User login
AHA: It’s best to have a cardiac arrest in Midwest
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr. Aiham Albaeni reported at the American Heart Association scientific sessions.
He presented an analysis of 155,592 adults who survived at least until hospital admission following non–trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer U.S. inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr. Albaeni of Johns Hopkins University, Baltimore.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care – that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission – were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10%-12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr. Albaeni.
He reported having no financial conflicts of interest regarding his study.
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr. Aiham Albaeni reported at the American Heart Association scientific sessions.
He presented an analysis of 155,592 adults who survived at least until hospital admission following non–trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer U.S. inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr. Albaeni of Johns Hopkins University, Baltimore.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care – that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission – were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10%-12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr. Albaeni.
He reported having no financial conflicts of interest regarding his study.
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr. Aiham Albaeni reported at the American Heart Association scientific sessions.
He presented an analysis of 155,592 adults who survived at least until hospital admission following non–trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer U.S. inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr. Albaeni of Johns Hopkins University, Baltimore.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care – that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission – were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10%-12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr. Albaeni.
He reported having no financial conflicts of interest regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Substantial and as-yet unexplained regional differences in survival and total hospital charges following out-of-hospital cardiac arrest exist across the United States.
Major finding: Mortality among adults hospitalized after experiencing out-of-hospital cardiac arrest was 14% lower in the Midwest than in the Northeast.
Data source: A retrospective analysis of data from the Nationwide Inpatient Sample for 2002-2012 that included 155,592 adults with out-of-hospital cardiac arrest who survived to hospital admission.
Disclosures: The presenter reported having no financial conflicts of interest.
Postsurgical Analgesic Found to Decrease Opioid Use, Hospital Stay, and Readmission Rates After Knee Replacement Surgery
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
Cutaneous Leishmaniasis: An Emerging Infectious Disease in Travelers
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
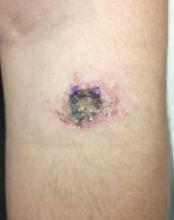
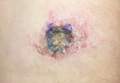
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
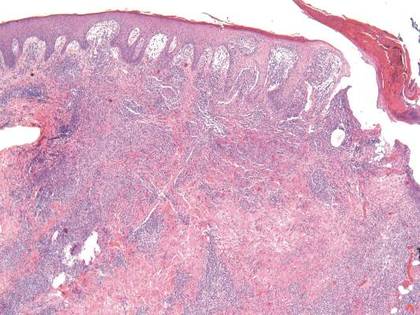
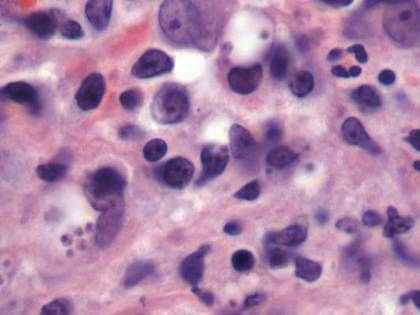
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.
| 
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.

| 
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.

| 
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
Practice Points
- Cutaneous leishmaniasis is an emerging infectious disease that may be misdiagnosed due to its rarity and varied clinical presentation as well as the limited use of tissue biopsy in general practice.
- United States health care practitioners who evaluate patients with new isolated skin lesions and a history of recent travel to Mexico or South or Central Americas should consider cutaneous leishmaniasis in the differential diagnosis.
- Whenever possible, travelers to rural areas of Mexico and South and Central Americas should be educated about strategies to avoid arthropod bites, such as wearing protective clothing and using insect repellents.
Companies abuse orphan drug designation, team says

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()
Group uses lettuce to produce clotting factor on large scale

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()
FDA approves ixazomib for MM

The US Food and Drug Administration (FDA) has approved ixazomib (Ninlaro), the first oral proteasome inhibitor, for use in combination with lenalidomide and dexamethasone to treat multiple myeloma (MM) patients who have received at least 1 prior therapy.
The FDA previously granted ixazomib priority review and orphan designation.
The regulatory submission for ixazomib was primarily based on results from the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior progression-free survival when compared to patients who received placebo plus lenalidomide and dexamethasone.
Results from TOURMALINE-MM1 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 727) in December.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Ixazomib is marketed by Takeda Pharmaceuticals. ![]()

The US Food and Drug Administration (FDA) has approved ixazomib (Ninlaro), the first oral proteasome inhibitor, for use in combination with lenalidomide and dexamethasone to treat multiple myeloma (MM) patients who have received at least 1 prior therapy.
The FDA previously granted ixazomib priority review and orphan designation.
The regulatory submission for ixazomib was primarily based on results from the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior progression-free survival when compared to patients who received placebo plus lenalidomide and dexamethasone.
Results from TOURMALINE-MM1 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 727) in December.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Ixazomib is marketed by Takeda Pharmaceuticals. ![]()

The US Food and Drug Administration (FDA) has approved ixazomib (Ninlaro), the first oral proteasome inhibitor, for use in combination with lenalidomide and dexamethasone to treat multiple myeloma (MM) patients who have received at least 1 prior therapy.
The FDA previously granted ixazomib priority review and orphan designation.
The regulatory submission for ixazomib was primarily based on results from the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior progression-free survival when compared to patients who received placebo plus lenalidomide and dexamethasone.
Results from TOURMALINE-MM1 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 727) in December.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Ixazomib is marketed by Takeda Pharmaceuticals. ![]()
EC approves carfilzomib for relapsed MM

Photo courtesy of Amgen
The European Commission (EC) has granted marketing authorization for carfilzomib (Kyprolis) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
Carfilzomib is the first irreversible proteasome inhibitor approved in the European Union (EU) as part of combination treatment for patients with relapsed MM.
Carfilzomib was granted orphan designation in the EU in 2008. The drug also received accelerated assessment, which shortened the review period from 210 days to 150 days.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan.
ASPIRE trial
The EC approved carfilzomib based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. The median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Global development
In the US, carfilzomib is approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval in the US as a single agent to treat MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of the completion of last therapy.
Amgen plans to submit data from the phase 3 ENDEAVOR trial for potential authorization of carfilzomib in combination with dexamethasone in the EU.
This data serves as the basis of the supplemental new drug application of carfilzomib in combination with dexamethasone for patients with relapsed MM, which has been accepted for priority review in the US.
In addition to the US and EU, carfilzomib is approved for use in Argentina, Israel, Kuwait, Mexico, Thailand, and Colombia. Additional regulatory applications for the drug have been submitted to health authorities worldwide. ![]()

Photo courtesy of Amgen
The European Commission (EC) has granted marketing authorization for carfilzomib (Kyprolis) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
Carfilzomib is the first irreversible proteasome inhibitor approved in the European Union (EU) as part of combination treatment for patients with relapsed MM.
Carfilzomib was granted orphan designation in the EU in 2008. The drug also received accelerated assessment, which shortened the review period from 210 days to 150 days.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan.
ASPIRE trial
The EC approved carfilzomib based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. The median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Global development
In the US, carfilzomib is approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval in the US as a single agent to treat MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of the completion of last therapy.
Amgen plans to submit data from the phase 3 ENDEAVOR trial for potential authorization of carfilzomib in combination with dexamethasone in the EU.
This data serves as the basis of the supplemental new drug application of carfilzomib in combination with dexamethasone for patients with relapsed MM, which has been accepted for priority review in the US.
In addition to the US and EU, carfilzomib is approved for use in Argentina, Israel, Kuwait, Mexico, Thailand, and Colombia. Additional regulatory applications for the drug have been submitted to health authorities worldwide. ![]()

Photo courtesy of Amgen
The European Commission (EC) has granted marketing authorization for carfilzomib (Kyprolis) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
Carfilzomib is the first irreversible proteasome inhibitor approved in the European Union (EU) as part of combination treatment for patients with relapsed MM.
Carfilzomib was granted orphan designation in the EU in 2008. The drug also received accelerated assessment, which shortened the review period from 210 days to 150 days.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan.
ASPIRE trial
The EC approved carfilzomib based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. The median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Global development
In the US, carfilzomib is approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval in the US as a single agent to treat MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of the completion of last therapy.
Amgen plans to submit data from the phase 3 ENDEAVOR trial for potential authorization of carfilzomib in combination with dexamethasone in the EU.
This data serves as the basis of the supplemental new drug application of carfilzomib in combination with dexamethasone for patients with relapsed MM, which has been accepted for priority review in the US.
In addition to the US and EU, carfilzomib is approved for use in Argentina, Israel, Kuwait, Mexico, Thailand, and Colombia. Additional regulatory applications for the drug have been submitted to health authorities worldwide. ![]()
Do Heavier Patients Require Fewer Blood Transfusions In Hip, Knee Replacement Surgery?
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
Transient Reactive Papulotranslucent Acrokeratoderma: A Report of 3 Cases Showing Excellent Response to Topical Calcipotriene
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.
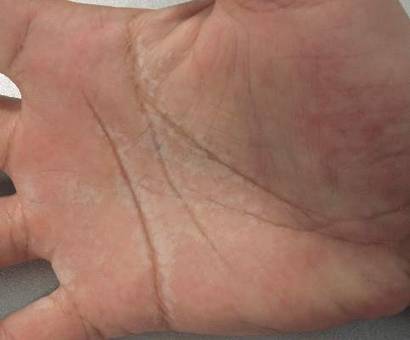
| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||
| 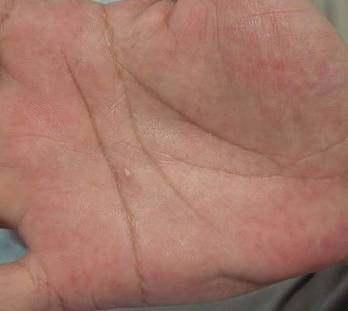
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.

| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||

| 
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.

| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||

| 
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
Sleep medicine specialists issue statement on drowsy driving
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.
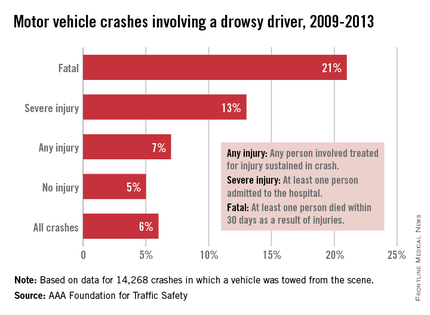
AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.

AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.

AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
FROM JOURNAL OF CLINICAL SLEEP MEDICINE