12.5 | 65 126.2 | 0.046 |
| Low‐sodium diet order | 65% | 83% | 0.002 |
| Ordering service | |||
| Cardiology | 14% | 19% | |
| Internal medicine | 49% | 47% | |
| Nephrology | 0% | 6% | |
| Other services | 37% | 28% | |
| Prescriber type | |||
| Resident | 30% | 49% | |
| Physician extender | 53% | 35% | |
| Attending | 17% | 16% | |
| 1‐time‐only order | 5% | 19% | |
| As‐needed order | 95% | 81% | |

Prescribing Patterns
Medical residents prescribed nearly half (49%) of the orders for episodic IV antihypertensives. Attending physicians were responsible for 16% of episodic antihypertensive orders and physician extenders (physician's assistants and nurse practitioners) for 35%. A total of 321 orders were prescribed for the 246 patients in the study. Hydralazine was the preferred antihypertensive agent (80.1%), with IV ‐blockers prescribed less frequently (labetalol 15.6% and metoprolol 4.4%). There were no orders for IV enalaprilat. BP parameters were included in 181 (56%) of the episodic IV antihypertensive orders. Of the IV antihypertensive orders containing criteria, 153 (84.5%) had systolic BP threshold for administration <180 mm Hg (Table 2).
| BP Criteria for Administration of IV Antihypertensive Contained in Order, mm Hg | Did Not Receive IV Antihypertensive, n (%), n = 71* | Did Receive IV Antihypertensive, n (%), n = 133* |
|---|---|---|
| ||
| SBP >120 | 2 (2.8) | 1 (0.7) |
| SBP >130 | 2 (2.8) | 9 (6.8) |
| SBP >140 | 2 (2.8) | 5 (3.8) |
| SBP >150 | 4 (5.6) | 8 (6) |
| SBP >160 | 27 (38) | 58 (43.7) |
| SBP >170 | 26 (36.6) | 29 (21.8) |
| SBP >180 | 8 (11.4) | 18 (13.5) |
| SBP >200 | 4 (3) | |
| DBP >100 | 1 (0.7) | |
Drug Administration and Short‐term Data
Table 2 indicates the BP criteria specified in the episodic IV antihypertensive orders. For the 74 patients who did not receive an episodic IV antihypertensive agent, despite having an order, the nurses caring for the patients determined that their BPs never met the criteria for administration of the IV antihypertensive agent. The remainder of the results apply only to the 172 patients who actually received episodic IV antihypertensive therapy. Two of these patients did not have BP data available and were not included in the short‐term BP analysis. Almost half (48%) of the patients received 1 dose of episodic IV antihypertensive, 26% received 2 doses, and 11% received 3 doses. One patient received 10 doses. Hydralazine significantly lowered BP, whereas metoprolol did not (Figure 2).

The number of IV antihypertensive doses (for which BP data are available) administered during the night shift (n = 75) was numerically higher than the morning (n = 54) and the afternoon (n = 41) shifts. The mean BPs that triggered administration of IV antihypertensives did not differ among shifts (night shift 183/93, morning shift 184/99, afternoon shift 182/97).
Changes to Oral Antihypertensive Regimen After Administration of IV Antihypertensive Drugs
After administration of an episodic IV antihypertensive, the inpatient oral medication regimen was intensified in only 89 patients (52%). The BP reduction from admission to discharge in patients who had their inpatient oral medication regimen adjusted versus those who did not have an inpatient oral regimen adjustment after receiving IV antihypertensive medication is shown in Figure 3. Patients with intensification of their oral medications had a greater reduction in systolic BP from admission to discharge, compared to patients who received episodic IV antihypertensives but had no subsequent change to their inpatient oral antihypertensive regimen (Figure 3).

Adverse Events
Fifty‐six patients (32.6%) demonstrated BP reductions of more than 25% within 6 hours of antihypertensive administration. Of these patients, 2 received IV fluids, and 6 (3.5%) had a scheduled oral BP medication held. Of the patients who received IV hydralazine, 13 (4.4%) had an increase in heart rate >20 bpm, with 7 having a heart rate >100 bpm. One patient who received labetolol experienced bradycardia. No patient required a higher level of care (transfer to an intensive care unit) because of hemodynamic instability. In addition, no patient experienced a change in mental status, transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration.
DISCUSSION
The overwhelming majority of administrations of costly episodic IV antihypertensive drugs among this low‐risk population were in patients with modest BP elevations who may have merited no more, at most, than intensification of their oral antihypertensive drug regimen or observation. Such administration was infrequently followed by intensification of the oral antihypertensive drug regimen, and a significant number of patients experienced a potentially adverse clinical event. Excessive reduction of BP resulting in withholding of oral agents or administering IV fluids (as seen in 8 patients) is clinically relevant, especially in a setting where rapid lowering of BP with IV antihypertensives have no proven clinical benefit. There were differences between patients who did and did not received IV antihypertensive drug therapy, as those receiving therapy were higher‐risk patients. Of the patients initially evaluated for inclusion in this analysis, approximately half had a clear indication for IV antihypertensive therapy and were not included in this analysis. It should also be noted that one‐third of the patients included in the study did not subsequently receive an IV antihypertensive agent.
Recently updated hypertensive guidelines do not address the treatment of hypertensive urgency and emergency, whereas the JNC 7 addressed hypertensive urgency but did not provide a specific BP definition or goals because of concerns about overly aggressive management of severe asymptomatic hypertension.[2, 6] For patients with chronically elevated BP, its rapid reduction, even to levels that remain in the frankly hypertensive range, can be associated with negative clinical sequelae, attributable to decreased target organ perfusion causing clinically manifest ischemia.[3] Accordingly, there have been reports of ischemic events related to unwarranted and overzealous BP lowering.[12, 13, 14] In such patients, resistance vessel remodeling causes a rightward shift of the entire pressure/flow auto regulatory curve in critical arterial beds (eg, cerebral, coronary, and renal). Higher systemic pressure is necessary to maintain adequate perfusion in the target organ, at least over the short‐term. Thus, rapid, aggressive BP reduction can result in the aforementioned negative sequelae because remodeled resistance arterioles are not capable of vasodilating enough to ensure adequate blood flow when systemic pressure falls precipitously.
The patients in this study had no evidence of new or worsening pressure‐related end‐organ damage; therefore, there appeared to be no medical justification for emergent BP lowering via the IV route (a very small minority may have had BP high enough to have justified being diagnosed with hypertensive urgency in which fast‐acting oral therapy would be used). Despite the paucity of data to support this practice, it does, however, appear to be relatively common.[9] The high prevalence of IV hydralazine use in this inpatient study is consistent with the retrospective study reported by Weder and Erickson at the University of Michigan.[9]
Even among those with hypertensive urgencies, oral medication is the preferred route (assuming the patient can eat and swallow without difficulty and does not manifest an altered sensorium). Furthermore, the risks associated with overzealous BP lowering can be devastating. The likelihood of target‐organ ischemia (eg, angina pectoris, myocardial infarction, azotemia, stroke, transient ischemic attack) is most strongly correlated to the rapidity of the BP reduction, even to levels within the hypertensive range, in patients with persistent poor BP control.[4, 15, 16] Thus, the justification for considering a >25% drop in systolic BP within 6 hours of the administration of the IV antihypertensive agent as a potential adverse event, especially because there was only a very small immediate risk for adverse cardiovascular sequelae at the BP levels triggering administration of IV antihypertensive drug therapy.
Although we found that residents and physician assistants prescribed most IV antihypertensives, the practice of prescribing IV antihypertensive therapy appears to be common among all prescriber types. A recent survey assessing the attitudes and practices of resident physicians regarding hypertension in the inpatient setting found that 44% of respondents would treat acute asymptomatic, moderately elevated BP (182/100 mm Hg) with either an oral or intravenous agent.[17]
In addition to there being no proven clinical benefit in this setting, the use of unnecessary IV antihypertensives is associated with unnecessary risks and excess cost. Another report of IV hydralazine in asymptomatic patients found that 17 of 94 patients experienced an adverse effect after administration.[18] Not only is the drug acquisition cost for IV antihypertensives greater than their oral counterparts, often by a factor of 10 to 100, the intravenous route requires additional care to monitor their effects, adding to the human resource expense. Finally, the onset of action of intravenous agents is generally more rapid, which increases the risk of inducing hypotension and therefore target‐organ ischemia.
This study does, however, have limitations. This is a single‐center study, so the findings may not be generalizable to different hospital settings. The findings of this study depend on the accuracy and completeness of the medical record as recorded during routine clinical care; therefore, errors and omissions of data input and documentation may affect the quality of the data. Omissions and errors in the medication history can affect inpatient management as well as appropriateness of discharge medications. BP values before and after administration of an IV antihypertensive were not always available, limiting some of the short‐term outcomes data that were available. The impact of acuity of illness and concomitant disease states of patients were not assessed, which could also affect outcomes. The outcomes measured in this investigation were all short‐term outcomes and did not include important clinical outcomes (long‐term BP control, rehospitalization rates, or patient morbidity or mortality).
We speculate that the practice of episodic IV antihypertensive therapy has developed out of convenience for the practitioner and is likely commonplace across the country.[17] Healthcare systems should examine practices locally and address them as appropriate. To assist in promoting evidence‐based practice that is safe, prudent, and clinically appropriate, we propose that national BP organizations and consensus development groups consider placing priority on developing recommendations for inpatient hypertension treatment algorithms beyond those for hypertensive emergencies. In many cases, adjustments to a patient's oral regimen or observation of the patient are the only interventions that are needed. In addition, appropriate coordination of ambulatory follow‐up care upon discharge is prudent. Finally, individual healthcare systems might need to identify formal programs to modify institutional behavior of both medical and nursing staff to eliminate or limit this practice that is not supported by clinical evidence and potentially places the patient at risk.
CONCLUSIONS
Our study found that the practice of prescribing episodic IV antihypertensive agents at our institution occurred across all prescriber types. Hydralazine was the most frequently ordered agent. The majority of orders containing systolic BP criteria for administration of an episodic IV antihypertensive agent were well below the BP level associated with immediate or near‐immediate cardiovascular risk. Administration of episodic IV antihypertensive agents, without subsequent intensification of the patient's chronic oral antihypertensive regimen was nearly as likely to occur as subsequent intensification of the oral regimen in our study. The absence of evidence‐based guidelines, combined with the results of this evaluation, provide a rationale for implementing hospital‐ and health systembased policies limiting the use of episodic IV antihypertensive agents in asymptomatic patients with uncontrolled BP in noncritical care settings in the absence of new or worsening target‐organ injury.
Disclosure: Nothing to report.
Current recommendations for blood pressure (BP) control focus on chronic management of ambulatory patients; however, treatment guidelines for hospitalized patients who have acute increases in BP or simply uncontrolled BP lack clarity regarding appropriate therapeutic options and short‐term treatment goals.[1, 2] For patients with a history of hypertension, management in the hospital setting typically involves continuation of home therapies. In the inpatient setting, uncontrolled hypertension can be categorized as hypertensive emergency, hypertensive urgency, or asymptomatic poor BP control.[3] Asymptomatic BP elevations occur when the BP is not at goal (but not inordinately high) and the patient has no signs of new or worsening end‐organ damage.[4, 5, 6]
Published data have not demonstrated that aggressive treatment of asymptomatic hypertension in the inpatient setting improves short‐ or long‐term outcomes; however, such aggressive treatment may be associated with iatrogenic adverse effects.[5, 7, 8] Despite the lack of evidence of patient benefit, there is a tendency to treat hospitalized patients with asymptomatic BP elevations aggressively by prescribing IV antihypertensive agents on an as‐needed basis.[9] Intravenous hydralazine and labetalol are frequently used, although these agents are not recommended as initial therapy in consensus recommendations for asymptomatic uncontrolled hypertension in either the inpatient or outpatient setting.[10]
We therefore undertook the present study to determine the type and frequency of ordered and administered episodic intravenous (IV) antihypertensive drug therapy, the BP thresholds triggering such administration, and subsequent in‐hospital clinical outcomes after administration of IV antihypertensive drugs. Accordingly, we evaluated a series of hospitalized patients, in noncritical care settings with no evidence of new or worsening target‐organ injury, who were treated with episodic (either as needed or 1 time only) IV antihypertensive therapy.
METHODS
This study is a retrospective review. Between November 1, 2010 and January 31, 2011 we reviewed the charts of all patients who had at least 1 dose of IV hydralazine, enalaprilat, labetalol, or metoprolol ordered, regardless of previous oral antihypertensive treatment or hypertension diagnosis. Other IV antihypertensive agents were not evaluated in this study, as they are only available in critical care units at our institution. This study took place at an 806‐bed urban hospital that utilizes 100% computer prescriber order entry and bar code technology to document medication administration. The institutional review boards of the Detroit Medical Center and Wayne State University, Detroit, Michigan approved this study.
Patient Identification
Patients were identified through a list of all 1‐time‐only and as‐needed orders for IV hydralazine, enalaprilat, labetolol, or metoprolol. The list was generated daily through the hospital electronic medical record system (Cerner Powerchart, North Kansas City, MO). Patients were excluded if they were younger than 18 or older than 89 years of age, admitted to the intensive care or coronary care unit, were receiving nothing by mouth, pregnant, received a renal transplant in the past 3 months, or if there was any clinical manifestation of new or worsening target‐organ injury consistent with the diagnosis of hypertensive emergency.
Data Collection
The following data were collected for all patients: basic demographic information including factors that have been specifically associated with differences in hypertension risk (ie, age, sex, race, weight, and renal function), antihypertensive regimen (if any) prior to admission, changes to oral antihypertensive therapy during admission, order for sodium‐restricted diet, baseline and discharge laboratory values and vital signs. In addition, the details of their antihypertensive therapy order and administration were collected, including prescriber type (attending, resident, or physician extender), service of prescriber, criteria for use, and date and time of drug administration categorized by shift (morning shift, 7 am to 3 pm; afternoon shift, 3 pm to 11 pm; and night shift, 11 pm to 7 am). To analyze the outcomes of administering episodic IV antihypertensive therapy, the following data were collected: changes in average BP within 30 minutes to 6 hours after drug administration and occurrence of antihypertensive therapy‐related adverse events, including any interventions required after administration and adjustments to oral antihypertensive therapy during admission or upon discharge. In cases where BP data were not available (either just prior to or within 6 hours following administration of an IV antihypertensive), the data were not included in the analysis. To determine whether an antihypertensive drug regimen had been intensified, a therapeutic intensity score (TIS) was calculated for the oral antihypertensive regimen on admission and again at discharge. The antihypertensive TIS was calculated by dividing the total daily dose of each antihypertensive medication by the maximum US Food and Drug Administrationapproved daily dose.[11]
Adverse Outcomes Definition
We defined an adverse outcome as a 25% decrease in systolic or diastolic BP within 6 hours and/or intervention to treat symptoms of hypotension. This definition is consistent with Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommendations to assure safety when lowering BP in the setting of hypertensive emergency.[6] Although the patients in this study were not experiencing hypertensive emergency, this definition is supported by reports of negative sequelae from overzealous lowering of BP,[12, 13, 14] and it reflects criteria used in other trials.[10] Hypotension was deemed to have occurred if any of the following were documented: as IV fluid administration; scheduled BP medication held (at either the nurses discretion or per physician order); change in level of care; change in mental status; or transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration. Heart rate changes were also considered to be adverse outcomes, including tachycardia (heart rate >100 beats per minute [bpm] or increase 20 bpm from baseline) or bradycardia (heart rate <50 bpm).
Analysis
Descriptive statistics were performed for all variables. Continuous data were summarized using means and standard deviations. Categorical variables were summarized as counts and percentages. Paired t tests were used to contrast changes from baseline for continuous variables pre‐ and post‐BP, and heart rate changes were evaluated only for the first episode of IV antihypertensive drug administration in patients receiving multiple doses of antihypertensive medication to avoid the bias created by repeated or clustered measures in a given patient. 2 tests were used to test differences in categorical variables. All statistical testing was considered significant when 2‐tailed P values were <0.05. Analyses were generated using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patients
During the study period, there were 6133 inpatient adult admissions. Of 495 patients who had at least 1 order for IV hydralazine, enalaprilat, labetolol, or metoprolol, 246 were included in the analysis after applying the exclusion criteria (Figure 1). Patients were divided into 2 groups. One group had an order for an IV antihypertensive that was not administered (n = 74), and the other had an order for an IV antihypertensive and received at least 1 dose (n = 172). The demographic characteristics of the 2 groups are compared in Table 1. Patients who had their chronic oral antihypertensive regimens intensified after receiving IV antihypertensive medications were more often African American, leaner, more intensively treated, and had higher baseline BP.
| Did Not Receive IV Antihypertensive (n = 74) | Did Receive IV Antihypertensive (n = 172) | P | |
|---|---|---|---|
| |||
| Age, y | 61.6 13.9 | 60.6 13.7 | |
| Male sex | 51% | 47% | |
| African American | 74% | 87% | 0.008 |
| Weight, kg | 94.6 33.2 | 88.5 27.7 | |
| Admit systolic BP | 148 23 | 163 32 | <0.0001 |
| Admit diastolic BP | 82 13 | 87 18 | 0.009 |
| Admit heart rate | 87 18 | 82 20 | 0.069 |
| Admit TIS | 0.84 0.72 | 1.08 0.88 | 0.026 |
| Baseline SCr | 1.78 2.00 | 2.74 3.30 | 0.006 |
| Baseline AST | 26.5 12.5 | 65 126.2 | 0.046 |
| Low‐sodium diet order | 65% | 83% | 0.002 |
| Ordering service | |||
| Cardiology | 14% | 19% | |
| Internal medicine | 49% | 47% | |
| Nephrology | 0% | 6% | |
| Other services | 37% | 28% | |
| Prescriber type | |||
| Resident | 30% | 49% | |
| Physician extender | 53% | 35% | |
| Attending | 17% | 16% | |
| 1‐time‐only order | 5% | 19% | |
| As‐needed order | 95% | 81% | |

Prescribing Patterns
Medical residents prescribed nearly half (49%) of the orders for episodic IV antihypertensives. Attending physicians were responsible for 16% of episodic antihypertensive orders and physician extenders (physician's assistants and nurse practitioners) for 35%. A total of 321 orders were prescribed for the 246 patients in the study. Hydralazine was the preferred antihypertensive agent (80.1%), with IV ‐blockers prescribed less frequently (labetalol 15.6% and metoprolol 4.4%). There were no orders for IV enalaprilat. BP parameters were included in 181 (56%) of the episodic IV antihypertensive orders. Of the IV antihypertensive orders containing criteria, 153 (84.5%) had systolic BP threshold for administration <180 mm Hg (Table 2).
| BP Criteria for Administration of IV Antihypertensive Contained in Order, mm Hg | Did Not Receive IV Antihypertensive, n (%), n = 71* | Did Receive IV Antihypertensive, n (%), n = 133* |
|---|---|---|
| ||
| SBP >120 | 2 (2.8) | 1 (0.7) |
| SBP >130 | 2 (2.8) | 9 (6.8) |
| SBP >140 | 2 (2.8) | 5 (3.8) |
| SBP >150 | 4 (5.6) | 8 (6) |
| SBP >160 | 27 (38) | 58 (43.7) |
| SBP >170 | 26 (36.6) | 29 (21.8) |
| SBP >180 | 8 (11.4) | 18 (13.5) |
| SBP >200 | 4 (3) | |
| DBP >100 | 1 (0.7) | |
Drug Administration and Short‐term Data
Table 2 indicates the BP criteria specified in the episodic IV antihypertensive orders. For the 74 patients who did not receive an episodic IV antihypertensive agent, despite having an order, the nurses caring for the patients determined that their BPs never met the criteria for administration of the IV antihypertensive agent. The remainder of the results apply only to the 172 patients who actually received episodic IV antihypertensive therapy. Two of these patients did not have BP data available and were not included in the short‐term BP analysis. Almost half (48%) of the patients received 1 dose of episodic IV antihypertensive, 26% received 2 doses, and 11% received 3 doses. One patient received 10 doses. Hydralazine significantly lowered BP, whereas metoprolol did not (Figure 2).

The number of IV antihypertensive doses (for which BP data are available) administered during the night shift (n = 75) was numerically higher than the morning (n = 54) and the afternoon (n = 41) shifts. The mean BPs that triggered administration of IV antihypertensives did not differ among shifts (night shift 183/93, morning shift 184/99, afternoon shift 182/97).
Changes to Oral Antihypertensive Regimen After Administration of IV Antihypertensive Drugs
After administration of an episodic IV antihypertensive, the inpatient oral medication regimen was intensified in only 89 patients (52%). The BP reduction from admission to discharge in patients who had their inpatient oral medication regimen adjusted versus those who did not have an inpatient oral regimen adjustment after receiving IV antihypertensive medication is shown in Figure 3. Patients with intensification of their oral medications had a greater reduction in systolic BP from admission to discharge, compared to patients who received episodic IV antihypertensives but had no subsequent change to their inpatient oral antihypertensive regimen (Figure 3).

Adverse Events
Fifty‐six patients (32.6%) demonstrated BP reductions of more than 25% within 6 hours of antihypertensive administration. Of these patients, 2 received IV fluids, and 6 (3.5%) had a scheduled oral BP medication held. Of the patients who received IV hydralazine, 13 (4.4%) had an increase in heart rate >20 bpm, with 7 having a heart rate >100 bpm. One patient who received labetolol experienced bradycardia. No patient required a higher level of care (transfer to an intensive care unit) because of hemodynamic instability. In addition, no patient experienced a change in mental status, transient ischemic attack, stroke, or chest pain within 30 minutes to 6 hours after administration.
DISCUSSION
The overwhelming majority of administrations of costly episodic IV antihypertensive drugs among this low‐risk population were in patients with modest BP elevations who may have merited no more, at most, than intensification of their oral antihypertensive drug regimen or observation. Such administration was infrequently followed by intensification of the oral antihypertensive drug regimen, and a significant number of patients experienced a potentially adverse clinical event. Excessive reduction of BP resulting in withholding of oral agents or administering IV fluids (as seen in 8 patients) is clinically relevant, especially in a setting where rapid lowering of BP with IV antihypertensives have no proven clinical benefit. There were differences between patients who did and did not received IV antihypertensive drug therapy, as those receiving therapy were higher‐risk patients. Of the patients initially evaluated for inclusion in this analysis, approximately half had a clear indication for IV antihypertensive therapy and were not included in this analysis. It should also be noted that one‐third of the patients included in the study did not subsequently receive an IV antihypertensive agent.
Recently updated hypertensive guidelines do not address the treatment of hypertensive urgency and emergency, whereas the JNC 7 addressed hypertensive urgency but did not provide a specific BP definition or goals because of concerns about overly aggressive management of severe asymptomatic hypertension.[2, 6] For patients with chronically elevated BP, its rapid reduction, even to levels that remain in the frankly hypertensive range, can be associated with negative clinical sequelae, attributable to decreased target organ perfusion causing clinically manifest ischemia.[3] Accordingly, there have been reports of ischemic events related to unwarranted and overzealous BP lowering.[12, 13, 14] In such patients, resistance vessel remodeling causes a rightward shift of the entire pressure/flow auto regulatory curve in critical arterial beds (eg, cerebral, coronary, and renal). Higher systemic pressure is necessary to maintain adequate perfusion in the target organ, at least over the short‐term. Thus, rapid, aggressive BP reduction can result in the aforementioned negative sequelae because remodeled resistance arterioles are not capable of vasodilating enough to ensure adequate blood flow when systemic pressure falls precipitously.
The patients in this study had no evidence of new or worsening pressure‐related end‐organ damage; therefore, there appeared to be no medical justification for emergent BP lowering via the IV route (a very small minority may have had BP high enough to have justified being diagnosed with hypertensive urgency in which fast‐acting oral therapy would be used). Despite the paucity of data to support this practice, it does, however, appear to be relatively common.[9] The high prevalence of IV hydralazine use in this inpatient study is consistent with the retrospective study reported by Weder and Erickson at the University of Michigan.[9]
Even among those with hypertensive urgencies, oral medication is the preferred route (assuming the patient can eat and swallow without difficulty and does not manifest an altered sensorium). Furthermore, the risks associated with overzealous BP lowering can be devastating. The likelihood of target‐organ ischemia (eg, angina pectoris, myocardial infarction, azotemia, stroke, transient ischemic attack) is most strongly correlated to the rapidity of the BP reduction, even to levels within the hypertensive range, in patients with persistent poor BP control.[4, 15, 16] Thus, the justification for considering a >25% drop in systolic BP within 6 hours of the administration of the IV antihypertensive agent as a potential adverse event, especially because there was only a very small immediate risk for adverse cardiovascular sequelae at the BP levels triggering administration of IV antihypertensive drug therapy.
Although we found that residents and physician assistants prescribed most IV antihypertensives, the practice of prescribing IV antihypertensive therapy appears to be common among all prescriber types. A recent survey assessing the attitudes and practices of resident physicians regarding hypertension in the inpatient setting found that 44% of respondents would treat acute asymptomatic, moderately elevated BP (182/100 mm Hg) with either an oral or intravenous agent.[17]
In addition to there being no proven clinical benefit in this setting, the use of unnecessary IV antihypertensives is associated with unnecessary risks and excess cost. Another report of IV hydralazine in asymptomatic patients found that 17 of 94 patients experienced an adverse effect after administration.[18] Not only is the drug acquisition cost for IV antihypertensives greater than their oral counterparts, often by a factor of 10 to 100, the intravenous route requires additional care to monitor their effects, adding to the human resource expense. Finally, the onset of action of intravenous agents is generally more rapid, which increases the risk of inducing hypotension and therefore target‐organ ischemia.
This study does, however, have limitations. This is a single‐center study, so the findings may not be generalizable to different hospital settings. The findings of this study depend on the accuracy and completeness of the medical record as recorded during routine clinical care; therefore, errors and omissions of data input and documentation may affect the quality of the data. Omissions and errors in the medication history can affect inpatient management as well as appropriateness of discharge medications. BP values before and after administration of an IV antihypertensive were not always available, limiting some of the short‐term outcomes data that were available. The impact of acuity of illness and concomitant disease states of patients were not assessed, which could also affect outcomes. The outcomes measured in this investigation were all short‐term outcomes and did not include important clinical outcomes (long‐term BP control, rehospitalization rates, or patient morbidity or mortality).
We speculate that the practice of episodic IV antihypertensive therapy has developed out of convenience for the practitioner and is likely commonplace across the country.[17] Healthcare systems should examine practices locally and address them as appropriate. To assist in promoting evidence‐based practice that is safe, prudent, and clinically appropriate, we propose that national BP organizations and consensus development groups consider placing priority on developing recommendations for inpatient hypertension treatment algorithms beyond those for hypertensive emergencies. In many cases, adjustments to a patient's oral regimen or observation of the patient are the only interventions that are needed. In addition, appropriate coordination of ambulatory follow‐up care upon discharge is prudent. Finally, individual healthcare systems might need to identify formal programs to modify institutional behavior of both medical and nursing staff to eliminate or limit this practice that is not supported by clinical evidence and potentially places the patient at risk.
CONCLUSIONS
Our study found that the practice of prescribing episodic IV antihypertensive agents at our institution occurred across all prescriber types. Hydralazine was the most frequently ordered agent. The majority of orders containing systolic BP criteria for administration of an episodic IV antihypertensive agent were well below the BP level associated with immediate or near‐immediate cardiovascular risk. Administration of episodic IV antihypertensive agents, without subsequent intensification of the patient's chronic oral antihypertensive regimen was nearly as likely to occur as subsequent intensification of the oral regimen in our study. The absence of evidence‐based guidelines, combined with the results of this evaluation, provide a rationale for implementing hospital‐ and health systembased policies limiting the use of episodic IV antihypertensive agents in asymptomatic patients with uncontrolled BP in noncritical care settings in the absence of new or worsening target‐organ injury.
Disclosure: Nothing to report.
- , , , et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115(21):2761–2788.
- , , , et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520.
- , . Hypertensive crises: challenges and management. Chest. 2007;131(6):1949–1962.
- , . Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4):513–529.
- , , , , ; American College of Emergency Physicians Clinical Policies Subcommittee on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med.2006;47(3):237–249.
- , , , et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- , . Best Evidence on management of asymptomatic hypertension in ED patients. J Emerg Nurs. 2011;37(2):174–178.
- , , , et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150–160.
- , . Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29–33.
- , , , et al. Patterns of antihypertensive treatment in patients with acute severe hypertension from a nonneurologic cause: Studying the Treatment of Acute Hypertension (STAT) registry. Pharmacotherapy. 2010;30(11):1087–1096.
- , , , , . Does earlier attainment of blood pressure goal translate into fewer cardiovascular events? Curr Hypertens Rep. 2008;10(5):398–404.
- . Symptomatic hypotension induced by nifedipine in the acute treatment of severe hypertension. Arch Intern Med. 1987;147(3):556–558.
- , , . Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186–2189.
- , , . Nifedipine‐associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185–186.
- , , , , . Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;9(4):339–346.
- , , , . Adverse events associated with aggressive treatment of increased blood pressure. Int J Clin Prac. 2004;58(5):517–519.
- , , , et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens. 2010;12(9):698–705.
- , , , . Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473–477.
- , , , et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115(21):2761–2788.
- , , , et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520.
- , . Hypertensive crises: challenges and management. Chest. 2007;131(6):1949–1962.
- , . Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4):513–529.
- , , , , ; American College of Emergency Physicians Clinical Policies Subcommittee on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med.2006;47(3):237–249.
- , , , et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- , . Best Evidence on management of asymptomatic hypertension in ED patients. J Emerg Nurs. 2011;37(2):174–178.
- , , , et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150–160.
- , . Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29–33.
- , , , et al. Patterns of antihypertensive treatment in patients with acute severe hypertension from a nonneurologic cause: Studying the Treatment of Acute Hypertension (STAT) registry. Pharmacotherapy. 2010;30(11):1087–1096.
- , , , , . Does earlier attainment of blood pressure goal translate into fewer cardiovascular events? Curr Hypertens Rep. 2008;10(5):398–404.
- . Symptomatic hypotension induced by nifedipine in the acute treatment of severe hypertension. Arch Intern Med. 1987;147(3):556–558.
- , , . Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186–2189.
- , , . Nifedipine‐associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185–186.
- , , , , . Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;9(4):339–346.
- , , , . Adverse events associated with aggressive treatment of increased blood pressure. Int J Clin Prac. 2004;58(5):517–519.
- , , , et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens. 2010;12(9):698–705.
- , , , . Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473–477.
© 2015 Society of Hospital Medicine
Appropriateness of Antibiotics for UTIs
After pneumonia, urinary tract infection (UTI) is the second most commonly diagnosed infection leading to hospitalization.[1] However, a large proportion of those admitted with a diagnosis of UTI do not meet diagnostic criteria and receive inappropriate antibiotic therapy.[2, 3] Because antibiotic treatment often begins in the emergency department (ED), we conducted a study to determine the rate of initiation of inappropriate antibiotic treatment for UTIs in the ED and the rate of continuation of inappropriate antibiotics after admission to the hospital.
METHODS
We retrospectively identified all patients admitted from the ED of Johns Hopkins Bayview Medical Center, a tertiary, acute care hospital during 4 nonconsecutive weeks in the winter of 2012 to 2013. We reviewed ED and hospital records of all patients with positive urinalyses who initiated antibiotic treatment in the ED for a diagnosis of UTI. A positive urinalysis was defined as the presence of more than 5 leukocytes per high‐power field, leukocyte esterase, or nitrites. In the ED, approximately two‐thirds of urinalyses were ordered via order sets, and the majority of patients were evaluated by nurse practitioners and physician assistants.
In the absence of specific guidelines for the treatment of UTIs in the ED, criteria for this study were based on the Centers for Disease Control and Prevention (CDC) surveillance definitions,[4] the Infectious Diseases Society of America guidelines for asymptomatic bacteriuria,[5] and the Society for Healthcare Epidemiology of America (SHEA) criteria for diagnosing and treating UTIs in long‐term care facilities.[6, 7] We defined initiation of antibiotic treatment in the ED for a potential UTI as appropriate only if the patient had a positive urinalysis and 1 or more of the following: (1) fever (temperature >38C), (2) a urinary symptom or sign (urgency, frequency, dysuria, suprapubic tenderness, or costovertebral angle pain or tenderness), (3) an indication for treating asymptomatic bacteriuria (pregnancy or a planned invasive urologic procedure), or (4) altered mental status in the presence of a chronic urinary catheter.[6, 7] Continuation of antibiotics was considered inappropriate if 1 or more doses were given after admission to patients who did not meet the above criteria for appropriate initiation of antibiotics (regardless of urine culture results). For patients who met the above criteria, continuation was considered inappropriate if the urine culture grew no organisms or only grew nonpathogenic urogenital flora and the patient received antibiotics for 3 or more days.
Urine culture results were reported as positive if >104 organisms per milliliter grew on semiquantitative culture. The following were considered potential uropathogens: enteric gram‐negative rods (GNRs), nonlactose fermenting GNRs, Corynebacterium urealyticum, yeast, group B streptococci, Enterococcus spp., Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus lugdunensis, and Aerococcus urinae. More than 2 potential uropathogens were reported as mixed fecal flora. The following were considered to be nonpathogenic urogenital flora: coagulase‐negative staphylococci not designated as potential uropathogens, Lactobacillus spp, urease‐negative Corynebacterium, viridans group streptococci, and Gardnerella vaginalis. Specimens with mixed fecal flora and specimens with 1 to 2 uropathogens were grouped together as containing a potential uropathogen. Cultures that grew no organisms or only nonpathogenic urogenital flora were labeled as containing no uropathogen.
RESULTS
Of 1163 patients admitted to the hospital from the ED, 138 began antibiotic therapy for either a presumed UTI (94 patients) or another infection (44) (Figure 1). Non‐UTI infections included pneumonia (23), skin and soft tissue infection (9), intra‐abdominal infection (8), and other (4).
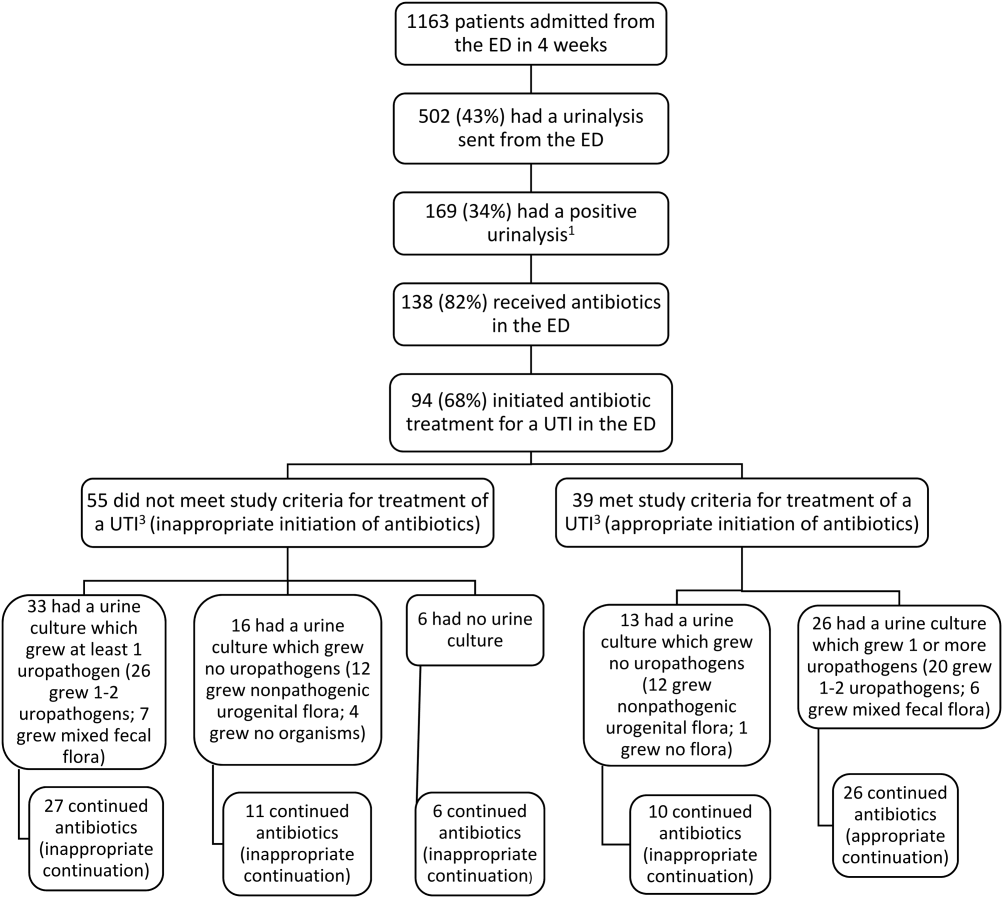
For the 94 patients treated for a UTI in the ED, the mean age was 67 years, and 77% were women. Ten had a chronic urinary catheter, and 13 came from a long‐term care facility. Eighty of these patients continued antibiotics after admission.
According to study criteria, 55 of the 94 patients (59%) who initiated treatment for a UTI in the ED had no indication to do so. These 55 patients had a variety of admitting diagnoses other than UTI (Table 1); 25% were admitted with altered mental status. Forty‐four of these 55 (80%) continued antibiotics (inappropriately) after admission, including 11 patients whose urine cultures grew no uropathogens.
| Admission Diagnosis | No. of Patients With Diagnosis (%) | Mean Age, y | No. of Women (%) | No. of Patients Continuing Antibiotics After Admission (%) |
|---|---|---|---|---|
| ||||
| Altered mental status* | 14 (25) | 76 | 11 (79) | 14 (100) |
| Syncope or near syncope | 7 (13) | 72 | 6 (86) | 5 (71) |
| Other neurologic conditions | 6 (11) | 64 | 5 (83) | 6 (100) |
| Mechanical falls | 6 (11) | 85 | 5 (83) | 4 (67) |
| Gastrointestinal conditions | 8 (15) | 53 | 6 (75) | 3 (38) |
| Psychiatric conditions | 3 (5) | 32 | 4 (100) | 4 (100) |
| Other | 11 (20) | 72 | 9 (79) | 9 (79) |
| All patients | 55 (100) | 69 | 46 (84) | 45 (82) |
Of the 39 patients with an indication to initiate treatment for a possible UTI, 13 had urine cultures (taken before antibiotics were administered) that grew no uropathogens, yet 10 of these patients continued antibiotics inappropriately after admission (Figure 1).
In summary, initiation of antibiotics in the ED was inappropriate for 55 of 94 patients (59% [95% confidence interval {CI}, 48%‐69%]), and continuation after admission was inappropriate for 54 of 80 patients (68% [95% CI, 57%‐78%]).
DISCUSSION
According to study criteria, the majority of patients treated for a UTI in the ED before admission initiated antibiotic treatment inappropriately in the ED and continued antibiotics inappropriately after admission. Our findings suggest several points where intervention could interrupt this chain of events.
Reducing the number of urinalyses ordered in the ED could reduce inappropriate treatment.[8] In this study, 43% of patients admitted from the ED had urinalyses, many obtained via order sets before evaluation by a clinician. Although triage order sets improve ED throughput,[9] they also produce extraneous results that may lead to unnecessary interventions. We suggest removing urinalyses from order sets for conditions for which a UTI is unlikely to contribute.
In this study altered mental status was a common diagnosis among patients categorized as receiving inappropriate antibiotics in the ED. All patients with altered mental status continued antibiotic treatment after admission. According to the study definition (and CDC and SHEA criteria[4, 6, 7]), bacteriuria and altered mental status without additional criteria (urinary symptoms or signs, fever, or an indwelling urinary catheter) are insufficient for the diagnosis of a symptomatic UTI. Since the study was conducted, the CDC surveillance definition for UTIs in long‐term care has been updated and now includes the new onset of confusion in catheterized individuals only if leukocytosis is also present.[10] Because patients at the greatest risk of developing altered mental statusthe frail elderlyalso have high rates of asymptomatic bacteriuria (up to 40%50% in nursing home residents[5]), the 2 conditions co‐occur frequently by chance alone. Although it is common to attribute altered mental status in a patient with pyuria or bacteriuria to a UTI, there are no convincing data to support a causal relationship for patients who are otherwise asymptomatic.[11] An alternative approach for stable patients is careful observation while withholding antibiotics and looking for other causes of altered mental status.[11]
Inappropriate treatment may also stem from misunderstanding the significance of asymptomatic pyuria and bacteriuria, common findings in certain populations. The only evidence‐based indications for treatment of asymptomatic bacteriuria are pregnancy and planned invasive urinary tract procedures.[5] For several other populations, strong randomized trials show no benefit.[5]
Obtaining a good specimen for urinalysis and culture is often problematic. In this study, 37 of 88 cultures (42%) grew mixed fecal or nonpathogenic urogenital flora and appeared to be contaminated. Reporting techniques can be influential.[12] Microbiology reports could state that mixed urogenital flora and mixed fecal flora often represent contamination.
Human factors may also contribute to the inappropriate continuation of antibiotic therapy started in the ED. Hospital providers may not question a diagnosis made by another provider, especially if no alternative diagnosis emerges. Coincidental improvement with antimicrobial treatment may be mistaken as evidence of efficacy. Clinicians may be reluctant to tell a patient or family that the initial diagnosis and treatment plan were incorrect.
This study has several limitations. First, this review was retrospective. Omission of undocumented symptoms could lead to an overestimation of inappropriate antibiotic treatment. Alternatively, several factors could lead to an underestimation: patients treated for a UTI in the ED were identified only among those with positive urinalyses; cultures with mixed fecal flora were accepted as containing a potential uropathogen in spite of the high likelihood of contamination. Also, the study definition of appropriate antibiotic treatment was less stringent than guidelines on which it was based. The generalizability is limited by the single‐center design, and results may not apply to centers with different staffing in their EDs or less utilization of order sets. Finally, the study definition was derived from guidelines that were not developed specifically for use in the ED.
In conclusion, we found a high rate of inappropriate antibiotic administration for UTIs that began in the ED and continued after admission. Overall, providers in the ED should aim not to detect or treat asymptomatic pyuria, and clinicians in the hospital should reevaluate the need for antibiotic treatment started in the ED. Specific guidelines should be developed and validated to direct diagnosis and treatment of UTIs in the ED and hospital.
Disclosures: The information in this article was presented in part at the Society of Hospital Medicine annual meeting on March 2427, 2014. No financial support was provided, and no conflicts of interest exist for any author.
- , , , , , . Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035.
- , . Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clin Infect Dis. 2007;45:1335–1337.
- , , , , . Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol. 2009;30:193–195.
- Centers for Disease Control and Prevention. CDC/NHSN surveillance definition of healthcare‐associated infection and criteria for specific types of infections in the acute care setting. 2013. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed August 2014.
- , , , et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Development of minimum criteria for the initiation of antibiotics in residents of long‐term–care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–124.
- , , , et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.
- , , . Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med. 2015;175(10):1711–1713.
- , , , , . The effect of triage diagnostic standing orders on emergency department treatment time. Ann Emerg Med. 2011;57:89–99.
- Centers for Disease Control and Prevention. Urinary tract infection (UTI) event for long‐term care facilities. Available at: http://www.cdc.gov/nhsn/PDFs/LTC/LTCF‐UTI‐protocol_FINAL_8‐24‐2012.pdf. Accessed September 2015.
- , , , . Bacteriuria in patients who become delirious. Am J Med. 2014;127:255–257.
- , , , et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof‐of‐concept study. Clin Infect Dis. 2014;58:980–983.
After pneumonia, urinary tract infection (UTI) is the second most commonly diagnosed infection leading to hospitalization.[1] However, a large proportion of those admitted with a diagnosis of UTI do not meet diagnostic criteria and receive inappropriate antibiotic therapy.[2, 3] Because antibiotic treatment often begins in the emergency department (ED), we conducted a study to determine the rate of initiation of inappropriate antibiotic treatment for UTIs in the ED and the rate of continuation of inappropriate antibiotics after admission to the hospital.
METHODS
We retrospectively identified all patients admitted from the ED of Johns Hopkins Bayview Medical Center, a tertiary, acute care hospital during 4 nonconsecutive weeks in the winter of 2012 to 2013. We reviewed ED and hospital records of all patients with positive urinalyses who initiated antibiotic treatment in the ED for a diagnosis of UTI. A positive urinalysis was defined as the presence of more than 5 leukocytes per high‐power field, leukocyte esterase, or nitrites. In the ED, approximately two‐thirds of urinalyses were ordered via order sets, and the majority of patients were evaluated by nurse practitioners and physician assistants.
In the absence of specific guidelines for the treatment of UTIs in the ED, criteria for this study were based on the Centers for Disease Control and Prevention (CDC) surveillance definitions,[4] the Infectious Diseases Society of America guidelines for asymptomatic bacteriuria,[5] and the Society for Healthcare Epidemiology of America (SHEA) criteria for diagnosing and treating UTIs in long‐term care facilities.[6, 7] We defined initiation of antibiotic treatment in the ED for a potential UTI as appropriate only if the patient had a positive urinalysis and 1 or more of the following: (1) fever (temperature >38C), (2) a urinary symptom or sign (urgency, frequency, dysuria, suprapubic tenderness, or costovertebral angle pain or tenderness), (3) an indication for treating asymptomatic bacteriuria (pregnancy or a planned invasive urologic procedure), or (4) altered mental status in the presence of a chronic urinary catheter.[6, 7] Continuation of antibiotics was considered inappropriate if 1 or more doses were given after admission to patients who did not meet the above criteria for appropriate initiation of antibiotics (regardless of urine culture results). For patients who met the above criteria, continuation was considered inappropriate if the urine culture grew no organisms or only grew nonpathogenic urogenital flora and the patient received antibiotics for 3 or more days.
Urine culture results were reported as positive if >104 organisms per milliliter grew on semiquantitative culture. The following were considered potential uropathogens: enteric gram‐negative rods (GNRs), nonlactose fermenting GNRs, Corynebacterium urealyticum, yeast, group B streptococci, Enterococcus spp., Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus lugdunensis, and Aerococcus urinae. More than 2 potential uropathogens were reported as mixed fecal flora. The following were considered to be nonpathogenic urogenital flora: coagulase‐negative staphylococci not designated as potential uropathogens, Lactobacillus spp, urease‐negative Corynebacterium, viridans group streptococci, and Gardnerella vaginalis. Specimens with mixed fecal flora and specimens with 1 to 2 uropathogens were grouped together as containing a potential uropathogen. Cultures that grew no organisms or only nonpathogenic urogenital flora were labeled as containing no uropathogen.
RESULTS
Of 1163 patients admitted to the hospital from the ED, 138 began antibiotic therapy for either a presumed UTI (94 patients) or another infection (44) (Figure 1). Non‐UTI infections included pneumonia (23), skin and soft tissue infection (9), intra‐abdominal infection (8), and other (4).

For the 94 patients treated for a UTI in the ED, the mean age was 67 years, and 77% were women. Ten had a chronic urinary catheter, and 13 came from a long‐term care facility. Eighty of these patients continued antibiotics after admission.
According to study criteria, 55 of the 94 patients (59%) who initiated treatment for a UTI in the ED had no indication to do so. These 55 patients had a variety of admitting diagnoses other than UTI (Table 1); 25% were admitted with altered mental status. Forty‐four of these 55 (80%) continued antibiotics (inappropriately) after admission, including 11 patients whose urine cultures grew no uropathogens.
| Admission Diagnosis | No. of Patients With Diagnosis (%) | Mean Age, y | No. of Women (%) | No. of Patients Continuing Antibiotics After Admission (%) |
|---|---|---|---|---|
| ||||
| Altered mental status* | 14 (25) | 76 | 11 (79) | 14 (100) |
| Syncope or near syncope | 7 (13) | 72 | 6 (86) | 5 (71) |
| Other neurologic conditions | 6 (11) | 64 | 5 (83) | 6 (100) |
| Mechanical falls | 6 (11) | 85 | 5 (83) | 4 (67) |
| Gastrointestinal conditions | 8 (15) | 53 | 6 (75) | 3 (38) |
| Psychiatric conditions | 3 (5) | 32 | 4 (100) | 4 (100) |
| Other | 11 (20) | 72 | 9 (79) | 9 (79) |
| All patients | 55 (100) | 69 | 46 (84) | 45 (82) |
Of the 39 patients with an indication to initiate treatment for a possible UTI, 13 had urine cultures (taken before antibiotics were administered) that grew no uropathogens, yet 10 of these patients continued antibiotics inappropriately after admission (Figure 1).
In summary, initiation of antibiotics in the ED was inappropriate for 55 of 94 patients (59% [95% confidence interval {CI}, 48%‐69%]), and continuation after admission was inappropriate for 54 of 80 patients (68% [95% CI, 57%‐78%]).
DISCUSSION
According to study criteria, the majority of patients treated for a UTI in the ED before admission initiated antibiotic treatment inappropriately in the ED and continued antibiotics inappropriately after admission. Our findings suggest several points where intervention could interrupt this chain of events.
Reducing the number of urinalyses ordered in the ED could reduce inappropriate treatment.[8] In this study, 43% of patients admitted from the ED had urinalyses, many obtained via order sets before evaluation by a clinician. Although triage order sets improve ED throughput,[9] they also produce extraneous results that may lead to unnecessary interventions. We suggest removing urinalyses from order sets for conditions for which a UTI is unlikely to contribute.
In this study altered mental status was a common diagnosis among patients categorized as receiving inappropriate antibiotics in the ED. All patients with altered mental status continued antibiotic treatment after admission. According to the study definition (and CDC and SHEA criteria[4, 6, 7]), bacteriuria and altered mental status without additional criteria (urinary symptoms or signs, fever, or an indwelling urinary catheter) are insufficient for the diagnosis of a symptomatic UTI. Since the study was conducted, the CDC surveillance definition for UTIs in long‐term care has been updated and now includes the new onset of confusion in catheterized individuals only if leukocytosis is also present.[10] Because patients at the greatest risk of developing altered mental statusthe frail elderlyalso have high rates of asymptomatic bacteriuria (up to 40%50% in nursing home residents[5]), the 2 conditions co‐occur frequently by chance alone. Although it is common to attribute altered mental status in a patient with pyuria or bacteriuria to a UTI, there are no convincing data to support a causal relationship for patients who are otherwise asymptomatic.[11] An alternative approach for stable patients is careful observation while withholding antibiotics and looking for other causes of altered mental status.[11]
Inappropriate treatment may also stem from misunderstanding the significance of asymptomatic pyuria and bacteriuria, common findings in certain populations. The only evidence‐based indications for treatment of asymptomatic bacteriuria are pregnancy and planned invasive urinary tract procedures.[5] For several other populations, strong randomized trials show no benefit.[5]
Obtaining a good specimen for urinalysis and culture is often problematic. In this study, 37 of 88 cultures (42%) grew mixed fecal or nonpathogenic urogenital flora and appeared to be contaminated. Reporting techniques can be influential.[12] Microbiology reports could state that mixed urogenital flora and mixed fecal flora often represent contamination.
Human factors may also contribute to the inappropriate continuation of antibiotic therapy started in the ED. Hospital providers may not question a diagnosis made by another provider, especially if no alternative diagnosis emerges. Coincidental improvement with antimicrobial treatment may be mistaken as evidence of efficacy. Clinicians may be reluctant to tell a patient or family that the initial diagnosis and treatment plan were incorrect.
This study has several limitations. First, this review was retrospective. Omission of undocumented symptoms could lead to an overestimation of inappropriate antibiotic treatment. Alternatively, several factors could lead to an underestimation: patients treated for a UTI in the ED were identified only among those with positive urinalyses; cultures with mixed fecal flora were accepted as containing a potential uropathogen in spite of the high likelihood of contamination. Also, the study definition of appropriate antibiotic treatment was less stringent than guidelines on which it was based. The generalizability is limited by the single‐center design, and results may not apply to centers with different staffing in their EDs or less utilization of order sets. Finally, the study definition was derived from guidelines that were not developed specifically for use in the ED.
In conclusion, we found a high rate of inappropriate antibiotic administration for UTIs that began in the ED and continued after admission. Overall, providers in the ED should aim not to detect or treat asymptomatic pyuria, and clinicians in the hospital should reevaluate the need for antibiotic treatment started in the ED. Specific guidelines should be developed and validated to direct diagnosis and treatment of UTIs in the ED and hospital.
Disclosures: The information in this article was presented in part at the Society of Hospital Medicine annual meeting on March 2427, 2014. No financial support was provided, and no conflicts of interest exist for any author.
After pneumonia, urinary tract infection (UTI) is the second most commonly diagnosed infection leading to hospitalization.[1] However, a large proportion of those admitted with a diagnosis of UTI do not meet diagnostic criteria and receive inappropriate antibiotic therapy.[2, 3] Because antibiotic treatment often begins in the emergency department (ED), we conducted a study to determine the rate of initiation of inappropriate antibiotic treatment for UTIs in the ED and the rate of continuation of inappropriate antibiotics after admission to the hospital.
METHODS
We retrospectively identified all patients admitted from the ED of Johns Hopkins Bayview Medical Center, a tertiary, acute care hospital during 4 nonconsecutive weeks in the winter of 2012 to 2013. We reviewed ED and hospital records of all patients with positive urinalyses who initiated antibiotic treatment in the ED for a diagnosis of UTI. A positive urinalysis was defined as the presence of more than 5 leukocytes per high‐power field, leukocyte esterase, or nitrites. In the ED, approximately two‐thirds of urinalyses were ordered via order sets, and the majority of patients were evaluated by nurse practitioners and physician assistants.
In the absence of specific guidelines for the treatment of UTIs in the ED, criteria for this study were based on the Centers for Disease Control and Prevention (CDC) surveillance definitions,[4] the Infectious Diseases Society of America guidelines for asymptomatic bacteriuria,[5] and the Society for Healthcare Epidemiology of America (SHEA) criteria for diagnosing and treating UTIs in long‐term care facilities.[6, 7] We defined initiation of antibiotic treatment in the ED for a potential UTI as appropriate only if the patient had a positive urinalysis and 1 or more of the following: (1) fever (temperature >38C), (2) a urinary symptom or sign (urgency, frequency, dysuria, suprapubic tenderness, or costovertebral angle pain or tenderness), (3) an indication for treating asymptomatic bacteriuria (pregnancy or a planned invasive urologic procedure), or (4) altered mental status in the presence of a chronic urinary catheter.[6, 7] Continuation of antibiotics was considered inappropriate if 1 or more doses were given after admission to patients who did not meet the above criteria for appropriate initiation of antibiotics (regardless of urine culture results). For patients who met the above criteria, continuation was considered inappropriate if the urine culture grew no organisms or only grew nonpathogenic urogenital flora and the patient received antibiotics for 3 or more days.
Urine culture results were reported as positive if >104 organisms per milliliter grew on semiquantitative culture. The following were considered potential uropathogens: enteric gram‐negative rods (GNRs), nonlactose fermenting GNRs, Corynebacterium urealyticum, yeast, group B streptococci, Enterococcus spp., Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus lugdunensis, and Aerococcus urinae. More than 2 potential uropathogens were reported as mixed fecal flora. The following were considered to be nonpathogenic urogenital flora: coagulase‐negative staphylococci not designated as potential uropathogens, Lactobacillus spp, urease‐negative Corynebacterium, viridans group streptococci, and Gardnerella vaginalis. Specimens with mixed fecal flora and specimens with 1 to 2 uropathogens were grouped together as containing a potential uropathogen. Cultures that grew no organisms or only nonpathogenic urogenital flora were labeled as containing no uropathogen.
RESULTS
Of 1163 patients admitted to the hospital from the ED, 138 began antibiotic therapy for either a presumed UTI (94 patients) or another infection (44) (Figure 1). Non‐UTI infections included pneumonia (23), skin and soft tissue infection (9), intra‐abdominal infection (8), and other (4).

For the 94 patients treated for a UTI in the ED, the mean age was 67 years, and 77% were women. Ten had a chronic urinary catheter, and 13 came from a long‐term care facility. Eighty of these patients continued antibiotics after admission.
According to study criteria, 55 of the 94 patients (59%) who initiated treatment for a UTI in the ED had no indication to do so. These 55 patients had a variety of admitting diagnoses other than UTI (Table 1); 25% were admitted with altered mental status. Forty‐four of these 55 (80%) continued antibiotics (inappropriately) after admission, including 11 patients whose urine cultures grew no uropathogens.
| Admission Diagnosis | No. of Patients With Diagnosis (%) | Mean Age, y | No. of Women (%) | No. of Patients Continuing Antibiotics After Admission (%) |
|---|---|---|---|---|
| ||||
| Altered mental status* | 14 (25) | 76 | 11 (79) | 14 (100) |
| Syncope or near syncope | 7 (13) | 72 | 6 (86) | 5 (71) |
| Other neurologic conditions | 6 (11) | 64 | 5 (83) | 6 (100) |
| Mechanical falls | 6 (11) | 85 | 5 (83) | 4 (67) |
| Gastrointestinal conditions | 8 (15) | 53 | 6 (75) | 3 (38) |
| Psychiatric conditions | 3 (5) | 32 | 4 (100) | 4 (100) |
| Other | 11 (20) | 72 | 9 (79) | 9 (79) |
| All patients | 55 (100) | 69 | 46 (84) | 45 (82) |
Of the 39 patients with an indication to initiate treatment for a possible UTI, 13 had urine cultures (taken before antibiotics were administered) that grew no uropathogens, yet 10 of these patients continued antibiotics inappropriately after admission (Figure 1).
In summary, initiation of antibiotics in the ED was inappropriate for 55 of 94 patients (59% [95% confidence interval {CI}, 48%‐69%]), and continuation after admission was inappropriate for 54 of 80 patients (68% [95% CI, 57%‐78%]).
DISCUSSION
According to study criteria, the majority of patients treated for a UTI in the ED before admission initiated antibiotic treatment inappropriately in the ED and continued antibiotics inappropriately after admission. Our findings suggest several points where intervention could interrupt this chain of events.
Reducing the number of urinalyses ordered in the ED could reduce inappropriate treatment.[8] In this study, 43% of patients admitted from the ED had urinalyses, many obtained via order sets before evaluation by a clinician. Although triage order sets improve ED throughput,[9] they also produce extraneous results that may lead to unnecessary interventions. We suggest removing urinalyses from order sets for conditions for which a UTI is unlikely to contribute.
In this study altered mental status was a common diagnosis among patients categorized as receiving inappropriate antibiotics in the ED. All patients with altered mental status continued antibiotic treatment after admission. According to the study definition (and CDC and SHEA criteria[4, 6, 7]), bacteriuria and altered mental status without additional criteria (urinary symptoms or signs, fever, or an indwelling urinary catheter) are insufficient for the diagnosis of a symptomatic UTI. Since the study was conducted, the CDC surveillance definition for UTIs in long‐term care has been updated and now includes the new onset of confusion in catheterized individuals only if leukocytosis is also present.[10] Because patients at the greatest risk of developing altered mental statusthe frail elderlyalso have high rates of asymptomatic bacteriuria (up to 40%50% in nursing home residents[5]), the 2 conditions co‐occur frequently by chance alone. Although it is common to attribute altered mental status in a patient with pyuria or bacteriuria to a UTI, there are no convincing data to support a causal relationship for patients who are otherwise asymptomatic.[11] An alternative approach for stable patients is careful observation while withholding antibiotics and looking for other causes of altered mental status.[11]
Inappropriate treatment may also stem from misunderstanding the significance of asymptomatic pyuria and bacteriuria, common findings in certain populations. The only evidence‐based indications for treatment of asymptomatic bacteriuria are pregnancy and planned invasive urinary tract procedures.[5] For several other populations, strong randomized trials show no benefit.[5]
Obtaining a good specimen for urinalysis and culture is often problematic. In this study, 37 of 88 cultures (42%) grew mixed fecal or nonpathogenic urogenital flora and appeared to be contaminated. Reporting techniques can be influential.[12] Microbiology reports could state that mixed urogenital flora and mixed fecal flora often represent contamination.
Human factors may also contribute to the inappropriate continuation of antibiotic therapy started in the ED. Hospital providers may not question a diagnosis made by another provider, especially if no alternative diagnosis emerges. Coincidental improvement with antimicrobial treatment may be mistaken as evidence of efficacy. Clinicians may be reluctant to tell a patient or family that the initial diagnosis and treatment plan were incorrect.
This study has several limitations. First, this review was retrospective. Omission of undocumented symptoms could lead to an overestimation of inappropriate antibiotic treatment. Alternatively, several factors could lead to an underestimation: patients treated for a UTI in the ED were identified only among those with positive urinalyses; cultures with mixed fecal flora were accepted as containing a potential uropathogen in spite of the high likelihood of contamination. Also, the study definition of appropriate antibiotic treatment was less stringent than guidelines on which it was based. The generalizability is limited by the single‐center design, and results may not apply to centers with different staffing in their EDs or less utilization of order sets. Finally, the study definition was derived from guidelines that were not developed specifically for use in the ED.
In conclusion, we found a high rate of inappropriate antibiotic administration for UTIs that began in the ED and continued after admission. Overall, providers in the ED should aim not to detect or treat asymptomatic pyuria, and clinicians in the hospital should reevaluate the need for antibiotic treatment started in the ED. Specific guidelines should be developed and validated to direct diagnosis and treatment of UTIs in the ED and hospital.
Disclosures: The information in this article was presented in part at the Society of Hospital Medicine annual meeting on March 2427, 2014. No financial support was provided, and no conflicts of interest exist for any author.
- , , , , , . Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035.
- , . Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clin Infect Dis. 2007;45:1335–1337.
- , , , , . Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol. 2009;30:193–195.
- Centers for Disease Control and Prevention. CDC/NHSN surveillance definition of healthcare‐associated infection and criteria for specific types of infections in the acute care setting. 2013. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed August 2014.
- , , , et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Development of minimum criteria for the initiation of antibiotics in residents of long‐term–care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–124.
- , , , et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.
- , , . Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med. 2015;175(10):1711–1713.
- , , , , . The effect of triage diagnostic standing orders on emergency department treatment time. Ann Emerg Med. 2011;57:89–99.
- Centers for Disease Control and Prevention. Urinary tract infection (UTI) event for long‐term care facilities. Available at: http://www.cdc.gov/nhsn/PDFs/LTC/LTCF‐UTI‐protocol_FINAL_8‐24‐2012.pdf. Accessed September 2015.
- , , , . Bacteriuria in patients who become delirious. Am J Med. 2014;127:255–257.
- , , , et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof‐of‐concept study. Clin Infect Dis. 2014;58:980–983.
- , , , , , . Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035.
- , . Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clin Infect Dis. 2007;45:1335–1337.
- , , , , . Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol. 2009;30:193–195.
- Centers for Disease Control and Prevention. CDC/NHSN surveillance definition of healthcare‐associated infection and criteria for specific types of infections in the acute care setting. 2013. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed August 2014.
- , , , et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Development of minimum criteria for the initiation of antibiotics in residents of long‐term–care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–124.
- , , , et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.
- , , . Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med. 2015;175(10):1711–1713.
- , , , , . The effect of triage diagnostic standing orders on emergency department treatment time. Ann Emerg Med. 2011;57:89–99.
- Centers for Disease Control and Prevention. Urinary tract infection (UTI) event for long‐term care facilities. Available at: http://www.cdc.gov/nhsn/PDFs/LTC/LTCF‐UTI‐protocol_FINAL_8‐24‐2012.pdf. Accessed September 2015.
- , , , . Bacteriuria in patients who become delirious. Am J Med. 2014;127:255–257.
- , , , et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof‐of‐concept study. Clin Infect Dis. 2014;58:980–983.
© 2015 Society of Hospital Medicine
Ordering Patterns in Shift‐Based Care
Duty‐hour restrictions were implemented by the Accreditation Council for Graduate Medical Education (ACGME) in 2003 in response to data showing that sleep deprivation was correlated with serious medical errors.[1] In 2011, the ACGME required more explicit restrictions in the number of hours worked and the maximal shift length.[2] These requirements have necessitated a transition from a traditional q4 call model for interns to one in which shifts are limited to a maximum of 16 hours.
Studies of interns working these shorter shifts have had varied results, and comprehensive reviews have failed to demonstrate consistent improvements.[3, 4, 5] Studies of shift‐length limitation initially suggested improvements in patient safety (decreased length of stay,[6, 7] cost of hospitalization,[6] medication errors,[7] serious medical errors,[8] and intensive care unit [ICU] admissions[9]) and resident quality of life.[10] However, other recent studies have reported an increased number of self‐reported medical errors[11] and either did not detect change[12] or reported perceived decreases[13] in quality of care and continuity of care.
We previously reported decreased length of stay and decreased cost of hospitalization in pediatric inpatients cared for in a day/night‐shiftbased care model.[6] An hypothesized reason for those care improvements is the restructured care model led to increased active clinical management during both day and night hours. Here we report the findings of a retrospective analysis to investigate this hypothesis.
PATIENTS AND METHODS
Study Population
We reviewed the charts of pediatric patients admitted to University of California, San Francisco Benioff Children's Hospital, a 175‐bed tertiary care facility, over a 2‐year period between September 15, 2007 and September 15, 2008 (preintervention) and September 16, 2008 and September 16, 2009 (postintervention). During this study period, our hospital was still dependent on paper orders. Admission order sets were preprinted paper forms that were unchanged for the study period. Using International Classification of Diseases, 9th Revision coding, we identified patients on the general pediatrics service with 1 of 6 common diagnosesdehydration, community‐acquired pneumonia, aspiration pneumonia, upper respiratory infection, asthma, and bronchiolitis. These diagnoses were chosen because it was hypothesized that their length of inpatient stay could be impacted by active clinical management. We excluded patients admitted to the ICU or transferred between services.
A list of medical record numbers (MRNs) corresponding to admissions for 1 of the 6 above diagnoses during the pre‐ and postintervention periods was compiled. MRNs were randomized and then sequentially reviewed until 50 admissions in each time period were obtained. After data collection was completed, we noted that 2 patients had been in the ICU for part of their hospitalization, and these were excluded, leaving 48 admissions from prior to the intervention and 50 admissions from after intervention who were examined.
Intervention
During the preintervention period, patients were cared for by interns who took call every sixth night (duty periods up to 30 hours), with cross‐coverage of patients on multiple teams. Cross‐coverage was defined as coverage of patients cared for during nonconsecutive shifts and for whom residents did not participate in attending rounds. Noncall shifts were typically 10 to 11 hours. They were supervised by senior residents who took call every fourth or fifth night and who provided similar cross‐coverage.
During the postintervention period, interns worked day and night shifts of 13 hours (1 hour overlap time between shifts for handoffs), with increased night staffing to eliminate intern‐level cross‐coverage of multiple teams and maintain interns as the primary providers. Interns covered the same team for 5 to 7 consecutive days on either the day or night shifts. Interns remained on the same teams when they switched from day shifts to night shifts to preserve continuity. There were some 24‐hour shifts for senior residents on weekends. Senior residents maintained supervisory responsibility for all patients (both hospitalist teams and a subspecialty team). They also worked 7 consecutive nights.
There were changes in the staffing ratios associated with the change to day and night teams (Table 1, Figure 1). In the preintervention period, general pediatrics patients were covered by a single hospitalist and cohorted on a single team (team A), which also covered several groups of subspecialty patients with subspecialty attendings. The team consisted of 2 interns and 1 senior resident, who shared extended (30‐hour) call in a cycle with 2 other inpatient teams. In the postintervention period, general pediatrics patients were split between 2 teams (teams D and E) and mixed with subspecialty patients. Hospitalist continued to be the attendings, and these hospitalists also covered specialty patients with subspecialists in consulting roles. The teams consisted of 3 interns on the day shift, and 1 on the night shift. There was 1 senior resident per team on day shift, and a single senior resident covering all teams at night.
| Preintervention | Postintervention | |||||
|---|---|---|---|---|---|---|
| ||||||
| General Pediatrics | Team A | Team B | Team C | Team D | Team E | Team F |
| Patient Distribution | General Pediatrics | GI/Liver | Renal | General Pediatrics | General Pediatrics | Liver |
| Pulmonary | Neurology | Rheumatology | Mixed Specialty | Mixed Specialty | Renal | |
| Adolescent | Endocrine | |||||
| Team membersa | 2 interns (q6 call) | 4 interns (3 on day shift/1 on night shift) | ||||
| 1 senior resident (q5 call) | 1 senior resident | |||||
| Night‐shift coveragea | 1 intern and 1 senior resident together covered all 3 teams. | 1 night intern per team (teams D/E) working 7 consecutive night shifts | ||||
| 1 supervising night resident covering all 3 teams | ||||||
| Intern cross‐coverage of other teams | Nights/clinic afternoons | None | ||||
| Length of night shift | 30 hours | 13 hours | ||||
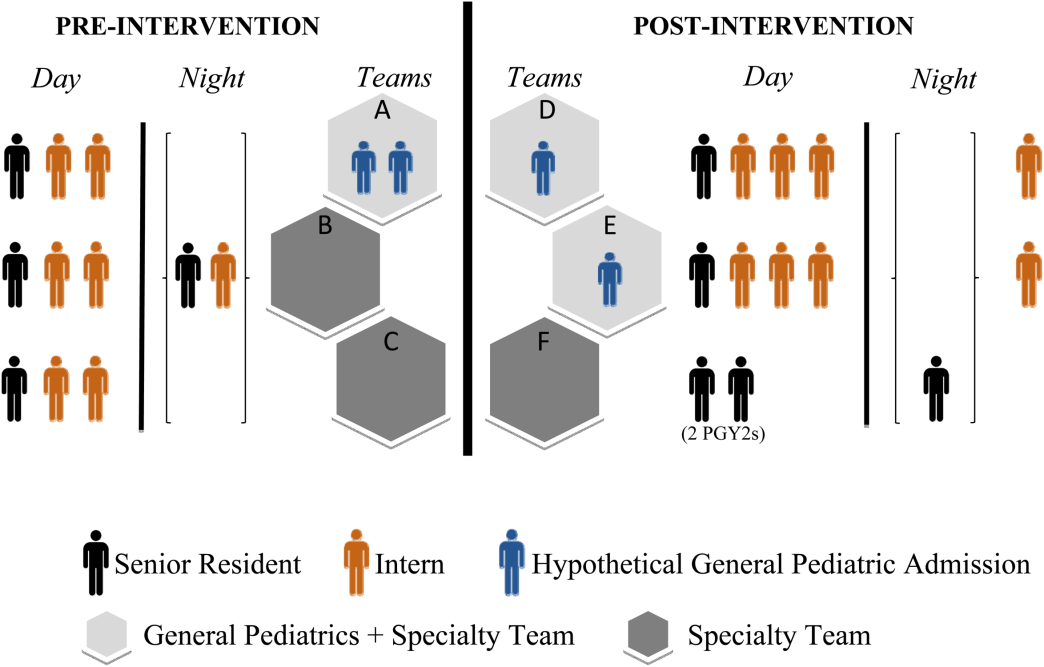
There was no change in the paper‐order system, the electronic health record, timing of the morning blood draw, use of new facilities for patient care, or protocol for emergency department admission. Concomitant with the restructuring, most subspecialty patients were consolidated onto the hospitalist service, necessitating creation of a second hospitalist team. However, patients admitted with the diagnoses identified above would have been on the hospitalist service before and after the restructuring.
Data Collection/Analysis
We reviewed specific classes of orders and categorized by type: respiratory medication, oxygen, intravenous (IV) fluids, diet, monitoring, and activity, time of day (day vs night‐shift), and whether they were an escalation or de‐escalation of care. De‐escalation of care was defined as orders that decreased patient care such as weaning a patient off nebulized albuterol or decreasing their IV fluids. Orders between 07:00 to 18:00 were considered day‐shift orders and between 18:01 and 06:59 were classified as night‐shift orders. Only orders falling into 1 of the aforementioned categories were recorded. Admission order sets were not included. Initially, charts were reviewed by both investigators together; after comparing results for 10 charts to ensure consistency of methodology and criteria, the remaining charts were reviewed by 1 of the study investigators.
To compare demographics, diagnoses, and ordering patterns, t tests and 2 (SAS version 9.2 [SAS Institute, Cary, NC], Stata version 13.1 [StataCorp, College Station, TX]) were used. Multivariate gamma models (SAS version 9.2 [SAS Institute]) that adjusted for clustering at the attending level and patient age were used to compare severity of illness before and after the intervention. This study was approved by the University of California, San Francisco Committee on Human Research.
RESULTS
We analyzed data for 48 admissions preintervention and 50 postintervention. With the exception of insurance type, there was no difference in baseline demographics, diagnoses, or severity of illness between the groups (Table 2). Within the order classes above, we identified 212 orders preintervention and 231 orders postintervention.
| Preintervention,n = 48, N (%) | Postintervention, n = 50, N (%) | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 4.8 (4.6) | 5.5 (4.7) | 0.4474 |
| Race/ethnicity | 0.1953 | ||
| NH white | 12 (25.0%) | 9 (18.0%) | |
| NH black | 11 (22.9%) | 7 (14.0%) | |
| Hispanic | 16 (33.3%) | 13 (26.0%) | |
| Asian | 6 (12.5%) | 10 (20.0%) | |
| Other | 3 (6.3%) | 10 (20.0%) | |
| Missing | 0 | 1 (2.0%) | |
| Gender | 0.6577 | ||
| Female | 19 (39.6%) | 22 (44.0%) | |
| Male | 29 (60.4%) | 28 (56.0%) | |
| Primary language | 0.2601 | ||
| English | 38 (79.2%) | 45 (90.0%) | |
| Spanish | 9 (18.8%) | 5 (10.0%) | |
| Other | 1 (2.1%) | 0 | |
| Insurance | 0.0118 | ||
| Private | 13 (27.1%) | 26 (52.0%) | |
| Medical | 35 (72.9%) | 24 (48.0%) | |
| Other | 0 | 0 | |
| Admit source | 0.6581 | ||
| Referral | 20 (41.7%) | 18 (36.0%) | |
| ED | 26 (54.2%) | 31 (62.0%) | |
| Transfer | 2 (4.2%) | 1 (2.0%) | |
| Severity of illness | 0.1926 | ||
| Minor | 15 (31.3%) | 24 (48.0%) | |
| Moderate | 23 (47.9%) | 16 (32.0%) | |
| Severe | 10 (20.8%) | 10 (20.0%) | |
| Extreme | 0 | 0 | |
| Diagnoses | 0.562 | ||
| Asthma | 21 | 19 | |
| Bronchiolitis | 2 | 4 | |
| Pneumonia | 17 | 19 | |
| Dehydration | 6 | 7 | |
| URI | 0 | 1 | |
| Aspiration pneumonia | 2 | 0 | |
After the intervention, there was a statistically significant increase in the average number of orders written within the first 12 hours (pre: 0.58 orders vs post: 1.12, P = 0.009) and 24 hours (pre: 1.52 vs post: 2.38, P = 0.004) following admission (Table 3), not including the admission order set. The fraction of orders written at night was not significantly different (27% at night preintervention, 33% postintervention, P = 0.149). The fraction of admissions on the day shift compared to the night shift did not change (P = 0.72). There was no difference in the ratio of de‐escalation to escalation orders written during the night (Table 2).
| Preintervention, 48 Admissions | Postintervention, 50 Admissions | P Value | |
|---|---|---|---|
| |||
| Total no. of orders | 212 | 231 | |
| Mean no. of orders per admission | 4.42 | 4.62 | |
| Day shift orders, n (%) | 155 (73) | 155 (67) | 0.149 |
| Night shift orders, n (%) | 57 (27) | 76 (33) | |
| Mean no. of orders within first 12 hours* | 0.58 | 1.12 | 0.009 |
| Mean no. of orders within first 24 hours* | 1.52 | 2.38 | 0.004 |
| Night shift escalation orders (%) | 27 (47) | 33 (43) | 0.491 |
| Night shift de‐escalation orders (%) | 30 (53) | 43 (57) | |
DISCUSSION
In this study, we demonstrate increased patient care management early in the hospitalization, measured in this study by the mean number of orders written per patient in the first 12 and 24 hours after admission, after transition from a call schedule with extended (>16 hours) shifts to one with shorter shifts compliant with current ACGME duty‐hour restrictions and an explicit focus on greater continuity of care. We did not detect a change in the proportion of total orders written on the night shift compared to the day shift. Earlier active medical management, such as weaning nebulized albuterol or supplemental oxygen, can speed the time to discharge.[14]
Our failure to detect a significant change in the proportion or type of orders written at night may have been due to our small sample size. Anecdotally, after the intervention, medical students reported to us that they noticed a difference between our service, in which we expect night teams to advance care, and other services at our institution, in which nights are a time to focus on putting out fires. This was not something that had been reported to us prior. It is likely reflective of the overall approach to patient care taken by residents working a night shift as part of a longitudinal care team.
This study builds on previous findings that demonstrated lower costs and shorter length of stay after implementing a schedule based on day and night teams.[7] The reasons for such improvements are likely multifactorial. In our model, which was purposefully designed to create night‐team continuity and minimize cross‐coverage, it is likely that residents also felt a greater sense of responsibility for and familiarity with the patients[15] and therefore felt more comfortable advancing care. Not only were interns likely better rested, the patient‐to‐provider ratio was also lower than in the preintervention model. Increases in staffing are often necessary to eliminate cross‐coverage while maintaining safe, 24‐hour care. These findings suggest that increases in cost from additional staffing may be at least partially offset by more active patient management early in the hospitalization, which has the potential to lead to shorter hospital stays.
There are several limitations to our research. We studied a small sample, including a subset of general pediatrics diagnoses that are amenable to active management, limiting generalizability. We did not calculate a physician‐to‐patient ratio because this was not possible with the retrospective data we collected. Staffing ratios likely improved, and we consider that part of the overall improvements in staffing that may have contributed to the observed changes in ordering patterns. Although intern‐level cross‐coverage was eliminated, the senior resident continued to cover multiple teams overnight. This senior covered the same 3 teams for 7 consecutive nights. The addition of a hospitalist team, with subspecialists being placed in consultant roles, may have contributed to the increase in active management, though our study population did not include subspecialty patients. There was a difference in insurance status between the 2 groups. This was unlikely to affect resident physician practices as insurance information is not routinely discussed in the course of patient care. In the context of the ongoing debate about duty‐hour restrictions, it will be important for future studies to elucidate whether sleep or other variables are the primary contributors to this finding. Our data are derived solely from 1 inpatient service at a single academic medical center; however, we do feel there are lessons that may be applied to other settings.
CONCLUSION
A coverage system with improved nighttime resident coverage was associated with a greater number of orders written early in the hospitalization, suggesting more active management of clinical problems to advance care.
Acknowledgements
The authors thank Dr. I. Elaine Allen, John Kornak, and Dr. Derek Pappas for assistance with biostatistics, and Dr. Diana Bojorquez and Dr. Derek Pappas for assistance with review of the manuscript and creation of the figures.
Disclosures: None of the authors have financial relationships or other conflicts of interest to disclose. No external funding was secured for this study. Dr. Auerbach was supported by grant K24HL098372 during the course of this study. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through University of California San FranciscoClinical and Translational Sciences Institute grant UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. Rosenbluth had access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- , , . New requirements for resident duty hours. JAMA. 2002;288(9):1112–1114.
- Accreditation Council for Graduate Medical Education. Common program requirements. 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed November 28, 2011.
- , , . Patient safety, resident education and resident well‐being following implementation of the 2003 ACGME duty hour rules. J Gen Intern Med. 2011;26(8):907–919.
- , , , et al. A systematic review of the effects of resident duty hour restrictions in surgery: impact on resident wellness, training, and patient outcomes. Ann Surg. 2014;259(6):1041–1053.
- , , , . Duty‐hour limits and patient care and resident outcomes: can high‐quality studies offer insight into complex relationships? Annu Rev Med. 2013;64:467–483.
- , , , , , . Association between adaptations to ACGME duty hour requirements, length of stay, and costs. Sleep. 2013;36(2):245–248.
- , , , . Effect of a change in house staff work schedule on resource utilization and patient care. Arch Intern Med. 1991;151(10):2065–2070.
- , , , et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848.
- , , , . Changes in outcomes for internal medicine inpatients after work‐hour regulations. Ann Intern Med. 2007;147(2):97–103.
- , , . Effects of reducing or eliminating resident work shifts over 16 hours: a systematic review. Sleep. 2010;33(8):1043–1053.
- , , , et al. Effects of the 2011 duty hour reforms on interns and their patients: a prospective longitudinal cohort study. JAMA Intern Med. 2013;173(8):657–662; discussion 663.
- , , , , . Effect of 16‐hour duty periods on patient care and resident education. Mayo Clin Proc. 2011;86(3):192–196.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
- , , , . Resident perceptions of autonomy in a complex tertiary care environment improve when supervised by hospitalists. Hosp Pediatr. 2012;2(4):228–234.
Duty‐hour restrictions were implemented by the Accreditation Council for Graduate Medical Education (ACGME) in 2003 in response to data showing that sleep deprivation was correlated with serious medical errors.[1] In 2011, the ACGME required more explicit restrictions in the number of hours worked and the maximal shift length.[2] These requirements have necessitated a transition from a traditional q4 call model for interns to one in which shifts are limited to a maximum of 16 hours.
Studies of interns working these shorter shifts have had varied results, and comprehensive reviews have failed to demonstrate consistent improvements.[3, 4, 5] Studies of shift‐length limitation initially suggested improvements in patient safety (decreased length of stay,[6, 7] cost of hospitalization,[6] medication errors,[7] serious medical errors,[8] and intensive care unit [ICU] admissions[9]) and resident quality of life.[10] However, other recent studies have reported an increased number of self‐reported medical errors[11] and either did not detect change[12] or reported perceived decreases[13] in quality of care and continuity of care.
We previously reported decreased length of stay and decreased cost of hospitalization in pediatric inpatients cared for in a day/night‐shiftbased care model.[6] An hypothesized reason for those care improvements is the restructured care model led to increased active clinical management during both day and night hours. Here we report the findings of a retrospective analysis to investigate this hypothesis.
PATIENTS AND METHODS
Study Population
We reviewed the charts of pediatric patients admitted to University of California, San Francisco Benioff Children's Hospital, a 175‐bed tertiary care facility, over a 2‐year period between September 15, 2007 and September 15, 2008 (preintervention) and September 16, 2008 and September 16, 2009 (postintervention). During this study period, our hospital was still dependent on paper orders. Admission order sets were preprinted paper forms that were unchanged for the study period. Using International Classification of Diseases, 9th Revision coding, we identified patients on the general pediatrics service with 1 of 6 common diagnosesdehydration, community‐acquired pneumonia, aspiration pneumonia, upper respiratory infection, asthma, and bronchiolitis. These diagnoses were chosen because it was hypothesized that their length of inpatient stay could be impacted by active clinical management. We excluded patients admitted to the ICU or transferred between services.
A list of medical record numbers (MRNs) corresponding to admissions for 1 of the 6 above diagnoses during the pre‐ and postintervention periods was compiled. MRNs were randomized and then sequentially reviewed until 50 admissions in each time period were obtained. After data collection was completed, we noted that 2 patients had been in the ICU for part of their hospitalization, and these were excluded, leaving 48 admissions from prior to the intervention and 50 admissions from after intervention who were examined.
Intervention
During the preintervention period, patients were cared for by interns who took call every sixth night (duty periods up to 30 hours), with cross‐coverage of patients on multiple teams. Cross‐coverage was defined as coverage of patients cared for during nonconsecutive shifts and for whom residents did not participate in attending rounds. Noncall shifts were typically 10 to 11 hours. They were supervised by senior residents who took call every fourth or fifth night and who provided similar cross‐coverage.
During the postintervention period, interns worked day and night shifts of 13 hours (1 hour overlap time between shifts for handoffs), with increased night staffing to eliminate intern‐level cross‐coverage of multiple teams and maintain interns as the primary providers. Interns covered the same team for 5 to 7 consecutive days on either the day or night shifts. Interns remained on the same teams when they switched from day shifts to night shifts to preserve continuity. There were some 24‐hour shifts for senior residents on weekends. Senior residents maintained supervisory responsibility for all patients (both hospitalist teams and a subspecialty team). They also worked 7 consecutive nights.
There were changes in the staffing ratios associated with the change to day and night teams (Table 1, Figure 1). In the preintervention period, general pediatrics patients were covered by a single hospitalist and cohorted on a single team (team A), which also covered several groups of subspecialty patients with subspecialty attendings. The team consisted of 2 interns and 1 senior resident, who shared extended (30‐hour) call in a cycle with 2 other inpatient teams. In the postintervention period, general pediatrics patients were split between 2 teams (teams D and E) and mixed with subspecialty patients. Hospitalist continued to be the attendings, and these hospitalists also covered specialty patients with subspecialists in consulting roles. The teams consisted of 3 interns on the day shift, and 1 on the night shift. There was 1 senior resident per team on day shift, and a single senior resident covering all teams at night.
| Preintervention | Postintervention | |||||
|---|---|---|---|---|---|---|
| ||||||
| General Pediatrics | Team A | Team B | Team C | Team D | Team E | Team F |
| Patient Distribution | General Pediatrics | GI/Liver | Renal | General Pediatrics | General Pediatrics | Liver |
| Pulmonary | Neurology | Rheumatology | Mixed Specialty | Mixed Specialty | Renal | |
| Adolescent | Endocrine | |||||
| Team membersa | 2 interns (q6 call) | 4 interns (3 on day shift/1 on night shift) | ||||
| 1 senior resident (q5 call) | 1 senior resident | |||||
| Night‐shift coveragea | 1 intern and 1 senior resident together covered all 3 teams. | 1 night intern per team (teams D/E) working 7 consecutive night shifts | ||||
| 1 supervising night resident covering all 3 teams | ||||||
| Intern cross‐coverage of other teams | Nights/clinic afternoons | None | ||||
| Length of night shift | 30 hours | 13 hours | ||||

There was no change in the paper‐order system, the electronic health record, timing of the morning blood draw, use of new facilities for patient care, or protocol for emergency department admission. Concomitant with the restructuring, most subspecialty patients were consolidated onto the hospitalist service, necessitating creation of a second hospitalist team. However, patients admitted with the diagnoses identified above would have been on the hospitalist service before and after the restructuring.
Data Collection/Analysis
We reviewed specific classes of orders and categorized by type: respiratory medication, oxygen, intravenous (IV) fluids, diet, monitoring, and activity, time of day (day vs night‐shift), and whether they were an escalation or de‐escalation of care. De‐escalation of care was defined as orders that decreased patient care such as weaning a patient off nebulized albuterol or decreasing their IV fluids. Orders between 07:00 to 18:00 were considered day‐shift orders and between 18:01 and 06:59 were classified as night‐shift orders. Only orders falling into 1 of the aforementioned categories were recorded. Admission order sets were not included. Initially, charts were reviewed by both investigators together; after comparing results for 10 charts to ensure consistency of methodology and criteria, the remaining charts were reviewed by 1 of the study investigators.
To compare demographics, diagnoses, and ordering patterns, t tests and 2 (SAS version 9.2 [SAS Institute, Cary, NC], Stata version 13.1 [StataCorp, College Station, TX]) were used. Multivariate gamma models (SAS version 9.2 [SAS Institute]) that adjusted for clustering at the attending level and patient age were used to compare severity of illness before and after the intervention. This study was approved by the University of California, San Francisco Committee on Human Research.
RESULTS
We analyzed data for 48 admissions preintervention and 50 postintervention. With the exception of insurance type, there was no difference in baseline demographics, diagnoses, or severity of illness between the groups (Table 2). Within the order classes above, we identified 212 orders preintervention and 231 orders postintervention.
| Preintervention,n = 48, N (%) | Postintervention, n = 50, N (%) | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 4.8 (4.6) | 5.5 (4.7) | 0.4474 |
| Race/ethnicity | 0.1953 | ||
| NH white | 12 (25.0%) | 9 (18.0%) | |
| NH black | 11 (22.9%) | 7 (14.0%) | |
| Hispanic | 16 (33.3%) | 13 (26.0%) | |
| Asian | 6 (12.5%) | 10 (20.0%) | |
| Other | 3 (6.3%) | 10 (20.0%) | |
| Missing | 0 | 1 (2.0%) | |
| Gender | 0.6577 | ||
| Female | 19 (39.6%) | 22 (44.0%) | |
| Male | 29 (60.4%) | 28 (56.0%) | |
| Primary language | 0.2601 | ||
| English | 38 (79.2%) | 45 (90.0%) | |
| Spanish | 9 (18.8%) | 5 (10.0%) | |
| Other | 1 (2.1%) | 0 | |
| Insurance | 0.0118 | ||
| Private | 13 (27.1%) | 26 (52.0%) | |
| Medical | 35 (72.9%) | 24 (48.0%) | |
| Other | 0 | 0 | |
| Admit source | 0.6581 | ||
| Referral | 20 (41.7%) | 18 (36.0%) | |
| ED | 26 (54.2%) | 31 (62.0%) | |
| Transfer | 2 (4.2%) | 1 (2.0%) | |
| Severity of illness | 0.1926 | ||
| Minor | 15 (31.3%) | 24 (48.0%) | |
| Moderate | 23 (47.9%) | 16 (32.0%) | |
| Severe | 10 (20.8%) | 10 (20.0%) | |
| Extreme | 0 | 0 | |
| Diagnoses | 0.562 | ||
| Asthma | 21 | 19 | |
| Bronchiolitis | 2 | 4 | |
| Pneumonia | 17 | 19 | |
| Dehydration | 6 | 7 | |
| URI | 0 | 1 | |
| Aspiration pneumonia | 2 | 0 | |
After the intervention, there was a statistically significant increase in the average number of orders written within the first 12 hours (pre: 0.58 orders vs post: 1.12, P = 0.009) and 24 hours (pre: 1.52 vs post: 2.38, P = 0.004) following admission (Table 3), not including the admission order set. The fraction of orders written at night was not significantly different (27% at night preintervention, 33% postintervention, P = 0.149). The fraction of admissions on the day shift compared to the night shift did not change (P = 0.72). There was no difference in the ratio of de‐escalation to escalation orders written during the night (Table 2).
| Preintervention, 48 Admissions | Postintervention, 50 Admissions | P Value | |
|---|---|---|---|
| |||
| Total no. of orders | 212 | 231 | |
| Mean no. of orders per admission | 4.42 | 4.62 | |
| Day shift orders, n (%) | 155 (73) | 155 (67) | 0.149 |
| Night shift orders, n (%) | 57 (27) | 76 (33) | |
| Mean no. of orders within first 12 hours* | 0.58 | 1.12 | 0.009 |
| Mean no. of orders within first 24 hours* | 1.52 | 2.38 | 0.004 |
| Night shift escalation orders (%) | 27 (47) | 33 (43) | 0.491 |
| Night shift de‐escalation orders (%) | 30 (53) | 43 (57) | |
DISCUSSION
In this study, we demonstrate increased patient care management early in the hospitalization, measured in this study by the mean number of orders written per patient in the first 12 and 24 hours after admission, after transition from a call schedule with extended (>16 hours) shifts to one with shorter shifts compliant with current ACGME duty‐hour restrictions and an explicit focus on greater continuity of care. We did not detect a change in the proportion of total orders written on the night shift compared to the day shift. Earlier active medical management, such as weaning nebulized albuterol or supplemental oxygen, can speed the time to discharge.[14]
Our failure to detect a significant change in the proportion or type of orders written at night may have been due to our small sample size. Anecdotally, after the intervention, medical students reported to us that they noticed a difference between our service, in which we expect night teams to advance care, and other services at our institution, in which nights are a time to focus on putting out fires. This was not something that had been reported to us prior. It is likely reflective of the overall approach to patient care taken by residents working a night shift as part of a longitudinal care team.
This study builds on previous findings that demonstrated lower costs and shorter length of stay after implementing a schedule based on day and night teams.[7] The reasons for such improvements are likely multifactorial. In our model, which was purposefully designed to create night‐team continuity and minimize cross‐coverage, it is likely that residents also felt a greater sense of responsibility for and familiarity with the patients[15] and therefore felt more comfortable advancing care. Not only were interns likely better rested, the patient‐to‐provider ratio was also lower than in the preintervention model. Increases in staffing are often necessary to eliminate cross‐coverage while maintaining safe, 24‐hour care. These findings suggest that increases in cost from additional staffing may be at least partially offset by more active patient management early in the hospitalization, which has the potential to lead to shorter hospital stays.
There are several limitations to our research. We studied a small sample, including a subset of general pediatrics diagnoses that are amenable to active management, limiting generalizability. We did not calculate a physician‐to‐patient ratio because this was not possible with the retrospective data we collected. Staffing ratios likely improved, and we consider that part of the overall improvements in staffing that may have contributed to the observed changes in ordering patterns. Although intern‐level cross‐coverage was eliminated, the senior resident continued to cover multiple teams overnight. This senior covered the same 3 teams for 7 consecutive nights. The addition of a hospitalist team, with subspecialists being placed in consultant roles, may have contributed to the increase in active management, though our study population did not include subspecialty patients. There was a difference in insurance status between the 2 groups. This was unlikely to affect resident physician practices as insurance information is not routinely discussed in the course of patient care. In the context of the ongoing debate about duty‐hour restrictions, it will be important for future studies to elucidate whether sleep or other variables are the primary contributors to this finding. Our data are derived solely from 1 inpatient service at a single academic medical center; however, we do feel there are lessons that may be applied to other settings.
CONCLUSION
A coverage system with improved nighttime resident coverage was associated with a greater number of orders written early in the hospitalization, suggesting more active management of clinical problems to advance care.
Acknowledgements
The authors thank Dr. I. Elaine Allen, John Kornak, and Dr. Derek Pappas for assistance with biostatistics, and Dr. Diana Bojorquez and Dr. Derek Pappas for assistance with review of the manuscript and creation of the figures.
Disclosures: None of the authors have financial relationships or other conflicts of interest to disclose. No external funding was secured for this study. Dr. Auerbach was supported by grant K24HL098372 during the course of this study. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through University of California San FranciscoClinical and Translational Sciences Institute grant UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. Rosenbluth had access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Duty‐hour restrictions were implemented by the Accreditation Council for Graduate Medical Education (ACGME) in 2003 in response to data showing that sleep deprivation was correlated with serious medical errors.[1] In 2011, the ACGME required more explicit restrictions in the number of hours worked and the maximal shift length.[2] These requirements have necessitated a transition from a traditional q4 call model for interns to one in which shifts are limited to a maximum of 16 hours.
Studies of interns working these shorter shifts have had varied results, and comprehensive reviews have failed to demonstrate consistent improvements.[3, 4, 5] Studies of shift‐length limitation initially suggested improvements in patient safety (decreased length of stay,[6, 7] cost of hospitalization,[6] medication errors,[7] serious medical errors,[8] and intensive care unit [ICU] admissions[9]) and resident quality of life.[10] However, other recent studies have reported an increased number of self‐reported medical errors[11] and either did not detect change[12] or reported perceived decreases[13] in quality of care and continuity of care.
We previously reported decreased length of stay and decreased cost of hospitalization in pediatric inpatients cared for in a day/night‐shiftbased care model.[6] An hypothesized reason for those care improvements is the restructured care model led to increased active clinical management during both day and night hours. Here we report the findings of a retrospective analysis to investigate this hypothesis.
PATIENTS AND METHODS
Study Population
We reviewed the charts of pediatric patients admitted to University of California, San Francisco Benioff Children's Hospital, a 175‐bed tertiary care facility, over a 2‐year period between September 15, 2007 and September 15, 2008 (preintervention) and September 16, 2008 and September 16, 2009 (postintervention). During this study period, our hospital was still dependent on paper orders. Admission order sets were preprinted paper forms that were unchanged for the study period. Using International Classification of Diseases, 9th Revision coding, we identified patients on the general pediatrics service with 1 of 6 common diagnosesdehydration, community‐acquired pneumonia, aspiration pneumonia, upper respiratory infection, asthma, and bronchiolitis. These diagnoses were chosen because it was hypothesized that their length of inpatient stay could be impacted by active clinical management. We excluded patients admitted to the ICU or transferred between services.
A list of medical record numbers (MRNs) corresponding to admissions for 1 of the 6 above diagnoses during the pre‐ and postintervention periods was compiled. MRNs were randomized and then sequentially reviewed until 50 admissions in each time period were obtained. After data collection was completed, we noted that 2 patients had been in the ICU for part of their hospitalization, and these were excluded, leaving 48 admissions from prior to the intervention and 50 admissions from after intervention who were examined.
Intervention
During the preintervention period, patients were cared for by interns who took call every sixth night (duty periods up to 30 hours), with cross‐coverage of patients on multiple teams. Cross‐coverage was defined as coverage of patients cared for during nonconsecutive shifts and for whom residents did not participate in attending rounds. Noncall shifts were typically 10 to 11 hours. They were supervised by senior residents who took call every fourth or fifth night and who provided similar cross‐coverage.
During the postintervention period, interns worked day and night shifts of 13 hours (1 hour overlap time between shifts for handoffs), with increased night staffing to eliminate intern‐level cross‐coverage of multiple teams and maintain interns as the primary providers. Interns covered the same team for 5 to 7 consecutive days on either the day or night shifts. Interns remained on the same teams when they switched from day shifts to night shifts to preserve continuity. There were some 24‐hour shifts for senior residents on weekends. Senior residents maintained supervisory responsibility for all patients (both hospitalist teams and a subspecialty team). They also worked 7 consecutive nights.
There were changes in the staffing ratios associated with the change to day and night teams (Table 1, Figure 1). In the preintervention period, general pediatrics patients were covered by a single hospitalist and cohorted on a single team (team A), which also covered several groups of subspecialty patients with subspecialty attendings. The team consisted of 2 interns and 1 senior resident, who shared extended (30‐hour) call in a cycle with 2 other inpatient teams. In the postintervention period, general pediatrics patients were split between 2 teams (teams D and E) and mixed with subspecialty patients. Hospitalist continued to be the attendings, and these hospitalists also covered specialty patients with subspecialists in consulting roles. The teams consisted of 3 interns on the day shift, and 1 on the night shift. There was 1 senior resident per team on day shift, and a single senior resident covering all teams at night.
| Preintervention | Postintervention | |||||
|---|---|---|---|---|---|---|
| ||||||
| General Pediatrics | Team A | Team B | Team C | Team D | Team E | Team F |
| Patient Distribution | General Pediatrics | GI/Liver | Renal | General Pediatrics | General Pediatrics | Liver |
| Pulmonary | Neurology | Rheumatology | Mixed Specialty | Mixed Specialty | Renal | |
| Adolescent | Endocrine | |||||
| Team membersa | 2 interns (q6 call) | 4 interns (3 on day shift/1 on night shift) | ||||
| 1 senior resident (q5 call) | 1 senior resident | |||||
| Night‐shift coveragea | 1 intern and 1 senior resident together covered all 3 teams. | 1 night intern per team (teams D/E) working 7 consecutive night shifts | ||||
| 1 supervising night resident covering all 3 teams | ||||||
| Intern cross‐coverage of other teams | Nights/clinic afternoons | None | ||||
| Length of night shift | 30 hours | 13 hours | ||||

There was no change in the paper‐order system, the electronic health record, timing of the morning blood draw, use of new facilities for patient care, or protocol for emergency department admission. Concomitant with the restructuring, most subspecialty patients were consolidated onto the hospitalist service, necessitating creation of a second hospitalist team. However, patients admitted with the diagnoses identified above would have been on the hospitalist service before and after the restructuring.
Data Collection/Analysis
We reviewed specific classes of orders and categorized by type: respiratory medication, oxygen, intravenous (IV) fluids, diet, monitoring, and activity, time of day (day vs night‐shift), and whether they were an escalation or de‐escalation of care. De‐escalation of care was defined as orders that decreased patient care such as weaning a patient off nebulized albuterol or decreasing their IV fluids. Orders between 07:00 to 18:00 were considered day‐shift orders and between 18:01 and 06:59 were classified as night‐shift orders. Only orders falling into 1 of the aforementioned categories were recorded. Admission order sets were not included. Initially, charts were reviewed by both investigators together; after comparing results for 10 charts to ensure consistency of methodology and criteria, the remaining charts were reviewed by 1 of the study investigators.
To compare demographics, diagnoses, and ordering patterns, t tests and 2 (SAS version 9.2 [SAS Institute, Cary, NC], Stata version 13.1 [StataCorp, College Station, TX]) were used. Multivariate gamma models (SAS version 9.2 [SAS Institute]) that adjusted for clustering at the attending level and patient age were used to compare severity of illness before and after the intervention. This study was approved by the University of California, San Francisco Committee on Human Research.
RESULTS
We analyzed data for 48 admissions preintervention and 50 postintervention. With the exception of insurance type, there was no difference in baseline demographics, diagnoses, or severity of illness between the groups (Table 2). Within the order classes above, we identified 212 orders preintervention and 231 orders postintervention.
| Preintervention,n = 48, N (%) | Postintervention, n = 50, N (%) | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 4.8 (4.6) | 5.5 (4.7) | 0.4474 |
| Race/ethnicity | 0.1953 | ||
| NH white | 12 (25.0%) | 9 (18.0%) | |
| NH black | 11 (22.9%) | 7 (14.0%) | |
| Hispanic | 16 (33.3%) | 13 (26.0%) | |
| Asian | 6 (12.5%) | 10 (20.0%) | |
| Other | 3 (6.3%) | 10 (20.0%) | |
| Missing | 0 | 1 (2.0%) | |
| Gender | 0.6577 | ||
| Female | 19 (39.6%) | 22 (44.0%) | |
| Male | 29 (60.4%) | 28 (56.0%) | |
| Primary language | 0.2601 | ||
| English | 38 (79.2%) | 45 (90.0%) | |
| Spanish | 9 (18.8%) | 5 (10.0%) | |
| Other | 1 (2.1%) | 0 | |
| Insurance | 0.0118 | ||
| Private | 13 (27.1%) | 26 (52.0%) | |
| Medical | 35 (72.9%) | 24 (48.0%) | |
| Other | 0 | 0 | |
| Admit source | 0.6581 | ||
| Referral | 20 (41.7%) | 18 (36.0%) | |
| ED | 26 (54.2%) | 31 (62.0%) | |
| Transfer | 2 (4.2%) | 1 (2.0%) | |
| Severity of illness | 0.1926 | ||
| Minor | 15 (31.3%) | 24 (48.0%) | |
| Moderate | 23 (47.9%) | 16 (32.0%) | |
| Severe | 10 (20.8%) | 10 (20.0%) | |
| Extreme | 0 | 0 | |
| Diagnoses | 0.562 | ||
| Asthma | 21 | 19 | |
| Bronchiolitis | 2 | 4 | |
| Pneumonia | 17 | 19 | |
| Dehydration | 6 | 7 | |
| URI | 0 | 1 | |
| Aspiration pneumonia | 2 | 0 | |
After the intervention, there was a statistically significant increase in the average number of orders written within the first 12 hours (pre: 0.58 orders vs post: 1.12, P = 0.009) and 24 hours (pre: 1.52 vs post: 2.38, P = 0.004) following admission (Table 3), not including the admission order set. The fraction of orders written at night was not significantly different (27% at night preintervention, 33% postintervention, P = 0.149). The fraction of admissions on the day shift compared to the night shift did not change (P = 0.72). There was no difference in the ratio of de‐escalation to escalation orders written during the night (Table 2).
| Preintervention, 48 Admissions | Postintervention, 50 Admissions | P Value | |
|---|---|---|---|
| |||
| Total no. of orders | 212 | 231 | |
| Mean no. of orders per admission | 4.42 | 4.62 | |
| Day shift orders, n (%) | 155 (73) | 155 (67) | 0.149 |
| Night shift orders, n (%) | 57 (27) | 76 (33) | |
| Mean no. of orders within first 12 hours* | 0.58 | 1.12 | 0.009 |
| Mean no. of orders within first 24 hours* | 1.52 | 2.38 | 0.004 |
| Night shift escalation orders (%) | 27 (47) | 33 (43) | 0.491 |
| Night shift de‐escalation orders (%) | 30 (53) | 43 (57) | |
DISCUSSION
In this study, we demonstrate increased patient care management early in the hospitalization, measured in this study by the mean number of orders written per patient in the first 12 and 24 hours after admission, after transition from a call schedule with extended (>16 hours) shifts to one with shorter shifts compliant with current ACGME duty‐hour restrictions and an explicit focus on greater continuity of care. We did not detect a change in the proportion of total orders written on the night shift compared to the day shift. Earlier active medical management, such as weaning nebulized albuterol or supplemental oxygen, can speed the time to discharge.[14]
Our failure to detect a significant change in the proportion or type of orders written at night may have been due to our small sample size. Anecdotally, after the intervention, medical students reported to us that they noticed a difference between our service, in which we expect night teams to advance care, and other services at our institution, in which nights are a time to focus on putting out fires. This was not something that had been reported to us prior. It is likely reflective of the overall approach to patient care taken by residents working a night shift as part of a longitudinal care team.
This study builds on previous findings that demonstrated lower costs and shorter length of stay after implementing a schedule based on day and night teams.[7] The reasons for such improvements are likely multifactorial. In our model, which was purposefully designed to create night‐team continuity and minimize cross‐coverage, it is likely that residents also felt a greater sense of responsibility for and familiarity with the patients[15] and therefore felt more comfortable advancing care. Not only were interns likely better rested, the patient‐to‐provider ratio was also lower than in the preintervention model. Increases in staffing are often necessary to eliminate cross‐coverage while maintaining safe, 24‐hour care. These findings suggest that increases in cost from additional staffing may be at least partially offset by more active patient management early in the hospitalization, which has the potential to lead to shorter hospital stays.
There are several limitations to our research. We studied a small sample, including a subset of general pediatrics diagnoses that are amenable to active management, limiting generalizability. We did not calculate a physician‐to‐patient ratio because this was not possible with the retrospective data we collected. Staffing ratios likely improved, and we consider that part of the overall improvements in staffing that may have contributed to the observed changes in ordering patterns. Although intern‐level cross‐coverage was eliminated, the senior resident continued to cover multiple teams overnight. This senior covered the same 3 teams for 7 consecutive nights. The addition of a hospitalist team, with subspecialists being placed in consultant roles, may have contributed to the increase in active management, though our study population did not include subspecialty patients. There was a difference in insurance status between the 2 groups. This was unlikely to affect resident physician practices as insurance information is not routinely discussed in the course of patient care. In the context of the ongoing debate about duty‐hour restrictions, it will be important for future studies to elucidate whether sleep or other variables are the primary contributors to this finding. Our data are derived solely from 1 inpatient service at a single academic medical center; however, we do feel there are lessons that may be applied to other settings.
CONCLUSION
A coverage system with improved nighttime resident coverage was associated with a greater number of orders written early in the hospitalization, suggesting more active management of clinical problems to advance care.
Acknowledgements
The authors thank Dr. I. Elaine Allen, John Kornak, and Dr. Derek Pappas for assistance with biostatistics, and Dr. Diana Bojorquez and Dr. Derek Pappas for assistance with review of the manuscript and creation of the figures.
Disclosures: None of the authors have financial relationships or other conflicts of interest to disclose. No external funding was secured for this study. Dr. Auerbach was supported by grant K24HL098372 during the course of this study. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through University of California San FranciscoClinical and Translational Sciences Institute grant UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. Rosenbluth had access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- , , . New requirements for resident duty hours. JAMA. 2002;288(9):1112–1114.
- Accreditation Council for Graduate Medical Education. Common program requirements. 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed November 28, 2011.
- , , . Patient safety, resident education and resident well‐being following implementation of the 2003 ACGME duty hour rules. J Gen Intern Med. 2011;26(8):907–919.
- , , , et al. A systematic review of the effects of resident duty hour restrictions in surgery: impact on resident wellness, training, and patient outcomes. Ann Surg. 2014;259(6):1041–1053.
- , , , . Duty‐hour limits and patient care and resident outcomes: can high‐quality studies offer insight into complex relationships? Annu Rev Med. 2013;64:467–483.
- , , , , , . Association between adaptations to ACGME duty hour requirements, length of stay, and costs. Sleep. 2013;36(2):245–248.
- , , , . Effect of a change in house staff work schedule on resource utilization and patient care. Arch Intern Med. 1991;151(10):2065–2070.
- , , , et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848.
- , , , . Changes in outcomes for internal medicine inpatients after work‐hour regulations. Ann Intern Med. 2007;147(2):97–103.
- , , . Effects of reducing or eliminating resident work shifts over 16 hours: a systematic review. Sleep. 2010;33(8):1043–1053.
- , , , et al. Effects of the 2011 duty hour reforms on interns and their patients: a prospective longitudinal cohort study. JAMA Intern Med. 2013;173(8):657–662; discussion 663.
- , , , , . Effect of 16‐hour duty periods on patient care and resident education. Mayo Clin Proc. 2011;86(3):192–196.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
- , , , . Resident perceptions of autonomy in a complex tertiary care environment improve when supervised by hospitalists. Hosp Pediatr. 2012;2(4):228–234.
- , , . New requirements for resident duty hours. JAMA. 2002;288(9):1112–1114.
- Accreditation Council for Graduate Medical Education. Common program requirements. 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed November 28, 2011.
- , , . Patient safety, resident education and resident well‐being following implementation of the 2003 ACGME duty hour rules. J Gen Intern Med. 2011;26(8):907–919.
- , , , et al. A systematic review of the effects of resident duty hour restrictions in surgery: impact on resident wellness, training, and patient outcomes. Ann Surg. 2014;259(6):1041–1053.
- , , , . Duty‐hour limits and patient care and resident outcomes: can high‐quality studies offer insight into complex relationships? Annu Rev Med. 2013;64:467–483.
- , , , , , . Association between adaptations to ACGME duty hour requirements, length of stay, and costs. Sleep. 2013;36(2):245–248.
- , , , . Effect of a change in house staff work schedule on resource utilization and patient care. Arch Intern Med. 1991;151(10):2065–2070.
- , , , et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848.
- , , , . Changes in outcomes for internal medicine inpatients after work‐hour regulations. Ann Intern Med. 2007;147(2):97–103.
- , , . Effects of reducing or eliminating resident work shifts over 16 hours: a systematic review. Sleep. 2010;33(8):1043–1053.
- , , , et al. Effects of the 2011 duty hour reforms on interns and their patients: a prospective longitudinal cohort study. JAMA Intern Med. 2013;173(8):657–662; discussion 663.
- , , , , . Effect of 16‐hour duty periods on patient care and resident education. Mayo Clin Proc. 2011;86(3):192–196.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
- , , , . Resident perceptions of autonomy in a complex tertiary care environment improve when supervised by hospitalists. Hosp Pediatr. 2012;2(4):228–234.
Linear Bluish Black Papules on the Shoulder
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1
|
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1


|
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1


|
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
A 57-year-old woman presented with an asymptomatic, unchanging, 3.5×0.7-cm linear plaque on the right shoulder composed of dozens of clustered, bluish black papules that had been present for several decades. The skin between the papules was unaffected. The patient’s medical and family histories were unremarkable. A deep shave biopsy from the center of the plaque was performed.
10 Apps for Veterans to Improve Health and Wellness
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.