User login
In reply: Blood pressure targets
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
Interpreting SPRINT: How low should you go?
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
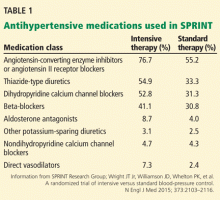
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
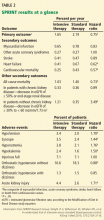
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
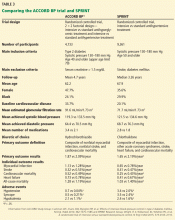
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
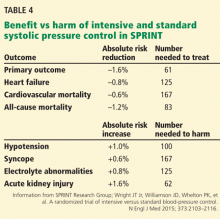
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
KEY POINTS
- SPRINT is the first large prospective randomized trial to show evidence of cardiovascular and mortality benefit for intensive lowering of systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk, but without a history of diabetes mellitus or stroke.
- A similar trial in patients with type 2 diabetes mellitus did not show significant benefit of intensive treatment.
- Intensive treatment was associated with more adverse events, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury.
- It is unclear if these results can be extrapolated to patients with a history of diabetes or stroke, younger patients, or those with low cardiovascular risk.
- Healthcare providers should engage patients in a shared decision-making process, with discussion of the benefits and risks associated with intensive lowering of blood pressure.
Should all patients with significant proteinuria take a renin-angiotensin inhibitor?
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
New hypertension guidelines: One size fits most?
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
KEY POINTS
- JNC 8 focuses on three main questions: when to begin treatment, how low to aim for, and which antihypertensive medications to use. It does not cover many topics that were included in JNC 7.
- In patients age 60 or older, JNC 8 recommends starting antihypertensive treatment if the blood pressure is 150/90 mm Hg or higher, with a goal of less than 150/90.
- For everyone else, including people with diabetes or chronic kidney disease, the threshold is 140/90 mm Hg, and the goal is less than 140/90.
- The recommended classes of drugs for initial therapy in nonblack patients without chronic kidney disease are thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs), although the last two classes should not be used in combination.
- For black patients, the initial classes of drugs are diuretics and calcium channel blockers; patients with chronic kidney disease should receive an ACE inhibitor or ARB.
Albuminuria: When urine predicts kidney and cardiovascular disease
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
KEY POINTS
- Albuminuria is best measured by the albumin-to-creatinine ratio.
- In several studies, albuminuria has been independently associated with a higher risk of death, cardiovascular events, heart failure, stroke, and progression of chronic kidney disease.
- Despite strong evidence linking albuminuria to adverse outcomes, evidence is limited in favor of routinely screening for it in the general population.
- Evaluating and managing albuminuria require understanding the limits of its clinical measures, controlling other risk factors for progression of renal disease, managing it medically, and referring to a specialist in certain situations.
Finding the cause of acute kidney injury: Which index of fractional excretion is better?
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
KEY POINTS
- Finding the cause of acute kidney injury is important, as management strategies differ.
- Although cutoff values differ among studies, in a patient with acute kidney injury, an FENa lower than 1% suggests a prerenal cause, whereas a value higher than 3% suggests an intrinsic cause.
- Similarly, an FEU less than 35% suggests a prerenal cause of acute kidney injury, whereas a value higher than 50% suggests an intrinsic one.
- The FENa can be falsely high in patients taking a diuretic; it can be falsely low in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis.