User login
Veteran Cancer Research Highlighted in ASCO Posters and Abstracts
At the 2016 American Society of Clinical Oncology in Chicago, Illinois, researchers from the VA submitted new abstracts on more than 20 different research projects focused on veteran’s oncology care. The researchers conducted studies with the scope of veteran diagnosis, treatment, and quality of care. The abstracts were presented at the annual ASCO meeting during poster sessions and online.
General Oncology
The Impact of a Veterans Administration NURSE Disease Management Program: the Oncology Aspect
Author(s): George Anthony Dawson, et al.
Background: In an effort to decrease time to diagnosis and/or treatment intervention in newly diagnosed veteran cancer patients, Hudson Valley administrators chose the disease case management team model as a potential useful solution to enhance and / or expedite health care delivery. This disease management program began in 2008. The team consists of 3 registered nurses who work under the auspices of the quality management division rather than a specific oncology clinic. This report will show the clinical impact of Hudson Valley VA’s disease management team.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161764-176
Implementation of a Nurse Communication Strategy to Improve Perception of Nurse-Patient Communication at the Bedside on an Inpatient Oncology Unit
Author(s): Lyn Cain Zehner, et al.
Background: Patients spend more time with nurses when in the hospital than with any other health care professional. Patient experience scores have been used as a measure of quality patient outcomes. The Hospital Consumer Assessment of Healthcare Providers and Systems Survey (HCAHPS) score communication with nurses, correlates with improvements in 4 other dimensions: pain, communication about medications, responsiveness of staff, and overall rating. As patient experience scores are tied to Medicare reimbursement, nurses have an impact on reimbursement. The nurse communication scores on the inpatient 29 bed oncology unit reflected an opportunity for improvement. The purpose of this project was to develop a communication strategy that staff would implement at the bedside. The goal was to achieve an improvement in the HCAHPS Communication with Nurses question.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166250-176
Modified Ketogenic Diet in Advanced Malignancies–Final Results of a Safety and Feasibility Trial Within the Veterans Affairs Healthcare System
Author(s): Jocelyn Tan-Shalaby, et al.
Final report of a safety and feasibility trial within the Pittsburgh Veteran Affairs HealthCare System- Modified Ketogenic Diet in Advanced Cancer.
Background: Malignant cells have dysfunctional mitochondria, which limit their ability to use energy from fatty acids and ketones. Preclinical animal studies using a ketogenic diet in cancer show encouraging results. The authors tested safety and feasibility of the modified ketogenic diet in a veteran population of advanced cancer patients across a variety of solid tumors.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170081-176
[Click through for : Prostate Cancer, Liver, Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Prostate Cancer
Improving Risk Stratification Among Veterans With Newly Diagnosed, Clinically Low-Risk Prostate Cancer Using the 17-Gene Genomic Prostate Score Assay.
Author(s): Julie Ann Lynch, et al.
Background: Active surveillance (AS) is a recommended treatment option for low risk prostate cancer (PCa). Studies have shown high rates of AS in the VA yet treatment variation exists between VAMCs, likely due to concern about missing aggressive disease. The 17-gene Genomic Prostate Score (GPS) has been validated to predict likelihood of favorable pathology in men with low risk PCa. This study compares treatment patterns before and after introduction of the GPS to determine if the assay assists in risk-stratification. Men with PCa who met National Comprehensive Cancer Network criteria for very low, low, or intermediate risk PCa were eligible. Chart review of men across 6 VAMCs established baseline treatment in untested patients in 2013-2014. In 2015, Veterans at the same VAMCs were offered the assay in a prospective study. Treatment recommendations and treatment implemented were captured.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170703-176
Trends in Utilization of Novel Oral Therapeutic Agents for Men With Metastatic Castrate-Resistant Prostate Cancer Within the United States Veteran’s Affairs Health System.
Author(s): Elizabeth Henry, et al.
Background: Therapeutic options for men with metastatic castrate resistant prostate cancer (mCRPC) have expanded significantly over the past 5 years with several new agents demonstrating improved survival, including two oral agents. Abiraterone acetate, a CYP-17 androgen synthesis inhibitor, obtained initial FDA approval in 2011 for post-docetaxel (D) use, and gained expanded approval in 2012 for pre-D use. Enzalutamide, an androgen receptor signaling inhibitor, also gained FDA approval in 2012 (post-D) and indication was expanded in 2014 (pre-D). Due to the relatively recent approvals of these agents, there is limited data on rates of uptake and prescribing patterns for patients with mCRPC.
To read the full abstract click here: http://meetinglibrary.asco.org/content/157970-172
Liver
A Comparison of Liver Cancer Patients Receiving Palliative Care at Two Veteran Affairs Medical Centers.
Author(s): Sarah Lee, Zhen Wang, et al.
Background: Liver cancer is a leading cause of death. Lack of data exists on palliative care in this group, and care varies by location. The authors aim to determine if there are differences in palliative care for liver cancer patients by VA site.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171726-176
[Click through for : Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Radiation Oncology
A Cross-Sectional View of Radiation Dose Fractionation Schemes Used for Treating Painful Bone Metastases (PBM) Within Veterans Health Administration’s Radiation Oncology Centers.
Author(s): George Anthony Dawson, et al.
Background: The use of single fraction radiotherapy in the treatment of PBM in the United States is now gaining clinical acceptance, especially at large institutional levels. In this cross-sectional report, the authors report the varying dose fractionation schemes used to treat PBM by VHA radiation oncology centers.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161496-176
Will Academic and Community Physicians Engage and Share Knowledge in an Online Physician Social Network? Lessons from the Radiation Oncology Community.
Author(s): Nadine Housri, et al.
Background: The exponential growth of medical knowledge has made it increasingly difficult for clinicians to make sense of new information and recognize how to incorporate new research results into clinical practice. The authors sought to determine whether a social question and answer website designed to connect academic and community physicians would consistently engage radiation oncologists to share evidence based information and clinical insights with each other.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171497-176
Lung Cancer
Non–Small Cell Lung Cancer in Veterans: Disparities in Prevalence and Survival Among Different Histologic Subtypes.
Author(s): Hussein Assi, Efstratios Koutroumpakis, et al.
Background: Since the 1980s, adenocarcinoma (AC) of the lung has become more common than squamous cell carcinoma (SCC). Given the differences in smoking patterns, it is unclear if the same holds true among veterans. In this study, the authors compare the distribution of lung AC and SCC in the veterans’ population to that in the general population. They also looked at the survival of patients with different histologies in the VA population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167505-176
VA Oncologists’ Attitudes and Behaviors Regarding Genomic-Based Targeted Therapy for the Management of Advanced Lung Cancer.
Author(s): Jennifer Arney, et al.
Background: Genomic-based targeted therapy (GBTT) has emerged as a treatment option for patients with advanced lung cancer. Clinical practice guidelines of the National Comprehensive Cancer Network and the American Society of Clinical Oncology recommend testing all metastatic adenocarcinomas for EGFR mutation, and use of EGFR-TKIs (erlotinib) as first-line therapy for advanced adenocarcinoma lung cancer patients with EGFR mutation positive. Little is known about how oncologists utilize genomic testing and GBTT in a clinical setting. Drawing from the Cabana and colleagues theoretical framework on providers’ adherence to guidelines, this study aims to elicit provider and facility-level barriers and facilitators to using GBTT in VA healthcare delivery system.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166517-176
[Click through for: Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Colorectal
Survival Advantage for Hepatectomy With or Without Hemicolectomy in Colorectal Liver Metastases.
Author(s): Dalia A. Mobarek, et al.
Background: Surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study survival in patients presenting with hepatic metastasis at the outset and rates of surgical intervention in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171640-176
Colorectal Liver Metastases Management in the Veterans Health Administration: Geographic Disparity.
Author(s): Dalia A. Mobarek, et al.
Background: Multidisciplinary management including surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study surgical intervention types, rates and factors affecting the decision making in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/160233-173
HIV and Cancer
Gleason Grade in HIV+ Versus Uninfected Prostate Cancer Patients in the Veterans Aging Cohort Study.
Author(s): Roxanne Jimmy Wadia, et al.
Background: Little is known about how the biology of prostate cancer in HIV+ men compares to that of uninfected men. The purpose of this study was to compare the Gleason grade (GL) of prostate adenocarcinoma (PrCA) in HIV+ vs uninfected patients in the combination antiretroviral therapy (ART) era (1997-present).
To read the full abstract click here: http://meetinglibrary.asco.org/content/162980-176
Disparities in AIDS-Related Kaposi Sarcoma Incidence and Survival.
Author(s): Firas El Chaer, et al.
Background: With advances in combined antiretroviral therapy (ART), Kaposi’s sarcoma (KS) incidence has decreased over the past decade. Geographical and racial disparities may contribute to variation in the incidence and outcomes of multiple cancers in the U.S., including KS. Using the Surveillance, Epidemiology, and End Results (SEER) database, the authors analyzed KS incidence and survival by race and geographical region in the ART era.
To read the full abstract click here: http://meetinglibrary.asco.org/content/168953-176
[Click through for : Palliative Care, Esophageal]
Palliative Care
Palliative Care Interventions and EOL Care Outcomes for Hepatocellular Patients (pts) at 2 VA Medical Centers.
Author(s): Zhen Wang, et al.
Background: Palliative care interventions and its effect on EOL outcomes for liver cancer pts have not been described. The authors investigated the association between palliative care intervention and EOL care outcomes.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167081-176
Esophageal
Characteristics of Esophageal Carcinoma in a Veteran Population.
Author(s): Theresa Ratajczak, et al.
Background: Esophageal cancer is projected to increase by approximately 35% through 2025 in the U.S. The authors investigated the characteristics of esophageal carcinoma in a veteran population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/162712-176
At the 2016 American Society of Clinical Oncology in Chicago, Illinois, researchers from the VA submitted new abstracts on more than 20 different research projects focused on veteran’s oncology care. The researchers conducted studies with the scope of veteran diagnosis, treatment, and quality of care. The abstracts were presented at the annual ASCO meeting during poster sessions and online.
General Oncology
The Impact of a Veterans Administration NURSE Disease Management Program: the Oncology Aspect
Author(s): George Anthony Dawson, et al.
Background: In an effort to decrease time to diagnosis and/or treatment intervention in newly diagnosed veteran cancer patients, Hudson Valley administrators chose the disease case management team model as a potential useful solution to enhance and / or expedite health care delivery. This disease management program began in 2008. The team consists of 3 registered nurses who work under the auspices of the quality management division rather than a specific oncology clinic. This report will show the clinical impact of Hudson Valley VA’s disease management team.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161764-176
Implementation of a Nurse Communication Strategy to Improve Perception of Nurse-Patient Communication at the Bedside on an Inpatient Oncology Unit
Author(s): Lyn Cain Zehner, et al.
Background: Patients spend more time with nurses when in the hospital than with any other health care professional. Patient experience scores have been used as a measure of quality patient outcomes. The Hospital Consumer Assessment of Healthcare Providers and Systems Survey (HCAHPS) score communication with nurses, correlates with improvements in 4 other dimensions: pain, communication about medications, responsiveness of staff, and overall rating. As patient experience scores are tied to Medicare reimbursement, nurses have an impact on reimbursement. The nurse communication scores on the inpatient 29 bed oncology unit reflected an opportunity for improvement. The purpose of this project was to develop a communication strategy that staff would implement at the bedside. The goal was to achieve an improvement in the HCAHPS Communication with Nurses question.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166250-176
Modified Ketogenic Diet in Advanced Malignancies–Final Results of a Safety and Feasibility Trial Within the Veterans Affairs Healthcare System
Author(s): Jocelyn Tan-Shalaby, et al.
Final report of a safety and feasibility trial within the Pittsburgh Veteran Affairs HealthCare System- Modified Ketogenic Diet in Advanced Cancer.
Background: Malignant cells have dysfunctional mitochondria, which limit their ability to use energy from fatty acids and ketones. Preclinical animal studies using a ketogenic diet in cancer show encouraging results. The authors tested safety and feasibility of the modified ketogenic diet in a veteran population of advanced cancer patients across a variety of solid tumors.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170081-176
[Click through for : Prostate Cancer, Liver, Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Prostate Cancer
Improving Risk Stratification Among Veterans With Newly Diagnosed, Clinically Low-Risk Prostate Cancer Using the 17-Gene Genomic Prostate Score Assay.
Author(s): Julie Ann Lynch, et al.
Background: Active surveillance (AS) is a recommended treatment option for low risk prostate cancer (PCa). Studies have shown high rates of AS in the VA yet treatment variation exists between VAMCs, likely due to concern about missing aggressive disease. The 17-gene Genomic Prostate Score (GPS) has been validated to predict likelihood of favorable pathology in men with low risk PCa. This study compares treatment patterns before and after introduction of the GPS to determine if the assay assists in risk-stratification. Men with PCa who met National Comprehensive Cancer Network criteria for very low, low, or intermediate risk PCa were eligible. Chart review of men across 6 VAMCs established baseline treatment in untested patients in 2013-2014. In 2015, Veterans at the same VAMCs were offered the assay in a prospective study. Treatment recommendations and treatment implemented were captured.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170703-176
Trends in Utilization of Novel Oral Therapeutic Agents for Men With Metastatic Castrate-Resistant Prostate Cancer Within the United States Veteran’s Affairs Health System.
Author(s): Elizabeth Henry, et al.
Background: Therapeutic options for men with metastatic castrate resistant prostate cancer (mCRPC) have expanded significantly over the past 5 years with several new agents demonstrating improved survival, including two oral agents. Abiraterone acetate, a CYP-17 androgen synthesis inhibitor, obtained initial FDA approval in 2011 for post-docetaxel (D) use, and gained expanded approval in 2012 for pre-D use. Enzalutamide, an androgen receptor signaling inhibitor, also gained FDA approval in 2012 (post-D) and indication was expanded in 2014 (pre-D). Due to the relatively recent approvals of these agents, there is limited data on rates of uptake and prescribing patterns for patients with mCRPC.
To read the full abstract click here: http://meetinglibrary.asco.org/content/157970-172
Liver
A Comparison of Liver Cancer Patients Receiving Palliative Care at Two Veteran Affairs Medical Centers.
Author(s): Sarah Lee, Zhen Wang, et al.
Background: Liver cancer is a leading cause of death. Lack of data exists on palliative care in this group, and care varies by location. The authors aim to determine if there are differences in palliative care for liver cancer patients by VA site.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171726-176
[Click through for : Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Radiation Oncology
A Cross-Sectional View of Radiation Dose Fractionation Schemes Used for Treating Painful Bone Metastases (PBM) Within Veterans Health Administration’s Radiation Oncology Centers.
Author(s): George Anthony Dawson, et al.
Background: The use of single fraction radiotherapy in the treatment of PBM in the United States is now gaining clinical acceptance, especially at large institutional levels. In this cross-sectional report, the authors report the varying dose fractionation schemes used to treat PBM by VHA radiation oncology centers.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161496-176
Will Academic and Community Physicians Engage and Share Knowledge in an Online Physician Social Network? Lessons from the Radiation Oncology Community.
Author(s): Nadine Housri, et al.
Background: The exponential growth of medical knowledge has made it increasingly difficult for clinicians to make sense of new information and recognize how to incorporate new research results into clinical practice. The authors sought to determine whether a social question and answer website designed to connect academic and community physicians would consistently engage radiation oncologists to share evidence based information and clinical insights with each other.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171497-176
Lung Cancer
Non–Small Cell Lung Cancer in Veterans: Disparities in Prevalence and Survival Among Different Histologic Subtypes.
Author(s): Hussein Assi, Efstratios Koutroumpakis, et al.
Background: Since the 1980s, adenocarcinoma (AC) of the lung has become more common than squamous cell carcinoma (SCC). Given the differences in smoking patterns, it is unclear if the same holds true among veterans. In this study, the authors compare the distribution of lung AC and SCC in the veterans’ population to that in the general population. They also looked at the survival of patients with different histologies in the VA population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167505-176
VA Oncologists’ Attitudes and Behaviors Regarding Genomic-Based Targeted Therapy for the Management of Advanced Lung Cancer.
Author(s): Jennifer Arney, et al.
Background: Genomic-based targeted therapy (GBTT) has emerged as a treatment option for patients with advanced lung cancer. Clinical practice guidelines of the National Comprehensive Cancer Network and the American Society of Clinical Oncology recommend testing all metastatic adenocarcinomas for EGFR mutation, and use of EGFR-TKIs (erlotinib) as first-line therapy for advanced adenocarcinoma lung cancer patients with EGFR mutation positive. Little is known about how oncologists utilize genomic testing and GBTT in a clinical setting. Drawing from the Cabana and colleagues theoretical framework on providers’ adherence to guidelines, this study aims to elicit provider and facility-level barriers and facilitators to using GBTT in VA healthcare delivery system.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166517-176
[Click through for: Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Colorectal
Survival Advantage for Hepatectomy With or Without Hemicolectomy in Colorectal Liver Metastases.
Author(s): Dalia A. Mobarek, et al.
Background: Surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study survival in patients presenting with hepatic metastasis at the outset and rates of surgical intervention in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171640-176
Colorectal Liver Metastases Management in the Veterans Health Administration: Geographic Disparity.
Author(s): Dalia A. Mobarek, et al.
Background: Multidisciplinary management including surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study surgical intervention types, rates and factors affecting the decision making in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/160233-173
HIV and Cancer
Gleason Grade in HIV+ Versus Uninfected Prostate Cancer Patients in the Veterans Aging Cohort Study.
Author(s): Roxanne Jimmy Wadia, et al.
Background: Little is known about how the biology of prostate cancer in HIV+ men compares to that of uninfected men. The purpose of this study was to compare the Gleason grade (GL) of prostate adenocarcinoma (PrCA) in HIV+ vs uninfected patients in the combination antiretroviral therapy (ART) era (1997-present).
To read the full abstract click here: http://meetinglibrary.asco.org/content/162980-176
Disparities in AIDS-Related Kaposi Sarcoma Incidence and Survival.
Author(s): Firas El Chaer, et al.
Background: With advances in combined antiretroviral therapy (ART), Kaposi’s sarcoma (KS) incidence has decreased over the past decade. Geographical and racial disparities may contribute to variation in the incidence and outcomes of multiple cancers in the U.S., including KS. Using the Surveillance, Epidemiology, and End Results (SEER) database, the authors analyzed KS incidence and survival by race and geographical region in the ART era.
To read the full abstract click here: http://meetinglibrary.asco.org/content/168953-176
[Click through for : Palliative Care, Esophageal]
Palliative Care
Palliative Care Interventions and EOL Care Outcomes for Hepatocellular Patients (pts) at 2 VA Medical Centers.
Author(s): Zhen Wang, et al.
Background: Palliative care interventions and its effect on EOL outcomes for liver cancer pts have not been described. The authors investigated the association between palliative care intervention and EOL care outcomes.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167081-176
Esophageal
Characteristics of Esophageal Carcinoma in a Veteran Population.
Author(s): Theresa Ratajczak, et al.
Background: Esophageal cancer is projected to increase by approximately 35% through 2025 in the U.S. The authors investigated the characteristics of esophageal carcinoma in a veteran population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/162712-176
At the 2016 American Society of Clinical Oncology in Chicago, Illinois, researchers from the VA submitted new abstracts on more than 20 different research projects focused on veteran’s oncology care. The researchers conducted studies with the scope of veteran diagnosis, treatment, and quality of care. The abstracts were presented at the annual ASCO meeting during poster sessions and online.
General Oncology
The Impact of a Veterans Administration NURSE Disease Management Program: the Oncology Aspect
Author(s): George Anthony Dawson, et al.
Background: In an effort to decrease time to diagnosis and/or treatment intervention in newly diagnosed veteran cancer patients, Hudson Valley administrators chose the disease case management team model as a potential useful solution to enhance and / or expedite health care delivery. This disease management program began in 2008. The team consists of 3 registered nurses who work under the auspices of the quality management division rather than a specific oncology clinic. This report will show the clinical impact of Hudson Valley VA’s disease management team.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161764-176
Implementation of a Nurse Communication Strategy to Improve Perception of Nurse-Patient Communication at the Bedside on an Inpatient Oncology Unit
Author(s): Lyn Cain Zehner, et al.
Background: Patients spend more time with nurses when in the hospital than with any other health care professional. Patient experience scores have been used as a measure of quality patient outcomes. The Hospital Consumer Assessment of Healthcare Providers and Systems Survey (HCAHPS) score communication with nurses, correlates with improvements in 4 other dimensions: pain, communication about medications, responsiveness of staff, and overall rating. As patient experience scores are tied to Medicare reimbursement, nurses have an impact on reimbursement. The nurse communication scores on the inpatient 29 bed oncology unit reflected an opportunity for improvement. The purpose of this project was to develop a communication strategy that staff would implement at the bedside. The goal was to achieve an improvement in the HCAHPS Communication with Nurses question.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166250-176
Modified Ketogenic Diet in Advanced Malignancies–Final Results of a Safety and Feasibility Trial Within the Veterans Affairs Healthcare System
Author(s): Jocelyn Tan-Shalaby, et al.
Final report of a safety and feasibility trial within the Pittsburgh Veteran Affairs HealthCare System- Modified Ketogenic Diet in Advanced Cancer.
Background: Malignant cells have dysfunctional mitochondria, which limit their ability to use energy from fatty acids and ketones. Preclinical animal studies using a ketogenic diet in cancer show encouraging results. The authors tested safety and feasibility of the modified ketogenic diet in a veteran population of advanced cancer patients across a variety of solid tumors.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170081-176
[Click through for : Prostate Cancer, Liver, Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Prostate Cancer
Improving Risk Stratification Among Veterans With Newly Diagnosed, Clinically Low-Risk Prostate Cancer Using the 17-Gene Genomic Prostate Score Assay.
Author(s): Julie Ann Lynch, et al.
Background: Active surveillance (AS) is a recommended treatment option for low risk prostate cancer (PCa). Studies have shown high rates of AS in the VA yet treatment variation exists between VAMCs, likely due to concern about missing aggressive disease. The 17-gene Genomic Prostate Score (GPS) has been validated to predict likelihood of favorable pathology in men with low risk PCa. This study compares treatment patterns before and after introduction of the GPS to determine if the assay assists in risk-stratification. Men with PCa who met National Comprehensive Cancer Network criteria for very low, low, or intermediate risk PCa were eligible. Chart review of men across 6 VAMCs established baseline treatment in untested patients in 2013-2014. In 2015, Veterans at the same VAMCs were offered the assay in a prospective study. Treatment recommendations and treatment implemented were captured.
To read the full abstract click here: http://meetinglibrary.asco.org/content/170703-176
Trends in Utilization of Novel Oral Therapeutic Agents for Men With Metastatic Castrate-Resistant Prostate Cancer Within the United States Veteran’s Affairs Health System.
Author(s): Elizabeth Henry, et al.
Background: Therapeutic options for men with metastatic castrate resistant prostate cancer (mCRPC) have expanded significantly over the past 5 years with several new agents demonstrating improved survival, including two oral agents. Abiraterone acetate, a CYP-17 androgen synthesis inhibitor, obtained initial FDA approval in 2011 for post-docetaxel (D) use, and gained expanded approval in 2012 for pre-D use. Enzalutamide, an androgen receptor signaling inhibitor, also gained FDA approval in 2012 (post-D) and indication was expanded in 2014 (pre-D). Due to the relatively recent approvals of these agents, there is limited data on rates of uptake and prescribing patterns for patients with mCRPC.
To read the full abstract click here: http://meetinglibrary.asco.org/content/157970-172
Liver
A Comparison of Liver Cancer Patients Receiving Palliative Care at Two Veteran Affairs Medical Centers.
Author(s): Sarah Lee, Zhen Wang, et al.
Background: Liver cancer is a leading cause of death. Lack of data exists on palliative care in this group, and care varies by location. The authors aim to determine if there are differences in palliative care for liver cancer patients by VA site.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171726-176
[Click through for : Radiation Oncology, Lung Cancer, Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Radiation Oncology
A Cross-Sectional View of Radiation Dose Fractionation Schemes Used for Treating Painful Bone Metastases (PBM) Within Veterans Health Administration’s Radiation Oncology Centers.
Author(s): George Anthony Dawson, et al.
Background: The use of single fraction radiotherapy in the treatment of PBM in the United States is now gaining clinical acceptance, especially at large institutional levels. In this cross-sectional report, the authors report the varying dose fractionation schemes used to treat PBM by VHA radiation oncology centers.
To read the full abstract click here: http://meetinglibrary.asco.org/content/161496-176
Will Academic and Community Physicians Engage and Share Knowledge in an Online Physician Social Network? Lessons from the Radiation Oncology Community.
Author(s): Nadine Housri, et al.
Background: The exponential growth of medical knowledge has made it increasingly difficult for clinicians to make sense of new information and recognize how to incorporate new research results into clinical practice. The authors sought to determine whether a social question and answer website designed to connect academic and community physicians would consistently engage radiation oncologists to share evidence based information and clinical insights with each other.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171497-176
Lung Cancer
Non–Small Cell Lung Cancer in Veterans: Disparities in Prevalence and Survival Among Different Histologic Subtypes.
Author(s): Hussein Assi, Efstratios Koutroumpakis, et al.
Background: Since the 1980s, adenocarcinoma (AC) of the lung has become more common than squamous cell carcinoma (SCC). Given the differences in smoking patterns, it is unclear if the same holds true among veterans. In this study, the authors compare the distribution of lung AC and SCC in the veterans’ population to that in the general population. They also looked at the survival of patients with different histologies in the VA population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167505-176
VA Oncologists’ Attitudes and Behaviors Regarding Genomic-Based Targeted Therapy for the Management of Advanced Lung Cancer.
Author(s): Jennifer Arney, et al.
Background: Genomic-based targeted therapy (GBTT) has emerged as a treatment option for patients with advanced lung cancer. Clinical practice guidelines of the National Comprehensive Cancer Network and the American Society of Clinical Oncology recommend testing all metastatic adenocarcinomas for EGFR mutation, and use of EGFR-TKIs (erlotinib) as first-line therapy for advanced adenocarcinoma lung cancer patients with EGFR mutation positive. Little is known about how oncologists utilize genomic testing and GBTT in a clinical setting. Drawing from the Cabana and colleagues theoretical framework on providers’ adherence to guidelines, this study aims to elicit provider and facility-level barriers and facilitators to using GBTT in VA healthcare delivery system.
To read the full abstract click here: http://meetinglibrary.asco.org/content/166517-176
[Click through for: Colorectal, HIV and Cancer, Palliative Care, Esophageal]
Colorectal
Survival Advantage for Hepatectomy With or Without Hemicolectomy in Colorectal Liver Metastases.
Author(s): Dalia A. Mobarek, et al.
Background: Surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study survival in patients presenting with hepatic metastasis at the outset and rates of surgical intervention in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/171640-176
Colorectal Liver Metastases Management in the Veterans Health Administration: Geographic Disparity.
Author(s): Dalia A. Mobarek, et al.
Background: Multidisciplinary management including surgical resection of colorectal liver metastases offers the greatest chance of long-term survival. The authors aimed to study surgical intervention types, rates and factors affecting the decision making in the VHA.
To read the full abstract click here: http://meetinglibrary.asco.org/content/160233-173
HIV and Cancer
Gleason Grade in HIV+ Versus Uninfected Prostate Cancer Patients in the Veterans Aging Cohort Study.
Author(s): Roxanne Jimmy Wadia, et al.
Background: Little is known about how the biology of prostate cancer in HIV+ men compares to that of uninfected men. The purpose of this study was to compare the Gleason grade (GL) of prostate adenocarcinoma (PrCA) in HIV+ vs uninfected patients in the combination antiretroviral therapy (ART) era (1997-present).
To read the full abstract click here: http://meetinglibrary.asco.org/content/162980-176
Disparities in AIDS-Related Kaposi Sarcoma Incidence and Survival.
Author(s): Firas El Chaer, et al.
Background: With advances in combined antiretroviral therapy (ART), Kaposi’s sarcoma (KS) incidence has decreased over the past decade. Geographical and racial disparities may contribute to variation in the incidence and outcomes of multiple cancers in the U.S., including KS. Using the Surveillance, Epidemiology, and End Results (SEER) database, the authors analyzed KS incidence and survival by race and geographical region in the ART era.
To read the full abstract click here: http://meetinglibrary.asco.org/content/168953-176
[Click through for : Palliative Care, Esophageal]
Palliative Care
Palliative Care Interventions and EOL Care Outcomes for Hepatocellular Patients (pts) at 2 VA Medical Centers.
Author(s): Zhen Wang, et al.
Background: Palliative care interventions and its effect on EOL outcomes for liver cancer pts have not been described. The authors investigated the association between palliative care intervention and EOL care outcomes.
To read the full abstract click here: http://meetinglibrary.asco.org/content/167081-176
Esophageal
Characteristics of Esophageal Carcinoma in a Veteran Population.
Author(s): Theresa Ratajczak, et al.
Background: Esophageal cancer is projected to increase by approximately 35% through 2025 in the U.S. The authors investigated the characteristics of esophageal carcinoma in a veteran population.
To read the full abstract click here: http://meetinglibrary.asco.org/content/162712-176
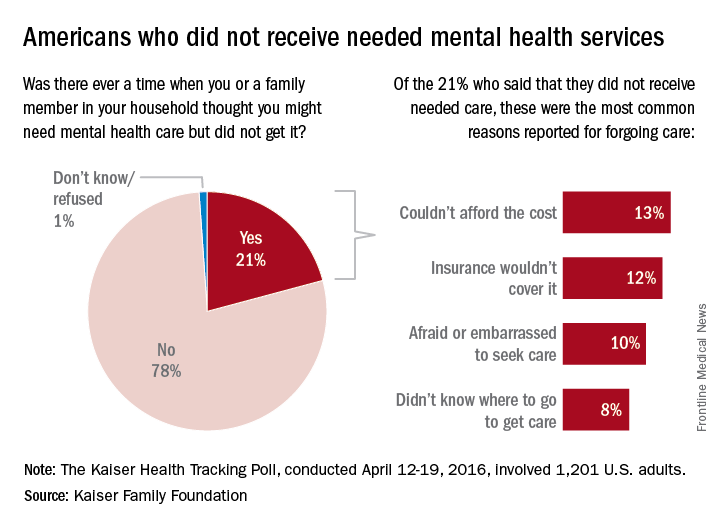
Antibodies cut factor VIII half-life in patients with hemophilia A
Non-neutralizing, factor VIII–specific IgG antibodies can contribute significantly to reductions in factor VIII half-life in patients with hemophilia A, according to a study published online in Blood.
Screening for factor VIII–specific IgG may aid in tailoring factor VIII prophylactic regimens for hemophilia A patients, said Christoph J. Hofbauer, Ph.D., of the biopharmaceutical company Baxalta in Vienna, and his associates at the Medical University of Vienna.
The researchers examined the effect of factor VIII–specific IgG antibodies on factor VIII half-life in 42 adult patients with hemophilia A without inhibitors. Patients ranged in age from 18-61 years, and 37 of them had severe disease. Of the cohort, 31 received recombinant factor VIII concentrates and 11 received plasma-derived factor VIII concentrates (Blood. 2016 May 23 [Epub ahead of print])
In the initial antibody screen, 15 patients tested positive for factor VIII–binding IgG with titers of at least 1:20. Factor VIII–specific antibodies were found at titers of at least 1:40 in 9 of the 15 subjects. Most had low- to moderate-affinity IgG1 and IgG3 antibodies. One patient with high-affinity IgG4 antibodies went on to develop low titers of factor VIII inhibitors.
Patients with factor VIII–specific antibodies had a shorter factor VIII half-life (median 7.8 hours) than did patients without antibodies (median 10.4 hours).
Dr. Hofbauer is employed by Baxalta, which makes a variety of antihemophilic factors.
On Twitter @maryjodales
Non-neutralizing, factor VIII–specific IgG antibodies can contribute significantly to reductions in factor VIII half-life in patients with hemophilia A, according to a study published online in Blood.
Screening for factor VIII–specific IgG may aid in tailoring factor VIII prophylactic regimens for hemophilia A patients, said Christoph J. Hofbauer, Ph.D., of the biopharmaceutical company Baxalta in Vienna, and his associates at the Medical University of Vienna.
The researchers examined the effect of factor VIII–specific IgG antibodies on factor VIII half-life in 42 adult patients with hemophilia A without inhibitors. Patients ranged in age from 18-61 years, and 37 of them had severe disease. Of the cohort, 31 received recombinant factor VIII concentrates and 11 received plasma-derived factor VIII concentrates (Blood. 2016 May 23 [Epub ahead of print])
In the initial antibody screen, 15 patients tested positive for factor VIII–binding IgG with titers of at least 1:20. Factor VIII–specific antibodies were found at titers of at least 1:40 in 9 of the 15 subjects. Most had low- to moderate-affinity IgG1 and IgG3 antibodies. One patient with high-affinity IgG4 antibodies went on to develop low titers of factor VIII inhibitors.
Patients with factor VIII–specific antibodies had a shorter factor VIII half-life (median 7.8 hours) than did patients without antibodies (median 10.4 hours).
Dr. Hofbauer is employed by Baxalta, which makes a variety of antihemophilic factors.
On Twitter @maryjodales
Non-neutralizing, factor VIII–specific IgG antibodies can contribute significantly to reductions in factor VIII half-life in patients with hemophilia A, according to a study published online in Blood.
Screening for factor VIII–specific IgG may aid in tailoring factor VIII prophylactic regimens for hemophilia A patients, said Christoph J. Hofbauer, Ph.D., of the biopharmaceutical company Baxalta in Vienna, and his associates at the Medical University of Vienna.
The researchers examined the effect of factor VIII–specific IgG antibodies on factor VIII half-life in 42 adult patients with hemophilia A without inhibitors. Patients ranged in age from 18-61 years, and 37 of them had severe disease. Of the cohort, 31 received recombinant factor VIII concentrates and 11 received plasma-derived factor VIII concentrates (Blood. 2016 May 23 [Epub ahead of print])
In the initial antibody screen, 15 patients tested positive for factor VIII–binding IgG with titers of at least 1:20. Factor VIII–specific antibodies were found at titers of at least 1:40 in 9 of the 15 subjects. Most had low- to moderate-affinity IgG1 and IgG3 antibodies. One patient with high-affinity IgG4 antibodies went on to develop low titers of factor VIII inhibitors.
Patients with factor VIII–specific antibodies had a shorter factor VIII half-life (median 7.8 hours) than did patients without antibodies (median 10.4 hours).
Dr. Hofbauer is employed by Baxalta, which makes a variety of antihemophilic factors.
On Twitter @maryjodales
FROM BLOOD
Key clinical point: Non-neutralizing, factor VIII–specific IgG antibodies can contribute significantly to reductions in factor VIII half-life in patients with hemophilia A.
Major finding: Patients with factor VIII–specific antibodies had a shorter factor VIII half-life (median 7.8 hours) than did patients without antibodies (median 10.4 hours).
Data source: 42 adult patients, ranging in age from 18-61 years, with hemophilia A without inhibitors, 37 of whom had severe disease.
Disclosures: Dr. Hofbauer is employed by Baxalta, which makes a variety of antihemophilic factors.
VIDEO: Surgical quality measures boost survival in cancer patients
BALTIMORE – Surgeons’ adherence to select quality measures when treating stage IIIA non–small-cell lung cancer patients led to improved patient survival, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
Researchers at Washington University in St. Louis identified 10,323 patients who received surgery for Stage IIIA NSCLC in the National Cancer Data Base from 2006 to 2010, and chose four quality measures that should have been met by surgeons: delivery of neoadjuvant multiagent chemotherapy (with or without radiation therapy); performing a lobectomy or greater resection; obtaining more than 10 lymph nodes, and achieving an R0 resection.
The researchers said 12.8% of patients met all four quality measures. Kaplan-Meier analysis demonstrated improved overall median survival by number of quality measures obtained: 0 quality measures, 12.7 months; 1 quality measure, 25.0 months; 2 quality measures, 31.4 months; 3 quality measures, 36.6 months; and 4 quality measures, 43.5 months.
In an interview, Dr. Mark S. Allen, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said the most striking result of the study was that such a low percentage of patients had all four quality measures performed for stage IIIA cancer. He called that finding “disappointing.”
“In general, [the study] shows there is still some work to be done to improve the quality when we operate on stage IIIA patients,” Dr. Allen said. “I’m not sure we do the greatest job of staging them clinically. When they are staged properly they probably do need preoperative chemotherapy, and I’m not sure we do that all the time.” He added that surgeon education about quality outcomes was critical to process improvement and patient outcomes.
Dr. Allen reported no relevant financial disclosures.
On Twitter @richpizzi
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BALTIMORE – Surgeons’ adherence to select quality measures when treating stage IIIA non–small-cell lung cancer patients led to improved patient survival, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
Researchers at Washington University in St. Louis identified 10,323 patients who received surgery for Stage IIIA NSCLC in the National Cancer Data Base from 2006 to 2010, and chose four quality measures that should have been met by surgeons: delivery of neoadjuvant multiagent chemotherapy (with or without radiation therapy); performing a lobectomy or greater resection; obtaining more than 10 lymph nodes, and achieving an R0 resection.
The researchers said 12.8% of patients met all four quality measures. Kaplan-Meier analysis demonstrated improved overall median survival by number of quality measures obtained: 0 quality measures, 12.7 months; 1 quality measure, 25.0 months; 2 quality measures, 31.4 months; 3 quality measures, 36.6 months; and 4 quality measures, 43.5 months.
In an interview, Dr. Mark S. Allen, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said the most striking result of the study was that such a low percentage of patients had all four quality measures performed for stage IIIA cancer. He called that finding “disappointing.”
“In general, [the study] shows there is still some work to be done to improve the quality when we operate on stage IIIA patients,” Dr. Allen said. “I’m not sure we do the greatest job of staging them clinically. When they are staged properly they probably do need preoperative chemotherapy, and I’m not sure we do that all the time.” He added that surgeon education about quality outcomes was critical to process improvement and patient outcomes.
Dr. Allen reported no relevant financial disclosures.
On Twitter @richpizzi
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BALTIMORE – Surgeons’ adherence to select quality measures when treating stage IIIA non–small-cell lung cancer patients led to improved patient survival, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
Researchers at Washington University in St. Louis identified 10,323 patients who received surgery for Stage IIIA NSCLC in the National Cancer Data Base from 2006 to 2010, and chose four quality measures that should have been met by surgeons: delivery of neoadjuvant multiagent chemotherapy (with or without radiation therapy); performing a lobectomy or greater resection; obtaining more than 10 lymph nodes, and achieving an R0 resection.
The researchers said 12.8% of patients met all four quality measures. Kaplan-Meier analysis demonstrated improved overall median survival by number of quality measures obtained: 0 quality measures, 12.7 months; 1 quality measure, 25.0 months; 2 quality measures, 31.4 months; 3 quality measures, 36.6 months; and 4 quality measures, 43.5 months.
In an interview, Dr. Mark S. Allen, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said the most striking result of the study was that such a low percentage of patients had all four quality measures performed for stage IIIA cancer. He called that finding “disappointing.”
“In general, [the study] shows there is still some work to be done to improve the quality when we operate on stage IIIA patients,” Dr. Allen said. “I’m not sure we do the greatest job of staging them clinically. When they are staged properly they probably do need preoperative chemotherapy, and I’m not sure we do that all the time.” He added that surgeon education about quality outcomes was critical to process improvement and patient outcomes.
Dr. Allen reported no relevant financial disclosures.
On Twitter @richpizzi
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AATS ANNUAL MEETING
Enlist appropriate psychological consults for gender dysphoria
ORLANDO – Old notions about transgender and transsexual individuals are changing, driven by the emergence of transgender identity, scientific evidence, political activism in those communities, and the gay and lesbian rights movement, as well as patients demanding to be part of the decision-making process.
The terminology has evolved as well. What was called transsexualism in the DSM-III in 1980 became gender identity disorder in the DSM-IV (1994; 2000), and in the current DSM-5 is gender dysphoria. Terminology is still in flux with the possibility that terminology may evolve to “gender incongruence.”
The umbrella term “transgender” now covers transsexual, crossdressing, bigender, drag queen/king, female/male impersonator, and gender queer, and probably more, according to Eli Coleman, Ph.D., professor and director of the program in human sexuality at the University of Minnesota, Minneapolis. “Probably the most proper term you hear now is ‘trans,’ ” he said during a session on transgender medicine at the annual meeting of the American Association of Clinical Endocrinologists.
The DSM-5 criteria for gender dysphoria include a marked incongruence between one’s experienced/expressed gender and the assigned gender for at least 6 months with at least two of the following: an incongruence between one’s felt gender identity and one’s primary and secondary sex characteristics, a strong desire to be rid of one’s gender, a strong desire for the primary and/or secondary sex characteristics of the other gender, a strong desire to be of the other gender (or an alternative gender from the assigned one), a strong desire to be treated as such, and a strong conviction that one has the typical feeling and reactions of the other gender. Dr. Coleman said a further criterion of the DSM-5 is that “the condition is associated with clinically significant distress or impairment in social, occupational, or other areas of functioning.”
A new view of trans
There is now an awareness of a spectrum of gender identity and an affirmation of the right of individuals to express that identity as they would like. Many treatment options exist, and it is up to the individual to decide which way and how far they want to go. Dr. Coleman quoted the late Virginia Prince, a transvestite and a pioneering transgender activist: “If you get on a train in Los Angeles bound for New York, you don’t have to go all the way to New York. If you want, you can get off in Chicago.” So even if a person wants to undergo medical transitioning, hormone therapy does not necessarily have to be followed by sex-reassignment surgery.
Dr. Coleman is past president of the World Professional Association for Transgender Health (WPATH, www.wpath.org), which has published “Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People,” Version 7, a set of guidelines for health care providers. The guidelines are designed help patients achieve comfort with their gendered selves, maximize their overall health and psychological well-being, and achieve self-fulfillment. If untreated or undertreated, gender dysphoria is associated with increased morbidity and mortality, according to WPATH. Hormone treatment may reduce gender dysphoria symptoms by reducing the characteristics of the original sex and inducing ones of the opposite sex. Hormones can be used before or after sex-reassignment surgery, or on their own for patients not seeking surgery who “want [to] … get off in Chicago,” Dr. Coleman said.
Gender nonconformity is not pathological
“Gender nonconformity is not pathological, yet gender dysphoria is a specific distress that can be alleviated through medically necessary treatment,” Dr. Coleman said. “Gender dysphoria may be classified as a mental disorder [but] not necessarily a lifetime diagnosis.” It is the stress of the dysphoria that may be diagnosable and treated, but being gender nonconforming in itself is not the target of treatment. He said mental health professionals therefore have an important role in addressing the negative effects of stigma and helping the individuals become comfortable with a form of gender expression that suits them. This role requires a mental health professional who is skilled in this area.
Treatments to try to change gender identity and expression to become congruent with the sex assigned at birth have failed in the past and are no longer considered ethical, he said. Dr. Coleman suggested that intervening with medical treatments early to suppress puberty and then offering feminizing or masculinizing hormone therapy at the appropriate time may be the best course of action to avoid or alleviate gender dysphoria. He said studies have shown that sex-reassignment surgery can provide “an undeniable effect” on outcomes such as “subjective well-being, cosmesis, and sexual function.”
He recommended that health care professionals become familiar with transgender health care issues, examine their own attitudes and beliefs about these issues, seek out educational opportunities, and get to know and consult with experts in transgender health care. But that is not enough.
“You’ll never understand the condition if you just listen to your patients in the office,” he said. “You’ve got to get into a car and talk to somebody or have coffee with them and really get to know them outside of the office.” That includes meeting with transgender people several years after they have transitioned, who can give insights into the process that are not apparent from just seeing patients in the office in the midst of their gender dysphoria.
The knowledge gained must also be imbued in the health care professional’s staff as well. “You’ve got to have the right attitudes, but you’ve got to train your whole staff about this … that [patients] cannot be treated badly at all,” he said.
ORLANDO – Old notions about transgender and transsexual individuals are changing, driven by the emergence of transgender identity, scientific evidence, political activism in those communities, and the gay and lesbian rights movement, as well as patients demanding to be part of the decision-making process.
The terminology has evolved as well. What was called transsexualism in the DSM-III in 1980 became gender identity disorder in the DSM-IV (1994; 2000), and in the current DSM-5 is gender dysphoria. Terminology is still in flux with the possibility that terminology may evolve to “gender incongruence.”
The umbrella term “transgender” now covers transsexual, crossdressing, bigender, drag queen/king, female/male impersonator, and gender queer, and probably more, according to Eli Coleman, Ph.D., professor and director of the program in human sexuality at the University of Minnesota, Minneapolis. “Probably the most proper term you hear now is ‘trans,’ ” he said during a session on transgender medicine at the annual meeting of the American Association of Clinical Endocrinologists.
The DSM-5 criteria for gender dysphoria include a marked incongruence between one’s experienced/expressed gender and the assigned gender for at least 6 months with at least two of the following: an incongruence between one’s felt gender identity and one’s primary and secondary sex characteristics, a strong desire to be rid of one’s gender, a strong desire for the primary and/or secondary sex characteristics of the other gender, a strong desire to be of the other gender (or an alternative gender from the assigned one), a strong desire to be treated as such, and a strong conviction that one has the typical feeling and reactions of the other gender. Dr. Coleman said a further criterion of the DSM-5 is that “the condition is associated with clinically significant distress or impairment in social, occupational, or other areas of functioning.”
A new view of trans
There is now an awareness of a spectrum of gender identity and an affirmation of the right of individuals to express that identity as they would like. Many treatment options exist, and it is up to the individual to decide which way and how far they want to go. Dr. Coleman quoted the late Virginia Prince, a transvestite and a pioneering transgender activist: “If you get on a train in Los Angeles bound for New York, you don’t have to go all the way to New York. If you want, you can get off in Chicago.” So even if a person wants to undergo medical transitioning, hormone therapy does not necessarily have to be followed by sex-reassignment surgery.
Dr. Coleman is past president of the World Professional Association for Transgender Health (WPATH, www.wpath.org), which has published “Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People,” Version 7, a set of guidelines for health care providers. The guidelines are designed help patients achieve comfort with their gendered selves, maximize their overall health and psychological well-being, and achieve self-fulfillment. If untreated or undertreated, gender dysphoria is associated with increased morbidity and mortality, according to WPATH. Hormone treatment may reduce gender dysphoria symptoms by reducing the characteristics of the original sex and inducing ones of the opposite sex. Hormones can be used before or after sex-reassignment surgery, or on their own for patients not seeking surgery who “want [to] … get off in Chicago,” Dr. Coleman said.
Gender nonconformity is not pathological
“Gender nonconformity is not pathological, yet gender dysphoria is a specific distress that can be alleviated through medically necessary treatment,” Dr. Coleman said. “Gender dysphoria may be classified as a mental disorder [but] not necessarily a lifetime diagnosis.” It is the stress of the dysphoria that may be diagnosable and treated, but being gender nonconforming in itself is not the target of treatment. He said mental health professionals therefore have an important role in addressing the negative effects of stigma and helping the individuals become comfortable with a form of gender expression that suits them. This role requires a mental health professional who is skilled in this area.
Treatments to try to change gender identity and expression to become congruent with the sex assigned at birth have failed in the past and are no longer considered ethical, he said. Dr. Coleman suggested that intervening with medical treatments early to suppress puberty and then offering feminizing or masculinizing hormone therapy at the appropriate time may be the best course of action to avoid or alleviate gender dysphoria. He said studies have shown that sex-reassignment surgery can provide “an undeniable effect” on outcomes such as “subjective well-being, cosmesis, and sexual function.”
He recommended that health care professionals become familiar with transgender health care issues, examine their own attitudes and beliefs about these issues, seek out educational opportunities, and get to know and consult with experts in transgender health care. But that is not enough.
“You’ll never understand the condition if you just listen to your patients in the office,” he said. “You’ve got to get into a car and talk to somebody or have coffee with them and really get to know them outside of the office.” That includes meeting with transgender people several years after they have transitioned, who can give insights into the process that are not apparent from just seeing patients in the office in the midst of their gender dysphoria.
The knowledge gained must also be imbued in the health care professional’s staff as well. “You’ve got to have the right attitudes, but you’ve got to train your whole staff about this … that [patients] cannot be treated badly at all,” he said.
ORLANDO – Old notions about transgender and transsexual individuals are changing, driven by the emergence of transgender identity, scientific evidence, political activism in those communities, and the gay and lesbian rights movement, as well as patients demanding to be part of the decision-making process.
The terminology has evolved as well. What was called transsexualism in the DSM-III in 1980 became gender identity disorder in the DSM-IV (1994; 2000), and in the current DSM-5 is gender dysphoria. Terminology is still in flux with the possibility that terminology may evolve to “gender incongruence.”
The umbrella term “transgender” now covers transsexual, crossdressing, bigender, drag queen/king, female/male impersonator, and gender queer, and probably more, according to Eli Coleman, Ph.D., professor and director of the program in human sexuality at the University of Minnesota, Minneapolis. “Probably the most proper term you hear now is ‘trans,’ ” he said during a session on transgender medicine at the annual meeting of the American Association of Clinical Endocrinologists.
The DSM-5 criteria for gender dysphoria include a marked incongruence between one’s experienced/expressed gender and the assigned gender for at least 6 months with at least two of the following: an incongruence between one’s felt gender identity and one’s primary and secondary sex characteristics, a strong desire to be rid of one’s gender, a strong desire for the primary and/or secondary sex characteristics of the other gender, a strong desire to be of the other gender (or an alternative gender from the assigned one), a strong desire to be treated as such, and a strong conviction that one has the typical feeling and reactions of the other gender. Dr. Coleman said a further criterion of the DSM-5 is that “the condition is associated with clinically significant distress or impairment in social, occupational, or other areas of functioning.”
A new view of trans
There is now an awareness of a spectrum of gender identity and an affirmation of the right of individuals to express that identity as they would like. Many treatment options exist, and it is up to the individual to decide which way and how far they want to go. Dr. Coleman quoted the late Virginia Prince, a transvestite and a pioneering transgender activist: “If you get on a train in Los Angeles bound for New York, you don’t have to go all the way to New York. If you want, you can get off in Chicago.” So even if a person wants to undergo medical transitioning, hormone therapy does not necessarily have to be followed by sex-reassignment surgery.
Dr. Coleman is past president of the World Professional Association for Transgender Health (WPATH, www.wpath.org), which has published “Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People,” Version 7, a set of guidelines for health care providers. The guidelines are designed help patients achieve comfort with their gendered selves, maximize their overall health and psychological well-being, and achieve self-fulfillment. If untreated or undertreated, gender dysphoria is associated with increased morbidity and mortality, according to WPATH. Hormone treatment may reduce gender dysphoria symptoms by reducing the characteristics of the original sex and inducing ones of the opposite sex. Hormones can be used before or after sex-reassignment surgery, or on their own for patients not seeking surgery who “want [to] … get off in Chicago,” Dr. Coleman said.
Gender nonconformity is not pathological
“Gender nonconformity is not pathological, yet gender dysphoria is a specific distress that can be alleviated through medically necessary treatment,” Dr. Coleman said. “Gender dysphoria may be classified as a mental disorder [but] not necessarily a lifetime diagnosis.” It is the stress of the dysphoria that may be diagnosable and treated, but being gender nonconforming in itself is not the target of treatment. He said mental health professionals therefore have an important role in addressing the negative effects of stigma and helping the individuals become comfortable with a form of gender expression that suits them. This role requires a mental health professional who is skilled in this area.
Treatments to try to change gender identity and expression to become congruent with the sex assigned at birth have failed in the past and are no longer considered ethical, he said. Dr. Coleman suggested that intervening with medical treatments early to suppress puberty and then offering feminizing or masculinizing hormone therapy at the appropriate time may be the best course of action to avoid or alleviate gender dysphoria. He said studies have shown that sex-reassignment surgery can provide “an undeniable effect” on outcomes such as “subjective well-being, cosmesis, and sexual function.”
He recommended that health care professionals become familiar with transgender health care issues, examine their own attitudes and beliefs about these issues, seek out educational opportunities, and get to know and consult with experts in transgender health care. But that is not enough.
“You’ll never understand the condition if you just listen to your patients in the office,” he said. “You’ve got to get into a car and talk to somebody or have coffee with them and really get to know them outside of the office.” That includes meeting with transgender people several years after they have transitioned, who can give insights into the process that are not apparent from just seeing patients in the office in the midst of their gender dysphoria.
The knowledge gained must also be imbued in the health care professional’s staff as well. “You’ve got to have the right attitudes, but you’ve got to train your whole staff about this … that [patients] cannot be treated badly at all,” he said.
EXPERT ANALYSIS FROM AACE 2016
Secukinumab may slow structural ankylosing spondylitis progression
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients maintained on open-label secukinumab treatment for 2 years showed a low level of structural spinal progression in an uncontrolled study. Also, in a placebo-controlled phase II study, 12 weeks’ treatment of patients with active ankylosing spondylitis with 5 mg tofacitinib bid led to significant clinical responses, compared with placebo.
Major finding: Little to no radiographic progression occurred in about 80% of ankylosing spondylitis patients maintained on secukinumab for 104 weeks.
Data source: The secukinumab study involved an open-label, nonrandomized extension of treatment in 168 of the 371 patients originally enrolled in MEASURE 1. The tofacitinib study included 208 patients.
Disclosures: Patients in the secukinumab study had been enrolled in MEASURE 1, a study sponsored by Novartis, the company that markets secukinumab (Cosentyx). Dr. Braun has been a consultant to Novartis, and several other drug companies, and three of his coauthors are Novartis employees. The tofacitinib study was sponsored by Pfizer, the company that markets tofacitinib (Xeljanz). Dr. van der Heijde has been a consultant to Pfizer and several other drug companies, and five of her coauthors are Pfizer employees.
Growths on neck and beard area
The FP diagnosed flat warts based on their typical appearance and location. Flat warts are frequently found on the face and neck and can easily be spread when men shave their beards or women shave their legs. Flat warts can also occur on the face of young children.
The FP suggested cryotherapy for the largest warts and prescribed topical imiquimod (3 times per week) for home use. The FP suggested that the patient wait 2 weeks before starting the imiquimod to allow the cryotherapy to do its work and for any blistering or irritation to subside. A follow-up appointment was set for 6 weeks.
At the follow-up visit, there was little improvement and the patient wanted to try an alternative therapy. The risks and benefits of Candida antigen intralesional injection were discussed and the patient decided to proceed with this approach. Intralesional injections with Candida antigen induce a localized, cell-mediated, and human papillomavirus (HPV)-specific response that may target the injected wart as well as more distant warts. This method has moderate effectiveness (60% cure rates) for treatment of recalcitrant warts. The Candida antigen must be diluted before use, and 0.1 to 0.3 mL should be injected into the largest warts using a 30-gauge needle (up to 1 mL per treatment). Warn patients to expect itching in the area, burning, or peeling, and repeat the procedure every 4 weeks with up to 3 treatments or until warts are gone.
For this patient, the Candida antigen was diluted with normal saline (0.25 mL of generic Candida antigen with 0.75 mL of normal saline). The mixture was injected with a 30-gauge needle into approximately 10 of the largest flat warts. One month later, all of the flat warts were gone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Flat warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:755-758.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed flat warts based on their typical appearance and location. Flat warts are frequently found on the face and neck and can easily be spread when men shave their beards or women shave their legs. Flat warts can also occur on the face of young children.
The FP suggested cryotherapy for the largest warts and prescribed topical imiquimod (3 times per week) for home use. The FP suggested that the patient wait 2 weeks before starting the imiquimod to allow the cryotherapy to do its work and for any blistering or irritation to subside. A follow-up appointment was set for 6 weeks.
At the follow-up visit, there was little improvement and the patient wanted to try an alternative therapy. The risks and benefits of Candida antigen intralesional injection were discussed and the patient decided to proceed with this approach. Intralesional injections with Candida antigen induce a localized, cell-mediated, and human papillomavirus (HPV)-specific response that may target the injected wart as well as more distant warts. This method has moderate effectiveness (60% cure rates) for treatment of recalcitrant warts. The Candida antigen must be diluted before use, and 0.1 to 0.3 mL should be injected into the largest warts using a 30-gauge needle (up to 1 mL per treatment). Warn patients to expect itching in the area, burning, or peeling, and repeat the procedure every 4 weeks with up to 3 treatments or until warts are gone.
For this patient, the Candida antigen was diluted with normal saline (0.25 mL of generic Candida antigen with 0.75 mL of normal saline). The mixture was injected with a 30-gauge needle into approximately 10 of the largest flat warts. One month later, all of the flat warts were gone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Flat warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:755-758.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed flat warts based on their typical appearance and location. Flat warts are frequently found on the face and neck and can easily be spread when men shave their beards or women shave their legs. Flat warts can also occur on the face of young children.
The FP suggested cryotherapy for the largest warts and prescribed topical imiquimod (3 times per week) for home use. The FP suggested that the patient wait 2 weeks before starting the imiquimod to allow the cryotherapy to do its work and for any blistering or irritation to subside. A follow-up appointment was set for 6 weeks.
At the follow-up visit, there was little improvement and the patient wanted to try an alternative therapy. The risks and benefits of Candida antigen intralesional injection were discussed and the patient decided to proceed with this approach. Intralesional injections with Candida antigen induce a localized, cell-mediated, and human papillomavirus (HPV)-specific response that may target the injected wart as well as more distant warts. This method has moderate effectiveness (60% cure rates) for treatment of recalcitrant warts. The Candida antigen must be diluted before use, and 0.1 to 0.3 mL should be injected into the largest warts using a 30-gauge needle (up to 1 mL per treatment). Warn patients to expect itching in the area, burning, or peeling, and repeat the procedure every 4 weeks with up to 3 treatments or until warts are gone.
For this patient, the Candida antigen was diluted with normal saline (0.25 mL of generic Candida antigen with 0.75 mL of normal saline). The mixture was injected with a 30-gauge needle into approximately 10 of the largest flat warts. One month later, all of the flat warts were gone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Flat warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:755-758.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Lesions on foot
The FP diagnosed this patient with common warts. While any child can develop a large cluster of warts, the FP decided to ask more about the family’s health in case this was a sign of immunosuppression. The mother acknowledged that she was positive for human immunodeficiency virus (HIV) and that the father had passed away from acquired immune deficiency syndrome (AIDS). She consented to HIV testing for her son and he tested positive. Unfortunately, the mother did not have antiretroviral therapy during her pregnancy with this child.
While there were resources within the village for HIV treatment, there were no resources for wart treatment. Because the child could receive treatment for HIV, the warts could resolve on their own as his immune system improved. Even immunocompetent children will often see their warts completely disappear over time.
In settings with greater resources, typical treatments for common warts include: cryotherapy, topical salicylic acid, topical cantharidin, topical imiquimod, and intralesional immunotherapy. Intralesional immunotherapy can be performed with Candida antigens, the measles, mumps, and rubella vaccine, and a purified protein derivative (as used for tuberculosis testing). Other less commonly used treatments include lasers, photodynamic therapy, contact immunotherapy, hypnosis, oral cimetidine, duct tape, and surgery.
The reason there are so many treatments is that no single treatment works well enough in most cases. Based on a Cochrane review, the strongest evidence supports topical salicylic acid and cryotherapy.1 Immunosuppressed patients can sometimes have the most recalcitrant warts, but treatment of the immune system, along with typical treatment options, can still be successful.
1. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001;(2):CD001781.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Common warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:749-754.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with common warts. While any child can develop a large cluster of warts, the FP decided to ask more about the family’s health in case this was a sign of immunosuppression. The mother acknowledged that she was positive for human immunodeficiency virus (HIV) and that the father had passed away from acquired immune deficiency syndrome (AIDS). She consented to HIV testing for her son and he tested positive. Unfortunately, the mother did not have antiretroviral therapy during her pregnancy with this child.
While there were resources within the village for HIV treatment, there were no resources for wart treatment. Because the child could receive treatment for HIV, the warts could resolve on their own as his immune system improved. Even immunocompetent children will often see their warts completely disappear over time.
In settings with greater resources, typical treatments for common warts include: cryotherapy, topical salicylic acid, topical cantharidin, topical imiquimod, and intralesional immunotherapy. Intralesional immunotherapy can be performed with Candida antigens, the measles, mumps, and rubella vaccine, and a purified protein derivative (as used for tuberculosis testing). Other less commonly used treatments include lasers, photodynamic therapy, contact immunotherapy, hypnosis, oral cimetidine, duct tape, and surgery.
The reason there are so many treatments is that no single treatment works well enough in most cases. Based on a Cochrane review, the strongest evidence supports topical salicylic acid and cryotherapy.1 Immunosuppressed patients can sometimes have the most recalcitrant warts, but treatment of the immune system, along with typical treatment options, can still be successful.
1. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001;(2):CD001781.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Common warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:749-754.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with common warts. While any child can develop a large cluster of warts, the FP decided to ask more about the family’s health in case this was a sign of immunosuppression. The mother acknowledged that she was positive for human immunodeficiency virus (HIV) and that the father had passed away from acquired immune deficiency syndrome (AIDS). She consented to HIV testing for her son and he tested positive. Unfortunately, the mother did not have antiretroviral therapy during her pregnancy with this child.
While there were resources within the village for HIV treatment, there were no resources for wart treatment. Because the child could receive treatment for HIV, the warts could resolve on their own as his immune system improved. Even immunocompetent children will often see their warts completely disappear over time.
In settings with greater resources, typical treatments for common warts include: cryotherapy, topical salicylic acid, topical cantharidin, topical imiquimod, and intralesional immunotherapy. Intralesional immunotherapy can be performed with Candida antigens, the measles, mumps, and rubella vaccine, and a purified protein derivative (as used for tuberculosis testing). Other less commonly used treatments include lasers, photodynamic therapy, contact immunotherapy, hypnosis, oral cimetidine, duct tape, and surgery.
The reason there are so many treatments is that no single treatment works well enough in most cases. Based on a Cochrane review, the strongest evidence supports topical salicylic acid and cryotherapy.1 Immunosuppressed patients can sometimes have the most recalcitrant warts, but treatment of the immune system, along with typical treatment options, can still be successful.
1. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001;(2):CD001781.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Common warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:749-754.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Bevacizumab offers best value of anti-VEGF drugs to treat DME
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
FROM JAMA OPHTHALMOLOGY
Key clinical point: Bevacizumab remains the best option, in terms of price and value, over aflibercept and ranibizumab for treatment of diabetic macular edema.
Major finding: Based on incremental cost-effectiveness ratios (ICERs) over 1- and 10-year periods, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, to match the cost of bevacizumab over the same time periods.
Data source: Post hoc analysis of patients enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial at 1-year follow-up.
Disclosures: The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures.
Weighty issues: Exploring the connection between diabetes, depression
“You’re wearing an Unna boot – what happened?”
“Doc, my wife made too many tempting desserts while we were in Florida, and when we got back, I had an infected toe. My doctor gave me antibiotics, but the toe turned blue, so they had to amputate.”
I had been treating this man for depression for many years and did not know about his having diabetes, so I asked, “Are you diabetic?”
“No,” he answered. “My doctor says I’ve been prediabetic for 20 years, and he’s put me on a low dose of metformin. … My friends are on twice as much. I don’t even have to have one of those meters.
“I can just go to the senior center or stop by my doctor’s office once a week and have my sugar checked. My wife says she won’t tempt me with any more desserts; she’s buying fruit, which I’m not used to, and I’m not eating bread anymore.
“Hey, those pills you’re giving me for my depression are working great. I am eating and sleeping and doing things I love to do. The wife and I are getting over the kids’ divorces, and we are still able to help out with the grandchild, who has been so sick. When we got back home, I tried to get back with my poker buddies, but one of them is in cardiac rehab; he had a heart attack, and another one, his wife says he’s got Alzheimer’s – he did lose a lot last year and that wasn’t like him. … Yeah, I guess I’m OK. As soon as this toe heals, I’ll be 100%.”
This dialogue is a composite; the names have been changed to protect the innocent, but unfortunately, it is an oft-told tale. The relationship between diabetes and depression has been known for a long time.1Each one is a risk factor for the other, and together and separately, they are a risk factor for dementia.
For quite a while, it was thought that having a diabetes diagnosis and having to manage it in and of itself was depressing, and that therefore, people would become depressed. It was also thought that people who are depressed might try to soothe themselves with copious amounts of comfort food and alcohol, and would thereby develop diabetes. Certainly, many people’s routes to depression and diabetes are just that – psychological reactions to having the other disease. But research shows that there is a much deeper physiologic relationship between the two.
Since diabetes and depression or their sequelae are among the 14 leading causes of death in the United States, psychiatrists and other medical professionals need to collaborate in the treatment of these diseases in their patients. Medical homes are good, but most patients continue to receive treatment for all disorders separately and in isolation. If it were not for the medical professional asking directly, or discovering some medication after the patient has given permission for an electronic medication prescribing overview of all his medications, treatment for diabetes or depression might be unknown by the other medical professional. Our noncommunicative EHRs will not help here. The only thing that will help is open communication between the patient and all of his medical treaters.
Now that I am educated and alarmed about the diabetes-depression connection, I send a note to the primary care physician and follow up with a few articles from Science Daily such as “Depression, early death among seniors with diabetes: Strong link found by research,”2 or “Treating major depression in older adults with diabetes may lower risk of death”3, or the clincher, “Treatment for diabetes and depression improves both, researchers say.”4
For patients with type 2 diabetes, the form of the illness usually referred to in research on diabetes and depression, the body becomes insensitive to insulin, i.e., insulin resistance develops. We now know that insulin resistance occurs throughout the body, including the brain. Insulin receptors are present in all organs of the body, including the brain. We also know that the higher fasting glucose level seen in prediabetes is an indication of the development of insulin resistance. Insulin’s job is to get glucose into cells for ready availability of energy and into muscle for backup energy.
If glucose is too plentiful, as it is when sugary foods are overconsumed, insulin directs the rest of the glucose to be stored as fat in the liver, inside blood vessels, around organs, and subcutaneously. Ultimately, there is nowhere else to store the excess energy, and insulin resistance develops. The pancreas, which secretes insulin, keeps on pumping insulin and can poop out, requiring exogenous insulin to keep things moving.5Treatments can include insulin itself, medications that increase insulin sensitivity, diet, and exercise to deplete the energy stores, or bariatric surgery, which, by the way, is said to cure both diabetes and depression within 3 weeks after surgery (this effect is negated if patients regain their weight.)
What the research shows
Clinical research from the University of Pennsylvania6 and Massachusetts General Hospital7shows that having a third, nonphysician treater work with patients diagnosed with both disorders improves outcomes. Both of those protocols used cognitive-behavioral therapy (CBT) and motivational interviewing, group treatment, and telephone contact as modalities. One also used electronic monitoring of medication dosing and the record of the glucometer to follow patients’ progress.
In both studies, patients in the protocol groups did better than the treatment-as-usual groups in terms of relief of depression and control of diabetes. In the private primary care physician and psychiatrist office setting, a third party is not practical, but psychiatrists can add motivational interviewing and some aspects of CBT. Also, both psychiatrists and primary care physicians can use electronic medication monitoring and blood glucose monitoring. Recently, Apple released apps that the company said will make it easy for patients with those devices8, but the old glucometer and pharmacy follow-up for prescriptions also can be useful. Medication (bottle cap) monitors can be expensive and may not be practical for some patients.
A prospective study of 2,525 patients showed that those with depression and metabolic risk factors were more than six times more likely to develop diabetes than patients who had depression alone, metabolic risk factors alone, or neither. These results allow for gross sorting out of which people with depression are more likely to develop diabetes.9This can provide an opportunity to intervene before diabetes sets in – and would have saved the toe of the patient I described earlier.
At the cellular level, at least in mice, it appears that insulin resistance in the brain alters dopamine turnover and causes behavioral disorders that look like anxiety and depression.10Mice with a brain-specific knockout of the insulin receptor showed “mitochondrial dysfunction and oxidative distress in the dorsal striatum and the nucleus accumbens. Increased levels of MAO A and B leading to increased turnover of dopamine in the mesolimbic system were also observed.”
The depression in these mice was relieved with the use of imipramine and phenelzine, and the researchers also noted that previous research had shown a decrease in depressive-like behavior with the insulin sensitizer rosiglitazone, which reduces glucose in the brain when given to obese, diabetic mice. Certainly, further research is necessary, as is research in humans. But this demonstrates what might be happening to our patients who have metabolic syndrome or diabetes and depression, and may offer suggestions for appropriate treatments.
“If you see something, say something.”
In short, early effective intervention in the metabolic/prediabetes state is best. Taking weights and heights, calculating BMIs, and either measuring or observing waist circumference, can give us a hunch that metabolic syndrome exists. We do our patients a favor if we mention this – and enlist their curiosity and efforts in avoiding or mitigating the ravages of diabetes and worsening depression.
Dr. Harris, a diplomate of the American Board of Obesity Medicine, is in private practice and adult and geriatric psychiatry in Hartford, Conn. She also works as a psychiatric consultant to continuing care retirement organizations and professional groups. Dr. Harris, a former president of the Black Psychiatrists of America, is a Distinguished Fellow of the American Psychiatric Association. Besides psychotherapy, her major clinical interests include geriatrics, and the interface between general medicine and psychiatry.
References
1. U.S. Medicine, November 2009.
2. Science Daily, March 29, 2014.
3. Science Daily, Jan. 27, 2016.
4. Science Daily, Jan. 18, 2012.
5. “Diabetes Facts and Guidelines,” Yale Diabetes Center, 2011.
6. Ann Fam Med. 2012 Jan-Feb;10(1):15-22.
7. Diabetes Care. 2014;37(3):625-33.
8. Macworld, May 10, 2016.
9. Mol Psychiatry. 2016 Feb 23. doi: 10:1038/mp 2016.7.
10. Proc Natl Acad Sci USA. 2014 Mar 17;112(11):3463-8.
“You’re wearing an Unna boot – what happened?”
“Doc, my wife made too many tempting desserts while we were in Florida, and when we got back, I had an infected toe. My doctor gave me antibiotics, but the toe turned blue, so they had to amputate.”
I had been treating this man for depression for many years and did not know about his having diabetes, so I asked, “Are you diabetic?”
“No,” he answered. “My doctor says I’ve been prediabetic for 20 years, and he’s put me on a low dose of metformin. … My friends are on twice as much. I don’t even have to have one of those meters.
“I can just go to the senior center or stop by my doctor’s office once a week and have my sugar checked. My wife says she won’t tempt me with any more desserts; she’s buying fruit, which I’m not used to, and I’m not eating bread anymore.
“Hey, those pills you’re giving me for my depression are working great. I am eating and sleeping and doing things I love to do. The wife and I are getting over the kids’ divorces, and we are still able to help out with the grandchild, who has been so sick. When we got back home, I tried to get back with my poker buddies, but one of them is in cardiac rehab; he had a heart attack, and another one, his wife says he’s got Alzheimer’s – he did lose a lot last year and that wasn’t like him. … Yeah, I guess I’m OK. As soon as this toe heals, I’ll be 100%.”
This dialogue is a composite; the names have been changed to protect the innocent, but unfortunately, it is an oft-told tale. The relationship between diabetes and depression has been known for a long time.1Each one is a risk factor for the other, and together and separately, they are a risk factor for dementia.
For quite a while, it was thought that having a diabetes diagnosis and having to manage it in and of itself was depressing, and that therefore, people would become depressed. It was also thought that people who are depressed might try to soothe themselves with copious amounts of comfort food and alcohol, and would thereby develop diabetes. Certainly, many people’s routes to depression and diabetes are just that – psychological reactions to having the other disease. But research shows that there is a much deeper physiologic relationship between the two.
Since diabetes and depression or their sequelae are among the 14 leading causes of death in the United States, psychiatrists and other medical professionals need to collaborate in the treatment of these diseases in their patients. Medical homes are good, but most patients continue to receive treatment for all disorders separately and in isolation. If it were not for the medical professional asking directly, or discovering some medication after the patient has given permission for an electronic medication prescribing overview of all his medications, treatment for diabetes or depression might be unknown by the other medical professional. Our noncommunicative EHRs will not help here. The only thing that will help is open communication between the patient and all of his medical treaters.
Now that I am educated and alarmed about the diabetes-depression connection, I send a note to the primary care physician and follow up with a few articles from Science Daily such as “Depression, early death among seniors with diabetes: Strong link found by research,”2 or “Treating major depression in older adults with diabetes may lower risk of death”3, or the clincher, “Treatment for diabetes and depression improves both, researchers say.”4
For patients with type 2 diabetes, the form of the illness usually referred to in research on diabetes and depression, the body becomes insensitive to insulin, i.e., insulin resistance develops. We now know that insulin resistance occurs throughout the body, including the brain. Insulin receptors are present in all organs of the body, including the brain. We also know that the higher fasting glucose level seen in prediabetes is an indication of the development of insulin resistance. Insulin’s job is to get glucose into cells for ready availability of energy and into muscle for backup energy.
If glucose is too plentiful, as it is when sugary foods are overconsumed, insulin directs the rest of the glucose to be stored as fat in the liver, inside blood vessels, around organs, and subcutaneously. Ultimately, there is nowhere else to store the excess energy, and insulin resistance develops. The pancreas, which secretes insulin, keeps on pumping insulin and can poop out, requiring exogenous insulin to keep things moving.5Treatments can include insulin itself, medications that increase insulin sensitivity, diet, and exercise to deplete the energy stores, or bariatric surgery, which, by the way, is said to cure both diabetes and depression within 3 weeks after surgery (this effect is negated if patients regain their weight.)
What the research shows
Clinical research from the University of Pennsylvania6 and Massachusetts General Hospital7shows that having a third, nonphysician treater work with patients diagnosed with both disorders improves outcomes. Both of those protocols used cognitive-behavioral therapy (CBT) and motivational interviewing, group treatment, and telephone contact as modalities. One also used electronic monitoring of medication dosing and the record of the glucometer to follow patients’ progress.
In both studies, patients in the protocol groups did better than the treatment-as-usual groups in terms of relief of depression and control of diabetes. In the private primary care physician and psychiatrist office setting, a third party is not practical, but psychiatrists can add motivational interviewing and some aspects of CBT. Also, both psychiatrists and primary care physicians can use electronic medication monitoring and blood glucose monitoring. Recently, Apple released apps that the company said will make it easy for patients with those devices8, but the old glucometer and pharmacy follow-up for prescriptions also can be useful. Medication (bottle cap) monitors can be expensive and may not be practical for some patients.
A prospective study of 2,525 patients showed that those with depression and metabolic risk factors were more than six times more likely to develop diabetes than patients who had depression alone, metabolic risk factors alone, or neither. These results allow for gross sorting out of which people with depression are more likely to develop diabetes.9This can provide an opportunity to intervene before diabetes sets in – and would have saved the toe of the patient I described earlier.
At the cellular level, at least in mice, it appears that insulin resistance in the brain alters dopamine turnover and causes behavioral disorders that look like anxiety and depression.10Mice with a brain-specific knockout of the insulin receptor showed “mitochondrial dysfunction and oxidative distress in the dorsal striatum and the nucleus accumbens. Increased levels of MAO A and B leading to increased turnover of dopamine in the mesolimbic system were also observed.”
The depression in these mice was relieved with the use of imipramine and phenelzine, and the researchers also noted that previous research had shown a decrease in depressive-like behavior with the insulin sensitizer rosiglitazone, which reduces glucose in the brain when given to obese, diabetic mice. Certainly, further research is necessary, as is research in humans. But this demonstrates what might be happening to our patients who have metabolic syndrome or diabetes and depression, and may offer suggestions for appropriate treatments.
“If you see something, say something.”
In short, early effective intervention in the metabolic/prediabetes state is best. Taking weights and heights, calculating BMIs, and either measuring or observing waist circumference, can give us a hunch that metabolic syndrome exists. We do our patients a favor if we mention this – and enlist their curiosity and efforts in avoiding or mitigating the ravages of diabetes and worsening depression.
Dr. Harris, a diplomate of the American Board of Obesity Medicine, is in private practice and adult and geriatric psychiatry in Hartford, Conn. She also works as a psychiatric consultant to continuing care retirement organizations and professional groups. Dr. Harris, a former president of the Black Psychiatrists of America, is a Distinguished Fellow of the American Psychiatric Association. Besides psychotherapy, her major clinical interests include geriatrics, and the interface between general medicine and psychiatry.
References
1. U.S. Medicine, November 2009.
2. Science Daily, March 29, 2014.
3. Science Daily, Jan. 27, 2016.
4. Science Daily, Jan. 18, 2012.
5. “Diabetes Facts and Guidelines,” Yale Diabetes Center, 2011.
6. Ann Fam Med. 2012 Jan-Feb;10(1):15-22.
7. Diabetes Care. 2014;37(3):625-33.
8. Macworld, May 10, 2016.
9. Mol Psychiatry. 2016 Feb 23. doi: 10:1038/mp 2016.7.
10. Proc Natl Acad Sci USA. 2014 Mar 17;112(11):3463-8.
“You’re wearing an Unna boot – what happened?”
“Doc, my wife made too many tempting desserts while we were in Florida, and when we got back, I had an infected toe. My doctor gave me antibiotics, but the toe turned blue, so they had to amputate.”
I had been treating this man for depression for many years and did not know about his having diabetes, so I asked, “Are you diabetic?”
“No,” he answered. “My doctor says I’ve been prediabetic for 20 years, and he’s put me on a low dose of metformin. … My friends are on twice as much. I don’t even have to have one of those meters.
“I can just go to the senior center or stop by my doctor’s office once a week and have my sugar checked. My wife says she won’t tempt me with any more desserts; she’s buying fruit, which I’m not used to, and I’m not eating bread anymore.
“Hey, those pills you’re giving me for my depression are working great. I am eating and sleeping and doing things I love to do. The wife and I are getting over the kids’ divorces, and we are still able to help out with the grandchild, who has been so sick. When we got back home, I tried to get back with my poker buddies, but one of them is in cardiac rehab; he had a heart attack, and another one, his wife says he’s got Alzheimer’s – he did lose a lot last year and that wasn’t like him. … Yeah, I guess I’m OK. As soon as this toe heals, I’ll be 100%.”
This dialogue is a composite; the names have been changed to protect the innocent, but unfortunately, it is an oft-told tale. The relationship between diabetes and depression has been known for a long time.1Each one is a risk factor for the other, and together and separately, they are a risk factor for dementia.
For quite a while, it was thought that having a diabetes diagnosis and having to manage it in and of itself was depressing, and that therefore, people would become depressed. It was also thought that people who are depressed might try to soothe themselves with copious amounts of comfort food and alcohol, and would thereby develop diabetes. Certainly, many people’s routes to depression and diabetes are just that – psychological reactions to having the other disease. But research shows that there is a much deeper physiologic relationship between the two.
Since diabetes and depression or their sequelae are among the 14 leading causes of death in the United States, psychiatrists and other medical professionals need to collaborate in the treatment of these diseases in their patients. Medical homes are good, but most patients continue to receive treatment for all disorders separately and in isolation. If it were not for the medical professional asking directly, or discovering some medication after the patient has given permission for an electronic medication prescribing overview of all his medications, treatment for diabetes or depression might be unknown by the other medical professional. Our noncommunicative EHRs will not help here. The only thing that will help is open communication between the patient and all of his medical treaters.
Now that I am educated and alarmed about the diabetes-depression connection, I send a note to the primary care physician and follow up with a few articles from Science Daily such as “Depression, early death among seniors with diabetes: Strong link found by research,”2 or “Treating major depression in older adults with diabetes may lower risk of death”3, or the clincher, “Treatment for diabetes and depression improves both, researchers say.”4
For patients with type 2 diabetes, the form of the illness usually referred to in research on diabetes and depression, the body becomes insensitive to insulin, i.e., insulin resistance develops. We now know that insulin resistance occurs throughout the body, including the brain. Insulin receptors are present in all organs of the body, including the brain. We also know that the higher fasting glucose level seen in prediabetes is an indication of the development of insulin resistance. Insulin’s job is to get glucose into cells for ready availability of energy and into muscle for backup energy.
If glucose is too plentiful, as it is when sugary foods are overconsumed, insulin directs the rest of the glucose to be stored as fat in the liver, inside blood vessels, around organs, and subcutaneously. Ultimately, there is nowhere else to store the excess energy, and insulin resistance develops. The pancreas, which secretes insulin, keeps on pumping insulin and can poop out, requiring exogenous insulin to keep things moving.5Treatments can include insulin itself, medications that increase insulin sensitivity, diet, and exercise to deplete the energy stores, or bariatric surgery, which, by the way, is said to cure both diabetes and depression within 3 weeks after surgery (this effect is negated if patients regain their weight.)
What the research shows
Clinical research from the University of Pennsylvania6 and Massachusetts General Hospital7shows that having a third, nonphysician treater work with patients diagnosed with both disorders improves outcomes. Both of those protocols used cognitive-behavioral therapy (CBT) and motivational interviewing, group treatment, and telephone contact as modalities. One also used electronic monitoring of medication dosing and the record of the glucometer to follow patients’ progress.
In both studies, patients in the protocol groups did better than the treatment-as-usual groups in terms of relief of depression and control of diabetes. In the private primary care physician and psychiatrist office setting, a third party is not practical, but psychiatrists can add motivational interviewing and some aspects of CBT. Also, both psychiatrists and primary care physicians can use electronic medication monitoring and blood glucose monitoring. Recently, Apple released apps that the company said will make it easy for patients with those devices8, but the old glucometer and pharmacy follow-up for prescriptions also can be useful. Medication (bottle cap) monitors can be expensive and may not be practical for some patients.
A prospective study of 2,525 patients showed that those with depression and metabolic risk factors were more than six times more likely to develop diabetes than patients who had depression alone, metabolic risk factors alone, or neither. These results allow for gross sorting out of which people with depression are more likely to develop diabetes.9This can provide an opportunity to intervene before diabetes sets in – and would have saved the toe of the patient I described earlier.
At the cellular level, at least in mice, it appears that insulin resistance in the brain alters dopamine turnover and causes behavioral disorders that look like anxiety and depression.10Mice with a brain-specific knockout of the insulin receptor showed “mitochondrial dysfunction and oxidative distress in the dorsal striatum and the nucleus accumbens. Increased levels of MAO A and B leading to increased turnover of dopamine in the mesolimbic system were also observed.”
The depression in these mice was relieved with the use of imipramine and phenelzine, and the researchers also noted that previous research had shown a decrease in depressive-like behavior with the insulin sensitizer rosiglitazone, which reduces glucose in the brain when given to obese, diabetic mice. Certainly, further research is necessary, as is research in humans. But this demonstrates what might be happening to our patients who have metabolic syndrome or diabetes and depression, and may offer suggestions for appropriate treatments.
“If you see something, say something.”
In short, early effective intervention in the metabolic/prediabetes state is best. Taking weights and heights, calculating BMIs, and either measuring or observing waist circumference, can give us a hunch that metabolic syndrome exists. We do our patients a favor if we mention this – and enlist their curiosity and efforts in avoiding or mitigating the ravages of diabetes and worsening depression.
Dr. Harris, a diplomate of the American Board of Obesity Medicine, is in private practice and adult and geriatric psychiatry in Hartford, Conn. She also works as a psychiatric consultant to continuing care retirement organizations and professional groups. Dr. Harris, a former president of the Black Psychiatrists of America, is a Distinguished Fellow of the American Psychiatric Association. Besides psychotherapy, her major clinical interests include geriatrics, and the interface between general medicine and psychiatry.
References
1. U.S. Medicine, November 2009.
2. Science Daily, March 29, 2014.
3. Science Daily, Jan. 27, 2016.
4. Science Daily, Jan. 18, 2012.
5. “Diabetes Facts and Guidelines,” Yale Diabetes Center, 2011.
6. Ann Fam Med. 2012 Jan-Feb;10(1):15-22.
7. Diabetes Care. 2014;37(3):625-33.
8. Macworld, May 10, 2016.
9. Mol Psychiatry. 2016 Feb 23. doi: 10:1038/mp 2016.7.
10. Proc Natl Acad Sci USA. 2014 Mar 17;112(11):3463-8.
Over 20% of Americans skipped needed mental health care
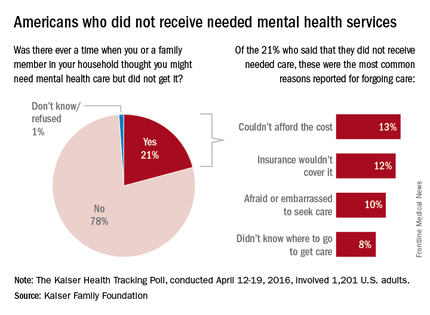
About one in five Americans report that there was a time when they or a family member in their household thought they needed mental health care but did not receive it for various reasons, according to a poll conducted by the Kaiser Family Foundation.
Among the 21% of Americans who did not get such care, the most common reason was “couldn’t afford the cost” (13%), which was closely followed by “insurance wouldn’t cover it” at 12%. “Afraid or embarrassed to seek care” was cited by 10% of those who had forgone mental health care, and 8% said that the reason was “didn’t know where to go to get care,” the Kaiser report said.
The Kaiser Health Tracking Poll was conducted April 12-19, 2016, among a nationally representative sample of 1,201 adults aged 18 years and older.

About one in five Americans report that there was a time when they or a family member in their household thought they needed mental health care but did not receive it for various reasons, according to a poll conducted by the Kaiser Family Foundation.
Among the 21% of Americans who did not get such care, the most common reason was “couldn’t afford the cost” (13%), which was closely followed by “insurance wouldn’t cover it” at 12%. “Afraid or embarrassed to seek care” was cited by 10% of those who had forgone mental health care, and 8% said that the reason was “didn’t know where to go to get care,” the Kaiser report said.
The Kaiser Health Tracking Poll was conducted April 12-19, 2016, among a nationally representative sample of 1,201 adults aged 18 years and older.

About one in five Americans report that there was a time when they or a family member in their household thought they needed mental health care but did not receive it for various reasons, according to a poll conducted by the Kaiser Family Foundation.
Among the 21% of Americans who did not get such care, the most common reason was “couldn’t afford the cost” (13%), which was closely followed by “insurance wouldn’t cover it” at 12%. “Afraid or embarrassed to seek care” was cited by 10% of those who had forgone mental health care, and 8% said that the reason was “didn’t know where to go to get care,” the Kaiser report said.
The Kaiser Health Tracking Poll was conducted April 12-19, 2016, among a nationally representative sample of 1,201 adults aged 18 years and older.