User login
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
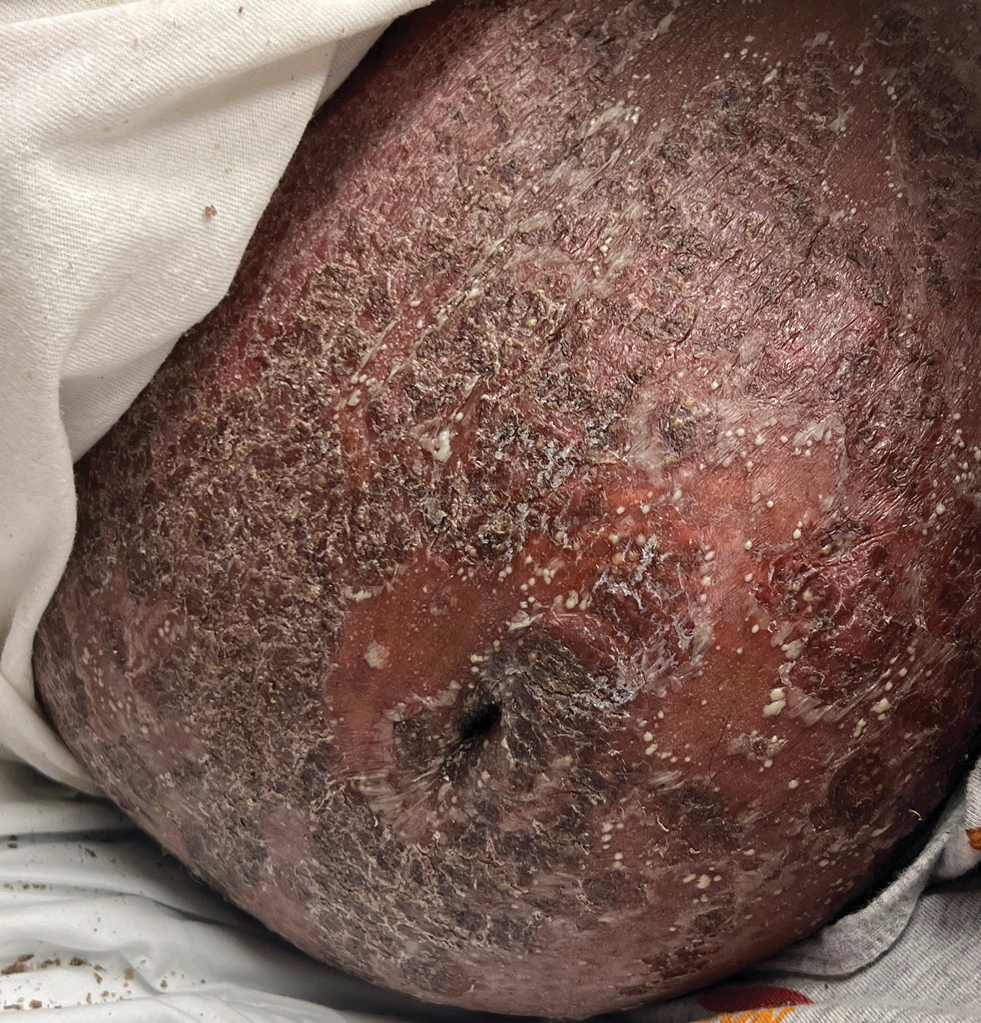
A 17-year-old girl was admitted to the hospital at 19 weeks' gestation for a widespread eruption of erythematous plaques with pustules covering more than 60% of the body and signs of sepsis. The rash initially appeared as a few small spots on the upper chest and under the breasts 5 weeks prior to hospital admission with subsequent spread to the abdomen and groin. At admission, the patient had a mild fever and tachycardia. She reported a history of eczema, herpes simplex virus, and intertrigo. Physical examination performed by dermatology revealed generalized erythematous plaques with pustule-studded margins and overlying scale involving the neck, torso, arms, and legs favoring the flexural areas. There was no involvement of the face, eyes, oral mucosa, palms, soles, or nails. Laboratory testing revealed hypoalbuminemia (2.4 g/dL [reference range, 3.5-5.5 g/dL]) and elevated inflammatory markers, including leukocytosis (15.83×103μL [reference range, 4.50- 11.00×103/μL]), absolute neutrophil count (12.87×103/μL [reference range, 1.50-8.00×103/μL]), and erythrocyte sedimentation rate (124 mm/h [reference range, 0-20 mm/h]). A culture from an abdominal pustule grew 1 colony of taphylococcus epidermidis, a suspected contaminant. A biopsy from a lesion on the right chest was performed.
Managing Residual Limb Hyperhidrosis in Wounded Warriors
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
Practice Points
- Hyperhidrosis of residual limbs often is induced by the occlusive effects of the water-resistant prosthetic liner that fits snugly over the limb.
- Commonly accepted treatments of hyperhidrosis often are less effective or poorly tolerated by these patients. The microwave thermal ablation device is a promising tool that may provide long-term relief of symptoms.

