User login
Finger-prick blood tests may need repeating

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.” ![]()

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.” ![]()

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.” ![]()
Device linked to anemia in blood donors

A device used to measure hemoglobin can deliver inaccurate results and may have prompted an increase in anemia among blood donors in Ireland, according to the Irish Blood Transfusion Service (IBTS).
The IBTS began using the device—called haemospect—in July 2014 but recently discovered that it cannot detect anemia accurately in all cases.
This may have resulted in anemic individuals donating blood or caused individuals to become anemic by donating.
The IBTS said this issue likely affected female donors more than males, so the organization has decided to temporarily stop taking blood donations from women who have donated in the last 18 months.
The IBTS also said it will perform a full blood count on all male and female donors going forward, until this issue has been resolved.
“Until we have a resolution to the problem that has arisen, we are asking male donors to attend our clinics and give blood,” said IBTS Medical and Scientific Director William Murphy, MD.
“We need male donors to make an extra effort to donate and maintain the blood supply. We will be double-checking all hemoglobin results on these donors.”
Discovering the problem
Haemospect is a noninvasive device that uses white light to detect hemoglobin levels. The device is made by the German company MBR Optical Systems.
IBTS learned of the potential for inaccuracies with haemospect after a blood donor contacted the organization. She had been diagnosed as severely anemic by her doctor but had given blood the week before.
“We have now discovered that the device gives inaccurate results in some individuals with anemia down to, and probably below, 8.4 g/dL,” Dr Murphy said. “As a result of the issue . . . , some women, and probably a much smaller number of men, could have been rendered iron-deficient and anemic from blood donation in the past 18 months.”
Steps to resolution
Since the issue was discovered, more than 30 women with anemia have been identified and contacted. The IBTS said it will contact all donors who are found to be anemic.
“Over the next few weeks, we will introduce new software to reanalyze all the electronic results from all donors who have been tested and accepted for donation since we introduced this device,” Dr Murphy said. “Any discrepant results will be notified to the donors involved.”
Dr Murphy also said donors who think they might be anemic or iron-deficient should visit their doctors for testing, and the IBTS will foot the bill. Concerned donors can contact the IBTS at 1850 731137.
The IBTS plans to replace haemospect with an alternative device as soon as possible. The organization has contacted the Health Products Regularity Authority about the issue with haemospect, which is used in Austria and Germany as well.
“We are determined to have this matter resolved as soon as possible,” Dr Murphy said. “We are confident that this issue . . . has not had any impact on blood received by patients.” ![]()

A device used to measure hemoglobin can deliver inaccurate results and may have prompted an increase in anemia among blood donors in Ireland, according to the Irish Blood Transfusion Service (IBTS).
The IBTS began using the device—called haemospect—in July 2014 but recently discovered that it cannot detect anemia accurately in all cases.
This may have resulted in anemic individuals donating blood or caused individuals to become anemic by donating.
The IBTS said this issue likely affected female donors more than males, so the organization has decided to temporarily stop taking blood donations from women who have donated in the last 18 months.
The IBTS also said it will perform a full blood count on all male and female donors going forward, until this issue has been resolved.
“Until we have a resolution to the problem that has arisen, we are asking male donors to attend our clinics and give blood,” said IBTS Medical and Scientific Director William Murphy, MD.
“We need male donors to make an extra effort to donate and maintain the blood supply. We will be double-checking all hemoglobin results on these donors.”
Discovering the problem
Haemospect is a noninvasive device that uses white light to detect hemoglobin levels. The device is made by the German company MBR Optical Systems.
IBTS learned of the potential for inaccuracies with haemospect after a blood donor contacted the organization. She had been diagnosed as severely anemic by her doctor but had given blood the week before.
“We have now discovered that the device gives inaccurate results in some individuals with anemia down to, and probably below, 8.4 g/dL,” Dr Murphy said. “As a result of the issue . . . , some women, and probably a much smaller number of men, could have been rendered iron-deficient and anemic from blood donation in the past 18 months.”
Steps to resolution
Since the issue was discovered, more than 30 women with anemia have been identified and contacted. The IBTS said it will contact all donors who are found to be anemic.
“Over the next few weeks, we will introduce new software to reanalyze all the electronic results from all donors who have been tested and accepted for donation since we introduced this device,” Dr Murphy said. “Any discrepant results will be notified to the donors involved.”
Dr Murphy also said donors who think they might be anemic or iron-deficient should visit their doctors for testing, and the IBTS will foot the bill. Concerned donors can contact the IBTS at 1850 731137.
The IBTS plans to replace haemospect with an alternative device as soon as possible. The organization has contacted the Health Products Regularity Authority about the issue with haemospect, which is used in Austria and Germany as well.
“We are determined to have this matter resolved as soon as possible,” Dr Murphy said. “We are confident that this issue . . . has not had any impact on blood received by patients.” ![]()

A device used to measure hemoglobin can deliver inaccurate results and may have prompted an increase in anemia among blood donors in Ireland, according to the Irish Blood Transfusion Service (IBTS).
The IBTS began using the device—called haemospect—in July 2014 but recently discovered that it cannot detect anemia accurately in all cases.
This may have resulted in anemic individuals donating blood or caused individuals to become anemic by donating.
The IBTS said this issue likely affected female donors more than males, so the organization has decided to temporarily stop taking blood donations from women who have donated in the last 18 months.
The IBTS also said it will perform a full blood count on all male and female donors going forward, until this issue has been resolved.
“Until we have a resolution to the problem that has arisen, we are asking male donors to attend our clinics and give blood,” said IBTS Medical and Scientific Director William Murphy, MD.
“We need male donors to make an extra effort to donate and maintain the blood supply. We will be double-checking all hemoglobin results on these donors.”
Discovering the problem
Haemospect is a noninvasive device that uses white light to detect hemoglobin levels. The device is made by the German company MBR Optical Systems.
IBTS learned of the potential for inaccuracies with haemospect after a blood donor contacted the organization. She had been diagnosed as severely anemic by her doctor but had given blood the week before.
“We have now discovered that the device gives inaccurate results in some individuals with anemia down to, and probably below, 8.4 g/dL,” Dr Murphy said. “As a result of the issue . . . , some women, and probably a much smaller number of men, could have been rendered iron-deficient and anemic from blood donation in the past 18 months.”
Steps to resolution
Since the issue was discovered, more than 30 women with anemia have been identified and contacted. The IBTS said it will contact all donors who are found to be anemic.
“Over the next few weeks, we will introduce new software to reanalyze all the electronic results from all donors who have been tested and accepted for donation since we introduced this device,” Dr Murphy said. “Any discrepant results will be notified to the donors involved.”
Dr Murphy also said donors who think they might be anemic or iron-deficient should visit their doctors for testing, and the IBTS will foot the bill. Concerned donors can contact the IBTS at 1850 731137.
The IBTS plans to replace haemospect with an alternative device as soon as possible. The organization has contacted the Health Products Regularity Authority about the issue with haemospect, which is used in Austria and Germany as well.
“We are determined to have this matter resolved as soon as possible,” Dr Murphy said. “We are confident that this issue . . . has not had any impact on blood received by patients.” ![]()
Group quantifies cardiotoxicity risk with HL treatment

Photo by Rhoda Baer
European researchers say they have quantified the risk of cardiovascular disease associated with treatments for Hodgkin lymphoma (HL).
The group analyzed the risks associated with specific doses of radiation and anthracycline exposure.
They believe their results, published in The Lancet Haematology, could help clinicians identify the optimal treatment regimen for each individual HL patient.
“These study results are exciting,” said Maja V. Maraldo, PhD, of Rigshospitalet in Copenhagen, Denmark.
“They should allow physicians to optimize the combination of systemic therapy and radiation and thereby balance the risks and benefits of different regimens in individual patients.”
Study details
Dr Maraldo and her colleagues analyzed data from patients who participated in 9 trials conducted by the European Organisation for Research and Treatment of Cancer (EORTC) and the Groupe d’Etude des Lymphomes de l’Adulte (GELA, now renamed LYSA) between 1964 and 2004.
In 2009 and 2010, the researchers mailed a Life Situation Questionnaire (LSQ) to the trial participants. The goal was to determine late-onset effects of HL and its treatment.
The team also reconstructed patients’ mean radiation doses to the heart and carotid arteries and the cumulative doses of anthracyclines and vinca-alkaloids they received. The incidence of cardiovascular disease was reported during follow-up and updated through the LSQ.
Patient data
The researchers were able to collect complete information on primary treatment for 6039 HL survivors. Of these patients, 2923 received the LSQ, and 1919 responded. The median follow-up was 9 years, and the patients’ median age at diagnosis was 30.
There were 1238 cardiovascular events in 703 patients, including 46 patients who died from such an event.
The events included ischemic heart disease (24%), congestive heart failure (21%), arrhythmia (17%), valvular disease (14%), disease of the arterial vessels (9%), stroke (6%), venous thromboembolism (5%), pericarditis (3%), peripheral vasculopathy (1%), other vascular events (1%), and other cardiac events (<1%).
Predictors of risk
The researchers found that the mean radiation dose to the heart, per 1 Gy increase, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.015 (P=0.0014).
However, the mean radiation doses to the left internal carotid artery and the right internal carotid artery were not significant predictors of cardiovascular events (P=0.41 and 0.70, respectively).
The dose of anthracyclines, per 50 mg/m2 increase in cumulative dose, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.077 (P=0.0064).
But the cumulative dose of vinblastine or vincristine was not (P=0.77 and 0.36, respectively).
The researchers said a limitation of this study is that they were not able to assess the impact of cardiovascular risk factors such as smoking, hypertension, and diabetes because that information was not consistently available.
Still, the team believes their analyses quantified the effect of radiotherapy and anthracyclines on the risk of cardiovascular disease, and the findings should aid treatment decisions in HL. ![]()

Photo by Rhoda Baer
European researchers say they have quantified the risk of cardiovascular disease associated with treatments for Hodgkin lymphoma (HL).
The group analyzed the risks associated with specific doses of radiation and anthracycline exposure.
They believe their results, published in The Lancet Haematology, could help clinicians identify the optimal treatment regimen for each individual HL patient.
“These study results are exciting,” said Maja V. Maraldo, PhD, of Rigshospitalet in Copenhagen, Denmark.
“They should allow physicians to optimize the combination of systemic therapy and radiation and thereby balance the risks and benefits of different regimens in individual patients.”
Study details
Dr Maraldo and her colleagues analyzed data from patients who participated in 9 trials conducted by the European Organisation for Research and Treatment of Cancer (EORTC) and the Groupe d’Etude des Lymphomes de l’Adulte (GELA, now renamed LYSA) between 1964 and 2004.
In 2009 and 2010, the researchers mailed a Life Situation Questionnaire (LSQ) to the trial participants. The goal was to determine late-onset effects of HL and its treatment.
The team also reconstructed patients’ mean radiation doses to the heart and carotid arteries and the cumulative doses of anthracyclines and vinca-alkaloids they received. The incidence of cardiovascular disease was reported during follow-up and updated through the LSQ.
Patient data
The researchers were able to collect complete information on primary treatment for 6039 HL survivors. Of these patients, 2923 received the LSQ, and 1919 responded. The median follow-up was 9 years, and the patients’ median age at diagnosis was 30.
There were 1238 cardiovascular events in 703 patients, including 46 patients who died from such an event.
The events included ischemic heart disease (24%), congestive heart failure (21%), arrhythmia (17%), valvular disease (14%), disease of the arterial vessels (9%), stroke (6%), venous thromboembolism (5%), pericarditis (3%), peripheral vasculopathy (1%), other vascular events (1%), and other cardiac events (<1%).
Predictors of risk
The researchers found that the mean radiation dose to the heart, per 1 Gy increase, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.015 (P=0.0014).
However, the mean radiation doses to the left internal carotid artery and the right internal carotid artery were not significant predictors of cardiovascular events (P=0.41 and 0.70, respectively).
The dose of anthracyclines, per 50 mg/m2 increase in cumulative dose, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.077 (P=0.0064).
But the cumulative dose of vinblastine or vincristine was not (P=0.77 and 0.36, respectively).
The researchers said a limitation of this study is that they were not able to assess the impact of cardiovascular risk factors such as smoking, hypertension, and diabetes because that information was not consistently available.
Still, the team believes their analyses quantified the effect of radiotherapy and anthracyclines on the risk of cardiovascular disease, and the findings should aid treatment decisions in HL. ![]()

Photo by Rhoda Baer
European researchers say they have quantified the risk of cardiovascular disease associated with treatments for Hodgkin lymphoma (HL).
The group analyzed the risks associated with specific doses of radiation and anthracycline exposure.
They believe their results, published in The Lancet Haematology, could help clinicians identify the optimal treatment regimen for each individual HL patient.
“These study results are exciting,” said Maja V. Maraldo, PhD, of Rigshospitalet in Copenhagen, Denmark.
“They should allow physicians to optimize the combination of systemic therapy and radiation and thereby balance the risks and benefits of different regimens in individual patients.”
Study details
Dr Maraldo and her colleagues analyzed data from patients who participated in 9 trials conducted by the European Organisation for Research and Treatment of Cancer (EORTC) and the Groupe d’Etude des Lymphomes de l’Adulte (GELA, now renamed LYSA) between 1964 and 2004.
In 2009 and 2010, the researchers mailed a Life Situation Questionnaire (LSQ) to the trial participants. The goal was to determine late-onset effects of HL and its treatment.
The team also reconstructed patients’ mean radiation doses to the heart and carotid arteries and the cumulative doses of anthracyclines and vinca-alkaloids they received. The incidence of cardiovascular disease was reported during follow-up and updated through the LSQ.
Patient data
The researchers were able to collect complete information on primary treatment for 6039 HL survivors. Of these patients, 2923 received the LSQ, and 1919 responded. The median follow-up was 9 years, and the patients’ median age at diagnosis was 30.
There were 1238 cardiovascular events in 703 patients, including 46 patients who died from such an event.
The events included ischemic heart disease (24%), congestive heart failure (21%), arrhythmia (17%), valvular disease (14%), disease of the arterial vessels (9%), stroke (6%), venous thromboembolism (5%), pericarditis (3%), peripheral vasculopathy (1%), other vascular events (1%), and other cardiac events (<1%).
Predictors of risk
The researchers found that the mean radiation dose to the heart, per 1 Gy increase, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.015 (P=0.0014).
However, the mean radiation doses to the left internal carotid artery and the right internal carotid artery were not significant predictors of cardiovascular events (P=0.41 and 0.70, respectively).
The dose of anthracyclines, per 50 mg/m2 increase in cumulative dose, was a significant predictor of cardiovascular disease, with a hazard ratio of 1.077 (P=0.0064).
But the cumulative dose of vinblastine or vincristine was not (P=0.77 and 0.36, respectively).
The researchers said a limitation of this study is that they were not able to assess the impact of cardiovascular risk factors such as smoking, hypertension, and diabetes because that information was not consistently available.
Still, the team believes their analyses quantified the effect of radiotherapy and anthracyclines on the risk of cardiovascular disease, and the findings should aid treatment decisions in HL. ![]()
Coconut oil may prevent bloodstream infection

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis. ![]()

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis. ![]()

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis. ![]()
Fillers in Dermatology: From Past to Present
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
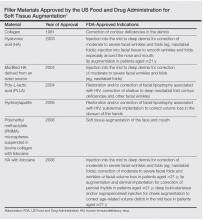
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.
Study: Pediatric cancer patients have high rate of germline mutations in predisposition genes
Children and adolescents with cancer were found to have a significantly higher rate of germline mutations in cancer predisposing genes compared with individuals with no known cancer; however, family history of cancer did not predict the presence of a predisposition syndrome for most patients.
Of the 1,120 children with cancer, 8.5% had mutations in predisposition genes, compared with 1.1% in the control group. Mutations in TP53 were most common (50 patients), followed by APC (6), BRCA2 (6), NF1 (4), PMS2 (4), RB1 (3), and RUNX1 (3). Germline TP53 mutations were present in 27 of 39 patients (69%) with adrenocortical tumors, 9 of 47 (19%) with hypodiploid acute lymphoblastic leukemia, and 1 of 4 (25%) with choroid plexus carcinoma (N Engl J Med. 2015 Nov. 18. doi: 10.1056/NEJMoa1508054).
Only 40% of the pediatric patients with pathogenic germline mutations had a family history of cancer, and in just half of those cases the history was consistent with a known cancer-predisposition syndrome. Among patients without predisposition germline mutations, a similar proportion (42%) had a family history of cancer.
“On the basis of these observations, family history cannot be the sole indication used to guide the provision of genetic testing,” wrote Jinghui Zhang, Ph.D., of the department of computational biology, St. Jude’s Research Hospital, Memphis, Tennessee, and her colleagues.
Unexpected germline mutations were found in several cases. Six patients with Ewing’s sarcoma had unexpected pathogenic germline mutations (TP53 in four patients, PMS2 in one and RET in one). Eight patents had heterozygous mutations in BRCA1, BRCA2, or PALB2, supporting the notion that mutations in these genes may play a role in pediatric as well as adult cancer. Other new associations included germline APC and SDHB mutations with neuroblastoma, and APC, VHL, CDH1, PTCH1, and SDHA germline mutations with leukemia.
The St. Jude–Washington University Pediatric Cancer Genome Project (PCGP) included 1,120 patients representing the major types of pediatric cancer, including 53% with leukemia, 22% with CNS tumors, and 26% with non-CNS tumors. Whole genomes were sequenced from 595 patients, whole exomes (coding regions only) from 456 patients, and both whole genomes and exomes from 69 patients. Whole exomes were sequenced from two control cohorts of individuals with no known cancer: 966 from the 1,000 Genome project and 723 from the National Database for Autism Research (cancer predisposition genes known to be associated with autism, NF1 and PTEN, were excluded from analysis with this control set).
The study sequenced whole genomes and exomes, but focused most of the analysis on 60 autosomal dominant cancer predisposition genes. Tumor types with the highest prevalence of germline mutations in these genes were non-CNS solid tumors (48 of 287 patients, 17%) and CNS tumors (21 of 245, 9%). Among patients with adrenocortical tumors, 69% had germline mutations. Despite inclusion of hypodiploid acute lymphoblastic leukemia, the lowest germline mutation prevalence was found in leukemia (26 of 588, 4%).
Dr. Zhang reported having no disclosures. One coauthor reported financial ties to an industry source.
The sequencing study by Zhang et al. found that 8.5% of 1,120 participants with pediatric cancer had pathogenic mutations in an autosomal dominant cancer-predisposition gene, and four of the mutations were mosaic (i.e., present in only a subset of normal cells, probably indicating the defect was not inherited). The four mosaic mutations probably would not be detected by standard genetic testing strategies.
Not surprisingly, more than 50% of the mutations were in the tumor suppressor gene TP53. A long list of other genes identified as potentially disease causing each occurred at a prevalence of less than 6%.
Although the study’s inclusion of certain high-risk childhood cancers could bias the results toward overestimating the proportion of germline cancer predisposition mutations, more likely the results are an underestimate. By evaluating mutations in only a small subset of candidate autosomal dominant genes, the findings to not reflect a thorough assessment of most genes in the genome. In addition, focusing only on the exome ignores DNA mutations in noncoding regions, especially in tissue-specific enhancers, which may have a role in cancer susceptibility. So-called epimutations may affect cancer susceptibility in a nonmendelian fashion. Finally, the ability to study the interaction of several of these events may contribute to our understanding of tumor initiation.
The study raises several important questions. Are children with mutations in APC, BRCA1, or BRCA2 at risk for childhood cancers? How does germline mosaicism influence disease penetrance, and how many of the mosaic mutations were inherited? How do the mutations identified interact with less well known gene mutations elsewhere in the genome to influence malignant transformation? How can the findings translate to the clinic?
The study highlights the fact that family history is insufficient to assess the likelihood of a cancer-predisposition syndrome in any patient with a newly diagnosed cancer.
Dr. John Maris is a pediatric oncologist in the division of oncology and Center for Childhood Cancer Research, Children’s Hospital of Philadelphia, and in the department of pediatrics at the Perelman School of Medicine, University of Pennsylvania. These remarks were part of an editorial accompanying the report (N Engl J Med. 2015 Nov. 18 doi: 10.1056/NEJMoa1508054). Dr. Maris reported having no disclosures.
The sequencing study by Zhang et al. found that 8.5% of 1,120 participants with pediatric cancer had pathogenic mutations in an autosomal dominant cancer-predisposition gene, and four of the mutations were mosaic (i.e., present in only a subset of normal cells, probably indicating the defect was not inherited). The four mosaic mutations probably would not be detected by standard genetic testing strategies.
Not surprisingly, more than 50% of the mutations were in the tumor suppressor gene TP53. A long list of other genes identified as potentially disease causing each occurred at a prevalence of less than 6%.
Although the study’s inclusion of certain high-risk childhood cancers could bias the results toward overestimating the proportion of germline cancer predisposition mutations, more likely the results are an underestimate. By evaluating mutations in only a small subset of candidate autosomal dominant genes, the findings to not reflect a thorough assessment of most genes in the genome. In addition, focusing only on the exome ignores DNA mutations in noncoding regions, especially in tissue-specific enhancers, which may have a role in cancer susceptibility. So-called epimutations may affect cancer susceptibility in a nonmendelian fashion. Finally, the ability to study the interaction of several of these events may contribute to our understanding of tumor initiation.
The study raises several important questions. Are children with mutations in APC, BRCA1, or BRCA2 at risk for childhood cancers? How does germline mosaicism influence disease penetrance, and how many of the mosaic mutations were inherited? How do the mutations identified interact with less well known gene mutations elsewhere in the genome to influence malignant transformation? How can the findings translate to the clinic?
The study highlights the fact that family history is insufficient to assess the likelihood of a cancer-predisposition syndrome in any patient with a newly diagnosed cancer.
Dr. John Maris is a pediatric oncologist in the division of oncology and Center for Childhood Cancer Research, Children’s Hospital of Philadelphia, and in the department of pediatrics at the Perelman School of Medicine, University of Pennsylvania. These remarks were part of an editorial accompanying the report (N Engl J Med. 2015 Nov. 18 doi: 10.1056/NEJMoa1508054). Dr. Maris reported having no disclosures.
The sequencing study by Zhang et al. found that 8.5% of 1,120 participants with pediatric cancer had pathogenic mutations in an autosomal dominant cancer-predisposition gene, and four of the mutations were mosaic (i.e., present in only a subset of normal cells, probably indicating the defect was not inherited). The four mosaic mutations probably would not be detected by standard genetic testing strategies.
Not surprisingly, more than 50% of the mutations were in the tumor suppressor gene TP53. A long list of other genes identified as potentially disease causing each occurred at a prevalence of less than 6%.
Although the study’s inclusion of certain high-risk childhood cancers could bias the results toward overestimating the proportion of germline cancer predisposition mutations, more likely the results are an underestimate. By evaluating mutations in only a small subset of candidate autosomal dominant genes, the findings to not reflect a thorough assessment of most genes in the genome. In addition, focusing only on the exome ignores DNA mutations in noncoding regions, especially in tissue-specific enhancers, which may have a role in cancer susceptibility. So-called epimutations may affect cancer susceptibility in a nonmendelian fashion. Finally, the ability to study the interaction of several of these events may contribute to our understanding of tumor initiation.
The study raises several important questions. Are children with mutations in APC, BRCA1, or BRCA2 at risk for childhood cancers? How does germline mosaicism influence disease penetrance, and how many of the mosaic mutations were inherited? How do the mutations identified interact with less well known gene mutations elsewhere in the genome to influence malignant transformation? How can the findings translate to the clinic?
The study highlights the fact that family history is insufficient to assess the likelihood of a cancer-predisposition syndrome in any patient with a newly diagnosed cancer.
Dr. John Maris is a pediatric oncologist in the division of oncology and Center for Childhood Cancer Research, Children’s Hospital of Philadelphia, and in the department of pediatrics at the Perelman School of Medicine, University of Pennsylvania. These remarks were part of an editorial accompanying the report (N Engl J Med. 2015 Nov. 18 doi: 10.1056/NEJMoa1508054). Dr. Maris reported having no disclosures.
Children and adolescents with cancer were found to have a significantly higher rate of germline mutations in cancer predisposing genes compared with individuals with no known cancer; however, family history of cancer did not predict the presence of a predisposition syndrome for most patients.
Of the 1,120 children with cancer, 8.5% had mutations in predisposition genes, compared with 1.1% in the control group. Mutations in TP53 were most common (50 patients), followed by APC (6), BRCA2 (6), NF1 (4), PMS2 (4), RB1 (3), and RUNX1 (3). Germline TP53 mutations were present in 27 of 39 patients (69%) with adrenocortical tumors, 9 of 47 (19%) with hypodiploid acute lymphoblastic leukemia, and 1 of 4 (25%) with choroid plexus carcinoma (N Engl J Med. 2015 Nov. 18. doi: 10.1056/NEJMoa1508054).
Only 40% of the pediatric patients with pathogenic germline mutations had a family history of cancer, and in just half of those cases the history was consistent with a known cancer-predisposition syndrome. Among patients without predisposition germline mutations, a similar proportion (42%) had a family history of cancer.
“On the basis of these observations, family history cannot be the sole indication used to guide the provision of genetic testing,” wrote Jinghui Zhang, Ph.D., of the department of computational biology, St. Jude’s Research Hospital, Memphis, Tennessee, and her colleagues.
Unexpected germline mutations were found in several cases. Six patients with Ewing’s sarcoma had unexpected pathogenic germline mutations (TP53 in four patients, PMS2 in one and RET in one). Eight patents had heterozygous mutations in BRCA1, BRCA2, or PALB2, supporting the notion that mutations in these genes may play a role in pediatric as well as adult cancer. Other new associations included germline APC and SDHB mutations with neuroblastoma, and APC, VHL, CDH1, PTCH1, and SDHA germline mutations with leukemia.
The St. Jude–Washington University Pediatric Cancer Genome Project (PCGP) included 1,120 patients representing the major types of pediatric cancer, including 53% with leukemia, 22% with CNS tumors, and 26% with non-CNS tumors. Whole genomes were sequenced from 595 patients, whole exomes (coding regions only) from 456 patients, and both whole genomes and exomes from 69 patients. Whole exomes were sequenced from two control cohorts of individuals with no known cancer: 966 from the 1,000 Genome project and 723 from the National Database for Autism Research (cancer predisposition genes known to be associated with autism, NF1 and PTEN, were excluded from analysis with this control set).
The study sequenced whole genomes and exomes, but focused most of the analysis on 60 autosomal dominant cancer predisposition genes. Tumor types with the highest prevalence of germline mutations in these genes were non-CNS solid tumors (48 of 287 patients, 17%) and CNS tumors (21 of 245, 9%). Among patients with adrenocortical tumors, 69% had germline mutations. Despite inclusion of hypodiploid acute lymphoblastic leukemia, the lowest germline mutation prevalence was found in leukemia (26 of 588, 4%).
Dr. Zhang reported having no disclosures. One coauthor reported financial ties to an industry source.
Children and adolescents with cancer were found to have a significantly higher rate of germline mutations in cancer predisposing genes compared with individuals with no known cancer; however, family history of cancer did not predict the presence of a predisposition syndrome for most patients.
Of the 1,120 children with cancer, 8.5% had mutations in predisposition genes, compared with 1.1% in the control group. Mutations in TP53 were most common (50 patients), followed by APC (6), BRCA2 (6), NF1 (4), PMS2 (4), RB1 (3), and RUNX1 (3). Germline TP53 mutations were present in 27 of 39 patients (69%) with adrenocortical tumors, 9 of 47 (19%) with hypodiploid acute lymphoblastic leukemia, and 1 of 4 (25%) with choroid plexus carcinoma (N Engl J Med. 2015 Nov. 18. doi: 10.1056/NEJMoa1508054).
Only 40% of the pediatric patients with pathogenic germline mutations had a family history of cancer, and in just half of those cases the history was consistent with a known cancer-predisposition syndrome. Among patients without predisposition germline mutations, a similar proportion (42%) had a family history of cancer.
“On the basis of these observations, family history cannot be the sole indication used to guide the provision of genetic testing,” wrote Jinghui Zhang, Ph.D., of the department of computational biology, St. Jude’s Research Hospital, Memphis, Tennessee, and her colleagues.
Unexpected germline mutations were found in several cases. Six patients with Ewing’s sarcoma had unexpected pathogenic germline mutations (TP53 in four patients, PMS2 in one and RET in one). Eight patents had heterozygous mutations in BRCA1, BRCA2, or PALB2, supporting the notion that mutations in these genes may play a role in pediatric as well as adult cancer. Other new associations included germline APC and SDHB mutations with neuroblastoma, and APC, VHL, CDH1, PTCH1, and SDHA germline mutations with leukemia.
The St. Jude–Washington University Pediatric Cancer Genome Project (PCGP) included 1,120 patients representing the major types of pediatric cancer, including 53% with leukemia, 22% with CNS tumors, and 26% with non-CNS tumors. Whole genomes were sequenced from 595 patients, whole exomes (coding regions only) from 456 patients, and both whole genomes and exomes from 69 patients. Whole exomes were sequenced from two control cohorts of individuals with no known cancer: 966 from the 1,000 Genome project and 723 from the National Database for Autism Research (cancer predisposition genes known to be associated with autism, NF1 and PTEN, were excluded from analysis with this control set).
The study sequenced whole genomes and exomes, but focused most of the analysis on 60 autosomal dominant cancer predisposition genes. Tumor types with the highest prevalence of germline mutations in these genes were non-CNS solid tumors (48 of 287 patients, 17%) and CNS tumors (21 of 245, 9%). Among patients with adrenocortical tumors, 69% had germline mutations. Despite inclusion of hypodiploid acute lymphoblastic leukemia, the lowest germline mutation prevalence was found in leukemia (26 of 588, 4%).
Dr. Zhang reported having no disclosures. One coauthor reported financial ties to an industry source.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Children and adolescents with cancer have a significantly higher rate of germline mutations in cancer predisposition genes compared with individuals with no known cancer.
Major finding: Of the 1,120 children with cancer, 8.5% had mutations in predisposition genes, compared with 1.1% in the control group.
Data source: The St. Jude–Washington University Pediatric Cancer Genome Project (PCGP) sequenced whole genomes of 595 patients, whole exomes of 456 patients, and both whole genomes and exomes of 69 patients; whole exomes were sequenced from two control cohorts of 966 and 723 individuals.
Disclosures: Dr. Zhang reported having no disclosures. One coauthor reported financial ties to an industry source.
AGA convened GI and hepatology societies to develop consensus principles on MOC
AGA, in its role as a leader and reformer in the dialogue with the American Board of Internal Medicine (ABIM) on reforming the maintenance of certification (MOC) process, convened the gastroenterology and hepatology societies, including ACG, ASGE, AASLD, ANMS and NASPGHAN to develop a set of consensus principles on this issue at the end of October. AGA shared the following consensus principles with ABIM:
• MOC needs to be simpler, less intrusive, and less expensive.
• We, the societies listed above, support ending the high-stakes, every-10-years exam.
• We, the societies listed above, do not support closed-book assessments as they do not represent the current realities of medicine in the digital age.
• We, the societies listed above, support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
• We, the societies listed above, support the concept that, for the many diplomates who specialize in certain areas of gastroenterology and hepatology, MOC should not need to include high-stakes assessments of areas where the diplomate may not practice.
For more information, review AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP, which was authored by AGA’s education and training councilor, Dr. Suzanne Rose, and a group of AGA colleagues.
AGA, in its role as a leader and reformer in the dialogue with the American Board of Internal Medicine (ABIM) on reforming the maintenance of certification (MOC) process, convened the gastroenterology and hepatology societies, including ACG, ASGE, AASLD, ANMS and NASPGHAN to develop a set of consensus principles on this issue at the end of October. AGA shared the following consensus principles with ABIM:
• MOC needs to be simpler, less intrusive, and less expensive.
• We, the societies listed above, support ending the high-stakes, every-10-years exam.
• We, the societies listed above, do not support closed-book assessments as they do not represent the current realities of medicine in the digital age.
• We, the societies listed above, support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
• We, the societies listed above, support the concept that, for the many diplomates who specialize in certain areas of gastroenterology and hepatology, MOC should not need to include high-stakes assessments of areas where the diplomate may not practice.
For more information, review AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP, which was authored by AGA’s education and training councilor, Dr. Suzanne Rose, and a group of AGA colleagues.
AGA, in its role as a leader and reformer in the dialogue with the American Board of Internal Medicine (ABIM) on reforming the maintenance of certification (MOC) process, convened the gastroenterology and hepatology societies, including ACG, ASGE, AASLD, ANMS and NASPGHAN to develop a set of consensus principles on this issue at the end of October. AGA shared the following consensus principles with ABIM:
• MOC needs to be simpler, less intrusive, and less expensive.
• We, the societies listed above, support ending the high-stakes, every-10-years exam.
• We, the societies listed above, do not support closed-book assessments as they do not represent the current realities of medicine in the digital age.
• We, the societies listed above, support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
• We, the societies listed above, support the concept that, for the many diplomates who specialize in certain areas of gastroenterology and hepatology, MOC should not need to include high-stakes assessments of areas where the diplomate may not practice.
For more information, review AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP, which was authored by AGA’s education and training councilor, Dr. Suzanne Rose, and a group of AGA colleagues.
Law & Medicine: To whom do doctors owe a duty?
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Adalimumab posts strong early results in refractory JIA-associated uveitis
SAN FRANCISCO – The monoclonal antibody adalimumab was more than twice as effective as was placebo for treating methotrexate-refractory uveitis in children with idiopathic arthritis, according to early results from the largest-ever double-blind, randomized, controlled trial of the disease.
Because the difference in time to treatment failure met the prespecified efficacy endpoint, investigators halted the trial after enrolling 90 patients, said Dr. Athimalaipet V. Ramanan, professor of pediatric rheumatology at the University of Bristol (England). But adalimumab also was associated with about three times more infections and other serious adverse events, compared with placebo, he reported in a late-breaker session at the annual meeting of the American College of Rheumatology. “We will, of course, be looking at the long-term data to evaluate cost-effectiveness, as well as safety,” he added.
About one in five children with juvenile idiopathic arthritis (JIA) develops uveitis, which threatens sight and is the most common type of pediatric intraocular inflammation. First-line methotrexate can substantially improve articular inflammation in JIA, but had not been studied in randomized, controlled trials of JIA-associated uveitis. Based on adalimumab’s success in treating the articular aspects of JIA, the investigators studied the biologic in patients aged 2-18 years who had active JIA-associated uveitis despite at least 12 weeks of stable methotrexate therapy. Most cases were mild to moderate, including mild flare and little vitreous haze. The average age of the patients was 9 years, about three-quarters were girls, and about half tested positive for antinuclear antibodies. All patients continued on methotrexate and were randomized 1:2 to subcutaneous, biweekly injections of placebo or weight-based adalimumab.
Patients in the adalimumab arm were about 75% less likely than were controls to fail treatment – that is, to meet Standardization of the Uveitis Nomenclature (SUN) criteria for progressive anterior segment inflammation (hazard ratio, 0.25; 95% confidence interval, 0.12-0.49; P less than .0001). The median time to treatment failure was about 20 weeks for the control arm, and nearly 60 weeks for the adalimumab arm. Specific definitions of treatment failure included a consecutive, two-step rise from baseline in the SUN cell activity score, sustained failure to improve from baseline grade 3 inflammation, persistent grade 1 or 2 inflammation after 6 months of treatment, and worsening ocular comorbidities, Dr. Ramanan said. Adalimumab also was associated with improved SUN scores, although the researchers are still analyzing those data, he added.
Serious adverse events occurred in 22% of patients who received adalimumab, Dr. Ramanan reported. These most often consisted of streptococcal disease, varicella, and other infections for which the immunomodulator has a black box warning. Although only 7% of the control group experienced serious adverse events, these included two flares of uveitis that caused patients to withdraw.
The National Institute for Health Research Health Technology Assessment Programme and Arthritis Research UK funded the study. Dr. Ramanan and one coauthor reported having received consulting fees from AbbVie, maker of Humira. AbbVie reviewed and commented on the abstract, but did not participate in trial design or data analysis.
SAN FRANCISCO – The monoclonal antibody adalimumab was more than twice as effective as was placebo for treating methotrexate-refractory uveitis in children with idiopathic arthritis, according to early results from the largest-ever double-blind, randomized, controlled trial of the disease.
Because the difference in time to treatment failure met the prespecified efficacy endpoint, investigators halted the trial after enrolling 90 patients, said Dr. Athimalaipet V. Ramanan, professor of pediatric rheumatology at the University of Bristol (England). But adalimumab also was associated with about three times more infections and other serious adverse events, compared with placebo, he reported in a late-breaker session at the annual meeting of the American College of Rheumatology. “We will, of course, be looking at the long-term data to evaluate cost-effectiveness, as well as safety,” he added.
About one in five children with juvenile idiopathic arthritis (JIA) develops uveitis, which threatens sight and is the most common type of pediatric intraocular inflammation. First-line methotrexate can substantially improve articular inflammation in JIA, but had not been studied in randomized, controlled trials of JIA-associated uveitis. Based on adalimumab’s success in treating the articular aspects of JIA, the investigators studied the biologic in patients aged 2-18 years who had active JIA-associated uveitis despite at least 12 weeks of stable methotrexate therapy. Most cases were mild to moderate, including mild flare and little vitreous haze. The average age of the patients was 9 years, about three-quarters were girls, and about half tested positive for antinuclear antibodies. All patients continued on methotrexate and were randomized 1:2 to subcutaneous, biweekly injections of placebo or weight-based adalimumab.
Patients in the adalimumab arm were about 75% less likely than were controls to fail treatment – that is, to meet Standardization of the Uveitis Nomenclature (SUN) criteria for progressive anterior segment inflammation (hazard ratio, 0.25; 95% confidence interval, 0.12-0.49; P less than .0001). The median time to treatment failure was about 20 weeks for the control arm, and nearly 60 weeks for the adalimumab arm. Specific definitions of treatment failure included a consecutive, two-step rise from baseline in the SUN cell activity score, sustained failure to improve from baseline grade 3 inflammation, persistent grade 1 or 2 inflammation after 6 months of treatment, and worsening ocular comorbidities, Dr. Ramanan said. Adalimumab also was associated with improved SUN scores, although the researchers are still analyzing those data, he added.
Serious adverse events occurred in 22% of patients who received adalimumab, Dr. Ramanan reported. These most often consisted of streptococcal disease, varicella, and other infections for which the immunomodulator has a black box warning. Although only 7% of the control group experienced serious adverse events, these included two flares of uveitis that caused patients to withdraw.
The National Institute for Health Research Health Technology Assessment Programme and Arthritis Research UK funded the study. Dr. Ramanan and one coauthor reported having received consulting fees from AbbVie, maker of Humira. AbbVie reviewed and commented on the abstract, but did not participate in trial design or data analysis.
SAN FRANCISCO – The monoclonal antibody adalimumab was more than twice as effective as was placebo for treating methotrexate-refractory uveitis in children with idiopathic arthritis, according to early results from the largest-ever double-blind, randomized, controlled trial of the disease.
Because the difference in time to treatment failure met the prespecified efficacy endpoint, investigators halted the trial after enrolling 90 patients, said Dr. Athimalaipet V. Ramanan, professor of pediatric rheumatology at the University of Bristol (England). But adalimumab also was associated with about three times more infections and other serious adverse events, compared with placebo, he reported in a late-breaker session at the annual meeting of the American College of Rheumatology. “We will, of course, be looking at the long-term data to evaluate cost-effectiveness, as well as safety,” he added.
About one in five children with juvenile idiopathic arthritis (JIA) develops uveitis, which threatens sight and is the most common type of pediatric intraocular inflammation. First-line methotrexate can substantially improve articular inflammation in JIA, but had not been studied in randomized, controlled trials of JIA-associated uveitis. Based on adalimumab’s success in treating the articular aspects of JIA, the investigators studied the biologic in patients aged 2-18 years who had active JIA-associated uveitis despite at least 12 weeks of stable methotrexate therapy. Most cases were mild to moderate, including mild flare and little vitreous haze. The average age of the patients was 9 years, about three-quarters were girls, and about half tested positive for antinuclear antibodies. All patients continued on methotrexate and were randomized 1:2 to subcutaneous, biweekly injections of placebo or weight-based adalimumab.
Patients in the adalimumab arm were about 75% less likely than were controls to fail treatment – that is, to meet Standardization of the Uveitis Nomenclature (SUN) criteria for progressive anterior segment inflammation (hazard ratio, 0.25; 95% confidence interval, 0.12-0.49; P less than .0001). The median time to treatment failure was about 20 weeks for the control arm, and nearly 60 weeks for the adalimumab arm. Specific definitions of treatment failure included a consecutive, two-step rise from baseline in the SUN cell activity score, sustained failure to improve from baseline grade 3 inflammation, persistent grade 1 or 2 inflammation after 6 months of treatment, and worsening ocular comorbidities, Dr. Ramanan said. Adalimumab also was associated with improved SUN scores, although the researchers are still analyzing those data, he added.
Serious adverse events occurred in 22% of patients who received adalimumab, Dr. Ramanan reported. These most often consisted of streptococcal disease, varicella, and other infections for which the immunomodulator has a black box warning. Although only 7% of the control group experienced serious adverse events, these included two flares of uveitis that caused patients to withdraw.
The National Institute for Health Research Health Technology Assessment Programme and Arthritis Research UK funded the study. Dr. Ramanan and one coauthor reported having received consulting fees from AbbVie, maker of Humira. AbbVie reviewed and commented on the abstract, but did not participate in trial design or data analysis.
AT THE ACR ANNUAL MEETING
Key clinical point: Adalimumab may effectively treat methotrexate-refractory JIA-associated uveitis.
Major finding: Patients in the adalimumab arm were about 75% less likely than were controls to meet SUN criteria for progressive anterior segment inflammation (HR, 0.25; P less than .0001). A total of 21.7% experienced a serious adverse event, compared with 6.7% of the control group.
Data source: A randomized, double-blinded trial of 90 patients who received optimized methotrexate plus either placebo or weight-based adalimumab.
Disclosures: The National Institute for Health Research Health Technology Assessment Programme and Arthritis Research UK funded the study. Dr. Ramanan and one coauthor reported having received consulting fees from AbbVie, maker of Humira. AbbVie reviewed and commented on the abstract, but did not participate in trial design or data analysis.
Blistering Diseases in Newborns
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which congenital blistering condition is caused by a mast cell growth factor receptor (KIT) mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome
1. Which congenital blistering condition is caused by a mast cell growth factor (KIT) receptor mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which congenital blistering condition is caused by a mast cell growth factor receptor (KIT) mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome
1. Which congenital blistering condition is caused by a mast cell growth factor (KIT) receptor mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which congenital blistering condition is caused by a mast cell growth factor receptor (KIT) mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome
1. Which congenital blistering condition is caused by a mast cell growth factor (KIT) receptor mutation?
a. aplasia cutis congenita
b. bullous mastocytosis
c. congenital erosive and vesicular dermatosis
d. epidermolysis bullosa simplex
e. ichthyosis bullosa of Siemens
2. What gene mutation is present in acrodermatitis enteropathica?
a. collagen VII
b. keratin 2e
c. mast cell growth factor receptor
d. NF-κB essential modulator
e. solute carrier family 39 (zinc transporter), member 4
3. Which congenital blistering disease is associated with an increased risk of squamous cell carcinoma in adult patients?
a. aplasia cutis congenita
b. bullous mastocytosis
c. Kindler syndrome
d. pemphigus syphiliticus
e. varicella
4. Which congenital blistering condition can be caused by prenatal exposure to methimazole?
a. aplasia cutis congenita
b. bullous mastocytosis
c. dystrophic epidermolysis bullosa
d. Kindler syndrome
e. pemphigus syphiliticus
5. Which congenital blistering condition is caused by a mutation in transglutaminase 5?
a. acral peeling skin syndrome
b. aplasia cutis congenita
c. bullous mastocytosis
d. dystrophic epidermolysis bullosa
e. Kindler syndrome