User login
Candida Esophagitis Associated With Adalimumab for Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
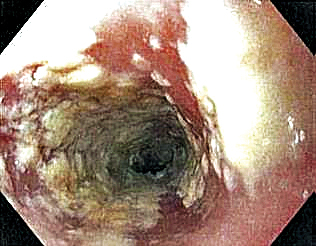
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
Practice Points
- Adalimumab is an effective treatment for patients with hidradenitis suppurativa.
- There is risk for opportunistic infections with adalimumab, and patients should be monitored closely.
Skin Tightening
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Practice Points
- There are a multitude of noninvasive modalities available to treat skin laxity.
- Understanding the mechanisms of each modality is crucial to selecting the appropriate treatment for your patients.
- Treatments should be tailored to the individual patient based on desired outcome, possible adverse events, patient preferences, and cost.
The Role of Vitamins and Supplements on Skin Appearance
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).
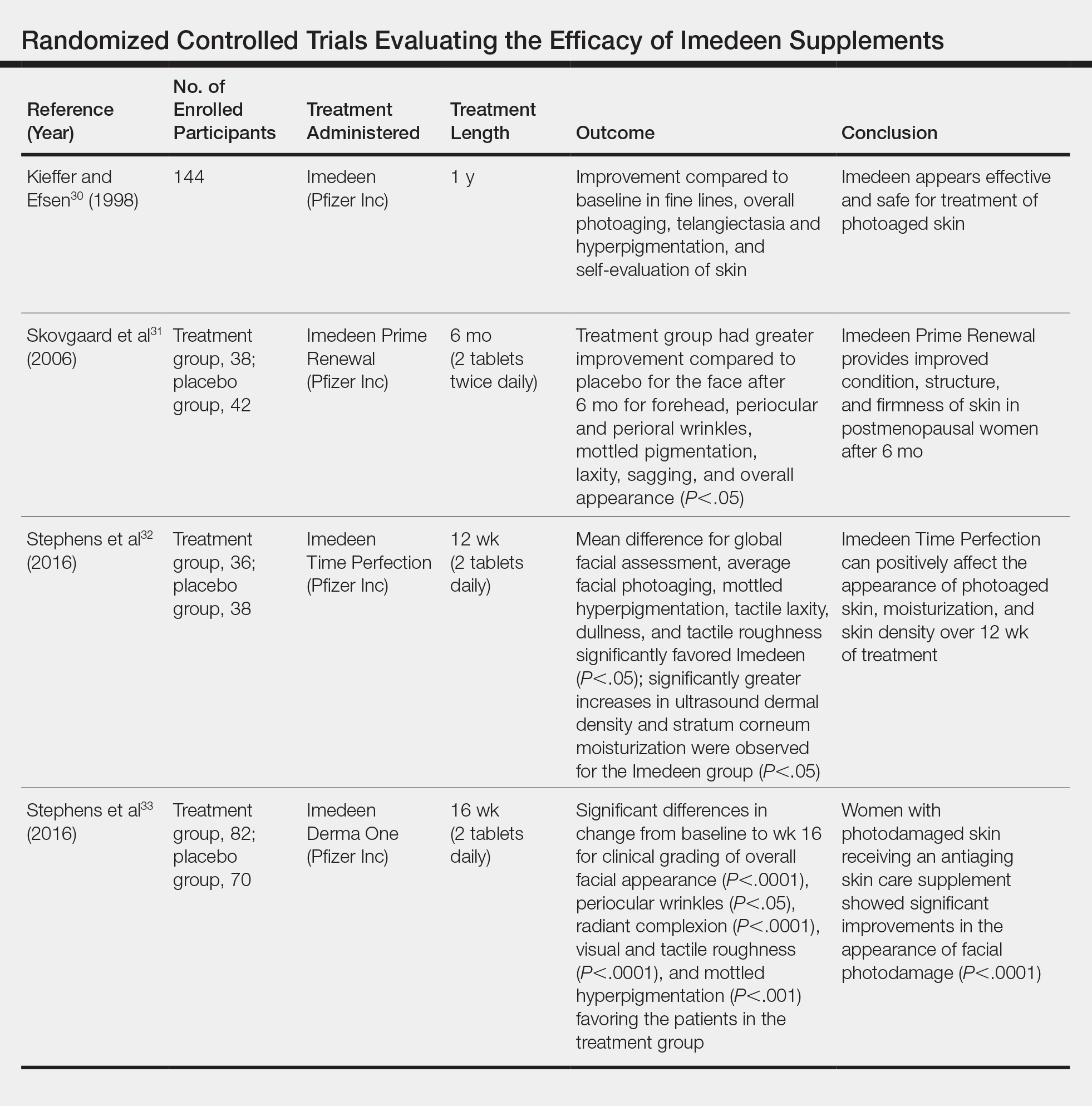
A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).

A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).

A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
Practice Points
- Multiple vitamins and supplements have demonstrated evidence in improving skin appearance.
- Carotenoids, along with vitamins C and E, have been shown to protect skin from UV-induced photodamage, while supplements containing collagen decrease the appearance of wrinkles.
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
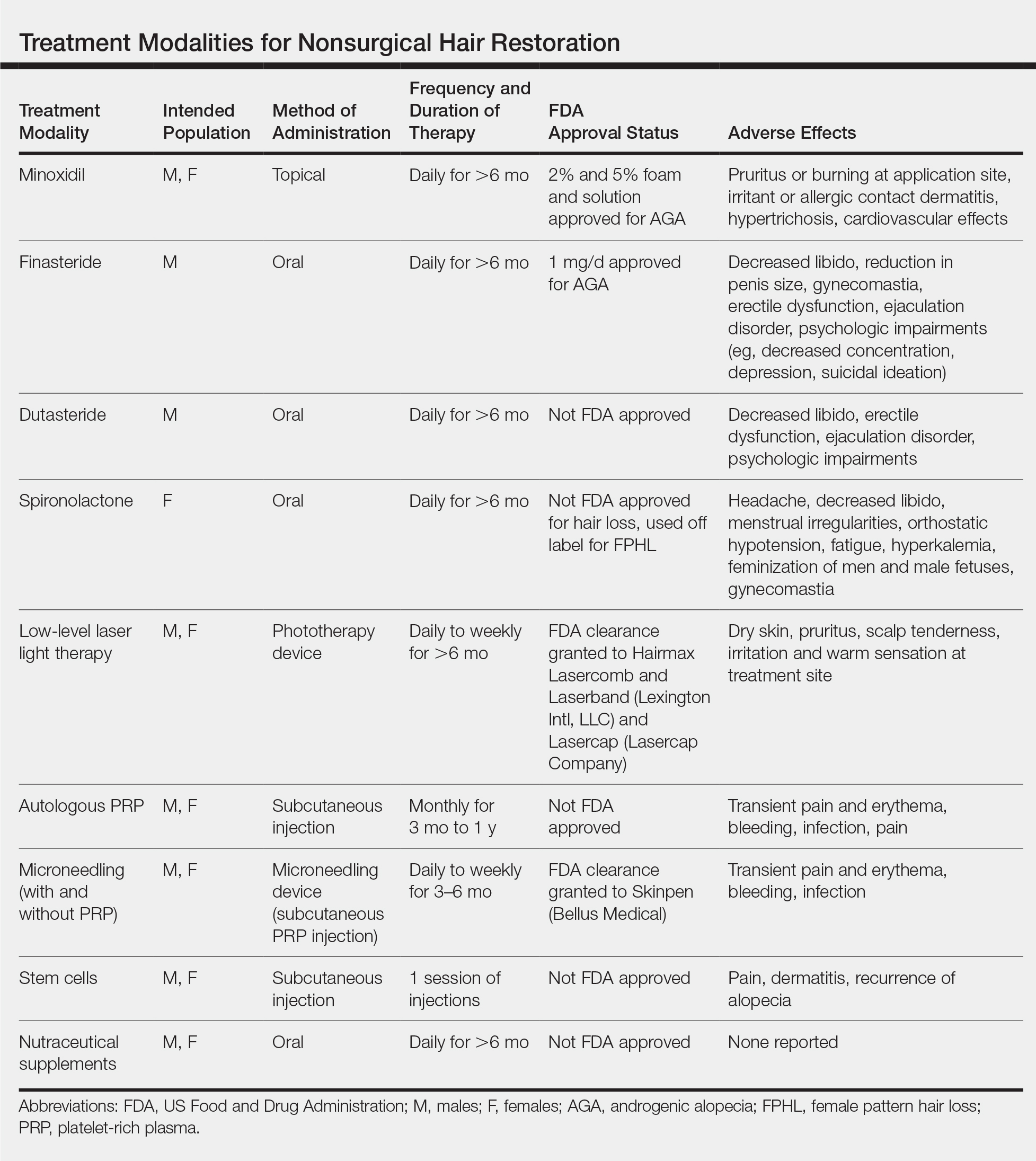
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
The Role of Diet in Preventing Photoaging and Treating Common Skin Conditions
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
Practice Points
- Growing evidence indicates that diet plays a role in overall skin health as well as the pathophysiology of several common cutaneous diseases.
- Broadly, we advocate for a low-glycemic diet that is rich in fruits and vegetables. In addition, dietary supplements of beta-carotene, collagen peptides, zinc, and fat-soluble vitamins (eg, vitamins D and E) have shown promising results in various conditions.
Noninvasive Vaginal Rejuvenation
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Practice Points
- Noninvasive vaginal rejuvenation represents a growing area of cosmetic dermatology.
- Radiofrequency and ablative laser devices have demonstrated promising results in treating vaginal laxity and genitourinary syndrome of menopause, but US Food and Drug Administration approval has yet to be obtained.
Update on Acne Scar Treatment
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26
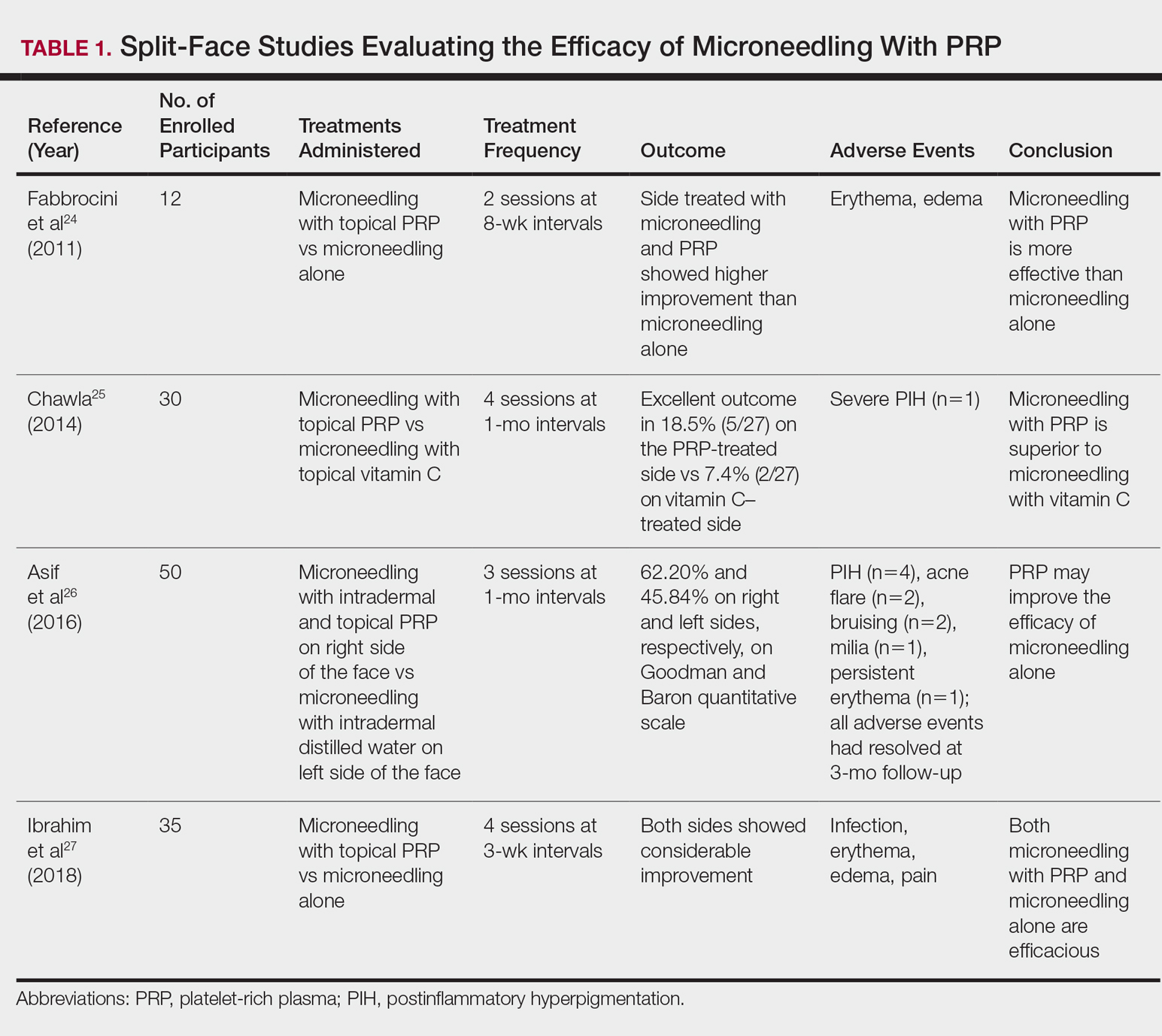
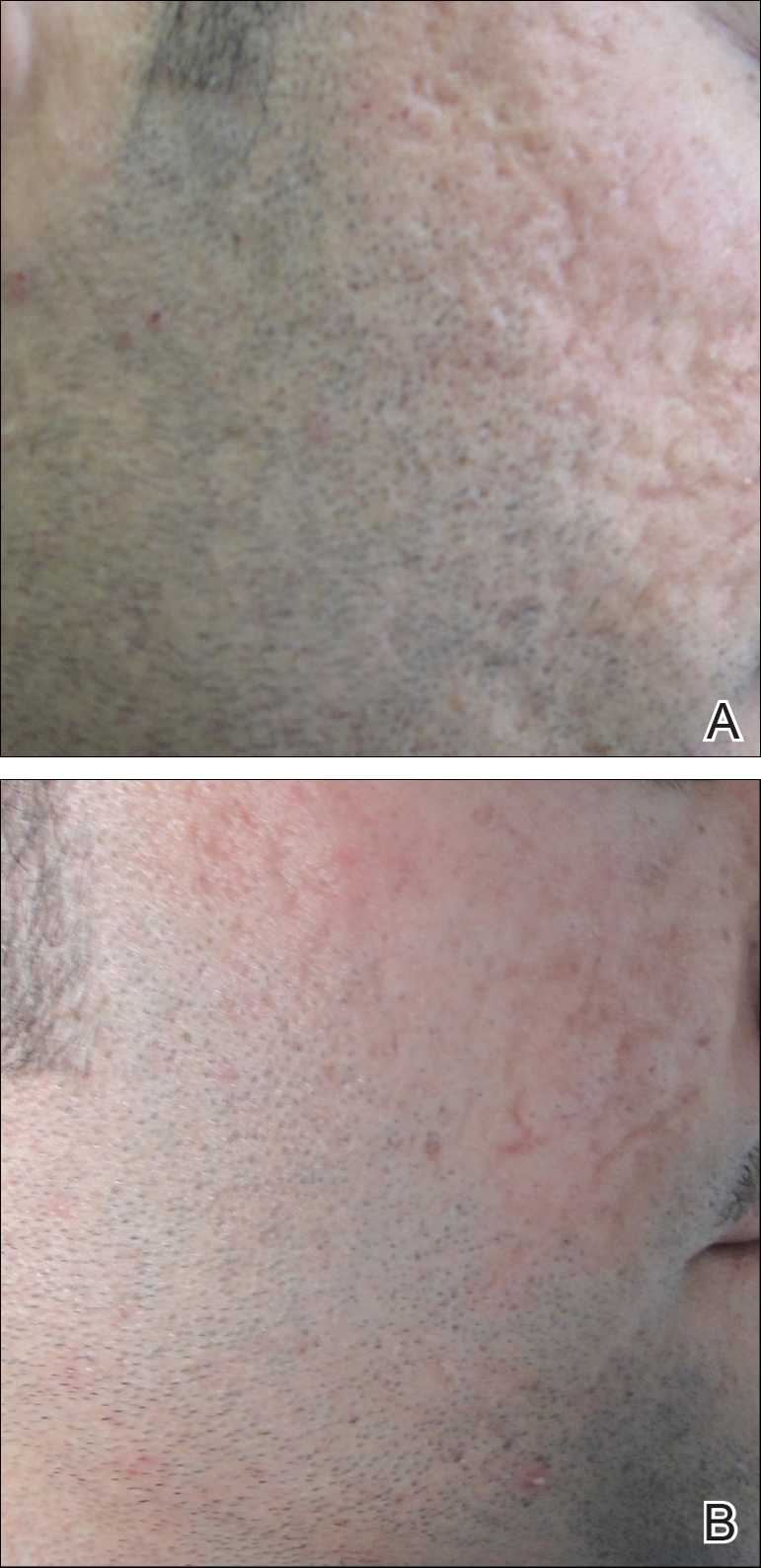
Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
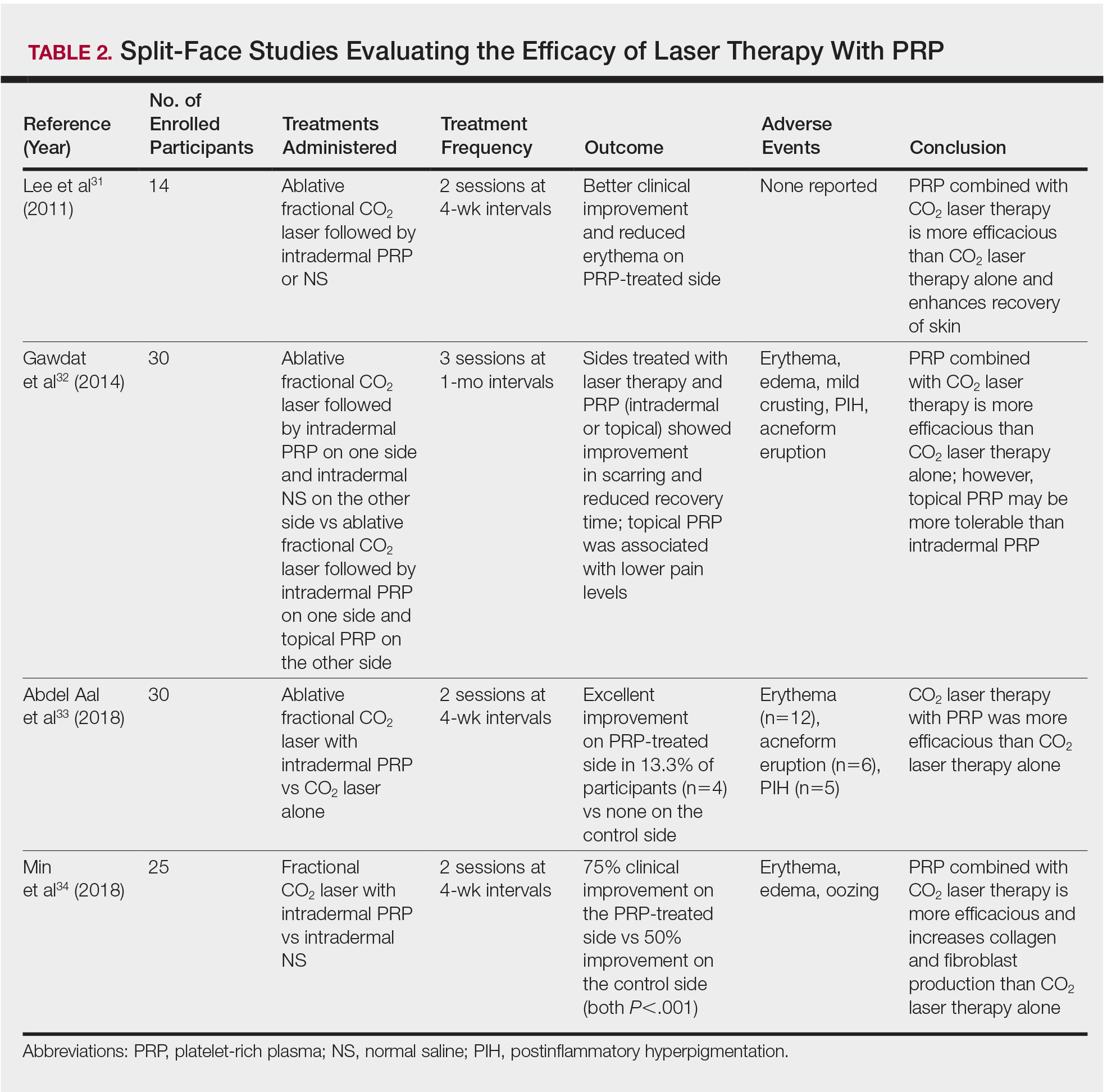
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Practice Points
- Acne scarring affects millions of Americans and can lead to poor psychological sequelae such as low self-esteem.
- Multiple modalities for acne scarring treatment exist including microneedling, lasers, chemical peels, and dermal fillers.
- Consider patient-desired outcome, cost, and adverse events when choosing a specific treatment modality.
Enhanced Melanoma Diagnosis With Multispectral Digital Skin Lesion Analysis
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
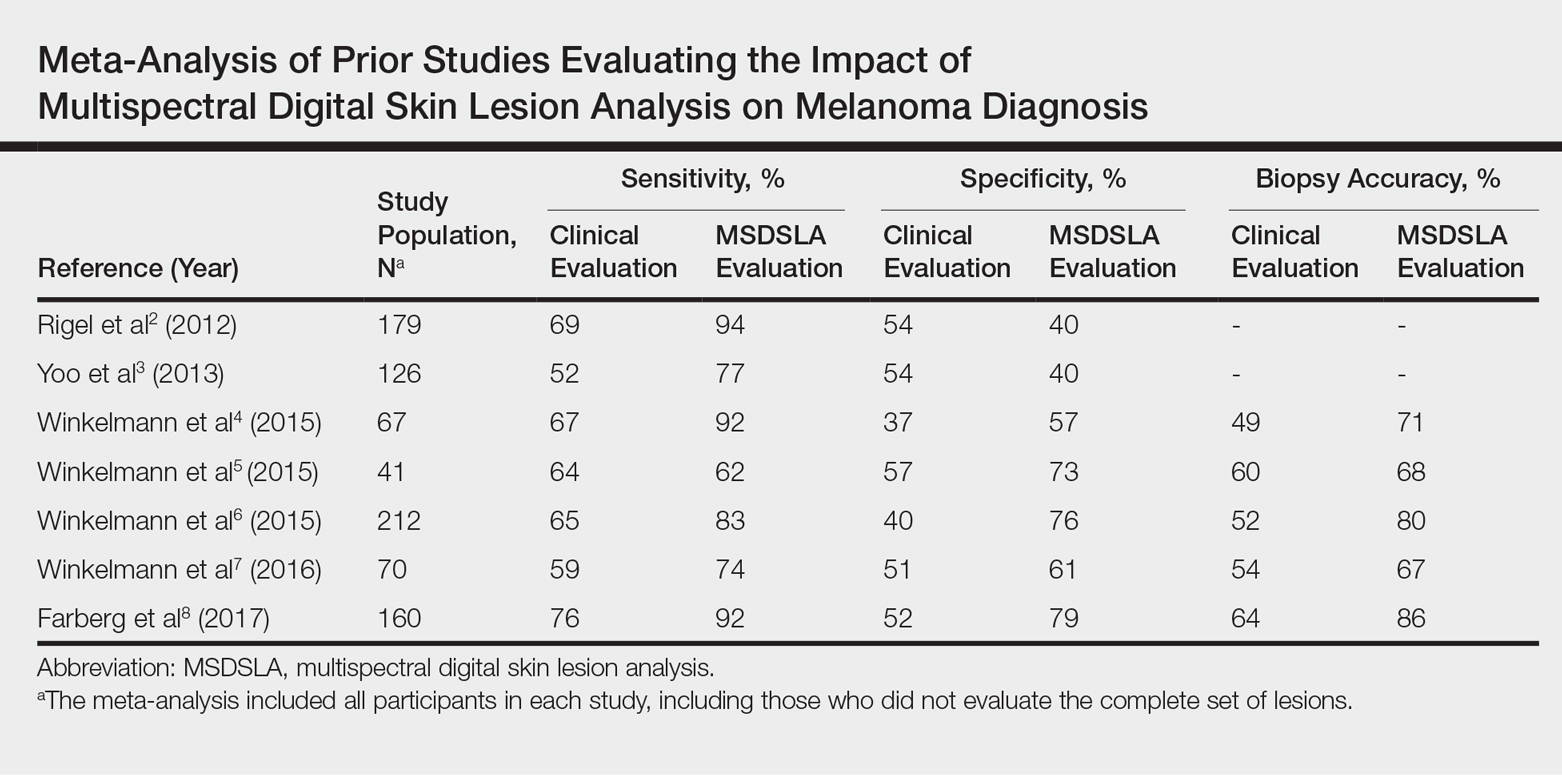
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
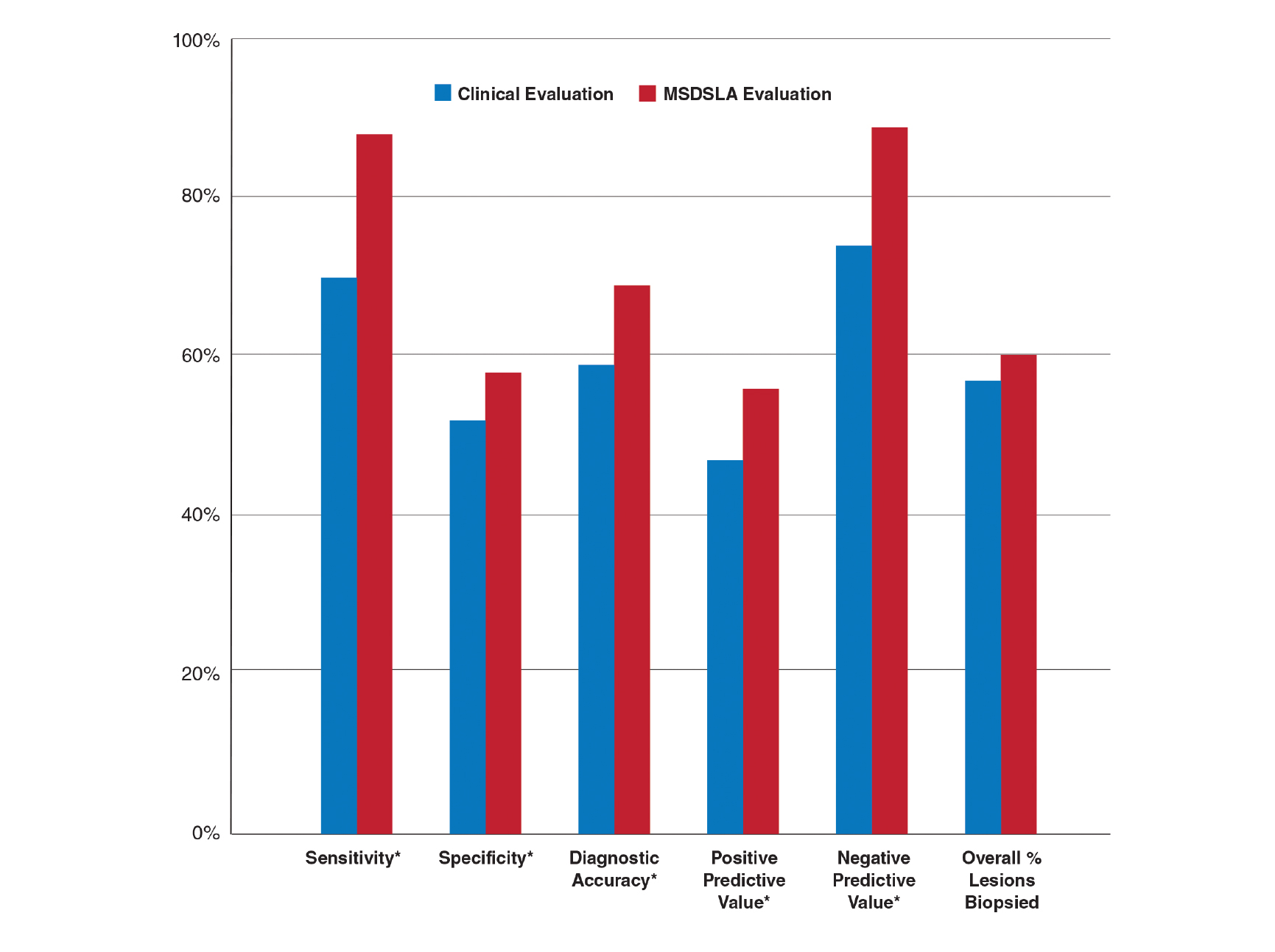
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Practice Points
- Multispectral digital skin lesion analysis (MSDSLA) can be a valuable tool in the evaluation of pigmented skin lesions (PSLs).
- MSDSLA may help to better identify high-risk PSLs and improve cost of care.
New Guidelines of Care for the Management of Nonmelanoma Skin Cancer
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
Update on Noninvasive Body Contouring Techniques
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.
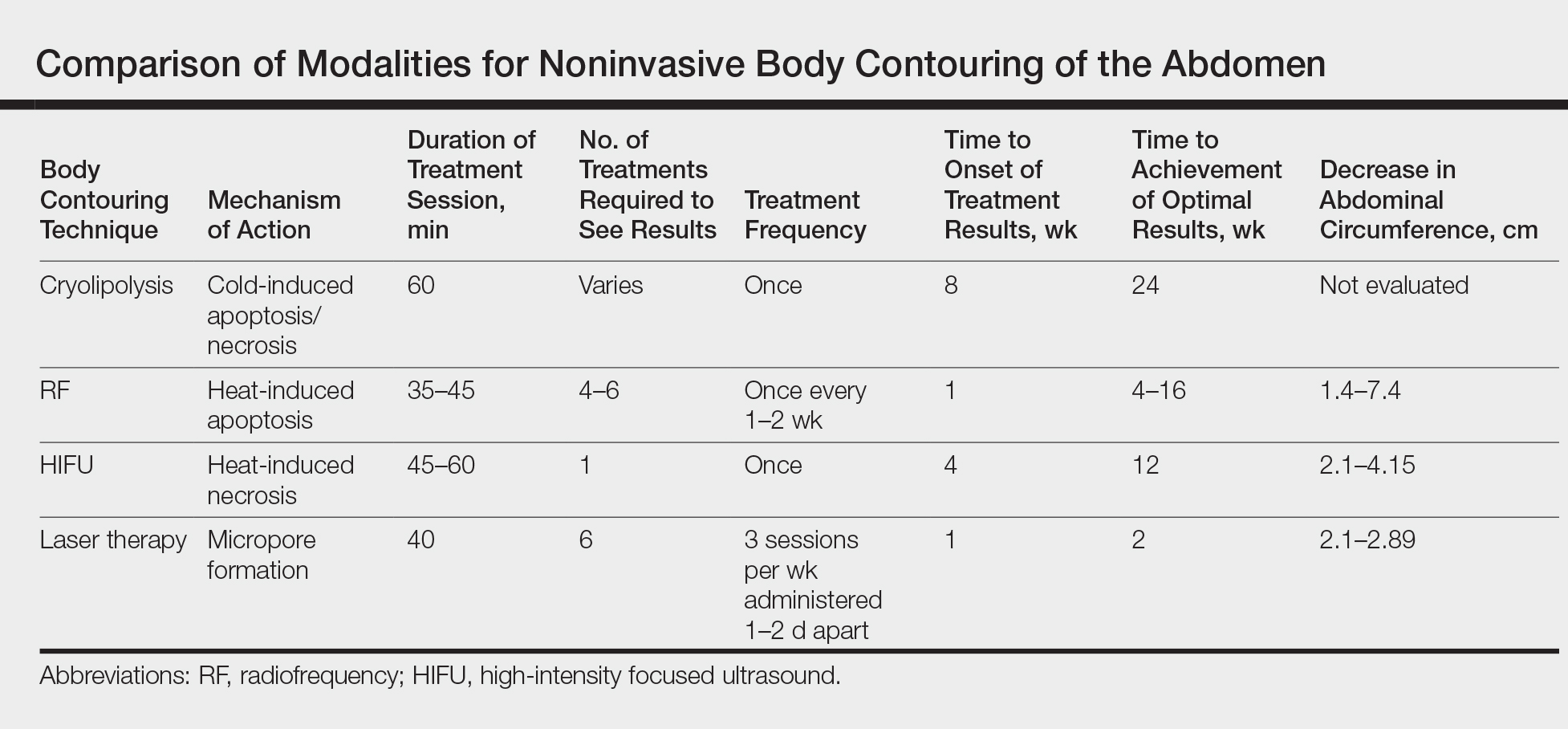
Cryolipolysis
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.

Cryolipolysis
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.

Cryolipolysis
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
Practice Points
- There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency, high-intensity focused ultrasound, and laser therapy.
- Devices utilizing these 4 modalities have been found to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.
- Dermatologists utilizing body contouring treatments need to be familiar with available devices to determine which treatment is appropriate for each patient.