User login
Metacognition to Reduce Medical Error
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
The Puzzle of Posthospital Recovery
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
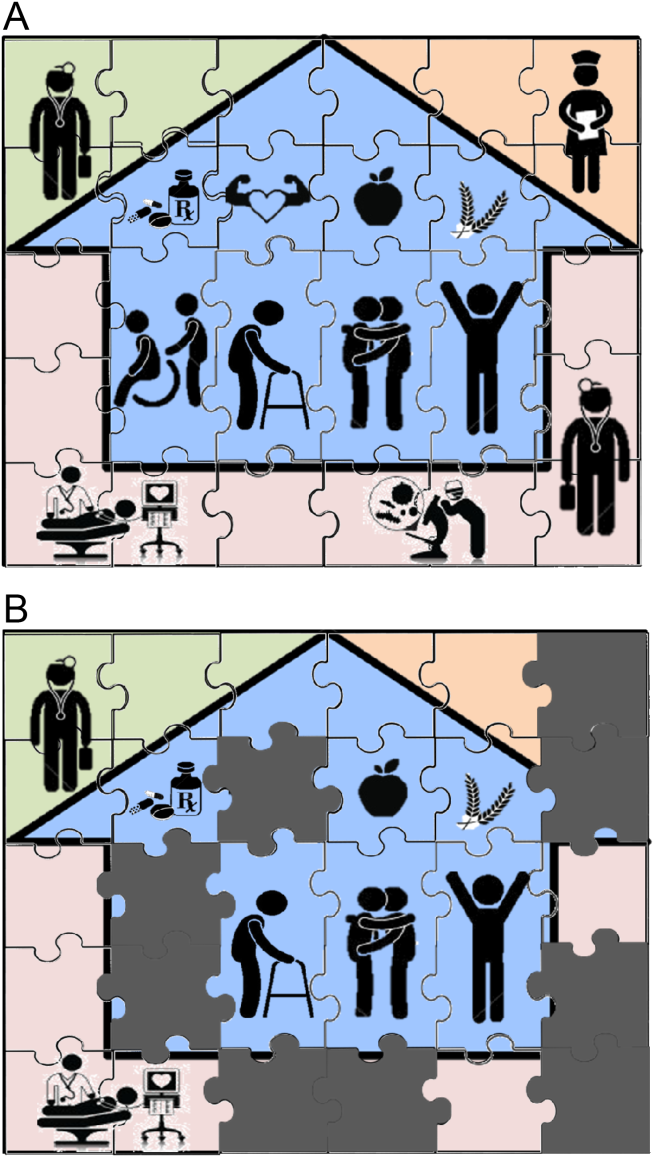
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.
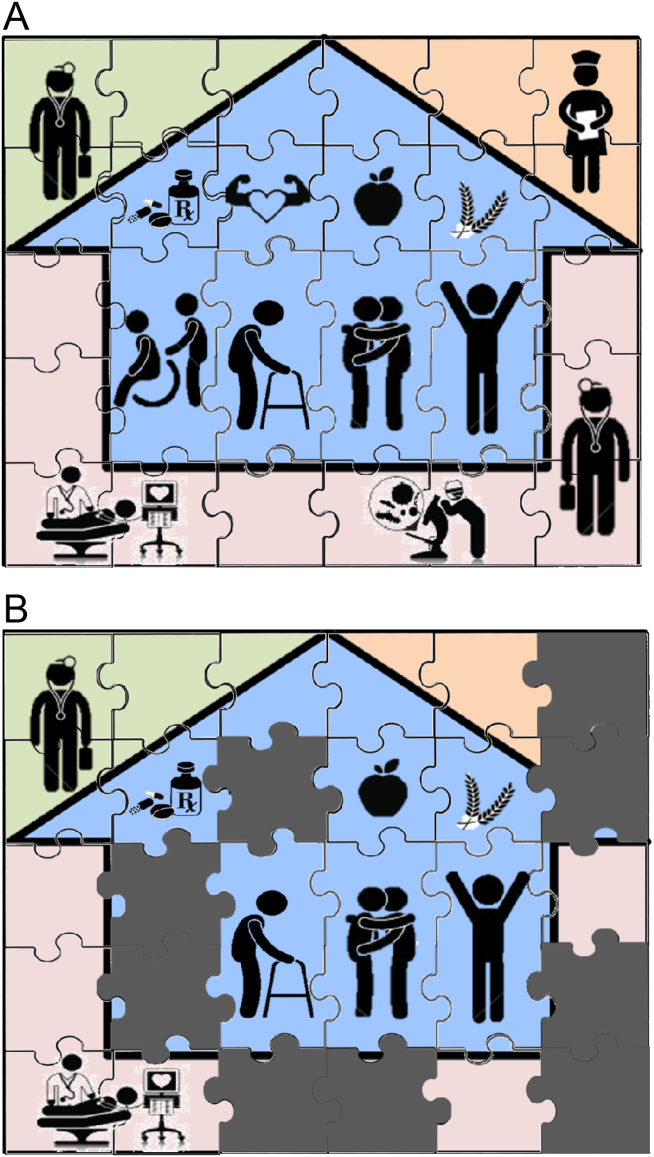
Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.

Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.

Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
Elements of Confusion
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).
The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).
The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.
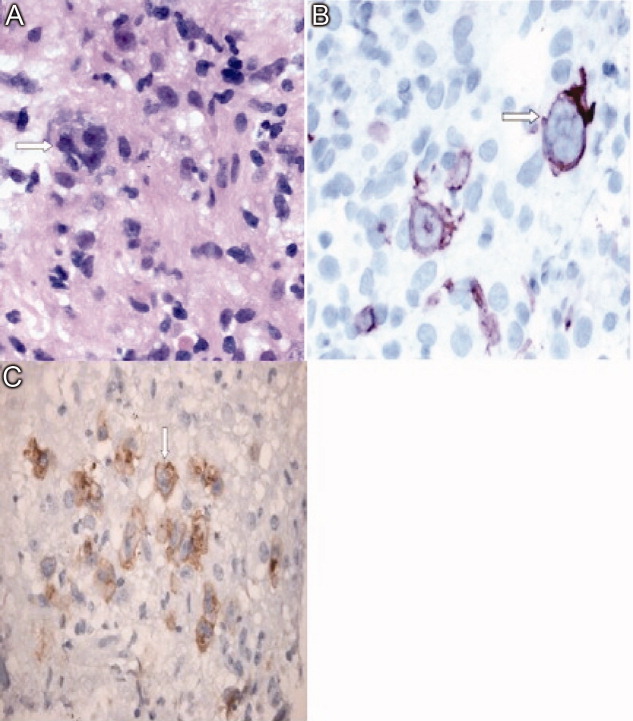
Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).


The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).

The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.

Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).


The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).

The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.

Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
Opacity Overlying Vertebral Column on CRX
In the evaluation of patients presenting with complaints referable to the chest, the chest radiograph (CXR) is an important and almost universal component of the initial assessment.
Chest radiography is normally performed with both posterior‐anterior (PA) and lateral projections.1 The lateral projection is generally accepted as an indispensable component because it allows better visualization of certain structures including the lower lobes, areas of which are partially obscured by the heart or hemidiaphragms on the PA projection. As such, some radiographic findings are only apparent on the lateral projection. As well, when an abnormality is discovered on the PA projection, the orthogonal orientation of the lateral projection often allows lesion localization.
Together with information gleaned from a thorough history and physical examination, the results of chest radiography often inform initial management when a diagnosis has been established, and the need for additional investigations when the diagnosis remains in question. In the hospital setting, the CXR is often reviewed first by physicians who are not radiologists (eg, internists, emergency physicians, and trainees at various stages of training) when evaluating a patient.
We undertook the current study to investigate the test characteristics (sensitivity, specificity, and likelihood ratio [LR]), and precision of 1 particular finding on lateral chest radiography as interpreted by nonradiologist physicians in the hospital setting. On a normal lateral CXR, one should observe progressive superior‐inferior vertebral radiolucency (Figure 1A). Observed opacity overlying the vertebral column obscuring this progression is usually abnormal and suggestive of pathology in the lower lobes of the lungs or associated structures (Figure 1B). A review of the literature yielded only 1 study of this finding,2 which used a case‐control design and lacked a true gold standard investigation necessary for calculation of meaningful test characteristics. In fact, few studies have compared findings on chest radiography with more definitive investigations,3, 4 and none have examined the predictive value of this finding by nonradiologist observers using a reference standard investigation such as computed tomography (CT) of the chest.
Methods
The radiology Picture Archiving and Communication System (PACS) used at our institution allows us to search for exams by date and study type. We retrospectively identified all patients seen at 1 of 3 university‐affiliated tertiary care adult teaching hospitals (Toronto General, Toronto Western, and Mount Sinai Hospitals) within an 8‐month period (January 1, 2006 to August 31, 2006) who underwent a 2‐view CXR (PA and lateral views). (Note that in this study, the terms radiograph, x‐ray, and plain film are used synonymously.) We then determined which of these patients had a subsequent CT within 24 hours of the x‐ray, resulting in a sample of 370 patients for this study. These patients primarily included patients presenting to the emergency department, and inpatients, with a very small number of outpatients. The majority of the index CXRs were performed for chief complaints of dyspnea, chest pain, cough, or for follow‐up of a previous CXR. However, many were simply performed routinely for admission. Patients with prosthetic devices or appliances obscuring the vertebral column were excluded.
After several training sessions by an experienced internist (A.S.D.), 2 authors (D.R.M., M.E.D.) independently reviewed each lateral CXR using standard 17‐inch displays and documented the presence or absence of abnormal radioopacity obscuring the superior to inferior progression of vertebral radiolucency. These 2 authors were fourth‐year medical students at the time the study began and first‐year trainees in internal medicine when it ended. The presence of abnormal opacity overlying the vertebral column was recorded as a positive test while the absence of this finding was recorded as a negative test.
Observed opacity overlying the vertebral column on lateral CXRs was considered abnormal when it did not represent manifestations of normal anatomical structures. However, the finding of opacity overlying the vertebral column of little diagnostic significance, such as prominent pulmonary vessels, degenerative bony changes, or the finding of a tortuous aorta, were considered normal in this study. Corresponding PA CXRs were also available for viewing. In most cases, the authors viewed both the lateral and PA CXRs, reflecting their use in clinical practice. However, in cases of obvious abnormality on the lateral CXR, only that projection was viewed. No clinical information was made available to the observers of the lateral CXR and they were blinded to the results of CT imaging of the chest. All 370 cases were reviewed by both observers (D.R.M. and M.E.D.). For the purpose of calculating test characteristics and LRs, cases of disagreement between the 3 lateral CXR observers were resolved by independent review by a third author (A.S.D.), a general internist with over 20 years of experience interpreting the lateral CXR.
A fourth author (M.O.B) reviewed the chest CT reports for each patient and recorded the mention of the presence or absence of various pathologies in the lower lobes of the lungs and associated structures in those reports. No clinical information was made available to this author and he was blinded to the results of lateral CXR. All CT investigations were originally interpreted by a university‐affiliated chest radiology faculty member at the time of the investigation. Table 1 lists all relevant chest CT findings in our sample that were recorded as disease‐positive for the purpose of dichotomizing the results of the reference standard, and enabling calculation of test characteristics (Table 2). Notable chest CT findings that were not recorded as disease‐positive for this purpose included mediastinal lymphadenopathy, subpleural density, lytic vertebral lesions, cystic or emphysematous changes, and pneumothorax. Dependent atelectasis was included within the disease‐positive category, though some cases may not have been pathological. It should be pointed out that there may be some variation in terminology used between staff radiologists (eg, reticulation by one radiologist may be called minor densities by another radiologist).
| Number of Cases | CXR (+) | CXR () | LR (+)* | LR ()* | |
|---|---|---|---|---|---|
| |||||
| Disease‐positive/abnormal findings | |||||
| Atelectasis or fibrosis including usual interstitial pneumonitis | 215 | 191 | 24 | 3.1 | 0.16 |
| Effusion, loculated effusion, empyema or fluid collections in fissures | 83 | 79 | 4 | 3.3 | 0.07 |
| Consolidation, airspace disease, mucous plugging or postradiation opacities | 57 | 54 | 3 | 3.3 | 0.07 |
| Ground glass opacity | 50 | 42 | 8 | 2.9 | 0.23 |
| Nodule or mass >5 mm | 48 | 44 | 4 | 3.1 | 0.12 |
| Pulmonary embolus | 22 | 18 | 4 | 2.8 | 0.26 |
| Bronchiectasis or bronchial dilation | 14 | 13 | 1 | 3.2 | 0.10 |
| Reticulation | 10 | 9 | 1 | 3.1 | 0.14 |
| Sclerotic bone lesion | 10 | 10 | 0 | 3.4 | 0 |
| Pulmonary edema or septal thickening | 8 | 8 | 0 | 3.4 | 0 |
| Interlobular septal thickening | 8 | 7 | 1 | 3.0 | 0.18 |
| Pleural plaque or calcification | 6 | 5 | 1 | 2.9 | 0.24 |
| Abnormal hemidiaphragm | 5 | 5 | 0 | 3.4 | 0 |
| Hydrothorax | 3 | 3 | 0 | 3.4 | 0 |
| Cavitary lesion | 2 | 2 | 0 | 3.4 | 0 |
| Pleural thickening | 1 | 1 | 0 | 3.4 | 0 |
| Vertebral compression fracture(s) | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial obstruction | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial wall thickening | 1 | 1 | 0 | 3.4 | 0 |
| Any abnormal CT finding | 289 | 251 | 38 | 2.9 | 0.19 |
| Disease‐negative/normal findings | |||||
| Normal | 81 | 24 | 57 | ||
| Overall LR | 2.9 (2.14.1) | 0.19 (0.130.26) | |||
| Abnormal Chest CT | Normal Chest CT | |
|---|---|---|
| ||
| Abnormal lateral CXR | 251 | 24 |
| Normal lateral CXR | 38 | 57 |
Using the chest CT report as the reference standard for abnormal opacity overlying the vertebral column on lateral chest radiography, we calculated the sensitivity, specificity, and positive and negative LRs (LR+ and LR, respectively) with 95% confidence intervals (CIs) for individual and summary CT‐documented pathologies.5 For this purpose, we constructed a 2 2 table (Table 2) for summary CT‐documented abnormal findings, in which patients with any abnormal CT finding were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. We also constructed 2 2 tables for each of the individual CT‐documented pathologies using data from Table 1, in which only the patients with the abnormal CT finding of interest (eg, consolidation) were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. In this case, patients with abnormal CT findings (eg, atelectasis, effusion) other than the finding of interest were excluded from the analysis. This secondary analysis is an attempt to estimate the variability of the accuracy of the finding in question across different diagnoses, and not to derive precise estimates of LRs given the small sample sizes for some individual findings.
Of the 370 original patients, we selected a sample of 100 patients by random number assignment whose lateral CXRs were reviewed a second time by the same observers to quantify intraobserver variability. Interobserver variability was quantified by comparing the data of the 2 independent lateral CXR observers on all 370 patients. In both cases, we calculated simple agreement and kappa statistics as measures of precision.6 Our chest CT observer also identified a sample of 10 CT investigations by random number assignment and reviewed the images in a blinded fashion to quantify interobserver variability in CT findings (ie, a comparison of the original CT report with our chest CT observer's interpretation).
We obtained approval from the relevant research ethics boards for the hospitals in which our study population was identified and have endeavored to comply with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.7 All statistical analyses were performed using R version 2.018 (Free Software Foundation, Boston, MA) and WinBUGS version 1.4. (MRC Biostatistics Unit, Cambridge, UK)9
Results
The identified study sample of 370 patients was 52% male and had an average age of 58 17 years (range, 18 to 96 years). Of the 370 patients, 81 (21.9%) were found to have a normal chest CT, 118 (31.9%) had a single CT finding in the lower lobes designated as disease‐positive, and 171 (46.2%) had 2 or more lower‐lobe CT findings. Overall, 78.1% had 1 or more CT findings considered disease‐positive.
Abnormal opacity overlying the vertebral column on lateral chest radiography had a sensitivity of 86.9% (95% CI, 82.5%‐90.3%) and specificity of 70.4% (95% CI, 59.7%‐79.2%) for CT‐documented lower‐lobe and associated structural pathology (Table 2). The summary LR+ for abnormal opacity overlying the vertebral column on lateral chest radiography was 2.9 (95% CI, 2.1‐4.1) and the summary LR for the absence of this finding was 0.19 (95% CI, 0.13‐0.26). LRs for individual CT‐documented pathologies were very similar to the summary LRs, with a range for LR+s between 2.8 and 3.4, and a range for LRs between 0 and 0.26 (Table 1).
Intraobserver simple agreement and kappa statistics for each of the lateral CXR observers were 79% ( = 0.56) and 81% ( = 0.58), respectively. Interobserver simple agreement between the lateral CXR observers, as well as the associated kappa statistic, were similar at 77% ( = 0.52). Compared with the original chest CT reports generated by university‐affiliated radiology faculty members, the blinded review of 10 randomly‐identified CT investigations by our chest CT observer (M.O.B.) yielded 100% agreement.
Discussion
This study fills a gap in the literature by providing evidence of the accuracy and precision of a particular finding on lateral chest radiography: namely, observed radioopacity obscuring the normal succession of superior‐inferior vertebral radiolucency.
Our investigation of this finding's test characteristics reveal that abnormal opacity overlying the vertebral column on lateral chest radiography is a more sensitive than specific finding, and thus in general more useful for ruling out the presence of disease than ruling it in. But it is our calculated LRs that allow application of this finding's predictive value to clinical scenarios in practice.
LRs are a powerful method of applying new information to the pretest probability of disease, to arrive at the posttest probability. If the summary point estimate LRs of our study are applied to a hypothetical pretest probability of 50% for any CT‐documented pathology, abnormal opacity overlying the vertebral column (LR+ 2.9) gives a posttest probability of 75%, and the absence of this finding (LR 0.19) gives a posttest probability of 16%. In some cases, these posttest probabilities may be high enough to stop investigating and start treating, or low enough to stop investigating.
We also calculated LRs for each subgroup of CT‐documented pathology by comparing only patients with the CT finding of interest and patients with CTs interpreted as normal. While the validity of these calculations is compromised by ignoring the patients in the other subgroups of diagnoses in the calculation, the stability of these LR estimates suggests that the finding and summary LRs can be used for a variety of diagnoses. The individual LRs, however, should not be used in arriving at posttest probabilities of individual pathologies.
Our calculated kappa statistics, a measure of chance‐corrected agreement, quantified the precision of abnormal opacity overlying the vertebral column noted by nonradiologist observers. The kappa statistics associated with intraobserver and interobserver variability for abnormal opacity overlying the vertebral column are indicative of moderate agreement, which is similar to the precision of many other investigational findings in common usage.
This study does have some limitations related to its design. First, CT was used as the gold standard in this study. Ideally, a combination of CT and more invasive measures such as lung biopsy would have been used; however, for ethical and logistical reasons this was obviously not possible. Second, when designing the study we had to decide whether or not to repeat the interpretation of CT images with observers we could ensure were blinded to the corresponding CXRs. We chose not to repeat the interpretation of CT images, and instead used the report of the staff chest radiologist who read the imaging study at the time it was performed. The person reviewing the report of the CT was blinded to the CXR. Our reasons for not rereading each of the CT images with a blinded study radiologist are as follows. First, the chest radiologists who reviewed the CT images at the time they were done were completely unaware of our hypothesis regarding the utility of the lateral CXR (our study took place after the CTs were interpreted). Second, the radiologists tell us that when they interpret CTs they rarely rely on findings in the CXR to help with those interpretations. For these 2 reasons, the original interpretation is very close to complete blinding. In addition, the individuals who interpret and write reports on chest CTs are all expert staff radiologists with considerable experience in this area. A study radiologist (likely a radiology resident) would not have been as proficient. Finally, in performing any study one must weigh the costs with the benefits of any methodological decision, reinterpretation of 370 chest CTs would have required an enormous amount of time. Finally, our small sample of 10 comparing official reports to the reinterpretation of the scans themselves supported the view that we did not need to review all 370 cases again.
Approximately three‐quarters of our study population was found to have CT‐documented disease. However, this is not surprising given our method of patient selection. Because the sample was collected from clinical practice, it is likely that only patients who exhibited a finding on the CXR that required delineation went on to have the reference standard investigation (CT). This study is therefore subject to workup bias. Workup bias in this scenario could work in 1 of 2 directions. In one situation, some patients would have a clear pathology or diagnosis based on the CXR, such that a CT was unnecessary and therefore not performed. In this case, our study would have underestimated the sensitivity of the sign being studied because a group of true positives would have been left out of the sample. In the second situation, patients with true pathology and a normal CXR (false negatives) fail to undergo CT. In this case, our study would have overestimated the sensitivity. We are not sure which effect of workup bias predominates in the study, but in either case an independent, prospective comparison of these imaging modalities in all patients who had CXRs was not feasible for ethical reasons. If we were to apply the reference standard investigation to all those patients, the potential for harm from excess radiation10 would be too great. As such, our cohort of patients is the best possible sample that can be studied.
Another feature of this study is that it intentionally used nonradiologist (budding internist) interpreters of the lateral CXRs, thus defining its generalizability. We did so for 2 reasons. First, the sign studied is likely too basic to be of relevance to radiologists. Second, it is intended to be used by internists, emergency physicians, and nonradiology trainees at all levels, who are required to make initial treatment decisions based on their preliminary interpretation of x‐rays, particularly in the hospital setting. Therefore, we decided our results would be more externally valid and applicable if the interpreters of the x‐rays and use of the x‐ray sign in this study was by trainees.
Abnormal opacity overlying the vertebral column on lateral chest radiography is a clinically useful finding that can help nonradiologist physicians determine initial management or the need for further investigation when diagnostic uncertainty remains. This study provides evidence that this finding is both reliable and useful for ruling the presence of lower‐lobe and associated structural pathology out, and somewhat useful for ruling the presence of such pathology in.
Acknowledgements
The authors thank Dr. Meyer Balter for his comments on an earlier version of this work.
- ,,,.Efficacy of routine screening and lateral chest radiographs in a hospital‐based population.N Engl J Med.1974;291:1001–1004.
- ,,, et al.Diagnosing left lower lobe pneumonia: usefulness of the ‘spine sign’ on lateral chest radiographs.J Fam Pract.1996;43:242–248.
- ,,, et al.Digital storage phosphor imaging versus conventional film radiography in CT‐documented chest disease.Radiology.1990;174:207–210.
- ,,,,.Chest imaging with a selenium detector versus conventional film radiography: a CT‐controlled study.Radiology.1996;200:687–690.
- .A primer on the precision and accuracy of the clinical examination.JAMA.1992;267:2638–2644.
- ,,, et al.Tips for learners of evidence‐based medicine: 3. Measures of observer variability (kappa statistic).CMAJ.2004;171:1369–1373.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Ann Intern Med.2003;138:40–44.
- R Development Core Team.R: A Language and Environment for Statistical Computing.Vienna, Austria:R Foundation for Statistical Computing;2004.
- ,,,.WinBUGS Version 1.4.1 User Manual.Cambridge, England:MRC Biostatistics Unit;2004.
- ,.Computed tomography—an increasing source of radiation exposure.N Engl J Med.2007;357(22):2277–2284.
In the evaluation of patients presenting with complaints referable to the chest, the chest radiograph (CXR) is an important and almost universal component of the initial assessment.
Chest radiography is normally performed with both posterior‐anterior (PA) and lateral projections.1 The lateral projection is generally accepted as an indispensable component because it allows better visualization of certain structures including the lower lobes, areas of which are partially obscured by the heart or hemidiaphragms on the PA projection. As such, some radiographic findings are only apparent on the lateral projection. As well, when an abnormality is discovered on the PA projection, the orthogonal orientation of the lateral projection often allows lesion localization.
Together with information gleaned from a thorough history and physical examination, the results of chest radiography often inform initial management when a diagnosis has been established, and the need for additional investigations when the diagnosis remains in question. In the hospital setting, the CXR is often reviewed first by physicians who are not radiologists (eg, internists, emergency physicians, and trainees at various stages of training) when evaluating a patient.
We undertook the current study to investigate the test characteristics (sensitivity, specificity, and likelihood ratio [LR]), and precision of 1 particular finding on lateral chest radiography as interpreted by nonradiologist physicians in the hospital setting. On a normal lateral CXR, one should observe progressive superior‐inferior vertebral radiolucency (Figure 1A). Observed opacity overlying the vertebral column obscuring this progression is usually abnormal and suggestive of pathology in the lower lobes of the lungs or associated structures (Figure 1B). A review of the literature yielded only 1 study of this finding,2 which used a case‐control design and lacked a true gold standard investigation necessary for calculation of meaningful test characteristics. In fact, few studies have compared findings on chest radiography with more definitive investigations,3, 4 and none have examined the predictive value of this finding by nonradiologist observers using a reference standard investigation such as computed tomography (CT) of the chest.
Methods
The radiology Picture Archiving and Communication System (PACS) used at our institution allows us to search for exams by date and study type. We retrospectively identified all patients seen at 1 of 3 university‐affiliated tertiary care adult teaching hospitals (Toronto General, Toronto Western, and Mount Sinai Hospitals) within an 8‐month period (January 1, 2006 to August 31, 2006) who underwent a 2‐view CXR (PA and lateral views). (Note that in this study, the terms radiograph, x‐ray, and plain film are used synonymously.) We then determined which of these patients had a subsequent CT within 24 hours of the x‐ray, resulting in a sample of 370 patients for this study. These patients primarily included patients presenting to the emergency department, and inpatients, with a very small number of outpatients. The majority of the index CXRs were performed for chief complaints of dyspnea, chest pain, cough, or for follow‐up of a previous CXR. However, many were simply performed routinely for admission. Patients with prosthetic devices or appliances obscuring the vertebral column were excluded.
After several training sessions by an experienced internist (A.S.D.), 2 authors (D.R.M., M.E.D.) independently reviewed each lateral CXR using standard 17‐inch displays and documented the presence or absence of abnormal radioopacity obscuring the superior to inferior progression of vertebral radiolucency. These 2 authors were fourth‐year medical students at the time the study began and first‐year trainees in internal medicine when it ended. The presence of abnormal opacity overlying the vertebral column was recorded as a positive test while the absence of this finding was recorded as a negative test.
Observed opacity overlying the vertebral column on lateral CXRs was considered abnormal when it did not represent manifestations of normal anatomical structures. However, the finding of opacity overlying the vertebral column of little diagnostic significance, such as prominent pulmonary vessels, degenerative bony changes, or the finding of a tortuous aorta, were considered normal in this study. Corresponding PA CXRs were also available for viewing. In most cases, the authors viewed both the lateral and PA CXRs, reflecting their use in clinical practice. However, in cases of obvious abnormality on the lateral CXR, only that projection was viewed. No clinical information was made available to the observers of the lateral CXR and they were blinded to the results of CT imaging of the chest. All 370 cases were reviewed by both observers (D.R.M. and M.E.D.). For the purpose of calculating test characteristics and LRs, cases of disagreement between the 3 lateral CXR observers were resolved by independent review by a third author (A.S.D.), a general internist with over 20 years of experience interpreting the lateral CXR.
A fourth author (M.O.B) reviewed the chest CT reports for each patient and recorded the mention of the presence or absence of various pathologies in the lower lobes of the lungs and associated structures in those reports. No clinical information was made available to this author and he was blinded to the results of lateral CXR. All CT investigations were originally interpreted by a university‐affiliated chest radiology faculty member at the time of the investigation. Table 1 lists all relevant chest CT findings in our sample that were recorded as disease‐positive for the purpose of dichotomizing the results of the reference standard, and enabling calculation of test characteristics (Table 2). Notable chest CT findings that were not recorded as disease‐positive for this purpose included mediastinal lymphadenopathy, subpleural density, lytic vertebral lesions, cystic or emphysematous changes, and pneumothorax. Dependent atelectasis was included within the disease‐positive category, though some cases may not have been pathological. It should be pointed out that there may be some variation in terminology used between staff radiologists (eg, reticulation by one radiologist may be called minor densities by another radiologist).
| Number of Cases | CXR (+) | CXR () | LR (+)* | LR ()* | |
|---|---|---|---|---|---|
| |||||
| Disease‐positive/abnormal findings | |||||
| Atelectasis or fibrosis including usual interstitial pneumonitis | 215 | 191 | 24 | 3.1 | 0.16 |
| Effusion, loculated effusion, empyema or fluid collections in fissures | 83 | 79 | 4 | 3.3 | 0.07 |
| Consolidation, airspace disease, mucous plugging or postradiation opacities | 57 | 54 | 3 | 3.3 | 0.07 |
| Ground glass opacity | 50 | 42 | 8 | 2.9 | 0.23 |
| Nodule or mass >5 mm | 48 | 44 | 4 | 3.1 | 0.12 |
| Pulmonary embolus | 22 | 18 | 4 | 2.8 | 0.26 |
| Bronchiectasis or bronchial dilation | 14 | 13 | 1 | 3.2 | 0.10 |
| Reticulation | 10 | 9 | 1 | 3.1 | 0.14 |
| Sclerotic bone lesion | 10 | 10 | 0 | 3.4 | 0 |
| Pulmonary edema or septal thickening | 8 | 8 | 0 | 3.4 | 0 |
| Interlobular septal thickening | 8 | 7 | 1 | 3.0 | 0.18 |
| Pleural plaque or calcification | 6 | 5 | 1 | 2.9 | 0.24 |
| Abnormal hemidiaphragm | 5 | 5 | 0 | 3.4 | 0 |
| Hydrothorax | 3 | 3 | 0 | 3.4 | 0 |
| Cavitary lesion | 2 | 2 | 0 | 3.4 | 0 |
| Pleural thickening | 1 | 1 | 0 | 3.4 | 0 |
| Vertebral compression fracture(s) | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial obstruction | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial wall thickening | 1 | 1 | 0 | 3.4 | 0 |
| Any abnormal CT finding | 289 | 251 | 38 | 2.9 | 0.19 |
| Disease‐negative/normal findings | |||||
| Normal | 81 | 24 | 57 | ||
| Overall LR | 2.9 (2.14.1) | 0.19 (0.130.26) | |||
| Abnormal Chest CT | Normal Chest CT | |
|---|---|---|
| ||
| Abnormal lateral CXR | 251 | 24 |
| Normal lateral CXR | 38 | 57 |
Using the chest CT report as the reference standard for abnormal opacity overlying the vertebral column on lateral chest radiography, we calculated the sensitivity, specificity, and positive and negative LRs (LR+ and LR, respectively) with 95% confidence intervals (CIs) for individual and summary CT‐documented pathologies.5 For this purpose, we constructed a 2 2 table (Table 2) for summary CT‐documented abnormal findings, in which patients with any abnormal CT finding were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. We also constructed 2 2 tables for each of the individual CT‐documented pathologies using data from Table 1, in which only the patients with the abnormal CT finding of interest (eg, consolidation) were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. In this case, patients with abnormal CT findings (eg, atelectasis, effusion) other than the finding of interest were excluded from the analysis. This secondary analysis is an attempt to estimate the variability of the accuracy of the finding in question across different diagnoses, and not to derive precise estimates of LRs given the small sample sizes for some individual findings.
Of the 370 original patients, we selected a sample of 100 patients by random number assignment whose lateral CXRs were reviewed a second time by the same observers to quantify intraobserver variability. Interobserver variability was quantified by comparing the data of the 2 independent lateral CXR observers on all 370 patients. In both cases, we calculated simple agreement and kappa statistics as measures of precision.6 Our chest CT observer also identified a sample of 10 CT investigations by random number assignment and reviewed the images in a blinded fashion to quantify interobserver variability in CT findings (ie, a comparison of the original CT report with our chest CT observer's interpretation).
We obtained approval from the relevant research ethics boards for the hospitals in which our study population was identified and have endeavored to comply with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.7 All statistical analyses were performed using R version 2.018 (Free Software Foundation, Boston, MA) and WinBUGS version 1.4. (MRC Biostatistics Unit, Cambridge, UK)9
Results
The identified study sample of 370 patients was 52% male and had an average age of 58 17 years (range, 18 to 96 years). Of the 370 patients, 81 (21.9%) were found to have a normal chest CT, 118 (31.9%) had a single CT finding in the lower lobes designated as disease‐positive, and 171 (46.2%) had 2 or more lower‐lobe CT findings. Overall, 78.1% had 1 or more CT findings considered disease‐positive.
Abnormal opacity overlying the vertebral column on lateral chest radiography had a sensitivity of 86.9% (95% CI, 82.5%‐90.3%) and specificity of 70.4% (95% CI, 59.7%‐79.2%) for CT‐documented lower‐lobe and associated structural pathology (Table 2). The summary LR+ for abnormal opacity overlying the vertebral column on lateral chest radiography was 2.9 (95% CI, 2.1‐4.1) and the summary LR for the absence of this finding was 0.19 (95% CI, 0.13‐0.26). LRs for individual CT‐documented pathologies were very similar to the summary LRs, with a range for LR+s between 2.8 and 3.4, and a range for LRs between 0 and 0.26 (Table 1).
Intraobserver simple agreement and kappa statistics for each of the lateral CXR observers were 79% ( = 0.56) and 81% ( = 0.58), respectively. Interobserver simple agreement between the lateral CXR observers, as well as the associated kappa statistic, were similar at 77% ( = 0.52). Compared with the original chest CT reports generated by university‐affiliated radiology faculty members, the blinded review of 10 randomly‐identified CT investigations by our chest CT observer (M.O.B.) yielded 100% agreement.
Discussion
This study fills a gap in the literature by providing evidence of the accuracy and precision of a particular finding on lateral chest radiography: namely, observed radioopacity obscuring the normal succession of superior‐inferior vertebral radiolucency.
Our investigation of this finding's test characteristics reveal that abnormal opacity overlying the vertebral column on lateral chest radiography is a more sensitive than specific finding, and thus in general more useful for ruling out the presence of disease than ruling it in. But it is our calculated LRs that allow application of this finding's predictive value to clinical scenarios in practice.
LRs are a powerful method of applying new information to the pretest probability of disease, to arrive at the posttest probability. If the summary point estimate LRs of our study are applied to a hypothetical pretest probability of 50% for any CT‐documented pathology, abnormal opacity overlying the vertebral column (LR+ 2.9) gives a posttest probability of 75%, and the absence of this finding (LR 0.19) gives a posttest probability of 16%. In some cases, these posttest probabilities may be high enough to stop investigating and start treating, or low enough to stop investigating.
We also calculated LRs for each subgroup of CT‐documented pathology by comparing only patients with the CT finding of interest and patients with CTs interpreted as normal. While the validity of these calculations is compromised by ignoring the patients in the other subgroups of diagnoses in the calculation, the stability of these LR estimates suggests that the finding and summary LRs can be used for a variety of diagnoses. The individual LRs, however, should not be used in arriving at posttest probabilities of individual pathologies.
Our calculated kappa statistics, a measure of chance‐corrected agreement, quantified the precision of abnormal opacity overlying the vertebral column noted by nonradiologist observers. The kappa statistics associated with intraobserver and interobserver variability for abnormal opacity overlying the vertebral column are indicative of moderate agreement, which is similar to the precision of many other investigational findings in common usage.
This study does have some limitations related to its design. First, CT was used as the gold standard in this study. Ideally, a combination of CT and more invasive measures such as lung biopsy would have been used; however, for ethical and logistical reasons this was obviously not possible. Second, when designing the study we had to decide whether or not to repeat the interpretation of CT images with observers we could ensure were blinded to the corresponding CXRs. We chose not to repeat the interpretation of CT images, and instead used the report of the staff chest radiologist who read the imaging study at the time it was performed. The person reviewing the report of the CT was blinded to the CXR. Our reasons for not rereading each of the CT images with a blinded study radiologist are as follows. First, the chest radiologists who reviewed the CT images at the time they were done were completely unaware of our hypothesis regarding the utility of the lateral CXR (our study took place after the CTs were interpreted). Second, the radiologists tell us that when they interpret CTs they rarely rely on findings in the CXR to help with those interpretations. For these 2 reasons, the original interpretation is very close to complete blinding. In addition, the individuals who interpret and write reports on chest CTs are all expert staff radiologists with considerable experience in this area. A study radiologist (likely a radiology resident) would not have been as proficient. Finally, in performing any study one must weigh the costs with the benefits of any methodological decision, reinterpretation of 370 chest CTs would have required an enormous amount of time. Finally, our small sample of 10 comparing official reports to the reinterpretation of the scans themselves supported the view that we did not need to review all 370 cases again.
Approximately three‐quarters of our study population was found to have CT‐documented disease. However, this is not surprising given our method of patient selection. Because the sample was collected from clinical practice, it is likely that only patients who exhibited a finding on the CXR that required delineation went on to have the reference standard investigation (CT). This study is therefore subject to workup bias. Workup bias in this scenario could work in 1 of 2 directions. In one situation, some patients would have a clear pathology or diagnosis based on the CXR, such that a CT was unnecessary and therefore not performed. In this case, our study would have underestimated the sensitivity of the sign being studied because a group of true positives would have been left out of the sample. In the second situation, patients with true pathology and a normal CXR (false negatives) fail to undergo CT. In this case, our study would have overestimated the sensitivity. We are not sure which effect of workup bias predominates in the study, but in either case an independent, prospective comparison of these imaging modalities in all patients who had CXRs was not feasible for ethical reasons. If we were to apply the reference standard investigation to all those patients, the potential for harm from excess radiation10 would be too great. As such, our cohort of patients is the best possible sample that can be studied.
Another feature of this study is that it intentionally used nonradiologist (budding internist) interpreters of the lateral CXRs, thus defining its generalizability. We did so for 2 reasons. First, the sign studied is likely too basic to be of relevance to radiologists. Second, it is intended to be used by internists, emergency physicians, and nonradiology trainees at all levels, who are required to make initial treatment decisions based on their preliminary interpretation of x‐rays, particularly in the hospital setting. Therefore, we decided our results would be more externally valid and applicable if the interpreters of the x‐rays and use of the x‐ray sign in this study was by trainees.
Abnormal opacity overlying the vertebral column on lateral chest radiography is a clinically useful finding that can help nonradiologist physicians determine initial management or the need for further investigation when diagnostic uncertainty remains. This study provides evidence that this finding is both reliable and useful for ruling the presence of lower‐lobe and associated structural pathology out, and somewhat useful for ruling the presence of such pathology in.
Acknowledgements
The authors thank Dr. Meyer Balter for his comments on an earlier version of this work.
In the evaluation of patients presenting with complaints referable to the chest, the chest radiograph (CXR) is an important and almost universal component of the initial assessment.
Chest radiography is normally performed with both posterior‐anterior (PA) and lateral projections.1 The lateral projection is generally accepted as an indispensable component because it allows better visualization of certain structures including the lower lobes, areas of which are partially obscured by the heart or hemidiaphragms on the PA projection. As such, some radiographic findings are only apparent on the lateral projection. As well, when an abnormality is discovered on the PA projection, the orthogonal orientation of the lateral projection often allows lesion localization.
Together with information gleaned from a thorough history and physical examination, the results of chest radiography often inform initial management when a diagnosis has been established, and the need for additional investigations when the diagnosis remains in question. In the hospital setting, the CXR is often reviewed first by physicians who are not radiologists (eg, internists, emergency physicians, and trainees at various stages of training) when evaluating a patient.
We undertook the current study to investigate the test characteristics (sensitivity, specificity, and likelihood ratio [LR]), and precision of 1 particular finding on lateral chest radiography as interpreted by nonradiologist physicians in the hospital setting. On a normal lateral CXR, one should observe progressive superior‐inferior vertebral radiolucency (Figure 1A). Observed opacity overlying the vertebral column obscuring this progression is usually abnormal and suggestive of pathology in the lower lobes of the lungs or associated structures (Figure 1B). A review of the literature yielded only 1 study of this finding,2 which used a case‐control design and lacked a true gold standard investigation necessary for calculation of meaningful test characteristics. In fact, few studies have compared findings on chest radiography with more definitive investigations,3, 4 and none have examined the predictive value of this finding by nonradiologist observers using a reference standard investigation such as computed tomography (CT) of the chest.
Methods
The radiology Picture Archiving and Communication System (PACS) used at our institution allows us to search for exams by date and study type. We retrospectively identified all patients seen at 1 of 3 university‐affiliated tertiary care adult teaching hospitals (Toronto General, Toronto Western, and Mount Sinai Hospitals) within an 8‐month period (January 1, 2006 to August 31, 2006) who underwent a 2‐view CXR (PA and lateral views). (Note that in this study, the terms radiograph, x‐ray, and plain film are used synonymously.) We then determined which of these patients had a subsequent CT within 24 hours of the x‐ray, resulting in a sample of 370 patients for this study. These patients primarily included patients presenting to the emergency department, and inpatients, with a very small number of outpatients. The majority of the index CXRs were performed for chief complaints of dyspnea, chest pain, cough, or for follow‐up of a previous CXR. However, many were simply performed routinely for admission. Patients with prosthetic devices or appliances obscuring the vertebral column were excluded.
After several training sessions by an experienced internist (A.S.D.), 2 authors (D.R.M., M.E.D.) independently reviewed each lateral CXR using standard 17‐inch displays and documented the presence or absence of abnormal radioopacity obscuring the superior to inferior progression of vertebral radiolucency. These 2 authors were fourth‐year medical students at the time the study began and first‐year trainees in internal medicine when it ended. The presence of abnormal opacity overlying the vertebral column was recorded as a positive test while the absence of this finding was recorded as a negative test.
Observed opacity overlying the vertebral column on lateral CXRs was considered abnormal when it did not represent manifestations of normal anatomical structures. However, the finding of opacity overlying the vertebral column of little diagnostic significance, such as prominent pulmonary vessels, degenerative bony changes, or the finding of a tortuous aorta, were considered normal in this study. Corresponding PA CXRs were also available for viewing. In most cases, the authors viewed both the lateral and PA CXRs, reflecting their use in clinical practice. However, in cases of obvious abnormality on the lateral CXR, only that projection was viewed. No clinical information was made available to the observers of the lateral CXR and they were blinded to the results of CT imaging of the chest. All 370 cases were reviewed by both observers (D.R.M. and M.E.D.). For the purpose of calculating test characteristics and LRs, cases of disagreement between the 3 lateral CXR observers were resolved by independent review by a third author (A.S.D.), a general internist with over 20 years of experience interpreting the lateral CXR.
A fourth author (M.O.B) reviewed the chest CT reports for each patient and recorded the mention of the presence or absence of various pathologies in the lower lobes of the lungs and associated structures in those reports. No clinical information was made available to this author and he was blinded to the results of lateral CXR. All CT investigations were originally interpreted by a university‐affiliated chest radiology faculty member at the time of the investigation. Table 1 lists all relevant chest CT findings in our sample that were recorded as disease‐positive for the purpose of dichotomizing the results of the reference standard, and enabling calculation of test characteristics (Table 2). Notable chest CT findings that were not recorded as disease‐positive for this purpose included mediastinal lymphadenopathy, subpleural density, lytic vertebral lesions, cystic or emphysematous changes, and pneumothorax. Dependent atelectasis was included within the disease‐positive category, though some cases may not have been pathological. It should be pointed out that there may be some variation in terminology used between staff radiologists (eg, reticulation by one radiologist may be called minor densities by another radiologist).
| Number of Cases | CXR (+) | CXR () | LR (+)* | LR ()* | |
|---|---|---|---|---|---|
| |||||
| Disease‐positive/abnormal findings | |||||
| Atelectasis or fibrosis including usual interstitial pneumonitis | 215 | 191 | 24 | 3.1 | 0.16 |
| Effusion, loculated effusion, empyema or fluid collections in fissures | 83 | 79 | 4 | 3.3 | 0.07 |
| Consolidation, airspace disease, mucous plugging or postradiation opacities | 57 | 54 | 3 | 3.3 | 0.07 |
| Ground glass opacity | 50 | 42 | 8 | 2.9 | 0.23 |
| Nodule or mass >5 mm | 48 | 44 | 4 | 3.1 | 0.12 |
| Pulmonary embolus | 22 | 18 | 4 | 2.8 | 0.26 |
| Bronchiectasis or bronchial dilation | 14 | 13 | 1 | 3.2 | 0.10 |
| Reticulation | 10 | 9 | 1 | 3.1 | 0.14 |
| Sclerotic bone lesion | 10 | 10 | 0 | 3.4 | 0 |
| Pulmonary edema or septal thickening | 8 | 8 | 0 | 3.4 | 0 |
| Interlobular septal thickening | 8 | 7 | 1 | 3.0 | 0.18 |
| Pleural plaque or calcification | 6 | 5 | 1 | 2.9 | 0.24 |
| Abnormal hemidiaphragm | 5 | 5 | 0 | 3.4 | 0 |
| Hydrothorax | 3 | 3 | 0 | 3.4 | 0 |
| Cavitary lesion | 2 | 2 | 0 | 3.4 | 0 |
| Pleural thickening | 1 | 1 | 0 | 3.4 | 0 |
| Vertebral compression fracture(s) | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial obstruction | 1 | 1 | 0 | 3.4 | 0 |
| Bronchial wall thickening | 1 | 1 | 0 | 3.4 | 0 |
| Any abnormal CT finding | 289 | 251 | 38 | 2.9 | 0.19 |
| Disease‐negative/normal findings | |||||
| Normal | 81 | 24 | 57 | ||
| Overall LR | 2.9 (2.14.1) | 0.19 (0.130.26) | |||
| Abnormal Chest CT | Normal Chest CT | |
|---|---|---|
| ||
| Abnormal lateral CXR | 251 | 24 |
| Normal lateral CXR | 38 | 57 |
Using the chest CT report as the reference standard for abnormal opacity overlying the vertebral column on lateral chest radiography, we calculated the sensitivity, specificity, and positive and negative LRs (LR+ and LR, respectively) with 95% confidence intervals (CIs) for individual and summary CT‐documented pathologies.5 For this purpose, we constructed a 2 2 table (Table 2) for summary CT‐documented abnormal findings, in which patients with any abnormal CT finding were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. We also constructed 2 2 tables for each of the individual CT‐documented pathologies using data from Table 1, in which only the patients with the abnormal CT finding of interest (eg, consolidation) were considered disease‐positive and compared with patients whose CTs were interpreted as normal, considered disease‐negative. In this case, patients with abnormal CT findings (eg, atelectasis, effusion) other than the finding of interest were excluded from the analysis. This secondary analysis is an attempt to estimate the variability of the accuracy of the finding in question across different diagnoses, and not to derive precise estimates of LRs given the small sample sizes for some individual findings.
Of the 370 original patients, we selected a sample of 100 patients by random number assignment whose lateral CXRs were reviewed a second time by the same observers to quantify intraobserver variability. Interobserver variability was quantified by comparing the data of the 2 independent lateral CXR observers on all 370 patients. In both cases, we calculated simple agreement and kappa statistics as measures of precision.6 Our chest CT observer also identified a sample of 10 CT investigations by random number assignment and reviewed the images in a blinded fashion to quantify interobserver variability in CT findings (ie, a comparison of the original CT report with our chest CT observer's interpretation).
We obtained approval from the relevant research ethics boards for the hospitals in which our study population was identified and have endeavored to comply with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.7 All statistical analyses were performed using R version 2.018 (Free Software Foundation, Boston, MA) and WinBUGS version 1.4. (MRC Biostatistics Unit, Cambridge, UK)9
Results
The identified study sample of 370 patients was 52% male and had an average age of 58 17 years (range, 18 to 96 years). Of the 370 patients, 81 (21.9%) were found to have a normal chest CT, 118 (31.9%) had a single CT finding in the lower lobes designated as disease‐positive, and 171 (46.2%) had 2 or more lower‐lobe CT findings. Overall, 78.1% had 1 or more CT findings considered disease‐positive.
Abnormal opacity overlying the vertebral column on lateral chest radiography had a sensitivity of 86.9% (95% CI, 82.5%‐90.3%) and specificity of 70.4% (95% CI, 59.7%‐79.2%) for CT‐documented lower‐lobe and associated structural pathology (Table 2). The summary LR+ for abnormal opacity overlying the vertebral column on lateral chest radiography was 2.9 (95% CI, 2.1‐4.1) and the summary LR for the absence of this finding was 0.19 (95% CI, 0.13‐0.26). LRs for individual CT‐documented pathologies were very similar to the summary LRs, with a range for LR+s between 2.8 and 3.4, and a range for LRs between 0 and 0.26 (Table 1).
Intraobserver simple agreement and kappa statistics for each of the lateral CXR observers were 79% ( = 0.56) and 81% ( = 0.58), respectively. Interobserver simple agreement between the lateral CXR observers, as well as the associated kappa statistic, were similar at 77% ( = 0.52). Compared with the original chest CT reports generated by university‐affiliated radiology faculty members, the blinded review of 10 randomly‐identified CT investigations by our chest CT observer (M.O.B.) yielded 100% agreement.
Discussion
This study fills a gap in the literature by providing evidence of the accuracy and precision of a particular finding on lateral chest radiography: namely, observed radioopacity obscuring the normal succession of superior‐inferior vertebral radiolucency.
Our investigation of this finding's test characteristics reveal that abnormal opacity overlying the vertebral column on lateral chest radiography is a more sensitive than specific finding, and thus in general more useful for ruling out the presence of disease than ruling it in. But it is our calculated LRs that allow application of this finding's predictive value to clinical scenarios in practice.
LRs are a powerful method of applying new information to the pretest probability of disease, to arrive at the posttest probability. If the summary point estimate LRs of our study are applied to a hypothetical pretest probability of 50% for any CT‐documented pathology, abnormal opacity overlying the vertebral column (LR+ 2.9) gives a posttest probability of 75%, and the absence of this finding (LR 0.19) gives a posttest probability of 16%. In some cases, these posttest probabilities may be high enough to stop investigating and start treating, or low enough to stop investigating.
We also calculated LRs for each subgroup of CT‐documented pathology by comparing only patients with the CT finding of interest and patients with CTs interpreted as normal. While the validity of these calculations is compromised by ignoring the patients in the other subgroups of diagnoses in the calculation, the stability of these LR estimates suggests that the finding and summary LRs can be used for a variety of diagnoses. The individual LRs, however, should not be used in arriving at posttest probabilities of individual pathologies.
Our calculated kappa statistics, a measure of chance‐corrected agreement, quantified the precision of abnormal opacity overlying the vertebral column noted by nonradiologist observers. The kappa statistics associated with intraobserver and interobserver variability for abnormal opacity overlying the vertebral column are indicative of moderate agreement, which is similar to the precision of many other investigational findings in common usage.
This study does have some limitations related to its design. First, CT was used as the gold standard in this study. Ideally, a combination of CT and more invasive measures such as lung biopsy would have been used; however, for ethical and logistical reasons this was obviously not possible. Second, when designing the study we had to decide whether or not to repeat the interpretation of CT images with observers we could ensure were blinded to the corresponding CXRs. We chose not to repeat the interpretation of CT images, and instead used the report of the staff chest radiologist who read the imaging study at the time it was performed. The person reviewing the report of the CT was blinded to the CXR. Our reasons for not rereading each of the CT images with a blinded study radiologist are as follows. First, the chest radiologists who reviewed the CT images at the time they were done were completely unaware of our hypothesis regarding the utility of the lateral CXR (our study took place after the CTs were interpreted). Second, the radiologists tell us that when they interpret CTs they rarely rely on findings in the CXR to help with those interpretations. For these 2 reasons, the original interpretation is very close to complete blinding. In addition, the individuals who interpret and write reports on chest CTs are all expert staff radiologists with considerable experience in this area. A study radiologist (likely a radiology resident) would not have been as proficient. Finally, in performing any study one must weigh the costs with the benefits of any methodological decision, reinterpretation of 370 chest CTs would have required an enormous amount of time. Finally, our small sample of 10 comparing official reports to the reinterpretation of the scans themselves supported the view that we did not need to review all 370 cases again.
Approximately three‐quarters of our study population was found to have CT‐documented disease. However, this is not surprising given our method of patient selection. Because the sample was collected from clinical practice, it is likely that only patients who exhibited a finding on the CXR that required delineation went on to have the reference standard investigation (CT). This study is therefore subject to workup bias. Workup bias in this scenario could work in 1 of 2 directions. In one situation, some patients would have a clear pathology or diagnosis based on the CXR, such that a CT was unnecessary and therefore not performed. In this case, our study would have underestimated the sensitivity of the sign being studied because a group of true positives would have been left out of the sample. In the second situation, patients with true pathology and a normal CXR (false negatives) fail to undergo CT. In this case, our study would have overestimated the sensitivity. We are not sure which effect of workup bias predominates in the study, but in either case an independent, prospective comparison of these imaging modalities in all patients who had CXRs was not feasible for ethical reasons. If we were to apply the reference standard investigation to all those patients, the potential for harm from excess radiation10 would be too great. As such, our cohort of patients is the best possible sample that can be studied.
Another feature of this study is that it intentionally used nonradiologist (budding internist) interpreters of the lateral CXRs, thus defining its generalizability. We did so for 2 reasons. First, the sign studied is likely too basic to be of relevance to radiologists. Second, it is intended to be used by internists, emergency physicians, and nonradiology trainees at all levels, who are required to make initial treatment decisions based on their preliminary interpretation of x‐rays, particularly in the hospital setting. Therefore, we decided our results would be more externally valid and applicable if the interpreters of the x‐rays and use of the x‐ray sign in this study was by trainees.
Abnormal opacity overlying the vertebral column on lateral chest radiography is a clinically useful finding that can help nonradiologist physicians determine initial management or the need for further investigation when diagnostic uncertainty remains. This study provides evidence that this finding is both reliable and useful for ruling the presence of lower‐lobe and associated structural pathology out, and somewhat useful for ruling the presence of such pathology in.
Acknowledgements
The authors thank Dr. Meyer Balter for his comments on an earlier version of this work.
- ,,,.Efficacy of routine screening and lateral chest radiographs in a hospital‐based population.N Engl J Med.1974;291:1001–1004.
- ,,, et al.Diagnosing left lower lobe pneumonia: usefulness of the ‘spine sign’ on lateral chest radiographs.J Fam Pract.1996;43:242–248.
- ,,, et al.Digital storage phosphor imaging versus conventional film radiography in CT‐documented chest disease.Radiology.1990;174:207–210.
- ,,,,.Chest imaging with a selenium detector versus conventional film radiography: a CT‐controlled study.Radiology.1996;200:687–690.
- .A primer on the precision and accuracy of the clinical examination.JAMA.1992;267:2638–2644.
- ,,, et al.Tips for learners of evidence‐based medicine: 3. Measures of observer variability (kappa statistic).CMAJ.2004;171:1369–1373.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Ann Intern Med.2003;138:40–44.
- R Development Core Team.R: A Language and Environment for Statistical Computing.Vienna, Austria:R Foundation for Statistical Computing;2004.
- ,,,.WinBUGS Version 1.4.1 User Manual.Cambridge, England:MRC Biostatistics Unit;2004.
- ,.Computed tomography—an increasing source of radiation exposure.N Engl J Med.2007;357(22):2277–2284.
- ,,,.Efficacy of routine screening and lateral chest radiographs in a hospital‐based population.N Engl J Med.1974;291:1001–1004.
- ,,, et al.Diagnosing left lower lobe pneumonia: usefulness of the ‘spine sign’ on lateral chest radiographs.J Fam Pract.1996;43:242–248.
- ,,, et al.Digital storage phosphor imaging versus conventional film radiography in CT‐documented chest disease.Radiology.1990;174:207–210.
- ,,,,.Chest imaging with a selenium detector versus conventional film radiography: a CT‐controlled study.Radiology.1996;200:687–690.
- .A primer on the precision and accuracy of the clinical examination.JAMA.1992;267:2638–2644.
- ,,, et al.Tips for learners of evidence‐based medicine: 3. Measures of observer variability (kappa statistic).CMAJ.2004;171:1369–1373.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Ann Intern Med.2003;138:40–44.
- R Development Core Team.R: A Language and Environment for Statistical Computing.Vienna, Austria:R Foundation for Statistical Computing;2004.
- ,,,.WinBUGS Version 1.4.1 User Manual.Cambridge, England:MRC Biostatistics Unit;2004.
- ,.Computed tomography—an increasing source of radiation exposure.N Engl J Med.2007;357(22):2277–2284.
Copyright © 2009 Society of Hospital Medicine
In the Eye of the Storm
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.
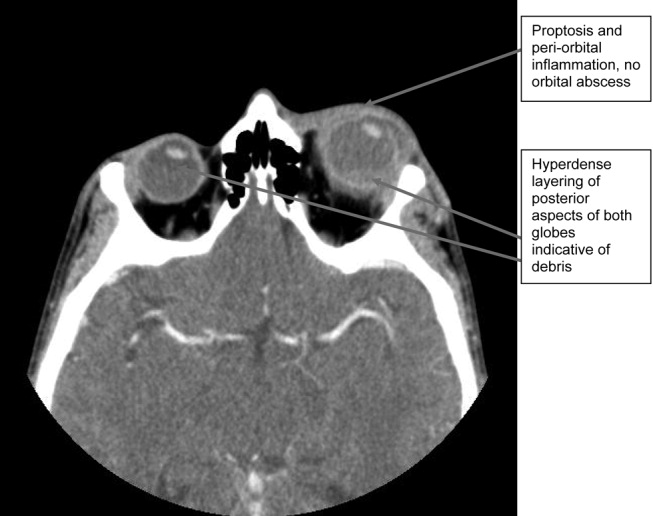
Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
Clinical Conundrum
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.
A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.
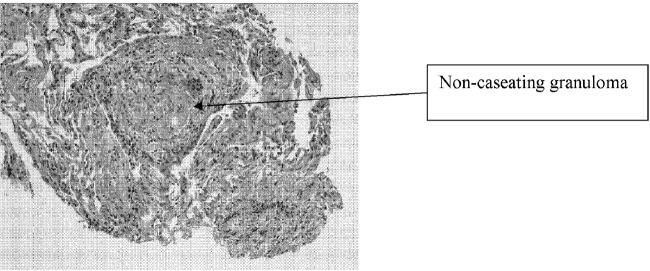
The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.
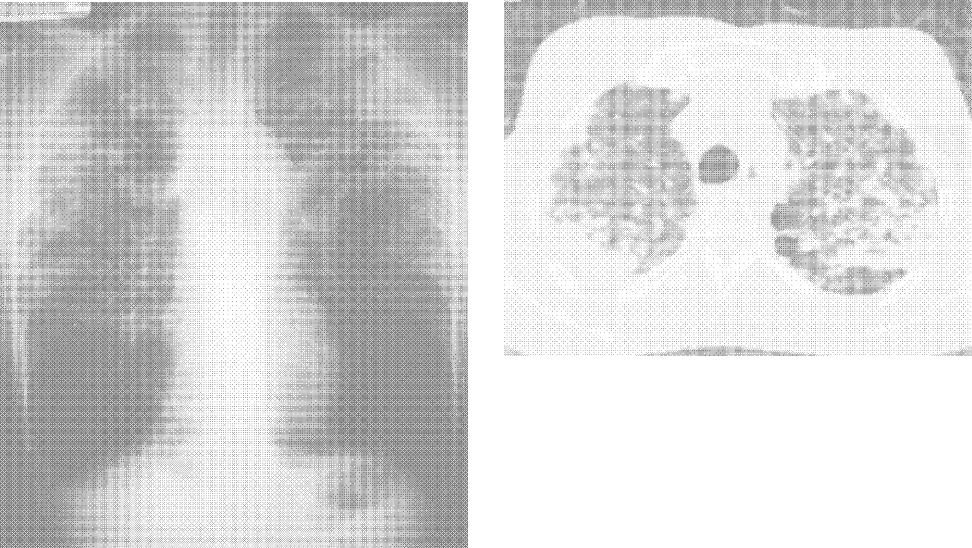
The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.
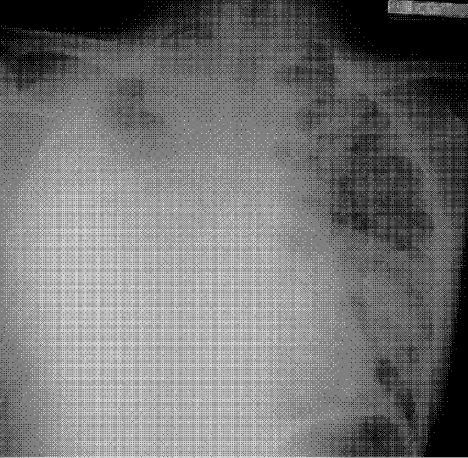
He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.
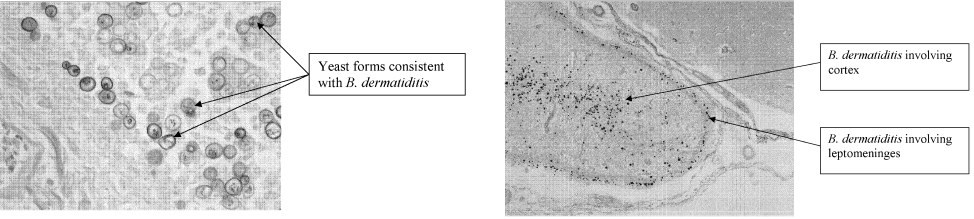
COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.


A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.

The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.

The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.

He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.

COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.


A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.

The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.

The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.

He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.

COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.