User login
HSC self-renewal depends on surroundings

in the bone marrow
Scientists say that, using a model of the hematopoietic system, they have determined which signaling pathways play an essential role in the self-renewal of hematopoietic stem cells (HSCs).
They found that a particularly important role in this process is the interactive communication with surrounding tissue cells in the bone marrow.
Robert Oostendorp, PhD, of Klinikum Rechts der Isar der Technischen Universität München in Munich, Germany, and his colleagues described these findings in Stem Cell Reports.
The team noted that, in steady-state conditions, HSCs are maintained as slow-dividing clones of quiescent cells. However, when stress occurs, such as an accident that leads to substantial blood loss or the defense against a pathogen requires more blood cells in the course of an infection, HSCs are activated.
In response, the entire hematopoietic system switches from “standby” mode into a state of alert. The activated HSCs generate new blood cells to counteract the blood loss or combat the pathogen. At the same time, self-renewal keeps the stem cell pool replenished.
This switch is accompanied by a complex communication process between the HSCs and tissue cells—an area that had not previously been examined in depth.
“In our study, we set out to establish which tissue signals are important to stem cell maintenance and functionality, and which HSC signals influence the microenvironment,” Dr Oostendorp said.
He and his colleagues used mixed cultures of tissue cells and HSCs to investigate how these cell types interact.
The scientists analyzed factors that are upregulated or downregulated in the interplay between tissue cells and HSCs. And they linked these findings with the signaling pathways described in existing literature.
The team then consolidated this information in a bioinformatics computer model. And they conducted extensive cell experiments to confirm the computer-generated signaling pathway model.
“The outcome was very interesting indeed,” Dr Oostendorp said. “The entire system operates in a feedback loop. In ‘alert’ mode, the stem cells first influence the behavior of the tissue cells, which, in turn, impact on the stem cells, triggering the self-renewal step.”
In alert mode, HSCs emit signaling substances, which induce tissue cells to release the connective tissue growth factor (CTGF) messenger. This is essential to maintain the HSCs through self-renewal. In the absence of CTGF, HSCs age and cannot replenish the stem cell pool.
“Our findings could prove significant in treating leukemia,” Dr Oostendorp noted. “In this condition, the stem cells are hyperactive, and their division is unchecked. Leukemic blood cells are in a constant state of alert, so we would expect a similar interplay with the tissue cells.”
To date, however, the focus here has been limited to stem cells as the actual source of the defect.
“Given what we know now about feedback loops, it would be important to integrate the surrounding cells in therapeutic approaches too, since they exert a strong influence on stem cell division,” Dr Oostendorp concluded. ![]()

in the bone marrow
Scientists say that, using a model of the hematopoietic system, they have determined which signaling pathways play an essential role in the self-renewal of hematopoietic stem cells (HSCs).
They found that a particularly important role in this process is the interactive communication with surrounding tissue cells in the bone marrow.
Robert Oostendorp, PhD, of Klinikum Rechts der Isar der Technischen Universität München in Munich, Germany, and his colleagues described these findings in Stem Cell Reports.
The team noted that, in steady-state conditions, HSCs are maintained as slow-dividing clones of quiescent cells. However, when stress occurs, such as an accident that leads to substantial blood loss or the defense against a pathogen requires more blood cells in the course of an infection, HSCs are activated.
In response, the entire hematopoietic system switches from “standby” mode into a state of alert. The activated HSCs generate new blood cells to counteract the blood loss or combat the pathogen. At the same time, self-renewal keeps the stem cell pool replenished.
This switch is accompanied by a complex communication process between the HSCs and tissue cells—an area that had not previously been examined in depth.
“In our study, we set out to establish which tissue signals are important to stem cell maintenance and functionality, and which HSC signals influence the microenvironment,” Dr Oostendorp said.
He and his colleagues used mixed cultures of tissue cells and HSCs to investigate how these cell types interact.
The scientists analyzed factors that are upregulated or downregulated in the interplay between tissue cells and HSCs. And they linked these findings with the signaling pathways described in existing literature.
The team then consolidated this information in a bioinformatics computer model. And they conducted extensive cell experiments to confirm the computer-generated signaling pathway model.
“The outcome was very interesting indeed,” Dr Oostendorp said. “The entire system operates in a feedback loop. In ‘alert’ mode, the stem cells first influence the behavior of the tissue cells, which, in turn, impact on the stem cells, triggering the self-renewal step.”
In alert mode, HSCs emit signaling substances, which induce tissue cells to release the connective tissue growth factor (CTGF) messenger. This is essential to maintain the HSCs through self-renewal. In the absence of CTGF, HSCs age and cannot replenish the stem cell pool.
“Our findings could prove significant in treating leukemia,” Dr Oostendorp noted. “In this condition, the stem cells are hyperactive, and their division is unchecked. Leukemic blood cells are in a constant state of alert, so we would expect a similar interplay with the tissue cells.”
To date, however, the focus here has been limited to stem cells as the actual source of the defect.
“Given what we know now about feedback loops, it would be important to integrate the surrounding cells in therapeutic approaches too, since they exert a strong influence on stem cell division,” Dr Oostendorp concluded. ![]()

in the bone marrow
Scientists say that, using a model of the hematopoietic system, they have determined which signaling pathways play an essential role in the self-renewal of hematopoietic stem cells (HSCs).
They found that a particularly important role in this process is the interactive communication with surrounding tissue cells in the bone marrow.
Robert Oostendorp, PhD, of Klinikum Rechts der Isar der Technischen Universität München in Munich, Germany, and his colleagues described these findings in Stem Cell Reports.
The team noted that, in steady-state conditions, HSCs are maintained as slow-dividing clones of quiescent cells. However, when stress occurs, such as an accident that leads to substantial blood loss or the defense against a pathogen requires more blood cells in the course of an infection, HSCs are activated.
In response, the entire hematopoietic system switches from “standby” mode into a state of alert. The activated HSCs generate new blood cells to counteract the blood loss or combat the pathogen. At the same time, self-renewal keeps the stem cell pool replenished.
This switch is accompanied by a complex communication process between the HSCs and tissue cells—an area that had not previously been examined in depth.
“In our study, we set out to establish which tissue signals are important to stem cell maintenance and functionality, and which HSC signals influence the microenvironment,” Dr Oostendorp said.
He and his colleagues used mixed cultures of tissue cells and HSCs to investigate how these cell types interact.
The scientists analyzed factors that are upregulated or downregulated in the interplay between tissue cells and HSCs. And they linked these findings with the signaling pathways described in existing literature.
The team then consolidated this information in a bioinformatics computer model. And they conducted extensive cell experiments to confirm the computer-generated signaling pathway model.
“The outcome was very interesting indeed,” Dr Oostendorp said. “The entire system operates in a feedback loop. In ‘alert’ mode, the stem cells first influence the behavior of the tissue cells, which, in turn, impact on the stem cells, triggering the self-renewal step.”
In alert mode, HSCs emit signaling substances, which induce tissue cells to release the connective tissue growth factor (CTGF) messenger. This is essential to maintain the HSCs through self-renewal. In the absence of CTGF, HSCs age and cannot replenish the stem cell pool.
“Our findings could prove significant in treating leukemia,” Dr Oostendorp noted. “In this condition, the stem cells are hyperactive, and their division is unchecked. Leukemic blood cells are in a constant state of alert, so we would expect a similar interplay with the tissue cells.”
To date, however, the focus here has been limited to stem cells as the actual source of the defect.
“Given what we know now about feedback loops, it would be important to integrate the surrounding cells in therapeutic approaches too, since they exert a strong influence on stem cell division,” Dr Oostendorp concluded. ![]()
Experiment reveals new method of RBC production

Photo courtesy of
Josh Barney/University of
Virginia Health System
An unexpected result of a lab experiment has led researchers to a new way to trigger red blood cell (RBC) production.
They found that engaging a stress receptor on type 1 conventional dendritic cells can induce stress erythropoiesis in mice.
The team believes that, eventually, this method could be used to turn on RBC production in humans when necessary, perhaps to replace a blood transfusion or as a stop-gap measure when a transfusion is delayed.
Thomas J. Braciale, MD, PhD, of the University of Virginia in Charlottesville, and his colleagues conducted this research and reported the results in The Journal of Clinical Investigation.
The team was not investigating RBC production when they made their discovery. They were looking into the role of dendritic cells in the lungs.
Dendritic cells have traditionally been thought to be sensors of infection and inflammation, but a lab test involving the flu virus produced an unexpected effect in mice that ultimately revealed a new aspect to the cells’ function.
The researchers injected mice with the flu virus and an αCD24 monoclonal antibody, which resulted in splenomegaly. The team was baffled at this outcome, so they repeated the experiment, only to get the same results.
“We did it again, and I didn’t believe it, and we did it again, and I didn’t believe it,” Dr Braciale recalled. “I asked whether you needed flu to infect the mice when you injected this antibody. So the postdoc did the experiment, and he just injected the antibody without flu-injecting the mice—giant spleens. After much consultation, after talking with my colleagues in pathology, we decided we were inducing stress erythropoiesis.”
Specifically, the researchers found that engaging CD24 on type 1 conventional dendritic cells upregulates expression of the Kit ligand stem cell factor, which results in Kit-mediated proliferative expansion of early erythroid progenitors and transient reticulocytosis.
“In a very basic way, what we’ve discovered is that the process of regulating stress in the body is mediated—certainly in part, at least—by these dendritic cells,” Dr Braciale explained. “And stress can be a variety of different stresses.”
“It doesn’t have to be infection. It doesn’t have to be inflammation. It can be anemia. It can be hemorrhage. And these cells act to initiate this response that, until this report, there’s been really no evidence that [dendritic] cells ever participate in making red blood cells.”
More work is needed before this approach to RBC production can be tested in humans. However, Dr Braciale is optimistic, based on the findings so far.
“We’re very excited to see where this goes,” he said. “We know that the same things can be done in humans in the following sense. There are mice called humanized mice. These are mice that are engineered so they have a human blood system. And if you inject these mice with this antibody, they’ll make red blood cells.” ![]()

Photo courtesy of
Josh Barney/University of
Virginia Health System
An unexpected result of a lab experiment has led researchers to a new way to trigger red blood cell (RBC) production.
They found that engaging a stress receptor on type 1 conventional dendritic cells can induce stress erythropoiesis in mice.
The team believes that, eventually, this method could be used to turn on RBC production in humans when necessary, perhaps to replace a blood transfusion or as a stop-gap measure when a transfusion is delayed.
Thomas J. Braciale, MD, PhD, of the University of Virginia in Charlottesville, and his colleagues conducted this research and reported the results in The Journal of Clinical Investigation.
The team was not investigating RBC production when they made their discovery. They were looking into the role of dendritic cells in the lungs.
Dendritic cells have traditionally been thought to be sensors of infection and inflammation, but a lab test involving the flu virus produced an unexpected effect in mice that ultimately revealed a new aspect to the cells’ function.
The researchers injected mice with the flu virus and an αCD24 monoclonal antibody, which resulted in splenomegaly. The team was baffled at this outcome, so they repeated the experiment, only to get the same results.
“We did it again, and I didn’t believe it, and we did it again, and I didn’t believe it,” Dr Braciale recalled. “I asked whether you needed flu to infect the mice when you injected this antibody. So the postdoc did the experiment, and he just injected the antibody without flu-injecting the mice—giant spleens. After much consultation, after talking with my colleagues in pathology, we decided we were inducing stress erythropoiesis.”
Specifically, the researchers found that engaging CD24 on type 1 conventional dendritic cells upregulates expression of the Kit ligand stem cell factor, which results in Kit-mediated proliferative expansion of early erythroid progenitors and transient reticulocytosis.
“In a very basic way, what we’ve discovered is that the process of regulating stress in the body is mediated—certainly in part, at least—by these dendritic cells,” Dr Braciale explained. “And stress can be a variety of different stresses.”
“It doesn’t have to be infection. It doesn’t have to be inflammation. It can be anemia. It can be hemorrhage. And these cells act to initiate this response that, until this report, there’s been really no evidence that [dendritic] cells ever participate in making red blood cells.”
More work is needed before this approach to RBC production can be tested in humans. However, Dr Braciale is optimistic, based on the findings so far.
“We’re very excited to see where this goes,” he said. “We know that the same things can be done in humans in the following sense. There are mice called humanized mice. These are mice that are engineered so they have a human blood system. And if you inject these mice with this antibody, they’ll make red blood cells.” ![]()

Photo courtesy of
Josh Barney/University of
Virginia Health System
An unexpected result of a lab experiment has led researchers to a new way to trigger red blood cell (RBC) production.
They found that engaging a stress receptor on type 1 conventional dendritic cells can induce stress erythropoiesis in mice.
The team believes that, eventually, this method could be used to turn on RBC production in humans when necessary, perhaps to replace a blood transfusion or as a stop-gap measure when a transfusion is delayed.
Thomas J. Braciale, MD, PhD, of the University of Virginia in Charlottesville, and his colleagues conducted this research and reported the results in The Journal of Clinical Investigation.
The team was not investigating RBC production when they made their discovery. They were looking into the role of dendritic cells in the lungs.
Dendritic cells have traditionally been thought to be sensors of infection and inflammation, but a lab test involving the flu virus produced an unexpected effect in mice that ultimately revealed a new aspect to the cells’ function.
The researchers injected mice with the flu virus and an αCD24 monoclonal antibody, which resulted in splenomegaly. The team was baffled at this outcome, so they repeated the experiment, only to get the same results.
“We did it again, and I didn’t believe it, and we did it again, and I didn’t believe it,” Dr Braciale recalled. “I asked whether you needed flu to infect the mice when you injected this antibody. So the postdoc did the experiment, and he just injected the antibody without flu-injecting the mice—giant spleens. After much consultation, after talking with my colleagues in pathology, we decided we were inducing stress erythropoiesis.”
Specifically, the researchers found that engaging CD24 on type 1 conventional dendritic cells upregulates expression of the Kit ligand stem cell factor, which results in Kit-mediated proliferative expansion of early erythroid progenitors and transient reticulocytosis.
“In a very basic way, what we’ve discovered is that the process of regulating stress in the body is mediated—certainly in part, at least—by these dendritic cells,” Dr Braciale explained. “And stress can be a variety of different stresses.”
“It doesn’t have to be infection. It doesn’t have to be inflammation. It can be anemia. It can be hemorrhage. And these cells act to initiate this response that, until this report, there’s been really no evidence that [dendritic] cells ever participate in making red blood cells.”
More work is needed before this approach to RBC production can be tested in humans. However, Dr Braciale is optimistic, based on the findings so far.
“We’re very excited to see where this goes,” he said. “We know that the same things can be done in humans in the following sense. There are mice called humanized mice. These are mice that are engineered so they have a human blood system. And if you inject these mice with this antibody, they’ll make red blood cells.” ![]()
EC expands indication for azacitidine in AML

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()
France to lift lifetime ban on MSM blood donors
Photo by Михаило Јовановић
France is planning to lift its lifetime ban on blood donations from men who have sex with men (MSM), and the change is set to take effect next year.
French officials say that, beginning in Spring 2016, an MSM will be able to donate whole blood in France if he has not had sex with another man in the last 12
months.
An MSM will be allowed to donate plasma if he has been abstinent or had only 1 sexual partner in the last 4 months.
Experts will analyze the impact of this policy change for about a year. If there has been no increase in health risks, the policy may be relaxed further, and MSM donors may be treated the same as non-MSM donors.
Non-MSM individuals in France are deferred from donating blood if they have had more than 1 sexual partner in the last 4 months.
Studies recently conducted in Canada and the UK have suggested that lifting restrictions on MSM blood donors does not
increase the risk of sexually transmitted infections affecting the blood
supply.
France’s lifetime ban on MSM blood donation was enacted in 1983 in an attempt to counter the spread of HIV/AIDS.
Hundreds of people died in France in the 1980s after HIV-tainted blood was distributed by the French National Blood Transfusion Center. HIV-positive blood donations were exported as well, which led to hundreds of additional deaths in other countries.
Bans lifting worldwide
In lifting its lifetime ban on MSM blood donors, France is following the lead of other countries within and outside of Europe.
England, Scotland, Wales, Sweden, and the Netherlands have all lifted their bans on MSM blood donors and changed to a 12-month deferral period. Australia, Japan, and New Zealand also have 12-month deferral periods for MSM blood donors.
South Africa has a 6-month deferral period for MSMs, while Italy and Spain have no policy specific to MSM blood donors. All donors are screened for high-risk sexual practices, and deferrals are made accordingly.
The US and Canada are currently considering adopting a 12-month deferral policy for MSM blood donors. The lifetime ban is still in place in the US, but Canada has a 5-year deferral period for MSMs. ![]()

Photo by Михаило Јовановић
France is planning to lift its lifetime ban on blood donations from men who have sex with men (MSM), and the change is set to take effect next year.
French officials say that, beginning in Spring 2016, an MSM will be able to donate whole blood in France if he has not had sex with another man in the last 12
months.
An MSM will be allowed to donate plasma if he has been abstinent or had only 1 sexual partner in the last 4 months.
Experts will analyze the impact of this policy change for about a year. If there has been no increase in health risks, the policy may be relaxed further, and MSM donors may be treated the same as non-MSM donors.
Non-MSM individuals in France are deferred from donating blood if they have had more than 1 sexual partner in the last 4 months.
Studies recently conducted in Canada and the UK have suggested that lifting restrictions on MSM blood donors does not
increase the risk of sexually transmitted infections affecting the blood
supply.
France’s lifetime ban on MSM blood donation was enacted in 1983 in an attempt to counter the spread of HIV/AIDS.
Hundreds of people died in France in the 1980s after HIV-tainted blood was distributed by the French National Blood Transfusion Center. HIV-positive blood donations were exported as well, which led to hundreds of additional deaths in other countries.
Bans lifting worldwide
In lifting its lifetime ban on MSM blood donors, France is following the lead of other countries within and outside of Europe.
England, Scotland, Wales, Sweden, and the Netherlands have all lifted their bans on MSM blood donors and changed to a 12-month deferral period. Australia, Japan, and New Zealand also have 12-month deferral periods for MSM blood donors.
South Africa has a 6-month deferral period for MSMs, while Italy and Spain have no policy specific to MSM blood donors. All donors are screened for high-risk sexual practices, and deferrals are made accordingly.
The US and Canada are currently considering adopting a 12-month deferral policy for MSM blood donors. The lifetime ban is still in place in the US, but Canada has a 5-year deferral period for MSMs. ![]()

Photo by Михаило Јовановић
France is planning to lift its lifetime ban on blood donations from men who have sex with men (MSM), and the change is set to take effect next year.
French officials say that, beginning in Spring 2016, an MSM will be able to donate whole blood in France if he has not had sex with another man in the last 12
months.
An MSM will be allowed to donate plasma if he has been abstinent or had only 1 sexual partner in the last 4 months.
Experts will analyze the impact of this policy change for about a year. If there has been no increase in health risks, the policy may be relaxed further, and MSM donors may be treated the same as non-MSM donors.
Non-MSM individuals in France are deferred from donating blood if they have had more than 1 sexual partner in the last 4 months.
Studies recently conducted in Canada and the UK have suggested that lifting restrictions on MSM blood donors does not
increase the risk of sexually transmitted infections affecting the blood
supply.
France’s lifetime ban on MSM blood donation was enacted in 1983 in an attempt to counter the spread of HIV/AIDS.
Hundreds of people died in France in the 1980s after HIV-tainted blood was distributed by the French National Blood Transfusion Center. HIV-positive blood donations were exported as well, which led to hundreds of additional deaths in other countries.
Bans lifting worldwide
In lifting its lifetime ban on MSM blood donors, France is following the lead of other countries within and outside of Europe.
England, Scotland, Wales, Sweden, and the Netherlands have all lifted their bans on MSM blood donors and changed to a 12-month deferral period. Australia, Japan, and New Zealand also have 12-month deferral periods for MSM blood donors.
South Africa has a 6-month deferral period for MSMs, while Italy and Spain have no policy specific to MSM blood donors. All donors are screened for high-risk sexual practices, and deferrals are made accordingly.
The US and Canada are currently considering adopting a 12-month deferral policy for MSM blood donors. The lifetime ban is still in place in the US, but Canada has a 5-year deferral period for MSMs. ![]()
Evaluation of Spinal Epidural Abscess
Spinal epidural abscess (SEA) is caused by a suppurative infection in the epidural space. The mass effect of the abscess can compress and reduce blood flow to the spinal cord, conus medullaris, or cauda equina. Left untreated, the infection can lead to sensory loss, muscle weakness, visceral dysfunction, sepsis, and even death. Early diagnosis is essential to limit morbidity and neurologic injury. The classic triad of fever, axial pain, and neurological deficit occurs in as few as 13% of patients, highlighting the diagnostic challenge associated with SEA.[1]
This investigation reviews the current literature on SEA epidemiology, clinical findings, laboratory data, and treatment methods, with particular focus on nonsurgical versus surgical treatment. Our primary objective was to educate the clinician on when to suspect SEA and how to execute an appropriate diagnostic evaluation.
INCIDENCE AND EPIDEMIOLOGY
In 1975, the incidence of SEA was reported to be 0.2 to 2.0 per 10,000 hospital admissions.[2] Two decades later, a study of tertiary referral centers documented a much higher rate of 12.5 per 10,000 admissions.[3] The reported incidence continues to rise and has doubled in the past decade, with SEA currently representing approximately 10% of all primary spine infections.[4, 5] Potential explanations for this increasing incidence include aging of the population, an increasing prevalence of diabetes, increasing intravenous drug abuse, more widespread use of advanced immunosuppressive regimens, and an increased rate of invasive spinal procedures.[6, 7, 8] Other factors contributing to the rising incidence include increased detection due to the greater accessibility of magnetic resonance imaging (MRI) and increased reporting as a result of the concentration of cases at tertiary referral centers.
SEA is most common in patients older than 60 years[7] and those with multiple medical comorbidities. A review of over 30,000 patients found that the average number of comorbidities in patients who underwent surgical intervention for SEA was 6, ranging from 0 to 20.[5] The same study noted that diabetes was the most frequently associated disease (30% of patients), followed by chronic lung disease (19%), renal failure (13%), and obesity (13%) (Table 1).[5] A history of invasive spine interventions is an additional risk factor; between 14% and 22% occur as a result of spine surgery or percutaneous spine procedures (eg, epidural steroid injections).[8, 9] Regardless of pathogenesis, the rate of permanent neurologic injury after SEA is 30% to 50%, and the mortality rate ranges from 10% to 20%.[4, 5, 8, 10]
| Medical Comorbidity | Prevalence (%) |
|---|---|
| |
| Diabetes mellitus | 1546 |
| IV drug use | 437 |
| Spinal trauma | 533 |
| End‐stage renal disease | 213 |
| Immunosuppressant therapy | 716 |
| Cancer | 215 |
| HIV/AIDS | 29 |
MISSED DIAGNOSIS
Despite the availability of advanced imaging, rates of misdiagnosis at initial presentation remain substantial, with current estimates ranging from 11% to 75%.[4, 9] Back and neck pain symptoms are ubiquitous and nonspecific, often making the diagnosis difficult. Repeated emergency room visits for pain are common in patients who are eventually diagnosed with SEA. Davis et al. found that 51% of 63 patients present to the emergency room at least 2 or more times prior to diagnosis; 11% present 3 or more times.[1]
PATHOPHYSIOLOGY
Microbiology
Staphylococcus aureus, including methicillin resistant S aureus (MRSA), accounts for two‐thirds of all infections.[3] S aureus infection of the spine may occur in the setting of surgical intervention or concomitant skin infection, although it often occurs without an identifiable source. Historically, MRSA has been reported to be responsible for 15% of all staphylococcal infections of the epidural space; some institutions report MRSA rates as high as 40%.[4] S epidermidis is another common pathogen, which is most often encountered following spinal surgery, epidural catheter insertion, and spinal injections.[11] Gram‐negative infections are less common. Escherichia coli is characteristically isolated in patients with active urinary tract infections, and Pseudomonas aeruginosa is more common in intravenous (IV) drug users.[12] Rare causes of SEA include anaerobes such as Bacteroides,[13] various parasites, and fungal organisms such as actinomyces, nocardia, and mycobacteria.[8]
Mechanism of Inoculation
Infections may enter the epidural space by 4 mechanisms: hematogenous spread, direct extension, inoculation via spinal procedure, and trauma. Hematogenous spread from an existing infection is the most common mechanism.[14] Seeding of the epidural space from transient bacteremia after dental procedures has also been reported.[13]
The second most common mechanism of infection is direct spread from an infected component of the vertebral column or paraspinal soft tissues. Most commonly, this takes the form of an anterior SEA in association with vertebral body osteomyelitis. Septic arthritis of a facet joint can also cause a secondary infection of the posterior epidural space.[4, 15] Direct spread from other posterior structures (eg, retropharyngeal, psoas, or paraspinal muscle abscess) may cause SEA as well.[16]
Less‐frequent mechanisms include direct inoculation and trauma. Infection of the epidural space can occur in association with spinal surgery, placement of an epidural catheter, or spinal injections. Grewal et al. reported in 2006 that 1 in 1000 surgical and 1 in 2000 obstetric patients develop SEA following epidural nerve block.[17] Hematoma secondary to an osseous or ligamentous injury can become seeded by bacteria, leading to abscess formation.[18]
Development of Neurologic Symptoms
There are several proposed mechanisms by which SEA can produce neurologic dysfunction. The first theory is that the compressive effect of an expanding abscess decreases blood flow to the neuronal tissue.[4] Improvement of neurologic function following surgical decompression lends credence to this theory. A second potential mechanism is a loss of blood flow due to local vascular inflammation from the infection. Local arteritis may decrease inflow to the cord parenchyma. This theoretical mechanism offers an explanation for the rapid onset of profound neurologic compromise in some cases.[19, 20] Infection can also result in venous thrombophlebitis, which produces ischemic injury due to impaired outflow. Postmortem examination supports this hypothesis; autopsy has revealed local thrombosis of the leptomeningeal vessels adjacent to the level of SEA.[4] All of these mechanisms are probably involved to some degree in any given case of neurologic compromise.
PATIENT HISTORY
Patients present with a wide variety of complaints and confounding variables that complicate the diagnosis (eg, medical comorbidities, psychiatric disease, chronic pain, dementia, or preexistent nonambulatory status). Ninety‐five percent of patients with SEA have a chief complaint of axial spinal pain.[1] Approximately half of patients report fever, and 47% complain of weakness in either the upper or lower extremities.[9] The classic triad of fever, spine pain, and neurological deficits presents in only a minority of patients, with rates ranging from 13% to 37%.[1, 3] Additionally, 1 study found that the sensitivity of this triad was a mere 8%.[1]
The physician should inquire about comorbid conditions associated with SEA, including diabetes, kidney disease, and history of drug use. Any recent infection at a remote site, such as cellulitis or urinary tract infection, should also be investigated. As many as 44% of patients with vertebral osteomyelitis have an associated SEA; conversely, osteomyelitis may be present in up to 80% of patients with SEA.[2, 13] Spinal procedures such as epidural[13] or facet joint injections,[14] placement of hemodialysis catheters,[21] acupuncture,[22] and tattoos[23] have also been implicated as risk factors.
PHYSICAL EXAM
Physical exam findings range from subtle back tenderness to severe neurologic deficits and complete paralysis. Spinal tenderness is elicited in up to 75% of patients, with equal rates of focal and diffuse back tenderness.[1, 4] Radicular symptoms are evident in 12% to 47% of patients, presenting as weakness identified in 26% to 60% and altered sensation in up to 67% of patients.[1, 4, 8, 11, 19] One study revealed that 71% of patients have an abnormal neurologic exam at presentation, including paresthesias (39%), motor weakness (39%), and loss of bladder and bowel control (27%).[3] Thus, a thorough neurologic exam is vital in SEA evaluation.
Heusner staged patients based on their clinical findings (Table 2). Because the evolution of symptoms can be variable and rapidly progressive[4, 24] to stage 3 or 4, documenting subtle abnormalities on initial exam and monitoring for changes are important. Many patients initially present in stage 1 or 2 but remain undiagnosed until progression to stage 3 or 4.[24]
| Stage | Symptoms |
|---|---|
| I | Back pain |
| II | Radiculopathy, neck pain, reflex changes |
| III | Paresthesia, weakness, bladder symptoms |
| IV | Paralysis |
DIAGNOSTIC WORKUP
Laboratory Testing
Routine tests should include a white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP). ESR has been shown to be the most sensitive and specific marker of SEA (Table 3). In a study of 63 patients matched with 126 controls, ESR was greater than 20 in 98% of cases and only 21% of controls.[1] Another study of 55 patients found the sensitivity and specificity of ESR to be 100% and 67%, respectively.[25] White count is less specific, with leukocytosis present in approximately two‐thirds of patients.[1, 25] CRP level rises faster than ESR in the setting of inflammation and also returns to baseline faster. As such, CRP is a useful means of monitoring the response to treatment.[1, 19]
|
| 1. Patient assessment |
| Evaluate for symptom triad (back pain, fever, neurologic dysfunction) |
| Assess for common comorbidities (diabetes, IV drug abuse, spinal trauma, ESRD, immunosuppressant therapy, recent spine procedure, systemic or local infection) |
| 2. Laboratory evaluation |
| Essential: ESR (most sensitive and specific), CRP, WBCs, blood cultures, UA |
| : echocardiogram, TB evaluation |
| No role: lumbar puncture |
| 3. Imaging |
| Gold standard: MRI w/gadolinium (90% sensitive) |
| If MRI contraindicated: CT myelogram |
| 4. Obtain tissue sample |
| Gold standard: open surgical biopsy, debridement fixation. |
| If patient unstable, diagnosis indeterminate, or no neuro symptoms: IR‐guided biopsy before surgery |
| 5. Antibiotics (once tissue sample obtained) |
| Empiric vancomycin plus third‐generation cephalosporin or aminoglycoside |
| Vancomycin only acceptable as monotherapy in patient with early diagnosis and no neurologic symptoms |
| 6. Surgical management (only if not done as step 4) |
| Early surgical consult recommended. Pursue surgical intervention if patient is stable for OR and neuro symptoms progressing or abscess refractory to antibiotics. Best outcomes occur with combined antibiotics and surgical debridement. |
Blood cultures are important to obtain at initial evaluation. Bacteremia from the offending organism may be present in up to 60% of cases; 60% to 90% of cases of bacteremia are caused by MRSA.[26] Urine culture should also be obtained in all patients. Sputum cultures should not be routinely obtained, but it may be beneficial in select patients with history of chronic obstructive pulmonary disease, new cough, or abnormality on chest radiographs. Transthoracic or transesophageal echocardiogram may be recommended for bacteremic patients, those with new heart murmur, or those with a history of IV drug abuse.
In patients in whom tuberculosis may be a potential risk, a tuberculin test with purified protein derivate can help rule out this organism. However, false‐negative testing may occur in up to 15% of patients, particularly the immunocompromised.[27] Patients with false positive results or prior vaccination with bacille Calmette‐Gurin (BCG) can be tested with QuantiFERON gold testing. These time‐consuming and expensive tests should not delay treatment with an empiric agent if tuberculosis is suspected due to patient risk factors or exposure, though an infectious disease specialist should be consulted before initiating treatment for tuberculosis.
Lumbar puncture should not be performed routinely in cases of suspected SEA, primarily due to the risk of bacterial contamination of the cerebrospinal fluid (CSF). Additionally, the increased protein and inflammatory cells seen in the CSF are nonspecific markers of parameningeal inflammation.[15]
Imaging
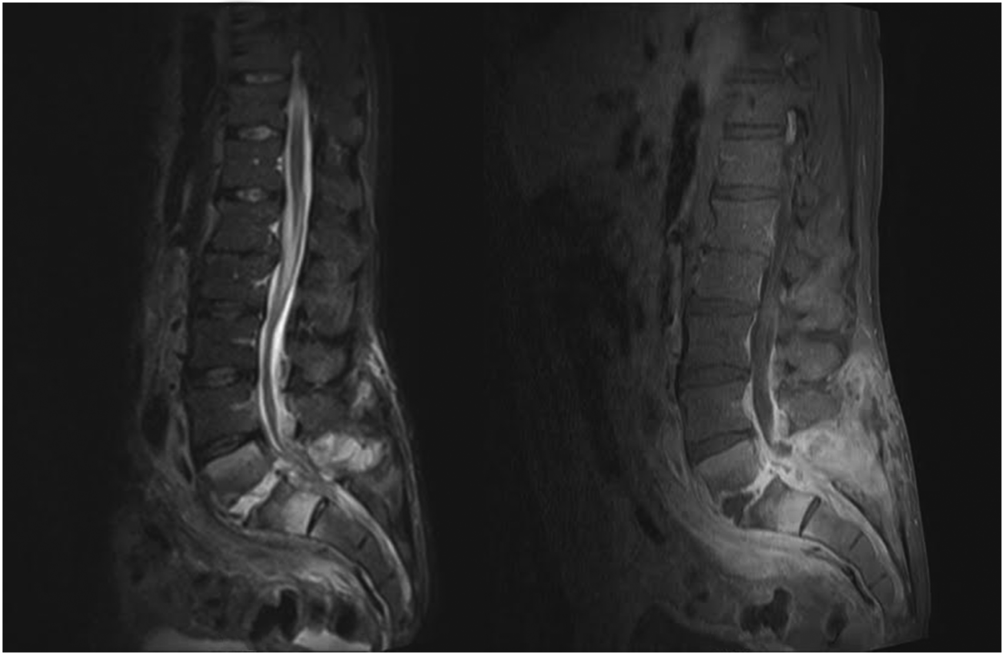
When the diagnosis of SEA is suspected based on clinical findings, MRI with gadolinium should be obtained first, as it is 90% sensitive for diagnosing SEA (Figure 1).[3] In patients unable to undergo MRI (eg, pacemaker), a computed tomography (CT) myelogram should be obtained instead. The study should be performed on an emergent basis, and the entire spine should be imaged due to the risk of noncontiguous lesions. Patients with plain radiograph and/or CT scan findings of bone lysis suggestive of vertebral osteomyelitis should also be evaluated with MRI if able.
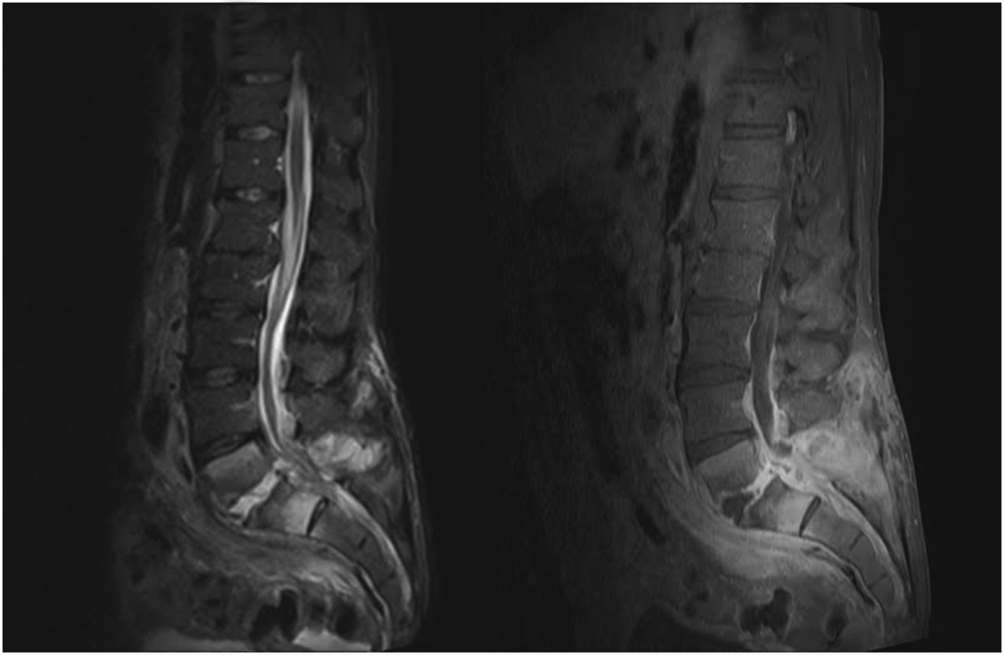
MRI with contrast can often differentiate SEA from malignancy and other space‐occupying lesions.[28] Plain radiography can rule out other causes of back pain, such as trauma and degenerative disc disease, but it cannot demonstrate SEA. One study found that x‐rays showed pathology in only 16.6% of patients with SEA.[29]
Patients with exam findings and laboratory studies concerning for SEA who present to a community hospital without MRI capability should be transferred to a tertiary care center for advanced imaging and potential emergent treatment.
Tissue Culture
Though MRI is essential to the workup of SEA, biopsy with cultures allows for a definitive diagnosis.[30] Cultures may be obtained in the operating room or interventional radiology (IR) suite via fine‐needle aspiration or core‐needle biopsy under CT guidance, if available.[30, 31] CT‐guided bone biopsy, which has a sensitivity of 81% and specificity of 100%, may also be performed when vertebral osteomyelitis is present.[32] Biopsy by IR should be considered before surgical intervention, when the patient has no evidence of progressive neurologic deficit, the diagnosis is unclear, or the patient is too high risk for surgical intervention. In very high‐risk surgical patients, IR aspiration may be curative. Lyu et al. describe a case of refractory SEA treated with percutaneous CT‐guided needle aspiration alone, though they note that surgical debridement is preferred when possible.[31]
NONSURGICAL VERSUS SURGICAL MANAGEMENT
SEA may be treated without surgery in carefully selected patients. Savage et al. studied 52 patients, and found that nonsurgical management was often effective in patients who were completely neurologically intact at initial presentation. In their study, only 3 patients needed to undergo surgery due to development of new neurologic symptoms.[33] However, other studies report neurologic symptoms in 71% of patients at initial diagnosis.[3]
Adogwa et al. reviewed surgical versus nonsurgical management in elderly patients (>50 years old) over 15 years.[10] Their study included 30 patients treated operatively and 52 who received antibiotics alone. The decision for surgical management was at the surgeons' discretion; however, most patients with grade 2 or 3 symptoms underwent surgery, and those with paraplegia or quadriplegia for >48 hours did not. The authors found no clinically significant difference in outcome between these 2 groups and cautioned against surgical intervention for elderly patients with multiple comorbidities. It is worth noting, however, that 7/30 (23%) patients treated with surgery versus 5/52 (10%) treated conservatively had improved neurological outcomes (P = 0.03).[10] Numerous other studies have also found that neurologic status at presentation is the most important predictor of surgical outcomes.[7, 15, 34]
Patel et al. performed a study analyzing risk factors for failure of medical management, and found that diabetes mellitus, CRP >115, WBC >12.5, and positive blood cultures were predictors of failure.[9] Patients with >3 of these risk factors required surgery 76.9% of the time compared to 40.2% with 2, 35.4% with 1, and 8.3% with none.[9] The authors also found that surgical patients experienced better mean improvement than patients who failed nonsurgical treatment and subsequently underwent surgical decompression.[9]
In a follow‐up study by Alton et al., 62 patients were treated with either nonsurgical or surgical management. Indications for surgery in their study included a neurologic deficit at initial presentation or the development of a new neurological deficit while undergoing treatment. Twenty‐four patients presented without deficits and underwent nonsurgical management, but only 6 (25%) were treated successfully, as defined by stable or improved neurological status following therapy. In contrast, none of the 38 patients who underwent therapy with both IV antibiotics and emergent surgical management within 24 hours experienced deterioration in the neurologic status. For the 18 patients who failed nonsurgical management, surgery was performed within an average of 7 days. This group experienced improvement following surgery, but their neurological status improved less than those who underwent early surgery.[35]
These recent studies demonstrate that medical management may be an option for patients who are diagnosed early and present without neurologic deficits, but surgery is the mainstay of treatment for most patients. Additionally, patients who experience a delay in operative management often do not recover function as well as those patients who undergo emergent surgical debridement.[35] A delay in diagnosis has also been shown to lead to increase in lawsuits against providers. French et al. found an increase in verdicts against the provider when a delay in diagnosis >48 hours was present, irrespective of the degree of permanent neurologic dysfunction.[36]
TREATMENT AND FOLLOW‐UP
Unless the patient is septic and hemodynamically unstable, antibiotics should be held until a tissue sample is obtained for speciation and culture, either in the IR suite or the operating room. Once cultures have been obtained, broad‐spectrum IV antibiotics, usually vancomycin for gram‐positive coverage in combination with a third‐generation cephalosporin or aminoglycoside for additional gram‐negative coverage, should be started until organism sensitivities are determined and specific antibiotics can be administered.[3] Antibiotic therapy should continue for 4 to 6 weeks, though therapy may be administered for 8 weeks or longer in patients with vertebral osteomyelitis or immunocompromised patients.[19] An infectious disease specialist should be involved to determine the duration of therapy and transition to oral antibiotics.
As noted earlier, biopsy by IR should be considered when a trial of nonsurgical management is planned for a patient with no evidence of neurologic involvement, when the diagnosis is unclear after imaging, or when the patient is too high risk for surgery. Otherwise, surgical management is standard and should involve extensive surgical debridement with possible fusion if there is considerable structural compromise to the spinal column. After definitive treatment, repeat MRI is not recommended in the follow‐up of SEA; however, it is useful if there is concern for recurrence, as indicated by new fevers or a rising leukocyte count or inflammatory markers, especially CRP. Inflammatory labs should be obtained every 1 to 2 weeks to monitor for resolution of the infectious process.
CONCLUSION
Spinal epidural abscess is a potentially devastating condition that can be difficult to diagnose. Although uncommon, the triad of axial spine pain, fever, and new‐onset neurologic dysfunction are concerning. Other factors that increase the likelihood of SEA include a history of diabetes, IV drug abuse, spinal trauma, end‐stage renal disease, immunosuppressant therapy, recent invasive spine procedures, and concurrent infection of the skin or urinary tract. In patients with suspected SEA, inflammatory laboratory studies should be obtained along with gadolinium‐enhanced MRI of the entire spinal axis. Once the diagnosis is established, spine surgery and infectious disease consultation is mandatory, and IR biopsy may be appropriate in some cases. The rapidity of diagnosis and initiation of treatment are critical factors in optimizing patient outcome.
Disclosures
Mark A. Palumbo, MD, receives research funding from Globus Medical and is a paid consultant for Stryker. Alan H. Daniels, MD, is a paid consultant for Stryker and Osseous. No funding was obtained in support of this work.
- , , , et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285–291.
- , , , . Spinal epidural abscess. N Engl J Med. 1975;293:463–468.
- , , , et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189–196; discussion 197.
- . Spinal epidural abscess. N Engl J Med. 2006;355:2012–2020.
- , . Mortality, complication risk, and total charges after the treatment of epidural abscess. Spine J. 2015;15:249–255.
- , . Spinal epidural abscess. Curr Infect Dis Rep. 2004;16:436.
- , , , . Spinal epidural abscess: a ten‐year perspective. Neurosurgery. 1980;27:177–184.
- , , . Spinal epidural abscess: a meta‐analysis of 915 patients. Neurosurg Rev. 2000;23:175–204; discussion 205.
- , , , , , . Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14:326–330.
- , , , et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15‐year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 2014;20:344–349.
- , . Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79–85; discussion 86–87.
- , , . Infectious agents in spinal epidural abscesses. Neurology. 1980;30:844–850.
- , , , , , . Cervical epidural abscess after epidural steroid injection. Spine (Phila Pa 1976). 2004;29:E7–E9.
- , , . Spinal epidural abscesses in adults: review and report of iatrogenic cases. Scand J Infect Dis. 1990;22:249–257.
- , , , , . Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71:369–385.
- , , , , . Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212.
- , , . Epidural abscesses. Br J Anaesth. 2006;96:292–302.
- , . Spinal epidural abscess. Med Clin North Am. 1985;69;375–384.
- , , , . Spinal epidural abscess. J Emerg Med. 2010;39:384–390.
- , , . Spinal epidural abscess: clinical presentation, management and outcome [comment on Curry WT, Hoh BL, Hanjani SA, et al. Surg Neurol. 2005;63:364–371). Surg Neurol. 2005;64:279.
- , , , , . Routine replacement of tunneled, cuffed, hemodialysis catheters eliminates paraspinal/vertebral infections in patients with catheter‐associated bacteremia. Am J Nephrol. 2003;23:202–207.
- , . Paraplegia caused by spinal infection after acupuncture. Spinal Cord. 2006;44:258–259.
- , , , . Spinal epidural abscess after tattooing. Clin Infect Dis. 1999;29:225–226.
- . Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845–854.
- , , , . Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine. 2011;14:765–770.
- , , , . Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63:364–371; discussion 371.
- , . Bone and joint tuberculosis. Eur Spine J. 2013;22:556–566.
- , . Surgical management of spinal epidural abscess: selection of approach based on MRI appearance. J Clin Neurosci. 2004;11:130–133.
- , . Infection as a cause of spinal cord compression: a review of 36 spinal epidural abscess cases. Acta Neurochir (Wien). 2000;142:17–23.
- , , , et al. Spondyloarthropathy from long‐term hemodialysis. Radiology. 1988;167:761–764.
- , , , . Spinal epidural abscess successfully treated with percutaneous, computed tomography‐guided, needle aspiration and parenteral antibiotic therapy: case report and review of the literature. Neurosurgery. 2002;51:509–512; discussion 512.
- , , , . CT‐guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol. 2006;186:977–980.
- , , . Spinal epidural abscess: early clinical outcome in patients treated medically. Clin Orthop. 2005;439:56–60.
- , . Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9:265–274.
- , , , , , . Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015;15:10–17.
- , , , . Medicolegal cases for spinal epidural hematoma and spinal epidural abscess. Orthopedics. 2013;36:48–53.
- , , , . Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76–81.
Spinal epidural abscess (SEA) is caused by a suppurative infection in the epidural space. The mass effect of the abscess can compress and reduce blood flow to the spinal cord, conus medullaris, or cauda equina. Left untreated, the infection can lead to sensory loss, muscle weakness, visceral dysfunction, sepsis, and even death. Early diagnosis is essential to limit morbidity and neurologic injury. The classic triad of fever, axial pain, and neurological deficit occurs in as few as 13% of patients, highlighting the diagnostic challenge associated with SEA.[1]
This investigation reviews the current literature on SEA epidemiology, clinical findings, laboratory data, and treatment methods, with particular focus on nonsurgical versus surgical treatment. Our primary objective was to educate the clinician on when to suspect SEA and how to execute an appropriate diagnostic evaluation.
INCIDENCE AND EPIDEMIOLOGY
In 1975, the incidence of SEA was reported to be 0.2 to 2.0 per 10,000 hospital admissions.[2] Two decades later, a study of tertiary referral centers documented a much higher rate of 12.5 per 10,000 admissions.[3] The reported incidence continues to rise and has doubled in the past decade, with SEA currently representing approximately 10% of all primary spine infections.[4, 5] Potential explanations for this increasing incidence include aging of the population, an increasing prevalence of diabetes, increasing intravenous drug abuse, more widespread use of advanced immunosuppressive regimens, and an increased rate of invasive spinal procedures.[6, 7, 8] Other factors contributing to the rising incidence include increased detection due to the greater accessibility of magnetic resonance imaging (MRI) and increased reporting as a result of the concentration of cases at tertiary referral centers.
SEA is most common in patients older than 60 years[7] and those with multiple medical comorbidities. A review of over 30,000 patients found that the average number of comorbidities in patients who underwent surgical intervention for SEA was 6, ranging from 0 to 20.[5] The same study noted that diabetes was the most frequently associated disease (30% of patients), followed by chronic lung disease (19%), renal failure (13%), and obesity (13%) (Table 1).[5] A history of invasive spine interventions is an additional risk factor; between 14% and 22% occur as a result of spine surgery or percutaneous spine procedures (eg, epidural steroid injections).[8, 9] Regardless of pathogenesis, the rate of permanent neurologic injury after SEA is 30% to 50%, and the mortality rate ranges from 10% to 20%.[4, 5, 8, 10]
| Medical Comorbidity | Prevalence (%) |
|---|---|
| |
| Diabetes mellitus | 1546 |
| IV drug use | 437 |
| Spinal trauma | 533 |
| End‐stage renal disease | 213 |
| Immunosuppressant therapy | 716 |
| Cancer | 215 |
| HIV/AIDS | 29 |
MISSED DIAGNOSIS
Despite the availability of advanced imaging, rates of misdiagnosis at initial presentation remain substantial, with current estimates ranging from 11% to 75%.[4, 9] Back and neck pain symptoms are ubiquitous and nonspecific, often making the diagnosis difficult. Repeated emergency room visits for pain are common in patients who are eventually diagnosed with SEA. Davis et al. found that 51% of 63 patients present to the emergency room at least 2 or more times prior to diagnosis; 11% present 3 or more times.[1]
PATHOPHYSIOLOGY
Microbiology
Staphylococcus aureus, including methicillin resistant S aureus (MRSA), accounts for two‐thirds of all infections.[3] S aureus infection of the spine may occur in the setting of surgical intervention or concomitant skin infection, although it often occurs without an identifiable source. Historically, MRSA has been reported to be responsible for 15% of all staphylococcal infections of the epidural space; some institutions report MRSA rates as high as 40%.[4] S epidermidis is another common pathogen, which is most often encountered following spinal surgery, epidural catheter insertion, and spinal injections.[11] Gram‐negative infections are less common. Escherichia coli is characteristically isolated in patients with active urinary tract infections, and Pseudomonas aeruginosa is more common in intravenous (IV) drug users.[12] Rare causes of SEA include anaerobes such as Bacteroides,[13] various parasites, and fungal organisms such as actinomyces, nocardia, and mycobacteria.[8]
Mechanism of Inoculation
Infections may enter the epidural space by 4 mechanisms: hematogenous spread, direct extension, inoculation via spinal procedure, and trauma. Hematogenous spread from an existing infection is the most common mechanism.[14] Seeding of the epidural space from transient bacteremia after dental procedures has also been reported.[13]
The second most common mechanism of infection is direct spread from an infected component of the vertebral column or paraspinal soft tissues. Most commonly, this takes the form of an anterior SEA in association with vertebral body osteomyelitis. Septic arthritis of a facet joint can also cause a secondary infection of the posterior epidural space.[4, 15] Direct spread from other posterior structures (eg, retropharyngeal, psoas, or paraspinal muscle abscess) may cause SEA as well.[16]
Less‐frequent mechanisms include direct inoculation and trauma. Infection of the epidural space can occur in association with spinal surgery, placement of an epidural catheter, or spinal injections. Grewal et al. reported in 2006 that 1 in 1000 surgical and 1 in 2000 obstetric patients develop SEA following epidural nerve block.[17] Hematoma secondary to an osseous or ligamentous injury can become seeded by bacteria, leading to abscess formation.[18]
Development of Neurologic Symptoms
There are several proposed mechanisms by which SEA can produce neurologic dysfunction. The first theory is that the compressive effect of an expanding abscess decreases blood flow to the neuronal tissue.[4] Improvement of neurologic function following surgical decompression lends credence to this theory. A second potential mechanism is a loss of blood flow due to local vascular inflammation from the infection. Local arteritis may decrease inflow to the cord parenchyma. This theoretical mechanism offers an explanation for the rapid onset of profound neurologic compromise in some cases.[19, 20] Infection can also result in venous thrombophlebitis, which produces ischemic injury due to impaired outflow. Postmortem examination supports this hypothesis; autopsy has revealed local thrombosis of the leptomeningeal vessels adjacent to the level of SEA.[4] All of these mechanisms are probably involved to some degree in any given case of neurologic compromise.
PATIENT HISTORY
Patients present with a wide variety of complaints and confounding variables that complicate the diagnosis (eg, medical comorbidities, psychiatric disease, chronic pain, dementia, or preexistent nonambulatory status). Ninety‐five percent of patients with SEA have a chief complaint of axial spinal pain.[1] Approximately half of patients report fever, and 47% complain of weakness in either the upper or lower extremities.[9] The classic triad of fever, spine pain, and neurological deficits presents in only a minority of patients, with rates ranging from 13% to 37%.[1, 3] Additionally, 1 study found that the sensitivity of this triad was a mere 8%.[1]
The physician should inquire about comorbid conditions associated with SEA, including diabetes, kidney disease, and history of drug use. Any recent infection at a remote site, such as cellulitis or urinary tract infection, should also be investigated. As many as 44% of patients with vertebral osteomyelitis have an associated SEA; conversely, osteomyelitis may be present in up to 80% of patients with SEA.[2, 13] Spinal procedures such as epidural[13] or facet joint injections,[14] placement of hemodialysis catheters,[21] acupuncture,[22] and tattoos[23] have also been implicated as risk factors.
PHYSICAL EXAM
Physical exam findings range from subtle back tenderness to severe neurologic deficits and complete paralysis. Spinal tenderness is elicited in up to 75% of patients, with equal rates of focal and diffuse back tenderness.[1, 4] Radicular symptoms are evident in 12% to 47% of patients, presenting as weakness identified in 26% to 60% and altered sensation in up to 67% of patients.[1, 4, 8, 11, 19] One study revealed that 71% of patients have an abnormal neurologic exam at presentation, including paresthesias (39%), motor weakness (39%), and loss of bladder and bowel control (27%).[3] Thus, a thorough neurologic exam is vital in SEA evaluation.
Heusner staged patients based on their clinical findings (Table 2). Because the evolution of symptoms can be variable and rapidly progressive[4, 24] to stage 3 or 4, documenting subtle abnormalities on initial exam and monitoring for changes are important. Many patients initially present in stage 1 or 2 but remain undiagnosed until progression to stage 3 or 4.[24]
| Stage | Symptoms |
|---|---|
| I | Back pain |
| II | Radiculopathy, neck pain, reflex changes |
| III | Paresthesia, weakness, bladder symptoms |
| IV | Paralysis |
DIAGNOSTIC WORKUP
Laboratory Testing
Routine tests should include a white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP). ESR has been shown to be the most sensitive and specific marker of SEA (Table 3). In a study of 63 patients matched with 126 controls, ESR was greater than 20 in 98% of cases and only 21% of controls.[1] Another study of 55 patients found the sensitivity and specificity of ESR to be 100% and 67%, respectively.[25] White count is less specific, with leukocytosis present in approximately two‐thirds of patients.[1, 25] CRP level rises faster than ESR in the setting of inflammation and also returns to baseline faster. As such, CRP is a useful means of monitoring the response to treatment.[1, 19]
|
| 1. Patient assessment |
| Evaluate for symptom triad (back pain, fever, neurologic dysfunction) |
| Assess for common comorbidities (diabetes, IV drug abuse, spinal trauma, ESRD, immunosuppressant therapy, recent spine procedure, systemic or local infection) |
| 2. Laboratory evaluation |
| Essential: ESR (most sensitive and specific), CRP, WBCs, blood cultures, UA |
| : echocardiogram, TB evaluation |
| No role: lumbar puncture |
| 3. Imaging |
| Gold standard: MRI w/gadolinium (90% sensitive) |
| If MRI contraindicated: CT myelogram |
| 4. Obtain tissue sample |
| Gold standard: open surgical biopsy, debridement fixation. |
| If patient unstable, diagnosis indeterminate, or no neuro symptoms: IR‐guided biopsy before surgery |
| 5. Antibiotics (once tissue sample obtained) |
| Empiric vancomycin plus third‐generation cephalosporin or aminoglycoside |
| Vancomycin only acceptable as monotherapy in patient with early diagnosis and no neurologic symptoms |
| 6. Surgical management (only if not done as step 4) |
| Early surgical consult recommended. Pursue surgical intervention if patient is stable for OR and neuro symptoms progressing or abscess refractory to antibiotics. Best outcomes occur with combined antibiotics and surgical debridement. |
Blood cultures are important to obtain at initial evaluation. Bacteremia from the offending organism may be present in up to 60% of cases; 60% to 90% of cases of bacteremia are caused by MRSA.[26] Urine culture should also be obtained in all patients. Sputum cultures should not be routinely obtained, but it may be beneficial in select patients with history of chronic obstructive pulmonary disease, new cough, or abnormality on chest radiographs. Transthoracic or transesophageal echocardiogram may be recommended for bacteremic patients, those with new heart murmur, or those with a history of IV drug abuse.
In patients in whom tuberculosis may be a potential risk, a tuberculin test with purified protein derivate can help rule out this organism. However, false‐negative testing may occur in up to 15% of patients, particularly the immunocompromised.[27] Patients with false positive results or prior vaccination with bacille Calmette‐Gurin (BCG) can be tested with QuantiFERON gold testing. These time‐consuming and expensive tests should not delay treatment with an empiric agent if tuberculosis is suspected due to patient risk factors or exposure, though an infectious disease specialist should be consulted before initiating treatment for tuberculosis.
Lumbar puncture should not be performed routinely in cases of suspected SEA, primarily due to the risk of bacterial contamination of the cerebrospinal fluid (CSF). Additionally, the increased protein and inflammatory cells seen in the CSF are nonspecific markers of parameningeal inflammation.[15]
Imaging
When the diagnosis of SEA is suspected based on clinical findings, MRI with gadolinium should be obtained first, as it is 90% sensitive for diagnosing SEA (Figure 1).[3] In patients unable to undergo MRI (eg, pacemaker), a computed tomography (CT) myelogram should be obtained instead. The study should be performed on an emergent basis, and the entire spine should be imaged due to the risk of noncontiguous lesions. Patients with plain radiograph and/or CT scan findings of bone lysis suggestive of vertebral osteomyelitis should also be evaluated with MRI if able.

MRI with contrast can often differentiate SEA from malignancy and other space‐occupying lesions.[28] Plain radiography can rule out other causes of back pain, such as trauma and degenerative disc disease, but it cannot demonstrate SEA. One study found that x‐rays showed pathology in only 16.6% of patients with SEA.[29]
Patients with exam findings and laboratory studies concerning for SEA who present to a community hospital without MRI capability should be transferred to a tertiary care center for advanced imaging and potential emergent treatment.
Tissue Culture
Though MRI is essential to the workup of SEA, biopsy with cultures allows for a definitive diagnosis.[30] Cultures may be obtained in the operating room or interventional radiology (IR) suite via fine‐needle aspiration or core‐needle biopsy under CT guidance, if available.[30, 31] CT‐guided bone biopsy, which has a sensitivity of 81% and specificity of 100%, may also be performed when vertebral osteomyelitis is present.[32] Biopsy by IR should be considered before surgical intervention, when the patient has no evidence of progressive neurologic deficit, the diagnosis is unclear, or the patient is too high risk for surgical intervention. In very high‐risk surgical patients, IR aspiration may be curative. Lyu et al. describe a case of refractory SEA treated with percutaneous CT‐guided needle aspiration alone, though they note that surgical debridement is preferred when possible.[31]
NONSURGICAL VERSUS SURGICAL MANAGEMENT
SEA may be treated without surgery in carefully selected patients. Savage et al. studied 52 patients, and found that nonsurgical management was often effective in patients who were completely neurologically intact at initial presentation. In their study, only 3 patients needed to undergo surgery due to development of new neurologic symptoms.[33] However, other studies report neurologic symptoms in 71% of patients at initial diagnosis.[3]
Adogwa et al. reviewed surgical versus nonsurgical management in elderly patients (>50 years old) over 15 years.[10] Their study included 30 patients treated operatively and 52 who received antibiotics alone. The decision for surgical management was at the surgeons' discretion; however, most patients with grade 2 or 3 symptoms underwent surgery, and those with paraplegia or quadriplegia for >48 hours did not. The authors found no clinically significant difference in outcome between these 2 groups and cautioned against surgical intervention for elderly patients with multiple comorbidities. It is worth noting, however, that 7/30 (23%) patients treated with surgery versus 5/52 (10%) treated conservatively had improved neurological outcomes (P = 0.03).[10] Numerous other studies have also found that neurologic status at presentation is the most important predictor of surgical outcomes.[7, 15, 34]
Patel et al. performed a study analyzing risk factors for failure of medical management, and found that diabetes mellitus, CRP >115, WBC >12.5, and positive blood cultures were predictors of failure.[9] Patients with >3 of these risk factors required surgery 76.9% of the time compared to 40.2% with 2, 35.4% with 1, and 8.3% with none.[9] The authors also found that surgical patients experienced better mean improvement than patients who failed nonsurgical treatment and subsequently underwent surgical decompression.[9]
In a follow‐up study by Alton et al., 62 patients were treated with either nonsurgical or surgical management. Indications for surgery in their study included a neurologic deficit at initial presentation or the development of a new neurological deficit while undergoing treatment. Twenty‐four patients presented without deficits and underwent nonsurgical management, but only 6 (25%) were treated successfully, as defined by stable or improved neurological status following therapy. In contrast, none of the 38 patients who underwent therapy with both IV antibiotics and emergent surgical management within 24 hours experienced deterioration in the neurologic status. For the 18 patients who failed nonsurgical management, surgery was performed within an average of 7 days. This group experienced improvement following surgery, but their neurological status improved less than those who underwent early surgery.[35]
These recent studies demonstrate that medical management may be an option for patients who are diagnosed early and present without neurologic deficits, but surgery is the mainstay of treatment for most patients. Additionally, patients who experience a delay in operative management often do not recover function as well as those patients who undergo emergent surgical debridement.[35] A delay in diagnosis has also been shown to lead to increase in lawsuits against providers. French et al. found an increase in verdicts against the provider when a delay in diagnosis >48 hours was present, irrespective of the degree of permanent neurologic dysfunction.[36]
TREATMENT AND FOLLOW‐UP
Unless the patient is septic and hemodynamically unstable, antibiotics should be held until a tissue sample is obtained for speciation and culture, either in the IR suite or the operating room. Once cultures have been obtained, broad‐spectrum IV antibiotics, usually vancomycin for gram‐positive coverage in combination with a third‐generation cephalosporin or aminoglycoside for additional gram‐negative coverage, should be started until organism sensitivities are determined and specific antibiotics can be administered.[3] Antibiotic therapy should continue for 4 to 6 weeks, though therapy may be administered for 8 weeks or longer in patients with vertebral osteomyelitis or immunocompromised patients.[19] An infectious disease specialist should be involved to determine the duration of therapy and transition to oral antibiotics.
As noted earlier, biopsy by IR should be considered when a trial of nonsurgical management is planned for a patient with no evidence of neurologic involvement, when the diagnosis is unclear after imaging, or when the patient is too high risk for surgery. Otherwise, surgical management is standard and should involve extensive surgical debridement with possible fusion if there is considerable structural compromise to the spinal column. After definitive treatment, repeat MRI is not recommended in the follow‐up of SEA; however, it is useful if there is concern for recurrence, as indicated by new fevers or a rising leukocyte count or inflammatory markers, especially CRP. Inflammatory labs should be obtained every 1 to 2 weeks to monitor for resolution of the infectious process.
CONCLUSION
Spinal epidural abscess is a potentially devastating condition that can be difficult to diagnose. Although uncommon, the triad of axial spine pain, fever, and new‐onset neurologic dysfunction are concerning. Other factors that increase the likelihood of SEA include a history of diabetes, IV drug abuse, spinal trauma, end‐stage renal disease, immunosuppressant therapy, recent invasive spine procedures, and concurrent infection of the skin or urinary tract. In patients with suspected SEA, inflammatory laboratory studies should be obtained along with gadolinium‐enhanced MRI of the entire spinal axis. Once the diagnosis is established, spine surgery and infectious disease consultation is mandatory, and IR biopsy may be appropriate in some cases. The rapidity of diagnosis and initiation of treatment are critical factors in optimizing patient outcome.
Disclosures
Mark A. Palumbo, MD, receives research funding from Globus Medical and is a paid consultant for Stryker. Alan H. Daniels, MD, is a paid consultant for Stryker and Osseous. No funding was obtained in support of this work.
Spinal epidural abscess (SEA) is caused by a suppurative infection in the epidural space. The mass effect of the abscess can compress and reduce blood flow to the spinal cord, conus medullaris, or cauda equina. Left untreated, the infection can lead to sensory loss, muscle weakness, visceral dysfunction, sepsis, and even death. Early diagnosis is essential to limit morbidity and neurologic injury. The classic triad of fever, axial pain, and neurological deficit occurs in as few as 13% of patients, highlighting the diagnostic challenge associated with SEA.[1]
This investigation reviews the current literature on SEA epidemiology, clinical findings, laboratory data, and treatment methods, with particular focus on nonsurgical versus surgical treatment. Our primary objective was to educate the clinician on when to suspect SEA and how to execute an appropriate diagnostic evaluation.
INCIDENCE AND EPIDEMIOLOGY
In 1975, the incidence of SEA was reported to be 0.2 to 2.0 per 10,000 hospital admissions.[2] Two decades later, a study of tertiary referral centers documented a much higher rate of 12.5 per 10,000 admissions.[3] The reported incidence continues to rise and has doubled in the past decade, with SEA currently representing approximately 10% of all primary spine infections.[4, 5] Potential explanations for this increasing incidence include aging of the population, an increasing prevalence of diabetes, increasing intravenous drug abuse, more widespread use of advanced immunosuppressive regimens, and an increased rate of invasive spinal procedures.[6, 7, 8] Other factors contributing to the rising incidence include increased detection due to the greater accessibility of magnetic resonance imaging (MRI) and increased reporting as a result of the concentration of cases at tertiary referral centers.
SEA is most common in patients older than 60 years[7] and those with multiple medical comorbidities. A review of over 30,000 patients found that the average number of comorbidities in patients who underwent surgical intervention for SEA was 6, ranging from 0 to 20.[5] The same study noted that diabetes was the most frequently associated disease (30% of patients), followed by chronic lung disease (19%), renal failure (13%), and obesity (13%) (Table 1).[5] A history of invasive spine interventions is an additional risk factor; between 14% and 22% occur as a result of spine surgery or percutaneous spine procedures (eg, epidural steroid injections).[8, 9] Regardless of pathogenesis, the rate of permanent neurologic injury after SEA is 30% to 50%, and the mortality rate ranges from 10% to 20%.[4, 5, 8, 10]
| Medical Comorbidity | Prevalence (%) |
|---|---|
| |
| Diabetes mellitus | 1546 |
| IV drug use | 437 |
| Spinal trauma | 533 |
| End‐stage renal disease | 213 |
| Immunosuppressant therapy | 716 |
| Cancer | 215 |
| HIV/AIDS | 29 |
MISSED DIAGNOSIS
Despite the availability of advanced imaging, rates of misdiagnosis at initial presentation remain substantial, with current estimates ranging from 11% to 75%.[4, 9] Back and neck pain symptoms are ubiquitous and nonspecific, often making the diagnosis difficult. Repeated emergency room visits for pain are common in patients who are eventually diagnosed with SEA. Davis et al. found that 51% of 63 patients present to the emergency room at least 2 or more times prior to diagnosis; 11% present 3 or more times.[1]
PATHOPHYSIOLOGY
Microbiology
Staphylococcus aureus, including methicillin resistant S aureus (MRSA), accounts for two‐thirds of all infections.[3] S aureus infection of the spine may occur in the setting of surgical intervention or concomitant skin infection, although it often occurs without an identifiable source. Historically, MRSA has been reported to be responsible for 15% of all staphylococcal infections of the epidural space; some institutions report MRSA rates as high as 40%.[4] S epidermidis is another common pathogen, which is most often encountered following spinal surgery, epidural catheter insertion, and spinal injections.[11] Gram‐negative infections are less common. Escherichia coli is characteristically isolated in patients with active urinary tract infections, and Pseudomonas aeruginosa is more common in intravenous (IV) drug users.[12] Rare causes of SEA include anaerobes such as Bacteroides,[13] various parasites, and fungal organisms such as actinomyces, nocardia, and mycobacteria.[8]
Mechanism of Inoculation
Infections may enter the epidural space by 4 mechanisms: hematogenous spread, direct extension, inoculation via spinal procedure, and trauma. Hematogenous spread from an existing infection is the most common mechanism.[14] Seeding of the epidural space from transient bacteremia after dental procedures has also been reported.[13]
The second most common mechanism of infection is direct spread from an infected component of the vertebral column or paraspinal soft tissues. Most commonly, this takes the form of an anterior SEA in association with vertebral body osteomyelitis. Septic arthritis of a facet joint can also cause a secondary infection of the posterior epidural space.[4, 15] Direct spread from other posterior structures (eg, retropharyngeal, psoas, or paraspinal muscle abscess) may cause SEA as well.[16]
Less‐frequent mechanisms include direct inoculation and trauma. Infection of the epidural space can occur in association with spinal surgery, placement of an epidural catheter, or spinal injections. Grewal et al. reported in 2006 that 1 in 1000 surgical and 1 in 2000 obstetric patients develop SEA following epidural nerve block.[17] Hematoma secondary to an osseous or ligamentous injury can become seeded by bacteria, leading to abscess formation.[18]
Development of Neurologic Symptoms
There are several proposed mechanisms by which SEA can produce neurologic dysfunction. The first theory is that the compressive effect of an expanding abscess decreases blood flow to the neuronal tissue.[4] Improvement of neurologic function following surgical decompression lends credence to this theory. A second potential mechanism is a loss of blood flow due to local vascular inflammation from the infection. Local arteritis may decrease inflow to the cord parenchyma. This theoretical mechanism offers an explanation for the rapid onset of profound neurologic compromise in some cases.[19, 20] Infection can also result in venous thrombophlebitis, which produces ischemic injury due to impaired outflow. Postmortem examination supports this hypothesis; autopsy has revealed local thrombosis of the leptomeningeal vessels adjacent to the level of SEA.[4] All of these mechanisms are probably involved to some degree in any given case of neurologic compromise.
PATIENT HISTORY
Patients present with a wide variety of complaints and confounding variables that complicate the diagnosis (eg, medical comorbidities, psychiatric disease, chronic pain, dementia, or preexistent nonambulatory status). Ninety‐five percent of patients with SEA have a chief complaint of axial spinal pain.[1] Approximately half of patients report fever, and 47% complain of weakness in either the upper or lower extremities.[9] The classic triad of fever, spine pain, and neurological deficits presents in only a minority of patients, with rates ranging from 13% to 37%.[1, 3] Additionally, 1 study found that the sensitivity of this triad was a mere 8%.[1]
The physician should inquire about comorbid conditions associated with SEA, including diabetes, kidney disease, and history of drug use. Any recent infection at a remote site, such as cellulitis or urinary tract infection, should also be investigated. As many as 44% of patients with vertebral osteomyelitis have an associated SEA; conversely, osteomyelitis may be present in up to 80% of patients with SEA.[2, 13] Spinal procedures such as epidural[13] or facet joint injections,[14] placement of hemodialysis catheters,[21] acupuncture,[22] and tattoos[23] have also been implicated as risk factors.
PHYSICAL EXAM
Physical exam findings range from subtle back tenderness to severe neurologic deficits and complete paralysis. Spinal tenderness is elicited in up to 75% of patients, with equal rates of focal and diffuse back tenderness.[1, 4] Radicular symptoms are evident in 12% to 47% of patients, presenting as weakness identified in 26% to 60% and altered sensation in up to 67% of patients.[1, 4, 8, 11, 19] One study revealed that 71% of patients have an abnormal neurologic exam at presentation, including paresthesias (39%), motor weakness (39%), and loss of bladder and bowel control (27%).[3] Thus, a thorough neurologic exam is vital in SEA evaluation.
Heusner staged patients based on their clinical findings (Table 2). Because the evolution of symptoms can be variable and rapidly progressive[4, 24] to stage 3 or 4, documenting subtle abnormalities on initial exam and monitoring for changes are important. Many patients initially present in stage 1 or 2 but remain undiagnosed until progression to stage 3 or 4.[24]
| Stage | Symptoms |
|---|---|
| I | Back pain |
| II | Radiculopathy, neck pain, reflex changes |
| III | Paresthesia, weakness, bladder symptoms |
| IV | Paralysis |
DIAGNOSTIC WORKUP
Laboratory Testing
Routine tests should include a white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP). ESR has been shown to be the most sensitive and specific marker of SEA (Table 3). In a study of 63 patients matched with 126 controls, ESR was greater than 20 in 98% of cases and only 21% of controls.[1] Another study of 55 patients found the sensitivity and specificity of ESR to be 100% and 67%, respectively.[25] White count is less specific, with leukocytosis present in approximately two‐thirds of patients.[1, 25] CRP level rises faster than ESR in the setting of inflammation and also returns to baseline faster. As such, CRP is a useful means of monitoring the response to treatment.[1, 19]
|
| 1. Patient assessment |
| Evaluate for symptom triad (back pain, fever, neurologic dysfunction) |
| Assess for common comorbidities (diabetes, IV drug abuse, spinal trauma, ESRD, immunosuppressant therapy, recent spine procedure, systemic or local infection) |
| 2. Laboratory evaluation |
| Essential: ESR (most sensitive and specific), CRP, WBCs, blood cultures, UA |
| : echocardiogram, TB evaluation |
| No role: lumbar puncture |
| 3. Imaging |
| Gold standard: MRI w/gadolinium (90% sensitive) |
| If MRI contraindicated: CT myelogram |
| 4. Obtain tissue sample |
| Gold standard: open surgical biopsy, debridement fixation. |
| If patient unstable, diagnosis indeterminate, or no neuro symptoms: IR‐guided biopsy before surgery |
| 5. Antibiotics (once tissue sample obtained) |
| Empiric vancomycin plus third‐generation cephalosporin or aminoglycoside |
| Vancomycin only acceptable as monotherapy in patient with early diagnosis and no neurologic symptoms |
| 6. Surgical management (only if not done as step 4) |
| Early surgical consult recommended. Pursue surgical intervention if patient is stable for OR and neuro symptoms progressing or abscess refractory to antibiotics. Best outcomes occur with combined antibiotics and surgical debridement. |
Blood cultures are important to obtain at initial evaluation. Bacteremia from the offending organism may be present in up to 60% of cases; 60% to 90% of cases of bacteremia are caused by MRSA.[26] Urine culture should also be obtained in all patients. Sputum cultures should not be routinely obtained, but it may be beneficial in select patients with history of chronic obstructive pulmonary disease, new cough, or abnormality on chest radiographs. Transthoracic or transesophageal echocardiogram may be recommended for bacteremic patients, those with new heart murmur, or those with a history of IV drug abuse.
In patients in whom tuberculosis may be a potential risk, a tuberculin test with purified protein derivate can help rule out this organism. However, false‐negative testing may occur in up to 15% of patients, particularly the immunocompromised.[27] Patients with false positive results or prior vaccination with bacille Calmette‐Gurin (BCG) can be tested with QuantiFERON gold testing. These time‐consuming and expensive tests should not delay treatment with an empiric agent if tuberculosis is suspected due to patient risk factors or exposure, though an infectious disease specialist should be consulted before initiating treatment for tuberculosis.
Lumbar puncture should not be performed routinely in cases of suspected SEA, primarily due to the risk of bacterial contamination of the cerebrospinal fluid (CSF). Additionally, the increased protein and inflammatory cells seen in the CSF are nonspecific markers of parameningeal inflammation.[15]
Imaging
When the diagnosis of SEA is suspected based on clinical findings, MRI with gadolinium should be obtained first, as it is 90% sensitive for diagnosing SEA (Figure 1).[3] In patients unable to undergo MRI (eg, pacemaker), a computed tomography (CT) myelogram should be obtained instead. The study should be performed on an emergent basis, and the entire spine should be imaged due to the risk of noncontiguous lesions. Patients with plain radiograph and/or CT scan findings of bone lysis suggestive of vertebral osteomyelitis should also be evaluated with MRI if able.

MRI with contrast can often differentiate SEA from malignancy and other space‐occupying lesions.[28] Plain radiography can rule out other causes of back pain, such as trauma and degenerative disc disease, but it cannot demonstrate SEA. One study found that x‐rays showed pathology in only 16.6% of patients with SEA.[29]
Patients with exam findings and laboratory studies concerning for SEA who present to a community hospital without MRI capability should be transferred to a tertiary care center for advanced imaging and potential emergent treatment.
Tissue Culture
Though MRI is essential to the workup of SEA, biopsy with cultures allows for a definitive diagnosis.[30] Cultures may be obtained in the operating room or interventional radiology (IR) suite via fine‐needle aspiration or core‐needle biopsy under CT guidance, if available.[30, 31] CT‐guided bone biopsy, which has a sensitivity of 81% and specificity of 100%, may also be performed when vertebral osteomyelitis is present.[32] Biopsy by IR should be considered before surgical intervention, when the patient has no evidence of progressive neurologic deficit, the diagnosis is unclear, or the patient is too high risk for surgical intervention. In very high‐risk surgical patients, IR aspiration may be curative. Lyu et al. describe a case of refractory SEA treated with percutaneous CT‐guided needle aspiration alone, though they note that surgical debridement is preferred when possible.[31]
NONSURGICAL VERSUS SURGICAL MANAGEMENT
SEA may be treated without surgery in carefully selected patients. Savage et al. studied 52 patients, and found that nonsurgical management was often effective in patients who were completely neurologically intact at initial presentation. In their study, only 3 patients needed to undergo surgery due to development of new neurologic symptoms.[33] However, other studies report neurologic symptoms in 71% of patients at initial diagnosis.[3]
Adogwa et al. reviewed surgical versus nonsurgical management in elderly patients (>50 years old) over 15 years.[10] Their study included 30 patients treated operatively and 52 who received antibiotics alone. The decision for surgical management was at the surgeons' discretion; however, most patients with grade 2 or 3 symptoms underwent surgery, and those with paraplegia or quadriplegia for >48 hours did not. The authors found no clinically significant difference in outcome between these 2 groups and cautioned against surgical intervention for elderly patients with multiple comorbidities. It is worth noting, however, that 7/30 (23%) patients treated with surgery versus 5/52 (10%) treated conservatively had improved neurological outcomes (P = 0.03).[10] Numerous other studies have also found that neurologic status at presentation is the most important predictor of surgical outcomes.[7, 15, 34]
Patel et al. performed a study analyzing risk factors for failure of medical management, and found that diabetes mellitus, CRP >115, WBC >12.5, and positive blood cultures were predictors of failure.[9] Patients with >3 of these risk factors required surgery 76.9% of the time compared to 40.2% with 2, 35.4% with 1, and 8.3% with none.[9] The authors also found that surgical patients experienced better mean improvement than patients who failed nonsurgical treatment and subsequently underwent surgical decompression.[9]
In a follow‐up study by Alton et al., 62 patients were treated with either nonsurgical or surgical management. Indications for surgery in their study included a neurologic deficit at initial presentation or the development of a new neurological deficit while undergoing treatment. Twenty‐four patients presented without deficits and underwent nonsurgical management, but only 6 (25%) were treated successfully, as defined by stable or improved neurological status following therapy. In contrast, none of the 38 patients who underwent therapy with both IV antibiotics and emergent surgical management within 24 hours experienced deterioration in the neurologic status. For the 18 patients who failed nonsurgical management, surgery was performed within an average of 7 days. This group experienced improvement following surgery, but their neurological status improved less than those who underwent early surgery.[35]
These recent studies demonstrate that medical management may be an option for patients who are diagnosed early and present without neurologic deficits, but surgery is the mainstay of treatment for most patients. Additionally, patients who experience a delay in operative management often do not recover function as well as those patients who undergo emergent surgical debridement.[35] A delay in diagnosis has also been shown to lead to increase in lawsuits against providers. French et al. found an increase in verdicts against the provider when a delay in diagnosis >48 hours was present, irrespective of the degree of permanent neurologic dysfunction.[36]
TREATMENT AND FOLLOW‐UP
Unless the patient is septic and hemodynamically unstable, antibiotics should be held until a tissue sample is obtained for speciation and culture, either in the IR suite or the operating room. Once cultures have been obtained, broad‐spectrum IV antibiotics, usually vancomycin for gram‐positive coverage in combination with a third‐generation cephalosporin or aminoglycoside for additional gram‐negative coverage, should be started until organism sensitivities are determined and specific antibiotics can be administered.[3] Antibiotic therapy should continue for 4 to 6 weeks, though therapy may be administered for 8 weeks or longer in patients with vertebral osteomyelitis or immunocompromised patients.[19] An infectious disease specialist should be involved to determine the duration of therapy and transition to oral antibiotics.
As noted earlier, biopsy by IR should be considered when a trial of nonsurgical management is planned for a patient with no evidence of neurologic involvement, when the diagnosis is unclear after imaging, or when the patient is too high risk for surgery. Otherwise, surgical management is standard and should involve extensive surgical debridement with possible fusion if there is considerable structural compromise to the spinal column. After definitive treatment, repeat MRI is not recommended in the follow‐up of SEA; however, it is useful if there is concern for recurrence, as indicated by new fevers or a rising leukocyte count or inflammatory markers, especially CRP. Inflammatory labs should be obtained every 1 to 2 weeks to monitor for resolution of the infectious process.
CONCLUSION
Spinal epidural abscess is a potentially devastating condition that can be difficult to diagnose. Although uncommon, the triad of axial spine pain, fever, and new‐onset neurologic dysfunction are concerning. Other factors that increase the likelihood of SEA include a history of diabetes, IV drug abuse, spinal trauma, end‐stage renal disease, immunosuppressant therapy, recent invasive spine procedures, and concurrent infection of the skin or urinary tract. In patients with suspected SEA, inflammatory laboratory studies should be obtained along with gadolinium‐enhanced MRI of the entire spinal axis. Once the diagnosis is established, spine surgery and infectious disease consultation is mandatory, and IR biopsy may be appropriate in some cases. The rapidity of diagnosis and initiation of treatment are critical factors in optimizing patient outcome.
Disclosures
Mark A. Palumbo, MD, receives research funding from Globus Medical and is a paid consultant for Stryker. Alan H. Daniels, MD, is a paid consultant for Stryker and Osseous. No funding was obtained in support of this work.
- , , , et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285–291.
- , , , . Spinal epidural abscess. N Engl J Med. 1975;293:463–468.
- , , , et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189–196; discussion 197.
- . Spinal epidural abscess. N Engl J Med. 2006;355:2012–2020.
- , . Mortality, complication risk, and total charges after the treatment of epidural abscess. Spine J. 2015;15:249–255.
- , . Spinal epidural abscess. Curr Infect Dis Rep. 2004;16:436.
- , , , . Spinal epidural abscess: a ten‐year perspective. Neurosurgery. 1980;27:177–184.
- , , . Spinal epidural abscess: a meta‐analysis of 915 patients. Neurosurg Rev. 2000;23:175–204; discussion 205.
- , , , , , . Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14:326–330.
- , , , et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15‐year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 2014;20:344–349.
- , . Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79–85; discussion 86–87.
- , , . Infectious agents in spinal epidural abscesses. Neurology. 1980;30:844–850.
- , , , , , . Cervical epidural abscess after epidural steroid injection. Spine (Phila Pa 1976). 2004;29:E7–E9.
- , , . Spinal epidural abscesses in adults: review and report of iatrogenic cases. Scand J Infect Dis. 1990;22:249–257.
- , , , , . Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71:369–385.
- , , , , . Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212.
- , , . Epidural abscesses. Br J Anaesth. 2006;96:292–302.
- , . Spinal epidural abscess. Med Clin North Am. 1985;69;375–384.
- , , , . Spinal epidural abscess. J Emerg Med. 2010;39:384–390.
- , , . Spinal epidural abscess: clinical presentation, management and outcome [comment on Curry WT, Hoh BL, Hanjani SA, et al. Surg Neurol. 2005;63:364–371). Surg Neurol. 2005;64:279.
- , , , , . Routine replacement of tunneled, cuffed, hemodialysis catheters eliminates paraspinal/vertebral infections in patients with catheter‐associated bacteremia. Am J Nephrol. 2003;23:202–207.
- , . Paraplegia caused by spinal infection after acupuncture. Spinal Cord. 2006;44:258–259.
- , , , . Spinal epidural abscess after tattooing. Clin Infect Dis. 1999;29:225–226.
- . Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845–854.
- , , , . Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine. 2011;14:765–770.
- , , , . Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63:364–371; discussion 371.
- , . Bone and joint tuberculosis. Eur Spine J. 2013;22:556–566.
- , . Surgical management of spinal epidural abscess: selection of approach based on MRI appearance. J Clin Neurosci. 2004;11:130–133.
- , . Infection as a cause of spinal cord compression: a review of 36 spinal epidural abscess cases. Acta Neurochir (Wien). 2000;142:17–23.
- , , , et al. Spondyloarthropathy from long‐term hemodialysis. Radiology. 1988;167:761–764.
- , , , . Spinal epidural abscess successfully treated with percutaneous, computed tomography‐guided, needle aspiration and parenteral antibiotic therapy: case report and review of the literature. Neurosurgery. 2002;51:509–512; discussion 512.
- , , , . CT‐guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol. 2006;186:977–980.
- , , . Spinal epidural abscess: early clinical outcome in patients treated medically. Clin Orthop. 2005;439:56–60.
- , . Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9:265–274.
- , , , , , . Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015;15:10–17.
- , , , . Medicolegal cases for spinal epidural hematoma and spinal epidural abscess. Orthopedics. 2013;36:48–53.
- , , , . Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76–81.
- , , , et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285–291.
- , , , . Spinal epidural abscess. N Engl J Med. 1975;293:463–468.
- , , , et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189–196; discussion 197.
- . Spinal epidural abscess. N Engl J Med. 2006;355:2012–2020.
- , . Mortality, complication risk, and total charges after the treatment of epidural abscess. Spine J. 2015;15:249–255.
- , . Spinal epidural abscess. Curr Infect Dis Rep. 2004;16:436.
- , , , . Spinal epidural abscess: a ten‐year perspective. Neurosurgery. 1980;27:177–184.
- , , . Spinal epidural abscess: a meta‐analysis of 915 patients. Neurosurg Rev. 2000;23:175–204; discussion 205.
- , , , , , . Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14:326–330.
- , , , et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15‐year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 2014;20:344–349.
- , . Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79–85; discussion 86–87.
- , , . Infectious agents in spinal epidural abscesses. Neurology. 1980;30:844–850.
- , , , , , . Cervical epidural abscess after epidural steroid injection. Spine (Phila Pa 1976). 2004;29:E7–E9.
- , , . Spinal epidural abscesses in adults: review and report of iatrogenic cases. Scand J Infect Dis. 1990;22:249–257.
- , , , , . Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71:369–385.
- , , , , . Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212.
- , , . Epidural abscesses. Br J Anaesth. 2006;96:292–302.
- , . Spinal epidural abscess. Med Clin North Am. 1985;69;375–384.
- , , , . Spinal epidural abscess. J Emerg Med. 2010;39:384–390.
- , , . Spinal epidural abscess: clinical presentation, management and outcome [comment on Curry WT, Hoh BL, Hanjani SA, et al. Surg Neurol. 2005;63:364–371). Surg Neurol. 2005;64:279.
- , , , , . Routine replacement of tunneled, cuffed, hemodialysis catheters eliminates paraspinal/vertebral infections in patients with catheter‐associated bacteremia. Am J Nephrol. 2003;23:202–207.
- , . Paraplegia caused by spinal infection after acupuncture. Spinal Cord. 2006;44:258–259.
- , , , . Spinal epidural abscess after tattooing. Clin Infect Dis. 1999;29:225–226.
- . Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845–854.
- , , , . Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine. 2011;14:765–770.
- , , , . Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63:364–371; discussion 371.
- , . Bone and joint tuberculosis. Eur Spine J. 2013;22:556–566.
- , . Surgical management of spinal epidural abscess: selection of approach based on MRI appearance. J Clin Neurosci. 2004;11:130–133.
- , . Infection as a cause of spinal cord compression: a review of 36 spinal epidural abscess cases. Acta Neurochir (Wien). 2000;142:17–23.
- , , , et al. Spondyloarthropathy from long‐term hemodialysis. Radiology. 1988;167:761–764.
- , , , . Spinal epidural abscess successfully treated with percutaneous, computed tomography‐guided, needle aspiration and parenteral antibiotic therapy: case report and review of the literature. Neurosurgery. 2002;51:509–512; discussion 512.
- , , , . CT‐guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol. 2006;186:977–980.
- , , . Spinal epidural abscess: early clinical outcome in patients treated medically. Clin Orthop. 2005;439:56–60.
- , . Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9:265–274.
- , , , , , . Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015;15:10–17.
- , , , . Medicolegal cases for spinal epidural hematoma and spinal epidural abscess. Orthopedics. 2013;36:48–53.
- , , , . Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76–81.
Lenalidomide plus rituximab achieves 87% response rate
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3 or 4 mantle cell lymphoma.
Major finding: The primary endpoint – overall response rate – was 87%, and the complete response rate was 61%.
Data source: A multicenter, industry-sponsored, open-label, phase II study involving 38 patients with mantle cell lymphoma followed for a median of 30 months.
Disclosures: This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
Guidelines: Follow low-risk benign thyroid nodules conservatively
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
EXPERT ANALYSIS FROM ITC 2015
Inflammatory loop drives rosacea
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY
Long working hours raised stroke risk
Individuals who worked at least 55 hours a week were about 33% more likely to have a stroke than were those who worked 35-40 hours, according to a large meta-analysis of prospective studies.
The finding is “robust,” wrote Mika Kivimäki, Ph.D., of University College London and his associates. “There was no evidence of between-study heterogeneity, reverse causation bias, or confounding. Furthermore, the association did not vary between men and women or by geographical region, and was independent of the method of stroke ascertainment” (Lancet 2015;386[10005]:1739-46).
“Long working hours were also associated with incident coronary heart disease, but this association was weaker than that for stroke,” they noted.
Past studies have linked long working hours to cardiovascular disease, but prospective data are “scarce, imprecise, and mostly limited to coronary heart disease,” the researchers wrote. To assess relationships between working hours, incident CHD, and stroke, they searched Medline and Embase for studies published through August 2014, and also acquired unpublished data from 20 cohort studies through the Individual-Participant-Data Meta-analysis in Working Populations (IPD-Work) Consortium and open-access databases. These efforts yielded 25 total studies of 24 cohorts in the United States, Europe, and Australia, comprising CHD risk data for almost 604,000 individuals and stroke risk data for almost 529,000, the researchers said.
The studies reported 1,722 strokes and 4,768 new episodes of CHD after an average of 7.2 and 8.5 years of follow-up, respectively, the investigators determined. After accounting for age, sex, and socioeconomic status, individuals who averaged a 55-hour work week were 33% more likely to have a stroke (relative risk, 1.33; 95% confidence interval, 1.11-1.61; P = .002) and a 13% greater risk of CHD (RR, 1.13; 95% CI, 1.02-1.26; P = .02) than were those who worked 35-40 hours per week.
“We recorded a dose-response association for stroke, with RR estimates of 1.10 (95% CI, 0.94 to 1.28; P = .24) for 41 to 48 working hours; 1.27 (1.03 to 1.56; P = .03) for 49 to 54 working hour; and 1.33 (1.11 to 1.61; P = .002) for 55 working hours or more per week, compared with standard working hours (P less than .0001),” the researchers wrote. Based on the findings, “more attention should be paid to the management of vascular risk factors in individuals who work long hours,” they concluded.
The study was funded by the U.K. Medical Research Council, the Economic and Social Research Council, the European Union New and Emerging Risks in Occupational Safety and Health research program, the Finnish Work Environment Fund, the Swedish Research Council for Working Life and Social Research, German Social Accident Insurance, the Danish National Research Centre for the Working Environment, the Academy of Finland, the Ministry of Social Affairs and Employment (Netherlands), the U.S. National Institutes of Health, and the British Heart Foundation. The investigators reported having no conflicts of interest.
Individuals who worked at least 55 hours a week were about 33% more likely to have a stroke than were those who worked 35-40 hours, according to a large meta-analysis of prospective studies.
The finding is “robust,” wrote Mika Kivimäki, Ph.D., of University College London and his associates. “There was no evidence of between-study heterogeneity, reverse causation bias, or confounding. Furthermore, the association did not vary between men and women or by geographical region, and was independent of the method of stroke ascertainment” (Lancet 2015;386[10005]:1739-46).
“Long working hours were also associated with incident coronary heart disease, but this association was weaker than that for stroke,” they noted.
Past studies have linked long working hours to cardiovascular disease, but prospective data are “scarce, imprecise, and mostly limited to coronary heart disease,” the researchers wrote. To assess relationships between working hours, incident CHD, and stroke, they searched Medline and Embase for studies published through August 2014, and also acquired unpublished data from 20 cohort studies through the Individual-Participant-Data Meta-analysis in Working Populations (IPD-Work) Consortium and open-access databases. These efforts yielded 25 total studies of 24 cohorts in the United States, Europe, and Australia, comprising CHD risk data for almost 604,000 individuals and stroke risk data for almost 529,000, the researchers said.
The studies reported 1,722 strokes and 4,768 new episodes of CHD after an average of 7.2 and 8.5 years of follow-up, respectively, the investigators determined. After accounting for age, sex, and socioeconomic status, individuals who averaged a 55-hour work week were 33% more likely to have a stroke (relative risk, 1.33; 95% confidence interval, 1.11-1.61; P = .002) and a 13% greater risk of CHD (RR, 1.13; 95% CI, 1.02-1.26; P = .02) than were those who worked 35-40 hours per week.
“We recorded a dose-response association for stroke, with RR estimates of 1.10 (95% CI, 0.94 to 1.28; P = .24) for 41 to 48 working hours; 1.27 (1.03 to 1.56; P = .03) for 49 to 54 working hour; and 1.33 (1.11 to 1.61; P = .002) for 55 working hours or more per week, compared with standard working hours (P less than .0001),” the researchers wrote. Based on the findings, “more attention should be paid to the management of vascular risk factors in individuals who work long hours,” they concluded.
The study was funded by the U.K. Medical Research Council, the Economic and Social Research Council, the European Union New and Emerging Risks in Occupational Safety and Health research program, the Finnish Work Environment Fund, the Swedish Research Council for Working Life and Social Research, German Social Accident Insurance, the Danish National Research Centre for the Working Environment, the Academy of Finland, the Ministry of Social Affairs and Employment (Netherlands), the U.S. National Institutes of Health, and the British Heart Foundation. The investigators reported having no conflicts of interest.
Individuals who worked at least 55 hours a week were about 33% more likely to have a stroke than were those who worked 35-40 hours, according to a large meta-analysis of prospective studies.
The finding is “robust,” wrote Mika Kivimäki, Ph.D., of University College London and his associates. “There was no evidence of between-study heterogeneity, reverse causation bias, or confounding. Furthermore, the association did not vary between men and women or by geographical region, and was independent of the method of stroke ascertainment” (Lancet 2015;386[10005]:1739-46).
“Long working hours were also associated with incident coronary heart disease, but this association was weaker than that for stroke,” they noted.
Past studies have linked long working hours to cardiovascular disease, but prospective data are “scarce, imprecise, and mostly limited to coronary heart disease,” the researchers wrote. To assess relationships between working hours, incident CHD, and stroke, they searched Medline and Embase for studies published through August 2014, and also acquired unpublished data from 20 cohort studies through the Individual-Participant-Data Meta-analysis in Working Populations (IPD-Work) Consortium and open-access databases. These efforts yielded 25 total studies of 24 cohorts in the United States, Europe, and Australia, comprising CHD risk data for almost 604,000 individuals and stroke risk data for almost 529,000, the researchers said.
The studies reported 1,722 strokes and 4,768 new episodes of CHD after an average of 7.2 and 8.5 years of follow-up, respectively, the investigators determined. After accounting for age, sex, and socioeconomic status, individuals who averaged a 55-hour work week were 33% more likely to have a stroke (relative risk, 1.33; 95% confidence interval, 1.11-1.61; P = .002) and a 13% greater risk of CHD (RR, 1.13; 95% CI, 1.02-1.26; P = .02) than were those who worked 35-40 hours per week.
“We recorded a dose-response association for stroke, with RR estimates of 1.10 (95% CI, 0.94 to 1.28; P = .24) for 41 to 48 working hours; 1.27 (1.03 to 1.56; P = .03) for 49 to 54 working hour; and 1.33 (1.11 to 1.61; P = .002) for 55 working hours or more per week, compared with standard working hours (P less than .0001),” the researchers wrote. Based on the findings, “more attention should be paid to the management of vascular risk factors in individuals who work long hours,” they concluded.
The study was funded by the U.K. Medical Research Council, the Economic and Social Research Council, the European Union New and Emerging Risks in Occupational Safety and Health research program, the Finnish Work Environment Fund, the Swedish Research Council for Working Life and Social Research, German Social Accident Insurance, the Danish National Research Centre for the Working Environment, the Academy of Finland, the Ministry of Social Affairs and Employment (Netherlands), the U.S. National Institutes of Health, and the British Heart Foundation. The investigators reported having no conflicts of interest.
FROM THE LANCET
Key clinical point: Long work weeks were tied to increased stroke risk in a large meta-analysis.
Major finding: Individuals worked an average of at least 55 hours a week were about 33% more likely to have a stroke than were those who worked 35-40 hours (P = .002).
Data source: Meta-analysis of 25 prospective cohort studies from Europe, the United States, and Australia.
Disclosures: The study was funded by a variety of governmental research councils, programs, funds, foundations, ministries, and academies in various countries. The investigators reported having no conflicts of interest.
Stem cell benefits endure 3 years in infants
Infants with congenital heart disease, specifically left heart syndrome, who had cardiac stem-cell therapy after surgery showed improved cardiac and brain function after 3 years when compared with infants who had standard therapy, according to latest results from a clinical trial of progenitor cell infusion in infants.
The prospective, controlled study, published in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150[5]:1198-1208), involved 14 infants with hypoplastic left heart syndrome (HLHS). Dr. Suguru Tarui and colleagues at Okayama University Hospital in Japan infused seven infants with intracoronary cardiosphere-derived cells (CDCs) 1 month after they had two- or three-stage palliative surgery. Seven controls had standard care alone. The trial is known TICAP (Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology) (ClinicalTrials.gov ID: NCT01273857).
Infants born with HLHS are known to have poor prognoses. HLHS has been associated with the highest mortality of all congenital heart lesions (Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18[1]:2-6).
The Okayama investigators conducted TICAP in 2011 and demonstrated the feasibility and safety of the intracoronary delivery of CDCs in infants with HLHS after staged procedures. The latest report looks at the secondary outcome of cardiac function through 36 months of follow-up, and is the first clinical trial to report on the mid-term results of cardiac progenitor therapy in congenital heart disease.
“CDC infusion could improve right ventricular function from 3 through 36 months of observation,” Dr. Tarui and colleagues said. “Patients treated by CDCs showed an increase in somatic growth and reduced heart failure status in mid-term follow-up.”
Upon entry into the study, all subjects were of similar age, body weight, and risk profiles. After 36 months, the CDC group showed no complications and significantly improved right ventricular injection fraction compared with controls. As a result, the CDC group had reduced brain natriuretic peptide levels, lower rates of unplanned catheterizations, and higher weight for age. No adverse events occurred in the CDC group, while two patients in the control group had complications (one developed heart failure, another developed enteropathy).
The investigators looked at predictors of cardiac functional efficacy in the CDC group and determined that younger age was associated with greater improvement of right ventricular ejection fraction, as measured on echocardiography. The lower weight-for-age Z score and reduced ejection fraction at the time of infusion may be predictors of cardiac function improvements at 3 years.
“This therapeutic strategy may merit somatic growth enhancement and reduce the incidence of heart failure,” Dr. Tarui and coauthors said.
They noted a number of limitations of their small clinical trial. The study was nonrandomized, and the cardiac interventions were not blinded, among others. “Larger phase II studies focusing on changes in cardiac function, myocardial fibrosis, and quality of life and clinical event rates are warranted to confirm these effects of CDC administration in patients with single ventricular physiology,” they said.
The government of Japan through its ministries of Health, Labor and Welfare, and Education, Culture Sports, Science and Technology provided funding for the study, as did the Research Foundation of Okayama University Hospital. The authors had no disclosures.
The benefits of stem cell treatments for cardiac function beyond the initial follow-up period have “remained an unanswered question,” Dr. Sunjay Kaushal of University of Maryland, Baltimore, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150[5]:1209-11). “The 3-year follow-up data from the TICAP trial, therefore, offers one of the first opportunities to examine the durability of the outcomes found in stem cell-treated patients,” Dr. Kaushal said.
While TICAP provides clues into independent predictors of success with stem cell treatments, the small study population of seven patients makes it difficult to confirm those predictors, he said. “Multivariate analysis of results from future trials with larger sample size will be needed to verify these preliminary findings,” he noted.
Nonetheless, the study adds to the emerging evidence that younger, sicker patients may respond better to stem cell infusion, a concept Dr. Kaushal termed “intriguing.”
“Meanwhile, the TICAP trial and its three-year follow-up should garner enthusiasm for stem cell therapy, and establish the basis for non-ischemic ventricular dysfunction in pediatric patients as an emerging indication for stem cell therapy,” he said.
The benefits of stem cell treatments for cardiac function beyond the initial follow-up period have “remained an unanswered question,” Dr. Sunjay Kaushal of University of Maryland, Baltimore, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150[5]:1209-11). “The 3-year follow-up data from the TICAP trial, therefore, offers one of the first opportunities to examine the durability of the outcomes found in stem cell-treated patients,” Dr. Kaushal said.
While TICAP provides clues into independent predictors of success with stem cell treatments, the small study population of seven patients makes it difficult to confirm those predictors, he said. “Multivariate analysis of results from future trials with larger sample size will be needed to verify these preliminary findings,” he noted.
Nonetheless, the study adds to the emerging evidence that younger, sicker patients may respond better to stem cell infusion, a concept Dr. Kaushal termed “intriguing.”
“Meanwhile, the TICAP trial and its three-year follow-up should garner enthusiasm for stem cell therapy, and establish the basis for non-ischemic ventricular dysfunction in pediatric patients as an emerging indication for stem cell therapy,” he said.
The benefits of stem cell treatments for cardiac function beyond the initial follow-up period have “remained an unanswered question,” Dr. Sunjay Kaushal of University of Maryland, Baltimore, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150[5]:1209-11). “The 3-year follow-up data from the TICAP trial, therefore, offers one of the first opportunities to examine the durability of the outcomes found in stem cell-treated patients,” Dr. Kaushal said.
While TICAP provides clues into independent predictors of success with stem cell treatments, the small study population of seven patients makes it difficult to confirm those predictors, he said. “Multivariate analysis of results from future trials with larger sample size will be needed to verify these preliminary findings,” he noted.
Nonetheless, the study adds to the emerging evidence that younger, sicker patients may respond better to stem cell infusion, a concept Dr. Kaushal termed “intriguing.”
“Meanwhile, the TICAP trial and its three-year follow-up should garner enthusiasm for stem cell therapy, and establish the basis for non-ischemic ventricular dysfunction in pediatric patients as an emerging indication for stem cell therapy,” he said.
Infants with congenital heart disease, specifically left heart syndrome, who had cardiac stem-cell therapy after surgery showed improved cardiac and brain function after 3 years when compared with infants who had standard therapy, according to latest results from a clinical trial of progenitor cell infusion in infants.
The prospective, controlled study, published in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150[5]:1198-1208), involved 14 infants with hypoplastic left heart syndrome (HLHS). Dr. Suguru Tarui and colleagues at Okayama University Hospital in Japan infused seven infants with intracoronary cardiosphere-derived cells (CDCs) 1 month after they had two- or three-stage palliative surgery. Seven controls had standard care alone. The trial is known TICAP (Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology) (ClinicalTrials.gov ID: NCT01273857).
Infants born with HLHS are known to have poor prognoses. HLHS has been associated with the highest mortality of all congenital heart lesions (Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18[1]:2-6).
The Okayama investigators conducted TICAP in 2011 and demonstrated the feasibility and safety of the intracoronary delivery of CDCs in infants with HLHS after staged procedures. The latest report looks at the secondary outcome of cardiac function through 36 months of follow-up, and is the first clinical trial to report on the mid-term results of cardiac progenitor therapy in congenital heart disease.
“CDC infusion could improve right ventricular function from 3 through 36 months of observation,” Dr. Tarui and colleagues said. “Patients treated by CDCs showed an increase in somatic growth and reduced heart failure status in mid-term follow-up.”
Upon entry into the study, all subjects were of similar age, body weight, and risk profiles. After 36 months, the CDC group showed no complications and significantly improved right ventricular injection fraction compared with controls. As a result, the CDC group had reduced brain natriuretic peptide levels, lower rates of unplanned catheterizations, and higher weight for age. No adverse events occurred in the CDC group, while two patients in the control group had complications (one developed heart failure, another developed enteropathy).
The investigators looked at predictors of cardiac functional efficacy in the CDC group and determined that younger age was associated with greater improvement of right ventricular ejection fraction, as measured on echocardiography. The lower weight-for-age Z score and reduced ejection fraction at the time of infusion may be predictors of cardiac function improvements at 3 years.
“This therapeutic strategy may merit somatic growth enhancement and reduce the incidence of heart failure,” Dr. Tarui and coauthors said.
They noted a number of limitations of their small clinical trial. The study was nonrandomized, and the cardiac interventions were not blinded, among others. “Larger phase II studies focusing on changes in cardiac function, myocardial fibrosis, and quality of life and clinical event rates are warranted to confirm these effects of CDC administration in patients with single ventricular physiology,” they said.
The government of Japan through its ministries of Health, Labor and Welfare, and Education, Culture Sports, Science and Technology provided funding for the study, as did the Research Foundation of Okayama University Hospital. The authors had no disclosures.
Infants with congenital heart disease, specifically left heart syndrome, who had cardiac stem-cell therapy after surgery showed improved cardiac and brain function after 3 years when compared with infants who had standard therapy, according to latest results from a clinical trial of progenitor cell infusion in infants.
The prospective, controlled study, published in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150[5]:1198-1208), involved 14 infants with hypoplastic left heart syndrome (HLHS). Dr. Suguru Tarui and colleagues at Okayama University Hospital in Japan infused seven infants with intracoronary cardiosphere-derived cells (CDCs) 1 month after they had two- or three-stage palliative surgery. Seven controls had standard care alone. The trial is known TICAP (Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology) (ClinicalTrials.gov ID: NCT01273857).
Infants born with HLHS are known to have poor prognoses. HLHS has been associated with the highest mortality of all congenital heart lesions (Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18[1]:2-6).
The Okayama investigators conducted TICAP in 2011 and demonstrated the feasibility and safety of the intracoronary delivery of CDCs in infants with HLHS after staged procedures. The latest report looks at the secondary outcome of cardiac function through 36 months of follow-up, and is the first clinical trial to report on the mid-term results of cardiac progenitor therapy in congenital heart disease.
“CDC infusion could improve right ventricular function from 3 through 36 months of observation,” Dr. Tarui and colleagues said. “Patients treated by CDCs showed an increase in somatic growth and reduced heart failure status in mid-term follow-up.”
Upon entry into the study, all subjects were of similar age, body weight, and risk profiles. After 36 months, the CDC group showed no complications and significantly improved right ventricular injection fraction compared with controls. As a result, the CDC group had reduced brain natriuretic peptide levels, lower rates of unplanned catheterizations, and higher weight for age. No adverse events occurred in the CDC group, while two patients in the control group had complications (one developed heart failure, another developed enteropathy).
The investigators looked at predictors of cardiac functional efficacy in the CDC group and determined that younger age was associated with greater improvement of right ventricular ejection fraction, as measured on echocardiography. The lower weight-for-age Z score and reduced ejection fraction at the time of infusion may be predictors of cardiac function improvements at 3 years.
“This therapeutic strategy may merit somatic growth enhancement and reduce the incidence of heart failure,” Dr. Tarui and coauthors said.
They noted a number of limitations of their small clinical trial. The study was nonrandomized, and the cardiac interventions were not blinded, among others. “Larger phase II studies focusing on changes in cardiac function, myocardial fibrosis, and quality of life and clinical event rates are warranted to confirm these effects of CDC administration in patients with single ventricular physiology,” they said.
The government of Japan through its ministries of Health, Labor and Welfare, and Education, Culture Sports, Science and Technology provided funding for the study, as did the Research Foundation of Okayama University Hospital. The authors had no disclosures.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: This is the first clinical trial to report the midterm safety and efficacy of cardiac progenitor therapy in congenital heart disease.
Major finding: After 36 months, infants who received stem cells for congenital heart failure had better outcomes than did infants who had standard treatment.
Data source: Prospective clinical trial of 14 infants enrolled in the Transcoronary Infusion of Cardiac Progenitor Cells in Hypoplastic Left Heat Syndrome (TICAP) trial, divided evenly between treatment and control groups.
Disclosures: The government of Japan through its ministries of Health, Labor and Welfare, and Education, Culture Sports, Science and Technology provided funding for the study, as did the Research Foundation of Okayama University Hospital. The authors had no disclosures.