User login
New antifungals effective with shorter treatment course for tinea pedis
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Newer apheresis system appears superior to standard
Photo by ec-jpr
ANAHEIM, CA—A newer, more streamlined apheresis system yields more CD34+ cells from stem cell transplant donors than a previous system, according to a new study.
Researchers used both tools—the COBE Spectra Apheresis System and the Spectra Optia Apheresis System—to collect mononuclear cells (MNCs) from healthy donors and found the collection efficiency and yield was superior with the Spectra Optia.
There were no unanticipated or serious adverse events with either system, and the frequency of treatment-emergent adverse events did not differ according to the system used.
Jose A. Cancelas, MD, PhD, of Hoxworth Blood Center in Cincinnati, Ohio, presented the results of this research at the 2015 AABB Annual Meeting (abstract S21-020A). The study was supported by Terumo BCT, the company that makes both systems.
The COBE Spectra Apheresis System collects MNCs via single-step processing and separation. It has been the gold standard for hematopoietic stem and progenitor cell collection since 1987, Dr Cancelas noted.
The newer Spectra Optia Apheresis System uses optical sensors for tracking the separation process and real-time electronic adjustment of plasma pump velocity (automatic interface management). A single-step, continuous MNC collection (CMNC) protocol, which was recently approved for use with this system in the US, is intended to increase automation and MNC collection reproducibility.
To compare the 2 systems, Dr Cancelas and his colleagues conducted a prospective, randomized, crossover study of 22 healthy donors. They had a mean age of 35 and a mean body mass index of 34.2 kg/m2.
The donors underwent 2 MNC collections, first with one apheresis system and then the other. Both times, the donors underwent apheresis on Days 5 and 6 after standard MNC mobilization with granulocyte colony-stimulating factor (G-CSF at 10 mg/kg/day) through Day 5. After the first collection, there was a 2-week washout period.
The study’s primary endpoint was CD34+ cell collection efficiency, which was the percentage of cells collected using the averaged pre/post-collection cell counts as the denominator (CE1). The secondary endpoint was also CD34+ cell collection efficiency, but this was the percentage of cells collected using only the pre-collection cell count as a denominator (CE2).
The researchers also assessed the CD34+ cell yield (CD34+ cells/kg), MNC product contamination/purity, procedure time, product volume, the need for operator involvement, and safety.
Results
All collections processed 1.5 times the total blood volume, and the procedures took nearly 2.5 hours, with no real time difference between the 2 systems.
The average flow rates were 66 mL/minute with the Spectra Optia and 68 mL/minute with COBE Spectra. Product volumes were 143 mL and 139 mL, respectively.
The Optia proved significantly superior to the COBE system with regard to CE1, CE2, and the CD34+ yield.
The mean CD34+ CE1 was 85% with Optia and 66.2% with COBE (P<0.001). The mean CD34+ CE2 was 62% and 48.4%, respectively (P<0.001). And the mean CD34+ yield (cells/kg) was 4.5 and 3.58, respectively (P=0.001).
In addition, granulocyte contamination was lower with the Optia system than the COBE system. The mean granulocyte yield was 7.7 x109 and 10.6 x109 granulocytes per unit, respectively (P=0.022).
However, red blood cell and platelet contaminations were similar between the systems. The mean red blood cell volume was 7.4 mL with Optia and 7.0 mL with COBE (P=0.660). And the mean platelet yield was 4.3 x1011 and 4.6 x1011, respectively (P=0.081).
Overall, there was no significant difference between the Optia and COBE systems in the need for operator adjustments, although there was a trend toward fewer adjustments with the Optia system. It required a median of 5.5 adjustments (range, 0-12), and the COBE system required a median of 6.5 adjustments (range, 1-14).
Dr Cancelas said the frequency of treatment-emergent adverse events did not differ according to the system used. And there were no unanticipated or serious adverse events.
The most frequently reported pre-collection treatment-emergent adverse events were back pain (n=10, 44%), bone pain (n=9, 39%), and fatigue (n=5, 22%).
“These results demonstrate that the Optia CMNC procedure is a safe and efficient means of collecting CD34+ cells in G-CSF mobilized donors,” Dr Cancelas said.
“The Optia collection efficiencies for CD34+ cells were significantly superior to the COBE . . . . And the Optia with automatic interface management system represents a technological advance in our ability to collect CD34+ cells.” ![]()

Photo by ec-jpr
ANAHEIM, CA—A newer, more streamlined apheresis system yields more CD34+ cells from stem cell transplant donors than a previous system, according to a new study.
Researchers used both tools—the COBE Spectra Apheresis System and the Spectra Optia Apheresis System—to collect mononuclear cells (MNCs) from healthy donors and found the collection efficiency and yield was superior with the Spectra Optia.
There were no unanticipated or serious adverse events with either system, and the frequency of treatment-emergent adverse events did not differ according to the system used.
Jose A. Cancelas, MD, PhD, of Hoxworth Blood Center in Cincinnati, Ohio, presented the results of this research at the 2015 AABB Annual Meeting (abstract S21-020A). The study was supported by Terumo BCT, the company that makes both systems.
The COBE Spectra Apheresis System collects MNCs via single-step processing and separation. It has been the gold standard for hematopoietic stem and progenitor cell collection since 1987, Dr Cancelas noted.
The newer Spectra Optia Apheresis System uses optical sensors for tracking the separation process and real-time electronic adjustment of plasma pump velocity (automatic interface management). A single-step, continuous MNC collection (CMNC) protocol, which was recently approved for use with this system in the US, is intended to increase automation and MNC collection reproducibility.
To compare the 2 systems, Dr Cancelas and his colleagues conducted a prospective, randomized, crossover study of 22 healthy donors. They had a mean age of 35 and a mean body mass index of 34.2 kg/m2.
The donors underwent 2 MNC collections, first with one apheresis system and then the other. Both times, the donors underwent apheresis on Days 5 and 6 after standard MNC mobilization with granulocyte colony-stimulating factor (G-CSF at 10 mg/kg/day) through Day 5. After the first collection, there was a 2-week washout period.
The study’s primary endpoint was CD34+ cell collection efficiency, which was the percentage of cells collected using the averaged pre/post-collection cell counts as the denominator (CE1). The secondary endpoint was also CD34+ cell collection efficiency, but this was the percentage of cells collected using only the pre-collection cell count as a denominator (CE2).
The researchers also assessed the CD34+ cell yield (CD34+ cells/kg), MNC product contamination/purity, procedure time, product volume, the need for operator involvement, and safety.
Results
All collections processed 1.5 times the total blood volume, and the procedures took nearly 2.5 hours, with no real time difference between the 2 systems.
The average flow rates were 66 mL/minute with the Spectra Optia and 68 mL/minute with COBE Spectra. Product volumes were 143 mL and 139 mL, respectively.
The Optia proved significantly superior to the COBE system with regard to CE1, CE2, and the CD34+ yield.
The mean CD34+ CE1 was 85% with Optia and 66.2% with COBE (P<0.001). The mean CD34+ CE2 was 62% and 48.4%, respectively (P<0.001). And the mean CD34+ yield (cells/kg) was 4.5 and 3.58, respectively (P=0.001).
In addition, granulocyte contamination was lower with the Optia system than the COBE system. The mean granulocyte yield was 7.7 x109 and 10.6 x109 granulocytes per unit, respectively (P=0.022).
However, red blood cell and platelet contaminations were similar between the systems. The mean red blood cell volume was 7.4 mL with Optia and 7.0 mL with COBE (P=0.660). And the mean platelet yield was 4.3 x1011 and 4.6 x1011, respectively (P=0.081).
Overall, there was no significant difference between the Optia and COBE systems in the need for operator adjustments, although there was a trend toward fewer adjustments with the Optia system. It required a median of 5.5 adjustments (range, 0-12), and the COBE system required a median of 6.5 adjustments (range, 1-14).
Dr Cancelas said the frequency of treatment-emergent adverse events did not differ according to the system used. And there were no unanticipated or serious adverse events.
The most frequently reported pre-collection treatment-emergent adverse events were back pain (n=10, 44%), bone pain (n=9, 39%), and fatigue (n=5, 22%).
“These results demonstrate that the Optia CMNC procedure is a safe and efficient means of collecting CD34+ cells in G-CSF mobilized donors,” Dr Cancelas said.
“The Optia collection efficiencies for CD34+ cells were significantly superior to the COBE . . . . And the Optia with automatic interface management system represents a technological advance in our ability to collect CD34+ cells.” ![]()

Photo by ec-jpr
ANAHEIM, CA—A newer, more streamlined apheresis system yields more CD34+ cells from stem cell transplant donors than a previous system, according to a new study.
Researchers used both tools—the COBE Spectra Apheresis System and the Spectra Optia Apheresis System—to collect mononuclear cells (MNCs) from healthy donors and found the collection efficiency and yield was superior with the Spectra Optia.
There were no unanticipated or serious adverse events with either system, and the frequency of treatment-emergent adverse events did not differ according to the system used.
Jose A. Cancelas, MD, PhD, of Hoxworth Blood Center in Cincinnati, Ohio, presented the results of this research at the 2015 AABB Annual Meeting (abstract S21-020A). The study was supported by Terumo BCT, the company that makes both systems.
The COBE Spectra Apheresis System collects MNCs via single-step processing and separation. It has been the gold standard for hematopoietic stem and progenitor cell collection since 1987, Dr Cancelas noted.
The newer Spectra Optia Apheresis System uses optical sensors for tracking the separation process and real-time electronic adjustment of plasma pump velocity (automatic interface management). A single-step, continuous MNC collection (CMNC) protocol, which was recently approved for use with this system in the US, is intended to increase automation and MNC collection reproducibility.
To compare the 2 systems, Dr Cancelas and his colleagues conducted a prospective, randomized, crossover study of 22 healthy donors. They had a mean age of 35 and a mean body mass index of 34.2 kg/m2.
The donors underwent 2 MNC collections, first with one apheresis system and then the other. Both times, the donors underwent apheresis on Days 5 and 6 after standard MNC mobilization with granulocyte colony-stimulating factor (G-CSF at 10 mg/kg/day) through Day 5. After the first collection, there was a 2-week washout period.
The study’s primary endpoint was CD34+ cell collection efficiency, which was the percentage of cells collected using the averaged pre/post-collection cell counts as the denominator (CE1). The secondary endpoint was also CD34+ cell collection efficiency, but this was the percentage of cells collected using only the pre-collection cell count as a denominator (CE2).
The researchers also assessed the CD34+ cell yield (CD34+ cells/kg), MNC product contamination/purity, procedure time, product volume, the need for operator involvement, and safety.
Results
All collections processed 1.5 times the total blood volume, and the procedures took nearly 2.5 hours, with no real time difference between the 2 systems.
The average flow rates were 66 mL/minute with the Spectra Optia and 68 mL/minute with COBE Spectra. Product volumes were 143 mL and 139 mL, respectively.
The Optia proved significantly superior to the COBE system with regard to CE1, CE2, and the CD34+ yield.
The mean CD34+ CE1 was 85% with Optia and 66.2% with COBE (P<0.001). The mean CD34+ CE2 was 62% and 48.4%, respectively (P<0.001). And the mean CD34+ yield (cells/kg) was 4.5 and 3.58, respectively (P=0.001).
In addition, granulocyte contamination was lower with the Optia system than the COBE system. The mean granulocyte yield was 7.7 x109 and 10.6 x109 granulocytes per unit, respectively (P=0.022).
However, red blood cell and platelet contaminations were similar between the systems. The mean red blood cell volume was 7.4 mL with Optia and 7.0 mL with COBE (P=0.660). And the mean platelet yield was 4.3 x1011 and 4.6 x1011, respectively (P=0.081).
Overall, there was no significant difference between the Optia and COBE systems in the need for operator adjustments, although there was a trend toward fewer adjustments with the Optia system. It required a median of 5.5 adjustments (range, 0-12), and the COBE system required a median of 6.5 adjustments (range, 1-14).
Dr Cancelas said the frequency of treatment-emergent adverse events did not differ according to the system used. And there were no unanticipated or serious adverse events.
The most frequently reported pre-collection treatment-emergent adverse events were back pain (n=10, 44%), bone pain (n=9, 39%), and fatigue (n=5, 22%).
“These results demonstrate that the Optia CMNC procedure is a safe and efficient means of collecting CD34+ cells in G-CSF mobilized donors,” Dr Cancelas said.
“The Optia collection efficiencies for CD34+ cells were significantly superior to the COBE . . . . And the Optia with automatic interface management system represents a technological advance in our ability to collect CD34+ cells.” ![]()
FDA grants drug priority review as FL therapy

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application for obinutuzumab (Gazyva) to treat patients with follicular lymphoma (FL) who have relapsed after or are refractory to a rituximab-containing regimen.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug is already FDA-approved for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
The FDA has accepted the supplemental application for obinutuzumab in FL based on results of the phase 3 GADOLIN study.
Interim results from this trial were presented at the 2015 ASCO Annual Meeting (abstract LBA8502). Additional data are scheduled to be presented at the 2015 ASH Annual Meeting in December (abstracts 1532 and 3978).
GADOLIN study
The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including FL, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab (OB) followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
In all, 156 patients completed induction in the OB arm, as did 129 patients in the control arm. Thirty-six patients completed maintenance with obinutuzumab, and 46 were still receiving maintenance at the time of the interim analysis.
According to an independent radiology facility, 69.2% of patients in the OB arm had responded to treatment at the end of induction, as had 63% of the control arm. The best overall response by the 12-month mark was 78.7% and 76.6%, respectively.
According to the radiology facility, the median progression-free survival (PFS) had not been reached in the OB arm at a median follow-up of 21 months. In the control arm, the median PFS was 14.9 months (P<0.0001).
According to investigators, the median PFS was 29.2 months and 14 months, respectively (P<0.0001).
The median overall survival had not been reached in either arm (P=0.4017). Thirty-four patients (18%) in the OB arm died, as did 41 (20%) in the control arm.
About 99% of patients in the OB arm experienced at least 1 adverse event (AE), as did 98% of patients in the control arm. Severe AEs occurred in 38.1% and 32.8% of patients, respectively, and grade 3/4 AEs occurred in 67% and 62.1%, respectively.
AEs leading to treatment withdrawal occurred in 18% and 15.7% of patients, respectively. And AEs leading to death occurred in 6.2% and 6.1%, respectively. ![]()

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application for obinutuzumab (Gazyva) to treat patients with follicular lymphoma (FL) who have relapsed after or are refractory to a rituximab-containing regimen.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug is already FDA-approved for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
The FDA has accepted the supplemental application for obinutuzumab in FL based on results of the phase 3 GADOLIN study.
Interim results from this trial were presented at the 2015 ASCO Annual Meeting (abstract LBA8502). Additional data are scheduled to be presented at the 2015 ASH Annual Meeting in December (abstracts 1532 and 3978).
GADOLIN study
The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including FL, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab (OB) followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
In all, 156 patients completed induction in the OB arm, as did 129 patients in the control arm. Thirty-six patients completed maintenance with obinutuzumab, and 46 were still receiving maintenance at the time of the interim analysis.
According to an independent radiology facility, 69.2% of patients in the OB arm had responded to treatment at the end of induction, as had 63% of the control arm. The best overall response by the 12-month mark was 78.7% and 76.6%, respectively.
According to the radiology facility, the median progression-free survival (PFS) had not been reached in the OB arm at a median follow-up of 21 months. In the control arm, the median PFS was 14.9 months (P<0.0001).
According to investigators, the median PFS was 29.2 months and 14 months, respectively (P<0.0001).
The median overall survival had not been reached in either arm (P=0.4017). Thirty-four patients (18%) in the OB arm died, as did 41 (20%) in the control arm.
About 99% of patients in the OB arm experienced at least 1 adverse event (AE), as did 98% of patients in the control arm. Severe AEs occurred in 38.1% and 32.8% of patients, respectively, and grade 3/4 AEs occurred in 67% and 62.1%, respectively.
AEs leading to treatment withdrawal occurred in 18% and 15.7% of patients, respectively. And AEs leading to death occurred in 6.2% and 6.1%, respectively. ![]()

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application for obinutuzumab (Gazyva) to treat patients with follicular lymphoma (FL) who have relapsed after or are refractory to a rituximab-containing regimen.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug is already FDA-approved for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
The FDA has accepted the supplemental application for obinutuzumab in FL based on results of the phase 3 GADOLIN study.
Interim results from this trial were presented at the 2015 ASCO Annual Meeting (abstract LBA8502). Additional data are scheduled to be presented at the 2015 ASH Annual Meeting in December (abstracts 1532 and 3978).
GADOLIN study
The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including FL, marginal zone lymphoma, small lymphocytic lymphoma, and Waldenstrom’s macroglobulinemia.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab (OB) followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
In all, 156 patients completed induction in the OB arm, as did 129 patients in the control arm. Thirty-six patients completed maintenance with obinutuzumab, and 46 were still receiving maintenance at the time of the interim analysis.
According to an independent radiology facility, 69.2% of patients in the OB arm had responded to treatment at the end of induction, as had 63% of the control arm. The best overall response by the 12-month mark was 78.7% and 76.6%, respectively.
According to the radiology facility, the median progression-free survival (PFS) had not been reached in the OB arm at a median follow-up of 21 months. In the control arm, the median PFS was 14.9 months (P<0.0001).
According to investigators, the median PFS was 29.2 months and 14 months, respectively (P<0.0001).
The median overall survival had not been reached in either arm (P=0.4017). Thirty-four patients (18%) in the OB arm died, as did 41 (20%) in the control arm.
About 99% of patients in the OB arm experienced at least 1 adverse event (AE), as did 98% of patients in the control arm. Severe AEs occurred in 38.1% and 32.8% of patients, respectively, and grade 3/4 AEs occurred in 67% and 62.1%, respectively.
AEs leading to treatment withdrawal occurred in 18% and 15.7% of patients, respectively. And AEs leading to death occurred in 6.2% and 6.1%, respectively. ![]()
Breast cancer drug may also work in MCL, myeloma
showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()

showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()

showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()
RIC can improve HSCT outcomes in older AML patients

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()
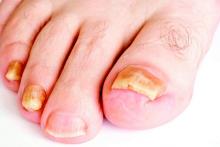
SDEF: Improved responses with newer topical onychomycosis treatments
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Providing ambivalent medical advice
When did some doctors become so wishy-washy?
A large part of what we do is guide people through an often-confusing maze of test results and treatment options. I respect patients’ right to make their own decisions, but it came as a surprise to me to find that some doctors are turning such things over to the patients. After all, doctors are the ones who went through over a decade of training to understand the risks, benefits, and goals of each step for them. Granted, I live in Arizona, where you don’t need a doctor’s order to have labs done. You can research whatever you want on Google, decide what work-up you need, and go get whatever labs you want done.
But back to my original point. Recently, one of my patients was admitted to the hospital, then followed up with me in the office. I looked through his test results and told him what I felt the next step should be, ordered a few things, and wrote an instruction sheet to start daily aspirin. I commented that it surprised me the last hadn’t been done as an inpatient.
His answer? “They said I could if I wanted to, but didn’t make a clear suggestion.” I figured this was a simple miscommunication, so I pulled up the hospital chart on my computer. There I found a note from the attending that said, “The patient was told he may or may not want to take a daily aspirin, and that doing so might or might not be to his benefit.” What on Earth?
I understand there are no guarantees in this job. There’s no crystal ball to know for sure that what we’re doing is right. Any drug can cause serious and unexpected complications. We take calculated risks and hope we come out ahead. But to phrase it like this? Where the patient isn’t given the guidance we’re supposed to provide? What’s the point of even being a doctor?
Since then, I’ve noticed similar phrasing in other charts: “We discussed doing a brain MRI, and she’ll let me know what she decides” and “I told her that starting Lamictal may or may not prevent seizures, and to consider it as something she should or shouldn’t do.”
I’m sure some of it is part of the hurried flight-of-ideas dictations we all do when we’re busy at the hospital. There’s also a component of legalese to make sure that we documented discussing risks with the patient.
But I still don’t get the ambivalence. In similar situations, I provide guidance and advice and tell people what I think they should do. I’m not going to force anyone to do anything they don’t want to. If they disagree, I note it and make whatever suggestions I think will help. In the end, it’s their decision. I get that.
When I take my car to get fixed, I don’t want the mechanic to tell me what may or may not need to be repaired, and I hope patients see me the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When did some doctors become so wishy-washy?
A large part of what we do is guide people through an often-confusing maze of test results and treatment options. I respect patients’ right to make their own decisions, but it came as a surprise to me to find that some doctors are turning such things over to the patients. After all, doctors are the ones who went through over a decade of training to understand the risks, benefits, and goals of each step for them. Granted, I live in Arizona, where you don’t need a doctor’s order to have labs done. You can research whatever you want on Google, decide what work-up you need, and go get whatever labs you want done.
But back to my original point. Recently, one of my patients was admitted to the hospital, then followed up with me in the office. I looked through his test results and told him what I felt the next step should be, ordered a few things, and wrote an instruction sheet to start daily aspirin. I commented that it surprised me the last hadn’t been done as an inpatient.
His answer? “They said I could if I wanted to, but didn’t make a clear suggestion.” I figured this was a simple miscommunication, so I pulled up the hospital chart on my computer. There I found a note from the attending that said, “The patient was told he may or may not want to take a daily aspirin, and that doing so might or might not be to his benefit.” What on Earth?
I understand there are no guarantees in this job. There’s no crystal ball to know for sure that what we’re doing is right. Any drug can cause serious and unexpected complications. We take calculated risks and hope we come out ahead. But to phrase it like this? Where the patient isn’t given the guidance we’re supposed to provide? What’s the point of even being a doctor?
Since then, I’ve noticed similar phrasing in other charts: “We discussed doing a brain MRI, and she’ll let me know what she decides” and “I told her that starting Lamictal may or may not prevent seizures, and to consider it as something she should or shouldn’t do.”
I’m sure some of it is part of the hurried flight-of-ideas dictations we all do when we’re busy at the hospital. There’s also a component of legalese to make sure that we documented discussing risks with the patient.
But I still don’t get the ambivalence. In similar situations, I provide guidance and advice and tell people what I think they should do. I’m not going to force anyone to do anything they don’t want to. If they disagree, I note it and make whatever suggestions I think will help. In the end, it’s their decision. I get that.
When I take my car to get fixed, I don’t want the mechanic to tell me what may or may not need to be repaired, and I hope patients see me the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When did some doctors become so wishy-washy?
A large part of what we do is guide people through an often-confusing maze of test results and treatment options. I respect patients’ right to make their own decisions, but it came as a surprise to me to find that some doctors are turning such things over to the patients. After all, doctors are the ones who went through over a decade of training to understand the risks, benefits, and goals of each step for them. Granted, I live in Arizona, where you don’t need a doctor’s order to have labs done. You can research whatever you want on Google, decide what work-up you need, and go get whatever labs you want done.
But back to my original point. Recently, one of my patients was admitted to the hospital, then followed up with me in the office. I looked through his test results and told him what I felt the next step should be, ordered a few things, and wrote an instruction sheet to start daily aspirin. I commented that it surprised me the last hadn’t been done as an inpatient.
His answer? “They said I could if I wanted to, but didn’t make a clear suggestion.” I figured this was a simple miscommunication, so I pulled up the hospital chart on my computer. There I found a note from the attending that said, “The patient was told he may or may not want to take a daily aspirin, and that doing so might or might not be to his benefit.” What on Earth?
I understand there are no guarantees in this job. There’s no crystal ball to know for sure that what we’re doing is right. Any drug can cause serious and unexpected complications. We take calculated risks and hope we come out ahead. But to phrase it like this? Where the patient isn’t given the guidance we’re supposed to provide? What’s the point of even being a doctor?
Since then, I’ve noticed similar phrasing in other charts: “We discussed doing a brain MRI, and she’ll let me know what she decides” and “I told her that starting Lamictal may or may not prevent seizures, and to consider it as something she should or shouldn’t do.”
I’m sure some of it is part of the hurried flight-of-ideas dictations we all do when we’re busy at the hospital. There’s also a component of legalese to make sure that we documented discussing risks with the patient.
But I still don’t get the ambivalence. In similar situations, I provide guidance and advice and tell people what I think they should do. I’m not going to force anyone to do anything they don’t want to. If they disagree, I note it and make whatever suggestions I think will help. In the end, it’s their decision. I get that.
When I take my car to get fixed, I don’t want the mechanic to tell me what may or may not need to be repaired, and I hope patients see me the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
TCT: Lower MI, thrombosis, higher bleeding with extended DAPT in patients with everolimus-eluting stents
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
AT TCT 2015
Key clinical point: The window of extended DAPT therapy may be narrow in patients stented with an everolimus-eluting stent.
Major finding: Patients randomly assigned to 18 months of thienopyridine plus aspirin after 1 year of DAPT had significantly reduced thrombosis (P = .04) and MI (P = .01) vs. placebo plus aspirin but more bleeding (P = .01).
Data source: Subanalysis of 4,703 patients from a double-blind, international, multisite, randomized, controlled trial of 25,682 patients.
Disclosures: Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
New therapies finding their place in management of follicular lymphoma
SAN FRANCISCO – A variety of emerging therapies are being incorporated into the management of follicular lymphoma, which is typically a long-term endeavor requiring strategic use of multiple treatments, Dr. Andrew D. Zelenetz said at the National Comprehensive Cancer Network (NCCN) 10th Annual Congress: Hematologic Malignancies.
“Follicular lymphoma is a disease of paradox. The reality is that overall survival is excellent, but patients are not going to be able to do that with one treatment; they are going to get a series of treatments,” he said. “Survival is the sum of your exposures to treatment, time on active therapy, time in remission, and actually time with relapse not needing treatment.”
Risk stratification
Overall survival for patients with follicular lymphoma diagnosed today and treated with modern therapy is only slightly inferior to that for age-matched controls, noted Dr. Zelenetz of the department of medicine at Memorial Sloan-Kettering Cancer Center and professor of medicine at Cornell University, both in New York.
But patients who are faring poorly at 12 months (American Society of Hematology [ASH] 2014, Abstract 1664) or at 24 months (J Clin Oncol. 2015;33[23]:2516-22) into care have a much worse prognosis. “This shows the importance of identifying those patients with poor biology, and [the question of] whether we can identify them without treating them first and having them progress,” he said.
Hematologists have historically looked to the Follicular Lymphoma International Prognostic Index (FLIPI) to estimate outcome. “The FLIPI clearly works; it’s an important clinical tool. But the FLIPI high-risk patients are still identifying more than those very high-risk patients,” he said.
Therefore, a clinicogenomic risk model was developed that incorporates seven mutations having poor prognostic impact, the m7-FLIPI (Lancet Oncol. 2015;16[9]:1111-22). Adding the mutations split the previously defined high-risk patients into a group with a prognosis similar to that of low-risk patients and a small group with a very poor prognosis.
“This actually represents something very close to the 20% of patients that we think have bad biology,” Dr. Zelenetz noted. “There will be some additional data at ASH looking at this exact question, because the holy grail is to know when you diagnose someone if they are in that bad-risk group because those are the patients you want to do novel clinical trials on. If your overall survival is equivalent to the general population, it’s going to be hard to ever prove an overall survival advantage for an intervention in an unselected group of patients.”
Advanced-stage disease with low tumor bulk
For patients who have advanced-stage follicular lymphoma but with low tumor bulk, the NCCN endorses a modification of criteria developed by the Follicular Lymphoma Study Group (GELF) in deciding when to start treatment (J Clin Oncol. 1998;16[7]:2332-8).
Roughly a fifth of patients who are eligible for and managed with a watch-and-wait approach will not need chemotherapy or die of their disease in the next 10 years (Lancet. 2003;362[9383]:516-22). Furthermore, this strategy nets a median delay in the need for chemotherapy of 2.6 years.
Compared with observation, treatment with the anti-CD20 antibody rituximab (Rituxan) improves progression-free survival but not overall survival in this setting (ASH 2010, Abstract 6). “Though in selected patients, rituximab may be appropriate as initial treatment for the observable patient, I would argue for the observable patient with no survival disadvantage, the standard of care remains observation,” Dr. Zelenetz said.
Advanced-stage disease requiring treatment
A meta-analysis has shown a clear survival benefit from adding rituximab to chemotherapy (R-chemo) in patients with advanced follicular lymphoma who need treatment (J Natl Cancer Inst. 2007;99[9]:706-14). “Based on the results, it is the standard of care to add rituximab to a chemotherapy backbone, but the optimum R-chemo actually remains undefined and would be customized to the individual clinical situation,” he commented.
A variety of emerging agents are being tested in this setting. Among the subset of patients with untreated follicular lymphoma in a single-center trial, the combination of rituximab with the immunomodulator lenalidomide (Revlimid) yielded an overall response rate of 98% and a complete response rate of 87%, as well as excellent progression-free and overall survival (Lancet Oncol. 2014;15[12]:1311-8). The main grade 3 or 4 toxicity was neutropenia, seen in 35% of all patients studied. Efficacy results were much the same in a multicenter trial (International Conference on Malignant Lymphoma [ICML] 2013, Abstract 63).
This combination is now being tested as front-line therapy for follicular lymphoma in the RELEVANCE (Rituximab and Lenalidomide Versus Any Chemotherapy) phase III trial. “The trial is now done, but we don’t have results, and we won’t have results until 2019, so don’t hold your breath,” Dr. Zelenetz commented. “That’s because this was an unselected trial; we took all patients, all comers. And if you don’t try to identify bad-risk patients, you actually have to do very large trials, and the effect size is relatively small.”
Relapsed and refractory disease
“Many times when patients with follicular lymphoma relapse, they are immediately started on treatment. It’s not necessary and probably in most cases not appropriate. If patients are asymptomatic and have a low tumor burden, they can have a second and a third and even a fourth period of observation, where they don’t need active treatment,” he said. “So I would encourage you to …wait until they actually meet GELF criteria again.”
A key question in this setting is whether patients previously given rituximab can derive benefit from an alternative anti-CD20 antibody. Taking on this question, the GADOLIN trial tested the addition of obinutuzumab (induction plus maintenance) to bendamustine among patients with rituximab-refractory disease (American Society of Clinical Oncology [ASCO] 2015, Abstract LBA8502; ICML 2015, Abstract 123).
Toxicities were generally similar by arm, except for a higher rate of infusion-related reactions with obinutuzumab. The overall response rates were comparable for the two arms, but progression-free survival was better with the combination (median event-free survival, not reached, vs. 14.9 months; hazard ratio, 0.55), and there was a trend for overall survival.
“These curves start separating after 6 months, and 6 months is the time of chemotherapy,” Dr. Zelenetz noted. “So I would argue from these data that the obinutuzumab didn’t add very much to the bendamustine backbone, but actually the obinutuzumab maintenance was effective even in rituximab-refractory patients.”
The combination of lenalidomide and rituximab has been compared with lenalidomide alone in patients with relapsed follicular lymphoma (ASCO 2012, Abstract 8000). The results showed a trend toward better median event-free survival with the combination (2.0 vs. 1.2 years; hazard ratio, 1.9; P = .061) but not overall survival.
In a phase II trial, idelalisib (Zydelig) was tested among patients with indolent non-Hodgkin lymphomas (60% with follicular lymphoma) that were refractory to both rituximab and an alkylator (N Engl J Med. 2014;370:1008-18). Noteworthy grade 3 or worse toxicities included pneumonia and transaminase elevations. The overall response rate was 57%, and the complete response rate was 6%; median progression-free survival was 11 months.
“Idelalisib can be safely combined with other agents including rituximab and bendamustine,” Dr. Zelenetz added (ASH 2014, Abstract 3063). “Interestingly, the overall response seems to be a little higher when you combine it, but it doesn’t seem to matter which drug you combine it with – rituximab, bendamustine, or [both] – you get good overall responses,” ranging from 71% to 85%.
The BCL-2 inhibitor venetoclax (formerly ABT-199/GDC-199) has been tested in non-Hodgkin lymphomas, where it has not been associated with the life-threatening tumor lysis syndrome seen in some other hematologic malignancies (European Hematology Association [EHA] 2015, Davis et al). It yielded an overall response rate of 31% in patients with relatively refractory follicular lymphoma. “This will lead to additional studies in this area,” he predicted.
Finally, nivolumab (Opdivo), a PD-1 immune checkpoint inhibitor, has been evaluated in a phase I study in relapsed or refractory hematologic malignancies, where it was well tolerated (ASH 2014, Abstract 291). Among the small subset of patients with follicular lymphoma, the overall response rate was 40%, prompting initiation of more trials.
Although chimeric antigen receptor (CAR) T-cell therapy is showing promise in various malignancies, Dr. Zelenetz said that other options are probably better avenues for research in follicular lymphoma at present.
“I’m much more interested in the tools that we have now, between the checkpoint inhibitors, the T-cell activators, and the bispecific monoclonal antibodies. I think I can [apply these therapies] with less money for probably less toxicity without the complexity of having to make a customized drug for the patient,” he said. “So I’m not very enthusiastic about CAR T cells in follicular lymphoma.”
SAN FRANCISCO – A variety of emerging therapies are being incorporated into the management of follicular lymphoma, which is typically a long-term endeavor requiring strategic use of multiple treatments, Dr. Andrew D. Zelenetz said at the National Comprehensive Cancer Network (NCCN) 10th Annual Congress: Hematologic Malignancies.
“Follicular lymphoma is a disease of paradox. The reality is that overall survival is excellent, but patients are not going to be able to do that with one treatment; they are going to get a series of treatments,” he said. “Survival is the sum of your exposures to treatment, time on active therapy, time in remission, and actually time with relapse not needing treatment.”
Risk stratification
Overall survival for patients with follicular lymphoma diagnosed today and treated with modern therapy is only slightly inferior to that for age-matched controls, noted Dr. Zelenetz of the department of medicine at Memorial Sloan-Kettering Cancer Center and professor of medicine at Cornell University, both in New York.
But patients who are faring poorly at 12 months (American Society of Hematology [ASH] 2014, Abstract 1664) or at 24 months (J Clin Oncol. 2015;33[23]:2516-22) into care have a much worse prognosis. “This shows the importance of identifying those patients with poor biology, and [the question of] whether we can identify them without treating them first and having them progress,” he said.
Hematologists have historically looked to the Follicular Lymphoma International Prognostic Index (FLIPI) to estimate outcome. “The FLIPI clearly works; it’s an important clinical tool. But the FLIPI high-risk patients are still identifying more than those very high-risk patients,” he said.
Therefore, a clinicogenomic risk model was developed that incorporates seven mutations having poor prognostic impact, the m7-FLIPI (Lancet Oncol. 2015;16[9]:1111-22). Adding the mutations split the previously defined high-risk patients into a group with a prognosis similar to that of low-risk patients and a small group with a very poor prognosis.
“This actually represents something very close to the 20% of patients that we think have bad biology,” Dr. Zelenetz noted. “There will be some additional data at ASH looking at this exact question, because the holy grail is to know when you diagnose someone if they are in that bad-risk group because those are the patients you want to do novel clinical trials on. If your overall survival is equivalent to the general population, it’s going to be hard to ever prove an overall survival advantage for an intervention in an unselected group of patients.”
Advanced-stage disease with low tumor bulk
For patients who have advanced-stage follicular lymphoma but with low tumor bulk, the NCCN endorses a modification of criteria developed by the Follicular Lymphoma Study Group (GELF) in deciding when to start treatment (J Clin Oncol. 1998;16[7]:2332-8).
Roughly a fifth of patients who are eligible for and managed with a watch-and-wait approach will not need chemotherapy or die of their disease in the next 10 years (Lancet. 2003;362[9383]:516-22). Furthermore, this strategy nets a median delay in the need for chemotherapy of 2.6 years.
Compared with observation, treatment with the anti-CD20 antibody rituximab (Rituxan) improves progression-free survival but not overall survival in this setting (ASH 2010, Abstract 6). “Though in selected patients, rituximab may be appropriate as initial treatment for the observable patient, I would argue for the observable patient with no survival disadvantage, the standard of care remains observation,” Dr. Zelenetz said.
Advanced-stage disease requiring treatment
A meta-analysis has shown a clear survival benefit from adding rituximab to chemotherapy (R-chemo) in patients with advanced follicular lymphoma who need treatment (J Natl Cancer Inst. 2007;99[9]:706-14). “Based on the results, it is the standard of care to add rituximab to a chemotherapy backbone, but the optimum R-chemo actually remains undefined and would be customized to the individual clinical situation,” he commented.
A variety of emerging agents are being tested in this setting. Among the subset of patients with untreated follicular lymphoma in a single-center trial, the combination of rituximab with the immunomodulator lenalidomide (Revlimid) yielded an overall response rate of 98% and a complete response rate of 87%, as well as excellent progression-free and overall survival (Lancet Oncol. 2014;15[12]:1311-8). The main grade 3 or 4 toxicity was neutropenia, seen in 35% of all patients studied. Efficacy results were much the same in a multicenter trial (International Conference on Malignant Lymphoma [ICML] 2013, Abstract 63).
This combination is now being tested as front-line therapy for follicular lymphoma in the RELEVANCE (Rituximab and Lenalidomide Versus Any Chemotherapy) phase III trial. “The trial is now done, but we don’t have results, and we won’t have results until 2019, so don’t hold your breath,” Dr. Zelenetz commented. “That’s because this was an unselected trial; we took all patients, all comers. And if you don’t try to identify bad-risk patients, you actually have to do very large trials, and the effect size is relatively small.”
Relapsed and refractory disease
“Many times when patients with follicular lymphoma relapse, they are immediately started on treatment. It’s not necessary and probably in most cases not appropriate. If patients are asymptomatic and have a low tumor burden, they can have a second and a third and even a fourth period of observation, where they don’t need active treatment,” he said. “So I would encourage you to …wait until they actually meet GELF criteria again.”
A key question in this setting is whether patients previously given rituximab can derive benefit from an alternative anti-CD20 antibody. Taking on this question, the GADOLIN trial tested the addition of obinutuzumab (induction plus maintenance) to bendamustine among patients with rituximab-refractory disease (American Society of Clinical Oncology [ASCO] 2015, Abstract LBA8502; ICML 2015, Abstract 123).
Toxicities were generally similar by arm, except for a higher rate of infusion-related reactions with obinutuzumab. The overall response rates were comparable for the two arms, but progression-free survival was better with the combination (median event-free survival, not reached, vs. 14.9 months; hazard ratio, 0.55), and there was a trend for overall survival.
“These curves start separating after 6 months, and 6 months is the time of chemotherapy,” Dr. Zelenetz noted. “So I would argue from these data that the obinutuzumab didn’t add very much to the bendamustine backbone, but actually the obinutuzumab maintenance was effective even in rituximab-refractory patients.”
The combination of lenalidomide and rituximab has been compared with lenalidomide alone in patients with relapsed follicular lymphoma (ASCO 2012, Abstract 8000). The results showed a trend toward better median event-free survival with the combination (2.0 vs. 1.2 years; hazard ratio, 1.9; P = .061) but not overall survival.
In a phase II trial, idelalisib (Zydelig) was tested among patients with indolent non-Hodgkin lymphomas (60% with follicular lymphoma) that were refractory to both rituximab and an alkylator (N Engl J Med. 2014;370:1008-18). Noteworthy grade 3 or worse toxicities included pneumonia and transaminase elevations. The overall response rate was 57%, and the complete response rate was 6%; median progression-free survival was 11 months.
“Idelalisib can be safely combined with other agents including rituximab and bendamustine,” Dr. Zelenetz added (ASH 2014, Abstract 3063). “Interestingly, the overall response seems to be a little higher when you combine it, but it doesn’t seem to matter which drug you combine it with – rituximab, bendamustine, or [both] – you get good overall responses,” ranging from 71% to 85%.
The BCL-2 inhibitor venetoclax (formerly ABT-199/GDC-199) has been tested in non-Hodgkin lymphomas, where it has not been associated with the life-threatening tumor lysis syndrome seen in some other hematologic malignancies (European Hematology Association [EHA] 2015, Davis et al). It yielded an overall response rate of 31% in patients with relatively refractory follicular lymphoma. “This will lead to additional studies in this area,” he predicted.
Finally, nivolumab (Opdivo), a PD-1 immune checkpoint inhibitor, has been evaluated in a phase I study in relapsed or refractory hematologic malignancies, where it was well tolerated (ASH 2014, Abstract 291). Among the small subset of patients with follicular lymphoma, the overall response rate was 40%, prompting initiation of more trials.
Although chimeric antigen receptor (CAR) T-cell therapy is showing promise in various malignancies, Dr. Zelenetz said that other options are probably better avenues for research in follicular lymphoma at present.
“I’m much more interested in the tools that we have now, between the checkpoint inhibitors, the T-cell activators, and the bispecific monoclonal antibodies. I think I can [apply these therapies] with less money for probably less toxicity without the complexity of having to make a customized drug for the patient,” he said. “So I’m not very enthusiastic about CAR T cells in follicular lymphoma.”
SAN FRANCISCO – A variety of emerging therapies are being incorporated into the management of follicular lymphoma, which is typically a long-term endeavor requiring strategic use of multiple treatments, Dr. Andrew D. Zelenetz said at the National Comprehensive Cancer Network (NCCN) 10th Annual Congress: Hematologic Malignancies.
“Follicular lymphoma is a disease of paradox. The reality is that overall survival is excellent, but patients are not going to be able to do that with one treatment; they are going to get a series of treatments,” he said. “Survival is the sum of your exposures to treatment, time on active therapy, time in remission, and actually time with relapse not needing treatment.”
Risk stratification
Overall survival for patients with follicular lymphoma diagnosed today and treated with modern therapy is only slightly inferior to that for age-matched controls, noted Dr. Zelenetz of the department of medicine at Memorial Sloan-Kettering Cancer Center and professor of medicine at Cornell University, both in New York.
But patients who are faring poorly at 12 months (American Society of Hematology [ASH] 2014, Abstract 1664) or at 24 months (J Clin Oncol. 2015;33[23]:2516-22) into care have a much worse prognosis. “This shows the importance of identifying those patients with poor biology, and [the question of] whether we can identify them without treating them first and having them progress,” he said.
Hematologists have historically looked to the Follicular Lymphoma International Prognostic Index (FLIPI) to estimate outcome. “The FLIPI clearly works; it’s an important clinical tool. But the FLIPI high-risk patients are still identifying more than those very high-risk patients,” he said.
Therefore, a clinicogenomic risk model was developed that incorporates seven mutations having poor prognostic impact, the m7-FLIPI (Lancet Oncol. 2015;16[9]:1111-22). Adding the mutations split the previously defined high-risk patients into a group with a prognosis similar to that of low-risk patients and a small group with a very poor prognosis.
“This actually represents something very close to the 20% of patients that we think have bad biology,” Dr. Zelenetz noted. “There will be some additional data at ASH looking at this exact question, because the holy grail is to know when you diagnose someone if they are in that bad-risk group because those are the patients you want to do novel clinical trials on. If your overall survival is equivalent to the general population, it’s going to be hard to ever prove an overall survival advantage for an intervention in an unselected group of patients.”
Advanced-stage disease with low tumor bulk
For patients who have advanced-stage follicular lymphoma but with low tumor bulk, the NCCN endorses a modification of criteria developed by the Follicular Lymphoma Study Group (GELF) in deciding when to start treatment (J Clin Oncol. 1998;16[7]:2332-8).
Roughly a fifth of patients who are eligible for and managed with a watch-and-wait approach will not need chemotherapy or die of their disease in the next 10 years (Lancet. 2003;362[9383]:516-22). Furthermore, this strategy nets a median delay in the need for chemotherapy of 2.6 years.
Compared with observation, treatment with the anti-CD20 antibody rituximab (Rituxan) improves progression-free survival but not overall survival in this setting (ASH 2010, Abstract 6). “Though in selected patients, rituximab may be appropriate as initial treatment for the observable patient, I would argue for the observable patient with no survival disadvantage, the standard of care remains observation,” Dr. Zelenetz said.
Advanced-stage disease requiring treatment
A meta-analysis has shown a clear survival benefit from adding rituximab to chemotherapy (R-chemo) in patients with advanced follicular lymphoma who need treatment (J Natl Cancer Inst. 2007;99[9]:706-14). “Based on the results, it is the standard of care to add rituximab to a chemotherapy backbone, but the optimum R-chemo actually remains undefined and would be customized to the individual clinical situation,” he commented.
A variety of emerging agents are being tested in this setting. Among the subset of patients with untreated follicular lymphoma in a single-center trial, the combination of rituximab with the immunomodulator lenalidomide (Revlimid) yielded an overall response rate of 98% and a complete response rate of 87%, as well as excellent progression-free and overall survival (Lancet Oncol. 2014;15[12]:1311-8). The main grade 3 or 4 toxicity was neutropenia, seen in 35% of all patients studied. Efficacy results were much the same in a multicenter trial (International Conference on Malignant Lymphoma [ICML] 2013, Abstract 63).
This combination is now being tested as front-line therapy for follicular lymphoma in the RELEVANCE (Rituximab and Lenalidomide Versus Any Chemotherapy) phase III trial. “The trial is now done, but we don’t have results, and we won’t have results until 2019, so don’t hold your breath,” Dr. Zelenetz commented. “That’s because this was an unselected trial; we took all patients, all comers. And if you don’t try to identify bad-risk patients, you actually have to do very large trials, and the effect size is relatively small.”
Relapsed and refractory disease
“Many times when patients with follicular lymphoma relapse, they are immediately started on treatment. It’s not necessary and probably in most cases not appropriate. If patients are asymptomatic and have a low tumor burden, they can have a second and a third and even a fourth period of observation, where they don’t need active treatment,” he said. “So I would encourage you to …wait until they actually meet GELF criteria again.”
A key question in this setting is whether patients previously given rituximab can derive benefit from an alternative anti-CD20 antibody. Taking on this question, the GADOLIN trial tested the addition of obinutuzumab (induction plus maintenance) to bendamustine among patients with rituximab-refractory disease (American Society of Clinical Oncology [ASCO] 2015, Abstract LBA8502; ICML 2015, Abstract 123).
Toxicities were generally similar by arm, except for a higher rate of infusion-related reactions with obinutuzumab. The overall response rates were comparable for the two arms, but progression-free survival was better with the combination (median event-free survival, not reached, vs. 14.9 months; hazard ratio, 0.55), and there was a trend for overall survival.
“These curves start separating after 6 months, and 6 months is the time of chemotherapy,” Dr. Zelenetz noted. “So I would argue from these data that the obinutuzumab didn’t add very much to the bendamustine backbone, but actually the obinutuzumab maintenance was effective even in rituximab-refractory patients.”
The combination of lenalidomide and rituximab has been compared with lenalidomide alone in patients with relapsed follicular lymphoma (ASCO 2012, Abstract 8000). The results showed a trend toward better median event-free survival with the combination (2.0 vs. 1.2 years; hazard ratio, 1.9; P = .061) but not overall survival.
In a phase II trial, idelalisib (Zydelig) was tested among patients with indolent non-Hodgkin lymphomas (60% with follicular lymphoma) that were refractory to both rituximab and an alkylator (N Engl J Med. 2014;370:1008-18). Noteworthy grade 3 or worse toxicities included pneumonia and transaminase elevations. The overall response rate was 57%, and the complete response rate was 6%; median progression-free survival was 11 months.
“Idelalisib can be safely combined with other agents including rituximab and bendamustine,” Dr. Zelenetz added (ASH 2014, Abstract 3063). “Interestingly, the overall response seems to be a little higher when you combine it, but it doesn’t seem to matter which drug you combine it with – rituximab, bendamustine, or [both] – you get good overall responses,” ranging from 71% to 85%.
The BCL-2 inhibitor venetoclax (formerly ABT-199/GDC-199) has been tested in non-Hodgkin lymphomas, where it has not been associated with the life-threatening tumor lysis syndrome seen in some other hematologic malignancies (European Hematology Association [EHA] 2015, Davis et al). It yielded an overall response rate of 31% in patients with relatively refractory follicular lymphoma. “This will lead to additional studies in this area,” he predicted.
Finally, nivolumab (Opdivo), a PD-1 immune checkpoint inhibitor, has been evaluated in a phase I study in relapsed or refractory hematologic malignancies, where it was well tolerated (ASH 2014, Abstract 291). Among the small subset of patients with follicular lymphoma, the overall response rate was 40%, prompting initiation of more trials.
Although chimeric antigen receptor (CAR) T-cell therapy is showing promise in various malignancies, Dr. Zelenetz said that other options are probably better avenues for research in follicular lymphoma at present.
“I’m much more interested in the tools that we have now, between the checkpoint inhibitors, the T-cell activators, and the bispecific monoclonal antibodies. I think I can [apply these therapies] with less money for probably less toxicity without the complexity of having to make a customized drug for the patient,” he said. “So I’m not very enthusiastic about CAR T cells in follicular lymphoma.”
AT NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
CHEST: Catheter-directed thrombolysis shows pulmonary embolism efficacy
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 U.S. patients.
The review also found evidence that U.S. pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 U.S. clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national U.S. registry of hospitalized patients, Dr. Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score–matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr. Saqib, a researcher at Staten Island (N.Y.) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,” Dr. Saqib said.
The U.S. data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
“We need to more systematically identify the patients with an acute PE who could benefit from catheter-directed thrombolysis, especially patients with a massive PE,” commented Dr. Muthiah P. Muthiah, a critical-care medicine physician at the University of Tennessee Health Science Center in Memphis. “This may be something to offer to patients who have an absolute contraindication for systemic thrombolysis, such as recent surgery, but it is not available everywhere,” Dr. Muthiah said in an interview.
Dr. Saqib and her associates used data collected by the Federal National Inpatient Sample. Among U.S. patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score–adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr. Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr. Saqib said.
Dr. Saqib and Dr. Muthiah had no disclosures.
On Twitter @mitchelzoler
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 U.S. patients.
The review also found evidence that U.S. pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 U.S. clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national U.S. registry of hospitalized patients, Dr. Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score–matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr. Saqib, a researcher at Staten Island (N.Y.) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,” Dr. Saqib said.
The U.S. data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
“We need to more systematically identify the patients with an acute PE who could benefit from catheter-directed thrombolysis, especially patients with a massive PE,” commented Dr. Muthiah P. Muthiah, a critical-care medicine physician at the University of Tennessee Health Science Center in Memphis. “This may be something to offer to patients who have an absolute contraindication for systemic thrombolysis, such as recent surgery, but it is not available everywhere,” Dr. Muthiah said in an interview.
Dr. Saqib and her associates used data collected by the Federal National Inpatient Sample. Among U.S. patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score–adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr. Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr. Saqib said.
Dr. Saqib and Dr. Muthiah had no disclosures.
On Twitter @mitchelzoler
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 U.S. patients.
The review also found evidence that U.S. pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 U.S. clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national U.S. registry of hospitalized patients, Dr. Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score–matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr. Saqib, a researcher at Staten Island (N.Y.) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,” Dr. Saqib said.
The U.S. data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
“We need to more systematically identify the patients with an acute PE who could benefit from catheter-directed thrombolysis, especially patients with a massive PE,” commented Dr. Muthiah P. Muthiah, a critical-care medicine physician at the University of Tennessee Health Science Center in Memphis. “This may be something to offer to patients who have an absolute contraindication for systemic thrombolysis, such as recent surgery, but it is not available everywhere,” Dr. Muthiah said in an interview.
Dr. Saqib and her associates used data collected by the Federal National Inpatient Sample. Among U.S. patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score–adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr. Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr. Saqib said.
Dr. Saqib and Dr. Muthiah had no disclosures.
On Twitter @mitchelzoler
AT CHEST 2015
Key clinical point: Catheter-directed thrombolysis was linked to reduced mortality, compared with systemic thrombolysis in patients with an acute pulmonary embolism.
Major finding: In-hospital mortality in acute pulmonary embolism patients ran 10% with catheter-directed thrombolysis and 17% with systemic thrombolysis.
Data source: Review of 1,521 U.S. patients treated for acute pulmonary embolism during 2010-2012 in the National Inpatient Sample.
Disclosures: Dr. Saqib and Dr. Muthiah had no disclosures.