User login
iPad Apps Help Inpatients Bridge Information Gaps
A study in the Journal of the American Medical Informatics Association found that giving hospitalized patients iPads with basic information about their care helped them engage more with physicians.
The patient portal application helped inpatients remember their doctors’ names, the study noted, but had no statistically significant impact on patients’ ability to remember scheduled procedures or active medications.
Hospitalist and lead author Kevin O’Leary, MD, MS, SFHM, of Northwestern University Feinberg School of Medicine in Chicago says the portals can be a way to improve the discharge process.
“We know that patients have a fairly poor comprehension of their care during hospitalization, which doesn’t set them up to do well after discharge,” says Dr. O’Leary, who has studied the potential of portals. “This patient portal has the opportunity to fill in the gaps.”
Although the study didn’t address qualitative issues around why patients didn’t have better recall of information about tests, procedures, or active medications, Dr. O’Leary says future work will address how to tweak portals to improve their efficacy.
“Our study had limited information purposely to just test the concept,” Dr. O’Leary says. “I predict that 10 years from now, this is going to be commonplace. Even five years from now, many hospitals will be offering portal access to inpatients.”
Dr. O’Leary says he believes that using the increasingly ubiquitous technology of smartphones and tablet computers to engage patients creates greater transparency, which could result in better retention of care instructions.
“You’re giving ownership to the patient over information, and it’s much earlier than right at discharge and then a phone call after discharge,” Dr. O’Leary adds. “Why not begin to educate the patient about their condition and their medications from the very first minute of their admission? And give them access to that information and have them identify their own needs and knowledge deficits?”
Visit our website for more information about iPad technology in medical practice.
A study in the Journal of the American Medical Informatics Association found that giving hospitalized patients iPads with basic information about their care helped them engage more with physicians.
The patient portal application helped inpatients remember their doctors’ names, the study noted, but had no statistically significant impact on patients’ ability to remember scheduled procedures or active medications.
Hospitalist and lead author Kevin O’Leary, MD, MS, SFHM, of Northwestern University Feinberg School of Medicine in Chicago says the portals can be a way to improve the discharge process.
“We know that patients have a fairly poor comprehension of their care during hospitalization, which doesn’t set them up to do well after discharge,” says Dr. O’Leary, who has studied the potential of portals. “This patient portal has the opportunity to fill in the gaps.”
Although the study didn’t address qualitative issues around why patients didn’t have better recall of information about tests, procedures, or active medications, Dr. O’Leary says future work will address how to tweak portals to improve their efficacy.
“Our study had limited information purposely to just test the concept,” Dr. O’Leary says. “I predict that 10 years from now, this is going to be commonplace. Even five years from now, many hospitals will be offering portal access to inpatients.”
Dr. O’Leary says he believes that using the increasingly ubiquitous technology of smartphones and tablet computers to engage patients creates greater transparency, which could result in better retention of care instructions.
“You’re giving ownership to the patient over information, and it’s much earlier than right at discharge and then a phone call after discharge,” Dr. O’Leary adds. “Why not begin to educate the patient about their condition and their medications from the very first minute of their admission? And give them access to that information and have them identify their own needs and knowledge deficits?”
Visit our website for more information about iPad technology in medical practice.
A study in the Journal of the American Medical Informatics Association found that giving hospitalized patients iPads with basic information about their care helped them engage more with physicians.
The patient portal application helped inpatients remember their doctors’ names, the study noted, but had no statistically significant impact on patients’ ability to remember scheduled procedures or active medications.
Hospitalist and lead author Kevin O’Leary, MD, MS, SFHM, of Northwestern University Feinberg School of Medicine in Chicago says the portals can be a way to improve the discharge process.
“We know that patients have a fairly poor comprehension of their care during hospitalization, which doesn’t set them up to do well after discharge,” says Dr. O’Leary, who has studied the potential of portals. “This patient portal has the opportunity to fill in the gaps.”
Although the study didn’t address qualitative issues around why patients didn’t have better recall of information about tests, procedures, or active medications, Dr. O’Leary says future work will address how to tweak portals to improve their efficacy.
“Our study had limited information purposely to just test the concept,” Dr. O’Leary says. “I predict that 10 years from now, this is going to be commonplace. Even five years from now, many hospitals will be offering portal access to inpatients.”
Dr. O’Leary says he believes that using the increasingly ubiquitous technology of smartphones and tablet computers to engage patients creates greater transparency, which could result in better retention of care instructions.
“You’re giving ownership to the patient over information, and it’s much earlier than right at discharge and then a phone call after discharge,” Dr. O’Leary adds. “Why not begin to educate the patient about their condition and their medications from the very first minute of their admission? And give them access to that information and have them identify their own needs and knowledge deficits?”
Visit our website for more information about iPad technology in medical practice.
Joint Commission Unveils Online Resource to Promote High-Reliability Healthcare
Zero preventable harm is the goal of every healthcare organization, and a new online application from the Joint Commission Center for Transforming Healthcare can help groups reach that goal. Oro 2.0 can assist hospital leaders with determining their organization’s status across multiple components of high reliability that will let them achieve zero preventable harm.
Hospitalists are integral to this process, says Coleen Smith, RN, BSN, MBA, CPHQ, director of high reliability initiatives for the Joint Commission. “Hospitalists are a crucial piece of improving the quality of an organization. Physicians routinely getting involved in high reliability and leading those activities are how we want high-reliability hospitals to look,” says Smith.
The Oro 2.0 application has two major elements. The first is an assessment tool for senior hospital leadership that covers 14 different performance areas, covering components such as safety culture and leadership. After an assessment is complete, the application issues a report that identifies strengths and opportunities for improvement and directs the user to specific resources, which are the application’s second element: a library of published materials about specific areas of high reliability offering more than 125 references and tools. These materials will help organizations educate themselves about the 14 components included in Oro 2.0’s high-reliability model.
This is an idea long overdue in healthcare, which lags behind other industries, according to Smith. “We know high-reliability industries like nuclear power and commercial aviation do very complex daily work but have far fewer bad things happening,” she adds. “Healthcare is nowhere near that state of reliability, but it needs to get there.”
Participation by hospitalists is required to make such a change, Smith says. She hopes they will access Oro 2.0’s resource library to understand the solutions the Joint Commission is proposing. “Then they can go to the senior leadership of their hospital and ask them to consider committing to zero patient harm,” she says. “They can support their senior leadership in this work and be vocal about their interest in pursuing high reliability and zero patient harm.”
Visit our website for more information on hospitalists and healthcare safety.
Zero preventable harm is the goal of every healthcare organization, and a new online application from the Joint Commission Center for Transforming Healthcare can help groups reach that goal. Oro 2.0 can assist hospital leaders with determining their organization’s status across multiple components of high reliability that will let them achieve zero preventable harm.
Hospitalists are integral to this process, says Coleen Smith, RN, BSN, MBA, CPHQ, director of high reliability initiatives for the Joint Commission. “Hospitalists are a crucial piece of improving the quality of an organization. Physicians routinely getting involved in high reliability and leading those activities are how we want high-reliability hospitals to look,” says Smith.
The Oro 2.0 application has two major elements. The first is an assessment tool for senior hospital leadership that covers 14 different performance areas, covering components such as safety culture and leadership. After an assessment is complete, the application issues a report that identifies strengths and opportunities for improvement and directs the user to specific resources, which are the application’s second element: a library of published materials about specific areas of high reliability offering more than 125 references and tools. These materials will help organizations educate themselves about the 14 components included in Oro 2.0’s high-reliability model.
This is an idea long overdue in healthcare, which lags behind other industries, according to Smith. “We know high-reliability industries like nuclear power and commercial aviation do very complex daily work but have far fewer bad things happening,” she adds. “Healthcare is nowhere near that state of reliability, but it needs to get there.”
Participation by hospitalists is required to make such a change, Smith says. She hopes they will access Oro 2.0’s resource library to understand the solutions the Joint Commission is proposing. “Then they can go to the senior leadership of their hospital and ask them to consider committing to zero patient harm,” she says. “They can support their senior leadership in this work and be vocal about their interest in pursuing high reliability and zero patient harm.”
Visit our website for more information on hospitalists and healthcare safety.
Zero preventable harm is the goal of every healthcare organization, and a new online application from the Joint Commission Center for Transforming Healthcare can help groups reach that goal. Oro 2.0 can assist hospital leaders with determining their organization’s status across multiple components of high reliability that will let them achieve zero preventable harm.
Hospitalists are integral to this process, says Coleen Smith, RN, BSN, MBA, CPHQ, director of high reliability initiatives for the Joint Commission. “Hospitalists are a crucial piece of improving the quality of an organization. Physicians routinely getting involved in high reliability and leading those activities are how we want high-reliability hospitals to look,” says Smith.
The Oro 2.0 application has two major elements. The first is an assessment tool for senior hospital leadership that covers 14 different performance areas, covering components such as safety culture and leadership. After an assessment is complete, the application issues a report that identifies strengths and opportunities for improvement and directs the user to specific resources, which are the application’s second element: a library of published materials about specific areas of high reliability offering more than 125 references and tools. These materials will help organizations educate themselves about the 14 components included in Oro 2.0’s high-reliability model.
This is an idea long overdue in healthcare, which lags behind other industries, according to Smith. “We know high-reliability industries like nuclear power and commercial aviation do very complex daily work but have far fewer bad things happening,” she adds. “Healthcare is nowhere near that state of reliability, but it needs to get there.”
Participation by hospitalists is required to make such a change, Smith says. She hopes they will access Oro 2.0’s resource library to understand the solutions the Joint Commission is proposing. “Then they can go to the senior leadership of their hospital and ask them to consider committing to zero patient harm,” she says. “They can support their senior leadership in this work and be vocal about their interest in pursuing high reliability and zero patient harm.”
Visit our website for more information on hospitalists and healthcare safety.
Acute Generalized Exanthematous Pustulosis Associated With Ranolazine
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
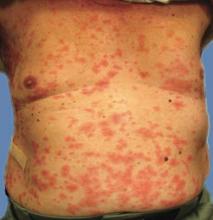
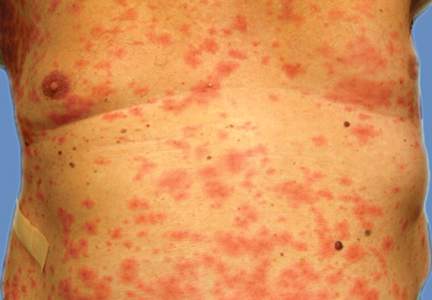
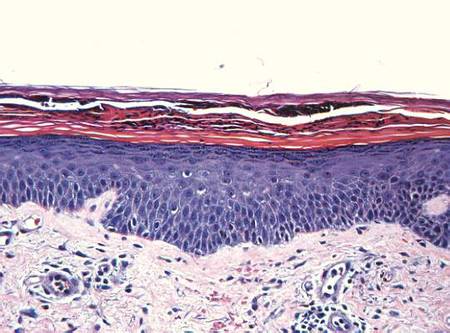
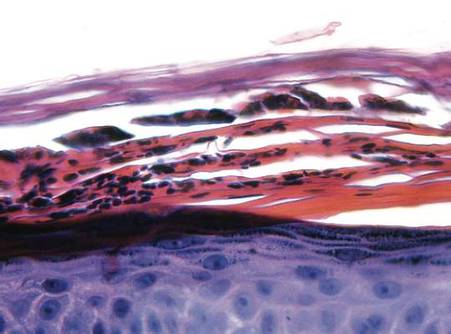
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.
| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
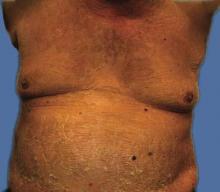
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Practice Points
- Encountering an acute pustular reaction pattern should trigger the clinician to rule out acute generalized exanthematous pustulosis (AGEP).
- Ranolazine, a new antianginal therapy, has been associated with AGEP.
- Upon confirmation of AGEP, the patient’s recent medication history should be reviewed so the potential causative agent can be identified and withdrawn.
Burden of psychiatric comorbidity higher in MS patients
The burden of psychiatric comorbidity is greater in patients with multiple sclerosis (MS), compared with the general population, reported Dr. Ruth Ann Marrie and coauthors from the departments of psychiatry and medicine at the University of Manitoba, Winnipeg.
A study of 44,452 MS patients and 220,849 controls in four Canadian provinces from 1995 to 2005 found that the incidence of depression in the MS group was 0.98% (95% CI; 0.81%-1.15%), compared with 0.72% (95% CI; 0.67%-0.76%) in the control group. The prevalence of depression was 20.1% in MS patients (19.5%-20.6%), compared with 11.9% (11.8%-12.1%) in the matched population, the authors noted.
Also, the incidence and prevalence of anxiety disorder in the MS population was 0.64% (0.54%-0.73%) and 8.7% (8.4%-9.1%), respectively, compared with 0.42% (0.39%-0.45%) and 5.1% (4.9%-5.2%) in controls .
For bipolar disorder, the MS group had an incidence of 0.33% (0.26%-0.39%), compared with 0.16% (0.14%-0.18%) in controls. Prevalence was 4.7% (4.4%-4.9%) in the MS group and 2.3% (2.2%-2.3%) in controls .
Lastly, in schizophrenia, MS patients had an incidence of 0.060% (0.031%-0.080%), compared with 0.018% (0.011%-0.024%) in controls. Prevalence was 1.28% (1.15%-1.41%), in the MS group and 1.03% (0.99%-1.08%) in controls, the investigators said.
The findings suggest a “nonspecific effect of MS on psychiatric comorbidity,” Dr. Marrie and colleagues said in the report.
“From a policy perspective, this implies the need for general psychiatric support rather than illness-specific strategies,” they concluded.
Read the study in Neurology.
The burden of psychiatric comorbidity is greater in patients with multiple sclerosis (MS), compared with the general population, reported Dr. Ruth Ann Marrie and coauthors from the departments of psychiatry and medicine at the University of Manitoba, Winnipeg.
A study of 44,452 MS patients and 220,849 controls in four Canadian provinces from 1995 to 2005 found that the incidence of depression in the MS group was 0.98% (95% CI; 0.81%-1.15%), compared with 0.72% (95% CI; 0.67%-0.76%) in the control group. The prevalence of depression was 20.1% in MS patients (19.5%-20.6%), compared with 11.9% (11.8%-12.1%) in the matched population, the authors noted.
Also, the incidence and prevalence of anxiety disorder in the MS population was 0.64% (0.54%-0.73%) and 8.7% (8.4%-9.1%), respectively, compared with 0.42% (0.39%-0.45%) and 5.1% (4.9%-5.2%) in controls .
For bipolar disorder, the MS group had an incidence of 0.33% (0.26%-0.39%), compared with 0.16% (0.14%-0.18%) in controls. Prevalence was 4.7% (4.4%-4.9%) in the MS group and 2.3% (2.2%-2.3%) in controls .
Lastly, in schizophrenia, MS patients had an incidence of 0.060% (0.031%-0.080%), compared with 0.018% (0.011%-0.024%) in controls. Prevalence was 1.28% (1.15%-1.41%), in the MS group and 1.03% (0.99%-1.08%) in controls, the investigators said.
The findings suggest a “nonspecific effect of MS on psychiatric comorbidity,” Dr. Marrie and colleagues said in the report.
“From a policy perspective, this implies the need for general psychiatric support rather than illness-specific strategies,” they concluded.
Read the study in Neurology.
The burden of psychiatric comorbidity is greater in patients with multiple sclerosis (MS), compared with the general population, reported Dr. Ruth Ann Marrie and coauthors from the departments of psychiatry and medicine at the University of Manitoba, Winnipeg.
A study of 44,452 MS patients and 220,849 controls in four Canadian provinces from 1995 to 2005 found that the incidence of depression in the MS group was 0.98% (95% CI; 0.81%-1.15%), compared with 0.72% (95% CI; 0.67%-0.76%) in the control group. The prevalence of depression was 20.1% in MS patients (19.5%-20.6%), compared with 11.9% (11.8%-12.1%) in the matched population, the authors noted.
Also, the incidence and prevalence of anxiety disorder in the MS population was 0.64% (0.54%-0.73%) and 8.7% (8.4%-9.1%), respectively, compared with 0.42% (0.39%-0.45%) and 5.1% (4.9%-5.2%) in controls .
For bipolar disorder, the MS group had an incidence of 0.33% (0.26%-0.39%), compared with 0.16% (0.14%-0.18%) in controls. Prevalence was 4.7% (4.4%-4.9%) in the MS group and 2.3% (2.2%-2.3%) in controls .
Lastly, in schizophrenia, MS patients had an incidence of 0.060% (0.031%-0.080%), compared with 0.018% (0.011%-0.024%) in controls. Prevalence was 1.28% (1.15%-1.41%), in the MS group and 1.03% (0.99%-1.08%) in controls, the investigators said.
The findings suggest a “nonspecific effect of MS on psychiatric comorbidity,” Dr. Marrie and colleagues said in the report.
“From a policy perspective, this implies the need for general psychiatric support rather than illness-specific strategies,” they concluded.
Read the study in Neurology.
New and Noteworthy Information—November 2015
HLA-DRB1*1501, adolescent summer sun habits, and BMI at the age of 20 independently affect age of multiple sclerosis (MS) onset, according to a study published online ahead of print October 7 in Neurology. This cross-sectional study included 1,161 Danish patients with MS. Lifestyle questionnaires and blood samples for genotyping were collected from all participants from 2009 to 2012. Information on age at onset was obtained from the Danish MS Treatment Registry. Younger age at onset was significantly associated with low exposure to summer sun in adolescence, higher BMI at age 20, and the HLA-DRB1*1501 risk allele in both univariate analyses and in a multivariable regression analysis. No association was found between age at onset and other single-nucleotide polymorphisms studied or vitamin D-associated environmental factors.
Treatment responses for autoimmune ataxia are more likely in patients with nonparaneoplastic disorders and those with exclusively plasma membrane protein (PMP) antibodies, according to a study published online ahead of print September 28 in JAMA Neurology. Investigators examined 118 patients with ataxia who were 18 or older, were seropositive for at least one neural autoantibody, had received at least one immunotherapy or cancer therapy, and had neurologist-reported outcomes documented from January 1, 1989, through December 31, 2013. Fifty-four patients had neurologic improvements. Kaplan-Meier analyses revealed that progression to wheelchair dependence occurred significantly faster among patients with neuronal nuclear or cytoplasmic antibody positivity only, although those with glutamic acid decarboxylase 65-kDa isoform autoimmunity progressed to wheelchair dependence at a rate similar to those with PMP autoimmunity.
Patients with celiac disease are not at increased risk for dementia overall, though they may be at increased risk for vascular dementia, according to a study published online ahead of print September 29 in Journal of Alzheimer’s Disease. Researchers compared the incidence of a subsequent dementia diagnosis among 8,846 older adults with celiac disease to that among 43,474 age- and gender-matched controls. The median age of the study population was 63, and 56% of participants were female. During a median follow-up time of 8.4 years, dementia was diagnosed in 4.3% of patients with celiac disease and 4.4% of controls. The researchers observed an increased risk of dementia in the first year following a diagnosis of celiac disease, but the increased risk was restricted to vascular dementia and was not present for Alzheimer’s dementia.
Infection may trigger childhood arterial ischemic stroke, while routine vaccinations appear to protect against it, according to a study published online ahead of print September 30 in Neurology. This international case–control study included 355 children with confirmed cases of arterial ischemic stroke and 354 controls without stroke. Median age was 7.6 for cases and 9.3 for controls. Infection in the week prior to stroke, or interview date for controls, was reported in 18% of cases versus 3% of controls. Infection thus conferred a 6.3-fold increased risk of arterial ischemic stroke. Children with some, few, or no routine vaccinations were at higher stroke risk than those receiving all or most vaccinations. Risk factors for arterial ischemic stroke included infection in the prior week, undervaccination, black race, and rural residence.
Amyloid PET and CSF biomarkers identify early Alzheimer’s disease with equal accuracy, according to a study published October 6 in Neurology. Researchers examined 122 healthy elderly people and 34 patients with mild cognitive impairment who developed Alzheimer’s disease dementia within three years (MCI-AD). They examined β-amyloid deposition in nine brain regions with [18F]-flutemetamol PET. CSF was analyzed with INNOTEST and EUROIMMUN ELISAs. CSF samples and PET scans each identified approximately 90% of patients who later received a diagnosis of Alzheimer’s disease. The best CSF measures for identifying MCI-AD were Aβ42/total tau and Aβ42/hyperphosphorylated tau, which performed better than CSF Aβ42 and Aβ42/40. CSF Aβ42/total tau had the highest accuracy of all CSF and PET biomarkers. The combination of CSF and PET was not better than either individual biomarker.
A combination of dextromethorphan and quinidine demonstrated clinically relevant efficacy for agitation in patients with probable Alzheimer’s disease and was generally well tolerated, according to a study published September 22 in JAMA. A total of 194 patients completed a preliminary 10-week phase II randomized clinical trial. In the sequential parallel comparison design, 152 patients received dextromethorphan–quinidine, and 127 received placebo. Analysis combining all patients and rerandomized placebo nonresponders showed significantly reduced agitation and aggression scores for dextromethorphan–quinidine versus placebo. Among all patients, mean agitation and aggression scores were reduced from 7.1 to 3.8 with dextromethorphan–quinidine and from 7.0 to 5.3 with placebo. Between-group treatment differences were significant. Among rerandomized placebo nonresponders, agitation and aggression scores were reduced from 5.8 to 3.8 with dextromethorphan–quinidine and from 6.7 to 5.8 with placebo.
The FDA has approved Betaconnect, an electronic autoinjector for the treatment of relapsing-remitting multiple sclerosis. Bayer HealthCare (Whippany, NJ) manufactures Betaconnect, which will be available to patients receiving Betaseron beginning in early 2016. The autoinjector, which was created based on feedback from patients and caregivers, offers customizable injection speed and depth settings that allow patients to administer injections quietly and precisely. Betaconnect also has an optional backup reminder function that tells patients the time of their next injection. In addition, the automatic needle insertion and retraction and a visual and audio end-of-dose indication tell patients when the injection is complete. Patients should speak with a healthcare provider before making any changes to injection depth or speed settings.
In patients with an intracranial pressure of more than 20 mmHg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure do not result in outcomes better than those associated with standard care alone, according to a study published online ahead of print October 7 in New England Journal of Medicine. Investigators enrolled 387 patients with TBI from November 2009 through October 2014 in a study. Barbiturates and decompressive craniectomy were required to control intracranial pressure in 54% of patients who received standard care and in 44% of patients who received hypothermia and standard care. The hypothermia group had worse outcomes in general than the standard-care group. A favorable outcome occurred in 26% of patients in the hypothermia group and in 37% of patients in the control group.
Differing manifestations of postconcussion symptoms on functional MRI (fMRI) between younger and older patients indicate that age influences the activation, modulation, and allocation of working memory processing resources after mild traumatic brain injury (MTBI), according to a study published online ahead of print October 6 in Radiology. Researchers performed fMRI exams on 13 young adults and 13 older adults with MTBI and 26 age- and gender-matched controls. Younger patients performing working-memory tasks had initial hyperactivation in the right precuneus and right inferior parietal gyrus, compared with younger controls. Older patients performing these tasks had hypoactivation in the right precuneus and right inferior frontal gyrus, compared with older controls. Younger patients, but not older patients, had partial recovery of activation pattern and decreased postconcussion symptoms at follow-up.
An immune system gene is associated with higher rates of amyloid plaque buildup in the brains of patients with Alzheimer’s disease and older adults at risk for the disease, according to a study published in the October issue of Brain. Investigators performed a genome-wide association study of longitudinal change in brain amyloid burden measured by 18F-florbetapir PET. They found that interleukin-1 receptor accessory protein (IL1RAP) was associated with higher rates of amyloid accumulation, independent of APOE ε4 status. This novel association was validated by deep sequencing. IL1RAP rs12053868-G carriers were more likely to progress from mild cognitive impairment to Alzheimer’s disease and exhibited greater longitudinal temporal cortex atrophy on MRI. In independent cohorts, rs12053868-G was associated with accelerated cognitive decline and lower cortical 11C-PBR28 PET signal.
For children with tuberous sclerosis complex and medically intractable epilepsy, a greater extent of resection is associated with a greater probability of seizure freedom, according to a study published in the October issue of Neurosurgery. Seventy-four patients were included in this retrospective chart review, and their median age at the time of surgery was 120 months. Engel Class I outcome was achieved in 65% and in 50% of patients at the one- and two-year follow-up, respectively. On univariate analyses, younger age at seizure onset, larger size of predominant tuber, and resection larger than a tuberectomy were associated with a longer duration of seizure freedom. In multivariate analyses, resection larger than a tuberectomy was independently associated with a longer duration of seizure freedom.
A new imaging method that uses a 7-T magnet shows promise in locating hard-to-find epileptic foci by visualizing the neurotransmitter glutamate, according to a study published October 14 in Science Translational Medicine. In a pilot study, researchers applied glutamate chemical exchange saturation transfer (GluCEST) to patients with nonlesional temporal lobe epilepsy based on conventional MRI. GluCEST correctly lateralized the temporal lobe seizure focus on visual and quantitative analyses in all patients. Hippocampal volumes were not significantly different between hemispheres. GluCEST allowed high-resolution functional imaging of brain glutamate and has the potential to identify the epileptic focus in patients previously deemed nonlesional. This method may lead to improved clinical outcomes for temporal lobe epilepsy as well as other localization-related epilepsies, according to the researchers.
—Kimberly Williams
HLA-DRB1*1501, adolescent summer sun habits, and BMI at the age of 20 independently affect age of multiple sclerosis (MS) onset, according to a study published online ahead of print October 7 in Neurology. This cross-sectional study included 1,161 Danish patients with MS. Lifestyle questionnaires and blood samples for genotyping were collected from all participants from 2009 to 2012. Information on age at onset was obtained from the Danish MS Treatment Registry. Younger age at onset was significantly associated with low exposure to summer sun in adolescence, higher BMI at age 20, and the HLA-DRB1*1501 risk allele in both univariate analyses and in a multivariable regression analysis. No association was found between age at onset and other single-nucleotide polymorphisms studied or vitamin D-associated environmental factors.
Treatment responses for autoimmune ataxia are more likely in patients with nonparaneoplastic disorders and those with exclusively plasma membrane protein (PMP) antibodies, according to a study published online ahead of print September 28 in JAMA Neurology. Investigators examined 118 patients with ataxia who were 18 or older, were seropositive for at least one neural autoantibody, had received at least one immunotherapy or cancer therapy, and had neurologist-reported outcomes documented from January 1, 1989, through December 31, 2013. Fifty-four patients had neurologic improvements. Kaplan-Meier analyses revealed that progression to wheelchair dependence occurred significantly faster among patients with neuronal nuclear or cytoplasmic antibody positivity only, although those with glutamic acid decarboxylase 65-kDa isoform autoimmunity progressed to wheelchair dependence at a rate similar to those with PMP autoimmunity.
Patients with celiac disease are not at increased risk for dementia overall, though they may be at increased risk for vascular dementia, according to a study published online ahead of print September 29 in Journal of Alzheimer’s Disease. Researchers compared the incidence of a subsequent dementia diagnosis among 8,846 older adults with celiac disease to that among 43,474 age- and gender-matched controls. The median age of the study population was 63, and 56% of participants were female. During a median follow-up time of 8.4 years, dementia was diagnosed in 4.3% of patients with celiac disease and 4.4% of controls. The researchers observed an increased risk of dementia in the first year following a diagnosis of celiac disease, but the increased risk was restricted to vascular dementia and was not present for Alzheimer’s dementia.
Infection may trigger childhood arterial ischemic stroke, while routine vaccinations appear to protect against it, according to a study published online ahead of print September 30 in Neurology. This international case–control study included 355 children with confirmed cases of arterial ischemic stroke and 354 controls without stroke. Median age was 7.6 for cases and 9.3 for controls. Infection in the week prior to stroke, or interview date for controls, was reported in 18% of cases versus 3% of controls. Infection thus conferred a 6.3-fold increased risk of arterial ischemic stroke. Children with some, few, or no routine vaccinations were at higher stroke risk than those receiving all or most vaccinations. Risk factors for arterial ischemic stroke included infection in the prior week, undervaccination, black race, and rural residence.
Amyloid PET and CSF biomarkers identify early Alzheimer’s disease with equal accuracy, according to a study published October 6 in Neurology. Researchers examined 122 healthy elderly people and 34 patients with mild cognitive impairment who developed Alzheimer’s disease dementia within three years (MCI-AD). They examined β-amyloid deposition in nine brain regions with [18F]-flutemetamol PET. CSF was analyzed with INNOTEST and EUROIMMUN ELISAs. CSF samples and PET scans each identified approximately 90% of patients who later received a diagnosis of Alzheimer’s disease. The best CSF measures for identifying MCI-AD were Aβ42/total tau and Aβ42/hyperphosphorylated tau, which performed better than CSF Aβ42 and Aβ42/40. CSF Aβ42/total tau had the highest accuracy of all CSF and PET biomarkers. The combination of CSF and PET was not better than either individual biomarker.
A combination of dextromethorphan and quinidine demonstrated clinically relevant efficacy for agitation in patients with probable Alzheimer’s disease and was generally well tolerated, according to a study published September 22 in JAMA. A total of 194 patients completed a preliminary 10-week phase II randomized clinical trial. In the sequential parallel comparison design, 152 patients received dextromethorphan–quinidine, and 127 received placebo. Analysis combining all patients and rerandomized placebo nonresponders showed significantly reduced agitation and aggression scores for dextromethorphan–quinidine versus placebo. Among all patients, mean agitation and aggression scores were reduced from 7.1 to 3.8 with dextromethorphan–quinidine and from 7.0 to 5.3 with placebo. Between-group treatment differences were significant. Among rerandomized placebo nonresponders, agitation and aggression scores were reduced from 5.8 to 3.8 with dextromethorphan–quinidine and from 6.7 to 5.8 with placebo.
The FDA has approved Betaconnect, an electronic autoinjector for the treatment of relapsing-remitting multiple sclerosis. Bayer HealthCare (Whippany, NJ) manufactures Betaconnect, which will be available to patients receiving Betaseron beginning in early 2016. The autoinjector, which was created based on feedback from patients and caregivers, offers customizable injection speed and depth settings that allow patients to administer injections quietly and precisely. Betaconnect also has an optional backup reminder function that tells patients the time of their next injection. In addition, the automatic needle insertion and retraction and a visual and audio end-of-dose indication tell patients when the injection is complete. Patients should speak with a healthcare provider before making any changes to injection depth or speed settings.
In patients with an intracranial pressure of more than 20 mmHg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure do not result in outcomes better than those associated with standard care alone, according to a study published online ahead of print October 7 in New England Journal of Medicine. Investigators enrolled 387 patients with TBI from November 2009 through October 2014 in a study. Barbiturates and decompressive craniectomy were required to control intracranial pressure in 54% of patients who received standard care and in 44% of patients who received hypothermia and standard care. The hypothermia group had worse outcomes in general than the standard-care group. A favorable outcome occurred in 26% of patients in the hypothermia group and in 37% of patients in the control group.
Differing manifestations of postconcussion symptoms on functional MRI (fMRI) between younger and older patients indicate that age influences the activation, modulation, and allocation of working memory processing resources after mild traumatic brain injury (MTBI), according to a study published online ahead of print October 6 in Radiology. Researchers performed fMRI exams on 13 young adults and 13 older adults with MTBI and 26 age- and gender-matched controls. Younger patients performing working-memory tasks had initial hyperactivation in the right precuneus and right inferior parietal gyrus, compared with younger controls. Older patients performing these tasks had hypoactivation in the right precuneus and right inferior frontal gyrus, compared with older controls. Younger patients, but not older patients, had partial recovery of activation pattern and decreased postconcussion symptoms at follow-up.
An immune system gene is associated with higher rates of amyloid plaque buildup in the brains of patients with Alzheimer’s disease and older adults at risk for the disease, according to a study published in the October issue of Brain. Investigators performed a genome-wide association study of longitudinal change in brain amyloid burden measured by 18F-florbetapir PET. They found that interleukin-1 receptor accessory protein (IL1RAP) was associated with higher rates of amyloid accumulation, independent of APOE ε4 status. This novel association was validated by deep sequencing. IL1RAP rs12053868-G carriers were more likely to progress from mild cognitive impairment to Alzheimer’s disease and exhibited greater longitudinal temporal cortex atrophy on MRI. In independent cohorts, rs12053868-G was associated with accelerated cognitive decline and lower cortical 11C-PBR28 PET signal.
For children with tuberous sclerosis complex and medically intractable epilepsy, a greater extent of resection is associated with a greater probability of seizure freedom, according to a study published in the October issue of Neurosurgery. Seventy-four patients were included in this retrospective chart review, and their median age at the time of surgery was 120 months. Engel Class I outcome was achieved in 65% and in 50% of patients at the one- and two-year follow-up, respectively. On univariate analyses, younger age at seizure onset, larger size of predominant tuber, and resection larger than a tuberectomy were associated with a longer duration of seizure freedom. In multivariate analyses, resection larger than a tuberectomy was independently associated with a longer duration of seizure freedom.
A new imaging method that uses a 7-T magnet shows promise in locating hard-to-find epileptic foci by visualizing the neurotransmitter glutamate, according to a study published October 14 in Science Translational Medicine. In a pilot study, researchers applied glutamate chemical exchange saturation transfer (GluCEST) to patients with nonlesional temporal lobe epilepsy based on conventional MRI. GluCEST correctly lateralized the temporal lobe seizure focus on visual and quantitative analyses in all patients. Hippocampal volumes were not significantly different between hemispheres. GluCEST allowed high-resolution functional imaging of brain glutamate and has the potential to identify the epileptic focus in patients previously deemed nonlesional. This method may lead to improved clinical outcomes for temporal lobe epilepsy as well as other localization-related epilepsies, according to the researchers.
—Kimberly Williams
HLA-DRB1*1501, adolescent summer sun habits, and BMI at the age of 20 independently affect age of multiple sclerosis (MS) onset, according to a study published online ahead of print October 7 in Neurology. This cross-sectional study included 1,161 Danish patients with MS. Lifestyle questionnaires and blood samples for genotyping were collected from all participants from 2009 to 2012. Information on age at onset was obtained from the Danish MS Treatment Registry. Younger age at onset was significantly associated with low exposure to summer sun in adolescence, higher BMI at age 20, and the HLA-DRB1*1501 risk allele in both univariate analyses and in a multivariable regression analysis. No association was found between age at onset and other single-nucleotide polymorphisms studied or vitamin D-associated environmental factors.
Treatment responses for autoimmune ataxia are more likely in patients with nonparaneoplastic disorders and those with exclusively plasma membrane protein (PMP) antibodies, according to a study published online ahead of print September 28 in JAMA Neurology. Investigators examined 118 patients with ataxia who were 18 or older, were seropositive for at least one neural autoantibody, had received at least one immunotherapy or cancer therapy, and had neurologist-reported outcomes documented from January 1, 1989, through December 31, 2013. Fifty-four patients had neurologic improvements. Kaplan-Meier analyses revealed that progression to wheelchair dependence occurred significantly faster among patients with neuronal nuclear or cytoplasmic antibody positivity only, although those with glutamic acid decarboxylase 65-kDa isoform autoimmunity progressed to wheelchair dependence at a rate similar to those with PMP autoimmunity.
Patients with celiac disease are not at increased risk for dementia overall, though they may be at increased risk for vascular dementia, according to a study published online ahead of print September 29 in Journal of Alzheimer’s Disease. Researchers compared the incidence of a subsequent dementia diagnosis among 8,846 older adults with celiac disease to that among 43,474 age- and gender-matched controls. The median age of the study population was 63, and 56% of participants were female. During a median follow-up time of 8.4 years, dementia was diagnosed in 4.3% of patients with celiac disease and 4.4% of controls. The researchers observed an increased risk of dementia in the first year following a diagnosis of celiac disease, but the increased risk was restricted to vascular dementia and was not present for Alzheimer’s dementia.
Infection may trigger childhood arterial ischemic stroke, while routine vaccinations appear to protect against it, according to a study published online ahead of print September 30 in Neurology. This international case–control study included 355 children with confirmed cases of arterial ischemic stroke and 354 controls without stroke. Median age was 7.6 for cases and 9.3 for controls. Infection in the week prior to stroke, or interview date for controls, was reported in 18% of cases versus 3% of controls. Infection thus conferred a 6.3-fold increased risk of arterial ischemic stroke. Children with some, few, or no routine vaccinations were at higher stroke risk than those receiving all or most vaccinations. Risk factors for arterial ischemic stroke included infection in the prior week, undervaccination, black race, and rural residence.
Amyloid PET and CSF biomarkers identify early Alzheimer’s disease with equal accuracy, according to a study published October 6 in Neurology. Researchers examined 122 healthy elderly people and 34 patients with mild cognitive impairment who developed Alzheimer’s disease dementia within three years (MCI-AD). They examined β-amyloid deposition in nine brain regions with [18F]-flutemetamol PET. CSF was analyzed with INNOTEST and EUROIMMUN ELISAs. CSF samples and PET scans each identified approximately 90% of patients who later received a diagnosis of Alzheimer’s disease. The best CSF measures for identifying MCI-AD were Aβ42/total tau and Aβ42/hyperphosphorylated tau, which performed better than CSF Aβ42 and Aβ42/40. CSF Aβ42/total tau had the highest accuracy of all CSF and PET biomarkers. The combination of CSF and PET was not better than either individual biomarker.
A combination of dextromethorphan and quinidine demonstrated clinically relevant efficacy for agitation in patients with probable Alzheimer’s disease and was generally well tolerated, according to a study published September 22 in JAMA. A total of 194 patients completed a preliminary 10-week phase II randomized clinical trial. In the sequential parallel comparison design, 152 patients received dextromethorphan–quinidine, and 127 received placebo. Analysis combining all patients and rerandomized placebo nonresponders showed significantly reduced agitation and aggression scores for dextromethorphan–quinidine versus placebo. Among all patients, mean agitation and aggression scores were reduced from 7.1 to 3.8 with dextromethorphan–quinidine and from 7.0 to 5.3 with placebo. Between-group treatment differences were significant. Among rerandomized placebo nonresponders, agitation and aggression scores were reduced from 5.8 to 3.8 with dextromethorphan–quinidine and from 6.7 to 5.8 with placebo.
The FDA has approved Betaconnect, an electronic autoinjector for the treatment of relapsing-remitting multiple sclerosis. Bayer HealthCare (Whippany, NJ) manufactures Betaconnect, which will be available to patients receiving Betaseron beginning in early 2016. The autoinjector, which was created based on feedback from patients and caregivers, offers customizable injection speed and depth settings that allow patients to administer injections quietly and precisely. Betaconnect also has an optional backup reminder function that tells patients the time of their next injection. In addition, the automatic needle insertion and retraction and a visual and audio end-of-dose indication tell patients when the injection is complete. Patients should speak with a healthcare provider before making any changes to injection depth or speed settings.
In patients with an intracranial pressure of more than 20 mmHg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure do not result in outcomes better than those associated with standard care alone, according to a study published online ahead of print October 7 in New England Journal of Medicine. Investigators enrolled 387 patients with TBI from November 2009 through October 2014 in a study. Barbiturates and decompressive craniectomy were required to control intracranial pressure in 54% of patients who received standard care and in 44% of patients who received hypothermia and standard care. The hypothermia group had worse outcomes in general than the standard-care group. A favorable outcome occurred in 26% of patients in the hypothermia group and in 37% of patients in the control group.
Differing manifestations of postconcussion symptoms on functional MRI (fMRI) between younger and older patients indicate that age influences the activation, modulation, and allocation of working memory processing resources after mild traumatic brain injury (MTBI), according to a study published online ahead of print October 6 in Radiology. Researchers performed fMRI exams on 13 young adults and 13 older adults with MTBI and 26 age- and gender-matched controls. Younger patients performing working-memory tasks had initial hyperactivation in the right precuneus and right inferior parietal gyrus, compared with younger controls. Older patients performing these tasks had hypoactivation in the right precuneus and right inferior frontal gyrus, compared with older controls. Younger patients, but not older patients, had partial recovery of activation pattern and decreased postconcussion symptoms at follow-up.
An immune system gene is associated with higher rates of amyloid plaque buildup in the brains of patients with Alzheimer’s disease and older adults at risk for the disease, according to a study published in the October issue of Brain. Investigators performed a genome-wide association study of longitudinal change in brain amyloid burden measured by 18F-florbetapir PET. They found that interleukin-1 receptor accessory protein (IL1RAP) was associated with higher rates of amyloid accumulation, independent of APOE ε4 status. This novel association was validated by deep sequencing. IL1RAP rs12053868-G carriers were more likely to progress from mild cognitive impairment to Alzheimer’s disease and exhibited greater longitudinal temporal cortex atrophy on MRI. In independent cohorts, rs12053868-G was associated with accelerated cognitive decline and lower cortical 11C-PBR28 PET signal.
For children with tuberous sclerosis complex and medically intractable epilepsy, a greater extent of resection is associated with a greater probability of seizure freedom, according to a study published in the October issue of Neurosurgery. Seventy-four patients were included in this retrospective chart review, and their median age at the time of surgery was 120 months. Engel Class I outcome was achieved in 65% and in 50% of patients at the one- and two-year follow-up, respectively. On univariate analyses, younger age at seizure onset, larger size of predominant tuber, and resection larger than a tuberectomy were associated with a longer duration of seizure freedom. In multivariate analyses, resection larger than a tuberectomy was independently associated with a longer duration of seizure freedom.
A new imaging method that uses a 7-T magnet shows promise in locating hard-to-find epileptic foci by visualizing the neurotransmitter glutamate, according to a study published October 14 in Science Translational Medicine. In a pilot study, researchers applied glutamate chemical exchange saturation transfer (GluCEST) to patients with nonlesional temporal lobe epilepsy based on conventional MRI. GluCEST correctly lateralized the temporal lobe seizure focus on visual and quantitative analyses in all patients. Hippocampal volumes were not significantly different between hemispheres. GluCEST allowed high-resolution functional imaging of brain glutamate and has the potential to identify the epileptic focus in patients previously deemed nonlesional. This method may lead to improved clinical outcomes for temporal lobe epilepsy as well as other localization-related epilepsies, according to the researchers.
—Kimberly Williams
Lymphedema Patients Benefit from Pneumatic Compression Devices
NEW YORK - Patients with lymphedema may reduce their risk of cellulitis, as well as the number of outpatient visits, by using an advanced pneumatic compression device (APCD), according to a new study.
"Our study demonstrates, for the first time, that receipt of an advanced pneumatic compression device is associated with significant improvements in key clinical endpoints for lymphedema patients, both for those with cancer and those without," Dr. Pinar Karaca-Mandic of the University of Minnesota School of Public Health in Minneapolis said by email.
"This finding has important implications for the patients who suffer from the disease, especially for those who have high rates of cellulitis. These devices serve as a viable self-management option and can reduce the need for more intensive outpatient care in rehabilitative settings," she added.
Advanced devices have more garment chambers and greater adjustability than earlier devices, the researchers wrote.
Dr. Karaca-Mandic and colleagues used a commercial insurance claims database to compare outcomes for 12 months before and 12 months after APCD purchase (Flexitouch System, Tactile Medical) by 718 patients (374 with cancer) between 2008 and 2012.
Lymphedema-related outcomes had either primary or secondary diagnosis codes.
The patients' mean age was 54.2, 84.8% were female, and 71.6% were non-Hispanic white. Just over half (52.2%) had hypertension, and breast cancer (39.6%) was the predominant disease in the cancer group.
As reported online October 7 in JAMA Dermatology, the adjusted rate of cellulitis diagnoses fell from 21.1% before APCD use to 4.5% afterward (p<0.001), a 79% decline. The noncancer group had a 75% decline, from 28.8% to 7.3% (p<0.001).
The noncancer group also had a 54% decline in adjusted rate of hospitalizations, from 7.0% to 3.2% (p=0.02), the authors reported.
Both groups had declines in receipt of manual therapy, from an adjusted rate of 35.6% before APCD use to 24.9% afterward for cancer patients (p<0.001) and from 32.3% to 21.2% for noncancer patients (p<0.001).
The adjusted rate of outpatient visits fell from 58.6% to 41.4% in the cancer cohort and from 52.6% to 31.4% in the noncancer group (p<0.001 for both).
Total costs per patient, excluding medical equipment, declined from $2597 to $1642 for cancer patients (p=0.002) and from $2937 to $1883 (p=0.007) for noncancer patients.
"While our findings are based upon the outcomes from one specific device, it is possible other such devices may also reduce patient burden. This warrants explorations in future studies. In addition, our study was not designed to assess the long term effectiveness of the device. That should be studied in future work," Dr. Karaca-Mandic explained.
Also, she pointed out, her team didn't look at nonmonetary expenses such as productivity loss and caretaker costs. "To the extent that device use improves physical functioning and lowers such costs as well, the impact is likely much larger than we can measure," she added.
Dr. Peter J. Franks, of the Center for Research and Implementation of Clinical Practice in London, UK, said by email, "We have these devices that appear to work. The problem is that the evidence on efficacy and cost effectiveness is so poor. The article gave some retrospective observational data that implied that the incidence of infection (cellulitis) was reduced. This is important, as infections lead to further deterioration of the lymphatic system, making the situation worse for the patient and increasing the risk of further infections."
"It is hard to say how generalizable the results are to other devices, though fundamentally they all work in similar ways," said Dr. Franks, who coauthored an accompanying editorial. "I think that this is an important step in how we consider the use of medical devices."
Cynthia Shechter, an occupational therapist in New York City who is a lymphedema specialist for cancer patients, said by email, "When looking for the right device, look for a pump that contains multiple chambers, operates on a short thirty-second cycle time, and applies graduated compression."
"The body operates on a pressure gradient system, so it is imperative to obtain a gradient or graduated compression pump. Pressure at the feet or hand is greater than the thigh or shoulder," she added.
"Clinicians practicing in the treatment of lymphedema need to be open-minded regarding less traditional treatment options for this insidious condition, including the use of traditional and advanced pneumatic compression devices," Shechter said.
"This study indicates that use of an APCD reduces the necessity for therapy. However, rehabilitation therapy for primary and secondary lymphedema, at least a short course of
treatment, is important, especially in order to ensure patients are adequately educated in lymphedema care, management, and precautions," she said.
"There should be a follow-up study performed to discuss a patient's ability to sustain use of the APCD versus a traditional pneumatic pump, and the long-term success in both preventing infection and in reduction of therapy visits," Shechter said.
Tactile Medical partially supported this research and employs one coauthor as chief medical officer. Dr. Karaca-Mandic, Dr. Franks, and his coauthor reported consulting for the company.
NEW YORK - Patients with lymphedema may reduce their risk of cellulitis, as well as the number of outpatient visits, by using an advanced pneumatic compression device (APCD), according to a new study.
"Our study demonstrates, for the first time, that receipt of an advanced pneumatic compression device is associated with significant improvements in key clinical endpoints for lymphedema patients, both for those with cancer and those without," Dr. Pinar Karaca-Mandic of the University of Minnesota School of Public Health in Minneapolis said by email.
"This finding has important implications for the patients who suffer from the disease, especially for those who have high rates of cellulitis. These devices serve as a viable self-management option and can reduce the need for more intensive outpatient care in rehabilitative settings," she added.
Advanced devices have more garment chambers and greater adjustability than earlier devices, the researchers wrote.
Dr. Karaca-Mandic and colleagues used a commercial insurance claims database to compare outcomes for 12 months before and 12 months after APCD purchase (Flexitouch System, Tactile Medical) by 718 patients (374 with cancer) between 2008 and 2012.
Lymphedema-related outcomes had either primary or secondary diagnosis codes.
The patients' mean age was 54.2, 84.8% were female, and 71.6% were non-Hispanic white. Just over half (52.2%) had hypertension, and breast cancer (39.6%) was the predominant disease in the cancer group.
As reported online October 7 in JAMA Dermatology, the adjusted rate of cellulitis diagnoses fell from 21.1% before APCD use to 4.5% afterward (p<0.001), a 79% decline. The noncancer group had a 75% decline, from 28.8% to 7.3% (p<0.001).
The noncancer group also had a 54% decline in adjusted rate of hospitalizations, from 7.0% to 3.2% (p=0.02), the authors reported.
Both groups had declines in receipt of manual therapy, from an adjusted rate of 35.6% before APCD use to 24.9% afterward for cancer patients (p<0.001) and from 32.3% to 21.2% for noncancer patients (p<0.001).
The adjusted rate of outpatient visits fell from 58.6% to 41.4% in the cancer cohort and from 52.6% to 31.4% in the noncancer group (p<0.001 for both).
Total costs per patient, excluding medical equipment, declined from $2597 to $1642 for cancer patients (p=0.002) and from $2937 to $1883 (p=0.007) for noncancer patients.
"While our findings are based upon the outcomes from one specific device, it is possible other such devices may also reduce patient burden. This warrants explorations in future studies. In addition, our study was not designed to assess the long term effectiveness of the device. That should be studied in future work," Dr. Karaca-Mandic explained.
Also, she pointed out, her team didn't look at nonmonetary expenses such as productivity loss and caretaker costs. "To the extent that device use improves physical functioning and lowers such costs as well, the impact is likely much larger than we can measure," she added.
Dr. Peter J. Franks, of the Center for Research and Implementation of Clinical Practice in London, UK, said by email, "We have these devices that appear to work. The problem is that the evidence on efficacy and cost effectiveness is so poor. The article gave some retrospective observational data that implied that the incidence of infection (cellulitis) was reduced. This is important, as infections lead to further deterioration of the lymphatic system, making the situation worse for the patient and increasing the risk of further infections."
"It is hard to say how generalizable the results are to other devices, though fundamentally they all work in similar ways," said Dr. Franks, who coauthored an accompanying editorial. "I think that this is an important step in how we consider the use of medical devices."
Cynthia Shechter, an occupational therapist in New York City who is a lymphedema specialist for cancer patients, said by email, "When looking for the right device, look for a pump that contains multiple chambers, operates on a short thirty-second cycle time, and applies graduated compression."
"The body operates on a pressure gradient system, so it is imperative to obtain a gradient or graduated compression pump. Pressure at the feet or hand is greater than the thigh or shoulder," she added.
"Clinicians practicing in the treatment of lymphedema need to be open-minded regarding less traditional treatment options for this insidious condition, including the use of traditional and advanced pneumatic compression devices," Shechter said.
"This study indicates that use of an APCD reduces the necessity for therapy. However, rehabilitation therapy for primary and secondary lymphedema, at least a short course of
treatment, is important, especially in order to ensure patients are adequately educated in lymphedema care, management, and precautions," she said.
"There should be a follow-up study performed to discuss a patient's ability to sustain use of the APCD versus a traditional pneumatic pump, and the long-term success in both preventing infection and in reduction of therapy visits," Shechter said.
Tactile Medical partially supported this research and employs one coauthor as chief medical officer. Dr. Karaca-Mandic, Dr. Franks, and his coauthor reported consulting for the company.
NEW YORK - Patients with lymphedema may reduce their risk of cellulitis, as well as the number of outpatient visits, by using an advanced pneumatic compression device (APCD), according to a new study.
"Our study demonstrates, for the first time, that receipt of an advanced pneumatic compression device is associated with significant improvements in key clinical endpoints for lymphedema patients, both for those with cancer and those without," Dr. Pinar Karaca-Mandic of the University of Minnesota School of Public Health in Minneapolis said by email.
"This finding has important implications for the patients who suffer from the disease, especially for those who have high rates of cellulitis. These devices serve as a viable self-management option and can reduce the need for more intensive outpatient care in rehabilitative settings," she added.
Advanced devices have more garment chambers and greater adjustability than earlier devices, the researchers wrote.
Dr. Karaca-Mandic and colleagues used a commercial insurance claims database to compare outcomes for 12 months before and 12 months after APCD purchase (Flexitouch System, Tactile Medical) by 718 patients (374 with cancer) between 2008 and 2012.
Lymphedema-related outcomes had either primary or secondary diagnosis codes.
The patients' mean age was 54.2, 84.8% were female, and 71.6% were non-Hispanic white. Just over half (52.2%) had hypertension, and breast cancer (39.6%) was the predominant disease in the cancer group.
As reported online October 7 in JAMA Dermatology, the adjusted rate of cellulitis diagnoses fell from 21.1% before APCD use to 4.5% afterward (p<0.001), a 79% decline. The noncancer group had a 75% decline, from 28.8% to 7.3% (p<0.001).
The noncancer group also had a 54% decline in adjusted rate of hospitalizations, from 7.0% to 3.2% (p=0.02), the authors reported.
Both groups had declines in receipt of manual therapy, from an adjusted rate of 35.6% before APCD use to 24.9% afterward for cancer patients (p<0.001) and from 32.3% to 21.2% for noncancer patients (p<0.001).
The adjusted rate of outpatient visits fell from 58.6% to 41.4% in the cancer cohort and from 52.6% to 31.4% in the noncancer group (p<0.001 for both).
Total costs per patient, excluding medical equipment, declined from $2597 to $1642 for cancer patients (p=0.002) and from $2937 to $1883 (p=0.007) for noncancer patients.
"While our findings are based upon the outcomes from one specific device, it is possible other such devices may also reduce patient burden. This warrants explorations in future studies. In addition, our study was not designed to assess the long term effectiveness of the device. That should be studied in future work," Dr. Karaca-Mandic explained.
Also, she pointed out, her team didn't look at nonmonetary expenses such as productivity loss and caretaker costs. "To the extent that device use improves physical functioning and lowers such costs as well, the impact is likely much larger than we can measure," she added.
Dr. Peter J. Franks, of the Center for Research and Implementation of Clinical Practice in London, UK, said by email, "We have these devices that appear to work. The problem is that the evidence on efficacy and cost effectiveness is so poor. The article gave some retrospective observational data that implied that the incidence of infection (cellulitis) was reduced. This is important, as infections lead to further deterioration of the lymphatic system, making the situation worse for the patient and increasing the risk of further infections."
"It is hard to say how generalizable the results are to other devices, though fundamentally they all work in similar ways," said Dr. Franks, who coauthored an accompanying editorial. "I think that this is an important step in how we consider the use of medical devices."
Cynthia Shechter, an occupational therapist in New York City who is a lymphedema specialist for cancer patients, said by email, "When looking for the right device, look for a pump that contains multiple chambers, operates on a short thirty-second cycle time, and applies graduated compression."
"The body operates on a pressure gradient system, so it is imperative to obtain a gradient or graduated compression pump. Pressure at the feet or hand is greater than the thigh or shoulder," she added.
"Clinicians practicing in the treatment of lymphedema need to be open-minded regarding less traditional treatment options for this insidious condition, including the use of traditional and advanced pneumatic compression devices," Shechter said.
"This study indicates that use of an APCD reduces the necessity for therapy. However, rehabilitation therapy for primary and secondary lymphedema, at least a short course of
treatment, is important, especially in order to ensure patients are adequately educated in lymphedema care, management, and precautions," she said.
"There should be a follow-up study performed to discuss a patient's ability to sustain use of the APCD versus a traditional pneumatic pump, and the long-term success in both preventing infection and in reduction of therapy visits," Shechter said.
Tactile Medical partially supported this research and employs one coauthor as chief medical officer. Dr. Karaca-Mandic, Dr. Franks, and his coauthor reported consulting for the company.
Endovascular thrombectomy vs tPA: better function, same mortality
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Endovascular mechanical thrombectomy yielded better function and angiographic revascularization but similar mortality and rate of intracranial hemorrhage compared with standard medical therapy (tPA) for acute ischemic stroke.
Major finding: Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8.
Data source: A meta-analysis of eight high-quality multicenter randomized clinical trials published during 2013-2015 involving 2,423 adults with acute ischemic stroke.
Disclosures: No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
ACOG plans consensus conference on uniform guidelines for breast cancer screening
The Susan G. Komen Foundation estimates that 84% of breast cancers are found through mammography.1 Clearly, the value of mammography is proven. But controversy and confusion abound on how much mammography, and beginning at what age, is best for women.
Currently, the United States Preventive Services Task Force (USPSTF), the American Cancer Society (ACS), and the American College of Obstetricians and Gynecologists (ACOG) all have differing recommendations about mammography and about the importance of clinical breast examinations. These inconsistencies largely are due to different interpretations of the same data, not the data itself, and tend to center on how harm is defined and measured. Importantly, these differences can wreak havoc on our patients’ confidence in our counsel and decision making, and can complicate women’s access to screening. Under the Affordable Care Act, women are guaranteed coverage of annual mammograms, but new USPSTF recommendations, due out soon, may undermine that guarantee.
On October 20, ACOG responded to the ACS’ new recommendations on breast cancer screening by emphasizing our continued advice that women should begin annual mammography screening at age 40, along with a clinical breast exam.2
Consensus conference plansIn an effort to address widespread confusion among patients, health care professionals, and payers, ACOG is convening a consensus conference in January 2016, with the goal of arriving at a consistent set of guidelines that can be agreed to, implemented clinically across the country, and hopefully adopted by insurers, as well. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail and to consider the available data in the broader context of patient care.
Without doubt, guidelines and recommendations will need to evolve as new evidence emerges, but our hope is that scientific and medical organizations can look at the same evidence and speak with one voice on what is best for women’s health. Our patients would benefit from that alone.
ACOG’s recommendations, summarized
- Clinical breast examination every year for women aged 19 and older.
- Screening mammography every year for women aged 40 and older.
- Breast self-awareness has the potential to detect palpable breast cancer and can be recommended.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Susan G. Komen Web site. Accuracy of mammograms. http://ww5.komen.org/BreastCancer/AccuracyofMammograms.html. Updated June 26, 2015. Accessed October 30, 2015.
- ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. American College of Obstetricians and Gynecologists Web site. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 30, 2015.
The Susan G. Komen Foundation estimates that 84% of breast cancers are found through mammography.1 Clearly, the value of mammography is proven. But controversy and confusion abound on how much mammography, and beginning at what age, is best for women.
Currently, the United States Preventive Services Task Force (USPSTF), the American Cancer Society (ACS), and the American College of Obstetricians and Gynecologists (ACOG) all have differing recommendations about mammography and about the importance of clinical breast examinations. These inconsistencies largely are due to different interpretations of the same data, not the data itself, and tend to center on how harm is defined and measured. Importantly, these differences can wreak havoc on our patients’ confidence in our counsel and decision making, and can complicate women’s access to screening. Under the Affordable Care Act, women are guaranteed coverage of annual mammograms, but new USPSTF recommendations, due out soon, may undermine that guarantee.
On October 20, ACOG responded to the ACS’ new recommendations on breast cancer screening by emphasizing our continued advice that women should begin annual mammography screening at age 40, along with a clinical breast exam.2
Consensus conference plansIn an effort to address widespread confusion among patients, health care professionals, and payers, ACOG is convening a consensus conference in January 2016, with the goal of arriving at a consistent set of guidelines that can be agreed to, implemented clinically across the country, and hopefully adopted by insurers, as well. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail and to consider the available data in the broader context of patient care.
Without doubt, guidelines and recommendations will need to evolve as new evidence emerges, but our hope is that scientific and medical organizations can look at the same evidence and speak with one voice on what is best for women’s health. Our patients would benefit from that alone.
ACOG’s recommendations, summarized
- Clinical breast examination every year for women aged 19 and older.
- Screening mammography every year for women aged 40 and older.
- Breast self-awareness has the potential to detect palpable breast cancer and can be recommended.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Susan G. Komen Foundation estimates that 84% of breast cancers are found through mammography.1 Clearly, the value of mammography is proven. But controversy and confusion abound on how much mammography, and beginning at what age, is best for women.
Currently, the United States Preventive Services Task Force (USPSTF), the American Cancer Society (ACS), and the American College of Obstetricians and Gynecologists (ACOG) all have differing recommendations about mammography and about the importance of clinical breast examinations. These inconsistencies largely are due to different interpretations of the same data, not the data itself, and tend to center on how harm is defined and measured. Importantly, these differences can wreak havoc on our patients’ confidence in our counsel and decision making, and can complicate women’s access to screening. Under the Affordable Care Act, women are guaranteed coverage of annual mammograms, but new USPSTF recommendations, due out soon, may undermine that guarantee.
On October 20, ACOG responded to the ACS’ new recommendations on breast cancer screening by emphasizing our continued advice that women should begin annual mammography screening at age 40, along with a clinical breast exam.2
Consensus conference plansIn an effort to address widespread confusion among patients, health care professionals, and payers, ACOG is convening a consensus conference in January 2016, with the goal of arriving at a consistent set of guidelines that can be agreed to, implemented clinically across the country, and hopefully adopted by insurers, as well. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail and to consider the available data in the broader context of patient care.
Without doubt, guidelines and recommendations will need to evolve as new evidence emerges, but our hope is that scientific and medical organizations can look at the same evidence and speak with one voice on what is best for women’s health. Our patients would benefit from that alone.
ACOG’s recommendations, summarized
- Clinical breast examination every year for women aged 19 and older.
- Screening mammography every year for women aged 40 and older.
- Breast self-awareness has the potential to detect palpable breast cancer and can be recommended.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Susan G. Komen Web site. Accuracy of mammograms. http://ww5.komen.org/BreastCancer/AccuracyofMammograms.html. Updated June 26, 2015. Accessed October 30, 2015.
- ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. American College of Obstetricians and Gynecologists Web site. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 30, 2015.
- Susan G. Komen Web site. Accuracy of mammograms. http://ww5.komen.org/BreastCancer/AccuracyofMammograms.html. Updated June 26, 2015. Accessed October 30, 2015.
- ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. American College of Obstetricians and Gynecologists Web site. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 30, 2015.
Post-ibrutinib management in MCL unclear, speaker says

NEW YORK—Despite an “unprecedented” single-agent response rate and progression-free survival (PFS) in previously treated mantle cell lymphoma (MCL) patients, those with multiple risk factors have a dismal outcome following ibrutinib failure.
So after ibrutinib, what’s next in MCL? That was the question asked at Lymphoma & Myeloma 2015.
Peter Martin, MD, of Weill Cornell Medical College in New York, New York, discussed some possibilities.
Ibrutinib (Imbruvica) was approved by the US Food and Drug Administration for MCL based on the PCYC-1104 trial, which showed an overall response rate of 68%. In the MCL2001 trial, the overall response rate was 63%.
The median PFS for MCL was 13 months in PCYC-1104 and 10.5 months in MCL2001. The median overall survival (OS) was close to 2 years in PCYC-1104 and 18 months in MCL2001.
“So this is where I think it starts to get interesting,” Dr Martin said. “People were able to live for several months after progressing on ibrutinib. [However,] our experience at Cornell was not necessarily consistent with that.”
Cornell investigators, along with colleagues from Ohio State University, compiled data on patients who had been treated in clinical trials at their institutions and reviewed their survival after progression on ibrutinib. These patients had a median OS of 4 months.
In reviewing the patients’ Mantle Cell Lymphoma International Prognostic Index (MIPI) scores, Dr Martin said they were arguably a higher-risk population.
Dr Martin collected data on 114 relapsed/refractory MCL patients from centers around the world and found they had a lower response rate (50%) with ibrutinib overall and a lower duration of ibrutinib therapy (4.7 months).
The median OS after stopping ibrutinib was 2.9 months for the entire group. For patients who did not receive any subsequent therapy after failure, it was 0.8 months. Patients who received treatment after ibrutinib failure had a median OS of 5.8 months.
“And it didn’t seem to matter what we gave,” Dr Martin said. “Those treatments were pretty short-lived.”
The median time to next treatment with the first subsequent therapy was 2.4 months. These therapies included bendamustine, cytarabine, and lenalidomide.
“There was no statistical association between survival and choice of therapy,” Dr Martin said.
What was significant, by univariate Cox regression analysis, was the patients’ MIPI prior to ibrutinib therapy (P=0.0002) and the duration of ibrutinib treatment (P=0.0465).
“So at this point in time, I think it’s fair to say that there is insufficient data to recommend any specific treatment following ibrutinib failure,” Dr Martin said.
However, he did make a few suggestions for treating high-risk patients.
Treatment suggestions after ibrutinib failure
Dr Martin’s first suggestion is to focus on symptom management rather than active therapy for the older, frailer patients. Second, consider allogeneic stem cell transplant in any high-risk patient responding to ibrutinib.
Third, consider continuing ibrutinib therapy while starting the next therapy. And fourth, consider some form of continuous therapy that does not depend on TP53.
Dr Martin admitted that what to do following ibrutinib failure remains cloudy.
“Conducting a clinical trial will be tricky,” he said, “because the median time from ibrutinib failure to the next therapy was 9 days, and we’re targeting a very high-risk patient population.”
In addition, on average 80% have expression of Ki67.
Currently, a phase 2 trial of copanlisib (NCT02455297) is the only post-ibrutinib clinical trial in MCL open. Copanlisib is a potent and reversible phosphatidylinositol-3-kinase (PI3K) inhibitor with activity against both alpha and delta isoforms of PI3K. Preliminary results of the trial demonstrated response in 5 of 7 MCL patients.
So perhaps the best approach, Dr Martin suggested, would be to improve response and prevent relapse while on ibrutinib using combination therapies.
A phase 1/1b trial of ibrutinib with bendamustine and rituximab (BR) is underway. Of 17 MCL patients treated thus far, 94% have responded, and 76% have achieved a CR. But 25% developed grade 3 rash.
Ibrutinib is also being studied in combination with rituximab in MCL. The combination has produced an overall response rate of 88%, with 40% of patients achieving a CR.
“My interpretation from all these studies is you can probably add ibrutinib to any other effective anti-MCL therapy and improve that therapy,” Dr Martin said. “But there are questions, obviously, that still arise.”
Overcoming ibrutinib resistance
Dr Martin explained that, to use combinations rationally, we need to understand mechanisms of ibrutinib resistance, “and that’s not so straightforward.”
Mutations in MCL likely have multiple mechanisms of resistance. Mutations occur predominantly in 3 groups of genes involving NF-kB, PIM/mTOR, and epigenetic modifiers.
A number of trials are underway to hit some of these pathways, Dr Martin said.
Researchers at Cornell are studying ibrutinib plus palbociclib, an inhibitor of CDK4/CDK6 approved for advanced breast cancer, in a phase 1 trial of MCL patients.
The combination “very early on, has seen a high number of complete responses, which have been exciting,” Dr Martin said.
There are many ongoing ibrutinib trials in previously treated patients, including ones with carfilzomib, palbociclib, bortezomib, venetoclax, lenalidomide, and TGR-1202. In addition, the frontline trial of BR +/- ibrutinib is expected to have results soon.
“[A]nd once that happens, my guess is that this frontline trial, once it’s read out, essentially, makes all these other trials irrelevant because the minute ibrutinib moves into the frontline setting, it makes it very difficult to evaluate in a subsequent setting,” Dr Martin said. “So within a couple of years, it will be standard in the frontline setting.”
Dr Martin is concerned that resources are insufficient—there are too many studies, too few patients, and too little time—to find another, potentially more effective agent or combination.
He said there won’t be a one-size-fits-all approach to MCL either before or after ibrutinib, and collaboration among institutions, companies, and cooperative groups will be needed.
“Management in the post-ibrutinib setting remains unclear,” he said, “and these patients should be treated in a clinical trial if possible.” ![]()

NEW YORK—Despite an “unprecedented” single-agent response rate and progression-free survival (PFS) in previously treated mantle cell lymphoma (MCL) patients, those with multiple risk factors have a dismal outcome following ibrutinib failure.
So after ibrutinib, what’s next in MCL? That was the question asked at Lymphoma & Myeloma 2015.
Peter Martin, MD, of Weill Cornell Medical College in New York, New York, discussed some possibilities.
Ibrutinib (Imbruvica) was approved by the US Food and Drug Administration for MCL based on the PCYC-1104 trial, which showed an overall response rate of 68%. In the MCL2001 trial, the overall response rate was 63%.
The median PFS for MCL was 13 months in PCYC-1104 and 10.5 months in MCL2001. The median overall survival (OS) was close to 2 years in PCYC-1104 and 18 months in MCL2001.
“So this is where I think it starts to get interesting,” Dr Martin said. “People were able to live for several months after progressing on ibrutinib. [However,] our experience at Cornell was not necessarily consistent with that.”
Cornell investigators, along with colleagues from Ohio State University, compiled data on patients who had been treated in clinical trials at their institutions and reviewed their survival after progression on ibrutinib. These patients had a median OS of 4 months.
In reviewing the patients’ Mantle Cell Lymphoma International Prognostic Index (MIPI) scores, Dr Martin said they were arguably a higher-risk population.
Dr Martin collected data on 114 relapsed/refractory MCL patients from centers around the world and found they had a lower response rate (50%) with ibrutinib overall and a lower duration of ibrutinib therapy (4.7 months).
The median OS after stopping ibrutinib was 2.9 months for the entire group. For patients who did not receive any subsequent therapy after failure, it was 0.8 months. Patients who received treatment after ibrutinib failure had a median OS of 5.8 months.
“And it didn’t seem to matter what we gave,” Dr Martin said. “Those treatments were pretty short-lived.”
The median time to next treatment with the first subsequent therapy was 2.4 months. These therapies included bendamustine, cytarabine, and lenalidomide.
“There was no statistical association between survival and choice of therapy,” Dr Martin said.
What was significant, by univariate Cox regression analysis, was the patients’ MIPI prior to ibrutinib therapy (P=0.0002) and the duration of ibrutinib treatment (P=0.0465).
“So at this point in time, I think it’s fair to say that there is insufficient data to recommend any specific treatment following ibrutinib failure,” Dr Martin said.
However, he did make a few suggestions for treating high-risk patients.
Treatment suggestions after ibrutinib failure
Dr Martin’s first suggestion is to focus on symptom management rather than active therapy for the older, frailer patients. Second, consider allogeneic stem cell transplant in any high-risk patient responding to ibrutinib.
Third, consider continuing ibrutinib therapy while starting the next therapy. And fourth, consider some form of continuous therapy that does not depend on TP53.
Dr Martin admitted that what to do following ibrutinib failure remains cloudy.
“Conducting a clinical trial will be tricky,” he said, “because the median time from ibrutinib failure to the next therapy was 9 days, and we’re targeting a very high-risk patient population.”
In addition, on average 80% have expression of Ki67.
Currently, a phase 2 trial of copanlisib (NCT02455297) is the only post-ibrutinib clinical trial in MCL open. Copanlisib is a potent and reversible phosphatidylinositol-3-kinase (PI3K) inhibitor with activity against both alpha and delta isoforms of PI3K. Preliminary results of the trial demonstrated response in 5 of 7 MCL patients.
So perhaps the best approach, Dr Martin suggested, would be to improve response and prevent relapse while on ibrutinib using combination therapies.
A phase 1/1b trial of ibrutinib with bendamustine and rituximab (BR) is underway. Of 17 MCL patients treated thus far, 94% have responded, and 76% have achieved a CR. But 25% developed grade 3 rash.
Ibrutinib is also being studied in combination with rituximab in MCL. The combination has produced an overall response rate of 88%, with 40% of patients achieving a CR.
“My interpretation from all these studies is you can probably add ibrutinib to any other effective anti-MCL therapy and improve that therapy,” Dr Martin said. “But there are questions, obviously, that still arise.”
Overcoming ibrutinib resistance
Dr Martin explained that, to use combinations rationally, we need to understand mechanisms of ibrutinib resistance, “and that’s not so straightforward.”
Mutations in MCL likely have multiple mechanisms of resistance. Mutations occur predominantly in 3 groups of genes involving NF-kB, PIM/mTOR, and epigenetic modifiers.
A number of trials are underway to hit some of these pathways, Dr Martin said.
Researchers at Cornell are studying ibrutinib plus palbociclib, an inhibitor of CDK4/CDK6 approved for advanced breast cancer, in a phase 1 trial of MCL patients.
The combination “very early on, has seen a high number of complete responses, which have been exciting,” Dr Martin said.
There are many ongoing ibrutinib trials in previously treated patients, including ones with carfilzomib, palbociclib, bortezomib, venetoclax, lenalidomide, and TGR-1202. In addition, the frontline trial of BR +/- ibrutinib is expected to have results soon.
“[A]nd once that happens, my guess is that this frontline trial, once it’s read out, essentially, makes all these other trials irrelevant because the minute ibrutinib moves into the frontline setting, it makes it very difficult to evaluate in a subsequent setting,” Dr Martin said. “So within a couple of years, it will be standard in the frontline setting.”
Dr Martin is concerned that resources are insufficient—there are too many studies, too few patients, and too little time—to find another, potentially more effective agent or combination.
He said there won’t be a one-size-fits-all approach to MCL either before or after ibrutinib, and collaboration among institutions, companies, and cooperative groups will be needed.
“Management in the post-ibrutinib setting remains unclear,” he said, “and these patients should be treated in a clinical trial if possible.” ![]()

NEW YORK—Despite an “unprecedented” single-agent response rate and progression-free survival (PFS) in previously treated mantle cell lymphoma (MCL) patients, those with multiple risk factors have a dismal outcome following ibrutinib failure.
So after ibrutinib, what’s next in MCL? That was the question asked at Lymphoma & Myeloma 2015.
Peter Martin, MD, of Weill Cornell Medical College in New York, New York, discussed some possibilities.
Ibrutinib (Imbruvica) was approved by the US Food and Drug Administration for MCL based on the PCYC-1104 trial, which showed an overall response rate of 68%. In the MCL2001 trial, the overall response rate was 63%.
The median PFS for MCL was 13 months in PCYC-1104 and 10.5 months in MCL2001. The median overall survival (OS) was close to 2 years in PCYC-1104 and 18 months in MCL2001.
“So this is where I think it starts to get interesting,” Dr Martin said. “People were able to live for several months after progressing on ibrutinib. [However,] our experience at Cornell was not necessarily consistent with that.”
Cornell investigators, along with colleagues from Ohio State University, compiled data on patients who had been treated in clinical trials at their institutions and reviewed their survival after progression on ibrutinib. These patients had a median OS of 4 months.
In reviewing the patients’ Mantle Cell Lymphoma International Prognostic Index (MIPI) scores, Dr Martin said they were arguably a higher-risk population.
Dr Martin collected data on 114 relapsed/refractory MCL patients from centers around the world and found they had a lower response rate (50%) with ibrutinib overall and a lower duration of ibrutinib therapy (4.7 months).
The median OS after stopping ibrutinib was 2.9 months for the entire group. For patients who did not receive any subsequent therapy after failure, it was 0.8 months. Patients who received treatment after ibrutinib failure had a median OS of 5.8 months.
“And it didn’t seem to matter what we gave,” Dr Martin said. “Those treatments were pretty short-lived.”
The median time to next treatment with the first subsequent therapy was 2.4 months. These therapies included bendamustine, cytarabine, and lenalidomide.
“There was no statistical association between survival and choice of therapy,” Dr Martin said.
What was significant, by univariate Cox regression analysis, was the patients’ MIPI prior to ibrutinib therapy (P=0.0002) and the duration of ibrutinib treatment (P=0.0465).
“So at this point in time, I think it’s fair to say that there is insufficient data to recommend any specific treatment following ibrutinib failure,” Dr Martin said.
However, he did make a few suggestions for treating high-risk patients.
Treatment suggestions after ibrutinib failure
Dr Martin’s first suggestion is to focus on symptom management rather than active therapy for the older, frailer patients. Second, consider allogeneic stem cell transplant in any high-risk patient responding to ibrutinib.
Third, consider continuing ibrutinib therapy while starting the next therapy. And fourth, consider some form of continuous therapy that does not depend on TP53.
Dr Martin admitted that what to do following ibrutinib failure remains cloudy.
“Conducting a clinical trial will be tricky,” he said, “because the median time from ibrutinib failure to the next therapy was 9 days, and we’re targeting a very high-risk patient population.”
In addition, on average 80% have expression of Ki67.
Currently, a phase 2 trial of copanlisib (NCT02455297) is the only post-ibrutinib clinical trial in MCL open. Copanlisib is a potent and reversible phosphatidylinositol-3-kinase (PI3K) inhibitor with activity against both alpha and delta isoforms of PI3K. Preliminary results of the trial demonstrated response in 5 of 7 MCL patients.
So perhaps the best approach, Dr Martin suggested, would be to improve response and prevent relapse while on ibrutinib using combination therapies.
A phase 1/1b trial of ibrutinib with bendamustine and rituximab (BR) is underway. Of 17 MCL patients treated thus far, 94% have responded, and 76% have achieved a CR. But 25% developed grade 3 rash.
Ibrutinib is also being studied in combination with rituximab in MCL. The combination has produced an overall response rate of 88%, with 40% of patients achieving a CR.
“My interpretation from all these studies is you can probably add ibrutinib to any other effective anti-MCL therapy and improve that therapy,” Dr Martin said. “But there are questions, obviously, that still arise.”
Overcoming ibrutinib resistance
Dr Martin explained that, to use combinations rationally, we need to understand mechanisms of ibrutinib resistance, “and that’s not so straightforward.”
Mutations in MCL likely have multiple mechanisms of resistance. Mutations occur predominantly in 3 groups of genes involving NF-kB, PIM/mTOR, and epigenetic modifiers.
A number of trials are underway to hit some of these pathways, Dr Martin said.
Researchers at Cornell are studying ibrutinib plus palbociclib, an inhibitor of CDK4/CDK6 approved for advanced breast cancer, in a phase 1 trial of MCL patients.
The combination “very early on, has seen a high number of complete responses, which have been exciting,” Dr Martin said.
There are many ongoing ibrutinib trials in previously treated patients, including ones with carfilzomib, palbociclib, bortezomib, venetoclax, lenalidomide, and TGR-1202. In addition, the frontline trial of BR +/- ibrutinib is expected to have results soon.
“[A]nd once that happens, my guess is that this frontline trial, once it’s read out, essentially, makes all these other trials irrelevant because the minute ibrutinib moves into the frontline setting, it makes it very difficult to evaluate in a subsequent setting,” Dr Martin said. “So within a couple of years, it will be standard in the frontline setting.”
Dr Martin is concerned that resources are insufficient—there are too many studies, too few patients, and too little time—to find another, potentially more effective agent or combination.
He said there won’t be a one-size-fits-all approach to MCL either before or after ibrutinib, and collaboration among institutions, companies, and cooperative groups will be needed.
“Management in the post-ibrutinib setting remains unclear,” he said, “and these patients should be treated in a clinical trial if possible.” ![]()
Interventions can treat, prevent iron deficiency in blood donors

ANAHEIM, CA—Data from the STRIDE study have revealed interventions that can mitigate iron deficiency in repeat blood donors.
The study showed that providing repeat blood donors with iron supplements significantly improved their iron status.
But informing donors about their ferritin levels and recommending they take iron pills also significantly improved their iron status.
Meanwhile, patients in control groups became more iron-deficient over the study period.
The study also revealed no difference in ferritin or hemoglobin levels between patients who took 19 mg of iron and those who took 38 mg.
Alan E. Mast, MD, PhD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the 2015 AABB Annual Meeting (abstract S34-030E).
Dr Mast said blood donation removes a lot of iron, and iron is used to make hemoglobin in new red blood cells. But the measurement of hemoglobin does not accurately reflect iron stores.
“That’s really important,” he said. “The only test we do to qualify a blood donor doesn’t tell us if they have iron deficiency or not. And because of that, many regular blood donors become iron-deficient and continue to donate blood.”
Dr Mast said the strategies that appear to mitigate iron deficiency in regular blood donors are oral iron supplements and delaying the donation interval for more than 6 months.
“[However,] the effectiveness of providing iron pills versus providing the donor with information about their iron status has not been previously examined,” he noted.
This was the goal of the STRIDE (Strategies to Reduce Iron Deficiency) study.
Study design
This blinded, randomized, placebo-controlled study enrolled 692 frequent blood donors from 3 blood centers. They were assigned to 1 of 5 arms for 2 years of follow-up.
In 3 arms, donors received pills for 60 days after each donation. They received 38 mg or 19 mg of elemental iron, or they received a placebo.
Donors in the remaining 2 arms received letters after each donation—either a letter informing them of their iron status or a “control” letter thanking them for donating blood and urging them to donate again.
Every iron status letter reported the donor’s ferritin level. If the level was >26 mg/L, the letter simply urged donors to donate again. If the ferritin level was ≤26 mg/L, the letter recommended taking a self-purchased iron supplement (17 mg to 38 mg) and/or delaying donation for 6 months. Donors were allowed to choose either option, both, or neither.
The researchers measured ferritin, soluble transferrin receptor, and complete blood count at each donation.
Study completion
Of the 692 subjects randomized, 393 completed a final visit. The researchers noted that a donor’s ferritin level at enrollment, race, or gender did not impact study completion. However, older subjects were more likely to complete the study.
In all, 116 subjects were lost to follow-up, and the numbers were similar between the study arms. Thirty-nine subjects discontinued due to adverse events—16 in the 38 mg iron group, 12 in the 19 mg iron group, and 11 in the placebo group.
And 144 subjects discontinued for “other reasons”—9 in the iron status letter arm, 10 in the control letter arm, 30 in the 38 mg iron arm, 42 in the 19 mg iron arm, and 53 in the placebo arm.
Subjects’ reasons for discontinuation included not wanting to take a pill every day, believing they are in the placebo group and wanting to take iron, and subjects’ physicians recommending they start taking iron.
“Donors in pill arms de-enrolled more frequently than those in the letter arms, and the important thing to remember is that this is a controlled, randomized study where the donors did not know what they were taking,” Dr Mast said. “And I think that, a lot of the time, if donors had known what they were taking, they might have continued to participate in the study or continued to take the pills.”
Results
Dr Mast noted that, at the study’s end, all measures of iron deficiency were statistically indistinguishable in the 3 intervention arms, which were statistically different from the 2 control arms.
Between study enrollment and the donors’ final visit, the prevalence of ferritin <26 mg/L was unchanged in the control groups. But it had declined by more than 50% in the 3 intervention groups—19 mg iron, 38 mg iron, and iron status letter (P<0.0001 for all 3).
The prevalence of ferritin <12 mg/L was unchanged in the 2 control arms, but it had declined by more than 70% in the 3 intervention arms—19 mg iron (P<0.0001), 38 mg iron (P<0.01), and iron status letter (P<0.0001).
The researchers also calculated the odds ratios for iron deficiency over all donor visits. The odds for ferritin <26 or <12 mg/L decreased more than 80% in the 19 mg iron group (P<0.01 for both ferritin measurements) and the 38 mg iron group (P<0.01 for both).
The odds for ferritin <26 or <12 mg/L decreased about 50% in the iron status letter arm (P<0.01 for both).
And the odds for ferritin <12 mg/L increased about 50% in the control groups (P<0.01 for both the placebo and control letter groups). However, there was no significant difference for ferritin <26 mg/L in either control group.
Lastly, the researchers performed longitudinal modeling of hemoglobin. They found that hemoglobin increased >0.03 g/dL in the 19 mg and 38 mg iron arms (P<0.01 for both), decreasing the odds for low hemoglobin deferral about 50%.
Hemoglobin decreased >0.3 g/dL in the control groups (P<0.0001 for both the placebo and control letter groups), increasing the odds for low hemoglobin deferral about 70%.
“Interestingly, [being] in the iron status letter group did not affect hemoglobin that much in the longitudinal modeling of the donors,” Dr Mast noted.
In closing, he pointed out that the 19 mg and 38 mg iron pills were equally effective for mitigating iron deficiency and improving hemoglobin in these blood donors.
“From a physiology point of view, I think this is one of the most important results of this study,” Dr Mast said. “There’s absolutely no difference. There was no trend for 38 mg to be better than 19 in any analysis that we did.”
“There’s lots of reasons that could be happening, but I think it’s scientifically interesting and operationally interesting. And it’s important because we can tell donors—ask them to take a multivitamin with 19 mg of iron, and that will be sufficient to treat iron deficiency.” ![]()

ANAHEIM, CA—Data from the STRIDE study have revealed interventions that can mitigate iron deficiency in repeat blood donors.
The study showed that providing repeat blood donors with iron supplements significantly improved their iron status.
But informing donors about their ferritin levels and recommending they take iron pills also significantly improved their iron status.
Meanwhile, patients in control groups became more iron-deficient over the study period.
The study also revealed no difference in ferritin or hemoglobin levels between patients who took 19 mg of iron and those who took 38 mg.
Alan E. Mast, MD, PhD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the 2015 AABB Annual Meeting (abstract S34-030E).
Dr Mast said blood donation removes a lot of iron, and iron is used to make hemoglobin in new red blood cells. But the measurement of hemoglobin does not accurately reflect iron stores.
“That’s really important,” he said. “The only test we do to qualify a blood donor doesn’t tell us if they have iron deficiency or not. And because of that, many regular blood donors become iron-deficient and continue to donate blood.”
Dr Mast said the strategies that appear to mitigate iron deficiency in regular blood donors are oral iron supplements and delaying the donation interval for more than 6 months.
“[However,] the effectiveness of providing iron pills versus providing the donor with information about their iron status has not been previously examined,” he noted.
This was the goal of the STRIDE (Strategies to Reduce Iron Deficiency) study.
Study design
This blinded, randomized, placebo-controlled study enrolled 692 frequent blood donors from 3 blood centers. They were assigned to 1 of 5 arms for 2 years of follow-up.
In 3 arms, donors received pills for 60 days after each donation. They received 38 mg or 19 mg of elemental iron, or they received a placebo.
Donors in the remaining 2 arms received letters after each donation—either a letter informing them of their iron status or a “control” letter thanking them for donating blood and urging them to donate again.
Every iron status letter reported the donor’s ferritin level. If the level was >26 mg/L, the letter simply urged donors to donate again. If the ferritin level was ≤26 mg/L, the letter recommended taking a self-purchased iron supplement (17 mg to 38 mg) and/or delaying donation for 6 months. Donors were allowed to choose either option, both, or neither.
The researchers measured ferritin, soluble transferrin receptor, and complete blood count at each donation.
Study completion
Of the 692 subjects randomized, 393 completed a final visit. The researchers noted that a donor’s ferritin level at enrollment, race, or gender did not impact study completion. However, older subjects were more likely to complete the study.
In all, 116 subjects were lost to follow-up, and the numbers were similar between the study arms. Thirty-nine subjects discontinued due to adverse events—16 in the 38 mg iron group, 12 in the 19 mg iron group, and 11 in the placebo group.
And 144 subjects discontinued for “other reasons”—9 in the iron status letter arm, 10 in the control letter arm, 30 in the 38 mg iron arm, 42 in the 19 mg iron arm, and 53 in the placebo arm.
Subjects’ reasons for discontinuation included not wanting to take a pill every day, believing they are in the placebo group and wanting to take iron, and subjects’ physicians recommending they start taking iron.
“Donors in pill arms de-enrolled more frequently than those in the letter arms, and the important thing to remember is that this is a controlled, randomized study where the donors did not know what they were taking,” Dr Mast said. “And I think that, a lot of the time, if donors had known what they were taking, they might have continued to participate in the study or continued to take the pills.”
Results
Dr Mast noted that, at the study’s end, all measures of iron deficiency were statistically indistinguishable in the 3 intervention arms, which were statistically different from the 2 control arms.
Between study enrollment and the donors’ final visit, the prevalence of ferritin <26 mg/L was unchanged in the control groups. But it had declined by more than 50% in the 3 intervention groups—19 mg iron, 38 mg iron, and iron status letter (P<0.0001 for all 3).
The prevalence of ferritin <12 mg/L was unchanged in the 2 control arms, but it had declined by more than 70% in the 3 intervention arms—19 mg iron (P<0.0001), 38 mg iron (P<0.01), and iron status letter (P<0.0001).
The researchers also calculated the odds ratios for iron deficiency over all donor visits. The odds for ferritin <26 or <12 mg/L decreased more than 80% in the 19 mg iron group (P<0.01 for both ferritin measurements) and the 38 mg iron group (P<0.01 for both).
The odds for ferritin <26 or <12 mg/L decreased about 50% in the iron status letter arm (P<0.01 for both).
And the odds for ferritin <12 mg/L increased about 50% in the control groups (P<0.01 for both the placebo and control letter groups). However, there was no significant difference for ferritin <26 mg/L in either control group.
Lastly, the researchers performed longitudinal modeling of hemoglobin. They found that hemoglobin increased >0.03 g/dL in the 19 mg and 38 mg iron arms (P<0.01 for both), decreasing the odds for low hemoglobin deferral about 50%.
Hemoglobin decreased >0.3 g/dL in the control groups (P<0.0001 for both the placebo and control letter groups), increasing the odds for low hemoglobin deferral about 70%.
“Interestingly, [being] in the iron status letter group did not affect hemoglobin that much in the longitudinal modeling of the donors,” Dr Mast noted.
In closing, he pointed out that the 19 mg and 38 mg iron pills were equally effective for mitigating iron deficiency and improving hemoglobin in these blood donors.
“From a physiology point of view, I think this is one of the most important results of this study,” Dr Mast said. “There’s absolutely no difference. There was no trend for 38 mg to be better than 19 in any analysis that we did.”
“There’s lots of reasons that could be happening, but I think it’s scientifically interesting and operationally interesting. And it’s important because we can tell donors—ask them to take a multivitamin with 19 mg of iron, and that will be sufficient to treat iron deficiency.” ![]()

ANAHEIM, CA—Data from the STRIDE study have revealed interventions that can mitigate iron deficiency in repeat blood donors.
The study showed that providing repeat blood donors with iron supplements significantly improved their iron status.
But informing donors about their ferritin levels and recommending they take iron pills also significantly improved their iron status.
Meanwhile, patients in control groups became more iron-deficient over the study period.
The study also revealed no difference in ferritin or hemoglobin levels between patients who took 19 mg of iron and those who took 38 mg.
Alan E. Mast, MD, PhD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the 2015 AABB Annual Meeting (abstract S34-030E).
Dr Mast said blood donation removes a lot of iron, and iron is used to make hemoglobin in new red blood cells. But the measurement of hemoglobin does not accurately reflect iron stores.
“That’s really important,” he said. “The only test we do to qualify a blood donor doesn’t tell us if they have iron deficiency or not. And because of that, many regular blood donors become iron-deficient and continue to donate blood.”
Dr Mast said the strategies that appear to mitigate iron deficiency in regular blood donors are oral iron supplements and delaying the donation interval for more than 6 months.
“[However,] the effectiveness of providing iron pills versus providing the donor with information about their iron status has not been previously examined,” he noted.
This was the goal of the STRIDE (Strategies to Reduce Iron Deficiency) study.
Study design
This blinded, randomized, placebo-controlled study enrolled 692 frequent blood donors from 3 blood centers. They were assigned to 1 of 5 arms for 2 years of follow-up.
In 3 arms, donors received pills for 60 days after each donation. They received 38 mg or 19 mg of elemental iron, or they received a placebo.
Donors in the remaining 2 arms received letters after each donation—either a letter informing them of their iron status or a “control” letter thanking them for donating blood and urging them to donate again.
Every iron status letter reported the donor’s ferritin level. If the level was >26 mg/L, the letter simply urged donors to donate again. If the ferritin level was ≤26 mg/L, the letter recommended taking a self-purchased iron supplement (17 mg to 38 mg) and/or delaying donation for 6 months. Donors were allowed to choose either option, both, or neither.
The researchers measured ferritin, soluble transferrin receptor, and complete blood count at each donation.
Study completion
Of the 692 subjects randomized, 393 completed a final visit. The researchers noted that a donor’s ferritin level at enrollment, race, or gender did not impact study completion. However, older subjects were more likely to complete the study.
In all, 116 subjects were lost to follow-up, and the numbers were similar between the study arms. Thirty-nine subjects discontinued due to adverse events—16 in the 38 mg iron group, 12 in the 19 mg iron group, and 11 in the placebo group.
And 144 subjects discontinued for “other reasons”—9 in the iron status letter arm, 10 in the control letter arm, 30 in the 38 mg iron arm, 42 in the 19 mg iron arm, and 53 in the placebo arm.
Subjects’ reasons for discontinuation included not wanting to take a pill every day, believing they are in the placebo group and wanting to take iron, and subjects’ physicians recommending they start taking iron.
“Donors in pill arms de-enrolled more frequently than those in the letter arms, and the important thing to remember is that this is a controlled, randomized study where the donors did not know what they were taking,” Dr Mast said. “And I think that, a lot of the time, if donors had known what they were taking, they might have continued to participate in the study or continued to take the pills.”
Results
Dr Mast noted that, at the study’s end, all measures of iron deficiency were statistically indistinguishable in the 3 intervention arms, which were statistically different from the 2 control arms.
Between study enrollment and the donors’ final visit, the prevalence of ferritin <26 mg/L was unchanged in the control groups. But it had declined by more than 50% in the 3 intervention groups—19 mg iron, 38 mg iron, and iron status letter (P<0.0001 for all 3).
The prevalence of ferritin <12 mg/L was unchanged in the 2 control arms, but it had declined by more than 70% in the 3 intervention arms—19 mg iron (P<0.0001), 38 mg iron (P<0.01), and iron status letter (P<0.0001).
The researchers also calculated the odds ratios for iron deficiency over all donor visits. The odds for ferritin <26 or <12 mg/L decreased more than 80% in the 19 mg iron group (P<0.01 for both ferritin measurements) and the 38 mg iron group (P<0.01 for both).
The odds for ferritin <26 or <12 mg/L decreased about 50% in the iron status letter arm (P<0.01 for both).
And the odds for ferritin <12 mg/L increased about 50% in the control groups (P<0.01 for both the placebo and control letter groups). However, there was no significant difference for ferritin <26 mg/L in either control group.
Lastly, the researchers performed longitudinal modeling of hemoglobin. They found that hemoglobin increased >0.03 g/dL in the 19 mg and 38 mg iron arms (P<0.01 for both), decreasing the odds for low hemoglobin deferral about 50%.
Hemoglobin decreased >0.3 g/dL in the control groups (P<0.0001 for both the placebo and control letter groups), increasing the odds for low hemoglobin deferral about 70%.
“Interestingly, [being] in the iron status letter group did not affect hemoglobin that much in the longitudinal modeling of the donors,” Dr Mast noted.
In closing, he pointed out that the 19 mg and 38 mg iron pills were equally effective for mitigating iron deficiency and improving hemoglobin in these blood donors.
“From a physiology point of view, I think this is one of the most important results of this study,” Dr Mast said. “There’s absolutely no difference. There was no trend for 38 mg to be better than 19 in any analysis that we did.”
“There’s lots of reasons that could be happening, but I think it’s scientifically interesting and operationally interesting. And it’s important because we can tell donors—ask them to take a multivitamin with 19 mg of iron, and that will be sufficient to treat iron deficiency.” ![]()