User login
A new sort of consultant: Advising doctors, patients on California’s aid-in-dying law
BERKELEY, Calif. – Few people have the unusual set of professional experiences that Dr. Lonny Shavelson does. He worked as an emergency room physician in Berkeley for years – while also working as a journalist. He has written several books and takes hauntingly beautiful photographs.
Now, just as California’s aid-in-dying law takes effect June 9, Dr. Shavelson has added another specialty: A consultant to physicians and terminally ill patients who have questions about how it works.
“Can I just sit back and watch?” Dr. Shavelson asked from his cottage office. “This is really an amazing opportunity to be part of establishing policy and initiating something in medicine. This is a major change … [that] very, very few people know anything about and how to do it.”
Dr. Shavelson is the author of the 1995 book, “A Chosen Death,” which followed five terminally ill people over 2 years as they determined whether to amass drugs on their own and end their lives at a time of their choosing. He was present at the death of all of them.
He followed the issue closely for several years, but ultimately moved on to other projects – among them a book about addiction and a documentary about people who identify as neither male nor female.
Then last fall came the surprising passage of California’s End of Life Option Act, giving terminally ill adults with 6 months to live the right to request lethal medication to end their lives. The law takes effect June 9.
Dr. Shavelson decided he had to act, adding that he feels “quite guilty” about having been away from the issue while others pushed it forward.
His website, Bay Area End of Life Options, went up in April, and he’s outlined the law at “grand rounds” at several Bay Area hospitals this spring. His practice will be focused on consulting not only with physicians whose patients request aid-in-dying, but also with patients themselves. As he indicates on his site, he will offer care to patients who choose him as their “attending End-of-Life physician.”
Dr. Shavelson is adamant that this is “something that has to be done right.” To him, that means starting every patient encounter with a one-word question: “Why?”
“In fact, it’s the only initial approach that I think is acceptable. If somebody calls me and says, ‘I want to take the medication, my first question is, ‘Why? Let me talk to you about all the various alternatives and all the ways that we can think about this.’ ”
Dr. Shavelson worries that patients may seek aid-in-dying because they are in pain. So first, he would like all his patients to be enrolled in hospice care.
“This can only work when you’re sure that the patients have been given the best end-of-life care, which to me is most guaranteed by being a part of hospice or at least having a good palliative care physician. Then this is a rational decision. If you’re doing it otherwise, it’s because of lack of good care.”
California is the fifth state to legalize aid-in-dying, joining Oregon, Washington, Vermont, and Montana. The option is very rarely used. For example, in 2014 in Oregon, just 155 lethal prescriptions were written under the state’s law, and 105 people ultimately took the medicine and died.
Under the California law, two doctors must agree that a patient has 6 months or less to live. The patient must be mentally competent. At least one of the meetings between the patient and his or her doctor must be private, with no one else present, to ensure the patient is acting independently.
Patients must be able to swallow the medication themselves and must affirm in writing, within the 48 hours before taking the medication, that they will do so.
Dr. Shavelson says he has been surprised by the poor understanding of the law among some health care providers. One insisted the law was not taking effect this year; another asked how the law would benefit his patients with Alzheimer’s disease. (Patients with dementia don’t qualify under the law because they are not mentally competent.)
The law does not require that health care providers participate in ending terminally ill patients’ lives. Many physicians are “queasy” about the law, Dr. Shavelson said, and are unwilling to prescribe to patients who request the lethal medication – even when they think having such a law in place is the right thing to do.
“My response to that is as health care providers, you might have been uncomfortable the first time you drew blood. You might have been uncomfortable the first time you took out somebody’s gall bladder,” he said. “If it’s a medical procedure you believe in and you believe it’s the patient’s right, then it’s your obligation to learn how to do it – and do it correctly.”
Dr. Shavelson predicts that many physicians who are initially reluctant to provide this option to their patients may become more comfortable after the law goes into effect and they see how it works.
Dr. Burt Presberg, an East Bay psychiatrist who works with cancer patients and their families, attended a talk by Dr. Shavelson, and it led to some soul searching.
He wrestles with his own comfort level in handling patient requests. When he talks, he often pivots from his initial point to “on the other hand.”
Dr. Presberg says he is concerned that patients suffer from clinical depression at the end of life. Sometimes they feel they are a burden to family members who could “really push for the end of life to happen a little sooner than the patient themselves.”
His experience is that terminally ill patients with clinical depression can be successfully treated. He said he believes Dr. Shavelson will be aware of the need to treat depression,”but I do have concerns about other physicians.”
“On the other hand,” he added, “I think it’s really good that this is an option.”
Dr. Shavelson says he’s already received a handful of calls from patients, but mostly he’s spent his time before the law takes effect talking to other physicians. He needs a consulting physician and a pharmacist who will accept prescriptions for a lethal dose of medicine.
Then his mind returns to the patient. “It’s important … that we’re moving forward,” he said. “It’s crucial that we do that because this is part of the rights of patient care to have a certain level of autonomy in how they die.”
To him, this type of care “isn’t so tangibly different” from other kinds of questions doctors address.
“I’m just one of those docs who sees dying as a process, and [the] method of death is less important than making sure it’s a good death.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. This story is part of a partnership that includes KQED, NPR, and Kaiser Health News.
BERKELEY, Calif. – Few people have the unusual set of professional experiences that Dr. Lonny Shavelson does. He worked as an emergency room physician in Berkeley for years – while also working as a journalist. He has written several books and takes hauntingly beautiful photographs.
Now, just as California’s aid-in-dying law takes effect June 9, Dr. Shavelson has added another specialty: A consultant to physicians and terminally ill patients who have questions about how it works.
“Can I just sit back and watch?” Dr. Shavelson asked from his cottage office. “This is really an amazing opportunity to be part of establishing policy and initiating something in medicine. This is a major change … [that] very, very few people know anything about and how to do it.”
Dr. Shavelson is the author of the 1995 book, “A Chosen Death,” which followed five terminally ill people over 2 years as they determined whether to amass drugs on their own and end their lives at a time of their choosing. He was present at the death of all of them.
He followed the issue closely for several years, but ultimately moved on to other projects – among them a book about addiction and a documentary about people who identify as neither male nor female.
Then last fall came the surprising passage of California’s End of Life Option Act, giving terminally ill adults with 6 months to live the right to request lethal medication to end their lives. The law takes effect June 9.
Dr. Shavelson decided he had to act, adding that he feels “quite guilty” about having been away from the issue while others pushed it forward.
His website, Bay Area End of Life Options, went up in April, and he’s outlined the law at “grand rounds” at several Bay Area hospitals this spring. His practice will be focused on consulting not only with physicians whose patients request aid-in-dying, but also with patients themselves. As he indicates on his site, he will offer care to patients who choose him as their “attending End-of-Life physician.”
Dr. Shavelson is adamant that this is “something that has to be done right.” To him, that means starting every patient encounter with a one-word question: “Why?”
“In fact, it’s the only initial approach that I think is acceptable. If somebody calls me and says, ‘I want to take the medication, my first question is, ‘Why? Let me talk to you about all the various alternatives and all the ways that we can think about this.’ ”
Dr. Shavelson worries that patients may seek aid-in-dying because they are in pain. So first, he would like all his patients to be enrolled in hospice care.
“This can only work when you’re sure that the patients have been given the best end-of-life care, which to me is most guaranteed by being a part of hospice or at least having a good palliative care physician. Then this is a rational decision. If you’re doing it otherwise, it’s because of lack of good care.”
California is the fifth state to legalize aid-in-dying, joining Oregon, Washington, Vermont, and Montana. The option is very rarely used. For example, in 2014 in Oregon, just 155 lethal prescriptions were written under the state’s law, and 105 people ultimately took the medicine and died.
Under the California law, two doctors must agree that a patient has 6 months or less to live. The patient must be mentally competent. At least one of the meetings between the patient and his or her doctor must be private, with no one else present, to ensure the patient is acting independently.
Patients must be able to swallow the medication themselves and must affirm in writing, within the 48 hours before taking the medication, that they will do so.
Dr. Shavelson says he has been surprised by the poor understanding of the law among some health care providers. One insisted the law was not taking effect this year; another asked how the law would benefit his patients with Alzheimer’s disease. (Patients with dementia don’t qualify under the law because they are not mentally competent.)
The law does not require that health care providers participate in ending terminally ill patients’ lives. Many physicians are “queasy” about the law, Dr. Shavelson said, and are unwilling to prescribe to patients who request the lethal medication – even when they think having such a law in place is the right thing to do.
“My response to that is as health care providers, you might have been uncomfortable the first time you drew blood. You might have been uncomfortable the first time you took out somebody’s gall bladder,” he said. “If it’s a medical procedure you believe in and you believe it’s the patient’s right, then it’s your obligation to learn how to do it – and do it correctly.”
Dr. Shavelson predicts that many physicians who are initially reluctant to provide this option to their patients may become more comfortable after the law goes into effect and they see how it works.
Dr. Burt Presberg, an East Bay psychiatrist who works with cancer patients and their families, attended a talk by Dr. Shavelson, and it led to some soul searching.
He wrestles with his own comfort level in handling patient requests. When he talks, he often pivots from his initial point to “on the other hand.”
Dr. Presberg says he is concerned that patients suffer from clinical depression at the end of life. Sometimes they feel they are a burden to family members who could “really push for the end of life to happen a little sooner than the patient themselves.”
His experience is that terminally ill patients with clinical depression can be successfully treated. He said he believes Dr. Shavelson will be aware of the need to treat depression,”but I do have concerns about other physicians.”
“On the other hand,” he added, “I think it’s really good that this is an option.”
Dr. Shavelson says he’s already received a handful of calls from patients, but mostly he’s spent his time before the law takes effect talking to other physicians. He needs a consulting physician and a pharmacist who will accept prescriptions for a lethal dose of medicine.
Then his mind returns to the patient. “It’s important … that we’re moving forward,” he said. “It’s crucial that we do that because this is part of the rights of patient care to have a certain level of autonomy in how they die.”
To him, this type of care “isn’t so tangibly different” from other kinds of questions doctors address.
“I’m just one of those docs who sees dying as a process, and [the] method of death is less important than making sure it’s a good death.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. This story is part of a partnership that includes KQED, NPR, and Kaiser Health News.
BERKELEY, Calif. – Few people have the unusual set of professional experiences that Dr. Lonny Shavelson does. He worked as an emergency room physician in Berkeley for years – while also working as a journalist. He has written several books and takes hauntingly beautiful photographs.
Now, just as California’s aid-in-dying law takes effect June 9, Dr. Shavelson has added another specialty: A consultant to physicians and terminally ill patients who have questions about how it works.
“Can I just sit back and watch?” Dr. Shavelson asked from his cottage office. “This is really an amazing opportunity to be part of establishing policy and initiating something in medicine. This is a major change … [that] very, very few people know anything about and how to do it.”
Dr. Shavelson is the author of the 1995 book, “A Chosen Death,” which followed five terminally ill people over 2 years as they determined whether to amass drugs on their own and end their lives at a time of their choosing. He was present at the death of all of them.
He followed the issue closely for several years, but ultimately moved on to other projects – among them a book about addiction and a documentary about people who identify as neither male nor female.
Then last fall came the surprising passage of California’s End of Life Option Act, giving terminally ill adults with 6 months to live the right to request lethal medication to end their lives. The law takes effect June 9.
Dr. Shavelson decided he had to act, adding that he feels “quite guilty” about having been away from the issue while others pushed it forward.
His website, Bay Area End of Life Options, went up in April, and he’s outlined the law at “grand rounds” at several Bay Area hospitals this spring. His practice will be focused on consulting not only with physicians whose patients request aid-in-dying, but also with patients themselves. As he indicates on his site, he will offer care to patients who choose him as their “attending End-of-Life physician.”
Dr. Shavelson is adamant that this is “something that has to be done right.” To him, that means starting every patient encounter with a one-word question: “Why?”
“In fact, it’s the only initial approach that I think is acceptable. If somebody calls me and says, ‘I want to take the medication, my first question is, ‘Why? Let me talk to you about all the various alternatives and all the ways that we can think about this.’ ”
Dr. Shavelson worries that patients may seek aid-in-dying because they are in pain. So first, he would like all his patients to be enrolled in hospice care.
“This can only work when you’re sure that the patients have been given the best end-of-life care, which to me is most guaranteed by being a part of hospice or at least having a good palliative care physician. Then this is a rational decision. If you’re doing it otherwise, it’s because of lack of good care.”
California is the fifth state to legalize aid-in-dying, joining Oregon, Washington, Vermont, and Montana. The option is very rarely used. For example, in 2014 in Oregon, just 155 lethal prescriptions were written under the state’s law, and 105 people ultimately took the medicine and died.
Under the California law, two doctors must agree that a patient has 6 months or less to live. The patient must be mentally competent. At least one of the meetings between the patient and his or her doctor must be private, with no one else present, to ensure the patient is acting independently.
Patients must be able to swallow the medication themselves and must affirm in writing, within the 48 hours before taking the medication, that they will do so.
Dr. Shavelson says he has been surprised by the poor understanding of the law among some health care providers. One insisted the law was not taking effect this year; another asked how the law would benefit his patients with Alzheimer’s disease. (Patients with dementia don’t qualify under the law because they are not mentally competent.)
The law does not require that health care providers participate in ending terminally ill patients’ lives. Many physicians are “queasy” about the law, Dr. Shavelson said, and are unwilling to prescribe to patients who request the lethal medication – even when they think having such a law in place is the right thing to do.
“My response to that is as health care providers, you might have been uncomfortable the first time you drew blood. You might have been uncomfortable the first time you took out somebody’s gall bladder,” he said. “If it’s a medical procedure you believe in and you believe it’s the patient’s right, then it’s your obligation to learn how to do it – and do it correctly.”
Dr. Shavelson predicts that many physicians who are initially reluctant to provide this option to their patients may become more comfortable after the law goes into effect and they see how it works.
Dr. Burt Presberg, an East Bay psychiatrist who works with cancer patients and their families, attended a talk by Dr. Shavelson, and it led to some soul searching.
He wrestles with his own comfort level in handling patient requests. When he talks, he often pivots from his initial point to “on the other hand.”
Dr. Presberg says he is concerned that patients suffer from clinical depression at the end of life. Sometimes they feel they are a burden to family members who could “really push for the end of life to happen a little sooner than the patient themselves.”
His experience is that terminally ill patients with clinical depression can be successfully treated. He said he believes Dr. Shavelson will be aware of the need to treat depression,”but I do have concerns about other physicians.”
“On the other hand,” he added, “I think it’s really good that this is an option.”
Dr. Shavelson says he’s already received a handful of calls from patients, but mostly he’s spent his time before the law takes effect talking to other physicians. He needs a consulting physician and a pharmacist who will accept prescriptions for a lethal dose of medicine.
Then his mind returns to the patient. “It’s important … that we’re moving forward,” he said. “It’s crucial that we do that because this is part of the rights of patient care to have a certain level of autonomy in how they die.”
To him, this type of care “isn’t so tangibly different” from other kinds of questions doctors address.
“I’m just one of those docs who sees dying as a process, and [the] method of death is less important than making sure it’s a good death.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. This story is part of a partnership that includes KQED, NPR, and Kaiser Health News.
Mental health disorders the leading cause of disease burden for females
Mental health and substance abuse disorders are the leading cause of disease burden among U.S. females and the fourth-leading cause for males, according to the Kaiser Family Foundation.
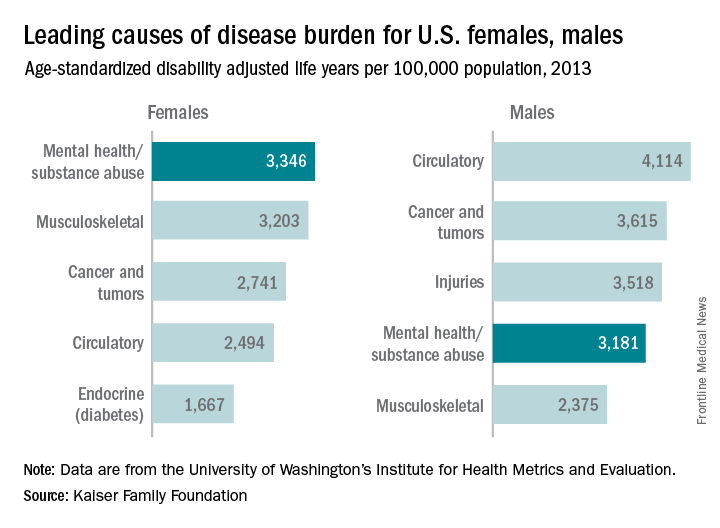
Mental health/substance abuse conditions caused 3,346 age-standardized disability adjusted life years (DALYs) per 100,000 population for females in 2013, putting those conditions ahead of musculoskeletal conditions (3,203 DALYs per 100,000), cancer and tumors (2,741 DALYs), circulatory conditions (2,494 DALYs), and diabetes and other endocrine conditions (1,667 DALYs), Kaiser reported.
Among males, the disease burden resulting from mental health/substance abuse – 3,181 DALYs per 100,000 population – was less than that from circulatory conditions (4,114 DALYs per 100,000), cancer and tumors (3,615 DALYs), and injuries (3,518 DALYs). Musculoskeletal disorders were fifth at 2,375 DALYs, according to data from the Institute for Health Metrics and Evaluation’s Global Burden of Disease Study 2013.
The World Health Organization defines DALYs as “the sum of years of potential life lost due to premature mortality and the years of productive life lost due to disability.”
Mental health and substance abuse disorders are the leading cause of disease burden among U.S. females and the fourth-leading cause for males, according to the Kaiser Family Foundation.
Mental health/substance abuse conditions caused 3,346 age-standardized disability adjusted life years (DALYs) per 100,000 population for females in 2013, putting those conditions ahead of musculoskeletal conditions (3,203 DALYs per 100,000), cancer and tumors (2,741 DALYs), circulatory conditions (2,494 DALYs), and diabetes and other endocrine conditions (1,667 DALYs), Kaiser reported.
Among males, the disease burden resulting from mental health/substance abuse – 3,181 DALYs per 100,000 population – was less than that from circulatory conditions (4,114 DALYs per 100,000), cancer and tumors (3,615 DALYs), and injuries (3,518 DALYs). Musculoskeletal disorders were fifth at 2,375 DALYs, according to data from the Institute for Health Metrics and Evaluation’s Global Burden of Disease Study 2013.
The World Health Organization defines DALYs as “the sum of years of potential life lost due to premature mortality and the years of productive life lost due to disability.”
Mental health and substance abuse disorders are the leading cause of disease burden among U.S. females and the fourth-leading cause for males, according to the Kaiser Family Foundation.
Mental health/substance abuse conditions caused 3,346 age-standardized disability adjusted life years (DALYs) per 100,000 population for females in 2013, putting those conditions ahead of musculoskeletal conditions (3,203 DALYs per 100,000), cancer and tumors (2,741 DALYs), circulatory conditions (2,494 DALYs), and diabetes and other endocrine conditions (1,667 DALYs), Kaiser reported.
Among males, the disease burden resulting from mental health/substance abuse – 3,181 DALYs per 100,000 population – was less than that from circulatory conditions (4,114 DALYs per 100,000), cancer and tumors (3,615 DALYs), and injuries (3,518 DALYs). Musculoskeletal disorders were fifth at 2,375 DALYs, according to data from the Institute for Health Metrics and Evaluation’s Global Burden of Disease Study 2013.
The World Health Organization defines DALYs as “the sum of years of potential life lost due to premature mortality and the years of productive life lost due to disability.”
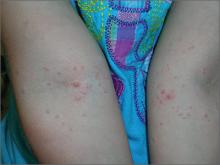
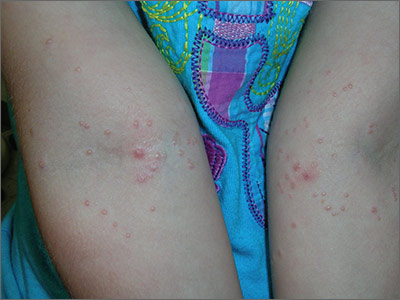
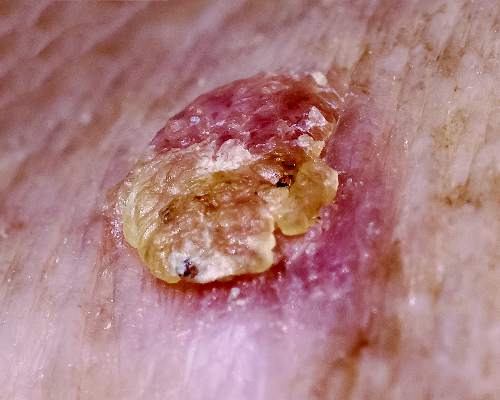
Bumps on arms
The FP diagnosed molluscum contagiosum because a few of the papules had central umbilication. While she noticed that many papules did not have central umbilication, she was aware that not all molluscum lesions would have this feature. Pearly papules are classic for molluscum, even when central umbilication is not visible.
Children with atopic dermatitis are more prone to molluscum infections and frequently get them in areas that have been, or presently are, involved with atopic dermatitis. In this case, the child had antecubital involvement with her atopic dermatitis (although her skin was relatively normal at the time). The altered barrier function found in atopic individuals makes them more prone to various viral and bacterial super infections, including molluscum, herpes, and bacterial impetigo.
In immunocompetent patients, lesions usually spontaneously resolve within 8 to 12 months. In a minority of cases, disease persists for a few years. Children do not have to be kept out of day care or school for this condition, even though it is somewhat contagious. Like warts, keeping kids out of school or day care is not useful to prevent the spread of disease and is not practical on a societal level.
The FP discussed cryotherapy with the mother and child, but the girl was not willing to allow it due to her fear of the pain. Other options included watch and wait, topical salicylic acid, tretinoin, and imiquimod—although none of these have been approved by the Food and Drug Administration. Cantharidin had also been used previously in this office, but it was not available because regulations have made it very difficult to obtain. Imiquimod is not suggested for children younger than 12; therefore, this costly medicine would not be covered by insurance.
The mother requested a prescription for tretinoin and stated that if the insurance would not cover it, she would go with over-the-counter salicylic acid. The FP wrote a prescription for 0.025% tretinoin cream to be applied daily and said to stop using it if irritation became too bothersome. Follow-up was to be done as needed, but was not completed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed molluscum contagiosum because a few of the papules had central umbilication. While she noticed that many papules did not have central umbilication, she was aware that not all molluscum lesions would have this feature. Pearly papules are classic for molluscum, even when central umbilication is not visible.
Children with atopic dermatitis are more prone to molluscum infections and frequently get them in areas that have been, or presently are, involved with atopic dermatitis. In this case, the child had antecubital involvement with her atopic dermatitis (although her skin was relatively normal at the time). The altered barrier function found in atopic individuals makes them more prone to various viral and bacterial super infections, including molluscum, herpes, and bacterial impetigo.
In immunocompetent patients, lesions usually spontaneously resolve within 8 to 12 months. In a minority of cases, disease persists for a few years. Children do not have to be kept out of day care or school for this condition, even though it is somewhat contagious. Like warts, keeping kids out of school or day care is not useful to prevent the spread of disease and is not practical on a societal level.
The FP discussed cryotherapy with the mother and child, but the girl was not willing to allow it due to her fear of the pain. Other options included watch and wait, topical salicylic acid, tretinoin, and imiquimod—although none of these have been approved by the Food and Drug Administration. Cantharidin had also been used previously in this office, but it was not available because regulations have made it very difficult to obtain. Imiquimod is not suggested for children younger than 12; therefore, this costly medicine would not be covered by insurance.
The mother requested a prescription for tretinoin and stated that if the insurance would not cover it, she would go with over-the-counter salicylic acid. The FP wrote a prescription for 0.025% tretinoin cream to be applied daily and said to stop using it if irritation became too bothersome. Follow-up was to be done as needed, but was not completed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed molluscum contagiosum because a few of the papules had central umbilication. While she noticed that many papules did not have central umbilication, she was aware that not all molluscum lesions would have this feature. Pearly papules are classic for molluscum, even when central umbilication is not visible.
Children with atopic dermatitis are more prone to molluscum infections and frequently get them in areas that have been, or presently are, involved with atopic dermatitis. In this case, the child had antecubital involvement with her atopic dermatitis (although her skin was relatively normal at the time). The altered barrier function found in atopic individuals makes them more prone to various viral and bacterial super infections, including molluscum, herpes, and bacterial impetigo.
In immunocompetent patients, lesions usually spontaneously resolve within 8 to 12 months. In a minority of cases, disease persists for a few years. Children do not have to be kept out of day care or school for this condition, even though it is somewhat contagious. Like warts, keeping kids out of school or day care is not useful to prevent the spread of disease and is not practical on a societal level.
The FP discussed cryotherapy with the mother and child, but the girl was not willing to allow it due to her fear of the pain. Other options included watch and wait, topical salicylic acid, tretinoin, and imiquimod—although none of these have been approved by the Food and Drug Administration. Cantharidin had also been used previously in this office, but it was not available because regulations have made it very difficult to obtain. Imiquimod is not suggested for children younger than 12; therefore, this costly medicine would not be covered by insurance.
The mother requested a prescription for tretinoin and stated that if the insurance would not cover it, she would go with over-the-counter salicylic acid. The FP wrote a prescription for 0.025% tretinoin cream to be applied daily and said to stop using it if irritation became too bothersome. Follow-up was to be done as needed, but was not completed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
VIDEO: The ins and outs of JAK ihibitors for alopecia
NEWPORT BEACH, CALIF. – The promise of Janus kinase (JAK) inhibitors for alopecia seems to be holding up in the practice of Dr. Natasha Mesinkovska, a dermatologist at the University of California, Irvine.
There’s been much excitement about JAK inhibitors since Yale researchers reported in 2014 that tofacitinib (Xeljanz), a JAK inhibitor approved in the United States for rheumatoid arthritis, appeared to grow a full head of hair, plus body hair, in an essentially hairless 25-year-old man with plaque psoriasis. JAK inhibitors have been under investigation for alopecia ever since. Meanwhile, they are being used off label for hair loss around the country.
In her own practice, Dr. Mesinkovska estimates that about two-thirds of patients have some degree of hair regrowth, with particularly satisfying results in men. About 40 of her alopecia patients have opted for JAK inhibitors so far.
In an interview at the Summit in Aesthetic Medicine, Dr. Mesinkovska shared her insights and tips, as well as promising alopecia results for the psoriasis biologic ustekinumab (Stelara), an interleukin-12 and -23 antagonist. “This is a very exciting time for alopecia areata,” she said.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – The promise of Janus kinase (JAK) inhibitors for alopecia seems to be holding up in the practice of Dr. Natasha Mesinkovska, a dermatologist at the University of California, Irvine.
There’s been much excitement about JAK inhibitors since Yale researchers reported in 2014 that tofacitinib (Xeljanz), a JAK inhibitor approved in the United States for rheumatoid arthritis, appeared to grow a full head of hair, plus body hair, in an essentially hairless 25-year-old man with plaque psoriasis. JAK inhibitors have been under investigation for alopecia ever since. Meanwhile, they are being used off label for hair loss around the country.
In her own practice, Dr. Mesinkovska estimates that about two-thirds of patients have some degree of hair regrowth, with particularly satisfying results in men. About 40 of her alopecia patients have opted for JAK inhibitors so far.
In an interview at the Summit in Aesthetic Medicine, Dr. Mesinkovska shared her insights and tips, as well as promising alopecia results for the psoriasis biologic ustekinumab (Stelara), an interleukin-12 and -23 antagonist. “This is a very exciting time for alopecia areata,” she said.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – The promise of Janus kinase (JAK) inhibitors for alopecia seems to be holding up in the practice of Dr. Natasha Mesinkovska, a dermatologist at the University of California, Irvine.
There’s been much excitement about JAK inhibitors since Yale researchers reported in 2014 that tofacitinib (Xeljanz), a JAK inhibitor approved in the United States for rheumatoid arthritis, appeared to grow a full head of hair, plus body hair, in an essentially hairless 25-year-old man with plaque psoriasis. JAK inhibitors have been under investigation for alopecia ever since. Meanwhile, they are being used off label for hair loss around the country.
In her own practice, Dr. Mesinkovska estimates that about two-thirds of patients have some degree of hair regrowth, with particularly satisfying results in men. About 40 of her alopecia patients have opted for JAK inhibitors so far.
In an interview at the Summit in Aesthetic Medicine, Dr. Mesinkovska shared her insights and tips, as well as promising alopecia results for the psoriasis biologic ustekinumab (Stelara), an interleukin-12 and -23 antagonist. “This is a very exciting time for alopecia areata,” she said.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE
Adding calcipotriene to 5-FU dramatically reduced AKs
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A combination of 5% 5-fluorouracil cream and 0.005% calcipotriene was significantly more effective at removing actinic keratoses at different anatomic sites as 5-FU monotherapy.
Major finding: At week 8, the combination group had an average 86% reduction in the number of facial AKs, compared with 26% with 5-FU monotherapy.
Data source: A randomized, double-blind, controlled study of 131 patients with at least four AKs on the face, scalp, and/or upper arms.
Disclosures: Dr. Demehri had no disclosures.
Nighttime extubations carry higher risks of reintubation, death
SAN FRANCISCO – Mechanically ventilated patients in the intensive care unit (ICU) have poorer outcomes if extubated during the night instead of during the day, finds a retrospective cohort study reported at an international conference of the American Thoracic Society.
Overall, 20.1% of the nearly 98,000 adult patients studied were extubated during nighttime hours, between 7:00 p.m. and 7:00 a.m., according to data presented in a session and a related press conference.
Compared with patients extubated during daytime hours, patients extubated during nighttime hours had higher rates of ICU and hospital death, with the absolute difference ranging from 1.0% to 5.1%. Additionally, among those mechanically ventilated for at least 12 hours, nighttime extubation was associated with an absolute 2% increase in the risk of reintubation.
“I think this is the first large-scale study that looks at a practice that, although not as common as we thought it was, is still done about a fifth of the time and even with decreasing rates, is not a rare practice on our units,” commented lead author Dr. Hayley B. Gershengorn of the department of medicine (critical care) and the Saul R. Korey department of neurology at the Albert Einstein College of Medicine, New York.
“As we have increasing staffing [overnight] and maybe an increasing push to move people through our ICUs, we need to probably take some care because although we can’t demonstrate a causal link, it is quite concerning, this consistent finding of increased mortality and reintubation in these folks,” she said.
There are several possible reasons for the observed heightened risks of death and reintubation with nighttime extubation that could not be fully explored in the study, Dr. Gershengorn said.
“We were not able to identify the indication for extubation or discontinuation of mechanical ventilation. So one of the concerns that we have is that it’s probably more common that folks unintentionally extubate themselves or someone unintentionally extubates them overnight, when staffing is less,” she explained. “The other part, which we tried to adjust for but we don’t have perfect data on, is what is the staffing overnight,” including factors such as the ratio of nurses to patients and how many units an intensivist is covering, not just whether he or she is present.
“In terms of the reintubation risk being higher in the [group with longer duration of mechanical ventilation], the question I have is whether or not there is less comfort with somebody looking less well when there is less staff around, and whether or not there may be a quicker trigger to reintubate them if they don’t look so great,” she said.
The majority of intubated patients are unlikely to improve enough physiologically to prompt nighttime extubation rather than waiting until daytime, according to Dr. Gershengorn. But there are at least two groups whom clinicians might want to extubate at night.
One group is those who underwent elective surgery during the day. “They are waiting to come out of anesthesia, and the plan is to discontinue mechanical ventilation at the time that that occurs,” she explained. Another group is those who are agitated on the ventilator, require more sedation than usual, and suddenly awake at night. “These patients are really hard to keep comfortable. I can [sedate them] again and try this problem all over again tomorrow morning, or I can just bite the bullet and pull the tube out,” she said.
The investigators analyzed data from the Project IMPACT critical care medicine database, in which data are prospectively collected for benchmarking purposes. In all, they studied 97,844 mechanically ventilated adults from 165 medical and surgical ICUs across the United States between 2000 and 2009.
Results showed that nighttime extubation was more common among elective surgical patients, those coming from the operating room or a postanesthesia care unit, and those mechanically ventilated for less than 12 hours.
In a finding that Dr. Gershengorn described as surprising, there was a temporal trend by which the adjusted proportion of extubations performed at night actually decreased in more recent years during the study period.
The investigators next looked at outcomes among 10,279 propensity-matched pairs of patients, one member of the pair having been extubated during the night and the other having been extubated during the day.
Among those mechanically ventilated for less than 12 hours, nighttime extubation was associated with higher ICU mortality (5.6% vs. 4.6%; P = .025) and hospital mortality (8.3% vs. 7.0%; P = .014). Findings were inconsistent for length of stay, with nighttime extubation associated with a shorter ICU stay but a longer hospital stay.
Among patients mechanically ventilated for 12 hours or longer, those extubated during the night had a higher rate of reintubation (14.6% vs. 12.4%; P less than .001), as well as higher ICU mortality (11.2% vs. 6.1%; P less than .001) and hospital mortality (16.0% vs. 11.1%; P less than .001). Lengths of stay did not differ by extubation time of day in this group.
In sensitivity analyses, findings were similar when the definition of nighttime extubation was altered to the hours of midnight to 5 a.m. and when analyses were restricted to nonpalliative patients, according to Dr. Gershengorn, who disclosed that she had no relevant conflicts of interest.
SAN FRANCISCO – Mechanically ventilated patients in the intensive care unit (ICU) have poorer outcomes if extubated during the night instead of during the day, finds a retrospective cohort study reported at an international conference of the American Thoracic Society.
Overall, 20.1% of the nearly 98,000 adult patients studied were extubated during nighttime hours, between 7:00 p.m. and 7:00 a.m., according to data presented in a session and a related press conference.
Compared with patients extubated during daytime hours, patients extubated during nighttime hours had higher rates of ICU and hospital death, with the absolute difference ranging from 1.0% to 5.1%. Additionally, among those mechanically ventilated for at least 12 hours, nighttime extubation was associated with an absolute 2% increase in the risk of reintubation.
“I think this is the first large-scale study that looks at a practice that, although not as common as we thought it was, is still done about a fifth of the time and even with decreasing rates, is not a rare practice on our units,” commented lead author Dr. Hayley B. Gershengorn of the department of medicine (critical care) and the Saul R. Korey department of neurology at the Albert Einstein College of Medicine, New York.
“As we have increasing staffing [overnight] and maybe an increasing push to move people through our ICUs, we need to probably take some care because although we can’t demonstrate a causal link, it is quite concerning, this consistent finding of increased mortality and reintubation in these folks,” she said.
There are several possible reasons for the observed heightened risks of death and reintubation with nighttime extubation that could not be fully explored in the study, Dr. Gershengorn said.
“We were not able to identify the indication for extubation or discontinuation of mechanical ventilation. So one of the concerns that we have is that it’s probably more common that folks unintentionally extubate themselves or someone unintentionally extubates them overnight, when staffing is less,” she explained. “The other part, which we tried to adjust for but we don’t have perfect data on, is what is the staffing overnight,” including factors such as the ratio of nurses to patients and how many units an intensivist is covering, not just whether he or she is present.
“In terms of the reintubation risk being higher in the [group with longer duration of mechanical ventilation], the question I have is whether or not there is less comfort with somebody looking less well when there is less staff around, and whether or not there may be a quicker trigger to reintubate them if they don’t look so great,” she said.
The majority of intubated patients are unlikely to improve enough physiologically to prompt nighttime extubation rather than waiting until daytime, according to Dr. Gershengorn. But there are at least two groups whom clinicians might want to extubate at night.
One group is those who underwent elective surgery during the day. “They are waiting to come out of anesthesia, and the plan is to discontinue mechanical ventilation at the time that that occurs,” she explained. Another group is those who are agitated on the ventilator, require more sedation than usual, and suddenly awake at night. “These patients are really hard to keep comfortable. I can [sedate them] again and try this problem all over again tomorrow morning, or I can just bite the bullet and pull the tube out,” she said.
The investigators analyzed data from the Project IMPACT critical care medicine database, in which data are prospectively collected for benchmarking purposes. In all, they studied 97,844 mechanically ventilated adults from 165 medical and surgical ICUs across the United States between 2000 and 2009.
Results showed that nighttime extubation was more common among elective surgical patients, those coming from the operating room or a postanesthesia care unit, and those mechanically ventilated for less than 12 hours.
In a finding that Dr. Gershengorn described as surprising, there was a temporal trend by which the adjusted proportion of extubations performed at night actually decreased in more recent years during the study period.
The investigators next looked at outcomes among 10,279 propensity-matched pairs of patients, one member of the pair having been extubated during the night and the other having been extubated during the day.
Among those mechanically ventilated for less than 12 hours, nighttime extubation was associated with higher ICU mortality (5.6% vs. 4.6%; P = .025) and hospital mortality (8.3% vs. 7.0%; P = .014). Findings were inconsistent for length of stay, with nighttime extubation associated with a shorter ICU stay but a longer hospital stay.
Among patients mechanically ventilated for 12 hours or longer, those extubated during the night had a higher rate of reintubation (14.6% vs. 12.4%; P less than .001), as well as higher ICU mortality (11.2% vs. 6.1%; P less than .001) and hospital mortality (16.0% vs. 11.1%; P less than .001). Lengths of stay did not differ by extubation time of day in this group.
In sensitivity analyses, findings were similar when the definition of nighttime extubation was altered to the hours of midnight to 5 a.m. and when analyses were restricted to nonpalliative patients, according to Dr. Gershengorn, who disclosed that she had no relevant conflicts of interest.
SAN FRANCISCO – Mechanically ventilated patients in the intensive care unit (ICU) have poorer outcomes if extubated during the night instead of during the day, finds a retrospective cohort study reported at an international conference of the American Thoracic Society.
Overall, 20.1% of the nearly 98,000 adult patients studied were extubated during nighttime hours, between 7:00 p.m. and 7:00 a.m., according to data presented in a session and a related press conference.
Compared with patients extubated during daytime hours, patients extubated during nighttime hours had higher rates of ICU and hospital death, with the absolute difference ranging from 1.0% to 5.1%. Additionally, among those mechanically ventilated for at least 12 hours, nighttime extubation was associated with an absolute 2% increase in the risk of reintubation.
“I think this is the first large-scale study that looks at a practice that, although not as common as we thought it was, is still done about a fifth of the time and even with decreasing rates, is not a rare practice on our units,” commented lead author Dr. Hayley B. Gershengorn of the department of medicine (critical care) and the Saul R. Korey department of neurology at the Albert Einstein College of Medicine, New York.
“As we have increasing staffing [overnight] and maybe an increasing push to move people through our ICUs, we need to probably take some care because although we can’t demonstrate a causal link, it is quite concerning, this consistent finding of increased mortality and reintubation in these folks,” she said.
There are several possible reasons for the observed heightened risks of death and reintubation with nighttime extubation that could not be fully explored in the study, Dr. Gershengorn said.
“We were not able to identify the indication for extubation or discontinuation of mechanical ventilation. So one of the concerns that we have is that it’s probably more common that folks unintentionally extubate themselves or someone unintentionally extubates them overnight, when staffing is less,” she explained. “The other part, which we tried to adjust for but we don’t have perfect data on, is what is the staffing overnight,” including factors such as the ratio of nurses to patients and how many units an intensivist is covering, not just whether he or she is present.
“In terms of the reintubation risk being higher in the [group with longer duration of mechanical ventilation], the question I have is whether or not there is less comfort with somebody looking less well when there is less staff around, and whether or not there may be a quicker trigger to reintubate them if they don’t look so great,” she said.
The majority of intubated patients are unlikely to improve enough physiologically to prompt nighttime extubation rather than waiting until daytime, according to Dr. Gershengorn. But there are at least two groups whom clinicians might want to extubate at night.
One group is those who underwent elective surgery during the day. “They are waiting to come out of anesthesia, and the plan is to discontinue mechanical ventilation at the time that that occurs,” she explained. Another group is those who are agitated on the ventilator, require more sedation than usual, and suddenly awake at night. “These patients are really hard to keep comfortable. I can [sedate them] again and try this problem all over again tomorrow morning, or I can just bite the bullet and pull the tube out,” she said.
The investigators analyzed data from the Project IMPACT critical care medicine database, in which data are prospectively collected for benchmarking purposes. In all, they studied 97,844 mechanically ventilated adults from 165 medical and surgical ICUs across the United States between 2000 and 2009.
Results showed that nighttime extubation was more common among elective surgical patients, those coming from the operating room or a postanesthesia care unit, and those mechanically ventilated for less than 12 hours.
In a finding that Dr. Gershengorn described as surprising, there was a temporal trend by which the adjusted proportion of extubations performed at night actually decreased in more recent years during the study period.
The investigators next looked at outcomes among 10,279 propensity-matched pairs of patients, one member of the pair having been extubated during the night and the other having been extubated during the day.
Among those mechanically ventilated for less than 12 hours, nighttime extubation was associated with higher ICU mortality (5.6% vs. 4.6%; P = .025) and hospital mortality (8.3% vs. 7.0%; P = .014). Findings were inconsistent for length of stay, with nighttime extubation associated with a shorter ICU stay but a longer hospital stay.
Among patients mechanically ventilated for 12 hours or longer, those extubated during the night had a higher rate of reintubation (14.6% vs. 12.4%; P less than .001), as well as higher ICU mortality (11.2% vs. 6.1%; P less than .001) and hospital mortality (16.0% vs. 11.1%; P less than .001). Lengths of stay did not differ by extubation time of day in this group.
In sensitivity analyses, findings were similar when the definition of nighttime extubation was altered to the hours of midnight to 5 a.m. and when analyses were restricted to nonpalliative patients, according to Dr. Gershengorn, who disclosed that she had no relevant conflicts of interest.
AT ATS 2016
Key clinical point: Mechanically ventilated ICU patients have poorer outcomes if they are extubated during the night instead of during the day.
Major finding: Compared with patients extubated during daytime hours, patients extubated during nighttime hours had higher rates of ICU and hospital death, with the absolute difference ranging from 1.0% to 5.1%.
Data source: A retrospective cohort study of 97,844 mechanically ventilated adult patients from 165 ICUs in the United States.
Disclosures: Dr. Gershengorn disclosed that she had no relevant conflicts of interest.
50 years of gynecologic surgery: A large dose of ingenuity, a small dose of controversy
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
LAA occlusion studied for stroke prevention in atrial fib with prior intracerebral hemorrhage
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
AT EUROPCR 2016
Key clinical point: Left atrial appendage occlusion appears to be better than medical management for stroke prevention in patients with AF and prior intracerebral hemorrhage.
Major finding: The risk of a combined endpoint of ischemic stroke, major bleeding, or death during follow-up was 81% lower in patients with atrial fibrillation and a prior intracerebral hemorrhage treated with a transcatheter left atrial appendage occlusion device than in controls on standard medical therapy.
Data source: This study included 147 patient pairs propensity-matched for stroke and bleeding risk.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to St. Jude Medical.
Does U.S. Healthcare Need More Diverse Leadership?
Throughout its history, the United States has been a nation of immigrants. From the early colonial settlements to the mid-20th century, most immigrants came from Western European countries. Since 1965, when the Immigration and Nationality Act abolished national-origin quotas, the diversity of immigrants has increased. “By the year 2043,” says Tomás León, president and CEO of the Institute for Diversity in Health Management in Chicago, “we will be a country where the majority of our population is comprised of racial and ethnic minorities.”
Those changing demographics, cited from the U.S. Census Bureau’s projections, already are evidenced in hospital patient populations. According to a benchmarking survey sponsored by the institute, which is an affiliate of the American Hospital Association, the percentage of minority patients seen in hospitals grew from 29% to 31% of patient census between 2011 and 2013.1 And yet, the survey found this increasing diversity is not currently reflected in leadership positions. During the same time period, underrepresented racial and ethnic minorities (UREM) on hospital boards of directors (14%) and in C-suite positions (14%) remained flat (see Figure 1).
Gender disparities in healthcare and academic leadership also have been slow to change. Periodic surveys conducted by the American College of Healthcare Executives indicate that women comprise only 11% of healthcare CEOs in the U.S.2 And despite the fact that women make up half of all medical students (and one-third of full-time faculty), the Association of American Medical Colleges (AAMC) finds that women still trail men when it comes to attaining full professorship and decanal positions at their academic institutions.3
The Hospitalist interviewed medical directors, researchers, diversity management professionals, and hospitalists to ascertain current solutions being pursued to narrow the gaps in leadership diversity.
Why Diversity in Leadership Matters
Eric E. Howell, MD, MHM, chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in the Hopkins School of Medicine in Baltimore, believes there is a need to encourage the advancement to leadership positions for female and UREM physicians.
“In medicine, it’s really about service. If we are really here for our patients, we need representation of diversity in our faculty and leadership,” says Dr. Howell, a past SHM president and faculty member of SHM’s Leadership Academy since its inception in 2005. In addition, he says, “Diversity adds incredible strength to an organization and adds to the richness of the ideas and solutions to overcome challenging problems.”
With the implementation of the Affordable Care Act, formerly uninsured people are now accessing the healthcare system; many are bilingual and bicultural, notes George A. Zeppenfeldt-Cestero, president and CEO, Association of Hispanic Healthcare Executives.
“You want to make sure that providers, whether they are physicians, nurses, dentists, or health executives that drive policy issues, are also reflective of that population throughout the organization,” he says. “The real definition of diversity is making sure you have diversity in all layers of the workforce, including the C-suite.”
León points to the coming “seismic demographic shifts” and wonders if healthcare is ready to become more reflective of the communities it serves.
“Increasing diversity in healthcare leadership and governance is essential for the delivery and provision of culturally competent care,” León says. “Now, more than ever, it’s important that we collectively accelerate progress in this area.”
Advancing in Academic and Hospital Medicine
Might hospital medicine offer additional opportunities for women and minorities to advance into leadership positions? Hospitalist Flora Kisuule, MD, SFHM, assistant professor of medicine at Johns Hopkins School of Medicine and associate division director of the Collaborative Inpatient Medicine Service (CIMS) at Johns Hopkins Bayview Medical Center, believes this may be the case. She was with Dr. Howell’s group when he needed to fill the associate director position.
“My advancement speaks to hospital medicine and the fact that we are growing as a field,” she says. “Because of that, opportunities are presenting themselves.”
Dr. Kisuule’s ability to thrive in her position speaks to her professionalism but also to a number of other intentional factors: Dr. Howell’s continuing sponsorship to include her in leadership opportunities, an emergency call system for parents with sick children, and a women’s task force whose agenda calls for transparency in hiring and advancement.
Intentional Structure Change
Cardiologist Hannah A. Valantine, MD, recognizes the importance of addressing the lack of women and people from unrepresented groups in the Science, Technology, Engineering, and Mathematics (STEM) workforce. While at Stanford University School of Medicine, she developed and put into place a set of strategies to understand and mitigate the drivers of gender imbalance. Since then, Dr. Valantine was recruited to bring her expertise to the National Institutes of Health in Bethesda, Md., where she is the inaugural chief officer for scientific workforce diversity. In this role, she is committed “to promoting biomedical workforce diversity as an opportunity, not a problem.”
Dr. Valantine is pushing NIH to pursue a wide range of evidence-based programming to eliminate career-transition barriers that keep women and individuals from underrepresented groups from attaining spots in the top echelons of science and health leadership. She believes that applying scientific rigor to the issue of workforce diversity can lead to quantifiable, translatable, and repeatable methods for recruitment and retention of talent in the biomedical workforce (see “Building Blocks").
Before joining NIH, Dr. Valantine and her colleagues at Stanford surveyed gender composition and faculty satisfaction several years after initiating a multifaceted intervention to boost recruitment and development of women faculty.4 After making a visible commitment of resources to support faculty, with special attention to women, Stanford rose from below to above national benchmarks in the representation of women among faculty. Yet significant work remains to be done, Dr. Valantine says. Her work predicts that the estimated time to achieve 50% occupancy of full professorships by women nationally approaches 50 years—“far too long using current approaches.”
In a separate review article, Dr. Valantine and co-author Christy Sandborg, MD, described the Stanford University School of Medicine Academic Biomedical Career Customization (ABCC) model, which was adapted from Deloitte’s Mass Career Customization framework and allows for development of individual career plans that span a faculty member’s total career, not just a year or two at a time. Long-term planning can enable better alignment between the work culture and values of the workforce, which will improve the outlook for women faculty, Dr. Valantine says.
The issues of work-life balance may actually be generational, Dr. Valantine explains. Veteran hospitalist Janet Nagamine, MD, BSN, SFHM, of Santa Clara, Calif., agrees.
“Nowadays, men as well as women are looking for work-life balance,” she says.
In hospital medicine, Dr. Nagamine points out, the structural changes required to effect a work-life balance for hospital leaders are often difficult to achieve.
“As productivity surveys show, HM group leaders are putting in as many RVUs as the staff,” the former SHM board member says. “There is no dedicated time for administrative duties.”
Construct a Pipeline
Barriers to advancement often are particular to characteristics of diverse populations. For example, the AAMC’s report on the U.S. physician workforce documents that in African-American physicians 40 and younger, women outnumber their male counterparts. Therefore, in the association’s Diversity in Medical Education: Facts and Figures 2012 report, the executive summary points out the need to strengthen the medical education pipeline to increase the number of African-American males who enter the premed track.
Despite the fast-growing percentage of Latino and Hispanic populations in the United States, the shortage of Latino/Hispanic physicians increased from 1980 to 2010. Latinos/Hispanics are greatly underrepresented in the medical student, resident, and faculty populations, according to John Paul Sánchez, MD, MPH, assistant dean for diversity and inclusion in the Office for Diversity and Community Engagement at Rutgers, The State University of New Jersey. Likewise, Zeppenfeldt-Cestero believes that efforts must begin much earlier with Latino and other minority and underrepresented students.
“We have to make sure our students pursue the STEM disciplines and that they also later have the education and preparation to be competitive at the MBA or MPH levels,” he says.
Dr. Sánchez, an associate professor of emergency medicine and a diversity activist since his med school days, is the recipient of last year’s Association of Hispanic Healthcare Executives’ academic leader of the year award. Since September 2014, he has been involved with Building the Next Generation of Academic Physicians Inc., which collaborates with more than 40 medical schools across the country. The initiative offers conferences designed to develop diverse medical students’ and residents’ interest in pursuing academic medicine. Open to all medical students and residents, the conference curriculum is tailored for women, UREMs, and trainees who identify as lesbian, gay, bisexual, or transgender (LGBT), he says. Seven conferences were held in 2015, 10 are planned for this year, and seven for 2017.
Healthcare Leadership Gaps
Despite their omnipresence in healthcare, there is a dearth of women in chief executive and governance roles, as has been noted by both the American College of Healthcare Executives and the National Center for Healthcare Leadership. As with academic leadership positions, the leadership gap in the administrative sector does not seem to be due to a lack of women entering graduate programs in health administration. On the contrary, since the mid-1980s women have comprised 50% to 60% of graduate students.
“This is absolutely not a pipeline issue,” says Christy Harris Lemak, PhD, FACHE, professor and chair of the Department of Health Services Administration at the University of Alabama at Birmingham School of Health Professions and lead investigator of the National Center for Healthcare Leadership’s study of women in healthcare executive positions. Other factors come into play.
In the study, she and her co-authors queried female healthcare CEOs to ascertain the critical career inflection points that led to their success.6 Those who were strategic about their careers, sought out mentors, and voiced their intentions about pursuing leadership positions were more likely to be successful in those efforts. However, individual career efforts must be coupled with overall organizational commitment to fostering inclusion (see “Path to the Top: Strategic Advice for Women").
Hospitals and healthcare organizations must pursue the development of human capital (and the diversity of their leaders) in a systematic way. “We recommended [in the study] that organizations set expectations that leaders who mentor other potential leaders be rewarded in the same way as those who hit financial targets or readmission rate targets,” Dr. Lemak says.
Leadership matters, agrees Deborah J. Bowen, FACHE, CAE, president and CEO of Chicago-based American College of Healthcare Executives.
“I think we’re getting a little smarter. Organizational leaders and trustees have a better understanding that talent development is one of the most important jobs,” she says. “If you don’t have the right people in the right places making good decisions on behalf of the patients and the populations in the communities they’re serving, the rest falls apart.”
Nuances of Mentoring
Many conversations about encouraging diversity in healthcare leadership converge around the role of effective mentoring and sponsorship. A substantial body of research supports the impact of mentoring on retention, research productivity, career satisfaction, and career development for women. It’s important to ensure that the institutional culture is geared toward mentoring junior faculty, says Jessie Kimbrough Marshall, MD, MPH, assistant professor in the Division of General Medicine Hospitalist Program at the University of Michigan Health System in Ann Arbor (UMHS).
Several of our sources pointed out that leaders must learn how to be effective mentors. More attention is being given to enhancing leaders’ mentorship skills. One example is at the Institute for Diversity in Health Management, which conducts an intensive 12-month certificate in diversity management program for practitioners. León says the program fosters ongoing networking and support through the American Leadership Council on Diversity in Healthcare by building leadership competencies.
Dr. Valantine points out that mentoring is hardly a one-style-fits-all proposition but that it is a crucial element to creating and retaining diversity. She says it should be viewed “much more broadly than it is today, and it should focus beyond the trainer-trainee relationship.”
The process is a two-way street. Denege Ward, MD, hospitalist, assistant professor of internal medicine, and director of the medical short stay unit at UMHS, says minorities need to be ready to take a leap of faith.
“Underrepresented faculty and staff should take the risk of possible failure in challenging situations but learn from it and do better and not succumb to fear in face of challenges,” Dr. Ward says.
Although mentoring is one important component in building diversity in academic medicine, Dr. Sánchez asserts that role models, champions, and sponsors are equally important.
“In addition and separate from role models, there must be in place policies and procedures that promote a climate for diverse individuals to succeed,” he says. “What’s needed is an institutional vision and strategic plan that recognizes the importance of diversity. [It] has to become a core principle.”
Dr. Marshall echoes that refrain, noting the recruitment and retention of a diverse set of leaders will take time and intentionality. She is actively engaged in organizing annual meeting mentoring panels at the Society of General Internal Medicine.
“There are still quite a few barriers for women and minorities to advance into hospital leadership roles,” she says. “We still have a long way to go. However, I’m seeing more women and people of color get into these positions. The numbers are increasing, and that encourages me.” TH
Gretchen Henkel is a freelance writer in California.
References
- Institute for Diversity in Health Management. The state of health care diversity and disparities: a benchmarking study of U.S. hospitals. Available at: http://www.diversityconnection.org/diversityconnection/leadership-conferences/Benchmarking-Survey.jsp?fll=S11.
- Top issues confronting hospitals in 2015. American College of Healthcare Executives website. Available at: https://www.ache.org/pubs/research/ceoissues.cfm. Accessed March 5, 2016.
- Association of American Medical Colleges. Diversity in the physician workforce: facts & figures 2014. Available at: http://aamcdiversityfactsandfigures.org/.
- Valantine HA, Grewal D, Ku MC, et al. The gender gap in academic medicine: comparing results from a multifaceted intervention for Stanford faculty to peer and national cohorts. Acad Med. 2014;89(6):904-911.
- Valantine H, Sandborg CI. Changing the culture of academic medicine to eliminate the gender leadership gap: 50/50 by 2020. Acad Med. 2013;88(10):1411-1413.
- Sexton DW, Lemak CH, Wainio JA. Career inflection points of women who successfully achieved the hospital CEO position. J Healthc Manag. 2014;59(5):367-383.
Throughout its history, the United States has been a nation of immigrants. From the early colonial settlements to the mid-20th century, most immigrants came from Western European countries. Since 1965, when the Immigration and Nationality Act abolished national-origin quotas, the diversity of immigrants has increased. “By the year 2043,” says Tomás León, president and CEO of the Institute for Diversity in Health Management in Chicago, “we will be a country where the majority of our population is comprised of racial and ethnic minorities.”
Those changing demographics, cited from the U.S. Census Bureau’s projections, already are evidenced in hospital patient populations. According to a benchmarking survey sponsored by the institute, which is an affiliate of the American Hospital Association, the percentage of minority patients seen in hospitals grew from 29% to 31% of patient census between 2011 and 2013.1 And yet, the survey found this increasing diversity is not currently reflected in leadership positions. During the same time period, underrepresented racial and ethnic minorities (UREM) on hospital boards of directors (14%) and in C-suite positions (14%) remained flat (see Figure 1).
Gender disparities in healthcare and academic leadership also have been slow to change. Periodic surveys conducted by the American College of Healthcare Executives indicate that women comprise only 11% of healthcare CEOs in the U.S.2 And despite the fact that women make up half of all medical students (and one-third of full-time faculty), the Association of American Medical Colleges (AAMC) finds that women still trail men when it comes to attaining full professorship and decanal positions at their academic institutions.3
The Hospitalist interviewed medical directors, researchers, diversity management professionals, and hospitalists to ascertain current solutions being pursued to narrow the gaps in leadership diversity.
Why Diversity in Leadership Matters
Eric E. Howell, MD, MHM, chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in the Hopkins School of Medicine in Baltimore, believes there is a need to encourage the advancement to leadership positions for female and UREM physicians.
“In medicine, it’s really about service. If we are really here for our patients, we need representation of diversity in our faculty and leadership,” says Dr. Howell, a past SHM president and faculty member of SHM’s Leadership Academy since its inception in 2005. In addition, he says, “Diversity adds incredible strength to an organization and adds to the richness of the ideas and solutions to overcome challenging problems.”
With the implementation of the Affordable Care Act, formerly uninsured people are now accessing the healthcare system; many are bilingual and bicultural, notes George A. Zeppenfeldt-Cestero, president and CEO, Association of Hispanic Healthcare Executives.
“You want to make sure that providers, whether they are physicians, nurses, dentists, or health executives that drive policy issues, are also reflective of that population throughout the organization,” he says. “The real definition of diversity is making sure you have diversity in all layers of the workforce, including the C-suite.”
León points to the coming “seismic demographic shifts” and wonders if healthcare is ready to become more reflective of the communities it serves.
“Increasing diversity in healthcare leadership and governance is essential for the delivery and provision of culturally competent care,” León says. “Now, more than ever, it’s important that we collectively accelerate progress in this area.”
Advancing in Academic and Hospital Medicine
Might hospital medicine offer additional opportunities for women and minorities to advance into leadership positions? Hospitalist Flora Kisuule, MD, SFHM, assistant professor of medicine at Johns Hopkins School of Medicine and associate division director of the Collaborative Inpatient Medicine Service (CIMS) at Johns Hopkins Bayview Medical Center, believes this may be the case. She was with Dr. Howell’s group when he needed to fill the associate director position.
“My advancement speaks to hospital medicine and the fact that we are growing as a field,” she says. “Because of that, opportunities are presenting themselves.”
Dr. Kisuule’s ability to thrive in her position speaks to her professionalism but also to a number of other intentional factors: Dr. Howell’s continuing sponsorship to include her in leadership opportunities, an emergency call system for parents with sick children, and a women’s task force whose agenda calls for transparency in hiring and advancement.
Intentional Structure Change
Cardiologist Hannah A. Valantine, MD, recognizes the importance of addressing the lack of women and people from unrepresented groups in the Science, Technology, Engineering, and Mathematics (STEM) workforce. While at Stanford University School of Medicine, she developed and put into place a set of strategies to understand and mitigate the drivers of gender imbalance. Since then, Dr. Valantine was recruited to bring her expertise to the National Institutes of Health in Bethesda, Md., where she is the inaugural chief officer for scientific workforce diversity. In this role, she is committed “to promoting biomedical workforce diversity as an opportunity, not a problem.”
Dr. Valantine is pushing NIH to pursue a wide range of evidence-based programming to eliminate career-transition barriers that keep women and individuals from underrepresented groups from attaining spots in the top echelons of science and health leadership. She believes that applying scientific rigor to the issue of workforce diversity can lead to quantifiable, translatable, and repeatable methods for recruitment and retention of talent in the biomedical workforce (see “Building Blocks").
Before joining NIH, Dr. Valantine and her colleagues at Stanford surveyed gender composition and faculty satisfaction several years after initiating a multifaceted intervention to boost recruitment and development of women faculty.4 After making a visible commitment of resources to support faculty, with special attention to women, Stanford rose from below to above national benchmarks in the representation of women among faculty. Yet significant work remains to be done, Dr. Valantine says. Her work predicts that the estimated time to achieve 50% occupancy of full professorships by women nationally approaches 50 years—“far too long using current approaches.”
In a separate review article, Dr. Valantine and co-author Christy Sandborg, MD, described the Stanford University School of Medicine Academic Biomedical Career Customization (ABCC) model, which was adapted from Deloitte’s Mass Career Customization framework and allows for development of individual career plans that span a faculty member’s total career, not just a year or two at a time. Long-term planning can enable better alignment between the work culture and values of the workforce, which will improve the outlook for women faculty, Dr. Valantine says.
The issues of work-life balance may actually be generational, Dr. Valantine explains. Veteran hospitalist Janet Nagamine, MD, BSN, SFHM, of Santa Clara, Calif., agrees.
“Nowadays, men as well as women are looking for work-life balance,” she says.
In hospital medicine, Dr. Nagamine points out, the structural changes required to effect a work-life balance for hospital leaders are often difficult to achieve.
“As productivity surveys show, HM group leaders are putting in as many RVUs as the staff,” the former SHM board member says. “There is no dedicated time for administrative duties.”
Construct a Pipeline
Barriers to advancement often are particular to characteristics of diverse populations. For example, the AAMC’s report on the U.S. physician workforce documents that in African-American physicians 40 and younger, women outnumber their male counterparts. Therefore, in the association’s Diversity in Medical Education: Facts and Figures 2012 report, the executive summary points out the need to strengthen the medical education pipeline to increase the number of African-American males who enter the premed track.
Despite the fast-growing percentage of Latino and Hispanic populations in the United States, the shortage of Latino/Hispanic physicians increased from 1980 to 2010. Latinos/Hispanics are greatly underrepresented in the medical student, resident, and faculty populations, according to John Paul Sánchez, MD, MPH, assistant dean for diversity and inclusion in the Office for Diversity and Community Engagement at Rutgers, The State University of New Jersey. Likewise, Zeppenfeldt-Cestero believes that efforts must begin much earlier with Latino and other minority and underrepresented students.
“We have to make sure our students pursue the STEM disciplines and that they also later have the education and preparation to be competitive at the MBA or MPH levels,” he says.
Dr. Sánchez, an associate professor of emergency medicine and a diversity activist since his med school days, is the recipient of last year’s Association of Hispanic Healthcare Executives’ academic leader of the year award. Since September 2014, he has been involved with Building the Next Generation of Academic Physicians Inc., which collaborates with more than 40 medical schools across the country. The initiative offers conferences designed to develop diverse medical students’ and residents’ interest in pursuing academic medicine. Open to all medical students and residents, the conference curriculum is tailored for women, UREMs, and trainees who identify as lesbian, gay, bisexual, or transgender (LGBT), he says. Seven conferences were held in 2015, 10 are planned for this year, and seven for 2017.
Healthcare Leadership Gaps
Despite their omnipresence in healthcare, there is a dearth of women in chief executive and governance roles, as has been noted by both the American College of Healthcare Executives and the National Center for Healthcare Leadership. As with academic leadership positions, the leadership gap in the administrative sector does not seem to be due to a lack of women entering graduate programs in health administration. On the contrary, since the mid-1980s women have comprised 50% to 60% of graduate students.
“This is absolutely not a pipeline issue,” says Christy Harris Lemak, PhD, FACHE, professor and chair of the Department of Health Services Administration at the University of Alabama at Birmingham School of Health Professions and lead investigator of the National Center for Healthcare Leadership’s study of women in healthcare executive positions. Other factors come into play.
In the study, she and her co-authors queried female healthcare CEOs to ascertain the critical career inflection points that led to their success.6 Those who were strategic about their careers, sought out mentors, and voiced their intentions about pursuing leadership positions were more likely to be successful in those efforts. However, individual career efforts must be coupled with overall organizational commitment to fostering inclusion (see “Path to the Top: Strategic Advice for Women").
Hospitals and healthcare organizations must pursue the development of human capital (and the diversity of their leaders) in a systematic way. “We recommended [in the study] that organizations set expectations that leaders who mentor other potential leaders be rewarded in the same way as those who hit financial targets or readmission rate targets,” Dr. Lemak says.
Leadership matters, agrees Deborah J. Bowen, FACHE, CAE, president and CEO of Chicago-based American College of Healthcare Executives.
“I think we’re getting a little smarter. Organizational leaders and trustees have a better understanding that talent development is one of the most important jobs,” she says. “If you don’t have the right people in the right places making good decisions on behalf of the patients and the populations in the communities they’re serving, the rest falls apart.”
Nuances of Mentoring
Many conversations about encouraging diversity in healthcare leadership converge around the role of effective mentoring and sponsorship. A substantial body of research supports the impact of mentoring on retention, research productivity, career satisfaction, and career development for women. It’s important to ensure that the institutional culture is geared toward mentoring junior faculty, says Jessie Kimbrough Marshall, MD, MPH, assistant professor in the Division of General Medicine Hospitalist Program at the University of Michigan Health System in Ann Arbor (UMHS).
Several of our sources pointed out that leaders must learn how to be effective mentors. More attention is being given to enhancing leaders’ mentorship skills. One example is at the Institute for Diversity in Health Management, which conducts an intensive 12-month certificate in diversity management program for practitioners. León says the program fosters ongoing networking and support through the American Leadership Council on Diversity in Healthcare by building leadership competencies.
Dr. Valantine points out that mentoring is hardly a one-style-fits-all proposition but that it is a crucial element to creating and retaining diversity. She says it should be viewed “much more broadly than it is today, and it should focus beyond the trainer-trainee relationship.”
The process is a two-way street. Denege Ward, MD, hospitalist, assistant professor of internal medicine, and director of the medical short stay unit at UMHS, says minorities need to be ready to take a leap of faith.
“Underrepresented faculty and staff should take the risk of possible failure in challenging situations but learn from it and do better and not succumb to fear in face of challenges,” Dr. Ward says.
Although mentoring is one important component in building diversity in academic medicine, Dr. Sánchez asserts that role models, champions, and sponsors are equally important.
“In addition and separate from role models, there must be in place policies and procedures that promote a climate for diverse individuals to succeed,” he says. “What’s needed is an institutional vision and strategic plan that recognizes the importance of diversity. [It] has to become a core principle.”
Dr. Marshall echoes that refrain, noting the recruitment and retention of a diverse set of leaders will take time and intentionality. She is actively engaged in organizing annual meeting mentoring panels at the Society of General Internal Medicine.
“There are still quite a few barriers for women and minorities to advance into hospital leadership roles,” she says. “We still have a long way to go. However, I’m seeing more women and people of color get into these positions. The numbers are increasing, and that encourages me.” TH
Gretchen Henkel is a freelance writer in California.
References
- Institute for Diversity in Health Management. The state of health care diversity and disparities: a benchmarking study of U.S. hospitals. Available at: http://www.diversityconnection.org/diversityconnection/leadership-conferences/Benchmarking-Survey.jsp?fll=S11.
- Top issues confronting hospitals in 2015. American College of Healthcare Executives website. Available at: https://www.ache.org/pubs/research/ceoissues.cfm. Accessed March 5, 2016.
- Association of American Medical Colleges. Diversity in the physician workforce: facts & figures 2014. Available at: http://aamcdiversityfactsandfigures.org/.
- Valantine HA, Grewal D, Ku MC, et al. The gender gap in academic medicine: comparing results from a multifaceted intervention for Stanford faculty to peer and national cohorts. Acad Med. 2014;89(6):904-911.
- Valantine H, Sandborg CI. Changing the culture of academic medicine to eliminate the gender leadership gap: 50/50 by 2020. Acad Med. 2013;88(10):1411-1413.
- Sexton DW, Lemak CH, Wainio JA. Career inflection points of women who successfully achieved the hospital CEO position. J Healthc Manag. 2014;59(5):367-383.
Throughout its history, the United States has been a nation of immigrants. From the early colonial settlements to the mid-20th century, most immigrants came from Western European countries. Since 1965, when the Immigration and Nationality Act abolished national-origin quotas, the diversity of immigrants has increased. “By the year 2043,” says Tomás León, president and CEO of the Institute for Diversity in Health Management in Chicago, “we will be a country where the majority of our population is comprised of racial and ethnic minorities.”
Those changing demographics, cited from the U.S. Census Bureau’s projections, already are evidenced in hospital patient populations. According to a benchmarking survey sponsored by the institute, which is an affiliate of the American Hospital Association, the percentage of minority patients seen in hospitals grew from 29% to 31% of patient census between 2011 and 2013.1 And yet, the survey found this increasing diversity is not currently reflected in leadership positions. During the same time period, underrepresented racial and ethnic minorities (UREM) on hospital boards of directors (14%) and in C-suite positions (14%) remained flat (see Figure 1).
Gender disparities in healthcare and academic leadership also have been slow to change. Periodic surveys conducted by the American College of Healthcare Executives indicate that women comprise only 11% of healthcare CEOs in the U.S.2 And despite the fact that women make up half of all medical students (and one-third of full-time faculty), the Association of American Medical Colleges (AAMC) finds that women still trail men when it comes to attaining full professorship and decanal positions at their academic institutions.3
The Hospitalist interviewed medical directors, researchers, diversity management professionals, and hospitalists to ascertain current solutions being pursued to narrow the gaps in leadership diversity.
Why Diversity in Leadership Matters
Eric E. Howell, MD, MHM, chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in the Hopkins School of Medicine in Baltimore, believes there is a need to encourage the advancement to leadership positions for female and UREM physicians.
“In medicine, it’s really about service. If we are really here for our patients, we need representation of diversity in our faculty and leadership,” says Dr. Howell, a past SHM president and faculty member of SHM’s Leadership Academy since its inception in 2005. In addition, he says, “Diversity adds incredible strength to an organization and adds to the richness of the ideas and solutions to overcome challenging problems.”
With the implementation of the Affordable Care Act, formerly uninsured people are now accessing the healthcare system; many are bilingual and bicultural, notes George A. Zeppenfeldt-Cestero, president and CEO, Association of Hispanic Healthcare Executives.
“You want to make sure that providers, whether they are physicians, nurses, dentists, or health executives that drive policy issues, are also reflective of that population throughout the organization,” he says. “The real definition of diversity is making sure you have diversity in all layers of the workforce, including the C-suite.”
León points to the coming “seismic demographic shifts” and wonders if healthcare is ready to become more reflective of the communities it serves.
“Increasing diversity in healthcare leadership and governance is essential for the delivery and provision of culturally competent care,” León says. “Now, more than ever, it’s important that we collectively accelerate progress in this area.”
Advancing in Academic and Hospital Medicine
Might hospital medicine offer additional opportunities for women and minorities to advance into leadership positions? Hospitalist Flora Kisuule, MD, SFHM, assistant professor of medicine at Johns Hopkins School of Medicine and associate division director of the Collaborative Inpatient Medicine Service (CIMS) at Johns Hopkins Bayview Medical Center, believes this may be the case. She was with Dr. Howell’s group when he needed to fill the associate director position.
“My advancement speaks to hospital medicine and the fact that we are growing as a field,” she says. “Because of that, opportunities are presenting themselves.”
Dr. Kisuule’s ability to thrive in her position speaks to her professionalism but also to a number of other intentional factors: Dr. Howell’s continuing sponsorship to include her in leadership opportunities, an emergency call system for parents with sick children, and a women’s task force whose agenda calls for transparency in hiring and advancement.
Intentional Structure Change
Cardiologist Hannah A. Valantine, MD, recognizes the importance of addressing the lack of women and people from unrepresented groups in the Science, Technology, Engineering, and Mathematics (STEM) workforce. While at Stanford University School of Medicine, she developed and put into place a set of strategies to understand and mitigate the drivers of gender imbalance. Since then, Dr. Valantine was recruited to bring her expertise to the National Institutes of Health in Bethesda, Md., where she is the inaugural chief officer for scientific workforce diversity. In this role, she is committed “to promoting biomedical workforce diversity as an opportunity, not a problem.”
Dr. Valantine is pushing NIH to pursue a wide range of evidence-based programming to eliminate career-transition barriers that keep women and individuals from underrepresented groups from attaining spots in the top echelons of science and health leadership. She believes that applying scientific rigor to the issue of workforce diversity can lead to quantifiable, translatable, and repeatable methods for recruitment and retention of talent in the biomedical workforce (see “Building Blocks").
Before joining NIH, Dr. Valantine and her colleagues at Stanford surveyed gender composition and faculty satisfaction several years after initiating a multifaceted intervention to boost recruitment and development of women faculty.4 After making a visible commitment of resources to support faculty, with special attention to women, Stanford rose from below to above national benchmarks in the representation of women among faculty. Yet significant work remains to be done, Dr. Valantine says. Her work predicts that the estimated time to achieve 50% occupancy of full professorships by women nationally approaches 50 years—“far too long using current approaches.”
In a separate review article, Dr. Valantine and co-author Christy Sandborg, MD, described the Stanford University School of Medicine Academic Biomedical Career Customization (ABCC) model, which was adapted from Deloitte’s Mass Career Customization framework and allows for development of individual career plans that span a faculty member’s total career, not just a year or two at a time. Long-term planning can enable better alignment between the work culture and values of the workforce, which will improve the outlook for women faculty, Dr. Valantine says.
The issues of work-life balance may actually be generational, Dr. Valantine explains. Veteran hospitalist Janet Nagamine, MD, BSN, SFHM, of Santa Clara, Calif., agrees.
“Nowadays, men as well as women are looking for work-life balance,” she says.
In hospital medicine, Dr. Nagamine points out, the structural changes required to effect a work-life balance for hospital leaders are often difficult to achieve.
“As productivity surveys show, HM group leaders are putting in as many RVUs as the staff,” the former SHM board member says. “There is no dedicated time for administrative duties.”
Construct a Pipeline
Barriers to advancement often are particular to characteristics of diverse populations. For example, the AAMC’s report on the U.S. physician workforce documents that in African-American physicians 40 and younger, women outnumber their male counterparts. Therefore, in the association’s Diversity in Medical Education: Facts and Figures 2012 report, the executive summary points out the need to strengthen the medical education pipeline to increase the number of African-American males who enter the premed track.
Despite the fast-growing percentage of Latino and Hispanic populations in the United States, the shortage of Latino/Hispanic physicians increased from 1980 to 2010. Latinos/Hispanics are greatly underrepresented in the medical student, resident, and faculty populations, according to John Paul Sánchez, MD, MPH, assistant dean for diversity and inclusion in the Office for Diversity and Community Engagement at Rutgers, The State University of New Jersey. Likewise, Zeppenfeldt-Cestero believes that efforts must begin much earlier with Latino and other minority and underrepresented students.
“We have to make sure our students pursue the STEM disciplines and that they also later have the education and preparation to be competitive at the MBA or MPH levels,” he says.
Dr. Sánchez, an associate professor of emergency medicine and a diversity activist since his med school days, is the recipient of last year’s Association of Hispanic Healthcare Executives’ academic leader of the year award. Since September 2014, he has been involved with Building the Next Generation of Academic Physicians Inc., which collaborates with more than 40 medical schools across the country. The initiative offers conferences designed to develop diverse medical students’ and residents’ interest in pursuing academic medicine. Open to all medical students and residents, the conference curriculum is tailored for women, UREMs, and trainees who identify as lesbian, gay, bisexual, or transgender (LGBT), he says. Seven conferences were held in 2015, 10 are planned for this year, and seven for 2017.
Healthcare Leadership Gaps
Despite their omnipresence in healthcare, there is a dearth of women in chief executive and governance roles, as has been noted by both the American College of Healthcare Executives and the National Center for Healthcare Leadership. As with academic leadership positions, the leadership gap in the administrative sector does not seem to be due to a lack of women entering graduate programs in health administration. On the contrary, since the mid-1980s women have comprised 50% to 60% of graduate students.
“This is absolutely not a pipeline issue,” says Christy Harris Lemak, PhD, FACHE, professor and chair of the Department of Health Services Administration at the University of Alabama at Birmingham School of Health Professions and lead investigator of the National Center for Healthcare Leadership’s study of women in healthcare executive positions. Other factors come into play.
In the study, she and her co-authors queried female healthcare CEOs to ascertain the critical career inflection points that led to their success.6 Those who were strategic about their careers, sought out mentors, and voiced their intentions about pursuing leadership positions were more likely to be successful in those efforts. However, individual career efforts must be coupled with overall organizational commitment to fostering inclusion (see “Path to the Top: Strategic Advice for Women").
Hospitals and healthcare organizations must pursue the development of human capital (and the diversity of their leaders) in a systematic way. “We recommended [in the study] that organizations set expectations that leaders who mentor other potential leaders be rewarded in the same way as those who hit financial targets or readmission rate targets,” Dr. Lemak says.
Leadership matters, agrees Deborah J. Bowen, FACHE, CAE, president and CEO of Chicago-based American College of Healthcare Executives.
“I think we’re getting a little smarter. Organizational leaders and trustees have a better understanding that talent development is one of the most important jobs,” she says. “If you don’t have the right people in the right places making good decisions on behalf of the patients and the populations in the communities they’re serving, the rest falls apart.”
Nuances of Mentoring
Many conversations about encouraging diversity in healthcare leadership converge around the role of effective mentoring and sponsorship. A substantial body of research supports the impact of mentoring on retention, research productivity, career satisfaction, and career development for women. It’s important to ensure that the institutional culture is geared toward mentoring junior faculty, says Jessie Kimbrough Marshall, MD, MPH, assistant professor in the Division of General Medicine Hospitalist Program at the University of Michigan Health System in Ann Arbor (UMHS).
Several of our sources pointed out that leaders must learn how to be effective mentors. More attention is being given to enhancing leaders’ mentorship skills. One example is at the Institute for Diversity in Health Management, which conducts an intensive 12-month certificate in diversity management program for practitioners. León says the program fosters ongoing networking and support through the American Leadership Council on Diversity in Healthcare by building leadership competencies.
Dr. Valantine points out that mentoring is hardly a one-style-fits-all proposition but that it is a crucial element to creating and retaining diversity. She says it should be viewed “much more broadly than it is today, and it should focus beyond the trainer-trainee relationship.”
The process is a two-way street. Denege Ward, MD, hospitalist, assistant professor of internal medicine, and director of the medical short stay unit at UMHS, says minorities need to be ready to take a leap of faith.
“Underrepresented faculty and staff should take the risk of possible failure in challenging situations but learn from it and do better and not succumb to fear in face of challenges,” Dr. Ward says.
Although mentoring is one important component in building diversity in academic medicine, Dr. Sánchez asserts that role models, champions, and sponsors are equally important.
“In addition and separate from role models, there must be in place policies and procedures that promote a climate for diverse individuals to succeed,” he says. “What’s needed is an institutional vision and strategic plan that recognizes the importance of diversity. [It] has to become a core principle.”
Dr. Marshall echoes that refrain, noting the recruitment and retention of a diverse set of leaders will take time and intentionality. She is actively engaged in organizing annual meeting mentoring panels at the Society of General Internal Medicine.
“There are still quite a few barriers for women and minorities to advance into hospital leadership roles,” she says. “We still have a long way to go. However, I’m seeing more women and people of color get into these positions. The numbers are increasing, and that encourages me.” TH
Gretchen Henkel is a freelance writer in California.
References
- Institute for Diversity in Health Management. The state of health care diversity and disparities: a benchmarking study of U.S. hospitals. Available at: http://www.diversityconnection.org/diversityconnection/leadership-conferences/Benchmarking-Survey.jsp?fll=S11.
- Top issues confronting hospitals in 2015. American College of Healthcare Executives website. Available at: https://www.ache.org/pubs/research/ceoissues.cfm. Accessed March 5, 2016.
- Association of American Medical Colleges. Diversity in the physician workforce: facts & figures 2014. Available at: http://aamcdiversityfactsandfigures.org/.
- Valantine HA, Grewal D, Ku MC, et al. The gender gap in academic medicine: comparing results from a multifaceted intervention for Stanford faculty to peer and national cohorts. Acad Med. 2014;89(6):904-911.
- Valantine H, Sandborg CI. Changing the culture of academic medicine to eliminate the gender leadership gap: 50/50 by 2020. Acad Med. 2013;88(10):1411-1413.
- Sexton DW, Lemak CH, Wainio JA. Career inflection points of women who successfully achieved the hospital CEO position. J Healthc Manag. 2014;59(5):367-383.
Venetoclax + LDAC has potential in older AML patients

site of ASCO Annual Meeting
© ASCO/Todd Buchanan
CHICAGO—Investigators are pursuing the combination of the selective BCL-2 inhibitor venetoclax plus low-dose cytarabine (LDAC) in older, treatment-naïve patients with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy.
These patients have few treatment options, and to date, the combination is achieving significant reduction in bone marrow and peripheral blast counts.
The combination has also achieved some complete responses, including those with incomplete marrow recovery, for a complete response (CR) rate of 54%. By comparison, expected CR rates with LDAC are about 10%.
Tara L. Lin, MD, of the University of Kansas Medical Center in Kansas City, reported the results of the non-randomized, open-label phase 1/2 dose-escalation/expansion study as abstract 7007* at the 2016 ASCO Annual Meeting.
Dr Lin reported on the 18 patients enrolled in the phase 1 portion and on an additional 8 patients treated in the phase 2 portion.
Objectives of the study were safety, efficacy, and exploratory for biomarkers predictive of outcome.
Dr Lin noted that the entire study had almost reached full enrollment early in May, and an additional 50 patients had been treated on the phase 2 portion to date.
Eligibility criteria
Patients 65 years or older with untreated AML were eligible to enroll. They could not be eligible for standard induction therapy, and they had to have ECOG performance status of 0 – 2.
Patients were excluded if they had received cytarabine previously for a pre-existing myeloid disorder, acute promyelocytic leukemia, or active central nervous system involvement with AML.
Dosing schedule
In the phase 1 portion, patients received venetoclax orally once daily on days 2 – 28 of cycle 1 and days 1 – 28 of subsequent cycles, which were 28 days.
They received LDAC at 20 mg/m2 subcutaneously on days 1 – 10 of all cycles.
The venetoclax dose escalated from 50 mg to 600 mg in 6 days for dose level 1, and from 100 mg to 800 mg in 6 days for dose level 2.
Every patient was hospitalized prior to the initiation of therapy and aggressive tumor lysis prophylaxis begun at least 48 hours prior to venetoclax administration during cycle 1 and 24 hours prior to start of LDAC.
Once the patients had received prophylaxis and had a white blood cell count <25,000/μL, they were able to begin therapy starting with LDAC on day 1 and continuing through day 10.
No patient received venetoclax on day 1, Dr Lin emphasized.
Instead venetoclax began 24 hours after the LDAC, starting on day 2, and dose escalated each day until patients reached the maximum dose that was designed for their cohort level, which was then continued on days 6 – 28.
“A dose-limiting toxicity of thrombocytopenia was identified in the phase 1 portion,” Dr Lin said, “which led to the phase 2 dose recommendation of 600 mg daily of venetoclax.”
Demographics
Twenty-six patients were evaluable at the time of the presentation, 16 in the venetoclax 600-mg dose group and 10 in the 800-mg dose group.
The patients were a median of 75 years (range 66 – 87).
Sixty-five percent were males, 62% were ECOG status 1, and 19% (5 patients) had received prior hypomethylating treatment for pre-existing myelodysplastic syndromes.
Thirty-eight percent had bone marrow blast counts of 51% or greater.
Safety
Treatment-emergent adverse events (TEAEs), excluding cytopenias, occurring in 30% or more of patients included nausea (77%), fatigue (42%), febrile neutropenia (38%), diarrhea (35%), and vomiting (31%).
Grade 3/4 TEAEs, excluding cytopenias, occurring in 10% or more of patients included febrile neutropenia (38%), hypertension (12%), hyponatremia (12%), and hypophosphatemia (12%).
“In general,” Dr Lin said, “the drug was very well tolerated and patients were not discontinuing therapy because of side effects.”
Pharmacokinetics
At day 10, which coincided with the end day of the co-administration of the 2 drugs, and again, at day 18, when patients were receiving venetoclax alone, no differences were seen in either the Cmax per dose or AUC per dose between day 10 and day 18.
So the co-administration of LDAC did not markedly affect venetoclax exposures.
Efficacy
The overall response rate, consisting of CR plus CRi plus partial responses (PR), totaled 58% (15/26) of all patients.
Nine patients (35%) had resistant or progressive disease; 2 had incomplete data due to discontinuation.
Most patients (79%)—19 of 24—had a decrease in bone marrow blast count of over 50%, and 88% (15/17) had a decrease in peripheral blast count of over 50%.
Responses of patients who had received hypomethylating agents did not differ from those who had not.
The investigators also evaluated the impact of a prior myeloproliferative neoplasm (MPN) (n=4) on outcome and found that none of these patients had a response to therapy.
However, patients who did not have a previous MPN had a response rate of 68%.
Survival
At 12 months, overall survival (OS) in all patients was 57.6%. If MPN patients were not included in the analysis, the OS increased to 70.5%.
The 11 non-responders had a median OS of 4 months, while for the 15 responders (CR, Cri, PR) the median OS has not yet been reached.
“This data, in terms of taking into account the safety data, how well it appears to have been tolerated by the patients, and these overall response data,” Dr Lin said, “certainly suggest that venetoclax plus low-dose araC appears to have significant activity in this older patient population and . . . is worth further study for this patient group.”
Venetoclax has demonstrated single-agent activity in heavily pretreated patients with relapsed/refractory AML. It received accelerated approval from the US Food and Drug Administration (FDA) for the treatment of chronic lymphocytic leukemia (CLL), and has 3 breakthrough therapy designations from the FDA—one in combination with hypomethylating agents for treatment-naïve AML, one in relapsed or refractory CLL, and one in combination with rituximab for relapsed/refractory CLL.
The European Commission also granted venetoclax orphan designation for AML.
Venetoclax is being developed by AbbVie in collaboration with Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data. ![]()
*Data in the abstract differ from the presentation.

site of ASCO Annual Meeting
© ASCO/Todd Buchanan
CHICAGO—Investigators are pursuing the combination of the selective BCL-2 inhibitor venetoclax plus low-dose cytarabine (LDAC) in older, treatment-naïve patients with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy.
These patients have few treatment options, and to date, the combination is achieving significant reduction in bone marrow and peripheral blast counts.
The combination has also achieved some complete responses, including those with incomplete marrow recovery, for a complete response (CR) rate of 54%. By comparison, expected CR rates with LDAC are about 10%.
Tara L. Lin, MD, of the University of Kansas Medical Center in Kansas City, reported the results of the non-randomized, open-label phase 1/2 dose-escalation/expansion study as abstract 7007* at the 2016 ASCO Annual Meeting.
Dr Lin reported on the 18 patients enrolled in the phase 1 portion and on an additional 8 patients treated in the phase 2 portion.
Objectives of the study were safety, efficacy, and exploratory for biomarkers predictive of outcome.
Dr Lin noted that the entire study had almost reached full enrollment early in May, and an additional 50 patients had been treated on the phase 2 portion to date.
Eligibility criteria
Patients 65 years or older with untreated AML were eligible to enroll. They could not be eligible for standard induction therapy, and they had to have ECOG performance status of 0 – 2.
Patients were excluded if they had received cytarabine previously for a pre-existing myeloid disorder, acute promyelocytic leukemia, or active central nervous system involvement with AML.
Dosing schedule
In the phase 1 portion, patients received venetoclax orally once daily on days 2 – 28 of cycle 1 and days 1 – 28 of subsequent cycles, which were 28 days.
They received LDAC at 20 mg/m2 subcutaneously on days 1 – 10 of all cycles.
The venetoclax dose escalated from 50 mg to 600 mg in 6 days for dose level 1, and from 100 mg to 800 mg in 6 days for dose level 2.
Every patient was hospitalized prior to the initiation of therapy and aggressive tumor lysis prophylaxis begun at least 48 hours prior to venetoclax administration during cycle 1 and 24 hours prior to start of LDAC.
Once the patients had received prophylaxis and had a white blood cell count <25,000/μL, they were able to begin therapy starting with LDAC on day 1 and continuing through day 10.
No patient received venetoclax on day 1, Dr Lin emphasized.
Instead venetoclax began 24 hours after the LDAC, starting on day 2, and dose escalated each day until patients reached the maximum dose that was designed for their cohort level, which was then continued on days 6 – 28.
“A dose-limiting toxicity of thrombocytopenia was identified in the phase 1 portion,” Dr Lin said, “which led to the phase 2 dose recommendation of 600 mg daily of venetoclax.”
Demographics
Twenty-six patients were evaluable at the time of the presentation, 16 in the venetoclax 600-mg dose group and 10 in the 800-mg dose group.
The patients were a median of 75 years (range 66 – 87).
Sixty-five percent were males, 62% were ECOG status 1, and 19% (5 patients) had received prior hypomethylating treatment for pre-existing myelodysplastic syndromes.
Thirty-eight percent had bone marrow blast counts of 51% or greater.
Safety
Treatment-emergent adverse events (TEAEs), excluding cytopenias, occurring in 30% or more of patients included nausea (77%), fatigue (42%), febrile neutropenia (38%), diarrhea (35%), and vomiting (31%).
Grade 3/4 TEAEs, excluding cytopenias, occurring in 10% or more of patients included febrile neutropenia (38%), hypertension (12%), hyponatremia (12%), and hypophosphatemia (12%).
“In general,” Dr Lin said, “the drug was very well tolerated and patients were not discontinuing therapy because of side effects.”
Pharmacokinetics
At day 10, which coincided with the end day of the co-administration of the 2 drugs, and again, at day 18, when patients were receiving venetoclax alone, no differences were seen in either the Cmax per dose or AUC per dose between day 10 and day 18.
So the co-administration of LDAC did not markedly affect venetoclax exposures.
Efficacy
The overall response rate, consisting of CR plus CRi plus partial responses (PR), totaled 58% (15/26) of all patients.
Nine patients (35%) had resistant or progressive disease; 2 had incomplete data due to discontinuation.
Most patients (79%)—19 of 24—had a decrease in bone marrow blast count of over 50%, and 88% (15/17) had a decrease in peripheral blast count of over 50%.
Responses of patients who had received hypomethylating agents did not differ from those who had not.
The investigators also evaluated the impact of a prior myeloproliferative neoplasm (MPN) (n=4) on outcome and found that none of these patients had a response to therapy.
However, patients who did not have a previous MPN had a response rate of 68%.
Survival
At 12 months, overall survival (OS) in all patients was 57.6%. If MPN patients were not included in the analysis, the OS increased to 70.5%.
The 11 non-responders had a median OS of 4 months, while for the 15 responders (CR, Cri, PR) the median OS has not yet been reached.
“This data, in terms of taking into account the safety data, how well it appears to have been tolerated by the patients, and these overall response data,” Dr Lin said, “certainly suggest that venetoclax plus low-dose araC appears to have significant activity in this older patient population and . . . is worth further study for this patient group.”
Venetoclax has demonstrated single-agent activity in heavily pretreated patients with relapsed/refractory AML. It received accelerated approval from the US Food and Drug Administration (FDA) for the treatment of chronic lymphocytic leukemia (CLL), and has 3 breakthrough therapy designations from the FDA—one in combination with hypomethylating agents for treatment-naïve AML, one in relapsed or refractory CLL, and one in combination with rituximab for relapsed/refractory CLL.
The European Commission also granted venetoclax orphan designation for AML.
Venetoclax is being developed by AbbVie in collaboration with Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data. ![]()
*Data in the abstract differ from the presentation.

site of ASCO Annual Meeting
© ASCO/Todd Buchanan
CHICAGO—Investigators are pursuing the combination of the selective BCL-2 inhibitor venetoclax plus low-dose cytarabine (LDAC) in older, treatment-naïve patients with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy.
These patients have few treatment options, and to date, the combination is achieving significant reduction in bone marrow and peripheral blast counts.
The combination has also achieved some complete responses, including those with incomplete marrow recovery, for a complete response (CR) rate of 54%. By comparison, expected CR rates with LDAC are about 10%.
Tara L. Lin, MD, of the University of Kansas Medical Center in Kansas City, reported the results of the non-randomized, open-label phase 1/2 dose-escalation/expansion study as abstract 7007* at the 2016 ASCO Annual Meeting.
Dr Lin reported on the 18 patients enrolled in the phase 1 portion and on an additional 8 patients treated in the phase 2 portion.
Objectives of the study were safety, efficacy, and exploratory for biomarkers predictive of outcome.
Dr Lin noted that the entire study had almost reached full enrollment early in May, and an additional 50 patients had been treated on the phase 2 portion to date.
Eligibility criteria
Patients 65 years or older with untreated AML were eligible to enroll. They could not be eligible for standard induction therapy, and they had to have ECOG performance status of 0 – 2.
Patients were excluded if they had received cytarabine previously for a pre-existing myeloid disorder, acute promyelocytic leukemia, or active central nervous system involvement with AML.
Dosing schedule
In the phase 1 portion, patients received venetoclax orally once daily on days 2 – 28 of cycle 1 and days 1 – 28 of subsequent cycles, which were 28 days.
They received LDAC at 20 mg/m2 subcutaneously on days 1 – 10 of all cycles.
The venetoclax dose escalated from 50 mg to 600 mg in 6 days for dose level 1, and from 100 mg to 800 mg in 6 days for dose level 2.
Every patient was hospitalized prior to the initiation of therapy and aggressive tumor lysis prophylaxis begun at least 48 hours prior to venetoclax administration during cycle 1 and 24 hours prior to start of LDAC.
Once the patients had received prophylaxis and had a white blood cell count <25,000/μL, they were able to begin therapy starting with LDAC on day 1 and continuing through day 10.
No patient received venetoclax on day 1, Dr Lin emphasized.
Instead venetoclax began 24 hours after the LDAC, starting on day 2, and dose escalated each day until patients reached the maximum dose that was designed for their cohort level, which was then continued on days 6 – 28.
“A dose-limiting toxicity of thrombocytopenia was identified in the phase 1 portion,” Dr Lin said, “which led to the phase 2 dose recommendation of 600 mg daily of venetoclax.”
Demographics
Twenty-six patients were evaluable at the time of the presentation, 16 in the venetoclax 600-mg dose group and 10 in the 800-mg dose group.
The patients were a median of 75 years (range 66 – 87).
Sixty-five percent were males, 62% were ECOG status 1, and 19% (5 patients) had received prior hypomethylating treatment for pre-existing myelodysplastic syndromes.
Thirty-eight percent had bone marrow blast counts of 51% or greater.
Safety
Treatment-emergent adverse events (TEAEs), excluding cytopenias, occurring in 30% or more of patients included nausea (77%), fatigue (42%), febrile neutropenia (38%), diarrhea (35%), and vomiting (31%).
Grade 3/4 TEAEs, excluding cytopenias, occurring in 10% or more of patients included febrile neutropenia (38%), hypertension (12%), hyponatremia (12%), and hypophosphatemia (12%).
“In general,” Dr Lin said, “the drug was very well tolerated and patients were not discontinuing therapy because of side effects.”
Pharmacokinetics
At day 10, which coincided with the end day of the co-administration of the 2 drugs, and again, at day 18, when patients were receiving venetoclax alone, no differences were seen in either the Cmax per dose or AUC per dose between day 10 and day 18.
So the co-administration of LDAC did not markedly affect venetoclax exposures.
Efficacy
The overall response rate, consisting of CR plus CRi plus partial responses (PR), totaled 58% (15/26) of all patients.
Nine patients (35%) had resistant or progressive disease; 2 had incomplete data due to discontinuation.
Most patients (79%)—19 of 24—had a decrease in bone marrow blast count of over 50%, and 88% (15/17) had a decrease in peripheral blast count of over 50%.
Responses of patients who had received hypomethylating agents did not differ from those who had not.
The investigators also evaluated the impact of a prior myeloproliferative neoplasm (MPN) (n=4) on outcome and found that none of these patients had a response to therapy.
However, patients who did not have a previous MPN had a response rate of 68%.
Survival
At 12 months, overall survival (OS) in all patients was 57.6%. If MPN patients were not included in the analysis, the OS increased to 70.5%.
The 11 non-responders had a median OS of 4 months, while for the 15 responders (CR, Cri, PR) the median OS has not yet been reached.
“This data, in terms of taking into account the safety data, how well it appears to have been tolerated by the patients, and these overall response data,” Dr Lin said, “certainly suggest that venetoclax plus low-dose araC appears to have significant activity in this older patient population and . . . is worth further study for this patient group.”
Venetoclax has demonstrated single-agent activity in heavily pretreated patients with relapsed/refractory AML. It received accelerated approval from the US Food and Drug Administration (FDA) for the treatment of chronic lymphocytic leukemia (CLL), and has 3 breakthrough therapy designations from the FDA—one in combination with hypomethylating agents for treatment-naïve AML, one in relapsed or refractory CLL, and one in combination with rituximab for relapsed/refractory CLL.
The European Commission also granted venetoclax orphan designation for AML.
Venetoclax is being developed by AbbVie in collaboration with Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data. ![]()
*Data in the abstract differ from the presentation.