User login
Esophageal perforation severity scoring system reliably stratifies patients
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Scoring system reliably stratifies patients into low-, intermediate-, and high-risk groups.
Major finding: Patients with a high perforation severity score were 3.37 times more likely to have operative management, compared with low-scoring patients.
Data source: A retrospective study was performed on 288 patients with esophageal perforation at 11 centers since 1990.
Disclosures: The authors presented no relevant disclosures.
Midlife interventions help preserve later cognitive function
Midlife interventions including physical activity, hypertension control, and maintaining healthy HDL cholesterol may ward off later cognitive decline, reported Cassandra Szoeke, Ph.D., of the Centre for Medical Research at the University of Melbourne, and her colleagues.
In a study of 387 patients from the Women’s Healthy Ageing Project, investigators collected neuropsychiatric assessments, clinical data, and biomarkers at baseline, with at least three follow-up visits and one cognitive reassessment over 20 years. Dr. Szoeke and her colleagues used mixed linear models to analyze the significance of risk factors on verbal memory. The mean age of participants was 49.6 years.
Results showed that cumulative mid- to late-life physical activity had the strongest effect on better verbal memory later in life, the authors said in the report. The next most likely contributors were the negative effect of cumulative hypertension and the beneficial effect of HDL cholesterol.
The findings indicate that “physical activity, hypertension control, and achieving optimal levels of HDL cholesterol will help maintain later-life verbal memory skills,” Dr. Szoeke and her colleagues wrote.
Read the full report in the American Journal of Geriatric Psychiatry: http://www.ajgponline.org/article/S1064-7481%2816%2930113-0/fulltext
Midlife interventions including physical activity, hypertension control, and maintaining healthy HDL cholesterol may ward off later cognitive decline, reported Cassandra Szoeke, Ph.D., of the Centre for Medical Research at the University of Melbourne, and her colleagues.
In a study of 387 patients from the Women’s Healthy Ageing Project, investigators collected neuropsychiatric assessments, clinical data, and biomarkers at baseline, with at least three follow-up visits and one cognitive reassessment over 20 years. Dr. Szoeke and her colleagues used mixed linear models to analyze the significance of risk factors on verbal memory. The mean age of participants was 49.6 years.
Results showed that cumulative mid- to late-life physical activity had the strongest effect on better verbal memory later in life, the authors said in the report. The next most likely contributors were the negative effect of cumulative hypertension and the beneficial effect of HDL cholesterol.
The findings indicate that “physical activity, hypertension control, and achieving optimal levels of HDL cholesterol will help maintain later-life verbal memory skills,” Dr. Szoeke and her colleagues wrote.
Read the full report in the American Journal of Geriatric Psychiatry: http://www.ajgponline.org/article/S1064-7481%2816%2930113-0/fulltext
Midlife interventions including physical activity, hypertension control, and maintaining healthy HDL cholesterol may ward off later cognitive decline, reported Cassandra Szoeke, Ph.D., of the Centre for Medical Research at the University of Melbourne, and her colleagues.
In a study of 387 patients from the Women’s Healthy Ageing Project, investigators collected neuropsychiatric assessments, clinical data, and biomarkers at baseline, with at least three follow-up visits and one cognitive reassessment over 20 years. Dr. Szoeke and her colleagues used mixed linear models to analyze the significance of risk factors on verbal memory. The mean age of participants was 49.6 years.
Results showed that cumulative mid- to late-life physical activity had the strongest effect on better verbal memory later in life, the authors said in the report. The next most likely contributors were the negative effect of cumulative hypertension and the beneficial effect of HDL cholesterol.
The findings indicate that “physical activity, hypertension control, and achieving optimal levels of HDL cholesterol will help maintain later-life verbal memory skills,” Dr. Szoeke and her colleagues wrote.
Read the full report in the American Journal of Geriatric Psychiatry: http://www.ajgponline.org/article/S1064-7481%2816%2930113-0/fulltext
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Disappointing results for GI bleed prevention in high-risk aspirin users
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
AT DDW® 2016
Key clinical point: Neither a PPI nor an H2 antagonist provided sufficient gastroprotection in high-risk aspirin users.
Major finding: The rate of recurrent bleeding or endoscopic ulcers was 7.9% with a PPI versus 12.4% with an H2 antagonist.
Data source: A randomized, controlled trial of 270 patients with a previous history of ulcers.
Disclosures: Dr. Chan has received financial support from Pfizer and Eisai.
Atopic Dermatitis in Children
Pulmonary function testing adds little to STS risk scores
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Additional pulmonary function testing adds little predictive value to STS risk scoring when available.
Major finding: There was no significant added benefit to the predictive ability of STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied, as determined by AUC analysis.
Data source: A retrospective, database analysis of 1,685 patients undergoing index cardiac surgery at a single center between April 2004 and January 2014.
Disclosures: The authors reported that they had no disclosures.
MS Misdiagnosis in the Era of McDonald Criteria
VANCOUVER—The misdiagnosis of multiple sclerosis (MS) is a problem with significant consequences for patients, as well as the health care system, according to Andrew J. Solomon, MD, Assistant Professor in the Department of Neurological Sciences at the University of Vermont in Burlington. “Overreliance on MRI abnormalities in the setting of atypical syndromes and unverified prior symptoms may be a major cause of misdiagnosis,” Dr. Solomon said at the 68th Annual Meeting of the American Academy of Neurology.
Dr. Solomon and colleagues conducted a simple survey in 2012. They asked 122 MS specialists if they recalled seeing a patient who was incorrectly diagnosed with MS over the last year. Nearly all (95%) had evaluated such patients. As a follow-up, Dr. Solomon and colleagues designed the present study, which evaluated the characteristics of a large population of patients who had been misdiagnosed with MS. “We wanted to determine what the diagnoses were that were being mistaken for MS and what the risks were that were associated with these misdiagnoses.” Their pilot study also probed the causes of misdiagnosis, particularly relating to the current McDonald MS diagnostic criteria.
Twenty-three MS specialists from the University of Vermont, Oregon Health and Science University, Washington University, and the Mayo Clinic participated in this study. The investigators defined two categories: definite and probable misdiagnosis. Patients were identified by participating neurologists during clinical evaluations either prospectively during the 13 months of the study or shortly prior to study initiation. Patients were classified as having definite misdiagnosis when an alternative diagnosis was definitively made based on clinical, laboratory, and neuroimaging evaluation and probable misdiagnosis when an alternative diagnosis was suspected and diagnostic criteria for MS were not met.
Migraine Was Mistaken for MS
The researchers identified 110 patients who were misdiagnosed with MS; 85% were women, and the mean age was 49. About one-quarter (24%) were initially misdiagnosed by a neurologist with fellowship training or a practice focus in MS, and 32% were initially misdiagnosed by a neurologist without such training. For 42%, it was difficult to determine the level of training of the initial neurologist. Nearly half (46%) were classified as definite misdiagnosis, and 54% fell into the probable misdiagnosis category. Neurologists informed 107 (97%) of the patients that their MS diagnosis was incorrect. Almost 30% had been misdiagnosed with MS for three to nine years; 33% had been misdiagnosed for 10 years or longer.
Of the syndromes and diagnoses that were commonly mistaken for MS, the top five represented two-thirds (66%) of all the misdiagnoses that were identified. Migraine, alone or in combination with other disorders, accounted for 22%. Fibromyalgia, along with another alternative cause of MRI abnormalities, accounted for 15%. A category labeled nonspecific or nonlocalized neurologic symptoms—symptoms that were not typical of demyelinating injury or CNS injury—with an abnormal MRI accounted for 12%. Conversion or psychogenic disorder accounted for 11%, and neuromyelitis optica (NMO) spectrum disorder accounted for 6%.
Disease-modifying therapy (DMT) had been initiated in 70% of these patients. About one-third (36%) had received more than one DMT for MS, and a number had received two, three, or four therapies. Thirteen percent had been exposed to natalizumab, a number had received oral therapies for MS, and a few patients had received mitoxantrone. Nearly one-quarter (24%) at some time were on a DMT with a known risk of PML. Almost 30% had been exposed to DMT for three to nine years. Almost 30% had been on DMT for 10 years or longer.
Cerebrospinal fluid (CSF) was available for 52 patients from the time of initial diagnosis, prior to study entry. In 54%, the CSF was normal, including oligoclonal bands and normal IgG. In eight patients, the study neurologists thought that there was an erroneous interpretation of CSF, meaning, for instance, oligoclonal bands were positive in CSF as well as serum.
The study neurologists concluded that 30% of the patients experienced some morbidity as a direct result of an MS misdiagnosis. Morbidities included inadequate treatment of their correct diagnosis, exposure to DMT, and other consequences. In 72% of all patients, study neurologists identified clear evidence of an earlier missed opportunity to make the correct diagnosis.
Imaging and Misdiagnosis
In 65% of cases, study neurologists concluded that inappropriate application of MS diagnostic criteria to a neurologic symptom atypical for a demyelinating attack contributed to misdiagnosis. Inappropriate application of diagnostic criteria to a historical episode of neurologic symptoms without any corroborating objective evidence of a lesion was noted in almost half the cases.
In more than 30% of cases, an erroneous determination of juxtacortical or periventricular lesion location was thought to have contributed to misdiagnosis. In 60%, the study neurologists cited misdiagnosis related to overreliance on MRI abnormalities, meaning dissemination in time, to confirm a diagnosis of MS in a patient with nonspecific neurologic symptoms.
“We all know the differential diagnosis of MS is broad, and a number of rare disorders can mimic MS,” Dr. Solomon said. “But here it was migraine, fibromyalgia, and a number of other disorders that are quite common that mimicked MS and were mistaken for MS.” Most of these diagnoses, with the exception of NMO, lack a specific biomarker. “What this means is that the correct diagnosis in many of these cases relies on our clinical skills and critical thinking, not just MRI. The problem is not confined to nonspecialists. MS specialists can also make mistakes.”
Study neurologists reported that in almost two-thirds of cases, atypical symptoms for a demyelinating attack contributed to misdiagnosis. “Perhaps this reflects a misunderstanding of what constitutes a typical demyelinating attack and when we should rely on our diagnostic criteria alone,” Dr. Solomon said. In half, historical episodes of neurologic dysfunction, without corroborating objective findings, contributed to misdiagnosis. “This means that patients came in and they had reported historical episodes—episodes of numbness or tingling or blurry vision—where there were no objective exam findings, evoked potentials, imaging findings, to corroborate those symptoms, yet perhaps these episodes were used to meet dissemination in time.”
MRI abnormalities incorrectly attributed to MS in patients without typical demyelinating symptoms contributed to more than half of the misdiagnoses. “It is important to highlight that our MRI criteria, as part of our MS diagnostic criteria, were not developed to differentiate MS from other disorders. The MRI criteria for MS were meant to identify patients at high risk for MS after typical clinical presentations for demyelination,” Dr. Solomon said.
The Importance of Diagnostic Criteria
“Making a diagnosis of MS is challenging. It is important to acknowledge that,” Dr. Solomon said. Common diagnoses and syndromes are often mistaken for MS. But there is significant risk and morbidity associated with misdiagnosis. “The best way to prevent misdiagnosis may be strict adherence and proper use of our MS diagnostic criteria. In patients with atypical clinical presentations or in patients with nonspecific MRI abnormalities, we may need to do more. We may need to monitor longer, do more imaging, make sure we get CSF. That may prevent misdiagnosis in a number of cases.”
Dr. Solomon also stressed the need for continued vigilance for misdiagnosis in patients with an existing MS diagnosis. “We should be thinking, ‘Is this really MS?’ each time we see a new patient, rather than simply accepting that diagnosis.” And lastly, Dr. Solomon recommended that future MS diagnostic criteria should balance the benefit of prompt diagnosis and initiation of DMT versus the potential risks of misdiagnosis.
—Glenn S. Williams
VANCOUVER—The misdiagnosis of multiple sclerosis (MS) is a problem with significant consequences for patients, as well as the health care system, according to Andrew J. Solomon, MD, Assistant Professor in the Department of Neurological Sciences at the University of Vermont in Burlington. “Overreliance on MRI abnormalities in the setting of atypical syndromes and unverified prior symptoms may be a major cause of misdiagnosis,” Dr. Solomon said at the 68th Annual Meeting of the American Academy of Neurology.
Dr. Solomon and colleagues conducted a simple survey in 2012. They asked 122 MS specialists if they recalled seeing a patient who was incorrectly diagnosed with MS over the last year. Nearly all (95%) had evaluated such patients. As a follow-up, Dr. Solomon and colleagues designed the present study, which evaluated the characteristics of a large population of patients who had been misdiagnosed with MS. “We wanted to determine what the diagnoses were that were being mistaken for MS and what the risks were that were associated with these misdiagnoses.” Their pilot study also probed the causes of misdiagnosis, particularly relating to the current McDonald MS diagnostic criteria.
Twenty-three MS specialists from the University of Vermont, Oregon Health and Science University, Washington University, and the Mayo Clinic participated in this study. The investigators defined two categories: definite and probable misdiagnosis. Patients were identified by participating neurologists during clinical evaluations either prospectively during the 13 months of the study or shortly prior to study initiation. Patients were classified as having definite misdiagnosis when an alternative diagnosis was definitively made based on clinical, laboratory, and neuroimaging evaluation and probable misdiagnosis when an alternative diagnosis was suspected and diagnostic criteria for MS were not met.
Migraine Was Mistaken for MS
The researchers identified 110 patients who were misdiagnosed with MS; 85% were women, and the mean age was 49. About one-quarter (24%) were initially misdiagnosed by a neurologist with fellowship training or a practice focus in MS, and 32% were initially misdiagnosed by a neurologist without such training. For 42%, it was difficult to determine the level of training of the initial neurologist. Nearly half (46%) were classified as definite misdiagnosis, and 54% fell into the probable misdiagnosis category. Neurologists informed 107 (97%) of the patients that their MS diagnosis was incorrect. Almost 30% had been misdiagnosed with MS for three to nine years; 33% had been misdiagnosed for 10 years or longer.
Of the syndromes and diagnoses that were commonly mistaken for MS, the top five represented two-thirds (66%) of all the misdiagnoses that were identified. Migraine, alone or in combination with other disorders, accounted for 22%. Fibromyalgia, along with another alternative cause of MRI abnormalities, accounted for 15%. A category labeled nonspecific or nonlocalized neurologic symptoms—symptoms that were not typical of demyelinating injury or CNS injury—with an abnormal MRI accounted for 12%. Conversion or psychogenic disorder accounted for 11%, and neuromyelitis optica (NMO) spectrum disorder accounted for 6%.
Disease-modifying therapy (DMT) had been initiated in 70% of these patients. About one-third (36%) had received more than one DMT for MS, and a number had received two, three, or four therapies. Thirteen percent had been exposed to natalizumab, a number had received oral therapies for MS, and a few patients had received mitoxantrone. Nearly one-quarter (24%) at some time were on a DMT with a known risk of PML. Almost 30% had been exposed to DMT for three to nine years. Almost 30% had been on DMT for 10 years or longer.
Cerebrospinal fluid (CSF) was available for 52 patients from the time of initial diagnosis, prior to study entry. In 54%, the CSF was normal, including oligoclonal bands and normal IgG. In eight patients, the study neurologists thought that there was an erroneous interpretation of CSF, meaning, for instance, oligoclonal bands were positive in CSF as well as serum.
The study neurologists concluded that 30% of the patients experienced some morbidity as a direct result of an MS misdiagnosis. Morbidities included inadequate treatment of their correct diagnosis, exposure to DMT, and other consequences. In 72% of all patients, study neurologists identified clear evidence of an earlier missed opportunity to make the correct diagnosis.
Imaging and Misdiagnosis
In 65% of cases, study neurologists concluded that inappropriate application of MS diagnostic criteria to a neurologic symptom atypical for a demyelinating attack contributed to misdiagnosis. Inappropriate application of diagnostic criteria to a historical episode of neurologic symptoms without any corroborating objective evidence of a lesion was noted in almost half the cases.
In more than 30% of cases, an erroneous determination of juxtacortical or periventricular lesion location was thought to have contributed to misdiagnosis. In 60%, the study neurologists cited misdiagnosis related to overreliance on MRI abnormalities, meaning dissemination in time, to confirm a diagnosis of MS in a patient with nonspecific neurologic symptoms.
“We all know the differential diagnosis of MS is broad, and a number of rare disorders can mimic MS,” Dr. Solomon said. “But here it was migraine, fibromyalgia, and a number of other disorders that are quite common that mimicked MS and were mistaken for MS.” Most of these diagnoses, with the exception of NMO, lack a specific biomarker. “What this means is that the correct diagnosis in many of these cases relies on our clinical skills and critical thinking, not just MRI. The problem is not confined to nonspecialists. MS specialists can also make mistakes.”
Study neurologists reported that in almost two-thirds of cases, atypical symptoms for a demyelinating attack contributed to misdiagnosis. “Perhaps this reflects a misunderstanding of what constitutes a typical demyelinating attack and when we should rely on our diagnostic criteria alone,” Dr. Solomon said. In half, historical episodes of neurologic dysfunction, without corroborating objective findings, contributed to misdiagnosis. “This means that patients came in and they had reported historical episodes—episodes of numbness or tingling or blurry vision—where there were no objective exam findings, evoked potentials, imaging findings, to corroborate those symptoms, yet perhaps these episodes were used to meet dissemination in time.”
MRI abnormalities incorrectly attributed to MS in patients without typical demyelinating symptoms contributed to more than half of the misdiagnoses. “It is important to highlight that our MRI criteria, as part of our MS diagnostic criteria, were not developed to differentiate MS from other disorders. The MRI criteria for MS were meant to identify patients at high risk for MS after typical clinical presentations for demyelination,” Dr. Solomon said.
The Importance of Diagnostic Criteria
“Making a diagnosis of MS is challenging. It is important to acknowledge that,” Dr. Solomon said. Common diagnoses and syndromes are often mistaken for MS. But there is significant risk and morbidity associated with misdiagnosis. “The best way to prevent misdiagnosis may be strict adherence and proper use of our MS diagnostic criteria. In patients with atypical clinical presentations or in patients with nonspecific MRI abnormalities, we may need to do more. We may need to monitor longer, do more imaging, make sure we get CSF. That may prevent misdiagnosis in a number of cases.”
Dr. Solomon also stressed the need for continued vigilance for misdiagnosis in patients with an existing MS diagnosis. “We should be thinking, ‘Is this really MS?’ each time we see a new patient, rather than simply accepting that diagnosis.” And lastly, Dr. Solomon recommended that future MS diagnostic criteria should balance the benefit of prompt diagnosis and initiation of DMT versus the potential risks of misdiagnosis.
—Glenn S. Williams
VANCOUVER—The misdiagnosis of multiple sclerosis (MS) is a problem with significant consequences for patients, as well as the health care system, according to Andrew J. Solomon, MD, Assistant Professor in the Department of Neurological Sciences at the University of Vermont in Burlington. “Overreliance on MRI abnormalities in the setting of atypical syndromes and unverified prior symptoms may be a major cause of misdiagnosis,” Dr. Solomon said at the 68th Annual Meeting of the American Academy of Neurology.
Dr. Solomon and colleagues conducted a simple survey in 2012. They asked 122 MS specialists if they recalled seeing a patient who was incorrectly diagnosed with MS over the last year. Nearly all (95%) had evaluated such patients. As a follow-up, Dr. Solomon and colleagues designed the present study, which evaluated the characteristics of a large population of patients who had been misdiagnosed with MS. “We wanted to determine what the diagnoses were that were being mistaken for MS and what the risks were that were associated with these misdiagnoses.” Their pilot study also probed the causes of misdiagnosis, particularly relating to the current McDonald MS diagnostic criteria.
Twenty-three MS specialists from the University of Vermont, Oregon Health and Science University, Washington University, and the Mayo Clinic participated in this study. The investigators defined two categories: definite and probable misdiagnosis. Patients were identified by participating neurologists during clinical evaluations either prospectively during the 13 months of the study or shortly prior to study initiation. Patients were classified as having definite misdiagnosis when an alternative diagnosis was definitively made based on clinical, laboratory, and neuroimaging evaluation and probable misdiagnosis when an alternative diagnosis was suspected and diagnostic criteria for MS were not met.
Migraine Was Mistaken for MS
The researchers identified 110 patients who were misdiagnosed with MS; 85% were women, and the mean age was 49. About one-quarter (24%) were initially misdiagnosed by a neurologist with fellowship training or a practice focus in MS, and 32% were initially misdiagnosed by a neurologist without such training. For 42%, it was difficult to determine the level of training of the initial neurologist. Nearly half (46%) were classified as definite misdiagnosis, and 54% fell into the probable misdiagnosis category. Neurologists informed 107 (97%) of the patients that their MS diagnosis was incorrect. Almost 30% had been misdiagnosed with MS for three to nine years; 33% had been misdiagnosed for 10 years or longer.
Of the syndromes and diagnoses that were commonly mistaken for MS, the top five represented two-thirds (66%) of all the misdiagnoses that were identified. Migraine, alone or in combination with other disorders, accounted for 22%. Fibromyalgia, along with another alternative cause of MRI abnormalities, accounted for 15%. A category labeled nonspecific or nonlocalized neurologic symptoms—symptoms that were not typical of demyelinating injury or CNS injury—with an abnormal MRI accounted for 12%. Conversion or psychogenic disorder accounted for 11%, and neuromyelitis optica (NMO) spectrum disorder accounted for 6%.
Disease-modifying therapy (DMT) had been initiated in 70% of these patients. About one-third (36%) had received more than one DMT for MS, and a number had received two, three, or four therapies. Thirteen percent had been exposed to natalizumab, a number had received oral therapies for MS, and a few patients had received mitoxantrone. Nearly one-quarter (24%) at some time were on a DMT with a known risk of PML. Almost 30% had been exposed to DMT for three to nine years. Almost 30% had been on DMT for 10 years or longer.
Cerebrospinal fluid (CSF) was available for 52 patients from the time of initial diagnosis, prior to study entry. In 54%, the CSF was normal, including oligoclonal bands and normal IgG. In eight patients, the study neurologists thought that there was an erroneous interpretation of CSF, meaning, for instance, oligoclonal bands were positive in CSF as well as serum.
The study neurologists concluded that 30% of the patients experienced some morbidity as a direct result of an MS misdiagnosis. Morbidities included inadequate treatment of their correct diagnosis, exposure to DMT, and other consequences. In 72% of all patients, study neurologists identified clear evidence of an earlier missed opportunity to make the correct diagnosis.
Imaging and Misdiagnosis
In 65% of cases, study neurologists concluded that inappropriate application of MS diagnostic criteria to a neurologic symptom atypical for a demyelinating attack contributed to misdiagnosis. Inappropriate application of diagnostic criteria to a historical episode of neurologic symptoms without any corroborating objective evidence of a lesion was noted in almost half the cases.
In more than 30% of cases, an erroneous determination of juxtacortical or periventricular lesion location was thought to have contributed to misdiagnosis. In 60%, the study neurologists cited misdiagnosis related to overreliance on MRI abnormalities, meaning dissemination in time, to confirm a diagnosis of MS in a patient with nonspecific neurologic symptoms.
“We all know the differential diagnosis of MS is broad, and a number of rare disorders can mimic MS,” Dr. Solomon said. “But here it was migraine, fibromyalgia, and a number of other disorders that are quite common that mimicked MS and were mistaken for MS.” Most of these diagnoses, with the exception of NMO, lack a specific biomarker. “What this means is that the correct diagnosis in many of these cases relies on our clinical skills and critical thinking, not just MRI. The problem is not confined to nonspecialists. MS specialists can also make mistakes.”
Study neurologists reported that in almost two-thirds of cases, atypical symptoms for a demyelinating attack contributed to misdiagnosis. “Perhaps this reflects a misunderstanding of what constitutes a typical demyelinating attack and when we should rely on our diagnostic criteria alone,” Dr. Solomon said. In half, historical episodes of neurologic dysfunction, without corroborating objective findings, contributed to misdiagnosis. “This means that patients came in and they had reported historical episodes—episodes of numbness or tingling or blurry vision—where there were no objective exam findings, evoked potentials, imaging findings, to corroborate those symptoms, yet perhaps these episodes were used to meet dissemination in time.”
MRI abnormalities incorrectly attributed to MS in patients without typical demyelinating symptoms contributed to more than half of the misdiagnoses. “It is important to highlight that our MRI criteria, as part of our MS diagnostic criteria, were not developed to differentiate MS from other disorders. The MRI criteria for MS were meant to identify patients at high risk for MS after typical clinical presentations for demyelination,” Dr. Solomon said.
The Importance of Diagnostic Criteria
“Making a diagnosis of MS is challenging. It is important to acknowledge that,” Dr. Solomon said. Common diagnoses and syndromes are often mistaken for MS. But there is significant risk and morbidity associated with misdiagnosis. “The best way to prevent misdiagnosis may be strict adherence and proper use of our MS diagnostic criteria. In patients with atypical clinical presentations or in patients with nonspecific MRI abnormalities, we may need to do more. We may need to monitor longer, do more imaging, make sure we get CSF. That may prevent misdiagnosis in a number of cases.”
Dr. Solomon also stressed the need for continued vigilance for misdiagnosis in patients with an existing MS diagnosis. “We should be thinking, ‘Is this really MS?’ each time we see a new patient, rather than simply accepting that diagnosis.” And lastly, Dr. Solomon recommended that future MS diagnostic criteria should balance the benefit of prompt diagnosis and initiation of DMT versus the potential risks of misdiagnosis.
—Glenn S. Williams
Obesity paradox extends to TAVR but not SAVR
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
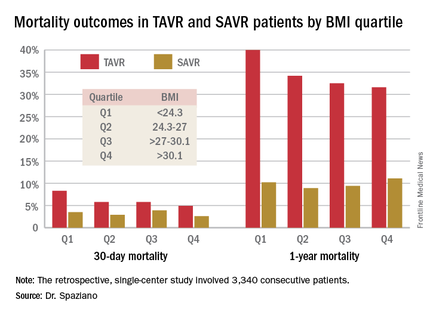
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.
The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.

The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.

The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
AT EUROPCR 2016
Key clinical point: Being overweight or obese may have a protective effect in patients undergoing transcatheter aortic valve replacement, but not in those who have surgical replacement.
Major finding: Thirty-day mortality was 4.9% in TAVR patients in the top BMI quartile and climbed stepwise with lowering BMIs.
Data source: A single-center retrospective study of 3,340 consecutive patients who underwent TAVR or SAVR.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Sleep strategies: Treating childhood OSA
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. But recent research suggests that a higher-than-realized number of children may grow out of their OSA. In fact, a recent study in CHEST identified factors associated with spontaneous resolution of sleep apnea that can help physicians identify children who can avoid surgery. For more on childhood OSA, see the article from Chest Physician, available at http://www.chestphysician.org/news-from-chest/article/sleep-strategies-treating-childhood-osa/d0566fd44f306901e0a1f3a3de7e57b2.html
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. But recent research suggests that a higher-than-realized number of children may grow out of their OSA. In fact, a recent study in CHEST identified factors associated with spontaneous resolution of sleep apnea that can help physicians identify children who can avoid surgery. For more on childhood OSA, see the article from Chest Physician, available at http://www.chestphysician.org/news-from-chest/article/sleep-strategies-treating-childhood-osa/d0566fd44f306901e0a1f3a3de7e57b2.html
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. But recent research suggests that a higher-than-realized number of children may grow out of their OSA. In fact, a recent study in CHEST identified factors associated with spontaneous resolution of sleep apnea that can help physicians identify children who can avoid surgery. For more on childhood OSA, see the article from Chest Physician, available at http://www.chestphysician.org/news-from-chest/article/sleep-strategies-treating-childhood-osa/d0566fd44f306901e0a1f3a3de7e57b2.html
Can hepatitis B and C be eliminated?
The United States has the “opportunity and responsibility” to lead a global action against the hepatitis B virus (HBV) and hepatitis C virus (HCV), according to a report published by the National Academies of Sciences, Engineering, and Medicine. While there is no vaccine for HCV, new therapies do offer a cure for most patients. But eradicating HCV requires a multipronged approach: ending transmission, eliminating chronic HCV, and reducing morbidity and mortality associated with the disease. For more information on the strategies that could meet these goals, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/hepatitis-c/article/can-hepatitis-b-and-c-be-eliminated/cc5f56c697746870f8da1c44dc7de26e.html.
The United States has the “opportunity and responsibility” to lead a global action against the hepatitis B virus (HBV) and hepatitis C virus (HCV), according to a report published by the National Academies of Sciences, Engineering, and Medicine. While there is no vaccine for HCV, new therapies do offer a cure for most patients. But eradicating HCV requires a multipronged approach: ending transmission, eliminating chronic HCV, and reducing morbidity and mortality associated with the disease. For more information on the strategies that could meet these goals, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/hepatitis-c/article/can-hepatitis-b-and-c-be-eliminated/cc5f56c697746870f8da1c44dc7de26e.html.
The United States has the “opportunity and responsibility” to lead a global action against the hepatitis B virus (HBV) and hepatitis C virus (HCV), according to a report published by the National Academies of Sciences, Engineering, and Medicine. While there is no vaccine for HCV, new therapies do offer a cure for most patients. But eradicating HCV requires a multipronged approach: ending transmission, eliminating chronic HCV, and reducing morbidity and mortality associated with the disease. For more information on the strategies that could meet these goals, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/hepatitis-c/article/can-hepatitis-b-and-c-be-eliminated/cc5f56c697746870f8da1c44dc7de26e.html.
Guidelines add 2 new heart failure treatments
The American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Failure Society of America (HFSA) recently issued joint recommendations on 2 new medications for stage C heart failure patients with a reduced ejection fraction. Valsartan/sacubitril, a combination angiotensin receptor–neprilysin inhibitor, and ivabradine, a sinoatrial node modulator, were both approved by the Food and Drug Administration in 2015, but ivabradine has been licensed for a decade in Europe. Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines. Find out what the guideline authors recommend at Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/guidelines-add-two-new-heart-failure-treatments/98c0b0a2a2e77dac550cdd52953033ea.html.
The American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Failure Society of America (HFSA) recently issued joint recommendations on 2 new medications for stage C heart failure patients with a reduced ejection fraction. Valsartan/sacubitril, a combination angiotensin receptor–neprilysin inhibitor, and ivabradine, a sinoatrial node modulator, were both approved by the Food and Drug Administration in 2015, but ivabradine has been licensed for a decade in Europe. Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines. Find out what the guideline authors recommend at Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/guidelines-add-two-new-heart-failure-treatments/98c0b0a2a2e77dac550cdd52953033ea.html.
The American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Failure Society of America (HFSA) recently issued joint recommendations on 2 new medications for stage C heart failure patients with a reduced ejection fraction. Valsartan/sacubitril, a combination angiotensin receptor–neprilysin inhibitor, and ivabradine, a sinoatrial node modulator, were both approved by the Food and Drug Administration in 2015, but ivabradine has been licensed for a decade in Europe. Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines. Find out what the guideline authors recommend at Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/guidelines-add-two-new-heart-failure-treatments/98c0b0a2a2e77dac550cdd52953033ea.html.