User login
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
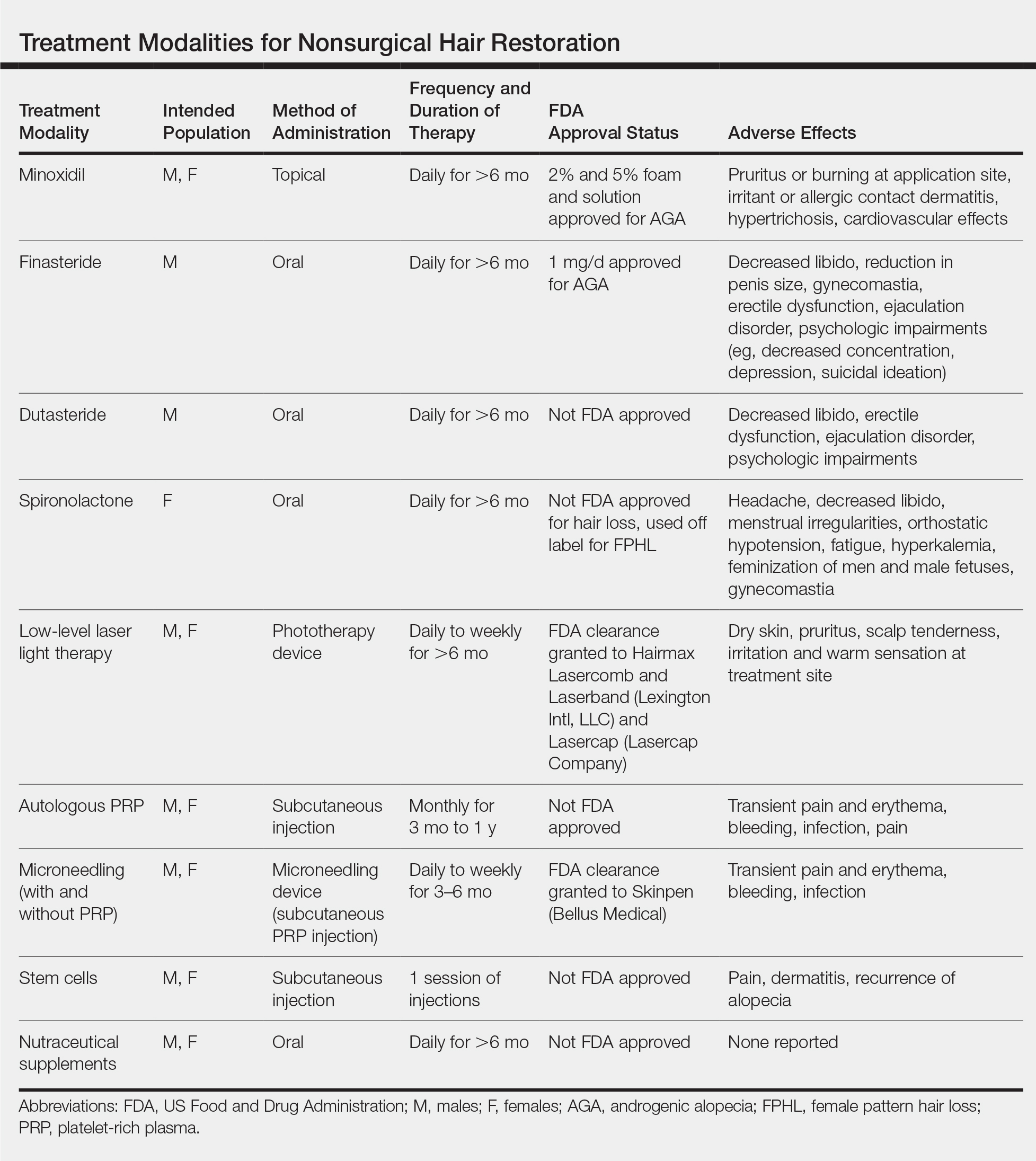
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
