User login
Real-world study: 8 weeks of two-drug combo highly effective against HCV
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Eight-week and 12-week regimens of ledipasvir/sofosbuvir achieved similarly high rates of sustained viral response (SVR) among noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients with viral loads under 6 million IU/mL.
Major finding: Twelve weeks after treatment, rates of SVR were 95.1% for the 8-week regimen and 95.8% for the 12-week regimen (P = .6).
Data source: A retrospective analysis of data from 17,487 patients with HCV infection.
Disclosures: The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
Fecal immunochemical testing, colonoscopy outperformed multitarget stool DNA testing
Fecal immunochemical testing and colonoscopy detected colorectal cancer (CRC) more effectively and cheaply than did multitarget stool DNA testing, based on a Markov model that assumed equal rates of participation in the three strategies, according to a report in the September issue of Gastroenterology.
Multitarget stool DNA testing (MT-sDNA) “may be a cost-effective alternative if it can achieve patient participation rates that are high enough compared with those of FIT [fecal immunochemical testing] that paying for its higher test cost can be justified,” wrote Uri Ladabaum, MD, and Ajitha Mannalithara, PhD, of Stanford (Calif.) University.
Studies have yielded mixed results about whether FIT or fecal DNA testing are preferable for CRC detection. In one recent large prospective study of patients at average CRC risk, a MT-sDNA test that included KRAS mutations, aberrant NDRG4 and BMP3 methylation, and hemoglobin outperformed FIT for detecting CRC and precancerous lesions but also yielded more false positives, the researchers noted.
Although many decision analyses have examined the efficacy and cost of CRC screening strategies, they have not delved into “the complex patterns of screening participation over time that are now being described (consistent screeners, late entrants, dropouts, intermittent screeners, consistent non-responders),” accounted for variable participation in organized and opportunistic screening programs, or controlled for differential reimbursement rates for public versus private insurance, they added (Gastroenterology 2016 Jun 7. doi: 10.1053/j.gastro.2016.06.003).
Dr. Ladabaum and Dr. Mannalithara therefore constructed a Markov model of patients at average risk for CRC to compare the efficacy and costs of MT-sDNA, colonoscopy, and FIT. The model included numerous variables, such as disease states ranging from a small adenomatous polyp to disseminated CRC, longitudinal changes in rates of participation for both opportunistic screening and organized screening programs, and different rates of commercial and Medicare reimbursement.
Assuming optimal adherence, an annual FIT test and colonoscopy every 10 years were more effective and less costly than MT-sDNA every 3 years, the researchers found. Compared with successful FIT screening programs – which have a 50% rate of consistent participation and a 27% rate of intermittent participation and cost about $153 per patient per testing cycle – an MT-sDNA program would need to have at least a 68% rate of consistent participation and a 32% rate of intermittent (every 3 years) participation, or would need to cost 60% less than it does now ($260 for commercial payment and $197 for Medicare payment in 2015) to be preferable when assuming a threshold of $100,000 for every extra quality-adjusted life year (QALY) gained. MT-sDNA every 3 years, however, would be more cost-effective than opportunistic FIT screening if participation rates were more than 1.7 times those that are typical of opportunistic FIT (15% consistent participation and 30% intermittent participation).
These results held up in various subgroup analyses, and FIT was preferred in 99% of iterations in a Monte Carlo simulation that assumed equal participation rates and the same $100,000 per QALY threshold. “For the MT-sDNA test to be cost-effective, the patient support program included in its cost would need to achieve substantially higher participation rates than those of FIT, whether in organized programs or under the opportunistic screening setting more common in the U.S. than in the rest of the world,” they concluded.
The study was funded by an unrestricted research grant from Exact Science Corp. Dr. Ladabaum reported consulting for ESC in 2014 and disclosed current consulting or advisory relationships with Given Imaging and Mauna Kea Technologies. Dr. Mannalithara had no disclosures.
Fecal immunochemical testing and colonoscopy detected colorectal cancer (CRC) more effectively and cheaply than did multitarget stool DNA testing, based on a Markov model that assumed equal rates of participation in the three strategies, according to a report in the September issue of Gastroenterology.
Multitarget stool DNA testing (MT-sDNA) “may be a cost-effective alternative if it can achieve patient participation rates that are high enough compared with those of FIT [fecal immunochemical testing] that paying for its higher test cost can be justified,” wrote Uri Ladabaum, MD, and Ajitha Mannalithara, PhD, of Stanford (Calif.) University.
Studies have yielded mixed results about whether FIT or fecal DNA testing are preferable for CRC detection. In one recent large prospective study of patients at average CRC risk, a MT-sDNA test that included KRAS mutations, aberrant NDRG4 and BMP3 methylation, and hemoglobin outperformed FIT for detecting CRC and precancerous lesions but also yielded more false positives, the researchers noted.
Although many decision analyses have examined the efficacy and cost of CRC screening strategies, they have not delved into “the complex patterns of screening participation over time that are now being described (consistent screeners, late entrants, dropouts, intermittent screeners, consistent non-responders),” accounted for variable participation in organized and opportunistic screening programs, or controlled for differential reimbursement rates for public versus private insurance, they added (Gastroenterology 2016 Jun 7. doi: 10.1053/j.gastro.2016.06.003).
Dr. Ladabaum and Dr. Mannalithara therefore constructed a Markov model of patients at average risk for CRC to compare the efficacy and costs of MT-sDNA, colonoscopy, and FIT. The model included numerous variables, such as disease states ranging from a small adenomatous polyp to disseminated CRC, longitudinal changes in rates of participation for both opportunistic screening and organized screening programs, and different rates of commercial and Medicare reimbursement.
Assuming optimal adherence, an annual FIT test and colonoscopy every 10 years were more effective and less costly than MT-sDNA every 3 years, the researchers found. Compared with successful FIT screening programs – which have a 50% rate of consistent participation and a 27% rate of intermittent participation and cost about $153 per patient per testing cycle – an MT-sDNA program would need to have at least a 68% rate of consistent participation and a 32% rate of intermittent (every 3 years) participation, or would need to cost 60% less than it does now ($260 for commercial payment and $197 for Medicare payment in 2015) to be preferable when assuming a threshold of $100,000 for every extra quality-adjusted life year (QALY) gained. MT-sDNA every 3 years, however, would be more cost-effective than opportunistic FIT screening if participation rates were more than 1.7 times those that are typical of opportunistic FIT (15% consistent participation and 30% intermittent participation).
These results held up in various subgroup analyses, and FIT was preferred in 99% of iterations in a Monte Carlo simulation that assumed equal participation rates and the same $100,000 per QALY threshold. “For the MT-sDNA test to be cost-effective, the patient support program included in its cost would need to achieve substantially higher participation rates than those of FIT, whether in organized programs or under the opportunistic screening setting more common in the U.S. than in the rest of the world,” they concluded.
The study was funded by an unrestricted research grant from Exact Science Corp. Dr. Ladabaum reported consulting for ESC in 2014 and disclosed current consulting or advisory relationships with Given Imaging and Mauna Kea Technologies. Dr. Mannalithara had no disclosures.
Fecal immunochemical testing and colonoscopy detected colorectal cancer (CRC) more effectively and cheaply than did multitarget stool DNA testing, based on a Markov model that assumed equal rates of participation in the three strategies, according to a report in the September issue of Gastroenterology.
Multitarget stool DNA testing (MT-sDNA) “may be a cost-effective alternative if it can achieve patient participation rates that are high enough compared with those of FIT [fecal immunochemical testing] that paying for its higher test cost can be justified,” wrote Uri Ladabaum, MD, and Ajitha Mannalithara, PhD, of Stanford (Calif.) University.
Studies have yielded mixed results about whether FIT or fecal DNA testing are preferable for CRC detection. In one recent large prospective study of patients at average CRC risk, a MT-sDNA test that included KRAS mutations, aberrant NDRG4 and BMP3 methylation, and hemoglobin outperformed FIT for detecting CRC and precancerous lesions but also yielded more false positives, the researchers noted.
Although many decision analyses have examined the efficacy and cost of CRC screening strategies, they have not delved into “the complex patterns of screening participation over time that are now being described (consistent screeners, late entrants, dropouts, intermittent screeners, consistent non-responders),” accounted for variable participation in organized and opportunistic screening programs, or controlled for differential reimbursement rates for public versus private insurance, they added (Gastroenterology 2016 Jun 7. doi: 10.1053/j.gastro.2016.06.003).
Dr. Ladabaum and Dr. Mannalithara therefore constructed a Markov model of patients at average risk for CRC to compare the efficacy and costs of MT-sDNA, colonoscopy, and FIT. The model included numerous variables, such as disease states ranging from a small adenomatous polyp to disseminated CRC, longitudinal changes in rates of participation for both opportunistic screening and organized screening programs, and different rates of commercial and Medicare reimbursement.
Assuming optimal adherence, an annual FIT test and colonoscopy every 10 years were more effective and less costly than MT-sDNA every 3 years, the researchers found. Compared with successful FIT screening programs – which have a 50% rate of consistent participation and a 27% rate of intermittent participation and cost about $153 per patient per testing cycle – an MT-sDNA program would need to have at least a 68% rate of consistent participation and a 32% rate of intermittent (every 3 years) participation, or would need to cost 60% less than it does now ($260 for commercial payment and $197 for Medicare payment in 2015) to be preferable when assuming a threshold of $100,000 for every extra quality-adjusted life year (QALY) gained. MT-sDNA every 3 years, however, would be more cost-effective than opportunistic FIT screening if participation rates were more than 1.7 times those that are typical of opportunistic FIT (15% consistent participation and 30% intermittent participation).
These results held up in various subgroup analyses, and FIT was preferred in 99% of iterations in a Monte Carlo simulation that assumed equal participation rates and the same $100,000 per QALY threshold. “For the MT-sDNA test to be cost-effective, the patient support program included in its cost would need to achieve substantially higher participation rates than those of FIT, whether in organized programs or under the opportunistic screening setting more common in the U.S. than in the rest of the world,” they concluded.
The study was funded by an unrestricted research grant from Exact Science Corp. Dr. Ladabaum reported consulting for ESC in 2014 and disclosed current consulting or advisory relationships with Given Imaging and Mauna Kea Technologies. Dr. Mannalithara had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Fecal immunochemical testing and colonoscopy were more effective and less costly than multitarget stool DNA testing for colorectal cancer screening.
Major finding: Annual fecal immunochemical testing and colonoscopy every 10 years were more efficacious and cost-effective than multitarget stool DNA testing, given optimal adherence rates.
Data source: A decision analytic health economic evaluation of the efficacy and cost-effectiveness of the three modalities.
Disclosures: The study was funded by an unrestricted research grant from Exact Science Corp. Dr. Ladabaum reported consulting for ESC in 2014 and disclosed current consulting or advisory relationships with Given Imaging and Mauna Kea Technologies. Dr. Mannalithara had no disclosures.
Study reinforced value of preconception IBD care
Targeted and regular outpatient care before conception helped prevent relapse of inflammatory bowel disease (IBD) during pregnancy, according to a single-center prospective observational study reported in the September issue of Clinical Gastroenterology and Hepatology.
Women who received such care had about 50% lower odds of relapse while pregnant compared with women seen only after conception, said Alison de Lima, MD, PhD, of Erasmus MC–University Medical Hospital Rotterdam (the Netherlands) and her associates. “Preconception care seems effective in achieving desirable behavioral modifications in IBD women in terms of folic acid intake, smoking cessation, and correct IBD medication adherence, eventually reducing disease relapse during pregnancy. Most importantly, preconception care positively influences birth outcomes,” the investigators concluded.
Several recent studies have reported “incorrect beliefs, unfounded fears, and insufficient knowledge” among women with IBD when it comes to pregnancy, the researchers noted. These beliefs can undermine medication adherence, potentially increasing the risk of complications and poor birth outcomes, they added. Studies have confirmed the value of preconception care for chronic diseases such as diabetes, but none had done so for IBD (Clin Gastroenterol Hepatol. 2016 Mar 18. doi: 10.1016/j.cgh.2016.03.018). Therefore, Dr. de Lima and her associates prospectively followed 317 women seen at the IBD preconception outpatient clinic at a tertiary referral hospital during 2008-2014. A total of 155 patients first visited the clinic before becoming pregnant, while the other 162 patients did so only after conception. New patient visits lasted about 30-45 minutes and included fecal calprotectin testing to assess disease activity, education about the need to avoid conceiving during a disease flare, and general advice about taking folic acid, quitting smoking, and avoiding alcohol during pregnancy. Follow-up visits, which occurred every 3 months before pregnancy and every 2 months thereafter, included clinical assessments of disease activity, maternal serum testing to assess compliance with antitumor necrosis factor and thiopurine therapy, and assessments of folic acid supplementation, smoking, and alcohol use.
Patients who received such care before conceiving tended to be younger (29.7 vs. 31.4 years; P = .001), were more often nulliparous (76% vs. 51%; P = .0001), and had a shorter history of IBD (5.1 vs. 8 years; P = .0001), compared with the postconception care group, the researchers said. However, after researchers controlled for parity, disease duration, and the number of relapses in the year before pregnancy, the preconception care group had a nearly sixfold greater odds of adhering to IBD medications during pregnancy (adjusted odds ratio, 5.7; 95% confidence interval, 1.9-17.3), about a fivefold greater odds of sufficient folic acid intake (aOR, 5.3; 95% CI, 2.7-10.3), and a more than fourfold odds of smoking cessation during pregnancy (aOR, 4.63; 95% CI, 1.2-17.6). Notably, preconception care was tied to a 49% lower odds of disease relapse during pregnancy (aOR, 0.51; 95% CI; 0.28-0.95) and to a nearly 50% lower rate of low birth weight (birth weight less than 2,500 g).
“To our surprise, this study did not detect an effect of preconception care on periconceptional disease activity,” the researchers said – even though they strove to educate patients on this concept. “We can only speculate about the explanation for this finding, but we believe this could be a result of a discrepancy between physician-declared disease remission and the patient’s own feeling of well-being combined with a strong reproductive desire.”
The investigators reported no funding sources, and Dr. de Lima had no disclosures. Two coinvestigators reported ties to Merck Sharp & Dohme, Abbott, Shire, and Ferring.
Targeted and regular outpatient care before conception helped prevent relapse of inflammatory bowel disease (IBD) during pregnancy, according to a single-center prospective observational study reported in the September issue of Clinical Gastroenterology and Hepatology.
Women who received such care had about 50% lower odds of relapse while pregnant compared with women seen only after conception, said Alison de Lima, MD, PhD, of Erasmus MC–University Medical Hospital Rotterdam (the Netherlands) and her associates. “Preconception care seems effective in achieving desirable behavioral modifications in IBD women in terms of folic acid intake, smoking cessation, and correct IBD medication adherence, eventually reducing disease relapse during pregnancy. Most importantly, preconception care positively influences birth outcomes,” the investigators concluded.
Several recent studies have reported “incorrect beliefs, unfounded fears, and insufficient knowledge” among women with IBD when it comes to pregnancy, the researchers noted. These beliefs can undermine medication adherence, potentially increasing the risk of complications and poor birth outcomes, they added. Studies have confirmed the value of preconception care for chronic diseases such as diabetes, but none had done so for IBD (Clin Gastroenterol Hepatol. 2016 Mar 18. doi: 10.1016/j.cgh.2016.03.018). Therefore, Dr. de Lima and her associates prospectively followed 317 women seen at the IBD preconception outpatient clinic at a tertiary referral hospital during 2008-2014. A total of 155 patients first visited the clinic before becoming pregnant, while the other 162 patients did so only after conception. New patient visits lasted about 30-45 minutes and included fecal calprotectin testing to assess disease activity, education about the need to avoid conceiving during a disease flare, and general advice about taking folic acid, quitting smoking, and avoiding alcohol during pregnancy. Follow-up visits, which occurred every 3 months before pregnancy and every 2 months thereafter, included clinical assessments of disease activity, maternal serum testing to assess compliance with antitumor necrosis factor and thiopurine therapy, and assessments of folic acid supplementation, smoking, and alcohol use.
Patients who received such care before conceiving tended to be younger (29.7 vs. 31.4 years; P = .001), were more often nulliparous (76% vs. 51%; P = .0001), and had a shorter history of IBD (5.1 vs. 8 years; P = .0001), compared with the postconception care group, the researchers said. However, after researchers controlled for parity, disease duration, and the number of relapses in the year before pregnancy, the preconception care group had a nearly sixfold greater odds of adhering to IBD medications during pregnancy (adjusted odds ratio, 5.7; 95% confidence interval, 1.9-17.3), about a fivefold greater odds of sufficient folic acid intake (aOR, 5.3; 95% CI, 2.7-10.3), and a more than fourfold odds of smoking cessation during pregnancy (aOR, 4.63; 95% CI, 1.2-17.6). Notably, preconception care was tied to a 49% lower odds of disease relapse during pregnancy (aOR, 0.51; 95% CI; 0.28-0.95) and to a nearly 50% lower rate of low birth weight (birth weight less than 2,500 g).
“To our surprise, this study did not detect an effect of preconception care on periconceptional disease activity,” the researchers said – even though they strove to educate patients on this concept. “We can only speculate about the explanation for this finding, but we believe this could be a result of a discrepancy between physician-declared disease remission and the patient’s own feeling of well-being combined with a strong reproductive desire.”
The investigators reported no funding sources, and Dr. de Lima had no disclosures. Two coinvestigators reported ties to Merck Sharp & Dohme, Abbott, Shire, and Ferring.
Targeted and regular outpatient care before conception helped prevent relapse of inflammatory bowel disease (IBD) during pregnancy, according to a single-center prospective observational study reported in the September issue of Clinical Gastroenterology and Hepatology.
Women who received such care had about 50% lower odds of relapse while pregnant compared with women seen only after conception, said Alison de Lima, MD, PhD, of Erasmus MC–University Medical Hospital Rotterdam (the Netherlands) and her associates. “Preconception care seems effective in achieving desirable behavioral modifications in IBD women in terms of folic acid intake, smoking cessation, and correct IBD medication adherence, eventually reducing disease relapse during pregnancy. Most importantly, preconception care positively influences birth outcomes,” the investigators concluded.
Several recent studies have reported “incorrect beliefs, unfounded fears, and insufficient knowledge” among women with IBD when it comes to pregnancy, the researchers noted. These beliefs can undermine medication adherence, potentially increasing the risk of complications and poor birth outcomes, they added. Studies have confirmed the value of preconception care for chronic diseases such as diabetes, but none had done so for IBD (Clin Gastroenterol Hepatol. 2016 Mar 18. doi: 10.1016/j.cgh.2016.03.018). Therefore, Dr. de Lima and her associates prospectively followed 317 women seen at the IBD preconception outpatient clinic at a tertiary referral hospital during 2008-2014. A total of 155 patients first visited the clinic before becoming pregnant, while the other 162 patients did so only after conception. New patient visits lasted about 30-45 minutes and included fecal calprotectin testing to assess disease activity, education about the need to avoid conceiving during a disease flare, and general advice about taking folic acid, quitting smoking, and avoiding alcohol during pregnancy. Follow-up visits, which occurred every 3 months before pregnancy and every 2 months thereafter, included clinical assessments of disease activity, maternal serum testing to assess compliance with antitumor necrosis factor and thiopurine therapy, and assessments of folic acid supplementation, smoking, and alcohol use.
Patients who received such care before conceiving tended to be younger (29.7 vs. 31.4 years; P = .001), were more often nulliparous (76% vs. 51%; P = .0001), and had a shorter history of IBD (5.1 vs. 8 years; P = .0001), compared with the postconception care group, the researchers said. However, after researchers controlled for parity, disease duration, and the number of relapses in the year before pregnancy, the preconception care group had a nearly sixfold greater odds of adhering to IBD medications during pregnancy (adjusted odds ratio, 5.7; 95% confidence interval, 1.9-17.3), about a fivefold greater odds of sufficient folic acid intake (aOR, 5.3; 95% CI, 2.7-10.3), and a more than fourfold odds of smoking cessation during pregnancy (aOR, 4.63; 95% CI, 1.2-17.6). Notably, preconception care was tied to a 49% lower odds of disease relapse during pregnancy (aOR, 0.51; 95% CI; 0.28-0.95) and to a nearly 50% lower rate of low birth weight (birth weight less than 2,500 g).
“To our surprise, this study did not detect an effect of preconception care on periconceptional disease activity,” the researchers said – even though they strove to educate patients on this concept. “We can only speculate about the explanation for this finding, but we believe this could be a result of a discrepancy between physician-declared disease remission and the patient’s own feeling of well-being combined with a strong reproductive desire.”
The investigators reported no funding sources, and Dr. de Lima had no disclosures. Two coinvestigators reported ties to Merck Sharp & Dohme, Abbott, Shire, and Ferring.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Preconception care with intensive follow-up seems to help prevent relapse of IBD during pregnancy.
Major finding: Women who were seen and followed before pregnancy had about a 50% lower odds of relapse while pregnant than did women who did not seek care until after becoming pregnant.
Data source: A single-center prospective observational study of 317 women with IBD seen at a university outpatient clinic.
Disclosures: The investigators reported no funding sources, and Dr. de Lima had no disclosures. Two coinvestigators reported ties to Merck Sharp & Dohme, Abbott, Shire, and Ferring.
Use these tips to choose the best neuroimaging modality for your pediatric patient
Social media: Potential pitfalls for psychiatrists
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
‘Enough!’ We need to take back our profession; More unresolved questions about psychiatry
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
The psychiatry workforce pool is shrinking. What are we doing about it?
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.
Neuroimaging in children and adolescents: When do you scan? With which modalities?
The first 15 years of the new millennium have seen a great increase in research on neuroimaging in children and adolescents who have a psychiatric disorder. In addition, imaging modalities continue to evolve, and are becoming increasingly accessible and informative. The literature is now replete with reports of neurostructural differences between patients and healthy subjects in a variety of common pediatric psychiatric conditions, including anxiety disorders, mood disorders, autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder (ADHD).
Historically, the clinical utility of neuroimaging was restricted to the identification of structural pathology. Today, accumulating data reveal novel roles for neuroimaging; these revelations are supported by studies demonstrating that treatment response for psychotherapeutic and psychopharmacotherapeutic interventions can be predicted by neurochemical and neurofunctional characteristics assessed by advanced imaging technologies, such as magnetic resonance spectroscopy (MRS) and functional MRI.
However, such advanced techniques are (at least at present) not ready for routine clinical use for this purpose. Instead, neuroimaging in the child and adolescent psychiatric clinic remains largely focused on ruling out neurostructural, neurologic, “nonpsychiatric” causes of our patients’ symptoms.
Understanding the role and limitations of major imaging modalities is key to guiding efficient and appropriate neuroimaging selection for pediatric patients. In this article, we describe and review:
- neuroimaging approaches for children and adolescents with psychiatric disorders
- the role of neuroimaging in (1) the differential diagnosis and workup of common psychiatric disorders and (2) urgent clinical situations
- how to determine what type of imaging to obtain.
Computed tomography
CT, which utilizes ionizing radiation, often is reserved, in the pediatric setting, for (1) emergency evaluation and (2) excluding potentially catastrophic neurologic injury resulting from:
- ischemic or hemorrhagic stroke
- herniation
- intracerebral hemorrhage
- subdural and epidural hematoma
- large intracranial mass with mass effect
- increased intracranial pressure
- acute skull fracture.
Although a CT scan is, typically, quick and has excellent sensitivity for acute bleeding and bony pathology, it exposes the patient to radiation and provides poor resolution compared with MRI.
In pediatrics, there has been practice-changing recognition of the importance of limiting lifetime radiation exposure incurred from medical procedures and imaging. As a result, most providers now agree that use of MRI in lieu of CT is appropriate in many, if not most, non-emergent situations. In an emergent situation, however, CT imaging is appropriate and should not be delayed. Moreover, in an emergent situation, you should not hesitate to use head CT in children, although timely discussion with the radiologist is recommended to review your differential diagnosis to better determine the preferred imaging modality.
Magnetic resonance imaging
Over the past several decades, MRI has been increasingly available in most pediatric health care facilities. The modality offers specific advantages for pediatric patients, including:
- better spatial resolution
- the ability to concurrently assess multiple pathologic processes
- lack of exposure to ionizing radiation.1
A number of MRI sequences, described below, can be used to assess vascular, inflammatory, structural, and metabolic processes.
A look inside. Comprehensive review of the physics that underlies MRI is beyond the scope of this article; several important principles are relevant to clinicians, however. Image contrast is dependent on intrinsic properties of tissue with regard to proton density, longitudinal relaxation time (T1), and transverse relaxation time (T2). Pulse sequences, which describe the strength and timing of the radiofrequency pulse and gradient pulses, define imaging acquisition parameters (eg, repetition time between the radio frequency pulse and echo time).
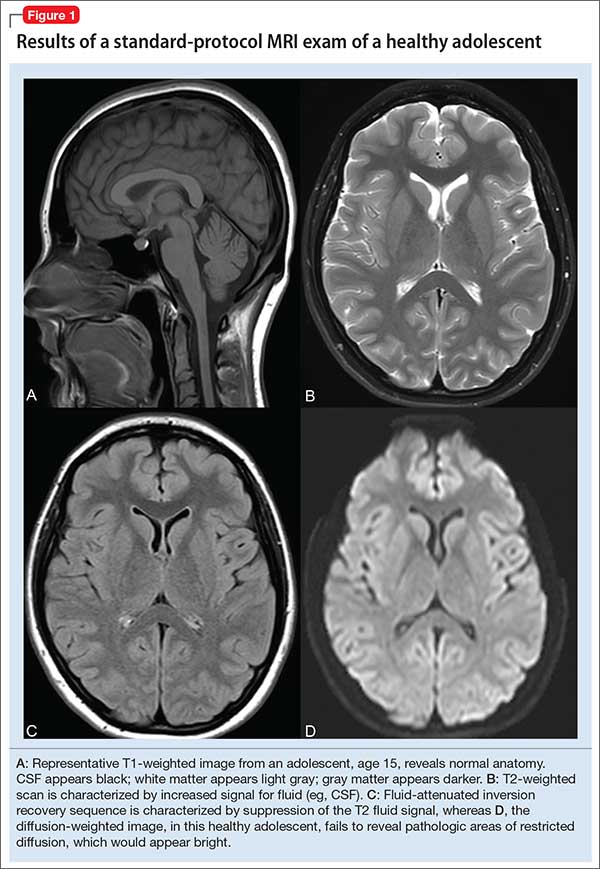
In turn, the intensity of the signal that is “seen” with various pulse sequences is differentially affected by intrinsic properties of tissue. At most pediatric institutions, the standard MRI-examination protocol includes: a T1-weighted image (Figure 1A); a T2-weighted scan (Figure 1B); fluid attenuated inversion recovery (FLAIR) (Figure 1C); and diffusion-weighted imaging (DWI) (Figure 1D).
Specific MRI sequences
T1 images. T1 sequences, or so-called anatomy sequences, are ideally suited for detailed neuroanatomic evaluations. They are generated in such a way that structures containing fluid are dark (hypo-intense), whereas other structures, with higher fat or protein content, are brighter (iso-intense, even hyper-intense). For this reason, CSF in the intracranial ventricles is dark, and white matter is brighter than the cortex because of lipid in myelin sheaths.
In addition, to view structural abnormalities that are characterized by altered vascular supply or flow, such as tumors and infections (abscesses), contrast imaging can be particularly helpful; such images generally are obtained as T1 sequences.
T2 images. By contrast to the T1-weighted sequence, the T2-weighted sequences emphasize fluid signal; structures such as the ventricles, which contain CSF, therefore will be bright (hyper-intense). Pathology that produces edema or fluid, such as edema surrounding demyelinating lesions or infections, also will show bright hyper-intense signal. In T2-weighted images of the brain, white matter shows lower signal intensity than the cortex because of the relatively lower water content in white matter tracts and myelin sheaths.
Fluid attenuation inversion recovery. FLAIR images are generated so that the baseline bright T2 signal seen in normal structures, such as the CSF, containing ventricles is cancelled out, or attenuated. In effect, this subtraction of typical background hyper-intense fluid signal leaves only abnormal T2 bright hyper-intense signal, such as vasogenic edema surrounding tumors, cytotoxic edema within an infarction, or extra-axial fluid collections such as a subarachnoid or subdural hemorrhage.
Diffusion-weighted imaging. DWI utilizes the random motion (ie, diffusion) of water molecules to generate contrast. In this regard, the diffusion of any molecule is influenced by its interaction with other molecules (eg, white-matter fibers and membranes, and macromolecules). Diffusion patterns therefore reflect details about tissue boundaries; as such, DWI is sensitive to a number of neurologic processes, such as ischemia, demyelinating disease, and some tumors, which restrict the free motion of water. DWI detects this so-called restricted diffusion and displays an area of bright signal.
Susceptibility-weighted imaging (SWI). In the pediatric population, SWI (Figure 2) utilizes a long-echo, 3-dimensional, velocity-compensated gradient recalled echo for image acquisition2 and, ultimately, leverages susceptibility differences across tissues by employing the phase image to identify these differences. SWI, which uses both magnitude and phase images and is remarkably sensitive to venous blood (and blood products), iron, and calcifications, therefore might be of increasing utility in pediatric patients with traumatic brain injury (TBI) (Figure 2B). As such, SWI has become a critical component of many pediatric MRI studies.3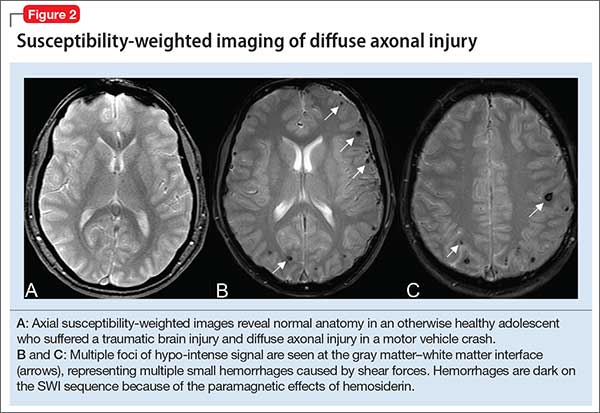
Magnetic resonance angiography (MRA) (Figure 3A) is helpful for assessing intracranial arteries and may be employed in the evaluation of:
- vessel pathology and injury underlying stroke, such as vessel occlusion or injury
- patterns of vessel involvement suggestive of vasculitis
- developmental or acquired structural vascular abnormalities, such as aneurysm or vascular malformations
- determination of tumor blood supply.
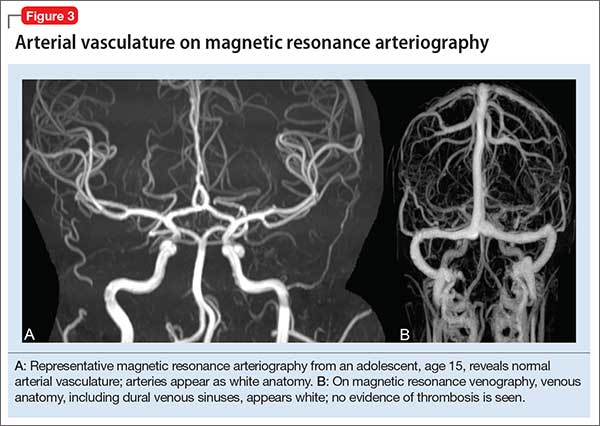
MRA can be performed without or with contrast, although MRA with contrast might provide a higher quality study and therefore be of greater utility. Of note: The spatial resolution of MRA is not as good as CT angiography; abnormalities, such as a small aneurysm, might not be apparent.
Magnetic resonance venography (MRV) (Figure 3B) is most commonly performed when the possibility of thrombosis of the dural venous sinuses is being considered; it also is employed to evaluate vascular malformations, tumor drainage patterns, and other pathologic states. As with MRA, MRV can be performed without or with contrast, although post-contrast MRV is generally of higher quality and might be preferred when assessing for sinus thrombosis.
Magnetic resonance spectroscopy (MRS) resides at the border between research and clinical practice. In children and adolescents, MRS provides data on neuronal and axonal viability as well as energetics and cell membranes.4 Pediatric neurologists often use MRS to evaluate for congenital neurometabolic disease; this modality also can help distinguish between an active intracranial tumor from an abscess or gliosis.5
Neuroimaging in pediatric neuropsychiatric conditions: Evidence, guidance
Delirium (altered mental status). The acute neuropsychiatric syndrome characterized by impaired attention and sensorium might have a broad underlying etiology, but it is always associated with alteration of CNS neurophysiology. Children with neurostructural abnormalities might have increased vulnerability to CNS insult and therefore be at increased risk of delirium.6 Additionally, delirium can present subtly in children, with the precise signs dependent on the individual patient’s developmental stage.
Neuroimaging may be helpful when an infectious, inflammatory, toxic, or a metabolic basis for delirium is suspected, or when a patient has new focal neurologic findings. In this regard, focal neurologic findings suggest an underlying localizable lesion and warrant dedicated neuroimaging to localize the lesion.
In general, the differential diagnosis should guide consideration of neuroimaging. When considering the possibility of an unwitnessed seizure in a child who presents with altered mental status, neuroimaging certainly is an important component of the workup.
As another example, when underlying trauma, intracranial hemorrhage, or mass is a possibility in acute delirium, urgent head CT is appropriate. In non-emergent cases, MRI is the modality of choice. In immunocompromised patients presenting with delirium, maintain a low threshold for neuroimaging with contrast to rule out opportunistic intracranial infection.
Last, in children who have hydrocephalus with a shunt, delirium could be a harbinger of underlying shunt malfunction, warranting a “shunt series.”
ADHD. The diagnosis of ADHD remains a clinical one; for the typical pediatric patient with ADHD but who does not have focal neurologic deficits, neuroimaging is unnecessary. Some structural MRI studies of youth with ADHD suggest diminished volume of the globus pallidus, putamen, and caudate7; other studies reveal changes in gyrification and cortical thickness8 in regions subserving attentional processes. However, intra-individual and developmental-related variability preclude routine use of neuroimaging in the standard diagnostic work-up of ADHD.
Nevertheless, neuroimaging should be strongly considered in a child with progressive worsening of inattention, especially if combined with other psychiatric or neurologic findings. In such a case, MRI should be obtained to evaluate for a progressive neurodegenerative leukoencephalopathy (eg, adrenoleukodystrophy).9
Depressive and anxiety disorders. In pediatric patients who exhibit depressive or anxiety symptoms, abnormalities have been observed in cortical thickness10 and gray matter volume,11,12 and functional signatures13 have been identified in the circuitry of the prefrontal amygdala. No data suggest that, in an individual patient, neuroimaging can be of diagnostic utility—particularly in the absence of focal neurologic findings.
That being said, headache and other somatic symptoms are common in pediatric patients with a mood or anxiety disorder. Evidence for neuroimaging in the context of pediatric headache suggests that MRI should be considered when headache is associated with neurologic signs or symptoms, such as aura, or accompanied by focal neurologic deficit.14
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS). Neuroimaging studies of patients with confirmed PANDAS or PANS are rare, but group analyses suggest a decreased average volume of the caudate, putamen, and globus pallidus in patients with PANDAS compared with healthy comparison subjects, although total cerebral volume does not appear to differ.15 Moreover, thalamic findings in patients with PANDAS have been noted to be similar to what is seen in patients with Sydenham’s chorea.
The most recent consensus statement regarding the treatment and assessment of PANDAS and PANS recommends ordering brain MRI when other conditions are suspected (eg, CNS, small vessel vasculitis, limbic encephalitis) or when the patient has severe headache, gait disturbance, cognitive deterioration, or psychosis.16 Furthermore, the consensus statement notes the potential utility of T2-weighted imaging with contrast to evaluate inflammatory changes in the basal ganglia.16
Autism spectrum disorder. Significant progress has been made during the past decade on the neuroanatomic characterization of ASD. Accumulating data indicate that, in pediatric patients with ASD, (1) development of white matter and gray matter is disrupted early in the course of the disorder and (2) cortical thickness is increased in regions subserving social cognition.17,18
Several studies have examined the presence of patient-level findings in samples of pediatric patients with ASD. Approximately 8% of pediatric patients with ASD were found to have some abnormality on routine brain MRI, the most common being white-matter signal abnormalities, dilated Virchow-Robin space, and temporal lobe abnormalities.19
Although abnormalities might be present in a large percentage of individual scans, routine screening MRI is unlikely to be of clinical utility in youth with ASD. In fact, no recommendation for routine MRI screening in patients with ASD has been made by the American Academy of Child & Adolescent Psychiatry, the Child Neurology Society of the American Academy of Neurology, or the American Academy of Pediatrics.
However, in patients with an underlying neurostructural disease that is phenotypically associated with ASD-like symptoms, imaging might be of use. In tuberous sclerosis, for example, MRI is especially important to classify intracranial lesions; determine burden and location; or identify treatment options (Figure 4). For patients with tuberous sclerosis—of whom more than one-third meet diagnostic criteria for ASD20—MRI study should include FLAIR, spin-echo, and gradient-echo sequences.
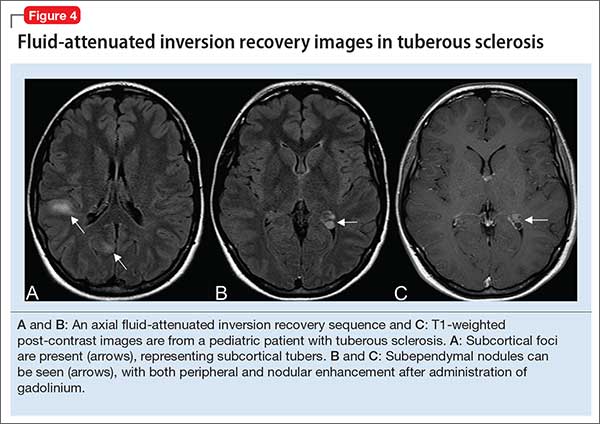
Movement disorders. Hyperkinetic movement disorders, including tic disorders and drug-induced movement disorders (eg, tremor) are common in pediatric patients. In pediatric patients with a tic disorder or Tourette’s disorder (TD), neuroimaging typically is unnecessary, despite the suggestion that the caudate nucleus volume is reduced in groups of patients with TD.
Many CNS-acting medications can exacerbate physiologic tremor; in pediatric patients with symptoms of a movement disorder, home medications should be carefully reviewed for potentially offending agents. When the patient is clinically and biochemically euthyroid and medication-induced movement disorder has been ruled out, or when the patient meets clinical diagnostic criteria for TD or a tic disorder, routine neuroimaging generally is unnecessary.
When tremor accompanies other cerebellar signs, such as ataxia or dysmetria, strongly consider MRI of the brain to evaluate pathology in the posterior fossa. In addition, neuroimaging should be considered for children with a new-onset abnormality on neurologic exam, including rapid onset of abnormal movements (other than common tics), continuous progressive worsening of symptoms, or any loss of developmental milestones.
Last, although tics and stereotypies often are transient and wane with age, other abnormal movements, such as dystonia, chorea, and parkinsonism (aside from those potentially associated with antipsychotic use), are never expected during typical development and warrant MRI.
Traumatic brain injury. Prompt evaluation and intervention for TBI can significantly affect overall outcome. Moreover, there has been increased enthusiasm around the pre-hospital assessment of TBI severity using (1) any of several proprietary testing systems (eg, Immediate Post-Concussion Assessment and Cognitive Testing [ImPACT]) plus (2) standard clinical staging, which is based on duration of loss of consciousness, persistence of memory loss, and the Glasgow Coma Scale score.
The goal for any TBI patient during the acute post-injury phase is to minimize continued neuronal injury from secondary effects of TBI, such as cerebral edema and herniation, and to optimize protection of surviving brain tissue; neuroimaging is a critical component of assessment during both acute and chronic recovery periods of TBI.21
The optimal imaging modality varies with the amount of time that has passed since initial injury.22 Urgent neuroimaging (the first 24 hours after brain injury) is typically obtained using head CT to assist decision-making in acute neurosurgical management. In this setting, head CT is fast and efficient; minimizes the amount of time that the patient is in the scanner; and provides valuable information on the acuity and extent of injury, degree of cerebral edema, and evidence or risk of pending herniation.
On the other hand, MRI is superior to CT during 48 to 72 hours after injury, given its higher resolution; superior imaging of the brainstem and deep gray nuclei; and ability to detect axonal injury, small contusions, and subtle neuronal damage. Specifically, SWI sequences can be particularly helpful in TBI for detecting diffuse axonal injury and micro-hemorrhages; several recent studies also suggest that SWI may be of particular value in pediatric patients with TBI.23
Additionally, given the increased sensitivity of MRI to detect subtle injuries, this modality can assist in identifying chronic sequelae of brain injury—thus contributing to determining of chronic therapy options and assisting with long-term prognosis. Gross structural changes resulting from TBI often are evident even in the acute post-injury phase; synaptic remodeling continues, however, for an indefinite period after injury, and this remodeling capacity is even more pronounced in the highly plastic brain of a young child.
Microstructural changes might not be detectable using traditional, readily available imaging sequences (CT, MRI). When those traditional modalities are used in concert with functional imaging techniques (eg, PET to evaluate cerebral metabolism and SPECT imaging which can detect abnormalities in cerebral blood flow), the combination of older and newer might provide a more complete picture of recovery after TBI.24
The important role of neuroimaging in severe TBI is intuitive. However, it is important to consider the role of neuroimaging in mild TBI in children, especially in the setting of repetitive mild injury.25 A growing body of evidence supports close, serial monitoring of children after even mild closed head injury for neurologic and psychiatric sequelae. Although it is rare that a child who is awake, interactive, and lacking focal neurologic deficits would need emergent (ie, CT) imaging after mild closed head injury, there might be a role for MRI later in the course of that child’s recovery—especially if recovery is complicated by clinical sequelae of mild TBI, such as cognitive impairment, headaches, or altered behavior.
When is additional neuroimaging needed?
It’s worthwhile briefly reviewing 4 scenarios that you might encounter, when you work with children and adolescents, in which urgent or emergent neuroimaging (often with consultation) should be obtained. The Table describes these situations and appropriate first- and second-line interventions.
1. When the presentation of your patient is consistent with an acute neurologic deficit, acute TBI, progressive neuropsychiatric decline, CNS infection, mass, demyelinating process, or toxic exposure, neuroimaging is likely critical.
2. In patients with progressive neurologic decline, including loss of developmental milestones, MRI, MRS, and referral to neurology should be part of the comprehensive evaluation.
3. In young children who exhibit a decrease in head circumference on growth curves, MRI is important to evaluate for underlying structural causes.
4. Pediatric patients with symptoms consistent with either stroke (ie, a new, persistent neurologic deficit) or a demyelinating process (eg, multiple episodes of variable transient focal neurologic symptoms), MRI should be obtained without compunction.
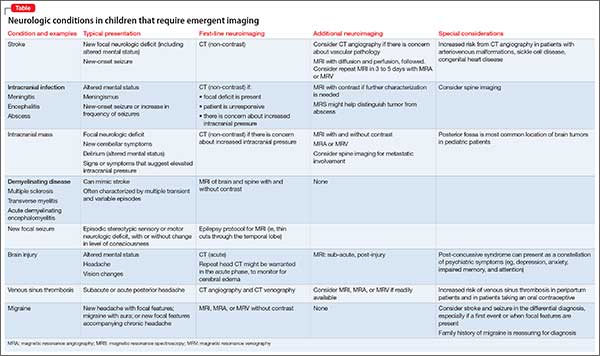
Consultation with pediatric neuroradiology
In deciding whether to obtain neuroimaging for a particular case, you should discuss your concerns with the pediatric radiologist or pediatric neuroradiologist, who will likely provide important guidance on key aspects of the study (eg, modifying slice thickness in a particular scan; recommending the use of contrast; including MRS in the order for imaging; performing appropriate vessel imaging). Consider asking 1 or more important questions when you discuss a patient’s presentation with the pediatric radiologist or pediatric neuroradiologist:
- “What neuroimaging studies are appropriate, based on my differential diagnosis?”
- “Are there specific imaging sequences that we should consider?”
- “Are there contraindications to the imaging modality for my patient?”
- “Is my patient likely to have difficulty tolerating the imaging procedure?”
- “Does my patient need sedation to tolerate this procedure?”
- “Should additional regions be included in the scan?” (Examples: In a child with stroke it might be important to include neck and chest vasculature and the heart. Other conditions might warrant imaging of the spinal cord.)
1. Abdelhalim AN, Alberico RA. Pediatric neuroimaging. Neurol Clin. 2009;27(1):285-301, x.
2. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22(4):439-450.
3. Bosemani T, Poretti A, Huisman TA. Susceptibility-weighted imaging in pediatric neuroimaging. J Magn Reson Imaging. 2014;40(3):530-544.
4. Cecil KM. Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clin N Am. 2013;23(3):381-392.
5. Panigrahy A, Nelson MD Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3-30.
6. Leentjens AF, Schieveld JN, Leonard M, et al. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res. 2008;64(2):219-223.
7. Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125(2):114-126.
8. Shaw P, Malek M, Watson B, et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191-197.
9. Phelan JA, Lowe LH, Glasier CM. Pediatric neurodegenerative white matter processes: leukodystrophies and beyond. Pediatr Radiol. 2008;38(7):729-749.
10. Strawn JR, Wegman CJ, Dominick KC, et al. Cortical surface anatomy in pediatric patients with generalized anxiety disorder. J Anxiety Disord. 2014;28(7):717-723.
11. Mueller SC, Aouidad A, Gorodetsky E, et al. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism [Erratum in J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195]? J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195.
12. Strawn JR, Hamm L, Fitzgerald DA, et al. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81-88.
13. Strawn JR, Dominick KC, Patino LR, et al. Neurobiology of pediatric anxiety disorders. Curr Behav Neurosci Reports. 2014;1(3):154-160.
14. Alexiou GA, Argyropoulou MI. Neuroimaging in childhood headache: a systematic review. Pediatr Radiol. 2013;43(7):777-784.
15. Giedd JN, Rapoport JL, Garvey MA, et al. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157(2):281-283.
16. Chang K, Frankovich J, Cooperstock M, et al; PANS Collaborative Consortium. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2014;25(1):3-13.
17. Wallace GL, Robustelli B, Dankner N, et al. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136(pt 6):1956-1967.
18. Libero LE, DeRamus TP, Deshpande HD, et al. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 2014;62:1-10.
19. Boddaert N, Zilbovicius M, Philipe A, et al. MRI findings in 77 children with non-syndromic autistic disorder. PLoS One. 2009;4:e445. doi: 10.1371/journal.pone.0004415.
20. Richards C, Jones C, Groves L, et al. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2(10):909-916.
21. Wilde EA, Hunter JV, Bigler ED. Pediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. NeuroRehabilitation. 2012;31(3):245-260.
22. Mechtler LL, Shastri KK, Crutchfield KE. Advanced neuroimaging of mild traumatic brain injury. Neurol Clin. 2014;32(1):31-58.
23. Ashwal S, Tong KA, Ghosh N, et al. Application of advanced neuroimaging modalities in pediatric traumatic brain injury. J Child Neurol. 2014;29(12):1704-1717.
24. Munson S, Schroth E, Ernst M. The role of functional neuroimaging in pediatric brain injury. Pediatrics. 2006;117(4):1372-1381.
25. Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555-568.
The first 15 years of the new millennium have seen a great increase in research on neuroimaging in children and adolescents who have a psychiatric disorder. In addition, imaging modalities continue to evolve, and are becoming increasingly accessible and informative. The literature is now replete with reports of neurostructural differences between patients and healthy subjects in a variety of common pediatric psychiatric conditions, including anxiety disorders, mood disorders, autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder (ADHD).
Historically, the clinical utility of neuroimaging was restricted to the identification of structural pathology. Today, accumulating data reveal novel roles for neuroimaging; these revelations are supported by studies demonstrating that treatment response for psychotherapeutic and psychopharmacotherapeutic interventions can be predicted by neurochemical and neurofunctional characteristics assessed by advanced imaging technologies, such as magnetic resonance spectroscopy (MRS) and functional MRI.
However, such advanced techniques are (at least at present) not ready for routine clinical use for this purpose. Instead, neuroimaging in the child and adolescent psychiatric clinic remains largely focused on ruling out neurostructural, neurologic, “nonpsychiatric” causes of our patients’ symptoms.
Understanding the role and limitations of major imaging modalities is key to guiding efficient and appropriate neuroimaging selection for pediatric patients. In this article, we describe and review:
- neuroimaging approaches for children and adolescents with psychiatric disorders
- the role of neuroimaging in (1) the differential diagnosis and workup of common psychiatric disorders and (2) urgent clinical situations
- how to determine what type of imaging to obtain.
Computed tomography
CT, which utilizes ionizing radiation, often is reserved, in the pediatric setting, for (1) emergency evaluation and (2) excluding potentially catastrophic neurologic injury resulting from:
- ischemic or hemorrhagic stroke
- herniation
- intracerebral hemorrhage
- subdural and epidural hematoma
- large intracranial mass with mass effect
- increased intracranial pressure
- acute skull fracture.
Although a CT scan is, typically, quick and has excellent sensitivity for acute bleeding and bony pathology, it exposes the patient to radiation and provides poor resolution compared with MRI.
In pediatrics, there has been practice-changing recognition of the importance of limiting lifetime radiation exposure incurred from medical procedures and imaging. As a result, most providers now agree that use of MRI in lieu of CT is appropriate in many, if not most, non-emergent situations. In an emergent situation, however, CT imaging is appropriate and should not be delayed. Moreover, in an emergent situation, you should not hesitate to use head CT in children, although timely discussion with the radiologist is recommended to review your differential diagnosis to better determine the preferred imaging modality.
Magnetic resonance imaging
Over the past several decades, MRI has been increasingly available in most pediatric health care facilities. The modality offers specific advantages for pediatric patients, including:
- better spatial resolution
- the ability to concurrently assess multiple pathologic processes
- lack of exposure to ionizing radiation.1
A number of MRI sequences, described below, can be used to assess vascular, inflammatory, structural, and metabolic processes.
A look inside. Comprehensive review of the physics that underlies MRI is beyond the scope of this article; several important principles are relevant to clinicians, however. Image contrast is dependent on intrinsic properties of tissue with regard to proton density, longitudinal relaxation time (T1), and transverse relaxation time (T2). Pulse sequences, which describe the strength and timing of the radiofrequency pulse and gradient pulses, define imaging acquisition parameters (eg, repetition time between the radio frequency pulse and echo time).
In turn, the intensity of the signal that is “seen” with various pulse sequences is differentially affected by intrinsic properties of tissue. At most pediatric institutions, the standard MRI-examination protocol includes: a T1-weighted image (Figure 1A); a T2-weighted scan (Figure 1B); fluid attenuated inversion recovery (FLAIR) (Figure 1C); and diffusion-weighted imaging (DWI) (Figure 1D).

Specific MRI sequences
T1 images. T1 sequences, or so-called anatomy sequences, are ideally suited for detailed neuroanatomic evaluations. They are generated in such a way that structures containing fluid are dark (hypo-intense), whereas other structures, with higher fat or protein content, are brighter (iso-intense, even hyper-intense). For this reason, CSF in the intracranial ventricles is dark, and white matter is brighter than the cortex because of lipid in myelin sheaths.
In addition, to view structural abnormalities that are characterized by altered vascular supply or flow, such as tumors and infections (abscesses), contrast imaging can be particularly helpful; such images generally are obtained as T1 sequences.
T2 images. By contrast to the T1-weighted sequence, the T2-weighted sequences emphasize fluid signal; structures such as the ventricles, which contain CSF, therefore will be bright (hyper-intense). Pathology that produces edema or fluid, such as edema surrounding demyelinating lesions or infections, also will show bright hyper-intense signal. In T2-weighted images of the brain, white matter shows lower signal intensity than the cortex because of the relatively lower water content in white matter tracts and myelin sheaths.
Fluid attenuation inversion recovery. FLAIR images are generated so that the baseline bright T2 signal seen in normal structures, such as the CSF, containing ventricles is cancelled out, or attenuated. In effect, this subtraction of typical background hyper-intense fluid signal leaves only abnormal T2 bright hyper-intense signal, such as vasogenic edema surrounding tumors, cytotoxic edema within an infarction, or extra-axial fluid collections such as a subarachnoid or subdural hemorrhage.
Diffusion-weighted imaging. DWI utilizes the random motion (ie, diffusion) of water molecules to generate contrast. In this regard, the diffusion of any molecule is influenced by its interaction with other molecules (eg, white-matter fibers and membranes, and macromolecules). Diffusion patterns therefore reflect details about tissue boundaries; as such, DWI is sensitive to a number of neurologic processes, such as ischemia, demyelinating disease, and some tumors, which restrict the free motion of water. DWI detects this so-called restricted diffusion and displays an area of bright signal.
Susceptibility-weighted imaging (SWI). In the pediatric population, SWI (Figure 2) utilizes a long-echo, 3-dimensional, velocity-compensated gradient recalled echo for image acquisition2 and, ultimately, leverages susceptibility differences across tissues by employing the phase image to identify these differences. SWI, which uses both magnitude and phase images and is remarkably sensitive to venous blood (and blood products), iron, and calcifications, therefore might be of increasing utility in pediatric patients with traumatic brain injury (TBI) (Figure 2B). As such, SWI has become a critical component of many pediatric MRI studies.3
Magnetic resonance angiography (MRA) (Figure 3A) is helpful for assessing intracranial arteries and may be employed in the evaluation of:
- vessel pathology and injury underlying stroke, such as vessel occlusion or injury
- patterns of vessel involvement suggestive of vasculitis
- developmental or acquired structural vascular abnormalities, such as aneurysm or vascular malformations
- determination of tumor blood supply.

MRA can be performed without or with contrast, although MRA with contrast might provide a higher quality study and therefore be of greater utility. Of note: The spatial resolution of MRA is not as good as CT angiography; abnormalities, such as a small aneurysm, might not be apparent.
Magnetic resonance venography (MRV) (Figure 3B) is most commonly performed when the possibility of thrombosis of the dural venous sinuses is being considered; it also is employed to evaluate vascular malformations, tumor drainage patterns, and other pathologic states. As with MRA, MRV can be performed without or with contrast, although post-contrast MRV is generally of higher quality and might be preferred when assessing for sinus thrombosis.
Magnetic resonance spectroscopy (MRS) resides at the border between research and clinical practice. In children and adolescents, MRS provides data on neuronal and axonal viability as well as energetics and cell membranes.4 Pediatric neurologists often use MRS to evaluate for congenital neurometabolic disease; this modality also can help distinguish between an active intracranial tumor from an abscess or gliosis.5
Neuroimaging in pediatric neuropsychiatric conditions: Evidence, guidance
Delirium (altered mental status). The acute neuropsychiatric syndrome characterized by impaired attention and sensorium might have a broad underlying etiology, but it is always associated with alteration of CNS neurophysiology. Children with neurostructural abnormalities might have increased vulnerability to CNS insult and therefore be at increased risk of delirium.6 Additionally, delirium can present subtly in children, with the precise signs dependent on the individual patient’s developmental stage.
Neuroimaging may be helpful when an infectious, inflammatory, toxic, or a metabolic basis for delirium is suspected, or when a patient has new focal neurologic findings. In this regard, focal neurologic findings suggest an underlying localizable lesion and warrant dedicated neuroimaging to localize the lesion.
In general, the differential diagnosis should guide consideration of neuroimaging. When considering the possibility of an unwitnessed seizure in a child who presents with altered mental status, neuroimaging certainly is an important component of the workup.
As another example, when underlying trauma, intracranial hemorrhage, or mass is a possibility in acute delirium, urgent head CT is appropriate. In non-emergent cases, MRI is the modality of choice. In immunocompromised patients presenting with delirium, maintain a low threshold for neuroimaging with contrast to rule out opportunistic intracranial infection.
Last, in children who have hydrocephalus with a shunt, delirium could be a harbinger of underlying shunt malfunction, warranting a “shunt series.”
ADHD. The diagnosis of ADHD remains a clinical one; for the typical pediatric patient with ADHD but who does not have focal neurologic deficits, neuroimaging is unnecessary. Some structural MRI studies of youth with ADHD suggest diminished volume of the globus pallidus, putamen, and caudate7; other studies reveal changes in gyrification and cortical thickness8 in regions subserving attentional processes. However, intra-individual and developmental-related variability preclude routine use of neuroimaging in the standard diagnostic work-up of ADHD.
Nevertheless, neuroimaging should be strongly considered in a child with progressive worsening of inattention, especially if combined with other psychiatric or neurologic findings. In such a case, MRI should be obtained to evaluate for a progressive neurodegenerative leukoencephalopathy (eg, adrenoleukodystrophy).9
Depressive and anxiety disorders. In pediatric patients who exhibit depressive or anxiety symptoms, abnormalities have been observed in cortical thickness10 and gray matter volume,11,12 and functional signatures13 have been identified in the circuitry of the prefrontal amygdala. No data suggest that, in an individual patient, neuroimaging can be of diagnostic utility—particularly in the absence of focal neurologic findings.
That being said, headache and other somatic symptoms are common in pediatric patients with a mood or anxiety disorder. Evidence for neuroimaging in the context of pediatric headache suggests that MRI should be considered when headache is associated with neurologic signs or symptoms, such as aura, or accompanied by focal neurologic deficit.14
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS). Neuroimaging studies of patients with confirmed PANDAS or PANS are rare, but group analyses suggest a decreased average volume of the caudate, putamen, and globus pallidus in patients with PANDAS compared with healthy comparison subjects, although total cerebral volume does not appear to differ.15 Moreover, thalamic findings in patients with PANDAS have been noted to be similar to what is seen in patients with Sydenham’s chorea.
The most recent consensus statement regarding the treatment and assessment of PANDAS and PANS recommends ordering brain MRI when other conditions are suspected (eg, CNS, small vessel vasculitis, limbic encephalitis) or when the patient has severe headache, gait disturbance, cognitive deterioration, or psychosis.16 Furthermore, the consensus statement notes the potential utility of T2-weighted imaging with contrast to evaluate inflammatory changes in the basal ganglia.16
Autism spectrum disorder. Significant progress has been made during the past decade on the neuroanatomic characterization of ASD. Accumulating data indicate that, in pediatric patients with ASD, (1) development of white matter and gray matter is disrupted early in the course of the disorder and (2) cortical thickness is increased in regions subserving social cognition.17,18
Several studies have examined the presence of patient-level findings in samples of pediatric patients with ASD. Approximately 8% of pediatric patients with ASD were found to have some abnormality on routine brain MRI, the most common being white-matter signal abnormalities, dilated Virchow-Robin space, and temporal lobe abnormalities.19
Although abnormalities might be present in a large percentage of individual scans, routine screening MRI is unlikely to be of clinical utility in youth with ASD. In fact, no recommendation for routine MRI screening in patients with ASD has been made by the American Academy of Child & Adolescent Psychiatry, the Child Neurology Society of the American Academy of Neurology, or the American Academy of Pediatrics.
However, in patients with an underlying neurostructural disease that is phenotypically associated with ASD-like symptoms, imaging might be of use. In tuberous sclerosis, for example, MRI is especially important to classify intracranial lesions; determine burden and location; or identify treatment options (Figure 4). For patients with tuberous sclerosis—of whom more than one-third meet diagnostic criteria for ASD20—MRI study should include FLAIR, spin-echo, and gradient-echo sequences.

Movement disorders. Hyperkinetic movement disorders, including tic disorders and drug-induced movement disorders (eg, tremor) are common in pediatric patients. In pediatric patients with a tic disorder or Tourette’s disorder (TD), neuroimaging typically is unnecessary, despite the suggestion that the caudate nucleus volume is reduced in groups of patients with TD.
Many CNS-acting medications can exacerbate physiologic tremor; in pediatric patients with symptoms of a movement disorder, home medications should be carefully reviewed for potentially offending agents. When the patient is clinically and biochemically euthyroid and medication-induced movement disorder has been ruled out, or when the patient meets clinical diagnostic criteria for TD or a tic disorder, routine neuroimaging generally is unnecessary.
When tremor accompanies other cerebellar signs, such as ataxia or dysmetria, strongly consider MRI of the brain to evaluate pathology in the posterior fossa. In addition, neuroimaging should be considered for children with a new-onset abnormality on neurologic exam, including rapid onset of abnormal movements (other than common tics), continuous progressive worsening of symptoms, or any loss of developmental milestones.
Last, although tics and stereotypies often are transient and wane with age, other abnormal movements, such as dystonia, chorea, and parkinsonism (aside from those potentially associated with antipsychotic use), are never expected during typical development and warrant MRI.
Traumatic brain injury. Prompt evaluation and intervention for TBI can significantly affect overall outcome. Moreover, there has been increased enthusiasm around the pre-hospital assessment of TBI severity using (1) any of several proprietary testing systems (eg, Immediate Post-Concussion Assessment and Cognitive Testing [ImPACT]) plus (2) standard clinical staging, which is based on duration of loss of consciousness, persistence of memory loss, and the Glasgow Coma Scale score.
The goal for any TBI patient during the acute post-injury phase is to minimize continued neuronal injury from secondary effects of TBI, such as cerebral edema and herniation, and to optimize protection of surviving brain tissue; neuroimaging is a critical component of assessment during both acute and chronic recovery periods of TBI.21
The optimal imaging modality varies with the amount of time that has passed since initial injury.22 Urgent neuroimaging (the first 24 hours after brain injury) is typically obtained using head CT to assist decision-making in acute neurosurgical management. In this setting, head CT is fast and efficient; minimizes the amount of time that the patient is in the scanner; and provides valuable information on the acuity and extent of injury, degree of cerebral edema, and evidence or risk of pending herniation.
On the other hand, MRI is superior to CT during 48 to 72 hours after injury, given its higher resolution; superior imaging of the brainstem and deep gray nuclei; and ability to detect axonal injury, small contusions, and subtle neuronal damage. Specifically, SWI sequences can be particularly helpful in TBI for detecting diffuse axonal injury and micro-hemorrhages; several recent studies also suggest that SWI may be of particular value in pediatric patients with TBI.23
Additionally, given the increased sensitivity of MRI to detect subtle injuries, this modality can assist in identifying chronic sequelae of brain injury—thus contributing to determining of chronic therapy options and assisting with long-term prognosis. Gross structural changes resulting from TBI often are evident even in the acute post-injury phase; synaptic remodeling continues, however, for an indefinite period after injury, and this remodeling capacity is even more pronounced in the highly plastic brain of a young child.
Microstructural changes might not be detectable using traditional, readily available imaging sequences (CT, MRI). When those traditional modalities are used in concert with functional imaging techniques (eg, PET to evaluate cerebral metabolism and SPECT imaging which can detect abnormalities in cerebral blood flow), the combination of older and newer might provide a more complete picture of recovery after TBI.24
The important role of neuroimaging in severe TBI is intuitive. However, it is important to consider the role of neuroimaging in mild TBI in children, especially in the setting of repetitive mild injury.25 A growing body of evidence supports close, serial monitoring of children after even mild closed head injury for neurologic and psychiatric sequelae. Although it is rare that a child who is awake, interactive, and lacking focal neurologic deficits would need emergent (ie, CT) imaging after mild closed head injury, there might be a role for MRI later in the course of that child’s recovery—especially if recovery is complicated by clinical sequelae of mild TBI, such as cognitive impairment, headaches, or altered behavior.
When is additional neuroimaging needed?
It’s worthwhile briefly reviewing 4 scenarios that you might encounter, when you work with children and adolescents, in which urgent or emergent neuroimaging (often with consultation) should be obtained. The Table describes these situations and appropriate first- and second-line interventions.
1. When the presentation of your patient is consistent with an acute neurologic deficit, acute TBI, progressive neuropsychiatric decline, CNS infection, mass, demyelinating process, or toxic exposure, neuroimaging is likely critical.
2. In patients with progressive neurologic decline, including loss of developmental milestones, MRI, MRS, and referral to neurology should be part of the comprehensive evaluation.
3. In young children who exhibit a decrease in head circumference on growth curves, MRI is important to evaluate for underlying structural causes.
4. Pediatric patients with symptoms consistent with either stroke (ie, a new, persistent neurologic deficit) or a demyelinating process (eg, multiple episodes of variable transient focal neurologic symptoms), MRI should be obtained without compunction.

Consultation with pediatric neuroradiology
In deciding whether to obtain neuroimaging for a particular case, you should discuss your concerns with the pediatric radiologist or pediatric neuroradiologist, who will likely provide important guidance on key aspects of the study (eg, modifying slice thickness in a particular scan; recommending the use of contrast; including MRS in the order for imaging; performing appropriate vessel imaging). Consider asking 1 or more important questions when you discuss a patient’s presentation with the pediatric radiologist or pediatric neuroradiologist:
- “What neuroimaging studies are appropriate, based on my differential diagnosis?”
- “Are there specific imaging sequences that we should consider?”
- “Are there contraindications to the imaging modality for my patient?”
- “Is my patient likely to have difficulty tolerating the imaging procedure?”
- “Does my patient need sedation to tolerate this procedure?”
- “Should additional regions be included in the scan?” (Examples: In a child with stroke it might be important to include neck and chest vasculature and the heart. Other conditions might warrant imaging of the spinal cord.)
The first 15 years of the new millennium have seen a great increase in research on neuroimaging in children and adolescents who have a psychiatric disorder. In addition, imaging modalities continue to evolve, and are becoming increasingly accessible and informative. The literature is now replete with reports of neurostructural differences between patients and healthy subjects in a variety of common pediatric psychiatric conditions, including anxiety disorders, mood disorders, autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder (ADHD).
Historically, the clinical utility of neuroimaging was restricted to the identification of structural pathology. Today, accumulating data reveal novel roles for neuroimaging; these revelations are supported by studies demonstrating that treatment response for psychotherapeutic and psychopharmacotherapeutic interventions can be predicted by neurochemical and neurofunctional characteristics assessed by advanced imaging technologies, such as magnetic resonance spectroscopy (MRS) and functional MRI.
However, such advanced techniques are (at least at present) not ready for routine clinical use for this purpose. Instead, neuroimaging in the child and adolescent psychiatric clinic remains largely focused on ruling out neurostructural, neurologic, “nonpsychiatric” causes of our patients’ symptoms.
Understanding the role and limitations of major imaging modalities is key to guiding efficient and appropriate neuroimaging selection for pediatric patients. In this article, we describe and review:
- neuroimaging approaches for children and adolescents with psychiatric disorders
- the role of neuroimaging in (1) the differential diagnosis and workup of common psychiatric disorders and (2) urgent clinical situations
- how to determine what type of imaging to obtain.
Computed tomography
CT, which utilizes ionizing radiation, often is reserved, in the pediatric setting, for (1) emergency evaluation and (2) excluding potentially catastrophic neurologic injury resulting from:
- ischemic or hemorrhagic stroke
- herniation
- intracerebral hemorrhage
- subdural and epidural hematoma
- large intracranial mass with mass effect
- increased intracranial pressure
- acute skull fracture.
Although a CT scan is, typically, quick and has excellent sensitivity for acute bleeding and bony pathology, it exposes the patient to radiation and provides poor resolution compared with MRI.
In pediatrics, there has been practice-changing recognition of the importance of limiting lifetime radiation exposure incurred from medical procedures and imaging. As a result, most providers now agree that use of MRI in lieu of CT is appropriate in many, if not most, non-emergent situations. In an emergent situation, however, CT imaging is appropriate and should not be delayed. Moreover, in an emergent situation, you should not hesitate to use head CT in children, although timely discussion with the radiologist is recommended to review your differential diagnosis to better determine the preferred imaging modality.
Magnetic resonance imaging
Over the past several decades, MRI has been increasingly available in most pediatric health care facilities. The modality offers specific advantages for pediatric patients, including:
- better spatial resolution
- the ability to concurrently assess multiple pathologic processes
- lack of exposure to ionizing radiation.1
A number of MRI sequences, described below, can be used to assess vascular, inflammatory, structural, and metabolic processes.
A look inside. Comprehensive review of the physics that underlies MRI is beyond the scope of this article; several important principles are relevant to clinicians, however. Image contrast is dependent on intrinsic properties of tissue with regard to proton density, longitudinal relaxation time (T1), and transverse relaxation time (T2). Pulse sequences, which describe the strength and timing of the radiofrequency pulse and gradient pulses, define imaging acquisition parameters (eg, repetition time between the radio frequency pulse and echo time).
In turn, the intensity of the signal that is “seen” with various pulse sequences is differentially affected by intrinsic properties of tissue. At most pediatric institutions, the standard MRI-examination protocol includes: a T1-weighted image (Figure 1A); a T2-weighted scan (Figure 1B); fluid attenuated inversion recovery (FLAIR) (Figure 1C); and diffusion-weighted imaging (DWI) (Figure 1D).

Specific MRI sequences
T1 images. T1 sequences, or so-called anatomy sequences, are ideally suited for detailed neuroanatomic evaluations. They are generated in such a way that structures containing fluid are dark (hypo-intense), whereas other structures, with higher fat or protein content, are brighter (iso-intense, even hyper-intense). For this reason, CSF in the intracranial ventricles is dark, and white matter is brighter than the cortex because of lipid in myelin sheaths.
In addition, to view structural abnormalities that are characterized by altered vascular supply or flow, such as tumors and infections (abscesses), contrast imaging can be particularly helpful; such images generally are obtained as T1 sequences.
T2 images. By contrast to the T1-weighted sequence, the T2-weighted sequences emphasize fluid signal; structures such as the ventricles, which contain CSF, therefore will be bright (hyper-intense). Pathology that produces edema or fluid, such as edema surrounding demyelinating lesions or infections, also will show bright hyper-intense signal. In T2-weighted images of the brain, white matter shows lower signal intensity than the cortex because of the relatively lower water content in white matter tracts and myelin sheaths.
Fluid attenuation inversion recovery. FLAIR images are generated so that the baseline bright T2 signal seen in normal structures, such as the CSF, containing ventricles is cancelled out, or attenuated. In effect, this subtraction of typical background hyper-intense fluid signal leaves only abnormal T2 bright hyper-intense signal, such as vasogenic edema surrounding tumors, cytotoxic edema within an infarction, or extra-axial fluid collections such as a subarachnoid or subdural hemorrhage.
Diffusion-weighted imaging. DWI utilizes the random motion (ie, diffusion) of water molecules to generate contrast. In this regard, the diffusion of any molecule is influenced by its interaction with other molecules (eg, white-matter fibers and membranes, and macromolecules). Diffusion patterns therefore reflect details about tissue boundaries; as such, DWI is sensitive to a number of neurologic processes, such as ischemia, demyelinating disease, and some tumors, which restrict the free motion of water. DWI detects this so-called restricted diffusion and displays an area of bright signal.
Susceptibility-weighted imaging (SWI). In the pediatric population, SWI (Figure 2) utilizes a long-echo, 3-dimensional, velocity-compensated gradient recalled echo for image acquisition2 and, ultimately, leverages susceptibility differences across tissues by employing the phase image to identify these differences. SWI, which uses both magnitude and phase images and is remarkably sensitive to venous blood (and blood products), iron, and calcifications, therefore might be of increasing utility in pediatric patients with traumatic brain injury (TBI) (Figure 2B). As such, SWI has become a critical component of many pediatric MRI studies.3
Magnetic resonance angiography (MRA) (Figure 3A) is helpful for assessing intracranial arteries and may be employed in the evaluation of:
- vessel pathology and injury underlying stroke, such as vessel occlusion or injury
- patterns of vessel involvement suggestive of vasculitis
- developmental or acquired structural vascular abnormalities, such as aneurysm or vascular malformations
- determination of tumor blood supply.

MRA can be performed without or with contrast, although MRA with contrast might provide a higher quality study and therefore be of greater utility. Of note: The spatial resolution of MRA is not as good as CT angiography; abnormalities, such as a small aneurysm, might not be apparent.
Magnetic resonance venography (MRV) (Figure 3B) is most commonly performed when the possibility of thrombosis of the dural venous sinuses is being considered; it also is employed to evaluate vascular malformations, tumor drainage patterns, and other pathologic states. As with MRA, MRV can be performed without or with contrast, although post-contrast MRV is generally of higher quality and might be preferred when assessing for sinus thrombosis.
Magnetic resonance spectroscopy (MRS) resides at the border between research and clinical practice. In children and adolescents, MRS provides data on neuronal and axonal viability as well as energetics and cell membranes.4 Pediatric neurologists often use MRS to evaluate for congenital neurometabolic disease; this modality also can help distinguish between an active intracranial tumor from an abscess or gliosis.5
Neuroimaging in pediatric neuropsychiatric conditions: Evidence, guidance
Delirium (altered mental status). The acute neuropsychiatric syndrome characterized by impaired attention and sensorium might have a broad underlying etiology, but it is always associated with alteration of CNS neurophysiology. Children with neurostructural abnormalities might have increased vulnerability to CNS insult and therefore be at increased risk of delirium.6 Additionally, delirium can present subtly in children, with the precise signs dependent on the individual patient’s developmental stage.
Neuroimaging may be helpful when an infectious, inflammatory, toxic, or a metabolic basis for delirium is suspected, or when a patient has new focal neurologic findings. In this regard, focal neurologic findings suggest an underlying localizable lesion and warrant dedicated neuroimaging to localize the lesion.
In general, the differential diagnosis should guide consideration of neuroimaging. When considering the possibility of an unwitnessed seizure in a child who presents with altered mental status, neuroimaging certainly is an important component of the workup.
As another example, when underlying trauma, intracranial hemorrhage, or mass is a possibility in acute delirium, urgent head CT is appropriate. In non-emergent cases, MRI is the modality of choice. In immunocompromised patients presenting with delirium, maintain a low threshold for neuroimaging with contrast to rule out opportunistic intracranial infection.
Last, in children who have hydrocephalus with a shunt, delirium could be a harbinger of underlying shunt malfunction, warranting a “shunt series.”
ADHD. The diagnosis of ADHD remains a clinical one; for the typical pediatric patient with ADHD but who does not have focal neurologic deficits, neuroimaging is unnecessary. Some structural MRI studies of youth with ADHD suggest diminished volume of the globus pallidus, putamen, and caudate7; other studies reveal changes in gyrification and cortical thickness8 in regions subserving attentional processes. However, intra-individual and developmental-related variability preclude routine use of neuroimaging in the standard diagnostic work-up of ADHD.
Nevertheless, neuroimaging should be strongly considered in a child with progressive worsening of inattention, especially if combined with other psychiatric or neurologic findings. In such a case, MRI should be obtained to evaluate for a progressive neurodegenerative leukoencephalopathy (eg, adrenoleukodystrophy).9
Depressive and anxiety disorders. In pediatric patients who exhibit depressive or anxiety symptoms, abnormalities have been observed in cortical thickness10 and gray matter volume,11,12 and functional signatures13 have been identified in the circuitry of the prefrontal amygdala. No data suggest that, in an individual patient, neuroimaging can be of diagnostic utility—particularly in the absence of focal neurologic findings.
That being said, headache and other somatic symptoms are common in pediatric patients with a mood or anxiety disorder. Evidence for neuroimaging in the context of pediatric headache suggests that MRI should be considered when headache is associated with neurologic signs or symptoms, such as aura, or accompanied by focal neurologic deficit.14
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS). Neuroimaging studies of patients with confirmed PANDAS or PANS are rare, but group analyses suggest a decreased average volume of the caudate, putamen, and globus pallidus in patients with PANDAS compared with healthy comparison subjects, although total cerebral volume does not appear to differ.15 Moreover, thalamic findings in patients with PANDAS have been noted to be similar to what is seen in patients with Sydenham’s chorea.
The most recent consensus statement regarding the treatment and assessment of PANDAS and PANS recommends ordering brain MRI when other conditions are suspected (eg, CNS, small vessel vasculitis, limbic encephalitis) or when the patient has severe headache, gait disturbance, cognitive deterioration, or psychosis.16 Furthermore, the consensus statement notes the potential utility of T2-weighted imaging with contrast to evaluate inflammatory changes in the basal ganglia.16
Autism spectrum disorder. Significant progress has been made during the past decade on the neuroanatomic characterization of ASD. Accumulating data indicate that, in pediatric patients with ASD, (1) development of white matter and gray matter is disrupted early in the course of the disorder and (2) cortical thickness is increased in regions subserving social cognition.17,18
Several studies have examined the presence of patient-level findings in samples of pediatric patients with ASD. Approximately 8% of pediatric patients with ASD were found to have some abnormality on routine brain MRI, the most common being white-matter signal abnormalities, dilated Virchow-Robin space, and temporal lobe abnormalities.19
Although abnormalities might be present in a large percentage of individual scans, routine screening MRI is unlikely to be of clinical utility in youth with ASD. In fact, no recommendation for routine MRI screening in patients with ASD has been made by the American Academy of Child & Adolescent Psychiatry, the Child Neurology Society of the American Academy of Neurology, or the American Academy of Pediatrics.
However, in patients with an underlying neurostructural disease that is phenotypically associated with ASD-like symptoms, imaging might be of use. In tuberous sclerosis, for example, MRI is especially important to classify intracranial lesions; determine burden and location; or identify treatment options (Figure 4). For patients with tuberous sclerosis—of whom more than one-third meet diagnostic criteria for ASD20—MRI study should include FLAIR, spin-echo, and gradient-echo sequences.

Movement disorders. Hyperkinetic movement disorders, including tic disorders and drug-induced movement disorders (eg, tremor) are common in pediatric patients. In pediatric patients with a tic disorder or Tourette’s disorder (TD), neuroimaging typically is unnecessary, despite the suggestion that the caudate nucleus volume is reduced in groups of patients with TD.
Many CNS-acting medications can exacerbate physiologic tremor; in pediatric patients with symptoms of a movement disorder, home medications should be carefully reviewed for potentially offending agents. When the patient is clinically and biochemically euthyroid and medication-induced movement disorder has been ruled out, or when the patient meets clinical diagnostic criteria for TD or a tic disorder, routine neuroimaging generally is unnecessary.
When tremor accompanies other cerebellar signs, such as ataxia or dysmetria, strongly consider MRI of the brain to evaluate pathology in the posterior fossa. In addition, neuroimaging should be considered for children with a new-onset abnormality on neurologic exam, including rapid onset of abnormal movements (other than common tics), continuous progressive worsening of symptoms, or any loss of developmental milestones.
Last, although tics and stereotypies often are transient and wane with age, other abnormal movements, such as dystonia, chorea, and parkinsonism (aside from those potentially associated with antipsychotic use), are never expected during typical development and warrant MRI.
Traumatic brain injury. Prompt evaluation and intervention for TBI can significantly affect overall outcome. Moreover, there has been increased enthusiasm around the pre-hospital assessment of TBI severity using (1) any of several proprietary testing systems (eg, Immediate Post-Concussion Assessment and Cognitive Testing [ImPACT]) plus (2) standard clinical staging, which is based on duration of loss of consciousness, persistence of memory loss, and the Glasgow Coma Scale score.
The goal for any TBI patient during the acute post-injury phase is to minimize continued neuronal injury from secondary effects of TBI, such as cerebral edema and herniation, and to optimize protection of surviving brain tissue; neuroimaging is a critical component of assessment during both acute and chronic recovery periods of TBI.21
The optimal imaging modality varies with the amount of time that has passed since initial injury.22 Urgent neuroimaging (the first 24 hours after brain injury) is typically obtained using head CT to assist decision-making in acute neurosurgical management. In this setting, head CT is fast and efficient; minimizes the amount of time that the patient is in the scanner; and provides valuable information on the acuity and extent of injury, degree of cerebral edema, and evidence or risk of pending herniation.
On the other hand, MRI is superior to CT during 48 to 72 hours after injury, given its higher resolution; superior imaging of the brainstem and deep gray nuclei; and ability to detect axonal injury, small contusions, and subtle neuronal damage. Specifically, SWI sequences can be particularly helpful in TBI for detecting diffuse axonal injury and micro-hemorrhages; several recent studies also suggest that SWI may be of particular value in pediatric patients with TBI.23
Additionally, given the increased sensitivity of MRI to detect subtle injuries, this modality can assist in identifying chronic sequelae of brain injury—thus contributing to determining of chronic therapy options and assisting with long-term prognosis. Gross structural changes resulting from TBI often are evident even in the acute post-injury phase; synaptic remodeling continues, however, for an indefinite period after injury, and this remodeling capacity is even more pronounced in the highly plastic brain of a young child.
Microstructural changes might not be detectable using traditional, readily available imaging sequences (CT, MRI). When those traditional modalities are used in concert with functional imaging techniques (eg, PET to evaluate cerebral metabolism and SPECT imaging which can detect abnormalities in cerebral blood flow), the combination of older and newer might provide a more complete picture of recovery after TBI.24
The important role of neuroimaging in severe TBI is intuitive. However, it is important to consider the role of neuroimaging in mild TBI in children, especially in the setting of repetitive mild injury.25 A growing body of evidence supports close, serial monitoring of children after even mild closed head injury for neurologic and psychiatric sequelae. Although it is rare that a child who is awake, interactive, and lacking focal neurologic deficits would need emergent (ie, CT) imaging after mild closed head injury, there might be a role for MRI later in the course of that child’s recovery—especially if recovery is complicated by clinical sequelae of mild TBI, such as cognitive impairment, headaches, or altered behavior.
When is additional neuroimaging needed?
It’s worthwhile briefly reviewing 4 scenarios that you might encounter, when you work with children and adolescents, in which urgent or emergent neuroimaging (often with consultation) should be obtained. The Table describes these situations and appropriate first- and second-line interventions.
1. When the presentation of your patient is consistent with an acute neurologic deficit, acute TBI, progressive neuropsychiatric decline, CNS infection, mass, demyelinating process, or toxic exposure, neuroimaging is likely critical.
2. In patients with progressive neurologic decline, including loss of developmental milestones, MRI, MRS, and referral to neurology should be part of the comprehensive evaluation.
3. In young children who exhibit a decrease in head circumference on growth curves, MRI is important to evaluate for underlying structural causes.
4. Pediatric patients with symptoms consistent with either stroke (ie, a new, persistent neurologic deficit) or a demyelinating process (eg, multiple episodes of variable transient focal neurologic symptoms), MRI should be obtained without compunction.

Consultation with pediatric neuroradiology
In deciding whether to obtain neuroimaging for a particular case, you should discuss your concerns with the pediatric radiologist or pediatric neuroradiologist, who will likely provide important guidance on key aspects of the study (eg, modifying slice thickness in a particular scan; recommending the use of contrast; including MRS in the order for imaging; performing appropriate vessel imaging). Consider asking 1 or more important questions when you discuss a patient’s presentation with the pediatric radiologist or pediatric neuroradiologist:
- “What neuroimaging studies are appropriate, based on my differential diagnosis?”
- “Are there specific imaging sequences that we should consider?”
- “Are there contraindications to the imaging modality for my patient?”
- “Is my patient likely to have difficulty tolerating the imaging procedure?”
- “Does my patient need sedation to tolerate this procedure?”
- “Should additional regions be included in the scan?” (Examples: In a child with stroke it might be important to include neck and chest vasculature and the heart. Other conditions might warrant imaging of the spinal cord.)
1. Abdelhalim AN, Alberico RA. Pediatric neuroimaging. Neurol Clin. 2009;27(1):285-301, x.
2. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22(4):439-450.
3. Bosemani T, Poretti A, Huisman TA. Susceptibility-weighted imaging in pediatric neuroimaging. J Magn Reson Imaging. 2014;40(3):530-544.
4. Cecil KM. Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clin N Am. 2013;23(3):381-392.
5. Panigrahy A, Nelson MD Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3-30.
6. Leentjens AF, Schieveld JN, Leonard M, et al. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res. 2008;64(2):219-223.
7. Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125(2):114-126.
8. Shaw P, Malek M, Watson B, et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191-197.
9. Phelan JA, Lowe LH, Glasier CM. Pediatric neurodegenerative white matter processes: leukodystrophies and beyond. Pediatr Radiol. 2008;38(7):729-749.
10. Strawn JR, Wegman CJ, Dominick KC, et al. Cortical surface anatomy in pediatric patients with generalized anxiety disorder. J Anxiety Disord. 2014;28(7):717-723.
11. Mueller SC, Aouidad A, Gorodetsky E, et al. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism [Erratum in J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195]? J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195.
12. Strawn JR, Hamm L, Fitzgerald DA, et al. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81-88.
13. Strawn JR, Dominick KC, Patino LR, et al. Neurobiology of pediatric anxiety disorders. Curr Behav Neurosci Reports. 2014;1(3):154-160.
14. Alexiou GA, Argyropoulou MI. Neuroimaging in childhood headache: a systematic review. Pediatr Radiol. 2013;43(7):777-784.
15. Giedd JN, Rapoport JL, Garvey MA, et al. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157(2):281-283.
16. Chang K, Frankovich J, Cooperstock M, et al; PANS Collaborative Consortium. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2014;25(1):3-13.
17. Wallace GL, Robustelli B, Dankner N, et al. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136(pt 6):1956-1967.
18. Libero LE, DeRamus TP, Deshpande HD, et al. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 2014;62:1-10.
19. Boddaert N, Zilbovicius M, Philipe A, et al. MRI findings in 77 children with non-syndromic autistic disorder. PLoS One. 2009;4:e445. doi: 10.1371/journal.pone.0004415.
20. Richards C, Jones C, Groves L, et al. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2(10):909-916.
21. Wilde EA, Hunter JV, Bigler ED. Pediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. NeuroRehabilitation. 2012;31(3):245-260.
22. Mechtler LL, Shastri KK, Crutchfield KE. Advanced neuroimaging of mild traumatic brain injury. Neurol Clin. 2014;32(1):31-58.
23. Ashwal S, Tong KA, Ghosh N, et al. Application of advanced neuroimaging modalities in pediatric traumatic brain injury. J Child Neurol. 2014;29(12):1704-1717.
24. Munson S, Schroth E, Ernst M. The role of functional neuroimaging in pediatric brain injury. Pediatrics. 2006;117(4):1372-1381.
25. Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555-568.
1. Abdelhalim AN, Alberico RA. Pediatric neuroimaging. Neurol Clin. 2009;27(1):285-301, x.
2. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22(4):439-450.
3. Bosemani T, Poretti A, Huisman TA. Susceptibility-weighted imaging in pediatric neuroimaging. J Magn Reson Imaging. 2014;40(3):530-544.
4. Cecil KM. Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clin N Am. 2013;23(3):381-392.
5. Panigrahy A, Nelson MD Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3-30.
6. Leentjens AF, Schieveld JN, Leonard M, et al. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res. 2008;64(2):219-223.
7. Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125(2):114-126.
8. Shaw P, Malek M, Watson B, et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191-197.
9. Phelan JA, Lowe LH, Glasier CM. Pediatric neurodegenerative white matter processes: leukodystrophies and beyond. Pediatr Radiol. 2008;38(7):729-749.
10. Strawn JR, Wegman CJ, Dominick KC, et al. Cortical surface anatomy in pediatric patients with generalized anxiety disorder. J Anxiety Disord. 2014;28(7):717-723.
11. Mueller SC, Aouidad A, Gorodetsky E, et al. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism [Erratum in J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195]? J Am Acad Child Adolesc Psychiatry. 2013;52(2):184-195.
12. Strawn JR, Hamm L, Fitzgerald DA, et al. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81-88.
13. Strawn JR, Dominick KC, Patino LR, et al. Neurobiology of pediatric anxiety disorders. Curr Behav Neurosci Reports. 2014;1(3):154-160.
14. Alexiou GA, Argyropoulou MI. Neuroimaging in childhood headache: a systematic review. Pediatr Radiol. 2013;43(7):777-784.
15. Giedd JN, Rapoport JL, Garvey MA, et al. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157(2):281-283.
16. Chang K, Frankovich J, Cooperstock M, et al; PANS Collaborative Consortium. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2014;25(1):3-13.
17. Wallace GL, Robustelli B, Dankner N, et al. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136(pt 6):1956-1967.
18. Libero LE, DeRamus TP, Deshpande HD, et al. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 2014;62:1-10.
19. Boddaert N, Zilbovicius M, Philipe A, et al. MRI findings in 77 children with non-syndromic autistic disorder. PLoS One. 2009;4:e445. doi: 10.1371/journal.pone.0004415.
20. Richards C, Jones C, Groves L, et al. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2(10):909-916.
21. Wilde EA, Hunter JV, Bigler ED. Pediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. NeuroRehabilitation. 2012;31(3):245-260.
22. Mechtler LL, Shastri KK, Crutchfield KE. Advanced neuroimaging of mild traumatic brain injury. Neurol Clin. 2014;32(1):31-58.
23. Ashwal S, Tong KA, Ghosh N, et al. Application of advanced neuroimaging modalities in pediatric traumatic brain injury. J Child Neurol. 2014;29(12):1704-1717.
24. Munson S, Schroth E, Ernst M. The role of functional neuroimaging in pediatric brain injury. Pediatrics. 2006;117(4):1372-1381.
25. Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555-568.
Pseudobulbar affect: When patients laugh or cry, but don’t know why
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.
Pregnant nearly a year? The patient has symptoms but evidence is lacking
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.