User login
Delaying therapy for HL/NHL likely safe for mom, baby

Photo credit: Vera Kratochvil
A single-center retrospective study of 39 pregnant women diagnosed with Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) during pregnancy indicates that delaying treatment until the second trimester is “likely safe and results in acceptable” outcomes for both mother and child.
Investigators also found that deferring therapy until after delivery did not adversely affect maternal outcomes.
In order to clarify the sometimes conflicting reports regarding management of lymphoma during pregnancy, investigators at MD Anderson Cancer Center in Houston, Texas, undertook the study to determine whether administering standard chemotherapy during the second and third trimesters has acceptable maternal and fetal outcomes.
Michelle A. Fanale, MD, and colleagues published their findings in JAMA Oncology.
Patient characteristics
Investigators identified 31 women with HL and 8 with NHL who were diagnosed between 1991 and 2014 and had sufficient pregnancy and follow-up data.
The women were a median age of 28 years (range 19-38). The patients with NHL were significantly older, P=0.004.
Approximately 20% of patients had B symptoms. Most were stage II disease (72%), and 80% were ECOG performance status 0 or 1.
About two thirds of patients had hemoglobin levels less than 12 g/dL.
Most patients did not have extranodal nonbone-marrow disease (82%), although there was a significant difference between those with HL (90%) and NHL (50%), P=0.03.
One third of patients had bulky disease, and 88% were in their second or third trimesters at diagnosis.
Three women electively terminated their pregnancies at diagnosis. Of the 36 remaining patients, 24 (61%) began antenatal therapy and 12 (31%) postponed therapy until after delivery.
Four patients received radiation therapy above the diaphragm at a median dose of 40.4 Gy.
Obstetric outcomes
Antenatal therapy was not associated with increased incidence of preterm delivery. Of the 24 women who received treatment during pregnancy, 7 (29%) gave birth prematurely compared with 5 of the 12 women (42%) who postponed treatment until after delivery, P=0.73.
The investigators noted that the miscarriage rate was approximately 10%, which was higher than previous studies.
Four patients had miscarriages, 2 during the first trimester and 2 during the second. Both patients who had miscarriages in the first trimester had received lymphoma treatment during that time.
And one of the patients who had antenatal therapy during the second trimester and had a miscarriage was critically ill, which the investigators believed was a contributing factor.
The second woman who miscarried after therapy in the second trimester had conceived twins through in vitro fertilization and miscarried at 23 weeks after ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy had been initiated at gestational week 15.
Investigators had fetal outcomes available for 31 of 32 patients who did not terminate or have a miscarriage. And these 31 infants had no anomalies at birth.
The investigators said that although the follow-up time is relatively short, they observed no malformations in the newborns.
Treatment responses and survival
The overall response rate (OR) was 91.7% and the complete response (CR) rate was 75.0% for the 24 patients who started treatment during pregnancy.
And for those 12 women who deferred therapy until delivery, both ORR and CR rates were 91.7%.
The 5-year progression-free survival (PFS) and OS were 74.7% and 82.4%, respectively, for all women. The median follow-up was 67.9 months (range 8.8 to 277.5).
For the 31 women with HL, the 5-year PFS and OS were 69.9% and 80%, respectively.
And for the 8 women with NHL, the 5-year PFS and OS were 85.7% and 83.3%, respectively.
Investigators found no difference in PFS or OS (P=0.84) based on undergoing antenatal lymphoma treatment among the 36 women who did not terminate their pregnancies at diagnosis.
The investigators conducted a univariate analysis and found that for the 36 women who did not electively terminate their pregnancies, the following were associated with increased risk of disease progression:
• Bulky disease—hazard ratio [HR] 3.6, P = 0.06
• Extranodal nonbone marrow involvement—HR 4.2, P = 0.04
• Poor ECOG performance status—HR 3.9, P = 0.005
Poor performance status was also associated with OS, HR 8.88, P = 0.004.
Multivariate analysis revealed significant associations in terms of OS and PFS for extranodal nonbone marrow involvement and performance status:
• Nonbone marrow involvement—OS HR, 73.5, P = 0.02; PFS HR 8.26, P = 0.01
• Performance status—OS HR, 26.7, P = 0.003; PFS HR, 4.89, P = 0.002
The investigators concluded that because they found no differences in PFS or OS according to whether patients received antenatal therapy, they believe that disease factors, rather than treatment-related factors, influence worse maternal outcomes.
They recommended delaying therapy until the second trimester if that can be accomplished without harm to the patient. ![]()

Photo credit: Vera Kratochvil
A single-center retrospective study of 39 pregnant women diagnosed with Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) during pregnancy indicates that delaying treatment until the second trimester is “likely safe and results in acceptable” outcomes for both mother and child.
Investigators also found that deferring therapy until after delivery did not adversely affect maternal outcomes.
In order to clarify the sometimes conflicting reports regarding management of lymphoma during pregnancy, investigators at MD Anderson Cancer Center in Houston, Texas, undertook the study to determine whether administering standard chemotherapy during the second and third trimesters has acceptable maternal and fetal outcomes.
Michelle A. Fanale, MD, and colleagues published their findings in JAMA Oncology.
Patient characteristics
Investigators identified 31 women with HL and 8 with NHL who were diagnosed between 1991 and 2014 and had sufficient pregnancy and follow-up data.
The women were a median age of 28 years (range 19-38). The patients with NHL were significantly older, P=0.004.
Approximately 20% of patients had B symptoms. Most were stage II disease (72%), and 80% were ECOG performance status 0 or 1.
About two thirds of patients had hemoglobin levels less than 12 g/dL.
Most patients did not have extranodal nonbone-marrow disease (82%), although there was a significant difference between those with HL (90%) and NHL (50%), P=0.03.
One third of patients had bulky disease, and 88% were in their second or third trimesters at diagnosis.
Three women electively terminated their pregnancies at diagnosis. Of the 36 remaining patients, 24 (61%) began antenatal therapy and 12 (31%) postponed therapy until after delivery.
Four patients received radiation therapy above the diaphragm at a median dose of 40.4 Gy.
Obstetric outcomes
Antenatal therapy was not associated with increased incidence of preterm delivery. Of the 24 women who received treatment during pregnancy, 7 (29%) gave birth prematurely compared with 5 of the 12 women (42%) who postponed treatment until after delivery, P=0.73.
The investigators noted that the miscarriage rate was approximately 10%, which was higher than previous studies.
Four patients had miscarriages, 2 during the first trimester and 2 during the second. Both patients who had miscarriages in the first trimester had received lymphoma treatment during that time.
And one of the patients who had antenatal therapy during the second trimester and had a miscarriage was critically ill, which the investigators believed was a contributing factor.
The second woman who miscarried after therapy in the second trimester had conceived twins through in vitro fertilization and miscarried at 23 weeks after ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy had been initiated at gestational week 15.
Investigators had fetal outcomes available for 31 of 32 patients who did not terminate or have a miscarriage. And these 31 infants had no anomalies at birth.
The investigators said that although the follow-up time is relatively short, they observed no malformations in the newborns.
Treatment responses and survival
The overall response rate (OR) was 91.7% and the complete response (CR) rate was 75.0% for the 24 patients who started treatment during pregnancy.
And for those 12 women who deferred therapy until delivery, both ORR and CR rates were 91.7%.
The 5-year progression-free survival (PFS) and OS were 74.7% and 82.4%, respectively, for all women. The median follow-up was 67.9 months (range 8.8 to 277.5).
For the 31 women with HL, the 5-year PFS and OS were 69.9% and 80%, respectively.
And for the 8 women with NHL, the 5-year PFS and OS were 85.7% and 83.3%, respectively.
Investigators found no difference in PFS or OS (P=0.84) based on undergoing antenatal lymphoma treatment among the 36 women who did not terminate their pregnancies at diagnosis.
The investigators conducted a univariate analysis and found that for the 36 women who did not electively terminate their pregnancies, the following were associated with increased risk of disease progression:
• Bulky disease—hazard ratio [HR] 3.6, P = 0.06
• Extranodal nonbone marrow involvement—HR 4.2, P = 0.04
• Poor ECOG performance status—HR 3.9, P = 0.005
Poor performance status was also associated with OS, HR 8.88, P = 0.004.
Multivariate analysis revealed significant associations in terms of OS and PFS for extranodal nonbone marrow involvement and performance status:
• Nonbone marrow involvement—OS HR, 73.5, P = 0.02; PFS HR 8.26, P = 0.01
• Performance status—OS HR, 26.7, P = 0.003; PFS HR, 4.89, P = 0.002
The investigators concluded that because they found no differences in PFS or OS according to whether patients received antenatal therapy, they believe that disease factors, rather than treatment-related factors, influence worse maternal outcomes.
They recommended delaying therapy until the second trimester if that can be accomplished without harm to the patient. ![]()

Photo credit: Vera Kratochvil
A single-center retrospective study of 39 pregnant women diagnosed with Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) during pregnancy indicates that delaying treatment until the second trimester is “likely safe and results in acceptable” outcomes for both mother and child.
Investigators also found that deferring therapy until after delivery did not adversely affect maternal outcomes.
In order to clarify the sometimes conflicting reports regarding management of lymphoma during pregnancy, investigators at MD Anderson Cancer Center in Houston, Texas, undertook the study to determine whether administering standard chemotherapy during the second and third trimesters has acceptable maternal and fetal outcomes.
Michelle A. Fanale, MD, and colleagues published their findings in JAMA Oncology.
Patient characteristics
Investigators identified 31 women with HL and 8 with NHL who were diagnosed between 1991 and 2014 and had sufficient pregnancy and follow-up data.
The women were a median age of 28 years (range 19-38). The patients with NHL were significantly older, P=0.004.
Approximately 20% of patients had B symptoms. Most were stage II disease (72%), and 80% were ECOG performance status 0 or 1.
About two thirds of patients had hemoglobin levels less than 12 g/dL.
Most patients did not have extranodal nonbone-marrow disease (82%), although there was a significant difference between those with HL (90%) and NHL (50%), P=0.03.
One third of patients had bulky disease, and 88% were in their second or third trimesters at diagnosis.
Three women electively terminated their pregnancies at diagnosis. Of the 36 remaining patients, 24 (61%) began antenatal therapy and 12 (31%) postponed therapy until after delivery.
Four patients received radiation therapy above the diaphragm at a median dose of 40.4 Gy.
Obstetric outcomes
Antenatal therapy was not associated with increased incidence of preterm delivery. Of the 24 women who received treatment during pregnancy, 7 (29%) gave birth prematurely compared with 5 of the 12 women (42%) who postponed treatment until after delivery, P=0.73.
The investigators noted that the miscarriage rate was approximately 10%, which was higher than previous studies.
Four patients had miscarriages, 2 during the first trimester and 2 during the second. Both patients who had miscarriages in the first trimester had received lymphoma treatment during that time.
And one of the patients who had antenatal therapy during the second trimester and had a miscarriage was critically ill, which the investigators believed was a contributing factor.
The second woman who miscarried after therapy in the second trimester had conceived twins through in vitro fertilization and miscarried at 23 weeks after ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy had been initiated at gestational week 15.
Investigators had fetal outcomes available for 31 of 32 patients who did not terminate or have a miscarriage. And these 31 infants had no anomalies at birth.
The investigators said that although the follow-up time is relatively short, they observed no malformations in the newborns.
Treatment responses and survival
The overall response rate (OR) was 91.7% and the complete response (CR) rate was 75.0% for the 24 patients who started treatment during pregnancy.
And for those 12 women who deferred therapy until delivery, both ORR and CR rates were 91.7%.
The 5-year progression-free survival (PFS) and OS were 74.7% and 82.4%, respectively, for all women. The median follow-up was 67.9 months (range 8.8 to 277.5).
For the 31 women with HL, the 5-year PFS and OS were 69.9% and 80%, respectively.
And for the 8 women with NHL, the 5-year PFS and OS were 85.7% and 83.3%, respectively.
Investigators found no difference in PFS or OS (P=0.84) based on undergoing antenatal lymphoma treatment among the 36 women who did not terminate their pregnancies at diagnosis.
The investigators conducted a univariate analysis and found that for the 36 women who did not electively terminate their pregnancies, the following were associated with increased risk of disease progression:
• Bulky disease—hazard ratio [HR] 3.6, P = 0.06
• Extranodal nonbone marrow involvement—HR 4.2, P = 0.04
• Poor ECOG performance status—HR 3.9, P = 0.005
Poor performance status was also associated with OS, HR 8.88, P = 0.004.
Multivariate analysis revealed significant associations in terms of OS and PFS for extranodal nonbone marrow involvement and performance status:
• Nonbone marrow involvement—OS HR, 73.5, P = 0.02; PFS HR 8.26, P = 0.01
• Performance status—OS HR, 26.7, P = 0.003; PFS HR, 4.89, P = 0.002
The investigators concluded that because they found no differences in PFS or OS according to whether patients received antenatal therapy, they believe that disease factors, rather than treatment-related factors, influence worse maternal outcomes.
They recommended delaying therapy until the second trimester if that can be accomplished without harm to the patient. ![]()
Tracking the Decline of AAA Repair in the United States
Has the advent of branched-fenestrated aortic endografts increased the number of abdominal aortic aneurysm (AAA) repairs due to patients who otherwise would not be offered open repair or infrarenal endovascular repair (EVAR)?
This is the question addressed by Dr. Bjoern D. Suckow, who will present the results of a study that he and his colleagues at the Dartmouth-Hitchcock Medical Center performed to evaluate trends in open AAA repair, EVAR, and branched-fenestrated EVAR (BEVAR) for AAA in Medicare beneficiaries from 2003 to 2013. They used a 100% sample of Medicare Part B claims to determine the number of each of these repair types and to compare them annually over the study period.
Between 2003 and 2013, the total number of AAA repairs significantly declined by 13% from 27,352 to 23,835, after a peak number of 29,924 repairs performed in 2005. The number of open AAA repairs significantly and steadily declined by 78%, from 15,385 in 2003 to 3,315 in 2013. In contrast, while the number of EVARs significantly increased from 11,967 in 2003 to 20,845 in 2008, that number has since declined a total of 13% to only 18,098 repairs in 2013, although this was not a significant difference.
Prior to 2011, there were no BEVAR cases among Medicare beneficiaries, according to Dr. Suckow. However, the number of BEVAR cases continuously and significantly rose more than 500% from 401 procedures in 2011 to 2,422 procedures in 2013. Over this same time period, the number of open repairs and the number of EVARs declined by 2,499 and 2,415 cases, respectively .
“The total number of AAA repairs in the Unites States has been declining annually since 2005,” said Dr. Suckow. “Our study shows that the continuous increase in BEVAR cases performed among Medicare patients over the last 3 years has not reversed the steady decline in overall numbers of AAA repairs, because of a concomitant decline in both open aneurysm repair and EVAR.”
Their results indicated that BEVAR was not likely responsible for the overall decline in open repair, as this trend was found to be present over a much longer time period, according to Dr. Suckow. Similarly, the rapid rise in BEVAR cases occurred when there was an almost equal decline in EVAR case numbers.
“These trends suggest a potential decline in the incidence of AAA in the U.S. Medicare population, with shifts in the type of repair based on available technology and perhaps patient preference,” said Dr. Suckow. “Future efforts are needed to determine the characteristics of patients treated with BEVAR as well as the long-term effectiveness of these new treatments,” he concluded.
Friday: Plenary Session 4
8:00 A.M. - 9:30 A.M.
Potomac Ballroom A/B
Has the advent of branched-fenestrated aortic endografts increased the number of abdominal aortic aneurysm (AAA) repairs due to patients who otherwise would not be offered open repair or infrarenal endovascular repair (EVAR)?
This is the question addressed by Dr. Bjoern D. Suckow, who will present the results of a study that he and his colleagues at the Dartmouth-Hitchcock Medical Center performed to evaluate trends in open AAA repair, EVAR, and branched-fenestrated EVAR (BEVAR) for AAA in Medicare beneficiaries from 2003 to 2013. They used a 100% sample of Medicare Part B claims to determine the number of each of these repair types and to compare them annually over the study period.
Between 2003 and 2013, the total number of AAA repairs significantly declined by 13% from 27,352 to 23,835, after a peak number of 29,924 repairs performed in 2005. The number of open AAA repairs significantly and steadily declined by 78%, from 15,385 in 2003 to 3,315 in 2013. In contrast, while the number of EVARs significantly increased from 11,967 in 2003 to 20,845 in 2008, that number has since declined a total of 13% to only 18,098 repairs in 2013, although this was not a significant difference.
Prior to 2011, there were no BEVAR cases among Medicare beneficiaries, according to Dr. Suckow. However, the number of BEVAR cases continuously and significantly rose more than 500% from 401 procedures in 2011 to 2,422 procedures in 2013. Over this same time period, the number of open repairs and the number of EVARs declined by 2,499 and 2,415 cases, respectively .
“The total number of AAA repairs in the Unites States has been declining annually since 2005,” said Dr. Suckow. “Our study shows that the continuous increase in BEVAR cases performed among Medicare patients over the last 3 years has not reversed the steady decline in overall numbers of AAA repairs, because of a concomitant decline in both open aneurysm repair and EVAR.”
Their results indicated that BEVAR was not likely responsible for the overall decline in open repair, as this trend was found to be present over a much longer time period, according to Dr. Suckow. Similarly, the rapid rise in BEVAR cases occurred when there was an almost equal decline in EVAR case numbers.
“These trends suggest a potential decline in the incidence of AAA in the U.S. Medicare population, with shifts in the type of repair based on available technology and perhaps patient preference,” said Dr. Suckow. “Future efforts are needed to determine the characteristics of patients treated with BEVAR as well as the long-term effectiveness of these new treatments,” he concluded.
Friday: Plenary Session 4
8:00 A.M. - 9:30 A.M.
Potomac Ballroom A/B
Has the advent of branched-fenestrated aortic endografts increased the number of abdominal aortic aneurysm (AAA) repairs due to patients who otherwise would not be offered open repair or infrarenal endovascular repair (EVAR)?
This is the question addressed by Dr. Bjoern D. Suckow, who will present the results of a study that he and his colleagues at the Dartmouth-Hitchcock Medical Center performed to evaluate trends in open AAA repair, EVAR, and branched-fenestrated EVAR (BEVAR) for AAA in Medicare beneficiaries from 2003 to 2013. They used a 100% sample of Medicare Part B claims to determine the number of each of these repair types and to compare them annually over the study period.
Between 2003 and 2013, the total number of AAA repairs significantly declined by 13% from 27,352 to 23,835, after a peak number of 29,924 repairs performed in 2005. The number of open AAA repairs significantly and steadily declined by 78%, from 15,385 in 2003 to 3,315 in 2013. In contrast, while the number of EVARs significantly increased from 11,967 in 2003 to 20,845 in 2008, that number has since declined a total of 13% to only 18,098 repairs in 2013, although this was not a significant difference.
Prior to 2011, there were no BEVAR cases among Medicare beneficiaries, according to Dr. Suckow. However, the number of BEVAR cases continuously and significantly rose more than 500% from 401 procedures in 2011 to 2,422 procedures in 2013. Over this same time period, the number of open repairs and the number of EVARs declined by 2,499 and 2,415 cases, respectively .
“The total number of AAA repairs in the Unites States has been declining annually since 2005,” said Dr. Suckow. “Our study shows that the continuous increase in BEVAR cases performed among Medicare patients over the last 3 years has not reversed the steady decline in overall numbers of AAA repairs, because of a concomitant decline in both open aneurysm repair and EVAR.”
Their results indicated that BEVAR was not likely responsible for the overall decline in open repair, as this trend was found to be present over a much longer time period, according to Dr. Suckow. Similarly, the rapid rise in BEVAR cases occurred when there was an almost equal decline in EVAR case numbers.
“These trends suggest a potential decline in the incidence of AAA in the U.S. Medicare population, with shifts in the type of repair based on available technology and perhaps patient preference,” said Dr. Suckow. “Future efforts are needed to determine the characteristics of patients treated with BEVAR as well as the long-term effectiveness of these new treatments,” he concluded.
Friday: Plenary Session 4
8:00 A.M. - 9:30 A.M.
Potomac Ballroom A/B
Learn Radiation Safety During Vascular Surgery
Practical tips for protecting your vascular surgery patients and yourselves from radiation exposure will be featured as part of this morning’s session “Radiation Safety for the Vascular Surgeon.”
“Since endovascular procedures became an essential part of the vascular field about 25 years ago, minimally invasive, radiation-guided procedures have become more sophisticated, more involved and more frequent while requiring better resolution,” said session co-moderator Dr. Peter Schneider of Kaiser Permanente Medical Group in Honolulu. “All of this means more radiation for the patient and for the physician. Going forward, as our field continues to develop, the likelihood is high that these requirements will continue and will intensify and that the radiation risk to patients and providers will increase.
“Radiation is a necessary tool in our ability to help our patients,” Dr. Schneider added. “Yet radiation has negative effects, such as radiation-induced cancers and cataracts. We need to do everything we can to minimize those negative effects.”
A good 60%-70% of vascular procedures involve endovascular techniques that utilize radiation, said session co-moderator Dr. Amy Reed of Rush University Medical Center in Chicago. “Even though your patient may be exposed multiple times in the future through CAT scans or cardiac catheterizations, you as the surgeon are going to be doing this for decades – so the long-term exposure to radiation is a concern. Usually it goes hand in hand that if you’re protecting the patient, you’re protecting yourself as well.”
There are a number of measures vascular surgeons can take to minimize radiation exposure, the moderators said. “These include simple things like the use of radiation filters during imaging, decreasing frame rates to the lowest level that gives the imaging required, being careful about maintaining distance from the image intensifier during fluoroscopy and use of radiation shielding,” Dr. Schneider said.
Additional steps can include collimating or focusing the view and changing table positions, Dr. Reed noted. Good radiation safety techniques are especially important for residency program directors, she added: “Trainees stand closer to the radiation, so it’s important to have good technique. Just like universal precautions measures such as wearing gloves and washing hands, trainees are going to learn radiation safety by emulating you.”
During the session, six expert clinicians knowledgeable about the effects of radiation will share their wisdom. Dr. Christopher Carsten of Greenville Health System in South Carolina will cover what is known about the clinically allowable limits of radiation to the provider and to the patient; Dr. Sunita Srivastava of the Cleveland Clinic will provide a basic understanding of the mechanism of the biological effects of radiation; Dr. Melissa Kirkwood of the University of Texas Southwestern Medical Center in Dallas will offer practical tips to the vascular surgeon for decreasing radiation; Dr. Audra Duncan of the University of Western Ontario will discuss the need for diagnostic tests or radiation-guided procedures in patients who are pregnant; Dr. Mark Farber of the University of North Carolina will discuss new technologies in radiation monitoring; and Dr. Lois Killewich of the University of Texas Medical Branch in Galveston will cover governmental regulations associated with the clinical use of radiation.
“We’re really trying to increase the awareness of radiation safety,” Dr. Reed said. “It’s not to go over all of the things radiation can cause – we know that – but what’s on the horizon that might be helpful for you, and what tips you can take back for protection if you’re not currently using them.”
Friday Breakfast Session B4
6:30 A.M. - 8:00 A.M.
Potomac Ballroom C
Practical tips for protecting your vascular surgery patients and yourselves from radiation exposure will be featured as part of this morning’s session “Radiation Safety for the Vascular Surgeon.”
“Since endovascular procedures became an essential part of the vascular field about 25 years ago, minimally invasive, radiation-guided procedures have become more sophisticated, more involved and more frequent while requiring better resolution,” said session co-moderator Dr. Peter Schneider of Kaiser Permanente Medical Group in Honolulu. “All of this means more radiation for the patient and for the physician. Going forward, as our field continues to develop, the likelihood is high that these requirements will continue and will intensify and that the radiation risk to patients and providers will increase.
“Radiation is a necessary tool in our ability to help our patients,” Dr. Schneider added. “Yet radiation has negative effects, such as radiation-induced cancers and cataracts. We need to do everything we can to minimize those negative effects.”
A good 60%-70% of vascular procedures involve endovascular techniques that utilize radiation, said session co-moderator Dr. Amy Reed of Rush University Medical Center in Chicago. “Even though your patient may be exposed multiple times in the future through CAT scans or cardiac catheterizations, you as the surgeon are going to be doing this for decades – so the long-term exposure to radiation is a concern. Usually it goes hand in hand that if you’re protecting the patient, you’re protecting yourself as well.”
There are a number of measures vascular surgeons can take to minimize radiation exposure, the moderators said. “These include simple things like the use of radiation filters during imaging, decreasing frame rates to the lowest level that gives the imaging required, being careful about maintaining distance from the image intensifier during fluoroscopy and use of radiation shielding,” Dr. Schneider said.
Additional steps can include collimating or focusing the view and changing table positions, Dr. Reed noted. Good radiation safety techniques are especially important for residency program directors, she added: “Trainees stand closer to the radiation, so it’s important to have good technique. Just like universal precautions measures such as wearing gloves and washing hands, trainees are going to learn radiation safety by emulating you.”
During the session, six expert clinicians knowledgeable about the effects of radiation will share their wisdom. Dr. Christopher Carsten of Greenville Health System in South Carolina will cover what is known about the clinically allowable limits of radiation to the provider and to the patient; Dr. Sunita Srivastava of the Cleveland Clinic will provide a basic understanding of the mechanism of the biological effects of radiation; Dr. Melissa Kirkwood of the University of Texas Southwestern Medical Center in Dallas will offer practical tips to the vascular surgeon for decreasing radiation; Dr. Audra Duncan of the University of Western Ontario will discuss the need for diagnostic tests or radiation-guided procedures in patients who are pregnant; Dr. Mark Farber of the University of North Carolina will discuss new technologies in radiation monitoring; and Dr. Lois Killewich of the University of Texas Medical Branch in Galveston will cover governmental regulations associated with the clinical use of radiation.
“We’re really trying to increase the awareness of radiation safety,” Dr. Reed said. “It’s not to go over all of the things radiation can cause – we know that – but what’s on the horizon that might be helpful for you, and what tips you can take back for protection if you’re not currently using them.”
Friday Breakfast Session B4
6:30 A.M. - 8:00 A.M.
Potomac Ballroom C
Practical tips for protecting your vascular surgery patients and yourselves from radiation exposure will be featured as part of this morning’s session “Radiation Safety for the Vascular Surgeon.”
“Since endovascular procedures became an essential part of the vascular field about 25 years ago, minimally invasive, radiation-guided procedures have become more sophisticated, more involved and more frequent while requiring better resolution,” said session co-moderator Dr. Peter Schneider of Kaiser Permanente Medical Group in Honolulu. “All of this means more radiation for the patient and for the physician. Going forward, as our field continues to develop, the likelihood is high that these requirements will continue and will intensify and that the radiation risk to patients and providers will increase.
“Radiation is a necessary tool in our ability to help our patients,” Dr. Schneider added. “Yet radiation has negative effects, such as radiation-induced cancers and cataracts. We need to do everything we can to minimize those negative effects.”
A good 60%-70% of vascular procedures involve endovascular techniques that utilize radiation, said session co-moderator Dr. Amy Reed of Rush University Medical Center in Chicago. “Even though your patient may be exposed multiple times in the future through CAT scans or cardiac catheterizations, you as the surgeon are going to be doing this for decades – so the long-term exposure to radiation is a concern. Usually it goes hand in hand that if you’re protecting the patient, you’re protecting yourself as well.”
There are a number of measures vascular surgeons can take to minimize radiation exposure, the moderators said. “These include simple things like the use of radiation filters during imaging, decreasing frame rates to the lowest level that gives the imaging required, being careful about maintaining distance from the image intensifier during fluoroscopy and use of radiation shielding,” Dr. Schneider said.
Additional steps can include collimating or focusing the view and changing table positions, Dr. Reed noted. Good radiation safety techniques are especially important for residency program directors, she added: “Trainees stand closer to the radiation, so it’s important to have good technique. Just like universal precautions measures such as wearing gloves and washing hands, trainees are going to learn radiation safety by emulating you.”
During the session, six expert clinicians knowledgeable about the effects of radiation will share their wisdom. Dr. Christopher Carsten of Greenville Health System in South Carolina will cover what is known about the clinically allowable limits of radiation to the provider and to the patient; Dr. Sunita Srivastava of the Cleveland Clinic will provide a basic understanding of the mechanism of the biological effects of radiation; Dr. Melissa Kirkwood of the University of Texas Southwestern Medical Center in Dallas will offer practical tips to the vascular surgeon for decreasing radiation; Dr. Audra Duncan of the University of Western Ontario will discuss the need for diagnostic tests or radiation-guided procedures in patients who are pregnant; Dr. Mark Farber of the University of North Carolina will discuss new technologies in radiation monitoring; and Dr. Lois Killewich of the University of Texas Medical Branch in Galveston will cover governmental regulations associated with the clinical use of radiation.
“We’re really trying to increase the awareness of radiation safety,” Dr. Reed said. “It’s not to go over all of the things radiation can cause – we know that – but what’s on the horizon that might be helpful for you, and what tips you can take back for protection if you’re not currently using them.”
Friday Breakfast Session B4
6:30 A.M. - 8:00 A.M.
Potomac Ballroom C
Enjoy a ‘Capitol’ Performance with the Capitol Steps
First, combine a Washington, D.C. venue and an upcoming and hotly debated presidential election. Add a date shortly before the GOP and Democratic national conventions.
What do you get? The perfect time and place for a political comedy show performed by the “Capitol Steps,” a group that’s been poking fun at politics, politicians and the headlines of the day since the Reagan administration.
The group will perform its particular brand of satirical humor – song parodies, standup comedy and more – at a special presentation for all VAM attendees from 8:30 to 9:30 p.m., Friday, in Potomac Ballroom A/B. The event is open at no charge to all registered attendees, guests and exhibitors.
No topic is safe, whether it’s the FBI’s fight earlier this year with Apple over a locked iPhone to Bill Clinton commenting on Hillary to Donald Trump and Sarah Palin. Perhaps the audience will hear Bernie Sanders sing a show tune or hear about “Deleter of the Facts.”
The group got its start in 1981. Senate staffers, planning entertainment for a Christmas party, created song parodies and skits from the headlines at the time and created the Capitol Steps in the process. The group has recorded more than 30 albums (the latest is “What to Expect When You’re Electing”) and has been featured on national television and radio.
“We are thrilled to offer the humor of the Capitol Steps to our members and guests,” said SVS President Dr. Bruce A. Perler, who was instrumental in bringing the “Steps” to VAM. “I am certain that, no matter what a particular member’s politics, in this most ‘unusual’ of presidential election campaigns, the Capitol Steps will provide plenty of laughs and remarkable satirical insights.”
First, combine a Washington, D.C. venue and an upcoming and hotly debated presidential election. Add a date shortly before the GOP and Democratic national conventions.
What do you get? The perfect time and place for a political comedy show performed by the “Capitol Steps,” a group that’s been poking fun at politics, politicians and the headlines of the day since the Reagan administration.
The group will perform its particular brand of satirical humor – song parodies, standup comedy and more – at a special presentation for all VAM attendees from 8:30 to 9:30 p.m., Friday, in Potomac Ballroom A/B. The event is open at no charge to all registered attendees, guests and exhibitors.
No topic is safe, whether it’s the FBI’s fight earlier this year with Apple over a locked iPhone to Bill Clinton commenting on Hillary to Donald Trump and Sarah Palin. Perhaps the audience will hear Bernie Sanders sing a show tune or hear about “Deleter of the Facts.”
The group got its start in 1981. Senate staffers, planning entertainment for a Christmas party, created song parodies and skits from the headlines at the time and created the Capitol Steps in the process. The group has recorded more than 30 albums (the latest is “What to Expect When You’re Electing”) and has been featured on national television and radio.
“We are thrilled to offer the humor of the Capitol Steps to our members and guests,” said SVS President Dr. Bruce A. Perler, who was instrumental in bringing the “Steps” to VAM. “I am certain that, no matter what a particular member’s politics, in this most ‘unusual’ of presidential election campaigns, the Capitol Steps will provide plenty of laughs and remarkable satirical insights.”
First, combine a Washington, D.C. venue and an upcoming and hotly debated presidential election. Add a date shortly before the GOP and Democratic national conventions.
What do you get? The perfect time and place for a political comedy show performed by the “Capitol Steps,” a group that’s been poking fun at politics, politicians and the headlines of the day since the Reagan administration.
The group will perform its particular brand of satirical humor – song parodies, standup comedy and more – at a special presentation for all VAM attendees from 8:30 to 9:30 p.m., Friday, in Potomac Ballroom A/B. The event is open at no charge to all registered attendees, guests and exhibitors.
No topic is safe, whether it’s the FBI’s fight earlier this year with Apple over a locked iPhone to Bill Clinton commenting on Hillary to Donald Trump and Sarah Palin. Perhaps the audience will hear Bernie Sanders sing a show tune or hear about “Deleter of the Facts.”
The group got its start in 1981. Senate staffers, planning entertainment for a Christmas party, created song parodies and skits from the headlines at the time and created the Capitol Steps in the process. The group has recorded more than 30 albums (the latest is “What to Expect When You’re Electing”) and has been featured on national television and radio.
“We are thrilled to offer the humor of the Capitol Steps to our members and guests,” said SVS President Dr. Bruce A. Perler, who was instrumental in bringing the “Steps” to VAM. “I am certain that, no matter what a particular member’s politics, in this most ‘unusual’ of presidential election campaigns, the Capitol Steps will provide plenty of laughs and remarkable satirical insights.”
Presidentially Speaking
Dr. Bruce Perler, Johns Hopkins Hospital, will give his SVS Presidential Address Friday after a short introduction by President-Elect Dr. Ronald Fairman.
Friday: 11 a.m. – 12:15 p.m. Potomac Ballroom A/B
Dr. Bruce Perler, Johns Hopkins Hospital, will give his SVS Presidential Address Friday after a short introduction by President-Elect Dr. Ronald Fairman.
Friday: 11 a.m. – 12:15 p.m. Potomac Ballroom A/B
Dr. Bruce Perler, Johns Hopkins Hospital, will give his SVS Presidential Address Friday after a short introduction by President-Elect Dr. Ronald Fairman.
Friday: 11 a.m. – 12:15 p.m. Potomac Ballroom A/B
Are You Going to the Fair? A Guide to What You Need to Know
There’s no Ferris Wheel or tilt-a-whirl at this fair – just useful information on 73 vascular training programs and the chance for aspiring vascular surgeons to find a good fit for their futures.
Programs are arranged on the floor by the types of offered paradigms: 0+5, 5+2 as well as those offering both training pathways. The configuration, introduced in 2015, makes it easier for attendees to locate the programs in which they are specifically interested.
This year residents/students will receive more data – such as if the program is academic- or community practice-based – to help them better locate the programs in which they are most interested in visiting in the time allotted. The change was made as the result of attendee feedback from earlier fairs.
The Residency Fair is the perfect way for residents and students to network and make connections. Program directors, faculty and current trainees from the 73 institutions will be on hand to talk with interested attendees.
More than 200 students and residents who have an active interest in vascular surgery are expected to participate.
A directory of all participating programs was distributed to students and residents earlier and also will be available at the fair. V
Friday: 5:00 P.M. - 6:30 P.M.
Exhibit Hall D
There’s no Ferris Wheel or tilt-a-whirl at this fair – just useful information on 73 vascular training programs and the chance for aspiring vascular surgeons to find a good fit for their futures.
Programs are arranged on the floor by the types of offered paradigms: 0+5, 5+2 as well as those offering both training pathways. The configuration, introduced in 2015, makes it easier for attendees to locate the programs in which they are specifically interested.
This year residents/students will receive more data – such as if the program is academic- or community practice-based – to help them better locate the programs in which they are most interested in visiting in the time allotted. The change was made as the result of attendee feedback from earlier fairs.
The Residency Fair is the perfect way for residents and students to network and make connections. Program directors, faculty and current trainees from the 73 institutions will be on hand to talk with interested attendees.
More than 200 students and residents who have an active interest in vascular surgery are expected to participate.
A directory of all participating programs was distributed to students and residents earlier and also will be available at the fair. V
Friday: 5:00 P.M. - 6:30 P.M.
Exhibit Hall D
There’s no Ferris Wheel or tilt-a-whirl at this fair – just useful information on 73 vascular training programs and the chance for aspiring vascular surgeons to find a good fit for their futures.
Programs are arranged on the floor by the types of offered paradigms: 0+5, 5+2 as well as those offering both training pathways. The configuration, introduced in 2015, makes it easier for attendees to locate the programs in which they are specifically interested.
This year residents/students will receive more data – such as if the program is academic- or community practice-based – to help them better locate the programs in which they are most interested in visiting in the time allotted. The change was made as the result of attendee feedback from earlier fairs.
The Residency Fair is the perfect way for residents and students to network and make connections. Program directors, faculty and current trainees from the 73 institutions will be on hand to talk with interested attendees.
More than 200 students and residents who have an active interest in vascular surgery are expected to participate.
A directory of all participating programs was distributed to students and residents earlier and also will be available at the fair. V
Friday: 5:00 P.M. - 6:30 P.M.
Exhibit Hall D
Study finds most antidepressants ineffective or harmful in children, adolescents
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
FROM THE LANCET
Key clinical point: The majority of antidepressant drugs prescribed to children and adolescents are ineffective, with only fluoxetine showing significant improvement.
Major finding: Fluoxetine showed the most promising results among all 14 antidepressants studied.
Data source: Retrospective review of 34 studies with 5,260 participants investigating 14 distinct drugs used to treat depression in children and adolescents.
Disclosures: The study was funded by the National Basic Research Program of China. Authors did not report any relevant financial disclosures.
Postpartum glycemic screening rates are low for women with GDM history
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Glycemic screening rates for women who had gestational diabetes mellitus are generally low and vary based on race, geography, and medication treatment in pregnancy.
Major finding: More than three-quarters of women with a history of gestational diabetes mellitus did not receive a glycemic screen in their first year postpartum.
Data sources: A retrospective analysis of medical records from women enrolled in a large, U.S. commercial health plan from 2000-2012.
Disclosures: The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
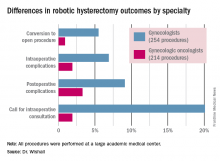
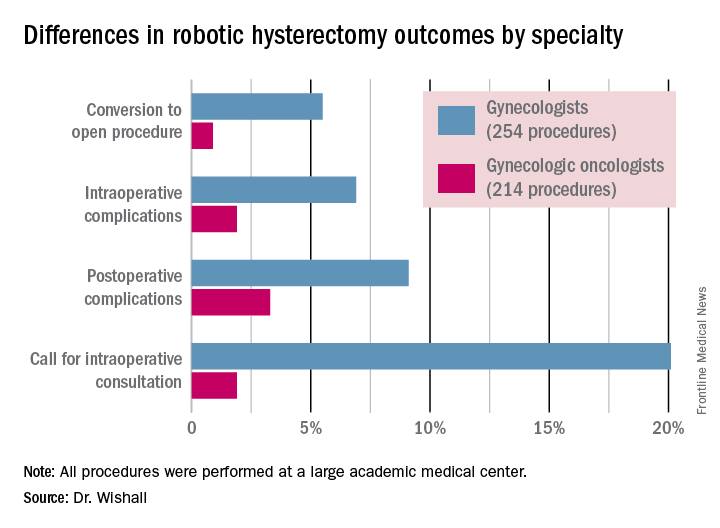
Gyn. oncologists are in demand for robotic hysterectomy
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.

|
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.

|
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.

|
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Gynecologic oncologists achieved better robotic hysterectomy outcomes than gynecologists despite challenging referrals.
Major finding: The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists.
Data source: A retrospective observational study conducted at a single center included 254 women whose robotic hysterectomies were performed by gynecologists and 214 done by gynecologic oncologists.
Disclosures: Dr. Wishall reported having no financial conflicts related to the study, which was conducted free of commercial support.
Marijuana use disorders in adolescents on the decline
New findings show that marijuana use disorders are on the decline among U.S. adolescents, according to Richard A. Grucza, Ph.D., and his associates.
They examined National Survey on Drug Use and Health data for 2002-2013 from 216,852 adolescents aged 12-17 years. They divided the data into two age groups: 12-14 and 15-17.
The prevalence of past-year marijuana use of the adolescents declined steadily from 15.8% in 2002 to 12.5% in 2007. After that it began to climb, peaking at 14.2% to 14.3% in 2010 and 2011. Then it dropped back to 13.2% in 2013. The decline was significant for both age groups, with no significant difference in trends between the groups (ages 12-14 years, odds ratio, 0.978 per year; ages 15-17 years, OR, 0.987).
In examining risk factors and protective factors, the researchers observed significant negative trends for the three risk factors and significant positive trends for four of the six protective factors. The two protective factors that decreased over time were drug education and religious commitment. “Thus, seven of the nine risk/protective factors changed over time in a manner that might partially explain the downward trend in the prevalence of marijuana use disorders,” they noted.
“Our findings underscore the importance of adolescent mental health in conferring resilience to risk for substance use disorders,” the researchers concluded. “There are one or more environmental factors – yet to be identified – that may be changing over time in a manner that leads to both lower risk for marijuana use disorders and for other behavioral problems.”
Read the study in the Journal of the American Academy of Child & Adolescent Psychiatry (doi: 10.1016/j.jaac.2016.04.002).
New findings show that marijuana use disorders are on the decline among U.S. adolescents, according to Richard A. Grucza, Ph.D., and his associates.
They examined National Survey on Drug Use and Health data for 2002-2013 from 216,852 adolescents aged 12-17 years. They divided the data into two age groups: 12-14 and 15-17.
The prevalence of past-year marijuana use of the adolescents declined steadily from 15.8% in 2002 to 12.5% in 2007. After that it began to climb, peaking at 14.2% to 14.3% in 2010 and 2011. Then it dropped back to 13.2% in 2013. The decline was significant for both age groups, with no significant difference in trends between the groups (ages 12-14 years, odds ratio, 0.978 per year; ages 15-17 years, OR, 0.987).
In examining risk factors and protective factors, the researchers observed significant negative trends for the three risk factors and significant positive trends for four of the six protective factors. The two protective factors that decreased over time were drug education and religious commitment. “Thus, seven of the nine risk/protective factors changed over time in a manner that might partially explain the downward trend in the prevalence of marijuana use disorders,” they noted.
“Our findings underscore the importance of adolescent mental health in conferring resilience to risk for substance use disorders,” the researchers concluded. “There are one or more environmental factors – yet to be identified – that may be changing over time in a manner that leads to both lower risk for marijuana use disorders and for other behavioral problems.”
Read the study in the Journal of the American Academy of Child & Adolescent Psychiatry (doi: 10.1016/j.jaac.2016.04.002).
New findings show that marijuana use disorders are on the decline among U.S. adolescents, according to Richard A. Grucza, Ph.D., and his associates.
They examined National Survey on Drug Use and Health data for 2002-2013 from 216,852 adolescents aged 12-17 years. They divided the data into two age groups: 12-14 and 15-17.
The prevalence of past-year marijuana use of the adolescents declined steadily from 15.8% in 2002 to 12.5% in 2007. After that it began to climb, peaking at 14.2% to 14.3% in 2010 and 2011. Then it dropped back to 13.2% in 2013. The decline was significant for both age groups, with no significant difference in trends between the groups (ages 12-14 years, odds ratio, 0.978 per year; ages 15-17 years, OR, 0.987).
In examining risk factors and protective factors, the researchers observed significant negative trends for the three risk factors and significant positive trends for four of the six protective factors. The two protective factors that decreased over time were drug education and religious commitment. “Thus, seven of the nine risk/protective factors changed over time in a manner that might partially explain the downward trend in the prevalence of marijuana use disorders,” they noted.
“Our findings underscore the importance of adolescent mental health in conferring resilience to risk for substance use disorders,” the researchers concluded. “There are one or more environmental factors – yet to be identified – that may be changing over time in a manner that leads to both lower risk for marijuana use disorders and for other behavioral problems.”
Read the study in the Journal of the American Academy of Child & Adolescent Psychiatry (doi: 10.1016/j.jaac.2016.04.002).
FROM JOURNAL OF THE AMERICAN ACADEMY OF CHILD & ADOLESCENT PSYCHIATRY