User login
Pimavanserin for psychosis in patients with Parkinson’s disease
Pimavanserin is a potent 5-HT2A inverse agonist and 5-HT2C inverse agonist, with 5-fold greater affinity for the 5-HT2A receptor.1 Although antagonists block agonist actions at the receptor site, inverse agonists reduce the level of baseline constitutive activity seen in many G protein-coupled receptors. This medication is FDA approved for treating hallucinations and delusions associated with Parkinson’s disease (PD) psychosis (Table 1).1
In the pivotal 6-week clinical trial, pimavanserin significantly reduced positive symptoms seen in PD patients with psychosis (effect size = 0.50), with no evident impairment of motor function.2 Only 2 adverse effects occurred in ≥5% of pimavanserin-treated patients and at ≥2 times the rate of placebo: peripheral edema (7% vs 3% for placebo) and confusion (6% vs 3% for placebo). There was a mean increase in the QTc of 7.3 milliseconds compared with placebo in the pivotal phase III study.
Clinical implications
Despite numerous developments in the pharmacotherapeutics of psychotic disorders, patients with psychosis related to PD previously responded in a robust manner to only 1 antipsychotic, low-dosage clozapine (mean effect size, 0.80),2 with numerous failed trials for other atypical antipsychotics, including quetiapine.3,4 The pathophysiology of psychosis in PD patients is not related to dopamine agonist treatment, but is caused by the accumulation of cortical Lewy body burden, which results in loss of serotonergic signaling from dorsal raphe neurons. The net effect is up-regulation of postsynaptic 5-HT2A receptors.5 Psychosis is the most common cause of nursing home placement among PD patients without dementia.6
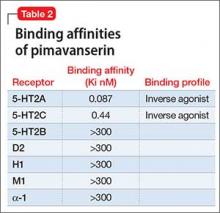
Receptor blocking. Based on the finding that clozapine in low dosages acts at 5-HT2A receptors,7 pimavanserin was designed to be a potent 5-HT2A inverse agonist, with more than 5-fold higher selectivity over 5-HT2C receptors, and no appreciable affinity for other serotonergic, adrenergic, dopaminergic, muscarinic, or histaminergic receptors8 (Table 2). The concept that 5-HT2A receptor stimulation can cause psychosis with prominent visual hallucinations is known from studies of LSD and other hallucinogenic compounds whose activity is blocked by 5-HT2A antagonists.
As an agent devoid of dopamine D2 antagonism, pimavanserin carries no risk of exacerbating motor symptoms, which was commonly seen with most atypical antipsychotics studied for psychosis in PD patients, except for clozapine and quetiapine.3 Although quetiapine did not cause motor effects, it proved ineffective in multiple studies (n = 153), likely because of the near absence of potent 5-HT2A binding.4
Pimavanserin also lacks:
- the hematologic monitoring requirement of clozapine
- clozapine’s risks of sedation, orthostasis, and anticholinergic and metabolic adverse effects.
Pimavanserin is significantly more potent than other non-antipsychotic psychotropics at the 5-HT2Areceptor, including doxepin (26 nM), trazodone (36 nM), and mirtazapine (60 nM).
Use in psychosis associated with PD. Recommended dosage is 34 mg once daily without titration (with or without food), based on results from a phase III clinical trial2 (because of the FDA breakthrough therapy designation for this compound, only 1 phase III trial was required). Pimavanserin produced significant improvement on the PD-adapted Scale for the Assessment of Positive Symptoms (SAPS-PD), a 9-item instrument extracted from the larger SAPS used in schizophrenia research. Specifically, pimavanserin was effective for both the hallucinations and delusions components of the SAPS-PD.
Pharmacologic profile, adverse effects. Pimavanserin lacks affinity for receptors other than 5-HT2A and 5-HT2C, leading to an absence of significant anticholinergic effects, orthostasis, or sedation in clinical trials.2 In all short-term clinical trials, the only common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were peripheral edema (7% vs 2% placebo) and confusional state (6% vs 3% placebo).2 More than 300 patients have been treated for >6 months, >270 have been treated for at least 12 months, and >150 have been treated for at least 24 months with no adverse effects other than those seen in the short-term trials.1
There is a measurable impact on cardiac conduction seen in phase III data and in the thorough QT study. In the thorough QT study, 252 healthy participants received multiple dosages in a randomized, double-blind manner with positive controls.1 The maximum mean change from baseline was 13.5 milliseconds at dosages twice the recommended dosage, and the upper limit of the 90% CI was only slightly greater at 16.6 milliseconds. Subsequent kinetic analyses suggested concentration-dependent QTc interval prolongation in the therapeutic range, with a recommendation to halve the daily dosage in patients taking potent cytochrome P450 (CYP) 3A4 inhibitors.
In the 6-week, placebo-controlled effectiveness studies, mean increases in QTc interval were in the range of 5 to 8 milliseconds. There were sporadic reports of QTcF values ≥500 milliseconds, or changes from baseline QTc values ≥60 milliseconds in pimavanserin-treated participants, although the incidence generally was the same for pimavanserin and placebo groups. There were no reports of torsades de pointes or any differences from placebo in the incidence of adverse reactions associated with delayed ventricular repolarization.
How it works
The theory behind development of pimavanserin rests in the finding that low-dosage clozapine (6.25 to 50 mg/d) was effective for PD patients with psychosis (effect size 0.80).8 Although clozapine has high affinity for multiple sites, including histamine H1 receptors (Ki = 1.13 nM), α-1A and a α-2C adrenergic receptors (Ki = 1.62 nM and 6 nM, respectively), 5-HT2A receptors (Ki = 5.35 nM), and muscarinic M1 receptors (Ki = 6 nM), the hypothesized primary mechanism of clozapine’s effectiveness for PD psychosis at low dosages focused on the 5-HT2Areceptor. This idea was based on the knowledge that hallucinogens such as mescaline, psilocybin, and LSD are 5-HT2A agonists.9 This hallucinogenic activity can be blocked with 5-HT2A antagonists. Because of pimavanserin’s binding profile, the compound was studied as a treatment for psychosis in PD patients.
Pharmacokinetics
Pimavanserin demonstrates dose-proportional pharmacokinetics after a single oral dose as much as 7.5 times the recommended dosage. The pharmacokinetics of pimavanserin were similar in study participants (mean age, 72.4) and healthy controls, and a high-fat meal had no impact on the maximum blood levels (Cmax) or total drug exposure (area under the curve [AUC]).
The mean plasma half-lives for pimavanserin and its metabolite N-desmethyl-pimavanserin (AC-279) are 57 hours and 200 hours, respectively. Although the metabolite appears active in in vitro assays, it does not cross the blood-brain barrier to any appreciable extent, therefore contributing little to the clinical effect. The median time to maximum concentration (Tmax) of pimavanserin is 6 hours with a range of 4 to 24 hours, while the median Tmax of the primary metabolite AC-279 is 6 hours. The bioavailability of pimavanserin in an oral tablet or solution essentially is identical.
Pimavanserin is primarily metabolized via CYP3A4 to AC-279, and strong CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin, indinavir) increase pimavanserin Cmax by 1.5-fold, and AUC by 3-fold. In patients taking strong CYP3A4 inhibitors, the dosage of pimavanserin should be reduced by 50% to 17 mg/d. Conversely, patients on CYP3A4 inducers (eg, rifampin, carbamazepine, phenytoin) should be monitored for lack of efficacy; consider a dosage increase as necessary. Neither pimavanserin nor its metabolite, AC-279, are inhibitors or inducers of major CYP enzymes or drug transporters.
Efficacy in PD psychosis
Study 1. This 6-week, fixed dosage, double-blind, placebo-controlled trial was performed in adult PD patients age ≥40 with PD psychosis.2 Participants had to have (1) a PD diagnosis for at least 1 year and (2) psychotic symptoms that developed after diagnosis. Psychotic symptoms had to be present for at least 1 month, occurring at least weekly in the month before screening, and severe enough to warrant antipsychotic treatment. Baseline Mini-Mental State Examination score had to be ≥21 out of 30, with no evidence of delirium. Patients with dementia preceding or concurrent with the PD diagnosis were excluded. Antipsychotic treatments were not permitted during the trial.
After a 2-week nonpharmacotherapeutic lead-in phase that included a brief, daily psychosocial intervention by a caregiver, 199 patients who still met severity criteria were randomly allocated in a 1:1 manner to pimavanserin (34 mg of active drug, reported in the paper as 40 mg of pimavanserin tartrate) or matched placebo. Based on kinetic modeling and earlier clinical data, lower dosages (ie, 17 mg) were not explored, because they achieved only 50% of the steady state plasma levels thought to be required for efficacy.
The primary outcome was assessed by central, independent raters using the PD-adapted SAPS-PD. The efficacy analysis included 95 pimavanserin-treated individuals and 90 taking placebo. Baseline SAPS-PD scores were 14.7 ± 5.55 in the placebo group, and 15.9 ± 6.12 in the pimavanserin arm. Participants had a mean age of 72.4 and 94% white ethnicity across both cohorts; 42% of the placebo group and 33% of the pimavanserin group were female. Antipsychotic exposure in the 21 days prior to study entry were reported in 17% (n = 15) and 19% (n = 18) of the placebo and pimavanserin groups, respectively, with the most common agent being quetiapine (13 of 15, placebo, 16 of 18, pimavanserin). Approximately one-third of all participants were taking a cholinesterase inhibitor throughout the study.
Efficacy outcome. Pimavanserin was associated with a 5.79-point decrease in SAPS-PD scores compared with 2.73-point decrease for placebo (difference −3.06, 95% CI −4.91 to −1.20; P = .001). The effect size for this difference (Cohen’s d) was 0.50. The significant effect of pimavanserin vs placebo also was seen in separate analyses of the SAPS-PD subscore for hallucinations and delusions (effect size 0.50), and individually for hallucinations (effect size 0.45) and delusions (effect size 0.33). Separation from placebo appeared after the second week of pimavanserin treatment, and continued through the end of the study. There is unpublished data showing efficacy through week 10, and longer term, uncontrolled data consistent with sustained response. An exploratory analysis of caregiver burden demonstrated an effect size of 0.50.
Tolerability
The discontinuation rate because of adverse events for pimavanserin and placebo-treated patients was 10 patients in the pimavanserin group (4 due to psychotic symptoms within 10 days of starting the study drug) compared with 2 in the placebo group. There was no evidence of motor worsening in either group, demonstrated by the score on part II of the Unified Parkinson’s Disease Rating Scale (UPDRS) that captures self-reported activities of daily living, or on UPDRS part III (motor examination). Pimavanserin has no contraindications.
Unique clinical issues
Binding properties. Pimavanserin possesses potent 5-HT2A inverse agonist properties required to manage psychosis in PD patients, but lacks clozapine’s affinities for α-1 adrenergic, muscarinic, or histaminergic receptors that contribute to clozapine’s poor tolerability. Moreover, pimavanserin has no appreciable affinity for dopaminergic receptors, and therefore does not induce motor adverse effects.
Clozapine aside, all available atypical antipsychotics have proved ineffective for psychosis in PD patients, and most caused significant motor worsening.3 Although quetiapine does not cause motor effects, it has been shown to be ineffective for psychosis in PD patients in multiple trials.4
The effect size for clozapine response is large (0.80) in PD patients with psychosis, but tolerability issues and administrative burdens regarding patient and prescriber registration and routine hematological monitoring pose significant clinical barriers. Clozapine also lacks an FDA indication for this purpose, which may pose a hurdle to its use in certain treatment settings.
Why Rx? The reasons to prescribe pimavanserin for PD patients with psychosis likely include:
- absence of tolerability issues seen with the only other effective agent, clozapine
- lack of motor effects
- lack of administrative and monitoring burden related to clozapine prescribing
- only agent with FDA approval for hallucinations and delusions in PD patients with psychosis.
Dosing
The recommended dosage of pimavanserin is 34 mg/d administered as a single dose with or without food. There is no need for titration, and none was performed in the pivotal clinical trial. Given the long half-life (57 hours), steady state is not achieved until day 12, therefore initiation with a lower dosage might prolong the time to efficacy. There is no dosage adjustment required in patients with mild or moderate renal impairment, but pimavanserin treatment is not recommended in patients with severe renal impairment. Pimavanserin has not been evaluated in patients with hepatic impairment (using Child-Pugh criteria), and is not recommended for these patients.
Other key aspects of dosing to keep in mind.
- Because pimavanserin is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a strong CYP3A4 inhibitor; the recommended dosage is 17 mg/d when administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of pimavanserin with CYP3A4 inducers, patients should be monitored for lack of efficacy during concomitant use with a CYP3A4 inducer, and consideration given to a dosage increase.
Use in pregnancy and lactation. There are no data on the use of pimavanserin in pregnant women, but no developmental effects were seen when the drug was administered orally at 10 or 12 times the maximum recommended human dosage to rats or rabbits during organogenesis. Pimavanserin was not teratogenic in pregnant rats and rabbits. There is no information regarding the presence of pimavanserin in human breast milk.
Geriatric patients. No dosage adjustment is required for older patients. The study population in the pivotal trial was mean age 72.4 years.
Summing up
Before development of pimavanserin, clozapine was the only effective treatment for psychosis in PD patients. Despite clozapine’s robust effects across several trials, patients often were given ineffective medications, such as quetiapine, because of the administrative and tolerability barriers posed by clozapine use. Because psychosis is the most common cause of nursing home placement in non-demented PD patients, an agent with demonstrated efficacy and without the adverse effect profile of clozapine or monitoring requirements represents an enormous advance in the treatment of psychosis in PD patients.
1. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; 2016.
2. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. [Erratum in Lancet. 2014;384(9937):28]. Lancet. 2014;383(9916):533-540.
3. Borek LL, Friedman JH. Treating psychosis in movement disorder patients: a review. Expert Opin Pharmacother. 2014;15(11):1553-1564.
4. Desmarais P, Massoud F, Filion J, et al. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol. 2016;29(4):227-236.
5. Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67(4):416-421.
6. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061-1068.
7. Nordström AL, Farde L, Nyberg S, et al. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152(10):1444-1449.
8. Hacksell U, Burstein ES, McFarland K, et al. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res. 2014;39(10):2008-2017.
9. Moreno JL, Holloway T, Albizu L, et al. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493(3):76-79.
Pimavanserin is a potent 5-HT2A inverse agonist and 5-HT2C inverse agonist, with 5-fold greater affinity for the 5-HT2A receptor.1 Although antagonists block agonist actions at the receptor site, inverse agonists reduce the level of baseline constitutive activity seen in many G protein-coupled receptors. This medication is FDA approved for treating hallucinations and delusions associated with Parkinson’s disease (PD) psychosis (Table 1).1
In the pivotal 6-week clinical trial, pimavanserin significantly reduced positive symptoms seen in PD patients with psychosis (effect size = 0.50), with no evident impairment of motor function.2 Only 2 adverse effects occurred in ≥5% of pimavanserin-treated patients and at ≥2 times the rate of placebo: peripheral edema (7% vs 3% for placebo) and confusion (6% vs 3% for placebo). There was a mean increase in the QTc of 7.3 milliseconds compared with placebo in the pivotal phase III study.
Clinical implications
Despite numerous developments in the pharmacotherapeutics of psychotic disorders, patients with psychosis related to PD previously responded in a robust manner to only 1 antipsychotic, low-dosage clozapine (mean effect size, 0.80),2 with numerous failed trials for other atypical antipsychotics, including quetiapine.3,4 The pathophysiology of psychosis in PD patients is not related to dopamine agonist treatment, but is caused by the accumulation of cortical Lewy body burden, which results in loss of serotonergic signaling from dorsal raphe neurons. The net effect is up-regulation of postsynaptic 5-HT2A receptors.5 Psychosis is the most common cause of nursing home placement among PD patients without dementia.6
Receptor blocking. Based on the finding that clozapine in low dosages acts at 5-HT2A receptors,7 pimavanserin was designed to be a potent 5-HT2A inverse agonist, with more than 5-fold higher selectivity over 5-HT2C receptors, and no appreciable affinity for other serotonergic, adrenergic, dopaminergic, muscarinic, or histaminergic receptors8 (Table 2). The concept that 5-HT2A receptor stimulation can cause psychosis with prominent visual hallucinations is known from studies of LSD and other hallucinogenic compounds whose activity is blocked by 5-HT2A antagonists.
As an agent devoid of dopamine D2 antagonism, pimavanserin carries no risk of exacerbating motor symptoms, which was commonly seen with most atypical antipsychotics studied for psychosis in PD patients, except for clozapine and quetiapine.3 Although quetiapine did not cause motor effects, it proved ineffective in multiple studies (n = 153), likely because of the near absence of potent 5-HT2A binding.4
Pimavanserin also lacks:
- the hematologic monitoring requirement of clozapine
- clozapine’s risks of sedation, orthostasis, and anticholinergic and metabolic adverse effects.
Pimavanserin is significantly more potent than other non-antipsychotic psychotropics at the 5-HT2Areceptor, including doxepin (26 nM), trazodone (36 nM), and mirtazapine (60 nM).
Use in psychosis associated with PD. Recommended dosage is 34 mg once daily without titration (with or without food), based on results from a phase III clinical trial2 (because of the FDA breakthrough therapy designation for this compound, only 1 phase III trial was required). Pimavanserin produced significant improvement on the PD-adapted Scale for the Assessment of Positive Symptoms (SAPS-PD), a 9-item instrument extracted from the larger SAPS used in schizophrenia research. Specifically, pimavanserin was effective for both the hallucinations and delusions components of the SAPS-PD.
Pharmacologic profile, adverse effects. Pimavanserin lacks affinity for receptors other than 5-HT2A and 5-HT2C, leading to an absence of significant anticholinergic effects, orthostasis, or sedation in clinical trials.2 In all short-term clinical trials, the only common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were peripheral edema (7% vs 2% placebo) and confusional state (6% vs 3% placebo).2 More than 300 patients have been treated for >6 months, >270 have been treated for at least 12 months, and >150 have been treated for at least 24 months with no adverse effects other than those seen in the short-term trials.1
There is a measurable impact on cardiac conduction seen in phase III data and in the thorough QT study. In the thorough QT study, 252 healthy participants received multiple dosages in a randomized, double-blind manner with positive controls.1 The maximum mean change from baseline was 13.5 milliseconds at dosages twice the recommended dosage, and the upper limit of the 90% CI was only slightly greater at 16.6 milliseconds. Subsequent kinetic analyses suggested concentration-dependent QTc interval prolongation in the therapeutic range, with a recommendation to halve the daily dosage in patients taking potent cytochrome P450 (CYP) 3A4 inhibitors.
In the 6-week, placebo-controlled effectiveness studies, mean increases in QTc interval were in the range of 5 to 8 milliseconds. There were sporadic reports of QTcF values ≥500 milliseconds, or changes from baseline QTc values ≥60 milliseconds in pimavanserin-treated participants, although the incidence generally was the same for pimavanserin and placebo groups. There were no reports of torsades de pointes or any differences from placebo in the incidence of adverse reactions associated with delayed ventricular repolarization.
How it works
The theory behind development of pimavanserin rests in the finding that low-dosage clozapine (6.25 to 50 mg/d) was effective for PD patients with psychosis (effect size 0.80).8 Although clozapine has high affinity for multiple sites, including histamine H1 receptors (Ki = 1.13 nM), α-1A and a α-2C adrenergic receptors (Ki = 1.62 nM and 6 nM, respectively), 5-HT2A receptors (Ki = 5.35 nM), and muscarinic M1 receptors (Ki = 6 nM), the hypothesized primary mechanism of clozapine’s effectiveness for PD psychosis at low dosages focused on the 5-HT2Areceptor. This idea was based on the knowledge that hallucinogens such as mescaline, psilocybin, and LSD are 5-HT2A agonists.9 This hallucinogenic activity can be blocked with 5-HT2A antagonists. Because of pimavanserin’s binding profile, the compound was studied as a treatment for psychosis in PD patients.
Pharmacokinetics
Pimavanserin demonstrates dose-proportional pharmacokinetics after a single oral dose as much as 7.5 times the recommended dosage. The pharmacokinetics of pimavanserin were similar in study participants (mean age, 72.4) and healthy controls, and a high-fat meal had no impact on the maximum blood levels (Cmax) or total drug exposure (area under the curve [AUC]).
The mean plasma half-lives for pimavanserin and its metabolite N-desmethyl-pimavanserin (AC-279) are 57 hours and 200 hours, respectively. Although the metabolite appears active in in vitro assays, it does not cross the blood-brain barrier to any appreciable extent, therefore contributing little to the clinical effect. The median time to maximum concentration (Tmax) of pimavanserin is 6 hours with a range of 4 to 24 hours, while the median Tmax of the primary metabolite AC-279 is 6 hours. The bioavailability of pimavanserin in an oral tablet or solution essentially is identical.
Pimavanserin is primarily metabolized via CYP3A4 to AC-279, and strong CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin, indinavir) increase pimavanserin Cmax by 1.5-fold, and AUC by 3-fold. In patients taking strong CYP3A4 inhibitors, the dosage of pimavanserin should be reduced by 50% to 17 mg/d. Conversely, patients on CYP3A4 inducers (eg, rifampin, carbamazepine, phenytoin) should be monitored for lack of efficacy; consider a dosage increase as necessary. Neither pimavanserin nor its metabolite, AC-279, are inhibitors or inducers of major CYP enzymes or drug transporters.
Efficacy in PD psychosis
Study 1. This 6-week, fixed dosage, double-blind, placebo-controlled trial was performed in adult PD patients age ≥40 with PD psychosis.2 Participants had to have (1) a PD diagnosis for at least 1 year and (2) psychotic symptoms that developed after diagnosis. Psychotic symptoms had to be present for at least 1 month, occurring at least weekly in the month before screening, and severe enough to warrant antipsychotic treatment. Baseline Mini-Mental State Examination score had to be ≥21 out of 30, with no evidence of delirium. Patients with dementia preceding or concurrent with the PD diagnosis were excluded. Antipsychotic treatments were not permitted during the trial.
After a 2-week nonpharmacotherapeutic lead-in phase that included a brief, daily psychosocial intervention by a caregiver, 199 patients who still met severity criteria were randomly allocated in a 1:1 manner to pimavanserin (34 mg of active drug, reported in the paper as 40 mg of pimavanserin tartrate) or matched placebo. Based on kinetic modeling and earlier clinical data, lower dosages (ie, 17 mg) were not explored, because they achieved only 50% of the steady state plasma levels thought to be required for efficacy.
The primary outcome was assessed by central, independent raters using the PD-adapted SAPS-PD. The efficacy analysis included 95 pimavanserin-treated individuals and 90 taking placebo. Baseline SAPS-PD scores were 14.7 ± 5.55 in the placebo group, and 15.9 ± 6.12 in the pimavanserin arm. Participants had a mean age of 72.4 and 94% white ethnicity across both cohorts; 42% of the placebo group and 33% of the pimavanserin group were female. Antipsychotic exposure in the 21 days prior to study entry were reported in 17% (n = 15) and 19% (n = 18) of the placebo and pimavanserin groups, respectively, with the most common agent being quetiapine (13 of 15, placebo, 16 of 18, pimavanserin). Approximately one-third of all participants were taking a cholinesterase inhibitor throughout the study.
Efficacy outcome. Pimavanserin was associated with a 5.79-point decrease in SAPS-PD scores compared with 2.73-point decrease for placebo (difference −3.06, 95% CI −4.91 to −1.20; P = .001). The effect size for this difference (Cohen’s d) was 0.50. The significant effect of pimavanserin vs placebo also was seen in separate analyses of the SAPS-PD subscore for hallucinations and delusions (effect size 0.50), and individually for hallucinations (effect size 0.45) and delusions (effect size 0.33). Separation from placebo appeared after the second week of pimavanserin treatment, and continued through the end of the study. There is unpublished data showing efficacy through week 10, and longer term, uncontrolled data consistent with sustained response. An exploratory analysis of caregiver burden demonstrated an effect size of 0.50.
Tolerability
The discontinuation rate because of adverse events for pimavanserin and placebo-treated patients was 10 patients in the pimavanserin group (4 due to psychotic symptoms within 10 days of starting the study drug) compared with 2 in the placebo group. There was no evidence of motor worsening in either group, demonstrated by the score on part II of the Unified Parkinson’s Disease Rating Scale (UPDRS) that captures self-reported activities of daily living, or on UPDRS part III (motor examination). Pimavanserin has no contraindications.
Unique clinical issues
Binding properties. Pimavanserin possesses potent 5-HT2A inverse agonist properties required to manage psychosis in PD patients, but lacks clozapine’s affinities for α-1 adrenergic, muscarinic, or histaminergic receptors that contribute to clozapine’s poor tolerability. Moreover, pimavanserin has no appreciable affinity for dopaminergic receptors, and therefore does not induce motor adverse effects.
Clozapine aside, all available atypical antipsychotics have proved ineffective for psychosis in PD patients, and most caused significant motor worsening.3 Although quetiapine does not cause motor effects, it has been shown to be ineffective for psychosis in PD patients in multiple trials.4
The effect size for clozapine response is large (0.80) in PD patients with psychosis, but tolerability issues and administrative burdens regarding patient and prescriber registration and routine hematological monitoring pose significant clinical barriers. Clozapine also lacks an FDA indication for this purpose, which may pose a hurdle to its use in certain treatment settings.
Why Rx? The reasons to prescribe pimavanserin for PD patients with psychosis likely include:
- absence of tolerability issues seen with the only other effective agent, clozapine
- lack of motor effects
- lack of administrative and monitoring burden related to clozapine prescribing
- only agent with FDA approval for hallucinations and delusions in PD patients with psychosis.
Dosing
The recommended dosage of pimavanserin is 34 mg/d administered as a single dose with or without food. There is no need for titration, and none was performed in the pivotal clinical trial. Given the long half-life (57 hours), steady state is not achieved until day 12, therefore initiation with a lower dosage might prolong the time to efficacy. There is no dosage adjustment required in patients with mild or moderate renal impairment, but pimavanserin treatment is not recommended in patients with severe renal impairment. Pimavanserin has not been evaluated in patients with hepatic impairment (using Child-Pugh criteria), and is not recommended for these patients.
Other key aspects of dosing to keep in mind.
- Because pimavanserin is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a strong CYP3A4 inhibitor; the recommended dosage is 17 mg/d when administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of pimavanserin with CYP3A4 inducers, patients should be monitored for lack of efficacy during concomitant use with a CYP3A4 inducer, and consideration given to a dosage increase.
Use in pregnancy and lactation. There are no data on the use of pimavanserin in pregnant women, but no developmental effects were seen when the drug was administered orally at 10 or 12 times the maximum recommended human dosage to rats or rabbits during organogenesis. Pimavanserin was not teratogenic in pregnant rats and rabbits. There is no information regarding the presence of pimavanserin in human breast milk.
Geriatric patients. No dosage adjustment is required for older patients. The study population in the pivotal trial was mean age 72.4 years.
Summing up
Before development of pimavanserin, clozapine was the only effective treatment for psychosis in PD patients. Despite clozapine’s robust effects across several trials, patients often were given ineffective medications, such as quetiapine, because of the administrative and tolerability barriers posed by clozapine use. Because psychosis is the most common cause of nursing home placement in non-demented PD patients, an agent with demonstrated efficacy and without the adverse effect profile of clozapine or monitoring requirements represents an enormous advance in the treatment of psychosis in PD patients.
Pimavanserin is a potent 5-HT2A inverse agonist and 5-HT2C inverse agonist, with 5-fold greater affinity for the 5-HT2A receptor.1 Although antagonists block agonist actions at the receptor site, inverse agonists reduce the level of baseline constitutive activity seen in many G protein-coupled receptors. This medication is FDA approved for treating hallucinations and delusions associated with Parkinson’s disease (PD) psychosis (Table 1).1
In the pivotal 6-week clinical trial, pimavanserin significantly reduced positive symptoms seen in PD patients with psychosis (effect size = 0.50), with no evident impairment of motor function.2 Only 2 adverse effects occurred in ≥5% of pimavanserin-treated patients and at ≥2 times the rate of placebo: peripheral edema (7% vs 3% for placebo) and confusion (6% vs 3% for placebo). There was a mean increase in the QTc of 7.3 milliseconds compared with placebo in the pivotal phase III study.
Clinical implications
Despite numerous developments in the pharmacotherapeutics of psychotic disorders, patients with psychosis related to PD previously responded in a robust manner to only 1 antipsychotic, low-dosage clozapine (mean effect size, 0.80),2 with numerous failed trials for other atypical antipsychotics, including quetiapine.3,4 The pathophysiology of psychosis in PD patients is not related to dopamine agonist treatment, but is caused by the accumulation of cortical Lewy body burden, which results in loss of serotonergic signaling from dorsal raphe neurons. The net effect is up-regulation of postsynaptic 5-HT2A receptors.5 Psychosis is the most common cause of nursing home placement among PD patients without dementia.6
Receptor blocking. Based on the finding that clozapine in low dosages acts at 5-HT2A receptors,7 pimavanserin was designed to be a potent 5-HT2A inverse agonist, with more than 5-fold higher selectivity over 5-HT2C receptors, and no appreciable affinity for other serotonergic, adrenergic, dopaminergic, muscarinic, or histaminergic receptors8 (Table 2). The concept that 5-HT2A receptor stimulation can cause psychosis with prominent visual hallucinations is known from studies of LSD and other hallucinogenic compounds whose activity is blocked by 5-HT2A antagonists.
As an agent devoid of dopamine D2 antagonism, pimavanserin carries no risk of exacerbating motor symptoms, which was commonly seen with most atypical antipsychotics studied for psychosis in PD patients, except for clozapine and quetiapine.3 Although quetiapine did not cause motor effects, it proved ineffective in multiple studies (n = 153), likely because of the near absence of potent 5-HT2A binding.4
Pimavanserin also lacks:
- the hematologic monitoring requirement of clozapine
- clozapine’s risks of sedation, orthostasis, and anticholinergic and metabolic adverse effects.
Pimavanserin is significantly more potent than other non-antipsychotic psychotropics at the 5-HT2Areceptor, including doxepin (26 nM), trazodone (36 nM), and mirtazapine (60 nM).
Use in psychosis associated with PD. Recommended dosage is 34 mg once daily without titration (with or without food), based on results from a phase III clinical trial2 (because of the FDA breakthrough therapy designation for this compound, only 1 phase III trial was required). Pimavanserin produced significant improvement on the PD-adapted Scale for the Assessment of Positive Symptoms (SAPS-PD), a 9-item instrument extracted from the larger SAPS used in schizophrenia research. Specifically, pimavanserin was effective for both the hallucinations and delusions components of the SAPS-PD.
Pharmacologic profile, adverse effects. Pimavanserin lacks affinity for receptors other than 5-HT2A and 5-HT2C, leading to an absence of significant anticholinergic effects, orthostasis, or sedation in clinical trials.2 In all short-term clinical trials, the only common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were peripheral edema (7% vs 2% placebo) and confusional state (6% vs 3% placebo).2 More than 300 patients have been treated for >6 months, >270 have been treated for at least 12 months, and >150 have been treated for at least 24 months with no adverse effects other than those seen in the short-term trials.1
There is a measurable impact on cardiac conduction seen in phase III data and in the thorough QT study. In the thorough QT study, 252 healthy participants received multiple dosages in a randomized, double-blind manner with positive controls.1 The maximum mean change from baseline was 13.5 milliseconds at dosages twice the recommended dosage, and the upper limit of the 90% CI was only slightly greater at 16.6 milliseconds. Subsequent kinetic analyses suggested concentration-dependent QTc interval prolongation in the therapeutic range, with a recommendation to halve the daily dosage in patients taking potent cytochrome P450 (CYP) 3A4 inhibitors.
In the 6-week, placebo-controlled effectiveness studies, mean increases in QTc interval were in the range of 5 to 8 milliseconds. There were sporadic reports of QTcF values ≥500 milliseconds, or changes from baseline QTc values ≥60 milliseconds in pimavanserin-treated participants, although the incidence generally was the same for pimavanserin and placebo groups. There were no reports of torsades de pointes or any differences from placebo in the incidence of adverse reactions associated with delayed ventricular repolarization.
How it works
The theory behind development of pimavanserin rests in the finding that low-dosage clozapine (6.25 to 50 mg/d) was effective for PD patients with psychosis (effect size 0.80).8 Although clozapine has high affinity for multiple sites, including histamine H1 receptors (Ki = 1.13 nM), α-1A and a α-2C adrenergic receptors (Ki = 1.62 nM and 6 nM, respectively), 5-HT2A receptors (Ki = 5.35 nM), and muscarinic M1 receptors (Ki = 6 nM), the hypothesized primary mechanism of clozapine’s effectiveness for PD psychosis at low dosages focused on the 5-HT2Areceptor. This idea was based on the knowledge that hallucinogens such as mescaline, psilocybin, and LSD are 5-HT2A agonists.9 This hallucinogenic activity can be blocked with 5-HT2A antagonists. Because of pimavanserin’s binding profile, the compound was studied as a treatment for psychosis in PD patients.
Pharmacokinetics
Pimavanserin demonstrates dose-proportional pharmacokinetics after a single oral dose as much as 7.5 times the recommended dosage. The pharmacokinetics of pimavanserin were similar in study participants (mean age, 72.4) and healthy controls, and a high-fat meal had no impact on the maximum blood levels (Cmax) or total drug exposure (area under the curve [AUC]).
The mean plasma half-lives for pimavanserin and its metabolite N-desmethyl-pimavanserin (AC-279) are 57 hours and 200 hours, respectively. Although the metabolite appears active in in vitro assays, it does not cross the blood-brain barrier to any appreciable extent, therefore contributing little to the clinical effect. The median time to maximum concentration (Tmax) of pimavanserin is 6 hours with a range of 4 to 24 hours, while the median Tmax of the primary metabolite AC-279 is 6 hours. The bioavailability of pimavanserin in an oral tablet or solution essentially is identical.
Pimavanserin is primarily metabolized via CYP3A4 to AC-279, and strong CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin, indinavir) increase pimavanserin Cmax by 1.5-fold, and AUC by 3-fold. In patients taking strong CYP3A4 inhibitors, the dosage of pimavanserin should be reduced by 50% to 17 mg/d. Conversely, patients on CYP3A4 inducers (eg, rifampin, carbamazepine, phenytoin) should be monitored for lack of efficacy; consider a dosage increase as necessary. Neither pimavanserin nor its metabolite, AC-279, are inhibitors or inducers of major CYP enzymes or drug transporters.
Efficacy in PD psychosis
Study 1. This 6-week, fixed dosage, double-blind, placebo-controlled trial was performed in adult PD patients age ≥40 with PD psychosis.2 Participants had to have (1) a PD diagnosis for at least 1 year and (2) psychotic symptoms that developed after diagnosis. Psychotic symptoms had to be present for at least 1 month, occurring at least weekly in the month before screening, and severe enough to warrant antipsychotic treatment. Baseline Mini-Mental State Examination score had to be ≥21 out of 30, with no evidence of delirium. Patients with dementia preceding or concurrent with the PD diagnosis were excluded. Antipsychotic treatments were not permitted during the trial.
After a 2-week nonpharmacotherapeutic lead-in phase that included a brief, daily psychosocial intervention by a caregiver, 199 patients who still met severity criteria were randomly allocated in a 1:1 manner to pimavanserin (34 mg of active drug, reported in the paper as 40 mg of pimavanserin tartrate) or matched placebo. Based on kinetic modeling and earlier clinical data, lower dosages (ie, 17 mg) were not explored, because they achieved only 50% of the steady state plasma levels thought to be required for efficacy.
The primary outcome was assessed by central, independent raters using the PD-adapted SAPS-PD. The efficacy analysis included 95 pimavanserin-treated individuals and 90 taking placebo. Baseline SAPS-PD scores were 14.7 ± 5.55 in the placebo group, and 15.9 ± 6.12 in the pimavanserin arm. Participants had a mean age of 72.4 and 94% white ethnicity across both cohorts; 42% of the placebo group and 33% of the pimavanserin group were female. Antipsychotic exposure in the 21 days prior to study entry were reported in 17% (n = 15) and 19% (n = 18) of the placebo and pimavanserin groups, respectively, with the most common agent being quetiapine (13 of 15, placebo, 16 of 18, pimavanserin). Approximately one-third of all participants were taking a cholinesterase inhibitor throughout the study.
Efficacy outcome. Pimavanserin was associated with a 5.79-point decrease in SAPS-PD scores compared with 2.73-point decrease for placebo (difference −3.06, 95% CI −4.91 to −1.20; P = .001). The effect size for this difference (Cohen’s d) was 0.50. The significant effect of pimavanserin vs placebo also was seen in separate analyses of the SAPS-PD subscore for hallucinations and delusions (effect size 0.50), and individually for hallucinations (effect size 0.45) and delusions (effect size 0.33). Separation from placebo appeared after the second week of pimavanserin treatment, and continued through the end of the study. There is unpublished data showing efficacy through week 10, and longer term, uncontrolled data consistent with sustained response. An exploratory analysis of caregiver burden demonstrated an effect size of 0.50.
Tolerability
The discontinuation rate because of adverse events for pimavanserin and placebo-treated patients was 10 patients in the pimavanserin group (4 due to psychotic symptoms within 10 days of starting the study drug) compared with 2 in the placebo group. There was no evidence of motor worsening in either group, demonstrated by the score on part II of the Unified Parkinson’s Disease Rating Scale (UPDRS) that captures self-reported activities of daily living, or on UPDRS part III (motor examination). Pimavanserin has no contraindications.
Unique clinical issues
Binding properties. Pimavanserin possesses potent 5-HT2A inverse agonist properties required to manage psychosis in PD patients, but lacks clozapine’s affinities for α-1 adrenergic, muscarinic, or histaminergic receptors that contribute to clozapine’s poor tolerability. Moreover, pimavanserin has no appreciable affinity for dopaminergic receptors, and therefore does not induce motor adverse effects.
Clozapine aside, all available atypical antipsychotics have proved ineffective for psychosis in PD patients, and most caused significant motor worsening.3 Although quetiapine does not cause motor effects, it has been shown to be ineffective for psychosis in PD patients in multiple trials.4
The effect size for clozapine response is large (0.80) in PD patients with psychosis, but tolerability issues and administrative burdens regarding patient and prescriber registration and routine hematological monitoring pose significant clinical barriers. Clozapine also lacks an FDA indication for this purpose, which may pose a hurdle to its use in certain treatment settings.
Why Rx? The reasons to prescribe pimavanserin for PD patients with psychosis likely include:
- absence of tolerability issues seen with the only other effective agent, clozapine
- lack of motor effects
- lack of administrative and monitoring burden related to clozapine prescribing
- only agent with FDA approval for hallucinations and delusions in PD patients with psychosis.
Dosing
The recommended dosage of pimavanserin is 34 mg/d administered as a single dose with or without food. There is no need for titration, and none was performed in the pivotal clinical trial. Given the long half-life (57 hours), steady state is not achieved until day 12, therefore initiation with a lower dosage might prolong the time to efficacy. There is no dosage adjustment required in patients with mild or moderate renal impairment, but pimavanserin treatment is not recommended in patients with severe renal impairment. Pimavanserin has not been evaluated in patients with hepatic impairment (using Child-Pugh criteria), and is not recommended for these patients.
Other key aspects of dosing to keep in mind.
- Because pimavanserin is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a strong CYP3A4 inhibitor; the recommended dosage is 17 mg/d when administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of pimavanserin with CYP3A4 inducers, patients should be monitored for lack of efficacy during concomitant use with a CYP3A4 inducer, and consideration given to a dosage increase.
Use in pregnancy and lactation. There are no data on the use of pimavanserin in pregnant women, but no developmental effects were seen when the drug was administered orally at 10 or 12 times the maximum recommended human dosage to rats or rabbits during organogenesis. Pimavanserin was not teratogenic in pregnant rats and rabbits. There is no information regarding the presence of pimavanserin in human breast milk.
Geriatric patients. No dosage adjustment is required for older patients. The study population in the pivotal trial was mean age 72.4 years.
Summing up
Before development of pimavanserin, clozapine was the only effective treatment for psychosis in PD patients. Despite clozapine’s robust effects across several trials, patients often were given ineffective medications, such as quetiapine, because of the administrative and tolerability barriers posed by clozapine use. Because psychosis is the most common cause of nursing home placement in non-demented PD patients, an agent with demonstrated efficacy and without the adverse effect profile of clozapine or monitoring requirements represents an enormous advance in the treatment of psychosis in PD patients.
1. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; 2016.
2. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. [Erratum in Lancet. 2014;384(9937):28]. Lancet. 2014;383(9916):533-540.
3. Borek LL, Friedman JH. Treating psychosis in movement disorder patients: a review. Expert Opin Pharmacother. 2014;15(11):1553-1564.
4. Desmarais P, Massoud F, Filion J, et al. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol. 2016;29(4):227-236.
5. Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67(4):416-421.
6. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061-1068.
7. Nordström AL, Farde L, Nyberg S, et al. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152(10):1444-1449.
8. Hacksell U, Burstein ES, McFarland K, et al. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res. 2014;39(10):2008-2017.
9. Moreno JL, Holloway T, Albizu L, et al. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493(3):76-79.
1. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; 2016.
2. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. [Erratum in Lancet. 2014;384(9937):28]. Lancet. 2014;383(9916):533-540.
3. Borek LL, Friedman JH. Treating psychosis in movement disorder patients: a review. Expert Opin Pharmacother. 2014;15(11):1553-1564.
4. Desmarais P, Massoud F, Filion J, et al. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol. 2016;29(4):227-236.
5. Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67(4):416-421.
6. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061-1068.
7. Nordström AL, Farde L, Nyberg S, et al. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152(10):1444-1449.
8. Hacksell U, Burstein ES, McFarland K, et al. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res. 2014;39(10):2008-2017.
9. Moreno JL, Holloway T, Albizu L, et al. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493(3):76-79.
Online dating and personal information: Pause before you post
Most adults want to have happy romantic relationships. But meeting eligible companions and finding the time to date can feel nearly impossible to many physicians, especially residents, whose 80-hour work weeks limit opportunities to meet potential partners.1
So it’s no surprise that Dr. R’s friends have suggested that she try online dating. If she does, she would be far from alone: 15% of U.S. adults have sought relationships online, and one-fourth of people in their 20s have used a mobile dating app.2,3 Online dating might work well for Dr. R, too. Between 2005 and 2012, more than one-third of U.S. marriages started online, and these marriages seemed happier and ended in separation or divorce less often than marriages that started in more traditional ways.4
Online dating is just one example of how “the permeation of online and social media into everyday life is placing doctors in new situations that they find difficult to navigate.”5 Many physicians—psychiatrists among them—date online. Yet, like Dr. R, physicians are cautious about using social media because of worries about public exposure and legal concerns.5 Moreover, medical associations haven’t developed guidelines that would help physicians reconcile their professional and personal lives if they seek companionship online.6
Although we don’t have complete answers to Dr. R’s questions, we have gathered some ideas and information that she might find helpful. Read on as we explore:
- potential benefits for psychiatrists who try online dating
- problems when physicians use social media
- how to minimize mishaps if you seek companionship online.
Advantages and benefits
Online dating is most popular among young adults. But singles and divorcees of all ages, sexual orientations, and backgrounds are increasingly seeking long-term relationships with internet-based dating tools rather than hoping to meet people through family, friends, church, and the workplace. It has become common—and no longer stigmatizing—for couples to say they met online.2,7
A dating Web site or app is a simple, fast, low-investment way to increase your opportunities to meet other singles and to make contact with more potential partners than you would meet otherwise. This is particularly helpful for people in thinner dating markets (eg, gays, lesbians, middle-age heterosexuals, and rural dwellers) or people seeking a companion of a particular type or lifestyle.7,8 Many internet dating tools claim that their matching algorithms can increase your chances of meeting someone you will find compatible (although research questions whether the algorithms really work8). Dating sites and apps also let users engage in brief, computer-mediated communications that can foster greater attraction and comfort before meeting for a first date.8
Appeal to psychiatrists
Online dating may have special appeal to young psychiatrists such as Dr. R. Oddly enough, being a mental health professional can leave you socially isolated. Many people react cautiously when they learn you are a psychiatrist—they think you are evaluating them (and let’s face it: often, this is true).9 Psychiatrists should be cordial but circumspect in conducting work relationships, which limits the type and amount of social life they might generate in the setting where many people meet their future spouses.10
Online dating can help single psychiatrists overcome these barriers. Scientifically minded physicians can find plenty of research-grounded advice for improving online dating chances.11-14 Two medical researchers even published a meta-analysis of evidence-based methods that can improve the chances of converting online contacts to a first date.15
Caution: Hazards ahead
When seeking romance online, psychiatrists shouldn’t forget their professional obligations, including the duty to maintain clear boundaries between their social and work lives.16 If Dr. R decides to try online dating, she will be making it possible for curious patients to gain access to some of her personal information. She will have to figure out how to avoid jeopardizing her professional reputation or inadvertently opening the door to sexual misconduct.17
Boundaries online. Psychiatrists use the term “boundaries” to refer to how they structure appointments and monitor their behavior during therapy to keep the treatment relationship free of personal, sexual, and romantic influences. Keeping one’s emotional life out of treatment helps prevent exploitation of patients and fosters a sense of safety and assurance that the physician is acting solely with the patient’s interest in mind. Breaching boundaries in ways that exploit patients or serve the doctor’s needs can undermine treatment, harm patients, and result in serious professional consequences.18
Maintaining appropriate boundaries can be challenging for psychiatrists who want to date online because the outside-the-office context can muddy the distinction between one’s professional and personal identity. Online dating environments make it easier for physicians to inadvertently initiate social or romantic interactions with people they have treated but don’t recognize (something the authors know has happened to colleagues). Additionally, the internet’s anonymity leaves users vulnerable to being lured into interactions with someone who is using a fictional online persona—an activity colloquially called “catfishing.”19
Although patients may play an active role in boundary breaches, the physician bears sole responsibility for maintaining proper limits within the therapeutic relationship.18 For many psychiatrists, innocuous but non-professional interactions with patients have been the first steps down a “slippery slope” toward serious boundary violations, including sexual contact—an activity that both the American Medical Association (AMA) and the American Psychiatric Association deem categorically unethical and that can lead to malpractice lawsuits, sanctions by medical license boards, and (in some jurisdictions) criminal prosecution.20 When using social media and online dating tools, psychiatrists should avoid even seemingly minor boundary violations as a safeguard against more serious transgressions.20,21
Reports of online misconduct by medical trainees and practitioners are plentiful.22,23 In response, several medical organizations, including the AMA and the American College of Physicians, have developed professional guidelines for appropriate behavior on social media by physicians.24,25 These guidelines stress the importance of maintaining a professional presence when one’s online activity is publicly viewable.
How much self-disclosure is appropriate?
Traditionally, psychiatrists (including psychoanalysts) have felt that occasional, limited, well-considered references to oneself are acceptable and even helpful in treatment.26 The majority of therapists report using therapy-relevant self-disclosure, but they are cautious about what they say. Conscientious therapists avoid self-disclosure to satisfy their own needs, and they avoid self-disclosure with patients for whom it would have detrimental effects.18,27
Dating Web sites contain a lot of personal information that physicians don’t usually share with patients. Although physicians who use social media are advised to be careful about the information they make available to the public,28 this is more difficult to do with dating applications, where revealing some information about yourself is necessary for making meaningful connections. Creating an online dating profile means that you are potentially letting patients or patients’ relatives know about your place of residence, income, sexual orientation, number of children, and interests. You will need to think about how you will respond if a patient unexpectedly comments on your dating profile during a session or asks you out.
Beyond creating awkward situations, self-disclosure can have treatment implications, and it’s impossible to know how a particular comment will affect a particular client in a particular situation.29 Psychiatrists who engage in online dating may want to limit their posted personal information only to what they would feel reasonably comfortable with having patients know about them, and hope this will suffice to capture the attention of potential partners.
Sustaining professionalism while remaining human. The term “medical professionalism” originally referred to ethical conduct during the practice of medicine30 and to sustaining one’s commitment to patients, fellow professionals, and the institutions within which health care is provided.31 More recently, however, discussions of medical professionalism have encompassed how physicians comport themselves away from work. Physicians’ actions outside the office or hospital—and especially what they say, do, or post online—have a powerful effect on perceptions of their institutions and the medical profession as a whole.25,32
Photos and comments posted by physicians can be seen by millions and can have major repercussions for employment prospects and public perceptions.25 Questionable postings by physicians on social media outlets have resulted in disciplinary actions by licensing authorities and have damaged physicians’ careers.23
What seems appropriate for a dating Web site varies from person to person. A suggestive smile or flirtatious joke that most people would find harmless may strike others as provocative. Derogatory language, depictions of intoxication or substance abuse, and inappropriate patient-related comments are clear-cut mistakes.32-34 But also keep in mind that what medical professionals find acceptable to post on social networking sites does not always match what the general public thinks.35
Bottom Line
1. Miller JA. Romance in residency: is dating even possible? Medscape. http://www.medscape.com/viewarticle/844059. Published May 5, 2016. Accessed June 27, 2016.
2. Smith A, Anderson M. 5 facts about online dating. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/02/29/5-facts-about-online-dating. Published February 29, 2016. Accessed June 27, 2016.
3. Smith A. 15% of American adults have used online dating sites or mobile dating apps. http://www.pewinternet.org/2016/02/11/15-percent-of-american-adults-have-used-online-dating-sites-or-mobile-dating-apps/. Published February 11, 2016. Accessed June 27, 2016.
4. Cacioppo JT, Cacioppo S, Gonzaga GC, et al. Marital satisfaction and break-ups differ across on-line and off-line meeting venues. Proc Natl Acad Sci U S A. 2013;110(25):10135-10140.
5. Brown J, Ryan C, Harris A. How doctors view and use social media: a national survey. J Med Internet Res. 2014;16(12):e267.
6. Berlin R. The professional ethics of online dating: need for guidance. J Am Acad Child Adolesc Psychiatry. 2014;53(9):935-937.
7. Rosenfeld MJ, Thomas RJ. Searching for a mate: the rise of the Internet as a social intermediary. Am Sociol Rev. 2012;77(4):523-547.
8. Finkel EJ, Eastwick PW, Karney BR, et al. Online dating: a critical analysis from the perspective of psychological science. Psychol Sci Public Interest. 2012;13(1):3-66.
9. Pierre J. A mad world: a diagnosis of mental illness is more common than ever—did psychiatrists create the problem, or just recognise it? Aeon.co. https://aeon.co/essays/do-psychiatrists-really-think-that-everyone-is-crazy. Published March 19, 2014. Accessed June 28, 2016.
10. Pearce A, Gambrell D. This chart shows who marries CEOs, doctors, chefs and janitors. Bloomberg. http://www.bloomberg.com/graphics/2016-who-marries-whom. February 11, 2016. Accessed June 28, 2016.
11. Lowin R. Proofread that text before sending! Bad grammar is a dating deal breaker, most say. Today. http://www.today.com/health/can-your-awesome-grammar-really-get-you-date-according-new-t77376. Published March 2, 2016. Accessed June 28, 2016.
12. Reilly K. This strategy will make your Tinder game much stronger. Time. http://time.com/4263598/tinder-gif-messages-response-rate. Published March 17, 2016. Accessed June 28, 2016.
13. Wotipka CD, High AC. Providing a foundation for a satisfying relationship: a direct test of warranting versus selective self-presentation as predictors of attraction to online dating profiles. Presentation at the 101st Annual Meeting of the National Communication Association; November 20, 2014; Chicago, IL.
14. Vacharkulksemsuk T, Reit E, Khambatta P, et al. Dominant, open nonverbal displays are attractive at zero-acquaintance. Proc Natl Acad Sci U S A. 2016;113(15):4009-4014.
15. Khan KS, Chaudhry S. An evidence-based approach to an ancient pursuit: systematic review on converting online contact into a first date. Evid Based Med. 2015;20(2):48-56.
16. Chretien KC, Tuck MG. Online professionalism: a synthetic review. Int Rev Psychiatry. 2015;27(2):106-117.
17. Jackson WC. When patients are normal people: strategies for managing dual relationships. Prim Care Companion J Clin Psychiatry. 2002;4(3):100-103.
18. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
19. D’Costa K. Catfishing: the truth about deception online. ScientificAmerican.com. http://blogs.scientificamerican.com/anthropology-in-practice/catfishing-the-truth-about-deception-online. Published April 25, 2014. Accessed June 29, 2016.
20. Sarkar SP. Boundary violation and sexual exploitation in psychiatry and psychotherapy: a review. Adv Psychiatr Treat. 2004;10(4):312-320.
21. Nadelson C, Notman MT. Boundaries in the doctor-patient relationship. Theor Med Bioeth. 2002;23(3):191-201.
22. Walton JM, White J, Ross S. What’s on YOUR Facebook profile? Evaluation of an educational intervention to promote appropriate use of privacy settings by medical students on social networking sites. Med Educ Online. 2015;20:28708. doi: 10.3402/meo.v20.28708.
23. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
24. Decamp M. Physicians, social media, and conflict of interest. J Gen Intern Med. 2013;28(2):299-303.
25. Farnan JM, Snyder Sulmasy L, Worster BK, et al; American College of Physicians Ethics, Professionalism and Human Rights Committee; American College of Physicians Council of Associates; Federation of State Medical Boards Special Committee on Ethics and Professionalism. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-627.
26. Meissner WW. The problem of self-disclosure in psychoanalysis. J Am Psychoanal Assoc. 2002;50(3):827-867.
27. Henretty JR, Levitt HM. The role of therapist self-disclosure in psychotherapy: a qualitative review. Clin Psychol Rev. 2010;30(1):63-77.
28. Ponce BA, Determann JR, Boohaker HA, et al. Social networking profiles and professionalism issues in residency applicants: an original study-cohort study. J Surg Educ. 2013;70(4):502-507.
29. Peterson ZD. More than a mirror: the ethics of therapist self-disclosure. Psychotherapy: Theory Research & Practice. 2002;39(1):21-31.
30. Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002;287(2):226-235.
31. Wass V. Doctors in society: medical professionalism in a changing world. Clin Med (Lond). 2006;6(1):109-113.
32. Langenfeld SJ, Cook G, Sudbeck C, et al. An assessment of unprofessional behavior among surgical residents on Facebook: a warning of the dangers of social media. J Surg Educ. 2014;71(6):e28-e32.
33. Chauhan B, George R, Coffin J. Social media and you: what every physician needs to know. J Med Pract Manage. 2012;28(3):206-209.
34. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
35. Jain A, Petty EM, Jaber RM, et al. What is appropriate to post on social media? Ratings from students, faculty members and the public. Med Educ. 2014;48(2):157-169.
36. Gabbard GO, Roberts LW, Crisp-Han H, et al. Professionalism in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2012.
Most adults want to have happy romantic relationships. But meeting eligible companions and finding the time to date can feel nearly impossible to many physicians, especially residents, whose 80-hour work weeks limit opportunities to meet potential partners.1
So it’s no surprise that Dr. R’s friends have suggested that she try online dating. If she does, she would be far from alone: 15% of U.S. adults have sought relationships online, and one-fourth of people in their 20s have used a mobile dating app.2,3 Online dating might work well for Dr. R, too. Between 2005 and 2012, more than one-third of U.S. marriages started online, and these marriages seemed happier and ended in separation or divorce less often than marriages that started in more traditional ways.4
Online dating is just one example of how “the permeation of online and social media into everyday life is placing doctors in new situations that they find difficult to navigate.”5 Many physicians—psychiatrists among them—date online. Yet, like Dr. R, physicians are cautious about using social media because of worries about public exposure and legal concerns.5 Moreover, medical associations haven’t developed guidelines that would help physicians reconcile their professional and personal lives if they seek companionship online.6
Although we don’t have complete answers to Dr. R’s questions, we have gathered some ideas and information that she might find helpful. Read on as we explore:
- potential benefits for psychiatrists who try online dating
- problems when physicians use social media
- how to minimize mishaps if you seek companionship online.
Advantages and benefits
Online dating is most popular among young adults. But singles and divorcees of all ages, sexual orientations, and backgrounds are increasingly seeking long-term relationships with internet-based dating tools rather than hoping to meet people through family, friends, church, and the workplace. It has become common—and no longer stigmatizing—for couples to say they met online.2,7
A dating Web site or app is a simple, fast, low-investment way to increase your opportunities to meet other singles and to make contact with more potential partners than you would meet otherwise. This is particularly helpful for people in thinner dating markets (eg, gays, lesbians, middle-age heterosexuals, and rural dwellers) or people seeking a companion of a particular type or lifestyle.7,8 Many internet dating tools claim that their matching algorithms can increase your chances of meeting someone you will find compatible (although research questions whether the algorithms really work8). Dating sites and apps also let users engage in brief, computer-mediated communications that can foster greater attraction and comfort before meeting for a first date.8
Appeal to psychiatrists
Online dating may have special appeal to young psychiatrists such as Dr. R. Oddly enough, being a mental health professional can leave you socially isolated. Many people react cautiously when they learn you are a psychiatrist—they think you are evaluating them (and let’s face it: often, this is true).9 Psychiatrists should be cordial but circumspect in conducting work relationships, which limits the type and amount of social life they might generate in the setting where many people meet their future spouses.10
Online dating can help single psychiatrists overcome these barriers. Scientifically minded physicians can find plenty of research-grounded advice for improving online dating chances.11-14 Two medical researchers even published a meta-analysis of evidence-based methods that can improve the chances of converting online contacts to a first date.15
Caution: Hazards ahead
When seeking romance online, psychiatrists shouldn’t forget their professional obligations, including the duty to maintain clear boundaries between their social and work lives.16 If Dr. R decides to try online dating, she will be making it possible for curious patients to gain access to some of her personal information. She will have to figure out how to avoid jeopardizing her professional reputation or inadvertently opening the door to sexual misconduct.17
Boundaries online. Psychiatrists use the term “boundaries” to refer to how they structure appointments and monitor their behavior during therapy to keep the treatment relationship free of personal, sexual, and romantic influences. Keeping one’s emotional life out of treatment helps prevent exploitation of patients and fosters a sense of safety and assurance that the physician is acting solely with the patient’s interest in mind. Breaching boundaries in ways that exploit patients or serve the doctor’s needs can undermine treatment, harm patients, and result in serious professional consequences.18
Maintaining appropriate boundaries can be challenging for psychiatrists who want to date online because the outside-the-office context can muddy the distinction between one’s professional and personal identity. Online dating environments make it easier for physicians to inadvertently initiate social or romantic interactions with people they have treated but don’t recognize (something the authors know has happened to colleagues). Additionally, the internet’s anonymity leaves users vulnerable to being lured into interactions with someone who is using a fictional online persona—an activity colloquially called “catfishing.”19
Although patients may play an active role in boundary breaches, the physician bears sole responsibility for maintaining proper limits within the therapeutic relationship.18 For many psychiatrists, innocuous but non-professional interactions with patients have been the first steps down a “slippery slope” toward serious boundary violations, including sexual contact—an activity that both the American Medical Association (AMA) and the American Psychiatric Association deem categorically unethical and that can lead to malpractice lawsuits, sanctions by medical license boards, and (in some jurisdictions) criminal prosecution.20 When using social media and online dating tools, psychiatrists should avoid even seemingly minor boundary violations as a safeguard against more serious transgressions.20,21
Reports of online misconduct by medical trainees and practitioners are plentiful.22,23 In response, several medical organizations, including the AMA and the American College of Physicians, have developed professional guidelines for appropriate behavior on social media by physicians.24,25 These guidelines stress the importance of maintaining a professional presence when one’s online activity is publicly viewable.
How much self-disclosure is appropriate?
Traditionally, psychiatrists (including psychoanalysts) have felt that occasional, limited, well-considered references to oneself are acceptable and even helpful in treatment.26 The majority of therapists report using therapy-relevant self-disclosure, but they are cautious about what they say. Conscientious therapists avoid self-disclosure to satisfy their own needs, and they avoid self-disclosure with patients for whom it would have detrimental effects.18,27
Dating Web sites contain a lot of personal information that physicians don’t usually share with patients. Although physicians who use social media are advised to be careful about the information they make available to the public,28 this is more difficult to do with dating applications, where revealing some information about yourself is necessary for making meaningful connections. Creating an online dating profile means that you are potentially letting patients or patients’ relatives know about your place of residence, income, sexual orientation, number of children, and interests. You will need to think about how you will respond if a patient unexpectedly comments on your dating profile during a session or asks you out.
Beyond creating awkward situations, self-disclosure can have treatment implications, and it’s impossible to know how a particular comment will affect a particular client in a particular situation.29 Psychiatrists who engage in online dating may want to limit their posted personal information only to what they would feel reasonably comfortable with having patients know about them, and hope this will suffice to capture the attention of potential partners.
Sustaining professionalism while remaining human. The term “medical professionalism” originally referred to ethical conduct during the practice of medicine30 and to sustaining one’s commitment to patients, fellow professionals, and the institutions within which health care is provided.31 More recently, however, discussions of medical professionalism have encompassed how physicians comport themselves away from work. Physicians’ actions outside the office or hospital—and especially what they say, do, or post online—have a powerful effect on perceptions of their institutions and the medical profession as a whole.25,32
Photos and comments posted by physicians can be seen by millions and can have major repercussions for employment prospects and public perceptions.25 Questionable postings by physicians on social media outlets have resulted in disciplinary actions by licensing authorities and have damaged physicians’ careers.23
What seems appropriate for a dating Web site varies from person to person. A suggestive smile or flirtatious joke that most people would find harmless may strike others as provocative. Derogatory language, depictions of intoxication or substance abuse, and inappropriate patient-related comments are clear-cut mistakes.32-34 But also keep in mind that what medical professionals find acceptable to post on social networking sites does not always match what the general public thinks.35
Bottom Line
Most adults want to have happy romantic relationships. But meeting eligible companions and finding the time to date can feel nearly impossible to many physicians, especially residents, whose 80-hour work weeks limit opportunities to meet potential partners.1
So it’s no surprise that Dr. R’s friends have suggested that she try online dating. If she does, she would be far from alone: 15% of U.S. adults have sought relationships online, and one-fourth of people in their 20s have used a mobile dating app.2,3 Online dating might work well for Dr. R, too. Between 2005 and 2012, more than one-third of U.S. marriages started online, and these marriages seemed happier and ended in separation or divorce less often than marriages that started in more traditional ways.4
Online dating is just one example of how “the permeation of online and social media into everyday life is placing doctors in new situations that they find difficult to navigate.”5 Many physicians—psychiatrists among them—date online. Yet, like Dr. R, physicians are cautious about using social media because of worries about public exposure and legal concerns.5 Moreover, medical associations haven’t developed guidelines that would help physicians reconcile their professional and personal lives if they seek companionship online.6
Although we don’t have complete answers to Dr. R’s questions, we have gathered some ideas and information that she might find helpful. Read on as we explore:
- potential benefits for psychiatrists who try online dating
- problems when physicians use social media
- how to minimize mishaps if you seek companionship online.
Advantages and benefits
Online dating is most popular among young adults. But singles and divorcees of all ages, sexual orientations, and backgrounds are increasingly seeking long-term relationships with internet-based dating tools rather than hoping to meet people through family, friends, church, and the workplace. It has become common—and no longer stigmatizing—for couples to say they met online.2,7
A dating Web site or app is a simple, fast, low-investment way to increase your opportunities to meet other singles and to make contact with more potential partners than you would meet otherwise. This is particularly helpful for people in thinner dating markets (eg, gays, lesbians, middle-age heterosexuals, and rural dwellers) or people seeking a companion of a particular type or lifestyle.7,8 Many internet dating tools claim that their matching algorithms can increase your chances of meeting someone you will find compatible (although research questions whether the algorithms really work8). Dating sites and apps also let users engage in brief, computer-mediated communications that can foster greater attraction and comfort before meeting for a first date.8
Appeal to psychiatrists
Online dating may have special appeal to young psychiatrists such as Dr. R. Oddly enough, being a mental health professional can leave you socially isolated. Many people react cautiously when they learn you are a psychiatrist—they think you are evaluating them (and let’s face it: often, this is true).9 Psychiatrists should be cordial but circumspect in conducting work relationships, which limits the type and amount of social life they might generate in the setting where many people meet their future spouses.10
Online dating can help single psychiatrists overcome these barriers. Scientifically minded physicians can find plenty of research-grounded advice for improving online dating chances.11-14 Two medical researchers even published a meta-analysis of evidence-based methods that can improve the chances of converting online contacts to a first date.15
Caution: Hazards ahead
When seeking romance online, psychiatrists shouldn’t forget their professional obligations, including the duty to maintain clear boundaries between their social and work lives.16 If Dr. R decides to try online dating, she will be making it possible for curious patients to gain access to some of her personal information. She will have to figure out how to avoid jeopardizing her professional reputation or inadvertently opening the door to sexual misconduct.17
Boundaries online. Psychiatrists use the term “boundaries” to refer to how they structure appointments and monitor their behavior during therapy to keep the treatment relationship free of personal, sexual, and romantic influences. Keeping one’s emotional life out of treatment helps prevent exploitation of patients and fosters a sense of safety and assurance that the physician is acting solely with the patient’s interest in mind. Breaching boundaries in ways that exploit patients or serve the doctor’s needs can undermine treatment, harm patients, and result in serious professional consequences.18
Maintaining appropriate boundaries can be challenging for psychiatrists who want to date online because the outside-the-office context can muddy the distinction between one’s professional and personal identity. Online dating environments make it easier for physicians to inadvertently initiate social or romantic interactions with people they have treated but don’t recognize (something the authors know has happened to colleagues). Additionally, the internet’s anonymity leaves users vulnerable to being lured into interactions with someone who is using a fictional online persona—an activity colloquially called “catfishing.”19
Although patients may play an active role in boundary breaches, the physician bears sole responsibility for maintaining proper limits within the therapeutic relationship.18 For many psychiatrists, innocuous but non-professional interactions with patients have been the first steps down a “slippery slope” toward serious boundary violations, including sexual contact—an activity that both the American Medical Association (AMA) and the American Psychiatric Association deem categorically unethical and that can lead to malpractice lawsuits, sanctions by medical license boards, and (in some jurisdictions) criminal prosecution.20 When using social media and online dating tools, psychiatrists should avoid even seemingly minor boundary violations as a safeguard against more serious transgressions.20,21
Reports of online misconduct by medical trainees and practitioners are plentiful.22,23 In response, several medical organizations, including the AMA and the American College of Physicians, have developed professional guidelines for appropriate behavior on social media by physicians.24,25 These guidelines stress the importance of maintaining a professional presence when one’s online activity is publicly viewable.
How much self-disclosure is appropriate?
Traditionally, psychiatrists (including psychoanalysts) have felt that occasional, limited, well-considered references to oneself are acceptable and even helpful in treatment.26 The majority of therapists report using therapy-relevant self-disclosure, but they are cautious about what they say. Conscientious therapists avoid self-disclosure to satisfy their own needs, and they avoid self-disclosure with patients for whom it would have detrimental effects.18,27
Dating Web sites contain a lot of personal information that physicians don’t usually share with patients. Although physicians who use social media are advised to be careful about the information they make available to the public,28 this is more difficult to do with dating applications, where revealing some information about yourself is necessary for making meaningful connections. Creating an online dating profile means that you are potentially letting patients or patients’ relatives know about your place of residence, income, sexual orientation, number of children, and interests. You will need to think about how you will respond if a patient unexpectedly comments on your dating profile during a session or asks you out.
Beyond creating awkward situations, self-disclosure can have treatment implications, and it’s impossible to know how a particular comment will affect a particular client in a particular situation.29 Psychiatrists who engage in online dating may want to limit their posted personal information only to what they would feel reasonably comfortable with having patients know about them, and hope this will suffice to capture the attention of potential partners.
Sustaining professionalism while remaining human. The term “medical professionalism” originally referred to ethical conduct during the practice of medicine30 and to sustaining one’s commitment to patients, fellow professionals, and the institutions within which health care is provided.31 More recently, however, discussions of medical professionalism have encompassed how physicians comport themselves away from work. Physicians’ actions outside the office or hospital—and especially what they say, do, or post online—have a powerful effect on perceptions of their institutions and the medical profession as a whole.25,32
Photos and comments posted by physicians can be seen by millions and can have major repercussions for employment prospects and public perceptions.25 Questionable postings by physicians on social media outlets have resulted in disciplinary actions by licensing authorities and have damaged physicians’ careers.23
What seems appropriate for a dating Web site varies from person to person. A suggestive smile or flirtatious joke that most people would find harmless may strike others as provocative. Derogatory language, depictions of intoxication or substance abuse, and inappropriate patient-related comments are clear-cut mistakes.32-34 But also keep in mind that what medical professionals find acceptable to post on social networking sites does not always match what the general public thinks.35
Bottom Line
1. Miller JA. Romance in residency: is dating even possible? Medscape. http://www.medscape.com/viewarticle/844059. Published May 5, 2016. Accessed June 27, 2016.
2. Smith A, Anderson M. 5 facts about online dating. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/02/29/5-facts-about-online-dating. Published February 29, 2016. Accessed June 27, 2016.
3. Smith A. 15% of American adults have used online dating sites or mobile dating apps. http://www.pewinternet.org/2016/02/11/15-percent-of-american-adults-have-used-online-dating-sites-or-mobile-dating-apps/. Published February 11, 2016. Accessed June 27, 2016.
4. Cacioppo JT, Cacioppo S, Gonzaga GC, et al. Marital satisfaction and break-ups differ across on-line and off-line meeting venues. Proc Natl Acad Sci U S A. 2013;110(25):10135-10140.
5. Brown J, Ryan C, Harris A. How doctors view and use social media: a national survey. J Med Internet Res. 2014;16(12):e267.
6. Berlin R. The professional ethics of online dating: need for guidance. J Am Acad Child Adolesc Psychiatry. 2014;53(9):935-937.
7. Rosenfeld MJ, Thomas RJ. Searching for a mate: the rise of the Internet as a social intermediary. Am Sociol Rev. 2012;77(4):523-547.
8. Finkel EJ, Eastwick PW, Karney BR, et al. Online dating: a critical analysis from the perspective of psychological science. Psychol Sci Public Interest. 2012;13(1):3-66.
9. Pierre J. A mad world: a diagnosis of mental illness is more common than ever—did psychiatrists create the problem, or just recognise it? Aeon.co. https://aeon.co/essays/do-psychiatrists-really-think-that-everyone-is-crazy. Published March 19, 2014. Accessed June 28, 2016.
10. Pearce A, Gambrell D. This chart shows who marries CEOs, doctors, chefs and janitors. Bloomberg. http://www.bloomberg.com/graphics/2016-who-marries-whom. February 11, 2016. Accessed June 28, 2016.
11. Lowin R. Proofread that text before sending! Bad grammar is a dating deal breaker, most say. Today. http://www.today.com/health/can-your-awesome-grammar-really-get-you-date-according-new-t77376. Published March 2, 2016. Accessed June 28, 2016.
12. Reilly K. This strategy will make your Tinder game much stronger. Time. http://time.com/4263598/tinder-gif-messages-response-rate. Published March 17, 2016. Accessed June 28, 2016.
13. Wotipka CD, High AC. Providing a foundation for a satisfying relationship: a direct test of warranting versus selective self-presentation as predictors of attraction to online dating profiles. Presentation at the 101st Annual Meeting of the National Communication Association; November 20, 2014; Chicago, IL.
14. Vacharkulksemsuk T, Reit E, Khambatta P, et al. Dominant, open nonverbal displays are attractive at zero-acquaintance. Proc Natl Acad Sci U S A. 2016;113(15):4009-4014.
15. Khan KS, Chaudhry S. An evidence-based approach to an ancient pursuit: systematic review on converting online contact into a first date. Evid Based Med. 2015;20(2):48-56.
16. Chretien KC, Tuck MG. Online professionalism: a synthetic review. Int Rev Psychiatry. 2015;27(2):106-117.
17. Jackson WC. When patients are normal people: strategies for managing dual relationships. Prim Care Companion J Clin Psychiatry. 2002;4(3):100-103.
18. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
19. D’Costa K. Catfishing: the truth about deception online. ScientificAmerican.com. http://blogs.scientificamerican.com/anthropology-in-practice/catfishing-the-truth-about-deception-online. Published April 25, 2014. Accessed June 29, 2016.
20. Sarkar SP. Boundary violation and sexual exploitation in psychiatry and psychotherapy: a review. Adv Psychiatr Treat. 2004;10(4):312-320.
21. Nadelson C, Notman MT. Boundaries in the doctor-patient relationship. Theor Med Bioeth. 2002;23(3):191-201.
22. Walton JM, White J, Ross S. What’s on YOUR Facebook profile? Evaluation of an educational intervention to promote appropriate use of privacy settings by medical students on social networking sites. Med Educ Online. 2015;20:28708. doi: 10.3402/meo.v20.28708.
23. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
24. Decamp M. Physicians, social media, and conflict of interest. J Gen Intern Med. 2013;28(2):299-303.
25. Farnan JM, Snyder Sulmasy L, Worster BK, et al; American College of Physicians Ethics, Professionalism and Human Rights Committee; American College of Physicians Council of Associates; Federation of State Medical Boards Special Committee on Ethics and Professionalism. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-627.
26. Meissner WW. The problem of self-disclosure in psychoanalysis. J Am Psychoanal Assoc. 2002;50(3):827-867.
27. Henretty JR, Levitt HM. The role of therapist self-disclosure in psychotherapy: a qualitative review. Clin Psychol Rev. 2010;30(1):63-77.
28. Ponce BA, Determann JR, Boohaker HA, et al. Social networking profiles and professionalism issues in residency applicants: an original study-cohort study. J Surg Educ. 2013;70(4):502-507.
29. Peterson ZD. More than a mirror: the ethics of therapist self-disclosure. Psychotherapy: Theory Research & Practice. 2002;39(1):21-31.
30. Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002;287(2):226-235.
31. Wass V. Doctors in society: medical professionalism in a changing world. Clin Med (Lond). 2006;6(1):109-113.
32. Langenfeld SJ, Cook G, Sudbeck C, et al. An assessment of unprofessional behavior among surgical residents on Facebook: a warning of the dangers of social media. J Surg Educ. 2014;71(6):e28-e32.
33. Chauhan B, George R, Coffin J. Social media and you: what every physician needs to know. J Med Pract Manage. 2012;28(3):206-209.
34. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
35. Jain A, Petty EM, Jaber RM, et al. What is appropriate to post on social media? Ratings from students, faculty members and the public. Med Educ. 2014;48(2):157-169.
36. Gabbard GO, Roberts LW, Crisp-Han H, et al. Professionalism in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2012.
1. Miller JA. Romance in residency: is dating even possible? Medscape. http://www.medscape.com/viewarticle/844059. Published May 5, 2016. Accessed June 27, 2016.
2. Smith A, Anderson M. 5 facts about online dating. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/02/29/5-facts-about-online-dating. Published February 29, 2016. Accessed June 27, 2016.
3. Smith A. 15% of American adults have used online dating sites or mobile dating apps. http://www.pewinternet.org/2016/02/11/15-percent-of-american-adults-have-used-online-dating-sites-or-mobile-dating-apps/. Published February 11, 2016. Accessed June 27, 2016.
4. Cacioppo JT, Cacioppo S, Gonzaga GC, et al. Marital satisfaction and break-ups differ across on-line and off-line meeting venues. Proc Natl Acad Sci U S A. 2013;110(25):10135-10140.
5. Brown J, Ryan C, Harris A. How doctors view and use social media: a national survey. J Med Internet Res. 2014;16(12):e267.
6. Berlin R. The professional ethics of online dating: need for guidance. J Am Acad Child Adolesc Psychiatry. 2014;53(9):935-937.
7. Rosenfeld MJ, Thomas RJ. Searching for a mate: the rise of the Internet as a social intermediary. Am Sociol Rev. 2012;77(4):523-547.
8. Finkel EJ, Eastwick PW, Karney BR, et al. Online dating: a critical analysis from the perspective of psychological science. Psychol Sci Public Interest. 2012;13(1):3-66.
9. Pierre J. A mad world: a diagnosis of mental illness is more common than ever—did psychiatrists create the problem, or just recognise it? Aeon.co. https://aeon.co/essays/do-psychiatrists-really-think-that-everyone-is-crazy. Published March 19, 2014. Accessed June 28, 2016.
10. Pearce A, Gambrell D. This chart shows who marries CEOs, doctors, chefs and janitors. Bloomberg. http://www.bloomberg.com/graphics/2016-who-marries-whom. February 11, 2016. Accessed June 28, 2016.
11. Lowin R. Proofread that text before sending! Bad grammar is a dating deal breaker, most say. Today. http://www.today.com/health/can-your-awesome-grammar-really-get-you-date-according-new-t77376. Published March 2, 2016. Accessed June 28, 2016.
12. Reilly K. This strategy will make your Tinder game much stronger. Time. http://time.com/4263598/tinder-gif-messages-response-rate. Published March 17, 2016. Accessed June 28, 2016.
13. Wotipka CD, High AC. Providing a foundation for a satisfying relationship: a direct test of warranting versus selective self-presentation as predictors of attraction to online dating profiles. Presentation at the 101st Annual Meeting of the National Communication Association; November 20, 2014; Chicago, IL.
14. Vacharkulksemsuk T, Reit E, Khambatta P, et al. Dominant, open nonverbal displays are attractive at zero-acquaintance. Proc Natl Acad Sci U S A. 2016;113(15):4009-4014.
15. Khan KS, Chaudhry S. An evidence-based approach to an ancient pursuit: systematic review on converting online contact into a first date. Evid Based Med. 2015;20(2):48-56.
16. Chretien KC, Tuck MG. Online professionalism: a synthetic review. Int Rev Psychiatry. 2015;27(2):106-117.
17. Jackson WC. When patients are normal people: strategies for managing dual relationships. Prim Care Companion J Clin Psychiatry. 2002;4(3):100-103.
18. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
19. D’Costa K. Catfishing: the truth about deception online. ScientificAmerican.com. http://blogs.scientificamerican.com/anthropology-in-practice/catfishing-the-truth-about-deception-online. Published April 25, 2014. Accessed June 29, 2016.
20. Sarkar SP. Boundary violation and sexual exploitation in psychiatry and psychotherapy: a review. Adv Psychiatr Treat. 2004;10(4):312-320.
21. Nadelson C, Notman MT. Boundaries in the doctor-patient relationship. Theor Med Bioeth. 2002;23(3):191-201.
22. Walton JM, White J, Ross S. What’s on YOUR Facebook profile? Evaluation of an educational intervention to promote appropriate use of privacy settings by medical students on social networking sites. Med Educ Online. 2015;20:28708. doi: 10.3402/meo.v20.28708.
23. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
24. Decamp M. Physicians, social media, and conflict of interest. J Gen Intern Med. 2013;28(2):299-303.
25. Farnan JM, Snyder Sulmasy L, Worster BK, et al; American College of Physicians Ethics, Professionalism and Human Rights Committee; American College of Physicians Council of Associates; Federation of State Medical Boards Special Committee on Ethics and Professionalism. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-627.
26. Meissner WW. The problem of self-disclosure in psychoanalysis. J Am Psychoanal Assoc. 2002;50(3):827-867.
27. Henretty JR, Levitt HM. The role of therapist self-disclosure in psychotherapy: a qualitative review. Clin Psychol Rev. 2010;30(1):63-77.
28. Ponce BA, Determann JR, Boohaker HA, et al. Social networking profiles and professionalism issues in residency applicants: an original study-cohort study. J Surg Educ. 2013;70(4):502-507.
29. Peterson ZD. More than a mirror: the ethics of therapist self-disclosure. Psychotherapy: Theory Research & Practice. 2002;39(1):21-31.
30. Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002;287(2):226-235.
31. Wass V. Doctors in society: medical professionalism in a changing world. Clin Med (Lond). 2006;6(1):109-113.
32. Langenfeld SJ, Cook G, Sudbeck C, et al. An assessment of unprofessional behavior among surgical residents on Facebook: a warning of the dangers of social media. J Surg Educ. 2014;71(6):e28-e32.
33. Chauhan B, George R, Coffin J. Social media and you: what every physician needs to know. J Med Pract Manage. 2012;28(3):206-209.
34. Greysen SR, Chretien KC, Kind T, et al. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142.
35. Jain A, Petty EM, Jaber RM, et al. What is appropriate to post on social media? Ratings from students, faculty members and the public. Med Educ. 2014;48(2):157-169.
36. Gabbard GO, Roberts LW, Crisp-Han H, et al. Professionalism in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2012.
Where to find guidance on using pharmacogenomics in psychiatric practice
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
Running for Rare Team Raises Funds for NORD Program for Undiagnosed Patients
NORD Announces 2016 Grant Opportunities
Register Now for the NORD Summit!
Collagen Meniscus Implant
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Setting Up Your New Physician for Success
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Surgical Pearls in Total Knee Arthroplasty: A Lifetime of Lessons Learned
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
Medical Issues in American Football: Eyes, Teeth, and Skin
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.